Abstract
Introduction
Several studies in developed and developing countries have analyzed the health risk factors associated with COVID-19 mortality. Comorbid diseases are a key explanatory factor behind COVID-19 mortality, but current studies treat comorbidities in isolation, at average-population values, and rarely assess how death risk varies for different health profiles across institutions. Estimating death risk variations for different interactions between comorbid diseases and across healthcare institutions is crucial to gaining a significant depth of understanding in relation to mortality during the pandemic.
Methods
This study relies on data from approximately half a million people in Mexico (of all recorded cases through August 15, 2020) and on Bayesian estimation to provide a more robust estimate of the combined effect of several comorbidities and institutional inequalities on COVID-19 mortality.
Results
The findings of the study illustrate the additive effects of several comorbid diseases, with the presence of obesity, diabetes, hypertension, and chronic kidney disease increasing the mortality risk of COVID-19. There are also variations in the risk of death across the heterogeneous Mexican health system.
Conclusions
This study shows that COVID-19 mortality risk sharply increases in patients with 2 or more comorbid diseases (obesity, diabetes, hypertension, and cardiovascular diseases) in Mexico. However, death risk varied significantly across institutions for patients with the same comorbidity profile.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) emerged in Wuhan, China, in December 2019 caused by the virus strain severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 Infection manifestations range from no symptoms to a severe acute respiratory disease that can lead to serious illness or death.2
International research in developed countries suggests that older age and comorbid diseases such as diabetes, hypertension, cardiovascular disease (CVD), chronic lung disease, and chronic kidney disease (CKD) are risk factors for severe illness—patients requiring hospitalization, intensive care unit admission, and invasive mechanical ventilation—and death.3, 4, 5, 6 These risk factors have also been correlated with other similar viral infections, such as influenza H1N1, severe acute respiratory syndrome, and Middle East respiratory syndrome.1 , 2 , 5 , 7 , 8
Hypertension has been the most prevalent underlying condition among hospitalized patients with COVID-19 across different countries.9 A total of 3 meta-analyses found a positive relationship between hypertension and COVID-19 severity (Y Chen, unpublished data, March 2020),10 , 11 but evidence on the effects of hypertension on mortality suggest that among nonsurvivors, it is the most common disease.12 , 13
CVD also correlates with the severity of COVID-19 infection. Patients in the U.S. with pre-existing heart disease are more likely to require invasive mechanical ventilation during hospitalization.14 In China, the second most prevalent underlying condition among hospitalized patients with COVID-19 is CVD.9 , 15 Findings from 2 meta-analyses of Chinese studies support the thesis that the presence of coronary heart disease and CVD increases the risk of developing severe illness almost threefold.11 , 12 In addition, 2 small retrospective studies in China suggest that patients with CVD have a significantly higher risk of death13 , 16; however, CVD does not have an effect in a sample of U.S. patients with COVID-19.17
Several studies have suggested that diabetes increases the severity of COVID-19.8 In China, the pooled estimate from 9 studies indicates that it increases the risk of severe illness almost 3-fold.11 , 17 Descriptive cross-tabulated data suggest that higher percentages of deaths are prevalent among those patients with a previous diagnosis of diabetes.11 , 13 , 18 , 19 A meta-analysis of 4 studies finds weak evidence of the effect of CKD,20 but a pooled-data model in the same study suggests that its effect on death risk is higher than reported in single studies.
Obesity has also been reported as another critical risk factor.4 Patients who are obese studied in China are more likely to progress to severe pneumonia owing to COVID-19.21 A small retrospective cohort study conducted in France indicates that the risk of receiving invasive mechanical ventilation was higher for those patients with a BMI >35 kg/m2.22 A large prospective cohort study in the U.S. reported that among people with COVID-19 admitted to hospital (n=5,279), those with a BMI >40 kg/m2 had a greater risk of critical illness and death.17 Similarly, another study determined that those aged <60 years with obesity (BMI 30–34.9 kg/m2) were more likely to be admitted to acute and critical care.23
Many of the extant studies model the effect of comorbid diseases in isolation at average-population values, and the emerging evidence from developing countries regarding COVID-19 risk factors echoes the same approach. This recent research forms an important addition to the literature because it analyzes data from countries where health and institutional profiles differ significantly from those in developed countries. In Mexico, recent studies have examined the risk factors associated with the risk of infection,24 severity, and mortality.25, 26, 27, 28 This literature is consistent with the international research but uses data that do not include the approximately 250,000 new cases that occurred during the peak of the pandemic in July–August. Furthermore, Mexico's epidemiologic profile includes a high prevalence of obesity (36.1% in adults in 201829), CVD, and diabetes—the 3 leading causes of mortality—combined with high levels of institutional inequality.30 Therefore, it is important to conduct more detailed analyses (using a most recently updated database) of the interactions of comorbidities (in such unequal institutional settings) and their impact on COVID-19 mortality risk.
