Abstract
COVID-19 is a recent pandemic that is still a major health problem of modern times and already more than 17.5 lakhs people succumbed to this deadly disease. This disease is caused by novel coronavirus which is named SARS-COV-2 by the International Committee on Taxonomy of Viruses. This virus originated from Wuhan city in Hubei province of China in December 2019 and within a short period spread across the many countries in the globe. There are a lot of basic as well as clinical research is going on to study the mode of transmission and the mechanism of action of SARS-COV-2 infection and its therapeutics. SARS-COV-2 is not only known to infect lungs, but it also infects other organs in the human body including the gastrointestinal (GI) tract, the liver, and the pancreas via the angiotensin-converting enzyme (ACE) 2, an important component of the renin-angiotensin system. In this short review, we are mainly discussing the mode of SARS-COV-2 transmission, physiological counterbalancing roles of ACE2 and ACE and the tissue patterns of ACE2 expression, and the overall effect of COVID19 on human gastrointestinal System. Therefore, this review sheds light on the possible mechanism of SARS-COV-2 infection in the GI system and its pathological symptoms raising a potential possibility of GI tract acting as a secondary site for SARS-CoV-2 tropism and infection. Finally, future studies to understand the fecal-oral transmission of the virus and the correlation of viral load and severity of GI symptoms are proposed to gain knowledge of the GI symptoms in COVID-19 to aid in early diagnosis and prognosis.
Keywords: SARS-COV-2, COVID-19, Virus, ACE2, Gastrointestinal system
The novel coronavirus (nCov) identified in 2019 belongs to a strain of human coronaviruses (CoVs) which include 229E, NL63, OC43, HKU1, Middle East respiratory syndrome (MERS-CoV), severe acute respiratory syndrome (SARS-CoV) [1]. Coronaviruses belong to the family Coronaviradae and further subdivided into alpha, beta, gamma, and delta genera coronaviruses. SARS-CoV, MERS-CoV, and SARS-CoV-2 belong to the genus beta-coronaviruses [1]. The International Committee on Taxonomy of Viruses named nCoV as SARS-CoV-2 because it shares more than 80% identity in nucleotide with the original SARS-CoV, and the disease caused by SARS-CoV-2 as COVID-19 [2]. Of the seven strains, SARS-CoV, MERS-CoV, and SARS-CoV-2 have a zoonotic origin and proven to be highly pathogenic: SARS in 2003 and MERS in 2012 and COVID-19 in 2019 [3]. The SARS outbreak of 2002 caused by the SARS-CoV lasted until 2004. SARS was first identified in Foshan in the Guangdong province of China in November 2002. The major phase of this outbreak lasted for about 8 months until July 2003 when WHO declared that the virus has been contained. Cases were continued to be reported until May 2004 [4]. 8422 people were infected with the virus and 916 people died worldwide [5]. A total of 29 countries were affected. China had the greatest number of cases and deaths with 5329 and 336, respectively [6]. The Middle Eastern respiratory syndrome (MERS) outbreak of 2012 caused by MERS-CoV was first originated in Saudi Arabia was mostly contained after it had caused 2521 cases and 866 deaths [7]. Most of the cases of MERS were confined to the Arabian Peninsula. The first case of COVID-19 was originated in the Hubei Province in the city of Wuhan in China in late 2019 [8]. Since then, the virus has spread at a rapid rate with more than 79.72 million infections and 1,749,340 fatalities globally as of December 25, 2020.
Mechanism of transmission
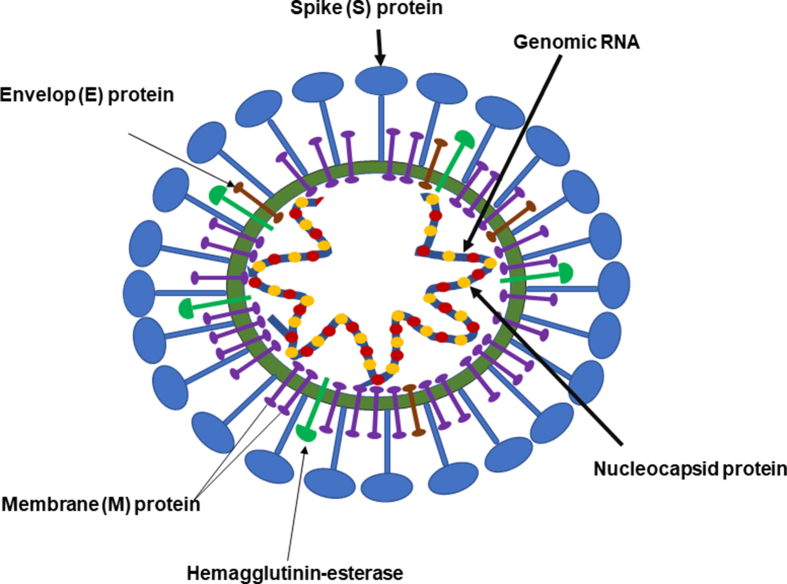
The genomics, epidemiology, and pathogenesis of SARS-CoV and SARS-Cov-2 are also similar [9]. In addition to the animal to human transmission, human to human transmission has been reported with SARS-CoV, MERS-CoV, and SARS-CoV-2. Akin to SARS-CoV, the transmission of SARS-CoV-2 is through droplet infection (respiratory secretions) and close person-to-person contact [[10], [11], [12]]. Transmission through asymptomatic cases appears to play a critical role in the transmission of SARS-CoV-2. The primary infection of coronaviruses involves the respiratory tract, and the infection causes the common cold, pneumonia, bronchiolitis, pharyngitis, sinusitis, and other symptoms such as diarrhea [13]. Coronaviruses (CoVs) are either pleomorphic or spherical and derive their name by the presence of characteristic club-shaped projections on the membrane envelope that contains a single-stranded RNA genome (size range between 26.2 and 31.7 kb, positive sense) that is packed in the nucleocapsid protein [14]. The RNA genome of CoV is the largest among all RNA viruses [15]. The genetic material of CoV is susceptible to frequent recombination processes resulting in new strains with altered virulence [16]. The spike (S) protein (trimeric), membrane (M) protein, envelop (E) protein, and the nucleocapsid (N) protein are the important structural proteins of SARS-CoV-2 [Fig. 1]. The surface S proteins play an important role in infecting the host cell [17,18].
