ABSTRACT
Pleurostoma species are wood-inhabiting fungi and emerging opportunistic pathogens causing phaeohyphomycosis. In this study, we isolated a dematiaceous fungus, HKU44T, from the subhepatic abscess pus and drain fluids of a liver transplant recipient with post-transplant biliary and hepatico-jejunostomy bypass strictures. Histology of the abscess wall biopsy showed abundant fungal hyphae. The patient survived after a second liver transplant and antifungal therapy. On SDA, HKU44T grew initially as white powdery colonies which turned beige upon maturation. Hyphae were septate and hyaline. Phialides were monophialidic and laterally located, generally closely associated to a cluster of conidia which were usually reniform. Phylogenetic analyses showed that HKU44T is most closely related to, but distinct from, Pleurostoma ootheca and Pleurostoma repens. These suggested that HKU44T is a novel Pleurostoma species, for which the name Pleurostoma hongkongense sp. nov. is proposed. Antifungal susceptibility testing showed that Pleurostoma species possessed high MICs/MECs for fluconazole, 5-flucytosine and the echinocandins; whereas they exhibited a high strain-to-strain variability to the susceptibilities to the other triazoles. As for amphotericin B, ∼65% of the Pleurostoma strains had low MICs (≤1 µg/mL). DNA sequencing should be performed to accurately identify fungi with Pleurostoma/Phialophora-like morphologies, so is antifungal susceptibility testing for patients with Pleurostoma infections.
KEYWORDS: Liver, phaeohyphomycosis, dematiaceous fungus, Pleurostoma, Pleurostoma hongkongense, novel species
Introduction
Pleurostoma species are emerging human pathogens causing phaeohyphomycosis. This historic genus was originally established in 1863 [1]. Following its merging with the asexual genus Pleurostomophora subsequent to the abolishment of dual nomenclature of the different morphs of the same fungus since 2013, it currently accommodates a total of five species, namely Pleurostoma candollei (type species), Pleurostoma ootheca, Pleurostoma ochraceum, Pleurostoma repens and Pleurostoma richardsiae [2, 3]. In nature, this group of fungi inhabits woods [4, 5]; and they have also been isolated from soil and sewage [4] and found as contaminants which cause blueing of ground wood pulp [6]. Infection may result from close contact with Pleurostoma-contaminated plants or direct inoculation of contaminated materials into the body [7–11]. Very often Pleurostoma infections are localized and manifest as cutaneous or subcutaneous lesions and/or nodules [7–9, 11–30], although infections of the bones/joints [9, 20, 22, 31–35], eyes [10, 35–37] and urinary tracts [38] are also observed. In more rare occasions especially in severely immunocompromised individuals, disseminated invasive infections affecting the bloodstreams, hearts and livers could also occur [35, 39, 40]. The aetiological agent for most of the cases were Pleurostoma richardsiae [7–25, 28–33, 35–40], while Pleurostoma ochraceum [26], Pleurostoma ootheca [27] and Pleurostoma repens [34] were also reported to cause infections in a few cases.
In 2017, we isolated a dematiaceous fungus from the subhepatic abscess pus and drain fluids of a patient. Microscopic examination of the fungal culture and preliminary internal transcribed spacer (ITS) region sequencing showed that it is a Pleurostoma species, although matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry (MALDI–TOF MS) failed to identify this fungus due to the absence of interpretable protein mass spectra. Further morphological and molecular characterizations showed that this fungus is closely related to, but distinct from, all the known Pleurostoma species. Therefore, we propose a new species, Pleurostoma hongkongense sp. nov., to describe this fungus. Antifungal susceptibilities of this novel species and the known Pleurostoma species were also performed to help guide patient management.
Materials and methods
Patient and strains
Clinical specimens, including pus from a subhepatic abscess as well as drain fluids, were collected from the patient, handled according to standard protocols, directly inoculated on Sabouraud dextrose agars (SDA; Difco, BD Diagnostics Systems, USA) supplemented with chloramphenicol (50 µg/mL; Calbiochem, USA) and incubated at 30°C to obtain the case isolate HKU44T. Fungal materials were harvested directly from a single colony for subsequent subcultures. Clinical data were collected by retrieving and analysing the hospital record of the patient, and the use of these data was approved by the Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Cluster. The ex-type strains of Pleurostoma ochraceum, Pleutostoma repens and Pleurostoma richardsiae, a reference strain of Pleurostoma ootheca as well as 23 reference strains of Pleurostoma richardsiae originating from clinical sources were obtained from the American Type Culture Collection (ATCC), USA; the Medical Mycology Research Center (MMRC) of Chiba University (IFM), Japan; the National Collection of Pathogenic Fungi (NCPF), Public Health England (PHE), UK; the UAMH Centre for Global Microfungal Biodiversity, Dalla Lana School of Public Health, University of Toronto, Canada as well as the Westerdijk Fungal Biodiversity Institute (CBS), the Netherlands (Table S1). The quality control strains for susceptibility testing Aspergillus flavus ATCC 204304, Aspergillus fumigatus ATCC 204305 and Candida parapsilosis ATCC 22019T were obtained from ATCC; Candida albicans CNM-CL F8555, Pichia kudriavzevii (synonym: Candida krusei) CNM-CL-3403 and Aspergillus flavus CNM-CM-1813 were obtained from Statens Serum Institut (SSI), Denmark; and Pichia kudriavzevii NRRL Y-413 (=ATCC 6258) was obtained from the Agricultural Research Service (ARS) Culture Collection (NRRL), Department of Agriculture, USA.
Phenotypic characterizations
The fungal isolate HKU44T and other Pleurostoma ex-type/reference strains were cultured on malt extract agar (MEA; Oxoid, UK), potato dextrose agar (PDA; BD Diagnostic Systems) and SDA (all supplemented with chloramphenicol [50 µg/mL]) for examination of growth rates and colony characteristics. Images of the fungal colonies were captured using the digital camera α5100 (Sony, Japan). For light microscopy, a slide culture was prepared by first placing an SDA block measuring 1 cm × 1 cm × 1 cm onto a glass slide. Each surface of the agar block was inoculated with the conidia of HKU44T (3 × 104 colony forming units) and a cover slip was then placed on top of the agar block. The slide culture was incubated at 25°C for 7 days after which the cover slip with the attaching fungal materials, mounted and stained with lactophenol cotton blue (BD Diagnostics System), was transferred on a fresh glass slide. Microscopic characteristics were observed using the microscope BX43 (Olympus, Japan) under an original magnification of 1000× and digital images were obtained by the attached camera DP80 (Olympus) and the software cellSens Dimension (Olympus). Measurement was made for 40–60 structures for each microscopic character. HKU44T was also microscopically examined by scanning electron microscopy, performed following a simplified protocol [41]. Briefly, polycarbonate filter membranes with a pore size of 0.8 µm were placed on tap water agar (1.5% agarose [w/v; BD Diagnostic Systems]). Strain HKU44T was then inoculated on the membranes and was incubated at 25°C for 14 days. Upon colony maturation, the culture was fixed using formalin vapour by adding 1 mL of 37% formaldehyde (in water, v/v; Sigma Aldrich, USA) onto the lid of the inverted Petri dish which was then securely sealed and incubated overnight. Membranes with fixed fungal materials were then collected and washed with absolute ethanol (Merck, Germany) twice and then sent to the Electron Microscope Unit of The University of Hong Kong for sample processing, where the fixed samples were dried by the critical point dryer CPD 030 (Bal-Tec, Liechtenstein) using liquid carbon dioxide as the transitional fluid, mounted on aluminium stubs and then coated with a thin layer (10–20 nm) of palladium for electrical conduction by the cool sputter coater SCD 005 (Bal-Tec). Coated specimens were then examined using the field emission scanning electron microscope S-4800 (Hitachi High-Technologies, Japan).
DNA extraction, ITS, partial 28S nrDNA, partial 18S nrDNA and partial β-tubulin gene sequencing, sequence identity analyses and phylogenetic analyses
Extraction of fungal DNA, polymerase chain reaction (PCR), and sequencing of the ITS, partial 28S nuclear ribosomal DNA (nrDNA), partial 18S nrDNA and partial β-tubulin gene for the case isolate HKU44T and the ex-type/reference strains of other Pleurostoma species were carried out following our previous publication [42] with the primer pairs ITS1/ITS4 [43] for the ITS, NL1/NL4 [44] for partial 28S nrDNA, NS1/NS4 [43] for partial 18S nrDNA as well as TUB2-F/TUB2-R [45] for partial β-tubulin gene. The DNA sequences obtained, together with those of other closely related species/strains accessioned in the DDBJ/ENA/GenBank databases, were then compared by pairwise alignment using BioEdit 7.2.0 [46]. These sequences were also analysed by multiple sequence alignment using MUSCLE 3.8 [47]. After end-trimming, divergent or poorly aligned regions of the DNA sequences were removed using Gblocks 0.91b [48, 49] with relaxed parameters. Test for substitution model and phylogenetic tree reconstruction for each DNA marker were performed by the maximum likelihood method using MEGA 6.0.6 [50]. Phylogenetic analyses included 531, 560, 822 and 397 nucleotide positions of the ITS, partial 28S nrDNA, partial 18S nrDNA and partial β-tubulin gene sequences, respectively. Phylogenetic trees were also reconstructed using the concatenated sequences of the four DNA markers (total 2340 nucleotide positions) of HKU44T and other Pleurostoma strains by the maximum likelihood method as described above and by the Bayesian evolutionary analysis using BEAST 1.10.4 [51]. For Bayesian inference, ten million generations were run with trees sampled every 1000th generation to yield 10,000 trees. The trees, after a 10% burn-in, were summarized as a single tree using TreeAnnotator 1.8.0 by choosing the tree with the maximum sum of posterior probabilities (maximum clade credibility) and viewed using FigTree 1.4.0.
