Abstract
Background
The outbreak of the coronavirus disease (COVID-19) has led to a major concern and caused a pandemic globally. The goal of this study was to clarify the clinical characteristics of recovery and death in patients with severe or critical COVID-19.
Materials and Methods
In this retrospective single-center study, clinical data were collected from 74 severe or critical COVID-19 patients in Wuhan Fourth Hospital between Jan. 25th and Feb. 26th, 2020. All patients were divided into a recovery group or a death group according to clinical outcomes, and the differences between the groups were compared.
Results
Of the 74 patients enrolled in the study, 48 (64.9%) were severe cases and 26 (35.1%) were critical cases. Sixty (81.1%) patients were recovered and 14 (18.9%) died. Compared with recovery patients, patients in the death group were older, and had higher incidences of hypertension, coronary disease and dyspnea at admission. Laboratory tests for lactate dehydrogenase, creatine kinase, myoglobin, brain natriuretic peptide and D-dimer indicated higher levels in the death group. The PaO2:FiO2 ratio and minimum SpO2 were lower in the death group, and a higher proportion of these patients received noninvasive mechanical ventilation, invasive mechanical ventilation and extracorporeal membrane oxygenation treatment.
Conclusions
Elderly patients with comorbidities are at higher risk of severe COVID-19 or death. Patients with a low blood gas index and poor coagulation function at admission had a high mortality rate. For such patients, comprehensive treatment should be performed as soon as possible to improve the prognosis and reduce mortality.
Key Indexing Terms: SARS-CoV-2, COVID-19, Coronavirus, Clinical features
INTRODUCTION
In December 2019, a novel coronavirus pneumonia (COVID-19) was identified in Wuhan, China, and quickly became a global pandemic.1, 2, 3, 4 Patients with serious cases of COVID-19 can develop severe pneumonia, acute respiratory distress syndrome (ARDS) and multiple organ failure leading to death, whereas mild patients present ordinary symptoms of respiratory system infection.5 As of April 21, 2020, a total of 2,402,250 cases have been confirmed and 163,097 deaths have been reported across 208 countries or regions.6 The source of infection is mainly through COVID-19 infected patients, some of whom may be asymptomatic. Evidence has been found that COVID-19 can be transmitted from human to human through respiratory droplets, contact and even via fecal-oral transmission.3 In addition, if exposed to a high concentration of aerosol for a long time, it also can be transmitted through aerosolization. All people are susceptible to the SARS-CoV-2 virus.7
This novel virus was confirmed to be a distinct clade from the β-coronaviruses associated with the Middle East Respiratory Syndrome and Severe Acute Respiratory Syndrome (SARS).8 , 9 It was officially named SARS-CoV-2 by the World Committee on Virus Classification,10 and disease caused by this virus was named COVID-19 by the World Health Organization.11, 12, 13 As a highly contagious acute respiratory infectious disease, COVID-19 has been included as a category B infectious disease stipulated in the Law of the People's Republic of China on the Prevention and Control of Infectious Diseases and is managed in accordance with category A infectious diseases.14
Presently, there is no effective drug or vaccine for COVID-19. To prevent and control the spread of the epidemic, many strategies are needed.15 On the one hand, strict control of the source of infection, personal protection precautions, early diagnosis and isolation are priorities. On the other hand, a focus on symptomatic treatment to improve the cure rate of the disease and reduce the mortality rate is also critical. Therefore, the treatment of severe and critical COVID-19 patients is important for prevention and control of the epidemic and is also a great challenge. Up to now, there have been reports on the clinical characteristics of patients with COVID-19,1 , 16 , 17 but reports about the clinical characteristics of severe or critical COVID-19 patients are scarce. This study retrospectively analyzed clinical data from 74 severe and critical COVID-19 patients admitted to Wuhan Fourth Hospital with a goal to provide useful information for the diagnosis and treatment of severe and critical COVID-19.
