Abstract
Background/purpose
The aim of this study was to systematically review all COVID-19 publications to summarize the clinical features, assess comorbidities, prevalence, and disease outcomes.
Methods
Included were all COVID-19 published studies between January 1 to July 20, 2020. The random effect model was used to calculate the pooled prevalence and corresponding 95% confidence interval (CI). Publication bias was assessed using the funnel plot for the standard error by logit event.
Results
The mean age of the patients was 46.8 years (95% CI, 41.0–52.6) and males comprised 54.0% (95% CI, 51.3–56.7). Total co-morbidities prevalence was 29.5% (95% CI, 19.0–36.6), with diabetes mellitus being the most prevalent 13.8% (95% CI, 8.7–21.1), followed by hypertension 11.7% (95% CI, 5.7–22.6), and cardiovascular disease 9.7% (95% CI, 6.5–14.2). The most common clinical manifestations were fever, 82.0% (95% CI, 67.7–90.8), cough 54.3% (95% CI, 45.5–62.9), fatigue 30.2% (95% CI, 23.3–38.1), sputum 28.5% (95% CI, 21.2–37.2), sore throat 21.7% (95% CI, 14.6–31.0), and headache 11.0% (95% CI, 7.9–15.2). The most common COVID-19 serious complications were RNA Anemia 98.2% (95% CI, 96.2–99.2), hospitalization 83.7% (95% CI, 76.0–89.3), bilateral pneumonia 70.9% (95% CI, 58.2–81.0); of those hospitalized 43.5% (95% CI, 24.9–64.2) were discharged. Fatality accounted for 10.5% (95% CI 6.8–16.1).
Conclusion
Patients infected with COVID-19 coronavirus showed a wide range of clinical presentation with non-specific symptoms.
Keywords: COVID-19, SARS-CoV-2, Clinical features, Epidemic, Meta-analysis
Introduction
The common human coronaviruses are usually harmless viruses causing mild illnesses, such as the common cold.1 However, certain types of coronaviruses infect the lower respiratory airways, and are causing pneumonia and bronchitis.2 Coronaviruses structure, as seen under an electron microscope, have large single-stranded RNA genomes at its center, surrounded by a spherical fatty outer layer, with a crown or “corona” of club-shaped spikes on its surface.2
The new coronavirus, SARS-CoV-2, which causes COVID-19 appears to have first emerged in Wuhan, China, in late 2019. The outbreak has since spread across China to other countries around the world. By the end of January 2020, the World Health Organization (WHO) declared the new coronavirus a public health emergency of international concern.3
Reported COVID-19 symptoms range from mild to severe. The most common symptoms include fever, dry cough, tiredness, runny nose, and sore throat.4 Severe COVID-19 cases may develop difficulty breathing, organ failure, and consequently requiring critical care respiratory ventilation with specialized management at intensive care units (ICU), and the most severe complication death.3 , 5, 6, 7, 8, 9, 10
Similarities in the epidemiology, clinical features, and management have been reported in SARS, MERS, and COVID-19 coronaviruses.5 , 7 , 11, 12, 13 These coronaviruses are enveloped by a positive-stranded RNA isolated from bats sharing sequence homology with isolates from humans, suggesting bats as the natural host and reservoir.11 , 12 , 14 Differences in these coronaviruses have been noted in their clinical picture. Cough frequency was reported highest in SARS (92.1%),15 than in MERS (72%) patients,16 and COVID-19 (57.6%).14 On the other hand, diarrhea was reported in 20–25% of SARS and MERS patients, and as low as 7% in COVID-19 patients.7 , 9 , 12 , 17 The case-fatality rate (3.5%) of COVID-19 has been reported to be much lower than that of SARS (9.6%) and MERS (34.4%).18
Several studies on the emergence of COVID-19 have been published in China and other countries.8 , 19, 20, 21 Studies have reported clinical findings, evolution, outcome of the disease, potential risk factors, laboratory and image findings. However, the literature still lacks systematic review that consolidates clinical and laboratory findings. The aim of this systematic review and meta-analysis is to summarize the currently available clinical features of COVID-19 and to examine the outcome of its cases.
Methods
Protocol
The protocol was developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P), the reporting used the PRISMA checklist and registered with PROSPERO register (registration number CRD42020199625).
Information sources and search strategy
The databases PubMed, EMBASE, Cochrane, Scopus, and Google scholars were reviewed from January 1 to July 20, 2020, to identify relevant COVID-19 English language published studies. The Medical Subject Headings (MeSH) terms selected included“ Novel coronavirus 2019”, “2019 nCoV”, “COVID-19”, “Wuhan coronavirus,” Wuhan pneumonia,” and “SARS-CoV-2.” The search strategy used the PICO framework: P (Population, children and adult patients with COVID-19), I (Intervention or exposure for observational studies, exposure to SARS-CoV), C (Comparison, recovered and non-recovered cases), and O (Outcome, cure rate).
Eligibility criteria
Included in the analysis were published peer-reviewed articles from January 1 to July 20, 2020, that reported cases of confirmed SARS-CoV-2. Excluded from the analysis were studies reporting COVID-19 cases with incomplete information.
Data collection
The following was retrieved from each published article: name of authors, date of publication, study location, aims and objectives, source of participants, number of patients, study design, population demographic data (gender and age), eligibility criteria, comorbidities, measurement of exposure and outcome; clinical, laboratory findings, CT imaging features, and follow-up data, and treatment outcomes.
Quality of the studies
The recommended Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist,22 was used to assess the risk of bias in all identified full-text articles. The quality of the included studies was assessed independently by two authors (FQ, KB). Twelve checklist criteria were selected and articles were classified into one of three categories of bias: (1) low-risk, where 9 out of 12 criteria were selected, (2) moderate risk, 6 to 8 criteria were selected, and (3) high-risk, only 5 criteria were selected (Table 1 ).
Table 1.
Quality assessment of the included articles (N = 35).
