Abstract
Background
Researchers are working at unprecedented speed to develop a SARS-CoV-2 vaccine. We aimed to assess the value of a hypothetical vaccine and its potential public health impact when prioritization is required due to supply constraints.
Methods
A Markov cohort model was used to estimate COVID-19 related direct medical costs and deaths in the United States (US), with and without implementation of a 60% efficacious vaccine. To prioritize the vaccine under constrained supply, the population was divided into tiers based on age; risk and age; and occupation and age; and outcomes were compared across one year under various supply assumptions. The incremental cost per quality-adjusted life-year (QALY) gained versus no vaccine was calculated for the entire adult population and for each tier in the three prioritization schemes.
Results
The incremental cost per QALY gained for the US adult population was $8,200 versus no vaccination. For the tiers at highest risk of complications from COVID-19, such as those ages 65 years and older, vaccination was cost-saving compared to no vaccination. The cost per QALY gained increased to over $94,000 for those with a low risk of hospitalization and death following infection. Results were most sensitive to infection incidence, vaccine price, the cost of treating COVID-19, and vaccine efficacy. Under the most optimistic supply scenario, the hypothetical vaccine may prevent 31% of expected deaths. As supply becomes more constrained, only 23% of deaths may be prevented. In lower supply scenarios, prioritization becomes more important to maximize the number of deaths prevented.
Conclusions
A COVID-19 vaccine is predicted to be good value for money (cost per QALY gained <$50,000). The speed at which an effective vaccine can be made available will determine how much morbidity and mortality may be prevented in the US.
Keywords: Coronavirus, COVID-19, Cost-effectiveness analysis, Economic analysis, SARS-CoV-2, Vaccine
1. Introduction
The novel coronavirus (SARS-CoV-2) was first identified in humans in late 2019. As of November 30, 2020 there were over 63 million cases of novel coronavirus disease 2019 (COVID-19) confirmed worldwide, with approximately 20% of these cases reported in the United States (US) [1]. The health and economic consequences of COVID-19 have been staggering. As of November 30, 2020, there were over 269,000 related deaths reported in the US [1], and a recent estimate has predicted that if 20% of the US population were to get infected, direct medical costs incurred, just during the course of the infection, could be as high as $163.4 billion [2]. This estimate does not include medical costs related to post-infection care or worsening of unrelated diseases due to postponement of preventive care and diagnosis, non-medical costs such as productivity losses due to absenteeism and premature mortality, or declines in economic activity.
In response to this global health emergency, researchers are working at unprecedented speed to find an effective vaccine and there are at least twenty potential candidates being tested in human clinical trials [3]. As of November 30, 2020, five of the candidates selected by the US government’s Operation Warp Speed are in Phase 3 trials, with two (Moderna’s mRNA-1273 and Pfizer/BioNTech’s BNT162) having applied for emergency use authorization. While clinical trials will determine whether the vaccine candidates are safe and efficacious against SARS-CoV-2 infection, important questions concerning the value of such vaccines remain: what role could a successful vaccine have in reducing the substantial burden of COVID-19 and, in a world where vaccine supply may initially be much lower than demand, which target groups should be prioritized for vaccination?
In the event of limited vaccine supply in the US, a strategy to target priority groups will likely be developed to guide the roll-out of vaccination programs. Groups may be prioritized based on a variety of criteria, including health benefit to the vaccinated individual and to others who may be protected indirectly, cost-effectiveness (i.e., where can we achieve the most health benefit per dose or per dollar spent?), and social and ethical considerations such as occupational priorities and attention to vulnerable populations [4], [5], [6].
To help address these important questions, we developed a mathematical model to assess the public health and economic impacts in the US of a hypothetical vaccine for SARS-CoV-2. Our focus was on estimating its potential value (based on cost-effectiveness) for vaccinated individuals, when distributed according to three different tier-based vaccination prioritization schemes. These include a simple age-based strategy, a risk-group-based strategy, and a strategy based on a combination of occupational groups and age. Outcomes predicted by our analysis, including estimates of clinical outcomes and vaccine cost-effectiveness by vaccination tier, should be helpful in guiding priority-setting decisions given constraints on vaccine availability as successful companies ramp up their production before and following regulatory approvals.
2. Methods
2.1. Study design
We used a Markov model with five health states (Fig. 1 ) to follow the US population for 1 year after vaccine supply is first available for use. The model compares various prioritization schemes for a hypothetical COVID-19 vaccine for adults aged 18 years and above to a no vaccine scenario.
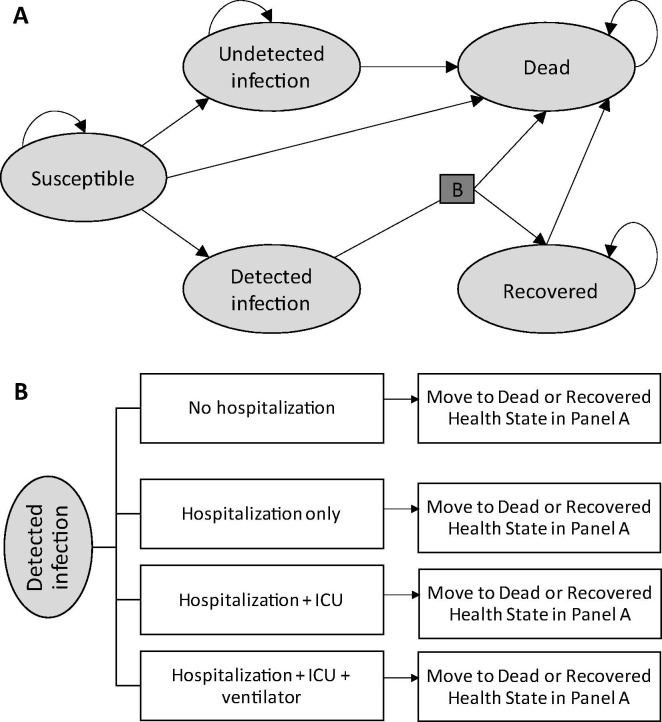
Fig. 1.
