Abstract
The world is currently facing the coronavirus disease (COVID-19) pandemic which places great pressure on health care systems and workers, often presents with severe clinical features, and sometimes requires admission into intensive care units. Derangements in nutritional status, both for obesity and malnutrition, are relevant for the clinical outcome in acute illness. Systemic inflammation, immune system impairment, sarcopenia, and preexisting associated conditions, such as respiratory, cardiovascular, and metabolic diseases related to obesity, could act as crucial factors linking nutritional status and the course and outcome of COVID-19. Nevertheless, vitamins and trace elements play an essential role in modulating immune response and inflammatory status. Overall, evaluation of the patient's nutritional status is not negligible for its implications on susceptibility, course, severity, and responsiveness to therapies, in order to perform a tailored nutritional intervention as an integral part of the treatment of patients with COVID-19. The aim of this study was to review the current data on the relevance of nutritional status, including trace elements and vitamin status, in influencing the course and outcome of the disease 3 mo after the World Health Organization's declaration of COVID-19 as a pandemic.
Keywords: COVID-19, Nutritional status, Sarcopenia, Vitamins, Trace elements
Introduction
First recognized in December 2019 in Wuhan, Hubei province, China, a novel coronavirus disease sustained by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), then named coronavirus disease (COVID-19), spread over six continents [1]. By March 11, 2020, the World Health Organization (WHO) declared COVID-19 a pandemic. To date, >22,400,000 cases of SARS-CoV-2 infection have been confirmed in 216 countries and >788,000 deaths have been reported [2].
COVID-19 presents with a broad clinical spectrum, ranging from asymptomatic and paucisymptomatic forms to mild upper respiratory tract infections (RTIs) to severe pneumonia, even resulting in acute respiratory distress syndrome (ARDS). Further symptoms such as fatigue, headache, diarrhea, nausea and vomiting, anorexia, and loss of taste and smell have been reported [3], [4], [5]. Extra-respiratory manifestations are frequent, and can include cardiac complications; renal dysfunction; and gastrointestinal, neurologic, and hematologic abnormalities [6], as SARS-CoV-2 has the ability to penetrate different organs, including the lungs, pharynx, heart, kidneys, liver, and brain [7].
Male sex, older age, comorbidities (mainly chronic lung diseases, hypertension, and diabetes) are described as the most important risk factors for outcome and mortality [4,[8], [9], [10]]. Although studies specifically assessing the role of a deranged nutritional status in this type of patient are still sparse, a high frequency of obesity in COVID-19 inpatients, with a median body mass index (BMI) 30 kg/m2, and a trend for disease severity with increased BMI values [11,12] has been reported. In this context, previous observational data were mostly extrapolated after the H1N1 pandemic in 2009 [13,14]. A meta-analysis performed with 3059 individuals reported a higher probability for admission to the intensive care unit (ICU) or death (odds ratio [OR], 2.01) for individuals with BMI >40 kg/m2 [15]. Similarly Moser et al. observed the OR increasing to 35.13 in individuals who were morbidly obese [16]. During the COVID-19 pandemic, Simmonet et al. reported that the need for mechanical ventilation reached nearly 90% in patients with a BMI >35 kg/m2, and in another study cohort of 5700 patients, 41.7% were obese [11,17].
Undernutrition appears to be elevated among these patients, with a ≤52.7% prevalence in elderly inpatients [18], acting as a negative prognostic factor, since it has previously been demonstrated that in-hospital malnutrition is associated to hospital length of stay (LOS), in-hospital mortality, and re-admission rate [19], [20], [21].
In addition to over- and undernutrition, the crucial role of trace elements must be considered as they are involved in the modulation of immune responses. Indeed, derangements in nutritional status have an important epidemiologic relevance: 1.9 billion individuals are affected by overweight (600 million by obesity), 800 million by chronic undernourishment, and >2 billion by micronutrient deficiencies [22].
These considerations lead to the assessment of overall nutritional status beyond merely anthropometric measurements, especially at the admission or first clinical evaluation, to identify the factors that could influence the course of the disease. In this context, ad hoc guidelines have been proposed to perform an adequate nutritional approach [23] as an integral part of the treatment regimen, particularly when an etiologic treatment has not been discovered or validated [24].
Aim
The aim of this study was to review the current data on the relevance of nutritional status, including trace elements and vitamin status, in influencing the course and outcome of the disease 3 mo after the WHO's declaration of COVID-19 as a pandemic.
Nutritional status, inflammation, immunity, and virus replication
As suggested by some Simonnet et al. [11], obesity could represent a major risk factor for SARS-CoV-2 infection, and could have a crucial role in the course of the disease. As previously reported, obesity may influence either the risk for infection or the outcome once it is established, as observed during the H1N1 influenza A pandemic in 2009 [25,26].
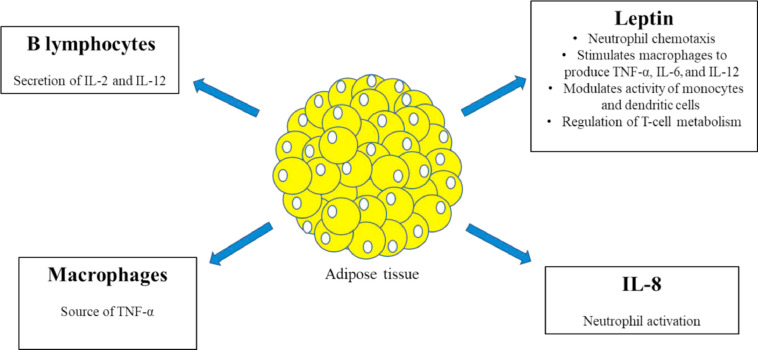
This could be mainly explained by the state of low-grade inflammation provided by adipose tissue (AT), which accounts for the modulation of immune responses (Fig. 1 ). AT itself contains a large amount of macrophages, which represent the most abundant immune cells, and therefore acts as a source of tumor necrosis factor (TNF) [27,28]. AT also produces interleukin (IL)-8, a chemokine of innate immunity. Plasma IL-8 levels are increased in obese individuals, and are related to fat mass (FM) and the TNF-α system. They then promote a stronger neutrophil activation and potential tissue damage [29]. Acting as an endocrine gland, AT stores and releases large amounts of molecules such as leptin, which has structural similarity to other proinflammatory cytokines such as IL-6, IL-12, and granulocyte colony-stimulating factor and plays a crucial role in modulating both innate and adaptative immunity [30]. Leptin has pleiotropic characteristics because it enhances neutrophil chemotaxis in macrophages, which are even stimulated for production of TNF, IL-6, and IL-12, and promotes modulation of monocytes and dendritic cells [31,32]. Moreover, leptin seems to regulate T-cell metabolism through the activation of mammalian target of rapamycin (mTOR) complex, in particular upregulating glucose uptake, and may drive CD4+ T cells to the production of T-helper (Th)1-type cells, which in turn favors the release of the proinflammatory cytokine interferon (IFN)-γ. B lymphocytes also accumulate in the AT and contribute to secretion of inflammatory cytokines, such as IL-2 and IL-12, responsible for T-cell differentiation [33], [34], [35], [36].
Fig. 1.
Adipose tissue as an endocrine and paracrine gland creates an inflammatory microenvironment, in which macrophages and B lymphocytes contribute to secretion of proinflammatory molecules, and is a source of IL-8. Indeed, leptin, which tends to be overexpressed in relation to fat mass, acts with pleiotropic activities enhancing inflammation cascade and modulating cell-mediated immunity. IL, interleukin; TNF, tumor necrosis factor.
In the COVID-19 scenario, patients show signs of an amplified and severe hyper-inflammation response due to macrophage activation, known with the evocative term cytokine storm. In particular, IL-6 seems to be overproduced, thus inducing lung injury with alveolar edema, hyalinosis (intraalveolar proteinosis), pneumocytes viral cytopathic change, and immune cell infiltration [37]. Moreover, IL-6 inhibits natural killer (NK) cell cytotoxicity, which are less able to kill target cells by perforin/granzyme-induced apoptosis, thus amplifying overproduction of proinflammatory cytokines [38].
Considering the lack of available data regarding the degree of inflammatory response in the specific obese population with COVID-19, we could speculate similar clinical findings described for H1N1 influenza, in which IL-6, IL-15, IL-8, and TNF-α levels correlated with worse outcome and were significantly higher in obese patients compared with lean ones [39].
In addition to obesity, malnutrition should be carefully evaluated. In fact, one should consider the lack of AT as source of adipocytokines, in which macrophages, cytotoxic T cells, and effector T cells are less represented. These aspects induce changes in immunometabolism and impaired protective response [36]. Regarding leptin levels, which are reduced in starvation, an association with immune defects was observed [40]. Moreover, T cells in fasting are less able to produce IL-2 and IFN-γ [35]. Other effects of undernutrition on the immune system are impaired complement activation and thymic atrophy (the primary site of T-cell development) [41]. Inflammatory pathways could be even more detrimental in the case of undernutrition as inflammation response has negative effects on protein stores because of the catabolic effects of acute phase proteins such as C-reactive protein and ILs, worsening a pre-existing state of frailty [42]. Indeed, if the energy requirement is not met, wasting will occur as mobilized amino acids are prioritized for the synthesis of protein related to immune response [41].
As mentioned, available data about prevalence of undernutrition in patients with SARS-CoV-2 infection are sparse. Li et al. evaluated the nutritional status of elderly inpatients with COVID-19 using the Mini Nutritional Assessment (MNA) and found that 27.5% were at risk for malnutrition and 52.7% were malnourished. However, the authors did not perform a statistical association with mortality or disease severity [18]. When considering the above cited analysis on H1N1 and respiratory viruses, some researchers have reported a fivefold increased risk for hospitalization in underweight individuals [16].
Taken together, these considerations should lead to accurate population screening in order to interrupt the vicious cycle in which inflammation status becomes abnormally overspread and leads to disruption of tissue integrity, with poor outcomes (Table 1 ).
Table 1.
Nutritional status and immunity derangements
| Overweight/Obesity | Role in infectious diseases |
|---|---|
|
| Undernutrition | Role in infectious diseases |
|---|---|
|
IFN, interferon; IL, interleukin; TFN, tumor necrosis factor; TLR, Toll-like receptor.
Numbers within brackets represent reference citation; see the Reference section.
It has been hypothesized that nutritional status could influence virus shedding and the potential for transmission. This hypothesis has been reinforced by a study from Moriconi et al., which observed that obese patients with COVID-19 require longer hospitalization and more intensive care and oxygen support; moreover, they seem to have longer SARS-CoV-2 shedding [43].
In a study by Maier et al., the authors described a shedding of influenza A virus 42% longer in symptomatic obese adults than in nonobese patients (5.23 versus 3.68 d). Consequently, it would be easier for virus to spread among other people [44]. This finding could be due to the microenvironment, which, as discussed, is characterized by low-grade inflammation. In animal models, a change in virus population diversity with emergence of a more virulent phenotype was observed. It can be due to less IFN pressure, which in normal status tends to inhibit viral replication [45].
In a study by Weger-Lucarelli et al. [46] in animal models infected with alphaviruses, an altered viral replication was observed and the authors hypothesized that the microenvironment in the obese can be involved in persistent alphavirus replication, which may evolve in chronic disease.
Conversely, undernutrition also can be associated with less responsiveness for vaccines. This is because of the crucial role of nutrition in modulating immune responses and lower production of antigens [47]. An emblematic example was reported by Bhattacharjee et al. [48], who evaluated the association between infections and the microbiome. In fact, the authors underlined the lower efficacy of oral vaccines in children living in resource-poor countries. This could be explained by diminution of antiviral T-cell responses due to lack of mucosal bacteria [49], which also express Toll-like receptor (TLR)-5, which is involved in vaccine responses [50].
An important role in immune function and responsiveness to vaccines is played by micronutrients, which is discussed further.
Obesity-associated diseases and effects on COVID-19
Respiratory comorbidities and complications
Several clinical conditions, frequently complicating or associated to obesity, act as independent risk factors for a more severe disease course in patients with COVID-19. According to the meta-analysis by Wang et al., the most important are chronic obstructive pulmonary disease (COPD; OR, 5.97), chronic cardiovascular disease (CVD; OR, 2.93), hypertension (OR, 2.29), diabetes (OR, 2.47), and cerebrovascular disease (OR, 3.89) [51].
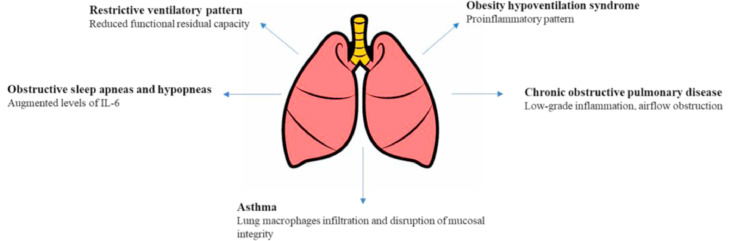
Several respiratory diseases could complicate an obesity condition, including obstructive sleep apneas and hypopneas (OSAHs), obesity hypoventilation syndrome (OHS), asthma, and COPD [52], [53], [54]. Under these conditions, the respiratory system is constantly under stress as it exhibits ≤16% increase in ventilatory work to meet oxygen demand, thus reducing the physiologic pulmonary reserve in acute episodes (eg, pneumonia, ARDS) [55,56]. In addition to these, he link between respiratory diseases and an increased susceptibility to influenza and bacterial pneumonia, with worse outcomes from these conditions, has been proven [15,57] (Fig. 2 ).
