Abstract
Background
Type 2 diabetes and obesity, as states of chronic inflammation, are risk factors for severe COVID-19. Metformin has cytokine-reducing and sex-specific immunomodulatory effects. Our aim was to identify whether metformin reduced COVID-19-related mortality and whether sex-specific interactions exist.
Methods
In this retrospective cohort analysis, we assessed de-identified claims data from UnitedHealth Group (UHG)'s Clinical Discovery Claims Database. Patient data were eligible for inclusion if they were aged 18 years or older; had type 2 diabetes or obesity (defined based on claims); at least 6 months of continuous enrolment in 2019; and admission to hospital for COVID-19 confirmed by PCR, manual chart review by UHG, or reported from the hospital to UHG. The primary outcome was in-hospital mortality from COVID-19. The independent variable of interest was home metformin use, defined as more than 90 days of claims during the year before admission to hospital. Covariates were comorbidities, medications, demographics, and state. Heterogeneity of effect was assessed by sex. For the Cox proportional hazards, censoring was done on the basis of claims made after admission to hospital up to June 7, 2020, with a best outcome approach. Propensity-matched mixed-effects logistic regression was done, stratified by metformin use.
Findings
6256 of the 15 380 individuals with pharmacy claims data from Jan 1 to June 7, 2020 were eligible for inclusion. 3302 (52·8%) of 6256 were women. Metformin use was not associated with significantly decreased mortality in the overall sample of men and women by either Cox proportional hazards stratified model (hazard ratio [HR] 0·887 [95% CI 0·782–1·008]) or propensity matching (odds ratio [OR] 0·912 [95% CI 0·777–1·071], p=0·15). Metformin was associated with decreased mortality in women by Cox proportional hazards (HR 0·785, 95% CI 0·650–0·951) and propensity matching (OR 0·759, 95% CI 0·601–0·960, p=0·021). There was no significant reduction in mortality among men (HR 0·957, 95% CI 0·82–1·14; p=0·689 by Cox proportional hazards).
Interpretation
Metformin was significantly associated with reduced mortality in women with obesity or type 2 diabetes who were admitted to hospital for COVID-19. Prospective studies are needed to understand mechanism and causality. If findings are reproducible, metformin could be widely distributed for prevention of COVID-19 mortality, because it is safe and inexpensive.
Funding
National Heart, Lung, and Blood Institute; Agency for Healthcare Research and Quality; Patient-Centered Outcomes Research Institute; Minnesota Learning Health System Mentored Training Program, M Health Fairview Institutional Funds; National Center for Advancing Translational Sciences; and National Cancer Institute.
Introduction
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread throughout the world. Despite exponential growth in COVID-19-related research, better understanding of this highly contagious and lethal virus is needed. An overall mortality rate of more than 5% for all patients admitted to hospital with COVID-19 highlights the urgent need for treatments while vaccines are developed.
Early on in the outbreak, male sex and older age were identified as leading risk factors for mortality and severe morbidity from COVID-19. Subsequent studies identified hypertension, diabetes, coronary artery disease, tobacco use, and obesity as risk factors for COVID-19 outcomes.1 People admitted to hospital with COVID-19 with a body-mass index (BMI) of 25 kg/m2 or more had a higher risk of needing mechanical ventilation, after controlling for diabetes, hypertension, and cardiovascular disease, and obesity appeared to be the most significant risk factor.1 Additionally, among individuals with COVID-19 and obesity, men had a higher risk of developing severe COVID-19 than women.1 This sex difference might be explained by visceral adiposity, which accumulates at a lower BMI in men.2
Visceral adipocytes secrete many of the proinflammatory and coagulopathic molecules that are implicated in COVID-19 morbidity, including interleukin (IL)-6, tumor necrosis factor α (TNFα), and D-dimer.3 High levels of TNFα have been found in lung tissue of people with COVID-19.4 TNFα contributes to insulin resistance, levels of TNFα are higher in individuals with type 2 diabetes, and both type 2 diabetes and obesity are associated with lower levels of the anti-inflammatory cytokine, IL-10.5 Metformin, a medication for type 2 diabetes, decreases TNFα and IL-6, boosts IL-10, and has been found to cause these beneficial effects significantly more in females than males in both animal and human studies.6, 7, 8
Research in context.
Evidence before this study
One of the first studies of COVID-19, controlling for body-mass index (BMI) and related comorbidities (ie, diabetes, hypertension, and coronary artery disease), showed that a BMI of more than 25 kg/m2 was a bigger risk factor for poor outcomes from COVID-19 than diabetes. Considering that both diabetes and obesity are states of chronic inflammation, and most individuals with obesity have some degree of insulin resistance and hyperglycaemia, we wanted to assess a medication that treated both obesity and diabetes and could realistically be given to a large number of people. Although metformin generally causes weight loss, it is generally small. Metformin has been shown to improve inflammation and cytokines associated with obesity in people with and without diabetes. In one of the largest such studies of metformin in humans without diabetes, the diabetes prevention programme, metformin was associated with a bigger reduction of inflammation in women than men.
Added value of this study
To the best of our knowledge, this is the first study to show reduced severity of COVID-19 with use of metformin before diagnosis with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Given the previously demonstrated effects of metformin on inflammation in women more than men, it is possible that the main mechanism of benefit from metformin is from reduced inflammation. Our study of home metformin use also highlights the effect that medications can have early on in the disease course (because metformin is stopped at hospitalisation in the USA). Our analysis supports the preventive use of metformin, before infection with SARS-CoV-2, to prevent severe COVID-19 in patients with diabetes or obesity. Our study also highlights the need to differentiate whether starting metformin at the time of diagnosis of SARS-CoV-2 infection will convey the same benefits as use of metformin before SARS-CoV-2 infection.