PATIENTS AND METHODS
Study Design and Participants
This retrospective study was approved by the institutional review board at Wuhan Fourth Hospital. Seventy-four patients with confirmed severe and critical COVID-19 admitted to Wuhan Fourth Hospital between Jan. 25th and Feb. 26th, 2020 were enrolled. All COVID-19 patients enrolled in this study were diagnosed according to World Health Organization interim guidance.18 Informed consent was waived as part of a public health outbreak investigation.
Clinical Diagnostic Criteria and Classification of Disease Severity
The diagnosis and clinical classification of all patients were performed strictly according to the “Diagnosis and Treatment Protocol for COVID-19 (Trial Version 5).”19 All patients were positive for the nucleic acid test 3 days before admission or on the day of admission.
The COVID-19 classification criteria for severe and critical illness are as follows; matches to any one of the conditions that is shown below can be included as a case. Severe COVID-19: (1) Respiratory distress, RR ≥ 30 times/minute; (2) oxygen saturation ≤93% in resting state; (3) partial pressure of arterial oxygen (PaO2)/oxygen concentration (FiO2 ≤ 300 mmHg (1 mmHg = 0.133 kPa). Critical COVID-19: (1) Respiratory failure requiring mechanical ventilation; (2) shock; (3) combined failure of other organs that requires ICU monitoring and treatment.
Data Collection
Two doctors in our team collected and checked clinical information from electronic medical records in Wuhan Fourth Hospital. Information recorded included demographic data, medical history, exposure history, underlying comorbidities, clinical manifestations, laboratory findings, chest CT scan and treatment measures (i.e., antiviral treatment, antibiotic treatment, hormone treatment, respiratory support). The data were reviewed by a trained team of physicians.
Imaging Analysis and Quantification
All patients underwent regular chest CT examination during admission and treatment. Images were reconstructed with a slice thickness of 1-1.5 mm using a lung kernel as part of the reconstruction process. All imaging features were reviewed and evaluated by 2 experienced radiologists. The CT features including ground glass opacity and mixed ground glass opacity, and consolidation were evaluated. Lesion size was described as grade 1 (diameter, <1 cm), grade 2 (diameter, 1-3 cm), grade 3 (diameter, 3 cm to 50% of the segment) or grade 4 (over 50% of the segment), and each segment was reviewed and scored.
Definitions
The recovery and discharge criteria were defined as follows: body temperature had returned to normal for more than 3 days, the respiratory symptoms had improved significantly and the chest CT imaging showed an obvious reduction in inflammation. Two consecutive nucleic acid tests from throat swabs must have been negative with a time interval between them of at least 1 day. Finally, after an evaluation by the expert team, a comprehensive evaluation was made to determine whether the patient could be discharged.
Statistical Analysis
Categorical variables were described using percentages, and continuous variables were described using median and interquartile ranges. Means for discrete variables were compared using independent Student's t tests when the data were normally distributed; otherwise, the Mann-Whitney test was used. Proportions for categorical variables were compared using the χ2 test, although the Fisher's exact test was used when the sample sizes were small. All statistical analyses were performed using SPSS software v.21 (SPSS Inc), and P < 0.05 was considered statistically significant.
RESULTS
Basic Characteristics
The baseline characteristics of the 74 patients are shown in Table 1 . Of the 74 patients, 48 were severe cases (64.8%) and 26 were critical cases (35.1%). Patients were 27-84 years old (mean = 63.6 ± 11.9) and included 44 males (59.5%) and 30 females (40.5%). Fifty-six patients had one or more comorbidities (75.7%). Hypertension (35 cases, 47.3%), diabetes (14 cases, 18.9%) and coronary heart disease (6 cases, 8.1%) were the most common. When compared with patients in the recovery group, patients in the death group were older (P = 0.002) and had a higher incidence of hypertension (P = 0.045), coronary disease (P = 0.002) and dyspnea (P = 0.020) at admission. The most common symptoms at onset of illness were fever (range: 37.4°C to 40.0°C, averaging 38.5 ± 0.6°C; 67 cases, 90.5%), cough and expectoration (47 cases, 63.5%), fatigue (49 cases, 66.2%), dyspnea (49 cases, 66.2%), anorexia (41 cases, 55.4%), muscle aches (23 cases, 31.1%) and diarrhea (6 cases, 8.1%).
