Abstract
Background and Aims
As community-based ambulatory endoscopy centers (AECs) across the nation are trying to reopen and safely resume outpatient endoscopic procedures after the unprecedented lockdown related to the coronavirus disease 2019 (COVID-19) pandemic, guidelines recommend pretesting and screening for COVID-19 along with other mitigation measures for the safety of patients and staff. The impact of such changes in the workflow of AECs on throughput and other performance indicators is largely unknown, although a significant reduction in revenue stream is expected.
Methods
A discrete event simulation–based model was developed in the setting of a small to medium community-based single-specialty AEC to quantify the impact of COVID-19–related workflow changes on performance indicators and cost per case compared with the pre–COVID-19 baseline.
Results
In the simulation model, post–COVID-19 recommended workflow changes significantly impacted the operational and productivity metrics and, in turn, adversely affected financial metrics. Overall, there was a significant decrease in staff utilization and consequent increase in total facility time, waiting time for patients, and cost per case because of a bottleneck at the time of preprocedure COVID-19 screening and testing while practicing social distancing. Strategies to minimize this adverse impact on productivity were assessed.
Conclusions
Pretesting and screening for COVID-19 as recommended by current guidelines will significantly impact the productivity and revenue stream of AECs. Urgent measures by payors are needed to adjust the facility reimbursement of endoscopy centers to ensure successful reopening and ramping up outpatient endoscopy services in these facilities already hit hard by the pandemic.
Abbreviations: AEC, ambulatory endoscopy center; COVID-19, novel coronavirus disease 2019; DES, discrete event simulation; POC, point of care; PPE, personal protective equipment; RN, registered nurse
Graphical abstract
The novel coronavirus disease 2019 (COVID-19) pandemic has led to millions of people being affected by illness and death and has disrupted life everywhere, including in the United States. Since the start of the pandemic, the rigorous mitigation and suppression efforts that were undoubtedly necessary to contain the pandemic also severely damaged our economy and, specifically, the healthcare sector. The specialty of gastroenterology, with its screening and diagnostic endoscopic procedures in ambulatory endoscopy centers (AECs), has been no exception, with most outpatient clinics and centers being locked down for extended periods.1 In addition to the short-term effects, it is expected that the COVID-19 pandemic will have a lasting and significant impact on the practice of outpatient endoscopy.2
As AECs struggle to reopen cautiously with the gradual resumption of diagnostic and elective endoscopic procedures, multiple governmental and society guidelines have been published. These guidelines have focused on the recommended changes in operational logistics and workflow, such as appropriate use of personal protective equipment (PPE), screening and pretesting of staff and patients, use of heightened sanitation protocols, and practice of social distancing to minimize the risk of spread of COVID-19 infection.2, 3, 4, 5
An AEC is typically a busy, high-throughput facility in which timely, safe, and cost-effective patient-centric care with an emphasis on outcomes is a must for successful operation. The extensive changes in the AEC workflow that will be needed in the post–COVID-19 era is sure to disrupt the regular workflow of most AECs and will have an unknown but significant impact on patient volumes, staff utilization, process times, and by extension the overall net revenue generated by the AEC.
Although most outpatient endoscopic procedures are performed in small- to medium-sized community-based stand-alone AECs, minimal quantitative information exists on the performance indicators of the workflow of these centers, and this makes an assessment of the overall impact of COVID-19–related workflow changes in these facilities even more challenging. In this study, the workflow of a community-based AEC was modeled to quantify the potential financial impact of COVID-19 on these facilities.
Methods
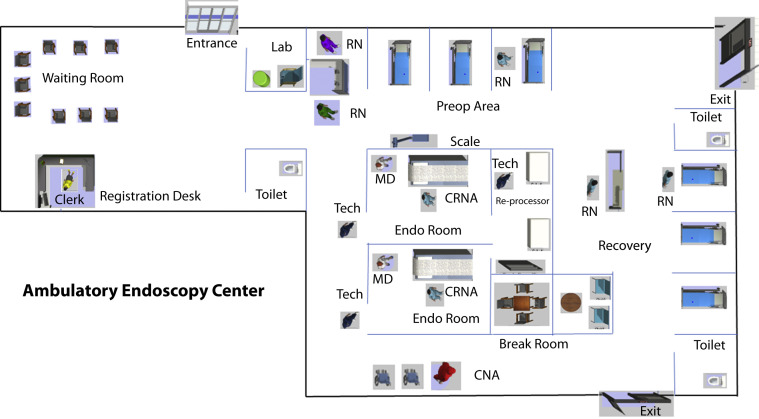
The workflow of a small to medium community-based, 2-room, 5700 square foot, single-specialty accredited AEC performing approximately 6000 endoscopic procedures annually was modeled using a discrete event simulation (DES) software (AnyLogic simulation software; AnyLogic Company, Oak Brook, Ill, USA). DES is a computerized virtual simulation technique where interactions between patients and environments of fixed and human resources can be modeled based on the different sequences of events that happen in actual practice in chronological order.6 Using the software, we created a virtual healthcare environment using realistic 3-dimensional visuals and guided patient process flow designed to simulate the movement of a patient as he or she moves through the AEC from entry to exit for undergoing specific procedures, interacting with staff and resources customized by patient attributes (Fig. 1 ). Milestones were embedded at different points of the workflow to monitor and quantify performance indicators. The various performance indicators considered in this model with definitions are shown in Table 1 .7 , 8 The different input variables used in this model are shown in Table 2 .
