ABSTRACT
Introduction
Traumatic peripheral nerve injuries (TPNIs) are increasingly prevalent in battlefield trauma, and the functional recovery with TPNIs depends on axonal continuity. Although the physical examination is the main tool for clinical diagnosis with diagnostic work up, there is no diagnostic tool available to differentiate nerve injuries based on axonal continuity. Therefore, treatment often relies on “watchful waiting,” and this leads to muscle weakness and further reduces the chances of functional recovery. 4-aminopyridine (4-AP) is clinically used in multiple sclerosis patients for walking performance improvement. Preliminary results in conscious mice suggested a diagnostic role of 4-AP in distinguishing axonal continuity. In this study, we thought to evaluate the diagnostic potential of 4-AP on the axonal continuity in unawake/sedated animals.
Materials and Methods
Rat sciatic nerve crush and transection injuries were used in this study. Briefly, rats were anesthetized with isoflurane and mechanically ventilated with oxygen-balanced vaporized isoflurane. Sciatic nerve and triceps surae muscles were exposed by blunt dissection, and a stimulating electrode was placed under a sciatic nerve proximal to the crush injury. A force transducer measured muscle tension response to electrical stimulation of sciatic nerve. Muscle response was measured before crush, after crush, and 30 minutes after systemic 4-AP (150 µg/kg) or local (4-AP)-poly(lactide-co-glycolide)-b-poly(ethylene glycol)-b-poly(lactide-co-glycolide) (PLGA-PEG) treatment.
Results
We found that both crush and transection injuries in sciatic nerve completely abolished muscle response to electrical stimulation. Single dose of systemic 4-AP and local (4-AP)-PLGA-PEG treatment with crush injury significantly restored muscle responses to electrical stimulation after 30 minutes of administration. However, systemic 4-AP treatment had no effect on muscle response after nerve transection. These results clearly demonstrate that 4-AP can restore nerve conduction and produce muscle response within minutes of administration only when there is a nerve continuity, even in the sedated animal.
Conclusions
We conclude that 4-AP could be a promising diagnostic agent in differentiating TPNI based on axonal continuity.
INTRODUCTION
Traumatic peripheral nerve injury (TPNI) is increasingly common in combat-related extremity injuries.1,2 And combat-injury-induced neurotrauma significantly contributes to the morbidity and mortality in military casualties.3 Traumatic peripheral nerve injury can be caused by gunshot wound, blast trauma, crush, stretching, laceration, and acute nerve compression. Traumatic peripheral nerve injury occurs along a spectrum in which some axonal continuity is maintained to complete nerve transection.4–6 The presence or absence of axonal continuity plays a pivotal role in deciding the treatment strategy for TPNI and improving functional recovery.7,8 Nerve injuries such as laceration have a complete loss of axonal continuity while axonal continuity is retained in crush injuries. Although the comprehensive physical examination of motor and sensory functions induced by the affected nerve is the mainstay for clinical diagnosis with diagnostic work up, there is no early diagnostic tool available to differentiate nerve injury based on axonal continuity. Advanced electro-diagnostics and imaging studies do not provide any diagnosis within weeks of injury and they are also impractical tools in the battlefield setting, thus “watchful waiting” of weeks or months increases the chances of poor functional recovery and drastic muscle loss because of TPNI.9,10 On the contrary, if the early surgical exploration of nerves was employed to decide axonal continuity, it might result in poor functional recovery in the case of crushed nerves. Therefore, there is an unmet need for a diagnostic tool which could address these issues for early diagnosis of closed-type nerve injuries and subsequent appropriate treatment strategies.
4-aminopyridine (4-AP) is a potent potassium channel blocker and a Food and Drug Administration-approved generic drug for symptomatic treatment of multiple sclerosis.11,12 In multiple sclerosis patients, 4-AP blocks potassium channels in demyelinated nerves, transiently improves nerve conduction and walking performance. 4-aminopyridine improves neurotransmitter release and increases the number of activated synaptic terminals, and it is also known to enhance nerve conduction in injured and demyelinated axons in awake/conscious mice.13–16 We consistently demonstrated that a single dose of systemic or transdermal 4-AP can significantly improve walking function in conscious mice with crushed nerve injury but not in mice with transected nerves.16–18 Although these findings suggest a diagnostic role of 4-AP in identifying lesions with axonal continuity in an awake/conscious animal, it is unknown whether this diagnostic criterion of 4-AP is applicable in an unawake/sedated animal. We thought to further investigate the diagnostic property of 4-AP in an anesthetized rat model of TPNI to mimic the clinical scenario of an unconscious/sedated TPNI patient.
