Abstract
Clinical manifestations of the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can include gastrointestinal signals and symptoms. Individuals with previous clinical conditions that usually enroll gut dysbiosis have been identified as being at high risk to develop more severe infectious phenotypes. Actually, intestinal dysbiosis has been observed in infected patients and potentially linked to systemic hyperinflammation. These observations suggest that a previous gut dysbiosis may be aggravated by SARS-CoV-2 infection and related to progression of the coronavirus disease 2019 (COVID-19) into more severe stages. While COVID-19’s pathophysiology is not fully understood, it seems relevant to consider the interactions of candidate therapeutic drugs with the host, gut microbiota, and SARS-CoV-2. Here we summarize scientific evidence supporting the potential relevance of these interactions and suggest that unfavorable clinical data on hydroxychloroquine administration in COVID-19 may have been influenced by the dose provided and its impact on gut dysbiosis. The proposition is based on preliminary data on gut microbiota composition from individuals with inactive systemic lupus erythematosus under exclusive continuous hydroxychloroquine treatment, displaying a direct correlation between drug doses and markers typically associated with gut dysbiosis.
Keywords: SARS-CoV-2, Gut microbiota, Gut dysbiosis, Hydroxychloroquine, COVID-19, Antimalarials
The new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the coronavirus disease 2019 (COVID-19) pandemic, which emerged in Wuhan, China, in December 2019 [1]. Beyond the classical respiratory symptoms, a significant proportion (50.5%) of people with COVID-19 can also have gastrointestinal symptomatology such as diarrhea, nausea, and vomiting [2]. These gastrointestinal features have been associated with increased disease susceptibility [3] and poor prognosis [2,4].
In fact, intestinal dysfunction is often associated with some respiratory infections or secondary to complications, suggesting lung–intestine crosstalk [5]. Despite the fact that the respiratory tract harbors its own microbial ecology, the gut microbiota has been shown to affect the host's systemic immune response, by influencing distant mucous membranes such as the lungs [6,7]. Importantly, a healthy gut microbiota profile can contribute to protecting the host against respiratory infections [8,9], whereas an unbalanced gut microbiota profile (dysbiosis) is associated with several infectious diseases and chronic inflammation [10,11].
Likewise, gut microbiota can be sensitive to respiratory viral infections, along with other stressors (such as exposure to antibiotics and chronic inflammation) [12,13]. In particular, SARS-CoV-2 binds its spike protein to the surface membrane angiotensin-converting enzyme 2 (ACE2) receptor to gain access and infect human cells. Similar to the lung, the intestinal epithelium also harbors ACE2 receptors, which seem to influence the composition of resident microbiota [14].
High-risk populations, such as people who are elderly or obese and people with diabetes, hypertension, or metabolic syndrome, are prone to the most severe forms of COVID-19, and often present gut dysbiosis [15], [16], [17], [18]. In these individuals, dysbiosis is associated with systemic low-grade inflammation, with increased intestinal bacterial translocation [15,16]. Disturbances in gut microbiota composition can contribute to epithelial barrier damage by mechanisms that may include an abnormal hyperactivation of intestinal toll-like receptors [18].
Therefore, low-grade inflammation due to increased bacteria translocation may be a predisposing factor for the unbalanced inflammatory responses to SARS-CoV-2 observed in more severe COVID-19 phenotypes [19]. Gou et al. have identified 20 unbalanced gut microbiota components that are predictive for COVID-19 progression to a severe phase [20]. SARS-CoV-2 translocation contributing to systemic reinfection is a possibility that cannot be ruled out, since this virus can be found in feces even when it is no longer detectable in the respiratory tract [21].
Of note, gut microbiota stabilization is one of the six components of a new therapy that effectively increased the rate of cure and reduced mortality for individuals with COVID-19 [22]. Inclusion of this intervention was based on a reduction in commensal Lactobacillus and Bifidobacterium observed in some people with COVID-19 [22]. Nutritional support and administration of prebiotics and probiotics to balance gut microbiota have also been suggested in COVID-19, to reduce the risk of secondary infection due to bacterial translocation [20,21].
While a specific and effective therapy for COVID-19 is lacking, alternative treatments have been proposed. One of the first therapies suggested for managing this new disease included the acute use of the antimalarial drugs chloroquine diphosphate (CQ) or hydroxychloroquine (HCQ). The rationale for testing CQ/HCQ in SARS-COV-2 infection was based on positive previous experience with these drugs against SARS-CoV-1 (which shares 79% of its genome with the new coronavirus) during the severe acute respiratory syndrome outbreak in 2003 [23].