TABLE 1.
Baseline characteristics of recovery and death patients with severe or critical COVID-19.
| Characteristics | Total (n = 74) | Recovery (n = 60) | Death (n = 14) | P Value |
|---|---|---|---|---|
| General information | ||||
| Age, years | 66 (55-72) | 62 (53-70) | 71 (69-77) | 0.002 |
| Sex, male | 44 (59.5%) | 33 (55.0%) | 11 (78.6%) | 0.106 |
| Sex, female | 30 (40.5%) | 27 (45.0%) | 3 (21.4%) | 0.106 |
| Smoking | 10 (13.5%) | 6 (10%) | 4 (28.6%) | 0.067 |
| Comorbidities | ||||
| Total number | 56 (75.7%) | 44 (73.3%) | 12 (85.7%) | 0.331 |
| Hypertension | 35 (47.3%) | 25 (41.7%) | 10 (71.4%) | 0.045 |
| Diabetes | 14 (18.9%) | 11 (18.3%) | 3 (21.4%) | 0.790 |
| Coronary disease | 6 (8.1%) | 2 (3.3%) | 4 (28.6%) | 0.002 |
| Tuberculosis | 6 (8.1%) | 5 (8.3%) | 1 (7.1%) | 0.883 |
| Chronic liver disease | 2 (2.7%) | 2 (3.3%) | 0 (0%) | 0.489 |
| Malignancy | 2 (2.7%) | 2 (3.3%) | 0 (0%) | 0.489 |
| Signs and symptoms | ||||
| Fever | 67 (90.5%) | 53 (88.3%) | 14 (100%) | 0.179 |
| Body temperature | 38.5 (38-39) | 38.5 (38-39) | 38.5 (38-39) | 0.435 |
| Dry cough | 34 (45.9%) | 29 (48.3%) | 5 (35.6%) | 0.720 |
| Expectoration | 13 (17.6%) | 11 (18.3%) | 2 (14.3%) | 0.349 |
| Fatigue | 49 (66.2%) | 39 (65.0%) | 10 (71.4%) | 0.647 |
| Dyspnea | 49 (66.2%) | 36 (60%) | 13 (92.9%) | 0.020 |
| Anorexia | 41 (55.4%) | 31 (51.7%) | 10 (71.4%) | 0.180 |
| Muscle ache | 23 (31.1%) | 19 (31.6%) | 4 (28.6%) | 0.822 |
| Hemoptysis | 6 (8.1%) | 4 (6.7%) | 2 (14.3%) | 0.347 |
| Diarrhea | 6 (8.1%) | 4 (6.7%) | 2 (14.3%) | 0.347 |
Data are reported as n (%) or median (IQR). The P value represents the difference between COVID-19 recovery and death patients. P value <0.05 was considered significant difference (Bold font).
Clinical Laboratory Parameters
The laboratory results of the 74 patients at hospital admission are shown in Table 2 . Most patients had lymphopenia and abnormalities of neutrophils, monocytes, alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, and inflammatory biomarkers C-reactive protein (CRP) and procalcitonin. Compared with the recovery group, the detection values of the 4 blood coagulation indicators were higher in patients who died, and D-dimer showed a significant difference. In addition, the death group had more cardiopulmonary injury indicators and significantly higher myoglobin levels (P = 0.005) and brain natriuretic peptide (P = 0.041) than the recovery group.
TABLE 2.