Fig. 2.
Obesity respiratory complications and their potential role in influencing COVID-19 course. Obese patients exhibit pulmonary alterations in which a proinflammatory environment promotes release of immunomodulatory molecules, and a structural alteration that could worsen the outcome of viral pneumonia in terms of respiratory exchanges and virus replication. IL, interleukin.
Restrictive ventilatory pattern
Obesity is typically associated with a restrictive ventilatory pattern, due to the excess AT hindering chest wall, diaphragmatic, and basal lung expansion movements, finally resulting in airway resistance, closure of peripheral lung units, ventilation-perfusion abnormalities, and arterial hypoxemia. On spirometry, they result in significantly lower functional residual capacity (FRC) and expiratory reserve volume (ERV), slightly reduced forced expiratory volume in 1 second (FEV1), and forced vital capacity (FVC) [52], whereas the FEV1/FVC ratio is usually unaffected except in the case of extreme BMI values (>62 kg/m2) [58].
In the COVID-19 context, severe pneumonia are connected with extensive pulmonary fibrosis (PF), where a further deleterious role has been hypothesized for pulmonary lipofibroblasts in the alveolar interstitium and closely similar to adipocytes, as they may transdifferentiate into myofibroblasts that play an integral part in the development of PF [59].
OSAHs
An obstructive component can be found in a whole series of respiratory diseases. Among these, OSAHs occur in ~40% of the obese population, as increased fat tissue deposition in the pharyngeal region and reduced operating lung volume lead to upper airway collapsibility and closures during sleep [60]. BMI, in addition to neck and abdominal circumferences, affects both risk for OSAHs (≤90% for BMI >40 kg/m2) [61], [62], [63] and variability in the apnea-hypopnea index, which defines the severity of the disease [64,65]. Notably, OSAH severity is associated with IL-6 levels in obese individuals [66], and likely is involved in the development of insulin resistance [67,68].
In the COVID-19 scenario, OSAH may lead to a worse course of the disease, even progressing to OHS and respiratory failure [69].
OHS
OHS presents with respiratory failure, severe hypoxemia, daytime hypercapnia (partial pressure of carbon dioxide [PaCO2] >6 kPa), and pulmonary hypertension [59]. It occurs in >11% of obese patients, with a proportional relationship between BMI and the hypercapnia severity [70].
Makinodan et al. demonstrated a significant association between PaCO2 values and higher serum leptin levels in obese patients with OSAH, so it has been suggested that a different sensitivity to circulating leptin could explain why many patients with OSAH do not necessarily progress to OHS [[71], [72], [73], [74]].
Although there is a lack of scientific data assessing OHS prevalence and incidence among COVID-19 patients, it is conceivable that OHS, in a much more complex context within chronic inflammation, metabolic, and cardiovascular alterations, could play an important role in aggravation of the prognosis of patients with COVID-19. In this regard, an emblematic case of OHS onset in a 23-y-old man with OSAH, fatty liver disease, and dyslipidemia has been reported [75].
Asthma
Asthma exhibits a typical obstructive ventilatory pattern and, according to the meta-analysis by Beuther et al., is prevalent in 38% of overweight patients and 92% in those who are obese [76]. An increased AT mass is associated with an increased infiltration by macrophages in obese individuals with asthma. Moreover visceral FM is an important source of mast cell progenitors, mediators of allergy [58]. However, it is still debated whether a direct relationship exists between obesity and the traditional biomarkers of airway inflammation in patients with asthma, as obesity does not necessarily worsen airway inflammation in asthma [77,78]. Rather, non-atopic mechanisms antagonizing therapies, such as the low-grade systemic inflammation associated with obesity, may affect glucocorticoid sensitivity [79,80].
Although data are limited, patients with severe and/or uncontrolled asthma and those with COPD appear to be at increased risk for a more severe course of SARS-CoV-2 infection [81,82]. Although asthma is not in the top 10 comorbidities associated with fatalities among all-aged COVID-19 patients [83], it has been reported among the most common comorbidities in younger patients with COVID-19, with obesity and diabetes [[84], [85]]. At a pathogenetic level, allergic sensitization and eosinophilic inflammation compromise the integrity of the airway mucosa, thus fostering viral infections in the lower airways and limiting the ability of the respiratory tract to clear viruses [86].
COPD
COPD is characterized by the progressive and largely irreversible airflow obstruction and occurs predominantly in smokers [87]. Overweight and obesity prevalence is higher in the early COPD population (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stages 1 and 2), compared with the general population [88,89], and low-grade inflammation and arterial hypoxemia lead to a loss in skeletal muscle tissue and respiratory muscle performance, thus predisposing the individual to increased morbidity and mortality [52,88,89]. Otherwise, undernutrition is more frequent in patients with moderate to severe COPD (GOLD stages 3 and 4), with an overall decline in BMI and decrease both in FM and fat-free mass (FFM). BMI values <25 kg/m2 seem to adversely affect prognosis [90]. Notably, COPD and obesity share a common association with tobacco smoking, as obese individuals smoke more than the general population, with a number of cigarettes smoked per day correlated with elevated BMI and/or visceral adiposity [91], [92], [93], [94]. In this regard, a common biological basis for the regulation of appetite for food and tobacco, and thus the vulnerability to obesity and nicotine addiction, has been suggested [95], [96], [97].
In the COVID-19 pandemic, the WHO has said that COPD and ongoing smoking history contribute to worse progression and outcome of disease [98], with higher severity and risk for death both in COPD patients (OR, ≤4.38) and current smokers (OR, 1.98) [99], [100], [101], [102].
Cardiovascular comorbidities and complications
The most common extrapulmonary complications of COVID-19 occur in the cardiovascular system because SARS-CoV-2 binds to the transmembrane angiotensin-converting enzyme (ACE)-2 to enter type 2 pneumocytes, cardiomyocytes, and perivascular pericytes, followed by a severe cytokine storm (IL-6, IL-7, IL-22, IL-17, etc). This may lead to myocardial, endothelial, and microvascular dysfunction, and plaque instability, with cardiac injury, myocardial infarction (MI), heart failure (HF), myocarditis, and arrhythmias [103], [104], [105], [106], [107], [108]. Katz et al. [109] reported that 28% of COVID-19 inpatients presented cardiac complications such as coronary artery disease (CAD; OR, 2.70), HF (OR, 2.48), and cardiac arrhythmias (OR, 1.95), all of which are associated with an increased risk for in-hospital death [110].
Ischemic heart disease
Overweight and obesity have been well recognized as major risk factors for CAD [111], [112], [113], which is associated with a high incidence rate, acute onset, and increased lethality, thereby posing a serious threat to the life of the patient [114,115]. Risk for CAD seems to be closely correlated with visceral obesity measures (waist circumference, waist-to-height ratio, waist-to-hip ratio, and the sagittal abdominal diameter) compared with BMI value alone [114,116].
A greater risk for severe COVID-19 and death in patients with pre-existing CVDs [117], [118], [119], [120], such as CAD (severe illness OR, 6.85), HF (OR, 9.77), CVD events (OR, 8.89), and hypertension (OR, 4.56) has been reported [121]. Even cardiac troponin I levels are higher in patients with severe COVID-19 than in those with milder forms of disease [117,122]. In this context, in addition to the systemic inflammation, the dangerous role of the epicardial adipose tissue (EAT), which is more abundant in obesity and induces pathophysiologic changes in cardiomyocytes, coronary artery endothelial cells, and monocytes through a local inflammatory pathway, has been suggested [123], [124], [125], [126], [127]. Similar effects on myocardial tissue from EAT have also been suggested for cardiovascular complications in patients with COVID-19 [128].
Pulmonary embolism and thrombotic events
Obesity has been consistently and independently associated with pulmonary embolism and thrombotic complications, even after adjustments for age and other risk factors (OR, 2.2) [129]. Thrombotic diathesis can be related to the systemic chronic inflammation and oxidative stress that cause the endothelium to lose its antithrombotic properties. Moreover, a role is played by impaired platelet reactivity, enhanced coagulation (elevated circulating levels of von Willebrand factor, tissue factor, factor VII and VIII, and fibrinogen), and impaired fibrinolytic system [130,131]. These effects may be further exacerbated by dysregulated expression and secretion of adipokines and microRNAs [131,132].
A high cumulative incidence of thrombotic complications in critically ill patients with COVID-19 admitted to the ICUs, with a higher risk for all-cause death (hazard ratio [HR], 5.4) has been reported [133]. In an Italian cohort of 388 patients, thromboembolic events occurred in 7.7% cases, corresponding to a cumulative rate of 21% (27.6% ICU; 6.6% general ward) [134]. Although the prevalence of obesity among these patients was not reported, it has been noted that inpatients with more severe disease from SARS-CoV-2 and with other risk factors, including obesity, have a higher risk for thromboembolic events [135]. Even postmortem examinations confirmed the high incidence of venous thromboembolism in deceased patients with COVID-19 [136], with a pulmonary picture characterized by small and mid-sized pulmonary artery thrombosis, massive capillary congestion often accompanied by microthrombi despite anticoagulation, diffuse alveolar damage, edema, hyaline membranes, and pneumocyte and fibroblast proliferation [137,138]. Elevated D-dimer and fibrinogen levels are commonly found in these patients [139,140], and an association between serum homocysteine levels and imaging progression of pulmonary disease on chest computed tomography has been found [141]. A direct role of SARS-CoV-2 in the pathogenesis of systemic coagulopathy [142,143] has been suggested and it has been hypothesized that heparin administration should be included in the treatment of severe forms [144].
Hypertension
In the COVID-19 scenario, hypertension has been associated with an increased risk for severe disease (OR, 2.49) as well as with a similarly significant higher mortality risk (OR, 2.42), especially in older individuals [145].
Excess weight gain, particularly when associated with increased visceral adiposity, is a major cause of hypertension, accounting for 65% to 75% of the risk for primary (essential) hypertension [146], with a proportional relationship between BMI and systolic and diastolic blood pressure (BP) values [147]. Among pathogenetic factors, a crucial role is played by increased sodium reabsorption [148], [149], [150], [151], [152], altered hemodynamics, renal dysfunction, autonomic nervous system imbalance, endocrine alterations, oxidative stress and inflammation, and vascular injury. Most of these factors interact with each other at multiple levels, and obesity-related hypertension has been proposed as a distinct form of hypertension [153].
A still controversial issue concerns the role of ACE inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) on the course of the COVID-19. An upregulation of the ACE-2 receptors, thus theoretically increasing the risk for infection and severity of the disease, has been suggested [154], [155], [156], [157]. However, a significant difference in the proportion of ACEI/ARBs medication between non-survivors and survivors among COVID-19 hospitalized patients has not been reported and no evidence that ACEI or ARBs affected the COVID-19 risk has been demonstrated [110,117,158,159]. In this regard, major cardiology associations discourage the discontinuation of anti-hypertensive therapy, as the acute risk for hypertension could exacerbate the clinical course and increase the mortality of patients with COVID-19 [10,160,161].
Metabolic comorbidities and complications
Type 2 diabetes
Type 2 diabetes is a major contributor to disease severity and mortality in all three known human pathogenic coronavirus infections, including Middle East respiratory syndrome (MERS-CoV), SARS-CoV, and SARS-CoV-2 [162,163].
Depending on the global region, 20% to 50% of patients with COVID-19 have diabetes, with an increased risk for severe complications such as ARDS, multiorgan failure (MOF) [163], need for mechanical ventilation [164], and as high as a 1.5-fold risk for a fatal outcome [165].
Several hypotheses could explain this increased severity of COVID-19 infection in patients with diabetes, including impairments in innate immunity (phagocytosis, neutrophil chemotaxis, and cell-mediated immunity), and both acute and chronic hyperglycemia (dysregulation in ACE-2 expression with higher cellular vulnerability to the damaging effect of the virus). Even dipeptidyl peptidase-4 enzyme, a functional receptor for the virus responsible for MERS, could increase inflammation in type 2 diabetes [163]. Moreover, chronic inflammation, increased coagulation activity, and potential direct pancreatic damage by SARS-CoV-2 might be among the underlying mechanisms of the association between diabetes and COVID-19 [166].
Most patients with type 2 diabetes are obese, and the global epidemic of obesity largely explains the dramatic increase in the incidence and prevalence of type 2 diabetes over the past 20 y [167,168], a phenomenon recently termed diabesity [169]. Visceral adiposity plays a crucial role in insulin resistance, as an increase in abdominal girth or waist-to-hip ratio is associated with risk for type 2 diabetes, metabolic syndrome, and CVD [170,171]. In the early stage of diabetes mellitus, AT can be further stimulated by a plethora of molecules such as acyl ghrelin [172]. In turn, AT affects metabolism by secreting higher levels of glycerol, leptin, cytokines, proinflammatory substances, and non-esterified fatty acids [171,173,174].