Fig. 1.
Schematic representation of the SARS-CoV-2. The major structural proteins of SARS-CoV-2 consists of a spike (S), membrane (M), and envelop proteins that are embedded in the lipid bilayers and nucleic capsid (N) proteins covering single-stranded RNA. The spike proteins of the SARS-CoV-2 for which they derived the name ‘corona’ are the key structures that attach to host cell receptor proteins angiotensin-converting enzyme 2 (ACE2). The S proteins consist of S1 and S2 subunits that attach to the ACE2 and the cell membrane, respectively. The membrane envelops a large single-stranded positive-sense RNA.
It was established that the angiotensin-converting enzyme (ACE2) was the host receptor for the SARS-CoV-1 virus [19]. It was also found that the many viruses belonging to coronaviridae also used ACE2 as a host receptor. The spike (S) proteins containing an S1 subunit and S2 subunit on the envelope of coronaviruses promote the entry of the virus into the host cells [20]. Similar to SARS-CoV, the receptor-binding domain (RBD) within the S1 protein in SARS-CoV-2 binds to the ACE2, albeit with much higher affinity and this correlates with greater infection rates and efficient spread of SARS-CoV-2 among humans [21]. Unlike SARS-CoV, the S protein of SARS-CoV-2 has a site that is activated by host cell protease furin that is found in several tissues including the lung [22]. The entry of the virus causes internalization of ACE2 thus reducing the availability of enzyme for cleavage of Angiotensin II, the main physiological function of ACE2 [23]. Upon entry of the virus via ACE2, the viral RNA that is released intracellularly manipulates the cellular programs of the host for viral replication. Open reading frames of viral RNA are translated resulting in RNA polymerase complex that is responsible for replication and transcription of viral RNA. Viral nucleocapsids, after assembly and budding off from the lumen of ER-Golgi complex encase viral RNA to produce new virions and that undergo exocytosis [24].
Physiology of ACE and ACE2
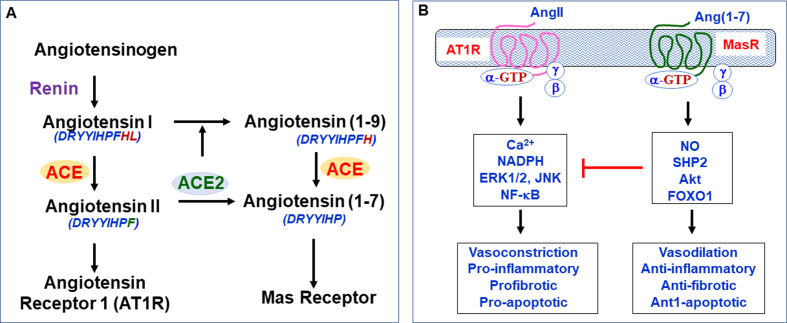
The Renin-Angiotensin-Aldosterone System (RAAS) plays a major in the control of blood pressure and electrolytes in the body [25]. Renin secreted from kidney converts angiotensinogen that is produced and secreted by the liver to angiotensin I (Ang I). Angiotensin-converting enzyme (ACE) converts Ang I to angiotensin II (Ang II) [26]. The major effects of Angiotensin II, acting mainly via its cognate AT1 receptor (AT1R), are vasoconstriction, reabsorption of sodium and excretion of potassium in the kidney, aldosterone synthesis, elevation of blood pressure and induction of pro-inflammatory pathways [27]. The octapeptide Ang II is cleaved by the peptidase activity of ACE2 to a heptapeptide called angiotensin (1–7) (Ang (1–7)) which has vasodilating, anti-inflammatory and anti-fibrotic effects acting mainly via its cognate Mas Receptor (MasR) [28]. There is also another pathway, which ACE2 participates where it converts Ang II to Ang (1–9) and later, under the action of ACE, it forms Ang (1–7), but this pathway is considered to be of less significance physiologically [29]. ACE2 is considered as a homolog of ACE, but they have opposite actions in the body, ACE2/Ang (1–7)/MasR pathway is considered anti-inflammatory and anti-fibrotic whereas ACE/AngII/AT1R pathway is pro-inflammatory and pro-fibrotic [Fig. 2]. The eventual function of RAAS activation depends on the balanced activities of ACE/AngII/AT1R and ACE2/Ang (−7)/MasR pathways. This balance is influenced by a lot of factors like the blockade of the RAAS using certain pharmacological drugs like ACE inhibitors for certain diseases. Studies have shown that increased dietary intake of sodium, fat, and fructose which are major risk factors for cardiovascular conditions distort the ACE/ACE2 balance and shift the curve more towards the ACE/Ang II/AT1R-mediated pro-inflammatory and pro-fibrotic effects [[30], [31], [32], [33]].
