Abstract
The incidence of esophagogastric junction (EGJ) adenocarcinoma has been gradually increasing in Asia, just like in Western countries a few decades ago. Despite recent advances in next‐generation sequencing and multimodal treatments, EGJ adenocarcinoma is still an aggressive malignancy with poor outcomes. Clinically, EGJ adenocarcinoma can be separated into Barrett's adenocarcinoma and cardiac adenocarcinoma, with frequent similarities observed. Barrett's adenocarcinoma is likely to be of gastric origin in terms of its premalignant background, risk factors, and stem cell regulators. Recent comprehensive genomic analyses suggest that immunotherapy may be essential for high‐level microsatellite instability (MSI‐H)‐ and Epstein‐Barr virus (EBV)‐associated subtypes, and against the immunosuppressive phenotype in genomically stable (GS) subtypes, in the treatment of EGJ and gastric adenocarcinoma. Although the chromosomal instability (CIN) subtype dominates EGJ adenocarcinoma, there is still a need to investigate the other molecular subtypes and their targets. Because of the distinctive characteristics of tumor location of EGJ adenocarcinoma, we also described the results of a multicenter cohort study of EGJ adenocarcinoma, comparing Siewert type I (distal esophagus), II (cardia of the stomach), and III (subcardia) tumors. We show that type I tumors were frequently accompanied by Barrett's esophagus (78%, P < .0001), with a significantly unfavorable outcome (multivariate EGJ‐cancer‐specific mortality hazard ratio = 1.81, 95% CI, 1.06‐2.97; P = .031). In addition, over half (56%) of these cases experienced disease recurrence in the lymph nodes. Our findings suggest that Barrett's adenocarcinoma may be an aggressive phenotype of EGJ adenocarcinoma due to the potential risk of tumor spread through the complex lympho‐vascular network of the esophagus.
Keywords: esophageal adenocarcinoma, esophagogastric junction, gastric cancer, molecular subtype, prognosis
In this review article, we discussed future therapeutic targets of adenocarcinoma of esophagogastric junction (EGJ) according to the four molecular subtypes by TCGA. We also referred to the current understanding of the similarity between Barrett's adenocarcinoma and cardiac carcinoma, and added our own analysis examining prognostic differences between those two tumors using a multicenter cohort in Japan.
1. BARRETT'S AND CARDIAC ADENOCARCINOMA
There has been a gradual increase in the incidence of esophagogastric junction (EGJ) adenocarcinoma in Asian countries, 1 , 2 , 3 , 4 including Japan. 5 Despite the recent advances in comprehensive genetic analyses as well as the progress in multimodal treatments, EGJ adenocarcinoma is still an aggressive malignancy with poor outcomes. EGJ adenocarcinoma includes Barrett's adenocarcinoma (also known as esophageal adenocarcinoma, or adenocarcinoma in distal esophagus) and cardiac adenocarcinoma (adenocarcinoma of gastric cardia) with esophageal invasion. 6 Barrett's and cardiac adenocarcinomas have been increasing in parallel since the late 1970s in Western countries, and are recognized as common upper gastrointestinal cancers. 7 There are numerous similarities between Barrett's and gastric adenocarcinomas in terms of tumor characteristics and background, with few differences highlighted to date.
In histological examinations, Barrett's adenocarcinoma and intestinal‐type gastric adenocarcinoma have a common premalignant background of intestinal metaplasia caused by chronic inflammation. Intestinal‐type gastric adenocarcinoma in the body and antrum of the stomach is mainly related to Helicobacter pylori‐induced chronic gastritis. 8 Helicobacter pylori infection can also induce both chronic inflammation and metaplasia in the gastric cardia and cardiac adenocarcinoma. 9 , 10 , 11 , 12 , 13 In addition, in terms of risk factors, both Barrett's and cardiac adenocarcinoma are associated with obesity in Western countries. 14 , 15 , 16 , 17 Adipokines produced from adipose tissue in association with metabolic syndrome can influence the development of chronic inflammation and cancer progression. 18 , 19 Finally, experimental data from transgenic mouse models suggest that Barrett's metaplasia may arise from gastric cardia progenitor cells in response to bile acid‐mediated inflammation via LGR5 expression and IL‐1β–IL‐6 signaling. 20 Cholecystokinin 2 receptor (CCK2R, also known as CCKBR), which regulates gastric stem cells in the cardia or antrum regions of the stomach, is also upregulated in Barrett's esophagus and in esophageal adenocarcinoma. 21 , 22 Although a few studies have proposed the esophageal submucosal gland as the origin of Barrett's adenocarcinoma, considering previous perspective studies, Barrett's adenocarcinoma is likely to be of gastric origin. 23 , 24
2. CANDIDATE MOLECULES OF THERAPEUTIC TARGETS
Previously, gastroesophageal tumors were predominantly classified by pathological classification; however, in recent years — in the era of next‐generation sequencing technology — a molecular taxonomy has emerged. 25 , 26 , 27 , 28 The Cancer Genome Atlas (TCGA) Network has shown that gastroesophageal adenocarcinoma can be categorized into four molecular subtypes: Epstein‐Barr virus (EBV)‐associated, high‐level microsatellite instability (MSI‐H), genomically stable (GS), and chromosomal instability (CIN) tumors. These subtypes are classified using a range of techniques, including somatic copy number aberration, whole‐genome and whole‐exon sequencing, RNA sequencing, methylation assays, and proteomics analysis. 26 , 28 In the multiomic data of EGJ adenocarcinoma acquired from TCGA from cBioPortal for Cancer Genomics (https://www.cbioportal.org), there were a total of 172 cases which were classified as gastroesophageal junctional categories, including seven cases (4.1%) of MSI‐H, six cases (3.5%) of EBV, 11 cases (6.4%) of GS, and 148 cases (86%) of CIN. 26 , 28 In this section, we discuss the potential therapeutic targets of adenocarcinoma from esophagogastric junction (EGJ) as well as gastric adenocarcinoma, according to these four molecular subtypes.
High‐level microsatellite instability tumors (MSI‐H), which are not common in EGJ, harbor hypermutations, hypermethylations, MLH1 silencing, immune reactivity, and demonstrate frequent mutations in various genes, including ARID1A, RNF43, PIK3CA, and KRAS. 5 , 26 , 28 Based on the recent exploratory analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial, MSI‐H was considered a favorable prognostic factor, and chemotherapy was deemed not beneficial to patients with operable MSI‐H gastroesophageal tumors. Hence, surgery alone would be sufficient to treat MSI‐H cases. 29 Although the MAGIC trial did not include any MSI‐H EGJ tumors, we have previously reported MSI‐H was detected in 7.6% of Siewert type II (11 cases of 145 patients), and 16.7% in Siewert type III EGJ adenocarcinoma (four cases of 24 patients). 5 Tumors with hypermutations tend to produce neoantigens in the tumor microenvironment; thus, MSI‐H tumors are commonly immunogenic, and the host will often activate anti‐tumor immunity against these neoantigens. 30 In addition, MSI‐H tumors can upregulate immunological checkpoints such as programmed death receptor‐1 (PD‐1), programmed death ligand‐1 (PD‐L1), or PD‐L2, in order to escape the host anti‐tumor immunity. In a randomized controlled clinical trial, Nivolumab treatment was shown to exert a significant survival benefit to patients with metastatic gastric or EGJ adenocarcinoma. 31 , 32 Nivolumab is an immune checkpoint inhibitor that blocks PD‐1. It may therefore be more effective for an MSI‐H population.
