Abstract
Droplets provide a well-known transmission media in the COVID-19 epidemic, and the particle size is closely related to the classification of the transmission route. However, the term “aerosol” covers most particle sizes of suspended particulates because of information asymmetry in different disciplines, which may lead to misunderstandings in the selection of epidemic prevention and control strategies for the public. In this review, the time when these droplets are exhaled by a patient was taken as the initial time. Then, all available viral loads and numerical distribution of the exhaled droplets was analyzed, and the evaporation model of droplets in the air was combined with the deposition model of droplet nuclei in the respiratory tract. Lastly, the perspective that physical spread affects the transmission risk of different size droplets at different times was summarized for the first time. The results showed that although the distribution of exhaled droplets was dominated by small droplets, droplet volume was proportional to the third power of particle diameter, meaning that the viral load of a 100 μm droplet was approximately 106 times that of a 1 μm droplet at the initial time. Furthermore, the exhaled droplets are affected by heat and mass transfer of evaporation, water fraction, salt concentration, and acid-base balance (the water fraction > 98%), which lead them to change rapidly, and the viral survival condition also deteriorates dramatically. The time required for the initial diameter (do) of a droplet to shrink to the equilibrium diameter (de, about 30% of do) is approximately proportional to the second power of the particle diameter, taking only a few milliseconds for a 1 μm droplet but hundreds of milliseconds for a 10 μm droplet; in other words, the viruses carried by the large droplets can be preserved as much as possible. Finally, the infectious droplet nuclei maybe inhaled by the susceptible population through different and random contact routes, and the droplet nuclei with larger de decompose more easily into tiny particles on account of the accelerated collision in a complex airway, which can be deposited in the higher risk alveolar region. During disease transmission, the infectious droplet particle size varies widely, and the transmission risk varies significantly at different time nodes; therefore, the fuzzy term “aerosol” is not conducive to analyzing disease exposure risk. Recommendations for epidemic prevention and control strategies are: 1) Large droplets are the main conflict in disease transmission; thus, even if they are blocked by a homemade mask initially, it significantly contains the epidemic. 2) The early phase of contact, such as close-contact and short-range transmission, has the highest infection risk; therefore, social distancing can effectively keep the susceptible population from inhaling active viruses. 3) The risk of the fomite route depends on the time in contact with infectious viruses; thus, it is important to promote good health habits (including frequent hand washing, no-eye rubbing, coughing etiquette, normalization of surface cleaning), although blind and excessive disinfection measures are not advisable. 4) Compared with the large droplets, the small droplets have larger numbers but carry fewer viruses and are more prone to die through evaporation.
Keywords: Respiratory tract infection, Transmission route, Droplet size, Evaporation, Infection risk
Graphical abstract
1. Introduction
Although significant progress has been achieved in medicine and personal hygiene, respiratory infections are still a major threat to human health. Especially after the outbreak of the COVID-19 epidemic at the end of 2019, severe acute respiratory coronavirus infection 2 (SARS-CoV-2) has again received widespread attention worldwide [[1], [2], [3], [4]]. Disease transmission is a complex subject involving many disciplines such as epidemiology, social management, and microbial survival environment.
Public awareness of respiratory infections was lacking at the beginning of the epidemic. To popularize the different transmission routes, guide the behavior of social groups, and formulate public intervention policies for epidemic prevention, it was necessary for relevant guidelines to roughly define the transmission routes of the disease. There are several possible modes for COVID-19 infection: contact and droplet transmission, fomite transmission, airborne transmission, and other modes of transmission [5]. The first three routes have been confirmed to be closely related to disease transmission. The other modes of transmission, like the vertical and fecal-oral routes are inconclusive and are not discussed in this study.
Scholars from different disciplines have conducted considerable research regarding the transmission routes of respiratory diseases. As environmental engineering scholars, Manuel [6] applied aerodynamic theory to analyze the different movement trajectories of droplets, due to the unbalanced force in the air, then explored different modes of disease transmission. Lei and Li et al. [7] also classified the different spatial patterns of secondary infection cases according to droplet size. While the term of “aerosol” was mostly preferred by scholars in the medical field for the transmission route of respiratory diseases [[8], [9], [10]], there are convincing reasons that the term “aerosol” in environmental engineering and medical science is inconsistent. Quoting from the Encyclopedia Britannica, true aerosol particles range in diameter from a few millimicrons to approximately 1 μm (equal to 10−4 cm) [11]; nevertheless, the term is commonly used, especially in the case of fog or cloud droplets, which can have diameters of over 100 μm [12,13]. The definition of “Aerosol” in Baidu is more ambiguous, covering the range of 0.001 μm–1000 μm [14]. The spray, droplets, and sputum of different sizes, exhaled by the patient are obscured by the vague term of “aerosol”, which may cause the results from the medical field, including those for COVID-19 disease, to be misread in other interdisciplinary fields. “Aerosol transmission” might be misunderstood as air-conditioning system transmission, or air transmission, erroneously magnifying the risk of transmission, and causing unnecessary panic and misunderstanding in the public [[15], [16], [17]].
It is the essence of disease transmission that infectious viruses are carried by droplets. After being exhaled from the host, the droplets will conduct heat, participate in mass transfer, and undergo an evaporation exchange with the environment. The viral survival condition, such as saltwater, nutrient, salt, pH, temperature, and humidity balance, are significantly influenced by various environmental factors [18,19]. The viruses can only enter the host “luckily” and cause infection when the concentration is sufficient and the virus is still alive, which is unfortunate for the susceptible population. Thus, the different risk of when and how to contact the infectious viruses is the most important question that should be answered, to provide a reference for early isolation and the mask dispute.
Therefore, this article refines the physical spreading process of respiratory tract infection diseases: from the initial time when the droplets are exhaled from the patient's mouth, the determination of the corresponding transmission route given the droplet size, and terminates when the droplets are directly finally inhaled by the susceptible population. The droplet diameter is not constant due to the effect of evaporation during transmission. Nicas et al. [20] found that the exhaled droplets were composed of 98.2% water and 1.8% non-volatile solid compounds. The evaporation regularity of the droplets is consistent with the D2 law (the total evaporation time is approximately proportional to the square of the particle diameter). Droplets of 10 μm and 100 μm evaporate to dryness in approximately 1 s or 10 s, respectively, under normal temperature conditions [21,22]. Large droplets can carry more viruses, and their longer evaporation times can also provide better survival conditions for the viruses [21,23]. The larger droplet nuclei are more easily decomposed into tiny particles due to accelerated collision in the complex airway, and could be deposited in the alveolar region (ALV); therefore, the exposure risk of the susceptible population is increased significantly [24].
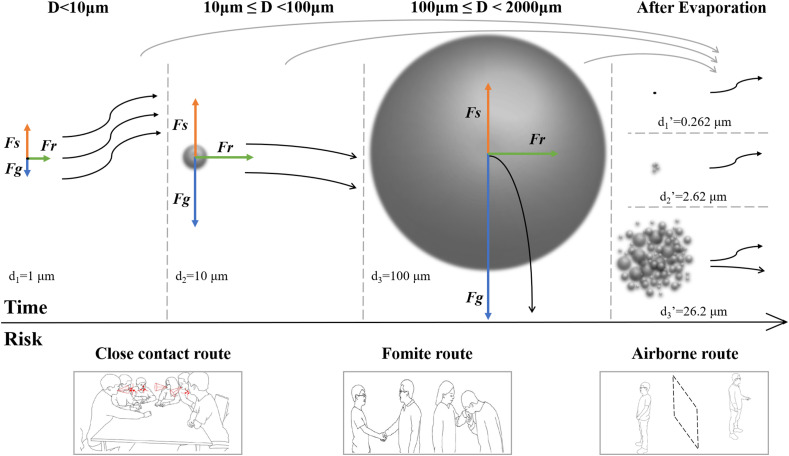
In brief, the influence of evaporation on particle size, viral survival, and personnel exposure risk at different times cannot be ignored, either from the perspective of physical spreading or disease transmission. In this review, the term of “aerosol” that is likely to be misinterpreted by scholars in different fields was diluted, and the main transmission risk caused by encounters with different size droplets at varying time and distance were analyzed (Fig. 1 ). The upper part of Fig. 1 shows the de of the droplets with different sizes after evaporation, and the corresponding movement trajectories caused by unbalanced forces. The lower part of Fig. 1 shows the different routes for virus contact in social activities. Infection risk is closely related to the time that people are exposed to the viruses.