Structure of the model of SARS-CoV-2 infection and COVID-19 progression. (A) Markov health states showing allowed transitions. (B) Probability tree linking transitions from the “Detected Infection” state in the Markov model. Arrows represent the movements between the health states. Death from “Detected infection” is due to COVID-19 while death from all other health states is due to other causes. ICU, intensive care unit.
2.2. Model structure
Individuals enter the model (Fig. 1, panel A) as either susceptible to SARS-CoV-2 infection (“Susceptible”), previously infected with SARS-CoV-2 that was never detected (“Undetected Infection”), or recovered following a detected SARS-CoV-2 infection (“Recovered”). At the end of each weekly cycle, individuals can transition to other health states (e.g., become infected, recover, or die) or remain in their current health state, as indicated by the arrows in Fig. 1. Patients in the “Detected Infection” health state remain there for only one cycle, during which they enter a probability tree which allocates patients through various levels of COVID-19 treatment (with or without hospitalization, +/- intensive care unit (ICU), +/- mechanical ventilation) to their ultimate resolution (recovered or dead) (Fig. 1, panel B). Individuals in the “Undetected Infection” and “Recovered” states remain in these states until they die or the end of the time horizon, as we assume that no reinfection occurs in the 1-year period. Each week, individuals in the “Susceptible”, “Undetected Infection” or “Recovered” health states may also die from non-COVID-19 causes.
We assumed that individuals in the “Recovered” state are not eligible for vaccination because they have all been diagnosed with a SARS-CoV-2 infection. However, individuals in the “Susceptible” and “Undetected Infection” states can receive vaccine, even though the latter group is assumed to have developed natural immunity that persists until at least the end of the 1-year time horizon. Vaccine efficacy was modeled as reducing the probabilities of transitioning from the “Susceptible” state to the “Undetected Infection” or “Detected Infection” states.
2.3. Model parameters
Additional details on model parameters are found in the Appendix.
2.3.1. Population characteristics and vaccination prioritization schemes
Every adult (18 years and older) in the US is eligible to receive the vaccine, but a tier-based approach was used to create three prioritization schemes to allocate the supply of vaccine as it becomes available over the 1-year time horizon. Within a prioritization scheme, target groups were created based on characteristics such as age and risk of COVID-19 complications. Each target group is then assigned to a priority tier (tier 1 is the highest priority to receive vaccination). When individuals fall within more than one defined target group, they are assumed to be vaccinated according to their highest priority tier. For the age-based prioritization scheme, adults are divided into three tiers and vaccinated sequentially from oldest (tier 1) to youngest (tier 3) [5]. For the risk-based prioritization scheme, priority is given to high-risk groups defined by residency in nursing homes (without consideration of age), presence of medical conditions that increase the risk of COVID-19 complications, and older age. For the occupational prioritization scheme, those considered to have a priority occupation in the Centers for Disease Control and Prevention’s (CDC’s) 2018 influenza pandemic plan [4] are placed in tier 1. The remaining adult population is placed into tiers based on age. The number of eligible individuals in each tier for each prioritization scheme is shown in Table 2.
Table 2.
Base case cost-effectiveness analysis results for the various tiers in each of the prioritization schemes.
| Vaccination Tier |
||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Age-based prioritization scheme | ||||
| Description* | 65+ yrs | 50–64 yrs | 18–49 yrs | n/a |
| # eligible for vaccination | 56,051,566 | 63,292,950 | 139,327,967 | – |
| Basecase ICER** | Vaccination Dominates† | $8,000 | $94,000 | n/a |
| Risk-based prioritization scheme | ||||
| Description* |
|
|
|
n/a |
| # eligible ICER for vaccination | 56,282,700 | 92,599,345 | 109,790,438 | – |
| Basecase** | Vaccination Dominates† | $10,000 | $340,000 | n/a |
| Occupational-based prioritization scheme | ||||
| Description* | Priority‡and other critical occupations§ | 65+ yrs | 50–64 yrs | 18–49 yrs |
| # eligible for vaccination | 21,700,000 | 54,706,166 | 57,390,550 | 124,875,767 |
| BasecaseICER** | $20,000 | Vaccination Dominates† | $8,000 | $94,000 |
ICER, incremental cost-effectiveness ratio; n/a, not applicable; QALY, quality-adjusted life-year; yrs, years.
For each prioritization scheme, individuals are assigned to one tier only; those qualifying for more than one tier are assigned to their highest priority tier. While those aged < 18 years are included in the model, they are not targeted for vaccination as current clinical trials target only those 18+ years.
Vaccination dominates: vaccination is less costly and more effective than no vaccination.
Includes: public health personnel; inpatient health care providers; outpatient and home health providers; health care providers in long-term care facilities; pharmacists and pharmacy technicians; community support and emergency management; and mortuary services personnel [4].
Includes: other health care personnel; emergency services and public safety sector personnel; manufacturers of pandemic vaccine and antiviral drugs; communications/information technology, electricity, nuclear, oil and gas, water sector personnel, and financial clearing and settlement personnel; critical government personnel; and other critical government personnel.
ICER= incremental cost per QALY gained. Base case vaccine price is $35 per dose ($70 per course).