One of the disadvantages of systemic 4-AP is its narrow therapeutic window and renal clearance.19,20 Systemic 4-AP also causes adverse effects like tremors and seizures at higher doses. We hypothesized that the enhanced conduction of non-severed nerves is as a result of local effects of 4-AP at the injury site. Therefore, we wanted to investigate whether a local formulation of 4-AP could diagnose non-severed nerves in continuity, as local delivery provides a more targeted approach. Additionally, a local formulation of 4-AP is optimal for battlefield diagnosis where multiple nerves may be injured in traumatized limbs. Moreover, battlefield diagnosis of nerve injury patients using an easily transportable injectable form of 4-AP would allow proper triage of patients when transporting them to the appropriate secondary facilities for timely surgical intervention. Therefore, we developed a locally deployable version of 4-AP, (4-AP)-poly(lactide-co-glycolide)-b-poly(ethyleneglycol)-b-poly(lactide-co-glycolide) (PLGA-PEG), consisting of two Food and Drug Administration-approved, biodegradable, and biocompatible hydrogels.
In this study, we investigated the transient stimulatory effect of both systemic and local 4-AP in nerve stimulation-induced muscle contraction in sedated rats in relation to nerve continuity.
MATERIALS AND METHODS
Animals
The experimental design and animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Penn State College of Medicine, Hershey. Eight- to ten-week old (350-400 g) male Sprague-Dawley (SD) rats (Charles River laboratories) were used in this study. The rats were housed at the animal facility, and the experimental animals were handled according to the IACUC guidelines for the care and use of laboratory animals.
Preparation and Administration of Systemic 4-AP
4-Aminopyridine was purchased from Sigma-Aldrich, and the stock solution was prepared in saline. Rats were given intraperitoneal (ip) injection of soluble 4-AP at a dose of 150 µg/kg or equivalent volume of saline. Because 4-AP is reported to cause adverse effects in humans if the serum concentration is more than 100 ng/mL, we performed high-performance liquid chromatography (HPLC) analysis of rat serum samples at different time points for different 4-AP doses (data not shown).20 We found that 4-AP at 150 µg/kg dose produces a serum concentration that is below the tolerable level of serum 4-AP concentration in humans and used this dose for our study (Appendix).
Formulation and Application of (4-AP)-PLGA-PEG at Nerve Crush Site
Poly(lactide-co-glycolide)-b-poly(ethylene glycol)-b-poly(lactide-co-glycolide) 1700-1500-1700 Da (lactic acid/glycolic acid 15:1 (94% lactic acid/6% glycolic acid) was purchased from PolySciTech (catalog # AK097). All of the chemicals were used without further purification. Twenty percent weight per volume solution was made using phosphate-buffered saline and PLGA-PEG block copolymer. A known amount of gelling solution was then vortexed with 4-AP (catalog # 275875-56; Sigma-Aldrich) to achieve the desired concentration. To locally administer the formulation, 75 µL of 5 µg/µL (4-AP)-PLGA-PEG was pipetted directly onto the sciatic nerve crush site, and this equals a total dose of 375 µg 4-AP at the injury site.
HPLC Analysis of Serum 4-AP in Rats
Circulating level of 4-AP in rat serum sample was determined using ABSciex 4000 Q Trap mass spectrometry (MS) coupled with a Waters Acquity ultra performance liquid chromatography (UPLC) separation system (UPLC/MS/MS) in the Mass Spectrometry Core Facility in College of Medicine of Penn State University.
Deuterium-labeled 4-AP (4-AP-d4) was used as an internal standard. The multiple reaction monitoring mode was used to analyze and quantify 4-AP and 4-AP-d4, with the transitions of m/z 95 > 78 for 4-AP and 99 > 82 for 4-AP-d4. 4-aminopyridine was quantified by using a standard curve which was constructed by plotting the ratio of the peak area of 4-AP to the peak area 4-AP-d4 versus 4-AP concentration (0.01-20 μM). All peaks were integrated and quantified by ABSciex Multiquan 2.1 software.
