Clinical Practice Points.
-
•
We describe the first case report of severe and irreversible bone marrow aplasia related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in a patient with Waldenstrom macroglobuninemia.
-
•
We report here the first evidence of SARS-CoV-2 infected cells and neutralizing antibodies in bone marrow samples despite the negative real-time polymerase chain reaction results.
-
•
Patients with compromised immunity or underlying hematologic malignancies have an elevated risk of severe and/or atypical forms of SARS-CoV-2 infection.
Introduction
Patients with underlying hematologic diseases have an elevated risk to develop severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection with significant morbidity and mortality. Waldenström macroglobulinemia (WM) is an indolent low-grade lymphoma accounting for 1% to 2% of lymphoproliferative disorders.1 WM is characterized by the infiltration of bone marrow (BM) by clonal lymphoplasmacytic cells that produce monoclonal immunoglobulin M (IgM).2 We report here a case of severe and irreversible bone marrow aplasia related to SARS-CoV-2 infection in a 61-year-old woman with WM.
Case Report
Smoldering WM diagnosis was initially made in March 2015 based on an IgM monoclonal component at 25 g/L, associated with 50% BM infiltration by lymphoplasmacytic cells. At the time of diagnosis, no tumoral syndrome had been identified on thoraco-abdominal scan, and initial International Prognostic Scoring System for MW3 (IPSS MW) was low. BM karyotype revealed in 8/20 metaphases a t(6;8)(q27;p12). Without treatment indication according to the Mayo Stratification of Macroglobulinemia and Risk-Adapted Therapy (mSMART) consensus,4 clinical and biological monitoring were initially proposed. In February 2017, the molecular biology analysis revealed a MYD88L265P mutation. The patient was monitored for almost 3 years. In October 2018, she developed pancytopenia (hemoglobin 8.5 g/dL, platelet count 81 × 109/L, and neutrophil count 0.81 × 109/L), whereas the IgM component remained stable around 30 g/L. A treatment with bendamustine 90 mg/m2 and rituximab was then initiated. In April 2019, after 6 cycles, a very good partial response was obtained, with normalization of blood counts and decreased monoclonal component at 0.9 g/L. In December 2019, the patient was still in very good partial response with a normal hematologic count, no clinical tumoral syndrome, no constitutional symptom, and an IgM component at 1.36 g/L.
Three months later, during the SARS-CoV-2 epidemic, the patient was admitted in the emergency department because of a fast deterioration of her general status with fever (39.1°C), dyspnea, and a high respiratory rate (> 30 breaths/minute) associated with an oxygen saturation of 89% in ambient air. At admission, a severe pancytopenia was discovered (hemoglobin 4 g/dL, platelets count 4 × 109/L, neutrophil count 0.01 × 109/L) associated with a lymphocytosis at 12 × 109/L. The patient also presented a major biological inflammatory syndrome (C-reactive protein 298 mg/L, serum ferritin 3965 μg/L, and fibrinogen 7.96 g/L) and increased plasma concentrations of interleukin 6 (110 pg/mL) and interferon gamma-induced protein 10 (1609 pg/mL). Besides, an endothelial injury was objective by an important elevation of the circulating endothelial cells (261 elements/mL, normal rate < 10) in the peripheral blood, consistent with a severe form of Coronavirus disease-2019 (COVID-19), as described in a previous study.5
This deep pancytopenia was not explained by any drugs or toxic exposure, leading to an exhaustive microbiological screening of putative responsible bacterial (repeated blood cultures), viral (cytomegalovirus, Epstein-Barr virus, enterovirus, parvorirus B19, adenovirus, Dengue, and hepatitis B, C, and E), and parasitologic (plasmodium, leishmania) organisms. All of these investigations were negative.
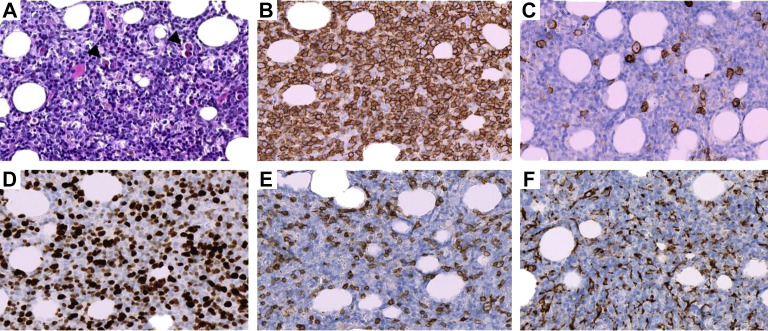
Histologically, BM biopsy showed a dense and diffuse interstitial infiltrate predominantly composed of relatively monotonous small lymphocytes and plasmacytoid lymphocytes (Figure 1A ) admixed with plasma cells and few large transformed cells. The neoplastic lymphocytes and plasmacytoid lymphocytes expressed the B-cell associated antigen CD20 (Figure 1B) and the Bcl2 protein, whereas neoplastic plasma cells expressed CD138 (Figure 1C) and a monotypic cytoplasmic kappa light chain. Several CD138-positive plasma cells, referred to as Mott cells, contained multiple round cytoplasmic hyaline inclusions. The Ki-67 proliferation index was high, > 50% (Figure 1D). CD3 and CD68 immunostaining highlighted an associated reactive T-cells and histiocytic infiltrate (Figure 1E and F). Reticulin staining showed a loose network of reticulin fibers with many intersections, corresponding to early-stage myelofibrosis. In parallel, the real time-polymerase chain reaction (RT-PCR) for SARS-CoV-2, mycobacterium, Histoplasma, and leishmania were all negative in the BM.