The aims of this study are 2-fold. First, it sets out to examine how the interaction effects of noncommunicable diseases affect mortality risk by analyzing a larger sample of patients with COVID-19 and using more robust statistical modeling. Second, it aims to estimate how the risk varies across institutions for different comorbidity profiles.
METHODS
Study Sample
Patient data came from official records of all confirmed COVID-19 cases and deaths in Mexico (August 15, 2020). There are 515,090 total cases in the patient data (n=55,963 registered COVID-19 deaths). The data are public and freely available.31
Measures
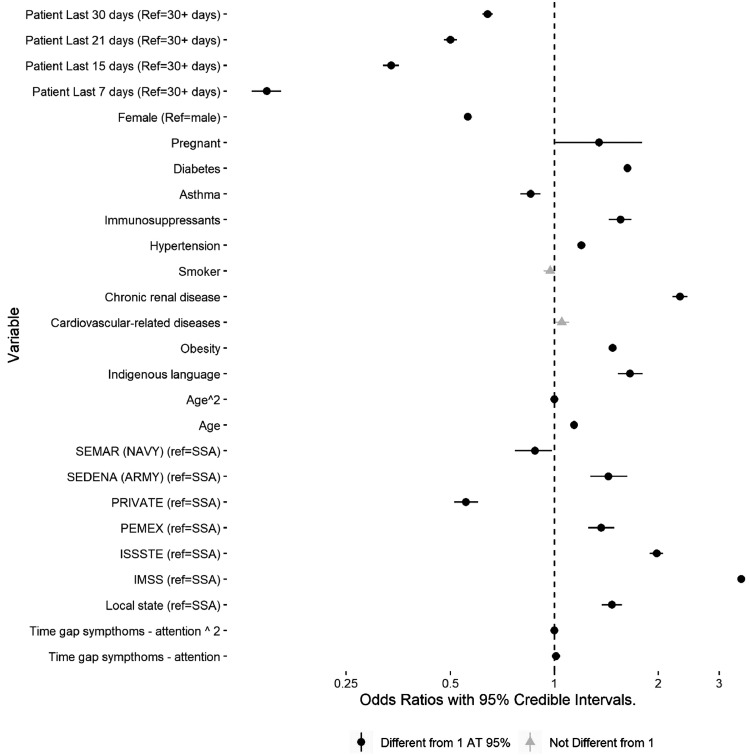
This study modeled the variation in COVID-19 death risk across several comorbidities and different healthcare institutions after adjusting for individual characteristics and other relevant auxiliary variables. The outcome variable was a binary indicator (COVID-related confirmed deaths versus COVID-19 positive but alive). The effects of both comorbid diseases being treated in a given institution on the risk of death were adjusted according to the following sets of variables: (1) sociodemographic information (age, sex, ethnic background), geographic data (state and municipality of residence), patient's ancillary variables (asthma, smoking, immunosuppressants, and other illnesses), and information on the time of diagnosis and healthcare intervention to control for time dependency. Table 1 and Appendix Table 1 (available online) describe these variables, and Figure 1 shows the coefficients from the model.
Table 1.
Descriptive Statistics of the Different Factors and Individual-Level Characteristics in the Sample
| Variable | With characteristic, % total sample (total) |
|---|---|
| Demographic | |
| Male | 53 (271,822) |
| Indigenous people | 1 (5,312) |
| Aged ≤49 years | 62 (320,936) |
| Illness | |
| Obesity | 19 (95,840) |
| Diabetes | 16 (82,062) |
| Asthma | 3 (13,634) |
| Smoker | 7 (37,021) |
| Immunosuppressants | 1 (5,988) |
| High blood pressure | 20 (101,806) |
| Chronic renal disease | 2 (10,107) |
| Heart disease | 2 (10,668) |
| Institution | |
| State (local) | 2 (11,196) |
| IMSS | 32 (164,732) |
| ISSSTE | 4 (22,294) |
| PEMEX | 1 (5,999) |
| Private | 3 (15,465) |
| SEMAR | 1 (4,033) |
| SEDENA | 1 (3,360) |
| SSA | 55 (283,028) |
Note: Age was included in the model as a continuous variable. Army health system includes SEDENA and SEMAR; Mexican Petroleum Institution includes PEMEX; open public health system includes SSE; and social security systems include IMSS and ISSSTE.
