Abstract
Objectives
Early in vitro studies have suggested that hydroxychloroquine (HCQ) is a potentially useful drug against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections. This study was conducted to determine whether HCQ had a preventive effect on coronavirus disease 2019 (COVID-19) in rheumatic disease patients who were taking HCQ.
Methods
We conducted a population-based retrospective cohort study using the records of the Korean Health Insurance Review and Assessment (HIRA) claim records. The clinical data of patients with rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE) who were tested for SARS-CoV-2 were investigated. We compared the attack rate of COVID-19 between those who underwent HCQ therapy within 14 days before the test for SARS-CoV-2 (HCQ users) and HCQ non-users. Data were analysed using logistic regression models, χ2, and Student's t-tests.
Results
As of 15th May 2020, 2066 patients with RA or SLE were tested for COVID-19. Among them, 31.4% (649/2066) were treated with HCQ. Most HCQ users (93.7%, 608/649) were taking 200–400 mg/day recommended for the treatment of rheumatic diseases. The attack rate of COVID-19 in the HCQ users (2.3%, 15/649) did not differ from that in the HCQ non-users (2.2%, 31/1417) (p 0.86).
Conclusions
HCQ prophylactic use at a usual dose did not prevent COVID-19 in patients with rheumatic disease.
Keywords: COVID-19, Hydroxychloroquine, Pre-exposure prophylaxis, Rheumatic diseases, Severe acute respiratory syndrome coronavirus 2
Introduction
In December 2019, coronavirus disease 2019 (COVID-19) outbreak caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) occurred in Wuhan, China [1], and it has developed into a devastating pandemic over several months. Researchers worldwide are trying to repurpose existing drugs known to have antiviral effects and to formulate new antiviral drugs and vaccines to combat this disease. Hydroxychloroquine (HCQ), used in patients with malaria or rheumatic disease, was regarded as one of the promising repurposed drugs early in the pandemic [2] because in vitro studies demonstrated the antiviral properties of HCQ on SARS-CoV-2 [3,4]. As expected, a favourable effect was observed in some earlier studies, including a small number of patients [[5], [6], [7]]; however, in subsequent clinical studies with larger numbers of patients no therapeutic effect has been observed at all [[8], [9], [10]]. In a recent meta-analysis, HCQ did not reduce the mortality rate of hospitalized adult COVID-19 patients [11].
Two additional clinical studies were performed to investigate the preventive effect of HCQ immediately after or before SARS-CoV-2 exposure [12,13]. In both studies, the effect of HCQ was not observed, but there were some important limitations such as late administration of HCQ [12], insufficient laboratory diagnosis [12], inclusion of only young and healthy participants [13], and small numbers of participants [13]. Therefore, to investigate the effect of HCQ on laboratory-confirmed COVID-19 incidence, another large-scale study is needed to compare outcomes between HCQ users before SARS-CoV-2 exposure and HCQ non-users, with a large number of participants, including older adults and those with chronic medical conditions. This study aimed to compare the attack rate of COVID-19 between rheumatic disease patients who were HCQ users before exposure to SARS-CoV-2 and HCQ non-users.
Methods
Data sources and study subjects
In this population-based retrospective study, we used the nationwide database of de-identified COVID-19 patients provided by the Korean government for domestic and international researchers. The database contains Health Insurance Review and Assessment (HIRA) claim records of 234 427 individuals tested for COVID-19 until 15th May 2020. The data include detailed information regarding patients' demographics, diagnoses, prescriptions, procedures, and outcomes. We used the Korean Classification of Diseases seventh revision (KCD-7)/International Classification of Diseases (ICD-10) codes and special codes provided by the Rare Intractable Diseases (RID) programme to identify systemic lupus erythematosus (SLE) from the database. The drugs used were identified using Anatomical Therapeutic Chemical (ATC) codes and HIRA general name codes.
