Abstract
We sequentially assessed the presence of SARS-CoV-2 IgG antibodies in 1253 hospital workers including 1026 HCWs at the University Medical Center Hamburg-Eppendorf at three time points during the early phase of the epidemic. By the end of the study in July 2020, the overall seroprevalence was 1.8% (n = 22), indicating the overall effectiveness of infection control interventions in mitigating coronavirus disease 2019 (COVID-19) in hospital workers.
Keywords: SARS-CoV-2, COVID-19, Seroprevalence, Hospital workers, Healthcare workers, Germany
1. Introduction
Health care workers (HCWs) are at the front line of the coronavirus disease 2019 (COVID-19) pandemic response and disproportionally at risk of contracting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) due to occupational exposure to droplets, aerosols and contaminated surfaces (Razzini et al., 2020). Reports about high infection rates of HCWs in many countries have illustrated the challenges of HCW protection, especially with regard to the risk of infections from pre-symptomatic COVID-19 patients (Arons et al., 2020; Zhan et al., 2020). Besides their personal health risk, infected HCWs may contribute to critical staff shortages and pose a risk for vulnerable patients and fellow HCWs. Serological surveillance of hospital workers allows for an estimation of the overall infection rate and for early identification of professional groups and hospital environments at increased risk for contracting COVID-19. This approach is paramount to evaluate and adjust infection control measures to mitigate nosocomial transmission of SARS-CoV-2. By now, several studies have demonstrated high seroprevalence in HCWs in countries most affected by COVID-19 (Garcia-Basteiro et al., 2020; Sotgiu et al., 2020; Rudberg et al., 2020; Houlihan et al., 2020; Martin et al., 2020; Moscola et al., 2020; Stubblefield et al., 2020) but data from German HCWs are scarce (Korth et al., 2020). By 17th July 2020, a total of 5232 cases had been confirmed amongst the 1.9 million inhabitants of the city of Hamburg, which constituted one of the highest infection rates in Germany (Robert Koch Institute, 2020). At the University Medical Center Hamburg-Eppendorf, more than 170 COVID-19 patients were treated during the same time period. The aim of our study was to longitudinally assess the SARS-CoV-2 seroprevalence and seroconversion rates in hospital workers at our tertiary care center at three time points during the early phase of the epidemic.
2. Material and methods
2.1. Recruitment of the study population
The study protocol was reviewed and approved by the Ethics Committee of the Medical Council of Hamburg (PV 7298). Participants were recruited by informing employees of the University Medical Center Hamburg-Eppendorf both in person and via an internal email newsletter and written informed consent was obtained by all study participants prior to recruitment. Both at baseline (Screening period 1 (SP 1): 20th March – 9th April) and during the first (SP 2: 20th April 20 – 8th May) and second (SP 3: 22nd June – 17th July) follow-up visit serum samples were drawn. Study participants were contacted by phone and email to remind them of the follow-up visits to reduce selection bias.
2.2. Data collection
On the day of recruitment, we collected demographic and general work-related data using a standardized questionnaire. Hospital workers were classified as HCWs if they reported regular occupational contact with patients. Study participants were asked to report only their main clinical role. Since some employees work in different departments in parallel, study participants could to assign themselves to different locations of work. In addition, we used another questionnaire to assess known and possible past contact to COVID-19 patients with or without sufficient personal protective equipment (PPE) as well as the presence of symptoms during the prior 4 weeks at SP 1 and SP 2 and during the prior 8 weeks at SP 3 respectively. Fever, cough, and dyspnea were classified as typical symptoms while rhinorrhea, sore throat, headache, stomach pain, joint and muscle pain, nausea, and diarrhea and were considered as uncharacteristic symptoms. Questionnaires were available both paper-based and on online REDcap electronic data capture tools hosted at our center. Study participants with positive SARS-CoV-2 IgG were contacted again by phone to assess the probable source of infection and to ask if nasopharyngeal swabs for RT-PCR had been performed.