Muscle Response Measurement Immediately After Sciatic Nerve Crush/Transection Injury
Sciatic nerve innervates the triceps surae muscle and thus sciatic nerve conduction can be measured in terms of triceps surae muscle contraction. As shown in this experimental setup, sciatic nerve was electrically stimulated proximal to crush site, and in situ triceps surae muscle tension was measured by a force transducer (FT10; Grass Instruments) (Fig. 1).
FIGURE 1.
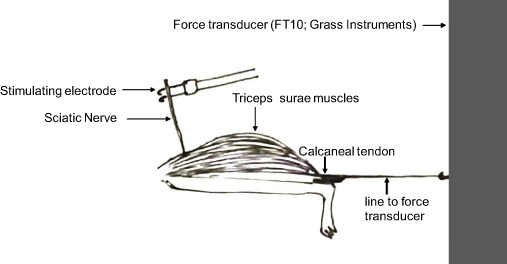
Experimental set-up for in situ muscle force measurement. Stimulating electrode is placed under the sciatic nerve and triceps surae muscle tension is measured by stimulating the sciatic nerve. Setup is equipped with a force transducer (FT10, Grass Instruments) to measure muscle tension.
Briefly, SD rats were anesthetized with 3%-4% isoflurane, the trachea was cannulated, and the lungs were mechanically ventilated (Harvard Apparatus) with oxygen balanced vaporized 2% isoflurane. Anesthesia was maintained throughout the experimental protocol with 1%-2% isoflurane balanced with oxygen supplied via a nose cone. Isoflurane was chosen for anesthesia because it has been reported to have the least effect on sciatic nerve conduction function compared to ketamine-xylazine.21 The area surrounding the site of surgical incision was shaved with a mechanical clipper. Then, the skin was cleaned with 70% ethanol and betadine using sterile gauze. Ophthalmic lubricant was applied to the eyes to prevent corneal abrasion. Isotonic saline (0.5 - 1 mL) was administered subcutaneously to prevent dehydration during the procedure. A heat lamp was utilized to maintain the animal’s body temperature. Surgery was done under aseptic condition using sterile drapes and instruments. The sciatic nerve and triceps surae muscles were exposed by blunt dissection and the calcaneal tendon was severed and linked with braided fishing line to a force transducer. A shielded stimulating electrode was placed under sciatic nerve and injury was performed distal to a stimulating electrode. The sciatic nerve underwent a standardized crush injury with blunt forceps (Miltex #18-1107) and jig (instant squeeze and release) or laceration injury with fine scissors. Sciatic nerve crush injury was done 3 mm proximal to trifurcation. The triceps surae muscles were contracted for 10 seconds at 40 Hz, 0.1 ms pulse, and a voltage 2 times above the motor threshold. The muscle response (tension/contraction) was measured before injury, after crush or laceration, and 30 minutes after ip injection of saline or 150 µg/kg 4-AP and or local (4-AP)-PLGA-PEG treatment.
Muscle Response Measurement 3 Days After Sciatic Nerve Crush Injury
The preparation of the rats and blunt dissection to expose the sciatic nerve were identical as described earlier except for the use of different forceps, the time of crush injury, and the subsequent steps for day 3 experiment. Crush injury was done using blunt forceps (Miltex #18-1107) and jig for 5 seconds. The segment of the crushed nerve corresponds to the 2 mm zone at the tip of the Miltex forceps. After the surgery, the muscle was sutured together with 6-0 monofilament and skin with 2-0 silk suture. Triple antibiotic cream was applied over the area as prophylactic against infection. Isoflurane was discontinued while still under supplemental oxygen with the nose cone until the rat awakens, and 1 mg/kg buprenorphine was given subcutaneously for pain management.