Laboratory findings of recovery and death patients with severe or critical COVID-19.
| Characteristics | Normal range | Recovery (n = 60) | Death (n = 14) | P Value |
|---|---|---|---|---|
| Blood routine | ||||
| White blood cell count, × 109/L | 3.5-9.5 | 6.3 (4.2-8.4) | 7.3 (3.7-11.3) | 0.815 |
| Lymphocyte count, × 109/L | 1.1-3.2 | 0.6 (0.5-1.0) | 0.6 (0.5-0.8) | 0.320 |
| Neutrophil count, × 109/L | 1.8-6.3 | 5.0 (2.9-7.1) | 6.3 (2.8-10.0) | 0.508 |
| Lymphocyte ratio, 100% | 0.2-0.5 | 0.1 (0.06-0.2) | 0.1 (0.05-0.16) | 0.258 |
| Neutrophil ratio, 100% | 0.4-0.75 | 0.8 (0.7-0.9) | 0.9 (0.8-0.9) | 0.495 |
| Monocyte ratio, 100% | 0.03-0.1 | 0.01 (0.03-0.06) | 0.01 (0.04-0.08) | 0.304 |
| Blood biochemistry | ||||
| Alanine aminotransferase, U/L | 9-40 | 31(18.5-47) | 34.5 (19-59) | 0.793 |
| Aspartate aminotransferase, U/L | 15-45 | 33 (20-45) | 37 (22-49) | 0.399 |
| Lactate dehydrogenase, U/L | 125-243 | 320.5 (255-409.5) | 485.5 (338-659) | 0.003 |
| Creatinine, μmol/L | 41-73 | 65.5 (55.5-82) | 78 (66-94) | 0.077 |
| Total cholesterol, mmol/L | 2.85-5.69 | 3.91 (3.36-4.26) | 3.86 (3.30-4.60) | 0.978 |
| Triacylglycerol, mmol/L | 0.45-1.69 | 1.37 (1.12-1.67) | 1.44 (1.15-1.96) | 0.783 |
| Creatine kinase, U/L | <171 | 73.3 (39.3-132.2) | 115 (62.4-179.8) | 0.031 |
| Creatine kinase-MB, U/L | <0.6 | 1.4 (1.0-2.4) | 1.4 (1.2-4.0) | 0.170 |
| Troponin I, μg/L | <0.2 | 0.03 (0.03-0.03) | 0.03 (0.03-0.1) | 0.418 |
| Myoglobin, ng/mL | 12-75 | 31.4 (17-48.4) | 71.6 (37.6-109.2) | 0.005 |
| Brain natriuretic peptide, pg/mL | <100 | 252 (57-1024) | 671 (373-1519) | 0.041 |
| Coagulation function | ||||
| Activated partial thromboplastin time, s | 22-36 | 34.5 (31.3-39.6) | 38.1 (32.8-41.5) | 0.217 |
| Prothrombin time, s | 10-13 | 13.2 (12.5-14.3) | 13.8 (12.3-15.7) | 0.412 |
| International normalized ratio | 0.8-1.25 | 1.1 (1.0-1.1) | 1.2 (1.0-1.2) | 0.285 |
| Fibrinogen, g/L | 2-4 | 4.8 (4.0-5.5) | 4.3 (4.1-5.4) | 0.995 |
| Thrombin time, s | 14-21 | 15.6 (15.0-16.5) | 15.1 (14.7-16.1) | 0.115 |
| D-dimer, mg/L | <0.05 | 0.46 (0.3-1.3) | 0.84 (0.5-5.1) | 0.033 |
| Infection-related biomarkers | ||||
| C-reaction protein, mg/L | <10.0 | 47.1 (28.6-88.8) | 60.4 (28.2-99.8) | 0.777 |
| Procalcitonin, ng/mL | <0.05 | 0.04 (0.04-0.07) | 0.07 (0.04-0.27) | 0.174 |
| Chest CT findings | ||||
| Bilateral distribution of patchy shadows or ground glass opacity | NA | 60 (100%) | 14 (100%) | 1.000 |
Abbreviations: NA, not available. Data are reported as n (%) or median (IQR). The P value represents the difference between COVID-19 recovery and death patients. P value <0.05 was considered significant difference (Bold font).
Blood gas analysis between the 2 groups of patients on admission was also performed. The results showed that the PaO2:FiO2, minimum PaO2:FiO2 and minimum SpO2 values of the death group were significantly lower, but no significant difference of pH, PaCO2 or PaO2 were found between these 2 groups (all P > 0.05, Table 3 ).