Epstein‐Barr virus‐associated tumors show hypermethylation, CDKN2A silencing, frequent mutations in PIK3CA and ARID1A, and immune reactivity. 26 , 28 EBV‐associated tumors seem to occupy only a small fraction of EGJ adenocarcinoma in TCGA data. Drugs that inhibit the PI3K pathway or methylation may be potentially beneficial for these tumors. Recent comprehensive genomic analyses revealed a significant mRNA expression of PD‐L1 and PD‐L2 in EBV‐associated gastric adenocarcinoma, suggesting that, like MSI‐H tumors, these tumors are also sensitive to immune checkpoint inhibitors, such as Nivolumab. 28 , 31
Genomically stable tumors are associated with diffuse histology, and present with frequent mutations in CDH1 and RHOA, and the CLDN18‐ARHGAP26 fusion gene. 26 , 28 Besides these major alterations, mutations in BRCA1, BRCA2, RAD51C, PALB2, and CTNNA1 are detected in diffuse‐type gastric adenocarcinoma. 33 , 34 , 35 , 36 A proteomics analysis of 84 cases of diffuse gastric adenocarcinoma proposed classification according to the following three groups: PX1, which describes tumors expressing cell‐cycle dysregulated proteins; PX2, which describes tumors expressing epithelial‐mesenchymal transition pathway proteins as well as cell‐cycle‐related proteins; and PX3, which describes tumors with an enrichment of immunological proteins. Of note, tumors in the PX3 group have overexpressed IDO1 and ARG1 immunosuppressive proteins, for which inhibitory agents are already actionable. 37
Finally, CIN tumors are described as having structural chromosomal aneuploidy without hypermutation, frequent TP53 mutations, whole‐genome doubling, and amplification of cell‐cycle genes and genes from the receptor tyrosine kinase (RTK)‐RAS signaling pathway. 26 , 28 , 38 , 39 Because the CIN subtype dominates EGJ adenocarcinoma as described earlier, an understanding of the characteristics of this tumor subtype is crucial to improve therapeutic outcomes for patients with EGJ adenocarcinoma. Liu et al described two novel CIN subtypes, designated by scoring the quantity and intensity of focal, high‐level amplicons and using a combined analysis of TCGA data, with 921 cases of gastrointestinal adenocarcinoma: CIN‐Focal (CIN‐F), defined as tumors with high‐amplitude focal amplicons; and CIN‐Broad (CIN‐B), defined as tumors with low‐amplitude, broad amplicons. 39 The authors reported that 74% of upper GI adenocarcinoma cases displayed CIN‐F. CIN‐F tumors frequently harbor mutations in TP53, and demonstrate amplifications in cell‐cycle‐related genes and RTK‐related pathway components, such as KRAS. We recently described a novel therapeutic strategy for KRAS‐amplified tumors. 40 We found that KRAS‐amplified gastric cancer cells showed overexpression of KRAS protein, possessing a large pool of inactive KRAS (KRAS‐GDP state). KRAS‐amplified tumor cells show insensitivity to MAPK blockade as they can adaptively respond by mobilization of their reserve inactive KRAS to increase KRAS‐GTP state. Such adaptive responses can be abrogated through inhibition of the guanine‐exchange factors SOS1 and SOS2 or the protein tyrosine phosphatase SHP2, which can lead to inhibition of tumor growth when combined with MEK blockade. In TCGA dataset, KRAS‐amplified tumor occupied 8.1% of CIN‐type EGJ adenocarcinoma and 11.3% of CIN‐type gastric adenocarcinoma, which were more frequently observed as compared to the other types of cancers (5.7% in non‐small cell lung adenocarcinoma, 4.4% in pancreatic adenocarcinoma, 3.7% in bladder urothelial carcinoma, 1.9% in uterine corpus endometrial carcinoma, 1.4% in breast invasive carcinoma, 1.0% in colorectal adenocarcinoma, 0.8% in liver hepatocellular carcinoma, and 0.6% in prostate adenocarcinoma). 26 , 28 , 41 Thus, combined inhibition of MEK and SHP2 may be one of the promising therapeutic approaches for CIN‐type EGJ adenocarcinoma as well as gastric adenocarcinoma.
Considering a recent emergence of nivolumab or pembrolizumab, PD‐L1 status is important molecular information in EGJ adenocarcinoma as well as gastric adenocarcinoma. There are several studies examining PD‐L1 positivity of EGJ adenocarcinoma separated from that of gastric adenocarcinoma (Table 1). 42 , 43 , 44 , 45 , 46 , 47 , 48 PD‐L1 positivity of EGJ adenocarcinoma seems to be similar to that of gastric adenocarcinoma, despite different definitions of PD‐L1 positivity by immunohistochemical staining across studies. 42 , 43 , 44 Focusing on EGJ adenocarcinoma, tumor PD‐L1 expression according to Siewert classification were conflicting across the studies. 45 , 46 , 48 Further study is needed to address whether PD‐L1 status differs according to tumor location in EGJ adenocarcinoma. 45 , 46 , 47 , 48
Table 1.
Previous studies examining PD‐L1 expression of EGJ adenocarcinoma separated from that of gastric adenocarcinoma
| No. | Ref. | Year | Author (Country) | EGJ/esophagus or gastric (No. of cases) | Method | Tumor PD‐L1 positivity (%) | Stromal PD‐L1 positivity | Definition of PD‐L1 positivity | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 41 | 2018 | Weinberg et al (USA) |
EGJ (N = 119) Gastric (N = 462) |
IHC |
EGJ, 9.7% Gastric, 7.6% |
No data | Tumor, ≥5% tumor cell membranous expression | ||
| 2 | 42 | 2017 | Xing et al (China) |
EGJ (N = 8) Gastric (N = 4) |
IHC | EGJ, 66.7% Gastric, 58.3% | EGJ, 75% Gastric, 50% |
≥1% tumor cell expression Stroma, any immune cell expression (≥1%) |
||
| 3 | 43 | 2015 | Thompson et al (USA) |
EGJ (N = 5) Gastric (N = 29) |
IHC |
EGJ, 0% Gastric, 13.8% |
EGJ, 20% Gastric, 48.3% |
Tumor, ≥5% tumor cell expression Stroma, any immune cell expression (≥1%) |
||
| 4 | 44 | 2015 | Derks et al (USA) | EGJ/esophagus (N = 344) | IHC | EGJ/esophagus, 18% (mid‐proximal esophagus, 0.6% distal esophagus, 4.7% EGJ, 18.3%) | No data | Tumor, ≥5% tumor cell membranous expression (by tumor tissue microarray) | ||
| 5 | 45 | 2018 | Kollmann et al (Austria, Czech, Switzerland and USA) | EGJ/esophagus (N = 168) | IHC | EGJ/esophagus, 18% (Siewert type I, 26.8%; type II, 8.3%; type III, 8.3%) | No data | Tumor, any tumor cell expression (≥1%) | ||
| 6 | 46 | 2019 | Knief et al (Germany) | EGJ/esophagus (N = 135) | IHC | EGJ/esophagus, 48.1% | Combined positive score ≥1% | |||
| 7 | 47 | 2020 | Wang et al (China) | EGJ/esophagus (N = 96) | IHC | EGJ/esophagus, 11.5% (Siewert type I, 0%; type II, 7.9%; type III, 15.7%) | No data | Tumor, ≥5 stained tumor cells in a 400x field | ||
Abbreviations: EGJ, esophagogastric junction; IHC, immunohistochemistry.