Implications of all the available evidence
Therapies to decrease the severity of COVID-19 are desperately needed. If our findings are replicated in other analyses and prospective trials, metformin should be widely distributed for prevention of severe COVID-19 in people with diabetes or a BMI of at least 30 kg/m2). Metformin is one of the few COVID-19 therapies that could be given to all adults, regardless of current or potential pregnancy status, as long as they do not have severe kidney disease. Follow-up is only needed after 1 year of metformin use, at which point kidney function should be assessed again. Metformin costs less than US$4 per month. Thus, with low cost and need for follow-up safety assessments, metformin could be distributed to people who do not have access to regular medical follow-up.
Metformin reduces TNFα and other inflammatory adipokines that are high in people with obesity and type 2 diabetes and contribute to COVID-19 severity. Given this and early findings of lower mortality among metformin users,9, 10 our primary objective was to understand whether home metformin use was associated with decreased mortality in people hospitalised for COVID-19 with obesity or type 2 diabetes, and our secondary objectives were to assess sex-specific effects. We hypothesised that metformin would be associated with decreased mortality from COVID-19 in people with type 2 diabetes or obesity, and that this benefit would be higher in women than men, given the sex-specific anti-inflammatory effects of metformin. We had an exploratory objective to explore home use of TNFα inhibitors and mortality from COVID-19.
Methods
Study design and participants
We did a retrospective cohort analysis of data from Jan 1 to June 7, 2020, from UnitedHealth Group (UHG)'s Clinical Discovery Claims Database. This database includes de-identified individual-level and state-level data for individuals with COVID-19 admissions in all 50 US states, covering a diverse range of ages, ethnicities, and regions, a limited set of SARS-CoV-2 laboratory tests from select third-party vendors and clinical sites, and historical data. The claims data include medical and pharmacy claims, laboratory results, and enrolment records. This study was approved by the University of Minnesota institutional review board (STUDY00001489), which provided a waiver of consent.
We included people with type 2 diabetes or obesity in our analysis because both populations have higher levels of cytokines that contribute to COVID-19 severity, as compared with those without type 2 diabetes and obesity, which might be reduced by metformin. Inclusion criteria were age 18 years or older; type 2 diabetes or obesity (defined based on claims of International Classification of Diseases codes; appendix p 7); at least 6 months of continuous enrolment in UHG in 2019; and admission to hospital for COVID-19 (COVID-19 diagnosis confirmed by PCR), or manual chart review by UHG, or reported from the hospital to UHG. Individuals with both commercial and Medicare Advantage insurance were included. Patients were excluded if they had type 1 diabetes or their age was missing from the database. An assumption was made that there was no missingness among the other variables.
Outcomes
Our primary outcome was in-hospital mortality defined using the hospital disposition indicator; individuals who remained in hospital on June 7, 2020, without a hospital disposition, were censored in Cox proportional hazards model analyses and excluded from mixed-model and propensity-matched analyses. The database did not include outcomes related to in-hospital complications, intensive care unit, or ventilator use, and thus an analysis on these endpoints was not possible.
We had an exploratory objective to explore home use of TNFα inhibitors and mortality from COVID-19.
Statistical analysis
The independent variable was metformin use, defined by filled pharmacy prescriptions (visible through prescription claims, matching the generic drug ingredient with the string “metformin”) of at least 90 days of metformin within 12 months of COVID-19 diagnosis. The exploratory independent variable of interest was home use of TNFα inhibitors.
Potential confounding medications hypothesised to be protective or harmful for patients with COVID-19 determined by a large evidence-based consortium were assessed, as were possible confounding comorbidities (appendix p 7).
Age was expressed by median (IQR). Categorical variables were expressed by percentages. Univariate analysis compared mortality for people without versus with home metformin use, and we were unable to do this by subsets of sex because of limits of the dataset.
To determine whether metformin use was independently associated with reduced mortality for patients admitted to hospital for COVID-19, multivariate models for mortality were developed with the use of the least absolute shrinkage and selection operator (LASSO) method,11 with the tuning parameter determined by the Akaike information criterion. We also considered clinically relevant pairwise interactions to determine whether their association with mortality differed according to metformin status. The non-linear effect of age was modelled in two manners, using restricted cubic splines or clinically relevant categories—ie, 0–55, 56–65, 66–75, 76–85, and 86 and over. Subgroup analyses of multivariate models were done by sex.
Logistic regression was done, controlling for all covariates in our conceptual framework and for LASSO variables and state, with and without specific disease–medication interaction terms (appendix p 2).2 Mixed-effects logistic regression was done with state-level random effects, controlling for LASSO variables, with and without specific disease–medication interaction terms. Only patients with known hospital disposition were included (appendix p 3).3 Cox proportional hazards regression was done by strata-specific and shared-frailty effects, with and without specific disease–medication interaction terms. Censoring was done on the basis of claims made after admission to hospital up to June 7, 2020. Using a best outcome approach, patients discharged not to hospice were assigned a censoring time equal to the longest observed hospital stay (169 days).12 Scaled Schoenfeld residual graphs and log–log plots were analysed to confirm adequacy of the proportional hazards assumption for home metformin use (appendix pp 4, 8).4 Propensity-matched mixed-effects logistic regression was done, stratified by metformin use. Propensity scores were estimated with logistic regression, with variables selected by the LASSO logistic model. Two evenly matched groups were formed with the common caliper set at 0·2 (appendix p 8), and a model with exact matching.13 Even distribution of propensity scores was confirmed between matched groups, with standardised differences less than 0·1 for all confounding variables (appendix p 9). Univariate logistic regression was then used to compare mortality for people who were receiving (vs not receiving) home metformin among the matched cohort. Kaplan-Meier survival curves were also estimated and compared using a log-rank test.
