Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spreads mainly by means of aerosols (microdroplets) in enclosed environments, especially those in which temperature and humidity are regulated by means of air-conditioning. About 30% of individuals infected with SARS-CoV-2 develop coronavirus disease 2019 (COVID-19) disease. Among them, approximately 25% require hospitalization. In medicine, cases are identified as those who become ill. During this pandemic, cases have been identified as those with a positive SARS-CoV-2 polymerase chain reaction test, including approximately 70% who were asymptomatic—this has caused unnecessary anxiety. Individuals more than 65 years old, those affected by obesity, diabetes, asthma, or are immune-depressed owing to cancer and other conditions, are at a higher risk of hospitalization and of dying of COVID-19. Healthy individuals younger than 40 years very rarely die of COVID-19. Estimates of the COVID-19 mortality rate vary because the definition of COVID-19–related deaths varies. Belgium has the highest death rate at 154.9 per 100,000 persons, because it includes anyone who died with symptoms compatible with COVID-19, even those never tested for SARS-CoV-2. The United States includes all patients who died with a positive test, whether they died because of, or with, SARS-CoV-2. Countries that include only patients in which COVID-19 was the main cause of death, rather than a cofactor, have lower death rates. Numerous therapies are being developed, and rapid improvements are anticipated. Because of disinformation, only approximately 50% of the U.S. population plans to receive a COVID-19 vaccine. By sharing accurate information, physicians, health professionals, and scientists play a key role in addressing myths and anxiety, help public health officials enact measures to decrease infections, and provide the best care for those who become sick. In this article, we discuss these issues.
Keywords: SARS-CoV-2, COVID-19, Coronavirus, COVID-19 transmission, pandemic
The Origin and the Initial Spreading of the Pandemic
The current pandemic started as an outbreak in Wuhan, the largest city in Hubei Province, in the People’s Republic of China. There, in the early December 2019, a person from rural People’s Republic of China arrived harboring an infection of a new coronavirus that the WHO initially termed “2019 novel coronavirus.”1 This virus, the cause of coronavirus disease 2019 (COVID-19), is now called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).2 It seems that the virus had infected people in rural People’s Republic of China between October and December 2019, but it was not capable of efficiently spreading from human to human. In December 2019, in Wuhan, a city of 11 million, the virus mutated, as viruses do, and became capable of easily spreading to other human hosts—and thus, the pandemic started.3 , 4 On January 23, the Chinese government quarantined Wuhan, but it was too late—an estimated five million people had left Wuhan to celebrate the Chinese New Year, whereas others left when it became known that the city would be locked down. A total of 5 million is way too many; the outbreak could no longer be contained.
The virus spread from the People’s Republic of China through international air travelers. One of them, a Chinese woman from Shanghai, flew to Germany on January 22, 2020, and while there, infected a 33-year-old German man. SARS-CoV-2 was now in Europe.5 From Germany or People’s Republic of China, in a possibly independent event, SARS-CoV-2 reached (probably by means of air travel) the region of Lombardia in Northern Italy, where the first case of COVID-19 was diagnosed on February 18, 2020.
A recent article claimed the widespread presence of SARS-CoV-2 in Italy before 2020. This article was based on serologic measurements in serum samples from 2019.6 However, the results presented were based on enzyme-linked immunosorbent assay (ELISA) tests that can produce false-positive results because cross-reactions with unrelated viral antigens were not ruled out. Indeed, an independent study7 based on the polymerase chain reaction (PCR) evaluation of nasal swabs collected in Rome, Italy in 2019 did not find any evidence of SARS-CoV-2 viral sequences.
After 22 days, on March 11, a total of 1028 patients infected with SARS-CoV-2 were in intensive care units (ICUs) in Lombardia, nearly saturating all ICU beds. Once ICU capacity was reached, the number of acutely ill patients became a health catastrophe because new patients could not be treated properly. Physicians had to make the difficult decision of who get into ICUs, thus, increasing their chances of survival, and who does not. An additional problem was that major elective surgeries, such as brain, cardiac, and various cancer-related surgeries, among others, can only be performed if ICU space was available. Given that ICU beds were close to saturation, elective surgeries had to stop. This caused additional deaths, indirectly caused by the viral epidemic, among patients with other diseases who cannot be treated properly. This happened in Wuhan, People’s Republic of China, in Italy, and elsewhere.8
Coronaviruses
Coronaviruses comprise a large group of related RNA viruses that cause diseases in birds and mammals. There are four common human coronaviruses that are considered seasonal, namely: (1) HCoV-HKU1, (2) HCoV-NL63, (3) HCoV-OC43, and (4) HCoV-229E.9 Each year, they account for about 15% of the incidence of common colds. In 2002, a more dangerous coronavirus emerged in the People’s Republic of China. This coronavirus, SARS-CoV-1, spread from horseshoe bats to palm civet cats and into humans. It infected about 8422 people, killing 774, and then “vanished.”10 The nucleotide similarity between SARS-CoV-1 and SARS-CoV-2 is about 79%. After 10 years, a new coronavirus, called the Middle East respiratory syndrome coronavirus (MERS-CoV), emerged. It originated in bats,11 , 12 spread to camels, then to humans, and caused a limited epidemic in the Middle East that is still ongoing and has caused about 858 deaths.13
Human infections from bat coronaviruses should not be seen as a surprise but rather as a catastrophe waiting to happen. Bats sold in several live markets in the Mekong Delta region in Vietnam were infected with coronaviruses.14 Anthony et al.15 estimated that there are at least 3204 species of coronaviruses circulating in bats. Whatever the accuracy of this prediction, it is obvious that there is a high risk for new coronaviruses to emerge from bats and infect other species. Several episodes of limited bats-to-human coronavirus infection have been documented.16 An additional source of potential infection is bat guano, which is dry bat excrement (urine and feces) used as fertilizer worldwide. Guano is also used as an ingredient of traditional Chinese medicine, and it can also be found on Amazon.com, in which the Grocery and Gourmet food category include a listing for 1 g priced at USD $2.95. In Asia, it is also used to make rudimental bandages for injured limbs. In his 1850 State of the Union address, U.S. President Fillmore stated: “guano has become so desirable an article to the agricultural interest of the United States that it is the duty of the Government to employ all the means properly in its power for the purpose of causing that article to be imported into the country at a reasonable price.”
From guano, scientists detected coronaviruses closely related to MERS-CoV and SARS-CoV-1 and -2, and there is at least one documented infection of a guano harvester.16 In short, given the extensive human exposure to bats and their excrements, it should be no surprise that every few years, a new coronavirus jumps from bats to humans.
Detection of SARS-CoV-2 in Clinical Specimens
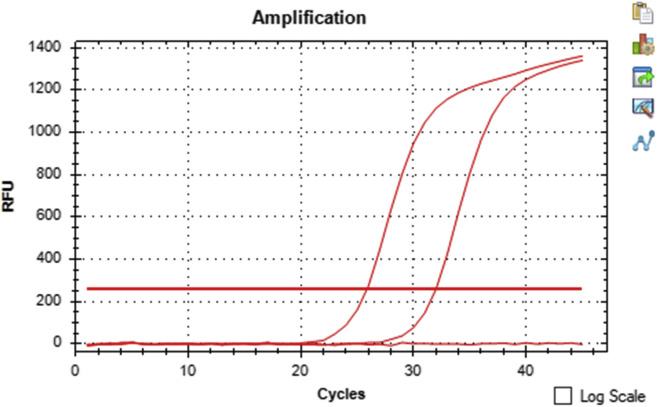
Measures taken to reduce the spread of COVID-19 critically depend on rapid and accurate identification of virus-infected individuals by the most sensitive and specific method available. At present, the most reliable and frequently used test is real-time reverse transcriptase–PCR (rRT-PCR), performed on upper respiratory tract specimens such as nasopharyngeal (or throat) swabs or saliva.17 SARS-CoV-2 viral genomic RNA (vRNA) levels are highest in the nasopharynx and oropharynx between 2 days before and 4 days after the onset of symptoms and remain detectable for a median duration of 15 to 18 days.18, 19, 20 Bronchial alveolar lavage fluids are more frequently positive but are not routinely collected.21 , 22 Saliva testing is replacing nasopharyngeal and bronchial testing because it is as sensitive and it is not painful to administer; thus, it is particularly helpful when testing children who can be given a straw and told to drool saliva in a test tube. SARS-CoV-2 remains viable in saliva for 4 days at room temperature, no chemicals are needed to be added and the sample can be mailed to a laboratory for RT-PCR testing or viral isolation (J. Lednicky, unpublished observations). RT-PCR testing of stool may hold value to identify patients who recovered from an infection that may shed apparently noninfectious viral particles for some time.18 , 20 Several RT-PCR tests have been developed, and the amplification targets vary, such as detection of SARS-CoV-2 env, N, S, and RdRp genes.17 The U.S. Centers of Disease Control and Prevention has developed two tests, the original version that tested for SARS-CoV-2 vRNA only, and a newer multiplex assay that also detects influenza A and B vRNAs.23 The analytical and clinical performance of seven commercial RT-PCR diagnostic kits from different manufacturers were judged as suitable for routine diagnostics of symptomatic COVID-19 patients.24 Several research teams have also developed their own tests. In the University of Florida, for example, an rRT-PCR test previously developed by J. Lednicky to detect beta coronavirus in bats is used in various research projects to detect SARS-CoV-2 vRNA in human and environmental samples.25 , 26 An example of a test run using the University of Florida test for detection of the RdRp gene sequence with a human specimen (from a patient with COVID-19), positive control SARS-CoV-2 vRNA, and negative control, is illustrated in Figure 1 .27
Figure 1.
rRT-PCR detection of SARS-CoV-2 vRNA in a nasopharyngeal swab from a patient with COVID-19. The Cq value of the patient specimen is 25.84, and the positive control 31.97. No signal was generated by the negative control. There is an inverse relationship between Cq and target amplification; the lower the Cq, the higher the amount of SARS-CoV-2 in the RT-PCR. The specificity of most of the frequently used RT-PCR tests is 100% because the primer design is specific to the genome sequence of SARS-CoV-2. Nevertheless, the sensitivity of detection varies between the RT-PCR tests. The cycle at which amplification exceeds the background fluorescence is expressed as the quantification cycle (which some refer to as Cq and some as Ct). This is the new standardized term for reporting rRT-PCR results on the basis of MIQE guidelines. COVID-19, coronavirus disease 2019; Cq, quantitation cycle; Ct, cycle threshold; MIQE, Minimum Information for Publication of Quantitative Real-Time PCR Experiments; RFU, relative fluorescence unit; rRT-PCR, real-time reverse transcriptase-polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; vRNA, virus genomic RNA.
Whereas PCR-based tests detect active infections, serology is used to detect past IgG or ongoing infections (IgM and IgG). Individuals who were infected with SARS-CoV-1 developed neutralizing antibody responses that lasted for several years. If reinfection occurred, the outcome was usually a mild illness or an asymptomatic presentation.28 Similarly, it has been assumed that individuals with antibodies against SARS-CoV-2 are protected from disease.
If individuals with SARS-CoV-2 antibodies are protected from disease, they could safely return to normal activities, help restart the economy, and may also help take care of the most vulnerable population. A number of serologic assays, such as ELISA and lateral flow assays, have been developed to detect SARS-CoV-2 antibodies. These assays may target the spike protein, the receptor-binding domain (RBD) part of the spike protein, or the SARS-CoV-2 nucleoprotein. These assays are relatively inexpensive and can be handled in a biosafety level 2 laboratory, making them preferable to classic virology neutralizing assays that require the use of a biosafety level 3 laboratory. However, the sensitivity of the serologic assays for SARS-CoV-2 has been questioned. Grandjean et al.29 reported that the half-life of the SARS-CoV-2 nucleoprotein antibody –which is the target of most commercial ELISA assays—is only 52 days, and for the spike protein, 82 days. Therefore, ELISA assays may be reliable only for recently infected patients. A parallel article by Fenwick et al.30 came to a similar conclusion and reported that nucleoprotein antibodies wane postinfection resulting in underestimation of the infected population and that tests using antibodies against the spike proteins are more sensitive.
Infections
SARS-CoV-2 infections are often referred to as cases, a term that, in other diseases, identifies those who became ill and require therapy. During the current pandemic, the term cases instead identifies those with a positive SARS-CoV-2 test, not the fraction (about 30% of infected individuals) who develop symptoms. This inaccurate terminology has caused confusion and has contributed to the anxiety that surrounds this infection.31 To put this issue in perspective, about 80% to 90% of sexually active people in the United States become infected with human papilloma viruses during their lifetimes, but we do not identify them as cases. Rather, cases are the 34,800 patients that develop human papilloma virus–related carcinomas each year in the United States, not the many millions with asymptomatic infections.32
Testing varies in different countries, within a country, and by region; some perform many tests, some only a few. Except in the People’s Republic of China, testing has been largely limited to symptomatic patients who seek medical care and to their immediate contacts; asymptomatic healthy people are largely not tested. Therefore, most countries are underestimating the total number of infected people, improperly called cases. As of December 12, 2020, there are about 70 million documented SARS-CoV-2 infections worldwide—this is an underestimate because asymptomatic individuals account for most infections, and they are seldom tested.
The percentage, rather than the actual number of positive tests, is a more relevant way to observe the pandemic, although because positive patients are often tested multiple times, even percentages are not accurate as they include new and ongoing infections. In some places, for example, in Wuhan during the initial phases of the outbreak, in Lombardia, and in many cities in the United States, among others, authorities tested almost exclusively those who were sick enough to seek hospitalization. This, of course, resulted in a much higher percentage of positives and, among them, of COVID-19 cases (here the term is used in its correct form), which required hospitalization, than in places where testing was random and included healthy volunteers.
Death Rates
When most of the positive tests are from hospital testing, as in the beginning of the pandemic, mortality is being measured in the fraction of patients most severely affected, and thus, will be higher than when measured across the population, which includes approximately 70% of asymptomatic individuals. The recent record numbers of infections in the United States revealed that the fraction of people who died because of SARS-CoV-2 infection is 1% or less, much smaller than previous estimates that had placed it at about 5% to 10%, percentages largely on the basis of hospital testing.
The overall number of deaths attributed to COVID-19 is inaccurate and overall represents an overestimation. As of December 12, 2020, there were an estimated 1.6 million deaths worldwide attributed to COVID-19, approximately 296,000 of them occurred in the United States.33 Why are these numbers not accurate? At the beginning of the pandemic, mortality counts may have underestimated the actual number of deaths because of COVID-19, as they did not include those who died at home or who died in a hospital but were not tested for SARS-CoV-2. In contrast, mortality will overestimate the number of deaths if all people infected with SARS-CoV-2 are counted as COVID-19 deaths regardless of the underlying pathological characteristics and cause of death.