2.3. ELISA
At all three screening periods, serum samples were drawn from all study participants. A semi-quantitative SARS-CoV-2 immunoglobin (Ig) G enzyme-linked immunosorbent assay (ELISA) targeting the S1-Domain of the S-protein spike protein subunit (Euroimmun Medizinische Labordiagnostika, Lübeck, Germany) was performed according to the manufacturer's protocol. Results are evaluated by calculation of a ratio of the extinction of the serum sample over the extinction of the calibrator. According to the manufacturer, a ratio <0.8 is considered negative, ≥0.8 and < 1.1 borderline, and ≥1.1 positive. The manufacturer reports a specificity 99.6% and sensitivity of 94.4% 10 days after the onset of symptoms. However, we used a more stringent cut-off value of ≥1.5 for positive results, which has been shown to display a specificity of 100% (Pflüger et al., 2020) to account for the low prevalence environment. Given the longitudinal character of our study, we do not expect a relevant loss of test sensitivity. Study participants were classified as seropositive in all future sampling periods if they had an IgG antibody titer ≥1.5 at least once, even if antibody titers waned over time.
2.4. Statistical analyses
Seroprevalence of antibodies against SARS-CoV-2 at different time points was calculated as proportions with 95% confidence interval (CI). Continuous variables were expressed as mean and interquartile range (IQR) and compared with student's t-test. Categorical variables were expressed as number (%) and compared by Chi-square or Fisher's exact test. P values less than 0.05 were considered statistically significant. Figures were designed using GraphPad Prism version 8 for macOS (GraphPad Software, La Jolla, California, USA). All other analyses were performed using SPSS, version 21.0 (IBM Corp., Armonk, New York, USA).
3. Results
3.1. Characterization of the study population
A total of 1253 individuals were included during SP 1, which represent around 11% of all employees at the University Medical Center Hamburg-Eppendorf. The majority of study participants were HCWs (n = 1026) who were recruited at different departments of our hospital including regular wards (n = 332), outpatient clinics (n = 234), the intensive care unit (n = 130), and the emergency department (n = 63) (Table 1 ). The remainder were researchers, administrative staff, or belonged to other occupational employee groups not directly involved in patient care. While we did not recruit a strictly representative sample of hospital workers at our institution, the relative proportion of different professional groups in our study cohort (35.4% nurses, 21.9% medical doctors, 42.7% others) fairly well matched the overall distribution at our hospital (30.1% nurses, 25.8% medical doctors, 44.1% others). Information on whether study participants had been knowingly in close contact with COVID-19 patients was available for 1163 (92.8%) study participants (Table 2 ). Of those, 25.4% (n = 295) reported to having been in direct contact with COVID-19 cases. A total of 226 individuals were only involved in the care of patients with diagnosed SARS-CoV-2 infections and were thus equipped with appropriate PPE. Another 69 study participants had contact with patients who were later diagnosed with COVID-19, which is why these HCWs did not wear adequate PPE at the time of exposure. Occupational contact to infected colleagues was reported by 12.0% (n = 140), community contact by 3.0% (n = 35) of participants.
Table 1.
Characterization of the study population.
| Total | Seropositive | p | |
|---|---|---|---|
| Total, n | 1253 | 22 (1.8) | |
| Age | |||
| Median | 36 | 33 | 0.33 |
| IQR | 29; 48 | 29; 40 | |
| Sex | |||
| Male | 308 | 8 (2.5) | 0.21 |
| Female | 934 | 14 (1.5) | 0.23 |
| Diverse | 11 | 0 (0) | 1.0 |
| Clinical role, n (%) | |||
| Nurse | 444 | 10 (2.3) | 0.37 |
| Physician | 275 | 9 (3.3) | 0.04 |
| Medical technician | 105 | 0 (0) | 0.25 |
| Medical student | 73 | 2 (2.7) | 0.37 |
| Physiotherapist | 15 | 0 (0) | 1.0 |
| Other HCW | 114 | 1 (0.3) | 0.25 |
| Non-HCW | 227 | 1 (0.4) | 0.16 |
| Location of work, n (%) | |||
| Regular ward | 332 | 9 (2.7) | 0.14 |
| Outpatient clinic | 234 | 5 (2.1) | 0.58 |
| Intensive care unit | 130 | 2 (1.5) | 1.0 |
| Operating room | 111 | 1 (0.9) | 0.71 |
| Emergency department | 63 | 1 (1.6) | 1.0 |
| Other | 354 | 4 (1.1) | 0.35 |
Baseline characterization of the study population and association of variables between seropositive and seronegative individuals; IQR = interquartile range.