Sensitivity analyses were done in individuals with COVID-19 confirmed by PCR. As another form of sensitivity analysis and assessment of residual confounding, we assessed the e value for each outcome of interest, using the method outlined by VanderWeele and colleagues,14 for frequent outcomes (as the percentage of people who died was >15% for both treatment groups). Statistical analyses were done using Stata, version 16. Statistical significance was defined as a two-tailed p value of less than 0·05.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the Article. CJT had full access to all the data in the study and had final responsibility for the decision to submit for publication. All aspects were supported by UHG, and CJT, SH, CM, and JG had access to raw data.
Results
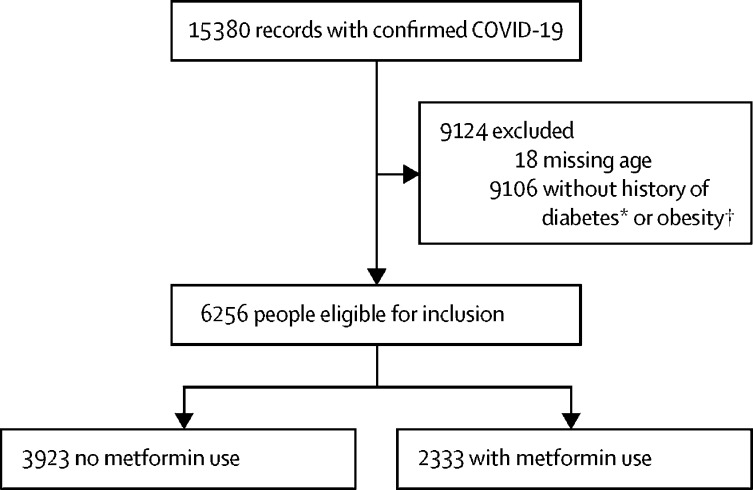
Using data from UHG's Clinical Discovery Database, 6256 of 15 380 individuals with pharmacy claims data and at least 6 months of enrolment were diagnosed and admitted to hospital with COVID-19 between Jan 1 and June 7, 2020, and were eligible for analysis (figure 1 ; analysed June 16, 2020). The characteristics of the two cohorts are shown in table 1 . 3302 (52·8%) of 6256 individuals were women, with a median age of 73 years (IQR 58–88). The median age of the 15 362 people included in the exploratory analyses was 70 years (IQR 58–80). Of the 2333 people in the metformin group, 394 (16·9%) died of COVID-19 during admission to hospital for COVID-19, compared with 791 (20·2%) of 3923 in the non-metformin group. In unadjusted analyses, metformin was associated with decreased mortality (table 2 ).
Figure 1.
Patient selection from UnitedHealthcare Group database between Jan 1 and June 7, 2020
*People with type 1 diabetics were excluded. †Obesity defined as a body-mass index of at least 30 kg/m2.
Table 1.
Demographic and clinical characteristics of patients admitted to hospital for COVID-19 with 6 months of continuous insurance coverage in 2019
| No metformin (n=3923) | Metformin (n=2333) | ||
|---|---|---|---|
| Demographics | |||
| Age, years | 76·0 (67·0–84·0) | 73·0 (66·0–80·0) | |
| <56 | 266 (6·8%) | 186 (8·0%) | |
| 56–65 | 535 (13·6%) | 387 (16·6%) | |
| 66–75 | 1112 (28·3%) | 808 (34·6%) | |
| 76–85 | 1234 (31·5%) | 694 (29·7%) | |
| >85 | 776 (19·8%) | 258 (11·1%) | |
| Sex | |||
| Male | 1750 (44·6%) | 1204 (51·6%) | |
| Female | 2173 (55·4%) | 1129 (48·4%) | |
| Transfer* | 705 (18·0%) | 418 (17·9%) | |
| Pre-existing conditions | |||
| Type 2 diabetes | 3719 (94·8%) | 2316 (99·3%) | |
| Type 1 diabetes | 278 (7·1%) | 108 (4·6%) | |
| Essential hypertension | 2370 (60·4%) | 1314 (56·3%) | |
| Tobacco | 8 (0·2%) | 5 (0·2%) | |
| Coronary artery disease | 864 (22·0%) | 456 (19·5%) | |
| Heart failure with preserved ejection fraction | 346 (8·8%) | 121 (5·2%) | |
| Heart failure with reduced ejection fraction | 344 (8·8%) | 133 (5·7%) | |
| Heart failure, unspecified | 659 (16·8%) | 239 (10·2%) | |
| Liver disease | 160 (4·1%) | 102 (4·4%) | |
| Venous thromboembolism | 161 (4·1%) | 62 (2·7%) | |
| Neutropenia | 9 (0·2%) | 5 (0·2%) | |
| Cancer | 441 (11·2%) | 281 (12·0%) | |
| Coagulation defect | 54 (1·4%) | 14 (0·6%) | |
| Valve repair | 33 (0·8%) | 17 (0·7%) | |
| Chronic obstructive pulmonary disease | 688 (17·5%) | 305 (13·1%) | |
| Interstitial lung disease | 70 (1·8%) | 33 (1·4%) | |
| Chronic kidney disease, stage 3, 4 | 729 (18·6%) | 147 (6·3%) | |
| Chronic kidney disease, unspecified | 527 (13·4%) | 219 (9·4%) | |
| End stage renal disease | 297 (7·6%) | 14 (0·6%) | |
| Atrial fibrillation | 630 (16·1%) | 290 (12·4%) | |
| Cerebrovascular accident or transient ischaemic attack | 432 (11·0%) | 207 (8·9%) | |
| Alcohol abuse | 43 (1·1%) | 17 (0·7%) | |
| HIV | 18 (0·5%) | 18 (0·8%) | |
