Abstract
By the end of May 2020, SARS-CoV-2 pandemic caused more than 350,000 deaths worldwide. In the first months, there have been uncertainties on almost any area: infection transmission route, virus origin and persistence in the environment, diagnostic tests, therapeutic approach, high-risk subjects, lethality, and containment policies. We provide an updated summary of the current knowledge on the pandemic, discussing the available evidence on the effectiveness of the adopted mitigation strategies.
Keywords: SARS-CoV-2, COVID-19, Epidemiology, Diagnostic tests, Mortality, Containment policies
1. Introduction
On December 31, 2019, an outbreak caused by a new human pathogen, lately named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was notified in Wuhan, China (Gorbalenya et al., 2020). The virus caused a severe respiratory syndrome, generically defined as coronavirus disease (COVID-19) (Huang et al., 2020). Human-to-human transmission was first reported on January 22, 2020 and the World Health Organization (WHO) declared that SARS-CoV-2 infection had become a pandemic on March 11, when the 118,319 infections and 4292 deaths had been reported in 113 countries (World Health Organization, 2019, 2020a). By the end of May 2020, SARS-CoV-2 pandemic caused more than 350,000 deaths worldwide (Worldometers.info, 2020).
In the first months of the pandemic, there have been uncertainties on almost any area: infection transmission route, virus origin and persistence in the environment, diagnostic tests, therapeutic approach, high-risk subjects, lethality, and containment policies. An updated summary of the current knowledge on SARS-CoV-2 pandemic is therefore needed.
2. Virus origin, transmission routes, and persistence in the environment
As other coronaviruses (229E, NL63, OC43, HKU1, SARS-CoV, MERS-CoV), SARS-CoV-2 has enveloped virions that measure approximately 120 nm in diameter, and its genome consists of a single strand of RNA (Cascella et al., 2020; Encyclopaedia Britannica., 2020). Due to wide genome affinities with coronaviruses affecting bats (Wu et al., 2020; Zhou et al., 2020a), the theories on the origin of the virus range from natural selection in an animal host before zoonotic transfer (possibly with an intermediate host), to selection during passage in laboratory cell cultures (this being the least accredited supposition). (Huang et al., 2020; Wu et al., 2020; Li et al., 2020a; Andersen et al., 2020; Xiao et al., 2020; Wang et al., 2020a).
SARS-CoV-2 genome has been detected in all of the following clinical specimens: nasopharingeal swabs, sputum, bronchoalveolar lavage fluid, fibrobronchoscope brush biopsy, stool, ocular fluid, and blood (Wang et al., 2020a; Cheng et al., 2004; Peng et al., 2019; Colavita et al., 2020; Backer et al., 2020). However, according to current evidence, the virus is most commonly transmitted from infected persons through respiratory droplets and contact routes (Huang et al., 2020; Li et al., 2020a; Liu et al., 2020a; Chan et al., 2020). Droplet transmission occurs when a person is within 1–1.5 m from a contagious individual and is therefore at risk of having his/her mucosae (mouth and nose) or conjunctiva exposed to the droplets.
Airborne transmission is considered unlikely even when aerosol-generating procedures (e.g. tracheal intubation, non-invasive ventilation, etc.) are performed (Liu et al., 2020b; Tran et al., 2012), but contagion may occur by direct contact of the mucosae or conjunctiva with infected surfaces, like the skin or objects that have been exposed to infected droplets, sputum or sneeze (Liu et al., 2020b).
No evidence exists of fecal-oral transmission (Xu et al., 2020; Zhang et al., 2020; Tang et al., 2020a; Lamers et al., 2020), and although viral RNA was detected in the plasma or serum of 15% of the infected subjects (Huang et al., 2020), a review on diagnostic testing concluded that viral nuclear acid cannot be detected in serum and urine (Cheng et al., 2020), and transmission by blood transfusion remains unproven (Chang et al., 2020).
Finally, uncertainties remain on sexual and vertical transmissions. The limited available data suggest that vertical transmission is rare, with very few newborns infected to date, and no evidence of placental transmission (Yang and Liu, 2020). COVID-19, however, increased the risk of delivery complications and preterm birth (Yang and Liu, 2020; World Association of Perinatal Medicine Study Group on COVID-19, 2020; Hosier et al., 2020; Zamaniyan et al., 2020).
There have also been controversies over who is contagious, when, and for how long. The viral load, and in turn contagiousness, is highest in the pharynx of symptomatic individuals in the first week after symptoms onset (Wang et al., 2020a; Tang et al., 2020a; Wölfel et al., 2020; Lauer et al., 2020; To et al., 2020; Byrne et al., 2020). It is thought, however, that the infection could be spread also by those who do not develop symptoms (He et al., 2020a; Aguilar et al., 2020), although the proportion of infective asymptomatic individuals is likely to be low (Gao et al., 2020). Once infected, persons usually remain contagious for approximately 14 days, although a low proportion of subjects remain infectious for more than 30 days (63 days being the longest documented duration of the infection). (Hosier et al., 2020; Byrne et al., 2020; Pan et al., 2020).
Because of the above characteristics, SARS-CoV-2 is considered highly contagious, with an estimated basic reproduction number (the now famous “R0″) ranging from 2 to 6 without containment (which means that, without protection, a single individual infects two to six persons on average), and a household secondary attack rate close to 12% (which means that household or close contacts of an infected persons have a 12% probability of being infected). (He et al., 2020a; Jing et al., 2020; Bi et al., 2020; Streeck et al., 2020).
Finally, when considering virus transmission, it cannot be ignored whether and how long the active virus can persist in the environment. It has been documented, under experimental conditions with high viral load, that SARS-CoV-2 may remain infectious in the aerosol for 3 h, and on the surfaces (especially plastic and stainless steel) for up to three days (van Doremalen et al., 2020).
3. Symptoms of disease
The clinical manifestations and lethality of SARS-CoV-2 infection widely vary across individuals, depending on a number of factors (already recognized or just hypothesized), that have been discussed in a dedicated paragraph. Approximately half of the infected subjects remain asymptomatic, and most of the remaining only experience influenza-like symptoms, including fever, cough, sore throat, runny nose, weakness, myalgia, headache and, less frequently, conjunctivitis, hemoptysis, olfactory and taste dysfunction, and diarrhea (Cascella et al., 2020; He et al., 2020a; Aguilar et al., 2020; ECDC, 2020; Whitcroft and Hummel, 2020). Without early treatment, approximately 10%–15% of the infected persons develop a severe disease (COVID-19), which may include any of the followings: pneumonia, acute respiratory distress syndrome, pulmonary embolism, cardiomiopathy, disseminated intravascular coagulation, ultimately leading to death in most critical cases (Huang et al., 2020; Tang et al., 2020b; Klok et al., 2020). Although the likelihood of severe disease is lower in children, the clinical scenario is similar (Su et al., 2020a; Liguoro et al., 2020).
The average time from exposure to symptom onset (incubation period) is 5 days, and the time from symptoms to death usually ranges between 10 and 18 days, with a median of 14 (He et al., 2020a; Wang et al., 2020b).
4. Diagnosis
The knowledge on the diagnostic criteria and tests to detect SARS-CoV-2 infection, and COVID-19, is continuously evolving (Sethuraman et al., 2020; Zhai et al., 2020). Although several novel or complementary diagnostic methods are being developed, including serologic and point-of-care molecular diagnostic tests, real-time reverse transcriptase polymerase chain reaction (RT-PCR)–based assays performed in the laboratory on respiratory specimens are currently the cornerstone of the diagnosis of SARS-CoV-2 infection (Cheng et al., 2020; Sethuraman et al., 2020; World Health Organization, 2020b). Various RT-PCR assays are used around the world: the CDC has developed an assay containing PCR primer–probe sets for 2 regions of the viral nucleocapsid gene (N1 and N2), and for the human RNase P gene to ensure the RNA extraction was successful. This assay differs from WHO primer–probe sets (originally proposed by the Charité-Universitätsmedizin Berlin Institute of Virology) (Corman et al., 2020a), which target the SARS–CoV-2 RNA dependent RNA polymerase (RdRP) and envelope (E) genes (World Health Organization, 2020b; Corman et al., 2020b). Both assays have high analytic sensitivity and specificity, with minimal cross reactivity with other circulating strains of coronaviruses. However, a number of laboratories also obtained an emergency use authorization from regulatory agencies for their own laboratory developed assays (Cheng et al., 2020). As of May 8, the European Union has granted the CE mark to 40 PCR and nine serology tests; the United States Food and Drug Administration has approved 74 PCR, twelve serology, and two isothermal amplification tests, while additional PCR tests have been approved by other competent institutions around the World (Garg et al., 2020). More recently, droplet digital PCR (ddPCR) has shown significantly better performances than RT-PCR, especially for the diagnosis of the subjects with low viral load (Liu et al., 2020c; Suo et al., 2020).