A well-written autopsy report distinguishes the main cause of death from underlying contributing factors and from the immediate cause of death. For example, mesothelioma (the main cause of death) in a patient with obesity and diabetes (contributing factors) who developed bronchopneumonia as he was lying in bed (immediate cause of death). It is easy to understand how the number of deaths attributed to COVID-19 will vary greatly depending on how we count them. In the United States, all patients who test positive for SARS-CoV-2 infection and die are counted as COVID-19 deaths, regardless of the underlying disease. It would be much more accurate instead to specify if these patients died with SARS-CoV-2 infection, which would clearly distinguish them from those who died because of its main cause of death, from those in which COVID-19 was a contributing factor or the immediate cause of death in an already terminally ill patient. Obviously, by using the current approach, the United States reports a higher number of deaths for COVID-19 than if we were to use the criteria we use for deaths caused by other diseases.34 The Istituto Superiore di Sanità of Italy, the equivalent of the National Institutes of Health in the United States, investigated 4942 death certificates and found that 89% of deaths attributed to COVID-19 were indeed caused by this disease: however, they did not distinguish whether COVID-19 was the main or the immediate cause of death. The remaining 11% of deaths were attributed to other causes in individuals infected with SARS-CoV-2 (passenger or cofactor).35 As to why people with COVID-19 die, a recent article reporting autopsy findings for 22 COVID-19 Italian cases (including four cases without comorbidities) found that COVID-19 caused a multisystem pathology. The main and most common pathology was in the lungs and in the cardiovascular system. In addition, hepatic, kidney, splenic, and bone marrow tissue damage and microvascular injury and thrombosis were detected.36
Viral Infection, Routes of Transmission, and Window of Infectivity
Cell entry requires binding of the SARS-CoV-2 spike protein to its cell surface receptor, angiotensin-converting enzyme 2 (ACE2), which is present on several cell types, including epithelial cells in the upper respiratory tract and type II pneumocytes.37, 38, 39
SARS-CoV-2 may spread through direct, indirect, or close contact with infected people through mouth and nose secretions. These include contact with secretion droplets (>5 μm) and inhalation of droplet nuclei (aerosol, <5 μm) or perhaps of airborne particles in fecal clouds.40 Droplets are expelled from the mouth when people talk, more so when they speak loudly or sing, and they fall to the floor or on objects within 1 to 3 feet from the source, thus, the recommendation for social distancing and hand washing. Although theoretically possible, this seems to be a rare source of infection.41 Most infections are instead linked to inhalation of droplet nuclei (i.e., in aerosols) as they float (i.e., are suspended) in the air. In a room in which the windows are closed and temperature maintained by means of air-conditioning (A/C) (thus, with low humidity), droplet nuclei can remain adrift for hours. Because SARS-CoV-2 remains infectious for at least 16 hours in aerosols, the risk of infection is high in crowded, enclosed spaces.41 , 42 Opening windows rapidly eliminate aerosols,43 reducing or eliminating the risk of inhaling SARS-CoV-2.42 , 44
Many, if not most, SARS-CoV-2 infections go undetected. Poletti et al.45 studied 5484 individuals infected with SARS-CoV-2 and found that 73.9% of them were less than 60 years old and did not develop symptoms (95% confidence interval [CI]: 71.8%–75.9%). The risk of symptoms increased with age—6.6% of infected patients older than 60 years had severe disease, with men at substantialy higher risk.
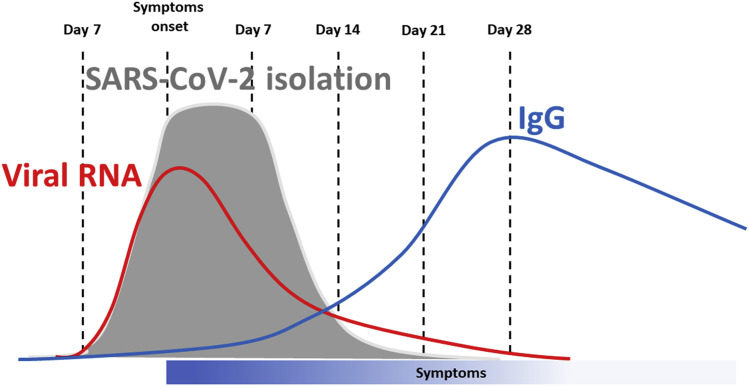
Infected individuals, regardless of whether they develop COVID-19, can spread the virus. It has been difficult to pinpoint the exact length of time during which infected individuals can spread the virus, the so-called “infectivity window”46 (Fig. 2 ). He et al.,47 studied 77 infectious-infected couples and proposed that the maximum infectivity occurs between 2 days before and 1 day after the onset of symptoms and that 8 days after the first symptoms, infectivity decreases significantly. The methodology of this work, however, has been criticized,48 although the authors made some corrections.49
Figure 2.
Infectivity window. The period of time during which infectious virus can be isolated and grown in cell culture is shown in gray, the detection of viral RNA by RT-PCR in red, the development of specific IgG in red. Virus isolation may vary significantly among different laboratories depending on the skills of those involved. Detection of viral sequences by RT-PCR may last longer than viral isolation as noninfectious viral particles are released. The length of time of IgG persistence is uncertain at this time. IgG, immunoglobulin G; RT-PCR, reverse transcriptase-polymerase chain reaction.
A more sensitive approach than relying on symptoms is to isolate viruses from patients (i.e., grow them in cell culture). If SARS-CoV-2 can be isolated from a nose or throat swab, or from saliva, it is likely that the patient is infectious. Indeed, an rRT-PCR test cannot establish infectivity. This is because rRT-PCR detects fragments of vRNA, the corresponding sequences of which are present in viable (“live”) and also in nonviable (“dead”) virus particles. In other words, the RT-PCR can be positive in the following settings: (1) exposed noninfected or noninfectious individuals who were in contact with the virus but resisted infection, and those who were infected but are no longer infectious and may release noninfectious viral RNA fragments; (2) infected individuals who did not develop COVID-19, that is, asymptomatic infectious carriers; and (3) those who have COVID-19 disease and for which the term “cases” should be reserved. RT-PCR cannot distinguish among these three categories.
Wölfel et al.,20 studied nine patients with COVID-19 and found that it was not possible to reliably isolate SARS-CoV-2 from throat swabs 8 days past the onset of symptoms. However, the estimated CIs were large: at 14 days from onset of symptoms, the probability of isolating SARS-CoV-2 varied between 0% and 20%. The authors proposed that when the viral load determined by PCR was less than 10,000 copies per reaction, the probability of isolating SARS-CoV-2 at a 95% CI varied between 0% and approximately 30%. Therefore, by correlating the viral load with SARS-CoV-2 isolation, it may be possible to establish a threshold value, below which the probability of a patient being infectious is small.
Singanayagam et al.,50 using a larger collection of samples, isolated SARS-CoV-2 up to 12 days from the initial symptoms. At day 14 after the initial symptoms, SARS-CoV-2 isolation dropped from 0% to 9.4%, and at day 15, to 0% to 6.7%. They found no difference in the probability of isolating SARS-CoV-2 from symptomatic or asymptomatic individuals, and they found no difference in the viral load of symptomatic and asymptomatic infected individuals—something that the authors of this review find it difficult to understand because it is contrary to most other viral infections. Singanayagam et al.50 further noted that, even for swabs with very low viral load (cycle threshold [Ct] >35), SARS-CoV-2 could be isolated in 5 of 60 samples (see Fig. 1 for an explanation of the Ct). Of note, these authors held their virus cultures for an observation period of 14 days, whereas many laboratories hold theirs for only 4 days. This partly explains their success at virus isolation with a Ct equal to 35 or higher. Many of the failures in viral isolation are caused by technical inadequacies. For example, the laboratory of J. Lednicky has an 85% success rate of SARS-CoV-2 isolation in samples with a Ct of 35. Moreover, SARS-CoV-2 replicates better in the upper respiratory tract than in the throat; thus, nasopharyngeal swabs (and saliva) are better specimens for evaluating infectivity (J. Lednicky personal observation, unpublished). A very recent study revealed that SARS-CoV-2 multiplies robustly in the oral cavity, and this likely explains why patients with COVID-19 express a range of oral manifestations such as ageusia and oral blisters.51
In summary, the following points are noted: (1) infectivity is higher immediately before and soon after the onset of symptoms; however infected patients can remain infectious for about 2 weeks; (2) ten days from the appearance of the initial symptoms, the risk that a patient is still infections varies between 1% to 10% at a 95% CI; (3) infectivity is related to the viral load, but not to the development and severity of COVID-19; (4) individuals with a low viral load, determined by Ct greater than or equal to 35, may be infectious; (5) the window of infectivity varies in different individuals and some may be infectious for several weeks (these “long-term spreaders” represent a fraction of the infected population, probably too small to significantly affect the spreading of the virus; thus, the quarantine length could ignore them. At lower levels of virus circulation, however, long-term spreaders may frustrate efforts to further curb the epidemic if the quarantine length is not long enough to account for their prolonged infectivity window); and (6) most importantly, asymptomatic individuals—who account for about 70% of infections—seem able to spread the virus as well as those who become ill50; if this is true, it will be much more difficult to contain the further spread of this pandemic.
Public Health Considerations
Public health measures initially focused on the role of respiratory droplets and fomites in the transmission of SARS-CoV-2.52 However, recent data underscore the role of aerosol transmission of SARS-CoV-2 in enclosed spaces where air recirculates as the main cause of the spreading of the pandemic.53, 54, 55 One of this article’s coauthors isolated SARS-CoV-2 from an air sample of a hospital room with a newly admitted patient with COVID-19.26 In contrast, the risk of transmission by means of fomites seems minimal.56 Qureshi et al.57 noted that the notion that SARS-CoV-2 is spread by droplets or fomites was based on old information related to other viruses, not on SARS-CoV-2 science. It has been estimated that the risk of SARS-CoV-2 transmission by means of fomite contamination of surfaces is less than 5 per 10,000.58
Person-to-person transmission of the virus in open spaces is also very rare.59 A recent study found very few examples of outdoor transmission of SARS-CoV-2 among 25,000 infections investigated, suggesting a very low risk.59 However, the risk of outdoor transmission increases when gathering density increases, particularly for an extended period of time in cold temperatures. Low humidity and cool temperatures result in aerosols remaining near our breathing zone. Instead, in warm temperatures, heat currents push small particles upward and away from our breathing zone. Think of a buzzard soaring on a hot day: the bird rides the upward draft from the heat currents and can stay aloft without moving its wings. Therefore, in cold temperatures, there may still be some risk outside if standing in a crowd.59
SARS-CoV-2 transmission occurs mostly in enclosed spaces wherein the temperature and humidity are maintained relatively low by means of A/C, and windows are kept closed, trapping airborne virus microdroplets (aerosol) within the space. Open the windows and move the air out, and the risk of infection is eliminated or at least drastically reduced.
In Wuhan, for example, many infections occurred in crowded emergency rooms and in physician’s offices where large numbers of infected people waited together with noninfected people. The high concentration of virus caused widespread infections, including physicians who treated these patients, despite wearing masks (Fig. 3 )60 Masks, except for N95 masks, and to a lesser degree, surgical masks, and cloth masks, seem to have limited capacity to protect in a crowded enclosed space with high viral concentration, such as a hospital waiting room; but of course, all masks are helpful to reduce transmission in enclosed spaces, including public transportation. The use of masks outside, in the open air, is unnecessary, except maybe in crowded settings.
Figure 3.
Wuhan. Left, crowded ER in Wuhan before the lockdown, patients with cough, fever, and other respiratory symptoms crowded the ER together with patients with other conditions. This is where the epidemic spread rapidly in the early phases of the pandemic. Right, the first day of lockdown in Wuhan, the main commercial street is desert. ER, emergency room.
The risk of SARS-CoV-2 transmission is high in crowded public transportation vehicles. Shen et al.,61 reported that during a 100 minutes bus drive, one passenger infected with SARS-CoV-2 infected 23 of the additional 67 passengers regardless of where they sat on the bus, providing strong evidence of transmission by means of aerosol. Passengers did not wear masks, and the temperature was regulated by means of A/C, which, by keeping the humidity low, allows the airborne virus to stay adrift longer. Moreover, when A/C recirculates the air, it favors the spreading of the aerosol.
The hypothesis that SARS-CoV-2 spread widely on airplanes was largely based on an older study on SARS-CoV-1 transmission in an airplane, which found that one individual infected 22 passengers who became ill within 4 days from the flight, including some seated far away from the infected case.62 In contrast, the spreading of SARS-CoV-2 in airplanes may be limited. First, there has not been a reported increase in SARS-CoV-2 infections among airplane hostesses, compared with the high rates of infections among health care workers. Second, the hypothesis that airline flight is relatively safe from infection is supported by a recent report of a flight from Tel Aviv to Frankfurt in March 2020, before any measures to prevent transmission, including wearing masks, had been introduced on commercial airlines.63 On this flight spanning 4 hours and 40 minutes, there were 102 passengers, including a tourist group of 24—four of them became sick during the flight. On arrival, 7 of 24 tested positive for SARS-CoV-2 infection. Among them, four were symptomatic, two were presymptomatic, and one never developed any symptoms. Follow-up testing of the other passengers revealed that only two, both within two rows of an infected passenger, had been infected. The low number of infections were limited to nearby passengers, despite the long flight duration with no masks, suggesting that the high-efficiency particulate air filters present in today’s airplanes, and the circulation system that replaces air every 2 to 3 minutes in the cabin, are effective in greatly reducing the spread of aerosol-containing virus throughout the whole cabin. Bae et al., 64 reported transmissions from one asymptomatic carrier to a woman sitting three rows away on an evacuation flight from Milano to South Korea. The infected woman wore an N95 mask throughout the flight—like every other passenger—except during meals and when she used the toilet. We do not know whether improvements in the air circulation and filtration in the cabin during the past 17 years, or differences among SARS-CoV-1 and SARS-CoV-2, or chance, or maybe a combination of all these variables, were responsible for these differences.