| Asthma | 166 (4·2%) | 96 (4·1%) | |
| General influenza | 65 (1·7%) | 35 (1·5%) | |
| Swine or avian influenza | 17 (0·4%) | 12 (0·5%) | |
| Inflammatory bowel disease | 28 (0·7%) | 11 (0·5%) | |
| Systemic lupus erythematosus or rheumatoid arthritis | 73 (1·9%) | 27 (1·2%) | |
| Dementia | 663 (16·9%) | 273 (11·7%) | |
| Charlson comorbidity index | 5·0 (3·0–7·0) | 4·0 (3·0–6·0) | |
| Diabetes complications severity index | 2·0 (1·0–4·0) | 2·0 (0·0–3·0) | |
| Body-mass index category | |||
| Absence of any weight-related code | 3548 (90·4%) | 2215 (94·9%) | |
| Obesity | 354 (9·0%) | 111 (4·8%) | |
| Medications | |||
| Ursodiol | 8 (0·2%) | 2 (0·1%) | |
| Angiotensin-converting enzyme inhibitors | 1069 (27·2%) | 912 (39·1%) | |
| Angiotensin II receptor blocker | 1003 (25·6%) | 731 (31·3%) | |
| Statin | 2591 (66·0%) | 1860 (79·7%) | |
| Antiplatelet | 618 (15·8%) | 342 (14·7%) | |
| Anticoagulation | 808 (20·6%) | 407 (17·4%) | |
| Tenofovir | 5 (0·1%) | 5 (0·2%) | |
| Highly active antiretroviral therapy | 16 (0·4%) | 14 (0·6%) | |
| Azithromycin | 541 (13·8%) | 321 (13·8%) | |
| GLP-1 receptor agonists | 27 (0·7%) | 35 (1·5%) | |
| Insulin | 1564 (39·9%) | 783 (33·6%) | |
| Steroids | 1010 (25·7%) | 546 (23·4%) | |
| Hydroxychloroquine | 47 (1·2%) | 14 (0·6%) | |
| Janus kinase inhibitors | 3 (0·1%) | 1 (0·04%) | |
| Calcineurin inhibitors | 85 (2·2%) | 31 (1·3%) | |
| mTor inhibitor | 1 (0·03%) | 0 (0·0%) | |
| β blocker | 2136 (54·4%) | 1213 (52·0%) | |
| Ivermectin | 35 (0·9%) | 13 (0·6%) | |
| β2 agonist | 1233 (31·4%) | 608 (26·1%) | |
| Allopurinol | 400 (10·2%) | 189 (8·1%) | |
| Azathioprine and mycophenolate mofetil | 42 (1·1%) | 13 (0·6%) | |
| Montelukast | 289 (7·4%) | 181 (7·8%) | |
| Non-steroidal anti-inflammatory | 362 (9·2%) | 316 (13·5%) | |
| Diuretics | 1604 (40·9%) | 670 (28·7%) | |
| Mast cell stabiliser | 65 (1·7%) | 41 (1·8%) | |
| Valacyclovir, acyclovir, or valgancyclovir | 129 (3·3%) | 72 (3·1%) | |
Data are median (IQR) or n (%). Terms are according to the International Classification of Diseases. 791 (21·3%) in the no metformin group and 394 (17·8%) in the metformin group died during hospitalisation (unadjusted proportions).
Transfer represents interhospital transfer during the hospitalisation for COVID-19.
Table 2.
Association between home metformin use and mortality in unadjusted and adjusted analyses in patients with type 2 diabetes or obesity hospitalised for COVID-19 (confirmed or presumed)
| Result of analysis | p value | |||
|---|---|---|---|---|
| Primary analyses, overall population | ||||
| Cox proportional hazards, stratified model*† | 0·887 (0·782–1·008) | 0·65 | ||
| Cox proportional hazards, shared frailty model*† | 0·884 (0·778–1·003) | 0·056 | ||
| Propensity matched model, exact matching‡§ | 0·912 (0·777–1·071) | 0·15¶ | ||
| Propensity matched model, caliper 0·2‡§ | 0·898 (0·768–1·051) | 0·10¶ | ||
| Subgroup analysis in women | ||||
| Cox proportional hazards, shared frailty model*† | 0·785 (0·650–0·951) | 0·013 | ||
| With disease-medication interaction terms‖ | 0·782 (0·646–0·947) | 0·012 | ||
| Propensity matched model, caliper 0·2 (n=2125)‡§ | 0·759 (0·601–0·960) | 0·02¶ | ||
| Sensitivity analyses in women with COVID-19 confirmed by PCR | ||||
| Cox proportional hazards, shared frailty model*† | 0·808 (0·651–1·003) | 0·053 | ||
| Cox proportional hazards, shared frailty model†** | 0·790 (0·637–0·978) | 0·031 | ||
| Propensity matched model (n=1416)‡§ | 0·744 (0·565–0·980) | 0·02¶ | ||
| Unadjusted analyses | 0·802 (0·701–0·917) | 0·001 | ||
Data are hazard ratio (95% CI) or odds ratio (95% CI). Patients had at least 6 months of continuous coverage in 2019 and 90 days of metformin use. ACEi=angiotensin-converting enzyme inhibitor. ARB=angiotensin receptor blocker.
Adjusted for variables selected by least absolute shrinkage and selection operator: age, sex (in overall, not in subgroups by sex), comorbidities (hypertension, tobacco use, venous thromboembolism, neutropenia, chronic obstructive pulmonary disease, chronic kidney disease, alcohol abuse, HIV, asthma, inflammatory bowel disease, dementia, Charlson comorbidity index, and the diabetes complications and severity index), and medications (ursodiol, ACEi, ARB, steroids, ivermectin, β2 agonists, mast cell stabilisers, allopurinol, azathioprine, mycophenolate mofetil), and state.
Hazard ratio.
Matched on the same variables as the logistic, mixed effects, and Cox models.
Odds ratio.
Log-rank test.
Hypertension with ACEi or ARB use; asthma with β2 agonist use.
Adjusted only for age and comorbidity indices.
Results of multivariate analyses are presented in table 2. Mortality associated with all variables in the multivariate analyses are given in the appendix (pp 2–4). The proportional hazards assumption was met; the distribution of the estimated propensity score and the standardised difference in propensity-matched covariates are given in the appendix (pp 8–9). Metformin use was not associated with significantly decreased mortality in the overall sample of men and women (table 2). Subgroup analyses of multivariate models were done by sex after an interaction between metformin and sex showed a decreased odds ratio (OR) for women on metformin (0·79, 95% CI 0·64–0·98, p=0·03).
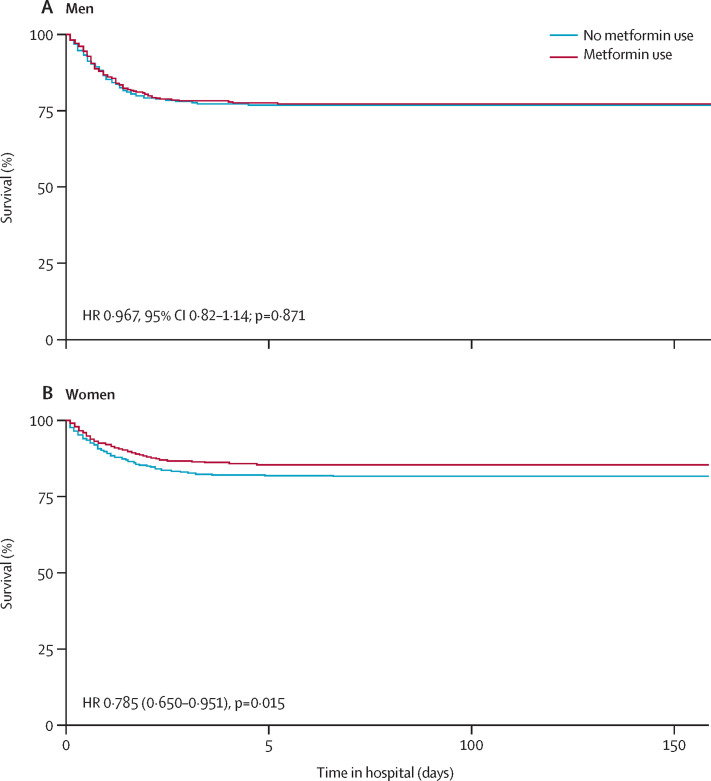
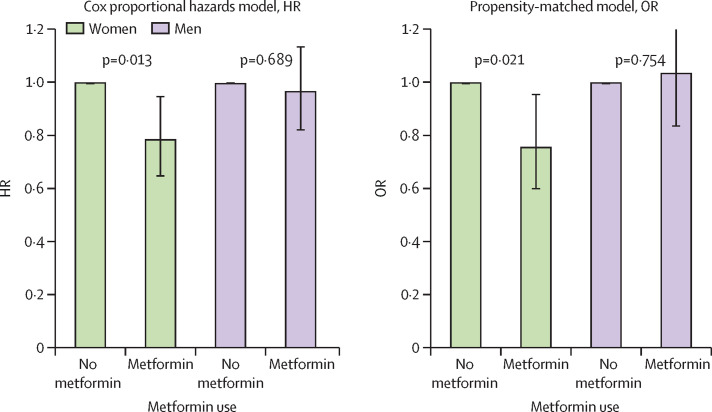
A Kaplan-Meier curve of survival by metformin use in men and women is shown in figure 2 . Metformin use was significantly associated with decreased mortality in women in the Cox proportional hazards model (hazard ratio [HR] 0·785, 95% CI 0·650–0·951), propensity-matched model (OR 0·759, 95% CI 0·601–0·960; table 2; figure 3 ), and by logistic regression (0·792, 0·640–0·979; mixed effects model 0·780, 0·631–0·965; appendix p 1).
Figure 2.
Kaplan-Meier survival curves in men (A) and women (B)
(A) Matching caliper 0·2.
Figure 3.
Survival among women and among men, comparing those without metformin to those with metformin
(A) Cox proportional hazards model. (B) Propensity-matched model. HR=hazard ratio. OR=odds ratio.
In the 1416 patients who remained in the propensity-matched model, among women with COVID-19 confirmed by PCR, metformin was significantly associated with decreased mortality in women with type 2 diabetes or obesity in the minimally adjusted Cox shared frailty model (OR 0·790, 95% CI 0·637–0·978), and the propensity-matched model (0·744, 0·565–0·980; log-rank p=0·019). However, in unadjusted analyses of men and women, metformin was not significantly associated with decreased mortality (0·859, 0·737–1·002). The e value for multivariate analyses ranged from 1·27 to 3·98 (appendix p 6).