The absence of an established reference standard, varying sample collection (including nasal, nasopharyngeal, oropharyngeal, mid-turbinate specimens, and endotracheal aspirates), differing preparation methods, and infection phase at the time of testing hamper rigorous assessment of the diagnostic accuracy of the many newly introduced SARS–CoV-2 assays (Tang et al., 2020a; Cheng et al., 2020; Mallapaty, 2020; Lippi et al., 2020a). In preliminary studies, the sensitivity and specificity widely varied, with four reports showing concerning rates of false negative results, higher than 20%, for some RT-PCR assays (Hu et al., 2020; He et al., 2020b; Fang et al., 2020; Meng et al., 2020; Wen et al., 2020; Zhifeng et al., 2020). The accuracy may also differ by time of infection: viral RNA becomes detectable as early as day 1 of symptoms and peaks within the first week of symptom onset, declining thereafter. Viral RNA has been detected even beyond week 6 following the first positive test, thus, at least two negative samples collected at ≥24-h intervals are required to document SARS–CoV-2 clearance. A few cases have been found positive after 2 consecutive negative PCR tests within 24 h, but it is unclear if these were testing errors, re-infections, or reactivations (Sethuraman et al., 2020; Zheng et al., 2020). Finally, as conventional RT-PCR tests could not be performed in low infrastructure settings (such as on cruise ships or in remote communities), rapid point-of-care molecular diagnostic tests have also been developed, although the sensitivity is likely to be lower than laboratory-based tests (Cheng et al., 2020; Sethuraman et al., 2020; Corman et al., 2020b).
With these limitations, RT-PCR tests are crucial to identify the subjects with circulating viruses, thus being essential to integrate the clinical diagnosis of severe COVID-19. However, they are unable to assess whether an individual was previously infected and has recovered, that could rather be detected using serologic tests, which identify antibodies to SARS-CoV-2 (IgA, IgM and IgG) from blood or saliva (Cheng et al., 2020; Perera et al., 2020). Serologic tests can be of four types: RDTs, chemiluminescent immunoassays, enzyme linked immunosorbent assays (ELISA), and neutralization assays. The Johns Hopkins University compiled a list of the tests approved for diagnostics or research, finding sensitivities ranging from 82% to 100%, and specificities ranging from 96% to 100%, with false negatives mainly due to cross-reactivity with other coronavirus proteins (Johns Hopkins Bloomberg School of Public Health - Center for Health Security, 2020).
Clearly, antibody responses to infection take weeks to be detectable (Johns Hopkins Bloomberg School of Public Health - Center for Health Security, 2020), thus serologic tests cannot be used for rapid diagnoses of symptomatic subjects (Cheng et al., 2020; Sethuraman et al., 2020). However, they will be of paramount importance for epidemiologic studies assessing SARS-CoV-2 lethality, vaccine studies, ongoing surveillance, and risk assessment and stratification of the population and health care workers (Cheng et al., 2020; Sethuraman et al., 2020; Stadlbauer et al., 2020; Hains et al., 2020; Goudsmit, 2020).
Besides RT-PCR and serologic tests, some biomarkers have been associated with COVID-19, including decreased albumin, elevated C-reactive protein, elevated lactate dehydrogenase, and lymphopenia (Rodriguez-Morales et al., 2020). To date, however, no biomarker or combination of biomarkers can be used to diagnose the infection or predict the clinical course (Cheng et al., 2020).
In light of the above mentioned limitations of the available tests, and of their accessibility, the diagnosis of COVID-19 is often made presumptively based on a compatible clinical presentation and the exclusion of other potential etiologies, in the setting of an exposure risk (residence in or travel to an area with widespread community transmission or known contact). (McIntosh, 2020).
For patients suffering from fever, sore throat, fatigue, coughing or dyspnea that is coupled with recent exposure, the diagnosis should be made using chest computerized tomography (CT) characteristics (typically, bilateral pulmonary parenchymal ground-glass and consolidative pulmonary opacities) despite negative RT-PCR results, which should be repeated 24–48 h after the initial test (Cheng et al., 2020; Zhai et al., 2020; Xie et al., 2020). Given that chest imaging is highly sensitive (78%–100%), but lacks specificity, the clinicians should always keep in mind the other pulmonary, cardiac, or systemic differentials because if missed, they could even lead to more severe consequences (Wu et al., 2007).
The same criteria are adopted to select the individuals to test. In fact, the characteristics of who should be tested have been controversially changing over time (European Centre for Disease Prevention and Control, 2020a; European Centre for Disease Prevention and Control, 2020b; Tsang et al., 2020). Although, theoretically, all symptomatic patients with suspected infection should undergo testing (Cheng et al., 2020; World Health Organization, 2020b), the CDC currently recommends to test the following, symptomatic subjects: hospital patients; residents in long-term care facilities or other congregate living settings, including prisons and shelters; healthcare workers; and workers in congregate living settings (CDC CfDCaP, 2020a). Testing is not recommended in asymptomatic individuals, unless they are prioritized by health departments or clinicians. Priority criteria may include, but are not limited to: the close contact with a confirmed or suspected COVID-19 case in the prior 14 days, residence in or travel to an area with widespread community transmission, and high risk of poor outcomes (CDC CfDCaP, 2020a).
5. Infection rates and lethality
By the end of May 2020, SARS-CoV-2 infection spread all over the world, with more than 5 million detected cases (0.7 × 1000 inhabitants worldwide) (Worldometers.info, 2020). The reported incidence rates ranged from 0.01 to approximately 16 cases per 1000 persons across nations with at least 100,000 residents (Worldometers.info, 2020). Besides a generally low incidence in African countries, no clear trends in the infection rates emerged by climate zone or per-capita domestic gross product (Worldometers.info, 2020; Berumen et al., 2020; Su et al., 2020b). Clearly, the incidence rates are influenced by the capacity to detect the infected persons through RT-PCR tests, the availability of which widely varied across nations. In fact, high income countries tested more than 4% of their population, while most African countries tested less than 5/1000 residents (Worldometers.info, 2020; Lachmann et al., 2020).
The variability in diagnostic testing does not only complicate the assessment of incidence rates of infection, it introduces an even higher likelihood of bias in the evaluation of the lethality. In many countries, during the weeks of the epidemic peak, because of the limited availability of PCR tests, these were administered only to the persons with severe symptoms (Wong et al., 2013). Thus, a large proportion of the infected subjects - most of those asymptomatic or with mild disease - remained undetected (Henegan et al., 2020; Ioannidis, 2020a). This creates a very large margin of uncertainty in the estimates of the case-fatality rate, as the numerator may be solid (n. of deaths), but the denominator (n. of infected persons) could be underestimated by a factor of 2, up to 50 (Ioannidis, 2020a; Oke and Henegan, 2020). In other terms, the real lethality of the disease, which is currently estimated at 6.2% worldwide, may range from 0.2% to 3.1%, and the summary estimates from two meta-analyses were 0.8% (Meyerowitz-Katz and Merone, 2020) and 3.3% (He et al., 2020a). Indeed, SARS-CoV-2 case-fatality rate widely varied across nations, even within the same continent and geographic area (Belgium, 16.2% and France, 15.5%, versus Germany, 4.7%, and Austria, 3.9%). To further complicate the correct estimation of the lethality, it has been shown that the risk of death largely depends upon age and comorbidities, thus the age distribution of a country, in addition to the detection rate, might have played a major role in determining current case-fatality rates. Also, the procedures of case recording (Iosa et al., 2020), the preparedness of the healthcare system, which in turn is affected by the intensity of the spread (Iosa et al., 2020; Boccia et al., 2020), and, especially, mitigation policies, certainly contributed to determine current case-fatality rates (Meyerowitz-Katz and Merone, 2020). Finally, some preliminary analyses are suggesting that, with current, more tailored therapies, COVID-19 lethality decreased over time (Flacco et al., 2020), and attenuated virus variants have been reported (Lau et al., 2020).