IMSS, Instituto Mexicano de Seguro Social; ISSSTE, Instituto de Seguridad y Servicios Sociales para los Trabajadores del Estado; PEMEX, Petróleos Mexicanos; SEDENA, Secretaría de la Defensa Nacional; SEMAR, Secretaría de Marina; SSA, Secretaría de Salubridad y Asistencia.
Figure 1.
Estimated coefficients from the hierarchical Bayesian model.
Note: ORs (95% CI) x-axis in log-scale. IMSS, Instituto Mexicano de Seguro Social; ISSSTE, Instituto de Seguridad y Servicios Sociales para los Trabajadores del Estado; PEMEX, Petróleos Mexicanos; SEDENA, Secretaría de la Defensa Nacional; SEMAR, Secretaría de Marina; SSA, Secretaría de Salubridad y Asistencia.
Statistical Analysis
A hierarchical Bayesian modeling approach was implemented to estimate the adjusted effects of both comorbid diseases and institutions on COVID-19 mortality risk. A total of 2 reasons underpin the selection of this approach. First, both large differences in population across states and municipalities and unknown contextual factors might influence the point estimates from a standard model. Hierarchical models result in better estimates than model uncertainty using random effects (i.e., intercepts for states and municipalities) and produce partially pooled estimates (i.e., shrink the point estimates toward average-population values).32 Therefore, state-level differences are conditional on the model. Second, random effects from hierarchical Bayesian models are more robust than those from maximum likelihood estimation, and the Bayesian model relies on the Hamiltonian Monte Carlo algorithm (M Betancourt, unpublished data, July 2018), which outperforms standard maximum likelihood estimators when using large and complex models in terms of both speed and accuracy.32
A 3-level hierarchical model (states, municipalities, and individuals) with a Bernoulli distribution was fitted to the data (Appendix, available online). The model uses weak priors () for all variables included in the model32 and was estimated in R, version 4.0.2, using Rstan in combination with the brms() package.33 , 34
A key objective of this paper was to obtain the interaction effect of comorbidities. However, the interaction of binary variables is difficult to interpret, especially for interactions with ≥3 variables. Hence, in the second step, marginal effects (i.e., adjusted probabilities for different population profiles) were obtained from the model for different combinations of factors to assess more clearly the variations in death risk probability for different combinations of comorbidities. This procedure uses the coefficients of the hierarchical model to calculate adjusted probabilities for a given combination of variables, such as the probability of death for patients with both obesity and diabetes (after adjusting by the mean population values of all other variables in the model). Hence, probabilities for all possible interactions of the 4 comorbidities were estimated (4 × 4=16).
The use of the procedure and the large sample size allowed the authors to go beyond average-population probabilities and look at the changes in the risk for specific population profiles. For the analysis of the institutional differences in risk, probabilities for 2 different profiles were produced: high and medium risks. The high-risk group comprises the population with ≥2 comorbidities, and the medium-risk group represents the population with average values in all variables, including comorbid diseases. Therefore, this study can assess how the risk varies according to each institution for both average/typical populations and high-risk populations.
The data were not prepared specifically for a fully randomized experiment, so it is important to consider the impact of different sources of error and bias: measurement/coding errors, sample/data dependency, and model dependency. To cross-validate the consistency of estimates from the Bayesian model, quasi-experimental analyses were undertaken as a second robustness check because they help to establish whether the effect holds for 2 otherwise similar patients. For each comorbidity, the nearest neighbor method, using the Mahalanobis distance, was implemented to obtain a well-matched subset of similar/comparable (given all observable characteristics in the data) patients.35 This subsample of equivalent patients was then used to estimate the effect of the comorbidity in question. The resulting effect was compared with that from the hierarchical Bayesian model (Appendix Figures 2–4, available online). R-package MatchIt() was used to conduct the quasi-experimental analyses.35
RESULTS
Table 1 shows the raw proportion of recorded deaths for each demographic and illness status and by institution. In almost all cases, there are observable differences within each group. Individuals who were male, indigenous (indigenous language speakers), and older were more likely to die from COVID-19. Those with diabetes, CVD, CKD, and hypertension, in addition to those taking immunosuppressants, were also at a higher risk of death. There was significant variation in death rates across institutions, ranging from 1% to 20%.
