A 9-year-old girl was admitted to the paediatric intensive care unit (PICU) with a history of high-grade fever for 14 days, throbbing frontal headache, vomiting, and progressive weakness on the right side of her body for 5 days. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA was detected on nasopharyngeal swab by RT-PCR on presentation. On admission to the PICU (day 1), she had bilateral non-purulent conjunctivitis, high-grade fever (axillary temperature using digital thermometer of 39·4°C), blood oxygen saturation of 98%, a heart rate of 64 bpm, tachypnoea, and hypertension (132/102 mm Hg). The patient had a Glasgow Coma Score (GCS) of 11 (E3, V2, M6), upper motor neuron type right-sided seventh cranial-nerve palsy, complete hemiplegia, brisk deep tendon reflexes, and extensor plantar response on the right. Her pupils were normal and she had no signs of meningeal irritation. Her paediatric quick sequential organ function assessment (qSOFA) score was 2 (of 3) on admission.1 The suspected causes for her neurological signs included COVID-19 associated encephalitis or stroke. She received tier 1 and tier 2 management for raised intracranial pressure—ie, mechanical ventilation, thermoregulation, sedation, and head-elevation to 30° (tier 1) and osmotherapy (glycerol, 3% hypertonic saline, and intermittent mannitol) and intermittent hyperventilation (tier 2), and other supportive care (eg, optimisation of intravascular volume, maintenance of normoxemia, and prevention and treatment of fever and seizures).2 Empirical antibiotics included ceftriaxone, vancomycin, and azithromycin. She had normal blood counts and renal function, mild transaminitis, high CRP, high ESR, high D-dimer, and increased triglyceride and ferritin concentrations, suggestive of a hyperinflammatory response (table ). Chest x-ray showed bilateral ground-glass opacification and reticulonodular opacity.
Table.
Laboratory results on admission
| Result | Normal value | |
|---|---|---|
| Haemoglobin, g/dL | 11·3 | 12–15 |
| Platelets, ×103 cells per mm3 | 2·5 | 1·5–4·5 |
| Leucocyte count, cells per mm3 | 6980 | 4000–11 000 |
| Serum bilirubin (total), mg/dL | 0·52 | 0·3–1·2 |
| Serum bilirubin (direct), mg/dL | 0·17 | <0·3 |
| Aspartate aminotransferase, IU/L | 53·4 | <31 |
| Alanine aminotransferase, IU/L | 126·1 | 10–28 |
| Alkaline phosphatase, IU/L | 208·9 | 100–290 |
| Triglycerides, mg/dL | 416·6 | <150 |
| Prothrombin time, s | 14·2 | <14 |
| Urea, mg/dL | 31·8 | 13–43 |
| Creatinine, mg/dL | 0·35 | 0·7–1·3 |
| Uric acid, mg/dL | 3·17 | 3·5–7·2 |
| Serum sodium, meq/L | 132·8 | 135–145 |
| Serum potassium, meq/L | 3·68 | 3·5–5 |
| LDH, U/L | 1000·8 | 230–460 |
| CBNAAT for Mycobacterium tuberculosis | Acid fast bacilli not shown | .. |
| CRP, mg/L | 64·9 | 0–5 |
| ESR, mm/h | 50 | 0–15 |
| D-dimer, μg/mL | 3·57 | <0·2 |
| Ammonia, μmol/L | 76·2 | 10–47 |
| Ferritin, ng/L | 614·6 | 10–291 |
| CPK-MB, U/L | 20·1 | 5–25 |
| Troponin-I, ng/mL | 0·006 | 0·02–0·06 |
| CSF cells, cells per mm3 | 50 (lymphocytes 80%, polymorphs 20%) | 0–5 |
| CSF glucose, mg/dL | 45 | 40–70 |
| CSF protein, mg/dL | 60·1 | 15–40 |
| CSF ADA, U/L | 2·7 | 0–5 |
| CSF LDH, U/L | 102·6 | 230–460 |
| CSF CBNAAT for Mycobacterium tuberculosis | Negative | Negative |
| CSF acid fast bacilli | Absent | Absent |
| CSF IgM for Japanese encephalitis and herpes simplex virus | Negative | Negative |
| CSF culture | Sterile | Sterile |
| Gastric aspirate for acid fast bacilli | Negative | Negative |
| Malarial parasite blood smear | Negative | Negative |
| ANA serology | Negative | Negative |
| Vasculitis profile | Negative | Negative |
| Sickling test | Negative | Negative |
| COVID-19 antibody test | Positive (week 4 of admission) | Negative |
| Widal test for salmonella typhi, dengue-NS1 antigen, dengue IgM and IgG antibody, rapid test for scrub typhus, leptospira IgM, HBsAg, hepatitis C antibody test | Negative | Negative |
ADA=adenosine deaminase. ANA=antinuclear antibody. CBNAAT=cartridge based nucleic acid amplification test. CPK-MB=creatine phosphokinase-MB. CSF=cerebrospinal fluid. LDH=lactate dehydrogenase. ESR=erythrocyte sedimentation rate.