Figure 1.
Schematic floor diagram (not drawn to scale) of a 2-room ambulatory endoscopy center showing work areas and staffing patterns. The area marked as “Lab” near the entrance is the area designated for COVID-19 testing and screening and would be physically isolated from the rest of the center. The seating capacity of the waiting room area may be modified to conform to the practice of social distancing. RN, Registered nurse; MD, endoscopist; CRNA, certified nurse anesthetist; CNA, certified nurse assistant; Tech, endoscopy technicians.
Table 1.
Selected performance indicators of an AEC
| Performance indicators | Definition |
|---|---|
| Operational indicators | |
| Facility time, min | The average total time spent by patients in AEC (time between initial entry and exit from the AEC) |
| Registration time, min | The average time between entry into AEC and completion of registration |
| Preoperative time, min | The average time between the end of registration and transfer out of the preoperative bay |
| Endoscopy room time, min | The average time between entry into an endoscopy room and transfer to a recovery room |
| Facility utilization, % | Percentage of time a specific location is used for providing patient care and maintenance/cleaning (instead of remaining unoccupied) |
| Waiting room | |
| Preoperative bays | |
| Endoscopy rooms | |
| Recovery bays | |
| COVID-19 testing/screening area | |
| Waiting time for patients, min | The average wait time for patients to acquire a specific location, which is currently not available (being used for another patient or undergoing maintenance/cleaning) |
| Waiting for preoperative bays | |
| Waiting for endoscopy rooms | |
| Waiting for recovery room | |
| Waiting for COVID-19 screening and testing | |
| Number of late discharges | Discharges that are completed after the scheduled closing time of the AEC (17:30) |
| Overtime | Daily average length of time a unit is operating past its operating hours (1730) |
| Productivity indicators | |
| Throughput | Number of patients/endoscopy room/day |
| Staffing utilization, % | The percentage of the time when staff is engaged in ongoing patient care and completing related paperwork/maintenance/cleaning (instead of remaining idle) |
| Physician utilization | |
| Nursing utilization | |
| Preoperative RN | |
| Recovery RNs | |
| CRNA | |
| Ancillary staff utilization | |
| Endoscopy technicians | |
| Certified nursing assistant | |
| Clerk | |
| Triage technician | |
| Total staff hours per case (excluding physicians and CRNAs∗) | Total staff hours divided by the number of patients per day |
| Financial indicators | |
| Net revenue per patient | Calculated by the total estimated reimbursement for facility fees per patient Calculated by the total staffing cost by hourly wage per patient Percentage of value-added time (based on utilization) divided by the total amount of staffing time |
| Staffing cost per patient (excluding physicians and CRNAs∗) | |
| Percent value-added† (activity-based costing) staffing time (excluding physicians and CRNAs∗) | |
AEC, Ambulatory endoscopy center; RN, registered nurse; CRNA, certified nurse anesthetist.
Physicians and CRNAs are reimbursed for billable professional services that are not included in the facility fees.
Value-added time was calculated using the average costing method by dividing total average staffing utilization time (when the staff was actively engaged in patient care or related activities) divided by the total staffing time.11
Table 2.
Input variables used in the simulation model
| Variables | Pre–COVID-19 | Post–COVID-19 |
|---|---|---|
| Facility-related variables | ||
| No. of endoscopy rooms | 2 | 2 |
| No. of preoperative bays | 3 | 3 |
| No. of recovery bays | 3 | 3 |
| No. of chairs in the waiting area | 9 | 6 |
| COVID-19 screening and testing area before entry into AEC | NA | Yes |
| Staffing variables | ||
| Endoscopists | 2 | 2 |
| Certified nurse anesthetist | 2 | 2 |
| Preoperative RN | 3 | 3 |
| Recovery RN | 3 | 3 |
| Certified nursing assistant | 1 | 1 |
| Registration clerk | 1 | 1 |
| COVID-19 triage staff | 0 | 1 (strategy I) 2 (strategy II) |
| Throughput | ||
| No. of cases scheduled per hour | ||
| 7 to 12 PM (patient arriving every 30 minutes) | 2 | 2 |
| 12:30 to 2:30 PM | 1 | 1 |
| Process times, min | ||
| COVID-19 screening and testing | 0 | 10-30 |
| Registration | 10 | 10 |
| Gown change, weighing, and preoperative check-in (including consent, intravenous cannula placement) | 23 | 23 |
| Anesthesia consent and assessment | 5 | 5 |
| Endoscopy procedure | 30 | 30 |
| Recovery time | 30 | 30 |
| Postprocedure discussion with the endoscopist | 5 | 5 |
| Enhanced sanitation: cleaning time after each patient, min | ||
| COVID-19 testing and screening area | NA | 5 |
| Preoperative area | NA | 5 |
| Endoscopy room | 3 | 10 |
| Recovery room | NA | 5 |
| Daily terminal cleaning | Yes | Yes |
| Cost variables | ||
| Average staffing cost per hour∗ (excluding endoscopist/certified nurse anesthetist who would bill for professional services) | $295 | $358 (strategy I) $358 (strategy II) |
| Cost of PPE per case† | 0 | $5 |
AEC, Ambulatory endoscopy center; RN, registered nurse; PPE, personal protective equipment; NA, not applicable.