Low doses of CQ/HCQ have been used to treat other diseases, such as systemic lupus, with relevant immunomodulatory effects and very low rates of adverse/side effects [24]. For some of these diseases, the CQ/HCQ therapeutic effect requires at least 3 mo of continuous use to reach a steady state [25]. Nevertheless, there has been a robust discussion on the safety and efficacy of CQ/HCQ in treating individuals with COVID-19, even for short periods.
Preliminary data for CQ/HCQ in COVID-19 came from non-randomized controlled trials or small sample-size trials with controversial results. Some benefits were reported with low daily doses (≤600 mg/d) [26,27], but increased toxicity was observed, particularly in the context of high doses (≥1200 mg/d) [28,29]. Importantly, in the Solidarity trial—a clinical study conducted with the participation of the World Health Organization (WHO), currently awaiting peer-reviewed publication—HCQ produced little or no reduction in mortality for people hospitalized with COVID-19 when compared to the standard of care [30]. Although evidence for increased mortality was not found, some associated safety signals in isolated clinical laboratory findings resulted in the exclusion of the HCQ treatment arm from the study [30].
According to the World Health Organization, observations from the Solidarity study do not preclude assessing the effect of HCQ in other studies with people who are not hospitalized or as pre- or postexposure prophylaxis for COVID-19 [30]. Considering that gut microbiota might play a role in COVID-19’s pathogenesis, therapies for its treatment or prophylaxis should avoid dysbiosis. As far as we can ascertain, the influence of HCQ use on human gut microbiota composition is unknown.
Our research group is investigating dysbiosis signatures (fecal-sample 16S genetic sequencing) associated with inactive (Systemic Lupus Erythematosus Disease Activity Index 2000 score = 0) systemic lupus erythematosus (SLE). In a preliminary analysis, it caught our attention that the abundance of some proinflammatory and commensal phyla and genera of bacteria occurred in a heterogeneous grade across participants. Once they were at same SLE activity grade and under exclusive HCQ treatment at a stable dose, ranging from 2.33 to 5.58 mg/kg/d for 2.62 ± 1.47 y, we searched for potential correlations of HCQ treatment with our negative findings on gut microbiota.
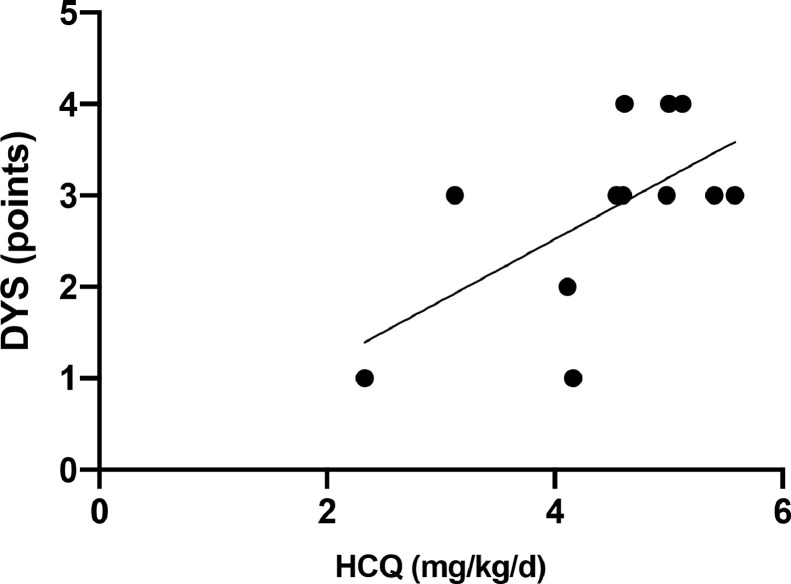
In our analysis, only individuals with SLE who had C-reactive protein levels ≤ 0.3 mg/dL (mean = 0.138 ± 0.097 mg/dL) were considered (n = 12), to avoid the influence of inflammation bias on gut microbiota composition [31]. With this rigorous parameter, we calculated the sum of markers typically associated with gut dysbiosis [32], [33], [34], [35] as an indirect measure of dysbiosis degree (Fig. 1 ) and tested it against different doses of HCQ. As can be seen in the figure, our data suggest that HCQ dosage is directly correlated with degree of dysbiosis in individuals with SLE (r = 0.61, CI = 0.04, P = 0.03). This correlation was observed despite the narrow dose range tested and the small sample size, reinforcing the relevance of further evaluating a larger number of participants.
Fig. 1.