Study population description
The study population included patients diagnosed with rheumatoid arthritis (RA) or SLE. Patients with RA were defined as those who were prescribed any of the disease-modifying anti-rheumatic drugs (DMARDs) with the following KCD-7/ICD-10 codes—M05 (seropositive RA) or M06 (other RA)—within 1 year before the COVID-19 test, regardless of age. DMARDs included conventional DMARDs (cDMARDs) such as methotrexate, sulfasalazine, leflunomide, tacrolimus, and HCQ, biological DMARDs (bDMARDs) such as etanercept, infliximab, adalimumab, golimumab, abatacept, tocilizumab, and rituximab, and targeted synthetic DMARDs (tsDMARDs) such as tofacitinib and baricitinib. A patient was considered to take DMARDs if an oral medication was prescribed for ≥30 days, or if an injection was administered more than once. Patients with SLE were defined as those who had both the KCD-7/ICD-10 code M32 (SLE) and the special code of the RID program (V136) within 1 year before the COVID-19 test, regardless of age.
HCQ users versus HCQ non-users
The HCQ users included those patients who took HCQ for at least one day during the 14-day (incubation) period before the COVID-19 test, and the HCQ non-users included those who did not. The COVID-19 attack rate was compared between the two groups repeatedly by changing the 14 days included in the HCQ user's definition to a 5-, 30- or 90-day period (sensitivity analysis). This was because there are no known regimens of HCQ that have proven to be effective for the prevention of SARS-CoV-2, and considering that the mean COVID-19 incubation period is 5 days [13] and the elimination half-life of HCQ is distributed up to more than a month [3].
Identification of COVID-19 cases
COVID-19 cases were identified using the following KCD-7/ICD-10 codes: B34.2 (coronavirus infection, unspecified site), B97.2 (coronavirus as the cause of diseases classified to other chapters), U18 (provisional assignment of new diseases of uncertain aetiology or emergency use), U18.1 (novel coronavirus infection), and U07.1 (coronavirus 2019). These diagnostic codes were given only if a definitive diagnosis of COVID-19 was made based on positive nasopharyngeal swab specimens tested with real-time reverse transcription polymerase chain reaction (rtPCR) assays.
Data collection and definitions
Comorbidities were defined based on claim codes within 1 year before the COVID-19 test and evaluated using the Charlson Comorbidity Index [14]. We included cDMARDs, bDMARDs/tsDMARDs, immunosuppressants such as cyclophosphamide, cyclosporine, azathioprine, tacrolimus, mycophenolate mofetil, and mizoribine, and corticosteroids, prescribed with HCQ within 1 year before the COVID-19 test.
Statistical analysis
The baseline characteristics between the two groups were tested using the χ2 test for categorical variables and Student's t-test for continuous variables. The difference in COVID-19 attack rate between the two groups was compared through a multivariable logistic model. To construct the multivariable logistic regression model, we performed univariate analysis, and only variables showing a significant difference (p < 0.05) between the two groups were included as covariates in the multivariable analysis. Due to the differences in sociodemographic characteristics, comorbidities, and comedications in our primary analysis, we conducted additional analyses applying propensity score matching and inverse probability treatment weighting (IPTW) to assess COVID-19 attack rate in a population with similar baseline characteristics. The propensity score, a conditional probability of receiving HCQ given a set of baseline covariates, was estimated using a non-parsimonious multivariable logistic regression model after adjusting for confounding factors such as age, sex, region, test month, hypertension, congestive heart failure, pulmonary disease, peptic ulcer disease, use of bDMARDs/tsDMARDs, number of healthcare claims, and RA- or SLE-related hospitalization in the previous 1 year. Propensity score matching was implemented with a strategy of 1:1 (HCQ users, n = 600 and HCQ non-users, n = 600, Supplementary Material Table S2) matching with a calliper width as 0.2 of the standard deviation. Standardized differences were estimated for all the baseline covariates before and after matching to assess prematch/preweighting and postmatch/postweighting balances. Standardized differences of <10.0% for a given covariate indicate a relatively small imbalance [15]. A sensitivity analysis for the attack rate of COVID-19, according to the window of HCQ use, was performed to evaluate the association between the date of HCQ administration and the occurrence of COVID-19.