Staffing cost was estimated by using national average hourly salaries of staff. The average salary per hour are as follows: RN, $37; endoscopy technicians, $24; registration clerk, $20; and certified nursing assistant, $18. Overtime pay was considered at 150% of regular pay.13
High-risk PPE was needed for all endoscopy room personnel and included an N95 mask, covered with a surgical mask, shield, gown, shoe covers, and a double set of gloves. For other staff, low-risk PPE was needed and included surgical masks and gloves.17
Initial observation and audit of the AEC
Before building the simulation model, the workflow of a community-based 2-room AEC was carefully observed, and an historical review of the staffing pattern and the number of cases performed each day over the last 2 quarters of 2019 was assessed for estimation of the model input parameters as part of an ongoing quality improvement effort.
Pre–COVID-19 baseline workflow of the AEC
The AEC was modeled with 2 endoscopy rooms, 3 preoperative bays, and 3 recovery bays, each bay with a bed that would be used to transport the patient to the procedure room and recovery area. The AEC was assumed to be a paperless electronic health record–based facility (including anesthesia record) with automated report generation and printing/faxing to referring providers. The AEC would be open every weekday from 0700 to 1730 or until the last patient left the facility. However, the model allowed overtime wages for staff in case of extended hours required for patient care related to late procedures and discharges. Every day there would be a 30-minute lunch break for the facility staff from noon during which staff would be expected to be unavailable except when taking care of patients already under the process flow. Patients would undergo 3 different types of endoscopic procedures, namely EGD, colonoscopy (including screening), and EGD and colonoscopy at the same time (a double). The distribution of the percentage of the 3 types of procedures was estimated based on the initial review (Table 3).
Table 3.
Input variables for Monte-Carlo analysis
| Variables | Median (interquartile range) |
|---|---|
| Distribution of types of procedure, % | |
| EGD | 30 (20-40) |
| Colonoscopy | 60 (20-80) |
| Double | 10 (5-15) |
| Proportion of screening colonoscopy, % | 35 (25-60) |
| National average Medicare reimbursement for facility fees,∗ U.S.$ | |
| EGD | 398 |
| Colonoscopy | 507 |
| Screening colonoscopy | 386 |
| Distribution of payor mix as a percent of cases, % | |
| Medicare | 31 (22-44) |
| Medicaid | 6 (4-12) |
| Commercial Insurance | 52 (41-62) |
| Other (self-pay, worker’s compensation, etc.) | 10 (6-18) |
| Reimbursement as a percentage of the Medicare fee schedule, % | |
| Commercial | 120 (90-200) |
| Medicaid | 90 (70-100) |
| Other | 100 (80-110) |
Reimbursement was calculated by facility fees payable for Healthcare Common Procedure Coding System codes 2020 final ambulatory surgery center (ASC) payment by Medicare. For EGD: Healthcare Common Procedure Coding System codes 43235-6, 43239; for screening colonoscopy: 44378, G0105, G1012; for colonoscopy: 144389, 44391-2, 44394, 44401, 44404-5 were used. For “double” procedures, modifier 51 is used for 2 procedures performed on the same day, for example, EGD and colonoscopy, listing the code with the greatest value first, as the multiple procedure rule applies.18
After entry into the facility, patients would either go to the registration desk directly or to the waiting room area until the registration clerk was available to help the patient register. Once registered, patients would wait in the waiting area until 1 of 3 preoperative registered nurses (RNs) would escort the patient to a restroom for changing into a patient gown and getting weighed on a scale and to a preoperative bay, in that order. In the preoperative bay, the patient would provide informed consent, baseline vitals would be taken, physical examination would be performed, and an intravenous cannula would be placed for use during the procedure. After the preoperative assessment, the designated certified nurse anesthetist would obtain anesthesia consent and reassess the patient. All procedures were assumed to be done with the patient under monitored anesthesia care without the need for endotracheal intubation, independently administered by the certified nurse anesthetist. Next, the certified nurse anesthetist would transport the patient to the endoscopy procedure room, and the endoscopist assisted by an endoscopy technician would perform the endoscopic procedure. Once the procedure is completed, the certified nurse anesthetist would transport the patient to an available recovery bay, where one of the recovery RNs would recover the patient over a period of at least 30 minutes. Once ready for discharge, the performing endoscopist would walk over to the recovery bay and discuss the endoscopy report with the patient and establish a plan for ongoing management. Next, a certified nursing assistant would escort the patient to one of the designated exits in a wheelchair for release from the facility.
After completion of each endoscopy procedure, an endoscopy technician would prepare the room for the next procedure, which included cleaning and setting up the equipment. Also, during the operating hours using a designated general task process flow, 1 of 3 endoscopy technicians would perform manual cleaning of the used endoscope and place it in the endoscopy reprocessors. Also, a separate general process workflow was established to answer incoming phone calls during the operating hours by the clerk, preoperative RN, and recovery RN in order of availability.
COVID-19–related changes in the workflow of the AEC
Long-term changes to the AEC workflow related to the COVID-19 pandemic were modeled using the trisociety guidelines and recent recommendations published by the American College of Gastroenterology.4 , 5 All patients scheduled for an endoscopic procedure would be assessed by a telehealth-based questionnaire screening by the performing physician’s office a few days before the procedure, preferably before any procedural preparation. This screening was not considered as a part of the AEC workflow.