As for the possibility that animals spread SARS-CoV-2, so far, there have been reports of minks passing the virus to people. SARS-CoV-2 replicates poorly in dogs, pigs, chickens, and ducks, but replicates well in cats and ferrets, as these animals are permissive to the infection; infected animals can infect other animals of the same species.65 , 66 Moreover, antibodies for SARS-CoV-2 were detected in cats in Wuhan, suggesting that humans can infect cats, raising the possibility that cats may infect humans.67 SARS-CoV-2–related coronaviruses have recently been isolated from Malayan pangolins, suggesting that these animals that are sold in some wet markets in Asia could represent an additional source of infection.68 , 69 In summary, SARS-CoV-2 might be spreading undetected among some animals. Whether this occurs and whether animals may contribute to some human infections remains to be investigated. Finally, SARS-CoV-2 causes a COVID-19–like disease in macaques, a good model to study this disease.70
Public Health Measures in Countries That Have Been Able to Contain the Pandemic
The People’s Republic of China is the only country (so far) that has been able to nearly block SARS -CoV-2 transmission by implementing very strict measures that require residents to give up any personal privacy. Initially, to stop the spread of SARS-CoV-2, Chinese authorities responded by locking down the city of Wuhan and by implementing a rapid multidisciplinary effort using all possible technologies, personnel, and resources that were based on their previous experience with SARS infection.71 , 72 At present, in the People’s Republic of China, there is, in addition, a ban on gatherings, and the requirement to wear masks; everyone is also “tagged” and traced on an app uploaded on their mobile phones. As people go about their lives, all those they meet and places they visit are identified and transmitted to the authorities by this app so that if they are considered at-risk, they are rapidly identified, tested, and quarantined. All public places, airports, train stations, hospitals require individuals to scan a barcode displaying a green code (indicating no contact with at-risk individuals) before admission is granted. Borders are sealed, and foreigners are subject to a 14 days quarantine. This means they are locked in a room in designated hotels with security guards; meals are brought to the door, and they are not allowed out of their room. With these strict control measures, the entire country is currently open for normal business.
Australia, New Zealand (NZ), and the Republic of China have also been able to contain the infection by implementing very strict measures that are similar to those used in the People’s Republic of China.73 , 74 Australia, NZ, and the Republic of China are islands and, therefore, can more easily monitor their borders. Moreover, they acted in the early phases of the pandemic, when relatively few had been infected. In NZ, the implementation of the lockdown was supported by a national state of emergency, declared on March 25, 2020, which, along with newly written law changes passed through parliament, enabled special powers to address the pandemic.75 The Republic of China established a National Health Command Center in 2004 after the SARS epidemic. This agency was dedicated to responding to emerging threats, such as pandemics, and given the power to coordinate work across government departments and draw on additional personnel in an emergency.
Equally important, however, is the willingness of the citizens to follow orders and the willingness or ability of the authorities to implement the orders. The same measures adopted in Australia, the People’s Republic of China, Republic of China, and NZ cannot be easily implemented in Western European democracies or even in the United States where many citizens continue to refuse to wear masks, do not accept to give up their privacy wearing an app that traces every step in their life, do not accept to seal the borders to refugees, do not accept bans on gatherings as illustrated, for example, by the many large demonstrations that occurred in the recent past in many U.S. cities and in France, Italy, Holland, and others. The culture is very different. For instance, Europe continued to accept thousands of asylum seekers from Africa during the past 12 months, some of them infected with SARS-CoV-2 on arrival. It would be illegal for European countries not to accept refugees. Australia, the People’s Republic of China, NZ, and Republic of China have a different, much tougher approach and were able to seal their borders.
As for the United States, different states have taken very different measures; therefore, it is not possible to generalize. Among the states that have taken stricter measures are Hawaii and New Mexico. Hawaii has remained, throughout the pandemic, the state with the lowest incidence of infections, of cases requiring hospitalizations and ICU, and of COVID-19–related deaths. In New Mexico, the hospitals are strained and the ICU at near capacity; as of December 5, 2020, the state governor “was considering to allow hospitals to move to crisis standards”—a move that frees them to ration care depending on a patient’s likelihood of surviving. There are only two differences we could identify. First is that Hawaii, being an island in the middle of the Pacific Ocean, can easily control its borders. New Mexico is unable to do this, as circulation is free among the U.S. states—except for Hawaii, where currently, there is the equivalent of a border-on-arrival for anybody, including residents who are required to quarantine for 2 weeks unless they upload the results of a COVID-19 negative test performed within 72 hours before departure. Second is the climate in Hawaii, which allows people to live outside and keep windows open. Although both geographic isolation and climate are important, the experience of Calabria, Italy (to be subsequently discussed) suggests that the favorable climate of Hawaii (almost never too hot and never too cold to require closed windows and A/C) is the most important.
Calabria is the poorest region in Italy, with a population of 1.85 million (this would seem an unlikely place to take as a model). It is the destination for thousands of tourists during the summer months because of the beautiful beaches and forests and a favorable climate. Therefore, Calabria shares many similarities with richer states, such as California, where the pandemic continues to spread rapidly, many are dying, and the economic impact has been devastating. Despite the recent COVID-19 epidemic in Northern Italy, during the summer of 2020 in Calabria, tourists were welcome, and hotels stayed open and at full capacity. The beaches and parks were and are open, and people were and are encouraged to live outside where COVID-19 infections occur rarely, and when they do, they do not spread widely. Different than most places in the United States and Europe, A/C is seldom used as most businesses, including schools and restaurants, and individuals in their homes, rely on open windows to regulate the temperature—this happens to be a very effective measure to eliminate SARS-CoV-2–contaminated aerosols. Businesses are open, and people wear masks (like everywhere else); only discos are/were closed. Over 150,000 people had been tested for SARS-CoV-2 as of September 30, and the rate of positivity had remained at 0.1% with no COVID-19 deaths in more than 4 months (June–September 2020). Many of those testing positives were people who escaped Africa and landed in Calabria already infected. These simple measures, together with the precaution of testing people at their homes to prevent spreading, contact tracing, and isolation of positive cases, have been successful in containing the spread of infection as long as the climate was favorable.
Calabria illustrates that, to some extent, it is possible to live with the epidemic and, at the same time, maintain our way of living by implementing simple, common-sense measures. However, as the weather is becoming colder, people congregate in enclosed spaces with windows and doors closed; the humidity is lower, and droplets float in the air longer. This set of circumstances creates the environment in which SARS-CoV-2 spreads; indeed, this autumn, we have seen the second wave of infections, especially in colder regions. Although Calabria remains the region in Italy with the lowest percentage of infections, there has been a significant increase of positive SARS-CoV-2 tests, from 0.1% during the summer months to 5.0% last autumn, and there have been a total of 379 deaths out of 19,333 documented infections as of December 12, 2020. In general, we tend to think that foreigners and visitors spread SARS-CoV-2; however, there were no tourists in Calabria past the early days of September and only refugees from Africa continue to land on its beaches at about the same rate as in the summer months, and masks have been used all year long. However, in autumn and winter, people in Calabria congregate inside and keep windows closed because of the cold climate, and this favors SARS-CoV-2 spread. Indeed, in Australia, the state of Victoria (6.3 million population) reported 20,352 SARS-CoV-2 infections and 820 deaths as of Dec 12, 2020, as compared with 1227 infections and six deaths in Queensland (5.0 million population). Both states have similar rules and immigration policies (actually, the lockdown was longer and stricter in the state of Victoria). The climate, however, is much warmer in Queensland.
COVID-19 Epidemiology and Susceptibility
About 30% of those infected develop COVID-19 and experience flulike symptoms, but 25% of them may require hospitalization, and one-third of hospitalized patients may require treatment in the ICU.76 Because of the large number of infections, the demand for ICU beds has exceeded the capacity in some regions.77 The concern that the capacity may be insufficient has led many hospitals to shut down elective surgeries and other procedures that may require ICU treatment, which causes additional deaths.
The overall hospital mortality has been about 20% and up to 81% among patients requiring mechanical ventilation—most are older patients with preexisting comorbidities.78, 79, 80, 81, 82 Men are much more likely to die and to require admission to ICU; in Lombardia, Italy, 82% of patients admitted in ICU were men.80
Individuals older than 65 years are at much greater risk of requiring hospitalization and death as are those affected by obesity, hypertension, congestive heart failure, diabetes, asthma, chronic kidney disease, and those who are immune-depressed, including patients with cancer.83 These preexisting diseases significantly increase the risk of hospitalization, ICU requirement, and death on SARS-CoV-2 infection, especially among older men.84 Among preexisting conditions, obesity stands out because of its frequency in the United States and its complexity, and because it seems to be the most common cofactor in patients with COVID-19 in the ICU.85 Because of the accumulation of abdominal fat, patients who are obese have reduced diaphragmatic excursion and ventilation. Moreover, patients who are obese have higher levels of inflammation, have compromised immune responses, and are often affected by additional comorbidities, such as diabetes, hypertension, congestive heart failure, and renal failure, that further increase their risk of developing life-threatening COVID-19.86 In addition, excessive secretion of proinflammatory adipokines by large deposits of epicardial fat among patients who are obese may have a negative inotropic effect and may also favor the cytokine storm, contributing to the patient’s demise.87
Cancer per se does not seem to increase the risk of death on SARS-CoV-2 infection; however, therapies that impair the immune response and surgery may increase the risk of COVID-19 disease and related death. Therefore, patients chemotherapy were largely stopped in the light of their immunosuppressive impact and potential adverse effects, and surgery was often postponed, partly because of pressures on hospital bed capacity in ICUs and also in an attempt to limit the spread of the virus to this vulnerable group.88 , 89 Luo et al.90 studied 102 patients with lung cancer who had a PCR-positive SARS-CoV-2 test—62% were hospitalized and 25% died. The median age was 68 years old, and 72% of all these patients had active and/or metastatic cancer. The determinants of severity were largely patient-specific and largely linked to smoking history, chronic obstructive pulmonary disease, and hypertension, which are common comorbidities in patients with lung cancer. Surgery was also delayed during active SARS-CoV-2 infection, and patients were treated with neoadjuvant therapy.90 The members of the Thoracic Cancers International COVID-19 Collaboration, a global consortium formed to study the effects of SARS-CoV-2 infection in patients with thoracic malignancies, reported a 33% mortality in what they characterized as an initial report of 200 patients, 151 of 200 with NSCLC, and the remaining 49 with other thoracic malignancies, all diagnosed with COVID-19. In multivariate analyses, only smoking habits maintained a statistical association with death. Because only 13 of 134 patients who met the criteria for admission to the ICU were actually admitted in the ICU, the authors suggested that reduced access to the ICU because of the COVID-19 pandemic might have influenced the high mortality. The data suggested that the type of systemic therapy, including immunotherapy and targeted therapies, did not influence the survival of these patients.91 A subsequent study conducted in Spain on 23 patients with lung cancer who developed COVID-19 also found that the type of systemic therapy did not influence the incidence of COVID-19 or mortality.92 These findings are still preliminary; however, they suggest that withholding or discontinuing therapy for patients with lung cancer out of fear of COVID-19 may not be warranted.
Epidemiologic studies have proposed an association between environmental pollutants measured as particulate matter (PM)2.5 concentrations in the air with the severity of COVID-19.93 Wu et al. 94 reported that even a small increase in exposure to PM2.5 leads to a large increase in COVID-19 death rate with the magnitude of increase 20 times that observed for PM2.5 and all-cause mortality. William et al., 95 reported that 50% of the variance of the predicted COVID-19 phenotype is because of genetic factors, and may reflect interindividual variation in the host immune response. Lucas et al. 96 reported that the immune profiles of patients that easily recovered from COVID-19 were different from those who did not. Zhang et al.97 found that loss of function at 13 human loci, which regulate TLR3- and IRF7-dependent type-1 immunity, transmitted either in an autosomal dominant or recessive fashion, underlie susceptibility to life-threatening COVID-19 pneumonia. They estimated that 3.55% of patients with life-threatening COVID-19 disease had genetic defects at eight of these 13 loci.97 In a parallel study, the same research team, estimated that 12.5% of men and 2.6% of women who develop life-threatening COVID-19 disease have autoantibodies against interferon; thus, their immune response against viral infections is impaired.98 The presence of autoantibodies in the population was estimated at 0.33% and patients with autoantibodies were 25 to 87 years old, with half of them older than 65 years. Therefore, these studies identified a subset of individuals with a genetic predisposition or acquired predisposition (autoantibodies) to develop severe COVID-19 disease. These findings help explain the higher prevalence of severe COVID-19 disease in men, and why, although rarely, apparently healthy and young individuals succumb to COVID 19. In summary, genetic susceptibility in some individuals together with exposure to pollutants exacerbates COVID-19 along with a model of gene and environment interaction.99
As for a possible increased susceptibility of African Americans to COVID-19, a recent study found that among patients treated in a hospital setting, mortality did not vary among whites and blacks after adjusting for comorbidities and sociodemographic factors, suggesting that the higher prevalence of comorbidities among African American patients is responsible for the observed increased mortality in this ethnic group.79 Accordingly, SARS-CoV-2 mortality among blacks in Haiti is much lower than among African Americans, possibly because of the differences in comorbidities (J. Lednicky, unpublished observations).
In contrast, the risk of death for young and healthy people is very small, and deaths outside the high-risk groups are quite rare.83 For example, in Northern Italy, on April 18, 2020, 20,000 people had already died of COVID-19. Among them, only eight were healthy and younger than 40 years. An additional six died in this age group, and no information was available about their previous health. Even assuming that some or all of these six were healthy, this would give a maximum of 14 healthy people younger than 40 dying of COVID 19 out of 20,000 COVID-19 deaths during a period of two months. To put this risk in perspective, in Italy, during the four weekends in the month of June 2019, a total of 72 people died of motorcycle accidents. Records of the COVID-19 pandemic in Italy revealed that patients younger than 40 very rarely died of COVID-19 and that less than 1% of those younger than 50 infected with SARS-CoV-2 died of COVID—about 4% to 5% of those between 50 and 70 years old, 20% of those older than 70, and 40% of those older than 80 died.100 Therefore, the reported average of 1% to 3% of the infection’s death rate is, as with many averages in medicine, a number of little relevance to the individual patient.