Table 2.
Contact to COVID-19 cases reported by the study participants.
| Total | Seropositive, n (%) | p | |
|---|---|---|---|
| Any known contact | 417 | 18 (4.3) | <0.001 |
|
226 | 5 (2.2) | 0.79 |
|
69 | 6 (8.7) | 0.001 |
|
140 | 3 (2.1) | 0.74 |
|
35 | 4 (11.4) | 0.003 |
Contact to COVID-19 cases reported by seropositive and -negative study participants and association of variables between seropositive and seronegative individuals. Information on contact to COVID-19 cases was available for 1163 study participants.
3.2. Serological results
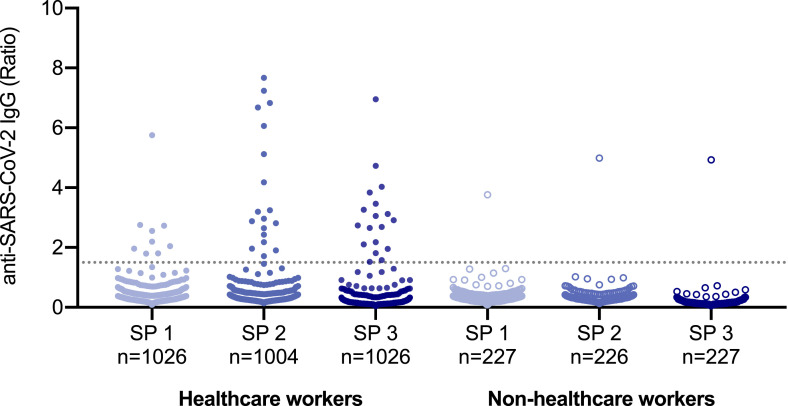
A total of 23 (1.8%) participants missed the first follow-up visit, but all study participants attended the second follow up visit. At the initial screening period, 0.8% (n = 10, 95% confidence interval [CI] 0.3 to 1.3) of the study participants were found to be SARS-CoV-2 seropositive (Fig. 1 ). Another ten individuals showed seroconversion at SP 2, giving a seroprevalence of 1.6% (95% CI 0.9 to 2.3). At SP 3, two more individuals had developed anti-SARS-CoV-2 IgG antibodies, thus the overall seroprevalence was 1.8% (n = 22, 95% CI 1.0 to 2.5) by the end of the study.
Fig. 1.
Serologic results of the study population.
No significant differences in age or the relative distribution of sex were observed between seronegative and seropositive study participants. Seropositivity was increased in physicians (3.3% vs 1.3%; p = 0,04), while none of the other professional categories and none of the different locations of work showed any association with the presence of antibodies to SARS-CoV-2.
The most probable source of infection could be identified for the majority of seropositive study participants (Table 2). Six HCWs presumably got infected when caring for pre-symptomatic patients who were later diagnosed with COVID-19 and had thus not used appropriate PPE at the time of exposure. Five HCWs most likely contracted SARS-CoV-2 while caring for COVID-19 patients with adequate PPE. Other probable infection routes in our cohort were close contact with infected colleagues (n = 3), community transmission in high-risk regions (n = 3), and infected household contacts (n = 1). The remainder of four seropositive employees did not report any known contact to COVID-19 patients, and the probable source of infection could not be established. Seroprevalence was higher in study participants who reported contact with COVID-19 patients (4.3%; p < 0.001). The subgroup of study participants who reported caring for pre-symptomatic COVID-19 and did not use appropriate PPE at the time of exposure showed the highest seroprevalence (5.4%; p = 0.001). Of note, no increased rate of anti-SARS-CoV-2 IgG-antibodies was observed in the subgroup of hospital workers who reported providing direct clinical care to diagnosed COVID-19 patients with appropriate PPE (2.2%; p = 0.79) or contact to SARS-CoV-2 infected colleagues (2.1%; n = 0.74). However, study participants who reported delivering care for pre-symptomatic COVID-19 and did therefore not use appropriate PPE at the time of exposure had a significantly higher seroprevalence (5.4% vs 27.3%; p = 0.001). Moreover, community contact with COVID-19 cases was a significant risk factor for seropositivity in our study cohort (2.7% vs 18.2%, p = 0.003).
