Abstract
Background
The practical experiences of active pharmacists involved in managing critically ill patients with coronavirus disease 2019 (COVID-19) have been rarely reported.
Objective
This work aimed to share professional experiences on medication optimization and provide a feasible reference for the pharmaceutical care of critically ill patients with COVID-19.
Methods
This study was conducted in a COVID-19-designated hospital in China. A group of dedicated clinical pharmacists participated in multidisciplinary rounds to optimize the treatments for critically ill patients with COVID-19. Consensus on medication recommendations was reached by a multidisciplinary team through bi-daily discussion. Related drug, classification, cause, and adjustment content for recommendations were recorded and reviewed.
Results
A total of 111 medication recommendations were supplied for 22 out of 33 (56.7%) critically ill patients from 1 February 2020 to 18 March 2020, and 106 (95.5%) of these were accepted. Among these recommendations, 64 (67.7%), 32 (28.8%), and 15 (13.5%) were related to antibiotics and antifungals, antiviral agents, and other drugs, respectively. Recommendation types significantly differed for different anti-infectives (p < 0.05). For antibiotics and antifungals, treatment effectiveness accounted for 60.9% of recommendation types, with 15 (38.5%) cases related to untreated infections. For antiviral agents, adverse drug events were the most common recommendation types (84.4%), with 20 (74.1%) cases related to liver function dysfunction. Discontinuation of suspected antiviral agents (66.7%) was usually recommended after the occurrence of adverse events that may progress and bring poor outcomes.
Conclusion
Forceful and extensive on-ward participation is recommended for clinical pharmacists in managing critically ill patients. Our experiences highlight the need for special attention toward untreated infections and adverse events related to antiviral agents.
Keywords: Pharmacists, Intensive care unit, Coronavirus disease 2019, Medication recommendation
Introduction
Since December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has globally spread and accounted for a considerable number of human morbidity and mortality in over 200 countries, areas, or territories worldwide.1 , 2 The disease caused by this virus was named as coronavirus disease 2019 (COVID-19) by the World Health Organization.3 Patients developing severe COVID-19 require hospitalization and approximately 5%–10% of patients need intensive care unit (ICU) admission.4 Critically ill patients generally have an advanced age and a high prevalence of comorbidities such as acute hypoxemic respiratory failure, liver function injury, and secondary infection.5 As an exceptional circumstance, repurposed and experimental therapies without strong evidence are offered to these patients.6 Multiple symptomatic medications, such as antibiotics, corticosteroids, and antifungal drugs, are also commonly used in ICUs.7 Owing to the complex pathophysiology of these patients, the limited experience of antiviral treatments, and the frequent changes in drug therapy, health care providers must deal with complex drug-related problems originating from either treatment effectiveness or potential adverse events.
Clinical pharmacists can monitor and evaluate medication therapy, provide dose adjustments, reduce multiple medical errors, and therefore have gradually been an essential part of health care teams in ICUs.8, 9, 10 However, their practical experiences in managing of critically ill patients with COVID-19 have been rarely reported. Most of the available guidelines didn't include detailed recommendations for pharmacist service on this particular population.1 , 11 , 12 As a rapid response to the pandemic, a multidisciplinary team was organized in a designated hospital in Zhejiang Province, China. As important members of this team, dedicated clinical pharmacists participated in daily multidisciplinary rounds to optimize the treatments for critically ill patients with COVID-19. In this work, medication recommendations supplied by clinical pharmacists, including related drug, classification, cause, and detailed adjustment content, were reviewed to share experiences on pharmacological optimization and provide a feasible reference for clinical pharmacists in other medical institutions.