Regardless of the age group and comorbidities, men are at much higher risk than women to require ICU treatment and to die. This increased risk has been linked to genetic predisposition and to the presence of autoantibodies97 , 98 and testosterone. Hoffman et al.101 discovered that the membrane-bound serine protease TMPRSS2 cleaves the spike protein, allowing the viral membrane to fuse with the cell membrane and enter the cell. Infections could be blocked in cell culture by a clinically approved inhibitor of TMPRSS2. TMPRSS2 is produced in response to the binding of testosterone to its receptor. Montopoli et al.102 reported that men treated with androgen deprivation therapy because of prostate cancer had a significantly lower risk of being infected with SARS-CoV-2 and, when infected, to require intensive care or to die than patients with prostate cancer who were not on androgen deprivation therapy. Subsequent studies supported that testosterone levels influence susceptibility to infection and COVID-19.103 , 104
An ongoing phase 2 clinical trial at the University of California in Los Angeles is testing whether temporary suppression of androgens reduces hospital stay, the rate of admission in the ICU, and death among patients with COVID-19. Several other trials aiming at interfering with androgens are in the works.
Children can be infected, but rarely reveal clinical symptoms and only exceptionally require hospitalization. However, a very small fraction of them may develop Kawasaki disease, or maybe a variant of it, characterized by a powerful immune response that causes a cytokine storm, which some have called multisystem inflammatory syndrome in children. Kawasaki disease can be caused by various pathogens, including coronaviruses, and occurred in one to three of every 10,000 children before the COVID-19 pandemic. A 30-fold increase in Kawasaki disease has been observed in Lombardia during the COVID-19 epidemic between March and June 2020. Genetic predisposition is suspected. In a few of the children affected by Kawasaki disease, their blood pressure drops and the child may go into shock. In these cases, steroid and immunoglobulin therapy is effective, and the children recovered.105 Overall, the risk that children infected with SARS-CoV-2 develop Kawasaki disease is very small. Although children infected with SARS-CoV-2 very rarely develop symptoms, they may infect other children or adults. This creates the dilemma of whether schools should be kept open or closed. The United Kingdom and several other countries plan to keep the schools open because of the lasting negative effects for children to miss out on their education. It may be advisable for teachers, school bus drivers, and other individuals with preexisting conditions and/or older than 65 years old to stay home and instead allow only healthy and younger individuals until the pandemic is under control.
Clinical Findings and Outcomes of COVID-19
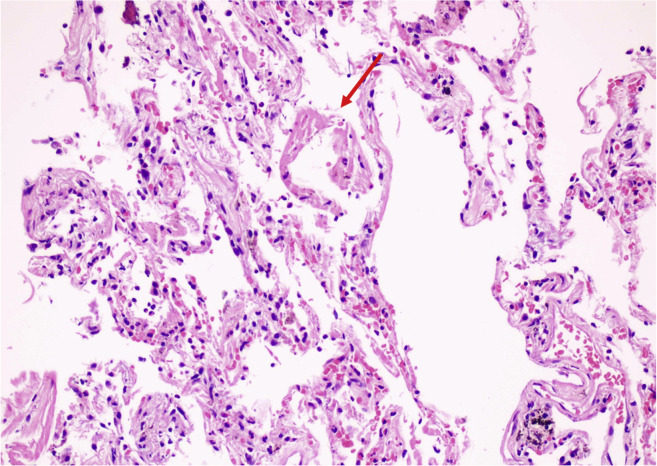
The pathophysiological mechanisms underlying these conditions are complex. The upper respiratory tract epithelial cells and alveolar type II pneumocytes, and endothelial cells throughout the body exhibit high densities of ACE2, the cell receptor for SARS-CoV-2 that allows viral infection.106, 107, 108 Patients with a higher risk for severe disease of fatal outcome do not exhibit increased ACE2 expression.39 Infected and damaged endothelial cells lead to vascular leakage, trigger blood clotting and cause inflammation and, at times, a lethal cytokine storm.109 The histologic appearance of the lungs of patients with COVID-19 (Fig. 4 ) revealed diffuse alveolar damage and pneumocyte hyperplasia, extravasation of fibrin and other proteins, focal patchy inflammatory infiltration, and massive congestion and the resulting thickened alveolar walls prevent oxygen exchange.31 , 110, 111, 112 Moreover, the endothelial damage leads to alveolar-capillary microthrombi and neoangiogenesis.113
Figure 4.
Lung pathology of a patient with COVID-19. Focal hyaline membrane (red arrow) indicating DAD, mild inflammatory infiltration, thickening of alveolar septa in adjacent areas. COVID-19, coronavirus disease 2019; DAD, diffuse alveolar damage.
The clinical spectrum of SARS-CoV-2 infection ranges from an asymptomatic condition to a multiorgan failure disease and includes life-threatening superinfections and long-term sequelae.114, 115, 116 Although the initial clinical manifestations are often those of an influenza-like illness, the infection is not limited to the lungs. SARS-CoV-2 causes viremia and binds the ACE2 receptors in the lungs, gastrointestinal tract, heart, vascular endothelium, kidneys, liver, and brain.117 The main symptoms include fever, cough, fatigue, myalgia, pharyngodynia, diarrhea, anosmia, and ageusia.118, 119, 120, 121, 122 More severe cases exhibit pneumonia, SARS, embolisms, diffuse intravascular coagulation, and eventually death.123
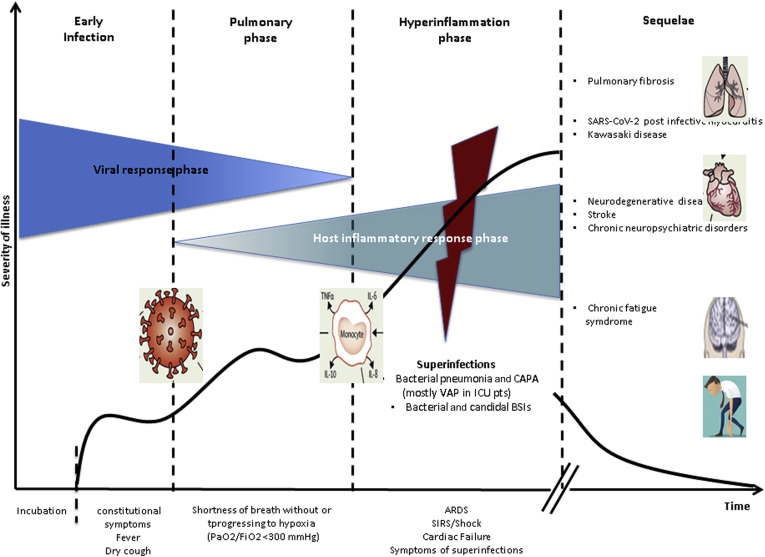
The early infection phase includes an asymptomatic incubation of 1 to 14 days, followed by disease manifestations (Fig. 5 ). A total of 7 to 14 days after the onset of the symptoms, some patients may develop a severe clinical condition. This fraction of patients (estimated at ∼5%, and as high as 20%–40% among those admitted to a hospital119 , 120 , 124) includes mostly elderly patients and/or patients with preexisting conditions (as previously mentioned.118, 119, 120 , 124 Clinical clues of a more aggressive and potentially fatal outcome include fever greater than 39°C, conjunctivitis, neurologic symptoms, evidence of a hypercoagulation state such as ecchymosis of the fingers and toes, and patients who underwent cytoreductive chemotherapy within 4 weeks before the onset of the symptoms.120 , 124, 125, 126, 127 As the severity of COVID-19 symptoms increases, some patients develop dyspnea with hypoxia, and chest imaging exhibits the appearance of ground-glass opacities in the lung that subsequently acquire a crazy-paving pattern and may progress from single to multiple and confluent, ensuing in lung consolidation (Fig. 6 A and B).114 , 125 , 128 In parallel, the peripheral blood T and B lymphocytes may significantly decrease, whereas laboratory markers of inflammation and organ involvement may either increase (C-reactive protein, lactate dehydrogenase, interleukin (IL)-6, prothrombin time, D-dimer, ferritin, liver transaminases, high-sensitivity troponin T, N-terminal pro-B-type natriuretic peptide) or decrease (albumin, fibrinogen, platelets count).118, 119, 120 , 124 , 125 , 129, 130, 131, 132 Clinicians must be aware that these laboratory markers indicate that these patients require immediate transfer to the ICU to prevent impending death. In fact, the clinical and laboratory findings outlined indicate that these patients may be developing acute respiratory distress syndrome, prothrombotic disseminated intravascular coagulation, septic shock, and/or cardiac failure. The latter may be caused by acute myocarditis with or without arrhythmias, acute myocardial infarction133 , 134 or pericardial effusion with tamponade.127 , 135 , 136 Some patients may suddenly deteriorate as a consequence of thromboembolism of large blood vessels, including pulmonary embolism.137 In this setting, some robust, though preliminary, evidence indicates that low molecular weight heparin helps to prevent and treat this hypercoagulation syndrome.138 To counter the systemic inflammation cascade before it causes multiorgan failure and death, therapy hinges on the use of immunomodulatory agents.125 In this phase, it may be helpful to use corticosteroids139 , 140 possibly in concert with cytokine inhibitors, such as tocilizumab, sarilumab, or clazakizumab (IL-6 inhibitors), anakinra or canakinumab (IL-1 inhibitors), and baricitinib or ruxolitinib (Jak inhibitors).141 A retrospective study142 found that tolicizumab (Roche) reduced the risk of death by 50% and reduced the requirement for mechanical ventilation in patients with COVID-19 pneumonia. Unfortunately, soon after, a Roche phase 3 clinical study with tocilizumab did not meet its primary end point of improved clinical status in adult hospitalized patients, although reporting a positive trend in time to hospital discharge.143 Recent data indicate that baricitinib has antiviral and anticytokine efficacy against SARS-CoV-2, reflecting a 71% mortality benefit in a propensity score–matched analyses of 166 patients with moderate to severe pneumonia.144 In vitro studies revealed that baricitinib is a Jak-1 inhibitor that interferes with SARS-CoV-2 infection with viral replication, and inhibits the cytokine storm.144
Figure 5.
SARS-CoV-2 infection stages over time: the relationship between disease severity and viral burden, host hyperinflammation response, bacterial and fungal superinfections, and postinfectious sequelae. ARDS, acute respiratory distress syndrome; BSIs, bloodstream infection; CAPA, COVID associated pulmonary aspergillosis; COVID, coronavirus disease; FiO2, fraction of inspired oxygen; ICU, intensive care unit; PaO2, partial pressure of oxygen; pt, patient; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; SIRS, systemic inflammatory response syndrome; VAP, ventilator-associated pneumonia.
Figure 6.
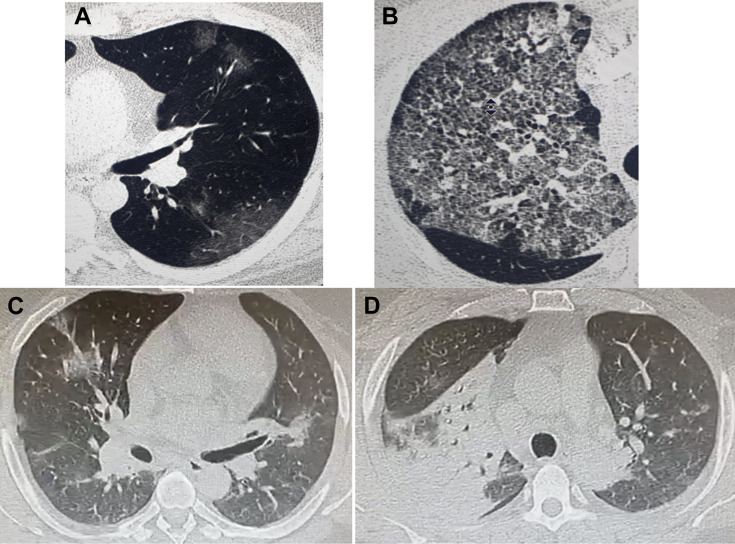
Thin-section computed tomography. (A) Initial subpleural GGO often found in most patients with COVID-19 requiring hospital admission. (B) diffuse GGOs going toward attenuation with superimposed interlobular septal thickening and intralobular lines (crazy-paving pattern). This imaging is often seen in patients with COVID-19 when they are developing, or have developed, ARDS and require treatment in the ICU. Similar GGOs can be found in various other pathologies, including lung cancer, lung fibrosis, drug injury, inflammation, and hemorrhage; however, in regions experiencing a COVID-19 epidemic, their presence alerts clinicians about possible COVID-19 pneumonia and should trigger PCR testing for SARS-CoV-2. C, D reveal different levels of thin-sections of computed tomography scans of the lungs on admission in ICU in a 44-year-old man requiring urgent intubation for SARS-Cov-2 infection. (C) multiple GGOs in both lungs. (D) large consolidation in the right upper lobe. In this patient, PCR nasopharyngeal swab testing was positive, pneumococcal and Legionella antigenuria were negative; cultures of the bronchoalveolar fluid yielded no microbial growth but a positive galactomannan index of 1,9. The final diagnosis was SARS-CoV-2 and invasive aspergillosis coinfection. ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; GGO, ground-glass opacity; ICU, intensive care unit; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
The potential advantages of immunomodulatory therapy in improving symptoms should, however, be balanced with a likely increase in the risk of severe bacterial and fungal superinfections that may cause the patient’s demise.145, 146, 147 Recent reports underscore the risk of COVID-19 associated pulmonary aspergillosis, which may be lethal in up to one-third of patients that require mechanical ventilation (Fig. 6 C and D).148 , 149
Moreover, cross-transmission and life-threatening infections with multiantibiotic resistant bacteria, such as carbapenem-resistant gram-negative bacilli and methicillin-resistant staphylococci, may be frequent in some ICU where optimal adoption of infection control procedures may be hampered by the massive nursing workload required by intubated patients with SARS-CoV-2 infection.147 A yet-to-be-defined fraction of patients who survive severe COVID-19 develops significant morbidity. These include pulmonary fibrosis, SARS-CoV-2 postinfective myocarditis, Kawasaki disease, stroke, and various neurodegenerative diseases, including subacute and chronic neuropsychiatric disorders (Fig. 5).150, 151, 152, 153, 154
Developing Therapies
A better understanding of the disease and some initial improvements in therapy may have helped reduce the death toll of COVID-19 to about 1% to 2% from initial estimates in the People’s Republic of China and Northern Italy of a 5% to 30% mortality. However, the initial estimates were an overestimation as they were based on the fraction of patients that required hospitalization.