In vitro antifungal susceptibility test
The in vitro susceptibilities against 11 antifungal drugs, including amphotericin B (Cayman Chemical, USA), anidulafungin (SelleckChem, USA), caspofungin (TargetMol, USA), micafungin (TargetMol), fluconazole (TargetMol), isavuconazole (TargetMol), itraconazole (TargetMol), posaconazole (Sigma-Aldrich), ravuconazole (Sigma-Aldrich), voriconazole (TargetMol) and flucytosine (Sigma-Aldrich), were determined by the microbroth dilution method according to the guidelines by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (test range: 0.0156–8 µg/mL for itraconazole and posaconazole; 0.0312–16 µg/mL for other drugs) [52] and our previous publication [53]. For echinocandins, minimum effective concentration (MEC) endpoints were recorded as the lowest drug concentrations in which abnormal, short and branched hyphal clusters were observed; whereas for the other antifungal agents, minimum inhibitory concentration (MIC) endpoints yielding no visible fungal growth by eyes were recorded. Strains Aspergillus flavus ATCC 204304 and CNM-CM-1813, Aspergillus fumigatus ATCC 204305, Candida albicans CNM-CL F8555, Candida parapsilosis ATCC 22019T as well as Pichia kudriavzevii CNM-CL-3403 and NRRL Y-413 were used as quality controls.
Data availability
A dried specimen of the case isolate HKU44T (the holotype) was deposited to UAMH whereas living cultures of HKU44T were deposited to UAMH and the Culture Collection of Switzerland (CCOS). The ITS, partial 28S nrDNA, partial 18S nrDNA and partial β-tubulin gene sequences of HKU44T and the ex-type/reference strains of other Pleurostoma species were deposited in the DDBJ/ENA/GenBank databases (Table S1).
Results
Patient
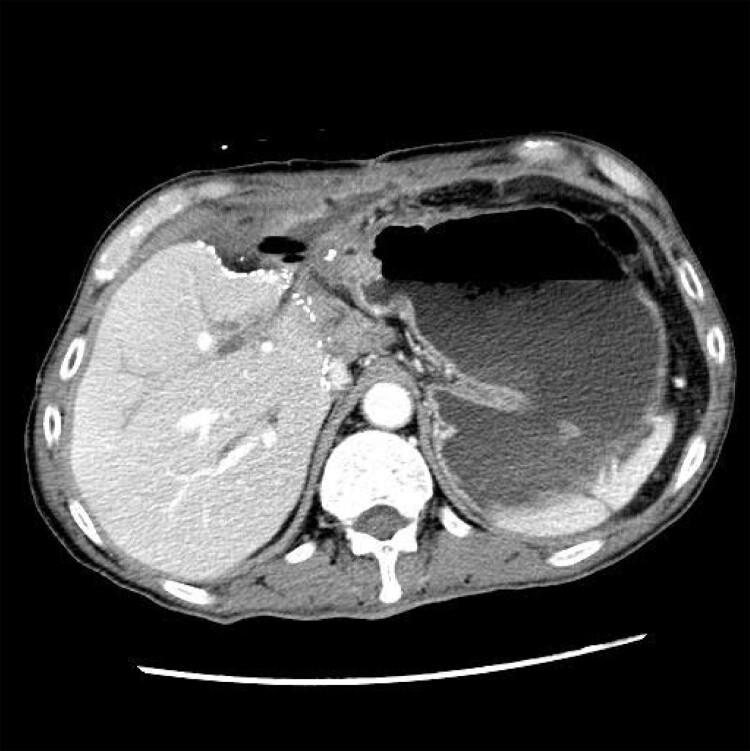
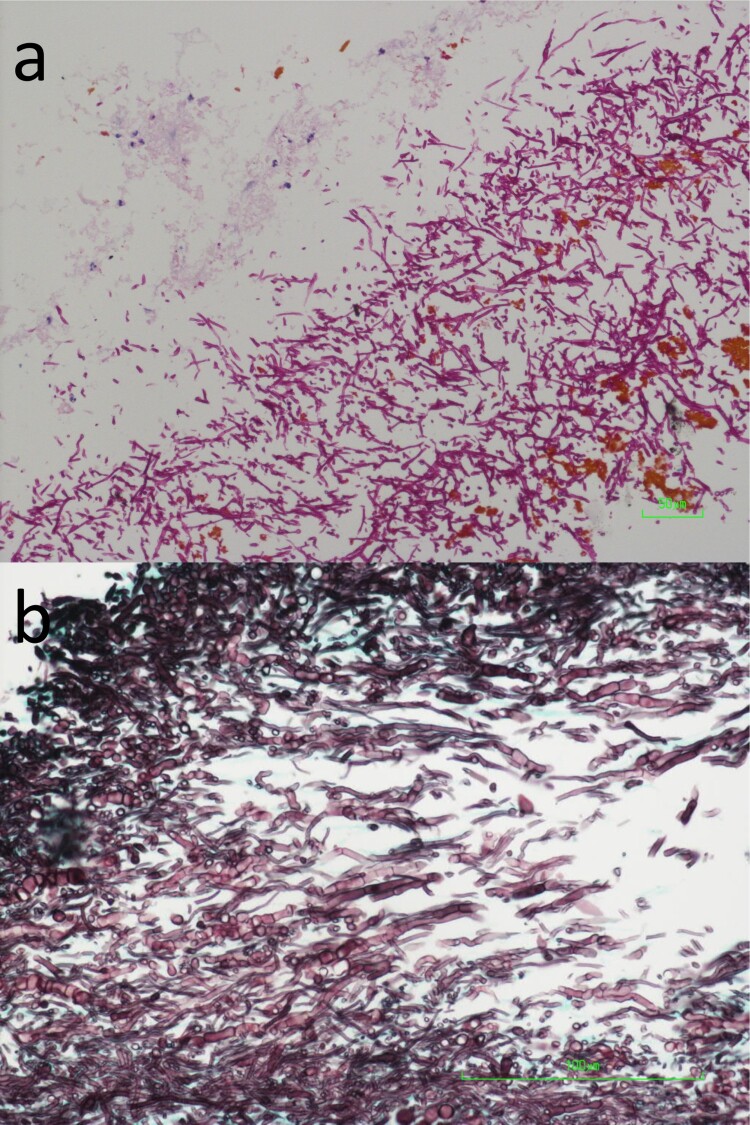
A 65-year-old Chinese man was admitted in March 2017 for management of post-liver transplant biliary stricture. The patient had hepatitis B virus-related liver failure and living donor liver transplantation was performed in November 2015. Biliary stricture developed and right hepatico-jejunostomy bypass was performed in August 2016. The patient developed stricture of the hepatico-jejunostomy bypass and was admitted in March 2017 for a second hepatico-jejunostomy bypass. Post-operatively, the patient developed increasing abdominal pain. Computed tomography examination of the abdomen revealed a 4.3 cm × 0.8 cm collection with gas pocket and rim enhancement was observed around the tubal drains in the upper abdomen (Figure 1). Laparotomy was performed in May 2017 and a loculated abscess with 5 mL of pus was observed in the subhepatic space. Drainage of the abscess was performed. Biopsy of the abscess wall showed abundant fungal hyphae (Figure 2). Bacterial and fungal cultures of the pus recovered Klebsiella pneumoniae, Enterococcus faecalis, Enterobacter cloacae and a dematiaceous fungus (strain HKU44T). Despite antimicrobial treatment including the use of anidulafungin, the same dematiaceous fungus was repeatedly recovered from the drain fluid eight more times. The patient developed progressive liver failure and cadaveric liver transplantation was performed again in July 2017. Post-operatively, amphotericin B and voriconazole were given for 16 days, followed by voriconazole maintenance therapy for three months. The function of the graft was normal and the dematiaceous fungus was not isolated from the patient again up to the time of writing, three years after the second liver transplantation.
Figure 1.
Computed tomography image of the upper abdomen. A small fluid collection with gas pocket was found and rim enhancement was observed around the tubal drains.
Figure 2.
Photomicrographs of the biopsied liver abscess wall tissue. (a) Abundant fungal hyphae, a small amount of necrotic tissue, brown bile pigments as well as a few acute inflammatory cells were observed (Periodic acid-Schiff staining, original magnification 200×). (b) The fungal hyphae were highly septate (Grocott methenamine-silver staining, original magnification 400×).