Within each prioritization scheme, tier groups were assumed to be vaccinated, starting with tier 1, on a weekly and uniform basis according to the weekly vaccine supply. Once the population in each tier has been vaccinated, individuals in the next tier become eligible for vaccination. Given that most of the vaccines expected to be first-to-market will involve a two-dose schedule [7], a two-dose vaccine with the second dose given at least four weeks after administration of the first dose was assumed. In all scenarios, second doses are prioritized ahead of vaccinating new individuals in a lower priority tier. This step-wise vaccination process continues until all tiers are vaccinated according to their predicted coverage rate or until the end of the analysis time horizon, whichever occurs first. Coverage rates by age, based on 2018–19 general population influenza coverage data [8], were applied to the first dose, and the ratio of second-dose coverage to first-dose coverage was based on data for childhood vaccinations [9] (Table 1 ).
Table 1.
Model parameters.
| Parameter | Base case value | Source |
|---|---|---|
| Vaccine coverage rates | ||
| First dose | ||
| 18 to 49 years | 34.9% | [8] |
| 50 to 64 years | 47.3% | [8] |
| 65+ years | 68.1% | [8] |
| Second dose (all ages) | 87.5% of proportion receiving first dose | [9] |
| Population distribution at baseline | ||
| Susceptible | 92.7% | |
| Undetected Infection | 5.2% | Estimated from IHME data [18] |
| Recovered | 2.1% | Estimated from IHME data [18] |
| SARS-CoV-2 incidence | ||
| Detected infection | Appendix Table A3 | Described in Appendix |
| Undetected infection | 1.05 times detected infection rates | Described in Appendix |
| Decision tree transition probabilities | Appendix Table A1 | Described in Appendix |
| Non-COVID-19 mortality rates | Appendix Table A1 | [19] |
| Vaccine efficacy (against detected and undetected SARS-CoV-2 infection) | ||
| First dose, age 18–49 years | 24.0% | Assumption |
| First dose, age 50–59 years | 19.5% | Assumption |
| First dose, age 60 + years | 15.0% | Assumption |
| Second dose, all ages | 60.0% | Assumption |
| Costs | ||
| Vaccine (per dose) | $35.00 | Assumption |
| Vaccine administration (per dose) | $14.44 | Code CPT90471 [26] |
| COVID-19 treatment: ambulatory care only (per event) | $228.98 | Physician visit ($112) + ED visit ($582 × 20.1% with visit*) [27] |
| COVID-19 treatment: hospitalization without ICU or ventilator (per event) | $16,924.00 | Physician visit ($112) + hospitalization ($16,812) [27] |
| COVID-19 treatment: hospitalization with ICU as highest level of care (per event) | $37,429.00 | Physician visit ($112) + midpoint of hospitalization and hospitalization with ventilator ($37,317) [27] |
| COVID-19 treatment: hospitalization with ICU + ventilator as highest level of care (per event) | $57,934.00 | Physician visit ($112) + hospitalization with ventilator ($57,822) [27] |
| Health state utility parameters | ||
| Detected infection symptoms disutility weight | 0.19 | Described in Appendix |
| Detected infection hospitalization as highest setting disutility weight | 0.30 | Described in Appendix |
| Detected infection hospitalization with ICU as highest setting disutility weight | 0.50 | Described in Appendix |
| Detected infection hospitalization with ICU + ventilator as highest setting disutility weight | 0.60 | Described in Appendix |
| Event durations | ||
| COVID-19 symptoms among all confirmed infections | 14 days | Described in Appendix |
| Hospitalization among detected infections not requiring ICU or ventilator | 6 days | Described in Appendix |
| Hospitalization among detected infections with ICU as highest level of care | 15 days | Described in Appendix |
| Hospitalization among detected infections with ventilator as highest level of care | 15 days | Described in Appendix |
ED, emergency department; ICU, intensive care unit; IHME, Institute for Health Metrics and Evaluation.
Proportion of patients who have an ED visit is assumed to be equal to 20.1% which is the average rate of hospitalization observed in our model, consistent with the approach utilized by Fiedler and Song, 2020.[27]
2.3.2. Vaccine supply
A hypothetical vaccine supply over time was estimated based on the scale and timing of four manufacturers’ public disclosures [10], [11], [12], [13]. Funding from programs such as Operation Warp Speed has allowed these companies to begin manufacturing doses at industrial scale before Phase 3 trials are complete, so that there is a stockpile of doses available when the vaccine receives regulatory approval. Exponential regression models were fit to each manufacturer’s disclosed estimates of US cumulative stock availability starting at launch to estimate weekly supply forecasts in the first year. For the “high supply” scenario, four vaccines are successfully launched and supply is fulfilled as estimated in these disclosures. For a “medium supply” scenario, manufacturers’ estimated final supply is delayed by one quarter, while for the “low supply” scenario, final supply is delayed by two quarters. The resultant curves, shown in the Appendix (Fig. A1), predict that a varying number of doses will be available at launch within the US, with a total of 529.2, 413.3, and 413.3 million doses available respectively after one year (i.e., sufficient to vaccinate 264.6, 206.7, and 206.7 million persons). In addition, a hypothetical “immediate supply” scenario wherein sufficient doses are available to vaccinate all individuals in the first week following launch was created in order to compare strategies without regard for delayed access.