All reported p values are two-sided and have not been adjusted for multiple testing. All the analyses were performed using the SAS software, version 9.4 (SAS Institute).
Ethical consideration
The study protocol was exempted from review by the Institutional Review Board of Chung-Ang University Hospital (2006-023-19319), because only anonymized data were used in this study.
Results
Baseline characteristics of the study population
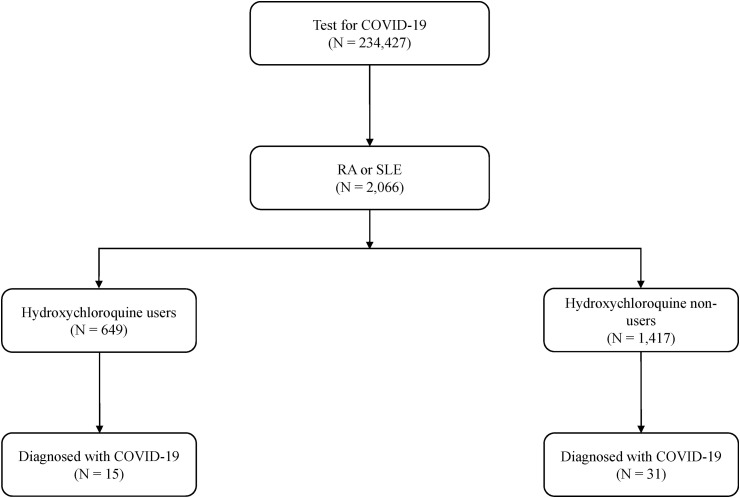
Of the 234 427 patients tested for COVID-19 during the study period, 2066 (0.9%) had RA or SLE. Of these, 31.4% (649/2066) of the patients constituted the HCQ users and 68.6% (1417/2066) the HCQ non-users (Fig. 1 ). The baseline characteristics of the study population are shown in Table 1 . More than 90% of the patients had RA. HCQ non-users were older, had higher comorbidity index scores, and used more comedications—including cDMARDs, bDMARDs/tsDMARDs, and immunosuppressants—than HCQ users. In the HCQ users, the daily mean HCQ dose was 252.5 ± 87.6 mg, and 93.7% (608/649) were taking doses between 200 mg/day and 400 mg/day, which were the usual recommended doses for the treatment of RA or SLE [[16], [17], [18]]. The majority of HCQ users (98.0%, 636/649) were taking HCQ at least for 3 months before the COVID-19 test. The baseline characteristics of IPTW analysis are presented in the Supplementary Material Table S5.
Fig. 1.
Study cohort, classification according to hydroxychloroquine use, and diagnosis of coronavirus disease 2019 (COVID-19). RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
Table 1.
Baseline characteristics of the study population
| Variable | Total (n = 2066) |
HCQ |
p | |
|---|---|---|---|---|
| Usera (n = 649) |
Nonuser (n = 1417) |
|||
| n (%) | n (%) | n (%) | ||
| Type of disease | ||||
| RA | 1877 (90.85) | 548 (84.44) | 1329 (93.79) | <0.0001 |
| SLE | 299 (14.47) | 182 (28.04) | 117 (8.26) | <0.0001 |
| Age, years | 0.0005 | |||
| Mean ± SD | 59.56 ± 16.79 | 57.60 ± 17.62 | 60.45 ± 16.33 | |
| Median (Q1, Q3) | 62 (48, 73) | 60 (43, 72) | 62 (50, 73) | |
| Age group (years) | 0.0013 | |||
| ≤19 | 14 (0.68) | 3 (0.46) | 11 (0.78) | |
| 20–39 | 302 (14.62) | 126 (19.41) | 176 (12.42) | |
| 40–49 | 236 (11.42) | 80 (12.33) | 156 (11.01) | |
| 50–59 | 382 (18.49) | 114 (17.57) | 268 (18.91) | |
| 60–69 | 475 (22.99) | 131 (20.18) | 344 (24.28) | |
| 70–79 | 429 (20.76) | 133 (20.49) | 296 (20.89) | |
| ≥80 | 228 (11.04) | 62 (9.55) | 166 (11.71) | |
| Sex | <0.0001 | |||
| Male | 574 (27.78) | 120 (18.49) | 454 (32.04) | |
| Female | 1492 (72.22) | 529 (81.51) | 963 (67.96) | |
| Charlson comorbidity index | <0.0001 | |||
| Mean ± SD | 4.24 ± 2.82 | 3.84 ± 2.49 | 4.42 ± 2.94 | |
| Median (Q1, Q3) | 4 (2, 6) | 3 (2, 5) | 4 (2, 6) | |
| No. of comorbidities | 0.0076 | |||