Phenotypic characterizations
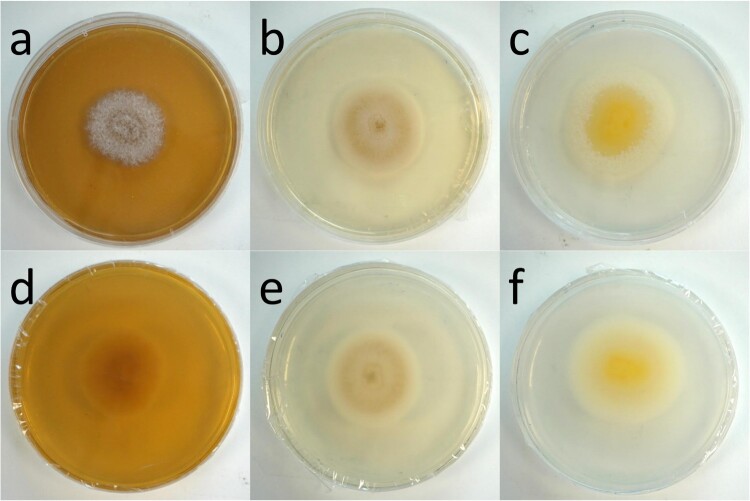
The colony macroscopy of HKU44T varied greatly when cultured on different media. On MEA, colonies were grey with creamy mycelial tufts on the surface. The reverse was beige (Figure 3(a,d)). On SDA, HKU44T initially grew white but developed a beige complexion on day 5. The colony surface was powdery but the outer colony border was floccose (Figure 3(b,e)). The reverse surface maintained the same coloration. Meanwhile on PDA, the colony surface was flat and creamy white. As the colony matured, a yellow pigment or hue appeared to diffuse from the initial point of inoculation, but remained confined to the mycelial mat (Figure 3(c,f)). Furthermore, short white creamy mycelial tufts began to emerge from the outer ring of the growing colony and later grew inwards as the colony matured. No diffusible pigment was observed for the fungus when cultured on all the three media tested. The growth rate of HKU44T on the three media tested also differed. After 7 days of incubation at 25°C, HKU44T grew significantly the fastest on PDA, attaining a colony diameter of approximately 50 mm; whereas on SDA and MEA the fungus grew slower, attaining colony diameters of around 35 mm and 31 mm, respectively (Figure S1(a)). In addition, growth of HKU44T was influenced by temperature. On SDA after 7 days of incubation, growth of HKU44T at 25°C and 30°C was comparable, with colony diameters of around 30–40 mm. When the temperature increased to 35–37C°, the fungus grew significantly faster, attaining colony diameters of around 60 mm (Figure S1(b)).
Figure 3.
Growth of Pleurostoma hongkongense HKU44T on various culture media after 7 days of incubation at 25°C. (a, d) Malt extract agar (MEA). (b, e) Sabouraud dextrose agar (SDA). (c, f) Potato dextrose agar (PDA). Brightness was adjusted individually for each panel.
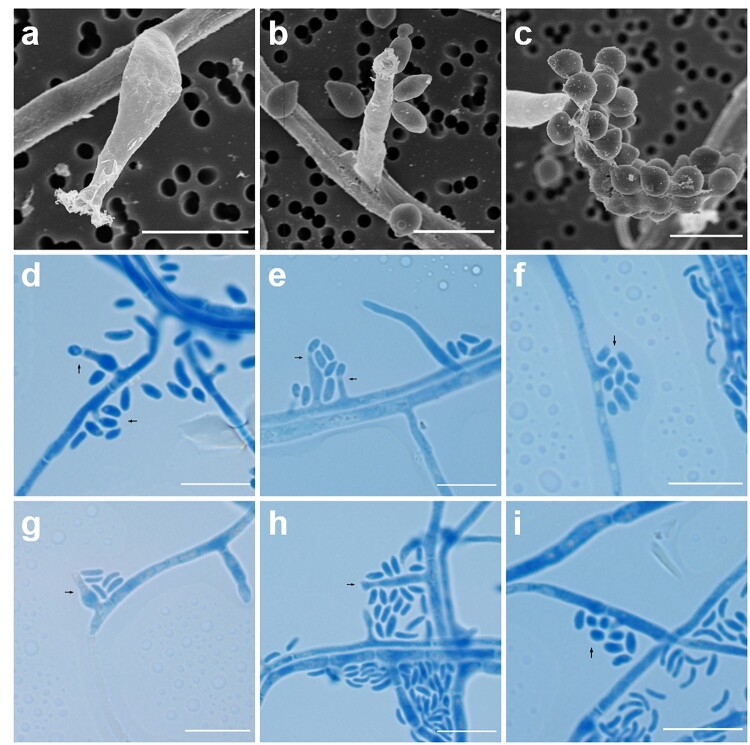
Microscopically, hyphae were septate and hyaline and hyphal walls were verruculose (Figure 4). Phialides appeared to be monophialidic, but were usually closely associated to a cluster of conidia and were usually laterally located rather than terminally on hyphae (Figure 4). Several distinguishable phialide structures were observed including swollen elongate-ampulliform phialides with constricted bases (Figure 4(a,d,g)), relatively long type I-like adelophialides which lacked basal septa (Figure 4(b,e,h)) as well as shorter and indistinct projection-like conidiophores associated with spherical to ellipsoidal conidia (Figure 4(c,f,i)). Collarettes were usually inconspicuous, though slightly cupped or tubular collarettes could be observed for the longer and larger conidiophores (Figure 4(e,g,h)). Conidia sported a wide variety of shapes and sizes including spherical, oblong-elliptical, allantoid and most commonly reniform. Notably, subspherical and oblong shaped conidia tended to associate more frequently with shorter conidiophores (Figure 4(c,f,i)), while reniform and allantoid conidia were found congregated to the other two types of conidiophores (Figure 4(a–h)).
Figure 4.
Microscopic features of Pleurostoma hongkongense HKU44T. Arrows indicate notable structures. (a–c) Scanning electron microscopy photographs of notable phialide and conidia features. Scale bars = 5 μm. (d–i) Bright-field microscopy of corresponding features using a slide culture preparation. Slides were prepared via wet mount and stained with lactophenol cotton blue (original magnification 1000×). Scale bars = 10 μm.
Molecular characterizations
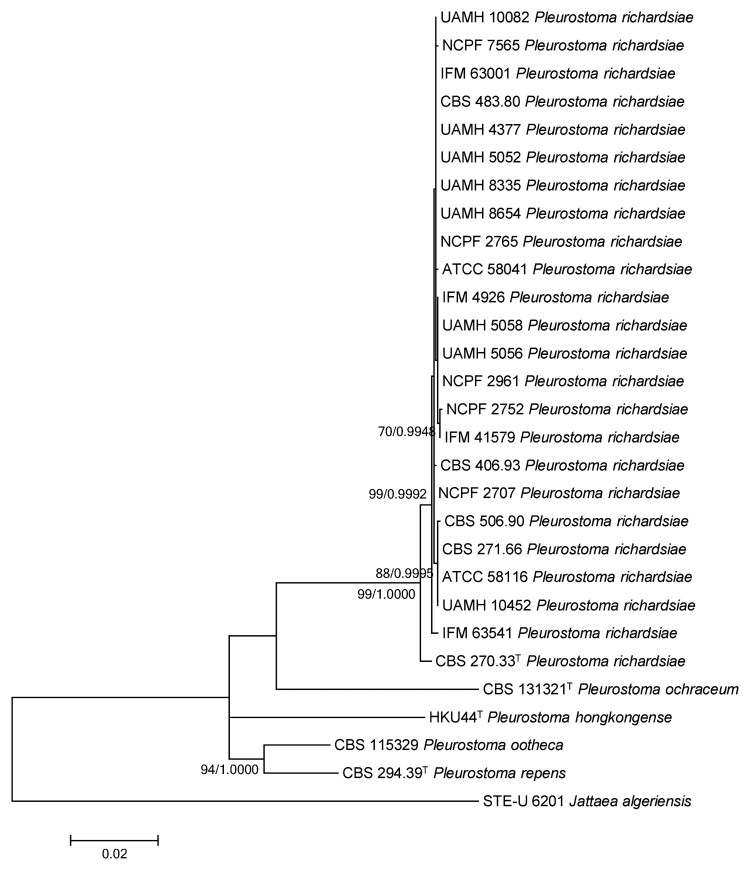
PCR of the ITS, partial 28S nrDNA, partial 18S nrDNA and partial β-tubulin gene of HKU44T and the reference strains yielded DNA products with lengths of about 600, 600, 1100 and 500 bp, respectively. Pairwise alignment showed that the partial ITS sequence of HKU44T possessed an 88.9% identity to Pleurostoma ootheca CBS 115329, an 88.6% identity to Pleurostoma repens CBS 294.39T, an 87.3% identity to Pleurostoma richardsiae CBS 270.33T and an 86.5% identity to Pleurostoma ochraceum CBS 131321T. The partial 28S nrDNA sequence of HKU44T possessed a 98.8% identity to Pleurostoma ootheca CBS 115329, a 98.2% identity to Pleurostoma repens CBS 294.39T, a 97.9% identity to Pleurostoma richardsiae CBS 270.33T and a 95.9% identity to Pleurostoma ochraceum CBS 131321T. The partial 18S nrDNA sequence of HKU44T possessed a 99.5% identity to Pleurostoma richardsiae CBS 270.33T, a 99.4% identity to Pleurostoma ootheca CBS 115329 and Pleurostoma repens CBS 294.39T and a 99.0% identity to Pleurostoma ochraceum CBS 131321T. The partial β-tubulin gene sequence of HKU44T possessed an 80.1% sequence identity to Pleurostoma repens CBS 294.39T, a 79.1% sequence identity to Pleurostoma ootheca CBS 115329, a 78.8% identity to Pleurostoma richardsiae CBS 270.33T and a 76.6% sequence identity to Pleurostoma ochraceum CBS 131321T. Phylogenetic analyses showed that HKU44T occupied a unique phylogenetic position in the ITS, partial 28S nrDNA, partial 18S nrDNA and partial β-tubulin gene trees (Figure S2) by the maximum-likelihood method as well as in the concatenated sequence trees by both the maximum-likelihood method and Bayesian evolutionary analysis (Figure 5). Although HKU44T is most closely related to Pleurostoma ootheca and Pleurostoma repens, it is distinct from all the Pleurostoma species characterized, suggesting it is a novel Pleurostoma species.