Metabolic-associated fatty liver disease
In the course of COVID-19, Zheng et al observed a higher severe illness risk (OR, 6.32) in obese patients with metabolic-associated fatty liver disease (MAFLD), even after adjusting for age, sex, smoking, diabetes, hypertension, and dyslipidemia, than those without MAFLD [175,176], and Hussain et al. provided similar results [177]. Notably, advanced liver disease represents an increased risk for infection and a severe course of COVID-19 [178], so that the European Association for the Study of the Liver (EASL) presented a position paper addressing this topic [179]. Although a clinically significant liver injury is rare and of relatively mild degree [180], unless in patients with severe SARS-CoV-2 illness [181], abnormal liver blood tests are common in the course of the disease [182]. A higher persistence of these abnormalities from admission to discharge in patients with MAFLD has been reported [173,183], whereas MAFLD seems not to be related to an increased liver SARS-CoV-2 uptake nor to changes in liver expression of genes implicated in virus infection [184]. Moreover, MAFLD is often associated with obesity and other metabolic risk factors, thus further aggravating the severity of respiratory illnesses [185], [186], [187], [188].
Role of sarcopenia
To our knowledge, no studies have specifically addressed the effects of sarcopenia on patients with COVID-19. It is conceivable that, as previously observed with other respiratory diseases, sarcopenia may heavily influence the course and outcome of COVID-19, especially in elderly patients. Individuals 60 to 70 y of age have a 5% to 13% prevalence of sarcopenia and those ≥80 y of age have as much as 50% [189]. According to this hypothesis, the Asian Working Group for Sarcopenia proposed recommendations to evaluate and manage secondary consequences of COVID-19 in the elderly [190].
Sarcopenia is a progressive, generalized skeletal muscle disorder associated with increased likelihood of adverse outcomes including falls, fractures, physical disability, poor quality of life, dependency in activities of daily living, and excess mortality [191,192]. The European Working Group on Sarcopenia in Older People performed a consensus on definition and diagnosis, stressing that the role of low muscle strength as the primary parameter of sarcopenia is better than lean mass in predicting adverse outcomes. Low muscle quantity or quality allows confirmation of the diagnosis, and low physical performance allows the determination of severity [193].
Primary (or age-related) sarcopenia occurs in the absence of specific causal factors and, at a pathogenetic level, systemic, chronic, sterile, low-grade inflammation acts as a major contributor. This is also known as inflamm-aging [194], [195], [196], [197] because senescent cells acquire a senescence-associated secretory phenotype, with slightly increased levels of proinflammatory cytokines (TNFα, IL-6, nuclear factor-κB overactivation), thus muscle protein breakdown and synthesis are affected [198], [199], [200], [201], [202].
Secondary sarcopenia occurs when causes other than (or in addition to) aging are evident, such as systemic diseases (e.g., malignancies) [203], loss of motor neuron units, diabetes mellitus, very low 25-hydroxyvitamin D levels [204], and physical inactivity (sedentary lifestyle or disease-related immobility) [203,205,206]. Furthermore, inadequate intake of energy or protein, as a result of anorexia, malabsorption, limited access to healthy foods, or limited ability to eat, can lead to sarcopenia [193]. In a critical care setting with endotracheal intubation required for mechanical ventilation, as many as 62% of patients develop post-extubation dysphagia due to tongue weakness and tube-related oropharyngeal mucosal inflammation, muscle atrophy, decreased proprioception, and laryngeal injury. Thus, post-extubation dysphagia could severely limit resumption of oral feeding [207], [208], [209], [210], [211], promoting undernutrition [212] and sarcopenia of the muscles used for swallowing, and further worsening dysphagia through a vicious cycle (sarcopenic dysphagia) [213], [214], [215], [216]. Notably, >3.2% of patients with COVID-19 required intubation [217]. Moreover, myopathies and rhabdomyolysis are common extrapulmonary manifestations in flu infections, as confirmed by the finding of atrophic/necrotic muscle fibers during muscle biopsies, thus further worsening sarcopenia. Elevated serum creatine kinase (CK) levels were found during the 2009 H1N1 flu pandemic, and were associated with higher LOS in ICUs, and increased both pulmonary and non-pulmonary complications [218].
The sarcopenic phenotype is typically associated with malnutrition due to low dietary intake (starvation, inability to eat), reduced nutrient bioavailability (e.g., diarrhea, vomiting), or high nutrient requirements (e.g., inflammatory diseases such as cancer or organ failure) [219,220]. However, sarcopenia can occur as a loss of lean body mass in the context of excess adiposity (sarcopenic obesity) [221], as obesity increases the fat infiltration into muscle and lowers physical functioning [222], [223], [224], [225].
The association between sarcopenia and respiratory function impairment has been widely described [226,227], with lower diaphragmatic muscle thickness and peak expiratory flow rate (PEFR) rates (245 versus 310 L/min in sarcopenic and non-sarcopenic patients, respectively) [228] leading to a specific definition of respiratory sarcopenia based on the PEFR values [229]. Moreover, frailty often overlaps with sarcopenia, although a broader syndrome characterized by vulnerability and a higher state for risk following minor stressor events has been described [226]. Even obesity could deteriorate respiratory function through an overburden on breathing associated with respiratory muscle mass loss [230,231].
In this context, body composition assessment plays a crucial role in differentiating lean mass from FM, thus grading the severity of sarcopenia in malnourished patients and recognizing sarcopenic obesity, with a reduced muscle mass despite a normal or even increased BMI [232].
Role of trace elements and vitamins
Trace elements and vitamins play a fundamental role in the modulation of immune response and their deficiency can influence the course of the disease (Table 2 ).
Table 2.
Roles of trace elements and vitamin deficiency in worsening COVID-19 course and outcome
| Trace element or vitamin | Deficiency and suggested role in COVID-19 |
|---|---|
| Selenium | |
|
|
| Zinc | |
|
|
| Copper | |
|
|
| Vitamin D | |
|
|
| Ascorbic acid | |
|
|
| Vitamin A | |
|
|
| Vitamin E | |
|
CVD, cardiovascular disease; DM, diabetes mellitus; FVC, forced vital capacity; NK, natural killer; ROS, reactive oxygen species.
Numbers within brackets represent reference citation; see the Reference section.
Selenium
It has been largely recognized that selenium has pleiotropic properties that are important for health status. Selenium exerts its functions through 25 selenoproteins that act as antioxidants, such as glutathione peroxidases (GPx) which is involved in control of reactive oxygen species (ROS) during inflammatory processes. Redox balance is recognized as a critical factor in the progression of viral infection [233], [234], [235].
Nutritional selenium deficiency effects the immune response, resulting in less proliferation of T cells, lymphocyte-mediated toxicity, and NK cell activity [236]. It appears to influence the course of viral infections by limiting elevation of ROS, whereas biosynthesis of antioxidant enzymes is reduced in the infected cells, and influencing virus replication by increasing the rate of the genome mutation, especially for RNA viruses [237]. It has been found that selenium status could influence the outcome of influenza A virus, as seen in studies that correlated lower selenium concentration and infection by the highly pathogenic H1N1 subtype of influenza A virus [238]. Other implications are severity of tissue inflammatory infiltration and emergence of more virulent virus subtypes [237].
As pointed out by Zhang et al. [239], selenium could be a choice for treatment of COVID-19, but it has also been hypothesized that it plays a crucial role in the emergence and spread of SARS-CoV-2. Because selenium concentrations in China vary between the lowest and the highest values in the world, some researchers have analyzed hair selenium concentrations, observing a much higher death rate in COVID-19 patients from low-selenium regions [240]. These findings should open discussion of considering eventual selenium supplementation, taking into account that a selenium overload could be detrimental in influencing immune response to vaccines [236].
Zinc
According to a report published by the WHO in 2013, it is estimated that zinc deficiency affects about 33% of the world's population, with estimates ranging from 4% to 73% across subregions and 1.4% of deaths worldwide (0.8 million) were attributed to zinc deficiency: 1.4% in men and 1.5% in women. It is also responsible for ~16% of lower RTIs [241].
A wide spectrum of immune response, both regarding innate and adaptive immunity derangements, occurs in zinc deficiency conditions. Zinc deficiency can result in reduced polymorphonuclear cell (PMNs) chemotaxis and phagocytosis and regulation of NADPH oxidase activity, involved in destruction of pathogens after phagocytosis. Zinc deficiency also causes increased production of proinflammatory cytokines such as IL-1B, IL-6, and TNF-α and compromised modulation of NK cell activity, especially in the setting of major histocompatibility complex (MHC) class I. Thymic atrophy and subsequent T-cell lymphopenia and reduction of premature and immature B cells with consequently reduced antibody production are other effects of zinc deficiency [242].
Zinc in binded as divalent cation to metallothioneins (MTs) and is released as a mechanism to reduce ROS generated by viral infections. Moreover, MTs have been classified as IFN-stimulating genes and their upregulation has been observed in response to measles virus, influenza, HIV, and hepatitis C virus. In the context of influenza and other RTIs sustained by coronavirus and metapneumovirus, the inhibition of RNA-dependent RNA polymerase, which inhibits viral replication, has been observed (in vitro) [243].
Various studies evaluated the effect of zinc supplementation in viral diseases, finding a prophylactic effect and reduced duration of symptoms [243], [244], [245], whereas other studies did not report a convincing effect on viral load or immune responses [242]. Some researchers have suggested a possible role of zinc supplementation in enhancing the clinical efficacy of chloroquine/hydroxychloroquine used for treatment of COVID-19, as chloroquine has characteristics of a zinc ionophore, specifically in lysosomes, which may result in a more efficient RNA-dependent RNA polymerase inhibition of intracellular SARS-CoV-2 replication [246]. However, the effective utility and recommendations for use of chloroquine/hydroxychloroquine must be effectively established and serious concerns remain, mostly about its cardiotoxicity [247], [248], [249].
Copper
Recently, some researchers have suggested a possible involvement of copper as an adjuvant in treatment of COVID-19. The rationale of this suggestion comes from the observation that severe copper deficiency has adverse effects on immune function, especially in elderly people where marginal or severe deficiency of copper is a strong possibility. Because copper and zinc are competitively absorbed from the jejunum via MT, high doses of zinc (>150 mg/d) can result in copper deficiency in healthy individuals [250].
The role of copper during infections has been previously investigated. Elevated levels of copper could attack microbes through copper toxicity [251], in particular during lung infections, in which an uptake in macrophages was observed [252]. Nearly all of copper content of the serum (95%) is bound to ceruloplasmin, whose levels are increased in response to inflammation, trauma, or infection, in order to facilitate copper delivery to sites of infection [253]. Moreover, there is also an activation of immune response, as seen by Kelley et al., who observed that after providing a diet with lower content of copper, peripheral blood mononuclear cell proliferation and secretion of the IL-2 receptor in the culture medium were reduced [254]. Interestingly, some researchers have reported a specific role of copper in viral replication, as in the case of influenza A/WSN/33 (H1N1) as a critical step in RNA and protein syntheses [255].
However, these observations should not be taken as advice for COVID-19 treatment, even in consideration of the possible toxic effect of copper overload.
Vitamin D
Considering the rate of vitamin D deficiency and insufficiency, it can be considered a global health problem that has characteristics as a pandemic. It has been estimated that ~30% of children and 60% of adults worldwide are vitamin D deficient and insufficient, respectively [256]. The severity of 25-hydroxyvitamin D deficiency is stratified into mild (<20 ng/mL), moderate (<10 ng/mL), and severe (<5 ng/mL) [257]. Pregnant women, individuals with increased skin melanin, abstinence from direct sun exposure (which explains the higher prevalence in higher latitudes), and obese children and adults are considered at high risk for deficiency. A prevalence of vitamin D deficiency is 35% higher in obese individuals regardless of latitude and age [256,258].
Vitamin D acts as an immunomodulator and as an antioxidant, with an important role in CVDs and diabetes mellitus [256]. It is also involved in protection against viral RTIs and acute lung injury, as observed in ARDS, in which there is reduced lung permeability by modulation of renin–angiotensin system activity and ACE-2 expression [259], [260], [261], [262]. Thus, vitamin D deficiency has been reasonably correlated to COVID-19 as a pathogenic factor. This hypothesis is corroborated by analysis of vitamin D prevalence and COVID-19 spreading and mortality observed in the Northern Hemisphere in contrast to the Southern Hemisphere [263]. Hastie et al. analyzed data available from 348 598 UK Biobank participants and found that median 25-hydroxyvitamin D concentration measured at recruitment was lower in patients who subsequently developed COVID-19 [264]. Daneshkhah et al. observed that the age-specific COVID-19 fatality rate was highest in Italy, Spain, and France, all of which are European countries with the highest incidence of severe vitamin D deficiency [265]. These findings suggest that measurement of serum 25-hydroxyvitamin D is necessary in patients infected with SARS-CoV-2 in order to identify the ones at highest risk. Once identified, a supplementation dose should be administered. Caccialanza et al. suggest cholecalciferol supplementation according to blood tests results (50 000 UI/wk and 25 000 UI/wk if 25-hydroxyvitamin D <20 ng/mL and ≥20 to <30 ng/mL, respectively) [266], whereas Ebady et al. propose a 100 000 IU start dose of cholecalciferol followed by 50 000 IU/wk for the second and third week [267]. According to the most recent guidelines for nutrition management in the ICU, a single high dose (500 000 IU) can be safely administered within the first week [268], and it could be reasonably applied for COVID-19 patients, although no evidence exists to date. To our knowledge, there is no clear consensus about the administration of cholecalciferol in COVID-19 patients, neither a proven efficacy as adjuvant therapy, even though some researchers suggest this is a possible application [269].