Figure 1 shows the estimated coefficients from the hierarchical Bayesian model. Females, leaving the other variables constant, had a lower fatality risk than males. Indigenous patients also had a higher risk than those with no declared indigenous background. Age also had a strong linear effect, with an OR of 1.14 (95% CI=1.13, 1.15) for each additional year of age. Patients taking immunosuppressants had a higher risk than those who do not (OR=1.60, 95% CI=1.40, 1.70). Smokers did not seem to have a greater risk once other factors were considered.
Duration of the disease was greater for sick patients at the peak of the pandemic (July), although there is, of course, a lag between diagnosis and mortality. The gap, measured in days, between developing symptoms and seeking medical attention had a small but positive effect. Hence, the longer it takes to receive medical attention, the higher the death risk.
Diabetes, obesity, hypertension, and chronic renal disease increased the odds of COVID-19–related death, with chronic renal disease having the highest effect (2.31, 95% CI=2.20, 2.42). Diabetes (OR=1.63, 95% CI=1.66, 1.59) and obesity (OR=1.48, 95% CI=1.44, 1.51) had similar effect sizes. CVD did not seem to influence death risk. The combined effect of these comorbidities is further explored and explained below.
There was also a clear relationship between the institution offering medical attention and death risk. Patients at private clinics and within the navy health system (Secretaría de Marina) had lower death risks than those in the open public health system (Secretaría de Salubridad y Asistencia). Yet, people receiving care at public local state hospitals, the army (Secretaría de la Defensa Nacional), and the 2 major social security systems (Instituto Mexicano de Seguro Social [IMSS] and Instituto de Seguridad y Servicios Sociales para los Trabajadores del Estado [ISSSTE]) had higher death risks. These findings reflect large interinstitutional inequalities in Mexico, and these effects are explored further in the following subsections.
After adjusting for individual-level factors, the state-level intercepts from the model suggest that the COVID-19–related death risk varied considerably across states and municipalities (Appendix Figure 4, available online). Around 12% of the unexplained variance was due to municipal differences and 8% due to state-level differences. This result indicates that there are other sources of risk across the Mexican territory affecting patients’ chances of overcoming the illness. States with lower average death risk tend to perform better across several key indicators, including gross domestic product per capita, infrastructure quality, and lower multidimensional poverty, than some of those with higher average death risk. However, the pattern is not conclusive, and this hypothesis requires further exploration to assess the contextual factors that explain interstate differences.
A second objective of the analysis was to estimate adjusted probabilities for different population profiles. These probabilities draw on the hierarchical model and were calculated to explore how interactions between the main risk factors can increase patient risk. The probabilities below consider the average value of the other variables so that these estimates approximate the mean probability for the typical sample population.
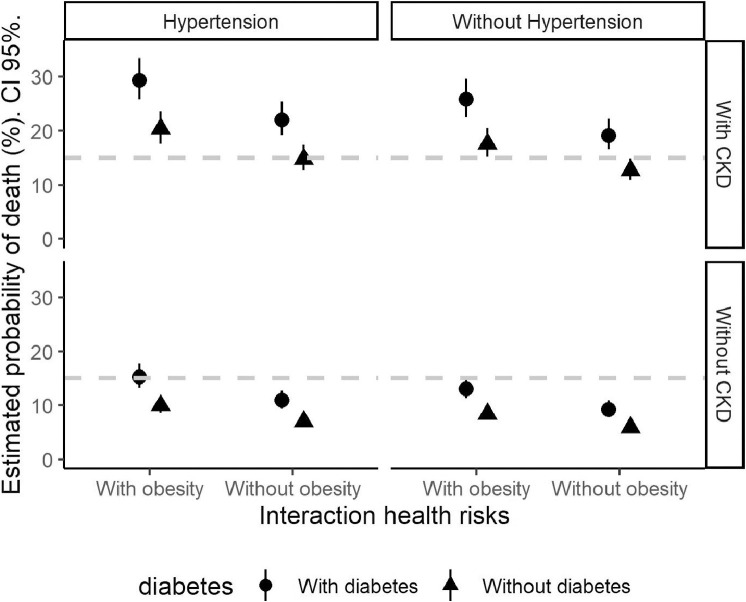
Figure 2 shows interactions among the 4 diseases (obesity, diabetes, hypertension, and CKD). Because the interaction is across binary variables (with and without the disease), this leads to 16 possible states. The circle in the bottom panel on the right shows the probability of COVID-19–related fatality for people without any of the 4 diseases (7%). In the same panel, it is possible to see the increase in probability for people with diabetes but not with the other 3 diseases. By contrast, the top panel on the left shows the probability of death for people with all the 4 diseases (26%), which, according to these findings, was 3.5 times higher for this second group than for those without them.