2.3.3. Transition probabilities
COVID-19-related transition probabilities were estimated using a two-stage calibration process. First, the age-specific risks of COVID-19-related hospitalization, admission to ICU with or without ventilation, and death from any location for individuals in a “Detected Infection” state were estimated separately for those with serious medical conditions versus those without, using data from the CDC case surveillance from January to May 2020 [14], the COVID-NET hospital surveillance system [15], and US studies of the outcomes of hospitalized COVID-19 patients [16], [17]. Second, the SARS-CoV-2 attack rates were estimated by calibrating to mortality targets generated using forecasts from the Institute for Health Metrics and Evaluation (IHME) model available on July 22, 2020 [18]. The base case scenario was based on the IHME’s reference scenario, while their best case (95% mask usage in public locations) and worst case (mandates easing) were used in sensitivity analyses. By November 2020, the incidence rates had increased beyond IHME’s worst case scenario so an additional sensitivity analysis where base case incidence rates where doubled has been added. Based on the IHME’s estimates of total and detected infections, the attack rate for undetected infections was assumed to be 1.05 times that for detected infections (i.e., 2.05 true infections per detected infection). The IHME’s projections were also used to estimate the proportion of people in the “Recovered” and “Undetected Infection” health states at the start of the model (Table 1). Finally, all-cause mortality from the “Susceptible”, “Recovered” or “Undetected Infection” states was applied [19].
2.3.4. Vaccine efficacy
Vaccine efficacy was modeled as the proportional reduction in the probability of SARS-CoV-2 infection (both detected and undetected). The World Health Organization (WHO) and the Food and Drug Administration (FDA) require a minimum efficacy of 50% for a COVID-19 vaccine, but the WHO prefers an efficacy of 70% [20], [21]. Therefore, two-dose efficacy was assumed to be 60% in the base case and varied between 50% and 70% in sensitivity analyses. A third sensitivity analysis of 90% efficacy was also conducted to reflect public disclosures of preliminary results from Phase 3 trials [22], [23]. Single-dose efficacy was assumed to be 40% and 25% of two-dose efficacy for those aged 18–54 years and 55 years and above, respectively, based on preliminary immunogenicity data [24] (Table 1). It was assumed that vaccine efficacy does not wane during the 1-year time horizon of the analysis.
2.3.5. Resource use, costs and health state utilities
A US health care system perspective was used and all costs are reported in 2020 US dollars [USD]. The base case cost of the hypothetical vaccine was assumed to be $35 per dose ($70 per course) [25], while the cost per administration was $14.44 [26]. A single cost was applied to each patient with a new detected infection, dependent on their highest level of care, based on the potential estimated Medicare costs as shown in Table 1 [27]. Disutility tolls (i.e., reductions in quality-of-life calculated using disutility weights and health event durations), reflecting impaired health-related quality-of-life during infection, were applied for those experiencing morbidity due to COVID-19 (Table 1). The expected quality-adjusted life-years (QALYs) lost due to death attributable to COVID-19 include both years of life lost and disutility owing to morbidity over a lifetime horizon (see the Technical Appendix for details) and were discounted at an annual rate of 3%. In assigning QALY-tolls for early death, we used average age-specific expected utility values and did not adjust the values for those with conditions and those without. Those with chronic conditions may gain fewer lifetime QALYs, but given the range of conditions that increase risk of COVID-19 complications, it was not possible to estimate what the difference may be. Therefore, we increased and decreased baseline age-specific utility values that affect this calculation by 10% overall to determine the potential effect of over- or under-estimating the gains in quality-adjusted life expectancy in various sub-populations.
2.4. Analyses
For estimation of the base case cost-effectiveness results, the “immediate supply” vaccination scenario was modeled to allow for a full year of benefits to be captured for all vaccinated individuals, regardless of when, in reality, they would have received their vaccination throughout the year. Given the uncertainty in estimating the model, a series of deterministic sensitivity analyses were conducted to explore the impact of alternative inputs and assumptions, including alternative sets of attack rates consistent with available data, and treatment costs for commercial payers versus Medicare (see Appendix). A second set of analyses was conducted to compare the deaths, hospitalizations, infections and costs across one year in the US population under the age-based, risk-based, and occupational prioritization schemes compared to no prioritization under the various vaccine-supply conditions.
3. Results
In the base case analysis, the incremental cost per QALY gained associated with vaccinating the US adult population is $8,200. The results of the cost-effectiveness analyses for each of the individual tiers in the three prioritization schemes are shown in Table 2. For the age-based and risk-based prioritization schemes, vaccination is less costly and more effective than no vaccination for the highest risk individuals in Tier 1. For both schemes, the incremental cost per QALY gained increases for Tiers that included individuals with lower risk of hospitalization and death due to COVID-19. For the occupation-based scheme, the incremental cost per QALY gained for Tier 1 (priority and critical occupations) is $20,000, while vaccination is cost-saving for Tier 2 (age 65 years and above). Cost savings are not seen in Tier 1 because the overall risks of hospitalization and deaths are lower in the younger age groups that comprise these occupations. In addition, the attack rate for the priority occupations (i.e., health care workers) was assumed to be the same as the general population. In the base case, the incremental cost per QALY gained when vaccinating individuals ages 18 to 49 years is high ($94,000), and even higher when considering only those in this age group who have no comorbid illness that increases risk of hospitalization and death ($340,000).