Some interesting mathematical models were developed to help estimating infection and fatality rates, and have been briefly summarized in a dedicated paragraph (Meyerowitz-Katz and Merone, 2020; Ferguson et al., 2020; Imperial College London MRC Centre for Global Infectious Disease Analysis, 2020; Remuzzi and Remuzzi, 2020; University of Washington Institute of Health Metrics and Evaluation (IHME), 2020). Although technically refined, they were made in the earlier phase of the pandemic, with an incomplete, varying set of rapidly evolving assumptions, the less tenable of which was the absence of mitigation strategies.
All considered, given the large variability among countries, age class, risk profile and, possibly, time, providing an overall, single estimate of SARS-CoV-2 case-fatality rate is currently impossible. It is however possible to compute lethality estimates stratified by the main risk factors, that are essential to support the clinicians in defining the prognosis and therapeutic strategies. We briefly discuss the main predictors of severe/lethal COVID-19 in the next paragraph.
6. Predictors of severe/lethal disease
In a number of descriptive studies, the infected persons who died were most frequently older than 70 years, males, hypertensive, diabetics, and affected by serious comorbidities such as congestive heart failure, chronic obstructive pulmonary diseases or renal diseases (Garg et al., 2020; Yang et al., 2020; Patel and Verma, 2020; Jordan et al., 2020; Castagnoli et al., 2020; Wu and McGoogan, 2020; Zhou et al., 2020b; Zuin et al., 2020; Bravi et al., 2020). Many of the unadjusted reports were confirmed when more reliable, adjusted estimates became available from cohort analyses, although the magnitudes of the associations were reduced (as typically happens) (Matsushita et al., 2020). As compared with the subjects younger than 50 years, those older than 70 years showed a 5- to 10-fold increase in the risk of severe/lethal COVID-19 (Bravi et al., 2020; Matsushita et al., 2020; Williamson et al., 2020). Males, hypertensive, diabetics, and those with COPD or major cardiovascular diseases all showed an approximately doubled risk of death or severe disease (Matsushita et al., 2020; Williamson et al., 2020; Mehra et al., 2020). Obesity is also emerging as a significant, independent predictor of severe/lethal disease (Garg et al., 2020; Williamson et al., 2020; Simonnet et al., 2020; Lighter et al., 2020), while fewer data are available on other potential risk factors such as tobacco smoking (Hu et al., 2020; Williamson et al., 2020; Alqahtani et al., 2020), socioeconomic status (Rose et al., 2020; Wadhera et al., 2020), ethnicity, (Williamson et al., 2020; Teo et al., 2020), immune system dysfunctions (ECDC, 2020; Tay et al., 2020), and exposure to pollution (Lippi et al., 2020b). Notably, no association was found between COVID-19 progression and use of Angiotensin converting enzyme inhibitors or Angiotensin II receptor blockers ( Bravi et al., 2020; Mancia et al., 2020; Mehta et al., 2020, Reynolds et al., 2020).
With regard to the children/adolescents, few deaths (n < 20) have been reported worldwide among children aged 0–9 years, and a low case-fatality rate (0.2%) has been observed for those aged 10–18 years (Worldometers.info, 2020; Castagnoli et al., 2020; CDC CfDCaP, 2020b). As for the adults, a higher risk of severe/lethal COVID-19 was observed among children with underlying comorbidities (Worldometers.info, 2020; Shekerdemian et al., 2020).
7. Accuracy of model predictions
As anticipated, a number of interesting statistical models were developed to predict (or just estimate the real) infection and lethality rates (Meyerowitz-Katz and Merone, 2020; Ferguson et al., 2020; Imperial College London MRC Centre for Global Infectious Disease Analysis, 2020; Remuzzi and Remuzzi, 2020; University of Washington Institute of Health Metrics and Evaluation (IHME), 2020). Given that the assumptions were inevitably affected by underreporting of cases (with test shortages, asymptomatic transmission, emergency situations that impaired rigorous contact tracing), and could not take into account unprecedented containment strategies (Li et al., 2020b), it was inevitable that most model estimates turned out to be incorrect.
As an example, a model predicted that all 5200 Italian ICU beds would have been occupied by March 14, when 1518 beds were actually employed for COVID-19 patients, and the peak in ICU beds (4068) was reached on April 3 (Remuzzi and Remuzzi, 2020). However, the model assumed no mitigation strategies, while lockdown measures were gradually implemented in Italy since February 21. Without the containment, the prediction might have been consistent. Another model, probably the most renowned, was developed by experts of the Imperial College of London (Ferguson et al., 2020). It predicted that, if the pandemic was left unmitigated, 81% of the population would be infected, with 510,000 deaths in the UK and 2.2 millions in the USA. Again, it is impossible to verify these estimates. However, 2009 H1N1 influenza pandemic infected no more than 27% of Americans, thus an overall infection rate of 81% may be overestimated (Yamey and Gonsalves, 2020; McKee, 2020; Van Kerkhove et al., 2013). Another model from China predicted that without implementation of any measures, China (without Hubei) would have had more than 800 million COVID-19 cases and an epidemic duration of 477 days (Chen et al., 2020).
In any case, as frequently occurs in preventive medicine (Sackett, 2002), a precise evaluation of these models cannot be made, which further complicates the choice of the most adequate containment strategies to adopt, in the next few months as well as for the next pandemics. Beyond accuracy, to increase their utility for public health decisions makers, the future models will have to incorporate separate estimates according to the most likely containment policies (including a strongly needed estimation of the health consequences of lockdown measures) and, possibly, produce estimates with smaller uncertainty margins (Ferguson et al., 2020; Karanikolos et al., 2013; Parmar et al., 2016; Fong et al., 2020). Notably, the last model from the Imperial College provided different estimates according to various combinations of mitigation strategies (Ferguson et al., 2020).
8. Therapy
There still is no evidence from randomized trials to recommend any specific drug or other therapeutic to prevent or treat COVID-19 (Zhai et al., 2020; CDC CfDCaP, 2020c). However, the U.S. National Institutes of Health (NIH) have published interim guidelines for the medical management of COVID-19, that are regularly updated and contain information about investigational therapeutics (National Institutes of Health, 2020). Besides supplemental oxygen and mechanical ventilatory support when indicated (CDC CfDCaP, 2020c), and although no drug has been proven to be safe and effective (National Institutes of Health, 2020), commonly tested treatments include antiviral agents (remdesivir, lopinavir, ritonavir - most of which, however, are discouraged by NIH if not administered in the context of a clinical trial), anticoagulants (mostly low molecular weight heparin - which is however not recommended by NIH for non-hospitalized subjects), and immune-based therapies (COVID-19 convalescent plasma, SARS-CoV-2 immune globulins, and interleukin 1 or 6 inhibitors such as sarilumab or tocilizumab) (National Institutes of Health, 2020; AIFA, 2020; Bhimraj et al., 2020; Sanders et al., 2020; American Society of Hematology, 2020). As for the other treatments, despite some preliminary, promising results (Capra et al., 2020; Di Giambenedetto et al., 2020; Mazzitelli et al., 2020), there are insufficient data to recommend either for or against the use of immune-based therapies (National Institutes of Health, 2020).
More than 800 randomized trials on treatments for COVID-19 have been registered worldwide (ECDC, 2020; Zhai et al., 2020; Covid-19 - living NMA initiative, 2020; World Health Organization, 2020c). Of them, more than 400 were actively recruiting patients by the end of May 2020. With such an unprecedented commitment, it is plausible to suppose that safe and effective therapeutic approaches will be available in a next future.
9. Containment policies
In the absence of vaccines and specific treatments, all the uncertainties on transmission routes, virus persistence in the environment, diagnostic tools accuracy, infection and lethality rates, therapies, and even the duration of the immunity (He et al., 2020b) contribute with a multiplier effect in delineating an extraordinary critical scenario at the end of the process, when adequate containment strategies have to be, or should have been chosen. Virtually all countries adopted preventive and containment measures directed to (a) reduce as much as possible human-to-human transmission, and (b) identify and isolate infected subjects through epidemiological investigations and extensive testing, and 14-day quarantine of COVID-19 cases and contacts (AIFA, 2020; Sanders et al., 2020; American Society of Hematology, 2020; Capra et al., 2020; Mazzitelli et al., 2020; European Centre for Disease Prevention and Control, 2020c).