Figure 2.
Adjusted probabilities of COVID-19 mortality by combinations of comorbidities (95% CI).
CKD, chronic kidney disease.
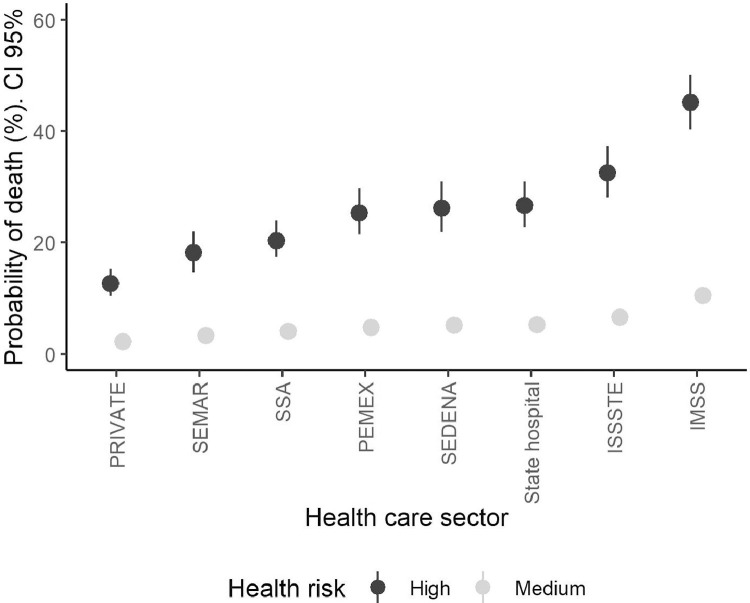
Figure 3 shows the estimated probabilities for each healthcare subsector. The estimates approximate 2 types of populations: typical and high risk (see Methods section). The results show that there was substantial variation across institutions. The probability of death ranged between 5% and almost 10% across institutions for the average population (medium risk). These values almost tripled once high health risks were included; however, the effect was much higher for patients at primary social security institutions (IMSS and ISSSTE) and local public hospitals (Secretaría de Salubridad y Asistencia). The lowest probability was for those patients in the private sector, whereas the probability was nearly the same as for relatively healthy patients at the IMSS.
Figure 3.
Adjusted probabilities of COVID-19 mortality according to healthcare sector by health risk group.
IMSS, Instituto Mexicano de Seguro Social; ISSSTE, Instituto de Seguridad y Servicios Sociales para los Trabajadores del Estado; PEMEX, Petróleos Mexicanos; SEDENA, Secretaría de la Defensa Nacional; SEMAR, Secretaría de Marina; SSA, Secretaría de Salubridad y Asistencia.
DISCUSSION
The study aimed to examine, after adjusting by different individual-level characteristics, how noncommunicable disease interactions affect mortality risk by COVID-19 in Mexico and how death risk varies across institutions for different comorbidity profiles. The findings of this study suggest that obesity, diabetes, hypertension, and CKD increase the mortality risk of COVID-19, in line with previously published research in Mexico26 and internationally.12 , 17, 18, 19, 20
When examining how death risk varies for different combinations (interactions) of comorbid diseases, it doubles when obesity interacts with diabetes, CKD, or hypertension. Obesity is a major health problem in the Mexican population, and the models show that COVID-19 mortality increases in line with this metric. The increased risk of death among patients who are obese is consistent with other studies in Mexico25 , 26 and 1 in the U.S.,17 which is expected considering that these 2 countries have the highest prevalence of obesity worldwide.36 For the case of Mexico, the results in this study raise questions about the specific mechanisms through which the Mexican obesity epidemic interacts with the current pandemic. These questions are of major importance for the clinical literature because they will not only help improve the healthcare protocols in situ for populations with obesity but also highlight the importance of strengthening obesity prevention strategies.