Materials and methods
Study design and participants
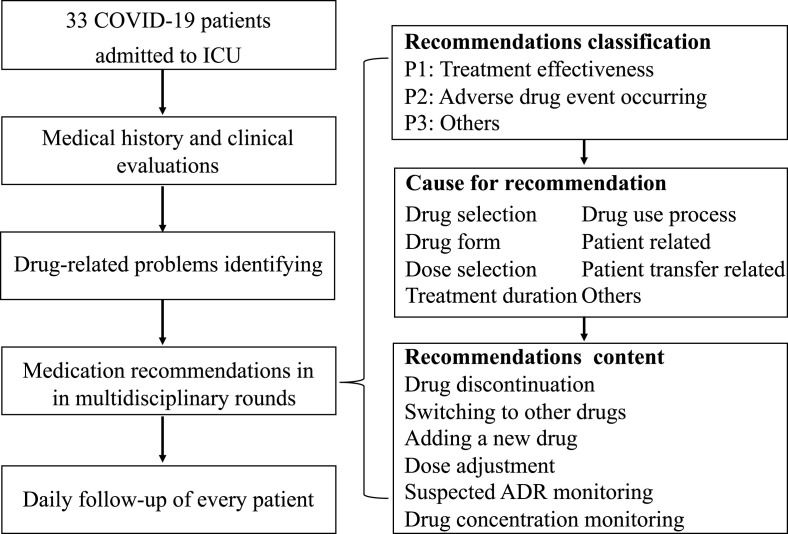
This study was conducted in the ICU of the First Affiliated Hospital of Zhejiang University (FAHZU), a designated hospital to treat patients with COVID-19 in Zhejiang Province, China. FAHZU is a university-affiliated tertiary hospital with 2500 beds and over 100,000 discharged patients each year. Laboratory confirmation of SARS-CoV-2 infection was performed using polymerase chain reaction testing. A multidisciplinary team, including senior experts in infection, respiratory diseases, laboratory department, Chinese medicine, psychiatry, imaging, and nursing, was organized to provide comprehensive and optimal treatment for these critically ill patients. In addition, a group of dedicated clinical pharmacists participated in daily multidisciplinary rounds to optimize the treatments for critically ill patients with COVID-19 (Fig. 1 ) through (1) comprehensive medical history and clinical evaluations, (2) identification of drug-related problems, (3) medication recommendations and discussion in multidisciplinary rounds, and (4) daily follow-up of every critically ill patient. Consensus on medication recommendations was reached by the multidisciplinary team through bi-daily discussion.
Fig. 1.
Study flow diagram and classification of medication recommendations.
This study was conducted in accordance with the Helsinki Declaration of 1975. Ethical approval was obtained from the authorized ethics committee of FAHZJ.
Data collection
From 1 February 2020 to 18 March 2020, medication recommendations by clinical pharmacists were collected and reviewed, and their classification (Fig. 1) was drawn on the concept the Pharmaceutical Care Network Europe Foundation Classification V 9.0.13 Related drug, classification, cause, and adjustment contents were recorded for each medication recommendation. The baseline characteristics of critically ill patients admitted to the ICU and the details for treatments and laboratory testing were collected from the electronic information system.
Statistical analysis
Categorical data were expressed as numbers and percentages, and continuous data were presented as mean (standard deviation [SD]) or the medians (interquartile range [IQR]). Classification of medication recommendation among different drugs were compared using Pearson χ2 of Fisher exact test (cell size<5). Data were analyzed through SPSS19.0 (www.spss.com). A value of p < 0.05 was considered statistically significant.
Results
Baseline and treatments of patients with medication recommendation
From 1 February 2020 to 18 March 2020, 33 patients diagnosed with COVID-19 were admitted to the ICU. Medication recommendations were supplied for 22 of these patients (66.7%). The baseline characteristics and treatments for patients with medication recommendations are shown in Table 1 . Their mean age was 66.3 (range, 36–91) years, and 17 (77.3%) patients were male. Hypertension was the most common coexisting condition (n = 16, 72.7%), followed by diabetes, cardiac disease, nephropathy, and liver disease. All these patients received antibiotics, 21 (95.5%) received antiviral agents, 21 (95.5%) received corticosteroids, and 15 (68.2%) received antifungal drugs. For organ support, 14 (63.6%) patients received invasive mechanical ventilation, 12 (54.5%) received continuous renal replacement therapy, and 8 (36.4%) received artificial liver support therapy.