| 0 | 199 (9.63) | 75 (11.56) | 124 (8.75) | |
| 1 | 352 (17.04) | 127 (19.57) | 225 (15.88) | |
| ≥2 | 1515 (73.33) | 447 (68.88) | 1068 (75.37) | |
| Hypertension | 1089 (52.71) | 329 (50.69) | 760 (53.63) | 0.2139 |
| Myocardial infarction | 74 (3.58) | 19 (2.93) | 55 (3.88) | 0.2788 |
| Congestive heart failure | 312 (15.10) | 84 (12.94) | 228 (16.09) | 0.0637 |
| Cerebral vascular accident | 325 (15.73) | 92 (14.18) | 233 (16.44) | 0.1889 |
| Pulmonary disease | 1051 (50.87) | 318 (49.00) | 733 (51.73) | 0.2492 |
| Peptic ulcer disease | 822 (39.79) | 236 (36.36) | 586 (41.35) | 0.0314 |
| Liver disease | 801 (38.77) | 235 (36.21) | 566 (39.94) | 0.1059 |
| Diabetes | 634 (30.69) | 167 (25.73) | 467 (32.96) | 0.0009 |
| Renal disease | 233 (11.28) | 70 (10.79) | 163 (11.50) | 0.6323 |
| Cancer | 304 (14.71) | 76 (11.71) | 228 (16.09) | 0.0091 |
| Type of insurance | 0.8701 | |||
| Health insurance | 1875 (90.76) | 590 (90.91) | 1285 (90.68) | |
| Medical aid | 191 (9.24) | 59 (9.09) | 132 (9.32) | |
| Medical history within 1 year | ||||
| Hydroxychloroquine | 978 (47.34) | 649 (100.00) | 329 (23.22) | <0.0001 |
| Methotrexate | 971 (47.00) | 218 (33.59) | 753 (53.14) | <0.0001 |
| Sulfasalazine | 425 (20.57) | 98 (15.10) | 327 (23.08) | <0.0001 |
| Leflunomide | 441 (21.35) | 74 (11.40) | 367 (25.90) | <0.0001 |
| bDMARDs/tsDMARDs | 204 (9.87) | 10 (1.54) | 194 (13.69) | <0.0001 |
| Immunosuppressants | 522 (25.27) | 138 (21.26) | 384 (27.10) | 0.0046 |
| Corticosteroids | 1891 (91.53) | 591 (91.06) | 1300 (91.74) | 0.6064 |
| Hydroxychloroquine dose (mg/day) | ||||
| ≤199 | 37 (5.70) | |||
| 200–299 | 364 (56.09) | |||
| 300-399 | 135 (20.80) | |||
| ≥400 | 113 (17.41) | |||
HCQ, hydroxychloroquine; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; bDMARDs, biological disease-modifying anti-rheumatic drugs; tsDMARDs, targeted synthetic disease-modifying anti-rheumatic drugs.
Hydroxychloroquine use within 14 days.
The attack rate of COVID-19 according to hydroxychloroquine use
COVID-19 occurred in 2.3% (15/649) and 2.2% (31/1417) of the individuals in the HCQ users and HCQ non-users, respectively (p 0.8598). After adjusting for confounders, the COVID-19 attack rate did not differ significantly between the two groups (adjusted odds ratio (OR) 1.131, 95% confidence interval (95%CI) 0.570–2.244, p 0.7248) (Table 2 ). Moreover, even when applied with the propensity score matching or IPTW analysis, the difference in the COVID-19 attack rate was not statistically significant between the two groups (2.33% in HCQ users versus 1.50% in HCQ non-users, OR 1.569, 95%CI 0.674–3.653, p 0.2963 in propensity score matching cohort, and 2.42% in HCQ users versus 2.41% in HCQ non-users, OR 1.006, 95%CI 0.643–1.574, p 0.9787 in the IPTW cohort) (Supplementary Material Tables S3 and S6).