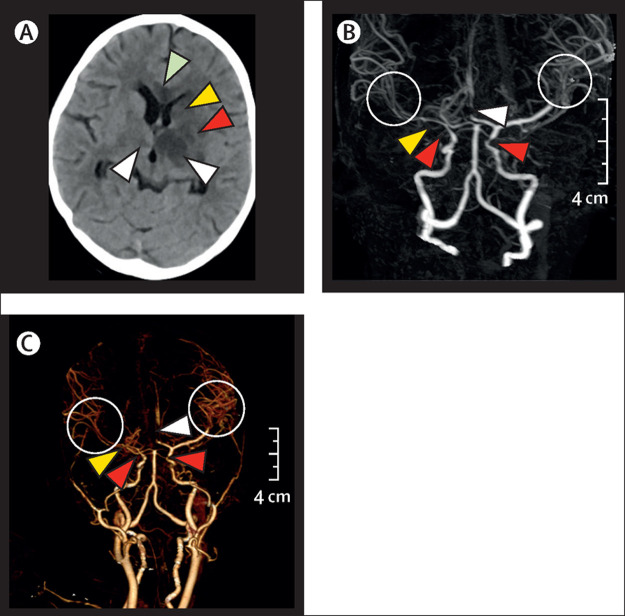
She deteriorated with worsening GCS, bradycardia, and cardiac arrest on day 3 of PICU admission. Return of spontaneous circulation was achieved after 2 mins of chest compression and ventilation. Her SOFA score worsened from 2 to 12, therefore requiring higher ventilatory, vasoactive, and inotropic support for respiratory failure, shock, and autonomic instability (appendix). Echocardiography did not show valvular defect or coronary artery dilatation. Her ECG, serum sample of myocardial creatine phosphokinase (CPK-MB), and troponin I measurements were normal. An unenhanced CT scan showed multifocal discrete and confluent hypodensities, suggestive of infarcts, in the genu and adjacent body of corpus callosum, left basal ganglia, and bilateral thalami, with mild oedema and mild mass effect over the lateral ventricle. Subsequently, a CT angiography showed multifocal smooth stenosis of both intracranial internal carotid arteries, right middle cerebral artery, and both A2 segments of the anterior cerebral arteries. Diffuse narrowing of the M2 and M3 segment branches of both middle cerebral arteries was also seen (figure ). To further investigate rarer causes of childhood stroke the following tests were done, all were negative: anti-nuclear antibody, vasculitis profile, and sickle cell screen (table). There was no history of neck trauma that suggested arterial dissection. She had no history of skin lesions that were suggestive of varicella (chickenpox) in the past or during current illness. Cerebrospinal fluid examination showed pleocytosis with slightly increased protein (table) but tested negative for SARS-CoV-2 using RT-PCR. No organism was detected in cerebrospinal fluid or blood despite extensive tests for possible organisms (table). Test for IgM and IgG antibody for varicella zoster virus could not be reported because the test kit was not available.
Figure.
CT scan and angiograph
(A) Unenhanced CT scan image of the axial section showing hypodensities in corpus callosum (green arrowhead), left caudate (yellow arrowhead), putamen (red arrowhead) and bilateral thalami (white arrowheads), with mild compression over the left lateral ventricle. (B) Minimum intensity projection image of CT angiography, showing substantial stenosis of the anterior cerebral artery (white arrowhead), stenosis of bilateral supraclinoid internal carotid artery segments (red arrowheads), diffuse stenosis of M1 segment of the right MCA (yellow arrowhead) and diffuse narrowing of the M2 and M3 segments of both MCA (circles). (C) Volume-rendered images of CT angiography showing multifocal narrowing involving both internal carotid arteries (red arrowheads), anterior cerebral artery (white arrowhead), and right MCA (yellow arrowhead). Note the diffuse narrowing of the segment M2 and M3 of both MCAs (circled); on the left side there is sudden tapering of the MCA at the M1–M2 junction. Scans taken on day 7 in paediatric intensive care unit. MCA=middle cerebral artery.