Fig. 1.
Transmission risk of droplets with different sizes at different times. Upper part: Typical trajectories of particles in the air (based from Ref. [6]). Lower part: Illustration of different transmission routes (based from Ref. [1,7]). Fs: aerodynamic forces, Fg: the force of gravity, Fr: the horizontal force when ejected from human mouth, d: diameter, d’: diameter after evaporation.
This review focuses on analyzing the risk of suspended droplets (<100 μm) carrying infectious viruses and aims to advance more appropriate recommendations for epidemic prevention and control. After the droplets leave an infected patient, the viral survival condition balance is broken due to the heat and mass transfer with the environment. How do these complex physical processes affect the transmission risk with infectious droplets? The main conflict should be grasped with the aim of accurately communicating the risk of droplet transmission to interdisciplinary scholars and focus on providing reliable prevention and control suggestions for the public.
2. Methods and data
Combined with the artificial screening of the subject and the content of this study, SpringerLink, ScienceDirect, and Web of Science (SCI) were used to identify and review the literature published before May 2020. We used the search keywords: “COVID-19”, “Respiratory tract infection”, “Disease transmission”, “Droplets”, “Viral load”, “Evaporation”, and “Deposition”. A total of 52 articles were selected from two main categories, virus transmission (28) and droplet evaporation (24), including 20 reference literature sources published in 2020.
The first subset involves the distribution and viral load of droplets exhaled by patients through different respiratory activities, and the deposition fraction of droplets in the respiratory tract after being inhaled by the susceptible population. Then, the cumulative volume of droplets was calculated, representing the ability of droplets to carry viruses. The deposition fraction in human airways were combined to indicate the initial transmission capacity of droplets of different sizes.
The second subset refers to the particle size variation of the suspended droplets evaporated in the air, which are affected by heat and mass transfer under different environmental conditions. The evaporation particle size variation data were screened, and the evolution and progress of the particle evaporation model were analyzed. The data were selected to show how environmental parameters like temperature and relative humidity, can be related to the evaporation rate.
Third, we combined the initial disease transmission capacity of droplets and the variable diameter of suspended droplets in the air, illustrated the main conflicts at different times, and answered how these complex physical and physiological changes affected the infection risk of infectious droplet sizes.
3. Results
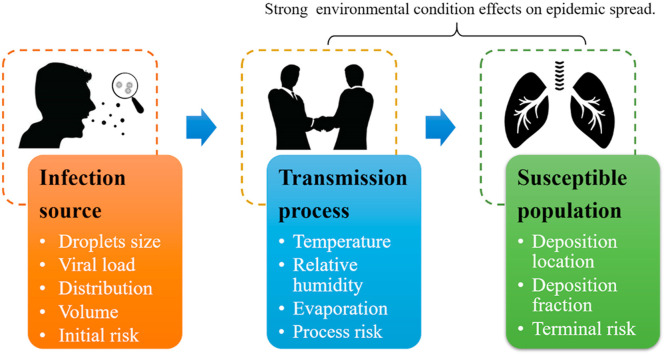
The process of occurrence, transmission, and termination of infectious diseases in the population is also known as the transmission process of infectious diseases, and its realization must have three basic links: infection source, transmission process, and susceptible population [25].
3.1. Infection source – the characteristics of exhaled droplets
To study the risk of transmission of infectious droplets, the first step is to fully understand the characteristics of exhaled droplets at the infection source, including the viral load, diameter range, and distribution exhaled by infected patients through different respiratory activities. RT-PCR viral nucleic acid detection is widely used for diagnosing respiratory infections. The throat swab of patient is usually collected as a test sample [26].
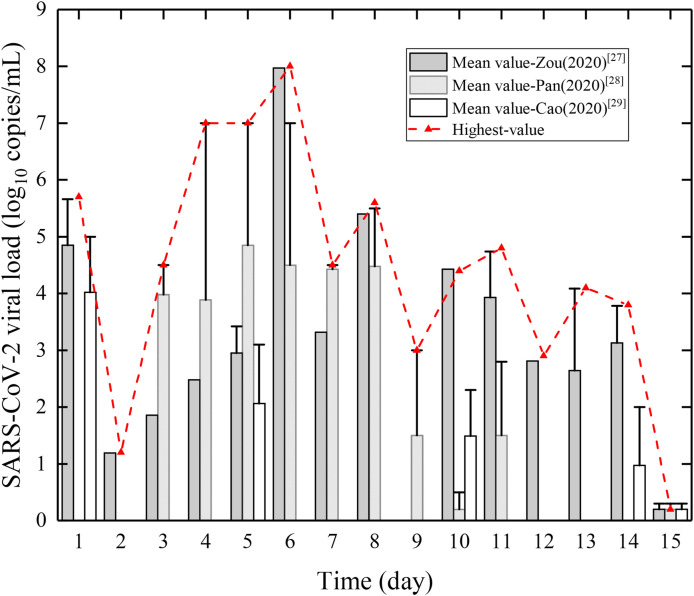
Since the outbreak of the epidemic, there have been many reports of SARS-CoV-2 patients' viral load data. We collected several clinical studies on the infection of pharyngeal swab by multiple scholars (see Fig. 2 ) [[27], [28], [29]]. Zou et al. [27] conducted viral load concentrations on throat swab specimens for six early stage patients, and found that the individual differences among the patients were notable, and the differences were obvious on different dates after admission. Pan et al. [28] conducted a specimen study on two cases in Beijing and found that throat swab samples with viral load peaked 5–6 days after the onset of symptoms, approximately 104–107 copies/ml. Cao et al. [29] targeted 199 SARS-CoV-2 patients who participated in drug therapy and standard therapy to conduct daily throat swab sampling, and found significant individual differences in virus load on different dates.
Fig. 2.
Viral load of SARS-CoV-2 in patient's body fluids. Only the first 15 days of data in each literature are shown. Bar chart: The mean viral load (from all the cases) in the literature; The upper error bar and red dotted line: The highest viral load (from only one case) on that day. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
It can be concluded that: 1) the viruses could exist in the human body for up to 2–3 weeks or more, and the viral load in body fluids decreased over time; the initial stage of infection was the highest risk period of contact, so early isolation measures were crucial. 2) The viral load of SARS-CoV-2 in patients' body fluids generally peaked approximately 4–6 days after onset, up to 108 copies/ml. This was unlike SARS, whose patients usually reached their peak around 10 days after onset. 3) There was a strong individual difference between COVID-19 patients, high-risk patients, whose viral load was approximately 103 copies/ml higher than the mean data over the same period, and those who might become super spreaders during disease transmission.