TABLE 3.
Blood gas analysis of recovery and death patients with severe or critical COVID-19.
| Characteristics | Normal range | Recovery (n = 60) | Death (n = 14) | P Value |
|---|---|---|---|---|
| pH | 7.35-7.45 | 7.44 (7.42-7.46) | 7.46 (7.41-7.47) | 0.197 |
| PaCO2, mm/Hg | 35-48 | 38 (34-43) | 34 (29-38) | 0.075 |
| PaO2, mm/Hg | 83-108 | 64 (52-77) | 57 (48-64) | 0.062 |
| PaO2:FiO2, mm/Hg (Admission) | 400-500 | 205 (145-263) | 131 (83-143) | <0.001 |
| PaO2:FiO2, mm/Hg (Discharged) | 400-500 | 303 (247-425) | 0 (0-0) | <0.001 |
| PaO2:FiO2, mm/Hg (Minimum) | 400-500 | 168 (116-227) | 59 (49-88) | <0.001 |
| SpO2, %, (Admission) | ≥ 93% | 93 (88-96) | 91 (85-94) | 0.116 |
| SpO2, %, (Minimum) | ≥ 93% | 90 (86-92) | 80.5 (63-85) | < 0.001 |
Data are reported as median (IQR). The P value represents the difference between COVID-19 recovery and death patients. P value <0.05 was considered significant difference (Bold font).
CT Imaging Results
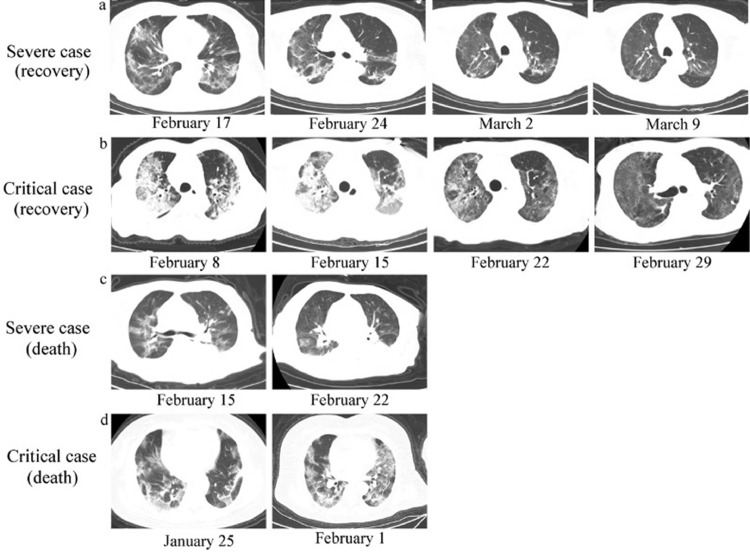
All patients included in this study completed CT imaging on the day before or within 48 hours of admission. The results were consistent with typical COVID-19 imaging results. All patients had diffuse lung lesions, which were mainly manifested by multiple patchy shadows and ground glass shadows with bronchial inflation signs. Most patients had large areas of consolidation with thickened lobular septa. The CT images of the lungs showed that critical cases were more serious than severe cases, and death cases were more serious than in patients who recovered. After treatment, lung inflammation in the recovery group was significantly improved, but the CT imaging results of the death group did not show improvement (Figure 1 ).
FIGURE 1.
Chest computed tomography (CT) imaging of COVID-19 patients. From top to bottom, the CT images of the lung are shown. A, Severe case (recovery), B, critical case (recovery), C, severe case (death), D, critical case (death). The brightness of patients’ lungs is decreased diffusely, presenting typical signs of viral infection and large areas of multiple ground-glass opacities or patchy shadows with an uneven density.