| Author | Date of Publication | Country | Study Design | Total Patients | Quality Assessment Score |
|---|---|---|---|---|---|
| Chung et al.24 | 2/04/2020 | China | Cross-Sectional | 21 | 10 |
| Chen et al.11 | 2/06/2020 | China | Cross-Sectional | 29 | 11 |
| Wang et al.25 | 2/07/2020 | China | Cross-Sectional | 138 | 9 |
| Kui et al.26 | 2/07/2020 | China | Cross-Sectional | 137 | 11 |
| Chang et al.27 | 2/07/2020 | China | Cross-Sectional | 13 | 9 |
| To et al.28 | 2/12/2020 | China | Cross-Sectional | 12 | 10 |
| COVID-19 team29 | 2/12/2020 | Australia | Cross-Sectional | 15 | 11 |
| Yueying et al.30 | 2/13/2020 | China | Cross-Sectional | 63 | 9 |
| Li et al.31 | 2/13/2020 | China | Case-Series | 24 | 11 |
| Feng et al.32 | 2/13/2020 | China | Case-Series | 21 | 9 |
| Liang et al.33 | 2/14/2020 | China | Cross-Sectional | 1590 | 10 |
| Zhang et al.34 | 2/15/2020 | China | Case-Series | 9 | 10 |
| Feng et al.35 | 2/17/2020 | China | Case-Series | 15 | 11 |
| Wang et al.17 | 2/17/2020 | China | Cross-Sectional | 34 | 9 |
| Xiaobo et al.36 | 2/21/2020 | China | Cross-Sectional | 52 | 10 |
| Zhou37 | 28/3/2020 | China | Cohort, Retrospective | 191 | 11 |
| Qiu H.38 | 25/3/2020 | China | Cohort, Prospective | 36 | 11 |
| Wang et al.39 | 12/3/2020 | China | Case-Study | 1 | 9 |
| Xia40 | 5/03/2020 | China | Cohort, Retrospective | 20 | 9 |
| Wan et al.41 | 21/3/2020 | China | Cohort, Retrospective | 135 | 10 |
| Liu et a.42 | 27/3/2020 | China | Cohort, Retrospective | 56 | 10 |
| Zhao43 | 12/3/2020 | China | Cohort, Retrospective | 19 | 10 |
| Zhou et al.10 | 05/3/2020 | China | Cohort, Retrospective | 62 | 11 |
| Wang 44 | 16/3/2020 | China | Cohort, Retrospective | 69 | 10 |
| Lei et al.45 | 5/4/2020 | China | Cross-Sectional | 20 | 10 |
| Lei Pan et al.46 | 14/4/2020 | China | Cross-Sectional | 204 | 9 |
| Fan et al.47 | 21/4/2020 | China | Cross-Sectional | 150 | 10 |
| Richardson et al.48 | 22/4/2020 | USA | Case Series | 5700 | 10 |
| Li et al.49 | 15/5/2020 | China | Cohort, Retrospective | 93 | 9 |
| Hong et al.50 | 7/4/2020 | South Korea | Cohort, Retrospective | 98 | 11 |
| Garazzino et al.51 | 7/5/2020 | Italy | Cross-Sectional | 168 | 10 |
| Zacharia et al.52 | 3/6/2020 | USA | Case series | 50 | 9 |
| Matos et al.53 | 26/6/2020 | Italy | Cross-Sectional | 106 | 11 |
| Li et al.54 | 11/6/2020 | China | Cohort, Retrospective | 102 | 10 |
| Alsofayan et al.55 | 31/5/2020 | KSA | Cross-Sectional | 1519 | 11 |
Statistical analysis
Meta-Analysis was used to analyse the data using the comprehensive Meta-Analysis (CMA) package, version 3. Percentages were calculated to describe the distribution of the categorical dichotomous variables. For continuous data, the mean and 95% confidence intervals (CI) were calculated. Studies reporting the mean with 95% CI, the formula (upper limit-lower limit)/4 was used to extract the standard deviation.
Meta-Analysis using the random-effect model was performed to estimate the pooled prevalence and 95% CI. Pooled percentage, prevalence, and corresponding 95% CI were calculated to summarize the weighted effect size for all binary variables. The measure of heterogeneity reported included the Cochran's Q statistics, I2 index with the level of heterogeneity defined as poor< 25, moderate >50, and high> 75, and the tau square (T2) test. Publication bias was assessed with a funnel plot and the Egger test.
Results
Study selection and characteristics
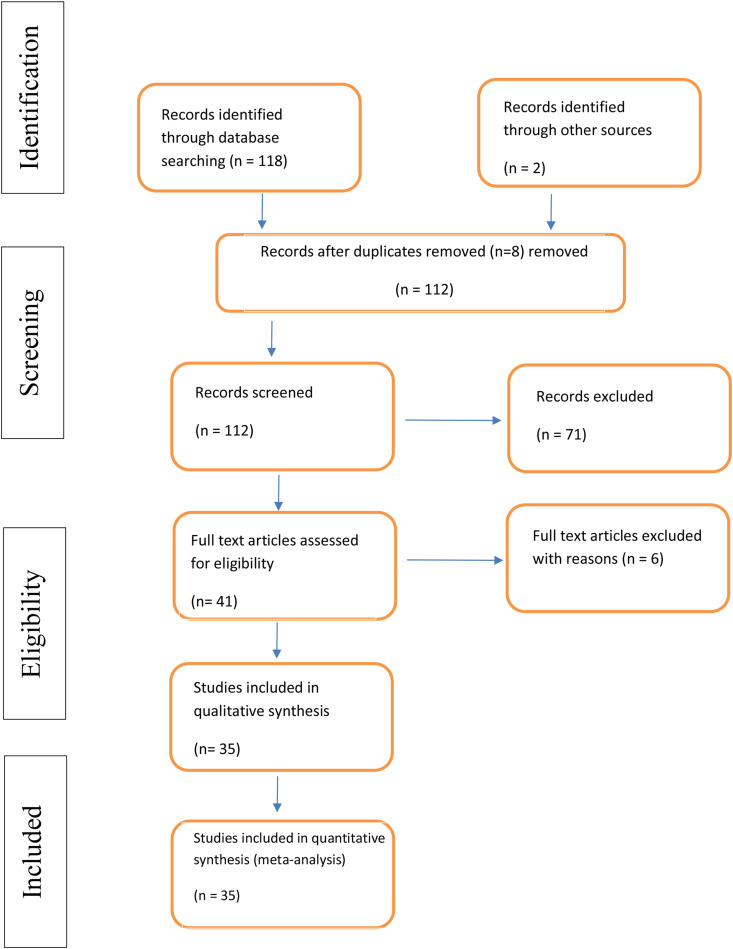
Figure 1 shows the literature retrieval flowchart. Five databases (PubMed, EMBASE, Cochrane database, Scopus, and Google Scholar) were searched from January 1 to July 20, 2020. One-hundred and eighteen studies were identified using the predefined search strategy and manual search. Eight duplicate studies were excluded and 71 did not meet the eligibility criteria, such as meta-analysis, review, or mechanism of COVID-19 infection. For this reason, forty-one studies were selected for full-text review. After revision, 6 studies were excluded due to lack of information, comment, or viewpoint.
Figure 1.
PRISMA chart of the selected studies.
The final analysis included 35 studies published between January 1 to July 20, 2020. Following the checklist criteria, all 35 screened articles were ranked as low risk of bias, with a weighted kappa statistic between author agreements of 87%. The study designs were either cross-sectional or cohort (Table 1).
Twenty-nine variables were included in the Meta-Analysis (Table 2, Table 3, Table 4, Table 5 ). Most of the studies showed considerable heterogeneity (I2>75%) (Table 6 ). No evidence of bias as demonstrated by Egger's test (P-value > 0.05) (Table 6).
Table 2.
Demographic details and comorbidities associated with COVID-19 patients (N = 35).