Beyond TCGA molecular subtypes, the Asian Cancer Research Group (ACRG) has also categorized gastroesophageal cancers, and proposed four subtypes based solely on gene expression signatures: MSI, MSS/epithelial‐to‐mesenchymal transition (EMT), MSS/TP53‐active, and MSS/TP53‐deficient tumors. 49 The MSS/TP53‐deficient subtype, which is enriched with TCGA CIN subtype due to substantial aneuploidy, is frequently observed in the EGJ cardiac adenocarcinoma. The ACRG study also performed a survival analysis comparing their subtyping scheme with that of TCGA subtyping. Under the ACRG subtyping scheme, the MSI cases showed the most favorable outcomes, followed by MSS/TP53‐active, MSS/TP53‐inactive, and MSS‐EMT. When classified by TCGA subtyping, MSI cases still showed the best outcomes, but there were no significant prognostic differences among the EBV, GS, and CIN subtypes. This discrepancy may be because the disease stage was biased across the molecular subtypes in both studies. Further analyses are needed to assess how molecular subtype confers prognostic impact in gastroesophageal adenocarcinoma.
3. CLINICAL AND PROGNOSTIC DIFFERENCES BY TUMOR LOCATION
Esophagogastric junction adenocarcinoma extends across the thorax and abdomen to varying degrees. For patients with EGJ adenocarcinoma, tumor location is specified by Siewert classification as follows: type I, defined as tumors of the distal esophagus, in which the epicenter of the tumor is located 1‐5 cm above the anatomical EGJ; type II, true junctional tumors, in which the epicenter is located 1 cm above and 2 cm below the EGJ; and type III, gastric tumors that infiltrate into the esophagus, for which the epicenter is located between 2 and 5 cm below the EGJ. 50 Although there does not seem to be any difference in terms of the carcinogenic origin of Barrett's and cardiac adenocarcinoma, as mentioned earlier, this anatomical classification may differentiate these two tumors in terms of clinicopathological or prognostic characteristics. Considering that Barrett's esophagus involves a replacement of normal squamous epithelium with metaplastic mucosa in response to gastroesophageal reflux disease (GERD), Barrett's adenocarcinoma is likely to be located more proximally (Siewert type I) than cardiac adenocarcinoma. 51 Furthermore, because tumor cells of EGJ adenocarcinoma can spread in various longitudinal pathways through the complex lympho‐vascular network in the submucosal layer, Siewert classification is likely to be associated with tumor aggressiveness. 52 , 53 Indeed, Siewert classification is frequently employed to determine a surgical approach: patients with Siewert type I tumors are often treated through a thoracic approach with mediastinal lymph node dissection, whereas patients with Siewert type II or III cases can be treated via a transabdominal approach (so‐called “transhiatal” approach) when technically possible.
A number of studies have examined the clinicopathological and prognostic characteristics of EGJ adenocarcinoma via a comparative analysis of Siewert type I‐III tumors. 54 , 55 , 56 , 57 , 58 , 59 , 60 However, the associations between Siewert type and surgical outcome are conflicting: Siewert type III tumors appear to have a worse clinical outcome, 56 , 61 but this difference is not significant when differentiated by tumor location. 54 , 55 , 57 Here, we sought to investigate the clinical features and prognostic outcomes of EGJ adenocarcinoma, using 395 patients with Siewert type I‐III tumors who underwent surgical resection without neoadjuvant chemotherapy or radiotherapy in Japan.
3.1. Patient cohort of EGJ adenocarcinoma
Our multicenter retrospective cohort included 464 patients with EGJ adenocarcinoma (Siewert type I, II, and III) who underwent surgical resection at four academic institutions in Japan between February 2000 and March 2015 (Figure 1). A total of 395 patients with EGJ adenocarcinoma were eligible for this study. Disease staging was based on the 7th edition of the Union for International Cancer Control (UICC) classification of esophageal cancer, which is applicable to Siewert type III tumors.
FIGURE 1.
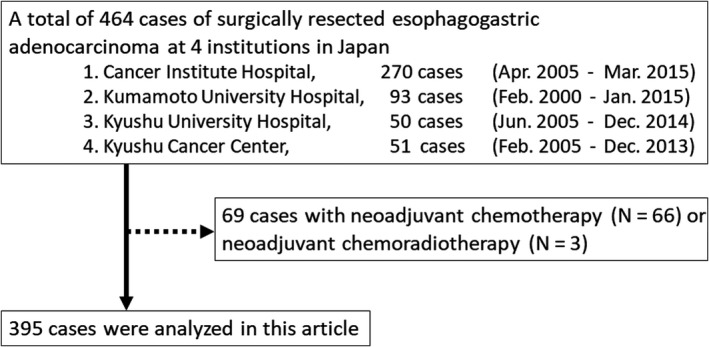
Flow diagram of our multicenter retrospective cohort of esophagogastric adenocarcinoma
3.2. Statistical analysis
All statistical analyses were performed using JMP 13 software (Version 13.2.1, SAS Institute). All P‐values were two‐sided. Univariate analyses were performed to investigate clinicopathological and molecular characteristics according to Siewert classification. Chi‐squared test or Fisher's exact test was used for categorical data, whereas the Wilcoxon test or Kruskal‐Wallis test was used for continuous data. The Kaplan‐Meier method was used to estimate survival distribution, and the log‐rank test was used to compare survival distributions. EGJ‐cancer‐specific survival time for each case was calculated from the date of surgical resection until death from EGJ adenocarcinoma, or 10 March 2020, whichever came first. Relapse‐free survival (RFS) time was from the date of surgical resection until recurrence or death from any cause, and overall survival (OS) until death from any cause. Cox proportional hazards models were used to estimate mortality hazard ratios (HRs). More details are provided in Appendix S1.