Fig. 2.
Simplified scheme of the renin-angiotensin system, and ACE/AngII/AT1R and ACE2/Ang (1–7)/MasR pathways. A. Renin derived from kidney converts angiotensin to decapeptide angiotensin I (Ang I). Angiotensin-converting enzyme (ACE) converts Ang I to octapeptide angiotensin II (Ang II). ACE2 converts Ang II to a heptapeptide Ang (1–7). ACE2 also acts on Ang I to form Ang (1–9) which then yields Ang (1–7) upon further cleavage by ACE. B. The effects of Ang II are mediated via activation of its cognate G protein-coupled receptor AT1 (AT1R), whereas the effects of Ang (1–7) are mediated via activation of its cognate G protein-coupled Mas receptor (MasR). The ACE2/Ang1-7/MasR pathway acts as a counter-regulatory pathway to the ACE/Ang II/AT1R pathway. The effects of ACE/Ang II/AT1R pathway involves an increase in Ca2+ and activation of NAD(P)H oxidase, extracellular regulated kinase1/2 (ERK1/2), Jun kinase (JNK) and the transcription factor NF-κB. The effects of the ACE2/Ang (1–7)/MasR pathway involves an increase in nitric oxide (NO) formation, and activation of Akt and the phosphatase SHP-2 and the transcription factor FOXO1.
ACE2 expression
Since ACE2 is the target protein of SARS-CoV-2, clinical symptoms of COVID-19 may be related to distribution and abundance of ACE2, which is ubiquitous in its expression in the body. The tissue distribution of ACE2 in the human body may also suggest potential infection routes and targets of SARS-CoV-2. ACE2 is predominantly expressed in the respiratory tract lining on the alveolar epithelial cells (type II alveolar cells) [34]. In the lungs, ACE2 acts as the entry point of the SARS-CoV-1 and SARS-CoV-2 viruses [29,35,36]. This makes a strong case for the respiratory tract as a route of transmission of SARS-CoV-2 virus and the GI tract being another possible route of transmission because it is also present on the small intestinal epithelial cells in the gastrointestinal tract (GI Tract) in the duodenum, jejunum, and ileum but not the colon [37,19,38]. In a study conducted by Hamming et al. it was found that ACE2 was present in endothelial cells from small and large arteries and veins in all the tissues studied in arterial smooth muscle cells [38]. ACE2 is also present in organs like the kidney, more so in the brush border of proximal tubular cells, and weakly in other areas like the epithelia in distal tubules and collecting ducts [38]. In the skin, ACE2 was present in the basal layer of the epidermis, and in the nasal and oral mucosa and nasopharynx, ACE2 was expressed in the basal layer of the epithelium [38]. GI manifestations are often associated with respiratory disorders in COVID-19 patients. Although the evidence for the involvement of the respiratory tract in the pathogenesis of COVID-19 is evident, the direct evidence for the involvement of GI in the pathogenesis is not clear. The GI manifestation could be due to the response of the immune system to viral infection. Recent studies using the rhesus monkey model demonstrated the relationship between GI dysfunction and lung infection induced by SARS-CoV-2. Intranasal inoculation with SARS-CoV-2 caused both lung infection and GI dysfunction that is associated with detectable viral RNA in the contents of swabs from nose, throat, and anus, and in the fecal samples. Intragastric inoculation with SARS-CoV-2 also caused both lung infection and GI dysfunction. These results indicate that both the GI tract and respiratory system play important roles in SARS-CoV-2 pathogenesis. The dysfunction of the GI tract with intranasal inoculation and dysfunction of the lung with intragastric inoculation could be due to the release of viruses from the infected tissue and/or via inflammatory cytokines. The bidirectional ‘gut–lung axis’ is also possible due to microbial metabolites and endotoxins resulting from the virus-induced changes in respiratory tract flora and the gut microbiome. Further studies are not needed to understand the variation in the GI symptoms, the relationship between severity of symptoms and route of infection, use of antibiotics and antiviral drugs on the gut microbiome and the role of microbiomes in the manifestation of GI dysfunction, the effect of COVID-19 on underlying chronic GI dysfunctions such as inflammatory bowel disorder, liver disorders, etc., and to define the importance of fecal testing for initial diagnosis and during discharge [39]. In the oral mucosa, ACE2 was highly expressed on the tongue and this could explain why COVID-19 patients experience altered taste sensation and a theory was suggested that it may be due to the binding of the virus to sialic acid receptors [40]. A hypothesis as to how COVID-19 causes an altered sense of smell was that it could directly damage the olfactory pathway [41]. It was present in the endothelial and smooth muscle cells in the brain and, ACE2 was absent in the spleen, thymus, lymph node, and the cells of the immune system [38].