To decrease the likelihood of transmission and slow the pandemic, a wide range of measures were gradually adopted: closure of the borders and block of passenger transportation across cities, obligation of social distancing (usually 1–1.5 m) and use of masks, respirators and/or gloves, shut down of schools, public services, social events, and inessential job activities, continuous indoor disinfection, up to complete lock-down with prohibition to leave the house, if not for essential needs (such as in Wuhan province and in Italy). (Sanders et al., 2020; Nussbaumer-Streit et al., 2020; Signorelli et al., 2020; Italian Prime MinisterItalian Government, 2020; Italian Prime MinisterGovernment I, 2020).
Besides some oddities, such as suspending 90% of the lawsuits and leaving tobacconists wide open (Italian Prime MinisterItalian Government, 2020; Italian Prime MinisterGovernment I, 2020), sending flawed diagnostic kits (Grady, 2020), or leaving healthcare workers untested and with no personal protective equipments in the first month of the pandemic, (Editor. Coronavirus, 2020; Errante, 2020), there are few doubts that the adopted containment measures were effective in reducing the spread of the infection and, in turn, drastically decreasing the death toll (Nussbaumer-Streit et al., 2020; Koo et al., 2020; Lai et al., 2020).
What is in fact entirely unknown, and urgently requires to be discerned, is which of the adopted strategies were more effective, and which were less, or even ineffective, and could thus be avoided in the next phases or next pandemics, in order to reduce as much as possible the tremendous socio-economic (and health) burden of an extensive lock-down (Nussbaumer-Streit et al., 2020; Ioannidis, 2020b; Parmet and Sinha, 2020; Morgan, 2020).
Unfortunately, no evidence is available except for modeling studies, but the analyses of the impact of various mitigation measures on other respiratory transmitted viral diseases, such as SARS, MERS or influenza, might certainly be helpful (Fong et al., 2020; Nussbaumer-Streit et al., 2020; Mahtani et al., 2020). In brief, there is evidence in favor of isolating symptomatic individuals and exposed contacts (as early as possible), avoiding crowds and restricting movements, social distancing and remote working (Fong et al., 2020; Nussbaumer-Streit et al., 2020; Lai et al., 2020; Mahtani et al., 2020; Rashid et al., 2015). However, controversies remain on the cost-benefit of school closures, methods for active case-finding (screening and contact tracing), tools for indoor disinfection, which masks to wear (and where), and types of quarantine (van Doremalen et al., 2020; Fong et al., 2020; Nussbaumer-Streit et al., 2020; Rashid et al., 2015; Radonovich et al., 2019; Smith et al., 2016; Setti et al., 2020; Patel et al., 2020).
Concerning screening tools, in Italy more than a million airport passengers were screened with thermal scanners in the months preceding the pandemic, with minimal or no effect over the course of the disease (Signorelli et al., 2020; Health Protection Agency IG, 2020). In fact, it is hard to support a screening test that misses almost half of the infected subjects (all those that are asymptomatic or in the incubation period), and inappropriately classifies as “dangerous” the much larger amount of persons with body temperature higher than 37.5 C° because of many other reasons (Quilty et al., 2020; O'Grady et al., 2008; Sund-Levander et al., 2002). Notwithstanding, the Italian National Institute for Insurance Against Accidents at Work recommended the use of thermal scanners in all workplaces (Italian National Institute for Insurance Against Accidents at Work (INAIL), 2020).
With regard to contact tracing, there are no doubts on its effectiveness, but limited evidence exists on cost-effectiveness, as considerable resources are required (Mahtani et al., 2020). One of the proposed solutions, a smartphone application to decrease health officials burden, raises concerns on its accuracy in detecting contacts, lack of standardization, potential danger for privacy and risk of use for social control in the future (Ioannidis, 2020b; McGee et al., 2020).
Even more important is the debate over the measures of social isolation for the general population (non-sick, non-exposed). A model has estimated - and may be logical to suppose - that the combination of case isolation, household quarantine, social distancing of the entire population, and school and university closures would achieve the greatest effect (Ferguson et al., 2020). However, besides some critiques on the plausibility of these estimates (Nussbaumer-Streit et al., 2020), the same model predicted that a less extreme combination of case isolation and voluntary quarantine for three months, and social distancing of people 70 years or older for four months, could prevent 49% of deaths. Other researchers suggested an alternative, lower-impact approach, that they called “inverse quarantine”, where isolation would be requested only to high-risk subjects (Standl et al., 2020). Also, an analysis of the official data indicate that the infection rates similarly decreased after the end of the lockdown in the vast majority of countries, thus suggesting that more stringent lockdown might have not substantially altered pandemic dynamics (Kolanovic and Kaplan, 2020).
This is not a trivial difference, as the most aggressive approaches are having huge economic consequences, are likely to cause psychological and health harms (potentially larger than the pandemic) (Kolanovic and Kaplan, 2020), and pose serious ethical issues (Ioannidis, 2020b; Parmet and Sinha, 2020; Gostin and Hodge, 2020). Italy represents a case study, as it was the first Western country to be seriously hit by the pandemic, and the most draconian restrictions were imposed (Italian Prime MinisterItalian Government, 2020; Italian Prime MinisterGovernment I, 2020). For almost two months, people throughout the country were not allowed to exit from their houses, unless for working (although only essential activities were maintained) or well-motivated and verifiable health needs or food supplying (Italian Prime MinisterItalian Government, 2020; Italian Prime MinisterGovernment I, 2020). Thousands of fines were imposed, with the support of drones and helicopters, and the prohibition was valid for any individual in any context, even alone, wearing masks, in open spaces (Ziniti, 2020). However, based on current evidence, the latter, extreme measures do not seem justified, as airborne transmission has not been proven (Liu et al., 2020b; Tran et al., 2012), the real infectivity of asymptomatic infected individuals appears low (Gao et al., 2020), public health authorities define a significant exposure to SARS-CoV-2 as face-to-face contact within 2 m with a symptomatic infected subject that is sustained for at least a few minutes, and the chances of catching SARS-CoV-2 in open spaces, wearing masks, are therefore minimal (or even negligible in many provinces, considering disease prevalence at the time of the lockdown). (Qian et al., 2020; ProtezioneCivile. COVID-19 Italia - Monitoraggio della situazione, 2020; Klompas et al., 2020).
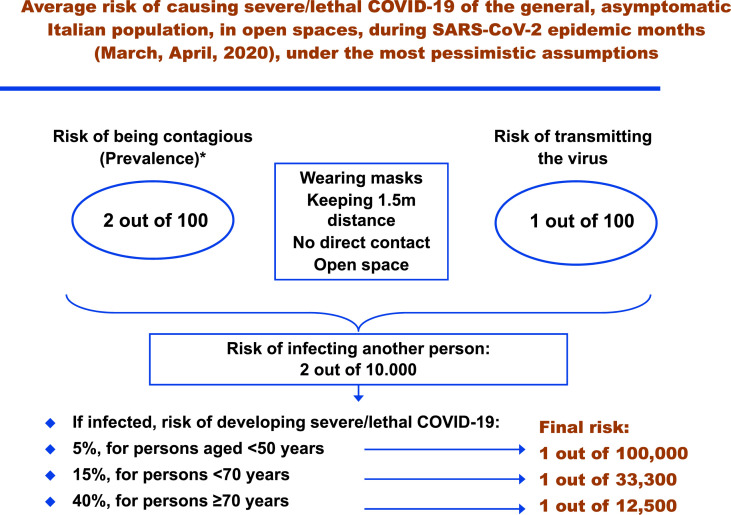
At the very end, the forced isolation of the entire population of a country for months is a quarantine on such an unprecedented scale to raise serious concerns on the balance between public health protection and civil liberties, requiring a particularly careful evaluation. The Constitutions of most Western countries require civil confinement to be temporary and applied only when individuals pose a significant risk of spreading dangerous, infectious diseases, with a use of coercion proportionate to the risk (Gostin and Hodge, 2020; Republic of ItalyItaly Ro, 1947; United States of AmericaAmerica USo, 1788). In this view, probability remains the beacon that should guide governments, as the precautionary principle can be applied in both directions in the case of a lockdown (Ioannidis, 2020b). Establishing whether a disease has a lethality of 3% or 0.3%; whether the risk of transmission in some situations is 10% or 0.1%; and whether the risk of encountering a contagious person is 1% or 0.1%, is not just an exercise for epidemiologists, it is the fundamental basis for achieving the most correct, effective and less restrictive measures to mitigate a pandemic, possibly saving billions, balancing health protection and civil rights (Parmet and Sinha, 2020; Gostin and Hodge, 2020). A good example is provided in Fig. 1 : even with the most pessimistic assumptions (altogether implausible), it is hard to conclude that the general Italian population forced in quarantine posed “a significant risk of spreading dangerous, infectious diseases, with a use of coercion proportionate to the risk”. It might have been so in some provinces (e.g. Bergamo or Brescia), certainly not in the entire country. With no blame for governments that faced an unprecedented challenge, let us learn from experience. For the next pandemic, and for the next phases of the current one.