Many scholars have conducted relevant analysis and research on the distribution of droplets exhaled by human respiratory activity. Most studies are based on the measurement of the human body exhaled droplets through a similar tiny droplet capture system [30]. In the early period, Duguid [31], a bacteriologist, conducted a series of measured studies on the diameter and particle number distribution of exhaled droplets. Later Lindsley and Morawska [32,33] compared the differences in droplets exhaled by different respiratory activities such as breathing, speaking, and coughing, and found that coughing released more droplets than breathing, then found that the type A detection rate of live influenza viruses bacteria in cough droplets were higher than that of breath droplets. Other studies [21,34,35] applied high-speed cameras and theoretical models of the exhaled airflow to simulate droplet distribution characteristics. Bourouiba et al. [34] visually tested severe respiratory events with high-speed cameras, to predict the range of droplet pollution for different particle sizes. Scholars at the University of Hong Kong [21,34] employed mathematical methods that assumed the cough physics as a turbulent jet, and analyzed the dispersion of particulate matter to estimate the relationship between the droplet diameter on the local spatial pollution range.
Due to the viral load from different patient samples, the values vary significantly at different times; therefore, it is not appropriate to determine a uniform representative value [[27], [28], [29]]. However, for a specific infected person, the concentration of the viruses in the oral, nose, and throat fluids can be considered as evenly distributed, and the viral load of the droplet is positively related to the droplet volume. Thus, the cumulative volume of droplets of different sizes may represent the number of viruses carried and the risk of disease transmission.
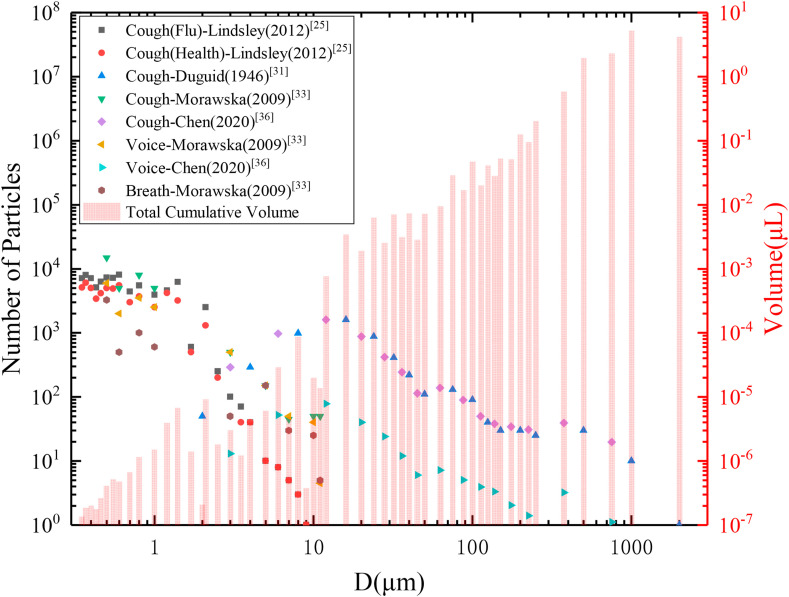
Fig. 3 summarizes the representative distribution data of exhaled droplets, and the calculated cumulative volume of droplets with different particle sizes. It can be seen that: 1) Generally, there are a large number of small droplets with little cumulative volume exhaled with respiratory activity, unlike large droplets. The more intense the respiratory activity, the higher the proportion of large droplets. Due to the randomness and complexity of the exhaled droplets, and the different methods and equipment employed by scholars, there was some difference observed among data sources. 2) Although the number of small droplets might be thousands of times larger than that of large droplets, the droplet volume was proportional to the third power of its particle size; thus, the volume of a 100 μm particle was at least three orders of magnitude larger than the volume of a 1 μm particle; therefore, the disease transmission ability of large droplets was much higher than that of small droplets.
Fig. 3.
Number distribution of exhaled droplets from different respiratory activities and the cumulative volume. The volume of a single droplet is multiplied by the total number data, obtaining the cumulative total volume of droplets with the specific diameter, and the average value will be employed if there are multi-group data.
3.2. Transmission process – evaporation model of suspended droplets
After the infectious droplets have been exhaled by the host through breathing, speaking, coughing, or sneezing, the small suspended droplets (<100 μm) form long-term suspended droplets or short-duration clusters of droplets in the air, and those droplets with large particle sizes (>100 μm) can land on the ground or on nearby objects [1,36]. Nevertheless, the droplet surface experiences intense heat and mass exchange with the environment. For droplets ejected from the patient's mouth at high speed, the moisture, nutrients, and balance of trace salts and acid-base in the droplets were altered during marked droplet shrinkage, which rapidly deteriorated the virus survival conditions. The evaporation process of suspended droplets is particularly important in analyzing the transmission risk of infectious droplets.
The evaporation process is a combination of heat and mass transfer between the droplet and the environment, and is strongly affected by the droplet diameter and the environmental conditions (including air temperature, humidity, and turbulence). To explore the mechanism of the droplet evaporation process, Kukkonen et al. [37] proposed an evaporation and dispersion model for pure water droplets, driven by the partial pressure difference between the droplet surface vapor and the ambient air. Currently, many evaporation studies are still based on this single component droplet model [[38], [39], [40], [41], [42], [43]].
The composition of infectious droplets from the mouth, nose, and throat fluids is very complicated. In addition to water, there are various nutrients, trace amounts of salt, and microbial bacteria in the droplets. Various respiratory activities are differently affected by the local airflow, air turbulence, temperature, humidity, and other environmental factors, and their evaporation progress is more specific. Therefore, it is difficult to establish a fully comprehensive evaporation model of human exhaled infectious droplets, and there remains a certain research gap. The development of a model, from simple to complex, also reflects the actual situation on the physical process of droplet evaporation.
Table 1 shows the comparison of representative evaporation models for different influence factors. It can be seen from Table 1 that most scholars took sodium chloride solution to represent body fluids. Based on the pure water model, a human exhalation droplet evaporation model was established, and the concentrations of non-volatile solutes in the evaporation process and the final formed de of the droplet nuclei were simulated [35,44]. In addition to the composition, it is also significant to consider the influence of factors such as exhaled airflow and air turbulence generated by human respiratory activity. Xie et al. [44] considered the influence of different respiratory activities on the exhaled jet, and found that different initial droplet velocities would affect the evaporation rate. Wei et al. [21] further found that the jet turbulence caused by the airflow would distribute the exhaled droplets more widely, and evaporation had the most significant effect on medium size droplets (approximately 50 μm). When the droplets evaporated into a saturated concentration, other solid components (bacteria, viruses, and salt crystals) would also be precipitated besides the sodium chloride (NaCl). Thus, Redrow et al. [23] based on Pruppacher's research [45], simulated the actual characteristics of exhaled sputum, and for the first time, established a droplet evaporation model, including sodium chloride, carbohydrate, lipid, and protein components.
Table 1.
Comparison and development of the droplet evaporation models.
| Country | Object | Factors | Model | Investigator |
|---|---|---|---|---|
| Israel | Pure water | Heat and mass transfer | Multi-shells droplet | Pariente et al. [46] |
| China | Pure water | Air turbulence | Single droplet | Wei et al. [21] |
| China | Pure water | Air thermal stratification | Single droplet | Liu et al. [47] |
| Australia | Pure water | Heat and mass transfer | Single droplet | Morawska et al. [48] |
| China | NaCl | Breathe speed | Single droplet | Xie et al. [44] |
| China | NaCl | Air turbulence | Single droplet | Liu et al. [35] |
| Australia | NaCl | Non-uniform humidity field | Mathematical model of droplet evaporation in cough | Li et al. [22] |
| USA | NaCl, carbohydrates, lipids, and proteins | Chemical composition | An improved multi-component droplet | Redrow et al. [23] |
The water in the droplets gradually evaporates completely, and de of the droplet nuclei is closely related to the initial do, and directly affects its deposition fraction in the respiratory tract [35]. Furthermore, it has been found by many scholars that the environmental parameters, such as temperature and humidity, also have a substantial influence on de, and are key factors to whether the survival condition balance of viruses carried in droplets could be guaranteed [49,50]. In conclusion, for susceptible populations, the exposure risk to disease in the local space are both determined by the de of the droplets after evaporation, and the amount of residual active viral load of the droplets.