| Author | Mean age (years) | Age range (years) | Gender | No. of patients in ICU (%) | Comorbidities No. (%) |
Diabetes No. (%) |
Hypertension No. (%) |
CVD No. (%) |
COPD No. (%) |
|---|---|---|---|---|---|---|---|---|---|
| Chung et al.24 | 51 | 29–77 | 13 M | a | a | a | a | a | a |
| Chen et al.11 | 56 | 26–79 | 21 M | a | 16 (55.2) | 5 (17.2) | 8 (27.6) | a | a |
| Wang et al.25 | 56 | 42–68 | 75 M | 36 (26.1) | 64 (46.4) | 14 (10.1) | 43 (31.2) | 20 (14.5) | 4 (2.9) |
| Kui et al.26 | 57 | 20–83 | 61 M | a | 27 (19.7) | 14 (10.2) | 13 (9.5) | 10 (7.3) | 2 (1.5) |
| Chang et al.27 | 34 | 34–48 | 10 M | a | a | a | a | a | a |
| To et al.28 | 62.5 | 37–75 | 7 M | a | a | a | a | a | a |
| COVID-19 team29 | 43 | 8–66 | 9 M | 1 (6.7) | a | a | a | a | a |
| Yueying et al.30 | a | 15.2 | 33 M | a | a | a | a | a | a |
| Li et al.31 | 43 | 412–48 | 8 M | a | a | a | a | a | a |
| Feng et al.32 | 40.9 | 25–63 | 6 M | a | a | a | a | a | a |
| Liang et al.33 | a | a | 911 M | 130 (8.2) | 18 (1.1) | 2 (0.1) | 2 (0.1) | a | 1 (0.06) |
| Zhang et al.34 | 36 | 15–49 | 5 M | a | 1 (11.1) | 1 (11.1) | a | a | a |
| Feng et al.35 | a | 4–14 | 5 M | a | a | a | a | a | a |
| Wang et al.17 | 8 | a | 14 M | a | a | a | a | a | a |
| Xiaobo et al.36 | 59.7 | 33.6–85.8 | 35 M | a | 21 (40.4) | 9 (17.3) | a | 5 (9.6) | 4 (7.7) |
| Zhou37 | 56 | 18–87 | 119 M 72 F |
50 (26) | 91 (48) | 36 (19) | 58 (30) | 15 (8) | 6 (3) |
| Qiu H.38 | 8.3 | 0–16 | 23 M 13 F |
a | a | a | a | a | a |
| Shaoshuai W et al.39 | a | a | 1 F, 1M (neonatal) | 0 | a | a | a | a | a |
| Xia40 | 2.15 | 0–14 | 13 M 7 F |
a | a | a | a | a | a |
| Wan et al.41 | 47 | 36–55 | 63 F 73 M |
a | a | 43 | 12 | 13 | 7 |
| Liu et a.42 | 47 | a | a | a | a | 19 | 4 | 10 | 2 |
| Zhao43 | 48 | 27–56 | 8 F 11 M |
a | 0 | 3 | 0 | 2 | 0 |
| Zhou et al.10 | 52.8 | 30–77 | 23 F 39 M |
a | a | 12 | 4 | 4 | a |
| Wang44 | 42 | a | 37 F 32 M |
a | a | 35 | 7 (10) | 9 (13) | 8 (12) |
| Lei et al.45 | 43.2 | 25–64 | 10 M 10 F |
1 (5.0) | 3 (15.0) | a | a | 5 (25.0) | 1 (5.0) |
| Lei Pan et al.46 | 52.9 | a | 107 M 97 F |
16 (7.8) | 13 (6.37) | a | a | 44 (21.6) | 9 (4.4) |
| Fan et al.47 | 56 | 17–90 | 68 M 82 F |
a | 69 (46.00) | a | a | a | a |
| Richardson et al.48 | 56 | 52–75 | 3437 M 2263 F | 373 (14.2) | 28% | 1808 (33.8) | 3026 (56.6) | 966 (18) | 287 (5.4) |
| Li et al.49 | 51.0 | a | 41 M 52F |
a | 32 (34) | 11 (12) | 5 (5) | 4 (4) | 8 (9) |
| Hong et al.50 | 55.4 | 21–65 | 38 M 60 F |
13 (13.3) | 38 (38.8) | 9 (9.2) | 30 (30.6) | 11 (11.2) | |
| Garazzino et al.51 | a | 1–17 | 94 M 74 F |
2 (11.1) | 32 (19.6) | a | a | a | a |
| Zacharia et al.52 | a | 6–21 | 27 M 23 F |
33 (60) | 3 (6) | a | a | a | |
| Matos et al.53 | a | 26–95 | 65M 41 F |
a | 40/106 (37.7) | a | a | a | a |
| Li et al.54 | a | 45–70 | 59 M 43 F |
44 (43) | 15 (15) | 31 (30) | 4 (4) | 2 (2) | |
| Alsofayan et al.55 | a | 14–66 | 825 M 694 F | 36 (4.7) | 1095 (72) | 83 (5.46) | 97 (6.4) | 25 (1.6) | 57 (3.75) |
No, number; ICU, intensive care unit; CVD, cardiovascular disease; COPD, chronic obstructive pulmonary disease; M, male; F, female.
Data not available.
Table 3.
Clinical feature of COVID-19 patients (N = 35).
| Author | No. | Fever No. (%) |
Cough No. (%) |
Sore Throat No. (%) |
Fatigue No. (%) |
Sputum No. (%) |
Headache No. (%) |
Hemoptysis No. (%) |
|---|---|---|---|---|---|---|---|---|
| Chung et al.24 | 21 | 14 (66.7) | 9 (42.9) | a | 6 (28.6) | a | 3 (14.3) | a |
| Chen et al.11 | 29 | 28 (96.6) | 21 (72.4) | a | 12 (41.4) | 21 (72.4) | 2 (6.9) | a |
| Wang et al.25 | 138 | 136 (98.6) | 82 (59.4) | 24 (17.4) | 138 (100) | 37 (26.8) | 9 (6.5) | a |
| Kui et al.26 | 137 | 112 (81.8) | 66 (48.2) | a | 44 (32.1) | 6 (4.4) | 13 (9.5) | 7 (5.1) |
| Chang et al.27 | 13 | 12 (9.3) | 6 (46.2) | a | 3 (23.1) | 2 (15.4) | 3 (23.1) | a |
| To et al.28 | 12 | a | a | a | a | a | a | a |
| COVID-19 team29 | 15 | 14 (93.3) | 11 (73.3) | a | a | a | a | a |
| Yueying et al.30 | 63 | a | a | a | a | a | a | a |
| Li et al.31 | 24 | 19 (79.2) | 6 (25) | a | 6 (25.0) | a | 4 (16.7) | a |
| Feng et al.32 | 21 | 18 (85.7) | 12 (57.1) | 4 (19.0) | 11 (52.4) | 6 (28.6) | a | a |
| Liang et al.33 | 1590 | a | a | a | a | a | a | a |
| Zhang et al.34 | 9 | 8 (88.9) | 5 (55.6) | 4 (44.4) | 4 (44.4) | a | a | a |
| Feng et al.35 | 15 | 5 (33.3) | 1 (6.7) | a | a | a | a | a |
| Wang et al.17 | 34 | 17 (50.0) | 13 (38.2) | a | a | a | a | a |
| Xiaobo et al.36 | 52 | 51 (98.1) | 40 (76.9) | a | 6 (76.9) | a | 3 (11.5) | a |
| Zhou37 | 191 | 180 (94) | 151 (79) | a | 44 (23) | 44 (23) | a | a |
| Qiu et al.38 | 36 | 13 (36) | 7 (21) | 2 (6) | a | a | 3 (8) | a |
| Shaoshuai W et al.39 | 2 | 1 (50) | 0 | 0 | 0 | 0 | 0 | 0 |
| Xia40 | 20 | 12 (60) | 13 (65) | 1 (5) | 1 (5) | a | a | a |
| Wan et al.41 | 135 | 120 | 102 | a | a | 12 | 34 | 4 |
| Liu et al.42 | 56 | 44 | 21 | a | 5 | 21 | a | a |
| Zhao43 | 19 | 15 | 15 | 4 | 2 | a | 2 | a |
| Zhou et al.10 | 62 | 53 | 28 | a | 14 | 28 | a | a |
| Wang44 | 69 | 40 (78) | 38 (55) | a | 29 (42) | a | a | a |
| Lei et al.45 | 20 | 16 (80.0) | 11 (55.0) | 4 (20.0) | 7 (35.0) | a | a | a |
| Lei Pan et al.46 | 204 | 95 (92.23) | a | a | 54 (52.42) | a | a | a |
| Fan et al.47 | 150 | 122 (81.33) | 99 (66.00) | 33 (22.0) | 6 (4.0) | |||
| Richardson et al.48 | 5700 | 5644 | a | a | a | a | a | a |
| Li et al.49 | 93 | 89 (96) | 66 (71) | a | 63 (68.0) | 29 (31) | a | a |
| Hong et al.50 | 98 | 62 (63.3) | 58 (59.2) | 39 (39.8) | ||||
| Garazzino et al.51 | 168 | 138 (82.1) | 82 (48.8) | a | a | a | a | a |
| Zacharia et al.52 | 50 | 40 (80) | 23 (46) | 6 (12) | a | a | a | a |
| Matos et al.53 | 106 | a | a | a | a | a | a | a |
| Li et al.54 | 102 | 94 (92) | 77 (75) | 35 (34) | 26 (25) | 5 (5) | ||
| Alsofayan et al.55 | 1519 | 333 (85.6) | 429 (89.4) | 257 (81.6) | 193 (27.3) |
No, number.