3.3. Clinicopathological and survival analysis in terms of tumor location
The baseline characteristics according to Siewert classification are shown in Table 2. Type I tumors were observed in 59 cases (15%), type II in 280 cases (71%), and type III in 56 cases (14%). Type I tumors were associated with recent cases (P < .0001), more frequently accompanied by Barrett's esophagus (78%, P < .0001), less frequently associated with Helicobacter pylori infection (P < .0001), and showed a less‐advanced disease stage as compared with type II or III tumors (P = .0002). Because surgeons often select a transthoracic approach for type I tumors (P < .0001), operative time was the longest for type I tumors (P < .0001), with less blood loss and a successful R0 resection rate (P = .0041). Patients with type III tumors exhibited a significantly larger size (P < .0001), because the radius of the tumor in type III tumors must be at least 2 cm to invade into the esophagus. Accordingly, type III cases included more advanced disease stage, longer operative time with more blood loss, and a lower R0 resection (Table 2).
Table 2.
Baseline characteristics according to Siewert classification (N = 395)
| Clinicopathological features | Total No. | Tumor location by Siewert classification | P (across 3 Siewert types) | ||
|---|---|---|---|---|---|
| Type I (N = 59) | Type II (N = 280) | Type III (N = 56) | |||
| Sex | |||||
| Female | 63 (16%) | 5 (9%) | 46 (16%) | 12 (21%) | .15 |
| Male | 332 (84%) | 54 (91%) | 234 (84%) | 44 (79%) | |
| Age at surgery (y, mean ± SD) | 65.0 ± 12.3 | 63.1 ± 11.5 | 64.7 ± 12.6 | 68.5 ± 11.3 | .060 |
| Year since surgery | |||||
| Before Dec. 2009 | 181 (46%) | 15 (25%) | 128 (46%) | 38 (68%) | <.0001 |
| Jan. 2010 to Mar. 2015 | 214 (54%) | 44 (75%) | 152 (54%) | 18 (32%) | |
| Body mass index (kg/m2, mean ± SD) | 22.7 ± 3.4 | 23.1 ± 4.3 | 22.8 ± 3.2 | 22.1 ± 3.4 | .28 |
| <22.6 (median) | 195 (50%) | 25 (43%) | 138 (50%) | 32 (57%) | .33 |
| ≥22.6 (median) | 196 (50%) | 33 (57%) | 139 (50%) | 24 (43%) | |
| Tumor diameter (mm, mean ± SD) | 54.9 ± 30.7 | 45.1 ± 28.0
|
51.4 ± 28.1
|
82.5 ± 31.1 | <.0001 |
| <50 (median) | 185 (47%) | 39 (66%) | 139 (50%) | 7 (13%) | <.0001 |
| ≥50 (median) | 210 (53%) | 20 (34%) | 141 (50%) | 49 (87%) | |
| Barrett's esophagus | |||||
| Absent | 271 (69%) | 13 (22%) | 204 (73%) | 54 (96%) | <.0001 |
| Present | 124 (31%) | 46 (78%) | 76 (27%) | 2 (4%) | |
| Helicobacter pylori infection (Limited to Cancer Institute Hospital cases) | |||||
| Negative | 100 (44%) | 35 (79%) | 64 (39%) | 1 (5%) | <.0001 |
| Positive | 128 (56%) | 9 (21%) | 100 (61%) | 19 (95%) | |
| pT Stage | |||||
| pT1 | 101 (26%) | 27 (46%) | 71 (25%) | 3 (5%) | <.0001 |
| pT2 | 54 (14%) | 6 (10%) | 47 (17%) | 1 (2%) | |
| pT3 | 137 (34%) | 23 (39%) | 95 (34%) | 19 (34%) | |
| pT4 | 103 (26%) | 3 (5%) | 67 (24%) | 33 (59%) | |
| pN Stage | |||||
| pN0 | 157 (39%) | 28 (47%) | 117 (41%) | 12 (21%) | .021 |
| pN1 | 79 (20%) | 10 (17%) | 58 (21%) | 11 (20%) | |
| pN2 | 65 (17%) | 8 (14%) | 47 (17%) | 10 (18%) | |
| pN3 | 94 (24%) | 13 (22%) | 58 (21%) | 23 (41%) | |
| No. of nodes harvested (mean ± SD) | 34.9 ± 17.8 | 37.3 ± 19.8 | 33.3 ± 16.1
|
40.6 ± 22.0 | .020 |
| No. of metastatic nodes (mean ± SD) | 4.2 ± 6.7 | 3.5 ± 5.3
|
3.7 ± 5.9
|
7.7 ± 9.9 | .0010 |
| M stage | |||||
| M0 | 336 (85%) | 54 (91%) | 242 (86%) | 40 (71%) | .0051 |
| M1 | 59 (15%) | 5 (9%) | 38 (14%) | 16 (29%) | |
| pStage | |||||
| I | 115 (29%) | 25 (42%) | 87 (31%) | 3 (5%) | .0002 |
| II | 51 (13%) | 7 (12%) | 37 (13%) | 7 (13%) | |
| III | 170 (43%) | 22 (37%) | 118 (42%) | 30 (53%) | |
| IV | 59 (15%) | 5 (9%) | 38 (14%) | 16 (29%) | |
| Adjuvant chemotherapy | |||||
| Absent | 237 (61%) | 35 (59%) | 176 (63%) | 26 (48%) | .10 |
| Present | 153 (39%) | 24 (41%) | 101 (37%) | 28 (52%) | |
| Histological subtypes | |||||
| Lauren classification | |||||
| Intestinal | 305 (77%) | 49 (83%) | 217 (77%) | 39 (70%) | .23 |
| Diffuse | 90 (23%) | 10 (17%) | 63 (23%) | 17 (30%) | |
| WHO classification | |||||
| Papillary | 7 (2%) | 1 (2%) | 4 (1%) | 2 (4%) | .30 |
| Tubular | 280 (70%) | 47 (79%) | 200 (71%) | 33 (59%) | |
| Mucinous | 18 (5%) | 1 (2%) | 13 (5%) | 4 (7%) | |
| Poorly cohesive | 90 (23%) | 10 (17%) | 63 (23%) | 17 (30%) | |
| Lymphatic invasion | |||||
| Absent | 123 (32%) | 26 (44%) | 82 (30%) | 15 (27%) | .077 |
| Present | 265 (68%) | 33 (56%) | 191 (70%) | 41 (73%) | |
| Venous invasion | |||||
| Absent | 130 (33%) | 27 (46%) | 84 (30%) | 19 (34%) | .076 |
| Present | 261 (67%) | 32 (54%) | 192 (70%) | 37 (66%) | |
| Surgical approach | |||||
| Transhiatal | 313 (79%) | 13 (22%) | 244 (87%) | 56 (100%) | <.0001 |
| Transthoracic | 82 (21%) | 46 (78%) | 36 (13%) | 0 | |
| Operative time (min, mean ± SD) | 346 ± 141 |
493 ± 149
|
321 ± 123 | 318 ± 127 | <.0001 |
| <320 (median) | 194 (50%) | 5 (9%) | 158 (57%) | 31 (56%) | <.0001 |
| ≥320 (median) | 194 (50%) | 52 (91%) | 118 (43%) | 24 (44%) | |
| Blood loss volume (g, mean ± SD) | 461 ± 414 | 450 ± 400
|
432 ± 391
|
613 ± 502 | .0084 |
| <350 (median) | 188 (49%) | 28 (49%) | 139 (50%) | 21 (38%) | .25 |
| ≥350 (median) | 199 (51%) | 29 (51%) | 136 (50%) | 34 (62%) | |
| Blood transfusion | |||||
| Absent | 343 (88%) | 51 (89%) | 248 (90%) | 44 (79%) | .056 |
| Present | 46 (12%) | 6 (11%) | 28 (10%) | 12 (21%) | |
| Resection margin | |||||
| R0 | 346 (87%) | 57 (96%) | 248 (89%) | 41 (73%) | .0041 |
| R1 | 26 (7%) | 1 (2%) | 17 (6%) | 8 (14%) | |
| R2 | 23 (6%) | 1 (2%) | 15 (5%) | 7 (13%) | |
| Preoperative complications | |||||
| None or Clavien‐Dindo < IIIa | 315 (80%) | 41 (69%) | 227 (81%) | 47 (84%) | .093 |
| Clavien‐Dindo ≥ IIIa | 80 (20%) | 18 (31%) | 53 (19%) | 9 (16%) | |
(%) Indicates the proportion of cases with specific clinicopathological features for each Siewert classification group. * P < .05, **P < .01, ***P < .001.