Table 1.
Baseline characteristics and treatments.
| Baseline characteristics (n = 22) | ||
|---|---|---|
| Age | ||
| Mean, SD, y | 66.3 | 15.1 |
| Range, y | 36–91 | |
| Gender | ||
| Female, n, % | 5 | 22.7% |
| Male, n, % | 17 | 77.3% |
| Chronic medical illness | ||
| Hypertension, n, % | 16 | 72.7% |
| Diabetes, n, % | 5 | 22.7% |
| Cardiac disease, n, % | 3 | 13.6% |
| Others, n, % | 4 | 18,2% |
| Laboratory results | ||
| ALT, mean, SD, U/L | 48.2 | 55.3 |
| AST, mean, SD, U/L | 41.9 | 25.6 |
| TBIL, mean, SD, μmol/L | 22.9 | 27.0 |
| Serum creatinine (μmol/L) | 176.3 | 244.7 |
| C-reactive protein (mg/L) | 75.2 | 58.6 |
| Leucocytes ( × 109/L) | 11.0 | 5.9 |
| Neutrophils (%) | 89.2 | 7.8 |
| Lymphocytes ( × 109/L) | 0.8 | 1.3 |
| Treatments (n=22) | ||
| Organ support | ||
| Invasive mechanical ventilation, n, % | 14 | 63.6% |
| Continuous renal replacement therapy, n, % | 12 | 54.5% |
| Artificial liver support therapy, n, % | 8 | 36.4% |
| Medicines | ||
| Antibiotics, n, % | 22 | 100% |
| Antiviral treatment, n, % | 21 | 95.5% |
| Corticosteroids, n, % | 21 | 95.5% |
| Antifungal drugs, n, % | 15 | 68.2% |
| Intravenous immunoglobin, n, % | 14 | 63.6% |
| Cardiovascular drugs, n, % | 12 | 54.5% |
| Psychotropic medication, n, % | 11 | 50.0% |
| Parenteral nutritional support, n, % | 8 | 36.4% |
Characteristics of medication recommendation
The characteristics of medication recommendations are shown in Table 2 . Clinical pharmacists supplied 111 medication recommendations, and 106 (95.5%) of which were accepted. The median number of recommendations per patient was 5 (IQR: 1–8). Most recommendations were related to anti-infectives. Among these recommendations, 64 (57.5%) were related to antibiotics, and 32 (28.8%) were related to antiviral agents against SARS-CoV-2. For recommendation classifications, 53 (47.7%) were advised for treatment effectiveness, and 58 (52.3%) were advised for adverse drug events (potential or existing).
Table 2.
Characteristics of medication recommendations.
| Characteristics | ||
|---|---|---|
| Number of recommendations (n, range) | 111 | 1–12 |
| Number of recommendations per patient (median, IQR) | 5 | 1–8 |
| Drugs | ||
| Antibiotics and antifungal drugs, n, % | 64 | 57.7% |
| Antiviral treatment, n, % | 32 | 28.8% |
| Drugs for digestive symptoms, n, % | 6 | 5.4% |
| Others, n, % | 9 | 8.1% |
| Acceptance | ||
| Recommendations accepted, n, % | 106 | 95.5% |
| Recommendation type | ||
| Treatment effectiveness, n, % | 53 | 47.7% |
| Adverse drug event occurring, n, % | 58 | 52.3% |
| Cause for recommendation | ||
| Drug selection, n, % | 79 | 71.2% |
| Dose selection, n, % | 20 | 18.0% |
| Treatment duration, n, % | 12 | 10.8% |
| Recommendations at drug level | ||
| Drug discontinuation, n, % | 35 | 31.5% |
| Switched to other drugs, n, % | 27 | 24.3% |
| Adding a new drug, n, % | 22 | 19.8% |
| Dose adjustment, n, % | 17 | 15.3% |
| Suspected ADR monitoring, n, % | 9 | 8.1% |
| Drug concentration monitoring, n, % | 1 | 0.9% |
ADR: adverse drug reactions.