3.3. Information on nasopharyngeal swabs of seropositive individuals
Out of all seropositive study participants, a total of 40.9% (n = 9) had been diagnosed with SARS-CoV-2 infection by RT-PCR and thus had been placed under quarantine during the study period. Another six seropositive HCWs reported that they had at least one nasopharyngeal swab performed due to symptoms or close contact with a COVID-19 case but were tested negative at that time. The remainder of seven seropositive individuals had not been tested by RT-PCR at all.
3.4. Symptoms
Complete information on symptoms throughout the study period was provided by 1094 (87.3%) of the study participants (Table 2). The overall rate of hospital workers who reported typical SARS-CoV-2 symptoms did not differ between seronegative and seropositive individuals (54.6% vs 63.6%; p = 0.52) (Table 3 ). Only fever (9.9% vs. 36.4%; p = 0.001) and muscle aches (22.6% vs 59.1%; p < 0.001) were more commonly reported in hospital workers with anti-SARS-CoV-2 antibodies.
Table 3.
Symptoms reported by seropositive and -negative study participants.
| Seronegative | Seropositive | p | |
|---|---|---|---|
| Typical symptoms, n (%) | 597 (54.6) | 14 (63.6) | 0.52 |
|
108 (9.9) | 8 (36.4) | 0.001 |
|
511 (46.7) | 11 (50.0) | 0.83 |
|
254 (23.2) | 8 (36.4) | 0.12 |
| Rhinorrhea, n (%) | 736 (67.3) | 16 (72.7) | 0.65 |
| Sore throat, n (%) | 576 (52.7) | 11 (50.0) | 0.83 |
| Headache, n (%) | 717 (65.5) | 15 (68.2) | 1.0 |
| Abdominal pain, n (%) | 297 (26.9) | 8 (36.4) | 0.34 |
| Muscle aches, n (%) | 247 (22.6) | 13 (59.1) | <0.001 |
| Nausea, n (%) | 211 (19.3) | 8 (36.4) | 0.06 |
| Diarrhea, n (%) | 299 (27.3) | 8 (36.4) | 0.34 |
Respective symptoms as stated by the study participants at SP 1 or SP 2 for the preceding month and at SP 3 for the preceding 2 months. Complete information was provided by a total of 1094 seronegative and 22 seropositive participants.
4. Discussion
The first results of our ongoing study demonstrate a low overall SARS-CoV-2 seroprevalence of 1.8% (n = 22, 95% CI 1.0 to 2.5) in a cohort of 1253 hospital workers at the University Medical Center Hamburg-Eppendorf at the early phase of the COVID-19 epidemic. Remarkably, seroprevalence was not significantly increased in HCWs directly involved in patient care compared to other hospital workers. These low overall infection rates are despite more than 170 patients with SARS-CoV-2 infections that had been treated at our center during the study period. Notwithstanding considerable exposure to COVID-19, infection rates in our study were substantially lower than in HCWs from Belgium (12.6%) (Martin et al., 2020), Spain (11.2%) (Garcia-Basteiro et al., 2020), Italy (14.4%) (Sotgiu et al., 2020), Sweden (19.1%) (Rudberg et al., 2020) the United Kingdom (10.8%–43.5%) (Martin et al., 2020; Houlihan et al., 2020) and the United Stated of America (7.6%–13.7%) (Moscola et al., 2020; Stubblefield et al., 2020). Those marked differences in infection rates may be partially explained by local differences in community transmission: population seroprevalence has been estimated to be 8.0% in Belgium, 5.5% in Spain, 4.6% in Italy, 3.7% in Sweden, 5.1% in the United Kingdom, and only 0.85% in Germany by May 2020 (Flaxman et al., 2020). However, our results confirm the findings of a previous cross-sectional study that detected anti-SARS-CoV-2 in 1.6% (n = 5) of 316 HCWs at another German tertiary care hospital (Korth et al., 2020) and are likely also an indicator that local infection control interventions were effective in protecting HCWs from COVID-19. A timeline of the most important infection control measures that were undertaken at our institution and in Hamburg, Germany are listed in Table 4 .