Additional treatment included intravenous immunoglobin (2 g/kg over 2 days), methylprednisolone (30 mg/kg per day intravenously for 5 days) followed by dexamethasone (0·15 mg/kg per day for 2 weeks), remdesivir (5·0 mg/kg by intravenous infusion over 1 h, followed by 2·5 mg/kg per day by intravenous infusion for 5 days), and low-molecular-weight heparin (1 mg/kg subcutaneously twice daily for 2 weeks followed by once daily for 1 week). Her clinical status improved progressively (appendix), and she was weaned off mechanical ventilation on day 12. Her serum CRP, ferritin, and lactate dehydrogenase values returned to normal. COVID-19 RT-PCR on nasopharyngeal swab and bronchoalveolar lavage was negative on day 12 of stay in the PICU. A serum COVID-19 antibody test (done after 4 weeks of admission) was positive. She showed slow but consistent improvement over the next 3 weeks, reaching a GCS of 13 (E4, V3, M6) and power of 2–3 of 5 on the affected side. She transferred from PICU to a paediatric ward where she continues to receive psychomotor rehabilitation for her residual illness.
COVID-19, a global health emergency, caused by the highly pathogenic SARS-CoV-2, has affected more than 28 million people and caused 900 000 deaths, as of Sept 13, 2020.3, 4 Most of the children infected with SARS-CoV-2 either are asymptomatic or have upper respiratory tract infection.5 Paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS-TS) or multisystem inflammatory syndrome in children (MIS-C) is an emerging life-threatening non-respiratory complication of COVID-19 that can present at any time during the course of illness, but commonly at 1–6 weeks after infection.6, 7, 8, 9 Early evidence suggests this syndrome is related to uncontrolled inflammatory response and cytokine storm following infection with SARS-CoV-2; however, understanding of the mechanism remains poor.6
Global and population-specific incidence of MIS-C is unknown. WHO has formulated a case definition and global registry for MIS-C.10 Our case of a 9-year-old girl with fever for 14 days, bilateral non-purulent conjunctivitis, shock, elevated D-dimer, ESR, CRP, sterile blood culture, positive COVID-19 RT-PCR, and no respiratory symptoms, satisfies WHO diagnostic criteria of MIS-C.10 This patient presented with initial clinical manifestations of acute ischaemic stroke, which has so far not been reported in children. In one of the first reports of PIMS-TS, the patient who died despite extensive organ support (mechanical ventilation, renal replacement therapy, and extracorporeal membrane oxygenation) had developed right middle cerebral artery and ACA ischaemic infarcts. That patient was confirmed as SARS-CoV-2 positive at post-mortem analysis.11 In our case, early manifestation of stroke and aggressive therapy to halt the cytokine storm seems to be crucial for reaching a positive outcome.