ACE2 in GI tract and GI symptoms of COVID19
Abundant expression of ACE2 in the epithelial cells of the GI tract is also reported and the expression levels are higher than lung [[37], [38], [39]]. The role of ACE2 in amino acid transport underscores the importance of ACE2 in the GI tract [42]. The C-terminal domain of ACE2 is a homolog of renal transmembrane glycoprotein which plays a role in the regulation of neutral amino acids. It is discovered that ACE2 shares about nearly 50% of its domains with collectrin [43]. Since both collectrin and ACE2 are 50% similar, it is suggested that they might have a similar function as well. Later, it is discovered that ACE2 does indeed play an important role in the transport of amino acids and this had a profound impact on the gut microbiota [44]. These studies have shown that ACE2 knockout animals had reduced levels of neutral amino acids in the serum and tryptophan is more significantly reduced compared to the other neutral amino acids [44]. This lack of tryptophan leads to decreased expression of antimicrobial peptides which altered gut microbiota. The gut microbiota is successfully restored following the administration of tryptophan [44]. An important study recently hypothesized that coronary artery disease (CAD) patients with decreased tryptophan levels were found to have a reduced life expectancy which could mean that ACE2 in the gut has a cardioprotective impact [45].
Expression of ACE2 on the luminal epithelial cells of the GI tract suggests the possibility of a secondary site for enteric SARS-CoV-2 infection [39]. Gastrointestinal symptoms such as nausea, vomiting, and diarrhea are common with infection of SARS-CoV and MERS-CoV [46]. Similarly, although, SARS-CoV-2 infection is manifested by respiratory symptoms suggesting droplet transmission, gastrointestinal manifestations such as vomiting, abdominal pain, and diarrhea are also reported [47]. The GI symptoms seem to precede the typical respiratory symptoms of COVID-19. SARS-CoV-2 RNA is identified in anal/rectal swabs and stool specimens of COVID-19 patients, suggesting also the fecal-oral route of transmission [48]. Immunohistochemical studies showed viral nucleocapsid protein in the cytoplasm of gastric, duodenal, and rectal epithelial cells [49]. Interaction of SARS-CoV-2 with ACE2 in the GI tract may lead to damage to the barrier function via disrupting barrier proteins ZO-1, occludin, and claudins, and increase in inflammatory cytokine production, which in turn may lead to dysbiosis and exacerbation of intestinal inflammation [50,51]. Besides, intestinal inflammation may augment dysbiosis and damage to the intestinal mucosal barrier function, and the intestinal lymphocytes, dendritic cells, and macrophages may perpetuate the cytokine storm. In animal models, leaky gut can be mitigated or exacerbated with either the gain or loss of ACE2 expression [51]. Co-morbid conditions such as diabetes, obesity, and hypertension may have adverse effects on gut microbiome [52], and infection with SARS-CoV-2 and decrease in ACE2 function may worsen the gut microbial dysbiosis [44]. Augmentation of diabetes-induced dysbiosis by ACE2 deficiency was reported [53].
ACE2 in the liver and liver symptoms of COVID19
In the liver, ACE2 was mainly expressed on the cholangiocytes rather than the hepatocytes [54]. The classical ACE/AngII/AT1R pathway of RAAS contributes to the development of nonalcoholic fatty liver disease (NAFLD) [55,56]. The non-conventional ACE2/Ang (1–7)/MasR pathway of RAAS shows effects opposite to that of the classical axis. The loss of ACE2 seemed to worsen liver fibrosis in chronic liver injury models. Importantly, the consequences of loss of the ACE2 gene in the liver become apparent only after chronic injury [57]. In a study conducted by Cao et al. it was discovered that the deletion of ACE2 aggravates the development of hepatic steatosis, oxidative stress, and inflammation. It was reported that overexpression of ACE2 decreased the hepatic steatosis in db/db mice [57,58]. These studies suggest that ACE2 has a protective role against inflammation. These studies also raise the possibility that COVID-19 patients with co-morbid liver dysfunction, the decrease in ACE2 expression and function due to infection may lead to exacerbated liver dysfunction. Impairment in liver function has been reported in patients infected with both SARS-CoV and MERS-CoV [59]. Liver impairment was also noted in several COVID-19 patients. Biochemical markers were elevated in these patients which signified mild-moderate hepatic dysfunction [60,61]. Markers like liver function tests (AST, ALT, GGT, and ALP) were elevated along with hypoalbuminemia, prolonged prothrombin time, and increased CRP, LDH, and hyperferritinemia which may indicate acute inflammation [60]. Patients with severe COVID-19 seem to have higher rates of liver dysfunction [61]. The exact mechanism by which liver injury occurs in COVID-19 is not clear, but it could involve direct infection or secondary infection due to prior liver injury and/or drug-mediated hepatotoxicity. Future studies on direct infection of liver and secretion of the virus into the bile are necessary to reveal see its route of presence in the GI tract. Studies on the replication of the virus in the hepatocytes or cholangiocytes and liver organoids are also important.
