Highlights
-
•
Asymptomatic patients with COVID-19 tend to be younger and may be more socially active.
-
•
Laboratory findings in most asymptomatic cases were unremarkable.
-
•
Around half of the cases had lung opacities, most frequently ground glass opacities.
-
•
Patients with normal CT were younger than patients with abnormal CT.
Keywords: Coronavirus disease-19 (COVID-19), SARS-CoV-2, Asymptomatic, Meta-analysis
Abstract
Background
Coronavirus Disease 2019 (COVID-19) is characterized by an unpredictable disease course, ranging from asymptomatic to severe, life-threatening infections. Asymptomatic COVID-19 infections have been described, and the aim of this systematic review was to summarise their presentation forms.
Methods
We searched PubMed® and Google® (1 December 2019 to 29 March 2020) and extracted age, laboratory findings, and computed tomography (CT) scans. Pooled incidence rates of clinical characteristics were analyzed using random-effect models.
Results
In total, 506 patients from 34 studies (68 single cases and 438 from case-series) with an asymptomatic course were identified. Patients with normal radiology were younger (19.59 ± 17.17 years) than patients with abnormal radiology (39.14 ± 26.70 years) (p-value = 0.013). Despite being asymptomatic, CT investigations revealed abnormalities in 62.2% of the cases; ground-glass opacities were most frequently observed (43.09% by meta-analysis). Most studies reported normal laboratory findings (61.74% by meta-analysis).
Conclusions
More than half of the patients without any symptoms present with CT abnormalities. Asymptomatic patients may be contagious and thus a potential source of transmission of COVID-19.
Introduction
Coronavirus Disease 2019 (COVID-19), caused by severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2), emerged as a global threat; it was declared a pandemic by the WHO on 11 March 2020. As of 12 June 2020, over 7,200,000 infected cases and 400,000 deaths have been reported by the World Health Organization (WHO).
The first reported infections had a direct relation to the Huanan seafood wholesale market in Wuhan, China; it was assumed that bats act as a reservoir, and Malayan pangolins might act as an intermediate to facilitate transfer to humans (Lam et al., 2020). Person-to-person transmission by inhalation or contact with droplets emerged as a leading source of infection, while more recently aerosols, the stability of SARS-CoV-2 on surfaces, and the potential of fecal-oral infection routes have been discussed (van Doremalen et al., 2020, Yeo et al., 2020).
The presentation of COVID-19 and its disease course are unpredictable and range from asymptomatic to mild respiratory infections to pneumonia and even to acute respiratory distress syndrome (ARDS). Among other factors, age has been described as a risk factor for a more severe disease course, while younger people seem to have mild or even asymptomatic presentations and thus might be crucial in further spreading of the disease (Guan et al., 2020). Young people are a majority of the workforce and are more likely to be socially active. Hence, younger, infected people may spread the disease unknowingly to a more substantial proportion of contacts. Given the lack of symptoms, they may not be screened and diagnosed with the infection.
To our knowledge, no systematic analysis of asymptomatic cases has been performed so far. Our systematic case-based review with meta-analysis summarised the current knowledge about these asymptomatic SARS-CoV-2 infections, taking into account reported infections and prediction models of undocumented or asymptomatic infections, to guide further strategies to reduce transmission.
Methods
Literature search strategy
Two investigators (D.K. and A.K.) searched the PubMed database from 1 December 2019 to 29 March 2020. The search terms used were as follows: (COVID-19 OR SARS-CoV-2 OR 2019-nCoV OR (coronavirus disease 2019)) AND (asymptomatic OR pre-symptomatic). Given the high number of publications on preprint servers, we decided to perform a Google® search to include all relevant articles. Each retrieved article was reviewed in detail, including the title, abstract, and the full-text. Inclusion and exclusion criteria were applied in the decision to include articles or not. Reports of children or adults with an asymptomatic disease course were included, while exclusion criteria were: (1) studies reporting "asymptomatic" cases with mild symptoms, or (2) insufficient documentation, and (3) if articles were not written in English. This systematic review was accomplished according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The PRISMA checklist is shown in Supplementary Table 1. Two of our investigators (D.K. and A.K.) individually performed the data search and extraction. Discrepancies were resolved via consensus. Information was gathered regarding the articles’ first author, age, sex, status during follow-up (remaining asymptomatic or clearance of the virus, which was defined as two sequential negative SARS-CoV-2 nucleic acid test results), whether others were infected, computed tomography (CT) and laboratory findings. In case-series, the percentage of the asymptomatic cases in comparison to the total cohort was given.