Figure 5.
Phylogenetic tree showing the relationship of Pleurostoma hongkongense HKU44T to its closely related species within the genus Pleurostoma. The tree was inferred from the concatenated sequence data of the internal transcribed spacer (ITS) region, partial β-tubulin gene, partial 18S nrDNA and partial 28S nrDNA by the maximum likelihood method with the substitution model T92 (Tamura 3-parameter model) + G (gamma-distributed rate variation) + I (estimated proportion of invariable sites). The scale bar indicates the estimated number of substitutions per base. Only nodes that were well supported by the maximum-likelihood method (≥70% bootstrap support) have their bootstrap support, calculated from 1,000 replicates, shown; and all these nodes were also well supported by the Bayesian inference method (posterior probabilities ≥0.99). The DDBJ/ENA/GenBank nucleotide accession numbers for Jattaea algeriensis STE-U 6201T are EU367446 (ITS), EU367466 (β-tubulin), EU367462 (18S nrDNA) and EU367456 (28S nrDNA).
In vitro antifungal susceptibilities
The in vitro susceptibilities of HKU44T and other Pleurostoma strains to 11 different antifungal agents are listed in Table 1. HKU44T possessed high MICs/MECs (>8 µg/mL) to all antifungal agents tested except amphotericin B (0.5 µg/mL). As for other Pleurostoma strains, all of them had high MICs to flucytosine and fluconazole (≥4 µg/mL) and most had high MECs (≥2 µg/mL) to the echinocandins, except Pleurostoma ochraceum CBS 131321T and Pleurostoma richardsiae NCPF 2765 which had low MECs (0.5 and 1 µg/mL, respectively) to caspofungin. In addition to fluconazole, in general these Pleutostoma strains possessed high MICs to other triazole agents as well. Only ten of them possessed low MICs (≤1 µg/mL) to posaconazole, seven to itraconazole, four to voriconazole and three to each of isavuconazole and ravuconazole. On the contrary, the majority of other Pleutostoma strains tested possessed low MICs (≤1 µg/mL) to amphotericin B, including Pleurostoma repens CBS 294.39, Pleurostoma ochraceum CBS 131321T, Pleurostoma ootheca CBS 115329 and 14 strains of Pleurostoma richardsiae.
Table 1.
Minimum inhibitory concentrations (MICs) or minimum effective concentrations (MECs) of different antifungal agents against Pleurostoma species.a
| Species | Strain | MICs/MECs (µg/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Triazoles | Echinocandins | Others | ||||||||||
| FLC | ISA | ITC | POS | RAV | VRC | AFG | CAS | MFG | 5FC | AMB | ||
| P. hongkongense | HKU44T | >16 | >16 | >8 | >8 | >16 | 16 | >16 | 16 | >16 | >16 | 0.5 |
| P. repens | CBS 294.39T | 16 | 1 | 0.5 | 0.5 | 1 | 0.25 | 4 | 2 | 16 | >16 | 0.25 |
| P. richardsiae | ATCC 58041 | >16 | 4 | >8 | 1 | 4 | 2 | >16 | 16 | >16 | >16 | 1 |
| ATCC 58116 | >16 | >16 | >8 | >8 | >16 | >16 | >16 | 16 | >16 | >16 | 2 | |
| CBS 270.33T | >16 | 1 | 0.5 | 0.25 | 1 | 2 | 4 | 8 | >16 | >16 | 0.5 | |
| CBS 271.66 | >16 | 2 | 0.5 | 2 | 2 | 4 | >16 | 16 | >16 | >16 | 1 | |
| CBS 406.93 | >16 | >16 | >8 | >8 | >16 | >16 | >16 | 16 | >16 | >16 | 2 | |
| CBS 483.80 | >16 | >16 | >8 | >8 | >16 | >16 | >16 | >16 | >16 | >16 | 2 | |
| CBS 506.90 | >16 | 4 | 8 | 1 | 8 | 2 | >16 | 4 | >16 | >16 | 2 | |
| IFM 4926 | >16 | 4 | 1 | 1 | 4 | 2 | >16 | 16 | >16 | >16 | 1 | |
| IFM 41579 | >16 | 4 | >8 | 8 | 4 | 4 | 2 | 8 | >16 | >16 | 1 | |
| IFM 63001 | >16 | >16 | >8 | >8 | >16 | >16 | >16 | 16 | >16 | >16 | 2 | |
| IFM 63541 | >16 | >16 | 8 | 8 | 16 | >16 | 4 | 8 | 2 | >16 | 1 | |
| UAMH 4377 | >16 | 2 | 1 | 1 | 2 | 1 | >16 | 16 | >16 | >16 | 1 | |
| UAMH 5052 | >16 | >16 | >8 | >8 | >16 | >16 | >16 | 8 | >16 | >16 | 1 | |
| UAMH 5056 | >16 | 2 | 1 | 1 | 4 | 4 | >16 | 16 | >16 | >16 | 0.5 | |
| UAMH 5058 | >16 | 2 | >8 | >8 | >16 | 1 | 4 | 4 | >16 | >16 | 1 | |
| UAMH 8335 | >16 | >16 | >8 | >8 | >16 | 8 | >16 | 8 | >16 | >16 | 2 | |
| UAMH 8654 | >16 | >16 | >8 | >8 | >16 | >16 | >16 | 16 | >16 | >16 | 2 | |
| UAMH 10082 | >16 | 16 | >8 | >8 | >16 | 16 | >16 | 8 | >16 | >16 | 2 | |
| UAMH 10452 | >16 | 16 | 8 | 8 | 16 | 16 | >16 | 8 | >16 | >16 | 1 | |
| NCPF 2707 | >16 | 8 | >8 | 8 | 4 | 8 | >16 | 2 | >16 | >16 | 1 | |
| NCPF 2752 | >16 | 4 | 2 | 1 | 4 | 4 | >16 | 16 | >16 | >16 | 2 | |
| NCPF 2765 | >16 | >16 | >8 | >8 | >16 | >16 | >16 | 1 | >16 | >16 | 4 | |
| NCPF 2961 | >16 | 8 | >8 | 8 | 4 | 2 | >16 | 8 | >16 | >16 | 1 | |
| NCPF 7565 | >16 | >16 | >8 | >8 | >16 | >16 | >16 | 4 | >16 | >16 | 1 | |
| P. ochraceum | CBS 131321T | 4 | 0.5 | 0.25 | 0.25 | 0.5 | 0.5 | 4 | 0.5 | 4 | >16 | 1 |
| P. ootheca | CBS 115329 | >16 | 16 | >8 | 1 | 8 | 8 | >16 | 8 | >16 | >16 | 1 |
AFG, anidulafungin; AMB, amphotericin B; CAS, caspofungin, FLC, fluconazole; ISA, isavuconazole; ITC, itraconazole; MFG, micafungin; POS, posaconazole; RAV, ravuconazole; VRC, voriconazole; 5FC, flucytosine.
Taxonomy
Pleurostoma hongkongense, C.-C. Tsang, K.-F. Chan, W. Chan, J.F.W. Chan, R.K.H. Au-Yeung, A.H.Y. Ngan, K.P.K. Lin, S.K.P. Lau, P.C.Y. Woo, sp. nov.
Mycobank accession number: MB 835850.
Etymology: of or belonging to Hong Kong, the place where the holotype was isolated.
Colonies on MEA grey, with creamy mycelial tufts and a beige reverse, reaching ∼31 mm in diameter after 7 days of incubation at 25°C. Colonies on SDA initially white, turning beige after 5 days of incubation, powdery, with a floccose border and same-coloured reverse, reaching ∼30–40 mm in diameter after 7 days of incubation at 25–30°C, and around 60 mm in diameter at 35–37C°. On PDA, colonies flat and creamy white, with a yellow hue at the centre and short, white creamy mycelial tufts emerging from the rims upon maturation, reaching ∼50 mm in diameter after 7 days of incubation at 25°C.