The interaction of diabetes with other comorbidities, namely CKD and hypertension, also doubles the risk of COVID-19 mortality. This is of utmost importance in the Mexican context because diabetes mellitus was the second cause of death in the country in 2018.30 Hence, the combination of the COVID-19 pandemic and the precarious health profile of the Mexican population has exacerbated the nation's death risk.
This paper examined the role of Mexican health institutions in managing the current health crisis. The analysis suggests that death risk significantly varies across institutions. The risk is higher for patients attending 2 of the main social security institutions (IMSS and ISSSTE): the death risk for an average patient is 2 times that of the national average and 3 times higher than that in the private sector. When considering critical health profiles (i.e., people suffering from ≥2 comorbidities), the risk increases across all institutions but remains low in the private health sector and triples for social security institutions. The differences in human and material resources and patient demand between private and public institutions might be key explanatory factors in these dramatic differences because they mirror longstanding health and spatial inequalities in Mexico.37, 38, 39
Limitations
This study has both strengths and limitations. First, this is the largest data set of patients with COVID-19 ever analyzed in Mexico to date, resulting in robust estimates of the effects of interactions between comorbidities on mortality. In addition, it is the first study to estimate how death risk varies across institutions in relation to different health profiles. One limitation is that under-reporting and bias could influence the model results in different ways. The impact of bias could be major if official records do not include a large population with a profile that is too different from those included in the data. This study employed a double robustness check to minimize the impact of any sources of biases.
The study did not model the state- or municipal-level effects, so research is required to cover this weakness. Another important consideration is the contrasting finding from this study in relation to the international literature (mostly Chinese studies) that CVD is not associated with mortality, as noted by Petrilli et al.17 in the U.S. A potential explanation for this similarity is that both countries share similar health profiles, defined in part by the obesity epidemic and health inequalities, which apparently seem to cancel out the potential effect of CVD on mortality. This requires further research with an even larger sample size to ensure that CVD has no role in increasing mortality in patients with COVID-19. Finally, this study is limited because it did not look at intrainstitutional variations. Empirical research on this matter is fundamental to understanding more precisely the performance of public health institutions.
CONCLUSIONS
This study shows that COVID-19 mortality risk sharply increases in patients with ≥2 comorbid diseases (obesity, diabetes, hypertension, and CVDs) in Mexico. However, the paper shows that death risk varies significantly across institutions for patients with the same comorbidity profile. Hence, it is very likely that 2 otherwise similar patients will have different outcomes if they are treated in different institutions. The study of the source of these variations in risk across institutions is central to understand the impact of differences in resources and provision on individuals’ mortality.
ACKNOWLEDGMENTS
The data used in this study are open, public, and free. The authors did not receive specific funding for this research. All authors contributed equally to the writing and analysis. The raw data and R-code will be made available on request.
No financial disclosures were reported by the authors of this paper.
Footnotes
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2020.10.015.
Appendix. SUPPLEMENTAL MATERIAL
REFERENCES
- 1.Luzi L, Radaelli MG. Influenza and obesity: its odd relationship and the lessons for COVID-19 pandemic. Acta Diabetol. 2020;57(6):759–764. doi: 10.1007/s00592-020-01522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sattar N, McInnes IB, McMurray JJV. Obesity a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020;142(1):4–6. doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- 4.Richardson S, Hirsch JS, Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area [published correction appears in JAMA. 2020;323(20):2098] JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puig-Domingo M, Marazuela M, Giustina A. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine. 2020;68(1):2–5. doi: 10.1007/s12020-020-02294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo T, Fan Y, Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietz W, Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity (Silver Spring) 2020;28(6):1005. doi: 10.1002/oby.22818. [DOI] [PubMed] [Google Scholar]