Analyses of cases
Each reported case and case-series data are provided in Table 1, Table 2 . To investigate the relationship between radiologic and laboratory abnormalities in case reports, radiologic findings of asymptomatic patients were compared between the patients with and without laboratory abnormalities. Also, various features of asymptomatic patients with radiologic abnormalities and normal radiology findings were analyzed.
Table 1.
Summary profiles of asymptomatic patients in case reports (N = 68).
| Variable | Total number of patients, n (%)a |
|---|---|
| Demographic characteristics | |
| Age (y), mean ± SD | 31.0 ± 23.8 |
| 0–9 years | 13 (19.1) |
| 10–19 years | 10 (14.7) |
| 20–29 years | 11 (16.2) |
| 30–39 years | 10 (14.7) |
| 40–49 years | 5 (7.4) |
| 50–59 years | 7 (10.3) |
| 60–69 years | 5 (7.4) |
| >70 years | 5 (7.4) |
| No information | 2 (2.9%) |
| Male | 31 (45.6) |
| Asymptomatic during follow-up | 63 (92.6) |
| Infected others | 6 (66.7) |
| Radiologic findings (n = 41) | |
| Normal | 18 (43.9) |
| Abnormal | 23 (56.1) |
| Typical ground-glass opacities with patchy shadows | 13 (31.7) |
| Typical ground-glass opacities with consolidation | 1 (2.4) |
| Typical ground-glass opacities only | 2 (4.9) |
| Stripe shadows | 5 (12.2) |
| Bronchitis | 1 (2.4) |
| Signs of pneumonia | 1 (2.4) |
| No information | 27 (39.7) |
| Laboratory findings (n = 47) | |
| Normal | 22 (46.8) |
| Abnormal | 25 (53.2) |
| Leukopenia | 6 (12.8) |
| Neutropenia | 1 (2.1) |
| Lymphocytopenia | 5 (10.6) |
| Thrombopenia | 1 (2.1) |
| Creatinine | 2 (4.3) |
| LDH elevation | 2 (4.3) |
| CRP elevation | 4 (8.5) |
| ALT elevation | 2 (4.3) |
| Procalcitonin elevationb | 6 (26.1) |
| D-dimer elevationb | 5 (21.7) |
| CK-MB elevationb | 5 (83.3) |
| No information | 21 (30.9) |
Abbreviations: LDH (lactose dehydrogenase), CRP (C-reactive protein), ALT (alanine aminotransferase), CK-MB (creatine kinase MB fraction).
Data are means ± SD or number (percentage) of patients.
The denominator is lower than the n number due to unavailable results/test is not done.
Table 2.
Summary profiles of asymptomatic patients in case-series (N = 438).
| Author | Cases | % of total cases | Age | Sex (female, %) | Number of Radiology abnormal | Radiology abnormal | Radiologic findings | Number of Laboratory abnormal | Laboratory abnormal | Laboratory findings | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shi et al. (2020) | 15 | 18.5 | 44.9 | 73 | 14 | 93% | Ground glass opacities | 3 | 20% | Lymphopenia (20%) | Including healthcare workers |
| Tian et al. (2020) | 13 | 5 | – | – | – | – | – | – | – | – | – |
| Mizumoto et al. (2020b) | 328 | 51.7 | – | – | – | – | – | – | – | – | – |
| Wang et al. (2020) | 55 | 100 | 49 | 60 | 39 | 70.9% | Signs of pneumonia | – | – | Lymphopenia (11 pts, 20%), leukopenia (11 pts, 20%), leukocytosis (1 pt, 2%), LDH elevation (13 pts, 24%), CRP elevation (10 pts, 18%), ESR elevation (20 pts, 36%) | – |
| Ling et al. (2020) | 4 | 1 | – | – | 0 | 0% | No | – | – | – | – |
| Tao et al. (2020) | 20 | 12 | 0−14 (2), 15−29 (2), 30−39 (2), 40−49 (6), 50−59 (5), >70 (3) | – | 8 | 40% | Ground glass opacity (6 pts, 30%), patchy bilateral shadowing (2 pts, 10%) | – | – | CRP elevation (3 pts, 15%), procalcitonin elevation (2 pts, 10%) | – |
| Kimball, (2020) | 3 (13)a | 13 (57)a | – | – | – | – | – | – | – | – | Nursing facility; 10 pts. turned positive one week after a positive test result |
Number of cases and % of total cases in brackets includes patients who developed symptoms one week after a positive test result.