Data not available.
Table 4.
Investigations of COVID-19 patients (N = 35).
| Author | No. | Leukocytosis No. (%) |
Leukopenia No. (%) |
Lymphopenia No. (%) |
High Creatinine No. (%) |
High LDH No. (%) |
High CR No. (%) |
ESR elevated No. (%) |
|---|---|---|---|---|---|---|---|---|
| Chung et al.24 | 21 | a | a | a | a | a | a | a |
| Chen et al.11 | 29 | 6 (20.7) | 6 (20.7) | 20 (69.0) | 2 (6.9) | 20 (6.9) | 27 (93.1) | a |
| Wang et al.25 | 138 | 0 | 0 | 97 (70.3) | a | 55 (39.9) | a | a |
| Kui et al.26 | 137 | 26 (19.0) | 51 (37.2) | 99 (72.3) | a | a | a | a |
| Chang et al.27 | 13 | a | a | a | a | a | a | a |
| To et al.28 | 12 | a | a | a | a | a | a | a |
| COVID-19 team29 | 15 | a | a | a | a | a | a | a |
| Yueying et al.30 | 63 | a | a | a | a | a | a | a |
| Li et al.31 | 24 | a | a | a | a | a | a | a |
| Feng et al.32 | 21 | a | a | a | a | a | a | a |
| Liang et al.33 | 1590 | a | a | a | a | a | a | a |
| Zhang et al.34 | 9 | 1 (11.1) | a | 2 (22.2) | a | a | 5 (55.6) | a |
| Feng et al.35 | 15 | a | 8 (53.3) | a | a | a | a | a |
| Wang et al.17 | 34 | 5 (14.7) | 1 (2.9) | 1 (2.9) | a | 10 (29.4) | 1 (2.9) | 5 (14.7) |
| Xiaobo et al.36 | 52 | a | a | a | a | a | a | a |
| Xia et al.40 | 20 | 2 (10) | 4 (20) | 7 (35) | a | a | 9 (45) | a |
| Zhou et al.37 | 191 | 40 (21) | 32 (17) | 77 (40) | 8 (4) | 123 (67) | a | a |
| Qiu et al.38 | 36 | a | 7 (19) | 11 (31) | 0 (0) | a | 1 (2.) | 0 (0) |
| Wan et al.41 | 135 | 9 (6.67) | 28 (20.) | 68 (50.4) | 6 (4.4) | 58 (43) | 40 (29.6) | a |
| Liu et al.42 | 56 | 4 (7.1) | 11 (19.6) | 17 (30.4) | a | a | a | a |
| Zhao et al.46 | 19 | 2 (5.9) | 11 (32.4) | 22 (64.7) | a | 6 (17.6) | 30 (88.2) | a |
| Zhou et al.10 | 62 | a | 6 (20.0) | 24 (80.0) | a | a | 27 (100) | 18 (66.7) |
| Wang et al.44 | 69 | 1 (1) | 36 (54) | 28 (42.0) | a | 25 (41) | 6 (10%) | 30 (52) |
| Lei et al.45 | 20 | a | a | a | 1 (5.0) | a | a | a |
| Lei Pan et al.46 | 204 | a | a | a | a | a | a | a |
| Fan et al.47 | 150 | 2 (1.33) | 34 (22.67) | 79 (52.67) | a | a | a | a |
| Richardson et al.48 | 5700 | a | a | 3387 (60) | a | 4003 | 4517 | a |
| Li et al.49 | 93 | a | a | a | a | a | a | a |
| Hong et al.50 | 98 | 9 (9.2) | 18 (18.4) | 40 (40.8) | 29 (29.6) | 47 (50.5) | 61 (67.8) | a |
| Garazzino et al.51 | 168 | a | a | a | a | a | a | a |
| Zacharia et al.52 | 50 | a | a | 36 (72) | a | a | a | a |
| Matos et al.53 | 106 | a | a | a | a | a | a | a |
| Li et al.54 | 102 | 16 (15) | 11 (11) | 66 (65) | 13 (13) | 75 (74) | 86 (84) | a |
| Alsofyan et al.55 | 1519 | 3 (3.5) | 9 (10.6) | 24 (37.5) | a | a | a | a |
No, number; LDH, Lactate dehydrogenase; ESR, erythrocyte sedimentation rate.
Data not available.
Table 5.
Clinical manifestations and complications from COVID-19 (N = 35).