On the day of the procedure, all patients scheduled for endoscopic procedures would undergo an in-facility point of care (POC) molecular testing, along with temperature, symptom, and exposure screening. The COVID-19 screening and testing would be done in an isolated, preferably externalized area of the AEC near the entrance, and only screened patients would be allowed to enter the waiting room area of the AEC. No family or escorts would be allowed in the center unless exceptional circumstances arise. Patients after recovery would exit through the separate exit(s) distinct from the entrance of the center.
A trained triage technician would perform POC testing and COVID-19 screening. Two strategies were modeled based on the initial optimization trials of the simulation model. Under strategy I, only 1 COVID-19 testing station was used, staffed by 1 triage technician. In strategy II, 2 separate COVID-19 testing stations were used and staffed by 2 triage technicians. Strict social distancing protocols would be followed, and the waiting room capacity would be restricted to maintain physical distancing by at least 6 feet. Patients with positive POC COVID-19 testing or positive screening would not be cared for in the AEC, and the affiliated practice would undertake alternate arrangements such as delaying the procedure or performing the procedure in a hospital setting according to established protocols.
All patients and staff would use surgical/loop masks at all times while in the AEC. All endoscopy room personnel would use high-risk PPE; other staff would use basic PPE. It was assumed that adequate PPE would be available and reusing PPE, such as N95 masks, would not be required.
Enhanced cleaning of COVID-19 testing area(s), preoperative and recovery bays, and the endoscopy room would be performed after each patient. Terminal cleaning of the procedure room at the end of the day would be done after the last procedure.
Monte-Carlo analysis
To ensure robustness and generalizability of this model applicable to small- and medium-sized AECs in most communities in the United States, a Monte-Carlo simulation of 1000 simulations, using a wide distribution of probabilities of payor mix, rates of payment by commercial insurance in terms of percentage of Medicare reimbursements, and procedural type mix of diagnostic and screening colonoscopies was performed (Table 3 ). During the simulation, instead of point estimates of these parameters, triangular distributions were used in the simulation model, with median values the most likely values and interquartile range values as the bounds of the distribution.6 Also, to simulate real-life scenarios, an exponential distribution was used to introduce stochastic randomness for each instance of the time variable requiring a discrete input in the model (such as arrival time, waiting times, procedure, and recovery time).9
Assumptions
Certain assumptions were necessary for modeling the workflow of the AEC. The number of patients scheduled per day was based on actual observation of a 2-room AEC, and results of the initial model optimization runs when the throughput of the system was optimized, taking into account the flexibility of human resources. Simulation length was fixed to 1 day and did not require any warm-up period because the system was reset each day. In this model, it was assumed that a finite number of staff, procedure rooms, and endoscopists would be available at any time, and patient flow was restricted based on their availability. Endoscopes, preprocessors, waiting room capacity, and ancillary equipment, such as weighing scale and wheelchairs for transport, were assumed to have unlimited availability for maximizing throughput.9
Occupancy costs; costs related to equipment, drugs, and materials; and fixed and overhead operational costs were not considered because they are unlikely to be affected by the COVID-19 pandemic. No significant capital outlay for COVID-19–related AEC workflow modifications was considered. Specific strategies of disinfection (such as the use of ultraviolet germicidal irradiation) or related to decreasing environmental risk dissemination (increasing number of air exchanges, external air exhaust) or interventions such as flipping (alternating) rooms, prolonged turnover times, and EGD-only rooms have not been widely studied or validated and are not routinely recommended. Standard reprocessing algorithms for endoscope reprocessing are considered adequate.10
It is expected that in the reopening phase, the administrative staff (typically the nursing administrator) of the AEC will bear additional responsibilities for patient and staff safety, appropriate use of PPE, retraining staff, infection control practices, scheduling and ensuring supply chain stability, and coordination with local regulatory authorities. However, no additional administrative cost was considered in this model. Follow-up phone calls within 24 to 48 hours after procedures is a standard practice in many endoscopy centers, and it was assumed that no additional follow-up phone calls would be required.
Considerable uncertainties exist regarding type of POC testing, performance characteristics, reimbursement amount, and, importantly, availability of POC testing.10 Although the guidelines currently do not explicitly recommend testing on the day of procedure because of issues of local prevalence, testing availability, sensitivity, and turn-around time, it is widely perceived that pretesting 48 to 72 hours before the procedure is impractical, particularly because of the need for self-quarantine after testing. Patients are unlikely to agree to self-quarantine on a routine basis given its potential impact on employment and lifestyle. Also, different options for POC testing for COVID-19 are rapidly becoming available. For example, the Sofia 2 SARS antigen fluorescent immunoassay (Quidel Corporation, San Diego, Calif, USA) can detect a COVID-19 infection in a sample within 15 minutes and has been recently approved by the U.S. Food and Drug Administration. Another recently approved molecular diagnostic test, Xpert Xpress SARS-CoV-2 (Cepheid, Sunnyvale, Calif, USA), can provide a result as soon as 30 minutes. Even if day-of-procedure testing is not performed, the recommended COVID-19 screening, which includes a check for daily temperature, symptom and exposure screening, and a temperature check on arrival to the endoscopy center in an isolated area performed by the triage technician, is estimated to require as much as 30 minutes per patient. It was assumed that although all patients would need POC pretesting, this would be cost-neutral to the endoscopy center. Although Medicare reimbursement for COVID-19 molecular diagnostics tests that use high-throughput technologies have gone up to $100 per test, the capital cost of establishing an in-house COVID-19 testing laboratory is currently unknown.