The proportion of CT and laboratory findings was calculated among patients with an asymptomatic disease course. All the data reported in case reports were combined and processed as one case-series. To estimate the proportion of radiologic and laboratory findings, data with median (ranges) were shown, and we presented meta-analysis results to estimate the summary effects with the proportion of radiologic and laboratory characteristics of asymptomatic COVID-19 patients. The overall estimation shows pooled analysis results, in which data are combined without being weighted.
Statistical analysis
Comparisons between groups were performed using Student’s t-test for continuous variables and Pearson’s chi-squared test or Fisher’s exact test for categorical variables. Descriptive statistics are expressed as mean ± standard deviation (SD). P values <0.05 were considered statistically significant. Statistical analyses were performed using SPSS v.25 (IBM Corp., Armonk, NY, USA). Radiologic and laboratory abnormalities of asymptomatic COVID-19 patients were meta-analyzed with the summary effects of 95% CI using random-effect models and the between-study heterogeneity using the I 2 metric of inconsistency and P-value of the χ2-based Cochran Q test. Random-effect models assume individual studies have substantial diversity and provide the weighted average of the effect sizes that may vary from study to study (DerSimonian and Kacker, 2007). I 2 values represent the ratio of the between-study variance and <25%, 25–50%, and >75% mean low, moderate (large), and high (very large) heterogeneity, respectively (Higgins et al., 2003). P values <0.10 were considered to have clinical heterogeneity because statistical tests for heterogeneity are not very powerful (Fletcher, 2007). The meta-analysis was done using MedCalc version 19.2.1 software (MedCalc Software, Ostend, trial version, Belgium).
Results
Search description
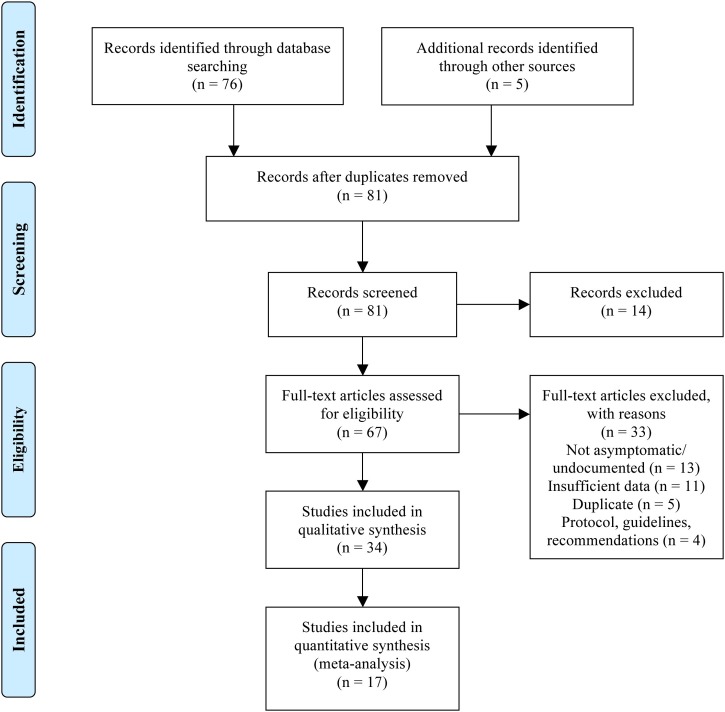
Eighty-one records reported patients with an asymptomatic disease course or provided a mathematical model to predict the rate of undocumented or asymptomatic infection, after duplicates were removed (Fig. 1 ) (Moher et al., 2009). Of those, 14 were excluded by title and abstract screening, and another 33 when the full-text was assessed (Fig. 1). Thirty-four studies were included in qualitative synthesis, including 18 case reports and seven case-series (506 asymptomatic patients). Among these, 17 studies (13 case reports and four case-series, in total 162 asymptomatic patients) were included in the meta-analysis that contained individual CT and laboratory test results. Such information was not available for the remaining 344 cases, which were subsequently excluded from the meta-analysis.