Correlation between hydroxychloroquine dosage (mg/kg/d) and gut dysbiosis in 12 individuals with inactive systemic lupus erythematosus. Spearman r = 0.61, CI = 0,04 to 0,88 (confidence interval), P = 0.03. Doses of hydroxychloroquine ranged from 2.33 to 5.58 mg/kg/d. Gut dysbiosis degree ranged from 0 to 6 points, determined based on empirical systematic criteria where one point was attributed for the presence of each of the following: low Firmicutes/Bacteroidetes ratio (<0.7); low Firmicutes + Bacteroidetes index (<85%); low richness (<360); low diversity (<5.5); presence of at least one pathological bacterium in abundance (Bacteroides fragilis >0.5%; others >0.1%); low Akkermansia muciniphila (<1.0%).
Although our observations are very preliminary and were obtained from a different clinical population than people with COVID-19, they raise the possibility that HCQ administration may influence previous gut dysbiosis in a dose-dependent fashion. We cannot determine whether low HCQ doses may alleviate gut dysbiosis, because data on gut microbiota composition without HCQ intervention are lacking. In a high-fat diet-induced mouse model of rheumatoid arthritis–associated atherosclerosis, a better gut microbiota profile was observed after HCQ administration, and this change was associated with clinical benefits of the treatment [36]. On the other hand, our data in people with lupus suggest that high HCQ doses may contribute to gut dysbiosis. In healthy C57BL/6J mice, despite the fact that intestinal integrity and immunologic responses were maintained, short-term high dose HCQ has been shown to affect gut microbiota composition and lactate dehydrogenase activity, an indicator for myocardial injury [37].
Different effects of HCQ doses on gut microbiota could explain, at least partially, why available clinical data in COVID-19 are quite heterogeneous on the benefits and side effects of HCQ according to the low and high doses provided [26], [27], [28]. In seeking a definitive treatment for COVID-19, researchers are concentrating their efforts on understanding mechanisms or biological predictors associated with prognosis. While we do not fully understand the pathophysiology of this new disease, our data draw attention to the potential relevance of considering the interactions of candidate drugs with the host, gut microbiota, and SARS-CoV-2.
Acknowledgments
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (#305068/2014-8 to E.B. and #306879/2018-2 to E.F.B.) and from the Fundação de Amparo a Pesquisa do Estado de São Paulo (#2015/03756-4 to E.B. and #2018/16162-3 to E.F.B.).
Footnotes
B.D.B. and R.S.T. conceptualized the study and wrote the original draft of the article. I.M.R., D.C.F., and F.F.C.F. reviewed and edited the article. E.S.D.O.B. reviewed and edited the article and acquired resources and funding. E.F.B. reviewed and edited the article, acquired resources and funding, and supervised. D.L.W. conceptualized the study, reviewed and edited the article, acquired funding, and supervised.
References
- 1.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nobel YR, Phipps M, Zucker J, Lebwohl B, Wang TC, Sobieszczyk ME, et al. Gastrointestinal symptoms and coronavirus disease 2019: a case-control study from the United States. Gastroenterology. 2020;159 doi: 10.1053/j.gastro.2020.04.017. P373–5.E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei XS, Wang X, Niu YR, Ye LL, Peng WB, Wang ZH, et al. Diarrhea is associated with prolonged symptoms and viral carriage in corona virus disease 2019. Clin Gastroenterol Hepatol. 2020;18 doi: 10.1016/j.cgh.2020.04.030. 1753–9.E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao QY, Chen YX, Fang JY. 2019 Novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020;21:125–126. doi: 10.1111/1751-2980.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budden KF, Gellatly SL, Wood DLA, Cooper MA, Morrison M, Hugenholtz P, et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15:55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 7.McAleer JP, Kolls JK. Contributions of the intestinal microbiome in lung immunity. Eur J Immunol. 2018;48:39–49. doi: 10.1002/eji.201646721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley KC, Finsterbusch K, Schnepf D, Crotta S, Llorian M, Davidson S, et al. Microbiota-driven tonic interferon signals in lung stromal cells protect from influenza virus infection. Cell Rep. 2019;28 doi: 10.1016/j.celrep.2019.05.105. 245–56.e4. [DOI] [PubMed] [Google Scholar]