Validation of the model
The simulation model was built by using observed (historical) data of procedures done in a community AEC from the last 2 quarters of 2019 extracted as part of a quality improvement process. The result of the baseline simulation, which was verified as logical by the author, was concordant with actual data. Also, the different processing times (as listed in Table 1) predicted by the baseline simulation model were internally validated with another set of data obtained from randomly selected electronic time-stamped records of 50 patients who underwent procedures in the AEC in the second week of February 2020 (a few weeks before the COVID-19 pandemic led to the temporary closure of the AEC). In addition, the results of the baseline simulation in terms of waiting times and staff utilization correlated well with published results from studies performed in academic medical centers and a nationwide survey of community-based AECs.6 , 11, 12, 13
Results
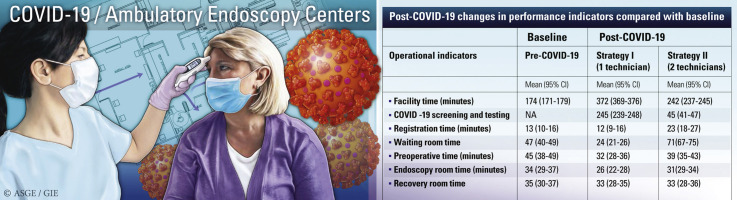
In the simulation model, post–COVID-19 recommended workflow changes significantly impacted the operational and productivity metrics and, in turn, adversely affected the financial metrics. The results of the changes in these metrics are listed in Table 4 . Overall, with the post–COVID-19 modifications, there was a significant increase in total processing times, waiting times with a consequent decrease in productivity, and financial metrics precisely because of a bottleneck at the time of preprocedure COVID-19 screening and testing while practicing social distancing (Fig. 2 ). Incorporating a day-of-procedure COVID-19 screening and testing protocol in the AEC led to a significant decrease in staff utilization and an increase in facility time, wait time for patients, and cost per case (Fig. 3 ). Also, if the additional time required for COVID-19 screening and testing exceeded 30 minutes, then the day-of-procedure screening was not practical and would have to be performed day(s) before the procedure. Such an arrangement would invariably lead to an increase in patient inconvenience and decreased compliance given the need for quarantine until the day of the procedure.
Table 4.
Results of the simulation showing post–COVID-19 changes in performance indicators compared with baseline
| Operational indicators | Baseline |
Post–COVID-19 |
|
|---|---|---|---|
| Pre–COVID-19 | Strategy I (1 technician) | Strategy II (2 technicians) | |
| Facility time, min | 174 (171-179) | 372 (369-376) | 242 (237-245) |
| COVID -19 screening and testing | NA | 245 (239-248) | 45 (41-47) |
| Registration time, min | 13 (10-16) | 12 (9-16) | 23 (18-27) |
| Waiting room time | 47 (40-49) | 24 (21-26) | 71(67-75) |
| Preoperative time, min | 45 (38-49) | 32 (28-36) | 39 (35-43) |
| Endoscopy room time, min | 34 (29-37) | 26 (22-28) | 31(29-34) |
| Recovery room time | 35 (30-37) | 33 (28-35) | 33 (28-36) |
| Facility utilization, % | |||
| Waiting room | 67 (63-67) | 36 (31-38) | 55 (51-59) |
| Preoperative bays | 63 (61-64) | 38 (33-41) | 54 (51-57) |
| Endoscopy rooms | 61 (59-66) | 52 (49-55) | 60 (58-64) |
| Recovery bays | 58 (53-59) | 41 (38-44) | 53 (51-56) |
| COVID-19 testing area | NA | 84 (79-87) | 54 (51-57) |
| Waiting time for patients, min | |||
| Waiting for preoperative bays | 36 (31-43) | 12 (8-13) | 55 (43-59) |
| Waiting for endoscopy rooms | 1 (1-4) | 3 (1-5) | 2 (1-4) |
| Waiting for recovery room | 2 (1-4) | 1(1-3) | 1(1-3) |
| Waiting for COVID-19 screening and testing | NA | 27 (19-37) | 9 (6-17) |
| Number of late discharges | 0 | 9 | 3 |
| Overtime, min | 0 | 180 | 60 |
| Productivity indicators | |||
| Throughput | |||
| Number of patients/endoscopy room/day | 12.5 | 12.5 | 12.5 |
| Staffing utilization, % | |||
| Physician utilization | 54 (51-57) | 42 (39-44) | 49 (47-51) |
| Nursing utilization | |||
| Preoperative RNs | 58 (53-60) | 56 (52-57) | 76 (72-78) |
| Recovery RNs | 48 (46-50) | 37 (34-40) | 44 (42-46) |
| Certified nurse anesthetist | 56 (53-58) | 43 (41-45) | 52 (49-55) |
| Ancillary staff utilization | |||
| Endoscopy technicians | 57 (55-59) | 41 (38-43) | 49 (47-52) |
| Certified nursing assistant | 16 (14-18) | 19 (17-21) | 22 (19-25) |
| Clerk | 37 (33-39) | 28%(26-30) | 31 (28-34) |
| COVID-19 lab triage technician | NA | 84 (81-87) | 51 (48-53) |
| Average (nonendoscopist/CRNA) staff hours per case | 4.2 (3.9-4.4) | 5.9 (5.7-6.2) | 5.5 (5.3-5.8) |
| Financial indicators | |||
| Reimbursement per patient, U.S.$ | 508 (447-574) | 504 (449-570) | 505 (445-578) |
| Nonphysician/CRNA staffing cost per patient, U.S.$ | 124 (121-127) | 177 (173-184) | 146 (142-152) |
| Percent value added nonphysician staffing time (activity-based costing) | 52 (48-55) | 45 (42-47) | 49 (46-51) |
RN, Registered nurse; CRNA, certified nurse anesthetist; NA, not Applicable.