Fig. 1.
PRISMA flow diagram for literature search.
Characteristics of asymptomatic cases
We identified 18 case reports of asymptomatic COVID-19 patients in the literature and summarized the data of 68 asymptomatic patients in Table 1. The mean age was 31.0 ± 23.8 years. Most asymptomatic COVID-19 cases were younger, with the highest number of single cases belonging to the age group between 0 and 9 years, followed by ten and eleven cases each in the age group between 10-to-19 and 20-to-29 years. Ten patients were identified in the age group between 30-to-39 years, while five, seven, five, and five cases were aged 40−49, 50−59, 60–69, and >70 years (Table 1). More female patients were recorded (54.4%; 33/62 cases), and the majority of patients (92.6%) remained asymptomatic during follow-up. Five patients developed symptoms, with mild fever (<38 ℃) recorded in all of them. Other symptoms such as cough, fatigue, arthralgia, dizziness, and nasal congestion were noted in single cases. Six out of nine patients infected others, while information about transmission in the other 59 patients was missing. Detailed patients' profiles in case reports are shown in Supplementary Table 2; patient data organized by case reports are shown in Supplementary Table 3. In case-series, the average age was 44.9 years among asymptomatic cases, and one study found most patients aged between 40-to-49 years (Table 2). Female preponderance was confirmed in two case-series, with a percentage of 73% and 60% (Table 1, Table 2). The average proportion of asymptomatic cases in case-series was 24.2% (SD 22.06), excluding one case-series with asymptomatic cases only (Table 2).
Radiologic and laboratory findings
Radiologic and laboratory findings in asymptomatic COVID-19 patients in case reports are summarized in Table 3, Table 4 . Patients with normal radiology were significantly younger (19.59 ± 17.17 years) than patients with abnormal radiology (39.14 ± 26.70 years) (p-value = 0.013). In all patients with normal radiology, symptoms appeared during the follow-up period, while five (21.7%) patients with abnormal radiology were asymptomatic during follow-up (Table 3). There were no significant differences in laboratory findings between patients with normal radiologic and abnormal radiologic findings (Table 3). The creatinine and D-dimer levels were elevated in more patients with abnormal radiology (0% versus 8.7%, and 0% versus 31.3%), while creatine kinase muscle-brain fraction (CK-MB) elevation was seen in more patients with normal radiology findings (100% versus 66.7%). The mean age of patients with normal and abnormal laboratory findings was 25.80 ± 20.00, and 33.36 ± 28.91 years. No significant differences in gender, asymptomatic disease course during follow-up, and radiologic findings were seen in patients with normal and abnormal laboratory findings (Table 4). More patients with abnormal laboratory findings showed typical ground-glass opacities with patchy shadows (25.0% versus 38.1%), while more patients with normal laboratory findings showed ground-glass opacities only and bronchitis (10.0% versus 0%, and 5.0% versus 0%, each).
Table 3.