Fig. 1.
Average risk of causing severe/lethal COVID-19 of the general, asymptomatic Italian population, in open spaces, during SARS-CoV-2 epidemic months (March, April 2020), under the most pessimistic assumptions.
* Official data indicate that, in the first three months of infection (up to May 25, 2020), the overall rate of infected subjects was 0.4 out of 100. Even increasing tenfold this value, asymptomatic persons are approximately half of all the infected subjects. Also, with an overestimated average disease duration of 30 days, only half of the overall number of infected subjects could be actively contagious on a single day. Importantly, these are intended as average prevalence values for the entire country, while some provinces had a completely different epidemiologic scenario, with official cumulative incidence rates as high as 10.8% (Bergamo), or 7.4% (Brescia); and as low as 0.05% (Taranto) or 0.08% (L'Aquila). As the lockdown was introduced in the entire country, these estimates are referred to the whole nation. In some provinces, the final risk of infecting other persons might have been 20 times higher, or 10 times lower.
Funding
This work was not funded.
CRediT authorship contribution statement
Cecilia Acuti Martellucci: Conceptualization, Investigation, Data curation, Writing - original draft. Maria Elena Flacco: Conceptualization, Investigation, Data curation, Validation, Writing - original draft. Rosaria Cappadona: Data curation, Writing - review & editing. Francesca Bravi: Data curation, Writing - review & editing. Lorenzo Mantovani: Data curation, Writing - review & editing. Lamberto Manzoli: Conceptualization, Methodology, Validation, Writing - review & editing, Visualization, Supervision, Project administration.
Declaration of competing interest
All authors declare they have no potential competing interests.
References
- Aguilar J.B., Faust J.S., Westafer L.M., et al. Investigating the impact of asymptomatic carriers on COVID-19 transmission. MedRxiv. 2020 2020.03.18.20037994. [Google Scholar]
- AIFA . Italian, Medicines, Agency. 2020. LMWH in adult COVID-19 patients [Eparine a basso peso molecolare nei pazienti adulti con COVID-19] Rome. [Google Scholar]
- Alqahtani J.S., Oyelade T., Aldhahir A.M., et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PloS One. 2020;15(5) doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Society of Hematology . 2020. COVID-19 and VTE/Anticoagulation. [Google Scholar]
- Andersen K.G., Rambaut A., Lipkin W.I., et al. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer J.A., Klinkenberg D., Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20-28 January 2020. Euro Surveill. : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2020;25(5) doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berumen J., Schmulson M., Guerrero G., et al. Trends of SARS-Cov-2 infection in 67 countries: role of climate zone, temperature, humidity and curve behavior of cumulative frequency on duplication time. MedRxiv. 2020 2020.04.18.20070920. [Google Scholar]
- Bhimraj A., Morgan R.L., Shumaker A.H., et al. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Clin. Infect. Dis. : an official publication of the Infectious Diseases Society of America. 2020 doi: 10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Q., Wu Y., Mei S., et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia S., Ricciardi W., Ioannidis J.P.A. What other countries can learn from Italy during the COVID-19 pandemic. JAMA Internal Medicine. 2020 doi: 10.1001/jamainternmed.2020.1447. [DOI] [PubMed] [Google Scholar]
- Bravi F., Flacco M.E., Carradori T., Volta C.A., Cosenza G., De Togni A., Acuti Martellucci C., Parruti G., Mantovani L., Manzoli L. Predictors of severe or lethal COVID-19, including Angiotensin converting enzyme inhibitors and Angiotensin II receptor blockers, in a sample of infected Italian citizens. MedRxiv. 2020 doi: 10.1371/journal.pone.0235248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne A.W., McEvoy D., Collins A., et al. Inferred duration of infectious period of SARS-CoV-2: rapid scoping review and analysis of available evidence for asymptomatic and symptomatic COVID-19 cases. MedRxiv. 2020 doi: 10.1136/bmjopen-2020-039856. 2020.04.25.20079889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra R., De Rossi N., Mattioli F., et al. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur. J. Intern. Med. 2020 doi: 10.1016/j.ejim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella M., Rajnik M., Cuomo A., et al. 2020. Features, Evaluation and Treatment Coronavirus (COVID-19) [PubMed] [Google Scholar]
- Castagnoli R., Votto M., Licari A., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatrics. 2020 doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- CDC CfDCaP Evaluating and testing persons for coronavirus disease 2019 (COVID-19). Secondary evaluating and testing persons for coronavirus disease 2019 (COVID-19) 2020. https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html
- CDC CfDCaP Provisional COVID-19 death counts by sex, age, and state. Secondary provisional COVID-19 death counts by sex, age, and state. 2020. https://data.cdc.gov/NCHS/Provisional-COVID-19-Death-Counts-by-Sex-Age-and-S/9bhg-hcku
- CDC CfDCaP Information for clinicians on investigational therapeutics for patients with COVID-19. Secondary information for clinicians on investigational therapeutics for patients with COVID-19. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html
- Chan J.F.-W., Yuan S., Kok K.-H., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Yan Y., Wang L. Coronavirus disease 2019: coronaviruses and blood safety. Transfus. Med. Rev. 2020 doi: 10.1016/j.tmrv.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Lan Y., Yuan X., et al. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg. Microb. Infect. 2020;9(1):469–473. doi: 10.1080/22221751.2020.1732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P.K.C., Wong D.A., Tong L.K.L., et al. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet (London, England) 2004;363(9422):1699–1700. doi: 10.1016/S0140-6736(04)16255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M.P., Papenburg J., Desjardins M., et al. Diagnostic testing for severe acute respiratory syndrome-related coronavirus-2: a narrative review. Ann. Intern. Med. 2020 doi: 10.7326/M20-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colavita F., Lapa D., Carletti F., et al. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann. Intern. Med. 2020 doi: 10.7326/M20-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covid-19 - living NMA initiative Living mapping and living systematic review of Covid-19 studies. Secondary Living mapping and living systematic review of Covid-19 studies. 2020. https://covid-nma.com
- Di Giambenedetto S., Ciccullo A., Borghetti A., et al. Off-label use of tocilizumab in patients with SARS-CoV-2 infection. J. Med. Virol. 2020 doi: 10.1002/jmv.25897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC . 2020. Disease Background of COVID-19. [Google Scholar]
- Editor Coronavirus . Il Fatto Quotidiano; 2020. Appelli in Tutta Italia: Mascherine Mancanti O Difettose. Il Ministro Speranza: “Garantire Protezioni Al Personale Sanitario”. [Google Scholar]
- Encyclopaedia Britannica. Coronavirus. Secondary coronavirus. 2020. https://www.britannica.com/science/coronavirus-virus-group
- Errante V. 2020. Coronavirus, boom di medici infettati: mancano i kit per proteggerli. [Google Scholar]
- European Centre for Disease Prevention and Control COVID-19: ECDC updates case definition for EU surveillance. Secondary COVID-19: ECDC updates case definition for EU surveillance. 2020. https://www.ecdc.europa.eu/en/news-events/covid-19-ecdc-updates-case-definition-eu-surveillance
- European Centre for Disease Prevention and Control Case definition and European surveillance for COVID-19, as of 2 March 2020. Secondary Case definition and European surveillance for COVID-19, as of 2 March 2020. 2020. https://www.ecdc.europa.eu/en/case-definition-and-european-surveillance-human-infection-novel-coronavirus-2019-ncov
- European Centre for Disease Prevention and Control . ECDC; Stockholm: 2020. Guidelines for the Use of Non-pharmaceutical Measures to Delay and Mitigate the Impact of 2019-nCoV. [Google Scholar]
- Fang Y., Zhang H., Xie J., et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020:200432. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson N.M., Laydon D., Nedjati-Gilani G., et al. Imperial College London; 2020. Impact of Non-pharmaceutical Interventions (NPIs) to Reduce COVID-19 Mortality and Healthcare Demand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flacco M., Acuti Martellucci C., Bravi F., Parruti G., Mascitelli A., Mantovani L., Manzoli L. SARS-CoV-2 lethality decreased over 1 time in two Italian Provinces. MedRxiv. 2020 doi: 10.1093/ofid/ofaa556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong M.W., Gao H., Wong J.Y., et al. Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings-social distancing measures. Emerg. Infect. Dis. 2020;26(5):976–984. doi: 10.3201/eid2605.190995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Yang L., Chen X., et al. A study on infectivity of asymptomatic SARS-CoV-2 carriers. Respir. Med. 2020:106026. doi: 10.1016/j.rmed.2020.106026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S., Kim L., Whitaker M., et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiology. 2020 doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostin L.O., Hodge J.G., Jr. US emergency legal responses to novel coronavirus: balancing public health and civil liberties. J. Am. Med. Assoc. 2020;323(12):1131–1132. doi: 10.1001/jama.2020.2025. [DOI] [PubMed] [Google Scholar]
- Goudsmit J. The paramount importance of serological surveys of SARS-CoV-2 infection and immunity. Eur. J. Epidemiol. 2020;35(4):331–333. doi: 10.1007/s10654-020-00635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady D. The New York Times; 2020. Coronavirus Test Kits Sent to States Are Flawed, C.D.C. Says. [Google Scholar]
- Hains D.S., Schwaderer A.L., Carroll A.E., et al. Asymptomatic seroconversion of immunoglobulins to SARS-CoV-2 in a pediatric dialysis unit. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W., Yi G.Y., Zhu Y. Estimation of the basic reproduction number, average incubation time, asymptomatic infection rate, and case fatality rate for COVID-19: meta-analysis and sensitivity analysis. MedRxiv. 2020 doi: 10.1002/jmv.26041. 2020.04.28.20083758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Lau E.H.Y., Wu P., et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- Health Protection Agency IG Coronavirus: controls in the airports continue [Coronavirus: proseguono i controlli sanitari negli aeroporti]. Secondary Coronavirus: controls in the airports continue [Coronavirus: proseguono i controlli sanitari negli aeroporti] 2020. http://www.protezionecivile.gov.it/media-comunicazione/comunicati-stampa/dettaglio/-/asset_publisher/default/content/coronavirus-proseguono-i-controlli-sanitari-negli-aeropo-12
- Henegan C., Brassey J., Jefferson T. COVID-19: what proportion are asymptomatic? Secondary COVID-19: what proportion are asymptomatic? 2020. https://www.cebm.net/covid-19/covid-19-what-proportion-are-asymptomatic/
- Hosier H., Farhadian S., Morotti R., et al. SARS-CoV-2 infection of the placenta. MedRxiv. 2020 doi: 10.1172/JCI139569. 2020.04.30.20083907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Chen S., Fu Y., et al. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperial College London MRC Centre for Global Infectious Disease Analysis Report 13 - estimating the number of infections and the impact of non-pharmaceutical interventions on COVID-19 in 11 European countries. Secondary Report 13 - estimating the number of infections and the impact of non-pharmaceutical interventions on COVID-19 in 11 European countries 30 March 2020. 2020. https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-13-europe-npi-impact/
- Ioannidis J.P.A. Coronavirus disease 2019: the harms of exaggerated information and non-evidence-based measures. Eur. J. Clin. Invest. 2020;50(4) doi: 10.1111/eci.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis J.P.A. Coronavirus disease 2019: the harms of exaggerated information and non-evidence-based measures. Eur. J. Clin. Invest. 2020;50(4) doi: 10.1111/eci.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosa M., Paolucci S., Morone G. Covid-19: a dynamic analysis of fatality risk in Italy. Front. Med. 2020;7:185. doi: 10.3389/fmed.2020.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italian National Institute for Insurance Against Accidents at Work (INAIL) 2020. Technical document on the possible remodulation of measures to contain SARS-CoV-2 contagion in the workplace, and prevention strategies [Documento tecnico sulla possibile rimodulazione delle misure di contenimento del contagio da SARS-CoV-2 nei luoghi di lavoro e strategie di prevenzione] Rome. [Google Scholar]
- Italian Prime Minister . In: Government I, editor. vol. 64. 2020. Ulteriori disposizioni attuative del decreto-legge 23 febbraio 2020, n. 6, recante misure urgenti in materia di contenimento e gestione dell’emergenza epidemiologica da COVID-19, applicabili sull’intero territorio nazionale. (Gazzetta Ufficiale Serie Generale). March 11, 2020. [Google Scholar]
- Italian Prime Minister . In: Italian Government, editor. vol. 59. 2020. Ulteriori disposizioni attuative del decreto-legge 23 febbraio 2020, n. 6, recante misure urgenti in materia di contenimento e gestione dell’emergenza epidemiologica da COVID-19. (Gazzetta Ufficiale Serie Generale). March 8, 2020. [Google Scholar]
- Jing Q.-L., Liu M.-J., Yuan J., et al. Household secondary attack rate of COVID-19 and associated determinants. MedRxiv. 2020 doi: 10.1016/S1473-3099(20)30471-0. 2020.04.11.20056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns Hopkins Bloomberg School of Public Health - Center for Health Security Serology-based tests for COVID-19. Secondary serology-based tests for COVID-19 10 may 2020. 2020. https://www.centerforhealthsecurity.org/resources/COVID-19/serology/Serology-based-tests-for-COVID-19.html
- Jordan R.E., Adab P., Cheng K.K. Covid-19: risk factors for severe disease and death. BMJ. 2020;368:m1198. doi: 10.1136/bmj.m1198. [DOI] [PubMed] [Google Scholar]
- Karanikolos M., Mladovsky P., Cylus J., et al. Financial crisis, austerity, and health in Europe. Lancet. 2013;381(9874):1323–1331. doi: 10.1016/S0140-6736(13)60102-6. [DOI] [PubMed] [Google Scholar]
- Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Thrombosis Research; 2020. Incidence of Thrombotic Complications in Critically Ill ICU Patients with COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompas M., Morris C.A., Sinclair J., et al. Universal masking in hospitals in the covid-19 era. N. Engl. J. Med. 2020;382(21):e63. doi: 10.1056/NEJMp2006372. [DOI] [PubMed] [Google Scholar]
- Kolanovic M., Kaplan B. 2020. Political Risks of Pandemic, Data Favors Further Reopening: J.P. Morgan - Global Quantitative and Derivative Strategy. [Google Scholar]
- Koo J.R., Cook A.R., Park M., et al. Interventions to mitigate early spread of SARS-CoV-2 in Singapore: a modelling study. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann A., Jagodnik K.M., Giorgi F.M., et al. Correcting under-reported COVID-19 case numbers: estimating the true scale of the pandemic. MedRxiv. 2020 2020.03.14.20036178. [Google Scholar]
- Lai S., Ruktanonchai N.W., Zhou L., et al. Effect of non-pharmaceutical interventions to contain COVID-19 in China. Nature. 2020 doi: 10.1038/s41586-020-2293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers M.M., Beumer J., van der Vaart J., et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020 doi: 10.1126/science.abc1669. eabc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.Y., Wang P., Mok B.W., et al. Attenuated SARS-CoV-2 variants with deletions at the S1/S2 junction. Emerg. Microb. Infect. 2020;9(1):837–842. doi: 10.1080/22221751.2020.1756700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer S.A., Grantz K.H., Bi Q., et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020 doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., et al. Early transmission dynamics in wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Pei S., Chen B., et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368(6490):489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighter J., Phillips M., Hochman S., et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin. Infect. Dis.: an official publication of the Infectious Diseases Society of America. 2020 doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Simundic A.M., Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19) Clin. Chem. Lab. Med. 2020 doi: 10.1515/cclm-2020-0285. [DOI] [PubMed] [Google Scholar]
- Liguoro I, Pilotto C, Bonanni M, et al. SARS-CoV-2 infection in children and newborns: a systematic review. European Journal of Pediatrics. 2020 doi: 10.1007/s00431-020-03684-7. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Sanchis-Gomar F., Henry B.M. Association between environmental pollution and prevalence of coronavirus disease 2019 (COVID-19) in Italy. MedRxiv. 2020 2020.04.22.20075986. [Google Scholar]