- 8.Singh AK, Gupta R, Misra A. Comorbidities in COVID-19: outcomes in hypertensive cohort and controversies with renin angiotensin system blockers. Diabetes Metab Syndr. 2020;14(4):283–287. doi: 10.1016/j.dsx.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodríguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lippi G, Wong J, Henry BM. Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020;130(4):304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Zheng Y, Gou X. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen T, Wu D, Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study [published correction appears in BMJ. 2020;368:m1295] BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published correction appears in Lancet. 2020;395(10229):1038] Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalligeros M, Shehadeh F, Mylona EK. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity (Silver Spring) 2020;28(7):1200–1204. doi: 10.1002/oby.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8(1):e35. [PMC free article] [PubMed] [Google Scholar]
- 16.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China [published correction appears in Intensive Care Med. 2020;46(6):1294–1297] Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrilli CM, Jones SA, Yang J. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo W, Li M, Dong Y. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020;36(7):e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy [published correction appears in JAMA. 2020;323(16):1619] JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 20.Henry BM, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol. 2020;52(6):1193–1194. doi: 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai Q, Chen F, Wang T. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020;43(7):1392–1398. doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- 22.Simonnet A, Chetboun M, Poissy J. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation [published correction appears in Obesity (Silver Spring). 2020;28(10):1994] Obesity (Silver Spring) 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lighter J, Phillips M, Hochman S. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020;71(15):896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernández-Garduño E. Obesity is the comorbidity more strongly associated for COVID-19 in Mexico. A case-control study. Obes Res Clin Pract. 2020;14(4):375–379. doi: 10.1016/j.orcp.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bello-Chavolla OY, Bahena-López JP, NE Antonio-Villa. Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metab. 2020;105(8):2752–2761. doi: 10.1210/clinem/dgaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernández-Galdamez DR, González-Block MÁ, Romo-Dueñas DK. Increased risk of hospitalization and death in patients with COVID-19 and pre-existing noncommunicable diseases and modifiable risk factors in Mexico. Arch Med Res. 2020;51(7):683–689. doi: 10.1016/j.arcmed.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giannouchos TV, Sussman RA, Mier JM, Poulas K, Farsalinos K. Characteristics and risk factors for COVID-19 diagnosis and adverse outcomes in Mexico: an analysis of 89,756 laboratory-confirmed COVID-19 cases. Eur Respir J. 2020;56(6) doi: 10.1183/13993003.02144-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denova-Gutiérrez E, López-Gatell H, Alomia-Zegarra JL. The association of obesity, type 2 diabetes, and hypertension with severe COVID-19 on admission among Mexicans. Obesity (Silver Spring) 2020;28(10):1826–1832. doi: 10.1002/oby.22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Encuesta de salud Y nutrición. Ensanut. https://ensanut.insp.mx/. Accessed June 1, 2020.
- 30.Mortalidad. INEGI.https://www.inegi.org.mx/temas/mortalidad/. Updated August 15, 2020. Accessed June 1, 2020.
- 31.Datos abiertos dirección general de epidemiología. Gobierno de México.https://www.gob.mx/salud/documentos/datos-abiertos-152127. Updated August 15, 2020. Accessed June 1, 2020.
- 32.Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. 3rd ed. Chapman and Hall/CRC; New York, NY: 2013. Bayesian Data Analysis. [DOI] [Google Scholar]
- 33.RStan: the R Interface to Stan. R package version 2.21.2. Stan Development Team. http://mc-stan.org/. Updated August 15, 2020. Accessed June 1, 2020.
- 34.Bürkner PC. Advanced Bayesian multilevel modeling with the R package brms. R J. 2018;10(1):395–411. doi: 10.32614/RJ-2018-017. [DOI] [Google Scholar]
- 35.Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42(8):1–28. doi: 10.18637/jss.v042.i08. [DOI] [Google Scholar]
- 36.Obesity update . 2017. Organisation for Economic Co-operation and Development.http://www.oecd.org/els/health-systems/obesity-update.htm PublishedAccessed November 4, 2020. [Google Scholar]
- 37.Jusidman C. Desigualdad y política social en México. Nueva Soc. 2009;220:190–207. https://search.proquest.com/openview/2d6692a85c6b7213e7d19e06d429ca1c/1.pdf?pq-origsite=gscholar&cbl=13322 Accessed June 1, 2020. [Google Scholar]
- 38.Ortíz-Hernández L, Pérez-Salgado D, Tamez-González S. Desigualdad socioeconómica y salud en Mexico. Rev Med Inst Mex Seguro Soc. 2015;53(3):336–347. https://www.redalyc.org/pdf/4577/457744937015.pdf Accessed June 1, 2020. [PubMed] [Google Scholar]
- 39.Cortés F, Nájera H, Vargas D, Valdez S. Las relaciones sociales y la difusión del contagio municipal por el SARS-CoV-2 en México. Econ UNAM. 2020;17(51):418–436. doi: 10.22201/fe.24488143e.2020.51.577. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.