Clinical characteristics and laboratory findings in radiology abnormal and normal patients in case reports.
| Radiology normal (n = 18) | Radiology abnormal (n = 23) | p-value | |
|---|---|---|---|
| Age | 19.59 ± 17.17 | 39.14 ± 26.70 | 0.013* |
| Male | 9 (50.0%) | 9 (39.1%) | 0.539 |
| Asymptomatic during follow-up | 0 (0%) | 5 (21.7%) | 0.056 |
| Suspected numbers | 3.00 ± 2.83 | – | – |
| Laboratory abnormal | 9 (50.0%) | 12 (52.2%) | 1.000 |
| Leukopenia | 1 (5.6%) | 2 (8.7%) | 1.000 |
| Neutropenia | 0 (0%) | 0 (0%) | – |
| Lymphocytopenia | 1 (5.6%) | 4 (17.4%) | 0.363 |
| Thrombopenia | 0 (0%) | 1 (4.3%) | 1.000 |
| Creatinine elevation | 0 (0%) | 2 (8.7%) | 0.495 |
| LDH elevation | 1 (5.6%) | 1 (4.3%) | 1.000 |
| CRP elevation | 2 (11.1%) | 2 (8.7%) | 1.000 |
| ALT elevation | 1 (5.6%) | 1 (4.3%) | 1.000 |
| Procalcitonin elevation | 2 of 7 (28.6%) | 4 of 16 (25.0%) | 1.000 |
| D-dimer elevation | 0 of 7 (0%) | 5 of 16 (31.3%) | 0.272 |
| CK-MB elevation | 3 of 3 (100%) | 2 of 3 (66.7%) | 1.000 |
Data are mean ± SD or number (percentage) of patients.
Abbreviations: LDH (lactose dehydrogenase), CRP (C-reactive protein), ALT (alanine aminotransferase), CK-MB (creatine kinase MB fraction).
Table 4.
Clinical characteristics and radiologic findings in laboratory abnormal and normal patients in case reports.
| Laboratory normal (n = 22) | Laboratory abnormal (n = 25) | p-value | |
|---|---|---|---|
| Age | 25.80 ± 20.00 | 33.36 ± 28.91 | 0.326 |
| Male | 10 (45.5%) | 11 (44.0%) | 1.000 |
| Asymptomatic during follow-up | 21 (95.5%) | 21 (84.0%) | 0.352 |
| Suspected numbers | 5 | 1 | – |
| Radiology abnormal | 11 (55.0%) | 12 (57.1%) | 1.000 |
| Typical ground-glass opacities with patchy shadows |
5 of 20 (25.0%) | 8 of 21 (38.1%) | 0.505 |
| Typical ground-glass opacities with consolidation |
0 of 20 (0%) | 1 of 21 (4.8%) | 1.000 |
| Typical ground-glass opacities only | 10 of 20 (10.0%) | 0 of 21 (0%) | 0.232 |
| Stripe shadows | 3 of 20 (15.0%) | 2 of 21 (9.5%) | 0.663 |
| Bronchitis | 1 of 20 (5.0%) | 0 of 21 (0%) | 0.488 |
| Signs of pneumonia | 0 of 20 (0%) | 1 of 21 (4.8%) | 1.000 |
Data are mean ± SD or number (percentage) of patients.
Meta-analysis results
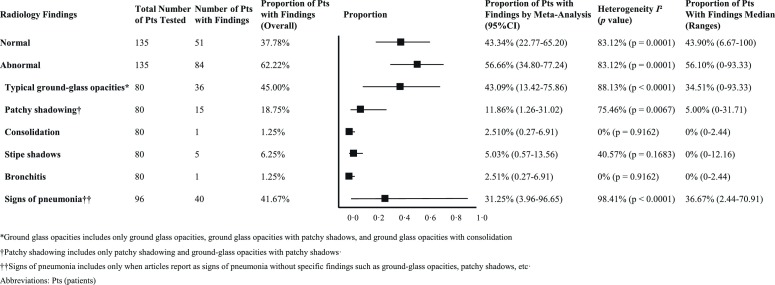
Of the eligible studies, 17 reported radiologic or laboratory findings in asymptomatic COVID-19 patients. The results of meta-analyses on the proportion of radiologic and laboratory findings in asymptomatic patients are presented in Supplementary Fig. 1a–h and 2a–i. In Fig. 2, Fig. 3, the lines in each forest plot do not represent the results of individual studies but represent the summary effect-sizes of the meta-analysis. Interestingly, most patients with an asymptomatic disease course underwent CT investigations (135 patients, 83.3%). A majority of individual cases had abnormalities (84/135 patients, 62.22%); the predominant lesion observed was ground-glass opacities with patchy shadows (45.00% by overall estimation and 43.09% by meta-analysis), then, to a lesser degree, signs of lower respiratory tract infections such as consolidations and bronchitis (both 1.25% by overall estimation and 2.51% by meta-analysis) (Fig. 2) . The proportion of patients with signs of pneumonia, not otherwise specified, varied from 2.44% to 70.91%, resulting in 41.67% by overall estimation and 31.25% by meta-analysis.
