Abstract
Introduction
National Institute for Health and Care Excellence (NICE) endorsed clinical frailty scale (CFS) to help with decision-making. However, this recommendation lacks an evidence basis and is controversial. This meta-analysis aims to quantify the dose-response relationship between CFS and mortality in COVID-19 patients, with a goal of supplementing the evidence of its use.
Methods
We performed a systematic literature search from several electronic databases up until 8 September 2020. We searched for studies investigating COVID-19 patients and reported both (1) CFS and its distribution (2) CFS and its association with mortality. The outcome of interest was mortality, defined as clinically validated death or non-survivor. The odds ratio (ORs) will be reported per 1% increase in CFS. The potential for a non-linear relationship based on ORs of each quantitative CFS was examined using restricted cubic splines with a three-knots model.
Results
There were a total of 3817 patients from seven studies. Mean age was 80.3 (SD 8.2), and 53% (48–58%) were males. The pooled prevalence for CFS 1–3 was 34% (32–36%), CFS 4–6 was 42% (40–45%), and CFS 7–9 was 23% (21–25%). Each 1-point increase in CFS was associated with 12% increase in mortality (OR 1.12 (1.04, 1.20), p = 0.003; I2: 77.3%). The dose-response relationship was linear (Pnon-linearity=0.116). The funnel-plot analysis was asymmetrical; Trim-and-fill analysis by the imputation of two studies on the left side resulted in OR of 1.10 [1.03, 1.19].
Conclusion
This meta-analysis showed that increase in CFS was associated with increase in mortality in a linear fashion.
Keywords: Age, Coronavirus, COVID-19, Frailty, Prognosis, Risk stratification
1. Introduction
Amid a coronavirus disease 2019 (COVID-19) pandemic, the surge in the number of patients has strained the healthcare system to its peak. Although the majority of cases are asymptomatic or mild, some patients with COVID-19 may develop into more severe forms accompanied by severe life-threatening complications, especially in those who are elderly and have pre-existing comorbidities (Huang & Pranata, 2020; Huang, Lim & Pranata, 2020; Yonas et al., 2020). When resources are strained, medical practitioners are faced with complex, challenging, and dilemmatic ethical decisions in determining to whom the allocation of intensive care and ventilator support will provide the most benefit (Truog, Mitchell & Daley, 2020). While it is evident for patients with a severe presentation, management of patients presenting with mild-moderate cases will benefit from triage and risk stratification.
Although age is associated with mortality (Bonanad et al., 2020; Mesas et al., 2020), age alone is not sufficient for risk stratification in COVID-19 patients, and is subject to ethical controversies (Lewis, Breckons, Lee, Dotchin & Walker, 2020; Montero-Odasso et al., 2020). Clinical frailty scale (CFS) emerges as a potentially useful and practical tool to enable efficient workflow even when faced with limited human resources and increasing demand for medical services (Cesari & Proietti, 2020). Recently, the National Institute for Health and Care Excellence (NICE) endorsed the use of clinical frailty scale (CFS) to help with decision-making (National Institute for Health & Care Excellence, 2020). However, this recommendation lacks evidence and is controversial (Chong, Chan, Tan & Lim, 2020; Lewis et al., 2020). This dose-response meta-analysis aims to quantify the dose-response relationship between CFS and mortality in COVID-19 patients, with the goal of supplementing the evidence for CFS use.
2. Methods
2.1. Search strategy and study selection
We performed a systematic literature search from PubMed, Scopus, EuropePMC, and medRvix from inception up until 8 September 2020 using the keywords (“Clinical Frailty Score” OR “Clinical Frailty Scale” OR “CFS”) AND (“COVID-19” OR “SARS-CoV-2” OR “Novel Coronavirus” OR “Ncov 2019”). After compiling records from the initial search, we proceed with duplicates exclusion. Two independent authors screened the title/abstract for potential articles. Unrelated records were then excluded, and the full-text of potential articles were assessed for relevance based on the eligibility criteria. This systematic review and meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. This study is registered in PROSPERO: ID CRD42020209294.
2.2. Eligibility criteria
The inclusion criteria were research articles (prospective and retrospective observational studies) investigating adult COVID-19 patients that reported both: (1) CFS and its distribution (2) CFS and its association with mortality. We excluded review articles, case reports, case-series <20 patients, letters, and non-English language papers.