Muscle response (tension/contraction) analysis in these animals after 3 days of surgery was performed as described earlier for “Muscle response measurement immediately after sciatic nerve crush/transection injury.” Briefly, a shielded stimulating electrode was placed under the sciatic nerve proximal to crush injury. The triceps surae muscles were contracted for 10 seconds at 40 Hz; 0.1 ms pulse, and minimum stimulating voltage (equivalent to the motor threshold). This initial muscle tension was considered as the baseline muscle tension on post crush injury day 3 for analysis. Rats were administered with ip Saline (0.9%) and muscle tension was measured after 30 minutes. The rats were then treated with ip 150 µg/kg 4-AP and muscle tension was measured 30 minutes after 4-AP treatment. To evaluate the effect of local 4-AP, (Sal)-PLGA-PEG was applied at the site of crush injury on the left sciatic nerve and (4-AP)-PLGA-PEG was applied at the site of crush injury on the right sciatic nerve.
Data Analysis
All bar graph results are presented as means ± SEM. Data were analyzed using GraphPad PRISM 7 (GraphPad Software, San Diego, CA, USA), and statistical analysis was performed using one-way ANOVA followed by Tukey’s post hoc test for multiple comparisons. A P value < 0.05 was con-sidered to be statistically significant.
RESULTS
Functional recovery after traumatic nerve injury depends on axonal continuity.22 To evaluate the effect of 4-AP in distinguishing axonal continuity in TPNI, we have used two different nerve injuries and two distinct time points for the study. The two different nerve injuries were nerve crush injury where axonal continuity was retained and nerve transection where axonal continuity was lost. Evaluation of nerve function after traumatic nerve injury in the clinical setting may be done within weeks or months of injury. Therefore, we used two different time points, immediately after injury and 3 days post-injury.
First, we investigated the effect of systemic (ip) and local 4-AP administrations on in situ muscle tension immediately after TPNI. Fig. 2A and C shows that sciatic nerve crush injury completely abolished the muscle response to electrical stimulation: 2,100 g muscle tension before the injury (black line) and no muscle tension after crush injury (red line). Both single dose of systemic and local 4-AP treatments significantly elicited muscle response (green lines) to electrical stimulation, and it was 900 and 1,700 g, respectively (Fig. 2A, C). Muscle response was increased by 800% and 1600% over the crush injury by systemic and local 4-AP treatment, respectively (Fig. 2B, D). Similarly, AUC and peak muscle tension were significantly increased by systemic and local 4-AP treatment respectively (Fig. 2E, F and G, H). In contrast, systemic 4-AP had no effect on muscle tension innervated by the transected sciatic nerve (Fig. 3A, B). This suggests that 4-AP improves nerve conduction, and thus muscle response upon electrical stimulation occurs only when there is an axonal continuity.
FIGURE 2.
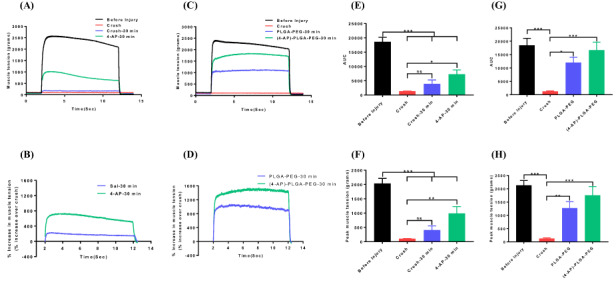
Representative muscle tension tracings of triceps surae muscles before and after crush injury with or without systemic 4-aminopyridine (4-AP) (150 μg/kg, ip) (A) and local 4-AP thermogel (C) treatments. Figures (B) and (D) show the muscle tension as % increase over crush after systemic and local 4-AP treatments, respectively. Bar graphs showing area under the curve (E, G) and peak muscle tension (F, H) before and after systemic or local 4-AP treatment, respectively. Figures E and F are for systemic, and figures G and H are for local 4-AP treatments. Data are represented as mean ± SEM. n = 6/group for systemic 4-AP, n = 3/group for local 4-AP. *P < 0.05, **P < 0.01, ***P < 0.001 by one-way ANOVA followed by Tukey’s multiple comparisons post hoc test.
FIGURE 3.
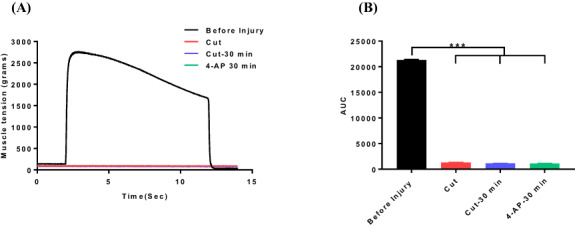
Representative muscle tension tracings of triceps surae muscles after nerve cut injury and 4-aminopyridine (4-AP) (150 μg/kg, ip) treatment (A) and area under the curve (B) derived from muscle tension experiments. Data are represented as mean ± SEM, n = 3/group. ***P < 0.001 by one-way ANOVA followed by Tukey’s multiple comparisons post hoc test.
Next, we evaluated the effect of 4-AP at a later time point of TPNI and it was on post crush injury day 3. For these experiments, we used a stimulation voltage far less than that used for maximum baseline contraction. This resulted in minimal to no response to electrical stimulation and thus minimal muscle tension on post injury day 3 (Fig. 4A, C). However, single dose of systemic and local 4-AP treatments significantly elicited muscle response (green lines) to electrical stimulation, and it was 250 and 500 g, respectively (Fig. 4A, C). Muscle response was increased by 80% and 250% over the crush injury by systemic and local 4-AP treatment, respectively (Fig. 4B, D). Similarly, AUC and peak muscle tension were significantly increased by systemic and local 4-AP treatment, respectively (Fig. 4E, F and G, H).
FIGURE 4.
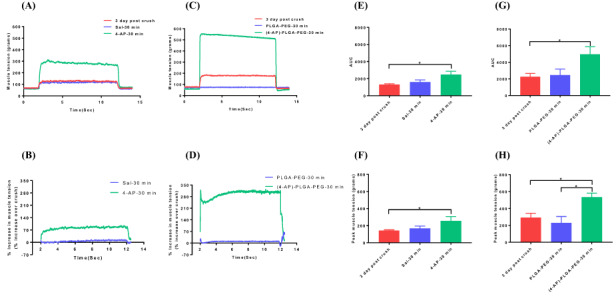
Representative muscle tension tracings of triceps surae muscles 3 days after crush injury with or without systemic 4-aminopyridine (4-AP) (150 μg/kg, ip) (A) and local 4-AP thermogel (C) treatments. Figures (B) and (D) show the muscle tension as % increase over crush after systemic and local 4-AP treatments, respectively. Bar graphs showing area under the curve (E, G) and peak muscle tension (F, H) before and after systemic or local 4-AP treatment, respectively. Figures E and F are for systemic, and figures G and H are for local 4-AP treatments. Data are represented as mean ± SEM. n = 6/group for systemic 4-AP, n = 3/group for local 4-AP. *P < 0.05 by one-way ANOVA followed by Tukey’s multiple comparisons post hoc test.
DISCUSSION
The main finding of this study is that both systemic and locally administered 4-AP can elicit in situ muscle contraction in sedated animals after sciatic nerve crush injury where axonal continuity is maintained, and this effect is absent with nerve transection. The diagnostic property of 4-AP was present both immediately and 3 days after peripheral neurotrauma. These findings suggest that 4-AP-induced muscle contraction innervated by the crushed nerve in this study is probably as a result of the local effects of 4-AP on non-severed nerves.
The rationale for using local 4-AP in the battle field setting is distinct for open injuries, and it may have tremendous impact in triage of patients with transection injuries for immediate nerve repair to the centers that perform these highly specialized microsurgical procedures. As civilian surgeons ourselves, we find that hospital transfers are often the result of ambiguity regarding nerve continuity after trauma. The key point is that the distinction of nerve continuity in patients with severe nerve injuries would be possible at times when decisions of care are made at specialized facilities without waiting for the relevant data on nerve continuity that could be too late or incorrectly guessed at the time of injury. That’s why we need an early nerve continuity test that could be readily deployed not only in the in the battlefield setting and forward areas but also in our own operating rooms first to diagnose and then to aid in electrodiagnostic studies to monitor the progress of long-term nerve repair outcomes.