ACE2 in the pancreas and pancreatic symptoms of COVID-19
A protective role for ACE2 was also reported in the pancreas. Wang et al. in a cell culture model of pancreatic damage showed that the non-conventional ACE2/Ang (1–7)/MasR pathway inhibits pancreatitis significantly by increasing the anti-inflammatory IL-10 cytokine and decreasing IL-6 and IL-8 which are pro-inflammatory [62]. Liu et al. showed that the expression of ACE2 was higher in the pancreas than lungs [63]. The expression of ACE2 was observed in both the exocrine glands and islets. These studies suggest that entry of SARS-CoV-2 into the pancreas may lead to β-cell dysfunction and glucose metabolism. Besides, in a subset of COVID-19 patients elevated levels of serum amylase and lipases were reported [64]. It was reported that patients who were positive for SARS-CoV-1 which caused SARS had elevated blood glucose levels [65,66] and this could be due to impairment of ACE2-mediated protective mechanism in energy metabolism. A study was done by Chhabra et al. where mice were infused with Ang II [67]. After 7 days of infusion with Ang II, it was seen that these mice had a significantly increased fasting blood glucose (FBG) compared to before the infusion of Ang II. And after 14 days, the effect of Ang II on FBG was more pronounced compared to after 7 days. The infusion of Ang II was shown to have significantly reduced the ACE2 expression in the islets and the pancreas. This shows that reduced ACE2 activity may adversely affect glycemic control. To test this relationship, on the 7th day of Ang II infusion, the mice were injected with intrapancreatic ACE2 and there was a significant improvement in glycemic control which does show that ACE2 does play an important role in glycemic control by improving insulin secretion and reversing the insulin resistance due to Ang II [67]. Future studies in COVID-19 patients involving imaging will provide insights into pancreatic inflammation in COVID-19 patients.
Conclusion
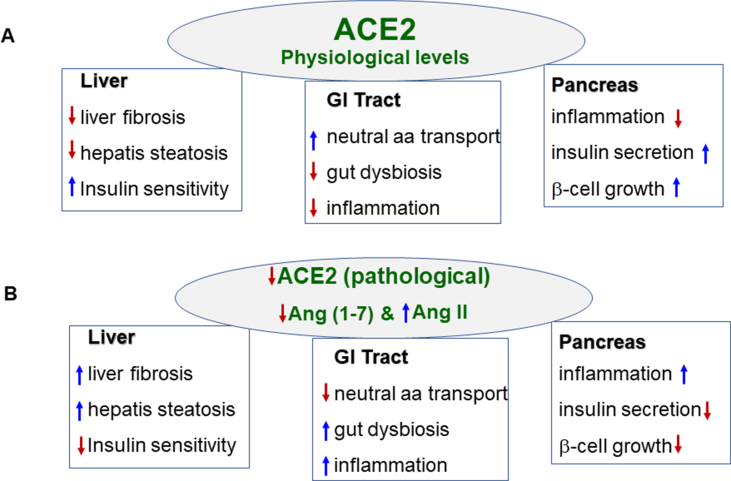
With the current COVID-19 pandemic taking center stage in the form of an unprecedented health crisis, we are learning new things about the SARS-CoV-2 daily. Although the infection with SARS-CoV-2 mostly affects the respiratory tract, the manifestation of gastrointestinal symptoms is common in many COVID-19 patients. Expression of SARS-CoV-2 receptor ACE2, presence of viral proteins in the cytoplasm, and presence of SARS-CoV-2 in the anal swabs and feces provided insights into the receptor-mediated entry into the host cells and basis for its possible transmission route through the fecal contents. The presence of ACE2 proteins in the GI tract, liver, and pancreas, the protective function of ACE2 in these organs against inflammation, the manifestation of GI symptoms in COVID-19 patients, and the possibility of possible fecal-oral transmission has important implications in understanding disease transmission, infection control, and disease management. Studies also suggest that patients with co-morbidity are likely to present severe symptoms. Imbalance in ACE/AngII/AT1R and ACE2/Ang (1–7)/MasR pathway to a predominant ACE/AngII/AT1R pathway due to the binding of SARS-CoV-2 to ACE2 resulting in attenuation of ACE2-mediated protective effects may augment morbidity and mortality in patients with co-morbid conditions associated with GI tract [Fig. 3]. Further studies on gut involvement and exit of SARS-CoV-2 in the feces and duration of virus viability in the environment are necessary to investigate the possibility of fecal-oral transmission of the virus, which is particularly relevant in regions of poor sanitation. Equally important are studies on the correlation between the amounts of fecal virus and the severity of GI symptoms. Establishing that GI symptoms precede respiratory symptoms in COVID-19 may improve early detection.