Abbreviation: SD, standard deviation.
In the survival analysis, there were 192 deaths, including 135 EGJ‐cancer‐specific deaths, over a median follow‐up of 5.3 years (interquartile range, 5.0‐6.6 years) for censored cases. Kaplan‐Meier analyses according to disease stage are provided in Figure 2A‐C. The 5‐year EGJ‐cancer‐specific survival rates were 95.1% for pStage I, 86.2% for pStage II, 59.1% for pStage III, and 8.7% for pStage IV (Figure 2A). Kaplan‐Meier analyses according to Siewert classification (N = 395, Figure 3A‐C) showed that the 5‐year EGJ‐cancer‐specific mortality of Siewert type III tumors was the worst (50.4%, Figure 3A) compared with that of type I (68.7%) or type II (67.3%) tumors. This result is consistent with previous reports, due to the bias of more advanced disease cases among type III tumors. 47 , 52 When adjusting for various clinical factors in the multivariate survival analysis, the significant dismal prognosis of type I tumors became evident using type II tumors as a reference (multivariate EGJ‐cancer‐specific mortality HR = 1.81, 95% confidence interval [CI], 1.06‐2.97; P = .031; Table 3). A subgroup analysis of pStage II‐III cases (N = 221, Figure 4A‐C) showed that the 5‐year EGJ‐cancer‐specific mortality of Siewert type I cases (58.9%, Figure 4A) was the highest as compared with that of type II (65.8%) or type III (68.9%) cases (multivariate mortality HR = 2.13, 95% confidence interval [CI], 1.09‐3.98; P = .028; N = 221, Table 4). Regarding recurrence among type I tumors after surgery, over half of type I cases (56%) experienced recurrent disease in lymph nodes, particularly in mediastinal or paraaortic nodes (Table S1). This raises a clinically important question, whether radical lymph node dissection, including removal of mediastinal or paraaortic node dissection, may be beneficial or not for patients with Siewert type I tumors. Further large‐scale study would be needed to address this issue.
FIGURE 2.
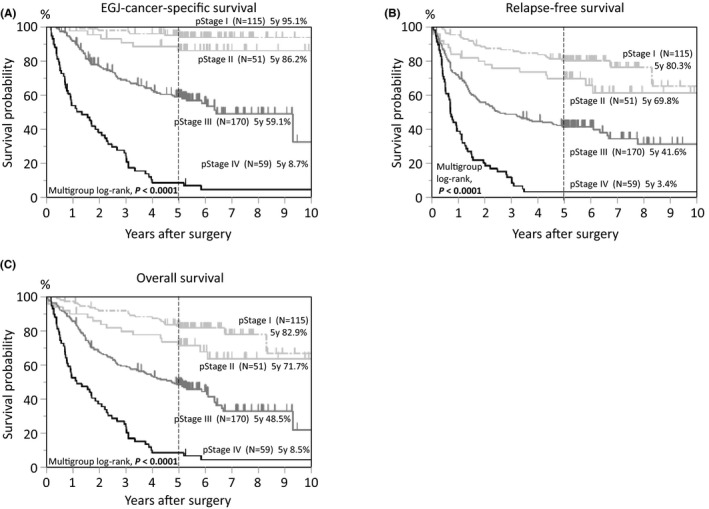
Kaplan‐Meier curves of 395 cases of esophagogastric junction (EGJ) adenocarcinoma, categorized according to disease stage (pStage I‐IV). A, EGJ‐cancer‐specific survival. B, Relapse‐free survival. C, Overall survival
FIGURE 3.
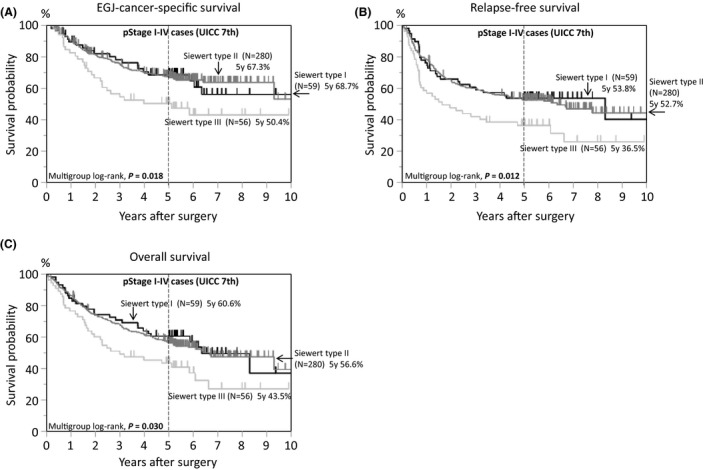
Kaplan‐Meier curves of 395 cases of esophagogastric junction (EGJ) adenocarcinoma according to tumor location by Siewert classification. A, EGJ‐cancer‐specific survival. B, Relapse‐free survival. C, Overall survival
Table 3.