Ascorbic acid
Overt vitamin C deficiency known as scurvy, is rare especially in high-income countries; however, a less pronounced deficiency (defined as a serum concentration <11.4 umol/L) is more common, with rates as low as 7.1% in the United States and up to 73.9% in northern India. Risk factors include alcohol intake, tobacco use, low income, male sex, patients on hemodialysis, and those with overall poor nutritional status [270].
Vitamin C mainly plays an essential role in protecting the cells from oxidative damage; improves neutrophil migration and chemotaxis; promotes the proliferation, differentiation, and maturation of T and possibly also B lymphocytes; and has an inhibitory effect on secretion of proinflammatory cytokines [271]. A link between vitamin C status and RTIs has been observed, as a lower mortality rate from pneumonia was reported in patients with higher serum vitamin C values [272]. Recently, Carr et al. evaluated vitamin C status in a cohort of patients with pneumonia and observed a depletion compared with healthy controls. In particular, the more severe patients in ICUs had significantly lower vitamin C levels [273]. This latter study was not performed in patients with COVID-19 and no data are available regarding vitamin C status in these patients. The potential role as an immunomodulator and antioxidant lead to administration of ascorbic acid in critically ill patients, and various studies have been performed, even if there are some discrepancies regarding the administered doses. A recent systematic review concluded that intravenous (IV) administration of vitamin C could reduce dependency on mechanical ventilation, possibly through the amelioration of lung injury, without affecting overall mortality [274].
To date, there is no consensus or proven efficacy of supplementation of ascorbic acid in COVID-19 patients but some researchers advise a possible use of IV supplementation as addressed by an expert panel document from the National Institiutes of Health that a regimen of 1.5 g/kg body weight could be considered safe and without major adverse events [275].
Vitamin A
Vitamin A (all-trans-retinol) deficiency is rarely seen in high-income countries. Nonetheless, the prevalence of vitamin A deficiency is ~30% among children <5 y of age worldwide and nearly 50% in young children in South Asia and sub-Saharan Africa [276].
Vitamin A acts through its metabolites and is involved in various processes from embryogenesis to adulthood, such as normal organogenesis, immune competence, tissue differentiation, and the visual cycle [277]. Deficiency of vitamin A can be caused by several conditions, including severe infections, malabsorption, liver disease, iron and zinc status, fat intake, xenobiotics, protein-energy malnutrition, and alcohol consumption [278]. Xerophthalmia is the hallmark of vitamin A deficiency and is the most common cause of preventable blindness in children, as well as other clinical manifestations including impairment of the humoral and cell-mediated immune system. In particular, mucosal integrity and Th2-mediated responses are compromised [277,279,280]. In cases of infections, during the acute phase response a decrease in serum retinol is observed, in a proportional manner to the severity of infection. This decrease is transitory and serum retinol typically returns to preinfection levels within a few days [280].
Regarding respiratory tract, vitamin A plays a central role during lung development and alveolar function in the prenatal period, although in the postnatal stage, it is essential for lung growth, alveolarization, and plays a main role in resistance and elasticity, and repair and remodeling of lung. Consequently, vitamin A deficiency can be associated with a low FVC, an indicator of airway obstruction and a strong predictor of mortality in asymptomatic adults without chronic respiratory conditions. It also can cause squamous metaplasia of the respiratory epithelium, with a decrease in mucus production, which increases the risk for invasive pathogens and severity of lower RTIs [278].
To our knowledge, there are no available data regarding vitamin A status in patients with COVID-19, thus its involvement in possible worsening of lung damage, virulence, and progression could only be hypothesized. Because administration reduced morbidity and mortality in different infectious diseases, such as measles, measles‐related pneumonia, HIV, and malaria, Zhang et al. suggest that vitamin A supplementation could be considered adjunctive to other medications for SARS-CoV-2 infection [239].
Vitamin E
Vitamin E is a family of related compounds, including α-, β-, γ-, and δ-tocopherol. Its most important role is as an antioxidant, as it limits the detrimental effects of peroxyl radicals on cellular surfaces, in particular for polyunsaturated fatty acids (PUFAs) [281]. Vitamin E also acts as a mediator in cell and humoral immune responses in animals and humans, increasing lymphocyte proliferation, immunoglobulin levels, antibody responses, NK cell activity, and IL-2 production. It has been described reduction of prostaglandin E2 production by the inhibition of cyclooxygenase activity mediated through decreasing nitric oxide production, modulation of Th1/Th2 balance, higher NK activity, and lower IL-12 production and migration [282]. Some researchers have reported a protective effect of vitamin E supplementation on upper respiratory infections in elderly patients [283], and there could be an increased virulence in cases of deficiency, as seen in animal models [284]. However, although to our knowledge no data concerning the association between vitamin E status and COVID-19 have been reported, it cannot be ruled out as a potential factor in diminishing the inflammatory state in these patients.
Conclusions
Although the nutritional status in patients with COVID-19 has been poorly investigated, preliminary reported evidence as nutritional derangements are associated with a worse course and outcome of the disease, as likely with a greater susceptibility to infection. Obesity plays a major role because it represents a prognostic risk factor and relates to worse outcomes independently from age, sex, and other comorbidities. Malnutrition and deficiency of trace elements likely act as factors strongly affecting the COVID-19 course, most likely because of their high prevalence estimated among the world population. Several pathogenetic mechanisms may explain the complex interaction between nutritional status and SARS-CoV-2 infection and disease, including systemic inflammation, immune system impairment, sarcopenia, and pre-existing associated diseases, such as respiratory, cardiovascular, and metabolic complications and comorbidities in obesity.
Because an etiologic and definitively effective treatment for COVID-19 has not yet been performed, the factors associated with the risk for disease severity should be recognized and managed, including nutritional status derangements, as the significant prognostic relevance of an early and adequate nutritional intervention, even in critical patients, has been widely demonstrated. Indeed, international guidelines recommend a thorough nutritional assessment at the first evaluation and then periodically during the course of the disease, in order to early perform a reasonable nutritional intervention as an integral part within the treatment and management of COVID-19 patients. Further studies will necessary to fully investigate these aspects, thus improving and tailoring the therapy in an individualized and precision medicine view. In this sense, the COVID-19 era may represent an opportunity to highlight the relevance of nutritional status as a critical point of host response to infections, therapies, and vaccines.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Available at: https://covid19.who.int/. Accessed September 11, 2020.
- 3.Zhu J., Zhong Z., Ji P., Li H., Li B., Pang J., et al. Clinicopathological characteristics of 8697 patients with COVID-19 in China: a meta-analysis. Fam Med Community Health. 2020;8 doi: 10.1136/fmch-2020-000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behzad S., Aghaghazvini L., Radmard A.R., Gholamrezanezhad A. Extrapulmonary manifestations of COVID-19: radiologic and clinical overview. Clin Imaging. 2020;66:35–41. doi: 10.1016/j.clinimag.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu L., Chen S., Fu Y., Gao Z., Long H., Wang J.M., et al. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China [Epub ahead of print] Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa539. ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakodkar P., Kaka N., Baig M.N. A Comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID-19) Cureus. 2020;12:e7560. doi: 10.7759/cureus.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., Lille Intensive Care COVID-19 and Obesity Study Group High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palaiodimos L., Kokkinidis D.G., Li W., Karamanis D., Ognibene J., Arora S., et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108 doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan O.W., Bramley A., Fowlkes A., Freedman D.S., Taylor T.H., Gargiullo P., et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS One. 2010;5:e9694. doi: 10.1371/journal.pone.0009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietz W., Santos-Burgoa C. Obesity and its Implications for COVID-19 mortality. Obesity (Silver Spring) 2020;28:1005. doi: 10.1002/oby.22818. [DOI] [PubMed] [Google Scholar]
- 15.Fezeu L., Julia C., Henegar A., Bitu J., Hu F.B., Grobbee D.E., et al. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: a systematic review and meta-analysis. Obes Rev. 2011;12:653–659. doi: 10.1111/j.1467-789X.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- 16.Moser J.S., Galindo-Fraga A., Ortiz-Hernández A.A., Gu W., Hunsberger S., Galan-Herrara J.F., et al. Underweight, overweight, and obesity as independent risk factors for hospitalization in adults and children from influenza and other respiratory viruses. Influenza Other Respir Viruses. 2019;13:3–9. doi: 10.1111/irv.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., and the Northwell COVID-19 Research Consortium Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li T., Zhang Y., Gong C., Wang J., Liu B., Shi L., et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020;22:1–5. doi: 10.1038/s41430-020-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hudson L., Chittams J., Griffith C., Compher C. Malnutrition identified by Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition is associated with more 30-day readmissions, greater hospital mortality, and longer hospital stays: a retrospective analysis of nutrition assessment data in a major medical center. JPEN J Parenter Enteral Nutr. 2018;42:892–897. doi: 10.1002/jpen.1021. [DOI] [PubMed] [Google Scholar]
- 20.Norman K., Pichard C., Lochs H., Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr. 2008;27:5–15. doi: 10.1016/j.clnu.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal E., Ferguson M., Banks M., Batterham M., Bauer J., Capra S., et al. Malnutrition and poor food intake are associated with prolonged hospital stay, frequent readmissions, and greater in-hospital mortality: results from the Nutrition Care Day Survey 2010. Clin Nutr. 2013;32:737–745. doi: 10.1016/j.clnu.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. General Assembly proclaims the Decade of Action on Nutrition. Available at: https://www.who.int/nutrition/GA_decade_action/en/. Accessed September 11, 2020.