Wang et al. [51] conducted a series of studies on the effects of ambient temperature, humidity, and initial particle size on evaporation, and their results showed that the impact of these three factors on the evaporation characteristics of droplet was not independent; therefore the influence of any factor on droplet evaporation cannot be discussed without the other factors. Additionally, Li et al. and Yan et al. [22,52] established an evaporation model of cough droplets under non-uniform humidity conditions, simulating the propagation distance and distribution characteristics of different size droplets. Liu [47] further considered the indoor thermal stratification factor to study droplets, and found that thermal stratification could significantly reduce evaporation. Xie [44] and Wei [21] both found in their respective studies that the larger the initial particle size, the longer the evaporation time required under the same environmental conditions. Yang et al. [53] believed that droplets with the same initial particle size would evaporate for a longer time under high humidity because air with higher relative humidity has less potential to absorb water vapor. Another scholar [54] found that the relative humidity not only affects the speed of evaporation, but also affects the diameter of the final droplet nuclei due to the Kelvin effect; the final normalized diameter de/do of small droplets is smaller than that of large droplets.
Under normal temperature conditions (5 °C-25 °C), and different relative humilities (0–90%), the evaporation time and final de of droplets with different sizes from the representative literature are summarized in Table 2 . The larger the initial droplet diameter, the longer it takes for the water to completely evaporate, which could create a more favorable survival condition for the viruses in large droplets, thus maintaining the transmission risk of disease. There were some differences in the final normalized diameter de/do by different scholars; however, de was roughly between 30% and 40% of do. While the moisture content of exhaled droplets is approximately 98%, the theoretical de should be approximately 26.2% of do [20]. Besides non-volatile salts, the existence of microorganisms and bacteria might be the reason the measured de is larger than the theoretical de. If these loose droplet nuclei are “luckily” inhaled into the small and tortuous airway, the large droplet nuclei might decompose into smaller particles due to acceleration and collision, causing a greater infection risk than the original particle.
Table 2.
Evaporation time and final de of the droplets under different environmental conditions.
| Initial diameter (μm) |
Environment |
Adjustment for evaporation | Evaporation τ (ms) |
final diameter (μm) |
References | |
|---|---|---|---|---|---|---|
| T (°C) | RH (%) | |||||
| 1 | 25 | 0 | equation 3 | 1 | 0.325 | Wei et al. [21] |
| 1 | 90 | 10 | 0.325 | |||
| 10 | 21 | 20 | equation 10 | 250 | 3.5 | Redrow et al. [23] |
| 10 | 50 | 300 | 3.5 | |||
| 10 | 80 | 550 | 3.5 | |||
| 12 | 25 | 0 | equation 14 | 300 | 3.25 | Li et al. [22] |
| 12 | 50 | 500 | 3.25 | |||
| 12 | 90 | 3100 | 3.25 | |||
| 12 | 5 | 50 | 1440 | 3.25 | ||
| 12 | 15 | 850 | 3.25 | |||
| 12 | 25 | 550 | 3.25 | |||
| 12 | 35 | 420 | 3.25 | |||
| 100 | 25 | 0 | – | 7900 | 0 | Morawska et al. [48] |
| 100 | 20 | 11,000 | 0 | |||
| 100 | 60 | 22,000 | 0 | |||
| 100 | 80 | 48,000 | 0 | |||
3.3. Susceptible population - droplets deposition in the respiratory tract
As mentioned above, as the infectious droplets exhaled by a patient drift in the air, the particle size changes drastically with time due to evaporation and concentration, and the infection risk depends on the inhalation time of the susceptible population. It is generally believed that the closer the droplet deposition site is to the lungs, or the greater the amount of viruses deposited, the greater the risk of disease infection [55]. The high-risk pathogenicity of the virus after it penetrates the lungs can be explained from the following two aspects.
Considering physiological characteristics, the upper respiratory tract is protective; for example, the cilia of endothelium in the trachea can adhere to the droplets, and cause cough reflexes to promote the expulsion of pathogens. The lower respiratory tract, such as the alveoli, is less protected and prone to infection. Considering pathogenicity, the alveolar is the smallest unit in the human body that communicates with the outside world. The S protein of the coronavirus directly contacts and binds with the ACE2 receptor of the alveolar cells, which may directly cause alveolar ventilation disorders. Even worse, with time, the viruses will penetrate the alveoli and enter the blood vessels, thereby entering various parts of body through the blood circulation along with the oxygen delivery path, and causing graver systemic infections.
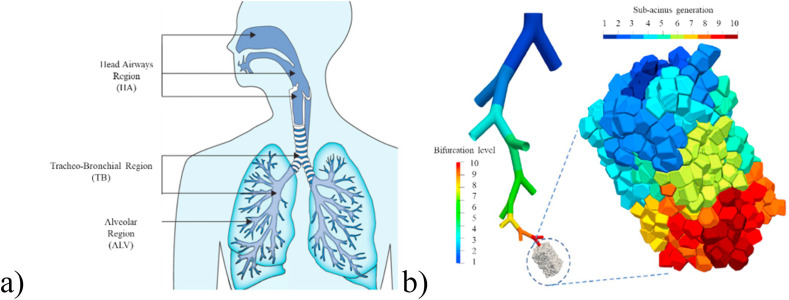
The respiratory tract and alveolar region of human body are shown in Fig. 4 a) and b), respectively. The human respiratory tract can be divided into the head airway (HA), tracheo-bronchial region (TB), and ALV, of which the nose pharynx, oropharynx, and laryngopharynx belong to the upper respiratory tract [26,56]; the trachea, bronchus, and ALV are also called the lower respiratory tract [57]. As shown in Fig. 4b), the schematic of the ALV is a bifurcated tree bionic model spanning ten generations, combined with multiple sub-spine models [58].
Fig. 4.
Schematic diagram of the human respiratory tract and lungs; a) the whole respiratory tract (cited from Ref. [57]); b) alveolar region (cited from Ref. [56]).
Most of the literature on the deposition of inhaled droplets in the respiratory tract has been conducted by medical scholars. When inhalation pulmonary drug delivery technology was employed to treat respiratory diseases, they found that the effect of drug particle size on the deposition location and fraction in the respiratory tract was critical [20,[59], [60], [61], [62], [63]]. Thus, the original intent was to enhance the deposition of drug particles in the lungs. This could be described as different tunes sung with equal skill with the work devoted to reducing the deposition of infectious pathogens in the lungs in the respiratory disease prevention and control work [64].
To obtain the deposition rules of droplets with different particle sizes in the respiratory tract, Guo et al. [65] employed a new type of breathing bag experimental device, established a real-life measurement system specifically for inhaled respiratory tract deposition, and observed a consistent trend of all participants that the deposition fraction increased with particle size decrease. However, due to the difficulty of performing human experiments on respiratory tract deposition, most of the literature was conducted based on mathematical models, such as aerodynamic, thermal balance, and Lagrangian tracking algorithms, or software models of the deposition fraction in the respiratory tract, such as ANSYS or the CFD [[66], [67], [68], [69], [70], [71]]. As early as 1994, the International Committee for Radiological Protection (ICRP), based on a nasal breathing and light movement deposition model, gave the total deposition and regional deposition prediction data related to the diameter of inhaled droplets during quiet breathing [57,72]. Euler simulations of transport and deposition in the respiratory tract are often used to explain that droplets have a U-shaped deposition curve [67,68]. Different respiratory activities could also cause differences in the deposition fraction of droplets of different sizes in different locations of the respiratory tract. Koullapis et al. [56]combined the deep lung and upper respiratory tract models to analyze the effects of different breathing strategies (deep breathing, shallow breathing, breath-holding) on the deposition of droplet particles, and their results showed that compared with calm breathing, the deposition in the lung area increased significantly during deep breathing.