Table 4.
Infection control interventions at the University Medical Center Hamburg-Eppendorf and in Hamburg, Germany.
| Interventions at the University Medical Center Hamburg-Eppendorf | |
|---|---|
| 27 February | Opening of an on-campus COVID-19 testing clinic |
| 11 March | Cancellation of all meetings not directly related to patient care |
| 18 March | Mandatory wearing of face masks in the emergency department |
| 19 March | Implementation of visitor restrictions |
| 22 March | Mandatory wearing of face masks in all clinical settings |
| 10 April | Mandatory wearing of FFP2 masks at all oncology and hematology wards and clinics |
| 20 April | Universal RT-PCR admission screening of patients |
| 11 May | Universal RT-PCR screening of employees caring for COVID-19 patients or vulnerable patients |
| Interventions in Hamburg, Germany | |
| 16 March | Closure of educational facilities |
| 22 March | Stay at home order |
| 27 April | Mandatory wearing of face masks |
Infection control interventions at the University Medical Center Hamburg-Eppendorf and in Hamburg, Germany.
It has been shown that stringent use of appropriate PPE by HCWs when providing direct care to patients with suspected or confirmed SARS-CoV-2 infections effectively prevents transmission (Wang et al., 2020). Indeed, we did not observe a significant association between the rate of anti-SARS-CoV-2 IgG-antibodies and contact with COVID-19 patients when using appropriate PPE. However, five HCWs in our study presumably contracted SARS-CoV-2 despite the use of appropriate PPE when caring for COVID-19 patients. We cannot determine with absolute certainty whether those HCWs contracted COVID-19 while caring for infected patients, and whether infection control standards were maintained by these individuals at all times. Our observations nevertheless demonstrate that awareness for SARS-CoV-2 infection is crucial even when appropriate PPE is used.
Moreover, another six HCWs presumably contracted SARS-CoV-2 infections when delivering care for pre-symptomatic patients who were not suspected COVID-19 cases at the time of exposure. Indeed, such contacts with yet undiagnosed patients without appropriate PPE were associated with seropositivity. Our observations are in line with the results of previous studies, which have demonstrated that pre-symptomatic patients play a pivotal role in both community and healthcare facility associated transmission of SARS-CoV-2 (Arons et al., 2020). To detect those pre-symptomatic patients pre-symptomat, universal admission screening for all hospitalized patients has been suggested (Sutton et al., 2020) and was also established at our institution by 20 April.
While exposure- and symptom-based SARS-CoV-2 RT-PCR is the mainstay of HCW surveillance, previous studies have demonstrated potential challenges and limitations of symptom-based screening efforts due to a lack of both sensitivity and specificity of symptoms suggestive of COVID-19 (Black et al., 2020). Our findings highlight those limitations. Indeed, only 68.2% of seropositive study participants had prior RT-PCR testing for SARS-CoV-2, and only 40.9% had a positive test result. Fever, cough, or dyspnea - symptoms generally considered suggestive of COVID 19 – were reported by only 63.3% of individuals with anti-SARS-CoV-2 antibodies. On the other hand, 54.6% of seronegative individuals reported at least one of those symptoms. Fever, but not cough or dyspnea were significantly associated with the detection of anti-SARS-CoV-2 antibodies. While testing of only symptomatic HCWs might miss a substantial number of COVID-19 cases, expanding RT-PCR surveillance to all asymptomatic HCWs might be a powerful, but resource- and cost-intensive tool to reducing the risk of nosocomial transmission.