Of the 15 362 people included in the exploratory analyses, 38 (0·25%) had claims for a TNFα inhibitor. In the Cox proportional hazards model, TNFα inhibitors were non-significantly associated with decreased mortality (HR 0·350, 95% CI 0·087–1·415). In a propensity-matched model, matched for the same variables as the metformin analyses, TNFα inhibitors were not associated with decreased mortality (0·483, 0·0821–2·845; p=0·421). In a propensity model matched only on age, sex, Charlson comorbidity index, inflammatory bowel disease, rheumatoid arthritis, and systemic lupus erythematosus, TNFα inhibitors were significantly associated with decreased mortality (OR 0·19, 95% CI 0·038–0·983; p=0·048; appendix pp 5, 10). A number of other variables were associated with increased or decreased risk of death from COVID-19, notably inflammatory bowel disease and asthma treated with β2 agonists (appendix pp 2–4).
Discussion
To our knowledge, this is the first study to report decreased mortality with outpatient metformin use in women with type 2 diabetes or obesity in a large cohort of patients admitted to hospital in the USA for COVID-19, and to describe a sex difference in this response to metformin. These findings could have wide-reaching effects, because more than 42% of women in the USA have obesity. We found that metformin use was associated with significantly lower mortality among women across all multivariate analyses, although these observational findings might still have residual confounding. Sensitivity analyses in those with PCR-confirmed COVID-19 showed similar ORs, with metformin use associated with decreased mortality in women with PCR confirmed by propensity matching and limited Cox model. CI was not significant in the full Cox model, potentially due to the reduced number of people in that sample. As noted, male sex is a risk factor for poor outcomes from COVID-19, and metformin did not appear to reduce mortality among men in this analysis.
The significant protective benefit in women compared with men might shed light on the mechanism by which metformin decreases mortality from COVID-19, because metformin has been shown to reduce TNFα to a greater extent in females than males in human and animal studies.6, 8 We also found that TNFα inhibitor use was associated with large decreases in odds of mortality, but these findings were only significant in a limited model, potentially due to the small sample size (n=38). Reduced mortality in people who use TNFα inhibitors would support previous research that TNFα plays a role in the pathology of COVID-19, probably due to macrophage activation and increased cytokine release.15
Reduced mortality from COVID-19 in patients taking metformin might be explained by several reasons. First, outpatient metformin use might indicate increased access to health care. The proportion of poor outcomes from COVID-19 is higher among people with low socioeconomic status and less access to health care, as compared with high socioeconomic status and better access to health care, and these issues need to be urgently addressed. Our population had the same insurer, so we felt that access to health care is probably not the reason we saw reduced mortality for those on metformin versus those not on metformin. Several possible mechanistic reasons why metformin might convey benefit are depicted in the appendix (p 11).
Besides TNFα reduction, we considered other (overlapping) mechanisms by which metformin could reduce the severity of SARS-CoV-2 infection; namely, angiotensin-converting enzyme 2 (ACE2) receptor modulation (via AMP-activated protein kinase [AMPK]), decreased inflammatory cytokine release (IL-6, TNFα, increased IL-10), improved neutrophil to lymphocyte ratio, decreased glycaemia (via AMPK), mast cell stabilisation, decreased thrombosis, and improved endothelial function.6, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 In patients with and without type 2 diabetes, metformin has been shown to decrease inflammatory mediators IL-6 and TNFα.23 These effects are notable, because IL-6 and TNFα are thought to contribute to COVID-19 pathology.4 The effects of metformin on these cytokines have been shown to differ by sex, with favourable effects in female mice over male mice, particularly for TNFα.6 Our findings of a strong sex-specific response to metformin in COVID-19 suggests that TNFα reduction might be the primary way by which metformin reduced mortality from COVID-19.
Our sex-specific findings are consistent with an earlier study8 showing mortality benefit with metformin use in women, but not men with colorectal cancer. Notably, TNFα is also a key inflammatory molecule in colorectal cancer pathophysiology.8 Possible reasons for sex-specific effects of metformin include the effect of sex hormones and epigenetic changes on the Y chromosome.26 There are two other potential ways in which metformin might cause sex-specific responses in COVID-19, namely metformin inhibits IgE-mediated and aryl hydrocarbon-mediated mast cell activation,27 and mast cell activation has been implicated as an early indicator of inflammatory response to SARS-CoV-2 and possibly a cytokine storm.28 Mast cells in female rats cause a greater increase in TNFα than in male rats, which could be one reason for greater benefit from metformin in women than men.29 Lastly, activation of AMPK by metformin can lead to increased expression of ACE2 and conformational changes to ACE2, and possibly decreased binding of SARS-CoV-2 to the ACE2 receptor.30 Recent work by Li and colleagues19 found that expression of the ACE2 receptor was equal in male and female human lungs, but that cytokine responses differed. This difference in subsequent inflammatory response and our sex-specific findings might support the anti-inflammatory effects of metformin as the primary means of benefit in COVID-19.
Additionally, in our multivariate analyses, β2 agonist use was associated with decreased mortality in patients with asthma across all analyses (appendix pp 2–4). This benefit might come from the effect of β2-agonists on boosting IL-10, which is a predominantly anti-inflammatory cytokine that can reduce levels of TNFα.31 Metformin has also been shown to boost levels of IL-10, more so in females than males.23 The mortality benefit in people with asthma on β2 agonists, combined with our finding of mortality benefit from metformin use, suggest that IL-10 might be important in COVID-19. The fact that these outpatient medications convey benefit in patients admitted to hospital for COVID-19 is interesting because metformin and TNFα inhibitors are universally stopped at hospital admission, suggesting its protective effects begin before hospitalisation.