Hyphae 1.3–6.5 µm (x̄ = 2.5 ± 0.9 µm, n = 58) wide, septate and hyaline, with verruculose walls. Phialides 1.5–11.0 µm × 0.7–3.0 µm (x̄ = 4.7 ± 2.7 µm × 1.7 ± 0.5 µm, n = 42), monophialidic, usually laterally located and closely associated to a cluster of conidia. Three types of phialide structures were observed, including swollen elongate-ampulliform phialides with constricted bases, relatively long type I-like adelophialides which lacked basal septa as well as shorter and indistinct projection-like conidiophores associated with spherical to ellipsoidal conidia. Collarettes inconspicuous, though slightly cupped or tubular collarettes were observable for the longer and larger conidiophores. Conidia 0.3–11.0 µm × 1.5–3.7 µm (x̄ = 2.6 ± 0.4 µm × 2.7 ± 0.6 µm, n = 49), most commonly reniform, occasionally spherical, oblong-elliptical or allantoid. Subspherical and oblong shaped conidia usually associated with shorter conidiophores and reniform and allantoid conidia usually congregated to the other two types of conidiophores.
Holotype: UAMH 12185. Ex-type culture: HKU44T (= CCOS 1933T = UAMH 12185T).
Pleurostoma hongkongense may be phenotypically difficult to differentiate from other Pleurostoma species due to the high variability observed in the conidiophore and conidia characteristics of each species (Table 2). Furthermore, colony age and maturity are also the confounding factors for microscopic identification. The asexual morphs of members of Pleurostoma are monophialidic and heavily sporulating. Pleurostoma richardsiae is likely the easiest to differentiate, as its conidia are most commonly spherical and aggregate as slimy masses at the apices of distinct flared collarettes (Figure S3(a)). The wide saucer shaped collarette observed in Pleurostoma richardsiae is not observed in other Pleurostoma species, which usually display either cupped or slightly rounded collarettes. Pleurostoma repens can be differentiated through its relatively long, branched and elongated conidiophores. In addition, spherical conidia have never been observed in Pleurostoma repens. Pleurostoma ootheca were observed to have the shortest phialides. In addition, spherical conidia were found to be tightly bunched at the apices of these short phialides (Figure S3(b)). Pleurostoma ochraceum and Pleurostoma hongkongense share remarkably similar phialide and hyphal structures, but a large noticeable difference is the degree of melanization of conidia. Pleurostoma ochraceum has distinctly melanized and thick-walled spherical conidia (Figure S3(c)). The asexual morph of P. candollei is not known [2].
Table 2.
Microscopic features of different Pleurostoma species during asexual life cycles.
| Species | Hyphae | Phialides | Conidia |
|---|---|---|---|
| P. hongkongense | 2–6 μm wide, septate, verruculose, and hyaline | 3–30 μm long, phialide variety similar to P. ochraceum | Microconidia 2–8 μm long, spherical, ellipsoidal or oblong; macroconidia most commonly allantoid and reniform |
| P. repens | 2–4 μm wide, septate, smooth and initially hyaline, pale brown when matured | 6–30 μm long, inconspicuous, slightly flared collarrettes on elongated phialides, conidiophores often branched | 3–6 μm long, cylindrical to allantoid only |
| P. richardsiae | 2–4 μm wide, septate and smooth when young, heavily septated, thick-walled and brown when matured | 10–35 μm long, flask shaped with distinct saucer-shaped flared collarette at apex | 2–6 μm long, commonly spherical or ellipsoidal |
| P. ochraceum | 1.2–3.2 μm wide, branched, septate, smooth and verruculose | 6–20 μm long, three types of phialides described: type 1 adelophialides, type 2 elongate-ampulliform, and larger, longer type 3 cyclindrical/ampulliform phialides | 1.5–6 μm long, melanised sub-spherical or ellipsoidal |
| P. ootheca | 1.8–3.3 μm wide, septate and hyaline | 6–19 μm long, inconspiciously flared collarettes, short phialides | 3–6 μm long, most commonly oblong, straight, or allantoid; shorter and ellipsoid conidia also present |
Discussion
We report the isolation of HKU44T from the subhepatic abscess as well as repeatedly from the drain fluid eight more times in a liver transplant recipient, who received prednisolone and tacrolimus that had suppressed his immune system, rendering him susceptible to invasive fungal infections. The clinical significance of the fungus was evident by the invasion of the fungus to the patient’s tissue as demonstrated by the presence of abundant fungal hyphae as well as necrotic tissue and acute inflammatory cells in histological sections of the biopsied specimen. Culture of the abscess pus and drain fluid specimens also showed colonies at the primary inoculum on all agar plates inoculated with the specimens, supporting that the isolate was from the clinical specimens, instead of due to environmental contamination. Sequencing of four different DNA regions commonly used for phylogenetic studies, including the ITS, partial 28S nrDNA, partial 18S nrDNA and partial β-tubulin gene [54], and phylogenetic analyses showed that HKU44T stood out as a unique branch distinct from the other Pleurostoma species in the concatenated trees (Figure 5) as well as all the individual DNA marker trees (Figure S2), despite being the most closely related to Pleurostoma ootheca and Pleurostoma repens. HKU44T also possessed morphological features different to those of other known Pleurostoma species (Figure 4 and Figure S3). All these results supported that HKU44T should belong to a novel Pleurostoma species distinct from any other member of this genus, and we propose a novel species, Pleurostoma hongkongense, to accommodate HKU44T.
In addition to the discovery of a novel Pleurostoma species, the present case also represents the first case of Pleurostoma subhepatic abscess in a liver transplant recipient. Among all the 37 cases of Pleurostoma infections reported, 33 (89.2%) were caused by Pleurostoma richardsiae, and the remaining four cases were due to Pleurostoma hongkongense, Pleurostoma ochraceum, Pleurostoma ootheca and Pleurostoma repens, respectively. For predisposing factors, more than 70% of Pleurostoma infections have major underlying diseases (Table 3). The commonest medical conditions leading to systemic immunosuppression are diabetes mellitus (33.3%), malignancies (18.5%) and renal transplant (18.5%). Of all the 37 cases, 27 (73.0%) had infections (osteomyelitis, cutaneous/subcutaneous nodules, masses, lesions, cysts or abscesses, bursitis, cellulitis, eumycetoma and chromoblastomycosis) of the extremities, with 18 cases (66.7%) involving the lower limbs only, six (22.2%) infecting the upper limbs only and three (11.1%) affecting both upper and lower extremities. The portal of entry of the fungus is believed to be direct inoculation of the dematiaceous fungus through the skin as a result of unnoticed minor trauma. Pleurostoma infection has only been reported in one other liver transplant recipient (Case 35, Table 3), who was a 57-year-old Thai man with disseminated phaeohyphomycosis and hepatic artery and portal vein thrombosis due to Pleurostoma richardsiae [40]. The fungus was recovered from the patient’s blood as well as the blood clot from the failed liver graft. Identification of the fungus was achieved through ITS sequencing. On the other hand, our present case had surgical site infection with subhepatic space infection. Although the route of infection cannot be ascertained, we speculate that the fungus has invaded through the wound of the surgical site and settled in the subhepatic space.
Table 3.
Infections caused by Pleurostoma species.