2.3. Data extraction
Two authors independently extracted data from the included studies with the help of extraction forms that contained the first author of the study, study design, age, gender, comorbidities, CFS, the effect estimate, and mortality. Discrepancies that arise was resolved by discussion
The outcome of interest was mortality, defined as clinically validated death or non-survivor in patients with COVID-19.
Risk of bias assessment was performed using the Newcastle-Ottawa Scale (NOS) by two independent authors and any discrepancies were resolved by discussion.
2.4. Statistical analysis
To perform meta-analysis STATA 16.0 (StataCorp LLC, Texas, US) was used. Meta-analysis of proportion was performed to estimate the prevalence of CFS 1-3, CFS 4-6, and CFS 7-9 across studies. A dose-response meta-analysis was then performed for studies that have at least three quantitative classifications to generate OR per 1-unit increment. Analyses were performed using a random-effects model regardless of heterogeneity. Due to the limited number of studies, HR was considered as an OR. The potential for a non-linear relationship based on ORs of each quantitative CFS was examined using restricted cubic splines with a three-knots model. A Wald-type test was performed to evaluate the non-linearity by testing the regression coefficient of the second spline. The p-values for pooled effect estimates were considered to be significant if below 0.05. Cochran's Q test and I2 statistic were used to assess the heterogeneity and I2 values >50% or p-value <0.10 indicated statistically significant heterogeneity.
3. Results
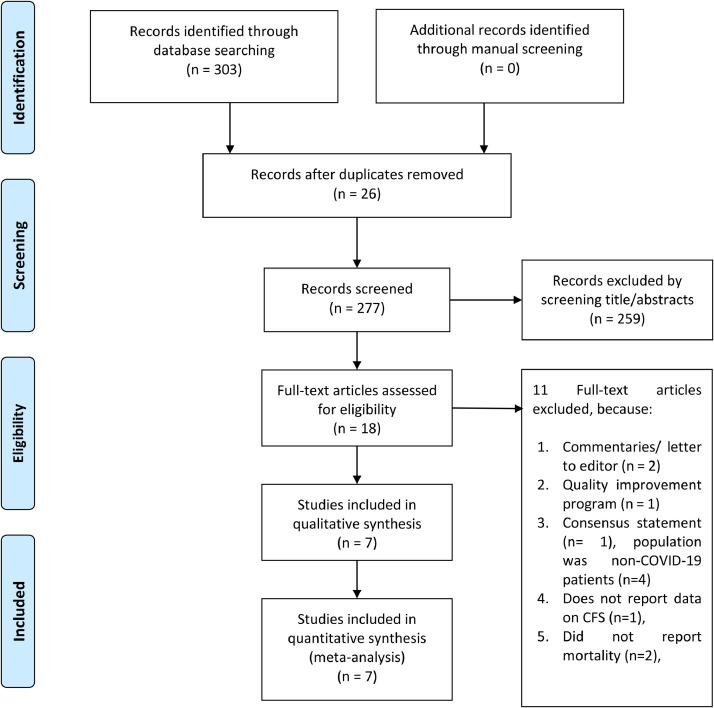
There were 303 results and 26 of them were duplicates. 259 of which, were excluded through title and abstract screening. Full-text articles from the remaining 18 records were assessed. We excluded 11 articles because of commentaries/ letter to editor (n = 2), quality improvement program (n = 2), consensus statement (n = 2), population was non-COVID-19 patients (n = 2), does not report data on CFS (n = 2), and did not report mortality (n = 2). Seven studies were included in the qualitative and quantitative synthesis (Aw, Woodrow, Ogliari & Harwood, 2020; De Smet et al., 2020; Hewitt et al., 2020; Knopp et al., 2020; Marengoni, Zucchelli, Grande, Fratiglioni & Rizzuto, 2020; Owen et al., 2020; Rawle, Bertfield & Brill, 2020). There were a total of 3817 patients from seven studies. [Fig. 1 ] Baseline characteristics of the included studies are displayed in Table 1 . Most of the studies, except Hewitt et al. enrolled only older patients. Mean age was 80.3 (SD 8.2) and 53% (48–58%) were males. The mean NOS of the included studies was 8.3 (SD 0.7).
Fig. 1.
PRISMA flow diagram.
Table 1.