Figure 1.
Histologic and Immunohistochemical Features of Bone Marrow Biopsy. A, Hematoxylin and Eosin Staining Showing a Dense Proliferation of Neoplastic Lymphocytes, Plasmacytoid Lymphocytes, and Plasma Cells including Mott Cells (Arrowhead). B, Diffuse CD20 Expression by Neoplastic Lymphocytes and Plasmacytoid Lymphocytes. C, CD138 Immunohistochemical Staining Highlighting Scattered Plasma Cells. D, Ki-67 Staining Reveals a High Proliferation Index. CD3 (E) and CD68 (F) Immunostaining Demonstrating Respectively an Associated Reactive T-Cells and Histiocytic Infiltrate. All the Pictures Were Taken at 400× Magnification Using the Hamamatsu Virtual Slide Scanner Nanozoomer 2.0-HT With the NDP.view2 Viewing Software (Ver. 2.6.17)
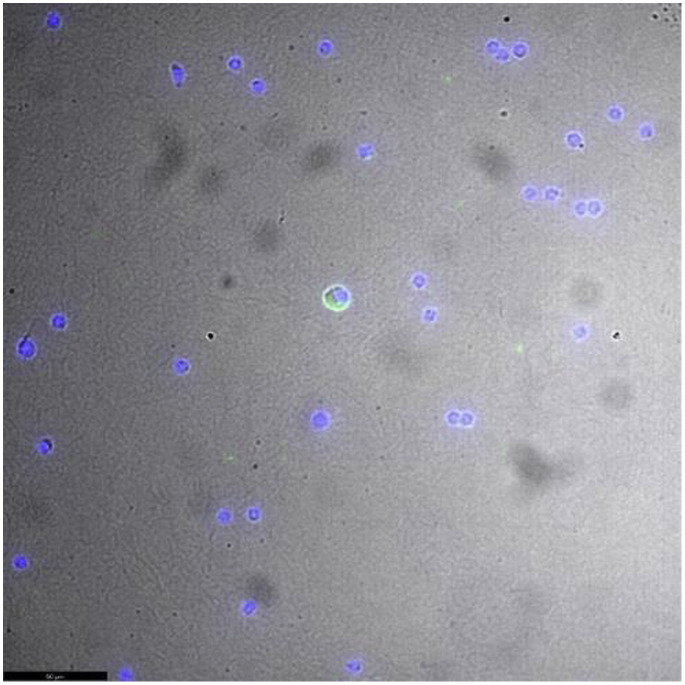
Because the patient has been confined at home for 3 weeks with her son and daughter who were both symptomatic and positive for SARS-CoV-2 (RT-PCR) via nasopharyngeal swab, a chest computed tomography scan was performed, showing typical images of COVID-19 in the intermediate to severe stage. Owing to the familial virus exposure, the blood, BM, and computed tomography scan results, and despite repeated negative SARS-CoV-2 RT-PCR tests in different samples (oropharynx, blood, BM, and urine), we considered BM aplasia accelerated by SARS-CoV-2 infection as likely and performed further investigations. First, the presence of anti-spike SARS-CoV-2 IgG antibodies was detected in both serum and BM samples by a commercial enzyme-linked immunosorbent assay (ELISA) test (Euroimmun, Luebeck, Germany). Then, a SARS-CoV-2-specific virus neutralization test was performed, and the presence of high neutralizing antibody titers (1/160 in BM and 1/80 in serum) confirmed that the patient had been previously exposed to SARS-CoV-2. Moreover, performing immunofluorescence using a known antisera obtained from an infected patient, we were able to detect, for the first time to our knowledge, the presence of infected cells by SARS-CoV-2 in the BM (Figure 2 ). These virologic investigations brought the direct and indirect proof of SARS-CoV-2 infection in this patient’s BM.
Figure 2.