- 23.Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Krznaric Z., Nitzan D., Pirlich M., Singer P., endorsed by the ESPEN Council ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39:1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laviano A., Koverech A., Zanetti M. Nutrition support in the time of SARS-CoV-2 (COVID-19) Nutrition. 2020;74 doi: 10.1016/j.nut.2020.110834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louie J.K., Acosta M., Samuel M.C., Schechter R., Vugia D.J., Harriman K., California Pandemic (H1N1) Working Group A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1) Clin Infect Dis. 2011;52:301–312. doi: 10.1093/cid/ciq152. [DOI] [PubMed] [Google Scholar]
- 26.Huttunen R., Syrjänen J. Obesity and the risk and outcome of infection. Int J Obes (Lond) 2013;37:333–340. doi: 10.1038/ijo.2012.62. [DOI] [PubMed] [Google Scholar]
- 27.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 29.Straczkowski M., Dzienis-Straczkowska S., Stêpieñ A., Kowalska I., Szelachowska M., Kinalska I. Plasma interleukin-8 concentrations are increased in obese subjects and related to fat mass and tumor necrosis factor-alpha system. J Clin Endocrinol Metab. 2002;87:4602–4606. doi: 10.1210/jc.2002-020135. [DOI] [PubMed] [Google Scholar]
- 30.Tilg H., Moschen A.R. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 31.Gainsford T., Willson T.A., Metcalf D., Handman E., McFarlane C., Ng A., et al. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc Natl Acad Sci U S A. 1996;93:14564–14568. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papathanassoglou E., El-Haschimi K., Li X.C., Matarese G., Strom T., Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006;176:7745–7752. doi: 10.4049/jimmunol.176.12.7745. [DOI] [PubMed] [Google Scholar]
- 33.MacIver N.J., Michalek R.D., Rathmell J.C. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Procaccini C., Jirillo E., Matarese G. Leptin as an immunomodulator. Mol Aspects Med. 2012;33:35–45. doi: 10.1016/j.mam.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Saucillo D.C., Gerriets V.A., Sheng J., Rathmell J.C., Maciver N.J. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J Immunol. 2014;192:136–144. doi: 10.4049/jimmunol.1301158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alwarawrah Y., Kiernan K., MacIver N.J. Changes in nutritional status impact immune cell metabolism and function. Front Immunol. 2018;9:1055. doi: 10.3389/fimmu.2018.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced Pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruscitti P., Berardicurti O., Iagnocco A., Giacomelli R. Cytokine storm syndrome in severe COVID-19. Autoimmun Rev. 2020 doi: 10.1016/j.autrev.2020.102562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagau N., Slavcovici A., Gonganau D.N., Oltean S., Dirzu D.S., Brezoszki E.S., et al. Clinical aspects and cytokine response in severe H1N1 influenza A virus infection. Crit Care. 2010;14:R203. doi: 10.1186/cc9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boden G., Chen X., Mozzoli M., Ryan I. Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab. 1996;81:3419–3423. doi: 10.1210/jcem.81.9.8784108. [DOI] [PubMed] [Google Scholar]
- 41.Gandy G., editor. Manual of dietetic practice. 6th ed. Wiley-Blackwell; Hoboken, NJ: 2019. [Google Scholar]
- 42.Jia H. Pulmonary angiotensin-converting enzyme 2 (ACE2) and inflammatory lung disease. Shock. 2016;46:239–248. doi: 10.1097/SHK.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 43.Moriconi D., Masi S., Rebelos E., Virdis A., Manca M.L., De Marco S., et al. Obesity prolongs the hospital stay in patients affected by COVID-19, and may impact on SARS-COV-2 shedding. Obes Res Clin Pract. 2020;14:205–209. doi: 10.1016/j.orcp.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maier H.E., Lopez R., Sanchez N., Ng S., Gresh L., Ojeda S., et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis. 2018;218:1378–1382. doi: 10.1093/infdis/jiy370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Honce R., Karlsson E.A., Wohlgemuth N., Estrada L.D., Meliopoulos V.A., Yao J., et al. Obesity-related microenvironment promotes emergence of virulent influenza virus strains. mBio. 2020;11 doi: 10.1128/mBio.03341-19. e03341–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weger-Lucarelli J., Carrau L., Levi L.I., Rezelj V., Vallet T., Blanc H., et al. Host nutritional status affects alphavirus virulence, transmission, and evolution. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1008089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoest C., Seidman J.C., Pan W., Ambikapathi R., Kang G., Kosek M., MAL-ED Network Investigators Evaluating associations between vaccine response and malnutrition, gut function, and enteric infections in the MAL-ED cohort study: methods and challenges. Clin Infect Dis. 2014;59(suppl 4):S273–S279. doi: 10.1093/cid/ciu611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhattacharjee A., Hand T.W. Role of nutrition, infection, and the microbiota in the efficacy of oral vaccines. Clin Sci (Lond) 2018;132:1169–1177. doi: 10.1042/CS20171106. [DOI] [PubMed] [Google Scholar]
- 49.Abt M.C., Osborne L.C., Monticelli L.A., Doering T.A., Alenghat T., Sonnenberg G.F., et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oh J.Z., Ravindran R., Chassaing B., Carvalho F.A., Maddur M.S., Bower M., et al. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity. 2014;41:478–492. doi: 10.1016/j.immuni.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang B., Li R., Lu Z., Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging. 2020;12:6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zammit C., Liddicoat H., Moonsie I., Makker H. Obesity and respiratory diseases. Int J Gen Med. 2010;3:335–343. doi: 10.2147/IJGM.S11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koenig S.M. Pulmonary complications of obesity. Am J Med Sci. 2001;321:249–279. doi: 10.1097/00000441-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Murugan A.T., Sharma G. Obesity and respiratory diseases. Chron Respir Dis. 2008;5:233–242. doi: 10.1177/1479972308096978. [DOI] [PubMed] [Google Scholar]
- 55.Chiumello D., Colombo A., Algieri I., Mietto C., Carlesso E., Crimella F., et al. Effect of body mass index in acute respiratory distress syndrome. Br J Anaesth. 2016;116:113–121. doi: 10.1093/bja/aev378. [DOI] [PubMed] [Google Scholar]
- 56.Bime C., Fiero M., Lu Z., Oren E., Berry C.E., Parthasarathy S., et al. High positive end-expiratory pressure is associated with improved survival in obese patients with acute respiratory distress syndrome. Am J Med. 2017;130:207–213. doi: 10.1016/j.amjmed.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ubags N.D., Stapleton R.D., Vernooy J.H., Burg E., Bement J., Hayes C.M., et al. Hyperleptinemia is associated with impaired pulmonary host defense. Version 2. JCI Insight. 2016;1:e82101. doi: 10.1172/jci.insight.82101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dixon A.E., Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12:755–767. doi: 10.1080/17476348.2018.1506331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kruglikov I.L., Scherer P.E. The role of adipocytes and adipocyte-like cells in the severity of COVID-19 infections. Obesity (Silver Spring) 2020;28:1187–1190. doi: 10.1002/oby.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Poulain M., Doucet M., Major G.C., Drapeau V., Sériès F., Boulet L.P., et al. The effect of obesity on chronic respiratory diseases: pathophysiology and therapeutic strategies. CMAJ. 2006;174:1293–1299. doi: 10.1503/cmaj.051299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Isono S. Obstructive sleep apnea of obese adults: pathophysiology and perioperative airway management. Anesthesiology. 2009;110:908–921. doi: 10.1097/ALN.0b013e31819c74be. [DOI] [PubMed] [Google Scholar]
- 62.Schäfer H., Pauleit D., Sudhop T., Gouni-Berthold I., Ewig S., Berthold H.K. Body fat distribution, serum leptin, and cardiovascular risk factors in men with obstructive sleep apnea. Chest. 2002;122:829–839. doi: 10.1378/chest.122.3.829. [DOI] [PubMed] [Google Scholar]
- 63.Candiotti K., Sharma S., Shankar R. Obesity, obstructive sleep apnoea, and diabetes mellitus: anaesthetic implications. Br J Anaesth. 2009;103(suppl 1):i23–i30. doi: 10.1093/bja/aep294. [DOI] [PubMed] [Google Scholar]
- 64.Mokhlesi B., Gozal D. Update in sleep medicine 2009. Am J Respir Crit Care Med. 2010;181:545–549. doi: 10.1164/rccm.200912-1948UP. [DOI] [PubMed] [Google Scholar]
- 65.Crummy F., Piper A.J., Naughton M.T. Obesity and the lung: 2. Obesity and sleep-disordered breathing. Thorax. 2008;63:738–746. doi: 10.1136/thx.2007.086843. [DOI] [PubMed] [Google Scholar]
- 66.Arnardottir E.S., Maislin G., Schwab R.J., Staley B., Benediktsdottir B., Olafsson I., et al. The interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: the Icelandic Sleep Apnea Cohort. Sleep. 2012;35:921–932. doi: 10.5665/sleep.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Makino S., Handa H., Suzukawa K., Fujiwara M., Nakamura M., Muraoka S., et al. Obstructive sleep apnoea syndrome, plasma adiponectin levels, and insulin resistance. Clin Endocrinol (Oxf) 2006;64:12–19. doi: 10.1111/j.1365-2265.2005.02407.x. [DOI] [PubMed] [Google Scholar]
- 68.Dumitrache-Rujinski S., Dinu I., Călcăianu G., Erhan I., Cocieru A., Zaharia D., et al. Metabolic profile in obese patients with obstructive sleep apnea. A comparison between patients with insulin resistance and with insulin sensitivity. Pneumologia. 2014;63:100–102. 104–6. [PubMed] [Google Scholar]
- 69.McSharry D., Malhotra A. Potential influences of obstructive sleep apnea and obesity on COVID-19 severity [Epub ahead of print] J Clin Sleep Med. 2020 doi: 10.5664/jcsm.8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laaban J.P., Chailleux E. Daytime hypercapnia in adult patients with obstructive sleep apnea syndrome in France, before initiating nocturnal nasal continuous positive airway pressure therapy. Chest. 2005;127:710–715. doi: 10.1378/chest.127.3.710. [DOI] [PubMed] [Google Scholar]
- 71.Makinodan K., Yoshikawa M., Fukuoka A., Tamaki S., Koyama N., Yamauchi M., et al. Effect of serum leptin levels on hypercapnic ventilatory response in obstructive sleep apnea. Respiration. 2008;75:257–264. doi: 10.1159/000112471. [DOI] [PubMed] [Google Scholar]
- 72.O'donnell C.P., Schaub C.D., Haines A.S., Berkowitz D.E., Tankersley C.G., Schwartz A.R., et al. Leptin prevents respiratory depression in obesity. Am J Respir Crit Care Med. 1999;159:1477–1484. doi: 10.1164/ajrccm.159.5.9809025. [DOI] [PubMed] [Google Scholar]
- 73.Shapiro S.D., Chin C.H., Kirkness J.P., McGinley B.M., Patil S.P., Polotsky V.Y., et al. Leptin and the control of pharyngeal patency during sleep in severe obesity. J Appl Physiol (1985) 2014;116:1334–1341. doi: 10.1152/japplphysiol.00958.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yee B.J., Cheung J., Phipps P., Banerjee D., Piper A.J., Grunstein R.R. Treatment of obesity hypoventilation syndrome and serum leptin. Respiration. 2006;73:209–212. doi: 10.1159/000088358. [DOI] [PubMed] [Google Scholar]
- 75.Huang J.F., Wang X.B., Zheng K.I., Liu W.Y., Chen J.J., et al. Letter to the Editor: obesity hypoventilation syndrome and severe COVID-19. Metabolism. 2020;108 doi: 10.1016/j.metabol.2020.154249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beuther D.A., Sutherland E.R. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sutherland E.R. Linking obesity and asthma. Ann N Y Acad Sci. 2014;1311:31–41. doi: 10.1111/nyas.12357. [DOI] [PubMed] [Google Scholar]
- 78.Kim S.H., Sutherland E.R., Gelfand E.W. Is there a link between obesity and asthma? Allergy Asthma Immunol Res. 2014;6:189–195. doi: 10.4168/aair.2014.6.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sin D.D., Sutherland E.R. Obesity and the lung: 4. Obesity and asthma. Thorax. 2008;63:1018–1023. doi: 10.1136/thx.2007.086819. [DOI] [PubMed] [Google Scholar]
- 80.Shore S.A. Obesity and asthma: implications for treatment. Curr Opin Pulm Med. 2007;13:56–62. doi: 10.1097/MCP.0b013e3280110196. [DOI] [PubMed] [Google Scholar]
- 81.Daccord C., Touilloux B., Von Garnier C. Asthma and COPD management during the COVID-19 pandemic. Rev Med Suisse. 2020;16:933–938. [PubMed] [Google Scholar]
- 82.Mahdavinia M., Foster K.J., Jauregui E., Moore D., Adnan D., Andy-Nweye A.B., et al. Asthma prolongs intubation in COVID-19. J Allergy Clin Immunol Pract. 2020 doi: 10.1016/j.jaip.2020.05.006. S2213–2198(20)30476–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hakim D. The New York Times; 2020. Asthma is absent among top Covid-19 risk factors, early data shows. [Google Scholar]
- 84.Garg S., Kim L., Whitaker M. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019- COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pennington E. Asthma increases risk of severity of COVID-19 [Epub ahead of print] Cleve Clin J Med. 2020 doi: 10.3949/ccjm.87a.ccc002. [DOI] [PubMed] [Google Scholar]
- 86.Akenroye A.T., Wood R., Keet C. Asthma, Biologics, Corticosteroids, and COVID-19. Ann Allergy Asthma Immunol. 2020;125:12–13. doi: 10.1016/j.anai.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vogelmeier C.F., Criner G.J., Martinez F.J., Anzueto A., Barnes P.J., Bourbeau J., et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 88.Steuten L.M., Creutzberg E.C., Vrijhoef H.J., Wouters E.F. COPD as a multicomponent disease: inventory of dyspnoea, underweight, obesity and fat free mass depletion in primary care. Prim Care Respir J. 2006;15:84–91. doi: 10.1016/j.pcrj.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eisner M.D., Blanc P.D., Sidney S., Yelin E.H., Lathon P.V., Katz P.P., et al. Body composition and functional limitation in COPD. Respir Res. 2007;8(1):7. doi: 10.1186/1465-9921-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Franssen F.M., O'Donnell D.E., Goossens G.H., Blaak E.E., Schols A.M. Obesity and the lung: 5. Obesity and COPD. Thorax. 2008;63:1110–1117. doi: 10.1136/thx.2007.086827. [DOI] [PubMed] [Google Scholar]
- 91.Bamia C., Trichopoulou A., Lenas D., Trichopoulos D. Tobacco smoking in relation to body fat mass and distribution in a general population sample. Int J Obes Relat Metab Disord. 2004;28:1091–1096. doi: 10.1038/sj.ijo.0802697. [DOI] [PubMed] [Google Scholar]
- 92.Chiolero A., Jacot-Sadowski I., Faeh D., Paccaud F., Cornuz J. Association of cigarettes smoked daily with obesity in a general adult population. Obesity (Silver Spring) 2007;15:1311–1318. doi: 10.1038/oby.2007.153. [DOI] [PubMed] [Google Scholar]
- 93.Clair C., Chiolero A., Faeh D., Cornuz J., Marques-Vidal P., Paccaud F., et al. Dose-dependent positive association between cigarette smoking, abdominal obesity and body fat: cross-sectional data from a population-based survey. BMC Public Health. 2011;11:23. doi: 10.1186/1471-2458-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Akbartabartoori M., Lean M.E., Hankey C.R. Relationships between cigarette smoking, body size and body shape. Int J Obes (Lond) 2005;29:236–243. doi: 10.1038/sj.ijo.0802827. [DOI] [PubMed] [Google Scholar]
- 95.Thorgeirsson T.E., Gudbjartsson D.F., Sulem P., Besenbacher S., Styrkarsdottir U., Thorleifsson G., TAG Consortium. Oxford-GSK Consortium. ENGAGE consortium. Furberg H., Sullivan P.F., Marchini J., McCarthy M.I., Steinthorsdottir V., Thorsteinsdottir U., et al. A common biological basis of obesity and nicotine addiction. Transl Psychiatry. 2013;3:e308. doi: 10.1038/tp.2013.81. et al; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tobacco and Genetics Consortium Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42:441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wills A.G., Hopfer C. Phenotypic and genetic relationship between BMI and cigarette smoking in a sample of UK adults. Addict Behav. 2019;89:98–103. doi: 10.1016/j.addbeh.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.World Health Organization. WHO statement: tobacco use and COVID-19. Available at: https://www.who.int/news-room/detail/11-05-2020-who-statement-tobacco-use-and-covid-19. Accessed September 14, 2020.