Patient mortality in all cases (N = 395)
| Clinicopathological factors | Total No. | EGJ‐cancer‐specific survival | Relapse‐free survival | Overall survival | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of events | Univariate HR (95% CI) | Multivariate HR (95% CI) | No. of events | Univariate HR (95% CI) | Multivariate HR (95% CI) | No. of events | Univariate HR (95% CI) | Multivariate HR (95% CI) | ||
| Siewert classification | ||||||||||
| Type I (vs Type II) | 59 | 20 |
1.02 (0.61‐1.62) P = .94 |
1.81 (1.06‐2.97) P = .031 |
28 |
0.96 (0.62‐1.41) P = .83 |
1.35 (0.87‐2.03) P = .18 |
27 |
0.93 (0.60‐1.38) P = .72 |
1.43 (0.91‐2.18) P = .12 |
| Type III (vs Type II) | 56 | 27 |
1.83 (1.17‐2.78) P = .0094 |
0.93 (0.59‐1.44) P = .76 |
37 |
1.69 (1.16‐2.41) P = .0071 |
0.95 (0.64‐1.38) P = .81 |
35 |
1.61 (1.09‐2.31) P = .018 |
0.92 (0.61‐1.33) P = .65 |
The multivariate, Cox proportional hazard regression model initially included gender, age, year of surgery, body mass index, tumor diameter, tumor location by Siewert classification, existence of Barrett's esophagus, disease stage, tumor differentiation, lymphatic invasion, venous invasion, surgical approach, operative time, blood loss volume, blood transfusion, resection margin, preoperative complication, adjuvant chemotherapy.
A backward elimination with a threshold of P = .20 was used to select variables in the final model.
Abbreviations: CI, confidence interval; HR, hazard ratio.
FIGURE 4.
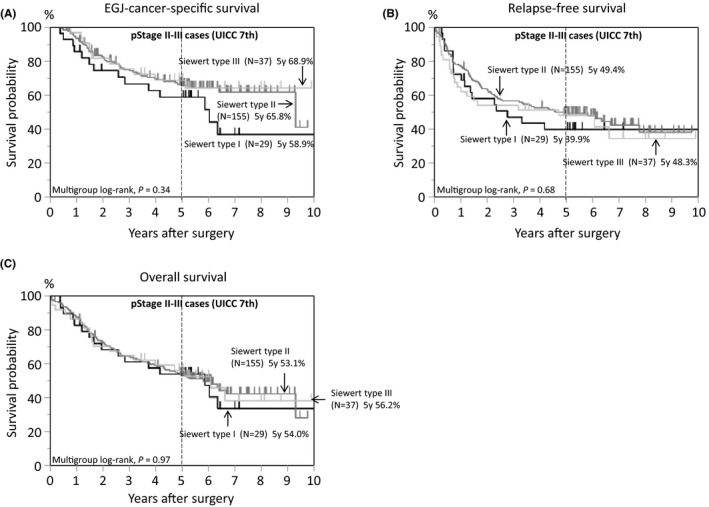
Kaplan‐Meier curves of pStage II‐III cases (N = 221) of esophagogastric junction (EGJ) adenocarcinoma according to tumor location by Siewert classification. A, EGJ‐cancer‐specific survival. B, Relapse‐free survival. C, Overall survival
Table 4.
Patient mortality according to Siewert classification in pStage II and III cases (N = 221)
| Total No. | EGJ‐cancer‐specific survival | Relapse‐free survival | Overall survival | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of events | Univariate HR (95% CI) | Multivariate HR (95% CI) | No. of events | Univariate HR (95% CI) | Multivariate HR (95% CI) | No. of events | Univariate HR (95% CI) | Multivariate HR (95% CI) | ||
| Tumor location (vs Siewert type II) | ||||||||||
| Siewert type I | 29 | 14 |
1.51 (0.80‐2.67) P = .19 |
2.13 (1.09‐3.98) P = .028 |
17 |
1.20 (0.69‐1.98) P = .50 |
1.64 (0.92‐2.78) P = .093 |
16 |
1.08 (0.61‐1.79) P = .79 |
1.57 (0.86‐2.70) P = .14 |
| Siewert type III | 37 | 11 |
0.93 (0.46‐1.73) P = .84 |
0.90 (0.44‐1.68) P = .75 |
21 |
1.18 (0.71‐1.86) P = .52 |
1.12 (0.67‐1.77) P = .66 |
19 |
1.01 (0.59‐1.62) P = .98 |
0.95 (0.56‐1.54) P = .85 |
The multivariate Cox regression model included the same set of covariates selected as in Table 2.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Because Siewert type I tumors were significantly associated with Barrett's esophagus, additional analyses were performed focusing on the presence or absence of Barrett's esophagus. Although the clinicopathological features of the cases accompanied by Barrett's esophagus was similar to those of Siewert type I tumors (Tables S2 and S3), there was no significant association between Barrett's esophagus and patient outcome in multivariate survival analysis (Tables S4 and S5).
4. CONCLUSION
Recent comprehensive genomic analyses suggest that immunotherapy may play an essential role in the treatment of MSI‐H, EBV, or GS molecular subtypes of EGJ adenocarcinoma. EGJ adenocarcinoma includes Barrett's adenocarcinoma and cardiac adenocarcinoma, and because of their extensive similarities, it is likely that Barrett's adenocarcinoma is of gastric origin. In our multicenter cohort study, we show that Siewert type I (distal esophagus) tumors are frequently accompanied with Barrett's esophagus and have significantly unfavorable outcomes, with more than half of patients experiencing disease recurrence in the lymph nodes. Thus, Barrett's adenocarcinoma may potentially be an aggressive clinical subtype of EGJ adenocarcinoma, with a potential risk of tumor spreading through the complex lympho‐vascular network of the esophagus.
CONFLICT OF INTERESTS
None of the authors has any conflict of interest related to this study.
Supporting information
Appendix S1
Table S1‐S5
ACKNOWLEDGEMENTS
We would like to express our sincere appreciation for data acquisition to Ikumi Haraguchi (Cancer Institute Hospital).
Imamura Y, Watanabe M, Oki E, Morita M, Baba H. Esophagogastric junction adenocarcinoma shares characteristics with gastric adenocarcinoma: Literature review and retrospective multicenter cohort study. Ann Gastroenterol Surg.2021;5:47–60. 10.1002/ags3.12406
Funding informationThis work was supported by JSPS KAKENHI Grant Number JP20K09046 (YI), and JP19K09231 (MW); Japan Research Foundation for Clinical Pharmacology (YI), and Foundation for Promotion of Cancer Research in Japan (Y.I).