Immunofluorescence Detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infected Cells in Bone Marrow. Detection of SARS CoV-2 (Green) in Bone-Marrow Lymphoid Cells Stained With Known Antisera From Infected Patient and Using a 400× Magnification. The Cell Nucleus was Stained by Hoechst 33,342 (Blue). Images Were Acquired Using a Leica DMi8
A therapeutic association of oral ibrutinib (140 mg a day) and 300 mg/d intravenous anakinra6 was initiated for 10 days within 48 hours of admission, leading to a rapid and significant decrease in both fever and blood inflammation, with a good clinical tolerance but without hematopoietic reconstitution. One month later, the patient was still in deep pancytopenia and developed a fatal invasive pulmonary fungal infection despite appropriate antifungal treatment.
Discussion
This unexpected hematologic complication of SARS-CoV-2 infection in our patient with WM is consistent with other recent reports of pancytopenia associated with SARS-CoV-2 infection in immunocompromised patients with hematologic diseases.7 , 8 Nevertheless, we noted significant differences between these reports, notably on the methods used to detect SARS-CoV-2 in BM. Issa and colleagues showed, for the first time, the persistence of SARS-CoV-2 nucleic acids in blood and BM at least 45 days in a patient with a medical history of mantle cell lymphoma. In contrast, in this report, we highlighted the presence of infected cells in BM by labeling of lymphoplasmacytic cells by an anti-SARS-CoV-2 serum.
Moreover, as with Hersby et al, we described nonspecific reactive T lymphocytes in the BM biopsy. Other hematologic cell morphologic changes such as pronounced granulocytic reaction with immaturity, dysmorphism, apoptotic-degenerative morphology, and circulating atypical reactive lymphocytes have been largely described in the subsequent phases of COVID-199, 10, 11 and particularly in the early phase of symptom aggravation.
Conclusion
To our knowledge, we report here the first evidence of SARS-CoV-2 infected cells and neutralizing antibodies in BM samples of a patient with WM despite negative RT-PCR results. This case confirms that patients with compromised immunity or underlying hematologic malignancies have an elevated risk of severe and/or atypical forms of SARS-CoV-2 infection and highlights the importance of BM investigations in case of severe and persistent pancytopenia, even if repeated SARS-CoV-2 RT-PCR tests are negative.
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Kapoor P., Ansell S.M., Fonseca R., et al. Diagnosis and management of Waldenström macroglobulinemia: Mayo Stratification of Macroglobulinemia and Risk-Adapted Therapy (mSMART) Guidelines 2016. JAMA Oncol. 2017;3:1257–1265. doi: 10.1001/jamaoncol.2016.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimopoulos M.A., Kyle R.A., Anagnostopoulos A., Treon S.P. Diagnosis and management of Waldenstroms macroglobulinemia. J Clin Oncol. 2005;23:1564–1577. doi: 10.1200/JCO.2005.03.144. [DOI] [PubMed] [Google Scholar]
- 3.Morel P., Duhamel A., Gobbi P., et al. International prognostic scoring system for Waldenstrom macroglobulinemia. Blood. 2009;113:4163–4170. doi: 10.1182/blood-2008-08-174961. [DOI] [PubMed] [Google Scholar]
- 4.Leblond V., Kastritis E., Advani R., et al. Treatment recommendations from the Eighth International Workshop on Waldenström’s Macroglobulinemia. Blood. 2016;128:1321–1328. doi: 10.1182/blood-2016-04-711234. [DOI] [PubMed] [Google Scholar]
- 5.Guervilly C., Burtey S., Sabatier F., et al. Circulating endothelial cells as a marker of endothelial injury in severe COVID -19. J Infect Dis. 2020;222:1789–1793. doi: 10.1093/infdis/jiaa528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cauchois R., Koubi M., Delarbre D., et al. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19 [published correction appears in Proc Natl Acad Sci U S A 2020; 117:22604] Proc Natl Acad Sci U S A. 2020;117:18951–18953. doi: 10.1073/pnas.2009017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Issa N., Lacassin F., Camou F. First case of persistent pancytopenia associated with SARS-CoV-2 bone marrow infiltration in an immunocompromised patient. Ann Oncol. 2020;31:1418–1419. doi: 10.1016/j.annonc.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hersby D.S., Do T.H., Gang A.O., Nielsen T.H. COVID-19-associated pancytopenia can be self-limiting and does not necessarily warrant bone marrow biopsy for the purposes of SARS-CoV-2 diagnostics. Ann Oncol. 2021;32:121–123. doi: 10.1016/j.annonc.2020.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zini G., Bellesi S., Ramundo F., d’Onofrio G. Morphological anomalies of circulating blood cells in COVID-19. Am J Hematol. 2020;95:870–872. doi: 10.1002/ajh.25824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foldes D., Hinton R., Arami S., Bain B.J. Plasmacytoid lymphocytes in SARS-CoV-2 infection (Covid-19) Am J Hematol. 2020;95:861–862. doi: 10.1002/ajh.25834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osman J., Lambert J., Templé M., et al. Rapid screening of COVID-19 patients using white blood cell scattergrams, a study on 381 patients. Br J Haematol. 2020;190:718–722. doi: 10.1111/bjh.16943. [DOI] [PMC free article] [PubMed] [Google Scholar]