- Liu J., Liao X., Qian S., et al. Community transmission of severe acute respiratory syndrome coronavirus 2, shenzhen, China, 2020. Emerg. Infect. Dis. 2020;26(6) doi: 10.3201/eid2606.200239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020 doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Liu X., Feng J., Zhang Q., et al. Analytical comparisons of SARS-COV-2 detection by qRT-PCR and ddPCR with multiple primer/probe sets. Emerg. Microb. Infect. 2020:1–12. doi: 10.1080/22221751.2020.1772679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahtani K., Heneghan C., Aronson J.K. What is the evidence for social distancing during global pandemics? A rapid summary of current knowledge. Secondary What is the evidence for social distancing during global pandemics? A rapid summary of current knowledge. 2020. https://www.cebm.net/covid-19/what-is-the-evidence-for-social-distancing-during-global-pandemics/
- Mallapaty S. Will antibody tests for the coronavirus really change everything? Nature. 2020;580(7805):571–572. doi: 10.1038/d41586-020-01115-z. [DOI] [PubMed] [Google Scholar]
- Mancia G., Rea F., Ludergnani M., et al. Renin-angiotensin-aldosterone system blockers and the risk of covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K., Ding N., Kou M., et al. The relationship of COVID-19 severity with cardiovascular disease and its traditional risk factors: a systematic review and meta-analysis. MedRxiv. 2020 doi: 10.5334/gh.814. 2020.04.05.20054155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzitelli M., Arrighi E., Serapide F., et al. Use of subcutaneous tocilizumab in patients with COVID-19 pneumonia. J. Med. Virol. 2020 doi: 10.1002/jmv.26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee P., Murphy H., Bradshaw T. Financial Times; 2020. Coronavirus Apps: the Risk of Slipping into a Surveillance State. [Google Scholar]
- McIntosh K. Coronavirus disease 2019 (COVID-19): epidemiology, virology, clinical features, diagnosis, and prevention. Secondary Coronavirus disease 2019 (COVID-19): epidemiology, virology, clinical features, diagnosis, and prevention. 2020. https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-epidemiology-virology-clinical-features-diagnosis-and-prevention
- McKee M. A European roadmap out of the covid-19 pandemic. BMJ. 2020;369:m1556. doi: 10.1136/bmj.m1556. [DOI] [PubMed] [Google Scholar]
- Mehra M.R., Desai S.S., Kuy S., et al. Cardiovascular disease, drug therapy, and mortality in covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mehta N., Kalra A., Nowacki A.S., et al. Association of use of angiotensin-converting enzyme inhibitors and Angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y., Wu P., Lu W., et al. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog. 2020;16(4) doi: 10.1371/journal.ppat.1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz-Katz G., Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection-fatality rates. MedRxiv. 2020 doi: 10.1016/j.ijid.2020.09.1464. 2020.05.03.20089854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J.P. COVID-19 impact across markets. Secondary COVID-19 impact across markets. 2020. https://www.jpmorgan.com/global/research/covid-19-across-markets
- National Institutes of Health COVID-19 treatment guidelines. Secondary COVID-19 treatment guidelines. 2020. https://www.covid19treatmentguidelines.nih.gov/whats-new/ [PubMed]
- Nussbaumer-Streit B., Mayr V., Dobrescu A.I., et al. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochrane Database Syst. Rev. 2020;4:CD013574. doi: 10.1002/14651858.CD013574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Grady N.P., Barie P.S., Bartlett J.G., et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit. Care Med. 2008;36(4):1330–1349. doi: 10.1097/CCM.0b013e318169eda9. [DOI] [PubMed] [Google Scholar]
- Oke J., Henegan C. Global covid-19 case fatality rates. Secondary global covid-19 case fatality rates. 2020. https://www.cebm.net/covid-19/global-covid-19-case-fatality-rates/
- Pan Y., Zhang D., Yang P., et al. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar D., Stavropoulou C., Ioannidis J.P.A. Health outcomes during the 2008 financial crisis in Europe: systematic literature review. BMJ. 2016;354:i4588. doi: 10.1136/bmj.i4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmet W.E., Sinha M.S. Covid-19 - the law and limits of quarantine. N. Engl. J. Med. 2020;382(15):e28. doi: 10.1056/NEJMp2004211. [DOI] [PubMed] [Google Scholar]
- Patel A.B., Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and Angiotensin receptor blockers: what is the evidence? J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.4812. [DOI] [PubMed] [Google Scholar]
- Patel K.P., Patel P.A., Vunnam S.R., et al. Patients with COVID-19: are current isolation guidelines effective enough? Publ. Health. 2020;183:38–39. doi: 10.1016/j.puhe.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Liu J., Xu W., et al. Novel Coronavirus can be detected in urine, blood, anal swabs and oropharyngeal swabs samples. MedRxiv. 2019 doi: 10.1002/jmv.25936. 2020:2020.02.21.20026179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera R.A., Mok C.K., Tsang O.T., et al. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Euro Surveill. : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2020;25(16) doi: 10.2807/1560-7917.ES.2020.25.16.2000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ProtezioneCivile Covid-19 Italia - Monitoraggio della situazione Secondary COVID-19 Italia - Monitoraggio della situazione. 2020. http://opendatadpc.maps.arcgis.com/apps/opsdashboard/index.html#/b0c68bce2cce478eaac82fe38d4138b1
- Qian H., Miao T., Liu L., Zheng X., Luo D., Li Y. Indoor transmission of SARS-CoV-2. MedRxiv. 2020 doi: 10.1111/ina.12766. [DOI] [PubMed] [Google Scholar]
- Quilty B.J., Clifford S., Flasche S., et al. Effectiveness of airport screening at detecting travellers infected with novel coronavirus (2019-nCoV) Euro Surveill. : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2020;25(5) doi: 10.2807/1560-7917.ES.2020.25.5.2000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radonovich L.J., Jr., Simberkoff M.S., Bessesen M.T., et al. N95 respirators vs medical masks for preventing influenza among health care personnel: a randomized clinical trial. J. Am. Med. Assoc. 2019;322(9):824–833. doi: 10.1001/jama.2019.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid H., Ridda I., King C., et al. Evidence compendium and advice on social distancing and other related measures for response to an influenza pandemic. Paediatr. Respir. Rev. 2015;16(2):119–126. doi: 10.1016/j.prrv.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395(10231):1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Republic of Italy . In: Italy Ro, editor. vol. 298. Italian Republic; 1947. Constitution of the Italian republic. (Gazzetta Ufficiale Serie Generale). December 12, 1947. [Google Scholar]
- Reynolds H.R., Adhikari S., Pulgarin C., et al. Renin-angiotensin-aldosterone system inhibitors and risk of covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutierrez-Ocampo E., et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Trav. Med. Infect. Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose T.C., Mason K., Pennington A., et al. Inequalities in COVID19 mortality related to ethnicity and socioeconomic deprivation. MedRxiv. 2020 2020.04.25.20079491. [Google Scholar]
- Sackett D.L. The arrogance of preventive medicine. CMAJ (Can. Med. Assoc. J.) : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2002;167(4):363–364. [PMC free article] [PubMed] [Google Scholar]