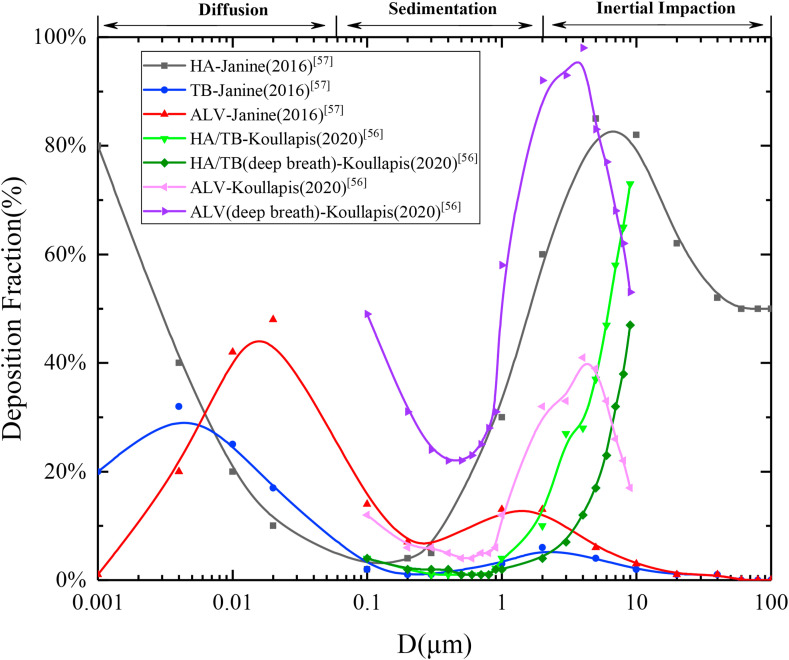
Due to the complex microstructure of various regions involved in the human respiratory tract, it is difficult to study the deposition fraction, and only a few literature studies were developed. Fig. 5 shows a comparison of different deposition fractions in the respiratory tract obtained by different scholars. The deposition fraction in the respiratory tract for normal and deep breathing are also displayed. It can be seen that: 1) The deposition mechanism of particulate matter was complicated. Large particles were mainly affected by gravity or impact deposition while small particles were mainly deposited through molecular diffusion; thus, the fraction of medium particle size droplet deposition was the lowest. In general, there were double peaks at 0.01 μm and 10 μm with the increase in particle size of the deposition fraction in the respiratory tract different areas; 2) Large droplets with particle sizes above 10 μm were mainly deposited in the upper respiratory tract (HA), and small droplets penetrated the human body and were deposited in the lower respiratory tract, including the TB and ALV; 3) Deep breathing could increase the deposition fraction of particulate matter in the ALV while reducing its deposition in the upper respiratory tract (HA/TB) area to increase the risk of disease infection. Although it is generally believed that as the particle size increases, the ALV deposition fraction will increasingly decline, it can be seen from Fig. 5 that the ALV also has a very high deposition fraction in the larger particle size range of 1 μm–10 μm. In other words, the large droplets carrying substantial virus load would develop into high-risk small droplet nuclei after evaporation and concentration in the air. Moreover, the dry, loose droplet nuclei are easily decomposed by the acceleration impacts in human airways; therefore, a large amount of irregular smaller solid particles could be deposited in alveolar region, which should be intercepted in the HA, thereby significantly increasing the transmission risk.
Fig. 5.
Deposition fraction of the droplets with different sizes in the respiratory tract.
4. Discussion
4.1. Transmission risk variation of droplets with evaporation
The infectious droplets may not be inhaled by the susceptible population immediately but will linger in the environmental space. Notably, cough-ejected droplets could move at up to 10 m/s [73], and these droplets sizes would be significantly affected by evaporation during transmission. The survival balance of viruses might also change drastically, causing the large differences in infection risk at different times.
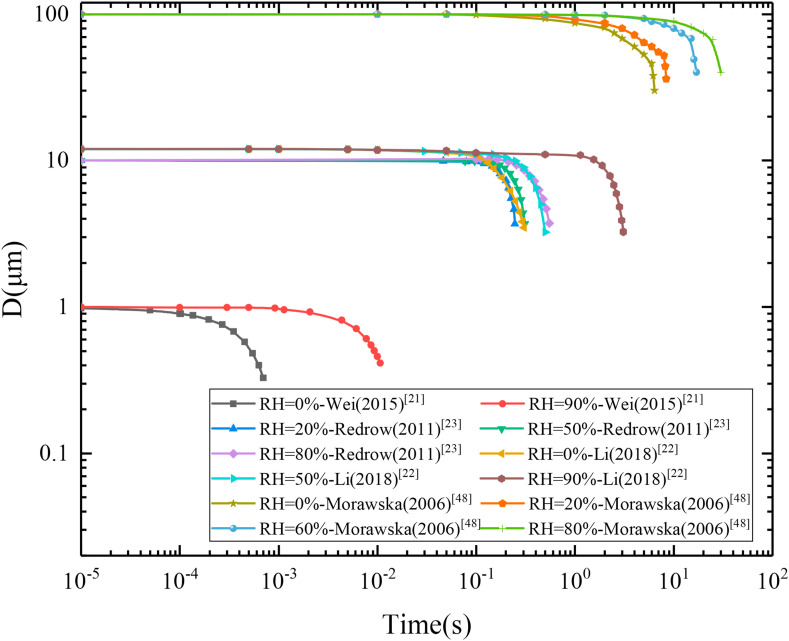
Based on the collected data in the previous studies, Fig. 6 shows the variations of different particle diameters (1 μm, 10 μm, 12 μm, 100 μm) for different relative humidities [[21], [22], [23],48]. The droplets with different initial sizes could be quickly evaporated in a short time, and the residual solid droplet nuclei de were only approximately 30% of the original droplet d0. Moreover, the porous nuclei of the droplet nuclei broke easily in the air turbulence, which made the density of fine suspended droplets easily inhaled locally increase significantly. Furthermore, small droplets were more likely to evaporate at low humidity, and the long moisture presence in large droplets was conducive to maintaining the balance of the viruses' survival condition; therefore, the disease infection capacity of large droplets remained high. In short, the high-risk droplet nuclei are formed after the large droplets evaporated, which with smaller diameters and a great number of residual active viruses, could penetrate the alveolar of the human body to cause infection. Therefore, it is necessary to control the infectious droplets at the early stage of the transmission process, and the earlier the better.
Fig. 6.
Changes in droplet evaporation particle size affected by relative humidity.