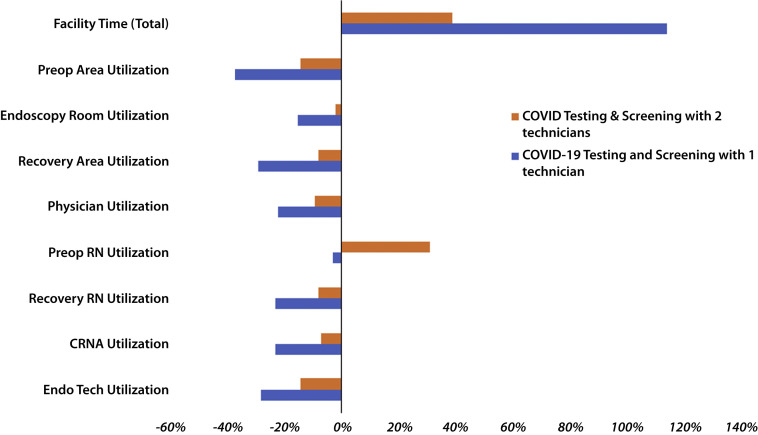
Figure 2.
Percentage of change (x-axis) from the pre–COVID-19 baseline in different performance indicators of the ambulatory endoscopy center (y-axis). The blue and orange bars represent the number of technicians performing COVID-19 testing and screening. Using only 1 technician (blue bars) leads to much more pronounced underutilization of space and staff resources and increased facility time compared with using 2 technicians (orange bars) for COVID-19 testing and screening. RN, Registered nurse; CRNA, certified nurse anesthetist; Endo Tech, endoscopy technicians.
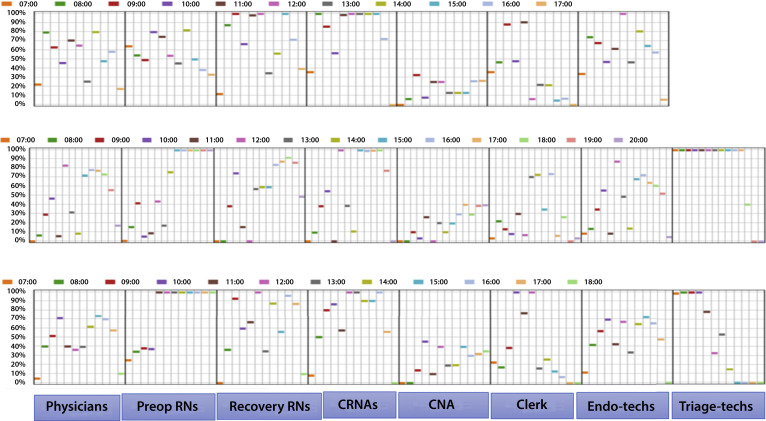
Figure 3.
Percentage of utilization (y-axis) of different staffing resources by the hours of the day as represented by colored boxes along the horizontal axis. The top panel represents the pre–COVID-19 baseline workflow of the center when no technician is needed for COVID-19 testing and screening. The middle panel represents COVID-19 testing and screening with 1 technician, and the bottom panel represents the use of 2 technicians for triage. To maintain throughput at baseline, the working hours of the endoscopy center are lengthened by COVID-19 testing and screening by 3 hours when 1 technician is used and by an hour when 2 technicians are used. Overall, both the middle and bottom panels show decreased staff utilization compared with baseline, except for the triage technicians(s) performing COVID-19 testing and screening. RN, Registered nurse; CRNA, certified nurse anesthetist; CNA, certified nurse assistant; Tech, technicians.
The overall facility time and wait time for patients were sensitive to the COVID-19 testing time needed for each patient. Using only 1 triage technician to perform COVID-19 testing and screening led to more than twice the total facility time and a 42% increase in cost per case related to payment of overtime salaries. Corrective interventions such as changing the interarrival time to every 15 minutes and adding more preoperative/recovery bays or more RNs did not decrease the total facility time or waiting time at the entry-level bottleneck related to COVID-19 testing. However, doubling the number of COVID-19 testing and screening to 2 triage technicians led to a more acceptable but still significantly increased total facility time and cost per case of $142. During Monte-Carlo simulations, the strategy of having 2 triage technicians for COVID-19 testing was always more efficient and cost-minimizing (Fig. 4 ).