Remdesivir, a drug developed by Gilead, is a broad-spectrum antiviral nucleotide prodrug with potent in vitro activity against a range of RNA viruses, including Ebola virus, Marburg, MERS-CoV, and SARS-CoV-1.155 On the basis of preliminary evidence from three randomized clinical trials,156, 157, 158 the Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19159 were recently updated (September 9, 2020), supporting therapy with this drug over no antiviral treatment for hospitalized patients with severe COVID 19 disease defined as those with oxygen saturation of less than or equal to 94% on room air, on supplemental oxygen, mechanical ventilation or extracorporeal membrane oxygenation. In crisis capacity settings (i.e., limited drug supply), Remdesivir was recommended only for those patients with hypoxia not requiring mechanical ventilation or extracorporeal membrane oxygenation.
In 1901, Emil von Behring received the Nobel prize for medicine for treating diphtheria with antiserum obtained from horses. Subsequently, convalescent plasma therapy using blood or plasma from recovered patients has been used for over a century to treat infectious diseases, including the infamous Spanish influenza in 1918 and, more recently, for Ebola virus infections. Shen et al.160 tried this approach during the initial epidemic in Wuhan. Duan et al.161 treated 10 patients with COVID-19 and found that plasma therapy was safe and seemed to improve the clinical outcome. Liu et al.162 studied 39 severely ill patients with COVID-19 and found that plasma therapy improved their clinical outcome. Patients treated with plasma in the early phases of COVID-19, before they required ventilation, fared better than those that were not treated or treated once on a respirator.163 This seems related to the inactivation of SARS-CoV-2 by antibodies during the early stages of the respiratory disease, before patients develop widespread COVID-19–related damage, including lung consolidation. In summary, plasma therapy is considered safe, although adverse effects may occur; therefore, the U.S. Food and Drug Administration (FDA) has recently approved its use as an emergency treatment for patients with COVID-19. However, the availability of convalescent plasma is limited, as each donation consists of about 690 to 880 mL of plasma, enough to treat only one or two patients. Moreover, the efficacy of the plasma and the antibody titer changes from donor to donor.163 Several companies are preparing hyperimmune globulins obtained by pooling the blood of hundreds of convalescent patients with COVID-19 and by a 10-fold concentration of antibodies. These preparations should be more effective while at the same time reducing the risk of adverse reactions, including transfusion-associated circulatory overload or transfusion-associated acute lung injury.
Neutralizing antibodies targeting the spike protein are a further improvement. Hansen et al.164 at Regeneron generated a large panel of such antibodies isolated from humanized mouse B-cells and from those of patients with COVID-19. This team subsequently revealed that SARS-CoV-2 grown in cell culture in the presence of any of these single antibodies developed mutations in the spike protein and became rapidly resistant to them. However, when they used a cocktail of four different antibodies directed to distinct and nonoverlapping regions of the RBD of the spike protein, SARS-CoV-2 was unable to develop resistance.165 These antibody cocktails are being produced by Regeneron and by several other companies and are in clinical trials. They are administered in the early phases of the disease, before COVID-19 patients require hospitalization, to reduce disease progression, ICU requirement and deaths.166
A novel experimental therapy uses adipose-derived mesenchymal-stroma cells (MSCs). MSC seems to modulate the immune response by reducing inflammation and is approved for the treatment of Crohn’s disease and graft-versus-host disease after hematopoietic transplantation.167 A clinical trial of MSC in 13 patients with COVID-19 under mechanical respiration reported no adverse effects, and 11 of 13 of them survived.168
Dai et al.169 made two drugs, 11a and 11b, that target the SARS-CoV-2 main protease, M0, required for viral replication and transcription. Both drugs had antiviral activity in cell culture, and 11a had low toxicity when tested in mice and dogs, making this compound a good drug candidate.
Nguyen et al.170 proposed an innovative approach using CRISPR/Cas13d technology to disrupt SARS-CoV-2 using guide RNAs that concomitantly target ORF1ab and S, which represent the replicase-transcriptase (ORF1ab) and the S component of the virus. This system will be used to specifically chew-up the SARS-CoV-2 genome, hence limiting its ability to reproduce.170
The rush to publish papers related to this pandemic has resulted in some inaccurate publications that have delayed progress and caused harm. In particular, three articles, all of them based on clinical data assembled by Surgisphere, a company based in Chicago, were retracted in short order. One was published in the New England Journal of Medicine in May 1, 2020, and claimed that therapy with ACE2 inhibitors did not increase the risk of death. A second one was published in The Lancet, May 22, 2020, which claimed that hydroxychloroquine treatment decreased survival in hospitalized patients with COVID-19. A third article published as a preprint claimed that a widely used antiparasitic drug, ivermectin, significantly reduced mortality in patients with COVID-19. This article has disappeared from the portal, and thus, it does not count as an official retraction. Apparently, the database collected by Surgisphere on which these three papers were based on contained a number of anomalies, and on questioning, Surgisphere declined to share the database; thus, the data could not be verified, and the papers were retracted.171 The consequences of these retractions were significant; in South America, some officials recommended the use of ivermectin, creating a shortage of the drug, and at the same time, patients with COVID-19 received a drug they did not need.172 Solidarity, the WHO organization that runs the COVID-19 trails, halted the recruitment of patients into the hydroxychloroquine arm. They anticipate that it will be difficult to restart the trial.
Herd Immunity and Vaccines
Infected people develop antibodies that protect them from developing illness from the same virus. A recent article studied the crew of a fishing boat of 122 fishermen, 104 of whom became infected with SARS-CoV-2 during the trip, as exhibited by RT-PCR and seroconversion.173 Before the trip, 120/122 had been tested for neutralizing antibodies against SARS-CoV-2, and 3 of 120 were positive. When the boat came ashore because one sailor had become sick with COVID-19, none of these three tested positive for SARS-CoV-2 by RT-PCR or developed any COVID-19–related symptoms, whereas 104 of 122 of the other sailors were infected (p = 0.002)—the authors did not know if and how many of them developed COVID-19. This report provides the first strong evidence in humans that neutralizing antibodies are protective and supports the hypothesis that a SARS-CoV-2 vaccine should work to prevent disease, but not infection. However, we do not know yet for how long those who have overcome SARS-CoV-2 infection are protected from developing the disease, and therefore we cannot predict how long immunity will last.
Protective antibodies can develop naturally after a viral infection or in response to vaccines. Some have suggested that we should let the virus run its course through the population to allow the natural development of herd immunity. When enough people—over 60%—are infected and develop antibodies, the so-called herd immunity protects the rest of the population, the epidemics slow down and eventually end because the virus cannot easily find new victims to infect. Brazil and Sweden have adopted this approach, assuming that a rapid, aggressive course of COVID-19 epidemic in their region will, in the end, cause less harm than a long-lasting one; time will tell if they were wise to do so.
So far mortality rate attributed to COVID-19 in Brazil and Sweden is 86.14 per 100,000 and 73.79 per 100,000 persons, respectively. This compares favorably, for example, to 104.89 per 100,000 and 86.09 per 100,000 in Italy and France, respectively, to 90.31 per 100,000 in the United States—countries that have adopted lockdowns and other measures to mitigate the spread of the pandemic (data as of December 12, 2020).33 In contrast, the nearby Sweden countries of Finland and Norway have experienced much lower mortality rates, of 8.21 per 100,000 and 7.28 per 100,000, respectively. So, the verdict is still out.
There are approximately 180 vaccines in production in the world. Among them, several are being produced in the United States, at least two in the Republic of China, one by a joint European effort known as the Oxford vaccine, among others.174 , 175 The Chinese SARS-CoV-2 vaccines are inactivated. One of them (PiCoVacc, recently renamed CoronaVac, Sinovac, China) provided partial or complete protection in macaques without causing the antibody-dependent enhancement of infection or detectable histologic damage in any organ and, therefore, is now being tested in human trials.176 These data are encouraging. The reason that there is no vaccine for SARS-CoV-1 is that animals that were given the vaccine developed a worse disease when challenged by the virus than those not vaccinated.
“Operation Warp Speed” is a $2 billion U.S. government–sponsored project that aims to test three to five potential vaccines in the fall of 2020 and start massive vaccinations in January of 2021. The first two of these vaccines are RNA vaccines coding for the spike protein of SARS-CoV-2 produced by Moderna and by Pfizer and BioNTech, the latter is known as BNT162b2, presently being tested in phase 2 to 3 trials. These vaccines use a technical approach never tried before; therefore, it will be very interesting to see how well they work as the results may impact all future vaccine preparations. Preliminary data from these companies suggest that they are 95% effective at generating neutralizing antibodies. They were largely tested in healthy adult volunteers younger than 55, and they seemed safe at least in the short term, although the BNT162b2 vaccine caused pain at the site of injection and headaches in greater than 90 of volunteers.177 These vaccines will soon become available for mass vaccination, and therefore, could play a major role in halting the epidemic. However, it remains to be seen if they are safe in older individuals with preexisting conditions and in children, how effective they are at preventing infection (especially in older individuals who mount less effective immune responses), and if they are (or for what length of time they are) protective. Distribution of these RNA vaccines worldwide will not be easy, as they require storage at –70°C (BNT162b2 vaccine) and at –20°C (Moderna vaccine).174 , 175 As this manuscript was in revision, on December 10, 2020, Polack et al. 178reported the interim results of a phase 2/3 part of a global phase 1/2/3 trial evaluating the safety, immunogenicity, and efficacy of 30 μg of BNT162b2 in preventing COVID-19 in persons 16 years of age or older. In this study, 21,720 individuals received two injections of BNT162b2, and 21,728 were injected with a placebo. There were eight cases of COVID-19 with onset at least seven days after the second dose among participants assigned to receive BNT162b2 and 162 cases among those assigned to placebo. Therefore, BNT162b2 was 95% effective in preventing COVID-19. The safety profile of BNT162b2 was characterized by short term, mild to moderate pain at the injection site, fatigue, and headache. These adverse effects were more pronounced in younger individuals and at the time of the second injection. The incidence of serious adverse events was low. There is, however, concern for possible allergic reactions; therefore, individuals with a history of anaphylaxis to a vaccine, medicine or food, are not candidates to receive the BNT162b2 vaccine. Moreover, despite alarming reports on the web, no causal relationship has been established between the vaccine and the two deaths reported among those who received this vaccine, nor the four Bell’s palsy cases during the trial. Therefore, BNT162b2 has been the first vaccine to receive approval for mass vaccination in the United Kingdom first, and soon after in Bahrain, whereas BNT162b2 should be distributed to the public in Saudi Arabia, Canada, and the United States within days. On December 11, the FDA advisory panel, with a 17 to 4 vote and one abstention, agreed that based on the totality of scientific evidence available, the benefits of BNT162b2 outweighed its risks for use in individuals 16 years of age and older. A concern was that in the 16- to 17-year-old age group, there were only 53 individuals who received the vaccine, and 26 of them reported adverse events. Therefore, some were worried that the FDA had too little data on which to evaluate the vaccine’s effect on patients between the ages of 16 to 17. In any case, the overall supportive vote set the stage for an emergency use authorization from the FDA, which was granted on December 12, 2020. Pfizer expects to produce 50 million doses of BNT162b2 in 2020 because two doses per person are required—they will suffice to vaccinate 25 million. Clearly, more vaccines need to become available to address the needs of the world.
Preliminary data from a phase 3 trial of the Oxford vaccine from AstraZeneca (ChADOx1nCoV-19)—which should also be available soon for mass vaccination—suggest that it is safe (at least in the short term), although 60% of volunteers developed headache and 70% fatigue. This vaccine was 70% effective at inducing neutralizing antibodies.175 Although apparently less effective than the Moderna and Pfizer vaccines, the ChADOx1nCoV-19 vaccine (which uses a genetically modified replication–incompetent chimpanzee adenovirus vector that expresses the full wild-type spike protein) can be stored at room temperature, making distribution much simpler. The estimated cost of this vaccine is three dollars per dose compared with 15 (BNT162b2) or 25 (Moderna) dollars per dose.
All vaccines in clinical trials are delivered intramuscularly to produce IgG; therefore, they cannot produce immunoglobulin A (IgA), which is instead produced by natural infections. For example, although ChADOx1nCoV-19 protected macaques that were challenged with SARS-CoV-2, there was no difference in SARS-CoV-2 nasal shedding from vaccinated and nonvaccinated infected macaques.179 , 180 This means that vaccinated subjects should be resistant to developing COVID-19, but they may still be infected in the nose, oral cavity, eyes, and intestine, and spread SARS-CoV-2. This will raise the issue of whether vaccinated people can safely return to a normal life without endangering others.
A problem faced by these trials is that to verify their ability to protect from infection, they need to be administered in areas where infections are spreading, and these are shifting. For example, the People’s Republic of China is testing their vaccines in Brazil, where the infections are still spreading rapidly. WHO is setting up vaccination teams capable of rapidly relocating to the areas in which the epidemic spreads.174
As for who should receive these vaccines, it can be argued that young people should receive it first because they should tolerate eventual side effects better and because they mount a stronger immune response. The opposite argument is that older people and those with preexisting health conditions should receive the vaccine first because they are at increased risk of developing a serious disease if infected. Moreover, it may be ethically questionable to vaccinate with a yet-to-be-proven safe vaccine for children (and maybe young, healthy people) who have a very small risk of becoming seriously ill and whose main reason to receive the vaccines is to protect high-risk groups.
We think that those who have already been infected with SARS-CoV-2 should not receive the vaccines because of the limited number of vaccines available versus the billions who need them. Moreover, on the basis of all we know from other viral infections and the extremely rare frequency of documented SARS-CoV-2 reinfections, those who have been naturally infected should be as or more (as they develop both IgA and IgG protecting antibodies) immune to a second infection than vaccinated people –who can only develop IgG. In addition, it is possible that SARS-CoV-2 vaccine adverse effects might be stronger in individuals already immune.
It has been estimated that if the vaccine is administered to about 20,000 in a region that has a 1% incidence of infection, it will take 6 months to validate its efficacy. Efficacy could be measured either as prevention of infection—but injectable vaccines do not induce IgA; thus, they cannot prevent infection, an asymptomatic infection, or in an attenuated disease course. Most trials have used the latter end point easier to achieve.174
An obvious problem is how to distribute vaccines to billions of people worldwide. The U.S. Operation War Speed program aims to distribute the vaccines first to U.S. citizens; instead, the access to COVID-19 tools accelerator sponsored by WHO has raised eight billion and plans to distribute vaccines equally among rich and poor countries.174
It is crucial to convince people to get vaccinated. The antivaccine movements in Europe and in the United States have raised skepticism in the population about vaccines, and up to 50% of the population in different countries does not plan to take a SARS-CoV-2 vaccine.181 Physicians, health professionals, and scientists shall play a critical role in educating the masses so that they understand the limited risks and large benefits of vaccination.