REFERENCES
- 1. Han WH, Eom BW, Yoon HM, Reim D, Kim YW, Kim MS, et al. The optimal extent of lymph node dissection in gastroesophageal junctional cancer: retrospective case control study. BMC Cancer. 2019;19:719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kusano C, Gotoda T, Khor CJ, Katai H, Kato H, Taniguchi H, et al. Changing trends in the proportion of adenocarcinoma of the esophagogastric junction in a large tertiary referral center in Japan. J Gastroenterol Hepatol. 2008;23:1662–5. [DOI] [PubMed] [Google Scholar]
- 3. Yamashita H, Seto Y, Sano T, Makuuchi H, Ando N, Sasako M, et al. Results of a nation‐wide retrospective study of lymphadenectomy for esophagogastric junction carcinoma. Gastric Cancer. 2017;20:69–83. [DOI] [PubMed] [Google Scholar]
- 4. Liu K, Yang K, Zhang W, Chen X, Chen X, Zhang B, et al. Changes of esophagogastric junctional adenocarcinoma and gastroesophageal reflux disease among surgical patients during 1988–2012: a single‐institution. High‐volume Experience in China. Ann Surg. 2016;263:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Imamura Y, Watanabe M, Toihata T, Takamatsu M, Kawachi H, Haraguchi I, et al. Recent incidence trend of surgically resected esophagogastric junction adenocarcinoma and microsatellite instability status in Japanese patients. Digestion. 2019;99:6–13. [DOI] [PubMed] [Google Scholar]
- 6. Yamada M, Kushima R, Oda I, Mojtahed K, Nonaka S, Suzuki H, et al. Different histological status of gastritis in superficial adenocarcinoma of the esophagogastric junction. Jpn J Clin Oncol. 2014;44:65–71. [DOI] [PubMed] [Google Scholar]
- 7. Devesa SS, Fraumeni JF Jr. The rising incidence of gastric cardia cancer. J Natl Cancer Inst. 1999;91:747–9. [DOI] [PubMed] [Google Scholar]
- 8. Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–31. [DOI] [PubMed] [Google Scholar]
- 9. Wu IC, Wu DC, Yu FJ, Wang JY, Kuo CH, Yang SF, et al. Association between Helicobacter pylori seropositivity and digestive tract cancers. World J Gastroenterol. 2009;15:5465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bornschein J, Dingwerth A, Selgrad M, Venerito M, Stuebs P, Frauenschlaeger K, et al. Adenocarcinomas at different positions at the gastro‐oesophageal junction show distinct association with gastritis and gastric preneoplastic conditions. Eur J Gastroenterol Hepatol. 2015;27:492–500. [DOI] [PubMed] [Google Scholar]
- 11. Bornschein J, Selgrad M, Warnecke M, Kuester D, Wex T, Malfertheiner P. Helicobacter pylori infection is a key risk factor for proximal gastric cancer. Dig Dis Sci. 2010;55:3124–31. [DOI] [PubMed] [Google Scholar]
- 12. Goldblum JR, Richter JE, Vaezi M, Falk GW, Rice TW, Peek RM. Helicobacter pylori infection, not gastroesophageal reflux, is the major cause of inflammation and intestinal metaplasia of gastric cardiac mucosa. Am J Gastroenterol. 2002;97:302–11. [DOI] [PubMed] [Google Scholar]
- 13. Goldblum JR, Vicari JJ, Falk GW, Rice TW, Peek RM, Easley K, et al. Inflammation and intestinal metaplasia of the gastric cardia: the role of gastroesophageal reflux and H. pylori infection. Gastroenterology. 1998;114:633–9. [DOI] [PubMed] [Google Scholar]
- 14. Thrift AP, Shaheen NJ, Gammon MD, Bernstein L, Reid BJ, Onstad L, et al. Obesity and risk of esophageal adenocarcinoma and Barrett's esophagus: a Mendelian randomization study. J Natl Cancer Inst. 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Merry AH, Schouten LJ, Goldbohm RA, van den Brandt PA. Body mass index, height and risk of adenocarcinoma of the oesophagus and gastric cardia: a prospective cohort study. Gut. 2007;56:1503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bae JM. Body Mass Index and risk of gastric cancer in Asian adults: a meta‐epidemiological meta‐analysis of population‐based cohort studies. Cancer Res Treat. 2020;52:369–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Y, Liu L, Wang X, Wang J, Yan Z, Cheng J, et al. Body mass index and risk of gastric cancer: a meta‐analysis of a population with more than ten million from 24 prospective studies. Cancer Epidemiol Biomarkers Prev. 2013;22:1395–408. [DOI] [PubMed] [Google Scholar]
- 18. Ericksen RE, Rose S, Westphalen CB, Shibata W, Muthupalani S, Tailor Y, et al. Obesity accelerates Helicobacter felis‐induced gastric carcinogenesis by enhancing immature myeloid cell trafficking and TH17 response. Gut. 2014;63:385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chandar AK, Devanna S, Lu C, Singh S, Greer K, Chak A, et al. Association of serum levels of adipokines and insulin with risk of Barrett's esophagus: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2015;13(13):2241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quante M, Bhagat G, Abrams JA, Marache F, Good P, Lee MD, et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett‐like metaplasia. Cancer Cell. 2012;21:36–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee Y, Urbanska AM, Hayakawa Y, Wang H, Au AS, Luna AM, et al. Gastrin stimulates a cholecystokinin‐2‐receptor‐expressing cardia progenitor cell and promotes progression of Barrett's‐like esophagus. Oncotarget. 2017;8:203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hayakawa Y, Jin G, Wang H, Chen X, Westphalen CB, Asfaha S, et al. CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut. 2015;64:544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abdulnour‐Nakhoul S, Nakhoul NL, Wheeler SA, Haque S, Wang P, Brown K, et al. Characterization of esophageal submucosal glands in pig tissue and cultures. Dig Dis Sci. 2007;52:3054–65. [DOI] [PubMed] [Google Scholar]
- 24. Nicholson AM, Graham TA, Simpson A, Humphries A, Burch N, Rodriguez‐Justo M, et al. Barrett's metaplasia glands are clonal, contain multiple stem cells and share a common squamous progenitor. Gut. 2012;61:1380–9. [DOI] [PubMed] [Google Scholar]
- 25. Liu Y, Sethi NS, Hinoue T, Schneider BG, Cherniack AD, Sanchez‐Vega F, et al. Comparative molecular analysis of gastrointestinal adenocarcinomas. Cancer Cell. 2018;33(721–735):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. The‐Cancer‐Genome‐Atlas‐Research‐Network . Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lauren P. The two histological main types of gastric carcinoma: diffuse and so‐called intestinal‐type carcinoma. an attempt at a histo‐clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. [DOI] [PubMed] [Google Scholar]
- 28. The‐Cancer‐Genome‐Atlas‐Research‐Network . Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smyth EC, Wotherspoon A, Peckitt C, Gonzalez D, Hulkki‐Wilson S, Eltahir Z, et al. Mismatch repair deficiency, microsatellite instability, and survival: an exploratory analysis of the medical research council adjuvant gastric infusional chemotherapy (MAGIC) Trial. JAMA Oncol. 2017;3:1197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site ‐ when a biomarker defines the indication. N Engl J Med. 2017;377:1409–12. [DOI] [PubMed] [Google Scholar]
- 31. Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, et al. Comprehensive molecular characterization of clinical responses to PD‐1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449–58. [DOI] [PubMed] [Google Scholar]