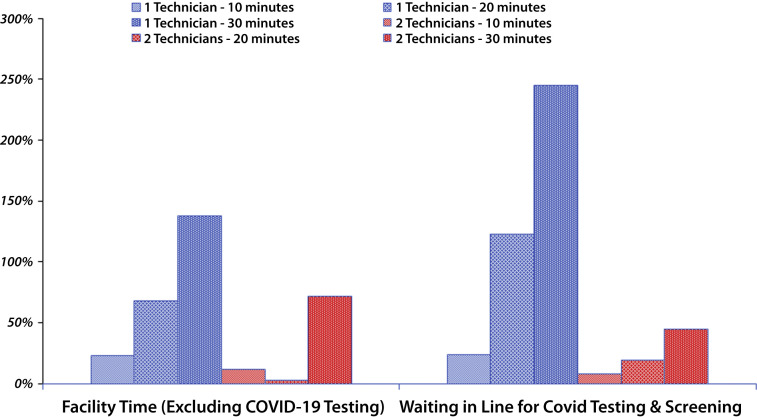
Figure 4.
Percentage of change (y-axis) from the pre–COVID-19 baseline in facility time and waiting time for patients (x-axis) for COVID-19 testing and screening. The blue and red columns represent the use of 1 technician compared with 2 technicians for COVID-19 testing and screening. In each bar group, the different fill patterns represent the time needed for COVOD-19 testing and screening (10 minutes, 20 minutes, and 30 minutes). If 30 minutes or more are needed for testing and screening each patient with only 1 technician, an unacceptable bottleneck of patients ensues waiting in line for testing and screening for more than 4 hours; this can be reduced by deploying 2 technicians for triage.
Incorporation of a day-of-procedure COVID-19 testing and screening led to late discharges and consequently increased hours of operation of the AEC and overtime pay to maintain baseline throughput of 12.5 procedures per day per room (Table 2). In terms of cost of care per patient, lengthening hours of the AEC operation and overtime pay for the staff were cost-minimizing compared with decreasing daily throughput.
Discussion
DES-based modeling is a novel method for formal quantitative assessment of resource utilization, throughput, and capacity constraints of complex systems by simulating dynamic interactions between individuals, populations, and their environments using a sequence of well-defined events and focusing on individual entities (eg, patients) moving through the system with changes in their states at discrete time points.9 Widely used in such diverse areas as in manufacturing, operational research, decision analysis, and production engineering, these simulation models are increasingly used in healthcare technology assessment at organizational levels. Multiple publications have used DES to understand and optimize healthcare system operation in settings such as operating rooms, intensive care units, emergency departments, and urgent care settings.14 Only a few published articles used DES techniques in the context of outpatient endoscopy centers. Berg et al6 used DES to assess the impact of procedure room-to-endoscopist ratio in improving efficiency. Day et al11 used DES to show the effects of minor changes such as reducing scheduling and recovery room times in improving staff utilization and other performance measures. All these studies were done in academic medical centers in an hospital outpatient department (HOPD) setting, which has different levels of reimbursement and often with the participation of trainees.11 , 12 , 15
In this study, incorporation of recommended post–COVID-19 related workflow modifications adversely impacted the efficiency and utilization of an AEC across a wide array of performance indicators and with significant financial implications. Although done in the setting of a community-based stand-alone AEC, the baseline results of this study are generally similar to the results published by other authors in terms of waiting time and staff utilization. Another strength of the study is that the simulation model was validated using historical survey data from other community-based AECs across the country.13 Also, to account for significant variations in procedure type, payor mix, and non-Medicare reimbursement and to introduce stochastic randomness in the process flows of the simulation model, an uncertainty analysis was used with the Monte-Carlo analysis to make the results more generalizable. The model was responsive to the calibration of parameters such as time and number of triage technicians for COVID-19 testing and screening to minimize the impact of COVID-19–related adjustments on baseline performance indicators. From the simulation model, it was clear that if more than 30 minutes is required for day-of-procedure COVID-19 testing and screening at the time of entry into the AEC, then it would lead to an unacceptable increase in facility time and wait times, causing a significant decrease in daily throughput.
Although this model was specifically developed to evaluate the impact of the COVID-19 pandemic on the operation of community-based AECs, to the author’s knowledge performance indicators of community-based AECs have not been published earlier using DES modeling techniques. Because reimbursements for facility fees for AECs are continuously decreasing, there is an urgent need to maximize the operational efficiency of community-based AECs and decrease the cost of care to offset the diminishing net revenue per case.16 A DES model using local data is an easily implantable strategy to pinpoint areas of inefficiency, establish a center-specific benchmark, and assess the impact of strategic interventions to improve throughput and efficiency.
There are certain important limitations to the results of this study. Significant uncertainty and ambiguity exist for the optimum process flow needed for screening and testing for COVID-19. As the pandemic evolves, likely these process flows will also change. The development of a safe and effective vaccine may drastically change this workflow. In addition to the expense related to the cost of PPE, the focus of the analysis was on the impact on staffing cost, which is the largest component of the total operating expense in a typical community-based AEC.13 Other fixed operational costs, which are unlikely to change in the post–COVID-19 period, were not assessed. Patient satisfaction metrics are one of the critical components of performance indicators of any endoscopy center. However, currently there are no data on patient satisfaction parameters in the post–COVID-19 period. Although real concern exists for loss of revenue related to the possibility of increased cancellation of procedures by patients because of COVID-19 infection fear, this was not considered in the model. Also, this model may not apply to large multispecialty ambulatory surgery centers or to hospital-based outpatient facilities.