Fig. 2.
Meta-analyses on the proportion of radiologic findings in asymptomatic patients.
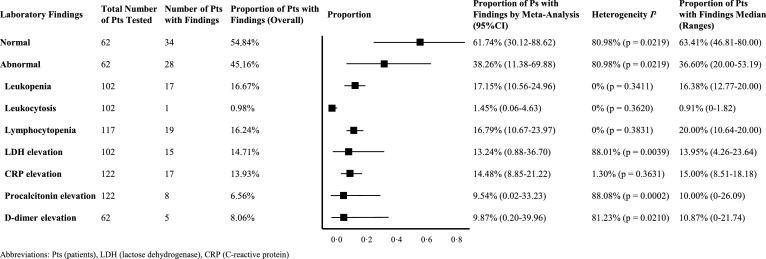
Fig. 3.
Meta-analyses on the proportion of laboratory findings in asymptomatic patients.
Among individual cases, laboratory findings were unremarkable in 54.84% (34/62 patients) of asymptomatic SARS-CoV-2 infections. A number of patients with abnormal laboratory values had leukopenia or lymphocytopenia (16.67% by overall estimation 17.15% by meta-analysis for leukopenia, 16.24% by overall estimation and 16.79% by meta-analysis for lymphocytopenia) (Fig. 3). C-reactive protein values were slightly above the upper range of normal in 13.93% of the patients by overall estimation and 14.48% by meta-analysis (Fig. 3 and Supplementary Table 2). Elevated lactate dehydrogenase (LDH) was also frequently found in individual cases and in case-series (14.71% and 13.24% by overall analysis and meta-analysis, each). In contrast, some had elevations in procalcitonin (6.56% and 9.54% by overall analysis and meta-analysis, respectively) and white blood cells (leucocytosis, 8.06%, and 9.87% by overall analysis and meta-analysis, respectively), which might be a sign of concomitant bacterial infection.
Discussion
Most patients with an asymptomatic disease course presented with normal laboratory parameters. Predominant laboratory findings were leukopenia, lymphopenia, LDH, and CRP elevation. Previous reports with symptomatic patients revealed more patients with leucocytosis than leukopenia, but other findings of our analysis were consistent with previous studies (Chen et al., 2020, Huang et al., 2020, Wang et al., 2020a). In contrast, findings from CT investigations revealed that over half of the 135 asymptomatic patients presented with abnormalities. Younger asymptomatic patients were significantly more likely to have normal CT findings. Therefore, we argue that ordering a CT in young patients under the age of 20 with a normal chest radiograph should be avoided. The predominant radiological finding in asymptomatic cases is lung opacities (all patients) and airway abnormalities in a third of the cases (Inui et al., 2020).
COVID-19 emerged as a global threat, affecting 7,200,000 people worldwide and causing about 400,000 deaths as of 12 June, 2020. Several ways of transmission are possible, with person-to-person transmission proposed as the leading route of infection. Also, experiments indicated that aerosol and fomite transmission is plausible since SARS-CoV-2 remains viable in aerosols for at least three hours. The virus was stable on plastic and stainless steel (van Doremalen et al., 2020), Fecal-oral transmission has been discussed, as SARS-CoV-2 RNA can be detected in stool samples of a proportion of patients with COVID-19, and gastrointestinal symptoms have been reported in about 2%–10% (Yeo et al., 2020). Urine might not be a source of transmission since an analysis of 72 samples revealed no positive test result (Wang et al., 2020b), which is in contrast to previous outbreaks caused by coronaviruses (Naicker et al., 2020). The highest risk of transmission is posed by individuals with undocumented infections, either those who are completely asymptomatic, those having mild symptoms and not seeking medical advice or those who are contagious during the incubation period. Almost all asymptomatic patients included in our meta-analysis were tested because they had close contact with a confirmed case, ranging from exposure of only 15s to sharing the same household (Bai et al., 2020, Han and Yang, 2020, Su et al., 2020). While few studies indicated whether these asymptomatic cases infected others, many reports observed transmission of the disease through asymptomatic carriers (Liu et al., 2020, Rothe et al., 2020). Analysis of the viral load indicated that among 18 COVID-19 infected patients, the viral load among 17 symptomatic and one asymptomatic patient was comparable, once again underlining the transmission possibility of asymptomatic individuals (Zou et al., 2020).