Our findings should be prospectively confirmed. With the median time to hospitalisation for COVID-19 being about 1 week, it is necessary to understand the duration of metformin use that conveys benefit, and whether it prevents SARS-CoV-2 infection. The ready availability and safety, even during pregnancy, of metformin make it appealing to providers, patients, and society.32 Additionally, because of the increased inflammation from adiposity, and the association of obesity with increased risk of poor outcomes from COVID-19, metformin should be assessed in patients of all BMI categories.
Our study has several limitations. Assessing outcomes other than mortality (eg, length of stay or need for mechanical ventilation) was beyond the scope of this retrospective analysis. Retrospective analyses are subject to biases and unmeasured confounding. Although claims data show metformin was prescribed as a home medication for at least 90 days within the last 12 months, they do not give information about adherence. Additionally, claims data are known to have a very low sensitivity for obesity, which was the case in this dataset, and thus we did not differentiate classes of obesity.33 Exposures and characteristics were not reported within subgroups because of privacy protection. We were also unable to compare demographic characteristics within each subgroup of sex. Metformin is sometimes purchased without insurance claims because of its low cost, thus some individuals in our control group might have been exposed to the treatment. This form of selection bias would reduce the observed effect size and bias towards the null. Selection bias limits the generalisability of these findings because, to be included in our analysis, insurance through UHG was required. There might be confounding by indication for metformin use in our analyses—examples of such confounding include an individual newly diagnosed with diabetes who might be in a trial of lifestyle modification before being prescribed metformin; an individual with heart failure or kidney failure causing their provider to discontinue metformin; or degree of diabetes control (although attempts were made to account for this by controlling for insulin use, GLP-1 agonist use, and the Diabetes Complications Severity Index, which was the same [2·0] between the two groups). Such attempts in claims data are limited by the fact that most chronic kidney disease is undiagnosed, so in a dataset that relies on diagnoses, there are probably people in both the metformin and no metformin groups that have chronic kidney disease.34 Researchers in France found no difference in COVID-19 outcomes based on glycated haemoglobin.10 Despite these attempts to control for confounding, there is probably residual confounding in our analyses. The e values indicate that an unmeasured confounder would need a risk ratio magnitude of association with the treatment and outcome, above and beyond the measured confounders, that is greater than or equal to the e value (1·27–3·98 in our analyses).14 These e values can be compared with the ORs of confounders such as age (appendix pp 2–5) to get a sense for how they compare with known confounders in COVID-19. Although multicollinearity among potential confounders makes interpreting their independent associations with in-hospital mortality potentially difficult, adjustments for these confounders is necessary to minimise bias for estimating the causal effect of home metformin use on in-hospital mortality.35 Finally, prescriptions for outpatient use of metformin cannot be extrapolated to starting metformin at COVID-19 diagnosis or exposure to SARS-CoV-2, and inpatient use would not be recommended.
In conclusion, in a large de-identified claims database of adults with type 2 diabetes or obesity, metformin was associated with significantly decreased mortality in women admitted to hospital with COVID-19, with no significant mortality reduction in men. Metformin has a good safety profile, availability, and needs to be prospectively assessed in patients with COVID-19 to understand mechanism, duration, and timing of treatment necessary for benefit. Given the proinflammatory effects of obesity which might contribute to COVID-19 pathology, and the potential anti-inflammatory benefit of metformin in COVID-19, metformin should also be assessed in all BMI categories.
Data sharing
The data are proprietary and are not available for public use but, under certain conditions, may be made available to editors and their approved auditors under a data use agreement to confirm the findings of the current study.
Acknowledgments
Acknowledgments
This research was supported by the National Heart, Lung, and Blood Institute (grant number T32HL07741 to NEI); the Agency for Healthcare Research and Quality and Patient-Centered Outcomes Research Institute (grant number K12HS026379 to CJT). This research was supposed by the Minnesota Learning Health System Mentored Training Program, M Health Fairview Institutional Funds (CTB). This research was supported by the National Center for Advancing Translational Sciences, grants KL2TR002492 and UL1TR002494 (CTB). This research was supported by the National Cancer Institute, grant P30CA077598 (TAM). TAM is grateful for the support of Medtronic, in the form of a faculty fellowship.
Contributors
CTB, NEI, and CJT contributed to all aspects of the Article except data collection. TAM contributed to data analyses, interpretation of data, preparation of figures, writing, and critical review. SM contributed to data analyses, interpretation of data, writing, and critical review. SH and JG contributed to study design, data collection, data analysis, data interpretation, writing, and critical review. CM contributed to study design, data collection, data analysis, data interpretation, and critical review. RF, GG, NA, and SK contributed to study design, data interpretation, and critical review. TM contributed to study design, writing, and critical review. LT contributed to the creation of the figure and critical review. KMP contributed to study design, data interpretation, writing, and critical review. BB contributed to study design, interpretation of results, and critical review. DV contributed to all aspects of the Article. CJT, SH, CM, and JG all had access to and verified the raw data.
Declaration of interests
CJT is currently running a COVID-19 randomised trial, not related to metformin. CTB has submitted an Investigational New Drug application for a COVID-19 randomised trial for metformin. All author authors declare no competing interest.
Supplementary Material
References
- 1.Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosch TA, Steinberger J, Sinaiko AR, et al. Identification of sex-specific thresholds for accumulation of visceral adipose tissue in adults. Obesity. 2015;23:375–382. doi: 10.1002/oby.20961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 4.Tufan A, Avanoğlu Güler A, Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci. 2020;50:620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blüher M, Fasshauer M, Tönjes A, Kratzsch J, Schön MR, Paschke R. Association of interleukin-6, C-reactive protein, interleukin-10 and adiponectin plasma concentrations with measures of obesity, insulin sensitivity and glucose metabolism. Exp Clin Endocrinol Diabetes. 2005;113:534–537. doi: 10.1055/s-2005-872851. [DOI] [PubMed] [Google Scholar]
- 6.Matsiukevich D, Piraino G, Lahni P, et al. Metformin ameliorates gender-and age-dependent hemodynamic instability and myocardial injury in murine hemorrhagic shock. Biochim Biophys Acta Mol Basis Dis. 2017;1863:2680–2691. doi: 10.1016/j.bbadis.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quan H, Zhang H, Wei W, Fang T. Gender-related different effects of a combined therapy of exenatide and metformin on overweight or obesity patients with type 2 diabetes mellitus. J Diabetes Complications. 2016;30:686–692. doi: 10.1016/j.jdiacomp.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Park JW, Lee JH, Park YH, et al. Sex-dependent difference in the effect of metformin on colorectal cancer-specific mortality of diabetic colorectal cancer patients. World J Gastroenterol. 2017;23:5196–5205. doi: 10.3748/wjg.v23.i28.5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo P, Qiu L, Liu Y, et al. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am J Trop Med Hyg. 2020;103:69–72. doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tibshirani R. Regression shrinkage and selection via the LASSO. J R Stat Soc B. 1996;58:267–288. [Google Scholar]
- 12.Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of COVID-19—preliminary report. Reply. N Engl J Med. 2020;383:994. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 13.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the e-value. Ann Intern Med. 2017;167:268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 15.Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60:1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma S, Ray A, Sadasivam B. Metformin in COVID-19: a possible role beyond diabetes. Diabetes Res Clin Pract. 2020;164 doi: 10.1016/j.diabres.2020.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M-Y, Li L, Zhang Y, Wang X-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jadhav S, Ferrell W, Greer IA, Petrie JR, Cobbe SM, Sattar N. Effects of metformin on microvascular function and exercise tolerance in women with angina and normal coronary arteries: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 2006;48:956–963. doi: 10.1016/j.jacc.2006.04.088. [DOI] [PubMed] [Google Scholar]
- 21.Chen R, Sang L, Jiang M, et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J Allergy Clin Immunol. 2020;146:89–100. doi: 10.1016/j.jaci.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng F, Li L, Zeng J, et al. Can we predict the severity of COVID-19 with a routine blood test? Pol Arch Intern Med. 2020;130:400–406. doi: 10.20452/pamw.15331. [DOI] [PubMed] [Google Scholar]
- 23.Cameron AR, Morrison VL, Levin D, et al. Anti-inflammatory effects of metformin irrespective of diabetes status. Circ Res. 2016;119:652–665. doi: 10.1161/CIRCRESAHA.116.308445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly B, Tannahill GM, Murphy MP, O'Neill LA. Metformin inhibits the production of reactive oxygen species from NADH:ubiquinone oxidoreductase to limit induction of interleukin-1β (IL-1β) and boosts interleukin-10 (IL-10) in lipopolysaccharide (LPS)-activated macrophages. J Biol Chem. 2015;290:20348–20359. doi: 10.1074/jbc.M115.662114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 27.Wang HC, Huang SK. Metformin inhibits IgE- and aryl hydrocarbon receptor-mediated mast cell activation in vitro and in vivo. Eur J Immunol. 2018;48:1989–1996. doi: 10.1002/eji.201847706. [DOI] [PubMed] [Google Scholar]
- 28.Kritas SK, Ronconi G, Caraffa A, Gallenga CE, Ross R, Conti P. Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J Biol Regul Homeost Agents. 2020;34:9–14. doi: 10.23812/20-Editorial-Kritas. [DOI] [PubMed] [Google Scholar]
- 29.Mackey E, Ayyadurai S, Pohl CS, D' Costa S, Li Y, Moeser AJ. Sexual dimorphism in the mast cell transcriptome and the pathophysiological responses to immunological and psychological stress. Biol Sex Differ. 2016;7:60. doi: 10.1186/s13293-016-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Li X, Lu Q, et al. AMPK: a balancer of the renin-angiotensin system. Biosci Rep. 2019;39 doi: 10.1042/BSR20181994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izeboud CA, Vermeulen RM, Zwart A, Voss HP, van Miert AS, Witkamp RF. Stereoselectivity at the beta2-adrenoceptor on macrophages is a major determinant of the anti-inflammatory effects of beta2-agonists. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:184–189. doi: 10.1007/s002100000281. [DOI] [PubMed] [Google Scholar]
- 32.Nascimento IBD, Sales WB, Dienstmann G, Souza MLR, Fleig R, Silva JC. Metformin for prevention of cesarean delivery and large-for-gestational-age newborns in non-diabetic obese pregnant women: a randomized clinical trial. Arch Endocrinol Metab. 2020;64:290–297. doi: 10.20945/2359-3997000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lloyd JT, Blackwell SA, Wei II, Howell BL, Shrank WH. Validity of a claims-based diagnosis of obesity among medicare beneficiaries. Eval Health Prof. 2015;38:508–517. doi: 10.1177/0163278714553661. [DOI] [PubMed] [Google Scholar]
- 34.US Centers for Disease Control and Prevention Chronic kidney disease basics. 2020. https://www.cdc.gov/kidneydisease/basics.html
- 35.O'Brien RM. Dropping highly collinear variables from a model: why it typically is not a good idea. Soc Sci Q. 2017;98:360–375. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are proprietary and are not available for public use but, under certain conditions, may be made available to editors and their approved auditors under a data use agreement to confirm the findings of the current study.