| Author | No. | Unilateral Pneumonia No. (%) |
Bilateral Pneumonia No. (%) |
Ground Glass opacity No. (%) |
Acute Respiratory distress No. (%) |
RNA Anemia No. (%) |
Shock No. (%) |
Hospitalization No. (%) |
Discharge No. (%) |
Death No. (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Chung et al.24 | 21 | 2 (1.5) | 16 (11.8) | 18 (13.2) | a | 21 (15.4) | a | 21 (15.4) | a | a |
| Chen et al.11 | 29 | a | a | 29 (100) | a | 29 (100) | a | 27 (9.1) | a | 2 (6.9) |
| Wang et al.25 | 138 | 0 (0) | 138 (100) | 138 (100) | 27 (19.6) | 138 (100) | 12 (8.7) | 138 (100) | 47 (34.1) | 6 (4.3) |
| Kui et al.26 | 137 | a | 36 (26.3) | 55 (40.1) | a | 137 (100) | a | 77 (56.6) | 44 (32.4) | 16 (11.8) |
| Chang et al.27 | 13 | 1 (7.7) | a | 6 (46.2) | a | 13 (100) | a | 12 (92.3) | 1 (7.7) | a |
| To et al.28 | 12 | a | a | a | a | 12 (100) | a | 12 (100) | a | a |
| COVID-19 team29 | 15 | a | a | a | a | 15 (100) | a | 11 (73.3) | a | a |
| Yueying et al.30 | 63 | a | 38 (60.3) | 14 (22.2) | a | 63 (100) | a | a | a | a |
| Li et al.31 | 24 | a | a | a | a | 24 (100) | a | a | a | a |
| Feng et al.32 | 21 | 18 (85.7) | a | a | a | 21 (100) | a | 21 (100) | a | a |
| Liang et al.33 | 1590 | a | a | a | a | a | a | 1590 (100) | a | a |
| Zhang et al.34 | 9 | 2 (2.22) | 5 (55.6) | 7 (77.8) | a | 9 (100) | a | 9 (100) | a | a |
| Feng et al.35 | 15 | 4 (26.7) | 8 (53.3) | a | a | 15 (100) | a | a | 15 (100) | a |
| Wang et al.17 | 34 | a | 34 (100) | 34 (100) | a | 34 (100) | a | 34 (100) | 34 (100) | a |
| Xiaobo et al.36 | 52 | a | a | a | 35 (67.3) | a | a | 52 (100) | a | 32 (61.5) |
| Xia et al.40 | 20 | 6 (30) | 10 (50) | 12 (60) | a | a | a | 20 (100) | a | a |
| Zhou et al.37 | 191 | a | 143 (75) | 136 (71) | 59 (31) | a | 38 (20) | 191 (100) | 137 (71.7) | 54 (28.3) |
| Wang et al.39 | 1 | 1 (100) | a | 1 (100) | a | a | a | 1 (100) | a | a |
| Qiu et al.38 | 36 | a | a | 19 (53) | a | a | 0 (0) | 36 (100) | a | a |
| Wan et al.41 | 135 | a | 135 (100.0) | a | 21 (15.6) | a | 1 (0.7) | 120 (88.9) | 15 (42.9) | 1 (0.7) |
| Liu et al.42 | 56 | 16 (28.6) | 40 (71.4) | a | a | a | 3 (5.4) | a | 53 (94.6) | 3 (5.4) |
| Zhao et al.43 | 19 | 15 (44.1) | 19 (55.9) | 18 (52.9) | a | a | a | a | a | a |
| Zhou et al.10 | 62 | 10 (16.1) | 52 (83.9) | 25 (40.3) | a | a | a | a | a | a |
| Wang et al.44 | 69 | a | a | a | a | a | a | 44 (65.7) | 18 (26.9) | 5 (7.5) |
| Lei et al.45 | 20 | 2 (10.0) | 18 (90.0) | 16 (80.0) | 1 (5.0) | a | 1 (5.0) | 6 (30.0) | 14 (70.0) | 0 (0.0) |
| Lei Pan et al.46 | 204 | a | a | a | a | a | a | a | 168 (82.35) | 36 (17.65) |
| Fan et al.47 | 150 | 67 (44.67) | 83 (55.33) | 93 (62.00) | a | a | a | a | a | a |
| Richardson et al.48 | 5700 | a | a | a | a | a | a | a | 82 (13.5) | 1381 (24.2) |
| Li et al.49 | 93 | 74 (80) | 17 (18) | a | a | a | a | a | 68 (73) | 25 (27) |
| Hong et al.50 | 98 | 14 (14.3) | 34 (34.7) | 42 (42.9) | 13 (13.3) | 9 (9.1) | 57 (58.2) | 30 (30.6) | 5 (5.1) | |
| Garazzino et al.51 | 168 | a | a | a | a | a | a | 110 (65.1) | a | a |
| Zacharia et al.52 | 50 | a | 25 (69) | a | a | a | a | a | a | 1 (2) |
| Matos et al.53 | 106 | 7 (6.6) | 99 (93.4) | 79 (74.5) | a | a | a | 97 (91.5) | 9 (8.5) | 25 (23.6) |
| Li et al.54 | 102 | a | a | a | a | a | a | 2 (1.96) | 85 (83.3) | 15 (14.7) |
| Alsofyan et al.55 | 1519 | a | a | a | a | a | a | 1087 (71.6) | a | 10 (0.65) |
No, number.
Data not available.
Table 6.
Meta-Analysis outcomes for COVID-19 patients (random-effects model) (N = 35).
| Item | No. of studies | Prevalence% | 95% CI | n | I2 | T2 | P-value for testing heterogeneity. | Egger's test P |
|---|---|---|---|---|---|---|---|---|
| Demographical characteristics | ||||||||
| Age (mean in years) | 21 | 46.8 | 41.0–52.6 | 20 | 99.78 | 180.467 | <0.001 | 0.0438 |
| Male | 33 | 54.0 | 51.3–56.7 | 32 | 69.535 | 0.040 | <0.001 | 0.0189 |
| Co-Morbidities | ||||||||
| Co-morbidities | 17 | 29.5 | 19.0–36.6 | 16 | 98.738 | 1.372 | <0.001 | 0.8677 |
| DM | 17 | 13.8 | 8.7–21.1 | 17 | 96.645 | 1.076 | <0.001 | 0.0183 |
| Hypertension | 15 | 11.7 | 5.7–22.6 | 14 | 98.66 | 2.241 | <0.001 | 0.0025 |
| CVD | 16 | 9.7 | 6.5–14.2 | 15 | 92.455 | 9.639 | <0.001 | 0.0441 |
| COPD | 14 | 3.9 | 2.9–5.3 | 13 | 62.282 | 0.132 | <0.001 | 0.1004 |
| Clinical manifestations | ||||||||
| Fever | 31 | 82.0 | 67.7–90.8 | 30 | 98.557 | 4.531 | <0.001 | 0.0117 |
| Cough | 29 | 54.3 | 45.5–62.9 | 28 | 93.792 | 0.779 | <0.001 | 0.0090 |
| Fatigue | 21 | 30.2 | 23.3–38.1 | 20 | 83.651 | 0.465 | <0.001 | 0.9873 |
| Sputum | 13 | 28.5 | 21.2–37.2 | 12 | 83.783 | 0.390 | <0.001 | 0.7643 |
| Sore Throat | 10 | 21.7 | 14.6–31.0 | 9 | 74.296 | 0.338 | <0.001 | 0.3871 |
| Headache | 13 | 11.0 | 7.9–15.2 | 12 | 68.412 | 0.227 | <0.001 | 0.4517 |
| Hemoptysis | 3 | 5.3 | 3.0–8.9 | 2 | 0 | 0 | 0.424 | 0.1580 |
| Laboratory findings | ||||||||
| Leukocytosis | 16 | 7.5 | 4.5–12 | 15 | 85.716 | 0.842 | <0.001 | 0.0093 |
| Leukopenia | 17 | 16.0 | 9.5–25.7 | 16 | 93.670 | 1.342 | <0.001 | 0.2359 |
| Lymphopenia | 17 | 41.9 | 30.1–54.7 | 16 | 97.302 | 1.074 | <0.001 | 0.0956 |
| High Creatinine | 7 | 7.7 | 3.2–17.3 | 6 | 86.947 | 1.135 | <0.001 | 0.1139 |
| High LDH | 10 | 51.5 | 40.3–62.6 | 9 | 94.679 | 0.463 | <0.001 | 0.0074 |
| CRP | 12 | 48.1 | 29.8–66.9 | 11 | 96.494 | 1.622 | <0.001 | 0.0215 |
| ESR | 4 | 23.5 | 11.1–42.9 | 3 | 79.739 | 0.752 | 0.002 | 0.0214 |
| Complications | ||||||||
| RNA Anemia | 13 | 98.2 | 96.2–99.2 | 12 | 0 | 0 | 0.974 | 0.0001 |
| Hospitalization | 23 | 83.7 | 76.0–89.3 | 22 | 89.67 | 0.784 | <0.001 | 0.0613 |
| Bilateral Pneumonia | 19 | 70.9 | 58.2–81.0 | 18 | 92.552 | 1.207 | <0.001 | 0.0485 |
| Unilateral Pneumonia | 15 | 26.2 | 14.6–42.2 | 14 | 91.829 | 1.723 | <0.001 | 0.2087 |
| ARDS | 6 | 23.6 | 12.9–39.2 | 5 | 91.623 | 0.703 | <0.001 | 0.7139 |
| Shock | 7 | 8.5 | 4.4–10.8 | 6 | 74.476 | 0.516 | <0.001 | 0.0254 |
| Discharge | 16 | 43.5 | 24.9–64.2 | 15 | 98.13 | 2.84 | <0.001 | 0.8226 |
| Death | 17 | 10.5 | 6.8–16.1 | 16 | 94.32 | 0.816 | <0.001 | 0.0214 |
No, number; CI, confidence interval; DM, diabetes mellitus; CVD, cardiovascular disease; COPD, chronic obstructive pulmonary disease ICU, intensive care unit. LDH, lactate dehydrogenase; ARDS, acute respiratory distress syndrome; CRP, C-reactive protein.
Q Cochran's Q statistic for heterogeneity. n, degree of freedom, I2 Index for the degree of heterogeneity. T2 Tau-squared measure of heterogeneity.
Demographic characteristics
The total number of patients analyzed was 10,972. The mean age of the patients across the studies was 46.8 years (95% CI, 41.0–52.6) and males comprised 54% (95% CI, 51.3–56.7) of the population (Table 6).
Co-morbidities
The total prevalence of co-morbidities in the 17 included studies was 29.5% (95% CI, 19.0–36.6), the most prevalent being diabetes mellitus (DM) 13.8% (95% CI, 8.7–21.1), hypertension 11.7% (95% CI, 5.7–22.6), cardiovascular disease (CVD) 9.7% (95% CI, 6.5–14.2), and chronic obstructive pulmonary disease (COPD) 3.9% (95% CI, 2.9–5.3) (Table 6).
Clinical manifestations
The clinical manifestations of patients diagnosed with COVID-19 included fever, 82.0% (95% CI, 67.7–90.8), cough 54.3% (95% CI, 45.5–62.9), fatigue 30.2% (95% CI, 23.3–38.1), sputum 28.5% (95% CI, 21.2–37.2), sore throat 21.7 (95% CI, 14.6–31.0), headache 11.0% (95% CI, 7.9–15.2), and hemoptysis 5.3% (95% CI, 3.0–8.9) (Table 6).
Laboratory findings
Results of blood investigations showed high levels of lactate dehydrogenase (LDH) 51.5% (95% CI, 40.3–62.6), lymphopenia 41.9% (95% CI, 30.1–54.7), C-reactive protein (CRP) 48.1% (95% CI 29.8–66.9), erythrocyte sedimentation rate (ESR) 23.5% (95% CI, 11.1–42.9), leukopenia 16% (95% CI, 9.5–25.7), leukocytosis 7.5% (95% CI, 4.5–12) and high levels of creatinine 7.7% (95% CI, 3.2–17.3) (Table 6).
Complications
RNA Anemia was the most dominant complication 98.2% (95% CI, 96.2–99.2), followed by hospitalization 83.7% (95%CI, 76.0–89.3), bilateral pneumonia 70.9% (95% CI, 58.2–81.0), unilateral pneumonia 26.2 (95% CI, 14.6–42.2), acute respiratory distress syndrome (ARDS) 23.6% (95%CI, 12.9–39.2), shock 8.5% (95% CI, 4.4–10.8) and death 10.5% (95% CI, 6.8–16.1). From those hospitalized 43.5% (95% CI, 24.9–64.2) were cured and discharged (Table 6).
Discussion
In this study, we analyzed the clinical characteristics of 10,972 confirmed cases infected with novel coronavirus (2019-nCoV) using systematic review and random-effects meta-analysis. The mean age in this study was similar to other published studies that showed that 2019-nCoV mainly infects middle-aged and elderly.7 , 56 , 57 Similar trends were reported for SARS patients where the median age was 52 (25, 78) years and age (per 1-year increase) was reported as a risk factor for death.58 Another study showed that the median age of MERS non-survivors was 62 (53, 73) which is older than the survivors of the disease 46 (35, 57) years.16 One of the possible reasons for this phenomenon is that lung aging is associated with an inability of lung cells to multiply resulting in structural and functional changes in the respiratory tract, giving rise to decreased lung function, altered pulmonary remodeling, diminished regeneration, and enhanced susceptibility to pulmonary disease.59 It is also reported that the older patients have a higher risk of acute respiratory distress syndrome (ARDS) development.60 This beta coronavirus has been reported to cause ARDS and can be transmitted between humans.5 It is speculated to use the angiotensin-converting enzyme (ACE) 2 as a receptor for cell invasion.41 For this reason, some of the 2019-nCoV patients showed rapid progression of lung lesions, which might have led to death.
In the current study, 29.5% of the patients presented with comorbidities, such as CVD (9.7%), hypertension (11.7%), DM (13.8%), and COPD (3.9%). The comorbidities, particularly the CVD and COPD, were considered by some authorities to predict the in-hospital mortality in critically ill patients.61 It is thought that diabetes may increase the risk of infection and can delay the recovery of the infectious illnesses. In this study, only, 13.8% of patients were found to have DM and there is no evidence that DM complicated their recovery status. Recent studies also showed that diabetes had no significant correlation with the initiation, progression, and prognosis of ARDS.62 , 63 A hypothesis is that there are more hypertensive patients who developed the 2019-CoV infection, which is related to the ACE inhibitors used in these patients which could indirectly increase the cellular ACE2 receptors, which may be the receptors for 2019-CoV. However, the exact roles of age and underlying disease played in the development and progression of novel coronavirus pneumonia require further investigation.
In this study, the clinical manifestations of patients diagnosed with COVID-19 infection most commonly includes fever (82.0%) and cough (54.3%). Other studies also reported that fever is the most observed symptom among the effected patients, furthermore, fever frequency is similar in SARS and MERS.64, 65, 66, 67 However, cough frequency was highest in SARS (92.1%),15 followed by MERS MERS (72%),16 and least in this COVID-19 study [54.3%]. Other clinical manifestations such as fatigue (30.2%), sputum (28.5%), sore throat (21.7%), headache (11.0%), and hemoptysis (5.3%) were also reported in this study. Similar observations have also been reported by other investigators,68 , 69 nevertheless, these clinical symptoms are rather non-specific and may mimic influenza or atypical pneumonia of other causes such as mycoplasma, chlamydia, and legionella.