Medication recommendations for different anti-infectives
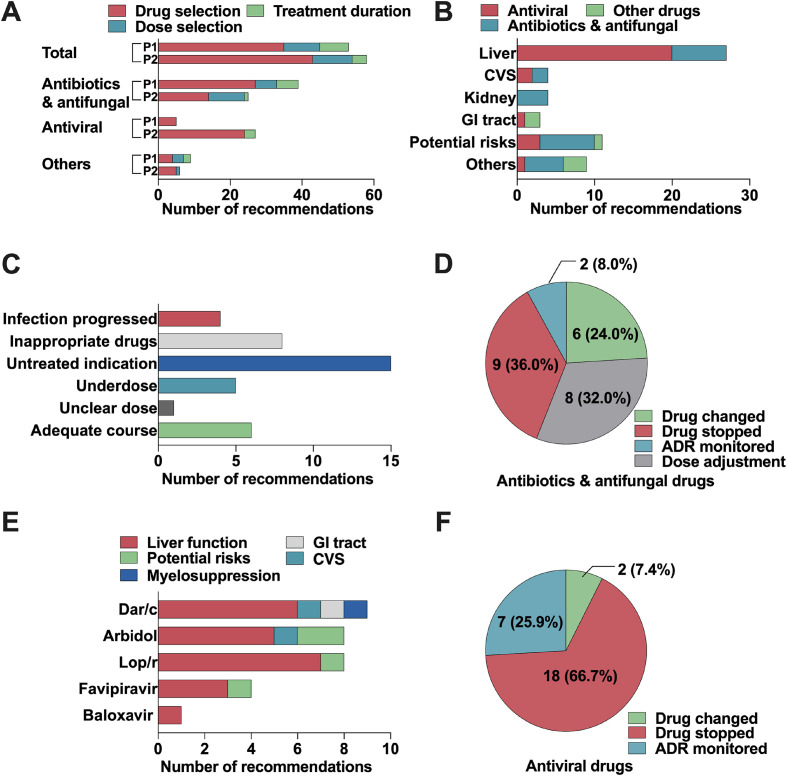
Significant difference (p < 0.05) in the classification of medication recommendation was observed among different anti-infectives (Fig. 2 ). Among the 64 recommendations related to antibiotics and antifungal drugs, 39 (60.9%) were advised for treatment effectiveness, and 25 (39.1%) were supplied for adverse drug events (Fig. 2A). For treatment effectiveness, untreated indications were the most common cause, followed by inappropriate drugs, adequate treatment duration, and under-dosage (Fig. 2C). Both drug discontinuation and dose adjustment were commonly advised after the identification of adverse events (Fig. 2D) involving liver and renal function dysfunction, irregular heart rate, and potential risks caused by over-high drug concentration (Fig. 2B). Examples of medication recommendations about antibiotics and antifungal agents are shown in Table Supplement 1.
Fig. 2.
Characteristics of medication recommendations for different drugs. A: Classification and cause of recommendations for different drugs. B: Adverse drug event-related recommendations for different drugs. C: Treatment effectiveness related-recommendations for antibiotics and antifungal drugs. D: Therapy adjustment recommendations for antibiotics and antifungal drugs after identifying adverse drug events. E: Adverse drug event related-recommendations for different antiviral agents. F: Therapy adjustment recommendations for antiviral agents after identifying adverse drug events. P1: treatment effectiveness; P2: adverse drug event occurring; CVS: cardiovascular system; GI: gastrointestinal; Dar/C: darunavir/cobicistat; Lop/r, lopinavir/ritonavir.