Treatment and Clinical Outcomes
After hospitalization, all patients received treatment according to the “Diagnosis and Treatment Protocol for COVID-19 (Trial Version 5)”.19 As Table 4 shows, all patients received antiviral therapy. The main antiviral drugs used were lopinavir and ritonavir (91.9%), ganciclovir (5.4%) and arbidol (2.7%). Fifty-eight (96.7%) patients in the recovery group and 14 (100%) patients in the death group received antibiotic therapy (P = 0.271). Immune enhancing treatment with intravenous immunoglobulin was given to 51.7% of the recovered cases and 71.4% of the death cases (P = 0.180). In terms of respiratory support, 67 (90.5%) patients received high flow nasal catheter oxygen therapy, 14 (18.9%) patients received noninvasive mechanical ventilation and 6 (8.1%) patients had invasive mechanical ventilation. Compared with patients in the recovery group, death patients had a higher proportion of invasive ventilation (P < 0.001), and 2 cases received extracorporeal membrane oxygenation as salvage therapy (P = 0.020). The median hospitalization period for patients with severe and critical COVID-19 pneumonia was 15 days. Seven days following admission was used as a time cut-off point for assessing outcomes. Fifty-five patients were discharged (80.8%).
TABLE 4.
Treatments and outcomes of recovery and death patients with severe or critical COVID-19.
| Characteristics | Total (n = 74) | Recovery (n = 60) | Death (n = 14) | P Value |
|---|---|---|---|---|
| Medical treatment | ||||
| Antiviral therapy | 74 (100%) | 60 (100%) | 14 (100%) | 1.000 |
| Lopinavir and Ritonavir | 68 (91.9%) | 57 (95.0%) | 11 (78.6%) | 0.043 |
| Ganciclovir | 4 (5.4%) | 2 (3.3%) | 2 (14.3%) | 0.103 |
| Arbidol | 2 (2.7%) | 1 (1.7%) | 1 (7.1%) | 0.255 |
| Antiviral drug atomization therapy | 68 (91.9%) | 56 (93.3%) | 12 (85.7%) | 0.347 |
| Antibiotic therapy | 68 (91.9%) | 54 (90.0%) | 14 (100%) | 0.271 |
| Corticosteroids therapy | 70 (94.6%) | 58 (96.7%) | 12 (85.7%) | 0.103 |
| Total duration of corticosteroids, days | 11 (8-15) | 11 (8-15) | 10 (5-15) | 0.369 |
| Intravenous immunoglobulin therapy | 41 (55.4%) | 31 (51.7%) | 10 (71.4%) | 0.180 |
| Oxygen inhalation | ||||
| High flow nasal catheter | 67 (90.5%) | 58 (96.7%) | 9 (64.3%) | <0.001 |
| Noninvasive mechanical ventilation | 14 (18.9%) | 5 (8.3%) | 9 (64.3%) | <0.001 |
| Invasive mechanical ventilation | 6 (8.1%) | 0 (0.0%) | 6 (42.9%) | <0.001 |
| Extracorporeal membrane oxygenation | 2 (2.7%) | 0 (0.0%) | 2 (14.3%) | 0.020 |
| Outcomes | ||||
| Hospital stay, days | 15 (11-22) | 16 (12-22) | 13 (10-23) | 0.267 |
| Severe cases | 48 (64.9%) | 47 (78.3%) | 1 (7.1%) | <0.001 |
| Critical cases | 26 (35.1%) | 13 (21.7%) | 13 (92.9%) | <0.001 |
Data are reported as n (%) or median (IQR). The P value represents the difference between COVID-19 recovery and death patients. P value <0.05 was considered significant different (Bold font).
DISCUSSION
This study retrospectively analyzed the clinical characteristics and outcomes of severe and critical COVID-19 patients. Currently, research on severe and critical COVID-19 patients is scarce, especially the clinical comparison of recovery cases to death cases.