Our study is subject to a number of limitations. Firstly, since various infection control measures were sequentially adopted and the study did not include a control group, we are not able to answer the question which of the described interventions are the most effective in preventing healthcare transmission. However, our results suggest that the overall approach in our institution was effective in mitigating SARS-CoV-2 transmission to HCWs. This is also supported by the fact that after the adoption of all infection control interventions, only two more seroconversions were observed in the two months between SP 2 and SP 3. Secondly, when analyzing serological data, we did not use the cut-off ≥1.1 as provided by the manufacturer, but an optimized IgG ratio of ≥1.5 to ensure maximum specificity of test results. Given the longitudinal character of our study and the expected increase of IgG ratios over time in infected individuals, we did not expect any relevant loss of test sensitivity. When analyzing serological data with the cut-off value of ≥1.1, the overall seroprevalence was 2.8% (n = 36). Thirdly, we did not recruit a strictly representative sample of hospital workers at our institution and participation was voluntary, which limits the overall generalizability of our results.
5. Conclusions
In conclusion, the SARS-CoV-2 seroprevalence of hospital workers at our tertiary care hospital in Germany was 1.8% during the early phase of the COVID-19 pandemic. Local infection control interventions appear to be generally effective in mitigating nosocomial transmission of SARS-CoV-2. However, our study also highlights the challenges and limitations of those measures. Awareness for SARS-CoV-2 infections remains crucial for HCWs on COVID-19 wards as well as for other hospital workers without known contact to SARS-CoV-2 infected patients.
Declaration of competing interest
AWL had a material transfer agreement with Euroimmun GmbH. Ten ELISA plates as well as technical help were provided by the company. All other authors declare no support from any organisation for the submitted work. All authors declare no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgments
We thank Sabrina Kreß, Jennifer Wigger, Martina Schulz, Corinna Eggers, Angelika Schmidt, Silke Kummer, Robin Woost, Nils Dittberner and Marcus Wurlitzer for excellent technical assistance. We thank all study participants and departments of the University Medical Center Hamburg-Eppendorf for active participation in the study. TTB, MML, ML, JKK, JSzW and AWL are funded by the German Center for Infection Research (DZIF). MMA is funded by the DZIF TTU 1.702 and the DZIF TTU 1.921 (First Steps to Fight SARS-CoV-2 (FITS)).
References
- Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R., Taylor J., Spicer K., Bardossy A.C., Oakley L.P., Tanwar S., Dyal J.W., Harney J., Chisty Z., Bell J.M., Methner M., Paul P., Carlson C.M., McLaughlin H.P., Thornburg N., Tong S., Tamin A., Tao Y., Uehara A., Harcourt J., Clark S., Brostrom-Smith C., Page L.C., Kay M., Lewis J., Montgomery P., Stone N.D., Clark T.A., Honein M.A., Duchin J.S., Jernigan J.A. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N. Engl. J. Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J.R.M., Bailey C., Przewrocka J., Dijkstra K.K., Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395:1418–1420. doi: 10.1016/S0140-6736(20)30917-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaxman S., Mishra S., Gandy A., Unwin H.J.T., Mellan T.A., Coupland H., Whittaker C., Zhu H., Berah T., Eaton J.W., Monod M., Ghani A.C., Donnelly C.A., Riley S., Vollmer M.A.C., Ferguson N.M., Okell L.C., Bhatt S. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020;584:257–261. doi: 10.1038/s41586-020-2405-7. [DOI] [PubMed] [Google Scholar]
- Garcia-Basteiro A.L., Moncunill G., Tortajada M., Vidal M., Guinovart C., Jiménez A., Santano R., Sanz S., Méndez S., Llupià A., Aguilar R., Alonso S., Barrios D., Carolis C., Cisteró P., Chóliz E., Cruz A., Fochs S., Jairoce C., Hecht J., Lamoglia M., Martínez M.J., Mitchell R.A., Ortega N., Pey N., Puyol L., Ribes M., Rosell N., Sotomayor P., Torres S., Williams S., Barroso S., Vilella A., Muñoz J., Trilla A., Varela P., Mayor A., Dobaño C. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat. Commun. 2020;11:3500. doi: 10.1038/s41467-020-17318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlihan C.F., Vora N., Byrne T., Lewer D., Kelly G., Heaney J., Gandhi S., Spyer M.J., Beale R., Cherepanov P., Moore D., Gilson R., Gamblin S., Kassiotis G., McCoy L.E., Swanton C., Crick C.-C., Hayward A., Nastouli E., Investigators S. Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers. Lancet. 2020;396:e6–e7. doi: 10.1016/S0140-6736(20)31484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert Koch Institute Coronavirus disease 2019 (COVID-19) daily situation report of the robert Koch Institute, 17.07.2020. 2020. https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/2020-07-17-en.pdf?__blob=publicationFile 01.09.20.