4.2. Disease transmission ability of droplets with different diameters
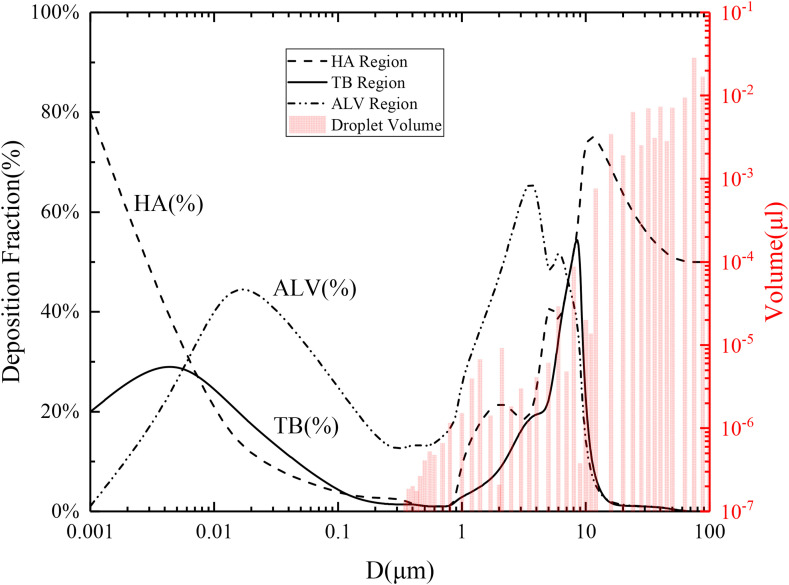
To explain the problem more intuitively, first, Fig. 7 shows the average deposition fraction (line) of different particle sizes, and the cumulative volume (columns) of exhaled droplets [57,74], to illustrate the transmission risks of droplets with different particle sizes. Additionally, it considers the randomness of how the infection source contacts susceptible individuals; infectious droplets might be immediately inhaled, or linger in the air. Evaporative concentration inevitably occurs, combined with the initial droplet's disease transmission ability and the change in droplet size during evaporation; therefore, the disease transmission risk at different times could be analyzed and judged.
Fig. 7.
Transmission risks of droplets with different diameters considering the deposition fraction.
As shown in Fig. 7, at the initial moment, the range of droplet diameters exhaled by patients was mainly distributed between 0.3 μm and 100 μm. The larger the accumulated volume, the more viruses might be carried. In the initial distribution of the droplets, the HA and ALV had higher deposition fraction peaks near 10 μm and 2 μm, respectively; however, the viral load of large droplets was several orders of magnitude higher than that of small droplets, which was the main conflict of disease prevention and control. It was assumed that these droplets were inhaled after evaporation, the final de distribution ranged from 0.0786 μm to 26.2 μm, and this de range was enough to include the deposition peak of the HA and the high-risk deposition peak of the ALV. Thus, it was evident that the risk of infection would increase dramatically. Therefore, first, it is necessary to take prevention and control measures at the patient source, and the sooner the better. Second, under the condition of the material restriction, the vulnerable groups should use homemade mask and other tools to prevent the large droplets that are dominant in disease transmission.
5. Mitigation
The above research results can provide useful hints for the prevention of COVID-19 disease:
-
1.
For the infection source (droplets), although the number of large droplets is relatively small, the amount of virus that may be carried is much larger than that by small droplets (several orders of magnitude), shown in Fig. 3. The measures to control the large droplets, such as wearing masks and social distancing, can significantly reduce the risk of disease transmission.
-
2.
For the transmission process (evaporation), the survival balance of viruses carried in droplets is rapidly destroyed after evaporation, shown in Table 2. Environments that are prone to evaporation, such as high temperature and low humidity, are more conducive to our epidemic prevention efforts.
-
3.
For the susceptible population (deposition), the virus deposition risk of small droplets is much smaller than that of large droplets, as shown in Fig. 7 (note: the right y-axis is logarithmic coordinate). However, the risk of small droplet nuclei (the original large droplets) must also be taken seriously, especially in environments where evaporation is easy.
6. Conclusions
Based on the different particle sizes, we conducted an in-depth review on the physical processes and transmission risks of viral infections caused by infectious droplets at different time points. We found that the viral load of large droplets was several orders of magnitude larger than that of small droplets. The disease transmission ability of large droplets at the initial moment is significantly higher than that of small droplets. Furthermore, during the transmission process, the original large droplets evaporation takes longer, and the survival condition balance of active viruses is better maintained, and becomes a high-risk small droplet nuclei carrying a massive number of active viruses after evaporation. Compared with the original small droplets, whose internal viruses are quickly evaporated and inactivated, the risk difference of disease transmission between the two is even greater, which also confirmed the dominant position of large droplets in disease transmission.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is supported by grant No.52078314 from the National Natural Science Foundation and grant No.2016YFC0700400 form the National Key R&D Program of China.
References
- 1.Zhang N., Chen W., Chan P.T., Yen H.L., Tang J.W., Li Y. Close contact behavior in indoor environment and transmission of respiratory infection. Indoor Air. 2020 doi: 10.1111/ina.12673. [DOI] [PubMed] [Google Scholar]
- 2.Singh S. Middle East respiratory syndrome virus pathogenesis. Semin. Respir. Crit. Care Med. 2016;37:572–577. doi: 10.1055/s-0036-1584796. 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23(2):130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J., Zhao S., Teng T., Abdalla A.E., Zhu W., Xie L., Wang Y., Guo X. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12(2) doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Transmission of SARS-CoV-2 . 2020. Implications for Infection Prevention Precautions.https://www.who.int/publications/i/item/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations 2020(accessed 14 August 2020) [Google Scholar]
- 6.Silva M.C.G.d. 2020. An Analysis of the Transmission Modes of COVID-19 in Light of the Concepts of Indoor Air Quality. [DOI] [Google Scholar]
- 7.Lei H., Li Y., Xiao S., Lin C.H., Norris S.L., Wei D., Hu Z., Ji S. Routes of transmission of influenza A H1N1, SARS CoV, and norovirus in air cabin: comparative analyses. Indoor Air. 2018;28(3):394–403. doi: 10.1111/ina.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chi M., Lou C., Zhao X., Sui X., Han F. A simple custom appliance against droplet and aerosol transmission of COVID-19 during advanced airway management. Crit. Care. 2020;24(1):319. doi: 10.1186/s13054-020-02985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge Z.Y., Yang L.M., Xia J.J., Fu X.H., Zhang Y.Z. Possible aerosol transmission of COVID-19 and special precautions in dentistry. J. Zhejiang Univ. - Sci. B. 2020;21(5):361–368. doi: 10.1631/jzus.B2010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mick P., Murphy R. Aerosol-generating otolaryngology procedures and the need for enhanced PPE during the COVID-19 pandemic: a literature review. J. Otolaryngol. Head Neck Surg. 2020;49(1):29. doi: 10.1186/s40463-020-00424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aerosol, Physics, Britannica. 2020. https://www.britannica.com/science/aerosol accessed 23 April 2020. [Google Scholar]
- 12.Valentini S., Barnaba F., Bernardoni V., Calzolai G., Costabile F., Di Liberto L., Forello A.C., Gobbi G.P., Gualtieri M., Lucarelli F., Nava S., Petralia E., Valli G., Wiedensohler A., Vecchi R. Classifying aerosol particles through the combination of optical and physical-chemical properties: results from a wintertime campaign in Rome (Italy) Atmos. Res. 2020;235 doi: 10.1016/j.atmosres.2019.104799. [DOI] [Google Scholar]