Table 2.
The attack rate of coronavirus disease 2019 (COVID-19) according to hydroxychloroquine (HCQ) use
| Variable | Patients | Events | Unadjusted OR |
Adjusted ORa |
||
|---|---|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | |||
| All | 2066 | 46 | ||||
| HCQ non-user | 1417 | 31 | (ref) | (ref) | ||
| HCQ userb | 649 | 15 | 1.058 (0.567, 1.973) | 0.8598 | 1.131 (0.570, 2.244) | 0.7248 |
| RA | 1877 | 46 | ||||
| HCQ non-user | 1329 | 31 | (ref) | (ref) | ||
| HCQ userb | 548 | 15 | 1.178 (0.631, 2.201) | 0.6065 | 1.252 (0.629, 2.493) | 0.5229 |
Events = number of COVID-19 diagnoses.
OR, odds ratio; RA, rheumatoid arthritis.
Adjusted for age, sex, region, a month of test, hypertension, congestive heart failure, pulmonary disease, peptic ulcer disease, the use of biological disease-modifying anti-rheumatic drug, the number of times healthcare used, and RA- or SLE-related hospitalization within 1 year.
Hydroxychloroquine use within 14 days.
When the COVID-19 attack rate was compared between HCQ users and HCQ non-users within 5, 14, 30, and 90 days, respectively, there was no significant difference (Supplementary Material Table S1). When the propensity score matching or IPTW analysis was implemented, no statistically significant differences were found (Supplementary Material Tables S4 and S7, respectively).
Subgroup analysis for the attack rate of COVID-19
Subgroup analyses showed no significant differences between the two groups (Table 3 ). The HCQ users also revealed no statistically significant benefits of HCQ on the reduction of the attack rate of COVID-19, after adjusting for the confounding factors. These results were similar when the propensity score matching or IPTW analyses were performed (data not shown).
Table 3.
Subgroup analysis of the attack rate of coronavirus disease 2019 (COVID-19)
| Variable | Patients | Events | Unadjusted OR |
Adjusted ORa |
||
|---|---|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | |||
| Age, years | ||||||
| <60 (n = 934) | ||||||
| HCQ non-user | 611 | 17 | (ref) | (ref) | ||
| HCQ userb | 323 | 6 | 0.661 (0.258, 1.694) | 0.3889 | 0.685 (0.245, 1.915) | 0.4702 |
| ≥60 (n = 1132) | ||||||
| HCQ non-user | 806 | 14 | (ref) | (ref) | ||
| HCQ userb |
326 |
9 |
1.606 (0.688, 3.748) |
0.2731 |
1.370 (0.542, 3.466) |
0.5060 |
| Sex | ||||||
| Male (n = 574) | ||||||
| HCQ non-user | 454 | 10 | (ref) | (ref) | ||
| HCQ userb | 120 | 4 | 1.531 (0.472, 4.970) | 0.4783 | 2.430 (0.606, 9.739) | 0.2101 |
| Female (n = 1492) | ||||||
| HCQ non-user | 963 | 21 | (ref) | (ref) | ||
| HCQ userb |
529 |
11 |
0.953 (0.456, 1.991) |
0.8972 |
0.909 (0.408, 2.026) |
0.8161 |
| Hypertension | ||||||
| Hypertension (n = 1089) | ||||||
| HCQ non-user | 760 | 12 | (ref) | (ref) | ||
| HCQ userb | 329 | 5 | 0.962 (0.336, 2.753) | 0.9428 | 1.109 (0.337, 3.646) | 0.8646 |
| No hypertension (n = 977) | ||||||
| HCQ non-user | 657 | 19 | (ref) | (ref) | ||
| HCQ userb |
320 |
10 |
1.083 (0.498, 2.357) |
0.8404 |
1.026 (0.434, 2.422) |
0.9537 |
| bDMARDs/tsDMARDs use within 1 year | ||||||
| bDMARDs/tsDMARDs use (n = 204) | ||||||
| HCQ non-user | 194 | 3 | (ref) | (ref) | ||
| HCQ userb | 10 | 0 | <0.001 (<0.001, >999.999) | 0.9793 | 0.049 (<0.001, >999.999) | 0.9624 |
| No bDMARDs/tsDMARDs use (n = 1802) | ||||||
| HCQ non-user | 1223 | 28 | (ref) | (ref) | ||
| HCQ userb | 639 | 15 | 1.026 (0.544, 1.935) | 0.9367 | 1.111 (0.556, 2.221) | 0.7661 |
Events = number of COVID-19 diagnoses.