- 99.Zhao Q., Meng M., Kumar R., Wu Y., Huang J., Lian N., et al. The impact of COPD and smoking history on the severity of COVID-19: a systemic review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alqahtani J.S., Oyelade T., Aldhahir A.M., Alghamdi S.M., Almehmadi M., Alqahtani A.S., et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vardavas C.I., Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob Induc Dis. 2020;18:20. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guo F.R. Smoking links to the severity of Covid-19: an update of a meta-analysis [Epub ahead of print] J Med Virol. 2020 doi: 10.1002/jmv.25967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guzik T.J., Mohiddin S.A., Dimarco A., Patel V., Savvatis K., Marelli-Berg F.M., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A., et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 106.Akhmerov A., Marbán E. COVID-19 and the heart. Circ Res. 2020;126:1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gupta M.D., Girish M.P., Yadav G., Shankar A., Yadav R. Coronavirus disease 2019 and the cardiovascular system: impacts and implications. Version 2. Indian Heart J. 2020;72:1–6. doi: 10.1016/j.ihj.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang Y., Roever L., Tse G., Liu T. 2019-Novel coronavirus-related acute cardiac injury cannot be ignored. Curr Atheroscler Rep. 2020;22:14. doi: 10.1007/s11883-020-00842-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Katz J.N., Sinha S.S., Alviar C.L., Dudzinski D.M., Gage A., Brusca S.B., et al. Disruptive modifications to cardiac critical care delivery during the Covid-19 pandemic: an international perspective. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.029. S0735–1097(20)35002–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007621. NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 111.Ndumele C.E., Matsushita K., Lazo M., Bello N., Blumenthal R.S., Gerstenblith G., et al. Obesity and subtypes of incident cardiovascular disease. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kang M.Y., Hong Y.C. Inter-correlation between working hours, sleep duration, obesity, and 10-year risk for CHD. Am J Ind Med. 2016;59:338–339. doi: 10.1002/ajim.22525. [DOI] [PubMed] [Google Scholar]
- 113.Kwagyan J., Retta T.M., Ketete M., Bettencourt C.N., Maqbool A.R., Xu S., et al. Obesity and cardiovascular diseases in a high-risk population: evidence-based approach to CHD risk reduction. Ethn Dis. 2015;25:208–213. [PMC free article] [PubMed] [Google Scholar]
- 114.Cao Q., Yu S., Xiong W., Li Y., Li H., Li J., Li F. Waist–hip ratio as a predictor of myocardial infarction risk: a systematic review and meta-analysis. Medicine. 2018;97:e11639. doi: 10.1097/MD.0000000000011639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van Dis I., Kromhout D., Geleijnse J.M., Boer J.M., Verschuren W.M. Body mass index and waist circumference predict both 10-year nonfatal and fatal cardiovascular disease risk: study conducted in 20,000 Dutch men and women aged 20-65 years. Eur J Cardiovasc Prev Rehabil. 2009;16:729–734. doi: 10.1097/HJR.0b013e328331dfc0. [DOI] [PubMed] [Google Scholar]
- 116.Yusuf S., Hawken S., Ounpuu S., Bautista L., Franzosi M.G., Commerford P., INTERHEART Study Investigators Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 117.Gupta R., Misra A. Contentious issues and evolving concepts in the clinical presentation and management of patients with COVID-19 infection with reference to use of therapeutic and other drugs used in Co-morbid diseases (hypertension, diabetes etc) Diabetes Metab Syndr. 2020;14:251–254. doi: 10.1016/j.dsx.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhu H., Rhee J.W., Cheng P., Waliany S., Chang A., Witteles R.M., et al. Cardiovascular complications in patients with COVID-19: consequences of viral toxicities and host immune response. Curr Cardiol Rep. 2020;22:32. doi: 10.1007/s11886-020-01292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Krittanawong C., Virk H.U.H., Narasimhan B., Wang Z., Narasimhan H., Zhang H.J., et al. Coronavirus disease 2019 (COVID-19) and cardiovascular risk: a meta-analysis [Epub ahead of print] Prog Cardiovasc Dis. 2020 doi: 10.1016/j.pcad.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lippi G., Lavie C.J., Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63:390–391. doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Karastergiou K., Evans I., Ogston N., Miheisi N., Nair D., Kaski J.C., et al. Epicardial adipokines in obesity and coronary artery disease induce atherogenic changes in monocytes and endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30:1340–1346. doi: 10.1161/ATVBAHA.110.204719. [DOI] [PubMed] [Google Scholar]
- 124.Sacks H.S., Fain J.N., Cheema P., Bahouth S.W., Garrett E., Wolf R.Y., et al. Depot-specific overexpression of proinflammatory, redox, endothelial cell, and angiogenic genes in epicardial fat adjacent to severe stable coronary atherosclerosis. Metab Syndr Relat Disord. 2011;9:433–439. doi: 10.1089/met.2011.0024. [DOI] [PubMed] [Google Scholar]
- 125.Langheim S., Dreas L., Veschini L., Maisano F., Foglieni C., Ferrarello S., et al. Increased expression and secretion of resistin in epicardial adipose tissue of patients with acute coronary syndrome. Am J Physiol Heart Circ Physiol. 2010;298:H746–H753. doi: 10.1152/ajpheart.00617.2009. [DOI] [PubMed] [Google Scholar]
- 126.Sacks H.S., Fain J.N. Human epicardial fat: what is new and what is missing? Clin Exp Pharmacol Physiol. 2011;38:879–887. doi: 10.1111/j.1440-1681.2011.05601.x. [DOI] [PubMed] [Google Scholar]
- 127.Sato F., Maeda N., Yamada T., Namazui H., Fukuda S., Natsukawa T., et al. Association of epicardial, visceral, and subcutaneous fat with cardiometabolic diseases. Circ J. 2018;82:502–508. doi: 10.1253/circj.CJ-17-0820. [DOI] [PubMed] [Google Scholar]
- 128.Zhao L. Obesity accompanying COVID-19: the role of epicardial fat [Epub ahead of print] Obesity (Silver Spring) 2020 doi: 10.1002/oby.22867. [DOI] [PubMed] [Google Scholar]
- 129.Movahed M.R., Khoubyari R., Hashemzadeh M., Hashemzadeh M. Obesity is strongly and independently associated with a higher prevalence of pulmonary embolism. Respir Investig. 2019;57:376–379. doi: 10.1016/j.resinv.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 130.Vilahur G., Ben-Aicha S., Badimon L. New insights into the role of adipose tissue in thrombosis. Cardiovasc Res. 2017;113:1046–1054. doi: 10.1093/cvr/cvx086. [DOI] [PubMed] [Google Scholar]
- 131.Schäfer K., Konstantinides S. Adipokines and thrombosis. Clin Exp Pharmacol Physiol. 2011;38:864–871. doi: 10.1111/j.1440-1681.2011.05589.x. [DOI] [PubMed] [Google Scholar]
- 132.Blokhin I.O., Lentz S.R. Mechanisms of thrombosis in obesity. Curr Opin Hematol. 2013;20:437–444. doi: 10.1097/MOH.0b013e3283634443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., et al. S; Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan. Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Khan I.H., Savarimuthu S., Leung M.S.T., Harky A. The need to manage the risk of thromboembolism in COVID-19 patients. J Vasc Surg. 2020 doi: 10.1016/j.jvs.2020.05.015. S0741–5214(20)31157–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020:M20–2003. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Menter T., Haslbauer J.D., Nienhold R., Savic S., Hopfer H., Deigendesch N., et al. Post-mortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction [Epub ahead of print] Histopathology. 2020 doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lax S.F., Skok K., Zechner P., Kessler H.H., Kaufmann N., Koelblinger C., et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020:M20–2566. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M., et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18:1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Griffin D.O., Jensen A., Khan M., Chin J., Chin K., Saad J., et al. Pulmonary embolism and increased levels of d-dimer in patients with coronavirus disease. Emerg Infect Dis. 2020;26:1941–1943. doi: 10.3201/eid2608.201477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang Z., Shi J., He Z., Lü Y., Xu Q., Ye C., et al. Predictors for imaging progression on chest CT from coronavirus disease 2019 (COVID-19) patients. Aging. 2020:126037–126048. doi: 10.18632/aging.102999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Müller M.C.A., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F., Lille ICU Haemostasis COVID-19 group Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 144.Cattaneo M., Bertinato E.M., Birocchi S., Brizio C., Malavolta D., Manzoni M., et al. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb Haemost. 2020;120:1230–1232. doi: 10.1055/s-0040-1712097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lippi G., Wong J., Henry B.M. Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020;130:304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 146.Hall J.E., do Carmo J.M., da Silva A.A., Wang Z., Hall M.E. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116:991–1006. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Linderman G.C., Lu J., Lu Y., Sun X., Xu W., Nasir K., et al. Association of body mass index with blood pressure among 1.7 million Chinese adults. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hall J.E., do Carmo J.M., da Silva A.A., Wang Z., Hall M.E. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol. 2019;15:367–385. doi: 10.1038/s41581-019-0145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kawarazaki W., Fujita T. The role of aldosterone in obesity-related hypertension. Am J Hypertens. 2016;29:415–423. doi: 10.1093/ajh/hpw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hall M.E., do Carmo J.M., da Silva A.A., Juncos L.A., Wang Z., Hall J.E. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis. 2014;7:75–88. doi: 10.2147/IJNRD.S39739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hall J.E., da Silva A.A., do Carmo J.M., Dubinion J., Hamza S., Munusamy S., et al. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285:17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Maimaituxun G., Fukuda D., Izaki H., Hirata Y., Kanayama H.O., Masuzaki H., et al. Levels of adiponectin expression in peri-renal and subcutaneous adipose tissue and its determinants in human biopsied samples. Front Endocrinol (Lausanne) 2020;10:897. doi: 10.3389/fendo.2019.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Susic D., Varagic J. Obesity: a perspective from hypertension. Med Clin North Am. 2017;101:139–157. doi: 10.1016/j.mcna.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 154.Li X.C., Zhang J., Zhuo J.L. The vasoprotective axes of the renin-angiotensin system:physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol Res. 2017;125:21–38. doi: 10.1016/j.phrs.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ishiyama E.Y., Gallagher B.P., Averill A.D., Tallant E.A., Brosnihan K.B., Ferrario C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 156.Klimas J., Olvedy M., Ochodnicka-Mackovicova K., Kruzliak P., Cacanyiova S., Kristek F., et al. Perinatally administered losartan augments renal ACE2 expression but not cardiac or renal Mas receptor in spontaneously hypertensive rats. J Cell Mol Med. 2015;19:1965–1974. doi: 10.1111/jcmm.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Furuhashi M., Moniwa N., Mita T., Fuseya T., Ishimura S., Ohno K., et al. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens. 2015;28:15–21. doi: 10.1093/ajh/hpu086. [DOI] [PubMed] [Google Scholar]
- 158.Peng Y.D., Meng K., Guan H.Q., Leng L., Zhu R.R., Wang B.Y., et al. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E004. doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 159.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of Covid–19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.European Society of Cardiology. Position statement of the ESC Council on Hypertension on ACE inhibitors and angiotensin receptor blockers. Available at: https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang. Accessed September 14, 2020.
- 161.Rico-Mesa J.S., White A., Anderson A.S. Outcomes in patients with COVID-19 infection taking ACEI/ARB. Curr Cardiol Rep. 2020;22:31. doi: 10.1007/s11886-020-01291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Memish Z.A., Perlman S., Van Kerkhove M.D., Zumla A. Middle East respiratory syndrome. Lancet. 2020;395:1063–1077. doi: 10.1016/S0140-6736(19)33221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bornstein S.R., Rubino F., Khunti K., Mingrone G., Hopkins D., Birkenfeld A.L., et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hur K., Price C.P.E., Gray E.L., Gulati R.K., Maksimoski M., Racette S.D., et al. Factors associated with intubation and prolonged intubation in hospitalized patients with COVID-19. Otolaryngol Head Neck Surg. 2020;163:170–178. doi: 10.1177/0194599820929640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395:1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hussain A., Bhowmik B., do Vale Moreira N.C. COVID-19 and diabetes: knowledge in progress. Diabetes Res Clin Pract. 2020;162 doi: 10.1016/j.diabres.2020.108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta. National Diabetes Fact Sheet. Available at: https://www.cdc.gov/diabetes/basics/quick-facts.html. Accessed September 14, 2020.