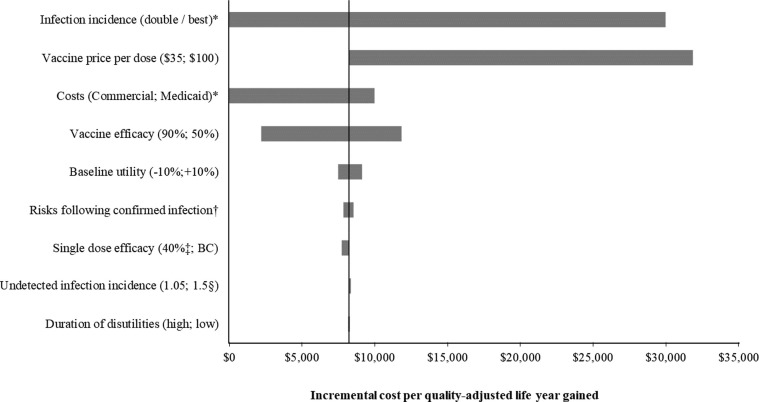
The incremental cost per QALY gained is most sensitive to changes in the attack rate, the vaccine price, and the costs of hospitalizations, but changes in the amount of disutility experienced by patients due to the morbidity associated with COVID-19 has minimal effect (Fig. 2 ). Varying the expected age-specific baseline utility has more impact on this outcome as this affects the QALY loss assigned to deaths from COVID-19. Under base case assumptions, a vaccine targeted at the entire population would have to be priced at over $150 per dose ($300 per course) in order to exceed an incremental cost per QALY of over $50,000 (Appendix, Table A7). The detailed results of the sensitivity analyses conducted for the schemes, stratified by tiers, are shown in the Appendix (Table A6). The incremental cost per QALY for the lowest risk individuals decreases as incidence of disease or vaccine efficacy decreases. For example, it is $25,000 for those aged 18 to 49 years and $110,000 for those aged 18 to 49 years with no comorbid conditions if the incidence pattern shifts and a higher number of deaths are seen in those under 50 years.
Fig. 2.
Tornado diagram showing the impact of the sensitivity analyses on the incremental cost per quality-adjusted life-year gained of vaccination compared to no vaccination (target population: all adults). BC, base case. *Vaccination dominates no vaccination (it is less costly and more effective) when the base case incidence of infection is doubled or the estimates of commercial costs are used as inputs. †Alternative values were used for the calibrated probabilities of hospitalization and death following detected infection as described in the Appendix. ‡For the base case, single dose efficacy was assumed to be 40% and 25% of full efficacy for those under 55 years and those 55+ years, respectively. This was increased to 40% of full efficacy for everyone in the sensitivity analysis. §Undetected infection incidence was assumed to be 1.05 times the incidence of detected infection in the base case. This was increased to 1.5 for the sensitivity analysis.
The numbers of infections, hospitalizations and deaths as well as costs, for scenarios without the vaccine and with a vaccine, and under different vaccine supply and prioritization schemes, are shown in Table 3 . With no vaccine, 264,600 deaths are expected in one year in the base case analysis. The number of deaths prevented ranges from 53,900 to 60,300 with the low vaccine supply scenario, 66,400 to 70,500 with the medium supply scenario, and 82,500 to 83,200 with the high supply scenario, depending on the prioritization schemes. The difference between the prioritization schemes narrows as the vaccine supply increases. While a scenario where there is no prioritization of the vaccine is not likely, it is included as a hypothetical anchor in the results table. For the low vaccine supply, the number of deaths prevented with no prioritization is 42,800, which increases to 60,300 deaths prevented under the age-based and risk-based prioritization schemes. For high supply, the difference narrows so that 77,000 deaths are prevented with no prioritization while up to 83,200 are prevented with the high-risk prioritization scheme. In all vaccine supply scenarios, the occupational prioritization scheme is expected to prevent fewer COVID-19-related outcomes than the age-based or risk-based scheme because of the younger ages reflected in the Tier 1 critical occupations target group in the model. The hypothetical “immediate” supply scenario, where there are no constraints in vaccine delivery, is included in Table 3 to serve an anchor point. It illustrates the maximum expected effect of a vaccine under base case efficacy and coverage assumptions in a perfect world without logistical and supply constraints.
Table 3.
Base case population-level outcomes under various vaccine supply scenarios.
| Vaccine supply scenario | Vaccination strategy | Deaths |
Hospitalizations |
Detected infections |
Costs (millions ) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Annual number | Difference from no vaccine | Annual number | Difference from no vaccine | Annual number | Difference from no vaccine | Hospitalizations | Vaccination | Total | ||
| No vaccine | n/a | 264,602 | 726,115 | 3,601,719 | $20,628 | $0 | $20,628 | |||
| Low | No priority | 221,785 | −16% | 621,556 | −14% | 3,171,221 | −12% | $17,653 | $10,823 | $28,476 |
| Low | Occupational/age-based | 210,668 | −20% | 604,383 | −17% | 3,156,372 | −12% | $17,161 | $10,823 | $27,984 |
| Low | Age-based | 204,253 | –23% | 595,040 | −18% | 3,149,627 | −13% | $16,895 | $10,823 | $27,718 |
| Low | Risk-group-based | 204,298 | –23% | 594,838 | −18% | 3,153,147 | −12% | $16,895 | $10,823 | $27,718 |
| Medium | No priority | 207,305 | –22% | 586,539 | −19% | 3,028,438 | −16% | $16,657 | $10,823 | $27,480 |
| Medium | Occupational/age-based | 198,237 | −25% | 572,768 | −21% | 3,017,299 | −16% | $16,263 | $10,823 | $27,086 |
| Medium | Age-based | 194,092 | −27% | 566,570 | –22% | 3,011,973 | −16% | $16,085 | $10,823 | $26,908 |
| Medium | Risk-group-based | 194,131 | −27% | 566,463 | –22% | 3,014,785 | −16% | $16,087 | $10,823 | $26,910 |
| High | No priority | 187,591 | −29% | 539,108 | −26% | 2,835,583 | −21% | $15,307 | $10,823 | $26,130 |
| High | Occupational/age-based | 182,097 | −31% | 529,569 | −27% | 2,821,455 | –22% | $15,030 | $10,823 | $25,854 |
| High | Age-based | 181,526 | −31% | 528,585 | −27% | 2,819,933 | –22% | $15,002 | $10,823 | $25,825 |
| High | Risk-group-based | 181,412 | −31% | 527,716 | −27% | 2,821,040 | –22% | $14,983 | $10,823 | $25,806 |
| Immediate | No priority | 179,775 | –32% | 520,452 | −28% | 2,760,399 | –23% | $14,776 | $10,823 | $25,599 |
| Immediate | Occupational/age-based | 179,775 | –32% | 520,452 | −28% | 2,760,399 | –23% | $14,776 | $10,823 | $25,599 |
| Immediate | Age-based | 179,775 | –32% | 520,452 | −28% | 2,760,399 | –23% | $14,776 | $10,823 | $25,599 |
| Immediate | Risk-group-based | 179,775 | –32% | 520,452 | −28% | 2,760,399 | –23% | $14,776 | $10,823 | $25,599 |
n/a, not applicable.
4. Discussion
Our analysis, based on available data, suggests that a COVID-19 vaccine that meets the target product profile of the WHO and the FDA has the potential to be good value for money. This conclusion holds even though the model considers only the benefits to vaccinated individuals (and not secondary benefits due to reduced transmission) and direct health care system costs (and not the value of economic productivity). Vaccination of persons over age 65 appears to be cost-saving because of the high cost and higher incidence of ICU care and ventilation. Except in the lowest-priority tier of each strategy, the incremental cost-effectiveness ratios (ICERs) are well under standard willingness-to-pay thresholds cited in the US, which range from $50,000 to $150,000 per QALY gained [28]. The results are consistent with an analysis that found vaccination ($100 per course; 90% efficacy) to be cost-saving overall considering societal costs [29].
One of the largest influencers of value is the attack rate for the year following the launch of the vaccine. Current models predict mortality only several months into the future, as changes in policy and individual behaviour may affect the course of the disease such that longer-term estimates are highly uncertain. In all three incidence scenarios considered in this analysis, the overall ICER for the hypothetical vaccine falls below $50,000 per QALY.
The ICERs for the different tiers in the prioritization schemes are primarily driven by the risk of hospitalization (which increases costs) and the risk of death (which leads to substantial loss of QALYs due to early death). For this reason, the value associated with the vaccination of priority occupations is lower than other tiers in our analysis. The WHO [5] and the CDC [6] are discussing ethical principles that will not be reflected in the ICER but should be considered when allocating vaccines during a pandemic, including the idea that those putting themselves at risk to serve others during the pandemic may be considered as high priority. Furthermore, our model does not estimate the impact of vaccination on the transmission of infection between individuals. As individuals in priority occupations are frequently in contact with COVID-19-infected persons and others at work, they may be at increased risk of infection or of spreading the disease if infected. Therefore, the value associated with vaccinating these individuals may be underestimated.
Our analysis predicts that the value associated with vaccinating individuals in the lower risk groups, primarily those under 50 years of age, is much lower than the value of vaccinating the older age groups. If a greater portion of the deaths occur in this age group due to shifting incidence patterns, then the cost per QALY of vaccinating these age groups will be lower. Furthermore, while these younger age groups are at low risk of developing more severe complications from COVID-19, they have been shown to be responsible for spreading the disease within the community [30]. The value of vaccinating these individuals is likely underestimated if vaccination prevents transmission; an analysis with a transmission model that includes the impact of herd immunity is required to understand the value of vaccinating younger persons to prevent community spread. In addition, this age group may be most impacted by many of the societal costs associated with this pandemic, which were not included in the analysis.
As data on COVID-19 are still emerging, assumptions were made to combine the sources of evolving data to create this model. The data on the risk of hospitalization and mortality used in this model were based on early experience with the pandemic. As many of the cases reported to the CDC were missing data on death and hospitalization status, Stokes [14] suggests there may be under-reporting of symptoms. On the other hand, as testing capabilities increase and the proportion of detected asymptomatic cases increases, the proportion of severe disease in detected cases will correspondingly decrease. We attempted to control for this by calibrating to predictions of mortality rather than to predictions of the number of detected infections when estimating the future SARS-CoV-2 attack rate. Furthermore, as the pandemic progresses, emerging therapeutics may decrease the mortality and morbidity of COVID-19. As the use and effectiveness of future therapies is uncertain, we conducted sensitivity analyses on incidence, mortality and cost of hospitalization rather than explicitly incorporating these therapies into the model.
Hospitalization for COVID-19 is another important driver of the value of the vaccine as the cost of ICU care and ventilation is expected to be high. These estimates were based on the current billing rules for COVID-19 patients [27], but the true cost of COVID-19 treatment will not be known until empirical health economic studies are conducted. We did not include the cost associated with diagnostic testing, as we assumed that testing behaviours will not change with vaccination but will continue until it is clear that the epidemic is controlled. When estimating the amount of QALYs lost due to death from COVID-19, we assigned a toll-based on average expected QALYs and did not adjust for presence or absence of comorbid conditions. To be conservative, we did not include the health system costs that may be unrelated to SARS-CoV-2 itself, such as mental illness, or of conditions that are exacerbated because care is delayed due to the pandemic. Our analysis does not consider the broader societal costs such as the productivity costs and patient out-of-pocket costs associated with the pandemic. Nor does it include the less tangible costs such as the value of reducing fear of contagion, the value of protecting against future productivity loss due to illness (insurance value), and improving equity, all of which have been proposed as part of future frameworks for cost-effectiveness analyses [31].