In this study, results of blood investigations showed lymphopenia (41.9%), CRP (48.1%), elevated ESR (23.5%), leukopenia (16.0%), leukocytosis (7.5%), and high creatinine (7.7%). The increased white blood cell (WBC) count suggesting that comorbid bacterial or fungal infection might have occurred in these patients. Similar to previous studies, this study found that patients had lower lymphocyte count and lymphocyte percentage. Previous studies found that during the acute phase of SARS-CoV infection in humans, the blood lymphocyte counts were decreased.70, 71, 72 Another study suggested that lymphocyte cells may play protective roles in coronavirus infection.73 Lower lymphocytes counts may be because the viral infection causes persistent consumption and/or insufficient regeneration of lymphocytes. Similar to previous study, the level of CRP in patients infected with 2019-nCoV is high.5 Moreover, CRP was a significant predictor for disease severity in SARS.74
In this study, RNA anemia (98.2%) was the most dominant complication of patients infected with COVID-19, followed by patients requiring hospitalization (83.7%), and bilateral pneumonia (70.9%). Furthermore, other studies reported that 45% of patients showed signs of pulmonary fibrosis within one month after being infected with SARS-CoV.75 Another study found lung fibrosis in 33% of patients who have recovered from MERS-CoV.76 It is possible that pulmonary fibrosis will become one of the serious complications in patients with 2019-nCoV infection.77
In this study 10.5% of patients died and similar numbers were recovered and discharged from the hospital. In comparison with data from other countries, for example, in South Korea the case fatality rate (10.4%) was in patients aged 80 years and over, in the 70 year-age group (5.4%), in the 60–69 years (1.51%), in the 50 year age-group (0.37%). Even lower rates were seen in the younger population, dropping to zero in those 29 years and younger,78 likewise, in China, the case fatality rate was higher in the early stages of the outbreak (17%) and reduced for patients with symptoms after first February (0.7%).79
Conclusion
Patients infected with the COVID-19 coronavirus had a wide clinical presentation with non-specific symptoms. With the major global outbreak of COVID-19, further research in the development of rapid diagnostic tests and an effective treatment are urgently needed.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Approval was not required.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
References
- 1.Mäkelä M., Puhakka T., Ruuskanen O., Leinonen M., Saikku P., Kimpimäki M. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36(2):539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jan M., Banach B., Susan C. Morphogenesis of coronavirus HCoV-NL63 in cell culture: a transmission electron microscopic study. Open Infect Dis J. 2008;2:52–58. doi: 10.2174/1874279300802010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adhikari S., Meng S., Wu Y., Yu-Ping M., Rui-Xue Y., Qing-Zhi W. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9:29. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Novel coronavirus (2019-nCoV) - situation report - 10 30. January 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200130-sitrep-10-ncov.pdf?sfvrsn=d0b2e480_2.2020
- 5.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N., Zhou M., Dong X., Jieming Q., Fengyun G., Yang H. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huangm C., Wang Y., Li X., Lili R., Jianping Z., Yi H. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bastola A., Sah R., Rodriguez-Morales A., Bibek Kumar L., Runa J., Hemant Chanda O. The first 2019 novel coronavirus case in Nepal. Lancet Infect Dis. 2020;20:279–280. doi: 10.1016/S1473-3099(20)30067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The L. Emerging understandings of 2019-nCoV. Lancet. 2020;395:311. doi: 10.1016/S0140-6736(20)30186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou P., Yang X., Wang X., Hu B., Zhang L., Zhang W. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. Nature. 2020 doi: 10.1038/s41586-020-2012-7. [DOI] [Google Scholar]
- 11.Chen L., Liu H.G., Liu W., Liu J., Liu K., Shang J. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jiehe He Huxi Zazhi. 2020;43:E005. doi: 10.3760/cma.j.issn.1001-0939.2020.0005. [DOI] [PubMed] [Google Scholar]
- 12.Al-Tawfiq J., Zumla A., Memish Z. Travel implications of emerging coronaviruses: SARS and MERS-CoV. Trav Med Infect Dis. 2014;12:422–428. doi: 10.1016/j.tmaid.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization Pneumonia of unknown cause – China. https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/.2020
- 14.Rodriguez-Morales A., Bonilla-Aldana D., Balbin-Ramon G., Ali A., Ranjit S., Alberto P. History is repeating itself, a probable zoonotic spillover as a cause of an epidemic: the case of 2019 novel Coronavirus. Infect Med. 2020;28:3–5. [PubMed] [Google Scholar]
- 15.Joseph S., Kwok Y., Albert D., Klaus S. The severe acute respiratory syndrome. N Engl J Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 16.Arabi Y., Al-Omari A., Mandourah Y., Fahad A., Anees A., Basem A. Critically ill patients with the Middle East respiratory syndrome: a multicenter retrospective cohort study. Crit Care Med. 2017;45:1683–1695. doi: 10.1097/CCM.0000000000002621. [DOI] [PubMed] [Google Scholar]
- 17.Wang X., Yuan J., Zheng Y., Chen J., Bao Y.M., Wang Y.R. Clinical and epidemiological characteristics of 34 children with 2019 novel coronavirus infection in Shenzhen. Zhonghua Er Ke Za Zhi. 2020;58:E00830. doi: 10.3760/cma.j.issn.0578-1310.2020.0008. [DOI] [PubMed] [Google Scholar]
- 18.Ye Z., Yuan S., Yuen K., Fung S., Chan C., Jin D. Zoonotic origins of human coronaviruses. Int J Biol Sci. 2020 Mar 15;16(10):1686–1697. doi: 10.7150/ijbs.45472. PMID: 32226286; PMCID: PMC7098031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pongpirul W., Pongpirul K., Ratnarathon A., Prasithsirikul W. Journey of a Thai taxi driver and novel coronavirus. N Engl J Med. 2020;382:1067–1068. doi: 10.1056/NEJMc2001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holshue M., DeBolt C., Lindquist S., Kathy L., John W., Hollianne B. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silverstein W., Stroud L., Cleghorn G., Leis J. First imported case of 2019 novel coronavirus in Canada, presenting as mild pneumonia. Lancet. 2020;95:734. doi: 10.1016/S0140-6736(20)30370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elm E., Altman D., Egger M., Pocock S., Gøtzsche P., Vandenbroucke P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Chung M., Bernheim A., Mei X., Ning Z., Mingqian H., Xianjun Z. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiol. 2020:200230. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D., Hu B., Hu C., Fangfang Z., Xing L., Jing Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kui L., Fang Y., Deng Y., Liu W., Wang M., Ma J.P. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang L., Wei L., Xie L., Lixin X., Guangfa Z., Charles S. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.To K., Tsang O., Chik-Yan Y.C., Kwok-Hung C., Tak-Chiu W., Jacky M.C. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Covid-19 National Incident Room Surveillance Team . 2020. COVID-19, Australia: epidemiology report 2. [Google Scholar]
- 30.Pan Yueying, Guan Hanxiong. Imaging changes in patients with 2019-nCov. Eur Radiol. 2020 doi: 10.1007/s00330-020-06713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q., Guan X., Wu P., Xiaoye W., Lei Z., Yeqing T. Early transmission dynamics in wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng K., Yun Y., Wang X., Yang G.D., Zheng Y.J., Lin C.M. Analysis of CT features of 15 Children with 2019 novel coronavirus infection. Zhonghua Er Ke Za Zhi. 2020;58:E007. doi: 10.3760/cma.j.issn.0578-1310.2020.0007. [DOI] [PubMed] [Google Scholar]
- 33.Liang W., Guan W., Chen R., Wang W., Li J., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang M., Wang X., Chen Y., Zhao K.L., Cai Y.Q., An C.L. Clinical features of 2019 novel coronavirus pneumonia in the early stage from a fever clinic in Beijing. Zhonghua Jiehe He Huxi Zazhi. 2020;43:E013. doi: 10.3760/cma.j.issn.1001-0939.2020.0013. [DOI] [PubMed] [Google Scholar]
- 35.Kai Feng, Yun Yongxing, Wang Xianfeng. CT image characteristics analysis of 15 cases of children with new coronavirus infection [J/OL] 2020. http://rs.yiigle.com/yufabiao/1181979.htm