An additional issue to consider because it is attracting a lot of media coverage and it is causing widespread anxiety is whether vaccines or the natural immunity that follows an infection will provide protection against all strains or only some strains of SARS-CoV-2. Recent articles revealed that it is possible to be reinfected by a different strain of SARS-CoV-2.182 However, considering that as of December 12, 2020, there have been about 70 million estimated infections and 1.6 estimated million COVID-related deaths worldwide, but only a few cases of documented reinfection, the risk that reinfection may pose a real public health problem seems unlikely.
How Long is the Epidemic Going to Last?
In Wuhan, where the epidemic started in December 2019, the authorities stopped it in its tracks in three months. The government literally sealed the doors of the homes and prohibited people from leaving their residences (Fig. 3). They used teams to deliver food and medicine to residents and quite literally stopped all movement of citizens. Before the Chinese government authorized the reopening of Wuhan to the rest of the country, every single person in Wuhan—a city of about 11 million people—had to be tested for SARS-CoV-2 with a nasopharyngeal swab. The sample collection and testing were completed in a 14-day window! People who were positive were placed in continuous quarantine until retests were negative (Xiao SY, personal observations, unpublished). Northern Italy, where the first case of COVID-19 was diagnosed a month later than in the People’s Republic of China, experienced an exponential crisis for 4 months that continues at a lower rate of infections up to now. Northern Italy implemented less restrictive measures than the People’s Republic of China did in Wuhan to lessen the negative economic impact, keeping so-called essential services open. Therefore, the virus continued to spread, although less rapidly.
On the basis of this evidence, by shutting everything down, the epidemics can be stopped. This is because the virus cannot travel on its own—it requires human-to-human transmission. Once all movements are restricted, the epidemic ends. Although the option of shutting down a city or a region, such as what the People’s Republic of China did in Wuhan, was a viable option during the early stages of the epidemics, at present, with approximately 70 million infected throughout the world as of December 12, 2020, this is no longer an option. It is not possible to paralyze the world without causing many more collateral deaths than SARS-CoV-2 could ever cause. In other words, the pandemic can no longer be stopped with a lockdown because we should lock down the entire world for this measure to be effective. It is possible, however, to slow down the rate of infections as we wait for more effective therapies to reduce the number of deaths, and eventually for an effective vaccine in sufficient amounts that can be delivered to the entire world population.
Conclusions
Until we find effective drugs or effective vaccines for all become available, the only option to stop or mitigate the pandemic is to stop the virus in its tracks by stopping our own movements. It is that simple. However, in doing so, we have to sacrifice our normal living routine, our jobs, our relations with friends and relatives, our religious functions, our passions, everything that defines our lives—and we have done most or all of that for the past several months. We have gone so far as to let the people we love die alone in the hospital, and their corpses burned without a funeral, all this for fear of infection. Is it worth it? And did it help?
It has been estimated that 1.2 million children and 55,000 women will die in the third world because of these restrictions, as children will not receive vaccines—as schools, where vaccines are administered are closed—and mothers will not receive gynecologic and obstetrical care. The U.S. National Cancer Institute estimated that public fear in engaging with health services and restricted access to diagnostic services would be responsible, so far, for approximately 10,000 additional colon and breast cancer deaths because early cancers are not diagnosed, as screening has largely been suspended. The National Cancer Institute stated that this analysis is conservative because it does not consider other cancer types, it does not account for the additional nonlethal morbidity from upstaging, and it assumed that all would revert to normal January 2021, which is impossible.183 A South Korean study found that the proportion of patients with advanced NSCLC significantly increased during the COVID-19 pandemic.184 In the United Kingdom, public health authorities estimated an additional 1372 lung cancer deaths because of delays in diagnosis caused by the pandemic, which results in more patients presenting with advanced disease.185 Therefore, reducing efforts to prevent and treat cancer and other critical diseases as we divert all our attention and resources to try to contain SARS-CoV-2 infections may end up costing many more lives.183 The price of humanity is paying for implementing strict restrictive measures on the basis of fear of contracting SARS-CoV-2 infection may outweigh any benefit of decreasing the rate of viral spreading.
So what can we do as we wait for effective therapies and for a vaccine?
Wearing facial masks, lockdowns, closing borders, among others, may mitigate SARS-CoV-2 transmission, but is not sufficient to significantly influence the pandemic in countries that already have hundreds of thousands or millions of infections, as it is obvious by the second wave of infections in the United States and Europe where these measures have been implemented. Living and meeting mostly outdoors and keeping windows open is very effective, probably more effective than any other measure, as exhibited by Hawaii and by the experience in Calabria during the summer months compared with the winter months, but this can be done only in Hawaii and in a few other places. Therefore, except for places like Hawaii where the weather allows people to live outdoors and keep windows open all year long because the temperature is neither too hot nor too cold, it is very difficult to contain a viral pandemic during cold months (or in places where temperature and humidity are too high and thus uncomfortable) without imposing strict measures that cause collateral deaths from other diseases, cripple the economy, and limit civil liberties.
Soon we should have more effective drugs to treat those who develop COVID-19. The science supporting the efficacy of therapies with anti–SARS-CoV-2 monoclonal antibody cocktails is strong, the very fast recovery of U.S. President Trump, who received this therapy in a clinical trial setting, makes us hope that this therapy - presently authorized for emergency use by the FDA to be administered before patients become severely ill, will be a game-changer. And eventually, it is hoped that there will be sufficient doses of effective vaccines, and we shall all go back to life the way it used to be.
Very recently, a new strain of SARS-CoV-2 designated SARS-CoV-2 VUI 202012/01 (Variant Under Investigation, year 2020, month 12, variant 01), lineage B.1.1.7, has generated much attention. The genome of this strain differs from that of the SARS-CoV-2 reference strain (Wuhan-Hu-1) by 23 mutations, eight of which are in the spike protein gene. First detected September 21, 2020 in Kent County in England, it started spreading widely in November. As of December 2020, it was the most common variant in England, representing more than 50% of new cases diagnosed between October and December 13 in the United Kingdom.186 This strain is referred to as a variant of concern (VOC). This virus strain may have mutated in a person who was immunocompromised.187 , 188 Unlike other RNA viruses, coronaviruses, including SARS-CoV-2, encode a 3'-to-5' exoribonuclease activity which provides proofreading functions that remove mismatched nucleotides during replication of the viral genomes. Therefore, these viruses tend to have a fairly stable genome. However, humans with weakened immune systems can harbor SARS-CoV-2 infectious virus for months, increasing the chance the virus can acquire adaptive mutations for replication in humans or to evade the immune system.189 It is still not certain how these 23 mutations affect the natural biology of SARS-CoV-2 VUI 202012/01. Most of the mutations likely arose by chance and may not affect the virus’s life cycle in humans. But three of these mutations may be significant: (1) a two amino-acid deletion (designated 69-70 Delta), which had previously been detected in a patient being treated with immunosuppressants who developed COVID-19. The patient received remdesivir, convalescent plasma and neutralizing antibodies, but died a few months later. Though the virus in that patient did not initially have this deletion, it acquired it over months.187 The authors of that report proposed that the mutation evolved so the virus could evade the immune system. Importantly, virus genomes with the 69-70 Delta mutation can test false negative for an RT-PCR test that targets the S-gene target (depending on the design of the test). Indeed, for laboratories that use a three-target assay (e.g., N, ORF1ab, S gene targets in the Thermo Fisher TaqPath system), the frequency of S-gene target negatives among PCR positives can be used as a proxy for frequency of the VOC190; (2) mutation N501Y is one of six key contact residues within the RBD, and RBD variants with this mutation bind more tightly to the ACE2 receptor than other versions of the coronavirus in vitro.191 Dozens of SARS-CoV-2 genomes from South Africa and Australia have tested positive for this mutation, but laboratory tests suggest the South African and U.K. variants separately evolved the same mutation. This suggests the N501Y mutation provides an evolutionary advantage to the virus; and (3) the third significant mutation is P681H, which is also in the RBD. This mutation sits next to the "furin cleavage site,"190 which is where the spike protein must be cleaved in order for the virus to enter cells. This new variant may be between 50% and 74% more transmissible than other dominant strains192 and has spread outside of the U.K., including the USA.193 , 194 Viruses with mutations of the spike protein gene like the British VOC have been detected elsewhere,195 , 196 , 197 including at the time of this writing in the laboratory of one of the co-authors (J.L.) in Florida. How are the new SARS-CoV-2 VOC detected? Because viruses mutate over time, genetic variants arise and circulate as a pandemic progresses. Sequencing of SARS-CoV-2 genomes provides the exact information needed, but is up to now impractical as a rapid diagnostic tool due to high costs, technical complexities, and relatively lengthy time requirements for bioinformatics analyses per sample. However, clues can be attained using molecular tests such as RT-PCR. These molecular tests are designed to detect specific targets within a virus genome, and false negative results may occur if a mutation occurs in the part of the virus’ genome assessed by that test. For that reason, molecular tests designed to detect multiple virus genetic targets are less susceptible to the effects of genetic variation than tests designed to detect a single genetic target. But by using a test such as the TaqPath COVID-19 Combo Kit, which is designed to detect three SARS-CoV-2 genomic regions (Orf-1ab, N, and S genes), it is possible to screen for SARS-CoV-2 B.1.1.7-type variants because the test generates positive PCR amplification signals for the Orf-1ab and N-gene targets, but no signal for the S-gene. This is because one of the primers used for the S-gene test targets the sequence corresponding to the 69-70Delta mutation. These types of SARS-CoV-2 RT-PCR profiles are referred to as “S-gene dropouts.” Thus, to screen for these particular SARS-CoV-2 VOC by RT-PCR, a proper test is needed. These variants cannot be screened for using the commonly used CDC test, which is used to detect two N-gene (N1, N2) target sequences.197 In summary, SARS-CoV-2 is now spreading very rapidly as 10% to 20% of tests globally are positive. Therefore, because of a combination of natural infections and vaccinations, herd immunity should be reached soon, by June we hope, when COVID-19 will decrease and, hopefully, almost disappear soon after. When this happens, we shall not forget that bats contain many more SARS-like viruses: unless we implement strict measures to close wet markets that sell bats and we stop collecting and selling bat guano as fertilizer, inevitably a new infection will spread from bats to humans along the lines of what we have seen in the past decade with SARS, MERS, and now SARS-CoV-2. To achieve this and thus reduce the chance of transmission of a new “SARS-like” virus from bat to humans, international cooperation is a must.
Acknowledgments
Dr. Carbone research is funded by the National Institute of Environmental Health Sciences (1R01ES030948-01), the National Cancer Institute (1R01CA237235-01A1 and 1R01CA198138), the U.S. Department of Defense (W81XWH-16-1-04400) by the UH Foundation through donations from the Riviera United-4-a Cure, the Melohn Family Endowment, the Honeywell International Inc., the Germaine Hope Brennan Foundation, and the Maurice and Joanna Sullivan Family Foundation. The authors thank Drs. Anna Nowak and Franco Vicario for a critical discussion regarding coronavirus disease 2019 in Australia and New Zealand.
Footnotes
Disclosure: Dr. Carbone has a patent issued for “Methods for Diagnosing a Predisposition to Develop Cancer,” for “Using Anti-HMGB1 Monoclonal Antibody or other HMGB1 Antibodies as a Novel Mesothelioma Therapeutic Strategy,” and for “HMGB1, As a Biomarker for Asbestos Exposure and Mesothelioma Early Detection;” and is also a board-certified pathologist who provides consultation for pleural pathology including medicolegal cases. Dr. Bucci is the owner of Resis Srl, a company dedicated to the analysis of biomedical data for public and private customers. The remaining authors declare no conflict of interest.
References
- 1.World Health Organization. Novel coronavirus (2019-nCoV): situation report, 10. https://apps.who.int/iris/handle/10665/330775. Accessed January 17, 2021.
- 2.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forster P., Forster L., Renfrew C., Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci U S A. 2020;117:9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Dorp L., Acman M., Richard D. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infect Genet Evol. 2020;83:104351. doi: 10.1016/j.meegid.2020.104351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Böhmer M.M., Buchholz U., Corman V.M. Investigation of a COVID-19 outbreak in Germany resulting from a single travel-associated primary case: a case series. Lancet Infect Dis. 2020;20:920–928. doi: 10.1016/S1473-3099(20)30314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apolone G, Montomoli E, Manenti A, et al. Unexpected detection of SARS-CoV-2 antibodies in the prepandemic period in Italy [e-pub ahead of print]. Tumori. https://doi.org/10.1177/0300891620974755. Accessed January 17, 2021. [DOI] [PMC free article] [PubMed]
- 7.Capalbo C., Bertamino E., Zerbetto A. No evidence of SARS-CoV-2 circulation in Rome (Italy) during the pre-pandemic period: results of a retrospective surveillance. Int J Environ Res Public Health. 2020;17:8461. doi: 10.3390/ijerph17228461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vestergaard L.S., Nielsen J., Richter L. Excess all-cause mortality during the COVID-19 pandemic in Europe - preliminary pooled estimates from the EuroMOMO network, March to April 2020. Euro Surveill. 2020;25:2001214. doi: 10.2807/1560-7917.ES.2020.25.26.2001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corman V.M., Muth D., Niemeyer D., Drosten C. Hosts and sources of endemic human coronaviruses. Adv Virus Res. 2018;100:163–188. doi: 10.1016/bs.aivir.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NHS. SARS (severe acute respiratory syndrome). https://www.nhs.uk/conditions/sars/. Accessed September 14, 2020.
- 11.Corman V.M., Ithete N.L., Richards L.R. Rooting the phylogenetic tree of middle East respiratory syndrome coronavirus by characterization of a conspecific virus from an African bat. J Virol. 2014;88:11297–11303. doi: 10.1128/JVI.01498-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Memish Z.A., Mishra N., Olival K.J. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013;19:1819–1823. doi: 10.3201/eid1911.131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV). https://www.who.int/emergencies/mers-cov/en/. Accessed September 14, 2020.
- 14.Berto A., Anh P.H., Carrique-Mas J.J. Detection of potentially novel paramyxovirus and coronavirus viral RNA in bats and rats in the Mekong Delta region of southern Viet Nam. Zoonoses Public Health. 2018;65:30–42. doi: 10.1111/zph.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anthony S.J., Johnson C.K., Greig D.J. Global patterns in coronavirus diversity. Virus Evol. 2017;3:vex012. doi: 10.1093/ve/vex012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afelt A., Frutos R., Bats D.C. Coronaviruses, and deforestation: toward the emergence of novel infectious diseases? Front Microbiol. 2018;9:702. doi: 10.3389/fmicb.2018.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323:2249–2251. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 18.Szymczak W.A., Goldstein D.Y., Orner E.P. Utility of stool PCR for the diagnosis of COVID-19: comparison of two commercial platforms. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.01369-20. e01369-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wölfel R., Corman V.M., Guggemos W. Virological assessment of hospitalized patients with COVID-2019 [published correction appears in Nature. 2020;588:E35. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 21.Wang W., Xu Y., Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin C., Xiang J., Yan M., Li H., Huang S., Shen C. Comparison of throat swabs and sputum specimens for viral nucleic acid detection in 52 cases of novel coronavirus (SARS-Cov-2)-infected pneumonia (COVID-19) Clin Chem Lab Med. 2020;58:1089–1094. doi: 10.1515/cclm-2020-0187. [DOI] [PubMed] [Google Scholar]
- 23.Center for Disease Control and Prevention. Diagnostic testing. https://www.cdc.gov/coronavirus/2019-ncov/lab/testing.html. Accessed September 14, 2020.
- 24.van Kasteren P.B., van der Veer B., van den Brink S. Comparison of seven commercial RT-PCR diagnostic kits for COVID-19. J Clin Virol. 2020;128:104412. doi: 10.1016/j.jcv.2020.104412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lednicky J.A., Shankar S.N., Elbadry M.A. Collection of SARS-CoV-2 virus from the air of a clinic within a University Student Health Care Center and analyses of the viral genomic sequence. Aerosol Air Qual Res. 2020;20:1167–1171. doi: 10.4209/aaqr.2020.02.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lednicky J.A., Lauzardo M., Fan Z.H. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int J Infect Dis. 2020;100:476–482. doi: 10.1016/j.ijid.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bustin S.A., Benes V., Garson J.A. Primer sequence disclosure: a clarification of the MIQE guidelines. Clin Chem. 2011;57:919–921. doi: 10.1373/clinchem.2011.162958. [DOI] [PubMed] [Google Scholar]
- 28.Huang A.T., Garcia-Carreras B., Hitchings M.D.T. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11:4704. doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grandjean L, Saso A, Ortiz A, et al. Humoral response dynamics following infection with SARS-CoV-2. https://www.medrxiv.org/content/10.1101/2020.07.16.20155663v2.full.pdf. Accessed January 17, 2021.
- 30.Fenwick C, Croxatto A, Coste AT, et al. Changes in SARS-CoV-2 antibody responses impact the estimates of infections in population-based seroprevalence studies [e-pub ahead of print]. J Virol. https://doi.org/10.1128/JVI.01828-20. Accessed January 17, 2021. [DOI] [PMC free article] [PubMed]
- 31.Carbone M., Green J.B., Bucci E.M., Lednicky J.A. Coronaviruses: facts, myths, and hypotheses. J Thorac Oncol. 2020;15:675–678. doi: 10.1016/j.jtho.2020.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saslow D., Andrews K.S., Manassaram-Baptiste D., Smith R.A., Fontham E.T.H., American Cancer Society Guideline Development Group Human papillomavirus vaccination 2020 guideline update: American Cancer Society guideline adaptation. CA Cancer J Clin. 2020;70:274–280. doi: 10.3322/caac.21616. [DOI] [PubMed] [Google Scholar]
- 33.John Hopkins University. Mortality analyses. https://coronavirus.jhu.edu/data/mortality. Accessed December 14, 2020.
- 34.Center for Disease Control and Prevention. Weekly updates by select demographic and geographic characteristics: comorbidities. https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm?fbclid=IwAR2-muRM3tB3uBdbTrmKwH1NdaBx6PpZo2kxotNwkUXlnbZXCwSRP2OmqsI#Comorbidities. Accessed September 14, 2020.
- 35.Instituto Nazionale di Staistica. Impact of the epidemic COVID-19 on mortality: causes of death in COVID-19 laboratory confirmed cases. https://www.istat.it/it/files//2020/07/Report_ISS_Istat_Inglese.pdf. Accessed September 28, 2020.
- 36.Falasca L., Nardacci R., Colombo D. Postmortem findings in Italian patients with COVID-19: a descriptive full autopsy study of cases with and without comorbidities. J Infect Dis. 2020;222:1807–1815. doi: 10.1093/infdis/jiaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shang J., Wan Y., Luo C. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang C., Li Y., Xiao S.Y. Differential expression of ACE2 in the respiratory tracts and its relationship to COVID-19 pathogenesis. EBioMedicine. 2020;60:103004. doi: 10.1016/j.ebiom.2020.103004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hindson J. COVID-19: faecal-oral transmission? Nat Rev Gastroenterol Hepatol. 2020;17:259. doi: 10.1038/s41575-020-0295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morawska L., Milton D.K. It is time to address airborne transmission of COVID-19. Clin Infect Dis. 2020;71:2311–2313. doi: 10.1093/cid/ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan M., Lednicky J.A., Wu C.Y. Collection, particle sizing and detection of airborne viruses. J Appl Microbiol. 2019;127:1596–1611. doi: 10.1111/jam.14278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie X., Li Y., Chwang A.T., Ho P.L., Seto W.H. How far droplets can move in indoor environments--revisiting the Wells evaporation-falling curve. Indoor Air. 2007;17:211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 44.Fears A.C., Klimstra W.B., Duprex P. Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerg Infect Dis. 2020;26:2168–2171. doi: 10.3201/eid2609.201806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poletti P, Tirani M, Cereda D, et al. Probability of symptoms and critical disease after SARS-CoV-2 infection. https://ui.adsabs.harvard.edu/abs/2020arXiv200608471P. Accessed June 1, 2020.
- 46.Savvides C, Siegel R. Asymptomatic and presymptomatic transmission of SARS-CoV-2: a systematic review. https://www.medrxiv.org/content/10.1101/2020.06.11.20129072v2.full.pdf. Accessed January 17, 2021.
- 47.He X., Lau E.H.Y., Wu P. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 48.Slifka M.K., Gao L. Is presymptomatic spread a major contributor to COVID-19 transmission? Nat Med. 2020;26:1531–1533. doi: 10.1038/s41591-020-1046-6. [DOI] [PubMed] [Google Scholar]
- 49.He X., Lau E.H.Y., Wu P. Author Correction: temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:1491–1493. doi: 10.1038/s41591-020-1016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singanayagam A., Patel M., Charlett A. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 2020;25:2001483. doi: 10.2807/1560-7917.ES.2020.25.32.2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang N, Perez P, Kato T, et al. Integrated single-cell atlases reveal an oral SARS-CoV-2 infection and transmission axis. https://www.medrxiv.org/content/10.1101/2020.10.26.20219089v1.full.pdf. Accessed January 17, 2021.
- 52.Center for Disease Control and Prevention. Frequently asked questions. https://www.cdc.gov/coronavirus/2019-ncov/faq.html. Accessed September 25, 2020.
- 53.Hamner L., Dubbel P., Capron I. High SARS-CoV-2 attack rate following exposure at a choir practice - Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:606–610. doi: 10.15585/mmwr.mm6919e6. [DOI] [PubMed] [Google Scholar]
- 54.Jayaweera M., Perera H., Gunawardana B., Manatunge J. Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ Res. 2020;188:109819. doi: 10.1016/j.envres.2020.109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J., Du G. COVID-19 may transmit through aerosol. Ir J Med Sci. 2020;189:1143–1144. doi: 10.1007/s11845-020-02218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldman E. Exaggerated risk of transmission of COVID-19 by fomites [published correction appears in Lancet Infect Dis. 2020;20:e215. Lancet Infect Dis. 2020;20:892–893. doi: 10.1016/S1473-3099(20)30561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qureshi Z, Jones N, Temple R, et al. What is the evidence to support the 2-metre social distancing rule to reduce COVID-19 transmission? The Centre for Evidence-Based Medicine. https://www.cebm.net/covid-19/what-is-the-evidence-to-support-the-2-metre-social-distancing-rule-to-reduce-covid-19-transmission/. Accessed September 25, 2020.
- 58.Harvey AP, Fuhrmeister ER, Cantrell M, et al. Longitudinal monitoring of SARS-CoV-2 RNA on high-touch surfaces in a community setting. https://www.medrxiv.org/content/10.1101/2020.10.27.20220905v1.full.pdf. Accessed January 17, 2021. [DOI] [PMC free article] [PubMed]
- 59.Weed M, Foad A. Rapid scoping review of evidence of outdoor transmission of COVID-19. https://www.medrxiv.org/content/10.1101/2020.09.04.20188417v2.full.pdf. Accessed January 17, 2021.
- 60.Xiao S.Y., Wu Y., Liu H. Evolving status of the 2019 novel coronavirus infection: proposal of conventional serologic assays for disease diagnosis and infection monitoring. J Med Virol. 2020;92:464–467. doi: 10.1002/jmv.25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen Y., Li C., Dong H. Community outbreak investigation of SARS-CoV-2 transmission among bus riders in Eastern China. JAMA Intern Med. 2020;180:1665–1671. doi: 10.1001/jamainternmed.2020.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olsen S.J., Chang H.L., Cheung T.Y. Transmission of the severe acute respiratory syndrome on aircraft. N Engl J Med. 2003;349:2416–2422. doi: 10.1056/NEJMoa031349. [DOI] [PubMed] [Google Scholar]
- 63.Hoehl S., Karaca O., Kohmer N. Assessment of SARS-CoV-2 transmission on an international flight and among a Tourist Group. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.18044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bae S.H., Shin H., Koo H.Y., Lee S.W., Yang J.M., Yon D.K. Asymptomatic transmission of SARS-CoV-2 on evacuation flight. Emerg Infect Dis. 2020;26:2705–2708. doi: 10.3201/eid2611.203353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi J., Wen Z., Zhong G. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020;368:1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muñoz-Fontela C., Dowling W.E., Funnell S.G.P. Animal models for COVID-19. Nature. 2020;586:509–515. doi: 10.1038/s41586-020-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Q, Zhang H, Huang K, et al. SARS-CoV-2 neutralizing serum antibodies in cats: a serological investigation. https://www.biorxiv.org/content/10.1101/2020.04.01.021196v1.full.pdf. Accessed January 17, 2021.
- 68.Lam T.T., Jia N., Zhang Y.W. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020;583:282–285. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- 69.Xiao K., Zhai J., Feng Y. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nature. 2020;583:286–289. doi: 10.1038/s41586-020-2313-x. [DOI] [PubMed] [Google Scholar]
- 70.Rockx B., Kuiken T., Herfst S. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 2020;368:1012–1015. doi: 10.1126/science.abb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zou H., Shu Y., Feng T. How Shenzhen,. China avoided widespread community transmission: a potential model for successful prevention and control of COVID-19. Infect Dis Poverty. 2020;9:89. doi: 10.1186/s40249-020-00714-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fan S., Wu M., Ma S., Zhao S. A preventive and control strategy for COVID-19 infection: an experience from a third-tier Chinese city. Front Public Health. 2020;8:562024. doi: 10.3389/fpubh.2020.562024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cook M.J., Dri G.G., Logan P., Tan J.B., Flahault A. COVID-19 Down Under: Australia’s initial pandemic experience. Int J Environ Res Public Health. 2020;17:8939. doi: 10.3390/ijerph17238939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang S.L., Harding N., Zachreson C., Cliff O.M., Prokopenko M. Modelling transmission and control of the COVID-19 pandemic in Australia. Nat Commun. 2020;11:5710. doi: 10.1038/s41467-020-19393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Summers J., Cheng H.Y., Lin H.H. Potential lessons from the Taiwan and New Zealand health responses to the COVID-19 pandemic. The Lancet Reg Health West Pacific. 2020;21:100044. doi: 10.1016/j.lanwpc.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abate S.M., Ahmed Ali S., Mantfardo B., Basu B. Rate of Intensive Care Unit admission and outcomes among patients with coronavirus: a systematic review and Meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0235653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Volpato S., Landi F., Incalzi R.A. A frail health care system for an old population: lesson form the COVID-19 outbreak in Italy. J Gerontol A Biol Sci Med Sci. 2020;75:e126–e127. doi: 10.1093/gerona/glaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yehia B.R., Winegar A., Fogel R. Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US hospitals. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.18039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region. Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study [published correction appears in Lancet Respir Med. 2020;8:e26. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhatraju P.K., Ghassemieh B.J., Nichols M. COVID-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mutti L., Pentimalli F., Baglio G. Coronavirus disease (COVID-19): what are we learning in a country with high mortality rate? Front Immunol. 2020;11:1208. doi: 10.3389/fimmu.2020.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ji D., Zhang D., Xu J. Prediction for progression risk in patients with COVID-19 pneumonia: the CALL score. Clin Infect Dis. 2020;71:1393–1399. doi: 10.1093/cid/ciaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ledford H. How obesity could create problems for a COVID vaccine. Nature. 2020;586:488–489. doi: 10.1038/d41586-020-02946-6. [DOI] [PubMed] [Google Scholar]
- 86.Falcone C., Caracciolo M., Correale P. Can adenosine fight COVID-19 acute respiratory distress syndrome? J Clin Med. 2020;9:3045. doi: 10.3390/jcm9093045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao L. Obesity accompanying COVID-19: the role of epicardial fat. Obesity (Silver Spring) 2020;28:1367. doi: 10.1002/oby.22867. [DOI] [PubMed] [Google Scholar]
- 88.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang L., Zhu F., Xie L. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luo J., Rizvi H., Preeshagul I.R. COVID-19 in patients with lung cancer. Ann Oncol. 2020;31:1386–1396. doi: 10.1016/j.annonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garassino M.C., Whisenant J.G., Huang L.C. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Calles A., Aparicio M.I., Alva M. Outcomes of COVID-19 in patients with lung cancer treated in a tertiary hospital in Madrid. Front Oncol. 2020;10:1777. doi: 10.3389/fonc.2020.01777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rosenquist N.A., Metcalf W.J., Ryu S.Y. Acute associations between PM2.5 and ozone concentrations and asthma exacerbations among patients with and without allergic comorbidities. J Expo Sci Environ Epidemiol. 2020;30:795–804. doi: 10.1038/s41370-020-0213-7. [DOI] [PubMed] [Google Scholar]
- 94.Wu X., Nethery R.C., Sabath B.M., Braun D., Dominici F. Exposure to air pollution and COVID-19 mortality in the United States: a nationwide cross-sectional study. Sci Adv. 2020;6 doi: 10.1126/sciadv.abd4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Williams FMK, Freydin M, Mangino M, et al. Self-reported symptoms of COVID-19 including symptoms most predictive of SARS-CoV-2 infection, are heritable. https://www.medrxiv.org/content/10.1101/2020.04.22.20072124v2.full.pdf. Accessed January 17, 2021. [DOI] [PubMed]
- 96.Lucas C., Wong P., Klein J. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Q., Bastard P., Liu Z. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bastard P., Rosen L.B., Zhang Q. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carbone M., Arron S.T., Beutler B. Tumour predisposition and cancer syndromes as models to study gene-environment interactions. Nat Rev Cancer. 2020;20:533–549. doi: 10.1038/s41568-020-0265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Infectious Diseases Department COVID. 19 Task Force IT Service Higher Institute of Health. COVID-19 epidemic. https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_15-settembre-2020.pdf. Accessed September 28, 2020.
- 101.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Montopoli M., Zumerle S., Vettor R. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) Ann Oncol. 2020;31:1040–1045. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goren A., Vano-Galván S., Wambier C.G. A preliminary observation: male pattern hair loss among hospitalized COVID-19 patients in Spain - A potential clue to the role of androgens in COVID-19 severity. J Cosmet Dermatol. 2020;19:1545–1547. doi: 10.1111/jocd.13443. [DOI] [PubMed] [Google Scholar]
- 104.Ghazizadeh Z., Majd H., Richter M. Androgen regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men. Cell Stem Cell. 2020;27:876–889. doi: 10.1016/j.stem.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Verdoni L., Mazza A., Gervasoni A. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lamers M.M., Beumer J., van der Vaart J. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50–54. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94 doi: 10.1128/JVI.00127-20. e00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein [published correction appears in Cell. 2020;183:1735. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tian S., Xiao S.Y. Pathology of 2019 novel coronavirus pneumonia: a dynamic disease process. J Thorac Oncol. 2020;15:e67–e68. doi: 10.1016/j.jtho.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tian S., Xiong Y., Liu H. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020;395:496. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cao J., Tu W.J., Cheng W. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:748–755. doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang Y., Geng X., Tan Y. New understanding of the damage of SARS-CoV-2 infection outside the respiratory system. Biomed Pharmacother. 2020;127:110195. doi: 10.1016/j.biopha.2020.110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutierrez-Ocampo E. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xie Y., Wang Z., Liao H., Marley G., Wu D., Tang W. Epidemiologic, clinical, and laboratory findings of the COVID-19 in the current pandemic: systematic review and meta-analysis. BMC Infect Dis. 2020;20:640. doi: 10.1186/s12879-020-05371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Del Sole F., Farcomeni A., Loffredo L. Features of severe COVID-19: a systematic review and meta-analysis. Eur J Clin Invest. 2020;50:e13378. doi: 10.1111/eci.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vargas-Gandica J., Winter D., Schnippe R. Ageusia and anosmia, a common sign of COVID-19? A case series from four countries. J Neurovirol. 2020;26:785–789. doi: 10.1007/s13365-020-00875-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Giacomelli A., Pezzati L., Conti F. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020;71:889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tian S., Liu H., Liao M. Analysis of mortality in patients with COVID-19: clinical and laboratory parameters. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa152. ofaa152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang K., Sheng Y., Huang C. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Loffredo L., Pacella F., Pacella E., Tiscione G., Oliva A., Violi F. Conjunctivitis and COVID-19: a meta-analysis. J Med Virol. 2020;92:1413–1414. doi: 10.1002/jmv.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang W., Sirajuddin A., Zhang X. The role of imaging in 2019 novel coronavirus pneumonia (COVID-19) Eur Radiol. 2020;30:4874–4882. doi: 10.1007/s00330-020-06827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Choi S., Jang W.J., Song Y.B. D-dimer levels predict myocardial injury in ST-segment elevation myocardial infarction: a cardiac magnetic resonance imaging study. PLoS One. 2016;11 doi: 10.1371/journal.pone.0160955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Violi F., Ceccarelli G., Cangemi R. Hypoalbuminemia, coagulopathy, and vascular disease in COVID-19. Circ Res. 2020;127:400–401. doi: 10.1161/CIRCRESAHA.120.317173. [DOI] [PubMed] [Google Scholar]
- 131.Violi F, Cangemi R, Romiti GF, et al. Is albumin predictor of mortality in COVID-19 [e-pub ahead of print]? Antioxid Redox Signal. https://doi.org/10.1089/ars.2020.8142. Accessed January 17, 2021. [DOI] [PubMed]
- 132.Zhang X., Tan Y., Ling Y. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583:437–440. doi: 10.1038/s41586-020-2355-0. [DOI] [PubMed] [Google Scholar]
- 133.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Siripanthong B., Nazarian S., Muser D. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17:1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Long B., Brady W.J., Koyfman A., Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38:1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hua A., O’Gallagher K., Sado D., Byrne J. Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur Heart J. 2020;41:2130. doi: 10.1093/eurheartj/ehaa253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Warkentin T.E., Kaatz S. COVID-19 versus HIT hypercoagulability. Thromb Res. 2020;196:38–51. doi: 10.1016/j.thromres.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Falcone M., Tiseo G., Barbieri G. Role of low-molecular weight heparin in hospitalized patients with SARS-CoV-2 pneumonia: a prospective observational study. Open Forum Infect Dis. 2020;7:ofaa563. doi: 10.1093/ofid/ofaa563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.WHO Rapid Evidence Appraisal for COVID-19 Thereapies (REACT) Working Group. Sterne J.A.C., Murthy S. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bartoletti M., Marconi L., Scudeller L. Efficacy of corticosteroid treatment for hospitalized patients with severe COVID-19: a multicenter study. Clin Microbiol Infect. 2020;27:105–111. doi: 10.1016/j.cmi.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rizk J.G., Kalantar-Zadeh K., Mehra M.R., Lavie C.J., Rizk Y., Forthal D.N. Pharmaco-immunomodulatory therapy in COVID-19. Drugs. 2020;80:1267–1292. doi: 10.1007/s40265-020-01367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guaraldi G., Meschiari M., Cozzi-Lepri A. Tocilizumab in patients with severe COVID-19: a retrospective cohort study [published correction appears in Lancet Rheumatol. 2020;2:e591. Lancet Rheumatol. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Roche. Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 associated pneumonia. https://www.roche.com/investors/updates/inv-update-2020-07-29.htm. Accessed September 14, 2020.
- 144.Stebbing J., Sanchez Nievas G., Falcone M. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2020;7 doi: 10.1126/sciadv.abe4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19 [e-pub ahead of print]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa954. Accessed January 17, 2021. [DOI] [PMC free article] [PubMed]
- 146.Garcia-Vidal C., Sanjuan G., Moreno-Garcia E. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2020;27:83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hoenigl M. Invasive Fungal Disease complicating COVID-19: when it rains it pours [e-pub ahead of print]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa1342. Accessed January 17, 2021. [DOI] [PMC free article] [PubMed]
- 148.White PL, Dhillon R, Cordey A, et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU [e-pub ahead of print]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa1298. Accessed January 17, 2021. [DOI] [PMC free article] [PubMed]
- 149.Bartoletti M, Pascale R, Cricca M, et al. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study [e-pub ahead of print]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa1065. Accessed January 17, 2021. [DOI] [PMC free article] [PubMed]
- 150.Spagnolo P., Balestro E., Aliberti S. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med. 2020;8:750–752. doi: 10.1016/S2213-2600(20)30222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tissiéres P., Teboul J.L. SARS-CoV-2 post-infective myocarditis: the tip of COVID-19 immune complications? Ann Intensive Care. 2020;10:98. doi: 10.1186/s13613-020-00717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mohammadi S., Moosaie F., Aarabi M.H. Understanding the immunologic characteristics of neurologic manifestations of SARS-CoV-2 and potential immunological mechanisms. Mol Neurobiol. 2020;57:5263–5275. doi: 10.1007/s12035-020-02094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34–39. doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Carfi A., Bernabei R., Landi F. Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Artese A., Svicher V., Costa G. Current status of antivirals and druggable targets of SARS CoV-2 and other human pathogenic coronaviruses. Drug Resist Updat. 2020;53:100721. doi: 10.1016/j.drup.2020.100721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the treatment of COVID-19 - final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang Y., Zhang D., Du G. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial [published correction appears in Lancet. 2020;395:1694. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Goldman J.D., Lye D.C.B., Hui D.S. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med. 2020;383:1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19 [e-pub ahead of print]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa478. Accessed January 17, 2021. [DOI] [PMC free article] [PubMed]
- 160.Shen C., Wang Z., Zhao F. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Duan K., Liu B., Li C. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu S.T.H., Lin H.M., Baine I. Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study. Nat Med. 2020;26:1708–1713. doi: 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- 163.Ibrahim D., Dulipsingh L., Zapatka L. Factors associated with good patient outcomes following convalescent plasma in COVID-19: a prospective phase II clinical trial. Infect Dis Ther. 2020;9:913–926. doi: 10.1007/s40121-020-00341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hansen J., Baum A., Pascal K.E. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020;369:1010–1014. doi: 10.1126/science.abd0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Baum A., Fulton B.O., Wloga E. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369:1014–1018. doi: 10.1126/science.abd0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Abraham J. Passive antibody therapy in COVID-19. Nat Rev Immunol. 2020;20:401–403. doi: 10.1038/s41577-020-0365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Galipeau J., Sensebe L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sánchez-Guijo F., García-Arranz M., López-Parra M. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine. 2020;25:100454. doi: 10.1016/j.eclinm.2020.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dai W., Zhang B., Jiang X.M. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368:1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nguyen T.M., Zhang Y., Pandolfi P.P. Virus against virus: a potential treatment for 2019-nCov (SARS-CoV-2) and other RNA viruses. Cell Res. 2020;30:189–190. doi: 10.1038/s41422-020-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Servick K., Enserink M. The pandemic’s first major research scandal erupts. Science. 2020;368:1041–1042. doi: 10.1126/science.368.6495.1041. [DOI] [PubMed] [Google Scholar]
- 172.Mega E.R. Latin America’s embrace of an unproven COVID treatment is hindering drug trials. Nature. 2020;586:481–482. doi: 10.1038/d41586-020-02958-2. [DOI] [PubMed] [Google Scholar]
- 173.Addetia A., Crawford K.H., Dingens A. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with high attack rate. J Clin Microbiol. 2020;58:e02107–e02120. doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cohen J. Pandemic vaccines are about to face the real test. Science. 2020;368:1295–1296. doi: 10.1126/science.368.6497.1295. [DOI] [PubMed] [Google Scholar]
- 175.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 176.Gao Q., Bao L., Mao H. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mulligan M.J., Lyke K.E., Kitchin N. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 178.Polack F.P., Thomas S.J., Kitchin N. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.van Doremalen N., Lambe T., Spencer A. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yu J., Tostanoski L.H., Peter L. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cornwall W. Officials gird for a war on vaccine misinformation. Science. 2020;369:14–15. doi: 10.1126/science.369.6499.14. [DOI] [PubMed] [Google Scholar]
- 182.Lee JS, Kim SY, Kim TS, et al. Evidence of severe acute respiratory syndrome coronavirus 2 reinfection after recovery from mild coronavirus disease 2019 [e-pub ahead of print]. Clin Infect Dis.https://doi.org/10.1093/cid/ciaa1421. Accessed January 17, 2021. [DOI] [PMC free article] [PubMed]
- 183.Sharpless NE COVID-19 and cancer. Science. 2020;368:1290. doi: 10.1126/science.abd3377. [DOI] [PubMed] [Google Scholar]
- 184.Park J.Y., Lee Y.J., Kim T. Collateral effects of the coronavirus disease 2019 pandemic on lung cancer diagnosis in Korea. BMC Cancer. 2020;20:1040. doi: 10.1186/s12885-020-07544-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gourd E. Lung cancer control in the UK hit badly by COVID-19 pandemic. Lancet Oncol. 2020;21:1559. doi: 10.1016/S1470-2045(20)30691-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.World Health organization SARS-CoV-2 Variant – United Kingdom of Great Britain and Northern Ireland. https://www.who.int/csr/don/21-december-2020-sars-cov2-variant-united-kingdom/en/
- 187.Kemp S.A., Collier D.A., Datir R. Neutralising antibodies in Spike mediated SARS-CoV-2 adaptation. https://www.medrxiv.org/content/10.1101/2020.12.05.20241927v3.full.pdf
- 188.Kupferschmidt K. U.K. variant puts spotlight on immunocompromised patients’ role in the COVID-19 pandemic. Science Magazine. https://www.sciencemag.org/news/2020/12/uk-variant-puts-spotlight-immunocompromised-patients-role-covid-19-pandemic
- 189.Rambaut A., Loman N., Pybus O. Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations. https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563
- 190.Public Health England Investigation of novel SARS-COV-2 variant - Variant of Concern 202012/01. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/947048/Technical_Briefing_VOC_SH_NJL2_SH2.pdf
- 191.Starr T.N., Greaney A.J., Hilton S.K. Deep mutational scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 binding. Cell. 2020;182:1295–1310.e20. doi: 10.1016/j.cell.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Davies N., Barnard R.C., Jarvis C.I. 2020. Estimated transmissibility and severity of novel SARS-CoV-2 Variant of Concern 202012/01 in England. Status Report - Status: report. Accessed December 30, 2020. [Google Scholar]
- 193.First U.S. Case of Highly Contagious Coronavirus Variant Is Found in Colorado New York Times. https://www.nytimes.com/2020/12/29/health/uk-coronavirus-variant-colorado.html
- 194.A more contagious coronavirus strain has been found in 8 states and 33 countries. Here's what we know. https://www.usatoday.com/story/news/health/2021/01/02/new-covid-strain-b-117-explained/4112125001/
- 195.Tegally H., Wilkinson E., Marta Giovanetti M. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. https://www.medrxiv.org/content/10.1101/2020.12.21.20248640v1
- 196.Mutated coronavirus variant from South Africa found in UK. EURACTIV. https://www.euractiv.com/section/coronavirus/news/mutated-coronavirus-variant-from-south-africa-found-in-uk/
- 197.Washington N.L., White S., Schiabor Barett K.M. S gene dropout patterns in SARS-CoV-2 tests suggest spread of the H69del/V70del mutation in the US. https://www.medrxiv.org/content/10.1101/2020.12.24.20248814v1.full.pdf