General measures to prevent the spread of COVID-19 include quarantine, isolation of infected cases, preventing close contact, and travel restrictions. Younger people are usually more active (social contacts, appointments, traveling), and thus a source of transmission. As of 27 March 2020, in countries with an effective screening policy such as South Korea, most confirmed infected individuals fall in the age group 20–29 years (29.9%). A second peak is seen in patients between 50 and 59 years of age (19.4%); only a few patients were between 70-to-79 years (5.4%) or over 80 years (2.7%) (Shim et al., 2020). In Italy, a country with a very high case fatality rate, most COVID-19 cases belonged to the age group older than 70 years (38.6%), followed by patients between 51 and 70 years (30.9%) (Informatica, 2020). These differences point towards different testing strategies among countries but argue that testing young adults, who represent a significant transmission source, is essential even though they might present with mild symptoms. Our systematic review and meta-analysis highlighted that most patients with an asymptomatic disease course belong to younger age groups, while there is no significant difference in gender distribution. Top-down draconian measures implemented in most countries resemble those successfully used to eradicate SARS-CoV, and proved successful in China to combat COVID-19, including active case detection, isolation of COVID-19 positive individuals, contact tracing and quarantine of all contacts, social distancing, and community quarantine (Wilder-Smith et al., 2020). In particular, transmission by asymptomatic cases may be contained by such rigorous measures.
The percentage of asymptomatic cases in case-series was 24.2%. Studies including results from different prediction models indicated the percentage of undocumented and asymptomatic cases ranging from 9.2% to 69%. Data came from 565 Japanese citizens evacuated out of Wuhan, with five out of eight positive tested passengers being asymptomatic during the follow-up period of over 30 days. The information was used to calculate the case ascertainment rate in Wuhan and found a 9.2% rate, using a conservative model (Nishiura et al., 2020a). The patients' asymptomatic ratio was calculated as 30.6% (Nishiura et al., 2020b). This rough estimate was confirmed by an analysis of passengers with COVID-19 on the “Diamond Princess” cruise ship (Mizumoto et al., 2020a). By implementing draconic measures, an increase in the rate of detected infections from 14% to 69% was predicted (Li et al., 2020). These calculations clearly argue that undocumented infected or asymptomatic patients are a primary source for the geographic spread of COVID-19. These estimates further highlight the importance of our study, since more than 20% of reported COVID-19 cases in case-series have no symptoms at the time of diagnosis and could be a relevant source of transmission.
Several limitations of our study should be considered. First, the number of studies included in our meta-analysis was relatively low (13 case reports and four case-series), although we also reviewed articles with asymptomatic patients that did not report CT or laboratory findings. Second, laboratory findings in many studies were reported only as means and standard deviation, especially in case-series. Thus, we were unable to identify the proportion of patients with a lower or higher-than-normal range of laboratory tests. Our findings should be interpreted carefully, considering that the representation of clinical characteristics in the summarised report may have been incomplete. Third, the article search was performed in PubMed® and Google®, since a vast majority of relevant publications had been deposited in preprint servers at the time of our literature search.