Our results demonstrate that local 4-AP administration could be used as an equally promising diagnostic tool as systemic 4-AP for the nerve continuity screening in the setting of acute neurotrauma. Local 4-AP may be an even more attractive diagnostic candidate since it enables targeted delivery to the injury site, minimizing renal clearance of the drug and exploiting its narrow therapeutic index. Moreover, systemic drug administration is suboptimal for battlefield diagnosis. A local injectable diagnostic tool could be an ideal diagnostic in the battlefield setting, where the extent of nerve injuries is complex, as it would enable multiple injury sites to be injected to observe whether the specific function returns. This could also be used in the most forward areas before transport to secondary medical facilities.
In conscious mice, we have repeatedly demonstrated that 4-AP administered either as oral, intraperitoneal, local, or transdermal route can distinctly differentiate a crush injury from a denervation injury, and the findings of this study in anesthetized rats are very consistent with our findings in conscious mice.16–18,23 In mice, we have shown that 4-AP treatment can improve nerve myelination, nerve conduction velocity, muscle atrophy, and ex vivo muscle contraction as compared to the denervation injury. In this study, the in situ muscle force enhancing effect of systemic and local 4-AP was present only in the crush injury group, but not in the transection group. Thus, we were able to confirm that both systemic and local administration of 4-AP in rodent model of TPNI can restore muscle contraction in injuries where axonal continuity is retained but not in injuries where axonal continuity is absent. Furthermore, this study provides the rationale for further investigation in the setting of peripheral neurotrauma where no diagnostic tool is currently available. The preliminary findings of this study were presented as an abstract at 2019 Military Health System Research Symposium.24 In the accepted abstract for 2020 Military Health System Research Symposium, we have shown that similar to systemic 4-AP, local (4-AP)-PLGA-PEG treatment has no effect on muscle contraction after nerve transection injury.25
In demyelinated axons, 4-AP inhibits voltage-gated potassium channels, restores the transmembrane potential and nerve conduction, prolongs action potential duration, and facilitates neuromuscular and synaptic transmission.8,11,13–15 Anesthesia slows or blocks nerve impulses and affects synaptic transmission and neuronal function, and this study was performed in anesthetized rats to assess the nerve injury diagnostic property of 4-AP in unconscious or sedated state.26 Although electrophysiological studies have shown that the synaptic transmission is more sensitive to the effects of general anesthetics than in axonal conduction and isoflurane inhibits 4-AP-evoked glutamate release, isoflurane (2.5%) is reported to have marginal effect on sciatic nerve conduction in rats and we used 1%-2% isoflurane for muscle tension measurements.27–30 Of note, isoflurane (1%-2%) is also reported to have minimal impact on the nerve conduction velocity in mice than with ketamine/xylazine.21 Importantly, our current findings in isoflurane-anesthetized rat model of nerve injury are comparable with our electrophysiological findings in ketamine/xylazine-anesthetized mice where 4-AP treatment improved functional recovery with improved nerve conduction velocity.16–18 Thus, it is apparent that the interaction of 4-AP and isoflurane, if any, is insignificant and negligible in our studies.
CONCLUSIONS
Our findings with 4-AP have tremendous clinical implications as a potential diagnostic tool in distinguishing axonal continuity in nerves at battlefield or trauma center because a timely assessment of an injured nerve determines the critical decision of the attending physician whether to explore and repair or continue with watchful waiting.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Mass Spectrometry Core Facility at the Penn State University College of Medicine, Hershey for UPLC/MS/MS measurement of 4-AP in rat serum samples.
Contributor Information
Anagha A Gurjar, Department of Orthopaedics and Rehabilitation, Center for Orthopaedics and Translational Science, The Pennsylvania State University College of Medicine, Milton S. Hershey Medical Center, Hershey, PA 17033, USA.
Kristen M Manto, Department of Orthopaedics and Rehabilitation, Center for Orthopaedics and Translational Science, The Pennsylvania State University College of Medicine, Milton S. Hershey Medical Center, Hershey, PA 17033, USA.
Juan A Estrada, Heart and Vascular Institute, The Pennsylvania State University College of Medicine, Milton S. Hershey Medical Center, Hershey, PA 17033, USA.
Marc Kaufman, Heart and Vascular Institute, The Pennsylvania State University College of Medicine, Milton S. Hershey Medical Center, Hershey, PA 17033, USA.