We have created hypothetical vaccine supply scenarios by assuming that vaccine will not get to market as quickly as currently predicted by manufacturers. Other challenges, including failure of products during clinical trials, may arise to reduce or delay the vaccine supply. During the H1N1 pandemic, supply was not sufficient to meet the demand from the identified priority groups [32]. Decisions were then made at the local level to prioritize vaccines further and the range of recommendations led to public confusion as to who was eligible for vaccination. In any prioritization system, there may be challenges in identifying and targeting high priority individuals; we have not considered those costs and challenges in our analysis and made simplifying assumptions to model allocation of vaccination by tiers.
Given the lack of data, we did not consider the long-term sequalae that may occur following COVID-19 [33], [34], [35]. For an infectious disease, it is typical that symptoms for acute infections have a small impact on cost-effectiveness because of their short durations, while any long-term consequences have a more significant impact. Finally, we examined the impact of vaccination only in a 1-year time horizon. If the vaccine provides protection for a longer time frame, its benefits will increase.
Despite the uncertainties, our analyses demonstrate that a hypothetical COVID-19 vaccine would be a cost-effective health care intervention compared to no vaccine. Under the base case conditions, the vaccine would have to be priced as high as $150 per dose to exceed an ICER of $50,000 per QALY gained when targeted to the entire adult population.
Contributors
MK, MM, and MCW designed the study. MM programmed the model with quality assurance and validation conducted by MK, DB, and MCW. Data collection and parameterization were completed by MK, MM, and DB. MM and MK conducted the analysis. DB and MK wrote the initial draft of the manuscript, and all remaining co-authors critically revised the manuscript and approved the final version.
Declaration of Competing Interest
MK and DB are shareholders in Quadrant Health Economics Inc. Quadrant Health Economics Inc. was contracted by Moderna, Inc. to conduct this study. MM and MCW are consultants at Quadrant Health Economics Inc.
Acknowledgments
Acknowledgements
This research was funded by Moderna, Inc. The authors would like to thank Amy Lee, PhD and Shannon Cartier, MSc, both from Quadrant Health Economics Inc., for their assistance with data collection and model programming quality assurance, respectively. The authors would also like to acknowledge Zach Piccioli from Moderna, Inc. who conceived of the study and provided references to inform several model inputs. IHME COVID-19 projections were use to estimate mortality over a 1-year period (Data used with permission. All rights reserved.)
Role of the sponsor
The study sponsor conceived of the study, provided references for data inputs and commented on the report. The funder was not involved in study design, data analysis, or data interpretation. The corresponding author had full access to all the data used in the study and had final responsibility for the decision to submit for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2020.12.078.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Johns Hopkins Coronavirus Resource Center. COVID-19 Map. Coronavirus COVID-19 global cases by the center for systems science and engineering; 2020. https://coronavirus.jhu.edu/ [accessed November 30, 2020].
- 2.Bartsch S.M., Ferguson M.C., McKinnell J.A., et al. The potential health care costs and resource use associated with COVID-19 in the United States. Health Affairs (Project Hope) 2020;39(6):927–935. doi: 10.1377/hlthaff.2020.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.J. Corum, D. Grady, S. Wee, C. Zimmer. The New York Times: coronavirus vaccine tracker. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html [accessed August 10, 2020].
- 4.Centers for Disease Control and Prevention. Interim updated planning guidance on allocating and targeting pandemic influenza vaccine during an influenza pandemic; 2018. https://www.cdc.gov/flu/pandemic-resources/pdf/2018-Influenza-Guidance.pdf [accessed June 25, 2020].
- 5.World Health Organization (WHO). Ethics and COVID-19: resource allocation and priority-setting (WHO/RFH/20.2). https://www.who.int/ethics/publications/ethics-and-covid-19-resource-allocation-and-priority-setting/en/ [accessed July 9, 2020].
- 6.S. Mbaeyi S. Considerations for COVID-19 vaccine prioritization. Presentation of the ACIP COVID-19 vaccines work group to the advisory committee on immunisation practices. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-06/COVID-08-Mbaey-508.pdf [accessed June 24, 2020].
- 7.Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382(21):1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2018–19 influenza season (FluVaxView, general population vaccination coverage). https://www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm [accessed July 11, 2020].
- 9.Hill H.A., Singleton J.A., Yankey D., Elam-Evans L.D., Pingali S.C., Kang Y. Vaccination coverage by age 24 months among children born in 2015 and 2016 – national immunization survey-child, United States, 2016–2018. Morbidity Mortal Weekly Rep (MMWR) 2019;68(41):913–918. doi: 10.15585/mmwr.mm6841e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfizer. Pfizer and BioNTech announce an agreement with the US government for up to 600 million doses of mRNA-based vaccine candiate against SARS-CoV-2. Press release July 22, 2020. https://investors.pfizer.com/investor-news/press-release-details/2020/Pfizer-and-BioNTech-Announce-an-Agreement-with-U.S.-Government-for-up-to-600-Million-Doses-of-mRNA-based-Vaccine-Candidate-Against-SARS-CoV-2/default.aspx [accessed July 24, 2020].
- 11.Moderna. Moderna and Catalent announce collaboration for fill-finish manufacturing of Moderna's COVID-19 vaccine candidate. Press release June 25, 2020. https://investors.modernatx.com/news-releases/news-release-details/moderna-and-catalent-announce-collaboration-fill-finish [accessed July 24, 2020].