Fig. 3.
Schematic diagram summarizing the role of ACE2/ANG-(1–7) in GI systems in normal physiology and presumably its involvement in SARS-CoV-2 infection. A. Physiologically, ACE2/Ang (1–7) mediates neutral amino acid transport and facilitates the release of antimicrobial peptides to decreases gut dysbiosis and prevent inflammation in the gastrointestinal (GI) tract, promotes insulin sensitivity and decrease fibrosis and steatosis in the liver, and promotes β cell growth and insulin secretion and decreases inflammation in the pancreas. B. Decrease in ACE2 function during SARS-Cov-2 infection may lead to an increase in Ang II and a decrease in Ang (1-) levels resulting in a decrease in neutral amino acid transport and an increase in gut dysbiosis and inflammation in the GI tract, decrease in insulin sensitivity and an increase in fibrosis and steatosis in the liver, and an increase in inflammation and decrease in β cell growth resulting in insulin secretion in the pancreas. A decrease in ACE2 expression and function of ACE2/Ang (1–7) may play a key role in the pathogenesis of SARS-CoV-2. A decrease in ACE2 expression and activity and loss of protective effects of ACE2/Ang (1–7) due to co-morbid conditions such as old-age, diabetes, liver diseases (e.g., NAFLD) and pancreatic inflammation may exacerbate the symptoms associated with SARS-CoV-2 infection. Arrows indicate an increase or a decrease.
Conflicts of interest
Authors do not have any conflicts of interest.
Acknowledgments
The work was supported by the funding from the Department of Biotechnology, Government of India, in the forms Ramalingaswami Fellowship grant to Prasanna K. Santhekadur.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Li H., Liu S.M., Yu X.H., Tang S.L., Tang C.K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrob Agents. 2020;55:105951. doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye Z.W., Yuan S., Yuen K.S., Fung S.Y., Chan C.P., Jin D.Y. Zoonotic origins of human coronaviruses. Int J Biol Sci. 2020;16:1686–1697. doi: 10.7150/ijbs.45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu R.H., He J.F., Evans M.R., Peng G.W., Field H.E., Yu D.W. Epidemiologic clues to SARS origin in China. Emerg Infect Dis. 2004;10:1030–1037. doi: 10.3201/eid1006.030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan-Yeung M., Xu R.H. SARS: epidemiology. Respirology. 2003;8:S9–S14. doi: 10.1046/j.1440-1843.2003.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hung L.S. The SARS epidemic in Hong Kong: what lessons have we learned? J R Soc Med. 2003;96:374–378. doi: 10.1258/jrsm.96.8.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabutti G., d'Anchera E., Sandri F., Savio M., Stefanati A. Coronavirus: update related to the current outbreak of COVID-19. Infect Dis Ther. 2020;9:1–13. doi: 10.1007/s40121-020-00295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K. Covid-19 in critically ill patients in the seattle region - case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu J., Zhao S., Teng T., Abdalla A.E., Zhu W., Xie L. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12:244. doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Health Protection Agency (HPA) UK Novel Coronavirus Investigation team Evidence of person-to-person transmission within a family cluster of novel coronavirus infections, United Kingdom, February 2013. Euro Surveill. 2013;18:20427. doi: 10.2807/ese.18.11.20427-en. [DOI] [PubMed] [Google Scholar]
- 11.Li C., Ji F., Wang L., Wang L., Hao J., Dai M. Asymptomatic and human-to-human transmission of SARS-CoV-2 in a 2-family cluster, xuzhou, China. Emerg Infect Dis. 2020;26:1626–1628. doi: 10.3201/eid2607.200718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yadav T., Saxena S.K. Transmission cycle of SARS-CoV and SARS-CoV-2. Coronavirus Disease 2019 (COVID-19) 2020; Aprl:33–42. [Google Scholar]
- 13.Raoult D., Zumla A., Locatelli F., Ippolito G., Kroemer G. Coronavirus infections: epidemiological, clinical, and immunological features and hypotheses. Cell Stress. 2020;4:66–75. doi: 10.15698/cst2020.04.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai M.M., Cavanagh D. The molecular biology of coronaviruses. Adv Virus Res. 1997;48:1–100. doi: 10.1016/S0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham R.L., Baric R.S. Recombination, reservoirs, and the modular spike: mechanisms of coronavirus cross-species transmission. J Virol. 2010;84:3134–3146. doi: 10.1128/JVI.01394-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snijder E.J., Bredenbeek P.J., Dobbe J.C., Thiel V., Ziebuhr J., Poon L.L. Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. J Mol Biol. 2003;331:991–1004. doi: 10.1016/S0022-2836(03)00865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiel V., Ivanov K.A., Putics A., Hertzig T., Schelle B., Bayer S. Mechanisms and enzymes involved in SARS coronavirus genome expression. J Gen Virol. 2003;84:2305–2315. doi: 10.1099/vir.0.19424-0. [DOI] [PubMed] [Google Scholar]