OR, odds ratio; HCQ, hydroxychloroquine; bDMARDs, biological disease-modifying anti-rheumatic drugs; tsDMARDs, targeted synthetic disease-modifying anti-rheumatic drugs.
Hydroxychloroquine use within 14 days.
Adjusted for age, sex, region, a month of test, hypertension, congestive heart failure, pulmonary disease, peptic ulcer disease, the use of biological disease-modifying anti-rheumatic drug, the number of healthcare uses, and RA- or SLE-related hospitalization within 1 year.
Prognosis of COVID-19 patients between HCQ users and HCQ non-users
After the attack, the 15 patients in the HCQ users and 31 in the HCQ non-users did not require admission to the intensive care unit (ICU) or ventilator therapy and did not have in-hospital fatality, with the exception of one patient who required admission to the ICU, received ventilator therapy, and expired (0/15 versus 1/31, p 1.000).
Discussion
In the unmatched, propensity score-matched and IPTW analyses, it was found that the use of HCQ before exposure to COVID-19 did not reduce the occurrence of COVID-19 among patients with rheumatic diseases. These results are consistent with the results of the two prospective clinical studies on HCQ prophylaxis introduced above [12,13]. Studies describing patients with rheumatic diseases infected with SARS-CoV-2 provide similar information [19,20]. Previous studies [[8], [9], [10], [11], [12], [13],19,20] estimated that HCQ has no protective effect against COVID-19 in the populations studied, including healthy individuals and patients with rheumatic disease. Although the earlier in vitro study showed an antiviral effect of HCQ on SARS-CoV-2 [4], more recent preclinical studies [21,22] support the results of the clinical studies, including ours.
It is still necessary to consider whether the duration, timing, and dose of HCQ administration affected the results in this study. First, since the HCQ user was defined as taking HCQ for more than 1 day, the duration of HCQ administration may be considered too short. However, in this study, 98.0% of HCQ users (636/649) were patients who were taking HCQ for at least 3 months. Additionally, considering that the elimination half-life of HCQ reaches 40–60 days [2], it is expected that the effect of HCQ lasted longer than the actual duration of administration.
Second, the timing of administration may not have been appropriate to prevent the occurrence of COVID-19. Since we were not aware of the proper timing of administration for prevention, we investigated the effect of HCQ by changing the 14 days to 5-, 30-, and 90-day periods. However, a preventive effect was not demonstrated in any of the cases.
Third, the dose administered in this study to demonstrate a prophylactic effect is questionable. Lê et al. reported the rationale of a loading dose initiation for HCQ treatment in COVID-19 infection [23]. Since initial HCQ loading has not been generally recommended in the treatment of RA or SLE, HCQ use without loading in this study might affect the result. However, considering that most of the patients in this study cohort had been taking HCQ for at least 3 months, it is thought that loading or not would have no effect or only a minimal effect on this study for the prophylactic effect of the use of HCQ at a usual dose.