- 32. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro‐oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO‐4538‐12, ATTRACTION‐2): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2017;390:2461–71. [DOI] [PubMed] [Google Scholar]
- 33. Hansford S, Kaurah P, Li‐Chang H, Woo M, Senz J, Pinheiro H, et al. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol. 2015;1:23–32. [DOI] [PubMed] [Google Scholar]
- 34. Fewings E, Larionov A, Redman J, Goldgraben MA, Scarth J, Richardson S, et al. Germline pathogenic variants in PALB2 and other cancer‐predisposing genes in families with hereditary diffuse gastric cancer without CDH1 mutation: a whole‐exome sequencing study. Lancet Gastroenterol Hepatol. 2018;3:489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sahasrabudhe R, Lott P, Bohorquez M, Toal T, Estrada AP, Suarez JJ, et al. Germline mutations in PALB2, BRCA1, and RAD51C, which regulate DNA recombination repair, in patients with gastric cancer. Gastroenterology. 2017;152(983–986):e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rokutan H, Hosoda F, Hama N, Nakamura H, Totoki Y, Furukawa E, et al. Comprehensive mutation profiling of mucinous gastric carcinoma. J Pathol. 2016;240:137–48. [DOI] [PubMed] [Google Scholar]
- 37. Mueller S, Engleitner T, Maresch R, Zukowska M, Lange S, Kaltenbacher T, et al. Evolutionary routes and KRAS dosage define pancreatic cancer phenotypes. Nature. 2018;554(7690):62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dulak AM, Schumacher SE, van Lieshout J, Imamura Y, Fox C, Shim B, et al. Gastrointestinal adenocarcinomas of the esophagus, stomach, and colon exhibit distinct patterns of genome instability and oncogenesis. Cancer Res. 2012;72:4383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu J, Lichtenberg T, Hoadley KA, Poisson LM, Lazar AJ, Cherniack AD, et al. An integrated TCGA pan‐cancer clinical data resource to drive high‐quality survival outcome analytics. Cell. 2018;173(400–416):e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wong GS, Zhou J, Liu JB, Wu Z, Xu X, Li T, et al. Targeting wild‐type KRAS‐amplified gastroesophageal cancer through combined MEK and SHP2 inhibition. Nat Med. 2018;24:968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sanchez‐Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, et al. Oncogenic signaling pathways in the cancer genome atlas. Cell. 2018;173(321–337):e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weinberg BA, Xiu J, Hwang JJ, Shields AF, Salem ME, Marshall JL. Immuno‐oncology biomarkers for gastric and gastroesophageal junction adenocarcinoma: why PD‐L1 testing may not be enough. Oncologist. 2018;23:1171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xing X, Jia S, Wu J, Feng Q, Dong B, Li B, et al. Clonality analysis of synchronous gastro‐oesophageal junction carcinoma and distal gastric cancer by whole‐exome sequencing. J Pathol. 2017;243:165–75. [DOI] [PubMed] [Google Scholar]
- 44. Thompson ED, Zahurak M, Murphy A, Cornish T, Cuka N, Abdelfatah E, et al. Patterns of PD‐L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 2017;66:794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Derks S, Nason KS, Liao X, Stachler MD, Liu KX, Liu JB, et al. Epithelial PD‐L2 expression marks Barrett's esophagus and esophageal adenocarcinoma. Cancer Immunol Res. 2015;3:1123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kollmann D, Ignatova D, Jedamzik J, Chang YT, Jomrich G, Baierl A, et al. PD‐L1 expression is an independent predictor of favorable outcome in patients with localized esophageal adenocarcinoma. Oncoimmunology. 2018;7:e1435226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Knief J, Lazar‐Karsten P, Hummel R, Wellner U, Thorns C. PD‐L1 expression in carcinoma of the esophagogastric junction is positively correlated with T‐cell infiltration and overall survival. Pathol Res Pract. 2019;215:152402. [DOI] [PubMed] [Google Scholar]
- 48. Wang Y, Wang S, Zhu C, Cao H, Zhang Z, Zhao E. The association between immune characteristic and clinical pathology in Chinese patients with adenocarcinoma of esophagogastric junction. Cancer Manag Res. 2020;12:3259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449–56. [DOI] [PubMed] [Google Scholar]
- 50. Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457–9. [DOI] [PubMed] [Google Scholar]
- 51. Spechler SJ, Souza RF. Barrett's esophagus. N Engl J Med. 2014;371:836–45. [DOI] [PubMed] [Google Scholar]
- 52. Schlottmann F, Barbetta A, Mungo B, Lidor AO, Molena D. Identification of the lymphatic drainage pattern of esophageal cancer with near‐infrared fluorescent imaging. J Laparoendosc Adv Surg Tech A. 2017;27:268–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ustaalioglu BBO, Tilki M, Surmelioglu A, Bilici A, Gonen C, Ustaalioglu R, et al. The clinicopathologic characteristics and prognostic factors of gastroesophageal junction tumors according to Siewert classification. Turk J Surg. 2017;33:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hosokawa Y, Kinoshita T, Konishi M, Takahashi S, Gotohda N, Kato Y, et al. Clinicopathological features and prognostic factors of adenocarcinoma of the esophagogastric junction according to Siewert classification: experiences at a single institution in Japan. Ann Surg Oncol. 2012;19:677–83. [DOI] [PubMed] [Google Scholar]
- 55. Hasegawa S, Yoshikawa T, Cho H, Tsuburaya A, Kobayashi O. Is adenocarcinoma of the esophagogastric junction different between Japan and western countries? The incidence and clinicopathological features at a Japanese high‐volume cancer center. World J Surg. 2009;33:95–103. [DOI] [PubMed] [Google Scholar]
- 56. Siewert JR, Feith M, Stein HJ. Biologic and clinical variations of adenocarcinoma at the esophago‐gastric junction: relevance of a topographic‐anatomic subclassification. J Surg Oncol. 2005;90:139–46. discussion 146. [DOI] [PubMed] [Google Scholar]
- 57. Leers JM, DeMeester SR, Chan N, Ayazi S, Oezcelik A, Abate E, et al. Clinical characteristics, biologic behavior, and survival after esophagectomy are similar for adenocarcinoma of the gastroesophageal junction and the distal esophagus. J Thorac Cardiovasc Surg. 2009;138(3):594–602. [DOI] [PubMed] [Google Scholar]
- 58. von Rahden BH, Stein HJ, Feith M, Becker K, Siewert JR. Lymphatic vessel invasion as a prognostic factor in patients with primary resected adenocarcinomas of the esophagogastric junction. J Clin Oncol. 2005;23:874–9. [DOI] [PubMed] [Google Scholar]
- 59. Parry K, Haverkamp L, Bruijnen RC, Siersema PD, Ruurda JP, van Hillegersberg R. Surgical treatment of adenocarcinomas of the gastro‐esophageal junction. Ann Surg Oncol. 2015;22:597–603. [DOI] [PubMed] [Google Scholar]
- 60. Ott K, Bader FG, Lordick F, Feith M, Bartels H, Siewert JR. Surgical factors influence the outcome after Ivor‐Lewis esophagectomy with intrathoracic anastomosis for adenocarcinoma of the esophagogastric junction: a consecutive series of 240 patients at an experienced center. Ann Surg Oncol. 2009;16:1017–25. [DOI] [PubMed] [Google Scholar]
- 61. Curtis NJ, Noble F, Bailey IS, Kelly JJ, Byrne JP, Underwood TJ. The relevance of the Siewert classification in the era of multimodal therapy for adenocarcinoma of the gastro‐oesophageal junction. J Surg Oncol. 2014;109:202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Table S1‐S5