There was no significant difference in the proportion of COVID-19 between male and female patients, but the mortality rate of male patients was higher (78.6% versus 21.4%), and the average age of patients who died was significantly older than those who recovered. In addition, 75.7% of all patients with COVID-19 had other chronic diseases, consistent with recent reports.20, 21, 22 Patients in the death group presented with a higher incidence of hypertension and coronary disease. Therefore, we conclude that elderly male patients with certain chronic diseases have a higher risk of death. The main clinical symptoms were fever, cough and expectoration, fatigue and dyspnea, and muscle aches, again consistent with the reported clinical features of typical COVID-19 patients.22 However, in this study, the proportion of patients with dyspnea was 66.2%, and 92.9% in patients who died. Compared with nonsevere or noncritical cases the incidence of dyspnea was higher in patients with severe or critical COVID-19 and was more pronounced in the complaints of the patients at the doctor's visit. Therefore, more attention should be paid to the emergence of ARDS during hospitalization.
Previous literature has reported that most patients with COVID-19 have fewer lymphocytes and elevatedCRP. In addition, they have fewer white blood cells, increased D-dimer and abnormal liver enzymes.23 In this study, the proportion of patients with abnormal white blood cells was 40.6%, 71.6% of patients had lymphopenia, 70.2% of patients had increased neutrophils, 93.2% had increased CRP and 33.8% had increased procalcitonin, which indicates that patients with severe and critical COVID-19 have obvious inflammatory reactions. Although these laboratory diagnostic parameters differed between recovery and death cases, there was no significant difference. In severe and critical COVID-19 patients, especially in death cases, D-dimer was significantly increased, the disease condition was prone to worsen, and sudden death could occur. Most patients had coagulation dysfunction, so it is recommended that the risk of venous thromboembolism be evaluated when preventing and treating COVID-19. Effective prevention should be implemented for high-risk patients. Physicians should be alert to patients with clinical manifestations such as sudden deterioration of oxygenation, respiratory distress and decreased blood pressure that can lead to a pulmonary embolism and treat them accordingly.
According to the CT images reviewed and scored, in severe and critical COVID-19 patients, imaging abnormalities are more pronounced in death cases than in recovery cases. The chest CT imaging of COVID-19 patients in death cases frequently shows a mixed pattern of consolidation in the center and ground glass opacity in the peripheral like a “halo sign,” and multiple patches or an integrated larger patch of ground glass opacity, consolidation or mixed consolidation and ground glass opacity might present in bilateral lungs as the disease progresses. This extensive consolidation and ground glass-like shadows are typical imaging manifestations of acute lung injury24 and suggest that patients with COVID-19 may progress rapidly to ARDS.21 However, the CT imaging of some patients has no obvious worsening symptoms, but according to the degree of respiratory distress, can lead the physician to measure oxygen saturation, blood gas analysis and other organ functions, which clearly meet the criteria of for a positive diagnosis of cases that are severe or of critical severity. This may be related to factors such as the patient's age, physical fitness, underlying disease and mood after illness. Therefore, CT imaging cannot be used as the sole criterion for assessing the severity of a patient's condition.
According to the opinion of the “Diagnosis and Treatment Protocol for COVID-19 (Trial Version 5),”19 patients with severe COVID-19 should receive a nasal cannula or an oxygen mask. When respiratory distress and/or hypoxemia cannot be alleviated after receiving standard oxygen therapy, nasal high-flow oxygen therapy or noninvasive ventilation can be considered. If the condition does not improve or worsens within a short time (1-2 hours), tracheal intubation and invasive mechanical ventilation should be performed. Most patients in this study (90.5%) received oxygen through nasal high-flow oxygen therapy. This allowed respiratory symptoms to improve and hypoxemia to be corrected. Some patients (18.9%) received noninvasive mechanical ventilation, and 8.1% of patients received invasive mechanical ventilation. However, compared with the recovery patients, the proportion of patients who received invasive mechanical ventilation was significantly greater in death cases, even when extracorporeal membrane oxygenation was performed as salvage therapy.
In summary, severe and critical COVID-19 poses a greater risk to the elderly and those with comorbidities. These patients had more severe clinical symptoms, especially dyspnea and lung inflammation identified by CT imaging. Most patients are generally in poor condition, prone to mixed infection, liver injury and high mortality. In view of this situation, comprehensive treatment including rational medication and professional care should be carried out early to improve the cure rate and reduce mortality.