- 19.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J. Angiotensin-converting enzyme-2 (ACE2), SARS-CoV-2 and pathophysiology of coronavirus disease 2019 (COVID-19) J Pathol. 2020;251:228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Follis K.E., York J., Nunberg J.H. Furin cleavage of the SARS coronavirus spike glycoprotein enhances cell-cell fusion but does not affect virion entry. Virology. 2006;350:358–369. doi: 10.1016/j.virol.2006.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.South A.M., Diz D.I., Chappell M.C. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318:H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Astuti I., Ysrafil Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes Metab Syndr. 2020;14:407–412. doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muñoz-Durango N., Fuentes C.A., Castillo A.E., González-Gómez L.M., Vecchiola A., Fardella C.E. Role of the renin-angiotensin-aldosterone system beyond blood pressure regulation: molecular and cellular mechanisms involved in end-organ damage during arterial hypertension. Int J Mol Sci. 2016;17:797. doi: 10.3390/ijms17070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voors A.A., Pinto Y.M., Buikema H., Urata H., Oosterga M., Rooks G. Dual pathway for angiotensin II formation in human internal mammary arteries. Br J Pharmacol. 1998;125:1028–1032. doi: 10.1038/sj.bjp.0702150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benigni A., Cassis P., Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology, and aging. EMBO Mol Med. 2010;2:247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchis G.F., Lavie C.J., Quilis C.P., Henry B.M., Lippi G. Angiotensin-converting enzyme 2 and antihypertensives (angiotensin receptor blockers and angiotensin-converting enzyme inhibitors) in coronavirus disease 2019. Mayo Clin Proc. 2020;95:1222–1230. doi: 10.1016/j.mayocp.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamming I., Cooper M.E., Haagmans B.L., Hooper N.M., Korstanje R., Osterhaus A.D.M.E. The emerging role of ACE2 in physiology and disease. J Pathol. 2007;212:1–11. doi: 10.1002/path.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernardi S., Toffoli B., Zennaro C., Tikellis C., Monticone S., Losurdo P. High-salt diet increases glomerular ACE/ACE2 ratio leading to oxidative stress and kidney damage. Nephrol Dial Transplant. 2012;27:1793–1800. doi: 10.1093/ndt/gfr600. [DOI] [PubMed] [Google Scholar]
- 31.Gupte M., Boustany Kari C.M., Bharadwaj K., Police S., Thatcher S., Gong M.C. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2008;295:R781–R788. doi: 10.1152/ajpregu.00183.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diázcouder A.H., Nava R.R., Carbó R., Lozada L.G.S., Muñoz F.S. High fructose intake and adipogenesis. Int J Mol Sci. 2019;20:E2787. doi: 10.3390/ijms20112787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamming I., van Goor H., Turner A.J., Rushworth C.A., Michaud A.A., Corvol P. Differential regulation of renal angiotensin converting enzyme (ACE) and ACE2 during ACE inhibition and dietary sodium restriction in healthy rats. Exp Physiol. 2008;93:631–638. doi: 10.1113/expphysiol.2007.041855. [DOI] [PubMed] [Google Scholar]
- 34.Mossel E.C., Wang J., Jeffers S., Edeen K.E., Wang S., Cosgrove G.P. SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells. Virology. 2008;372:127–135. doi: 10.1016/j.virol.2007.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ge X.Y., Li J.L., Yang X.L., Chmura A.A., Zhu G., Epstein J.H. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A. Angiotensin converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cipriano M., Ruberti E., Giacalone A. Gastrointestinal infection could Be new focus for coronavirus diagnosis. Cureus. 2020;12 doi: 10.7759/cureus.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamming I., Timens W., Bulthuis M., Lely A., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiao L., Li H., Xu J., Yang M., Ma C., Li J. The gastrointestinal tract is an alternative route for SARS-CoV-2 infection in a nonhuman primate model. Gastroenterology. 2021;160:1467–1469. doi: 10.1053/j.gastro.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tortorici M.A., Walls A.C., Lang Y., Wang C., Li Z., Koerhuis D. Structural basis for human coronavirus attachment to sialic acid receptors. Nat Struct Mol Biol. 2019;26:481–489. doi: 10.1038/s41594-019-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaira L.A., Salzano G., Fois A.G., Piombino P., De Riu G. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. Int Forum Allergy Rhinol. 2020;10:1103–1104. doi: 10.1002/alr.22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singer D., Camargo S.M. Collectrin and ACE2 in renal and intestinal amino acid transport. Channels. 2011;5:410–423. doi: 10.4161/chan.5.5.16470. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H., Wada J., Hida K., Tsuchiyama Y., Hiragushi K., Shikata K. Collectrin, a collecting duct-specific transmembrane glycoprotein, is a novel homolog of ACE2 and is developmentally regulated in embryonic kidneys. J Biol Chem. 2001;276:17132–17139. doi: 10.1074/jbc.M006723200. [DOI] [PubMed] [Google Scholar]
- 44.Hashimoto T., Perlot T., Rehman A., Trichereau J., Ishiguro H., Paolino M. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murr C., Grammer T.B., Kleber M.E., Meinitzer A., Marz W., Fuchs D. Low serum tryptophan predicts higher mortality in cardiovascular disease. Eur J Clin Invest. 2015;45:247–254. doi: 10.1111/eci.12402. [DOI] [PubMed] [Google Scholar]
- 46.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS, and MERS: are they closely related? Clin Microbiol Infect. 2020;26:729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian Y., Rong L., Nian W., He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y., Chen L., Deng Q., Zhang G., Wu K., Ni L. The presence of SARS-CoV-2 RNA in the faeces of COVID-19 patients. J Med Virol. 2020;92:833–840. doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- 49.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen L., Li L., Han Y., Lv B., Zou S., Yu Q. Tong-fu-li-fei decoction exerts a protective effect on intestinal barrier of sepsis in rats through upregulating ZO-1/occludin/claudin-1 expression. J Pharmacol Sci. 2020;143:89–96. doi: 10.1016/j.jphs.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Fernández Blanco J.A., Estévez J., Shea Donohue T., Martínez V., Vergara P. Changes in epithelial barrier function in response to parasitic infection: implications for IBD pathogenesis. J Crohn’s Colitis. 2015;9:463–476. doi: 10.1093/ecco-jcc/jjv056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vandana U.K., Barlaskar N.H., Gulzar A.B.M., Laskar I.H., Kumar D., Paul P. Linking gut microbiota with the human diseases. Bioinformation. 2020;16:196–208. doi: 10.6026/97320630016196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duan Y., Prasad R., Feng D., Beli E., Calzi S.L., Longhini A.L.F. Bone marrow-derived cells restore functional integrity of the gut epithelial and vascular barriers in a model of diabetes and ACE2 deficiency. Circ Res. 2019;125:969–988. doi: 10.1161/CIRCRESAHA.119.315743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu Y.Z., Zhang X., Wang L., Zhang F., Qiu Q., Liu M.L. An increased circulating angiotensin II concentration is associated with hypoadiponectinemia and postprandial hyperglycaemia in men with Nonalcoholic fatty liver disease. Intern Med. 2013;52:855–861. doi: 10.2169/internalmedicine.52.8839. [DOI] [PubMed] [Google Scholar]
- 56.Wei Y., Clark S.E., Morris E.M., Thyfault J.P., Uptergrove G.M.E., Connell A.T.W. Angiotensin II-induced non-alcoholic fatty liver disease is mediated by oxidative stress in transgenic TG (mRen2)27(Ren2) rats. J Hepatol. 2008;49:417–428. doi: 10.1016/j.jhep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Osterreicher C.H., Taura K., De Minicis S., Seki E., Osterreicher M.P., Kodama Y. Angiotensin-converting-enzyme 2 inhibits liver fibrosis in mice. Hepatology. 2009;50:929–938. doi: 10.1002/hep.23104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao X., Yang F., Shi T., Yuan M., Xin Z., Xie R. Angiotensin-converting enzyme 2/angiotensin-(1–7)/Mas axis activates Akt signalling to ameliorate hepatic steatosis. Sci Rep. 2016;6:21592. doi: 10.1038/srep21592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu L., Liu J., Lu M., Yang D., Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan Z., Chen L., Li J., Tian C., Zhang Y., Huang S. Clinical features of COVID-19 related liver damage. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang F., Wang H., Fan J., Zhang Y., Wang H., Zhao Q. Pancreatic injury patterns in patients with coronavirus disease 19 pneumonia. Gastroenterology. 2020;159:367–370. doi: 10.1053/j.gastro.2020.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu F., Long X., Zhang B., Zhang W., Chen X., Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2. Clin Gastroenterol Hepatol. 2020;18:2128–2130. doi: 10.1016/j.cgh.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mukherjee R., Smith A., Sutton R. Covid-19-related pancreatic injury. Br J Surg. 2020;107 doi: 10.1002/bjs.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang J.K., Feng Y., Yuan M.Y., Yuan S.Y., Fu H.J., Wu B.Y. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23:623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 66.Yang J.K., Lin S.S., Ji X.J., Guo L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chhabra K.H., Xia H., Pedersen K.B., Speth R.C., Lazartigues E. Pancreatic angiotensin converting enzyme 2 improves glycemia in angiotensin II-infused mice. Am J Physiol Endocrinol Metab. 2013;304:E874–E884. doi: 10.1152/ajpendo.00490.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]