- 168.Wilding J. Managing patients with type 2 diabetes and obesity. Practitioner. 2015;259:25–28. 3. [PubMed] [Google Scholar]
- 169.Kalra S. Diabesity. J Pak Med Assoc. 2013;63:532–534. [PubMed] [Google Scholar]
- 170.Eckel R.H., Kahn S.E., Ferrannini E., Goldfine A.B., Nathan D.M., Schwartz M.W., et al. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? J Clin Endocrinol Metab. 2011;96:1654–1663. doi: 10.1210/jc.2011-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen C.Y., Fujimiya M., Laviano A., Chang F.Y., Lin H.C., Lee S.D. Modulation of ingestive behavior and gastrointestinal motility by ghrelin in diabetic animals and humans. J Chin Med Assoc. 2010;73:225–229. doi: 10.1016/S1726-4901(10)70048-4. [DOI] [PubMed] [Google Scholar]
- 172.Karpe F., Dickmann J.R., Frayn K.N. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;6:2441–2449. doi: 10.2337/db11-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Al-Goblan A.S., Al-Alfi M.A., Khan M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014;7:587–591. doi: 10.2147/DMSO.S67400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kahn S.E. Clinical review 135: the importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab. 2001;86:4047–4058. doi: 10.1210/jcem.86.9.7713. [DOI] [PubMed] [Google Scholar]
- 175.Zheng K.I., Gao F., Wang X.B., Sun Q.F., Pan K.H., Wang T.Y., et al. Letter to the Editor: Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2009;108 doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gao F., Zheng K.I., Wang X.B., Yan H.D., Sun Q.F., Pan K.H., et al. Metabolic associated fatty liver disease increases coronavirus disease 2019 disease severity in nondiabetic patients [Epub ahead of print] J Gastroenterol Hepatol. 2020 doi: 10.1111/jgh.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hussain A., Vasas P., El-Hasani S. Letter to the Editor: obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108 doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ridruejo E., Soza A. The liver in times of COVID-19: what hepatologists should know. Ann Hepatol. 2020;19:353–358. doi: 10.1016/j.aohep.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Boettler T., Newsome P.N., Mondelli M.U., Maticic M., Cordero E., Cornberg M., et al. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2 doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Qian Z.P., Mei X., Zhang Y.Y., Zou Y., Zhang Z.G., Zhu H., et al. Analysis of baseline liver biochemical parameters in 324 cases with novel coronavirus pneumonia in Shanghai area. Zhonghua Gan Zang Bing Za Zhi. 2020;28:229–233. doi: 10.3760/cma.j.cn501113-20200229-00076. [DOI] [PubMed] [Google Scholar]
- 181.Parohan M., Yaghoubi S., Seraj A. Liver injury is associated with severe coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of retrospective studies. Hepatol Res. 2020;50:924–935. doi: 10.1111/hepr.13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Garrido I., Liberal R., Macedo G. Review article: COVID-19 and liver disease – what we know on 1st May 2020. Aliment Pharmacol Ther. 2020;52:267–275. doi: 10.1111/apt.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ji D., Qin E., Xu J., Zhang D., Cheng G., Wang Y., Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Biquard L., Valla D., Rautou P.E. No evidence for an increased liver uptake of SARS-CoV-2 in metabolic associated fatty liver disease. J Hepatol. 2020;73:717–718. doi: 10.1016/j.jhep.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Arias-Loste M.T., Fábrega E., López-Hoyos M., Crespo J. The crosstalk between hypoxia and innate immunity in the development of obesity-related nonalcoholic fatty liver disease. Biomed Res Int. 2015;2015 doi: 10.1155/2015/319745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lee C.H., Choi S.H., Chung G.E., Park B., Kwak M.S. Nonalcoholic fatty liver disease is associated with decreased lung function. Liver Int. 2018;38:2091–2100. doi: 10.1111/liv.13860. [DOI] [PubMed] [Google Scholar]
- 187.Nseir W.B., Mograbi J.M., Amara A.E., Abu Elheja O.H., Mahamid M.N. Non-alcoholic fatty liver disease and 30-day all-cause mortality in adult patients with community-acquired pneumonia. Qjm. 2019;112:95–99. doi: 10.1093/qjmed/hcy227. [DOI] [PubMed] [Google Scholar]
- 188.Peng T.C., Kao T.W., Wu L.W., Chen Y.J., Chang Y.W., Wang C.C., et al. Association between pulmonary function and nonalcoholic fatty liver disease in the NHANES III study. Medicine. 2015;94:e907. doi: 10.1097/MD.0000000000000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.von Haehling S., Morley J.E. Anker SDAn overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129–133. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lim W.S., Liang C.K., Assantachai P., Auyeung T.W., Kang L., Lee W.J., et al. COVID-19 and older people in Asia: AWGS calls to actions. Geriatr Gerontol Int. 2020;20:547–558. doi: 10.1111/ggi.13939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Delmonico M.J., Harris T.B., Lee J.S., Visser M., Nevitt M., Kritchevsky S.B., et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55:769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 192.Goodpaster B.H., Park S.W., Harris T.B., Kritchevsky S.B., Nevitt M., Schwartz A.V., et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 193.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2 Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chhetri J.K., de Souto Barreto P., Fougère B., Rolland Y., Vellas B., Cesari M. Chronic inflammation and sarcopenia: a regenerative cell therapy perspective. Exp Gerontol. 2018;103:115–123. doi: 10.1016/j.exger.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 195.Franceschi C., Garagnani P., Parini P., Giuliani C., Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14:576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 196.Franceschi C., Garagnani P., Vitale G., Capri M., Salvioli S. Inflammaging and 'Garb-aging'. Trends Endocrinol Metab. 2017;28:199–212. doi: 10.1016/j.tem.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 197.Roth S.M., Metter E.J., Ling S., Ferrucci L. Inflammatory factors in age-related muscle wasting. Curr Opin Rheumatol. 2006;18:625–630. doi: 10.1097/01.bor.0000245722.10136.6d. [DOI] [PubMed] [Google Scholar]
- 198.Dalle S., Rossmeislova L., Koppo K. The Role of inflammation in age-related sarcopenia. Front Physiol. 2017;8:1045. doi: 10.3389/fphys.2017.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rong Y.D., Bian A.L., Hu H.Y., Ma Y., Zhou X.Z. Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr. 2018 Dec 12;18(1):308. doi: 10.1186/s12877-018-1007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bano G., Trevisan C., Carraro S., Solmi M., Luchini C., Stubbs B., et al. Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas. 2017;96:10–15. doi: 10.1016/j.maturitas.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 201.Minciullo P.L., Catalano A., Mandraffino G., Casciaro M., Crucitti A., Maltese G., et al. Inflammaging and anti-inflammaging: the role of cytokines in extreme longevity. Arch Immunol Ther Exp (Warsz) 2016;64:111–126. doi: 10.1007/s00005-015-0377-3. [DOI] [PubMed] [Google Scholar]
- 202.Bian A.L., Hu H.Y., Rong Y.D., Wang J., Wang J.X., Zhou X.Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur J Med Res. 2017;22:25. doi: 10.1186/s40001-017-0266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mijnarends D.M., Koster A., Schols J.M., Meijers J.M., Halfens R.J., Gudnason V., et al. Physical activity and incidence of sarcopenia: the population-based AGES-Reykjavik Study. Age Ageing. 2016;45:614–620. doi: 10.1093/ageing/afw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.McKee A., Morley J.E., Matsumoto A.M., Vinik A. Sarcopenia: an endocrine disorder? Endocr Pract. 2017;23:1140–1149. doi: 10.4158/EP171795.RA. [DOI] [PubMed] [Google Scholar]
- 205.Alkahtani S., Aljuhani O., Alhussain M., Habib S.S. Association between physical activity patterns and sarcopenia in Arab men. J Int Med Res. 2020;48 doi: 10.1177/0300060520918694. 300060520918694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Alkahtani S., Aljaloud K., Yakout S., Al-Daghri N.M. Interactions between sedentary and physical activity patterns, lean mass, and bone density in Arab men. Dis Markers. 2019;2019 doi: 10.1155/2019/5917573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rassameehiran S., Klomjit S., Mankongpaisarnrung C., Rakvit A. Postextubation Dysphagia. Proc (Bayl Univ Med Cent) 2015;28:18–20. doi: 10.1080/08998280.2015.11929174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Park H.S., Koo J.H., Song S.H. Association of post-extubation dysphagia with tongue weakness and somatosensory disturbance in non-neurologic critically ill patients. Ann Rehabil Med. 2017;41:961–968. doi: 10.5535/arm.2017.41.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Su H., Hsiao T.Y., Ku S.C., Wang T.G., Lee J.J., Tzeng W.C., et al. Tongue weakness and somatosensory disturbance following oral endotracheal extubation. Dysphagia. 2015;30:188–195. doi: 10.1007/s00455-014-9594-x. [DOI] [PubMed] [Google Scholar]
- 210.Brodsky M.B., Pandian V., Needham D.M. Post-extubation dysphagia: a problem needing multidisciplinary efforts. Intensive Care Med. 2020;46:93–96. doi: 10.1007/s00134-019-05865-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zuercher P., Moret C.S., Dziewas R., Schefold J.C. Dysphagia in the intensive care unit: epidemiology, mechanisms, and clinical management. Crit Care. 2019;23(1):103. doi: 10.1186/s13054-019-2400-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Macht M., Wimbish T., Clark B.J., Benson A.B., Burnham E.L., Williams A., et al. Postextubation dysphagia is persistent and associated with poor outcomes in survivors of critical illness. Crit Care. 2011;15:R231. doi: 10.1186/cc10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fujishima I., Fujiu-Kurachi M., Arai H., Hyodo M., Kagaya H., Maeda K., et al. Sarcopenia and dysphagia: Position paper by four professional organizations. Geriatr Gerontol Int. 2019;19:91–97. doi: 10.1111/ggi.13591. [DOI] [PubMed] [Google Scholar]
- 214.214 Dellis S., Papadopoulou S., Krikonis K., Zigras F. Sarcopenic dysphagia. A narrative review. J Frailty Sarcopenia Falls. 2018;3:1–7. doi: 10.22540/JFSF-03-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Azzolino D., Damanti S., Bertagnoli L., Lucchi T., Cesari M. Sarcopenia and swallowing disorders in older people. Aging Clin Exp Res. 2019;31:799–805. doi: 10.1007/s40520-019-01128-3. [DOI] [PubMed] [Google Scholar]
- 216.Wakabayashi H., Takahashi R., Murakami T. The prevalence and prognosis of sarcopenic dysphagia in patients who require dysphagia rehabilitation. J Nutr Health Aging. 2019;23:84–88. doi: 10.1007/s12603-018-1117-2. [DOI] [PubMed] [Google Scholar]
- 217.Meng L., Qiu H., Wan L., Ai Y., Xue Z., Guo Q., D, et al. Intubation and ventilation amid the COVID-19 outbreak: Wuhan's experience. Anesthesiology. 2020;132:1317–1332. doi: 10.1097/ALN.0000000000003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bartley J.M., Pan S.J., Keilich S.R., Hopkins J.W., Al-Naggar I.M., Kuchel G.A., Haynes L. Aging augments the impact of influenza respiratory tract infection on mobility impairments, muscle-localized inflammation, and muscle atrophy. Aging. 2016;8:620–635. doi: 10.18632/aging.100882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Muscaritoli M., Anker S.D., Argilés J., Aversa Z., Bauer J.M., Biolo G., et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) "cachexia-anorexia in chronic wasting diseases" and "nutrition in geriatrics". Clin Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 220.Cederholm T., Barazzoni R., Austin P., Ballmer P., Biolo G., Bischoff S.C., et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36:49–64. doi: 10.1016/j.clnu.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 221.Prado C.M., Wells J.C., Smith S.R., Stephan B.C., Siervo M. Sarcopenic obesity: a Critical appraisal of the current evidence. Clin Nutr. 2012;31:583–601. doi: 10.1016/j.clnu.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 222.Kalinkovich A., Livshits G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. 2017;35:200–221. doi: 10.1016/j.arr.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 223.Barbat-Artigas S., Pion C.H., Leduc-Gaudet J.P., Rolland Y., Aubertin-Leheudre M. Exploring the role of muscle mass, obesity, and age in the relationship between muscle quality and physical function. J Am Med Dir Assoc. 2014;15 doi: 10.1016/j.jamda.2013.12.008. 303.e13–20. [DOI] [PubMed] [Google Scholar]
- 224.Tian S., Xu Y. Association of sarcopenic obesity with the risk of all-cause mortality: a meta-analysis of prospective cohort studies. Geriatr Gerontol Int. 2016;16:155–166. doi: 10.1111/ggi.12579. [DOI] [PubMed] [Google Scholar]