The differential diagnosis of stroke in children is broad. Common causes—such as intracranial bleed, prevalent viral encephalitis, bacterial meningoencephalitis, brain abscess, tubercular meningitis, and cardiac thromboembolism—were ruled out. There was no history to suggest varicella encephalitis, although antibodies for varicella zoster virus could not be tested. We also ruled out other rare causes of acute ischaemic stroke (table). Neurological presentations associated with COVID-19 and similar strains in adults include altered consciousness, headache, dizziness, anosmia, dysgeusia, seizures, stroke, Guillain-Barré syndrome, and neuropsychiatric symptoms.7, 8 SARS-CoV-2 is reported to have a 7·6 times increase (95% CI 2·3–25·2) in the risk of resulting in a stroke in comparison with other coronavirus and seasonal infections in adults.12 Pathophysiology of acute ischemic stroke in COVID-19 is not fully elucidated yet, although possible mechanisms could be immune mediated or para-infectious events, a hypercoagulable state from systemic inflammation and cytokine storm, viral mimicry of the host resulting in autoantibodies, viral super antigen sequences, antibody or T-cell recognition of viral antigens, or formation of immune complexes.6, 8, 13, 14 Direct viral-induced endothelitis or endotheliopathy, leading to angiopathic thrombosis is also seen in some neurotropic viruses such as Varicella zoster.15 Endothelial infection by SARS-CoV-2 in the kidney, heart, bowel, and lung at autopsy is reported but cerebral vessels have not yet been investigated.16 Initial neuroimaging findings, similar to our described case, are reported in a few adult cases of COVID-19 infection.7 In our case, RT-PCR for COVID-19 was positive on nasopharyngeal swab but negative in cerebrospinal fluid. Cerebrospinal fluid is often negative for viral genetic material in influenza-associated encephalopathy.8, 17 The negative RT-PCR for the cerebrospinal fluid sample could be attributed to low viral load in the sample, transient viraemia, and delay in testing after symptom appearance.18
MIS-C is rare; of 662 cases reported so far,19 only the case in this report has presented with acute ischaemic stroke. Considering the emerging evidence of this disease, clinicians should include SARS-CoV-2 in their differential diagnosis for children presenting with new neurological symptoms, positive inflammatory markers, and suggestive imaging findings while exploring other possible causes. Aggressive therapy to halt the cytokine storm and relevant supportive care while considering differential diagnoses is crucial for reaching positive outcomes in children. Further studies are required to understand the pathogenesis of ischaemic stroke and assess the neurological and cognitive outcomes in children with COVID-19.
Acknowledgments
Acknowledgment
Ethical clearance was granted by the All India Institute of Medical Sciences Patna ethics committee (approval no AIIMS/Pat/IEC/2020/535). Written informed consent for publication was obtained from the patient's parents. We thank Arun Prasad, Pradeep Kumar, Bhabesh Kant Chowdhary, Amit Sinha for contributing to the clinical management of the patient. All authors have access to all the data in the study. LT and SS have accessed and verified all the data in the study.
Contributors
LT made the treatment decisions, supervised the initial draft, reviewed the studies, and did the planning, analysis, and final drafting of the Case Report. SS and AB were involved in patient management, literature search, and writing of the initial draft. SK helped in synthesis and analysis of radiographical information. All the authors contributed to the final manuscript. LT and SS accessed and verified the data. LT had the final responsibility to submit for publication.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Tiwari L, Anand C, Kumar G, Chaturvedi J, Mishra NR. Abstract PCCLB-42: new predictive score for PICU: pediatric quick SOFA score—AIIMS Patna model. Pediatr Crit Care Med. 2018;19:256. [Google Scholar]
- 2.Singhi SC, Tiwari L. Management of intracranial hypertension. Indian J Pediatr. 2009;76:519–529. doi: 10.1007/s12098-009-0137-7. [DOI] [PubMed] [Google Scholar]
- 3.Zhu N, Zhang D, Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Coronavirus disease (COVID-19) outbreak situation. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (Sept 13, 2020).
- 5.Lu X, Zhang L, Du H. SARS-CoV-2 Infection in children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang L, Tang K, Levin M. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30651-4. published online Aug 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garg RK, Paliwal VK, Gupta A. Encephalopathy in patients with COVID-19: a review. J Med Virol. 2020 doi: 10.1002/jmv.26207. published online June 19. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Mannan O, Eyre M, Löbel U. Neurologic and radiographic findings associated with covid-19 infection in children. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.2687. published online July 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swann OV, Holden KA, Turtle L. Clinical characteristics of children and young people admitted to hospital with COVID-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370 doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19. May 15, 2020. https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19
- 11.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merkler AE, Parikh NS, Mir S. Risk of ischemic stroke in patients with COVID-19 versus patients with influenza. JAMA Neurol. 2020 doi: 10.1101/2020.05.18.20105494. published July 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellul MA, Benjamin L, Singh B. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paniz-Mondolfi A, Bryce C, Grimes Z. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Med Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;8:731–740. doi: 10.1016/S1474-4422(09)70134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varga Z, Flammer AJ, Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Destras G, Bal A, Escuret V, Morfin F, Lina B, Josset L. Systematic SARS-CoV-2 screening in cerebrospinal fluid during the COVID-19 pandemic. Lancet Microbe. 2020;1:e149. doi: 10.1016/S2666-5247(20)30066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheikhzadeh E, Eissa S, Ismail A. Diagnostic techniques for COVID-19 and new developments. Talanta. 2020;220 doi: 10.1016/j.talanta.2020.121392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed M, Advani S, Moreira A. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.