- Sanders J.M., Monogue M.L., Jodlowski T.Z., et al. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., et al. Airborne transmission route of COVID-19: why 2 meters/6 feet of inter-personal distance could not Be enough. Int. J. Environ. Res. Publ. Health. 2020;17(8) doi: 10.3390/ijerph17082932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekerdemian L.S., Mahmood N.R., Wolfe K.K., et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatrics. 2020 doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorelli C., Scognamiglio T., Odone A. COVID-19 in Italy: impact of containment measures and prevalence estimates of infection in the general population. Acta Biomed. : Atenei Parmensis. 2020;91(3-S):175–179. doi: 10.23750/abm.v91i3-S.9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonnet A., Chetboun M., Poissy J., et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020 doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.D., MacDougall C.C., Johnstone J., et al. Effectiveness of N95 respirators versus surgical masks in protecting health care workers from acute respiratory infection: a systematic review and meta-analysis. CMAJ (Can. Med. Assoc. J.) : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2016;188(8):567–574. doi: 10.1503/cmaj.150835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadlbauer D., Amanat F., Chromikova V., et al. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Current Protocols in Microbiology. 2020;57(1):e100. doi: 10.1002/cpmc.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standl F., Jockel K.H., Stang A. COVID-19 and the need of targeted inverse quarantine. Eur. J. Epidemiol. 2020;35(4):339–340. doi: 10.1007/s10654-020-00629-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeck H., Schulte B., Kümmerer . Heinsberg; 2020. BM Infection Fatality Rate of SARS-CoV-2 Infection in a German Community with a Super-spreading Event. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L., Ma X., Yu H., et al. The different clinical characteristics of corona virus disease cases between children and their families in China - the character of children with COVID-19. Emerg. Microb. Infect. 2020;9(1):707–713. doi: 10.1080/22221751.2020.1744483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su D., Chen Y., He K., et al. Influence of socio-ecological factors on COVID-19 risk: a cross-sectional study based on 178 countries/regions worldwide. MedRxiv. 2020 2020.04.23.20077545. [Google Scholar]
- Sund-Levander M., Forsberg C., Wahren L.K. Normal oral, rectal, tympanic and axillary body temperature in adult men and women: a systematic literature review. Scand. J. Caring Sci. 2002;16(2):122–128. doi: 10.1046/j.1471-6712.2002.00069.x. [DOI] [PubMed] [Google Scholar]
- Suo T., Liu X., Feng J., et al. ddPCR: a more accurate tool for SARS-CoV-2 detection in low viral load specimens. Emerg. Microb. Infect. 2020:1–30. doi: 10.1080/22221751.2020.1772678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A., Tong Z-d, Wang H-l, et al. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child. China Emerg Infect Dis. 2020;26(6) doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemostasis. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M.Z., Poh C.M., Rénia L., et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo J.T., Bean D., Bendeyan R., et al. Impact of ethnicity on outcome of severe COVID-19 infection. Data from an ethnically diverse UK tertiary centre. MedRxiv. 2020 2020.05.02.20078642. [Google Scholar]
- To K.K.-W., Tsang O.T.-Y., Leung W.-S., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran K., Cimon K., Severn M., et al. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PloS One. 2012;7(4) doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang T.K., Wu P., Lin Y., et al. Effect of changing case definitions for COVID-19 on the epidemic curve and transmission parameters in mainland China: a modelling study. Lancet Public Health. 2020;5(5):e289–e296. doi: 10.1016/S2468-2667(20)30089-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States of America . In: America USo, editor. 1788. Constitution of the United States. [Google Scholar]
- University of Washington Institute of Health Metrics and Evaluation (IHME) COVID-19 projections. Secondary COVID-19 projections 12 May 2020. 2020. https://covid19.healthdata.org/united-states-of-america
- van Doremalen N., Bushmaker T., Morris D.H., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kerkhove M.D., Hirve S., Koukounari A., et al. Estimating age-specific cumulative incidence for the 2009 influenza pandemic: a meta-analysis of A(H1N1)pdm09 serological studies from 19 countries. Influenza and Other Respiratory Viruses. 2013;7(5):872–886. doi: 10.1111/irv.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhera R.K., Wadhera P., Gaba P., et al. Variation in COVID-19 hospitalizations and deaths across New York city boroughs. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., et al. Detection of SARS-CoV-2 in different types of clinical specimens. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J. Med. Virol. 2020;92(4):441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z., Chi Y., Zhang L., Liu H., Du K., Li Z., Chen J., Cheng L., Wang D. Coronavirus disease 2019: initial detection on chest CT in a retrospective multicenter study of 103 Chinese subjects. Radiology: Cardiothoracic Imaging. 2020;2(2) doi: 10.1148/ryct.2020200092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcroft K.L., Hummel T. Olfactory dysfunction in COVID-19: diagnosis and management. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.8391. [DOI] [PubMed] [Google Scholar]
- Williamson E., Walker A.J., Bhaskaran K., et al. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. MedRxiv. 2020 [submitted] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., et al. Virological Assessment of Hospitalized Patients with COVID-2019. Nature. 2020 doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wong J.Y., Kelly H., Ip D.K.M., et al. Case fatality risk of influenza A (H1N1pdm09): a systematic review. Epidemiology. 2013;24(6):830–841. doi: 10.1097/EDE.0b013e3182a67448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Association of Perinatal Medicine Study Group on Covid-19 . 2020. Maternal and Perinatal Outcomes of Pregnant Women with Coronavirus Disease 2019 (COVID-19): the WAPM Study Group on COVID-19. submitted for publication. [Google Scholar]
- World Health Organization WHO timeline - COVID-19. Secondary WHO timeline - COVID-19. 2019. https://www.who.int/news-room/detail/08-04-2020-who-timeline-covid-19
- World Health Organization . WHO; 2020. Coronavirus Disease (COVID-2019) Situation Reports. [Google Scholar]
- World Health Organization . 2020. Laboratory Testing for Coronavirus Disease (COVID-19) in Suspected Human Cases - Interim Guidance. [Google Scholar]
- World Health Organization . 2020. Data Visualizations and Mapping of Registered Studies for COVID-19 Experimental Treatments. [Google Scholar]
- Worldometersinfo COVID-19 coronavirus outbreak. Secondary COVID-19 coronavirus outbreak 15 may. 2020 2020. https://www.worldometers.info/coronavirus/
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Wu L.P., Wang N.C., Chang Y.H., et al. Duration of antibody responses after severe acute respiratory syndrome. Emerg. Infect. Dis. 2007;13(10):1562–1564. doi: 10.3201/eid1310.070576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K., Zhai J., Feng Y., et al. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nature. 2020 doi: 10.1038/s41586-020-2313-x. [DOI] [PubMed] [Google Scholar]
- Xie X., Zhong Z., Zhao W., et al. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020:200343. doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Li X., Zhu B., et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26(4):502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamey G., Gonsalves G. Donald Trump: a political determinant of covid-19. BMJ. 2020;369:m1643. doi: 10.1136/bmj.m1643. [DOI] [PubMed] [Google Scholar]
- Yang Z., Liu Y. Vertical transmission of severe acute respiratory syndrome coronavirus 2: a systematic review. Am. J. Perinatol. 2020 doi: 10.1055/s-0040-1712161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zheng Y., Gou X., et al. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamaniyan M., Ebadi A., Aghajanpoor Mir S., et al. Preterm delivery in pregnant woman with critical COVID-19 pneumonia and vertical transmission. Prenat. Diagn. 2020 doi: 10.1002/pd.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai P., Ding Y., Wu X., et al. The epidemiology, diagnosis and treatment of COVID-19. Int. J. Antimicrob. Agents. 2020:105955. doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang S., Xue Y. Fecal specimen diagnosis 2019 novel coronavirus–infected pneumonia. J. Med. Virol. 2020;92(6):680–682. doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Fan J., Yu F., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhifeng J., Feng A., Li T. Consistency analysis of COVID-19 nucleic acid tests and the changes of lung CT. J. Clin. Virol. 2020;127:104359. doi: 10.1016/j.jcv.2020.104359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziniti A. La Repubblica; 2020. Cruel Fines, Borderline Cases and Errors: Here Is the Sheriff State [Multe Crudeli, Casi Limite Ed Errori: Ecco Lo Stato Sceriffo] [Google Scholar]
- Zuin M., Rigatelli G., Zuliani G., et al. Arterial hypertension and risk of death in patients with COVID-19 infection: systematic review and meta-analysis. J. Infect. 2020;S0163–4453(20) doi: 10.1016/j.jinf.2020.03.059. 30189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]