AUTHOR CONTRIBUTIONS
C.A. Cao had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: J.L. Li, Y.W. Luo, C.A. Cao. Acquisition, analysis or interpretation of data: J.L. Li, Y.W. Luo, X. Peng, G. Xu, H.P. Yu. Drafting of the manuscript: J.L. Li, Y.W. Luo. Critical revision of the manuscript for important intellectual content: C.A. Cao, X. Peng. Statistical analysis: J.L. Li. Administrative, technical, or material support: J.L. Li, X. Peng, G. Xu, H.P. Yu. Study supervision: C.A. Cao.
ACKNOWLEDGMENTS
We thank Dr. Zhi Wang of Beijing University of Technology for his help in data statistical analysis. We thank the patients and their family members for participating in our study.
Footnotes
Funding: The authors received no funding for this work.
Conflicts of Interest: The authors have no financial or other conflicts of interest to disclose.
REFERENCES
- 1.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Guan X, Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan JF, Yuan S, Kok KH. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Coronavirus disease (COVID-2019) situation reports. Apr. 21, 2020. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200421-sitrep-79-covid-19.pdf?sfvrsn=4796b143_4. Accessed April 21, 2020.
- 7.The General Office of the National Health Commission. Diagnosis and treatment protocol for COVID-19 (Trial Version 6). Available at: https://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf. Accessed April 21, 2020.
- 8.Malik YS, Sircar S, Bhat S. Emerging novel coronavirus (2019-nCoV)-current scenario, evolutionary perspective based on genome analysis and recent developments. Vet Q. 2020;40(1):68–76. doi: 10.1080/01652176.2020.1727993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu R, Zhao X, Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu K, Fang YY, Deng Y. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang X, Rayner S, Luo MH. Does SARS-CoV-2 has a longer incubation period than SARS and MERS? J Med Virol. 2020;92(5):476–478. doi: 10.1002/jmv.25708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorusso A, Calistri P, Petrini A. Novel coronavirus (SARS-CoV-2) epidemic: a veterinary perspective. Vet Ital. 2020 doi: 10.12834/VetIt.2173.11599.1. [DOI] [PubMed] [Google Scholar]
- 14.National Health Commission of the People's Republic of China. Announcement of the National Health Commission of the People's Republic of China (No. 1, 2020). (2020-01-20). Available at: https://www.gov.cn/xinwen/2020-01/21/content_5471158.htm. Accessed April 1, 2020.
- 15.Letko M, Munster V. Functional assessment of cell entry and receptor usage for lineage b β-coronaviruses, including 2019-nCoV. bioRxiv. 2020:2020.01.22.915660. [DOI] [PMC free article] [PubMed]
- 16.Yang W, Cao Q, Qin L. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80(4):388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu YH, Dong JH, An WM. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect. 2020;80(4):394–400. doi: 10.1016/j.jinf.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. Jan 11, 2020. Available at:https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf. Accessed April 5, 2020.
- 19.The General Office of the National Health Commission. Diagnosis and treatment protocol for COVID-19 (Trial Version 5). Available at: https://www.gov.cn/zhengce/zhengceku/2020-02/05/5474791/files/de44557832ad4be1929091dcbcfca891.pdf. Accessed April 5, 2020.
- 20.Wang D, Hu B, Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan WJ, Ni ZY, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen N, Zhou M, Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang XY, Miao CL, Jin MD. Status and progress of new coronavirus pneumonia in 2019. Chin Crit Care Emerg Med. 2020;32(02) doi: 10.3760/cma.j.issn.2095-4352.2020.0006. E006-E006. [DOI] [Google Scholar]
- 24.Harris RS. Acute respiratory distress syndrome: imaging of the injured lung. Clin Radiol. 2002;57(9):865. doi: 10.1016/s0009-9260(02)90999-2. Author reply 865. [DOI] [PubMed] [Google Scholar]