Abstract
Mammalian reproductive health affects the entire reproductive cycle starting with the ovarian function through implantation and fetal growth. Various environmental and physiological factors contribute to disturbed reproductive health status leading to infertility problems in mammalian species. In the last couple of decades a significant number of studies have been conducted to investigate the transcriptome of reproductive tissues and organs in relation to the various reproductive health issues including endometritis, polycystic ovarian syndrome (PCOS), intrauterine growth restriction (IUGR), preeclampsia, and various age-associated reproductive disorders. Among others, the post-transcriptional regulation of genes by small noncoding miRNAs contributes to the observed transcriptome dysregulation associated with reproductive pathophysiological conditions. MicroRNAs as a class of non-coding RNAs are also known to be involved in various pathophysiological conditions either in cellular cytoplasm or they can be released to the extracellular fluid via membrane-bounded extracellular vesicles and proteins. The present review summarizes the cellular and extracellular miRNAs and their association with the etiology of major reproductive pathologies including PCOS, endometritis, IUGR and age-associated disorders in various mammalian species.
Keywords: miRNAs, endometritis, PCOS, ageing, fertility
1. Introduction
Micro RNAs (miRNAs) are short noncoding RNA molecules and comprise 1–5% of animal genes [1]. While a typical miRNA could regulate the expression of hundreds of genes, a single target gene can be regulated by multiple miRNA [2]. Altogether they regulate 30% of the active human genes [3]. Changes in the expression of even a single miRNA found to have a profound effect on the outcome of diverse cellular functions. Advancements in library preparation and deep sequencing technologies have enabled the identification of several thousands of miRNAs in various mammalian species, as deposited in the public database, namely miRbase (http://www.mirbase.org). The importance of miRNAs in female fertility has been evidenced by conditional knockout of various miRNA-processing machinery genes using mouse models [4,5]. Accordingly, miRNAs were found to regulate various reproductive processes including germ cells proliferation and differentiation, oocyte growth and maturation, preimplantation embryo development and fetal growth and development. Moreover, miRNA mediated posttranscriptional regulation of epigenetic modifications including DNA methylation, RNA modification, and histone modifications has been reported [6].
Infertility is becoming a global health issue affecting 10–15% of women worldwide. Among the factors contributing to this phenomenon, lifestyle factors and reproductive health issues contribute significantly to infertility problems in humans. Polycystic ovary syndrome (PCOS) is one of the multifactorial reproductive and metabolic health issues that affects up to 5–10% of women in reproductive age and accounts for 75% of anovulatory infertility [7,8,9,10]. PCOS is characterized by follicle growth arrest, reduced granulosa cell proliferation, elevated levels of luteinizing hormone, reduced levels of follicle-stimulating hormone (FSH), and hyperandrogenemia [7]. These endocrine and metabolic imbalances due to inappropriate regulation of the hypothalamus–pituitary–gonadal axis commonly lead to ovulatory disorders in mammals. Different animal models have been used to understand the etiology of PCOS with translation potential to humans [11]. Recent molecular analysis using ovarian cortex, granulosa, and theca cells and follicular fluid has identified complex genetic pathways contributing to the etiology of PCOS.
Bacterial infection in the uterus is known to cause clinical or subclinical endometritis in 15–20% of cows postpartum with a significant impact on fertility. In the post-partum period since cows have multiple confounding conditions, the direct link between reduced fertility and endometritis is practically difficult. However, several in vitro or in vivo controlled experiments have been conducted to investigate the uterine infection-mediated changes in molecular signals in the endometrium and another reproductive tract with a significant impact on ovarian function and embryo implantation [12]. Despite the evidence of involvement of several classes of microorganisms ranging from Gram-negative (E. coli) or Gram-positive (T. pyogenes) bacteria and several anaerobes in endometritis, there are still controversial results from various model studies involving various pathogens. However, several studies have evidenced the dysregulation of various molecular signals in mammalian endometrium and in circulation, which mediate the negative impact of endometritis on oocyte and embryo growth. Moreover, intrauterine growth restriction (IUGR) as pregnancy-associated disorder remains a major problem in various farm animals and humans resulting in impaired fetal growth. Even though several maternal and fetal factors contribute to the development of IUGR, impaired placental function and growth is the main contributing factor. In addition to the gene transcripts, several classes of noncoding regulatory miRNA have been associated with this abnormality with a significant impact on fetal development and offspring health.
It is a well-established fact that increasing age incurred a pathophysiological condition to a growing human oocyte, which is mostly associated with aneuploidy [13,14]. This has led research on reproductive aging to focus on the importance of chromosomal abnormalities in reducing the developmental potential of female gametes. The biological reactions underlying aging are believed to occur spontaneously to give rise to cellular injuries with certain universality [15]. The most widely recognized biological reaction leading the modification of molecules during ovarian and oocyte aging is caused by oxidative stress [16]. The possible increase in the accumulation of reactive oxygen species (ROS) with aging in the ovarian environment can be attributed to reduced enzymatic antioxidant defense mechanisms [17]. Several studies have been conducted to investigate the mechanisms behind the age-associated oxidative stress damage and potential of antioxidant supplementation to avert those damages.
The non-coding miRNAs, which are active either in the cell or those released into the extracellular environment coupled with extracellular vesicles or proteins, have been evidenced to play a significant role in reproductive health. In this review data on the identification, characterization, and functional analysis of cellular and extracellular miRNAs in mammalian reproductive health associated with PCOS, endometritis, IUGR, and age-related disorders are presented.
2. MiRNAs and Polycystic Ovary Syndrome (PCOS)
Polycystic ovary syndrome (PCOS) is found to affect the ovarian follicular growth, granulosa cell proliferation and endocrine homeostasis [7,18]. Symptoms of PCOS include oligomenorrhea or amenorrhea, obstetrical complications, obesity, excess body and facial hair (hirsutism), acne, pelvic pain, low fertility, pregnant failure, and patches of thick and darker skin [18,19]. It is associated not only with infertility but also with increased risk of metabolic and other disorders including but not limited to insulin resistance, diabetes, obesity, mood disorders, obstructive sleep apnea, endometrial cancer, and cardiovascular diseases [7]. Complex genetic factors either heritable or non-heritable, which are evident at the onset of puberty, epigenetic, or/and environmental factors such as unhealthy diet and a lack of physical activity are known to contribute to the pathogenesis of PCOS [20]. Recent molecular genetic analyses including sequencing, expression profiling, and genome-wide association studies revealed the involvement of numerous miRNAs in the etiology of PCOS [21,22,23].
2.1. Expression and Regulation of Cellular miRNA in PCOS Ovary
Different animal models have been used to investigate the role of miRNAs in PCOS. A rat PCOS model has been established through the induction of dihydrotestosterone (DHT) to examine the expression of PCOS-associated ovarian miRNA [22]. The expression of miRNAs has also been examined in another study using the ovary of letrozole-induced PCOS rat through deep sequencing [21]. Compared to control rats, 129 and 44 miRNAs were found to be up- and downregulated in the ovary of PCOS rats, respectively. Wang et al. used a mouse model to study the role of miRNAs in the ovary with PCOS and increased expression of miR-27a-3p in the ovaries of mice with PCOS was observed [24]. Later on, using in vitro culture of primary mouse granulosa cells (mGCs) and the mouse granulosa-like tumor cell line it was found that miR-27a-3p represses CYP19A1 via targeting cyclic AMP response element (CRE)-binding protein 1 (Creb1) in granulosa cells from the PCOS mouse model [24]. The studies on miRNAs in the PCOS of the animal model mentioned above are mostly analysis of expression of miRNAs and no in depth functional study has been conducted yet. Moreover the results on the expression of miRNAs in the animal model with PCOS are not well matched to the findings (discussed below) from the studies in humans.
Expression of miRNAs in ovarian cortexes with PCOS was measured in a study using qRT-PCR and upregulation of miR-93 was observed [25]. The target gene of miR-93, CDKN1A was found to be downregulated in the ovary. Variation in the results of miRNAs expression profile among different studies might be due to the use of different models and methods, age of the animal, or women or inclusion criteria for the studies but could also exemplify the heterogeneous nature of PCOS itself.
Altered expression of ovarian miRNAs due to PCOS may have consequences on processes determining the fate of granulosa cells (proliferation, differentiation, and apoptosis). Ovarian granulosa and theca cells are the vital somatic cells that support follicular development; by producing and maintaining steroid, and sharing paracrine factors to favor oocyte growth and development. Extensive studies of miRNAs in granulosa cells both from normal ovaries [26,27,28] and ovaries with PCOS have been performed compared to that in other ovarian cell types. Expression and regulation of miRNAs in ovarian granulosa cell proliferation and apoptosis in various mammalian species are summarized in Table 1. A study on the expression of selected miRNAs in the granulosa cells of PCOS ovary by Cirillo et al. has identified the upregulation of MiR-146a, miR-155, miR-486, and downregulation of miR-320 and miR-370 in granulosa cells from PCOS patients [29]. Significantly increased expression of miR-200b was observed in the granulosa cells of PCOS patients compared to the controls revealed inhibition of cell proliferation through targeting PTEN [30].
Table 1.
Expression and regulation of miRNAs in the granulosa cells with known target genes in relation to PCOS.
| miRNAs | Species | Target Gene/Pathway | Biological Role | Expression in PCOS | Reference |
|---|---|---|---|---|---|
| miR-9 | Human | IL8, SYT1, IRS2 | Hinders testosterone release. | Up | [31] |
| miR-18b | Human | IL8, SYT1, IRS2 | Promotes progesterone release, inhibitstestosterone and estradiol release | Up | [31] |
| miR-21 | Human, Mouse, Rat | LATS1 | Reduce apoptosis. Progression of PCOS | Up | [31,32,33,34] |
| miR-29a-5p | Human | Klotho-associated signaling | Involved in cell apoptosis | Down | [35] |
| miR-30c | Human | Induced by FSH exposure | Up | [34] | |
| miR-93 | Human | CDKN1A | Promotes cell proliferation | Up | [25] |
| miR-126-5p | Human | Klotho-associated signaling | Involved in cell apoptosis | Down | [35] |
| miR-129 | Human | HMGB1 | Proliferation, apoptosis of granulosa cells | Up | [36] |
| miR-132 | Human Mouse Rat |
HMGA2, Ctbp1 | Promotes estradiol secretion, reduces progesterone and testosterone release | Down | [31,37] |
| miR-135a | Human | IL8, SYT1, IRS2 | Reduces progesterone and testosterone release | Up | [31] |
| miR-145 | Human | IRS1 | Inhibits cell proliferation | Down | [38] |
| miR-155 | Human | PDCD4 | Prevents testosterone release, promotes cell proliferation and migration | Up | [34,39,40] |
| miR-222 | Human Rat |
Estrogen receptor 1 | Positively correlated with serum insulin; increases estradiol secretion |
Up | [22,41] |
| miR-224 | Human Mouse |
PTX3, Smad4 | Induces GCs proliferation, increases estrogen release | Differentially expressed | [34,42,43] |
| miR-320 | Human Mouse |
RAB5B, E2F1, SF-1 | Increased in insulin resistance, Slow down cell proliferation and estradiol production | Down in serum, Up in granulosa cells | [44,45,46] |
| miR-320a | Human | RUNX2 | Related to the steroidogenesis | Down | [47] |
| miR-383 | Human Mouse |
RBMS1 | Enhances the release of estradiol | Up | [46,48] |
| miR-483-5p | Human | Notch3, MAPK3, IGF1 | Related to cell proliferation and apoptosis | Down | [23,49,50] |
| miR-509-3p | Human | MAP3K8 | Induces oestradiol (E2) secretion | Up | [51] |
Differential miRNA expression profiles of cumulus granulosa cells between the ovary of women with and without PCOS by next-generation sequencing revealed the upregulation of 21 and downregulation of 38 miRNAs [23]. In addition, through pathway analysis, it has been shown that Notch signaling pathway and the molecular pathways involved in regulation of hormones, and energy metabolism are targeted by the differentially expressed miRNAs. Both Notch3 and MAPK3, as members of Notch signaling and ERK-MAPK pathway, were demonstrated to be regulated by miR-483-5p. Downregulation of miR-483–5p and miR-486–5p was observed in the cumulus granulosa cells surrounding metaphase II oocytes of women with PCOS and the former was shown to be involved in the proliferation of granulosa cells of PCOS through induction of the PI3K/Akt pathway [49]. Similar to the granulosa cells, expression and regulation of miRNAs has been shown to be important in theca cells for the biosynthesis of androgen in ovaries. However, due to the difficulty to collect the theca cells from the PCOS ovary, studies on miRNAs in theca cells are limited compared to the granulosa cells. In situ hybridization of miRNAs in the ovary of a rat PCOS model revealed differential localization of miRNAs in the ovary undergoing or became cystic. Notably, miR-96, miR-31, and miR-222 were exclusively localized in the theca of cystic follicles [22]. Predominant localization of differentially expressed miRNAs in theca cells of the cystic ovary indicates their possible involvement in the abnormal androgenesis and hence might be important for the pathophysiology of PCOS.