- Korth J., Wilde B., Dolff S., Anastasiou O.E., Krawczyk A., Jahn M., Cordes S., Ross B., Esser S., Lindemann M., Kribben A., Dittmer U., Witzke O., Herrmann A. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J. Clin. Virol. 2020;128:104437. doi: 10.1016/j.jcv.2020.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Montesinos I., Dauby N., Gilles C., Dahma H., Van Den Wijngaert S., De Wit S., Delforge M., Clumeck N., Vandenberg O. Dynamics of SARS-CoV-2 RT-PCR positivity and seroprevalence among high-risk health care workers and hospital staff. J. Hosp. Infect. 2020;106(1):102–106. doi: 10.1016/j.jhin.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscola J., Sembajwe G., Jarrett M., Farber B., Chang T., McGinn T., Davidson K.W. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York city area. J. Am. Med. Assoc. 2020;324:893–895. doi: 10.1001/jama.2020.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflüger L.S., Bannasch J.H., Brehm T.T., Pfefferle S., Hoffmann A., Nörz D., van der Meirschen M., Kluge S., Haddad M., Pischke S., Hiller J., Addo M.M., Lohse A.W., Wiesch J.S.z., Peine S., Aepfelbacher M., Lütgehetmann M. Clinical evaluation of five different automated SARS-CoV-2 serology assays in a cohort of hospitalized COVID-19 patients. J. Clin. Virol. 2020;130 doi: 10.1016/j.jcv.2020.104549. [published online ahead of print, 2020 Jul 17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzini K., Castrica M., Menchetti L., Maggi L., Negroni L., Orfeo N.V., Pizzoccheri A., Stocco M., Muttini S., Balzaretti C.M. SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19 ward of a hospital in Milan, Italy. Sci. Total Environ. 2020;742:140540. doi: 10.1016/j.scitotenv.2020.140540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudberg A.S., Havervall S., Månberg A., Jernbom Falk A., Aguilera K., Ng H., Gabrielsson L., Salomonsson A.C., Hanke L., Murrell B., McInerney G., Olofsson J., Andersson E., Hellström C., Bayati S., Bergström S., Pin E., Sjöberg R., Tegel H., Hedhammar M., Phillipson M., Nilsson P., Hober S., Thålin C. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat. Commun. 2020;11:5064. doi: 10.1038/s41467-020-18848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotgiu G., Barassi A., Miozzo M., Saderi L., Piana A., Orfeo N., Colosio C., Felisati G., Davì M., Gerli A.G., Centanni S. SARS-CoV-2 specific serological pattern in healthcare workers of an Italian COVID-19 forefront hospital. BMC Pulm. Med. 2020;20:203. doi: 10.1186/s12890-020-01237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubblefield W.B., Talbot H.K., Feldstein L., Tenforde M.W., Rasheed M.A.U., Mills L., Lester S.N., Freeman B., Thornburg N.J., Jones I.D., Ward M.J., Lindsell C.J., Baughman A., Halasa N., Grijalva C.G., Rice T.W., Patel M.M., Self W.H. Seroprevalence of SARS-CoV-2 among frontline healthcare personnel during the first month of caring for COVID-19 patients - nashville, Tennessee. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa936. [published online ahead of print, 2020 Nov 19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton D., Fuchs K., D'Alton M., Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N. Engl. J. Med. 2020;382:2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Ferro E.G., Zhou G., Hashimoto D., Bhatt D.L. Association between universal masking in a health care system and SARS-CoV-2 positivity among health care workers. J. Am. Med. Assoc. 2020;324(7):703–704. doi: 10.1001/jama.2020.12897. [published online ahead of print, 2020 Jul 14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan M., Qin Y., Xue X., Zhu S. Death from covid-19 of 23 health care workers in China. N. Engl. J. Med. 2020;382(23):2267–2268. doi: 10.1056/NEJMc2005696. [DOI] [PMC free article] [PubMed] [Google Scholar]