Dongxiao Sun, Mass Spectrometry Core Facility, Penn State University College of Medicine, Hershey, PA 17033, USA.
M A Hassan Talukder, Department of Orthopaedics and Rehabilitation, Center for Orthopaedics and Translational Science, The Pennsylvania State University College of Medicine, Milton S. Hershey Medical Center, Hershey, PA 17033, USA.
John C Elfar, Department of Orthopaedics and Rehabilitation, Center for Orthopaedics and Translational Science, The Pennsylvania State University College of Medicine, Milton S. Hershey Medical Center, Hershey, PA 17033, USA.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Military Medicine online.
FUNDING
This work was supported by grants from the National Institutes of Health (K08 AR060164‐01A),and the Department of Defense (W81XWH‐16‐1‐0725) to John C. Elfar in addition to the institutional support from Pennsylvania State University Medical Center.
REFERENCES
- 1. Beltran MJ, Burns TC, Eckel TT, Potter BK, Wenke JC, Hsu JR: Fate of combat nerve injury. J Orthop Trauma 2012; 26(11): e198-203. [DOI] [PubMed] [Google Scholar]
- 2. Rivera JC, Glebus GP, Cho MS: Disability following combat-sustained nerve injury of the upper limb. Bone Joint J 2014; 96-b(2): 254-8. [DOI] [PubMed] [Google Scholar]
- 3. Belmont PJ, Owens BD, Schoenfeld AJ: Musculoskeletal injuries in Iraq and Afghanistan: epidemiology and outcomes following a decade of war. J Am Acad Orthop Surg 2016; 24(6): 341-8. [DOI] [PubMed] [Google Scholar]
- 4. Robinson LR: Traumatic injury to peripheral nerves. Suppl Clin Neurophysiol 2004; 57: 173-86. [DOI] [PubMed] [Google Scholar]
- 5. Campbell WW: Evaluation and management of peripheral nerve injury. Clin Neurophysiol 2008; 119(9): 1951-65. [DOI] [PubMed] [Google Scholar]
- 6. Menorca RM, Fussell TS, Elfar JC: Nerve physiology: mechanisms of injury and recovery. Hand Clin 2013; 29(3): 317-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mikesh M, Ghergherehchi CL, Hastings RL, et al. : Polyethylene glycol solutions rapidly restore and maintain axonal continuity, neuromuscular structures, and behaviors lost after sciatic nerve transections in female rats. J Neurosci Res 2018; 96(7): 1223-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Modrak M, Talukder MAH, Gurgenashvili K, Noble M, Elfar JC: Peripheral nerve injury and myelination: potential therapeutic strategies. J Neurosci Res 2020; 98(5): 780-95. doi: 10.1002/jnr.24538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aminoff MJ: Electrophysiologic testing for the diagnosis of peripheral nerve injuries. Anesthesiology 2004; 100(5): 1298-303. [DOI] [PubMed] [Google Scholar]
- 10. Chung T, Prasad K, Lloyd TE: Peripheral neuropathy: clinical and electrophysiological considerations. Neuroimaging Clin N Am 2014; 24(1): 49-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jensen HB, Ravnborg M, Dalgas U, Stenager E: 4-Aminopyridine for symptomatic treatment of multiple sclerosis: a systematic review. Ther Adv Neurol Disord 2014; 7(2): 97-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. King AM, Menke NB, Katz KD, Pizon AF: 4-Aminopyridine toxicity: a case report and review of the literature. J Medical Toxicology 2012; 8(3): 314-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith KJ, Felts PA, John GR: Effects of 4-aminopyridine on demyelinated axons, synapses and muscle tension. Brain 2000; 123(Pt.1): 171-84. [DOI] [PubMed] [Google Scholar]