A recent review suggested that 50% of the maximal effective concentration (EC50), 90% of the maximal effective concentration (EC90), and concentration for clearance of SARS-CoV-2 based on the current in vitro data, were ~242–1515 ng/mL, ~1679–5038 ng/mL, and ~6717 ng/mL, respectively [20]. The mean tertile of HCQ blood concentrations of 492 SLE patients who took long-term HCQ therapy (2.1–6.9 mg/kg) was reported as 0–741 ng/mL, 741.5–1176.5 ng/mL, and 1177–3513 ng/mL [17]. Therefore, it is considered that blood levels similar to the EC50 or EC90 will be reached when taking HCQ at a usual dosage for rheumatic diseases. Hence, it is difficult to argue that HCQ was ineffective in this study due to problems related to the duration, timing, and dose of HCQ.
The prevalence of RA in this cohort was 0.8%, which was similar to that in the previous reports in South Korea (0.26~1.1%) [24,25]. The prevalence of SLE was 127.55/100 000 people in this study population, which was higher than that reported in South Korea (35.45/100 000 people in 2015) from the previous report, where the method of defining SLE was the same as in our study [33]. This is somewhat high even considering that the prevalence of SLE is gradually increasing in South Korea [26]. The reason for this is unclear; however, it is possible that the patients with SLE may be tested more aggressively for COVID-19, as it has been emphasized publicly that the risk of COVID19 infection is higher for immunosuppressive patients with chronic diseases.
This study has a few limitations. First, considering the small number, the baseline characteristics differed between the HCQ users and HCQ non-users. The former group showed a lower comorbidity index score and lesser use of comedications than the latter group, which would be because HCQ is not generally recommended as an initial treatment for patients with RA and is rarely used in combination with bDMARDs/tsDMARDs [27]. However, since the attack rate was not different in the propensity score matching and IPTW analyses, this difference may not have a significant effect on the main result of this study. Second, although there seem to be no significant differences in the clinical course and outcomes of COVID-19 between patients of the two groups, the number of COVID-19-infected patients included in this study was small. Thus, it would be difficult to comment on the therapeutic effect of HCQ because the statistical power is low to observe a significant difference between the two groups.
In conclusion, prophylactic use of HCQ at a usual dose did not prevent the occurrence of COVID-19 in patients with rheumatic diseases. Prophylactic use of HCQ should not be recommended based on the current scientific knowledge.
Author contributions
Conceptualization: SYJ, MCK, SHC, JWC and STC. Methodology and software: SYJ and MSK. Validation: SYJ, SHC and STC. Formal analysis: SYJ, SHC and STC. Investigation: SYJ, MSK, SHC and STC. Data curation: SYJ and MSK. Writing—original draft: SYJ, SHC and STC. Writing—review and editing: SYJ, MSK, MCK, SHC, JWC and STC. Visualization: SYJ, SHC and STC. Project administration: SYJ, MCK, JWC and STC.
Transparency declaration
The authors declare that there are no competing interests. Seong-Ho Choi was supported by the National Research Foundation of Korea Grant funded by the Korea Government (Ministry of Science and ICT) (2019R1C1C1006417). Sang Tae Choi was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Republic of Korea (2018R1D1A1B07049248).
Acknowledgements
The authors appreciate the Ministry of Health and Welfare and the Health Insurance Review and Assessment Service of Korea for sharing invaluable national health insurance claims data in a prompt manner.