Supplement Table 1.
Example of recommendations for antibiotics and antifungal drugs
| Antibiotics and antifungal drugs | Recommendation examples |
|---|---|
| Treatment effectiveness | |
| Drug selection | A patient had gram-positive bacteria isolated from throat swabs, rising CRP, and CRRT treatment. Linezolid (0.6g q12h) was recommended for with (accepted). |
| Dose selection | A patient had less than 0.5 μg/ml blood concentration of voriconazole. Increasing the dose of voriconazole to 200 mg q8h was advised (accepted). |
| Treatment duration | A patient achieved near-normal indicators of bacterial infections. Considering the lack of evidence supporting infections caused by Gram positive bacteria, discontinuation of daptomycin was advised (accepted). |
| Adverse drug event occurring | |
| Drug selection | |
| Liver function | The blood concentration of voriconazole was 5.6 μg/ml for a patient whose TBL was 101.8 μmol/L. Adjustment of antifungal drugs was recommended (voriconazole ➝ micafungin 150 mg qd, accepted). |
| Central nervous system | A patient developed psychiatric symptoms, which may be caused by imipenem/cilastatin. Adjustment of antibiotics was recommended (imipenem/cilastatin ➝ cefoperazone/sulbactam 2g q8h, accepted). |
| Cardiovascular system | A patient with CRRT treatment developed arrhythmia and abnormal TBL, which may be caused by levofloxacin. Discontinuation of levofloxacin was recommended. |
| Skin | A patient developed skin rashes over the whole body, which may be caused by drugs. Discontinuation of caspofungin, linezolid, montelukast and olanzapine was recommended (accepted). |
| Urinary system | The Scr elevated to 143 μmol/L in a patient, which may be caused by teicoplanin. Discontinuation of teicoplanin was recommended. |
| Dose selection | The blood concentration of vancomycin was 33.34 μg/ml in a patient with hemodialysis treatment due to renal function failure. Discontinuation of vancomycin was recommended (accepted). |
| Treatment duration | The Scr was 129 μmol/L for a patient with polymyxin B treated for 2 weeks. Discontinuation of polymyxin B was recommended. |
For antiviral agents, the most common recommendations were related to adverse drug events, with 27 (84.4%) out of 32, and the remaining five (15.6%) were advised for treatment effectiveness. Among the 27 recommendations about adverse events, 20 (74.1%), two (11.1%), and three (5.3%) were related to liver function dysfunction, adverse event in cardiovascular system, and potential adverse events (Fig. 2B), respectively. After possible adverse events were identified, discontinuation of suspected antiviral agents was advised in 18 out of 27 (66.7%, Fig. 2F) recommendations. Darunavir/cobicistat, arbidol, and lopinavir/ritonavir were the antiviral agents constituting the majority of adverse event-related recommendations (Fig. 2E). Examples of medication recommendations about antiviral agents are shown in Table 3 .
Table 3.
Example of recommendations for antiviral regiments.