In summary, this study is the first to assess operational and financial performance indicators in a community-based stand-alone AEC performing GI endoscopic procedures using DES modeling. The results show that the workflow modifications currently recommended by professional societies and governmental authorities to reopen ambulatory endoscopic facilities in the post–COVID-19 period will have a significant impact on performance indicators with adverse financial consequences on these revenue-strained AECs. These results call for urgent action by governmental and commercial payors to adjust facility reimbursement fees for endoscopic procedures done in AECs to keep them open and financially viable in the post–COVID-19 era.
Footnotes
DISCLOSURE: The following author disclosed financial relationships: A. Das: Consultant for Boston Scientific.
See CME section, p. 960.
If you would like to chat with an author of this article, you may contact Dr Das at adas@azcdh.com.
References
- 1.Forbes N., Smith Z.L., Spitzer R.L. North American Alliance for the Study of Digestive Manifestations of COVID-19. Changes in gastroenterology and endoscopy practices in response to the COVID-19 pandemic: results from a North American Survey. Gastroenterology. Epub 2020 May 3. 2020 doi: 10.1053/j.gastro.2020.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sethi A., Swaminath A., Latorre M. Donning a new approach to the practice of gastroenterology: perspectives from the COVID-19 pandemic epicenter. Clin Gastroenterol Hepatol. 2020;18:1673–1681. doi: 10.1016/j.cgh.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitehouse/CDC guidelines: Opening up America again. Available at: https://www.whitehouse.gov/openingamerica/. Accessed May 2, 2020.
- 4.ASGE Updates for Members: Gastroenterology professional society guidance on endoscopic procedures during the COVID-19 pandemic. Available at: https://www.asge.org/home/advanced-education-training/covid-19-asge-updates-for-members. Accessed May 15, 2020.
- 5.American College of Gastroenterology Task Force on Endoscopic Resumption The American College of Gastroenterology (ACG) roadmap for safely resuming or ramping-up endoscopy in the COVID-19 pandemic updated as of May 12, 2020. https://webfiles.gi.org/docs/policy/2020resuming-endoscopy-fin-05122020.pdf Available at:
- 6.Berg B., Denton B., Nelson H. A discrete event simulation model to evaluate the operational performance of a colonoscopy suite. Med Decis Making. 2010;30:380-7. doi: 10.1177/0272989X09345890. [DOI] [PubMed] [Google Scholar]
- 7.Day L.W., Belson D. Studying and incorporating efficiency into gastrointestinal endoscopy centers. Gastroenterol Res Pract. 2015;2015:1–9. doi: 10.1155/2015/764153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gellad Z.F., Thompson C.P., Taheri J. Endoscopy unit efficiency: quality redefined. Clin Gastroenterol Hepatol. 2013;11:1046–1049. doi: 10.1016/j.cgh.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Kopach-Konrad R., Lawley M., Criswell M. Applying systems engineering principles in improving health care delivery. J Gen Intern Med. 2007;22(Suppl 3):431-7. doi: 10.1007/s11606-007-0292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Office of The Assistant Secretary For Health: SARS-CoV-2 (COVID-19) fact sheet. Guidance--proposed use of point-of-care (POC) testing platforms for SARS-CoV-2 (COVID-19). Available at: https://www.cdc.gov/coronavirus/2019-ncov/downloads/OASH-COVID-19-guidance-testing-platforms.pdf. Accessed May 29, 2020.
- 11.Day L.W., Belson D., Dessouky M. Optimizing efficiency and operations at a California safety-net endoscopy center: a modeling and simulation approach. Gastrointest Endosc. 2014;80:762–773. doi: 10.1016/j.gie.2014.02.1032. [DOI] [PubMed] [Google Scholar]
- 12.Sauer B.G., Singh K.P., Wagner B.L. Efficiency of endoscopy units can be improved with use of discrete event simulation modeling. Endosc Int Open. 2016;4:E1140–E1145. doi: 10.1055/s-0042-117217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.VMG Health 2018. Intellimaker 2018 multi-specialty ASC benchmarking study. https://intellimarker.com/content/intellimarker/VMG_Healths_Intellimarker/ Available at:
- 14.Salleh S., Thokala P., Brennan A. Discrete event simulation-based resource modelling in health technology assessment. Pharmacoeconomics. 2017;35:989–1006. doi: 10.1007/s40273-017-0533-1. [DOI] [PubMed] [Google Scholar]
- 15.Tayne S., Merrill C.A., Saxena R.C. Maximizing operational efficiency using an in-house ambulatory surgery model at an academic medical center. J Healthc Manag. 2018;63:118–129. doi: 10.1097/JHM-D-16-00008. [DOI] [PubMed] [Google Scholar]
- 16.Dorn S.D., Vesy C.J. Medicare's revaluation of gastrointestinal endoscopic procedures: implications for academic and community-based practices. Clin Gastroenterol Hepatol. 2016;14:924–928. doi: 10.1016/j.cgh.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 17.Joint GI Society statement: use of personal protective equipment in GI endoscopy. https://gi.org/2020/04/01/joint-gi-society-message-on-ppe-during-covid-19/ Available at:
- 18.CY 2020 Medicare Hospital Outpatient Prospective Payment System and Ambulatory Surgical Center Payment System Final Rule (CMS-1717-FC) https://www.asge.org/docs/default-source/practice-support/coding/2020-asc-medicarefacility-payment-for-gi-services.pdf?sfvrsnZ556df952_4 Available at: