Abstract
COVID-19 is a relatively new and rapidly emerging disease. Given current knowledge of the disease process, it is of the utmost importance to gain further insight into its different clinical manifestations. In this report we describe three cases involving Hispanic males with COVID-19 all of whom developed pneumomediastinum during their hospital course. We want to emphasize the importance of this adverse event despite their non-smoking history and the exclusion of positive pressure ventilation. Frequent chest radiographs help with early recognition of this disease process. Early detection of pneumomediastinum is important as this could lead to worse morbidity if left unrecognized despite its usually benign nature.
Keywords: Pneumomediastinum, SARS-CoV-2, ARDS, Critical care
Introduction
COVID-19 is an ever-evolving disease that the medical community continues to learn more about daily. It is important to highlight important clinical courses that are not common so that adverse events can be better predicted and treated appropriately. In a retrospective study done in a hospital out of Wuhan, China in February 2020 showed that out of its 99 patients, only one patient had pneumothorax.1 Later, in March 2020, a literature review published in the American Journal of Roentgenology of more than 900 patients described the incidence of pneumothorax as uncommon or rare.2 Pneumomediastinum is far less appreciated. A literature search of PubMed reveals only five case reports of pneumomediastinum, mostly originating from China, in the absence of positive pressure ventilation.3, 4, 5, 6, 7 Presented below is a case series from a county hospital in Long Island, New York on three patients diagnosed with pneumomediastinum in the absence of positive pressure ventilation. These cases are significant because they all involve non-smokers with no underlying pulmonary disease, the absence of positive pressure ventilation and delayed presentation (two weeks or more) after hospital admission.
Cases
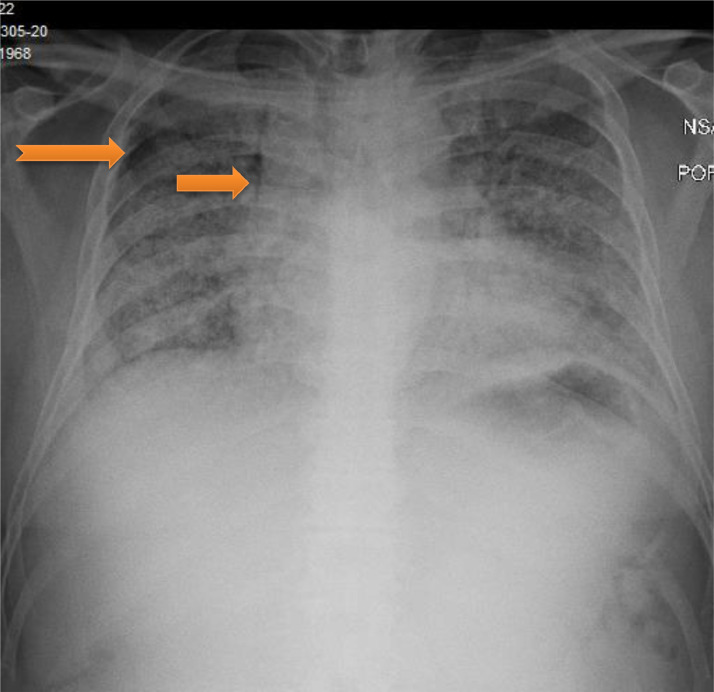
A 51-year-old obese Hispanic male, non-smoker with no significant medical history was admitted with three days of cough, chills and dyspnea. On admission, his temperature was 103 degrees Fahrenheit and oxygen saturation was 88% on non-rebreather mask (NRB) 15 L/min. He was found to have severe Acute Respiratory Distress Syndrome secondary to SARS-CoV-2 viral pneumonia, with a PaO2:FiO2 ratio of 68. Admission laboratory results revealed normal white blood cell count, C-reactive protein elevated at 10.1 mg/dL (Normal 0.0–0.9 mg/dL), lactate dehydrogenase elevated at 439 U/L (Normal 120–246 U/L), D-dimer elevated at 0.40 ug/mL (Normal < 0.4 ug/mL) and ferritin elevated at 489 ng/mL (Normal 7.3–270.7 ng/mL). His chest x-ray showed bilateral diffuse infiltrates. He was treated with a course of hydroxychloroquine, azithromycin, and methylprednisolone. He received tocilizumab and convalescent plasma. He continued to require 15 L/min NRB mask to maintain his oxygenation. On day 15 of his hospitalization, he developed sudden chest pain and worsening hypoxemia. Chest x-ray showed right sided apical pneumothorax and pneumomediastinum (Fig. 1 ). He had a chest tube placed with improvement in his oxygenation. His pneumothorax and pneumomediastinum resolved after which chest tube was removed. He was discharged to a rehabilitation facility on oxygen therapy 3 L/min nasal cannula. He had a CT thorax done 8 weeks later which showed peripheral ground glass opacities with bronchiectasis. No significant underlying bullous lung disease was noted.
Fig. 1.
Pneumomediastinum (arrow) with small right apical pneumothorax (notched arrow)
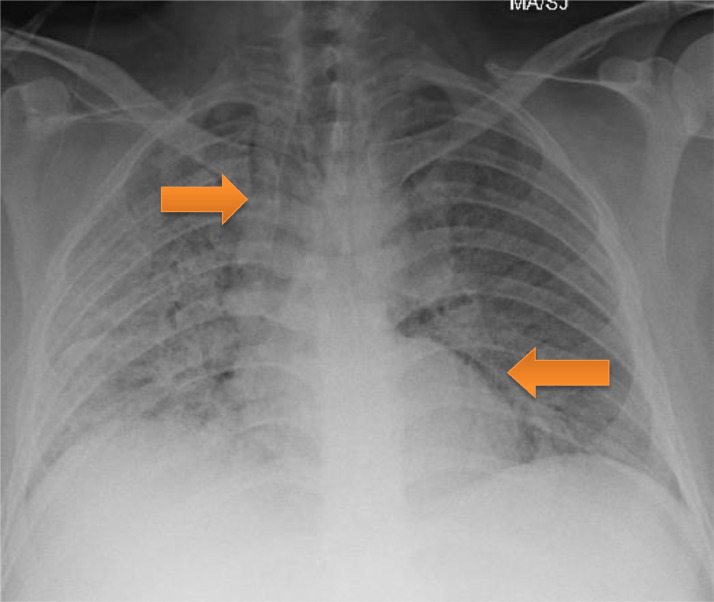
A 37-year-old obese, Hispanic male, non-smoker with no significant medical history was admitted with five days of cough, fever, and dyspnea. On admission, he was afebrile, tachycardic with a heart rate of 108 beats per minute, tachypneic with a respiratory rate of 31 breaths per minute and oxygen saturation was 92% on NRB 15 L/min. He was found to have severe Acute Respiratory Distress Syndrome secondary to SARS-CoV-2 viral pneumonia, with a PaO2:FiO2 ratio of 80. Admission laboratory results revealed normal white blood cell count, acute kidney injury with creatinine 1.7 mg/dL. C-reactive protein elevated at 5.5 mg/dL (Normal 0.0–0.9 mg/dL), lactate dehydrogenase elevated at 738 U/L (Normal 120–246 U/L), D-dimer elevated at 1.29 ug/mL dL (Normal < 0.4 ug/mL) and ferritin elevated at 480 ng/mL (Normal 7.3–270.7 ng/mL). His chest x-ray showed bilateral diffuse infiltrates. He was treated with a course of hydroxychloroquine, azithromycin, and methylprednisolone. He received tocilizumab and convalescent plasma. He continued to require 15 L/min NRB mask to maintain his oxygenation. On day 14 of his hospitalization, he had persistent hypoxemia requiring 15 L/min NRB and 6 L/min nasal cannula. Chest x-ray showed pneumomediastinum (Fig. 2 ). He had a CT thorax done which showed complete ground glass opacification of bilateral lungs, diffuse pneumomediastinum, and small amount of pneumopericardium. No significant underlying bullous lung disease was noted. He was managed conservatively, and was noted to have slowly decreasing oxygen requirements. He was discharged home after a 27-day hospitalization on 2 L/min nasal cannula oxygen. He was followed up in a pulmonary clinic with continued improvement in his symptoms. A repeat chest x-ray showed persistent pneumomediastinum and significant improvement in bilateral consolidations.
Fig. 2.
Pneumomediastinum (arrows)
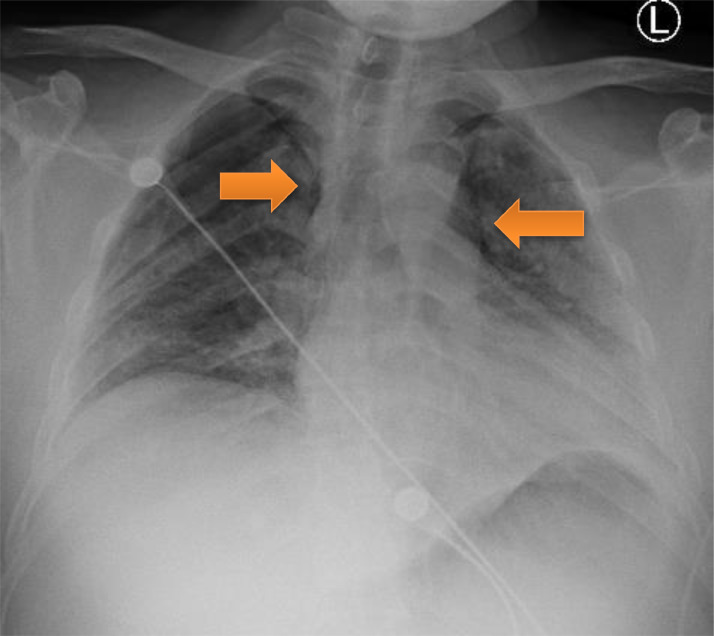
A 34-year-old Hispanic male, non-smoker with no significant medical history was admitted with two-week history of non-productive cough, diarrhea, non-bloody, non-bilious emesis and subjective fevers. On admission, he was afebrile, tachycardic with a heart rate of 140 beats per minute, and oxygen saturation 90% on room air and 99% on 4 L/min nasal cannula. He was found to have severe Acute Respiratory Distress Syndrome secondary to SARS-CoV-2 viral pneumonia, with a PaO2:FiO2 ratio of 59. Admission laboratory results revealed normal white blood cell count, lymphocytopenia of 6%. C-reactive protein elevated at 26.9 mg/dL (Normal 0.0–0.9 mg/dL), lactate dehydrogenase elevated at 353 U/L (Normal 120–246 U/L), D-dimer elevated at 0.66 ug/mL (Normal < 0.4 ug/mL) and ferritin elevated at 478 ng/mL (Normal 7.3–270.7 ng/mL). His chest x-ray revealed bilateral diffuse infiltrates. A CT scan for pulmonary embolism was done by the emergency department to rule out pulmonary embolism revealed patchy bilateral pulmonary consolidation and no evidence of filling defects. There was also no evidence of bullous lung disease. He was treated with a course of hydroxychloroquine, azithromycin, and methylprednisolone. He received tocilizumab and convalescent plasma. His hypoxia worsened during the hospital course, eventually requiring 15 L/min NRB mask and 6 L/min nasal cannula to maintain his oxygenation. On day 18 of his hospitalization, he had persistent hypoxemia with worsening respiratory distress. Patient refused intubation. During the hospital course, he was managed conservatively, and was noted to have slowly decreasing oxygen requirements. On hospital day 36, he developed pneumomediastinum (Fig. 3 ) likely secondary to violent coughing, which eventually improved with conservative management. Chest x-ray done prior to discharge revealed significant improvement in bilateral consolidations and resolution of pneumomediastinum. After six weeks since hospital admission, the patient was discharged home on 2 L/min nasal cannula.
Fig. 3.
Pneumomediastinum (arrows)
Discussion
COVID-19 is a complex disease and due to its recent emergence, continued research is being done to fully understand the pathophysiology behind it. Pneumomediastinum is thought to be caused by an increase in pressure in the alveoli, due to various etiologies, which in turn causes the alveoli to rupture. This air then travels through the perivascular sheath and toward the mediastinum.8 Current research shows that pneumomediastinum is a rare complication of COVID-19. Patients in our case series had no prior risk factors for developing pneumomediastinum and were not being treated by positive pressure ventilation. This is in contrast to a recent published case series by Volpi et al 9 where patients received positive pressure ventilation. Although usually benign, this can potentially lead to further problems including tension pneumothorax, cardiac tamponade, significant subcutaneous emphysema, or pneumorrhachis.8 Therefore, pneumomediastinum should be closely monitored since it can lead to cardiorespiratory compromise due to diminished cardiac output by direct cardiac compression or reduced venous return. We emphasize that pneumomediastinum should be in the differential diagnosis in patients with severe SARS-COV2 pneumonia, who develop new symptoms of chest pain and worsening shortness of breath. Repeat chest radiographs should be taken in these patients to recognize pneumomediastinum. Conservative management usually is the treatment of choice, although cardiorespiratory compromise can occur and caution should be warranted. The exact mechanism for which pneumomediastinum occurs in COVID-19 patients still remains unclear. Further research in this area should be conducted.
Declaration of Competing Interest
None.
References
- 1.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus Disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients [published online ahead of print, 2020 Mar 14] AJR Am J Roentgenol. 2020:1–7. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Gao R, Zheng Y, Jiang L. COVID-19 with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema [published online ahead of print, 2020 Apr 25] J Travel Med. 2020:taaa062. doi: 10.1093/jtm/taaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ucpinar BA, Sahin C, Yanc U. Spontaneous pneumothorax and subcutaneous emphysema in COVID-19 patient: case report [published online ahead of print, 2020 May 26] J Infect Public Health. 2020 doi: 10.1016/j.jiph.2020.05.012. S1876-0341(20)30478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou C, Gao C, Xie Y, Xu M. COVID-19 with spontaneous pneumomediastinum. Lancet Infect Dis. 2020;20(4):510. doi: 10.1016/S1473-3099(20)30156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohan V, Tauseen RA. Spontaneous pneumomediastinum in COVID-19. BMJ Case Rep. 2020;13(5) doi: 10.1136/bcr-2020-236519. Published 2020 May 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolani S, Nawfal H, Haloua M, et al. Spontaneous pneumomediastinum occurring in the SARS-COV-2 infection [published online ahead of print, 2020 May 11] IDCases. 2020;21:e00806. doi: 10.1016/j.idcr.2020.e00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kouritas VK, Papagiannopoulos K, Lazaridis G, et al. Pneumomediastinum. J Thorac Dis. 2015;7(Suppl 1):S44–S49. doi: 10.3978/j.issn.2072-1439.2015.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volpi Sara, Ali Jason M, Suleman Alishah. Rahim Nadeem Ahmed, Pneumomediastinum in COVID-19 patients: a case series of a rare complication. Eur J Cardio-Thorac Surg. September 2020;58(Issue 3):646–647. doi: 10.1093/ejcts/ezaa222. https://doi.org/10.1093/ejcts/ezaa222. [DOI] [PMC free article] [PubMed] [Google Scholar]