- 12.US Department of Health and Human Services. Trump administration's operation warp speed accelerates AstraZeneca COVID-19 vaccine to be available beginning in October. Press release May 21, 2020. https://www.hhs.gov/about/news/2020/05/21/trump-administration-accelerates-astrazeneca-covid-19-vaccine-to-be-available-beginning-in-october.html [accessed July 24, 2020].
- 13.US Department of Health and Human Services. HHS, DOD Collaborate with Novavax to Produce Millions of COVD-19 Investigational Vaccine Doses in Commercial-Scale Manufacturing Demonstration Projects; July 7, 2020. https://www.hhs.gov/about/news/2020/07/07/hhs-dod-collaborate-novavax-produce-millions-covid-19-investigational-vaccine-doses-commercial-scale-manufacturing-demonstration-projects.html [accessed July 24, 2020].
- 14.Stokes E.K., Zambrano L.D., Anderson K.N., et al. Coronavirus disease 2019 case surveillance — United States, January 22–May 30, 2020. Morbidity Mortal Weekly Rep (MMWR) 2020;69:759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC) COVID-NET. Laboratory-confirmed COVID-19-associated Hospitalizations. Preliminary cumulative rates as of June 6, 2020. https://gis.cdc.gov/grasp/COVIDNet/COVID19_3.html [accessed June 12, 2020].
- 16.C.M. Petrilli, S.A. Jones, J. Yang, et al. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. medRxiv; 2020. http://medrxiv.org/content/early/2020/04/11/2020.04.08.20057794.abstract [accessed July 22, 2020].
- 17.Auld S.C., Caridi-Scheible M., Blum J.M., et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020 doi: 10.1097/CCM.0000000000004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Institute for Health Metrics Evaluation (IMHE). COVID-19 projections. United States of America. Used with permission. All rights reserved. https://covid19.healthdata.org/united-states-of-america [accessed July 22, 2020].
- 19.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics. National vital statistics system – life expectancy; 2017. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/Publications/NVSR/68_07/ [accessed June 24, 2020].
- 20.World Health Organization (WHO). WHO target product profiles for COVID-19 vaccines. https://www.who.int/publications/m/item/who-target-product-profiles-for-covid-19-vaccines [accessed April 29, 2020].
- 21.US Department of Health and Human Services. Food and Drug Administration. Center for Biologics Evaluation and Research. Development and Licensure of Vaccines to Prevent COVID-19. Guidance for Industry; June 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-action-help-facilitate-timely-development-safe-effective-covid [accessed July 2, 2020].
- 22.Moderna. Moderna announce primary efficacy analysis in Phase 3 COVE study for its COVID-19 vaccine candidate and filing today with U.S. FDA for Emergency Use Authorization; November 30, 2020. https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-primary-efficacy-analysis-phase-3-cove-study [accessed November 30, 2020].
- 23.Pfizer. Pfizer and BioNTech conclude Phase 3 study of COVID-19 vaccine candidate, meeting all primary efficacy endpoints; November 18, 2020. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine [accessed November 30, 2020].
- 24.Jackson L.A., Anderson E.J., Rouphael N.G., et al. An mRNA vaccine against SARS-CoV-2 – preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2022483. https://www.ncbi.nlm.nih.gov/pubmed/32663912 [accessed July 14, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moderna. Business updates and second quarter 2020 financial results; August 5, 2020. https://investors.modernatx.com/ [accessed August 5, 2020].
- 26.Centers for Medicare and Medicaid Services. Physician fee schedule; 2020. https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx [accessed July 22, 2020].
- 27.M. Fiedler, Z. Song. Brookings report: estimating potential spending on COVID-19 care; 2020. https://www.brookings.edu/research/estimating-potential-spending-on-covid-19-care/ [accessed July 22, 2020].
- 28.Neumann P.J., Cohen J.T., Weinstein M.C. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 29.W.V. Padula, S.M. Malaviya, N.M. Reid, J. Tierce, G.C Alexander. U.S. cost-effectiveness and budget impact analysis Preprints with THE LANCET; 2020; https://ssrn.com/abstract=3586694.
- 30.Furuse Y., Sando E., Tsuchiya N., et al. Clusters of coronavirus disease in communities, Japan, January-April 2020. Emerg Infect Dis. 2020 doi: 10.3201/eid2609.202272. https://www.ncbi.nlm.nih.gov/pubmed/32521222 [accessed June 10, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakdawalla D.N., Doshi J.A., Garrison L.P., Jr., Phelps C.E., Basu A., Danzon P.M. Defining elements of value in health care-A health economics approach: an ISPOR special task force report [3] Value Health. 2018;21(2):131–139. doi: 10.1016/j.jval.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Rambhia K.J., Watson M., Sell T.K., Waldhorn R., Toner E. Mass vaccination for the 2009 H1N1 pandemic: approaches, challenges, and recommendations. Biosecur Bioterror. 2010;8(4):321–330. doi: 10.1089/bsp.2010.0043. [DOI] [PubMed] [Google Scholar]
- 33.V.O. Puntmann, M.L. Carerj, I. Wieters, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol; 2020. https://www.ncbi.nlm.nih.gov/pubmed/32730619 [accessed July 27, 2020]. [DOI] [PMC free article] [PubMed]
- 34.Wang Y., Dong C., Hu Y., et al. Temporal changes of CT findings in 90 Patients with COVID-19 Pneumonia: a longitudinal study. Radiology. 2020;296(2):E55–E64. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.A. Varatharaj, N. Thomas, M.A. Ellul, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry; 2020. https://www.ncbi.nlm.nih.gov/pubmed/32593341 [accessed June 25, 2020]. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.