| Antiviral treatment | Recommendation examples |
|---|---|
| Treatment effectiveness | |
| Drug selection | A patient with positive SARS-CoV-2 RNA received arbidol alone. Lopinavir/ritonavir combined with arbidol was proposed (accepted). |
| Adverse drug event occurring | |
| Drug selection | |
| Abnormal liver function risks | A patient had elevated ALT (114 U/L) and AST (236 U/L), which may be caused by darunavir/cobicistat. Discontinuation of darunavir/cobicistat was recommended (accepted). |
| Cardiovascular system risks | A patient had temporary sinus bradycardia, which may be caused by arbidol and should be closely monitored (accepted). |
| Drug-drug interactions | Serious drug-drug interaction exists between quetiapine and lopinavir/ritonavir. Discontinuation of darunavir/cobicistat was recommended (accepted). |
| Dose selection | A patient had 33.34 μg/ml blood concentration of vancomycin and received hemodialysis treatment due to renal function failure. Discontinuation of vancomycin was recommended (accepted). |
| Treatment duration | Three consecutive negative results with qPCR detection over an interval of 24 h. Discontinuation of favipiravir and arbidol was recommended (accepted). |
Discussion
The prevalence of COVID-19 and the high proportion of critically ill patients with this illness pose a global challenge for healthcare workers and increase the requirements for proper pharmacy care services.13, 14, 15, 16 In addition to the recognized difficulties for critically ill patients, such as multiple organ failure, severe infection, and complex medication therapy, the limited knowledge of COVID-19 and unproven interventions being used in practice bring additional uncertainty.5 , 6 Therefore, the pharmacy care services for such a special population must be adjusted and improved through clinical practice. To our knowledge, this study is the first to describe the specific classification of medication recommendations in critically ill patients with COVID-19. The sharing of drug-related problems, cause, and recommendation contents in detail may provide new insights into the management of critically ill patients with COVID-19.
The acceptance rate of medication recommendations was 95.5%, which was relatively high compared with that in previous studies.8 , 17, 18, 19 This finding indicates that our medication recommendations satisfy the clinical needs. The high acceptance rate may be related to the active pharmacists’ involvement. During the treatment period for patients with COVID-19, the dedicated pharmacist team in multidiscipline rounds conducted bi-daily discussion for 1.5 h each. Medical history, medication safety and efficacy, and possible drug-drug interactions were comprehensively assessed for every critically ill patient prior to discussion to guarantee the quality of medication recommendations. Variations on any treatment therapy were recorded and analyzed carefully. Medication recommendations were generated with the explicit, conscientious, and judicious use of best available evidence. Basing on our experience, we would recommend a forceful and extensive on-ward participation of clinical pharmacists in managing of critically ill patients.
Most recommendations were advised for anti-infectives. Different from previous studies,10 , 18 , 20 untreated indications, either inappropriate drugs or dose, were the most common cause of recommendations related to antibiotics and antifungal drugs. This finding indicates the active participation of pharmacists in clinical can expand the conventional scope of ICU pharmacists’ activities,9 , 17 , 19 such as drug information consultation, drug interactions identification, and adverse drug event monitoring, to more positive decision-making. Given that COVID-19 is an emerging pandemic with profound consequences, experimental and repurposed antiviral agents without strong evidence are used to improve patient outcomes.5 , 6 , 11 , 12 Recommendations on adverse drug events were most common for antiviral agents, with the majority related to liver function dysfunction. Antiviral agents that potentially induce liver injury in our center involved lopinavir/ritonavir,21 , 22 arbidol,23 darunavir/cobicistat,24 favipiravir,25 and baloxavir.26 High proportion of liver injury was also found in other studies, and hepatotoxic drugs were inferred as one of the potential risk factors.27 A latest study showed that lopinavir/ritonavir is a risk factor of liver injury in patients with COVID-19.28 Serum aminotransferase elevations or jaundice was observed in patients who received these antiviral agents in some reports about other diseases, which may be induced by either hepatotoxic effects or extensive drug to drug interactions related to antiviral agents, such as lopinavir/ritonavir29 and darunavir/cobicistat.30 After the unproven clinical benefits from these drugs and the potential risks from adverse effects were balanced out, the discontinuation of suspected antiviral agents was commonly recommended after the occurrence of adverse events that may progress and bring poor outcomes.
Our study has some limitations, which are mainly its retrospective design and lack of a control group. The effects of the active participation of clinical pharmacists cannot be accurately measured; however, the high acceptance of the medication recommendations may reflect the clinical value of our work to some extent. In addition, this study is the first to share the experience gained form real-world pharmacy care practice and provides a viable reference for clinical pharmacists in other medical institutions. Experimental and repurposed antiviral agents without strong evidence have been widely used in our center due to the urgent condition of COVID-19 pandemic and the lack of effective treatments. With the emergence of new treatments and evidence, the medication strategy for patients with COVID-19 should be adjusted and updated timely in practice. Due to these limitations, well-designed, controlled studies with a large sample size are required.
Conclusion
Clinical pharmacists in our center responded rapidly and imposed forceful and extensive on-ward participations for the management of critically ill patients to combat the COVID-19 pandemic. High acceptance rate was noted for medication recommendations provided by pharmacists, and most recommendations were advised for anti-infectives. The most common cause of recommendations for antibiotics and antifungal drugs was untreated indications, and that for antiviral agents was adverse drug events. Discontinuation of suspected antiviral agents was usually recommended after the occurrence of adverse events that may progress and bring poor outcomes. Our work serves as a feasible reference for clinical pharmacists in other medical institutions.
Statement of funding source
There's no funding for this study.
Declaration of competing interest
All authors declared that they have no financial relationships with any organizations or people that might influence or bias the content of the paper within 3 years of beginning of the work; no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgement
We thank the medical and nursing staff at our center.
References
- 1.Centers for Disease Control and Prevention . 2020. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines.https://covid19treatmentguidelines.nih.gov Accessed 20.05.29. [Google Scholar]
- 2.World Health Organization . 2020. Clinical Management of Severe Acute Respiratory Infection (SARI) when COVID-19 Disease Is Suspected.https://www.who.int/publicationsdetail/clinical-management-of-severe-acute-respiratory-infection-when-novelcoronavirus-(ncov)-infection-is-suspected Accessed 20.05.01. [Google Scholar]
- 3.World Health Organization . 2020. Naming the Coronavirus Disease (COVID-19) and the Virus that Causes it.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it Accessed 20.05.29. [Google Scholar]
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Phua J., Weng L., Ling L. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 7.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klopotowska J.E., Kuiper R., van Kan H.J. On-ward participation of a hospital pharmacist in a Dutch intensive care unit reduces prescribing errors and related patient harm: an intervention study. Crit Care. 2010;14:R174. doi: 10.1186/cc9278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chant C., Dewhurst N.F., Friedrich J.O. Do we need a pharmacist in the ICU? Intensive Care Med. 2015;41:1314–1320. doi: 10.1007/s00134-015-3718-0. [DOI] [PubMed] [Google Scholar]
- 10.Jiang S.P., Zhu Z.Y., Ma K.F., Zheng X., Lu X.Y. Impact of pharmacist antimicrobial dosing adjustments in septic patients on continuous renal replacement therapy in an intensive care unit. Scand J Infect Dis. 2013;45:891–899. doi: 10.3109/00365548.2013.827338. [DOI] [PubMed] [Google Scholar]
- 11.National Health Commission & State Administration of Traditional Chinese Medicine . 7th 2020. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia.http://en.nhc.gov.cn/index.html China. [Google Scholar]
- 12.World Health Organization Clinical Management of COVID-19. https://www.who.int/publications-detail/clinical-management-of-covid-19 Accessed 20.05.30.
- 13.Pharmaceutical Care Network Europe Foundation . 2019. Classification for Drug Related Problems.https://www.pcne.org/upload/files/334_PCNE_classification_V9-0.pdf Accessed 20.05.01. [Google Scholar]
- 14.Amariles P., Ledezma-Morales M., Salazar-Ospina A., Hincapié-García J.A. How to link patients with suspicious COVID-19 to health system from the community pharmacies? A route proposal. Res Soc Adm Pharm. 2020 doi: 10.1016/j.sapharm.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song Z., Hu Y., Zheng S., Yang L., Zhao R. Hospital pharmacists' pharmaceutical care for hospitalized patients with COVID-19: recommendations and guidance from clinical experience. Res Soc Adm Pharm. 2020 doi: 10.1016/j.sapharm.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahmoudjafari Z., Alexander M., Roddy J. American society for transplantation and cellular therapy pharmacy special interest group position statement on pharmacy practice management and clinical management for COVID-19 in hematopoietic cell transplantation and cellular therapy patients in the United States. Biol Blood Marrow Transplant. 2020;26:1043–1049. doi: 10.1016/j.bbmt.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaal R.J., Jansen M.M., Duisenberg-van Essenberg M., Tijssen C.C., Roukema J.A., van den Bemt P.M. Identification of drug-related problems by a clinical pharmacist in addition to computerized alerts. Int J Clin Pharm. 2013;35:753–762. doi: 10.1007/s11096-013-9798-4. [DOI] [PubMed] [Google Scholar]
- 18.Martins R.R., Silva L.T., Lopes F.M. Impact of medication therapy management on pharmacotherapy safety in an intensive care unit. Int J Clin Pharm. 2019;41:179–188. doi: 10.1007/s11096-018-0763-0. [DOI] [PubMed] [Google Scholar]
- 19.Khdour M.R., Jarab A.S., Adas H.O., Samaro E.Z., Mukattash T.L., Hallak H.O. Identification of drug-related problems: a prospective study in two general hospitals. Curr Clin Pharmacol. 2012;7:276–281. doi: 10.2174/157488412803305795. [DOI] [PubMed] [Google Scholar]
- 20.Dvořáčková E., Rychlíčková J., Pávek P., Hojný M., Vlček J. Analysis and management of drug related problems on a nephrology ward from a pharmacist's point of view. Pharmazie. 2019;74:625–629. doi: 10.1691/ph.2019.9568. [DOI] [PubMed] [Google Scholar]
- 21.Meraviglia P., Schiavini M., Castagna A. Lopinavir/ritonavir treatment in HIV antiretroviral-experienced patients: evaluation of risk factors for liver enzyme elevation. HIV Med. 2004;5:334–343. doi: 10.1111/j.1468-1293.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- 22.Lopinavir LiverTox . National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda (MD): 2020. Clinical and Research Information on Drug- Induced Liver Injury.https://www.ncbi.nlm.nih.gov/books/n/livertox/Lopinavir/ Accessed 20.05.01. [PubMed] [Google Scholar]
- 23.Deng L., Li C., Zeng Q. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yancheva N., Tzonev R. A case of late presentation of darunavir-related cholestatic hepatitis. Int J STD AIDS. 2019;30:620–622. doi: 10.1177/0956462419826723. [DOI] [PubMed] [Google Scholar]
- 25.Cai Q., Yang M., Liu D. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering (Beijing) 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LiverTox Baloxavir. National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda (MD): 2012. Clinical and Research Information on Drug-Induced Liver Injury.https://www.ncbi.nlm.nih.gov/books/n/livertox/Baloxavir/ Accessed 20.05.01. [PubMed] [Google Scholar]
- 27.Chao Zhang L.S., Wang Fu-Sheng. Liver injury in COVID-19 management and challenges. Lancet Gastroenterol Hepatol. 2020 doi: 10.1016/10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai Q., Huang D., Yu H. COVID-19: abnormal liver function tests. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandie M., Wiesner L., McIlleron H. Drug-drug interactions between bedaquiline and the antiretrovirals lopinavir/ritonavir and nevirapine in HIV-infected patients with drug-resistant TB. J Antimicrob Chemother. 2016;71:1037–1040. doi: 10.1093/jac/dkv447. [DOI] [PubMed] [Google Scholar]
- 30.Kakuda T.N., Crauwels H., Opsomer M. Darunavir/cobicistat once daily for the treatment of HIV. Expert Rev Anti Infect Ther. 2015;13:691–704. doi: 10.1586/14787210.2015.1033400. [DOI] [PubMed] [Google Scholar]