Abstract
Forty-three (n = 43) endophytic fungi with different morphologic characteristics were from a medicinal plant Sceletium tortuosum, were utilized to investigate their antifungal effectiveness against pathogenic fungi. All fungal isolates exhibited antifungal activity against one or more pathogens in the dual culture test whereas only 33 fungal culture filtrates (77%) showed decent antifungal effect. Fusaria and Aspergillus were the dominate genus that displayed significant antifungal activity. Isolates GG02, GG09, ND15, and ND17 showed the broadest spectrum of antifungal activity. Furthermore, culture filtrate of Fusarium sp. DR08 exhibited a broad range of antifungal activity against all the pathogens. The results suggest endophytic fungi isolated from medicinal plant might be a source of novel bioactive molecules. To the best our knowledge, this is the first report on endophytic fungi isolated from native kougoed exhibiting antifungal activity against plant fungal pathogens.
Keywords: Antifungal, fungal endophytes, fungal pathogens, fungicide, Sceletium tortuosum
Novel biotechnological approaches that focuses on the utilization of bioactive compounds isolated from endophytes for a broad spectrum of biological potentials, including antibiotics, antiviral, immunosuppressant, antiparasitic, and antifungal activities, have sparked tremendous interest in recent research [1]. Historically, ground breaking bioactive compounds isolated from endophytic fungi have been commercialized as pharmaceutical drugs from penicillin, camptothecin, podophyllotoxin to taxol [2]. Endophytic fungi live inside a plant tissue with no harmful symptoms observed. They are able to protect the plant from pathogens, to enhance their growth, and to avoid from herbivores [3]. It has been reported by various studies that plants colonized by endophytic fungi represent an essential source of fungal diversity and novel species [4].
The mutual co-exist in both the healing and unhealthy tissue highlights the uncertainty of boundaries in differentiating facultative pathogens, endophytes and latent pathogens. Fungi has a pathogenic nature that lead to plant decay and economic loss. A fungal outbreak can lead to a human disaster when it destroy the staple crops.
The survival spores, widespread by wind and massive abundance in soil, result in a rapid spread of fungal pathogens in nature. Plant pathogens not only affect the plant cultivation but also influence post-harvest storage and distribution before the consumption of agricultural products such as grains, roots, and fruits. Pathogenic fungi in the soil can result in difficulty for the same area to be used to grow vulnerable crops. They have caused terrible agricultural and economic impact. The aim of this present study is to investigate the antifungal potential of the endophytic fungal community isolated from native Sceletium tortuosum L. Bioactive compounds produced by endophytic fungi which are isolated from the host plant might be used for medicine and agriculture purposes [4]. In this study, a total of 43 endophytic fungi were isolated from healthy
S. tortuosum plants in South Africa and selected based on their biological activity. Deposit the ITS and EF1a DNA sequences to GenBank and provide GenBank accession numbers in Table 1.
Table 1.
Identification of endophytic fungi and activity against pathogenic fungi of plants according to the dual culture technique.
| Potential antifungal activity |
||||||||
|---|---|---|---|---|---|---|---|---|
| Strains | Distinguishing morphological characteristics on PDA | Identified as | A | B | C | D | E | F |
| GG01 | Brown green colony that looks like an algae swamp, white on reverse | Aspergillus sp | +++ | +++ | +++ | +++ | +++ | +++ |
| GG02 | Woolly growth resembling cotton candy. New growth is white in color but turns a grayish-brown with aging. The reverse is pale white | Mucor circinelloides | +++ | +++ | +++ | +++ | +++ | +++ |
| GG05 | Colonies are brown greenish in color with white edges | Fusarium solani | ++ | ++ | +++ | ++ | ++ | ++ |
| GG06 | Pale white colony, very thin colony | Ceratobasidium sp | +++ | +++ | ++ | +++ | ++ | ++ |
| GG09 | Cream white with mix of slightly yellow colonies | Neurospora sp | +++ | +++ | +++ | +++ | +++ | ++ |
| GG10 | Powdery colonies with a characteristic buff or cinnamon-brown color on the surface and a yellow to beige-brown color on the reverse | Aspergillus terreus | +++ | ++ | ++ | ++ | +++ | ++ |
| GG11 | White hairy cotton that grew up, fluffy like form | Fusarium solani | ++ | ++ | +++ | ++ | ++ | ++ |
| GG12 | White cottony colonies with the aerial mycelia becoming tinged in purple. The reverse was a rather non-descript pale to yellow | Fusarium oxysporum | + | ++ | +++ | ++ | ++ | ++ |
| GG13 | Powdery, showing various shades of green, blue-green to a grey-green with a narrow white border. The reverse is white to tan to pale yellowish | Aspergillus fumigatus | + | ++ | +++ | ++ | ++ | ++ |
| GG14 | White to yellowish felt-like mat of mycelia. Reverse is white to pale in color | Aspergillus niger | + | ++ | ++ | ++ | ++ | ++ |
| GG15.1 | Brown green colony that looks like an algae swamp, white on reverse | Aspergillus sp | ++ | ++ | ++ | ++ | ++ | + |
| GG15.2 | Brown green colony that looks like an algae swamp, white on reverse | Aspergillus sp | ++ | ++ | +++ | ++ | ++ | ++ |
| GG16 | Colony is brown/ green color with white and blue | Aspergillus niger | ++ | ++ | +++ | +++ | ++ | ++ |
| ND02 | The surface of the colony is velvety, downy or powdery, having different shades of green, blue-green with a narrow white border. Reverse is white to pale yellow | Aspergillus fumigatus | + | ++ | +++ | ++ | ++ | ++ |
| ND04 | White mycelium cotton in the center of the colony but grows to become green. | Penicillium echinulatum | + | ++ | ++ | ++ | ++ | ++ |
| ND06 | The colony is of a thin layer on the agar that is pale white | Penicillium sp | ++ | ++ | ++ | ++ | ++ | ++ |
| ND07 | Pale white with a thin layer on the agar | Geotrichum sp | ++ | ++ | +++ | + | +++ | ++ |
| ND08 | Grey to olive brown on the surface with short aerial hyphae, brown-black on reverse due to pigment production | Alternaria sp | + | ++ | +++ | ++ | ++ | ++ |
| ND09 | The colony has a white fur center that is surrounded by a blue-greenish part | Aspergillus sp | ++ | ++ | ++ | ++ | ++ | ++ |
| ND10 | White center with yellow bubbles and grows to become green with white fur | Alternaria sp | + | ++ | ++ | +++ | ++ | ++ |
| ND11 | White mycelium cotton in the center of the colony but grows to become green | + | ++ | ++ | + | + | ++ | |
| ND13 | White fluffy cotton like colony with a pink center | Fusarium oxysporum f. sp. ciceris | ++ | +++ | +++ | +++ | +++ | +++ |
| ND14 | The colony is green brownish with a white fur layer above | Aspergillus fumigatus | + | ++ | +++ | ++ | ++ | ++ |
| ND15 | Black spores held by yellowish hyphae with a white surface | Aspergillus niger | +++ | +++ | +++ | +++ | ++ | +++ |
| ND17 | Yellow colony on the surface with black spores shooting up from the colony supported a very thin hyphae like | Aspergillus sp | +++ | +++ | +++ | +++ | +++ | +++ |
| ND19 | The colony is green brownish with a white fur layer above | Fusarium oxysporum f. sp. lycoperscici | ++ | ++ | +++ | ++ | ++ | ++ |
| DR03 | White hairy cotton that has pink root like structure spreading out of it, in the surface with white reverse | Coniothyrium aleuritis | ++ | ++ | +++ | +++ | ++ | ++ |
| DR04 | Green brownish colony with blue and white edges | Fusarium oxysporum f. sp. ciceris | + | ++ | +++ | ++ | ++ | ++ |
| DR08 | White hairy cotton that grew up, fluffy like form | Fusarium sp. | ++ | ++ | ++ | ++ | ++ | + |
| DR09 | White thick cotton in the center and the edges of the cotton grew cream white like roots, growing out and long | Fusarium equiseti | ++ | ++ | ++ | ++ | ++ | + |
| DR10 | Green brownish colony with blue and white edges | Phomopsis columnaris | + | ++ | +++ | + | ++ | ++ |
| DR12 | Green brown colonies with gold bubbles on top | Pythium heterothallicum | + | ++ | ++ | + | ++ | +++ |
| DR14.1 | Green brownish colony with blue and white edges | Neonectria sp. | + | ++ | +++ | ++ | ++ | ++ |
| DR14.2 | Green brown colonies with gold bubbles on top | Neonectria sp. | ++ | +++ | +++ | ++ | ++ | ++ |
| DR16 | Green smooth colony with white fur at the edges | Cladosporium sp | +++ | +++ | +++ | +++ | ++ | ++ |
| DR17 | White fluffy cotton like colony | Fusarium solani | ++ | ++ | ++ | + | ++ | ++ |
| DR19 | Green brown colonies with gold bubbles on top | Fusarium penzigii | ++ | ++ | + | ++ | ++ | ++ |
| DR20 | The colony is green brown, with white and blue ends | Fusarium subglutinans | + | ++ | +++ | ++ | ++ | ++ |
| DR21 | Thick white cotton with fur growing upwards and reverse is light yellow | Fusarium equiseti | ++ | ++ | ++ | +++ | + | + |
| DR22 | White, pale thin layer of cotton | Fusarium sp. | ++ | ++ | ++ | ++ | ++ | ++ |
| DR23 | White cotton in the middle with cream looking for growing from it like roots | Fusarium oxysporum | ++ | ++ | +++ | +++ | ++ | ++ |
| DR24 | White fluffy colony also in reverse | Fusarium sp. | ++ | ++ | ++ | ++ | ++ | ++ |
Width of growth inhibition zone T = 0 mm; -, 0<T ≤ 3 mm; +, 3< T ≤ 5 ++, T > 5mm +++.
(A) 1 Aspergillus; (B) M Fusarium; (C) 2929 Fusarium oxysporum; (D) 13071 Borytis cinerea; (E) 10139 Fusarium graminearum; (F) 12517 Colletotrichum gleosporioides.
The identities of the fungal isolates were determined through morphological and molecular identification using internal transcribed spacer (ITS) and elongation factor 1-alpha (EF-1α) primers as reported in a previous finding [3].
Endophytic fungi were subjected to fermentation process in order to produce the secondary metabolites. Each of the fungal isolate was placed in a 50 mL of malt extract broth, 250 mL of Erlenmeyer flasks. Rotary shaker (Labcon FSVE-Spo8, Gauteng, South Africa) was set at 150 rpm and fungal isolates were incubated (Labcon FSVE-Spo8) for 5 days at 25 °C. The culture broth was filtered through a 0.45 μm sterile Acrodisc syringe filter (Pall Life Sciences, Ann Arbor, MI). In the dual culture method, the assay was conducted to check the antifungal activity of the endophytic fungi against selected pathogenic fungi. Potato dextrose agar (PDA; Merck Biolab, Gauteng, South Africa) media was used to perform a 3-point inoculation of 6 mm disks of the endophyte with a pathogenic fungus at the center. The plates were inoculated 5–8 days at 28 ± 10 °C. Incubation of the plates can result in the interference of the pathogen in the direction of endophytic fungal growth [5].
Fungal culture filtrates were subjected to antifungal screening against six selected pathogenic fungi. Thirty milliliter of PDA medium was poured into a sterilized 90 mm petri dishes and supplemented with 2 mL of the fungal filtrate
PDA only was then poured as a control. Upon solidification, plant pathogens were inoculated at the center of the plate and the growth was measured by mycelial growth inhibition and calculated according to the formula.
The activity of the culture filtrate was calculated using the formula given where = mean, = the measured diameter on each plate and = the number of plates representing a particular isolate. The standard deviation of each isolate was then calculated using the formula
where is the standard deviation, N is the number of plates, is the mean, and is the measured diameter on each plate. The reason for using these equations was to tell how diameters of the plates are spread out from the average (mean) or expected diameter. A low standard deviation meant that the diameters are close to the average. A high standard deviation means that the diameters are more spread out.
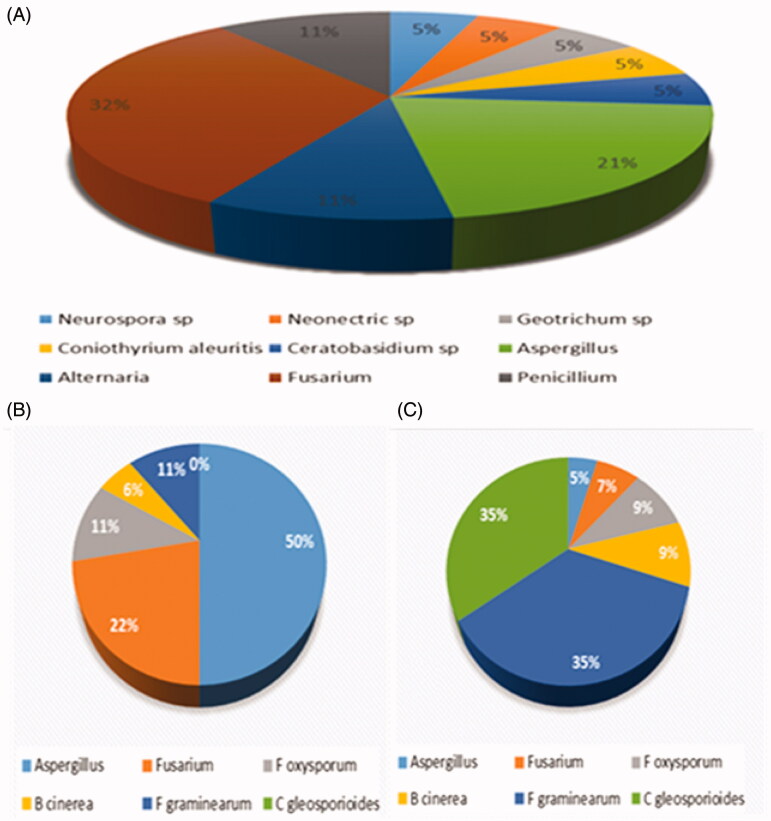
The dual culture assay showed that all forty-three endophytic fungi were inhibitory against one or more pathogens (Table 1). Aspergillus sp. (ND17), and A. niger (ND15) exhibited a broad spectrum of activity for all six pathogens. A. fumigatus isolates, GG13, ND02, and ND14, exhibited similar activity pattern against the same pathogens (Table 1). Diversity data show that Aspergillus genus attributed to 43%, followed by Fusaria with 29%, then we have 14% M. circinelloides and Neurospora respectively. Fusarium oxysporum with 37%, followed by Borytis cinerea 17%, then we had Fusarium sp. at 14% and F. graminearum with 10% was the least inhibited. Table 2 represents the antifungal effect of the selected endophytic fungi using filtrate culture assay. From a total of forty-three (n = 43) tested endophytic fungi, 77% showed inhibition activity against one or more pathogens (Table 2). Culture filtrates of the endophytic fungi DR08 exhibited a broad range of antifungal activity against all the pathogens. Least activity was displayed by DR04, DR18, DR14.1, and DR20. The greatest antifungal activity in dual culture was displayed by the isolate GG09, however, that was not the case in the culture filtrate where it showed almost no activity for pathogen Fusarium 2929 while showing complete inhibition for B. cinerea 10139 (D), F. graminearum 12517 (E), and Colletotrichum gleosporioides (F). Additionally, the results revealed that majority of the endophytic fungi extracts from the medicinal plant have partial antifungal activity. None of the isolates tested were able to control all six pathogenic fungi. From the diversity of the antifungal activity, Fusaria was dominate with 32%, followed by Aspergillus genus with 21% and Alternaria with 11% (Figure 1(A)). Endophytic fungi (DR08, Fusarium sp.) showed the most activity followed Fusarium sp. (DR24). Aspergillus sp. (ND9) showed the least activity against the pathogens. Among all the six pathogens, F. graminearum (G) and Colletotrichum gleosporioides (H) were less resistant. Aspergillus sp. which was isolated from maize was the most resistant pathogen. Figure 1(B,C) illustrates the activity that occurred between the six pathogens and the filtrates tested against. Aspergillus was the most resistant pathogen out of the six tested with 50% and F graminearum was the most inhibited pathogen with 35%. From a total of forty-three (n = 43) tested endophytic fungi, all (100%) exhibited inhibitory activity against one or more pathogens using dual diffusion assay. Fusarium and Aspergillus were the dominate genus that showed significate antifungal effect. Seventy-seven (77%) displayed inhibition activity against one or more pathogens. Endophytic fungi (DR08, Fusarium sp.) was the most effective and Aspergillus sp. (ND9) was the least activity.
Table 2.
Activity of culture filtrates of endophytic fungi against pathogenic fungi of plants.
| Fungi | Colony growth (mm) |
|||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| GG01 | 2.3 ± 0.10 | 3.65 ± 0.35 | 2.60 ± 0.20 | 4.00 ± 0.40 | 3.10 ± 0.14 | 4.00 ± 0.20 |
| GG02 | 2.35 ± 0.25 | 3.35 ± 0.55 | 5.25 ± 1.15 | 4.70 ± 0.61 | 2.65 ± 0.25 | 3.35 ± 0.45 |
| GG05 | 8.05 ± 0.05 | 3.95 ± 0.15 | 5.60 ± 0.30 | 3.15 ± 1.65 | 5.15 ± 0.21 | 4.95 ± 0.15 |
| GG06 | 1.60 ± 0.40 | 1.70 ± 0.10 | 3.00 ± 0.20 | 3.85 ± 0.25 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| GG09 | 1.60 ± 0.80 | 6.75 ± 0.25 | 3.80 ± 0.70 | 0.20 ± 0.20 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| GG10 | 7.60 ± 1.00 | 5.85 ± 1.05 | 7.20 ± 0.00 | 4.10 ± 0.50 | 3.95 ± 0.21 | 3.55 ± 0.05 |
| GG11 | 8.55 ± 0.05 | 3.55 ± 0.35 | 2.00 ± 0.10 | 4.35 ± 0.05 | 3.05 ± 0.21 | 2.00 ± 0.14 |
| GG12 | 6.95 ± 1.65 | 3.65 ± 1.15 | 6.30 ± 0.10 | 2.30 ± 0.00 | 4.90 ± 0.28 | 1.95 ± 0.21 |
| GG13 | 8.60 ± 0.10 | 1.20 ± 0.00 | 2.60 ± 0.00 | 5.50 ± 2.91 | 1.40 ± 0.14 | 1.00 ± 0.14 |
| GG14 | 7.35 ± 0.75 | 6.85 ± 0.75 | 7.20 ± 0.40 | 4.65 ± 0.45 | 3.65 ± 0.25 | 4.45 ± 0.45 |
| GG15 | 7.35 ± 1.25 | 4.30 ± 0.10 | 6.95 ± 0.05 | 2.65 ± 0.75 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| GG15.2 | 8.20 ± 0.10 | 8.35 ± 0.21 | 7.15 ± 0.05 | 5.40 ± 0.50 | 3.00 ± 0.10 | 2.95 ± 0.05 |
| GG16 | 7.95 ± 0.15 | 8.50 ± 0.10 | 6.55 ± 0.05 | 1.25 ± 0.35 | 0.10 ± 0.14 | 0.10 ± 0.14 |
| ND02 | 7.55 ± 0.35 | 7.25 ± 1.15 | 5.75 ± 0.25 | 8.70 ± 0.00 | 4.50 ± 0.40 | 5.95 ± 0.15 |
| ND04 | 2.00 ± 0.40 | 2.05 ± 0.05 | 8.70 ± 0.00 | 3.80 ± 0.40 | 0.10 ± 0.14 | 0.00 ± 0.00 |
| ND06 | 2.70 ± 0.28 | 3.90 ± 0.28 | 4.65 ± 0.21 | 3.80 ± 0.14 | 0.90 ± 0.80 | 0.40 ± 0.20 |
| ND07 | 3.20 ± 0.14 | 3.95 ± 0.07 | 5.00 ± 0.05 | 4.40 ± 0.14 | 0.65 ± 0.45 | 0.65 ± 0.45 |
| ND08 | 2.50 ± 0.60 | 3.45 ± 0.25 | 1.55 ± 0.35 | 3.50 ± 0.40 | 3.4 ± 0.14 | 0.95 ± 0.15 |
| ND09 | 2.40 ± 0.10 | 8.70 ± 0.00 | 8.70 ± 0.00 | 3.35 ± 0.25 | 1.30 ± 0.10 | 2.95 ± 0.05 |
| ND10 | 8.25 ± 0.35 | 7.30 ± 0.50 | 5.40 ± 0.50 | 0.80 ± 0.10 | 3.70 ± 0.10 | 3.80 ± 0.20 |
| ND11 | 8.45 ± 0.15 | 4.20 ± 0.10 | 5.45 ± 1.25 | 2.40 ± 1.20 | 0.00 ± 0.00 | 2.70 ± 0.20 |
| ND14 | 6.20 ± 0.00 | 8.60 ± 0.00 | 6.75 ± 0.15 | 0.85 ± 0.50 | 4.10 ± 0.20 | 3.65 ± 0.35 |
| ND17 | 1.75 ± 0.35 | 1.70 ± 0.10 | 0.50 ± 0.50 | 2.15 ± 0.05 | 1.50 ± 0.10 | 0.00 ± 0.00 |
| ND19 | 2.70 ± 0.10 | 3.30 ± 0.10 | 6.25 ± 0.65 | 2.50 ± 0.30 | 0.95 ± 0.05 | 2.30 ± 0.20 |
| DR03 | 0.00 ± 0.00 | 0.50 ± 0.10 | 1.65 ± 0.15 | 1.75 ± 0.35 | 1.35 ± 0.05 | 0.60 ± 0.92 |
| DR04 | 8.70 ± 0.00 | 6.80 ± 0.30 | 4.90 ± 0.10 | 7.40 ± 1.30 | 3.75 ± 0.15 | 5.80 ± 0.20 |
| DR08 | 2.45 ± 0.25 | 0.45 ± 0.45 | 1.45 ± 0.15 | 0.00 ± 0.00 | 0.70 ± 0.20 | 0.40 ± 0.10 |
| DR09 | 3.55 ± 0.45 | 2.3 ± 0.10 | 3.15 ± 0.15 | 4.40 ± 0.20 | 2.80 ± 0.10 | 1.85 ± 0.25 |
| DR10 | 5.74 ± 0.15 | 5.40 ± 1.00 | 4.90 ± 0.50 | 7.15 ± 0.25 | 5.15 ± 0.05 | 5.80 ± 0.20 |
| DR12 | 3.85 ± 0.25 | 7.05 ± 0.45 | 3.45 ± 0.45 | 2.90 ± 0.20 | 1.75 ± 0.35 | 2.00 ± 0.20 |
| DR14.1 | 7.55 ± 0.25 | 8.40 ± 0.10 | 7.85 ± 0.05 | 6.55 ± 0.05 | 8.70 ± 0.00 | 6.65 ± 0.45 |
| DR14.2 | 8.50 ± 0.20 | 2.45 ± 0.05 | 3.55 ± 0.45 | 5.45 ± 0.15 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| DR17 | 2.40 ± 0.20 | 5.45 ± 0.65 | 2.50 ± 0.30 | 3.00 ± 0.10 | 1.90 ± 0.00 | 1.50 ± 0.20 |
| DR18 | 7.35 ± 0.53 | 7.60 ± 1.00 | 5.15 ± 0.05 | 4.7 ± 0.16 | 4.60 ± 0.50 | 2.10 ± 0.00 |
| DR19 | 3.15 ± 0.75 | 0.60 ± 0.30 | 1.95 ± 0.05 | 1.00 ± 0.10 | 0.00 ± 0.00 | 1.60 ± 0.20 |
| DR20 | 7.50 ± 0.30 | 7.35 ± 0.25 | 4.60 ± 0.70 | 7.50 ± 1.20 | 8.70 ± 0.00 | 5.95 ± 0.05 |
| DR21 | 6.65 ± 1.05 | 3.00 ± 0.90 | 4.00 ± 0.20 | 4.80 ± 0.22 | 4.05 ± 0.07 | 3.75 ± 0.21 |
| DR22 | 1.95 ± 0.15 | 1.50 ± 0.16 | 0.75 ± 0.15 | 4.15 ± 0.35 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| DR23 | 8.00 ± 0.60 | 1.75 ± 0.15 | 0.00 ± 0.00g | 4.80 ± 0.40 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| DR24 | 0.85 ± 0.50 | 1.30 ± 0.20 | 0.00 ± 0.00 | 3.35 ± 0.15 | 0.00 ± 0.00 | 0.95 ± 0.95 |
(A) 1 Aspergillus; (B) M Fusarium; (C) 2929 Fusarium oxysporum; (D) 13071 Borytis cinerea; (E) 10139 Fusarium graminearum; (F) 12517 Colletotrichum gleosporioides.
0.99 ± 0.00 to 0.00 ± 0.00 = most active filtrate, 8.70 ± 0.20= least active filtrate.
Figure 1.
(A) Diversity of fungal extracts displaying antifungal activity, (B) resistant pathogens, and (C) least resistant pathogens; they were most inhibited by the filtrates.
Current global trend is moving toward a more sustainable, improved, safer and eco-friendly alternatives to the conventional resistant antimicrobial drugs on the market. Medicinal plants have shown to possess several biological activities that has tremendous health benefits. This set a tone in the investigation of significantly important microbial community that has a symbiotic interactions with plants [6]. S. tortuosum L. commonly known as kougoed is a native medicinal plant that has been used in indigenous tribes for stress relief, depression, as a painkiller, alleviate hunger and overall mood-enhancer [7]. Hence, S. tortuosum L. was selected in the current study for the screening of endophytic fungi that exhibit antifungal activity against fungal pathogens. Fungal pathogens cause diseases that pose serious concern in the agricultural sector. Subsequently leading to pathogenic fungi infects agricultural crop resulting in considerable losses of yield and economic losses which will have an impact on the global crop prices. Recent fungicide used for agricultural commodities are posing more shortcomings such as causing cancer, toxicity, and resistant [8]. Hence, more and more people are questioning the utilization of the current fungicide on the market and are looking for a more health benefit and eco-friendly approach. It was reported that approximately 12% of endophytic fungi have shown to exhibit antifungal activity against four plant fungal pathogens (B. cinerea, Sclerotinia sclerotiorum, Rhizoctonia solani and F. oxysporum) using dual-culture method. In the same study, Penicillium sp. and A. oryzae demonstrated to be the most effective [9]. In the current study, 100% of the endophytic fungi displayed inhibitory activity against one or more pathogens. Aspergillus strains were dominate in the antifungal bioassay using dual method. Fusaria genus was predominate followed by Aspergillus genus. It is not surprising, Fusaria genus are considered as an enormous group inhibiting plants, animals and humans as saprophytic, opportunistic and even symbiotic association [10]. Several studies have demonstrated that endophytic fungi producing a wealth of bioactive compounds have the potential to be an outstanding antifungal agent and fungicide [11–13].
In this study, we established that endophytic fungi isolated from medicinal plant of South Africa possess excellent antifungal properties against pathogenic fungi. Endophytic fungi are untapped territories filled with effective, novel bioactive compounds [2] to control fungal pathogens responsible for the mortality rate and high crop losses (Figure 2). In conclusion, these endophytic fungi have potential to be used as a natural biocontrol agent in the agricultural sector; antifungal agents in the medical as well as the pharmaceutical industries. To the best of our knowledge, this is the first report on antifungal activity of endophytic fungi isolated from S. tortuosum L. plants. Future research should focus on the isolation and characterization of these novel, lead bioactive compounds resulting in product development for a more efficacious, safer and eco-friendly fungicide and antifungal agent.
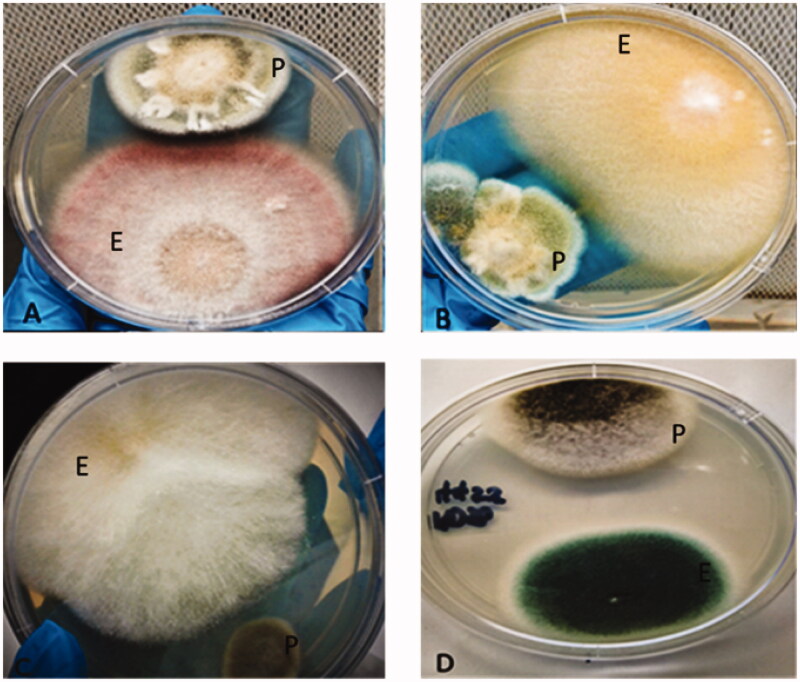
Figure 2.
Endophytic fungi from Sceletium tortuosum showing activity in dual culture against fungal pathogens, E: endophytic fungi; P: pathogenic fungi.
Disclosure statement
All authors declare there is no conflict of interest.
References
- 1.Jinxin Y, Ying W, Zheu H, et al. Diversity and antifungal activity of endophytic fungi associated with Camellia oleifera. Microbiology. 2018;46:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer J, Schueffler A.. Bioactive compounds from tree endophytes In: Pirttilä A, Frank A, editors. Endophytes of forest trees. Forestry sciences. Vol. 86 Cham: Springer; 2018. [Google Scholar]
- 3.Manganyi MC, Regnier T, Kumar A, et al. Phylogenetic analysis and diversity of novel endophytic fungi isolated from medicinal plant Sceletium tortuosum. Phytochem Lett. 2018;27:36–43. [Google Scholar]
- 4.Silva-Hughes AF, Wedge DE, Cantrell CL, et al. Diversity and antifungal activity of the endophytic fungi associated with the native medicinal cactus Opuntia humifusa (Cactaceae) from the United States. Microbiol Res. 2015;175:67–77. [DOI] [PubMed] [Google Scholar]
- 5.Luo ZP, Lin HY, Ding WB, et al. Phylogenetic diversity and antifungal activity of endophytic fungi associated with Tephrosia purpurea. Mycobiology. 2015;43(4):435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schafhauser T, Jahn L, Kirchner N, et al. Antitumor astins originate from the fungal endophyte Cyanodermella asteris living within the medicinal plant Aster tataricus. Proc Natl Acad Sci U S A. 2019;116(52):26909–26917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gericke N, Viljoen A.. Sceletium – a review update. J Ethnopharmacol. 2008;119(3):653–663. [DOI] [PubMed] [Google Scholar]
- 8.Newitt J, Prudence S, Hutchings M, et al. Biocontrol of cereal crop diseases using Streptomycetes. Pathogens. 2019;8(2):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdhary K, Kaushik N.. Diversity and antifungal activity of fungal endophytes of Asparagus racemosus Willd. Agric Res. 2019;8(1):27–35. [Google Scholar]
- 10.Manganyi M, Regnier T, Olivier E.. Antimicrobial activities of selected essential oils against Fusarium oxysporum isolates and their biofilms. S Afr J Bot. 2015;99:115–121. [Google Scholar]
- 11.Nurhaida N, Rashid S, Ibrahim D.. The potency of endophytic fungi from the bark of Syzygium cumini (L.) Skeels as antifungal agents. E3S Web Conf. 2020;151:01006. [Google Scholar]
- 12.Yu J, Wu Y, He Z, et al. Diversity and antifungal activity of endophytic fungi associated with Camellia oleifera. Mycobiology. 2018;46(2):85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suradkar KP, Hande DV, Kadu SR.. Screening of antifungal activity of endophytic fungi from Dioscorea bulbifera. Int J Life Sci. 2017;8:59–62. [Google Scholar]