- 36.Xiaobo Y., Yuan Y., Jiqian X., Huaqing S., Jia'an X., Hong L. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fei Z., Ting Y., Ronghui D., Guohui F., Ying L., Zhibo L. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiu H., Wu J., Hong L., Luo Y., Song Q., Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S., Guo Lili, Chen Ling, Feng Ling. A case report of neonatal COVID-19 infection in China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia W., Shao Jianbo, Peng Xuehua, Li Zhen, Hu Daoyu. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. 2020;55:1169–1174. doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol. 2020 doi: 10.1128/JVI.00127-20. pii:JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu W., Zhang Q., Chen J., Rong X., Huijuan S., Sainan S. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020;382:1370–1371. doi: 10.1056/NEJMc2003717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao D., Yao F., Wang L., Ling Z., Yongjun G., Jun Y. A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias. Clin Infect Dis. 2020:ciaa247. doi: 10.1093/cid/ciaa247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z., Yang B., Li Q., Lu W., Ruiguang Z. Clinical features of 69 cases with coronavirus disease 2019 in wuhan, China. J Clin Infect Dis. 2020;71:769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lei Z., Cao H., Jie Y., Zhanlian H., Xiaoyan G., Junfeng C. A cross-sectional comparison of epidemiological and clinical features of patients with coronavirus disease (COVID-19) in Wuhan and outside Wuhan, China. Travel Med Infect Dis. 2020:101664. doi: 10.1016/j.tmaid.2020.101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lei P., Mu M., Yang P., Yu S., Runsheng W., Junhong Y. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan N., Fan W., Li Z., Min S., Yi L. Imaging characteristics of initial chest computed tomography and clinical manifestations of patients with COVID-19 pneumonia. Jpn J Radiol. 2020;38:533–538. doi: 10.1007/s11604-020-00973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richardson S., Hirsch J., Narasimhan M., James M., Thomas M., Karina W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. J Am Med Assoc. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li L., Yang L., Gui S., Feng P., Tianhe Y., Bo L. Association of clinical and radiographic findings with the outcomes of 93 patients with COVID-19 in Wuhan, China. Theranostics. 2020;10:6113–6121. doi: 10.7150/thno.46569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong K., Lee K., Chung J., Kyeong C., Eun Y., Hyun J. Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in Daegu, South Korea: a brief descriptive study. Yonsei Med J. 2020;61:431–437. doi: 10.3349/ymj.2020.61.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garazzino S., Montagnani C., Donà D., Antonella M., Enrico F., Gianluca V. Multicentre Italian study of SARS-CoV-2 infection in children and adolescents, preliminary data as at 10 April 2020. Euro Surveill J. 2020;25:2000600. doi: 10.2807/1560-7917.ES.2020.25.18.2000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zachariah P., Johnson C., Halabi K., Danielle A., Anita I., Avital F. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children’s hospital in New York City, New York. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matos J., Paparo F., Mussetto I., Lorenzo B., Alessio V., Silvia P. Evaluation of novel coronavirus disease (COVID-19) using quantitative lung CT and clinical data: prediction of short-term outcome. Eur Radiol Exp. 2020;4:39. doi: 10.1186/s41747-020-00167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li K., Dian C., Shengchong C., Yuchen F., Chenli C., Zi W. Predictors of fatality including radiographic findings in adults with COVID-19. Respir Res. 2020;21:146. doi: 10.1186/s12931-020-01411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alsofayan Y., Althunayyan S., Khan A., Hakawi A., Assiri A. Clinical characteristics of COVID-19 in Saudi Arabia: a national retrospective study. J Infect Publ Health. 2020;13:920–925. doi: 10.1016/j.jiph.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xia J., Tong J., Liu M., Shen Y., Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020 doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao S., Lin Q., Ran J., Salihu S., Guangpu Y., Weiming W. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214–417. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lew T., Kwek T., Tai D., Arul A., Shi L., Kulgit S. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. J Am Med Assoc. 2003;290:374–380. doi: 10.1001/jama.290.3.374. [DOI] [PubMed] [Google Scholar]
- 59.Cho S.J., Stout-Delgado H.W. Aging and lung disease. Annu Rev Physiol. 2020;82:433–459. doi: 10.1146/annurev-physiol-021119-034610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ely E., Wheeler A., Thompson B., Ancukiewicz M., Steinberg K., Bernard G. Recovery rate and prognosis in older persons who develop acute lung injury and the acute respiratory distress syndrome. Ann Intern Med. 2002;136:25–36. doi: 10.7326/0003-4819-136-1-200201010-00004. [DOI] [PubMed] [Google Scholar]
- 61.Ladha K., Zhao K., Quraishi S., Tobias K., Matthias E., Haytham M. The Deyo-Charlson and Elixhauser-van Walraven Comorbidity Indices as predictors of mortality in critically ill patients. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-008990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boyle A., Madotto F., Laffey J., Giacomo B., Tài P., Antonio P. Identifying associations between diabetes and acute respiratory distress syndrome in patients with acute hypoxemic respiratory failure: an analysis of the LUNG SAFE database. Crit Care. 2018;22:268. doi: 10.1186/s13054-018-2158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ji M., Chen M., Hong X., Chen T., Zhang N. The effect of diabetes on the risk and mortality of acute lung injury/acute respiratory distress syndrome: a meta-analysis. Med (Baltimore) 2019;98 doi: 10.1097/MD.0000000000015095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan D., Wei L., Kui L., Fang Y.Y., Shang J., Zhou L. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chinese Med J. 2020 doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23:130–137. doi: 10.1111/resp.13196. Epub 2017 Oct 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Srikantiah P., Charles M.D., Reagan S., Clark T.A., Pletz M.W., Patel P.R. SARS clinical features, United States, 2003. Emerg Infect Dis. 2005;11:135–138. doi: 10.3201/eid1101.040585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Groot R., Baker S., Baric R., Caroline S., Christian D., Luis E. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol. 2013;87:7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections-more than just the common cold. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 69.Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of covid-19 - studies needed. N Engl J Med. 2020 doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 70.Wong R., Wu A., To K., Nelson L., Christopher W.K., Wong C.K. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ. 2003;326:1358–1362. doi: 10.1136/bmj.326.7403.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cui W., Fan Y., Wu W., Zhang F., Wang J.Y., Ni A.P. Expression of lymphocytes and lymphocyte subsets in patients with severe acute respiratory syndrome. Clin Infect Dis. 2003;37:857–859. doi: 10.1086/378587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li T., Qiu Z., Zhang L., Yang H., Wei H., Zhengyin L. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J Infect Dis. 2004;189:648–651. doi: 10.1086/381535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Channappanavar R., Zhao J., Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol Res. 2014;59:118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jang T., Yeh D., Shen S., Huang C., Jiang J., Kao S. Severe acute respiratory syndrome in Taiwan: analysis of epidemiological characteristics in 29 cases. J Infect. 2004;48:23–31. doi: 10.1016/j.jinf.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xie L., Liu Y., Xiao Y., Tian Q., Fan B., Zhao H. Follow-up study on pulmonary function and lung radiographic changes in rehabilitating severe acute respiratory syndrome patients after discharge. Chest. 2005;127:2119–2124. doi: 10.1378/chest.127.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Das K.M., Lee E.Y., Singh R., Enani M.A., Al Dossari K., Van Gorkom K. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J Radiol Imag. 2017;27:342–349. doi: 10.4103/ijri.IJRI_469_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun P., Qie S., Liu Z., Ren J., Li K., Xi J. Clinical characteristics of 50466 hospitalized patients with 2019-nCoV infection. J Med Virol. 2020 doi: 10.1002/jmv.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Korean Center for Disease Control (KCDC) The updates on COVID-19 in Korea as of 24 March 2020. https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030
- 79.Jason O., Carl H. Oxford COVID-19 Evidence Service; 25th March 2020. Global COVID-19 case fatality rates-CEBM. [Google Scholar]