- 13.Weijun Li L.L., Xu Liang, Zhang Jian, Qi Yuan, Ding Xiaokun, Hu Wei, Fu Pingqing. Science of the Total Environment; 2020. Daizhou Zhang Overview of Primary Biological Aerosol Particles from a Chinese Boreal Forest: Insight into Morphology, Size, and Mixing State at Microscopic Scale. [DOI] [PubMed] [Google Scholar]
- 14.Aerosol . 2020. Baidu Online Search Service.https://baike.baidu.com/item/%E6%B0%94%E6%BA%B6%E8%83%B6/1022460?fr=aladdin accessed 23 April 2020. [Google Scholar]
- 15.Li Z., Wang H., Zheng W., Li B., Wei Y., Zeng J., Lei C. A tracing method of airborne bacteria transmission across built environments. Build. Environ. 2019;164 doi: 10.1016/j.buildenv.2019.106335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z., Zheng W., Wei Y., Li B., Wang Y., Zheng H. Prevention of particulate matter and airborne culturable bacteria transmission between double-tunnel ventilation layer hen houses. Poultry Sci. 2019;98(6):2392–2398. doi: 10.3382/ps/pez019. [DOI] [PubMed] [Google Scholar]
- 17.Correia G., Rodrigues L., Gameiro da Silva M., Goncalves T. Airborne route and bad use of ventilation systems as non-negligible factors in SARS-CoV-2 transmission. Med. Hypotheses. 2020;141:109781. doi: 10.1016/j.mehy.2020.109781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pica N., Bouvier N.M. Environmental factors affecting the transmission of respiratory viruses. Curr. Opin. Virol. 2012;2(1):90–95. doi: 10.1016/j.coviro.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei J., Li Y. Airborne spread of infectious agents in the indoor environment. Am. J. Infect. Contr. 2016;44(9):S102–S108. doi: 10.1016/j.ajic.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicas M., Nazaroff W.W., Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J. Occup. Environ. Hyg. 2005;2(3):143–154. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei J., Li Y. Enhanced spread of expiratory droplets by turbulence in a cough jet. Build. Environ. 2015;93:86–96. doi: 10.1016/j.buildenv.2015.06.018. [DOI] [Google Scholar]
- 22.Li X., Shang Y., Yan Y., Yang L., Tu J. Modelling of evaporation of cough droplets in inhomogeneous humidity fields using the multi-component Eulerian-Lagrangian approach. Build. Environ. 2018;128:68–76. doi: 10.1016/j.buildenv.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redrow J., Mao S., Celik I., Posada J.A., Feng Z.-g. Modeling the evaporation and dispersion of airborne sputum droplets expelled from a human cough. Build. Environ. 2011;46(10):2042–2051. doi: 10.1016/j.buildenv.2011.04.011. [DOI] [Google Scholar]
- 24.Zhang H., Li D., Xie L., Xiao Y. Documentary research of human respiratory droplet characteristics. Procedia Eng. 2015;121:1365–1374. doi: 10.1016/j.proeng.2015.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindsley W.G., Pearce T.A., Hudnall J.B., Davis K.A., Davis S.M., Fisher M.A., Khakoo R., Palmer J.E., Clark K.E., Celik I., Coffey C.C., Blachere F.M., Beezhold D.H. Quantity and size distribution of cough-generated aerosol particles produced by influenza patients during and after illness. J. Occup. Environ. Hyg. 2012;9(7):443–449. doi: 10.1080/15459624.2012.684582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guarnieri M., Balmes J.R. Outdoor air pollution and asthma. Lancet. 2014;383(9928):1581–1592. doi: 10.1016/s0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H.L., Peiris M., Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20(4):411–412. doi: 10.1016/s1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao B., Wang Y., Wen D., Liu W., et al. A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milton D.K., Fabian M.P., Cowling B.J., Grantham M.L., McDevitt J.J. 2013. Influenza Virus Aerosols in Human Exhaled Breath: Particle Size, Culturability, and Effect of Surgical Masks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duguid M.B.J.P., Sc B. 1946. Expulsion of Pathogenic Organisms from the Respiratory Tract British Medical Journal. [DOI] [PubMed] [Google Scholar]
- 32.Lindsley W.G., Blachere F.M., Beezhold D.H., Thewlis R.E., Noorbakhsh B., Othumpangat S., Goldsmith W.T., McMillen C.M., Andrew M.E., Burrell C.N., Noti J.D. Viable influenza A virus in airborne particles expelled during coughs versus exhalations. Influenza Other Respir. Viruses. 2016;10(5):404–413. doi: 10.1111/irv.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morawska L., Johnson G.R., Ristovski Z.D., Hargreaves M., Mengersen K., Corbett S., Chao C.Y.H., Li Y., Katoshevski D. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol Sci. 2009;40(3):256–269. doi: 10.1016/j.jaerosci.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bourouiba L., Dehandschoewercker E., Bush John W.M. Violent expiratory events: on coughing and sneezing. J. Fluid Mech. 2014;745:537–563. doi: 10.1017/jfm.2014.88. [DOI] [Google Scholar]
- 35.Liu L., Wei J., Li Y., Ooi A. Evaporation and dispersion of respiratory droplets from coughing. Indoor Air. 2017;27(1):179–190. doi: 10.1111/ina.12297. [DOI] [PubMed] [Google Scholar]
- 36.Chen W., Zhang N., Wei J., Yen H.-L., Li Y. Short-range airborne route dominates exposure of respiratory infection during close contact. Build. Environ. 2020;176 doi: 10.1016/j.buildenv.2020.106859. [DOI] [Google Scholar]
- 37.Kukkonen J., Vesala T., Kulmala M. The interdependence of evaporation and settling for airborne freely falling droplets. J. Aerosol Sci. 1989:749–763. doi: 10.1016/0021-8502(89)90087-6. [DOI] [Google Scholar]
- 38.Chao C.Y., Wan M.P. A study of the dispersion of expiratory aerosols in unidirectional downward and ceiling-return type airflows using a multiphase approach. Indoor Air. 2006;16(4):296–312. doi: 10.1111/j.1600-0668.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 39.Chao C.Y.H., Wan M.P. Transport characteristics of expiratory droplets and droplet nuclei in indoor environments with different ventilation airflow patterns. J. Biomech. Eng. 2007;129(3):341. doi: 10.1115/1.2720911. [DOI] [PubMed] [Google Scholar]
- 40.Chen C., Zhao B. Some questions on dispersion of human exhaled droplets in ventilation room: answers from numerical investigation. Indoor Air. 2010;20(2):95–111. doi: 10.1111/j.1600-0668.2009.00626.x. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Yang Y., Zou Y., Cao Y., Ren X., Li Y. Evaporation and movement of fine water droplets influenced by initial diameter and relative humidity. Aerosol Air Qual. Res. 2016;16(2):301–313. doi: 10.4209/aaqr.2015.03.0191. [DOI] [Google Scholar]
- 42.Yang Y., Wang Y., Song B., Fan J., Cao Y., Duan M. Transport and control of droplets: a comparison between two types of local ventilation airflows. Powder Technol. 2019;345:247–259. doi: 10.1016/j.powtec.2019.01.008. [DOI] [Google Scholar]
- 43.Zhang Y., Feng G., Bi Y., Cai Y., Zhang Z., Cao G. Distribution of droplet aerosols generated by mouth coughing and nose breathing in an air-conditioned room. Sustain. Cities Soc. 2019;51 doi: 10.1016/j.scs.2019.101721. [DOI] [Google Scholar]