In this systematic review and meta-analysis, we provided evidence that the performance of CT scans in young asymptomatic patients should not be performed as a majority will have no relevant abnormalities. Our analysis found that asymptomatic cases could account for more than 20% of all COVID-19 patients. It is essential to test individuals in close contact with confirmed cases. Recent investigations from Iceland with random sampling indicate that over 6% of over 10,000 inhabitants tested positive. By increasing our testing capacities, we might identify asymptomatic cases earlier and thus prevent transmission. This is absolutely necessary to overcome the continuous spread of SARS-CoV-2 and eradicate this virus.
Author contributions
A. K., D. K., and S. Y. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. A. K., D. K., S. Y., K. L., M. E., and J. S. contributed substantially to the study design, data analysis and interpretation, and manuscript writing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interests
The authors declared that they have no conflicts of interest.
Ethical approval
This study does not require ethical approval because the meta‐analysis is based on published research, and the original data are anonymous.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.06.052.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R., Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp. Clin. Trials. 2007;28(2):105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Fletcher J. What is heterogeneity and is it important? BMJ. 2007;334(7584):94–96. doi: 10.1136/bmj.39057.406644.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Yang H. The transmission and diagnosis of 2019 novel coronavirus infection disease (COVID-19): A Chinese perspective. J. Med. Virol. 2020 doi: 10.1002/jmv.25749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel Coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui S., Fujikawa A., Jitsu M., Kunishima N., Watanabe S., Suzuki Y. 2020. Chest CT Findings in Cases from the Cruise Ship “Diamond Princess” with Coronavirus Disease 2019 (COVID-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball A. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility—King County, Washington, march 2020. MMWR. 2020;69 doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T.T.-Y., Shum M.H.-H., Zhu H.-C.-C., Tong Y.-G.-G., Ni X.-B.-B., Liao Y.-S.-S. Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins. Nature. 2020 doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- Li R., Pei S., Chen B., Song Y., Zhang T., Yang W. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) Science. 2020 doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Z., Xu X., Gan Q., Zhang L., Luo L., Tang X. Asymptomatic SARS-CoV-2 infected patients with persistent negative CT findings. Eur. J. Radiol. 2020;126 doi: 10.1016/j.ejrad.2020.108956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.C., Liao C.H., Chang C.F., Chou C.C., Lin Y.R. A locally transmitted case of SARS-CoV-2 infection in Taiwan. N. Engl. J. Med. 2020;382(11):1070–1072. doi: 10.1056/NEJMc2001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro. Surveill. 2020;25(10) doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro. Surveill. 2020;25(10) doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naicker S., Yang C.W., Hwang S.J., Liu B.C., Chen J.H., Jha V. The Novel Coronavirus 2019 epidemic and kidneys. Kidney Int. 2020 doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H., Kobayashi T., Miyama T., Suzuki A., Jung S.M., Hayashi K. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int. J. Inf. Dis. 2020 doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H., Kobayashi T., Yang Y., Hayashi K., Miyama T., Kinoshita R. The rate of underascertainment of novel coronavirus (2019-nCoV) infection: etimation using Japanese passengers data on evacuation flights. J. Clin. Med. 2020;9(2) doi: 10.3390/jcm9020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C. Transmission of 2019-nCoV Infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim E., Tariq A., Choi W., Lee Y., Chowell G. Transmission potential and severity of COVID-19 in South Korea. Int. J. Infect. Dis. 2020;93:339–344. doi: 10.1016/j.ijid.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L., Ma X., Yu H., Zhang Z., Bian P., Han Y. The different clinical characteristics of coronavirus disease cases between children and their families in China - the character of children with COVID-19. Emerg. Microbes Infect. 2020;9(1):707–713. doi: 10.1080/22221751.2020.1744483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., Cheng P., Chen W., Wan P., Chen Y., Yuan G. High incidence of asymptomatic SARS-CoV-2 infection, Chongqing, China. medRxiv. 2020 [Google Scholar]
- Tian S., Hu N., Lou J., Chen K., Kang X., Xiang Z. Characteristics of COVID-19 infection in Beijing. J. Infect. 2020;80(4):401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith A., Chiew C.J., Lee V.J. Can we contain the COVID-19 outbreak with the same measures as for SARS? Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is fecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol. Hepatol. 2020;5(4):335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.