| Case | Sexa/Age (year) | Ethnicity | Underlying medical conditions | Clinical syndrome | Isolation source | Fungal species | Antifungal treatment | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M/80 | American | Congestive heart failure | Subcutaneous cystic mass on proximal interphalangeal joint of left fifth finger | Cyst fluid | P. richardsiae | Surgical excision | Remission | [12] |
| 2 | F/50 | German | Not mentioned | Dacryocystitis | Lacrimal sac stone | P. richardsiae | Surgical excision | Remission | [36] |
| 3 | M/79 | African American | None | Chronic bursitis on right elbow | Cystic mass | P. richardsiae | Surgical excision | Remission | [31] |
| 4 | M/65 | American Puerto Rican | Diabetes mellitus | Right foot sole lesion | Purulent fluid from lesion | P. richardsiae | Surgical excision and drainage | Remission | [13] |
| 5 | M/43 | American Jamaican | Diabetes mellitus, adrenocortical carcinoma, multiple hepatic metastases | Ulcerative lesion on right leg | Lesion discharge | P. richardsiae | Not mentioned | Deceased | [13] |
| 6 | M/52 | American | None | Subcutaneous lesion on left index finger | Subcutaneous mass | P. richardsiae | Surgical excision | Remission | [7] |
| 7 | F/70 | American | Myeloproliferative disease | Osteomyelitis of right foot | Bone | P. richardsiae | Debridement of infected bone, amphotericin B for 1 week followed by flucytosine for 2.5 weeks | Deceased due to subsequent disseminated Nocardia infection | [32] |
| 8 | M/54 | American | Tendinitis, received methylprednisolone injection | Subcutaneous mass on right dorsal index finger | Not mentioned | P. richardsiae | Surgical excision | Not mentioned | [14] |
| 9 | M/30 | Malaysian Indian | Left femur fracture with skeletal traction and intramedullary nailing | Cutaneous lesions over left lower tibia & ankle | Lesion biopsy | P. richardsiae | Oral griseofulvin and topical naftifine for three weeks, surgical excision five months after recurrence | Persisted for two more months and then lost to follow-up | [15] |
| 10 | M/47 | French Senegalese | Hepatitis B virus carrier, recurrent duodenal ulcer | Cutaneous nodule at right patella | Cystic mass biopsy | P. richardsiae | Surgical excision | Remission | [16, 20] |
| 11 | M/30 | Japanese | Non-Hodgkin’s lymphoma, steroid-induced diabetes mellitus, osteoporosis | Subcutaneous abscess on right dorsal foot | Pus | P. richardsiae | Oral flucytosine, topical amphotericin B & surgical excision | Deceased due to disseminated intravascular coagulation | [17] |
| 12 | F/60 | American | Hypertension, diabetes mellitus, atherosclerotic heart disease, obesity | Subcutaneous cystic nodule on left medial foot | Cyst aspirate | P. richardsiae | Surgical excision | Remission | [18] |
| 13 | M/64 | Australian | Polymyalgia rheumatica, on prednisone & azathioprine therapy | Cutaneous nodules on right knee, right thigh and elbows | Skin biopsy | P. richardsiae & Exophiala jeanselmei | Oral flucytosine for 10 days, then ketoconazole for 1 week, then itraconazole for 3 months | Remission | [19] |
| 14 | M/39 | French | Renal transplant | Knee bursitis | Not mentioned | P. richardsiae | Surgical drainage and antifungal therapy, then surgical excision after recurrence | Not mentioned | [20] |
| 15 | F/15 | Indian | None | Cutaneous lesion near waistline | Skin scrapings & biopsy | P. richardsiae | Topical clotrimazole for 15 days | Remission | [8] |
| 16 | F/50 | Indian | None | Cutaneous lesion near waistline | Skin scrapings & biopsy | P. richardsiae | Topical clotrimazole for 15 days | Remission | [8] |
| 17 | M/52 | British | Diabetes mellitus, porcine mitral valve replacement | Endocarditis & fungaemia | Blood, aortic & porcine mitral valves | P. richardsiae | Aortic and mitral valves replacement, amphotericin B for nearly 6 weeks | Deceased | [39] |
| 18 | M/54 | British Afro-Caribbean | Renal transplant, hypertension, steroid-induced diabetes mellitus | Cutaneous lesions on right ring finger & left knee | Pus | P. richardsiae | Surgical excision | Remission | [21] |
| 19 | M/28 | French | HIV, hepatitis C, intravenous drug use, tuberculosis, orthopaedic reconstructive surgery of right foot | Osteomyelitis | Bone aspirates of left elbow & left foot | P. richardsiae | Amphotericin B and flucytosine followed by itraconazole | Not mentioned | [33] |
| 20 | M/41 | American | Surgical left ankle bone chip removal | Left ankle osteomyelitis | Surgical debridement specimens | P. repens | Surgical debridement, amphotericin B, oral ketoconazole for several months | Remission | [34] |
| 21 | M/36 | American | Metallic intraocular foreign body | Right eye endophthalmitis | Vitreous samples | P. richardsiae | Pars plana vitrectomy and ketoconazole, followed by a repeat vitrectomy with intraocular lens removal, intravitreal amphotericin B and miconazole | Recurred after 12 months post-second vitrectomy | [37] |
| 22 | M/77 | American | None | Septic infrapatellar bursitis & contiguous cellulitis | Bursal & superficial pustule aspirates, skin punch biopsy | P. richardsiae | Intravenous amphotericin B for 2 weeks, followed by oral itraconazole for 3 months | Remission | [22] |
| 23 | M/45 | New Zealander | Renal transplant, transplant-related diabetes mellitus | Subcutaneous left foot abscess | Lesion aspirates, tissues | P. richardsiae | Surgical excision and liposomal amphotericin B for 1 week, repeat debridement & liposomal amphotericin B for 2 weeks followed by itraconazole for 6 weeks after recurrence | Remission | [23] |
| 24 | M/59 | Japanese | Liver cirrhosis, hepatocellular carcinoma, hepatitis C | Eumycetoma on left foot | Lesion biopsy | P. richardsiae | Surgical excision | Remission | [24] |
| 25 | M/43 | Korean | None | Right shin chromoblastomycosis | Skin tissue biopsy | P. richardsiae | Oral itraconazole 3 months, then combined with terbinafine for 3 months | Remission | [25] |
| 26 | M/60 | Sudanese | None | Yellow-grain eumycetoma on right foot | Grains | P. ochraceum | Surgical debulking | Lost to follow-up | [26] |
| 27 | M/54 | Canadian | None | Subcutaneous nodule on right patella & prepatellar bursitis | Prepatellar fluid aspirate | P. richardsiae | Therapy declined by patient | Lost to follow-up | [9] |
| 28 | M/59 | Martinican | Renal transplant | Left ankle abscess | Cutaneous tissue | P. ootheca | Itraconazole for 1.5 months, then posaconazole for 2 months | Remission | [27] |
| 29 | M/43 | American | Alcoholic cardiomyopathy, porcine mitral valve replacement, intravenous drug abuse, on systemic corticosteroid therapy | Endogenous endophthalmitis of right eye associated with osteomyelitis and endocarditis | Vitreous biopsy | P. richardsiae | Intravitreal amphotericin B during initial diagnostic vitrectomy, topical natamycin & intravenous amphotericin B, intravenous voriconazole added later, followed by repeat vitrectomy and intravitreal amphotericin B | Deceased due to massive intracranial haemorrhage | [35] |
| 30 | F/75 | Dutch Chinese | Diabetes mellitus, chronic kidney disease, rheumatoid arthritis | Left arm infection | Pus and wound | P. richardsiae | Voriconazole for 6 months, repeated surgical eradication, discontinuation of immunosuppression | Deceased due to subsequent progressive multifocal leukoencephalopathy due to Human polyomavirus 2 reactivation | [28] |
| 31 | M/78 | Japanese | Diabetes mellitus, prostate & bladder cancers, myelodysplastic syndrome | Subcutaneous cyst on right crus | Pus | P. richardsiae | Local heat therapy with disposable chemical pocket warmers for 2 months, then combined with intracutaneous amphotericin B for 3 months | Deceased due to cancers | [29] |
| 32 | F/35 | Indian | None | Perilacrimal organised lesion with transient nasolacrimal duct obstruction | Perilacrimal mass | P. richardsiae | Surgical excision | Remission | [10] |
| 33 | M/72 | Turkish | Papillary urothelial carcinoma | Urinary tract infection | Urine | P. richardsiae | Amphotericin B for 4 days followed by itraconazole for 14 days | Remission | [38] |
| 34 | M/78 | Spaniard | None | Subcutaneous cyst nodule on right dorsal hand | Cyst biopsy | P. richardsiae | Therapy declined by patient | Not mentioned | [30] |
| 35 | M/57 | Thai | Hepatitis B virus-related cirrhosis, liver transplant | Fungaemia associated with hepatic artery and portal vein thrombosis | Blood and liver blood clot | P. richardsiae | Second liver transplant, amphotericin B for 4 weeks | Remission | [40] |
| 36 | M/74 | Singaporean Chinese | Renal transplant, bronchiectasis | Subcutaneous thigh nodules | Skin biopsy and pus | P. richardsiae | Surgical excisions, itraconazole for 10 months | Remission | [11] |
| 37 | M/65 | Hong Kong Chinese | Hepatitis B virus-related liver failure, liver transplant | Progressive liver failure | Subhepatic abscess pus and drain fluids | P. hongkongense | Second liver transplant, amphotericin B and voriconazole | Remission | Present case |
DNA sequencing should be performed to accurately identify Pleurostoma species from fungal cultures with Pleurostoma- or Phialophora-like morphologies. Microscopically, Pleurostoma species are characterized by the possession of Phialophora-like single, separate, hyaline to pigmented, monophialidic, short conidiophores with inconspicuous or flaring collarettes producing smooth, hyaline, dimorphic conidia, either straight to allantoid or short and ellipsoid, aggregated as slimy masses at the apices of conidiogenous cells during their asexual life cycles [55]. However, identification to the species level is best performed by DNA sequencing of one or more conserved markers, especially the ITS region [9–11, 25–27, 29, 30, 38, 40]. Notably, two early cases of subcutaneous nodules were originally reported as Pleurostoma repens-associated based on morphological identification of the filamentous fungi isolated [56, 57]. However, subsequent molecular studies revealed that the two fungal isolates were actually Phaeoacremonium krajdenii instead [58]. As for MALDI–TOF MS, although it has been increasingly used for the rapid identification of filamentous fungi in clinical microbiology laboratories, use of this technology for Pleurostoma identification is still not possible since reference protein mass spectra for Pleurostoma species are still lacking in the databases by the two major commercial platforms (Bruker Daltonics and Vitek, bioMérieux). Moreover, our attempt of MALDI–TOF MS analysis using the Bruker Daltonics platform for our case isolate HKU44T as well as other reference Pleurostoma strains also failed in generating interpretable protein mass spectra for database matching, probably due to difficulties in extracting cellular proteins during formic acid digestion. An optimal protein extraction protocol for sample preparation and expansion of the reference mass spectral libraries will be essential before MALDI–TOF MS could be utilized for the rapid identification of Pleurostoma species.