- 225.Newman A.B., Haggerty C.L., Goodpaster B., Harris T., Kritchevsky S., Nevitt M., Health Aging And Body Composition Research Group Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:323–330. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- 226.Bone A.E., Hepgul N., Kon S., Maddocks M. Sarcopenia and frailty in chronic respiratory disease. Chron Respir Dis. 2017;14:85–99. doi: 10.1177/1479972316679664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ohara D.G., Pegorari M.S., Oliveira Dos Santos N.L., de Fátima Ribeiro Silva C., Monteiro R.L., Matos A.P., et al. Respiratory muscle strength as a discriminator of sarcopenia in community-dwelling elderly: a cross-sectional study. J Nutr Health Aging. 2018;22:952–958. doi: 10.1007/s12603-018-1079-4. [DOI] [PubMed] [Google Scholar]
- 228.Deniz O., Coteli S., Karatoprak N.B., Pence M.C., Varan H.D., Kizilarslanoglu M.C., et al. Diaphragmatic muscle thickness in older people with and without sarcopenia [Epub ahead of print] Aging Clin Exp Res. 2020 May 13 doi: 10.1007/s40520-020-01565-5. [DOI] [PubMed] [Google Scholar]
- 229.Kera T., Kawai H., Hirano H., Kojima M., Watanabe Y., Motokawa K., et al. Definition of Respiratory Sarcopenia With Peak Expiratory Flow Rate. J Am Med Dir Assoc. 2019;20:1021–1025. doi: 10.1016/j.jamda.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 230.Gibson G.J. Obesity, respiratory function and breathlessness. Thorax. 2000;55(suppl 1):S41–S44. doi: 10.1136/thorax.55.suppl_1.s41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Laghi F., Tobin M.J. Disorders of the respiratory muscles. Am J Respir Crit Care Med. 2003;168:10–48. doi: 10.1164/rccm.2206020. [DOI] [PubMed] [Google Scholar]
- 232.Schols A.M., Soeters P.B., Dingemans A.M., Mostert R., Frantzen P.J., Wouters E.F. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis. 1993;147:1151–1156. doi: 10.1164/ajrccm/147.5.1151. [DOI] [PubMed] [Google Scholar]
- 233.Hariharan S., Dharmaraj S. Selenium and selenoproteins: it's role in regulation of inflammation. Inflammopharmacology. 2020;6:1–29. doi: 10.1007/s10787-020-00690-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Nencioni L., Sgarbanti R., Amatore D., Checconi P., Celestino I., Limongi D., et al. Intracellular redox signaling as therapeutic target for novel antiviral strategy. Curr Pharm Des. 2011;17:3898–3904. doi: 10.2174/138161211798357728. [DOI] [PubMed] [Google Scholar]
- 235.Williamson C.D., DeBiasi R.L., Colberg-Poley A.M. Viral product trafficking to mitochondria, mechanisms and roles in pathogenesis. Infect Disord Drug Targets. 2012;12:18–37. doi: 10.2174/187152612798994948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ivory K., Prieto E., Spinks C., Armah C.N., Goldson A.J., Dainty J.R., Nicoletti C. Selenium supplementation has beneficial and detrimental effects on immunity to influenza vaccine in older adults. Clin Nutr. 2017;36:407–415. doi: 10.1016/j.clnu.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Steinbrenner H., Al-Quraishy S., Dkhil M.A., Wunderlich F., Sies H. Dietary selenium in adjuvant therapy of viral and bacterial infections. Adv Nutr. 2015;6:73–82. doi: 10.3945/an.114.007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Erkekoğlu P., Aşçı A., Ceyhan M., Kızılgün M., Schweizer U., Ataş C., et al. Selenium levels, selenoenzyme activities and oxidant/antioxidant parameters in H1N1-infected children. Turk J Pediatr. 2013;55:271–282. [PubMed] [Google Scholar]
- 239.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhang J., Taylor E.W., Bennett K., Saad R., Rayman M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am J Clin Nutr. 2020;111:1297–1299. doi: 10.1093/ajcn/nqaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.World Health Organization. The world health report. Available at: https://www.who.int/whr/2002/chapter4/en/index3.html. Accessed September 14, 2020.
- 242.Gammoh N.Z., Rink L. Zinc in Infection and Inflammation. Nutrients. 2017;9:624. doi: 10.3390/nu9060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Adv Nutr. 2019;10:696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hulisz D. Efficacy of zinc against common cold viruses: an overview. J Am Pharm Assoc. 2004;44:594–603. doi: 10.1331/1544-3191.44.5.594.Hulisz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kurugöl Z., Akilli M., Bayram N., Koturoglu G. The prophylactic and therapeutic effectiveness of zinc sulphate on common cold in children. Acta Paediatr. 2006;95:1175–1181. doi: 10.1080/08035250600603024. [DOI] [PubMed] [Google Scholar]
- 246.Derwand R., Scholz M. Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win today's battle against COVID-19? Med Hypotheses. 2020;142 doi: 10.1016/j.mehy.2020.109815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lentini G., Cavalluzzi M.M., Habtemariam S. COVID-19, chloroquine repurposing, and cardiac safety concern: chirality might help. Molecules. 2020;25:1834. doi: 10.3390/molecules25081834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Chatre C., Roubille F., Vernhet H., Jorgensen C., Pers Y.M. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf. 2018;41:919–931. doi: 10.1007/s40264-018-0689-4. [DOI] [PubMed] [Google Scholar]
- 249.Tönnesmann E., Kandolf R., Lewalter T. Chloroquine cardiomyopathy – a review of the literature. Immunopharmacol Immunotoxicol. 2013;35:434–442. doi: 10.3109/08923973.2013.780078. [DOI] [PubMed] [Google Scholar]
- 250.Raha S., Mallick R., Basak S., Duttaroy A.K. Is copper beneficial for COVID-19 patients? Med Hypotheses. 2020;142 doi: 10.1016/j.mehy.2020.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Stafford S.L., Bokil N.J., Achard M.E., Kapetanovic R., Schembri M.A., McEwan A.G., et al. Metal ions in macrophage antimicrobial pathways: emerging roles for zinc and copper. Biosci Rep. 2013;33:e00049. doi: 10.1042/BSR20130014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.White C., Lee J., Kambe T., Fritsche K., Petris M.J. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem. 2009;284:33949–33956. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Besold A.N., Culbertson E.M., Culotta V.C. The Yin and Yang of copper during infection. J Biol Inorg Chem. 2016;21:137–144. doi: 10.1007/s00775-016-1335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kelley D.S., Daudu P.A., Taylor P.C., Mackey B.E., Turnlund J.R. Effects of low-copper diets on human immune response. Am J Clin Nutr. 1995;62:412–416. doi: 10.1093/ajcn/62.2.412. [DOI] [PubMed] [Google Scholar]
- 255.Rupp J.C., Locatelli M., Grieser A., Ramos A., Campbell P.J., Yi H., et al. Host cell copper transporters CTR1 and ATP7A are important for influenza A virus replication. Virol J. 2017;14:11. doi: 10.1186/s12985-016-0671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Holick M.F. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18:153–165. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- 257.Sizar O., Khare S., Goyal A., Bansal P., Givler A. Vitamin D deficiency. Treasure Island. FL: StatPearls Publishing. 2020 [PubMed] [Google Scholar]
- 258.Pereira-Santos M., Costa P.R., Assis A.M., Santos C.A., Santos D.B. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes Rev. 2015;16:341–349. doi: 10.1111/obr.12239. [DOI] [PubMed] [Google Scholar]
- 259.Hansdottir S., Monick M.M. Vitamin D effects on lung immunity and respiratory diseases. Vitam Horm. 2011;86:217–237. doi: 10.1016/B978-0-12-386960-9.00009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Xu J., Yang J., Chen J., Luo Q., Zhang Q., Zhang H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol Med Rep. 2017;16(5):7432–7438. doi: 10.3892/mmr.2017.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Martineau A.R., Jolliffe D.A., Greenberg L., Aloia J.F., Bergman P., Dubnov-Raz G., et al. Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Technol Assess. 2019;23:1–44. doi: 10.3310/hta23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zdrenghea M.T., Makrinioti H., Bagacean C., Bush A., Johnston S.L., Stanciu L.A. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol. 2017;27 doi: 10.1002/rmv.1909. [DOI] [PubMed] [Google Scholar]
- 263.Rhodes J.M., Subramanian S., Laird E., Kenny R.A. Editorial: low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment Pharmacol Ther. 2020;51:1434–1437. doi: 10.1111/apt.15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Hastie C.E., Mackay D.F., Ho F., Celis-Morales C.A., Katikireddi S.V., Niedzwiedz C.L., et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab Syndr. 2020;14:561–565. doi: 10.1016/j.dsx.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Daneshkhah A., Eshein A., Subramanian H. The possible role of vitamin D in suppressing cytokine storm and associated mortality of COVID-19 patients. MedRxiv. 2020 .04.08.20058578. [Google Scholar]
- 266.Caccialanza R., Laviano A., Lobascio F., Montagna E., Bruno R., Ludovisi S., et al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): rationale and feasibility of a shared pragmatic protocol. Nutrition. 2020;74 doi: 10.1016/j.nut.2020.110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ebadi M., Montano-Loza A.J. Perspective: improving vitamin D status in the management of COVID-19. Eur J Clin Nutr. 2020;74:856–859. doi: 10.1038/s41430-020-0661-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Singer P., Blaser A.R., Berger M.M., Alhazzani W., Calder P.C., Casaer M.P., et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38:48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 269.Jakovac H. COVID-19 and vitamin D–Is there a link and an opportunity for intervention? Am J Physiol Endocrinol Metab. 2020;318:E589. doi: 10.1152/ajpendo.00138.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Maxfield L., Crane J.S. StatPearls Publishing; FL: 2020 Jan. Vitamin C deficiency (Scurvy). Treasure Island. [Google Scholar]
- 271.Elmadfa I., Meyer A.L. The Role of the status of selected micronutrients in shaping the immune function. Endocr Metab Immune Disord Drug Targets. 2019;19:1100–1115. doi: 10.2174/1871530319666190529101816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Myint P.K., Wilson A.M., Clark A.B., Luben R.N., Wareham N.J., Khaw K.T. Plasma vitamin C concentrations and risk of incident respiratory diseases and mortality in the European Prospective Investigation into Cancer–Norfolk population–based cohort study. Eur J Clin Nutr. 2019;73:1492–1500. doi: 10.1038/s41430-019-0393-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Carr A.C., Spencer E., Dixon L., Chambers S.T. Patients with community acquired pneumonia exhibit depleted vitamin C status and elevated oxidative stress. Nutrients. 2020;12:E1318. doi: 10.3390/nu12051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Zhang M., Jativa D.F. Vitamin C supplementation in the critically ill: a systematic review and meta-analysis. SAGE Open Med. 2018;6 doi: 10.1177/2050312118807615. 2050312118807615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Cheng R.Z. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med Drug Discov. 2020;5 doi: 10.1016/j.medidd.2020.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.UNICEF . Author; New York, NY: 2018. Coverage at a Crossroads: New directions for vitamin A supplementation programmes. [Google Scholar]
- 277.Sommer A., Vyas K.S. A global clinical view on vitamin A and carotenoids. Am J Clin Nutr. 2012;96:1204S–12046. doi: 10.3945/ajcn.112.034868. [DOI] [PubMed] [Google Scholar]
- 278.Timoneda J., Rodríguez–Fernández L., Zaragozá R., Marín M.P., Cabezuelo M.T., Torres L., et al. Vitamin A deficiency and the lung. Nutrients. 2018;10 doi: 10.3390/nu10091132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Cantorna M.T., Nashold F.E., Hayes C.E. Vitamin A deficiency results in a priming environment conducive for Th1 cell development. Eur J Immunol. 1995;25:1673–1679. doi: 10.1002/eji.1830250629. [DOI] [PubMed] [Google Scholar]
- 280.Stephensen C.B. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 281.Traber M.G. Vitamin E regulatory mechanisms. Annu Rev Nutr. 2007;27:347–362. doi: 10.1146/annurev.nutr.27.061406.093819. [DOI] [PubMed] [Google Scholar]
- 282.Lee G.Y., Han S.N. The Role of Vitamin E in Immunity. Nutrients. 2018;10:1614. doi: 10.3390/nu10111614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Meydani S.N., Leka L.S., Fine B.C., Dallal G.E., Keusch G.T., Singh M.F., et al. Vitamin E and respiratory tract infections in elderly nursing home residents: a randomized controlled trial. JAMA. 2004;292:828–836. doi: 10.1001/jama.292.7.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Beck M.A., Kolbeck P.C., Rohr L.H., Shi Q., Morris V.C., Levander O.A. Vitamin E deficiency intensifies the myocardial injury of coxsackievirus B3 infection of mice. J Nutr. 1994;124:345–358. doi: 10.1093/jn/124.3.345. [DOI] [PubMed] [Google Scholar]