- 9.Steed AL, Christophi GP, Kaiko GE, Sun L, Goodwin VM, Jain U, et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science. 2017;357:498–502. doi: 10.1126/science.aam5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cani PD. Human gut microbiome: hopes, threats and promises. Gut. 2018;67:1716–1725. doi: 10.1136/gutjnl-2018-316723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shanahan F, Van Sinderen D, O'Toole PW, Stanton C. Feeding the microbiota: transducer of nutrient signals for the host. Gut. 2017;66:1709–1717. doi: 10.1136/gutjnl-2017-313872. [DOI] [PubMed] [Google Scholar]
- 12.Yildiz S, Mazel-Sanchez B, Kandasamy M, Manicassamy B, Schmolke M. Influenza A virus infection impacts systemic microbiota dynamics and causes quantitative enteric dysbiosis. Microbiome. 2018;6:9. doi: 10.1186/s40168-017-0386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groves HT, Cuthbertson L, James P, Moffatt MF, Cox MJ, Tregoning JS. Respiratory disease following viral lung infection alters the murine gut microbiota. Front Immunol. 2018;9:182. doi: 10.3389/fimmu.2018.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keebaugh ES, Ja WW. Breaking down walls: microbiota and the aging gut. Cell Host Microbe. 2017;21:417–418. doi: 10.1016/j.chom.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Viennois E, Gewirtz AT, Chassaing B. Chronic inflammatory diseases: are we ready for microbiota-based dietary intervention? Cell Mol Gastroenterol Hepatol. 2019;8:61–71. doi: 10.1016/j.jcmgh.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rius B, López-Vicario C, González-Périz A, Salvador EM, Alonso VG, Clária J, et al. Resolution of inflammation in obesity-induced liver disease. Front Immunol. 2012;3:257. doi: 10.3389/fimmu.2012.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 19.Torrinhas RS, Calder PC, Lemos GO, Waitzberg DL. Parenteral fish oil, an adjuvant pharmacotherapy for COVID-19? Nutrition. 2020;81 doi: 10.1016/j.nut.2020.110900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gou W, Fu Y, Yue L, Chen G, Cai X, Shuai M, et al. Gut microbiota may underlie the predisposition of healthy individuals to COVID-19. https://www.medrxiv.org/content/10.1101/2020.04.22.20076091v1. Accessed May 17, 2020.
- 21.Ianiro G, Mullish BH, Kelly CR, Sokol H, Kassam Z, Ng S, et al. Screening of faecal microbiota transplant donors during the COVID-19 outbreak: suggestions for urgent updates from an international expert panel. Lancet Gastroenterol Hepatol. 2020;5:430–432. doi: 10.1016/S2468-1253(20)30082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu K, Cai H, Shen Y, Ni Q, Chen Y, Hu S, et al. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:147–157. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases. Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69:20–28. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- 25.Browning DJ. Hydroxychloroquine and chloroquine retinopathy. Springer; New York: 2014. Pharmacology of chloroquine and hydroxychloroquine; pp. 35–63. [Google Scholar]
- 26.Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. https://www.medrxiv.org/content/10.1101/2020.03.22.20040758v3. Accessed May 17, 2020.
- 27.Gautret P, Lagier J-C, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19. https://www.who.int/news/item/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19. Accessed November 19, 2020.
- 31.Nehring SM, Goyal A, Bansal P, Patel BC. StatPearls [Internet. StatPearls Publishing; Treasure Island, FL: 2020. C reactive protein.https://www.ncbi.nlm.nih.gov/books/NBK441843/ Accessed November 19, 2020. [Google Scholar]
- 32.Belizário JE, Faintuch J. Microbiome and gut dysbiosis. Exp Suppl. 2018;109:459–476. doi: 10.1007/978-3-319-74932-7_13. [DOI] [PubMed] [Google Scholar]
- 33.Bello MG, Knight R, Gilbert JA, Blaser MJ. Preserving microbial diversity. Science. 2018;362:33–34. doi: 10.1126/science.aau8816. [DOI] [PubMed] [Google Scholar]
- 34.Falony G, Vieira-Silva S, Raes J. Richness and ecosystem development across faecal snapshots of the gut microbiota. Nat Microbiol. 2018;3:526–528. doi: 10.1038/s41564-018-0143-5. [DOI] [PubMed] [Google Scholar]
- 35.Zhang T, Li Q, Cheng L, Buch H, Zhang F. Akkermansia muciniphila is a promising probiotic. Microb Biotechnol. 2019;12:1109–1125. doi: 10.1111/1751-7915.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi N, Zhang S, Silverman G, Li M, Cai J, Niu H. Protective effect of hydroxychloroquine on rheumatoid arthritis-associated atherosclerosis. Animal Model Exp Med. 2019;2:98–106. doi: 10.1002/ame2.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan ZY, Chang YX, Han N, Hou FY, Lee BJY, Zhi FC, et al. Short-term high-dose gavage of hydroxychloroquine changes gut microbiota but not the intestinal integrity and immunological responses in mice. Life Sci. 2020;264 doi: 10.1016/j.lfs.2020.118450. [DOI] [PMC free article] [PubMed] [Google Scholar]