Baseline characteristics of the included studies.
| Author | Design | Sample | Population | Age (years) | Male (%) | Mortality (%) | NOS |
|---|---|---|---|---|---|---|---|
| Hewitt J 2020 | Prospective Cohort | 1559 | Hospitalised patients age ≥18 | <65 (31.2%) 65–79 (34.2%) ≥80 (34.6%) |
57.7 | 27.2 | 9 |
| Knopp P 2020 | Prospective Cohort | 217 | Hospitalised patients age ≥70 with frailty | 80 (SD 6.8) | 62 | – | 8 |
| Smet RD 2020 | Retrospective Cohort | 81 | Hospitalised older people in geriatric ward | 85 (81–90) | 41 | 23.5 | 8 |
| Rawle M 2020 | Retrospective Cohort | 134 | Hospitalised patients age ≥80 with frailty | 86 (SD 7.6) | 54.5 | 64.9 | 9 |
| Aw 2020 | Retrospective Cohort | 664 | Hospitalised older people (age ≥65) with frailty | 81.1 (SD 8.1) | 49 | 40.8 | 9 |
| Owen 2020 | Retrospective Cohort | 1071 | Hospitalised older people (age ≥65) with frailty | 78.8 (SD 8.3) | 46 | 30.5 | 8 |
| Marengoni 2020 | Retrospective Cohort | 91 | Hospitalised older people in geriatric ward | 79.5 (SD 6.1) | 60.4 | 42.9 | 7 |
NOS: Newcastle-Ottawa Scale.
3.1. Prevalence
The pooled prevalence for CFS 1-3 was 34% (32–36%), CFS 4-6 was 42% (40–45%), and CFS 7-9 was 23% (21–25%).
3.2. Dose-response meta-analysis
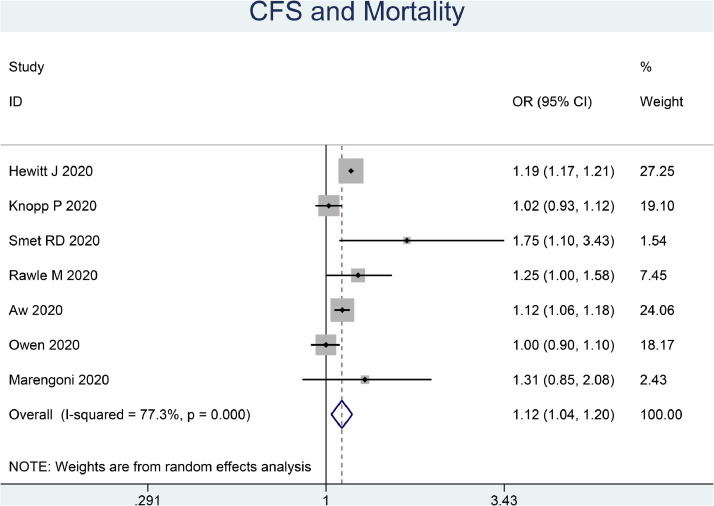
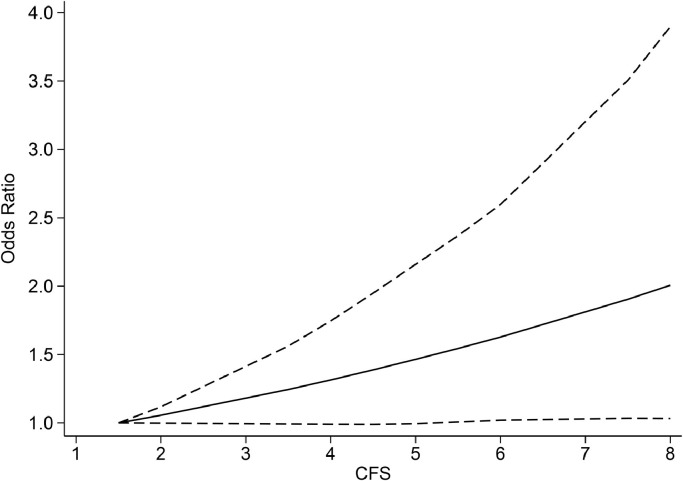
Each 1-point increase in CFS was associated with 12% increase in mortality (OR 1.12 (1.04, 1.20), p = 0.003; I2: 77.3%, p<0.001) [Fig. 2 ]. The dose-response relationship between CFS and increased mortality is linear (Pnon-linearity=0.116) [Fig. 3 ].
Fig. 2.
Clinical Frailty Scale and Mortality in COVID-19. The odds ratio is for per one-point increment in the scale.
Fig. 3.
Dose response meta-analysis between clinical frailty scale and mortality with restricted cubic splines in a random-effects dose-response model. Solid line indicates odds ratio and long dashed lines indicate its 95% confidence interval.
3.3. Publication bias
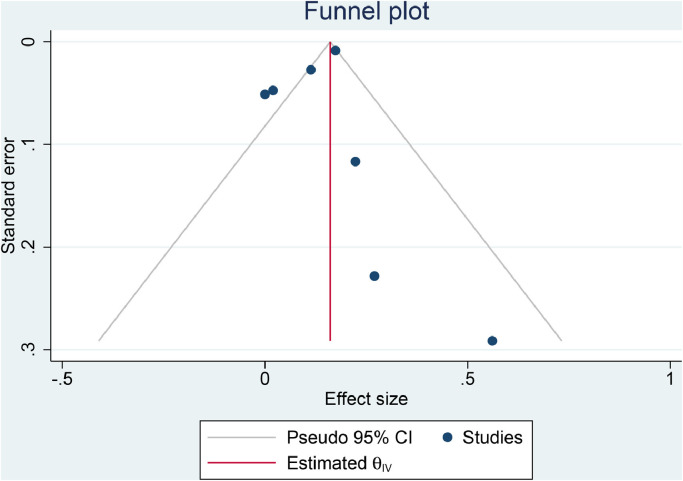
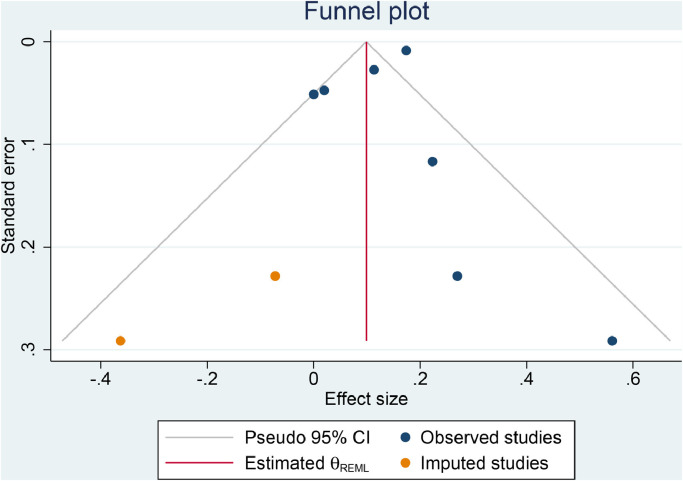
The funnel-plot analysis was asymmetrical, indicating publication bias [Fig. 4 A]. Trim-and-fill analysis by the imputation of two studies on the left side resulted in OR of 1.10 [1.03, 1.19] [Fig. 4B]. Regression-based Egger's test showed no indication of small-study effects (p = 0.191).
Fig. 4.
Funnel plot analysis (A) and trim-and-fill analysis (B).
4. Discussion
This dose-response meta-analysis showed that each 1-point increase in CFS was associated with 12% increase in mortality in a linear fashion. The largest proportion of patients have a CFS of 4–6, followed by CFS of 1–3, and CFS of 7–9.
There was a possibility of publication bias, indicated by the asymmetrical funnel-plot analysis. Trim-and-fill analysis was performed and showed that the imputation of two studies on the left side resulted in a similar effect estimate. This indicates that the addition of studies less positive studies was unlikely to change the effect estimate's direction. Analysis for heterogeneity was limited due to the fact that only two studies reported comorbidities that are associated with increased mortality in COVID-19 patients. These comorbidities are obesity (OR 1.55), hypertension (OR 2.21), diabetes (Risk Ratio [RR] 2.38), cardiovascular diseases (RR 2.25), and chronic obstructive pulmonary disease (OR 4.36) (Huang et al., 2020; Pranata et al., 2020b,c; Pranata, Lim, Huang, Raharjo & Lukito, 2020a; Pranata, Huang, Lim, Wahjoepramono & July, 2020d). Ideally, exploration of heterogeneity can be performed using meta-regression, however, it cannot be performed due to the lack of studies.
This finding bolsters the rapid guideline by NICE that endorse the use of CFS to identify and assess patients at high risk of poor outcomes and who might not benefit from critical care interventions (National Institute for Health & Care Excellence, 2020). CFS is a reliable predictor of outcomes in both acute care and critical care that can be undertaken by any trained healthcare professionals. Nevertheless, CFS is a supporting diagnostic tool that complements other assessment tools, and therefore, clinical decision making should be determined through shared, comprehensive, and holistic assessment. Since CFS has only been validated for people of advanced age (over 65), it may not be suitable for younger individuals or those with learning disabilities or progressive disability.
Although this systematic review and meta-analysis provide evidence for the use of CFS in elderly patients hospitalized with COVID-19, several important issues still need to be further elucidated. CFS cut-off value of 5 or more to guide critical care allocation in elderly patients, as recommended by NICE guideline, necessitates further research. A study by Darvall et al. in patients with pneumonia suggests that CFS of 5 or more alone is not useful for guiding the allocation of critical care resources (Darvall et al., 2020). Moreover, Chong et al. found that less frail individuals may also benefit from ICU care (Chong et al., 2020). The dichotomization of CFS at a specific cut-off point is required to be clinically useful in deciding patient's care. The current systematic review and meta-analysis cannot address this question. Hence, the evidence supporting this policy requires further investigation. Furthermore, relying solely in CFS to decide the need for critical care unit should be avoided. Not only is it oversimplifying a multifaceted matter, this one-dimensional approach is inconsistent with the core concept of geriatric medicine. At the very least, multi-comorbidities should as well be taken into account because it is undeniably contributed to the poor outcome among these elderly patients, as we have previously shown that per point increase in Charlson comorbidity index (CCI), could increase the mortality rate by 16% (Tuty Kuswardhani et al., 2020).
This systematic review's limitation is that most of the studies are retrospective in design, which are prone to biases. While it is unlikely that CFS is not associated with mortality, the magnitude might be over/underestimated due to the publication bias. Nevertheless, the trim-and-fill analysis indicates that the effect estimate is unlikely to change due to less positive studies. Most of the studies did not report comorbidities in their sample, and meta-regression analysis cannot be performed due to the lack of studies.
5. Conclusion
This meta-analysis showed that increase in CFS was associated with increase in mortality in a linear fashion. This parameter, combined with other clinical aspects, can help physicians determine the optimal allocation of valuable and limited medical resources.
Funding
None.
Authors' contribution
Raymond Pranata: This author helped in concept development, manuscript drafting, data acquisition, data analysis, and statistical analysis.
Joshua Henrina: This author helped in data acquisition, data analysis, extensive review, and editing of the manuscript.
Michael Anthonius Lim: This author helped in manuscript drafting, data acquisition, and data analysis.
Sherly Lawrensia: Investigation, Extensive review, and editing of the manuscript.
Emir Yonas: Investigation, Extensive review, and editing of the manuscript.
Rachel Vania: Investigation, Extensive review, and editing of the manuscript.
Ian Huang: Investigation, Extensive review, and editing of the manuscript.
Antonia Anna Lukito: Investigation, Extensive review, and editing of the manuscript.
Ketut Suastika: Investigation, Extensive review, and editing of the manuscript.
R.A. Tuty Kuswardhani: Investigation, Extensive review, and editing of the manuscript.
Siti Setiati: Investigation, Extensive review, and editing of the manuscript.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
None.
References
- Aw D., Woodrow L., Ogliari G., Harwood R. Association of frailty with mortality in older inpatients with Covid-19: A cohort study. Age and Ageing. 2020 doi: 10.1093/ageing/afaa184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanad C., García-Blas S., Tarazona-Santabalbina F., Sanchis J., Bertomeu-González V., Fácila L. The effect of age on mortality in patients with COVID-19: A meta-analysis with 611,583 subjects. Journal of the American Medical Directors Association. 2020;21(7):915–918. doi: 10.1016/j.jamda.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M., Proietti M. COVID-19 in Italy: Ageism and decision making in a pandemic. Journal of the American Medical Directors Association. 2020;21(5):576–577. doi: 10.1016/j.jamda.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong E., Chan M., Tan H.N., Lim W.S. COVID-19: Use of the clinical frailty scale for critical care decisions. Journal of the American Geriatrics Society. 2020;68(6) doi: 10.1111/jgs.16528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvall J.N., Bellomo R., Bailey M., Paul E., Young P.J., Rockwood K. Frailty and outcomes from pneumonia in critical illness: A population-based cohort study. British Journal of Anaesthesia. 2020 doi: 10.1016/j.bja.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet R., Mellaerts B., Vandewinckele H., Lybeert P., Frans E., Ombelet S. Frailty and mortality in hospitalized older adults with COVID-19: Retrospective observational study. Journal of the American Medical Directors Association. 2020;21(7):928–932. doi: 10.1016/j.jamda.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J., Carter B., Vilches-Moraga A., Quinn T.J., Braude P., Verduri A. The effect of frailty on survival in patients with COVID-19 (COPE): A multicentre, European, observational cohort study. The Lancet Public Health. 2020 doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia–A systematic review, meta-analysis, and meta-regression. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I., Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): Systematic review and meta-analysis. Journal of Intensive Care. 2020;8(1):36. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp P., Miles A., Webb T.E., Mcloughlin B.C., Mannan I., Raja N. European Geriatric Medicine; 2020. Presenting features of COVID-19 in older people: Relationships with frailty, inflammation and mortality. 2020.06.07.20120527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E.G., Breckons M., Lee R.P., Dotchin C., Walker R. Rationing care by frailty during the COVID-19 pandemic. Age and Ageing. 2020 doi: 10.1093/ageing/afaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengoni A., Zucchelli A., Grande G., Fratiglioni L., Rizzuto D. The impact of delirium on outcomes for older adults hospitalised with COVID-19. Age and Ageing. 2020 doi: 10.1093/ageing/afaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesas A.E., Cavero-Redondo I., Álvarez-Bueno C., Sarriá Cabrera M.A., Maffei de Andrade S., Sequí-Dominguez I. Predictors of in-hospital COVID-19 mortality: A comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions. PloS One. 2020;15(11) doi: 10.1371/journal.pone.0241742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Odasso M., Hogan D.B., Lam R., Madden K., MacKnight C., Molnar F. Age alone is not adequate to determine healthcare resource allocation during the COVID-19 pandemic. Canadian Geriatrics Journal. 2020;23(1):152–154. doi: 10.5770/cgj.23.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. (2020). COVID-19 rapid guideline: Critical care in adults. [PubMed]
- Owen R.K., Conroy S.P., Taub N., Jones W., Bryden D., Pareek M. Comparing associations between frailty and mortality in hospitalised older adults with or without COVID-19 infection: A retrospective observational study using electronic health records. Age and Ageing. 2020 doi: 10.1093/ageing/afaa167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Lim M.A., Huang I., Raharjo S.B., Lukito A.A. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: A systematic review, meta-analysis and meta-regression. JRAAS - Journal of the Renin-Angiotensin-Aldosterone System. 2020;21(2) doi: 10.1177/1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Lim M.A., Yonas E., Vania R., Lukito A.A., Siswanto B.B. Body mass index and outcome in patients with COVID-19: A dose–response meta-analysis. Diabetes and Metabolism. 2020 doi: 10.1016/j.diabet.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Soeroto A.Y., Huang I., Lim M.A., Santoso P., Permana H. Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. The International Journal of Tuberculosis and Lung Disease : The Official Journal of the International Union against Tuberculosis and Lung Disease. 2020;24(8) doi: 10.5588/ijtld.20.0278. [DOI] [PubMed] [Google Scholar]
- Pranata Raymond, Huang I., Lim M.A., Wahjoepramono E.J., July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19–systematic review, meta-analysis, and meta-regression. Journal of Stroke and Cerebrovascular Diseases. 2020;29(8) doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawle M.J., Bertfield D.L., Brill S.E. Atypical presentations of COVID-19 in care home residents presenting to secondary care: A UK single centre study. MedRxiv. 2020 doi: 10.1101/2020.07.07.20148148. 2020.07.07.20148148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truog R.D., Mitchell C., Daley G.Q. The toughest triage—Allocating ventilators in a pandemic. New England Journal of Medicine. 2020;382(21):1973–1975. doi: 10.1056/NEJMp2005689. [DOI] [PubMed] [Google Scholar]
- Tuty Kuswardhani R.A., Henrina J., Pranata R., Anthonius Lim M., Lawrensia S., Suastika K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: A systematic review and meta-analysis. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020;14(6):2103–2109. doi: 10.1016/j.dsx.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonas E., Alwi I., Pranata R., Huang I., Lim M.A., Gutierrez E.J. Effect of heart failure on the outcome of COVID-19—A meta analysis and systematic review. The American Journal of Emergency Medicine. 2020 doi: 10.1016/j.ajem.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]