- 44.Xie Y.L.X., Chwang A.T.Y., Ho P.L., Seto W.H. How far droplets can move in indoor environments – revisitingthe Wells evaporation–falling curve. Indoor Air. 2007 doi: 10.1111/j.1600-0668.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- 45.Pruppacher Hr K.J. D. Reidel; Dordrecht: 1978. Microphysics of Clouds and Precipitation; p. 714. [Google Scholar]
- 46.Parienta L.M.D., Johnson G.R., Ristovski Z.D., Hargreaves M., Mengersen K., Corbett S., Chao C.Y.H., Li Y., Katoshevski D. Theoretical analysis of the motion and evaporation of exhaled respiratory droplets of mixed composition. J. Aerosol Sci. 2011 doi: 10.1016/j.jaerosci.2010.10.005. [DOI] [Google Scholar]
- 47.Liu F., Qian H., Zheng X., Song J., Cao G., Liu Z. Evaporation and dispersion of exhaled droplets in stratified environment. IOP Conf. Ser. Mater. Sci. Eng. 2019;609 doi: 10.1088/1757-899x/609/4/042059. [DOI] [Google Scholar]
- 48.Morawska L. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air. 2006;16(5):335–347. doi: 10.1111/j.1600-0668.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- 49.Weber T.P., Stilianakis N.I. Inactivation of influenza A viruses in the environment and modes of transmission: a critical review. J. Infect. 2008;57(5):361–373. doi: 10.1016/j.jinf.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Metz J.A., Finn A. Influenza and humidity--Why a bit more damp may be good for you! J. Infect. 2015;71(Suppl 1):S54–S58. doi: 10.1016/j.jinf.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y., Wu S., Yang Y., Yang X., Song H., Cao Z., Huang Y. Evaporation and movement of fine droplets in non-uniform temperature and humidity field. Build. Environ. 2019;150:75–87. doi: 10.1016/j.buildenv.2019.01.003. [DOI] [Google Scholar]
- 52.Yan Y., Li X., Tu J. Thermal effect of human body on cough droplets evaporation and dispersion in an enclosed space. Build. Environ. 2019;148:96–106. doi: 10.1016/j.buildenv.2018.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang X., Ou C., Yang H., Liu L., Song T., Kang M., Lin H., Hang J. Transmission of pathogen-laden expiratory droplets in a coach bus. J. Hazard Mater. 2020;397 doi: 10.1016/j.jhazmat.2020.122609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang W., Marr L.C. Dynamics of airborne influenza A viruses indoors and dependence on humidity. PLoS One. 2011;6(6) doi: 10.1371/journal.pone.0021481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jahan F. Knowledge, attitude and practice towards droplet and airborne isolation precautions among pre-clinical medical students. J. Infect. Publ. Health. 2019;12(1) doi: 10.1016/j.jiph.2018.10.109. [DOI] [Google Scholar]
- 56.Koullapis P.G., Stylianou F.S., Sznitman J., Olsson B., Kassinos S.C. Towards whole-lung simulations of aerosol deposition: a model of the deep lung. J. Aerosol Sci. 2020;144 doi: 10.1016/j.jaerosci.2020.105541. [DOI] [Google Scholar]
- 57.Fröhlich-Nowoisky J., Kampf C.J., Weber B., Huffman J.A., Pöhlker C., Andreae M.O., Lang-Yona N., Burrows S.M., Gunthe S.S., Elbert W., Su H., Hoor P., Thines E., Hoffmann T., Després V.R., Pöschl U. Bioaerosols in the Earth system: climate, health, and ecosystem interactions. Atmos. Res. 2016;182:346–376. doi: 10.1016/j.atmosres.2016.07.018. [DOI] [Google Scholar]
- 58.Koshiyama K., Wada S. Mathematical model of a heterogeneous pulmonary acinus structure. Comput. Biol. Med. 2015;62:25–32. doi: 10.1016/j.compbiomed.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 59.Morrow P.E. Physics of airborne particles and their deposition in the lung. Ann. N. Y. Acad. Sci. 1980 doi: 10.1111/j.1749-6632.1980.tb18908.x. [DOI] [PubMed] [Google Scholar]
- 60.Knight V. Viruses as agents of airborne contagion. Ann. N. Y. Acad. Sci. 1980 doi: 10.1111/j.1749-6632.1980.tb18917.x. [DOI] [PubMed] [Google Scholar]
- 61.Yeh R.F.P.H.C., Raabe O.G. Environmental Health Perspectives; 1976. Factors Influencing the Deposition of Inhaled Particles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gralton J., Tovey E., McLaws M.-L., Rawlinson W.D. The role of particle size in aerosolised pathogen transmission: a review. J. Infect. 2011;62(1):1–13. doi: 10.1016/j.jinf.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Austin E., Brock J., Wissler E. A model for deposition of stable and unstable aerosols in the human respiratory tract. Am. Ind. Hyg. Assoc. J. 2010;40(12):1055–1066. doi: 10.1080/15298667991430703. [DOI] [PubMed] [Google Scholar]
- 64.Yung-Sung Cheng Y.Y., Yeh Hsu-Chi, Swift David L. Diffusional deposition of ultrafine aerosols in a human nasal cast. J. Aerosol Sci. 1988 doi: 10.1016/0021-8502(88)90009-2. [DOI] [Google Scholar]
- 65.Guo L., Johnson G.R., Hofmann W., Wang H., Morawska L. Deposition of ambient ultrafine particles in the respiratory tract of children: a novel experimental method and its application. J. Aerosol Sci. 2020;139 doi: 10.1016/j.jaerosci.2019.105465. [DOI] [Google Scholar]
- 66.Kim J.W., Xi J., Si X.A. Dynamic growth and deposition of hygroscopic aerosols in the nasal airway of a 5-year-old child. Int. J. Numer. Method Biomed. Eng. 2013;29(1):17–39. doi: 10.1002/cnm.2490. [DOI] [PubMed] [Google Scholar]
- 67.Pourhashem H., Owen M.P., Castro N.D., Rostami A.A. Eulerian modeling of aerosol transport and deposition in respiratory tract under thermodynamic equilibrium condition. J. Aerosol Sci. 2020;141 doi: 10.1016/j.jaerosci.2019.105501. [DOI] [Google Scholar]
- 68.Winkler-Heil R., Pichelstorfer L., Hofmann W. Aerosol dynamics model for the simulation of hygroscopic growth and deposition of inhaled NaCl particles in the human respiratory tract. J. Aerosol Sci. 2017;113:212–226. doi: 10.1016/j.jaerosci.2017.08.005. [DOI] [Google Scholar]
- 69.Xi J., Kim J., Si X.A., Zhou Y. Hygroscopic aerosol deposition in the human upper respiratory tract under various thermo-humidity conditions. J. Environ. Sci. Health, Part A. 2013;48(14):1790–1805. doi: 10.1080/10934529.2013.823333. [DOI] [PubMed] [Google Scholar]
- 70.Asgari M., Lucci F., Kuczaj A.K. Multispecies aerosol evolution and deposition in a bent pipe. J. Aerosol Sci. 2019;129:53–70. doi: 10.1016/j.jaerosci.2018.12.007. [DOI] [Google Scholar]
- 71.Xi J., Si X.A., Kim J.W. Characterizing respiratory airflow and aerosol condensational growth in children and adults using an imaging-CFD approach. Heat Transfer Fluid Flow Biol. Process. 2015:125–155. doi: 10.1016/B978-0-12-408077-5.00005-5. [DOI] [Google Scholar]
- 72.Summary description of respiratory tract dosimetry model. Ann. ICRP. 1994;24(1–3):106–120. doi: 10.1016/0146-6453(94)90038-8. [DOI] [Google Scholar]
- 73.van Doremalen N., Bushmaker T., Morris D., Holbrook M., Gamble A., Williamson B., Tamin A., Harcourt J., Thornburg N., Gerber S., Lloyd-Smith J., de Wit E., Munster V. 2020. Aerosol and Surface Stability of HCoV-19 (SARS-CoV-2) Compared to SARS-CoV-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Inthavong K., Ge Q.J., Li X.D., Tu J.Y. Detailed predictions of particle aspiration affected by respiratory inhalation and airflow. Atmos. Environ. 2012;62:107–117. doi: 10.1016/j.atmosenv.2012.07.071. [DOI] [Google Scholar]