2.2. Extracellular miRNAs in the PCOS Ovary
Follicular fluid is the result of secretory and metabolic activity of the oocyte, granulosa, and theca cells and the transfer of blood plasma components. This specialized fluid provides an appropriate intrafollicular environment that plays a pivotal role in the growth and development of oocyte [52]. Changes of the content of follicular fluid have been used as predictive factors or biomarkers of oocytes and even embryo quality and PCOS development [53]. Follicular fluid is also used in determining the relationship between abnormal miRNA expression and PCOS development [45]. In addition, the presence of numerous miRNAs as extracellular nucleotide and changes of their expression in the follicular fluid from different species signifies the potential role of miRNAs in steroidogenesis and PCOS.
Quantification of miRNAs through TaqMan miRNA assays revealed a significantly lower expression of miR-132 in the follicular fluid of PCOS patients compared to healthy controls [45]. In another study, analysis of miRNAs of human follicular fluid revealed significant increased expression of five miRNAs (miR-32, miR-34c, miR-135a, miR-18b, and miR-9) in the PCOS group compared to the healthy control. Three potential target genes of these miRNAs were found to be significantly downregulated in the PCOS group and pathway analysis implicated their association with carbohydrate metabolism, beta-cell function, and steroid synthesis [42]. Significant downregulation of let-7b and miR-140 and upregulation of miR-30a was found in PCOS follicular fluid [53]. In the same study, the FOX-2 has been identified as a target of miR-30a and the disruption of miR-30a revealed excessive androgen production by theca cells and PCOS related morphological changes of granulosa cells. Further studies are needed to determine whether extracellular miRNAs play a role in the etiology of PCOS or if they are downstream effects of other PCOS risk factors [54].
The expression of miR-320 and miR-383 was upregulated in the follicular fluid of PCOS patients and the expression of E2F1 and SF-1 as a target gene of those miRNAs were found to be downregulated in granulosa cells [46]. These results suggest that miR-320 suppresses the granulosa cells proliferation and steroid production by targeting E2F1 and SF-1, which might be associated with hyperandrogenemia in PCOS. The miRNA profile in PCOS follicular fluid found to be varied among different studies due to differences in the methods employed, inclusion criteria used, type of PCOS diagnostic criteria, control groups set up, and heterogenic morphology and physiological conditions of PCOS [55].
Similar to the miRNAs in the follicular fluid, circulating serum miRNAs can also be promising and non-invasive biomarkers for different diseases. Changes in cellular homeostasis lead to the modulation of the molecular function of cells and deregulated miRNAs bound with protein component AGO2 or encapsulated with a microvesicle may be released from cells and frequently circulate in extracellular body fluids [56]. Extracellular vesicles released from most cell types into the extracellular space including serum can be taken up by the neighboring or distant cells to facilitate genetic exchange between cells [57,58]. Several studies have been conducted to identify miRNAs in serum from PCOS patients as a potential molecular signature. Investigation on the expression of circulating miRNAs in PCOS during clinical diagnosis revealed the upregulation of let-7i-3p, miR-5706, miR-4463, miR-3665, and miR-638 and downregulation of miR-124-3p, miR-128, miR-29a-3p, and let-7c in PCOS patients. Further analysis showed that the predicted target genes of these miRNAs are involved in various disorders, including diabetes and celiac diseases [59]. It has been demonstrated that the downregulation of miR-592 is associated with a high level of luteinizing hormone/chorionic gonadotropin receptor (LHCGR), an important factor for hyperandrogenemia in PCOS [60,61]. MiR-146a as a suppressor of steroid secretion was shown to be a negative regulator of serum testosterone in PCOS [44]. The same study identified that miR-222 is positively associated with serum insulin from PCOS patients. In addition, miR-222 combined with miR-146a and miR-30c suggested to be used to distinguish women with PCOS from the healthy ones [44]. Similar to the miR-146a, miR-23a in PCOS serum was reported to be negatively correlated with the testosterone level and the likelihood of PCOS [62].
3. Involvement of Cellular and Extracellular microRNAs in Endometritis
During early postpartum stages, the uterine environment of dairy cows is susceptible to various uterine disorders [63], which reduces the reproductive and productive performance. Based on clinical signs, the nature and composition of uterine discharges and uterine cytology, uterine infections can be categorized as puerperal metritis, clinical metritis, clinical endometritis, and subclinical endometritis [64,65,66]. Endometritis is one of the leading uterine infections reported to impair the fertility of cows [67]. Endometritis is a phenomenon of endometrium inflammation without the manifestation of clear clinical signs. Multiple bacterial species including E. coli, A. pyogenes, F. necrophorum, and B. melaninogenicus are associated with endometritis, either individually or synergistically [68,69,70]. In bovine endometritis, the E. coli is one of the major pathogens that affect the uterine environment by producing a lipopolysaccharide (LPS) [71]. The initial defense mechanism against the pathogen invasion is through the activation of the animal’s innate immune system [72]. Following the activation of the innate immune system, the endometrial cells secrete cytokines and chemokines, which recruit neutrophils and macrophages to act on the pathogens [73]. In reaction to the pathogenic invasion, the neutrophils serve as the first line of defense and the number of the polynuclear neutrophils (PMN) is reported to increase in the uterine lumen [74]. As opposed to the clinical endometritis, the subclinical endometritis is characterized by inflammation of the uterus without visible clinical signs and an elevated number of PMN at a threshold of 5% and above [75].
Several studies have reported transcriptional changes resulting from uterine inflammation. The expression of inflammatory cytokines like the tumor necrosis factor-α (TNF-α), Toll-like receptor (TLR) 4, interleukins (IL), insulin-like growth factor-1 (IGF-1), and IGF-binding protein-2 (IGF-BP-2) were reported to be differentially expressed in the uterine of cows affected with endometritis [76,77]. In addition to the candidate genes screening approach, global transcriptome analysis in uterine cells obtained from cows with clinical and subclinical endometritis has been reported recently [78]. In that study about 203 and 28 genes were differentially expressed in cows with clinical and subclinical endometritis, respectively. This implies that the severity of infection significantly enhances the number of transcripts altered in the endometrial cells. Interestingly, these genes are involved in pathways related to the immune process, G-protein coupled receptor signaling, and chemotaxis.
MicroRNAs are thought to be involved in various uterine disorders [78,79,80]. Nevertheless, there are few in vivo experiments with respect to the expression profile of miRNAs in clinical and subclinical endometritis. Endometrial cytobrush samples derived from day 30 and 35 postpartum lactating cows with subclinical endometritis revealed 23 miRNAs to be differentially expressed in subclinical endometritis cows compared to the healthy cows [81]. In the same study, using an in vitro assay on epithelial and stromal cells, it was reported that the expression miR-24, miR-98, miR-223, miR-27, miR-196b, miR-503, miR-21, and miR-16 were upregulated in both epithelial and stromal cells treated with LPS. Moreover, the expression of miR-619, miR-215, and miR-643 was downregulated in response to LPS treatment. In another study, the miRNA expression of endometrial cytobrush derived from cows with clinical and subclinical endometritis at 40–60 days postpartum showed massive deregulation of miRNAs in both clinical and subclinical animals compared to the healthy counterparts [78]. As opposed to the less pronounced transcriptional deregulation impacts, the numbers of differentially regulated miRNAs were more pronounced in the subclinical groups than the clinical endometritis. This could be justified due to the negative transcriptional and posttranscriptional regulatory correlation between the miRNAs and the gene transcripts. Interestingly, 7 members of the let-7 family (let-7a, let-7c, let-7d, let-7d *, let-7e, let-7f, and let-7i) were among the highly abundant miRNAs preferentially enriched in the endometrial cells of the subclinical groups. Some miRNAs including let-7e, miR-92b, miR-337-3p, let-7f, and miR-145 were equally affected in both levels of the infection severity [78]. In addition to the genome-wide expression profiling of miRNAs associated with endometrial infection, understanding the functional implication of selected miRNAs during and post-infection could shed light to our understanding in the molecular mechanisms associated with etiology of the disease and development of diagnostic tools.
Other important miRNAs involved in establishment and progression of endometritis is miR-148a [78], which is also implicated in inflammatory disease [82]. Bovine endometrial epithelial cells treated with LPS to induce an inflammatory response revealed a significant downregulation of both the miR-148a and the associated proinflammatory cytokines, IL-1ß and the TNF-α. Moreover, overexpression of miR-148a suppresses the activation of the NF-κBp56 pathway by targeted suppression of TLR-mediated pathway [83]. In the same study, it was shown that overexpression of miR-148a alleviated the uterine inflammation, making the miR-148a a potential therapeutic molecule for uterine inflammation and endometritis. MiR-223 is another miRNA reported to be abundantly enriched in cows with subclinical endometritis and in endometrial cells treated with LPS [81]. Consistent with this finding, using an in vitro cell culture model of bovine endometrial epithelial cells treated with LPS, the transcription of miR-223 was elevated, which was promoted through the activation of the NF-κB and inhibition of the NLRP3, which in turn mediated the production of the cytokine IL-1ß [84]. This signifies the role of miR-223 in attenuating inflammatory conditions in the uterine environment. MiR-19a, which is a member of the miR-19-72 cluster, regulates the expression of TBK1 by negatively regulating the NF-κB pathway in LPS-induced endometritis [85]. These findings suggest that the proinflammatory pathways are negatively regulated by miRNAs expressed in endometrial epithelial cells.
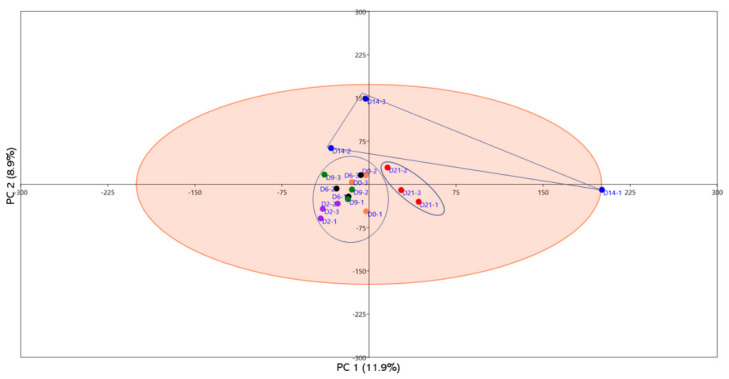
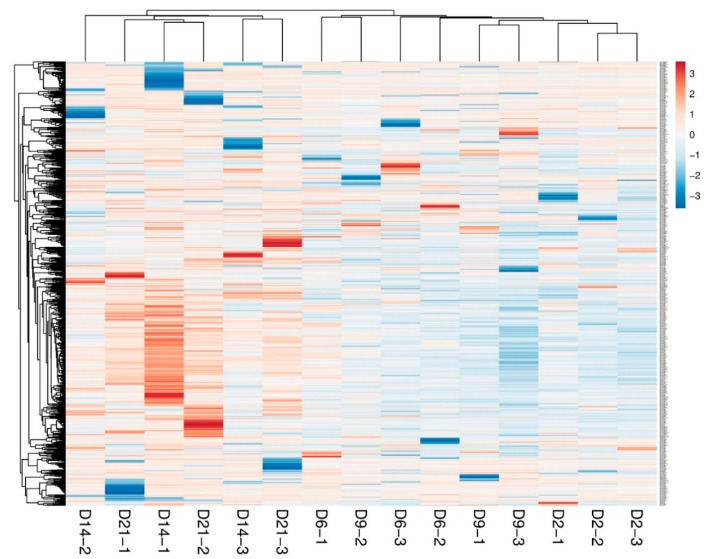
The biggest challenge in the diagnosis and treatment of uterine infections is the absence of non-invasive, reliable, and early diagnostic tools that can be used for early detection of the disease. Thus, the use of circulating miRNAs in animals could be an alternative approach. To address this, a pilot experiment on the clinical endometritis cow model was performed to determine the unique circulatory miRNAs associated with uterine infection. Briefly, lactating dairy cows were subjected to vaginal infusion of E. coli and T. pyogenes. The induction of uterine infection was verified according to previously described criteria [86] and the blood samples were collected and processed at days 2, 6, 9, 14, and 21 post-infection. The genome-wide miRNAs expression profile was assessed and compared with the preinfection status of the cows. Interestingly, no measurable differences were observed in the circulatory miRNA profile of cows until day 14 post-infection. However, starting day 14, the miRNA expression was more divergent, showing the sustained and severe endometrial infection (Figure 1). This was further verified by the hierarchical clustering analysis, where the number of upregulated miRNAs tends to be progressively increased starting day 14 towards day 21 post-infection (Figure 2). Taken together, the progression of endometrial infection can be diagnosed by measuring circulatory miRNAs as early as two weeks post infection. In a separate experiment, the serum circulatory miRNA profile in cows with underlying metritis and with no apparent uterine abnormality derived 1 week postpartum was performed. It was reported that bta-miR-15b, bta-miR-17-3p, bta-miR-16b, bta-miR-148a, bta-miR-26b, bta-miR-101, and bta-miR-29b were upregulated whereas bta-miR-148b, bta-miR-199a-3p, bta-miR-122, bta-miR-200b, and bta-miR-10a were downregulated in the serum of cows with metritis compared to healthy cows [87].
Figure 1.
Principal component analysis (PCA) of circulatory miRNAs of blood plasma samples derived from cows subjected to bacterial infection. Samples were collected at day 0 (orange dotes) pre-infection and days 2 (purple dotes), 6 (black dotes), 9 (green dotes), 14 (dark blue dotes), and 21 (red dotes) post-infection. It is shown that the groups from days 14 (dark blue dotes) and 21 (red dotes) showed clear separation from the earlier post-infection periods. Groups are indicated by different color in biological triplicates.
Figure 2.
Heatmap illustrating the hierarchical clustering of samples according to the expression pattern of the plasma circulatory miRNAs profile. Groups are indicated in biological triplicates.
Extracellular vesicles (EVs) are involved in immune activities affecting both the innate and adaptive immune responses [88], which are also mediated by miRNAs encapsulated in the EVs [89,90]. Recently, the correlation of between exosome-coupled miRNAs derived from serum of patients with endometritis and healthy counterparts revealed 24 miRNAs differentially expressed, of which the miR-320a and miR-22-3p, were significantly upregulated in exosomes of patients with endometritis and can be potential biomarkers of endometritis [91]. EV-derived miRNAs from the uterine fluid of cows had a negative impact on the blastocyst development when added to the embryo culture media [92]. Contrary to this, supplementation of embryos with EVs derived from conditioned embryo culture media and uterine fluid of healthy cows improved the cleavage rate and blastocyst formation [93,94]. It was also shown that miR-218 was one of the differentially expressed miRNAs and involved in the pathogenesis of bovine endometritis and bovine endometrial cells are reported to use the EVs to encapsulate and release the miR-218 into the uterine environment, which acts as an inhibitor of immune factors like the IL-6, IL-1b, and TNFα and inflammatory genes [95]. This signifies the fact that EV-coupled miRNAs could be one of the mechanisms for delivering immune regulating molecules in the uterus.
In conclusion, the current advancements in the EVs research and high-throughput sequencing technologies have provided the promising tools for the discovery and screening of clinical and subclinical endometritis with better precision and as early as possible, which could improve the fertility and welfare of animals. Moreover, the possibility of enrichment, loading, and delivering a bioactive molecule of our interest including miRNAs and proteins into cells could be a possible approach to understand the mechanistic molecular roles of selected miRNAs or proteins in endometrial health.
4. MicroRNAs in Intrauterine Growth Restriction
Intrauterine growth restriction (IUGR) is a pregnancy-related disorder in which a fetus cannot reach its maximum growth potential and the weight of the fetus is less than the 10th percentile of an appropriate weight for gestational age and fetal sex. Although different maternal and fetal factors can contribute to the development of IUGR, impaired placental development and function is the most common cause [96]. In recent years, miRNAs have emerged as major players in the pathogenesis of IUGR. miRNAs can be easily measured in tissue biopsies, blood, and other biological samples, making them potential biomarkers for early detection of various pathologies including IUGR [97,98,99]. Trophoblast proliferation and invasion is critical for placental health, therefore most of the miRNAs linked to trophoblast proliferation are also involved in the pathogenesis of IUGR [100].
Some unique species of miRNAs are exclusively expressed in human trophoblast cells and are called “trophomiRs” [101]. The majority of trophomiRs are expressed from chromosome 19 miRNA cluster (C19MC) [102]. C19MC locus generates 59 mature miRNAs, most of which are primarily expressed in the placenta [103,104]. Hromadnikova et al. demonstrated a correlation between C19MC miRNAs and pathogenesis of pregnancy complications by measuring the expression of 15 C19MC miRNAs in placental tissue from pregnancies complicated by preeclampsia (PE), IUGR, and gestational hypertension (GH) [105]. They reported that downregulation of six C19MC miRNAs (miR-517-5p, miR-518f-5p, miR-519a, miR-519d, miR-520a-5p, and miR-525) is associated with IUGR. Additionally, reduced expression of miR-517-5p, miR-519d, miR-520a-5p, and miR-525 is a common finding in PE, IUGR, and GH, whereas downregulation of miR-518f-5p and miR-519a is a common finding in preeclampsia and IUGR [105]. In contrast, according to Hromadnikova et al. increased circulating levels of C19MC miRNAs (miR-517-5p, miR-518b, and miR-520h) during the first trimester indicate increased risk of PE, but no circulating C19MC miRNAs can be used as biomarkers to predict IUGR [106]. Chromosome 14 miRNA cluster (C14MC) produces 63 mature miRNAs in humans, which are highly expressed in the placenta [107]. C14MC miRNAs play a critical role in placental development by regulating the expression of different genes involved in trophoblast proliferation, invasion, and migration [108,109]. From the first trimester to the third trimester of pregnancy in humans, the concentration of C19MC miRNAs increases whereas the concentration of C14MC miRNAs decreases in the placenta and maternal circulation [110,111,112]. Wommack et al. measured different miRNA clusters in maternal plasma and showed that increased expression of several C14MC miRNAs (miR-3373p, miR-4315p, miR-1363p, miR-1365p, miR-3803p, miR-323a3p, and miR-543) can be used as a marker for birth weight and gestation length [112].
Circulating placenta-specific miRNAs including miR-520a, miR-520h, miR-525, miR-526a, miR-516-5p, miR-517, and miR-518b in maternal plasma from IUGR pregnancies are significantly increased at week 12-16 of gestation, which later comes back to normal levels [110]. The temporary increase in these miRNAs can cause trophoblast apoptosis resulting in placental insufficiency and IUGR. Increased maternal plasma concentration of miR-206 can be used to predict IUGR [113]. miR-206 targets vascular endothelial growth factor (VEGF), which is critical for placental development. In a study conducted using 820 pregnant women (74 IUGR and 746 non-IUGR pregnancies), Li and Liu measured miR-206 and VEGF concentrations in maternal plasma at different stages of pregnancies. miR-206 was significantly increased whereas VEGF was significantly reduced in maternal blood at early, middle, and late gestation [113]. These findings suggest that miR-206 can lead to IUGR by targeting VEGF and its higher levels in maternal blood can be used to predict IUGR at the early stages of pregnancy.
miR-141 targets proliferation-associated genes and is downregulated in different types of cancers [114]. Tang et al. showed that miR-141 is associated with IUGR and its expression in placental tissue from IUGR pregnancies is higher compared to control pregnancies [115]. Moreover, expression of miR-141 target genes including E2F transcription factor 3 (E2F3) and pleiomorphic adenoma gene 1 (PLAG1) is significantly reduced in IUGR placentas [115]. E2F3 and PLAG1 are involved in cell proliferation and reduced expression of these genes can cause impaired trophoblast proliferation and contribute to the pathogenesis of IUGR [115]. According to Mouillet et al. the concentration of trophoblastic-hypoxia induced miRNAs (miR-27a-1, miR-30d, miR-93, miR-141, miR-200c, miR-205, miR-224, miR-335, mir-424, miR-451, and miR-491) is increased in maternal circulation with IUGR pregnancies compared to normal pregnancies [116]. Huang et al. also reported the role of miR-424 in the pathogenesis of IUGR by demonstrating that miR-424 is significantly increased and its target genes, mitogen-activated protein kinase 1 (MEK1) and fibroblast growth factor receptor 1 (FGFR1), are significantly reduced in placentas from IUGR pregnancies [117].
Using small RNA next-generation sequencing in fetal and maternal placental tissues, Rutkowska et al. demonstrated that 48 fetal and 22 maternal miRNAs are differentially expressed in IUGR pregnancies compared to normal pregnancies [118]. The change in miRNAs with a predicted role in fetal growth and development (miR-29a, miR-92b, miR-125b, miR-7641, miR-4321, miR-let-7g, and miR-2895) was also confirmed by RT-PCR [118]. The maximum change was witnessed in miR-4321, which was 4-fold higher in fetal placenta from IUGR pregnancies compared to control pregnancies [118]. An important miR-4321 target gene involved in placental and fetal growth is prostaglandin E receptor 3 (PTGER3), which has high expression in human trophoblast cells and plays a role in trophoblast invasion [118,119]. However, the concentration of miR-4321 in maternal circulation has never been studied, making it hard to use it as a potential biomarker for noninvasive diagnosis of IUGR.
The let-7 family of miRNAs (let-7a, let-7b, let-7c, let-7d, let-7e, let-7f, let-7g, let-7i, and miR-98), also referred to as differentiation-inducing miRNAs, play a profound role in trophoblast proliferation [120]. The expression of let-7 miRNAs is suppressed by oncoprotein LIN28, which has two paralogs LIN28A and LINB [121,122]. In 2019, Ali et al. demonstrated that the term human placentas from IUGR pregnancies have reduced expression of LIN28A and LIN28B and higher expression of let-7 miRNAs (let-7a, let-7b, let-7c, let-7d, let-7e, let-7f, let-7g, and let-7i), indicating a correlation between IUGR and let-7 miRNAs [120]. In trophoblast cells, let-7 miRNAs target several genes involved in cell proliferation, cell invasion, and angiogenesis including AT-hook 1 (HMGA1), MYC protooncogene (c-MYC), vascular endothelial growth factor A (VEGF-A), Wnt family member 1 (WNT1), AT-rich interaction domain (ARID)3A, and ARID3B [100,120,123]. CRISPR-Cas9 based LIN28 knockout in human trophoblast cells leads to increased expression of let-7 miRNAs and reduces cell proliferation [120]. Trophectoderm-specific knockdown of LIN28A or LIN28B in sheep increases let-7 miRNAs and reduces trophoblast cell proliferation and conceptus elongation in vivo [123]. Reduced conceptus elongation in domestic ruminants has been linked to IUGR and early pregnancy loss. Collectively, these findings indicate that a higher level of let-7 miRNAs in trophoblast cells can contribute to the pathogenesis of IUGR primarily by causing impaired placental development.
Awamleh et al. measured miRNAs and gene expression in human chorionic villi from pregnancies complicated by PE, IUGR, or PE+IUGR using next-generation sequencing. They showed that 11 miRNAs were upregulated in PE placenta samples, 25 miRNAs were upregulated, 12 miRNAs were downregulated in IUGR placentas, and 9 miRNAs were upregulated PE + IUGR placentas [124]. Similarly, 275 genes were differentially expressed in PE placentas, 155 genes were differentially expressed in IUGR placentas, and 556 genes were differentially expressed in PE + IUGR placentas. Six differentially expressed miRNAs (miR-193b-5p, miR-193b-3p, miR-210-3p, miR-365a-3p, miR-365b-3p, and miR-520a-3p) were common in all groups [124]. Hromadnikova et al. examined the expression of 32 miRNAs in placentas from pregnancies complicated with PR and IUGR that needed to be terminated before week 34 of gestation. They showed that 11 miRNAs (miR-16-5p, miR-100-5p, miR-122-5p, miR-125b-5p, miR-126-3p, miR-143-3p, miR-195-5p, miR-199a-5p, miR-221-3p, miR-342-3p, and miR-574-3p) were downregulated in pregnancies complicated by IUGR without PE [125]. Together these findings provide a set of miRNAs that are dysregulated in pregnancies complicated by just IUGR and can be used as potential biomarkers for the diagnosis of IUGR.
5. Potential of miRNAs in Aging and Related Disorders
The reproductive organ of the mammalian female exhibits a rate of aging that is much faster than any other organs in the body system. It has been speculated that the premature aging of the ovary, when compared with the somatic organs, might result from increased demand of energy for the maintenance and repair processes in the soma compartment during aging [126]. Based on the human biologic clock, the loss in female fertility becomes dramatic after the age of 35 years and results in menopause at 50–51 years of age [127]. The ovarian functional decline due to aging is related to the gradual loss of resting follicles and decreased biological competence of those surviving age-related atresia [128]. Throughout the life of female, follicles leave the resting pool of primordial stage to the growing pool on a regular basis and pass through various stages under the influence of stage-specific intraovarian regulators and endocrine factors [129,130]. This oocyte and follicle pool decline with increasing age, with a marked increase in the rate of disappearance from the age of 37 to 38 onwards. At the stage of menopause the follicle reserve decline to a number insufficient to sustain the cyclic hormonal process necessary for menstruation [131]. Moreover, in addition to the reduction in the follicular reserve, the phenomenon of ooplasm aging has been reported to be associated with various structural and morphological abnormalities including chromosome decondensation, chromosomal misalignment associated with anomalies in meiotic spindle, and highly compromised cellular machinery [132,133]. The proportion of poor quality oocytes increases with age, ranging from 50% at 20 years of age to 95% at 35 years of age [134]. All in all, decreased female fertility with advanced maternal age is well documented and it is widely recognized that the decline in oocyte quality is a key factor to explain age-associated infertility problems and related high risk of birth defects, genetic disorders and miscarriage [135,136]. Gene expression studies in oocytes showed that the presence and activity of gene products involved in cell cycle regulation, spindle formation, and organelle integrity may be altered in oocytes from older individuals from several species. Transcriptome analysis of human MII oocytes in relation to aging and ovarian reserve demonstrated that there is differential expression of coding and noncoding transcripts between young and old women [137]. Moreover, analysis of global gene expression profiles of MII oocytes from young (<35 years) and older (>37 years) women identified differential expression of genes associated with cell cycle regulation, cytoskeletal and chromosomal structure, energy pathways, transcription control, and stress response [138,139]. The differential expression of protein coding mRNA was found to be accompanied by the expression of noncoding regulatory RNA, including lncRNA, piRNA, and precursor miRNAs in oocytes between young and old women [137]. Similarly, transcriptome analysis of GV stage oocytes from young and aged mice showed differential expression of 160 endogenous small-interfering RNAs and 10 miRNAs [140].
Appropriate storage and utilization of maternal transcripts in oocytes are needed for maturation and early embryo development. A study by [141] showed that human MII stage oocytes obtained from women of advanced reproductive age showed altered expression of miRNAs regulating gene expression, pluripotency, chromatin remodeling, and early embryo development. In the same study evolutionary conserved miRNA (miR-29a-3p, miR-203a-3p, and miR-494-3p), which were found to be upregulated in aged mouse oocytes, to be correlated with downregulation of DNMT3a, DNMT3b, phosphatase and tensin homolog (PTEN), and mitochondrial transcription factor A (TFAM).
Cellular senescence is the biological consequence of aging, implicated in a variety of age-associated diseases. The increased vulnerability of oocytes to age-induced oxidative stress is associated with the attenuation of the efficacy of DNA double-strand break (DSB) repair mechanisms in aged oocytes [142]. Some studies have been conducted to correlate follicular fluid extracellular miRNA with advanced women age. MicroRNA expression profile of follicular fluid from younger (<31 years) and older (>38 years) women revealed a set of miRNAs involved in heparan-sulfate biosynthesis, extracellular matrix–receptor interaction, carbohydrate digestion, and absorption, p53 signaling, and cytokine–cytokine–receptor interaction [143]. With increased sample size and reduced age gap, the study by [144] revealed a differential expression of a single miRNA (hsa-mir-424). Differential plasma expression of miRNAs have been identified in cattle in an age- and genetic-dependent manner [145]. Using the PCR array platform, 306 plasma miRNA were assessed between calf and mature cows, and 26 miRNAs including miR-192, miR205, and miR-215, were enriched in mature animals.
Cellular senescence, which imposes permanent proliferative arrest in cells in response to stressors, is considered as a hallmark of aging and a major risk factor for the development of most common age-related diseases (ARDs) and it is an attractive target for therapeutic applications [146]. Senescence cells are characterized by a significantly reduced replicative potential and by the acquisition of a proinflammatory senescence-associated secretory phenotype (SASP) [147]. Considerable efforts have been devoted to distinguishing the effects of several epigenetic mechanisms namely: DNA methylation, and long and small non-coding RNA on the transcriptional and posttranscriptional programming leading to cellular senescence. Senescence modulation by microRNAs is a major senescence-associated epigenetic mechanism. This has been suggested by cellular miRNA signatures and by the release of extracellular vesicles, which contain different species and a number of miRNAs and proteins. The EVs population and type seem to reflect the molecular characteristics of their cells of origin and modulate the phenotype of recipient cells [148]. A recent study conducted to unravel the relative contribution of EVs released from senescence cells in spreading prosenescence signals to proliferating cells via their miRNA cargo [149]. In the same study it has been shown that senescence human umbilical endothelial cells release a greater number of EVs compared to their control counterparts. Among the 22 miRNAs differentially expressed, miR-21-5p and miR-217 were found to be enriched both in cells and EVs of senescence cells and are found to target DNMT1 and SIRT1 genes. Coculture of EVs from senescence with control cells resulted in induction of the senescence phenotype as evidenced by modulation of DNMT1 and SIRT1 genes, apoptosis, and cellular proliferation.
Considering mares as the best model to study reproductive aging in humans, a study on the identification of extracellular vesicles coupled miRNAs in relation to aging would provide knowledge on the mechanisms behind age-related fertility problems [150]. To investigate the effect of mare age on exosomal miRNA expression during follicular development, follicular fluid exosomes isolated from the normal follicle at deviation, mid-oestrus, and preovulatory stage of young (3–12 years) and old (20–26 years) were subjected to miRNA analysis [151]. In that study, exosomal miRNA expression differences were observed both across the developmental stages and between age groups. The abundance of miR-513a-3p, miR-181A, and miR-375 was higher in exosomes derived from follicular fluid of old compared to young mares. Among these, miR-181A was found to negatively regulate mouse granulosa cell proliferation by targeting ACVR2A [152].
Postovulatory oocyte aging in vitro or in vivo demonstrated that those aged oocytes frequently showed lower fertilization rate, polyspermy, chromosomal abnormalities, and abnormal embryo development [153]. These abnormalities in early embryo development result in decreased litter size, lower pregnancy rates, and an increase in the number of spontaneous miscarriages in humans [154]. In vitro and in vivo postovulatory aging oocytes are known to exhibit various cellular and molecular changes associated with intracellular signaling. These include morphological and organelle changes, reduction in the intracytoplasmic level of antioxidant GSH, elevated reactive oxygen species (ROS), reduction in the intracytoplasmic level of adenosine triphosphate (ATP), decrease in the expression of antiapoptotic factor Bcl-2, increased apoptosis, and abnormal Ca2+ regulation (reviewed by [155]). As a consequence of the progressive increase in ROS accumulation and the concomitant decline in antioxidant protection, the postovulatory aged oocytes experienced the state of oxidative stress. It has been long thought that the oxidative stress may act as a trigger for a cascade of factors that orchestrate postovulatory aging. Several in vitro studies have demonstrated that supplementation of antioxidants attenuates the process of postovulatory aging, however, there is a great variation between various antioxidants applied. This may be associated with the specificity of various antioxidants in scavenging the different types of reactive oxygen species. Considering the fact that cells exposed to various environmental stressors are known to release vesicles enriched with antioxidants [148], there is a great potential of supplementing such vesicles during in vitro oocyte maturation, which could lead to prevention of postovulatory oocyte aging phenotypes.
6. Conclusions
Reproductive disorders are a major cause of fetal and maternal morbidity and mortality in humans and cause huge economic losses to the cattle and sheep industry. Early diagnosis of these disorders can help in better management and treatment. Although several environmental and physiological factors can contribute to the pathophysiology of reproductive disorders, microRNAs have emerged as major players in the reproductive health of animals. One miRNA can target hundreds of different genes and hence several molecular pathways involved in reproductive health and efficiency of mammals are also regulated by miRNAs. In this review we summarized the miRNAs, which are differentially expressed in various reproductive disorders, suggesting the role of these miRNAs in pathogenesis of different reproductive disorders. Although the exact cause of miRNA dysregulation is unclear, epigenetic modifications, random genetic mutations, adverse uterine environment, oxidative stress, and malnutrition are some of the possible factors, which can cause dysregulation of miRNAs. MiRNAs are secreted by different cells and tissues in the extracellular environment either directly or via vesicles. MiRNAs can be detected and readily measured in different biological samples including peripheral blood, tissue biopsies, saliva, cerebrospinal fluid, and urine. Due to this reason, dysregulation of specific miRNAs can be used as a biomarker for early diagnosis of different reproductive disorders. As described in this review, dysregulation of more than one miRNA have been linked to various reproductive disorders. Therefore, using miRNAs as a biomarker for early diagnosis of different health conditions can be challenging and further studies are needed to identify the miRNAs that can be used as reliable biomarkers for each health condition.
Author Contributions
Conceptualization, S.G., D.T., R.V.A.; literature search, S.G., A.A., M.H. (Munir Hossain) writing—original draft preparation, S.G., A.A., M.H. (Munir Hossain), D.S.-W.; writing—review and editing, D.T., M.H. (Michael Hoelker), R.V.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
There is no conflict of interest in this article.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bartel D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Sood P., Krek A., Zavolan M., Macino G., Rajewsky N. Cell-type-specific signatures of microRNAs on target mRNA expression. Proc. Natl. Acad. Sci. USA. 2006;103:2746–2751. doi: 10.1073/pnas.0511045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim L.P., Lau N.C., Garrett-Engele P., Grimson A., Schelter J.M., Castle J., Bartel D.P., Linsley P.S., Johnson J.M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 4.Reza A., Choi Y.J., Han S.G., Song H., Park C., Hong K., Kim J.H. Roles of microRNAs in mammalian reproduction: From the commitment of germ cells to peri-implantation embryos. Biol. Rev. Camb. Philos. Soc. 2019;94:415–438. doi: 10.1111/brv.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tesfaye D., Gebremedhn S., Salilew-Wondim D., Hailay T., Hoelker M., Grosse-Brinkhaus C., Schellander K. MicroRNAs: Tiny molecules with a significant role in mammalian follicular and oocyte development. Reproduction. 2018;155:R121–R135. doi: 10.1530/REP-17-0428. [DOI] [PubMed] [Google Scholar]
- 6.Yao Q., Chen Y., Zhou X. The roles of microRNAs in epigenetic regulation. Curr. Opin. Chem. Biol. 2019;51:11–17. doi: 10.1016/j.cbpa.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Dunaif A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr. Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 8.Franks S. Polycystic ovary syndrome. N. Engl. J. Med. 1995;333:853–861. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 9.Moran L.J., Hutchison S.K., Norman R.J., Teede H.J. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2011 doi: 10.1002/14651858.CD007506.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Sirmans S.M., Parish R.C., Blake S., Wang X. Epidemiology and comorbidities of polycystic ovary syndrome in an indigent population. J. Investig. Med. 2014;62:868–874. doi: 10.1097/01.JIM.0000446834.90599.5d. [DOI] [PubMed] [Google Scholar]
- 11.Abedal-Majed M.A., Cupp A.S. Livestock animals to study infertility in women. Anim. Front. Rev. Mag. Anim. Agric. 2019;9:28–33. doi: 10.1093/af/vfz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piersanti R.L., Zimpel R., Molinari P.C.C., Dickson M.J., Ma Z., Jeong K.C., Santos J.E.P., Sheldon I.M., Bromfield J.J. A model of clinical endometritis in Holstein heifers using pathogenic Escherichia coli and Trueperella pyogenes. J. Dairy Sci. 2019;102:2686–2697. doi: 10.3168/jds.2018-15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuliev A., Cieslak J., Verlinsky Y. Frequency and distribution of chromosome abnormalities in human oocytes. Cytogenet. Genom. Res. 2005;111:193–198. doi: 10.1159/000086889. [DOI] [PubMed] [Google Scholar]
- 14.Pellestor F., Anahory T., Hamamah S. Effect of maternal age on the frequency of cytogenetic abnormalities in human oocytes. Cytogenet. Genom. Res. 2005;111:206–212. doi: 10.1159/000086891. [DOI] [PubMed] [Google Scholar]
- 15.Baynes J.W. The role of AGEs in aging: Causation or correlation. Exp. Gerontol. 2001;36:1527–1537. doi: 10.1016/S0531-5565(01)00138-3. [DOI] [PubMed] [Google Scholar]
- 16.Sohal R.S. Role of oxidative stress and protein oxidation in the aging process. Free Rad. Biol. Med. 2002;33:37–44. doi: 10.1016/S0891-5849(02)00856-0. [DOI] [PubMed] [Google Scholar]
- 17.Linton S., Davies M.J., Dean R.T. Protein oxidation and ageing. Exp. Gerontol. 2001;36:1503–1518. doi: 10.1016/S0531-5565(01)00136-X. [DOI] [PubMed] [Google Scholar]
- 18.Haqq L., McFarlane J., Dieberg G., Smart N. Effect of lifestyle intervention on the reproductive endocrine profile in women with polycystic ovarian syndrome: A systematic review and meta-analysis. Endocr. Connect. 2014;3:36–46. doi: 10.1530/EC-14-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ecklund L.C., Usadi R.S. Endocrine and reproductive effects of polycystic ovarian syndrome. Obstet. Gynecol. Clin. N. Am. 2015;42:55–65. doi: 10.1016/j.ogc.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Goodarzi M.O., Dumesic D.A., Chazenbalk G., Azziz R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat. Rev. Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 21.Fu L.L., Xu Y., Li D.D., Dai X.W., Xu X., Zhang J.S., Ming H., Zhang X.Y., Zhang G.Q., Ma Y.L., et al. Expression profiles of mRNA and long noncoding RNA in the ovaries of letrozole-induced polycystic ovary syndrome rat model through deep sequencing. Gene. 2018;657:19–29. doi: 10.1016/j.gene.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Hossain M.M., Cao M., Wang Q., Kim J.Y., Schellander K., Tesfaye D., Tsang B.K. Altered expression of miRNAs in a dihydrotestosterone-induced rat PCOS model. J. Ovarian Res. 2013;6:36. doi: 10.1186/1757-2215-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu B., Zhang Y.W., Tong X.H., Liu Y.S. Characterization of microRNA profile in human cumulus granulosa cells: Identification of microRNAs that regulate Notch signaling and are associated with PCOS. Mol. Cell. Endocrinol. 2015;404:26–36. doi: 10.1016/j.mce.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 24.Wang M., Liu M., Sun J., Jia L., Ma S., Gao J., Xu Y., Zhang H., Tsang S.Y., Li X. MicroRNA-27a-3p affects estradiol and androgen imbalance by targeting Creb1 in the granulosa cells in mouse polycytic ovary syndrome model. Reprod. Biol. 2017;17:295–304. doi: 10.1016/j.repbio.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Jiang L., Huang J., Li L., Chen Y., Chen X., Zhao X., Yang D. MicroRNA-93 promotes ovarian granulosa cells proliferation through targeting CDKN1A in polycystic ovarian syndrome. J. Clin. Endocrinol. Metab. 2015;100:E729–E738. doi: 10.1210/jc.2014-3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abd El Naby W.S., Hagos T.H., Hossain M.M., Salilew-Wondim D., Gad A.Y., Rings F., Cinar M.U., Tholen E., Looft C., Schellander K., et al. Expression analysis of regulatory microRNAs in bovine cumulus oocyte complex and preimplantation embryos. Zygote. 2013;21:31–51. doi: 10.1017/S0967199411000566. [DOI] [PubMed] [Google Scholar]
- 27.Gebremedhn S., Salilew-Wondim D., Ahmad I., Sahadevan S., Hossain M.M., Hoelker M., Rings F., Neuhoff C., Tholen E., Looft C., et al. MicroRNA Expression Profile in Bovine Granulosa Cells of Preovulatory Dominant and Subordinate Follicles during the Late Follicular Phase of the Estrous Cycle. PLoS ONE. 2015;10:e0125912. doi: 10.1371/journal.pone.0125912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salilew-Wondim D., Ahmad I., Gebremedhn S., Sahadevan S., Hossain M.D., Rings F., Hoelker M., Tholen E., Neuhoff C., Looft C., et al. The expression pattern of microRNAs in granulosa cells of subordinate and dominant follicles during the early luteal phase of the bovine estrous cycle. PLoS ONE. 2014;9:e106795. doi: 10.1371/journal.pone.0106795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cirillo F., Catellani C., Lazzeroni P., Sartori C., Nicoli A., Amarri S., La Sala G.B., Street M.E. MiRNAs Regulating Insulin Sensitivity Are Dysregulated in Polycystic Ovary Syndrome (PCOS) Ovaries and Are Associated With Markers of Inflammation and Insulin Sensitivity. Front. Endocrinol. (Lausanne) 2019;10:879. doi: 10.3389/fendo.2019.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He T., Sun Y., Zhang Y., Zhao S., Zheng Y., Hao G., Shi Y. MicroRNA-200b and microRNA-200c are up-regulated in PCOS granulosa cell and inhibit KGN cell proliferation via targeting PTEN. Reprod. Biol. Endocrinol. 2019;17:68. doi: 10.1186/s12958-019-0505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sirotkin A.V., Laukova M., Ovcharenko D., Brenaut P., Mlyncek M. Identification of microRNAs controlling human ovarian cell proliferation and apoptosis. J. Cell. Physiol. 2010;223:49–56. doi: 10.1002/jcp.21999. [DOI] [PubMed] [Google Scholar]
- 32.Carletti M.Z., Fiedler S.D., Christenson L.K. MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells. Biol. Reprod. 2010;83:286–295. doi: 10.1095/biolreprod.109.081448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang L., Li W., Wu M., Cao S. Ciculating miRNA-21 as a Biomarker Predicts Polycystic Ovary Syndrome (PCOS) in Patients. Clin. Lab. 2015;61:1009–1015. doi: 10.7754/Clin.Lab.2015.150122. [DOI] [PubMed] [Google Scholar]
- 34.Yao N., Yang B.Q., Liu Y., Tan X.Y., Lu C.L., Yuan X.H., Ma X. Follicle-stimulating hormone regulation of microRNA expression on progesterone production in cultured rat granulosa cells. Endocrine. 2010;38:158–166. doi: 10.1007/s12020-010-9345-1. [DOI] [PubMed] [Google Scholar]
- 35.Mao Z., Fan L., Yu Q., Luo S., Wu X., Tang J., Kang G., Tang L. Abnormality of Klotho Signaling Is Involved in Polycystic Ovary Syndrome. Reprod. Sci. 2017;25:372–383. doi: 10.1177/1933719117715129. [DOI] [PubMed] [Google Scholar]
- 36.Zhu H.L., Chen Y.Q., Zhang Z.F. Downregulation of lncRNA ZFAS1 and upregulation of microRNA-129 repress endocrine disturbance, increase proliferation and inhibit apoptosis of ovarian granulosa cells in polycystic ovarian syndrome by downregulating HMGB1. Genomics. 2020;112:3597–3608. doi: 10.1016/j.ygeno.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Fiedler S.D., Carletti M.Z., Hong X., Christenson L.K. Hormonal regulation of MicroRNA expression in periovulatory mouse mural granulosa cells. Biol. Reprod. 2008;79:1030–1037. doi: 10.1095/biolreprod.108.069690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai G., Ma X., Chen B., Huang Y., Liu S., Yang H., Zou W. MicroRNA-145 Negatively Regulates Cell Proliferation Through Targeting IRS1 in Isolated Ovarian Granulosa Cells From Patients With Polycystic Ovary Syndrome. Reprod. Sci. 2017;24:902–910. doi: 10.1177/1933719116673197. [DOI] [PubMed] [Google Scholar]
- 39.Murri M., Insenser M., Fernandez-Duran E., San-Millan J.L., Escobar-Morreale H.F. Effects of polycystic ovary syndrome (PCOS), sex hormones, and obesity on circulating miRNA-21, miRNA-27b, miRNA-103, and miRNA-155 expression. J. Clin. Endocrinol. Metab. 2013;98:E1835–E1844. doi: 10.1210/jc.2013-2218. [DOI] [PubMed] [Google Scholar]
- 40.Xia H., Zhao Y. miR-155 is high-expressed in polycystic ovarian syndrome and promotes cell proliferation and migration through targeting PDCD4 in KGN cells. Artif. Cells Nanomed. Biotechnol. 2019;48:197–205. doi: 10.1080/21691401.2019.1699826. [DOI] [PubMed] [Google Scholar]
- 41.Zhao J.J., Lin J., Yang H., Kong W., He L., Ma X., Coppola D., Cheng J.Q. MicroRNA-221/222 negatively regulates estrogen receptor alpha and is associated with tamoxifen resistance in breast cancer. J. Biol. Chem. 2008;283:31079–31086. doi: 10.1074/jbc.M806041200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Roth L.W., McCallie B., Alvero R., Schoolcraft W.B., Minjarez D., Katz-Jaffe M.G. Altered microRNA and gene expression in the follicular fluid of women with polycystic ovary syndrome. J. Assist. Reprod. Genet. 2014;31:355–362. doi: 10.1007/s10815-013-0161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao G., Liang M., Liang N., Yin M., Lu M., Lian J., Wang Y., Sun F. MicroRNA-224 is involved in the regulation of mouse cumulus expansion by targeting Ptx3. Mol. Cell. Endocrinol. 2014;382:244–253. doi: 10.1016/j.mce.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 44.Long W., Zhao C., Ji C., Ding H., Cui Y., Guo X., Shen R., Liu J. Characterization of serum microRNAs profile of PCOS and identification of novel non-invasive biomarkers. Cell. Physiol. Biochem. 2014;33:1304–1315. doi: 10.1159/000358698. [DOI] [PubMed] [Google Scholar]
- 45.Sang Q., Yao Z., Wang H., Feng R., Zhao X., Xing Q., Jin L., He L., Wu L., Wang L. Identification of microRNAs in human follicular fluid: Characterization of microRNAs that govern steroidogenesis in vitro and are associated with polycystic ovary syndrome in vivo. J. Clin. Endocrinol. Metab. 2013;98:3068–3079. doi: 10.1210/jc.2013-1715. [DOI] [PubMed] [Google Scholar]
- 46.Yin M., Wang X., Yao G., Lu M., Liang M., Sun Y., Sun F. Transactivation of micrornA-320 by microRNA-383 regulates granulosa cell functions by targeting E2F1 and SF-1 proteins. J. Biol. Chem. 2014;289:18239–18257. doi: 10.1074/jbc.M113.546044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang C.L., Wang H., Yan C.Y., Gao X.F., Ling X.J. Deregulation of RUNX2 by miR-320a deficiency impairs steroidogenesis in cumulus granulosa cells from polycystic ovary syndrome (PCOS) patients. Biochem. Biophys. Res. Commun. 2017;482:1469–1476. doi: 10.1016/j.bbrc.2016.12.059. [DOI] [PubMed] [Google Scholar]
- 48.Yin M., Lu M., Yao G., Tian H., Lian J., Liu L., Liang M., Wang Y., Sun F. Transactivation of microRNA-383 by steroidogenic factor-1 promotes estradiol release from mouse ovarian granulosa cells by targeting RBMS1. Mol. Endocrinol. 2012;26:1129–1143. doi: 10.1210/me.2011-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi L., Liu S., Zhao W., Shi J. miR-483-5p and miR-486-5p are down-regulated in cumulus cells of metaphase II oocytes from women with polycystic ovary syndrome. Reprod. Biomed. Online. 2015;31:565–572. doi: 10.1016/j.rbmo.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 50.Xiang Y., Song Y., Li Y., Zhao D., Ma L., Tan L. miR-483 is Down-Regulated in Polycystic Ovarian Syndrome and Inhibits KGN Cell Proliferation via Targeting Insulin-Like Growth Factor 1 (IGF1) Med. Sci. Monit. 2016;22:3383–3393. doi: 10.12659/MSM.897301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang X., Liu C., Hao C., Tang Q., Liu R., Lin S., Zhang L., Yan W. Identification of altered microRNAs and mRNAs in the cumulus cells of PCOS patients: miRNA-509-3p promotes oestradiol secretion by targeting MAP3K8. Reproduction. 2016;151:643–655. doi: 10.1530/REP-16-0071. [DOI] [PubMed] [Google Scholar]
- 52.Revelli A., Delle Piane L., Casano S., Molinari E., Massobrio M., Rinaudo P. Follicular fluid content and oocyte quality: From single biochemical markers to metabolomics. Reprod. Biol. Endocrinol. 2009;7:40. doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scalici E., Traver S., Mullet T., Molinari N., Ferrieres A., Brunet C., Belloc S., Hamamah S. Circulating microRNAs in follicular fluid, powerful tools to explore in vitro fertilization process. Sci. Rep. 2016;6:24976. doi: 10.1038/srep24976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGinnis L.K., Luense L.J., Christenson L.K. MicroRNA in Ovarian Biology and Disease. Cold Spring Harb. Perspect. Med. 2015;5:a022962. doi: 10.1101/cshperspect.a022962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen B., Xu P., Wang J., Zhang C. The role of MiRNA in polycystic ovary syndrome (PCOS) Gene. 2019;706:91–96. doi: 10.1016/j.gene.2019.04.082. [DOI] [PubMed] [Google Scholar]
- 56.Gould S.J., Raposo G. As we wait: Coping with an imperfect nomenclature for extracellular vesicles. J. Extracell. Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sato-Kuwabara Y., Melo S.A., Soares F.A., Calin G.A. The fusion of two worlds: Non-coding RNAs and extracellular vesicles--diagnostic and therapeutic implications (Review) Int. J. Oncol. 2015;46:17–27. doi: 10.3892/ijo.2014.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J.J., Lotvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 59.Ding C.F., Chen W.Q., Zhu Y.T., Bo Y.L., Hu H.M., Zheng R.H. Circulating microRNAs in patients with polycystic ovary syndrome. Hum. Fertil. 2015;18:22–29. doi: 10.3109/14647273.2014.956811. [DOI] [PubMed] [Google Scholar]
- 60.Comim F.V., Teerds K., Hardy K., Franks S. Increased protein expression of LHCG receptor and 17alpha-hydroxylase/17-20-lyase in human polycystic ovaries. Hum. Reprod. 2013;28:3086–3092. doi: 10.1093/humrep/det352. [DOI] [PubMed] [Google Scholar]
- 61.Song J., Luo S., Li S.W. miRNA-592 is downregulated and may target LHCGR in polycystic ovary syndrome patients. Reprod. Biol. 2015;15:229–237. doi: 10.1016/j.repbio.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 62.Xiong W., Lin Y., Xu L., Tamadon A., Zou S., Tian F., Shao R., Li X., Feng Y. Circulatory microRNA 23a and microRNA 23b and polycystic ovary syndrome (PCOS): The effects of body mass index and sex hormones in an Eastern Han Chinese population. J. Ovarian Res. 2017;10:10. doi: 10.1186/s13048-016-0298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toni F., Vincenti L., Ricci A., Schukken Y.H. Postpartum uterine diseases and their impacts on conception and days open in dairy herds in Italy. Theriogenology. 2015;84:1206–1214. doi: 10.1016/j.theriogenology.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 64.Kasimanickam R., Duffield T.F., Foster R.A., Gartley C.J., Leslie K.E., Walton J.S., Johnson W.H. Endometrial cytology and ultrasonography for the detection of subclinical endometritis in postpartum dairy cows. Theriogenology. 2004;62:9–23. doi: 10.1016/j.theriogenology.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 65.LeBlanc S.J., Duffield T.F., Leslie K.E., Bateman K.G., Keefe G.P., Walton J.S., Johnson W.H. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J. Dairy Sci. 2002;85:2223–2236. doi: 10.3168/jds.S0022-0302(02)74302-6. [DOI] [PubMed] [Google Scholar]
- 66.McDougall S., Macaulay R., Compton C. Association between endometritis diagnosis using a novel intravaginal device and reproductive performance in dairy cattle. Anim. Reprod. Sci. 2007;99:9–23. doi: 10.1016/j.anireprosci.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 67.Knutti B., Küpfer U., Busato A. Reproductive efficiency of cows with endometritis after treatment with intrauterine infusions or prostaglandin injections, or no treatment. J. Veter. Med. A Physiol. Pathol. Clin. Med. 2000;47:609–615. doi: 10.1046/j.1439-0442.2000.00324.x. [DOI] [PubMed] [Google Scholar]
- 68.Bonnett B.N., Martin S.W., Gannon V.P., Miller R.B., Etherington W.G. Endometrial biopsy in Holstein-Friesian dairy cows. III. Bacteriological analysis and correlations with histological findings. Can. J. Vet. Res. 1991;55:168–173. [PMC free article] [PubMed] [Google Scholar]
- 69.Messier S., Higgins R., Couture Y., Morin M. Comparison of swabbing and biopsy for studying the flora of the bovine uterus. Can. Vet. J. 1984;25:283–288. [PMC free article] [PubMed] [Google Scholar]
- 70.Studer E., Morrow D.A. Postpartum evaluation of bovine reproductive potential: Comparison of findings from genital tract examination per rectum, uterine culture, and endometrial biopsy. J. Am. Veter. Med. Assoc. 1978;172:489–494. [PubMed] [Google Scholar]
- 71.Swangchan-Uthai T., Lavender C.R.M., Cheng Z., Fouladi-Nashta A.A., Wathes D.C. Time course of defense mechanisms in bovine endometrium in response to lipopolysaccharide. Biol. Reprod. 2012;87:135. doi: 10.1095/biolreprod.112.102376. [DOI] [PubMed] [Google Scholar]
- 72.Wira C.R., Grant-Tschudy K.S., Crane-Godreau M.A. Epithelial cells in the female reproductive tract: A central role as sentinels of immune protection. Am. J. Reprod. Immunol. 2005;53:65–76. doi: 10.1111/j.1600-0897.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 73.Bromfield J.J., Santos J.E.P., Block J., Williams R.S., Sheldon I.M. PHYSIOLOGY AND ENDOCRINOLOGY SYMPOSIUM: Uterine infection: Linking infection and innate immunity with infertility in the high-producing dairy cow. J. Anim. Sci. 2015;93:2021–2033. doi: 10.2527/jas.2014-8496. [DOI] [PubMed] [Google Scholar]
- 74.Butt B.M., Senger P.L., Widders P.R. Neutrophil migration into the bovine uterine lumen following intrauterine inoculation with killed Haemophilus somnus. J. Reprod. Fertil. 1991;93:341–345. doi: 10.1530/jrf.0.0930341. [DOI] [PubMed] [Google Scholar]
- 75.Gilbert R.O., Shin S.T., Guard C.L., Erb H.N., Frajblat M. Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology. 2005;64:1879–1888. doi: 10.1016/j.theriogenology.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 76.Galvão K.N., Felippe M.J.B., Brittin S.B., Sper R., Fraga M., Galvão J.S., Caixeta L., Guard C.L., Ricci A., Gilbert R.O. Evaluation of cytokine expression by blood monocytes of lactating Holstein cows with or without postpartum uterine disease. Theriogenology. 2012;77:356–372. doi: 10.1016/j.theriogenology.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 77.Kasimanickam R., Kasimanickam V., Kastelic J.P. Mucin 1 and cytokines mRNA in endometrium of dairy cows with postpartum uterine disease or repeat breeding. Theriogenology. 2014;81:952–958.e952. doi: 10.1016/j.theriogenology.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 78.Salilew-Wondim D., Ibrahim S., Gebremedhn S., Tesfaye D., Heppelmann M., Bollwein H., Pfarrer C., Tholen E., Neuhoff C., Schellander K., et al. Clinical and subclinical endometritis induced alterations in bovine endometrial transcriptome and miRNome profile. BMC Genom. 2016;17:218. doi: 10.1186/s12864-016-2513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chegini N. Uterine microRNA signature and consequence of their dysregulation in uterine disorders. Anim. Reprod. 2010;7:117–128. [PMC free article] [PubMed] [Google Scholar]
- 80.Pan Q., Chegini N. MicroRNA signature and regulatory functions in the endometrium during normal and disease states. Semin. Reprod. Med. 2008;26:479–493. doi: 10.1055/s-0028-1096128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hailemariam D., Ibrahim S., Hoelker M., Drillich M., Heuwieser W., Looft C., Cinar M.U., Tholen E., Schellander K., Tesfaye D. MicroRNA-regulated molecular mechanism underlying bovine subclinical endometritis. Reprod. Fertil. Dev. 2014;26:898–913. doi: 10.1071/RD13027. [DOI] [PubMed] [Google Scholar]
- 82.Zhu Y., Gu L., Li Y., Lin X., Shen H., Cui K., Chen L., Zhou F., Zhao Q., Zhang J., et al. miR-148a inhibits colitis and colitis-associated tumorigenesis in mice. Cell Death Differ. 2017;24:2199–2209. doi: 10.1038/cdd.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiang K., Yang J., Yang C., Zhang T., Shaukat A., Yang X., Dai A., Wu H., Deng G. miR-148a suppresses inflammation in lipopolysaccharide-induced endometritis. J. Cell. Mol. Med. 2020;24:405–417. doi: 10.1111/jcmm.14744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao G., Jiang K., Yang Y., Zhang T., Wu H., Shaukat A., Qiu C., Deng G. The Potential Therapeutic Role of miR-223 in Bovine Endometritis by Targeting the NLRP3 Inflammasome. Front. Immunol. 2018;9:1916. doi: 10.3389/fimmu.2018.01916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yin N., Yang Y., Wang X., Yang C., Ma X., Shaukat A., Zhao G., Deng G. MiR-19a mediates the negative regulation of the NF-κB pathway in lipopolysaccharide-induced endometritis by targeting TBK1. Inflamm. Res. 2019;68:231–240. doi: 10.1007/s00011-019-01213-3. [DOI] [PubMed] [Google Scholar]
- 86.Sheldon I.M., Price S.B., Cronin J., Gilbert R.O., Gadsby J.E. Mechanisms of infertility associated with clinical and subclinical endometritis in high producing dairy cattle. Reprod. Domest. Anim. 2009;44(Suppl. 3):1–9. doi: 10.1111/j.1439-0531.2009.01465.x. [DOI] [PubMed] [Google Scholar]
- 87.Kasimanickam V., Kastelic J. Circulating cell-free mature microRNAs and their target gene prediction in bovine metritis. Sci. Rep. 2016;6:29509. doi: 10.1038/srep29509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang B., Yin Y., Lai R.C., Lim S.K. Immunotherapeutic potential of extracellular vesicles. Front. Immunol. 2014;5:518. doi: 10.3389/fimmu.2014.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Montecalvo A., Larregina A.T., Shufesky W.J., Stolz D.B., Sullivan M.L.G., Karlsson J.M., Baty C.J., Gibson G.A., Erdos G., Wang Z., et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Okoye I.S., Coomes S.M., Pelly V.S., Czieso S., Papayannopoulos V., Tolmachova T., Seabra M.C., Wilson M.S. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014;41:89–103. doi: 10.1016/j.immuni.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang L., Li H., Yuan M., Li D., Sun C., Wang G. Serum Exosomal MicroRNAs as Potential Circulating Biomarkers for Endometriosis. Dis. Markers. 2020;2020:2456340. doi: 10.1155/2020/2456340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang X., Tian F., Chen C., Feng Y., Sheng X., Guo Y., Ni H. Exosome-derived uterine microRNAs isolated from cows with endometritis impede blastocyst development. Reprod. Biol. 2019;19:204–209. doi: 10.1016/j.repbio.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 93.Qiao F., Ge H., Ma X., Zhang Y., Zuo Z., Wang M., Zhang Y., Wang Y. Bovine uterus-derived exosomes improve developmental competence of somatic cell nuclear transfer embryos. Theriogenology. 2018;114:199–205. doi: 10.1016/j.theriogenology.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 94.Qu P., Qing S., Liu R., Qin H., Wang W., Qiao F., Ge H., Liu J., Zhang Y., Cui W., et al. Effects of embryo-derived exosomes on the development of bovine cloned embryos. PLoS ONE. 2017;12:e0174535. doi: 10.1371/journal.pone.0174535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang X., Yao X., Xie T., Chang Z., Guo Y., Ni H. Exosome-derived uterine miR-218 isolated from cows with endometritis regulates the release of cytokines and chemokines. Microb. Biotechnol. 2020;13:1103–1117. doi: 10.1111/1751-7915.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hendrix N., Berghella V. Non-placental causes of intrauterine growth restriction. Semin. Perinatol. 2008;32:161–165. doi: 10.1053/j.semperi.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 97.Chirshev E., Oberg K.C., Ioffe Y.J., Unternaehrer J.J. Let-7 as biomarker, prognostic indicator, and therapy for precision medicine in cancer. Clin. Trans. Med. 2019;8:24. doi: 10.1186/s40169-019-0240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hanna J., Hossain G.S., Kocerha J. The Potential for microRNA Therapeutics and Clinical Research. Front. Genet. 2019;10:478. doi: 10.3389/fgene.2019.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsochandaridis M., Nasca L., Toga C., Levy-Mozziconacci A. Circulating microRNAs as clinical biomarkers in the predictions of pregnancy complications. BioMed. Res. Int. 2015;2015:294954. doi: 10.1155/2015/294954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ali A., Bouma G.J., Anthony R.V., Winger Q.A. The Role of LIN28-let-7-ARID3B Pathway in Placental Development. Int. J. Mol. Sci. 2020;21:3637. doi: 10.3390/ijms21103637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sadovsky Y., Mouillet J.-F., Ouyang Y., Bayer A., Coyne C.B. The Function of TrophomiRs and Other MicroRNAs in the Human Placenta. Cold Spring Harb. Perspect. Med. 2015;5:a023036. doi: 10.1101/cshperspect.a023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Donker R.B., Mouillet J.F., Chu T., Hubel C.A., Stolz D.B., Morelli A.E., Sadovsky Y. The expression profile of C19MC microRNAs in primary human trophoblast cells and exosomes. Mol. Hum. Reprod. 2012;18:417–424. doi: 10.1093/molehr/gas013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ouyang Y., Mouillet J.-F., Coyne C.B., Sadovsky Y. Placenta-specific microRNAs in exosomes—Good things come in nano-packages. Placenta. 2014;35:S69–S73. doi: 10.1016/j.placenta.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hossain M.M., Tesfaye D., Salilew-Wondim D., Held E., Proll M.J., Rings F., Kirfel G., Looft C., Tholen E., Uddin J., et al. Massive deregulation of miRNAs from nuclear reprogramming errors during trophoblast differentiation for placentogenesis in cloned pregnancy. BMC Genom. 2014;15:43. doi: 10.1186/1471-2164-15-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hromadnikova I., Kotlabova K., Ondrackova M., Pirkova P., Kestlerova A., Novotna V., Hympanova L., Krofta L. Expression Profile of C19MC microRNAs in Placental Tissue in Pregnancy-Related Complications. DNA Cell Biol. 2015;34:437–457. doi: 10.1089/dna.2014.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hromadnikova I., Kotlabova K., Ivankova K., Krofta L. First trimester screening of circulating C19MC microRNAs and the evaluation of their potential to predict the onset of preeclampsia and IUGR. PLoS ONE. 2017;12:e0171756. doi: 10.1371/journal.pone.0171756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mouillet J.-F., Ouyang Y., Coyne C.B., Sadovsky Y. MicroRNAs in placental health and disease. Am. J. Obs. Gynecol. 2015;213:S163–S172. doi: 10.1016/j.ajog.2015.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Farrokhnia F., Aplin J.D., Westwood M., Forbes K. MicroRNA regulation of mitogenic signaling networks in the human placenta. J. Biol. Chem. 2014;289:30404–30416. doi: 10.1074/jbc.M114.587295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fu G., Ye G., Nadeem L., Ji L., Manchanda T., Wang Y., Zhao Y., Qiao J., Wang Y.-L., Lye S., et al. MicroRNA-376c impairs transforming growth factor-β and nodal signaling to promote trophoblast cell proliferation and invasion. Hypertension. 2013;61:864–872. doi: 10.1161/HYPERTENSIONAHA.111.203489. [DOI] [PubMed] [Google Scholar]
- 110.Hromadnikova I., Kotlabova K., Doucha J., Dlouha K., Krofta L. Absolute and relative quantification of placenta-specific micrornas in maternal circulation with placental insufficiency-related complications. J. Mol. Diagn. JMD. 2012;14:160–167. doi: 10.1016/j.jmoldx.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 111.Morales-Prieto D.M., Ospina-Prieto S., Chaiwangyen W., Schoenleben M., Markert U.R. Pregnancy-associated miRNA-clusters. J. Reprod. Immunol. 2013;97:51–61. doi: 10.1016/j.jri.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 112.Wommack J.C., Trzeciakowski J.P., Miranda R.C., Stowe R.P., Ruiz R.J. Micro RNA clusters in maternal plasma are associated with preterm birth and infant outcomes. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0199029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li Y., Liu J. MicroRNA-206 predicts raised fetal growth retardation risk through the interaction with vascular endothelial growth factor in pregnancies. Medicine. 2020;99:e18897. doi: 10.1097/MD.0000000000018897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gao Y., Feng B., Han S., Zhang K., Chen J., Li C., Wang R., Chen L. The Roles of MicroRNA-141 in Human Cancers: From Diagnosis to Treatment. Cell. Physiol. Biochem. 2016;38:427–448. doi: 10.1159/000438641. [DOI] [PubMed] [Google Scholar]
- 115.Tang Q., Wu W., Xu X., Huang L., Gao Q., Chen H., Sun H., Xia Y., Sha J., Wang X., et al. miR-141 Contributes to Fetal Growth Restriction by Regulating PLAG1 Expression. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0058737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mouillet J.-F., Chu T., Hubel C.A., Nelson D.M., Parks W.A., Sadovsky Y. The levels of hypoxia-regulated microRNAs in plasma of pregnant women with fetal growth restriction. Placenta. 2010;31:781–784. doi: 10.1016/j.placenta.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang L., Shen Z., Xu Q., Huang X., Chen Q., Li D. Increased levels of microRNA-424 are associated with the pathogenesis of fetal growth restriction. Placenta. 2013;34:624–627. doi: 10.1016/j.placenta.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 118.Rutkowska K., Stachowiak M., Oprzadek J., Bauersachs S., Flisikowski K. Altered miRNA-4321 expression in maternal and foetal placenta of intrauterine growth restricted bovine foetuses. Placenta. 2018;70:50–52. doi: 10.1016/j.placenta.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 119.Ye Y., Vattai A., Ditsch N., Kuhn C., Rahmeh M., Mahner S., Ripphahn M., Immler R., Sperandio M., Jeschke U., et al. Prostaglandin E2 receptor 3 signaling is induced in placentas with unexplained recurrent pregnancy losses. Endocr. Connect. 2018;7:749–761. doi: 10.1530/EC-18-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ali A., Anthony R.V., Bouma G.J., Winger Q.A. LIN28-let-7 axis regulates genes in immortalized human trophoblast cells by targeting the ARID3B-complex. FASEB J. 2019;33:12348–12363. doi: 10.1096/fj.201900718RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mayr F., Heinemann U. Mechanisms of Lin28-mediated miRNA and mRNA regulation--a structural and functional perspective. Int. J. Mol. Sci. 2013;14:16532–16553. doi: 10.3390/ijms140816532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang J., Ratanasirintrawoot S., Chandrasekaran S., Wu Z., Ficarro S.B., Yu C., Ross C.A., Cacchiarelli D., Xia Q., Seligson M., et al. LIN28 Regulates Stem Cell Metabolism and Conversion to Primed Pluripotency. Cell Stem Cell. 2016;19:66–80. doi: 10.1016/j.stem.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 123.Ali A., Stenglein M.D., Spencer T.E., Bouma G.J., Anthony R.V., Winger Q.A. Trophectoderm-Specific Knockdown of LIN28 Decreases Expression of Genes Necessary for Cell Proliferation and Reduces Elongation of Sheep Conceptus. Int. J. Mol. Sci. 2020;21:2549. doi: 10.3390/ijms21072549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Awamleh Z., Gloor G.B., Han V.K.M. Placental microRNAs in pregnancies with early onset intrauterine growth restriction and preeclampsia: Potential impact on gene expression and pathophysiology. BMC Med. Genom. 2019;12:91. doi: 10.1186/s12920-019-0548-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hromadnikova I., Kotlabova K., Hympanova L., Krofta L. Cardiovascular and Cerebrovascular Disease Associated microRNAs Are Dysregulated in Placental Tissues Affected with Gestational Hypertension, Preeclampsia and Intrauterine Growth Restriction. PLoS ONE. 2015;10:e0138383. doi: 10.1371/journal.pone.0138383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kirkwood T.B.L. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 127.te Velde E.R., Pearson P.L. The variability of female reproductive ageing. Hum. Reprod. Update. 2002;8:141–154. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- 128.Faddy M.J. Follicle dynamics during ovarian ageing. Mol. Cell. Endocrinol. 2000;163:43–48. doi: 10.1016/S0303-7207(99)00238-5. [DOI] [PubMed] [Google Scholar]
- 129.Gilchrist R.B., Ritter L.J., Armstrong D.T. Oocyte-somatic cell interactions during follicle development in mammals. Anim. Reprod. Sci. 2004;82–83:431–446. doi: 10.1016/j.anireprosci.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 130.Pangas S. Growth Factors in Ovarian Development. Semin. Reprod. Med. 2007;25:225–234. doi: 10.1055/s-2007-980216. [DOI] [PubMed] [Google Scholar]
- 131.Faddy M.J., Gosden R.G., Gougeon A., Richardson S.J., Nelson J.F. Accelerated disappearance of ovarian follicles in mid-life: Implications for forecasting menopause. Hum. Reprod. 1992;7:1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 132.Liu L., Keefe D.L. Ageing-associated aberration in meiosis of oocytes from senescence-accelerated mice. Hum. Reprod. 2002;17:2678–2685. doi: 10.1093/humrep/17.10.2678. [DOI] [PubMed] [Google Scholar]
- 133.Battaglia D.E., Goodwin P., Klein N.A., Soules M.R. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum. Reprod. 1996;11:2217–2222. doi: 10.1093/oxfordjournals.humrep.a019080. [DOI] [PubMed] [Google Scholar]
- 134.Eldar-Geva T., Ben-Chetrit A., Spitz I.M., Rabinowitz R., Markowitz E., Mimoni T., Gal M., Zylber-Haran E., Margalioth E.J. Dynamic assays of inhibin B, anti-Mullerian hormone and estradiol following FSH stimulation and ovarian ultrasonography as predictors of IVF outcome. Hum. Reprod. 2005;20:3178–3183. doi: 10.1093/humrep/dei203. [DOI] [PubMed] [Google Scholar]
- 135.Hunt P.A., Hassold T.J. Human female meiosis: What makes a good egg go bad? Trends Genet. TIG. 2008;24:86–93. doi: 10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 136.Tatone C., Amicarelli F., Carbone M.C., Monteleone P., Caserta D., Marci R., Artini P.G., Piomboni P., Focarelli R. Cellular and molecular aspects of ovarian follicle ageing. Hum. Reprod. Update. 2008;14:131–142. doi: 10.1093/humupd/dmm048. [DOI] [PubMed] [Google Scholar]
- 137.Barragán M., Pons J., Ferrer-Vaquer A., Cornet-Bartolomé D., Schweitzer A., Hubbard J., Auer H., Rodolosse A., Vassena R. The transcriptome of human oocytes is related to age and ovarian reserve. Mol. Hum. Reprod. 2017;23:535–548. doi: 10.1093/molehr/gax033. [DOI] [PubMed] [Google Scholar]
- 138.Grondahl M.L., Yding Andersen C., Bogstad J., Nielsen F.C., Meinertz H., Borup R. Gene expression profiles of single human mature oocytes in relation to age. Hum. Reprod. 2010;25:957–968. doi: 10.1093/humrep/deq014. [DOI] [PubMed] [Google Scholar]
- 139.Steuerwald N.M., Bermudez M.G., Wells D., Munne S., Cohen J. Maternal age-related differential global expression profiles observed in human oocytes. Reprod. Biomed. Online. 2007;14:700–708. doi: 10.1016/S1472-6483(10)60671-2. [DOI] [PubMed] [Google Scholar]
- 140.Mihalas B.P., Camlin N.J., Xavier M.J., Peters A.E., Holt J.E., Sutherland J.M., McLaughlin E.A., Eamens A.L., Nixon B. The small non-coding RNA profile of mouse oocytes is modified during aging. Aging. 2019;11:2968–2997. doi: 10.18632/aging.101947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Battaglia R., Vento M.E., Ragusa M., Barbagallo D., La Ferlita A., Di Emidio G., Borzi P., Artini P.G., Scollo P., Tatone C., et al. MicroRNAs Are Stored in Human MII Oocyte and Their Expression Profile Changes in Reproductive Aging. Biol. Reprod. 2016;95:131. doi: 10.1095/biolreprod.116.142711. [DOI] [PubMed] [Google Scholar]
- 142.Sasaki H., Hamatani T., Kamijo S., Iwai M., Kobanawa M., Ogawa S., Miyado K., Tanaka M. Impact of Oxidative Stress on Age-Associated Decline in Oocyte Developmental Competence. Front. Endocrinol. 2019;10:811. doi: 10.3389/fendo.2019.00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Diez-Fraile A., Lammens T., Tilleman K., Witkowski W., Verhasselt B., Sutter P., Benoit Y., Espeel M., D’Herde K. Age-associated differential microRNA levels in human follicular fluid reveal pathways potentially determining fertility and success of in vitro fertilization. Hum. Fertil. 2014;17:90–98. doi: 10.3109/14647273.2014.897006. [DOI] [PubMed] [Google Scholar]
- 144.Moreno J.M., Nunez M.J., Quinonero A., Martinez S., La Orden M., Simon C., Pellicer A., Diaz-Garcia C., Dominguez F. Follicular fluid and mural granulosa cells microRNA profiles vary in in vitro fertilization patients depending on their age and oocyte maturation stage. Fertil. Steril. 2015;104:1037–1046.e1031. doi: 10.1016/j.fertnstert.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 145.Ioannidis J., Sánchez-Molano E., Psifidi A., Donadeu F.X., Banos G. Association of plasma microRNA expression with age, genetic background and functional traits in dairy cattle. Sci. Rep. 2018;8:12955. doi: 10.1038/s41598-018-31099-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Childs B.G., Durik M., Baker D.J., van Deursen J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015;21:1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Acosta J.C., Banito A., Wuestefeld T., Georgilis A., Janich P., Morton J.P., Athineos D., Kang T.-W., Lasitschka F., Andrulis M., et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013;15:978–990. doi: 10.1038/ncb2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saeed-Zidane M., Linden L., Salilew-Wondim D., Held E., Neuhoff C., Tholen E., Hoelker M., Schellander K., Tesfaye D. Cellular and exosome mediated molecular defense mechanism in bovine granulosa cells exposed to oxidative stress. PLoS ONE. 2017;12:e0187569. doi: 10.1371/journal.pone.0187569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mensa E., Guescini M., Giuliani A., Bacalini M.G., Ramini D., Corleone G., Ferracin M., Fulgenzi G., Graciotti L., Prattichizzo F., et al. Small extracellular vesicles deliver miR-21 and miR-217 as pro-senescence effectors to endothelial cells. J. Extracell. Vesicles. 2020;9:1725285. doi: 10.1080/20013078.2020.1725285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Carnevale E.M. The mare model for follicular maturation and reproductive aging in the woman. Theriogenology. 2008;69:23–30. doi: 10.1016/j.theriogenology.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 151.Da Silveira J.C., Winger Q.A., Bouma G.J., Carnevale E.M. Effects of age on follicular fluid exosomal microRNAs and granulosa cell transforming growth factor-β signalling during follicle development in the mare. Reprod. Fertil. Dev. 2015;27:897–905. doi: 10.1071/RD14452. [DOI] [PubMed] [Google Scholar]
- 152.Zhang Q., Sun H., Jiang Y., Ding L., Wu S., Fang T., Yan G., Hu Y. MicroRNA-181a suppresses mouse granulosa cell proliferation by targeting activin receptor IIA. PLoS ONE. 2013;8:e59667. doi: 10.1371/journal.pone.0059667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tarín J.J., Pérez-Albalá S., Cano A. Consequences on offspring of abnormal function in ageing gametes. Hum. Reprod. Update. 2000;6:532–549. doi: 10.1093/humupd/6.6.532. [DOI] [PubMed] [Google Scholar]
- 154.Wilcox A.J., Weinberg C.R., Baird D.D. Post-ovulatory ageing of the human oocyte and embryo failure. Hum. Reprod. 1998;13:394–397. doi: 10.1093/humrep/13.2.394. [DOI] [PubMed] [Google Scholar]
- 155.Takahashi T., Igarashi H., Amita M., Hara S., Matsuo K., Kurachi H. Molecular mechanism of poor embryo development in postovulatory aged oocytes: Mini review. J. Obstet. Gynaecol. Res. 2013;39:1431–1439. doi: 10.1111/jog.12111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.