Abstract
Aims
Metformin exerts anti-inflammatory and immunosuppressive effects. We addressed the impact of prior metformin use on prognosis in patients with type 2 diabetes hospitalised for COVID-19.
Methods
CORONADO is a nationwide observational study that included patients with diabetes hospitalised for COVID-19 between March 10 and April 10, 2020 in 68 French centres. The primary outcome combined tracheal intubation and/or death within 7 days of admission. A Kaplan-Meier survival curve was reported for death up to day 28. The association between metformin use and outcomes was then estimated in a logistic regression analysis after applying a propensity score inverse probability of treatment weighting approach.
Results
Among the 2449 patients included, 1496 were metformin users and 953 were not. Compared with non-users, metformin users were younger with a lower prevalence of diabetic complications, but had more severe features of COVID-19 on admission. The primary endpoint occurred in 28.0% of metformin users (vs 29.0% in non-users, P = 0.6134) on day 7 and in 32.6% (vs 38.7%, P = 0.0023) on day 28. The mortality rate was lower in metformin users on day 7 (8.2 vs 16.1%, P < 0.0001) and on day 28 (16.0 vs 28.6%, P < 0.0001). After propensity score weighting was applied, the odds ratios for primary outcome and death (OR [95%CI], metformin users vs non-users) were 0.838 [0.649−1.082] and 0.688 [0.470−1.007] on day 7, then 0.783 [0.615−0.996] and 0.710 [0.537−0.938] on day 28, respectively.
Conclusion
Metformin use appeared to be associated with a lower risk of death in patients with diabetes hospitalised for COVID-19.
Keywords: COVID-19, Death, Mechanical ventilation, Metformin, Propensity score, Type 2 diabetes
Introduction
Type 2 diabetes (T2D) and obesity are among the most important comorbidities linked to the severity of Coronavirus Disease 2019 (COVID-19) [1]. In the treatment of T2D, metformin is the recommended first-line pharmacologic agent according to most current guidelines [2]. Indeed, metformin has beneficial effects on glucose control (HbA1c) with no risk of hypoglycaemia or weight gain, is inexpensive, and may reduce the risk of cardiovascular events and death [3].
In fact, metformin is probably more than a “cardiometabolic” drug. Indeed, increasing evidence from both preclinical and clinical studies also points to the benefits of metformin in nephropathy [4], cancer prevention and/or treatment [5], neurodegenerative diseases [6] and ageing [7]. Ultimately, metformin has been recognized as a cellular protector independently of prevailing blood glucose concentration [8] since it enhances mitochondrial metabolism (thus attenuating the harmful effects of stress on mitochondrial function), potentiates autophagy through adenosine monophosphate-activated protein kinase (AMPK) activation, and scavenges reactive oxygen species [9]. This could explain why metformin has been shown to be associated with a relative reduction in mortality among patients with diabetes admitted to intensive care units (ICU) [10].
In the current context of COVID-19, it is important to remember that a dimethylbiguanide preparation (flumamine) was first launched to treat influenza virus infections (in the 1940s) [11]. Since then, metformin has demonstrated its adjuvant efficacy in malaria, tuberculosis, Legionella pneumonia, hepatitis C virus infection, and Zika virus infection, suggesting its additional potential as an antimicrobial therapy [12]. More specifically, metformin is reportedly one of the drugs that targets human host factors of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) via the mTOR pathway [13]. In addition, metformin exerts direct and indirect immunosuppressive effects [14] as illustrated by its ability to reduce the secretion of pro-inflammatory cytokines by macrophages, irrespective of diabetes status [15]. Of particular interest is the activity of metformin on the mitochondrial ROS/Ca2+ release-activated Ca2+ channels/IL-6 cascade that may mitigate the aggressive pro-inflammatory/pro-thrombotic nature of COVID-19 [16]. Finally, metformin is known to reverse established fibrosis in various lung models by facilitating the deactivation and apoptosis of myofibroblasts and accelerating fibrosis resolution by inducing myofibroblast-to-lipofibroblast transdifferentiation [17].
Considering all these effects, metformin may be a good drug candidate to attenuate the severity of COVID-19 [18]. Using the large nationwide CORONADO (Coronavirus SARS-CoV-2 and Diabetes Outcomes) study [19], we therefore aimed, in this post-hoc analysis, to assess whether prior metformin use was associated with improved prognosis in patients with T2D hospitalised for COVID-19 by using a propensity score approach.
Patients and methods
Population
The CORONADO (Coronavirus SARS-CoV-2 and Diabetes Outcomes) study is a nationwide multicentre observational study that was conducted in order to gather information on the phenotypic characteristics and main outcomes of COVID-19 in patients with diabetes who were admitted to the hospital for COVID-19 between March 10th, and April 10th, 2020. Interim results for the first 1317 patients, who were admitted to the hospital between March 10th and March 31st, have already been reported [19]. For the current post-hoc analysis of the CORONADO study, we restricted the analysis to all of the CORONADO participants with T2D and available information on routine metformin use.
Briefly, investigators in 68 French hospitals treating inpatients with COVID-19 were contacted to assess the possibility of participation in the CORONADO study. The main inclusion criteria were (i) inpatient admission to a dedicated COVID-19 unit with biologically or clinically/radiologically confirmed COVID-19 diagnosis and (ii) known diabetes or newly diagnosed diabetes on admission, defined as HbA1c ≥48 mmol/mol (≥ 6.5%). Biologically confirmed COVID-19 was defined as a nasopharyngeal swab specimen that tested positive in a reverse-transcriptase polymerase-chain-reaction assay and clinically / radiologically confirmed COVID-19 as clinical features and radiological findings that were compatible with COVID-19. The main exclusion criteria were (i) opposition to data collection by the patient, (ii) being under legal protection and (iii) age under 18 years.
The CORONADO (ClinicalTrials.gov Identifier: NCT04324736) study was sponsored by Nantes University Hospital (Centre Hospitalier Universitaire de Nantes). It was designed in accordance with the Declaration of Helsinki and conducted in accordance with good clinical practice guidelines and French legislation on clinical research and data protection. Approvals were obtained from an independent ethics committee (GNEDS: Groupe Nantais d'Ethique dans le Domaine de la Santé; Ref. CORONADOV2), the CEREES (Comité d'Expertise pour les Recherches, les Etudes et les Evaluations dans le Domaine de la Santé; n° INDS [Institut National des Données de Santé]:1544730) and the CNIL (Commission Nationale de l'Informatique et des Libertés; DR-2020-155/920129). Written informed consent was waived by the CNIL and the GNEDS but ‘oral non-opposition to participate’ was also collected when possible. Moreover, all living patients who were unable to give consent on admission received information about their inclusion in the CORONADO study before discharge and therefore had a clear and free choice to confirm their participation or opposition to the use of their data. Any patient who expressed his/her opposition to data collection, even after hospital discharge, was excluded from the study.
Data collection
Clinical research associates and trained physicians extracted data on demographics (age, sex, ethnicity, BMI), diabetes history (classification of diabetes type, including T2D, as per the medical file, duration of diabetes, recent glycaemic control – i.e. HbA1c measurement, microvascular and macrovascular complications), comorbidities, medications on admission as well as COVID-19 clinical, radiological and biological features on admission and during the hospital stay. The HbA1c value was that obtained during the first 7 days of hospitalisation or, if not available, the most recent value in up to 6 months before admission. Estimated glomerular filtration rate (eGFR, calculated using the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] formula) value was the most recent preceding hospitalisation. Microvascular complications were defined as (i) severe diabetic retinopathy (proliferative retinopathy and/or laser photocoagulation and/or clinically significant macular oedema requiring laser and/or intravitreal injections) and/or (ii) diabetic kidney disease [DKD] (proteinuria [albumin excretion rate ≥ 300 mg/24 h; urinary albumin/creatinine ratio ≥300 mg/g creatinine or > 30 mg/mmol creatinine; proteinuria ≥500 mg/24 h] and/or eGFR ≤60 ml/min/1.73 m²) and/or iii) history of diabetic foot ulcer. Macrovascular complications were defined as (i) ischaemic heart disease (acute coronary syndrome and/or coronary artery revascularisation) and/or (ii) cerebrovascular disease (stroke and/or transient ischemic attack) and/or (iii) peripheral artery disease (amputation owing to ischaemic disease and/or lower limb artery revascularisation).
Metformin exposure
All routine medications prescribed prior to hospitalisation were identified by noting prescription drugs on admission and through examination of the medical file, with possible questioning of GPs or pharmacists, if deemed necessary.
Outcomes
The primary endpoint was a composite of tracheal intubation for mechanical ventilation and death within 7 days of admission. Secondary endpoints included the same composite of tracheal intubation for mechanical ventilation and death within 28 days of admission; tracheal intubation up to day 7 and death up to day 7; tracheal intubation up to day 28 and death up to day 28. The aim of this study was to compare these major outcomes between metformin users (those who were taking metformin on admission) and patients without metformin on admission. Patients with T2D were therefore divided into two groups according to the use of metformin on admission.
Statistical analysis
Baseline demographics and clinical characteristics were expressed as mean ± standard deviation or as median [25th–75th percentile] for numerical variables and the frequency (percentage) for categorical variables. Between-group comparisons were performed with Student's t-test for numerical variables and a Fisher’s exact test for categorical variables. For non-normally distributed numerical variables, between-group comparisons were performed using Mann–Whitney–Wilcoxon test.
Kaplan-Meier survival curves were reported for death during hospital stay, up to day 28, and a log-rank test was used to evaluate significance of between-group difference of estimated survival functions.
We used a propensity score approach to limit confounding bias due to baseline characteristics in estimating the association between metformin treatment and outcomes. The use of a propensity score makes it possible to keep all patients in the analysis as opposed to matching, which can lead to a reduction in sample size owing to unmatched patients. First, a propensity score was calculated in order to control for confounding factors that could influence both metformin use and the study endpoints. Propensity score was defined as the probability of being treated with metformin on admission based on the participant's observed covariates. This probability was estimated using a logistic regression model with metformin treatment as the dependent variable and the following characteristics as independent variables: sex, age, body mass index (BMI), arterial hypertension, history of DKD, history of ischemic heart disease, history of heart failure, active cancer, treated obstructive sleep apnoea, use of any of the following drugs/drug classes on admission (renin-angiotensin system blockers including angiotensin-converting enzyme inhibitors [ACEIs], angiotensin receptor blockers [ARBs] and mineralocorticoid receptor antagonists, thiazide diuretics, beta-blockers, insulin, sulfonylurea, dipeptidyl peptidase 4 inhibitors [DPP4] inhibitors, statins, anti-platelet therapy and anticoagulant agents, and corticosteroids). Subsequently, the association between metformin treatment and the primary endpoint was estimated in a logistic regression analysis using the inverse probability of treatment weighting (IPTW) approach [20] with stabilized weights in order to limit the weights of the outliers in the estimate of the Average Treatment Effect (ATE). We decided to use the inverse probability of treatment to weight observations in models rather than other PS methods because it has been described elsewhere as the method of choice to limit bias and variance in the estimate of treatment-effect [21]. Although reported in some studies as providing similar results to balance baseline differences, IPTW has the advantage over propensity score matching by including all patients in the analysis, as discussed above [21]. The results (Model 1) were expressed as inverse probability of treatment-weighted odds ratios (OR) [95% confidence interval (CI)] which were adjusted to the HBA1c level in a sensitivity analysis (Model 2). In another sensitivity analysis, the results of Model 1 were adjusted to eGFR values prior to admission (Model 3) and to diabetes duration (Model 4). Balance between covariates before and after weighting was assessed by the standardized mean differences approach. In another sensitivity analysis, the association between study outcomes on day 28 and metformin treatment was computed using multivariate logistic regression models (without propensity score) adjusted on baseline covariates, baseline covariates + HbA1c, baseline covariates + prior eGFR and baseline covariates + diabetes duration.
The threshold for statistical significance was set 0.05. All statistical tests were two-sided and were performed with R software, version 3.6.2 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria; https://cran.r-project.org/bin/windows/base/old/3.6.2/). The PSW package was used for the propensity score analysis [22].
Results
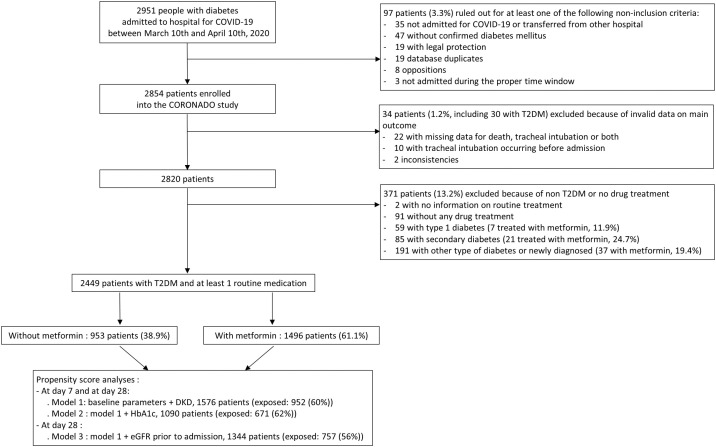
In the CORONADO study, 2951 patients with diabetes hospitalised for COVID-19 were recruited in 68 French centres between March 10th and April 10th, 2020. After further investigations, 97 patients (3.3%) were ruled out for not meeting inclusion criteria, while 34 patients (1.2%) were excluded because of at least one unavailable key clinical outcome. Finally, 2449 patients with T2D and who were taking at least one routine antidiabetic medication were identified and included in the present analysis (see Flow Chart in Fig. 1 ). In the interim analysis, 1166 patients with T2D (47.6%) were already described [19].
Fig. 1.
Flow chart of the study population showing the total population of the CORONADO study, the main reasons for exclusion from the present analysis and the main time points of the study.
Patient baseline characteristics are shown in Table 1 . In the study population, 1496 (61.1%) were treated with metformin before hospitalisation and 953 (38.9%) were not. Compared with metformin non-users, patients receiving metformin were younger and more often men. They were also characterized by a shorter duration of diabetes and a higher HbA1c level. The frequency of diabetic complications, including DKD and other comorbidities (hypertension, heart failure, liver cirrhosis, active cancer, and COPD) was lower in metformin users with the exception of non-alcoholic fatty liver disease (NAFLD) which was more prevalent. Insulin therapy was almost two times less prevalent in metformin users in contrast with a more frequent use of other oral antidiabetic drugs or glucagon-like peptide 1 receptor agonists (GLP-1 RAs).
Table 1.
Characteristics of CORONADO participants prior to admission, according to the use of metformin.
| Metformin use |
|||||
|---|---|---|---|---|---|
| Available data | All (N = 2449) | No (N = 953) | Yes (N = 1496) | P value | |
| Sex (female) | 2449 | 881/2449 (36%) | 385/953 (40.4%) | 496/1496 (33.2%) | 0.0003 |
| Age (years) | 2449 | 70.9 ± 12.5 | 74.6 ± 12.5 | 68.5 ± 11.9 | <0.0001 |
| Ethnicity | 2095 | 0.0001 | |||
| EU | 1229/2095 (58.7%) | 525/817 (64.3%) | 704/1278 (55.1%) | ||
| MENA | 446/2095 (21.3%) | 163/817 (20%) | 283/1278 (22.1%) | ||
| AC | 339/2095 (16.2%) | 101/817 (12.4%) | 238/1278 (18.6%) | ||
| AS | 81/2095 (3.9%) | 28/817 (3.4%) | 53/1278 (4.1%) | ||
| BMI (kg/m²) | 2150 | 28.7 [25.3−32.7] | 28.4 [24.8−32.4] | 28.8 [25.6−32.8] | 0.0683 |
| Diabetes duration (years) | 1483 | 13.9 ± 9.6 | 15.8 ± 10.3 | 12.7 ± 8.9 | <0.0001 |
| HbA1c (mmol/mol) | 1552 | 64.8 ± 20.1 | 62.5 ± 19.7 | 66.3 ± 20.3 | 0.0003 |
| HbA1c (%) | 1552 | 8.1 ± 1.8 | 7.9 ± 1.8 | 8.2 ± 1.9 | 0.0003 |
| eGFR (CKD-EPI), mL/min.1.73m² | 1606 | 68 ± 29.4 | 55.4 ± 31 | 77.6 ± 24.1 | <0.0001 |
| Hypertension | 2429 | 1947/2429 (80.2%) | 792/949 (83.5%) | 1155/1480 (78%) | 0.0012 |
| Dyslipidaemia | 2375 | 1173/2375 (49.4%) | 476/930 (51.2%) | 697/1445 (48.2%) | 0.1655 |
| Current tobacco use | 2005 | 113/2005 (5.6%) | 40/778 (5.1%) | 73/1227 (5.9%) | 0.4873 |
| Microvascular complications | 1724 | 782/1724 (45.4%) | 450/707 (63.6%) | 332/1017 (32.6%) | <0.0001 |
| Severe diabetic retinopathy | 1894 | 120/1894 (6.3%) | 73/736 (9.9%) | 47/1158 (4.1%) | <0.0001 |
| Diabetic kidney disease | 1990 | 668/1990 (33.6%) | 406/766 (53.0%) | 262/1224 (21.4%) | <0.0001 |
| Macrovascular complications | 2308 | 923/2308 (40%) | 463/911 (50.8%) | 460/1397 (32.9%) | <0.0001 |
| Ischemic heart disease | 2382 | 633/2382 (26.6%) | 312/927 (33.7%) | 321/1455 (22.1%) | <0.0001 |
| Cerebrovascular disease | 2394 | 309/2394 (12.9%) | 162/932 (17.4%) | 147/1462 (10.1%) | <0.0001 |
| Peripheral artery disease | 2425 | 276/2425 (11.4%) | 173/945 (18.3%) | 103/1480 (7%) | <0.0001 |
| Comorbidities | |||||
| Heart failure | 2329 | 280/2329 (12%) | 170/907 (18.7%) | 110/1422 (7.7%) | <0.0001 |
| NAFLD | 2078 | 158/2078 (7.6%) | 47/833 (5.6%) | 111/1245 (8.9%) | 0.0067 |
| Liver cirrhosis | 2301 | 62/2301 (2.7%) | 36/909 (4%) | 26/1392 (1.9%) | 0.0035 |
| Active cancer | 2405 | 233/2405 (9.7%) | 111/939 (11.8%) | 122/1466 (8.3%) | 0.0058 |
| COPD | 2394 | 233/2394 (9.7%) | 118/931 (12.7%) | 115/1463 (7.9%) | 0.0001 |
| Treated OSA | 2268 | 255/2268 (11.2%) | 105/894 (11.7%) | 150/1374 (10.9%) | 0.5413 |
| Routine treatment before admission | |||||
| Sulfonylurea/glinide | 2449 | 754/2449 (30.8%) | 255/953 (26.8%) | 499/1496 (33.4%) | 0.0005 |
| DPP-4 inhibitors | 2449 | 596/2449 (24.3%) | 148/953 (15.5%) | 448/1496 (29.9%) | <0.0001 |
| GLP1-RA | 2449 | 242/2449 (9.9%) | 59/953 (6.2%) | 183/1496 (12.2%) | <0.0001 |
| Insulin therapy | 2449 | 902/2449 (36.8%) | 495/953 (51.9%) | 407/1496 (27.2%) | <0.0001 |
| Thiazide diuretics | 2449 | 494/2449 (20.2%) | 147/953 (15.4%) | 347/1496 (23.2%) | <0.0001 |
| Loop diuretics | 2449 | 495/2449 (20.2%) | 329/953 (34.5%) | 166/1496 (11.1%) | <0.0001 |
| MRA | 2449 | 113/2449 (4.6%) | 46/953 (4.8%) | 67/1496 (4.5%) | 0.6937 |
| ARBs and/or ACE inhibitors | 2449 | 1422/2449 (58.1%) | 520/953 (54.6%) | 902/1496 (60.3%) | 0.0056 |
| β-blockers | 2449 | 919/2449 (37.5%) | 437/953 (45.9%) | 482/1496 (32.2%) | <0.0001 |
| Calcium channel-blockers | 2449 | 855/2449 (34.9%) | 363/953 (38.1%) | 492/1496 (32.9%) | 0.0091 |
| Statins | 2449 | 1192/2449 (48.7%) | 439/953 (46.1%) | 753/1496 (50.3%) | 0.0422 |
| Anti-platelet agent | 2449 | 1039/2449 (42.4%) | 432/953 (45.3%) | 607/1496 (40.6%) | 0.0211 |
| Anticoagulation therapy | 2449 | 460/2449 (18.8%) | 267/953 (28%) | 193/1496 (12.9%) | <0.0001 |
| Corticosteroid | 2449 | 129/2449 (5.3%) | 83/953 (8.7%) | 46/1496 (3.1%) | <0.0001 |
| COPD and/or asthma treatment | 2449 | 269/2449 (11%) | 115/953 (12.1%) | 154/1496 (10.3%) | 0.1850 |
Data are presented as numbers (%) and mean ± SD, or median [25th–75th percentile] if not normally distributed.
P values are calculated using Fisher’s exact test, unpaired Student t-test or Wilcoxon rank sum test (two-sided).
Ethnicity: EU (Europid), MENA (Middle East North Africa); AC (African or Caribbean), AS (Asian).
HbA1c corresponds to the glycated haemoglobin determined in the first 7 days following hospital admission or in the 6 months prior hospitalisation.
DKD: defined as eGFR ≤ 60 mL/min/1.73 m2 and/or proteinuria.
BMI: body mass index; eGFR (CKD-EPI): estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula; COPD, chronic obstructive pulmonary disease; OSA, obstructive sleep apnoea; NAFLD, non-alcoholic fatty liver disease; DPP4, dipeptidyl peptidase 4; GLP-1RA, glucagon-like peptide 1-receptor agonist; MRA, mineralocorticoid-receptor antagonist (i.e. spironolactone and eplerenone); ARB, angiotensin-2 receptor-blocker; ACE inhibitors, angiotensin converting enzyme inhibitors.
COVID-19 features on admission also revealed some differences between metformin users and non-users (Table 2 ). Indeed, a longer period between the onset of symptoms and hospital admission (6 vs 4 days) as well as more frequent COVID-19-related clinical symptoms characterised metformin users. Moreover, on admission, metformin users exhibited higher plasma glucose, liver transaminases, C-reactive protein and fibrinogen concentrations, eGFR and lymphocyte counts compared with non-users.
Table 2.
COVID-19-related clinical, radiological and biological characteristics on admission of CORONADO participants according to the use of metformin.
| Features | People with available data | All (N = 2449) | Metformin use before admission |
||
|---|---|---|---|---|---|
| No (N = 953) | Yes (N = 1496) | P value | |||
| Positive SARS-CoV-2 PCR | 2374 | 2245/2374 (94.6%) | 856/919 (93.1%) | 1389/1455 (95.5%) | 0.0198 |
| COVID-19 symptoms | 2448 | 2317/2448 (94.6%) | 896/953 (94%) | 1421/1495 (95.1%) | 0.2706 |
| Time between symptom onset and hospital admission (days) | 2399 | 5 [2], [3], [4], [5], [6], [7], [8] | 4 [1], [2], [3], [4], [5], [6], [7] | 6 [3], [4], [5], [6], [7], [8], [9] | <0.0001 |
| Clinical presentation | |||||
| Fever | 2414 | 1807/2414 (74.9%) | 682/941 (72.5%) | 1125/1473 (76.4%) | 0.0343 |
| Fatigue | 2337 | 1456/2337 (62.3%) | 508/900 (56.4%) | 948/1437 (66%) | <0.0001 |
| Cough | 2383 | 1606/2383 (67.4%) | 591/930 (63.5%) | 1015/1453 (69.9%) | 0.0015 |
| Cephalalgia | 2263 | 283/2263 (12.5%) | 88/882 (10%) | 195/1381 (14.1%) | 0.0041 |
| Dyspnoea | 2416 | 1562/2416 (64.7%) | 592/943 (62.8%) | 970/1473 (65.9%) | 0.1270 |
| Rhinitis and/or pharyngeal signs | 2227 | 181/2227 (8.1%) | 72/865 (8.3%) | 109/1362 (8%) | 0.8115 |
| Agueusia and/or Anosmia | 2129 | 298/2129 (14%) | 88/817 (10.8%) | 210/1312 (16%) | 0.0007 |
| Digestive disorders | 2336 | 775/2336 (33.2%) | 275/908 (30.3%) | 500/1428 (35%) | 0.0191 |
| Chest CT imaging | |||||
| Abnormal chest CT | 1735 | 1675/1735 (96.5%) | 609/639 (95.3%) | 1066/1096 (97.3%) | 0.0402 |
| Ground-glass opacity/ crazy paving |
1712 | 1548/1712 (90.4%) | 545/628 (86.8%) | 1003/1084 (92.5%) | 0.0002 |
| Biological findings | |||||
| Admission plasma glucose (mg/dl) | 1834 | 170 [127−236] | 162 [124−227] | 176 [129−241] | 0.0041 |
| eGFR (CKD-EPI) (mL/min/1.73 m²) |
2287 | 67.2 [41−88.5] | 49.6 [27−78.4] | 75.8 [51.5−92.7] | <0.0001 |
| ALT (%ULN) | 2056 | 0.61 [0.42−0.98] | 0.54 [0.37−0.88] | 0.66 [0.46−1.05] | <0.0001 |
| AST (%ULN) | 2023 | 1.06 [0.75−1.59] | 1 [0.69−1.48] | 1.11 [0.79−1.64] | 0.0005 |
| GGT (%ULN) | 1915 | 0.93 [0.55−1.73] | 0.95 [0.53−1.72] | 0.93 [0.58−1.73] | 0.7310 |
| Haemoglobin (g/dl) | 2387 | 12.7 [11.4−14.2] | 12.3 [10.9−13.9] | 12.9 [11.7−14.3] | <0.0001 |
| White cell count (103/mm3) | 2384 | 6600 [5000−8820] | 6450 [4932−8915] | 6600 [5030−8800] | 0.3658 |
| Lymphocyte count (103/mm3) | 2313 | 990 [690−1400] | 910 [620−1340] | 1020 [710−1420] | <0.0001 |
| Platelet count (103/mm3) | 2383 | 201 [155−258] | 191 [146−255] | 206 [160−262] | <0.0001 |
| d-dimers (µg/l) | 957 | 880 [328−1730] | 885 [334−1635] | 880 [306−1735] | 0.9600 |
| CRP (mg/l) | 2286 | 86 [40.8−146.9] | 76.9 [34.9−134.1] | 92.0 [45.0−152.1] | 0.0001 |
| LDH (UI/l) | 1253 | 350 [262−494] | 345 [256−479] | 350 [267−502] | 0.4398 |
| CPK (UI/l) | 1207 | 132 [66−302] | 137 [63−335] | 128 [67−282] | 0.4698 |
| Fibrinogen (g/l) | 1227 | 6.2 [5−7.4] | 6 [4.8−7.1] | 6.3 [5.1−7.5] | 0.0004 |
Data are presented as numbers (%) and mean ± SD, or median [25th–75th percentile] if not normally distributed.
P values are calculated using Fisher’s exact test, unpaired Student t-test or Wilcoxon rank sum test (two-sided).
PCR: reverse transcriptase polymerase chain reaction; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; CT, computed tomography; eGFR (CKD-EPI): estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula; ALT, Alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; LDH, Lactate dehydrogenase; CPK, creatinine phosphokinase.
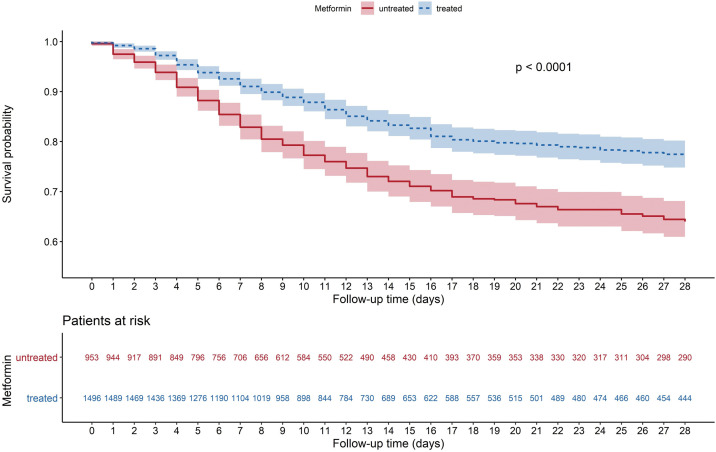
The primary composite endpoint (tracheal intubation for mechanical ventilation and/or death by day 7) developed in 695 (28.4%) patients with a similar rate in patients treated or not with metformin (Table 3 ). However, metformin users were less likely to meet this composite endpoint by day 28 compared with non-users (32.6% vs 38.7%, P = 0.0023). This favourable association was due to a lower rate of death in the metformin users (8.2% vs 16.1%, P < 0.0001 on day 7 and 16.0% vs 28.6%, P < 0.0001 on day 28) while the tracheal intubation was more frequent compared with non-users (21.1% vs 14.7%, P = 0.0001 on day 7 and 21.9% vs 15.6%, P = 0.0001 on day 28). As illustrated by Kaplan-Meier curves, the lower incidence of in-hospital death was observed in metformin users as early as in the first days of hospitalisation (Fig. 2 ).
Table 3.
Outcomes of patients according to the use of metformin before propensity score analysis.
| Metformin use |
||||
|---|---|---|---|---|
| All (N = 2449) | No (N = 953) | Yes (N = 1496) | P value | |
| Day 7 | ||||
| Composite endpoint | 695 (28.4%) | 276 (29.0%) | 419 (28.0%) | 0.6134 |
| IMV | 456 (18.6%) | 140 (14.7%) | 316 (21.1%) | 0.0001 |
| Death | 275 (11.2%) | 153 (16.1%) | 122 (8.2%) | <0.0001 |
| Day 28 | ||||
| Composite endpoint | 857 (35.0%) | 369 (38.7%) | 488 (32.6%) | 0.0023 |
| IMV | 477 (19.5%) | 149 (15.6%) | 328 (21.9%) | 0.0001 |
| Death | 512 (20.9%) | 273 (28.6%) | 239 (16.0%) | <0.0001 |
Composite endpoint combines tracheal intubation for mechanical ventilation (IMV) and death.
Fig. 2.
Kaplan-Meier survival curves showing the non-adjusted survival from hospital admission up to day 28 according to treatment with metformin.
In order to control for confounding factors linked to metformin use, we then applied inverse probability of treatment weighting according to the propensity score approach (Figure S1; see supplementary materials associated with this article on line). This analysis demonstrated a significant association between metformin use and the composite endpoint on day 28 (OR [95%CI]: 0.783 [0.615−0.996]) and also with death on day 28 (0.710 [0.537−0.938]) (Table 4 ). The results of the sensitivity analyses performed after adjustment for HbA1c values (Model 2), eGFR values prior to admission (Model 3) and diabetes duration (Model 4) were similar to that of Model 1 although with a loss of statistical significance owing to lower number of patients (Table 4 and Table S1 (see supplementary materials associated with this article on line) respectively). The results of a sensitivity analysis computed using multivariate logistic regression models without the propensity score are presented in Table S2: see supplementary materials associated with this article on line.
Table 4.
Outcomes of patients according to the use of metformin after propensity score analysis (odd ratio [CI]).
| Day 7 |
Day 28 |
|||
|---|---|---|---|---|
| Model 1: baseline parameters | Model 2: model 1 + HbA1c | Model 1: baseline parameters | Model 2: model 1 + HbA1c | |
| Population/exposed (%) | N = 1576 952 (60%) |
N = 1090 671 (62%) |
N = 1576 952 (60%) |
N = 1090 671 (62%) |
| Composite endpoint | 0.838 [0.649−1.082] | 0.824 [0.592−1.147] | 0.783 [0.615−0.996] | 0.822 [0.607−1.113] |
| IMV | 0.925 [0.694−1.233] | 0.901 [0.618−1.311] | 0.915 [0.691−1.212] | 0.932 [0.643−1.351] |
| Death | 0.688 [0.470−1.007] | 0.762 [0.465−1.248] | 0.710 [0.537−0.938] | 0.778 [0.549−1.102] |
Composite endpoint combines tracheal intubation for mechanical ventilation (IMV) and death.
Discussion
Since the outbreak of COVID-19 in December 2019, widely prescribed drugs such as renin-angiotensin system blockers [23] and statins [24] are scrutinized in order to determine their impact on outcomes in patients with COVID-19. Metformin is the first line anti-diabetic drug. In this observational study of a large number of patients with T2D hospitalised for COVID-19, a propensity score approach demonstrated that metformin use on admission was beneficial with a lower rate of the composite endpoint (tracheal intubation for mechanical ventilation and/or death) and death by day 28. Although observational, our data support evidence that metformin could exert some beneficial effects on the in-hospital course of COVID-19.
Of note, the lower risk of death was observed in metformin users as early as the first days of hospitalisation as illustrated by Kaplan-Meier curves. Surprisingly, the improved COVID-19 prognosis in metformin users occurred in spite of an apparently greater severity on admission regarding clinical, radiological, and biological features, compared with non-users. Such a difference in setting may merely reflect a more advanced stage of the inflammation state owing to COVID-19 than a more severe disease per se in metformin users. Indeed, although the time lag between the onset of COVID-19 symptoms and hospital admission was significantly longer in metformin users (a median of 6 days compared with 4 days in non-users), the rate of dyspnoea, a major severity criterion, was not more frequent in metformin users. With regard to the time lag for hospital admission between the two study groups, it could be hypothesised that metformin non-users may have been more rapidly hospitalised owing to their older age (74.6 ± 12.5 years vs 68.5 ± 11.9 years in metformin users) in association with more frequent comorbidities.
Importantly, the reduced rate of death observed on day 7 in metformin users remained significant until day 28, i.e. for almost one month of follow-up. However, because we are not aware if metformin treatment was continued during the hospital stay, the point is therefore raised as to whether metformin could have provided beneficial effects even after its withdrawal, in particular in case of worsening health. With regard to the persistent favourable impact of metformin, the elimination half-life of metformin from erythrocytes is rather long (nearly one day), so it takes nearly one week for total elimination of metformin from the body [25].
In addition, the beneficial effects on metformin on many cell types (e.g. endothelial cells, neurons and glial and cells, cardiomyocytes, hepatocytes, macrophages) (for a review, see [8], [9]) could persist and lead to favourable outcomes during hospital stay. In accordance with the present results, metformin use has also been shown to be associated with a reduction in mortality from sepsis in diabetic patients in the ICU [10].
Recently, several observational studies on diabetes and COVID-19 have reported an association between metformin and COVID-related outcomes in patients with type 2 diabetes (for reviews see [26], [27], [28]). In the interim analysis, which included 1166 patients with T2D, we reported a non-significant association between metformin treatment and better survival by day 7 [19]. In a retrospective observational study (n = 283 patients, including 104 on metformin) from China, in-hospital mortality was found to be lower in the metformin group [29] but important data were missing (including BMI, eGFR and routine treatment before admission). In another preprint from the USA, the authors reviewed claims data of 6256 COVID-19 patients with diabetes and obesity including 2333 metformin users. They found that metformin treatment was associated with decreased mortality only in women but not in the overall sample or in men. Importantly, data on BMI were missing in more than 90% of the patients [30]. Indeed, a large body of evidence suggests that obesity is associated with more severe clinical course of COVID-19 including higher mortality rate. Therefore, the missing information about BMI in these studies could be a source of bias in the reported associations between metformin use and mortality. A large retrospective electronic health record data analysis in > 25,000 subjects tested for COVID-19 (n = 604 positive cases) found that metformin use was associated with reduced mortality in 239 subjects with diabetes and COVID-19 (OR: 0.38 [0.17−0.87]) [31]. In contrast, some studies did not report such an association between metformin treatment and improved COVID-related outcomes in patients with type 2 diabetes. One retrospective observational study form China including 1213 patients with T2D hospitalised for COVID-19 (678 metformin users) found a neutral effect of metformin on 28-day mortality [32]. Another retrospective study from Korea, which was based on claims data, found no definite association between metformin use and COVID-19 outcomes [33]. There was however a disproportionate participant numbers (469 patients taking metformin and 95 taking other antidiabetic medications). A third retrospective study from Spain (n = 2666) has evaluated the association between glucose-lowering drugs and clinical outcomes after propensity score matching. No significant association between metformin treatment and mortality or other adverse outcomes was found but there were only 249 patients on metformin after propensity score matching [34]. Lastly, a small case-control study from China (n = 110 patients with T2D, 56 were treated with metformin and 54 were not) reported a four-fold increased risk of life‐threatening complications in the metformin group (admission to the ICU, acute respiratory distress syndrome, sepsis and septic shock, and organ dysfunction) but no analysis of mortality was performed [35].
While CORONADO is one of the largest studies so far that assessed the effect of metformin in COVID-19 outcomes, some limitations must be acknowledged in the current analysis: (i) those inherently associated with observational studies although the CORONADO study protocol imposed a uniform data collection strategy. However, as usual in such observational real-life studies, a significant amount of data was missing, despite the major efforts of the investigators to collect them. This may be tempered by the use of the propensity score but missing data led to a loss of power for statistical analyses (as for eGFR data, for instance). Moreover, although our propensity score was calculated with a large number of covariates captured by rigorous phenotyping in CORONADO, residual confounding cannot be completely ruled out; (ii) we are not aware of the duration and the dosage of the metformin treatment prior to admission. Regarding the duration of metformin treatment prior to admission, since metformin is the first-line treatment of T2D and owing to a mean diabetes duration of more than 10 years, we can hypothesise that patients were on metformin for a long time; (iii) we do not know if metformin was maintained after admission and it is highly probable that decisions about continuing/stopping metformin treatment were not homogenous between and within different centres. Moreover, information about glucose control during the hospitalisation period is missing. Good blood glucose control, as expressed by glycaemic variability between 3.9 and 10.0 mmol/L (70−180 mg/dl), was associated with markedly lower rate of mortality in inpatients with COVID-19 when compared to poorly controlled blood glucose [36]. It is therefore possible that glucose control during hospitalisation could affect COVID-19 outcomes [37]; (iv) proinflammatory mediators (such as interleukin-6) were not measured.
Nevertheless, some strengths should be highlighted: (i) we collected data from nearly seventy centres. Such a large number may circumvent biases owing to local specificities in COVID-19 management, such as that of ICU admission or intubation; (ii) the diagnosis of COVID-19 was confirmed by positive SARS-Cov-2 PCR in approximately 95% of the patients; (iii) a large number of covariates about comorbidities and routine medications was available; and (iv) the observational nature of the study reflects what could happen in ‘real life’.
We can summarise the issues that remain open as follows:
-
-
What is the minimal duration of metformin treatment that could offer protection?
-
-
What is the optimal dose of metformin for this putative protective effect on COVID-19?
-
-
What are the metformin prescription modalities for frail patients, in particular in the elderly with renal failure, considering on the one hand the increasing prevalence of its contraindication and on the other hand its potential pleiotropic beneficial effects? Indeed, at least on the basis of observational studies, metformin use is associated with reduced all-cause mortality in patients with chronic kidney disease, atherothrombosis, congestive heart failure, or chronic liver disease [38], [39].
-
-
What are the modalities for metformin treatment in hospital, knowing that metformin is associated with favourable outcomes in patients with diabetes in the ICU but also that, when worsening organs failure and hypoxia occur, kidney failure leads to metformin accumulation and liver failure reduces lactate elimination, increasing the risk of lactic acidosis?
-
-
To what extent could the beneficial impact of metformin be generalized to all patients with COVID-19, irrespective of diabetes status or health care settings, and that in terms of both incidence and severity of the disease?
Conclusion
In this nationwide observational study of a large number of patients with T2D admitted for COVID-19, metformin use was associated with a lower rate of a composite endpoint combining intubation and death within 28 days of hospitalisation and with a lower rate of death by days 28. Randomised, controlled studies are now needed in order to confirm the benefits associated with metformin and establish to what extent these protective effects, if any, can be generalised to non-diabetic patients with COVID-19.
Conflict of interest
JDL reports personal fees from AstraZeneca, Brothier, Lilly, MSD, Novo Nordisk, Pfizer, and Sanofi.
AAS reports personal fees from AstraZeneca and Novo Nordisk.
SH reports personal fees and non-financial support from AstraZeneca, grants and personal fees from Bayer, personal fees from Boehringer Ingelheim, grants from Dinno Santé, personal fees from Eli Lilly, non-financial support from LVL, personal fees and non-financial support from MSD, personal fees from Novartis, grants from Pierre Fabre Santé, personal fees and non-financial support from Sanofi, personal fees and non-financial support from Servier, and personal fees from Valbiotis.
MP reports personal fees and non-financial support from Novo Nordisk, non-financial support from Sanofi, and non-financial support from Amgen.
JFG reports personal fees and non-financial support from Eli Lilly, personal fees and non-financial support from Novo Nordisk, personal fees and non-financial support from Gilead, and personal fees and non-financial support from AstraZeneca.
MJ reports personal fees and non-financial support from Sanofi, personal fees and non-financial support from Eli Lilly, personal fees and non-financial support from Novo Nordisk, grants and personal fees from Boehringer Ingelheim, grants, personal fees and non-financial support from Medtronic, personal fees and non-financial support from Abbott, personal fees and non-financial support from BMS, personal fees and non-financial support from MSD, and grants, personal fees and non-financial support from AstraZeneca.
MM reports personal fees from Novo-Nordisk, Servier, and MSD.
RR reports grants, personal fees and non-financial support from Sanofi, grants, personal fees and non-financial support from Novo Nordisk, personal fees and non-financial support from Eli Lilly, personal fees from Mundipharma, personal fees from Janssen, personal fees from Servier, grants and personal fees from AstraZeneca, personal fees from MSD, personal fees from Medtronic, personal fees from Abbott, grants from Diabnext, and personal fees from Applied Therapeutics.
PG reports personal fees from Abbott, personal fees from Amgen, personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Eli Lilly, personal fees from MSD, personal fees from Mundipharma, grants and personal fees from Novo Nordisk, personal fees from Sanofi, and personal fees from Servier.
BC reports grants and personal fees from Amgen, personal fees from AstraZeneca, personal fees from Akcea, personal fees from Genfit, personal fees from Gilead, personal fees from Eli Lilly, personal fees from Novo Nordisk, personal fees from MSD, grants and personal fees from Sanofi, and grants and personal fees from Regeneron.
All other authors declare that there are no declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Contribution statement
Concept and design: JDL, AAS, PG and BC.
Full access to all of the data and responsibility for the integrity of the data and the accuracy of the data analysis: BC, SH, and MW.
Acquisition, analysis, or interpretation of data: all co-authors.
Statistical analysis: TG and MW.
Drafting the manuscript: JDL, AAS, BC, PG.
Critical revision of the manuscript: all co-authors.
Funding
This study received the following funding: the Fondation Francophone pour la Recherche sur le Diabète (FFRD), supported by Novo Nordisk, Merck Sharpe Dome, Abbott, AstraZeneca, Lilly and FFD (Fédération Française des Diabétiques) – CORONADO initiative emergency grant; Société Francophone du Diabète (SFD) – CORONADO initiative emergency grant; Fonds de dotation du CHU de Nantes (CORONADO project). All research facilities are acknowledged for providing clinical research associates and research technicians for clinical investigations pro bono. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Acknowledgements
We are grateful to P. Tucker (Trets, France) for editing the manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.diabet.2020.101216.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buse J.B., Wexler D.J., Tsapas A., Rossing P., Mingrone G., Mathieu C., et al. 2019 Update to: management of hyperglycemia in Type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2020;43:487–493. doi: 10.2337/dci19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maruthur N.M., Tseng E., Hutfless S., Wilson L.M., Suarez-Cuervo C., Berger Z., et al. Diabetes Medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016;164:740–751. doi: 10.7326/M15-2650. [DOI] [PubMed] [Google Scholar]
- 4.De Broe M.E., Kajbaf F., Lalau J.-D. Renoprotective effects of metformin. Nephron. 2018;138:261–274. doi: 10.1159/000481951. [DOI] [PubMed] [Google Scholar]
- 5.Heckman-Stoddard B.M., DeCensi A., Sahasrabuddhe V.V., Ford L.G. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia. 2017;60:1639–1647. doi: 10.1007/s00125-017-4372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rotermund C., Machetanz G., Fitzgerald J.C. The therapeutic potential of metformin in neurodegenerative diseases. Front Endocrinol. 2018;9:400. doi: 10.3389/fendo.2018.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulkarni A.S., Gubbi S., Barzilai N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. 2020;32:15–30. doi: 10.1016/j.cmet.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiernsperger N. Metformin as a cellular protector; a synoptic view of modern evidences. J Nephropharmacology. 2015;4:31–36. [PMC free article] [PubMed] [Google Scholar]
- 9.Foretz M., Guigas B., Viollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Rev Endocrinol. 2019;15:569–589. doi: 10.1038/s41574-019-0242-2. [DOI] [PubMed] [Google Scholar]
- 10.Liang H., Ding X., Li L., Wang T., Kan Q., Wang L., et al. Association of preadmission metformin use and mortality in patients with sepsis and diabetes mellitus: a systematic review and meta-analysis of cohort studies. Crit Care Lond Engl. 2019;23:50. doi: 10.1186/s13054-019-2346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey C.J. Metformin: historical overview. Diabetologia. 2017;60:1566–1576. doi: 10.1007/s00125-017-4318-z. [DOI] [PubMed] [Google Scholar]
- 12.Malik F., Mehdi S.F., Ali H., Patel P., Basharat A., Kumar A., et al. Is metformin poised for a second career as an antimicrobial? Diabetes Metab Res Rev. 2018;34:e2975. doi: 10.1002/dmrr.2975. [DOI] [PubMed] [Google Scholar]
- 13.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcucci F., Romeo E., Caserta C.A., Rumio C., Lefoulon F. Context-dependent pharmacological effects of metformin on the immune system. Trends Pharmacol Sci. 2020;41:162–171. doi: 10.1016/j.tips.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Cameron A.R., Morrison V.L., Levin D., Mohan M., Forteath C., Beall C., et al. Anti-inflammatory effects of metformin irrespective of diabetes status. Circ Res. 2016;119:652–665. doi: 10.1161/CIRCRESAHA.116.308445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menendez J.A. Metformin and SARS-CoV-2: mechanistic lessons on air pollution to weather the cytokine/thrombotic storm in COVID-19. Aging. 2020;12:8760–8765. doi: 10.18632/aging.103347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kheirollahi V., Wasnick R.M., Biasin V., Vazquez-Armendariz A.I., Chu X., Moiseenko A., et al. Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat Commun. 2019;10:2987. doi: 10.1038/s41467-019-10839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalau J.-D., Al-Salameh A. Management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:666–667. doi: 10.1016/S2213-8587(20)30231-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020 doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin P.C. The performance of different propensity-score methods for estimating differences in proportions (risk differences or absolute risk reductions) in observational studies. Stat Med. 2010;29:2137–2148. doi: 10.1002/sim.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin P.C. The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Med Decis Mak Int J Soc Med Decis Mak. 2009;29:661–677. doi: 10.1177/0272989X09341755. [DOI] [PubMed] [Google Scholar]
- 22.Mao H., Li L. 2018. Propensity score weighting methods for dichotomous treatments. R package version 1.1-3. [Google Scholar]
- 23.Messerli F., Siontis G., Rexhaj E. COVID-19 and renin angiotensin blockers: current evidence and recommendations. Circulation. 2020;141:2042–2044. doi: 10.1161/CIRCULATIONAHA.120.047022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cariou B., Goronflot T., Rimbert A., Boullu S., Le May C., Moulin P., et al. Routine use of statins and increased mortality related to COVID-19 in inpatients with type 2 diabetes: results from the CORONADO study. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robert F., Fendri S., Hary L., Lacroix C., Andréjak M., Lalau J.D. Kinetics of plasma and erythrocyte metformin after acute administration in healthy subjects. Diabetes Metab. 2003;29:279–283. doi: 10.1016/s1262-3636(07)70037-x. [DOI] [PubMed] [Google Scholar]
- 26.Scheen A.J. Metformin and COVID-19: from cellular mechanisms to reduced mortality. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh A.K., Singh R. Is metformin ahead in the race as a repurposed host-directed therapy for patients with diabetes and COVID-19? Diabetes Res Clin Pract. 2020;165 doi: 10.1016/j.diabres.2020.108268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hariyanto T.I., Kurniawan A. Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COVID-19) infection. Obes Med. 2020;19:100290. doi: 10.1016/j.obmed.2020.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo P., Qiu L., Liu Y., Liu X.-L., Zheng J.-L., Xue H.-Y., et al. Metformin treatment was associated with decreased mortality in Covid-19 patients with diabetes in a retrospective analysis. Am J Trop Med Hyg. 2020;103:69–72. doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bramante C., Ingraham N., Murray T., Marmor S., Hoversten S., Gronski J., et al. Observational study of metformin and risk of mortality in patients hospitalized with Covid-19. MedRxiv Prepr Serv Health Sci. 2020 doi: 10.1101/2020.06.19.20135095. [DOI] [Google Scholar]
- 31.Crouse A., Grimes T., Li P., Might M., Ovalle F., Shalev A. Metformin use is associated with reduced mortality in a diverse population with covid-19 and diabetes. MedRxiv Prepr Serv Health Sci. 2020 doi: 10.1101/2020.07.29.20164020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng X., Liu Y.-M., Li H., Zhang X., Lei F., Qin J.-J., et al. Metformin is associated with higher incidence of acidosis, but not mortality, in individuals with Covid-19 and pre-existing type 2 diabetes. Cell Metab. 2020;32:537–547. doi: 10.1016/j.cmet.2020.08.013. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Do J.Y., Kim S.W., Park J.W., Cho K.H., Kang S.H. Is there an association between metformin use and clinical outcomes in diabetes patients with COVID-19? Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pérez-Belmonte L.M., Torres-Peña J.D., López-Carmona M.D., Ayala-Gutiérrez M.M., Fuentes-Jiménez F., Huerta L.J., et al. Mortality and other adverse outcomes in patients with type 2 diabetes mellitus admitted for COVID-19 in association with glucose-lowering drugs: a nationwide cohort study. BMC Med. 2020;18:359. doi: 10.1186/s12916-020-01832-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao Y., Liu T., Zhong W., Liu R., Zhou H., Huang W., et al. Risk of metformin in patients with type 2 diabetes with Covid-19: a preliminary retrospective report. Clin Transl Sci. 2020 doi: 10.1111/cts.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu L., She Z.-G., Cheng X., Qin J.-J., Zhang X.-J., Cai J., et al. Association of Blood Glucose control and outcomes in patients with Covid-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–1077. doi: 10.1016/j.cmet.2020.04.021. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheen A.J., Marre M., Thivolet C. Prognostic factors in patients with diabetes hospitalized for COVID-19: findings from the CORONADO study and other recent reports. Diabetes Metab. 2020;46:265–271. doi: 10.1016/j.diabet.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crowley M.J., Diamantidis C.J., McDuffie J.R., Cameron C.B., Stanifer J.W., Mock C.K., et al. Clinical outcomes of metformin use in populations with chronic kidney disease, congestive heart failure, or chronic liver disease: a systematic review. Ann Intern Med. 2017;166:191–200. doi: 10.7326/M16-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roussel R., Travert F., Pasquet B., Wilson P.W.F., Smith S.C., Goto S., et al. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch Intern Med. 2010;170:1892–1899. doi: 10.1001/archinternmed.2010.409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.