Treatment of Pleurostoma infections is often achieved by a combination of surgical excision/debridement of the infected tissue and antifungal therapy. Of the 37 cases of Pleurostoma infections, 25 (67.6%) required surgery to remove the infected tissue and 22 (59.4%) involved the administration of antifungals (Table 3). For the present patient as well as the previous liver transplant recipient, they were both treated successfully with a second liver transplant and antifungal agents. As for the choice of antifungal agents, almost all the Pleurostoma strains tested in the present study had high MICs/MECs to fluconazole, 5-flucytosine and the echinocandins (Table 1). For amphotericin B, 18 (64.3%) of the 28 Pleurostoma strains tested, including our case isolate Pleurostoma hongkongense HKU44T, had MICs of ≤1 µg/mL, which is the general breakpoint for Candida species, Cryptococcus neoformans, Aspergillus fumigatus and Aspergillus niger [59]. As for the susceptibilities to the other triazoles, the results showed a high strain-to-strain variability (Table 1). In general, a strong Spearman correlation among the susceptibilities to different triazoles can be observed (Table S2). In view of the highly variable antifungal susceptibility profiles for Pleurostoma species and strains, MICs/MECs should be determined for deciding the optimal treatment regimen for individual patients. Remarkably, for the 36 Pleurostoma infection cases previously reported, seven of the isolates recovered were included in this study for susceptibility testing (ATCC 58041 [32], CBS 506.90 [8], CBS 131321T [26], UAMH 5052 [13], UAMH 5056 [12], UAMH 5058 [13] and UAMH 10082 [23]). Amongst these seven cases, antifungal therapies were only administered for three of the patients (Case 7, ATCC 58041 [32]; Case 16, CBS 506.90 [8] and Case 23, UAMH 10082 [23] in Table 3). For Case 7, the patient was treated with the debridement of infected bone and amphotericin B prescription for one week followed by flucytosine prescription for two and a half weeks [32], although susceptibility testing in this study showed that the isolate ATCC 58041 possessed MICs of 1 µg/mL and >16 µg/mL for these two antifungals, respectively. Similarly, for Case 23, the patient was first treated with surgical excision and prescription of liposomal amphotericin B for one week. However, due to recurrence a repeat debridement was performed and liposomal amphotericin B for two weeks followed by itraconazole for six weeks was prescribed [23]. Again, susceptibility testing in this study showed that isolate UAMH 10082 possessed high MICs of 2 µg/mL and >8 µg/mL for these two antifungals, respectively. For Case 16, the patient was treated with clotrimazole for 15 days [8]. Susceptibility to this drug was not tested in the present study and therefore, the activity of this drug to the fungal isolate (CBS 506.90) remains unknown. As for the present reported case due to Pleurostoma hongkongense, the patient was treated with antimicrobials including anidulafungin before the second liver transplant. However, this was not successful in curing the infection which may be partly due to a high MEC for anidulafungin (>16 µg/mL) for HKU44T. After the second liver transplant, the patient was treated with amphotericin B and voriconazole while HKU44T possessed MICs of 0.5 and 16 µg/mL for these two drugs, respectively. As a result, antifungal susceptibility testing should be performed for Pleurostoma species to guide patient management and to avoid the unnecessary use of less or ineffective drugs.
Supplementary Material
Acknowledgements
This work was partly supported by the Innovation and Technology Fund Midstream Research Programme for Universities (MRP/026/18) of the Innovation and Technology Commission, the Government of the Hong Kong Special Administrative Region. Any opinions, findings, conclusions or recommendations expressed in this material/event (or by members of the project team) do not reflect the views of the Government of the Hong Kong Special Administrative Region, the Innovation and Technology Commission or the Innovation and Technology Fund Research Projects Assessment Panel. We are grateful to Mrs Adrien Szekely of NCPF for providing the NCPF reference strains for free; Professor Maiken C. Arendrup and Dr Karin M. Jørgensen of SSI for providing the CNM-CL and CNM-CM reference strains for free; as well as the curators, especially Mr Travis W. Adkins, of NRRL for providing the NRRL reference strains for free. We also thank the Electron Microscope Unit of The University of Hong Kong which helped process the samples for scanning electron microscopy.
Funding Statement
This work was partly supported by the Innovation and Technology Fund Midstream Research Programme for Universities (grant number MRP/026/18) of the Innovation and Technology Commission, the Government of the Hong Kong Special Administrative Region. Any opinions, findings, conclusions or recommendations expressed in this material/event (or by members of the project team) do not reflect the views of the Government of the Hong Kong Special Administrative Region, the Innovation and Technology Commission or the Innovation and Technology Fund Research Projects Assessment Panel.
Disclosure statement
Patrick C. Y. Woo has provided scientific advisory/laboratory services for Gilead Sciences, Incorporated; International Health Management Associates, Incorporated; Merck & Corporation, Incorporated; Micología Molecular S.L. and Pfizer, Incorporated. The other authors report no conflicts of interest. The funding sources had no role in study design, data collection, analysis, interpretation, or writing of the report. The authors alone are responsible for the content and the writing of the manuscript. Part of this study has been presented as an abstract for the 30th European Congress of Clinical Microbiology and Infectious Diseases.
References
- 1.Tulasne LR, Tulasne C.. Selecta Fungorum Carpologia II. Paris: Imperiali Typographeo Excudebatur; 1863. [Google Scholar]
- 2.Réblová M, Jaklitsch WM, Réblová K, et al. . Phylogenetic reconstruction of the Calosphaeriales and Togniniales using five genes and predicted RNA secondary structures of ITS, and Flabellascus tenuirostris gen. et sp. nov. PLoS One. 2015;10(12):e0144616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Réblová M, Miller AN, Rossman AY, et al. . Recommendations for competing sexual-asexually typified generic names in Sordariomycetes (except Diaporthales, Hypocreales, and Magnaporthales). IMA Fungus. 2016;7(1):131–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schol-Schwarz MB. Revision of the genus Phialophora (Moniliales). Persoonia. 1970;6(1):59–94. [Google Scholar]
- 5.Barr ME. Notes on the Calosphaeriales. Mycologia. 1985;77(4):549–565. [Google Scholar]
- 6.Melin E, Nannfeldt JA.. Researches into the blueing of ground wood-pulp. Svenska Skogsvårdsför Tidskr. 1934;32:397–430. [Google Scholar]
- 7.Moskowitz LB, Cleary TJ, McGinnis MR, et al. . Phialophora richardsiae in a lesion appearing as a giant cell tumor of the tendon sheath. Arch Pathol Lab Med. 1983;107:374–376. [PubMed] [Google Scholar]
- 8.Singh SM, Agrawal A, Naidu J, et al. . Cutaneous phaeohyphomycosis caused by Phialophora richardsiae and the effect of topical clotrimazole in its treatment. Anton Leeuwenh. 1992;61(1):51–55. [DOI] [PubMed] [Google Scholar]
- 9.Levenstadt JS, Poutanen SM, Mohan S, et al. . Pleurostomophora richardsiae – an insidious fungus presenting in a man 44 years after initial inoculation: a case report and review of the literature. Can J Infect Dis Med Microbiol. 2012;23(3):110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alam MS, Vaidehi D, Therese KL, et al. . Rare Pleurostomophora richardsiae mass causing transient nasolacrimal duct obstruction. Ophthal Plast Reconstr Surg. 2017;33(6):e154–e156. [DOI] [PubMed] [Google Scholar]
- 11.Tee LY, Tan BH, Tan A-L, et al. . Subcutaneous phaeohyphomycosis caused by Pleurostomophora richardsiae in a renal transplant recipient. JAAD Case Rep. 2020;6(1):66–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz IS, Emmons CW.. Subcutaneous cystic granuloma caused by a fungus of wood pulp (Phialophora richardsiae). Am J Clin Pathol. 1968;49(4):500–505. [DOI] [PubMed] [Google Scholar]
- 13.Corrado ML, Weitzman I, Stanek A, et al. . Subcutaneous infection with Phialophora richardsiae and its susceptibility to 5-fluorocytosine, amphotericin B and miconazole. Sabouraudia. 1980;18(2):97–104. [PubMed] [Google Scholar]
- 14.Reyes FA, Buchman MT.. Phialophora richardsiae infection mimicking a soft tissue mass of a finger. J Hand Surg Br. 1986;11(2):274. [DOI] [PubMed] [Google Scholar]
- 15.Suppiah M, Chin CS, Keah KC.. Phialophora richardsiae isolated from a cutaneous lesion. Med J Malaysia. 1987;42(4):306–308. [PubMed] [Google Scholar]