- 14. Leung G, Sun W, Brookes S, Smith D, Shi R: Potassium channel blocker, 4-aminopyridine-3-methanol, restores axonal conduction in spinal cord of an animal model of multiple sclerosis. Exp Neurol 2011; 227(1): 232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Page JC, Shi R: Potassium channel blockers restore axonal conduction in CNS trauma and diseases. Neural Regen Res 2016; 11(8): 1226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tseng KC, Li H, Clark A, et al. : 4-Aminopyridine promotes functional recovery and remyelination in acute peripheral nerve injury. EMBO Mol Med 2016; 8(12): 1409-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Noble M, Tseng KC, Li H, Elfar JC: 4-Aminopyridine as a single agent diagnostic and treatment for severe nerve crush injury. J Mil Med 2019; 184(Suppl 1): 379-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clark AR, Hsu CG, Talukder MAH, Noble M, Elfar JC: Transdermal delivery of 4-aminopyridine accelerates motor functional recovery and improves nerve morphology following sciatic nerve crush injury in mice. Neural Regen Res 2020; 15(1): 136-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Diemen HA, Polman CH, Koetsier JC, Van Loenen AC, Nauta JJ, Bertelsmann FW: 4-Aminopyridine in patients with multiple sclerosis: dosage and serum level related to efficacy and safety. Clin Neuropharmacol 1993; 16(3): 195-204. [DOI] [PubMed] [Google Scholar]
- 20. Sindhurakar A, Mishra AM, Gupta D, Iaci JF, Parry TJ, Carmel JB: Clinically relevant levels of 4-aminopyridine strengthen physiological responses in intact motor circuits in rats, especially after pyramidal tract injury. Neurorehabil Neural Repair 2017; 31(4): 387-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oh SS, Hayes JM, Sims-Robinson C, Sullivan KA, Feldman EL: The effects of anesthesia on measures of nerve conduction velocity in male C57Bl6/J mice. Neurosci Lett 2010; 483(2): 127-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alvites R, Rita Caseiro A, Santos Pedrosa S, et al. : Peripheral nerve injury and axonotmesis: state of the art and recent advances. Cogent Med 2018; 5(1): 1466404. [Google Scholar]
- 23. Yue L, Talukder MAH, Gurjar A, et al. : 4-Aminopyridine attenuates muscle atrophy after sciatic nerve crush injury in mice. Muscle Nerve 2019; 60(2): 192-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gurjar AA, Estrada JA, Kaufman M, Talukder MA, Elfar JC: 4-Aminopyridine (4-AP): a single dose diagnostic agent to differentiate nerve crush and transection. Presentation at the 2019 Military Health System Research Symposium, Kissimmee, FL; MHSRS-19-01378. Available at https://mhsrs.amedd.army.mil/sites/mhsrs2019/Conference/SitePages/Accepted%20Abstracts.aspx?View={963AA4DE-EED5-4658-B4DB-03E4E851E4B1}&FilterField1=LinkTitle&FilterValue1=MHSRS%2D19%2D01378; accessed March 23, 2020. [Google Scholar]
- 25. Manto KM, Gurjar AA, Estrada JA, Kaufman M, Talukder MAH, Elfar JC: Local 4-aminopyridine thermogel: a promising point-of-care diagnostic tool to differentiate axonal continuity in peripheral nerve injuries. Accepted for 2020 Military Health System Research Symposium; MHSRS-20-00963. [Google Scholar]
- 26. Antkowiak B: How do general anaesthetics work? Naturwissenschaften 2001; 88(5): 201-13. [DOI] [PubMed] [Google Scholar]
- 27. Pocock G, Richards CD: Excitatory and inhibitory synaptic mechanisms in anaesthesia. Br J Anaesth 1993; 71(1): 134-47. [DOI] [PubMed] [Google Scholar]
- 28. Schlame M, Hemmings HC Jr.: Inhibition by volatile anesthetics of endogenous glutamate release from synaptosomes by a presynaptic mechanism. Anesthesiology 1995; 82(6): 1406-16. [DOI] [PubMed] [Google Scholar]
- 29. Westphalen RI, Hemmings HC Jr.: Volatile anesthetic effects on glutamate versus GABA release from isolated rat cortical nerve terminals: basal release. J Pharmacol Exp Ther 2006; 316(1): 208-15. [DOI] [PubMed] [Google Scholar]
- 30. Nowicki M, Baum P, Kosacka J, et al. : Effects of isoflurane anesthesia on F-waves in the sciatic nerve of the adult rat. Muscle Nerve 2014; 50(2): 257-61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