Editor: L. Scudeller
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.12.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Tang D., Comish P., Kang R. The hallmarks of COVID-19 disease. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pastick K.A., Okafor E.C., Wang F., Lofgren S.M., Skipper C.P., Nicol M.R. Review: hydroxychloroquine and chloroquine for treatment of SARS-CoV-2 (COVID-19) Open Forum Infect Dis. 2020;7:ofaa130. doi: 10.1093/ofid/ofaa130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv. 2020 (preprint) [Google Scholar]
- 7.Yu B., Li C., Chen P., Zhou N., Wang L., Li J. Low dose of hydroxychloroquine reduces fatality of critically ill patients with COVID-19. Sci China Life Sci. 2020;63(10):1515–1521. doi: 10.1007/s11427-020-1732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahevas M., Tran V.T., Roumier M., Chabrol A., Paule R., Guillaud C. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369:m1844. doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiolet T., Guihur A., Rebeaud M.E., Mulot M., Peiffer-Smadja N., Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis. Clin Microbiol Infect. 2020;27(1):19–27. doi: 10.1016/j.cmi.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abella B.S., Jolkovsky E.L., Biney B.T., Uspal J.E., Hyman M.C., Frank I. Efficacy and safety of hydroxychloroquine vs placebo for pre-exposure SARS-CoV-2 prophylaxis among health care workers: a Randomized clinical trial. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6319. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Austin P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marmor M.F., Kellner U., Lai T.Y., Melles R.B., Mieler W.F., American Academy of Ophthalmology Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 Revision) Ophthalmology. 2016;123:1386–1394. doi: 10.1016/j.ophtha.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 17.Petri M., Elkhalifa M., Li J., Magder L.S., Goldman D.W. Hydroxychloroquine blood levels predict hydroxychloroquine retinopathy. Arthritis Rheumatol. 2020;72:448–453. doi: 10.1002/art.41121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace D.J., Tse K., Hanrahan L., Davies R., Petri M.A. Hydroxychloroquine usage in US patients, their experiences of tolerability and adherence, and implications for treatment: survey results from 3127 patients with SLE conducted by the Lupus Foundation of America. Lupus Sci Med. 2019;6 doi: 10.1136/lupus-2019-000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathian A., Mahevas M., Rohmer J., Roumier M., Cohen-Aubart F., Amador-Borrero B. Clinical course of coronavirus disease 2019 (COVID-19) in a series of 17 patients with systemic lupus erythematosus under long-term treatment with hydroxychloroquine. Ann Rheum Dis. 2020;79:837–839. doi: 10.1136/annrheumdis-2020-217566. [DOI] [PubMed] [Google Scholar]
- 20.Konig M.F., Kim A.H.J., Scheetz M.H., Graef E.R., Liew J.W., Simard J. Baseline use of hydroxychloroquine in systemic lupus erythematosus does not preclude SARS-CoV-2 infection and severe COVID-19. Ann Rheum Dis. 2020;79:1386–1388. doi: 10.1136/annrheumdis-2020-217690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann M., Mosbauer K., Hofmann-Winkler H., Kaul A., Kleine-Weber H., Kruger N. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature. 2020;585:588–590. doi: 10.1038/s41586-020-2575-3. [DOI] [PubMed] [Google Scholar]
- 22.Maisonnasse P., Guedj J., Contreras V., Behillil S., Solas C., Marlin R. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020;585:584–587. doi: 10.1038/s41586-020-2558-4. [DOI] [PubMed] [Google Scholar]
- 23.Lê M.P., Peiffer-Smadja N., Guedj J., Neant N., Mentre F., Ader F. Rationale of a loading dose initiation for hydroxychloroquine treatment in COVID-19 infection in the DisCoVeRy trial—authors’ response. J Antimicrob Chemother. 2020:dkaa415. doi: 10.1093/jac/dkaa191. [Online ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Won S., Cho S.K., Kim D., Han M., Lee J., Jang E.J. Update on the prevalence and incidence of rheumatoid arthritis in Korea and an analysis of medical care and drug utilization. Rheumatol Int. 2018;38:649–656. doi: 10.1007/s00296-017-3925-9. [DOI] [PubMed] [Google Scholar]
- 25.Choi H.J., Han W.J., Im J.S., Baek H.J. The prevalence and clinical features of musculoskeletal diseases in Incheon: results from chronic disease management surveys. J Rheum Dis. 2009;16:281–290. [Google Scholar]
- 26.Bae E.H., Lim S.Y., Han K.D., Jung J.H., Choi H.S., Kim H.Y. Trend of prevalence and incidence of systemic lupus erythematosus in South Korea, 2005 to 2015: a nationwide population-based study. Korean J Intern Med. 2020;35:652–661. doi: 10.3904/kjim.2018.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smolen J.S., Landewe R.B.M., Bijlsma J.W.J., Burmester G.R., Dougados M., Kerschbaumer A. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:685–699. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.