- 16.Bonnefoy A, Luboinski J, Francoual S, et al. . Phaeohyphomycosis due to Phialophora richardsiae. Med Mal Infect. 1988;18(4):235–238. [Google Scholar]
- 17.Ikai K, Tomono H, Watanabe S.. Phaeohyphomycosis caused by Phialophora richardsiae. J Am Acad Dermatol. 1988;19(3):478–481. [DOI] [PubMed] [Google Scholar]
- 18.Pitrak DL, Koneman EW, Estupinan RC, et al. . Phialophora richardsiae infection in humans. Rev Infect Dis. 1988;10(6):1195–1203. [DOI] [PubMed] [Google Scholar]
- 19.Tam M, Freeman S.. Phaeohyphomycosis due to Phialophora richardsiae. Australas J Dermatol. 1989;30(1):37–40. [DOI] [PubMed] [Google Scholar]
- 20.Guého E, Bonnefoy A, Luboinski J, et al. . Subcutaneous granuloma caused by Phialophora richardiae: case report and review of the literature Phialophora richardsiae. Mycoses. 1989;32(5):219–223. [DOI] [PubMed] [Google Scholar]
- 21.Jumaa PA, Lightowler C, Baker LRI, et al. . Cutaneous infection caused by Phialophora richardsiae treated successfully by surgical excision in an immunocompromised patient. J Infect. 1995;30(3):261–262. [DOI] [PubMed] [Google Scholar]
- 22.Cornia PB, Raugi GJ, Miller RA.. Phialophora richardsiae bursitis treated medically. Am J Med. 2003;115(1):77–79. [DOI] [PubMed] [Google Scholar]
- 23.Yehia M, Thomas M, Pilmore H, et al. . Subcutaneous black fungus (phaeohyphomycosis) infection in renal transplant recipients: three cases. Transplantation. 2004;77(1):140–142. [DOI] [PubMed] [Google Scholar]
- 24.Sakayama K, Kidani T, Sugawara Y, et al. . Mycetoma of foot: a rare case report and review of the literature. Foot Ankle Int. 2004;25(10):763–767. [DOI] [PubMed] [Google Scholar]
- 25.Son Y-M, Kang H-K, Na S-Y, et al. . Chromoblastomycosis caused by Phialophora richardsiae. Ann Dermatol. 2010;22(3):362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mhmoud NA, Ahmed SA, Fahal AH, et al. . Pleurostomophora ochracea, a novel agent of human eumycetoma with yellow grains. J Clin Microbiol. 2012;50(9):2987–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amazan E, Desbois N, Fidelin G, et al. . First case of phaeohyphomycosis due to Pleurostoma ootheca in a kidney transplant recipient in Martinique (French West Indies). Med Sante Trop. 2014;24(3):323–325. [DOI] [PubMed] [Google Scholar]
- 28.de Regt MJA, Murk J-L, Schneider-Hohendorf T, et al. . Progressive multifocal leukoencephalopathy and black fungus in a patient with rheumatoid arthritis without severe lymphocytopenia. JMM Case Rep. 2016;3(4):e005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noguchi H, Hiruma M, Matsumoto T, et al. . Subcutaneous cystic phaeohyphomycosis due to Pleurostomophora richardsiae. J Dermatol. 2017;44(4):e62–e63. [DOI] [PubMed] [Google Scholar]
- 30.Cuenca-Barrales C, De Salazar A, Chueca N, et al. . Phaeohyphomycosis due to Pleurostomophora richardsiae: an uncommon cutaneous fungal infection. J Eur Acad Dermatol Venereol. 2018;32(10):e376–e377. [DOI] [PubMed] [Google Scholar]
- 31.Torstrick RF, Harrison K, Heckman JD, et al. . Chronic bursitis caused by Phialophora richardsiae. A case report. JBJS. 1979;61(5):772–774. [PubMed] [Google Scholar]
- 32.Yangco BG, TeStrake D, Okafor J.. Phialophora richardsiae isolated from infected human bone: morphological, physiological and antifungal susceptibility studies. Mycopathologia. 1984;86(2):103–111. [DOI] [PubMed] [Google Scholar]
- 33.Uberti-Foppa C, Fumagalli L, Gianotti N, et al. . First case of osteomyelitis due to Phialophora richardsiae in a patient with HIV infection. AIDS. 1995;9(8):975–976. [PubMed] [Google Scholar]
- 34.Rossmann SN, Cernoch PL, Davis JR.. Dematiaceous fungi are an increasing cause of human disease. Clin Infect Dis. 1996;22(1):73–80. [DOI] [PubMed] [Google Scholar]
- 35.Fox AR, Houser KH, Morris WR, et al. . Dematiaceous fungal endophthalmitis: report of a case and review of the literature. J Ophthalmic Inflamm Infect. 2016;6(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Listemann H. Die kulturelle untersuchung eines tranensteines mit isolierung des pilzes Phialophora richardsiae. Ernst Rodenwaldt Arc. 1975;2(2):45–52. [Google Scholar]
- 37.Lieb DF, Smiddy WE, Miller D, et al. . Case report: fungal endophthalmitis caused by Phialophora richardsiae. Retina. 2003;23(3):406–407. [DOI] [PubMed] [Google Scholar]
- 38.Polat SH, Kizilay F, Uyan A, et al. . Urinary tract infection due to Pleurostomophora richardsiae. Med Mycol. 2018;56(Suppl. 2):S91. [Google Scholar]
- 39.Juma A. Phialophora richardsiae endocarditis of aortic and mitral valves in a diabetic man with a porcine mitral valve. J Infect. 1993;27(2):173–175. [DOI] [PubMed] [Google Scholar]
- 40.Sribenjalux W, Chongtrakool P, Chayakulkeeree M.. Disseminated phaeohyphomycosis with hepatic artery and portal vein thrombosis caused by Pleurostomophora richardsiae in a liver transplant recipient: a case report. Transpl Infect Dis. 2019;21(3):e13075. [DOI] [PubMed] [Google Scholar]
- 41.Ngan AHY. Atlas of fungi. Hong Kong: June Just Printing; 2016. [Google Scholar]
- 42.Woo PCY, Ngan AHY, Tsang CCC, et al. . Clinical spectrum of Exophiala infections and a novel Exophiala species, Exophiala hongkongensis. J Clin Microbiol. 2013;51(1):260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White T, Bruns T, Lee S, et al. . Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Shinsky J, editor. PCR protocols: a guide to methods and applications. San Diego: Academic Press; 1990. p. 315–322. [Google Scholar]
- 44.O’Donnell K. Fusarium and its near relatives. In: Reynolds DR, Taylor JW, editor. The fungal holomorph: Mitotic, meiotic and pleomorphic speciation in fungal systematics. Wallingford: CAB International; 1993. p. 225–233. [Google Scholar]
- 45.Cruse M, Telerant R, Gallagher T, et al. . Cryptic species in Stachybotrys chartarum. Mycologia. 2002;94(5):814–822. [PubMed] [Google Scholar]
- 46.Hall TA. Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 47.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 2000;17(4):540–552. [DOI] [PubMed] [Google Scholar]
- 49.Talavera G, Castresana J.. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 2007;56(4):564–577. [DOI] [PubMed] [Google Scholar]
- 50.Tamura K, Stecher G, Peterson D, et al. . MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drummond AJ, Suchard MA, Xie D, et al. . Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012;29(8):1969–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arendrup MC, Meletiadis J, Mouton JW. EUCAST definitive Document E.Def 9.3.1. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds 2017. Available from: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_9_3_1_Mould_testing__definitive.pdf. [DOI] [PubMed]
- 53.Tsang C-C, Tang JYM, Chan K-F, et al. . Diversity of phenotypically non-dermatophyte, non-Aspergillus filamentous fungi causing nail infections: importance of accurate identification and antifungal susceptibility testing. Emerg Microbes Infect. 2019;8(1):531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tekpinar AD, Kalmer A.. Utility of various molecular markers in fungal identification and phylogeny. Nova Hedwigia. 2019;109(1–2):187–224. [Google Scholar]
- 55.Vijaykrishna D, Mostert L, Jeewon R, et al. . Pleurostomophora, an anamorph of Pleurostoma (Calosphaeriales), a new anamorph genus morphologically similar to Phialophora. Stud Mycol. 2004;50:387–395. [Google Scholar]
- 56.Hironaga M, Nakano K, Yokoyama I, et al. . Phialophora repens, an emerging agent of subcutaneous phaeohyphomycosis in humans. J Clin Microbiol. 1989;27(3):394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakano K, Yokoyama I, Kitajima J, et al. . A case of subcutaneous granuloma caused by Phialophora repens. Rinsho Hifuka. 1990;44(1):25–28. [Google Scholar]
- 58.Mostert L, Groenewald JZ, Summerbell RC, et al. . Species of Phaeoacremonium associated with infections in humans and environmental reservoirs in infected woody plants. J Clin Microbiol. 2005;43(4):1752–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.European Committee on Antimicrobial Susceptibility Testing . Overview of antifungal ECOFFs and clinical breakpoints for yeasts, moulds and dermatophytes using the EUCAST E.Def 7.3, E.Def 9.3 and E.Def 11.0 procedures. Version 2, valid from 2020-09-24. 2020. Available from: http://www.eucast.org/.
- 60.Meyers WM, Dooley JR, Kwon-Chung KJ.. Mycotic granuloma caused by Phialophora repens. Am J Clin Pathol. 1975;64(4):549–555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A dried specimen of the case isolate HKU44T (the holotype) was deposited to UAMH whereas living cultures of HKU44T were deposited to UAMH and the Culture Collection of Switzerland (CCOS). The ITS, partial 28S nrDNA, partial 18S nrDNA and partial β-tubulin gene sequences of HKU44T and the ex-type/reference strains of other Pleurostoma species were deposited in the DDBJ/ENA/GenBank databases (Table S1).