Abstract
Objectives
At the present time, there is an absence of any proven effective antiviral therapy for patients with coronavirus disease 2019 (COVID-19). The aim of this study was to assess the efficacy of intravenous immunoglobulin (IVIG) in non-severe patients with COVID-19.
Methods
A retrospective study based on propensity score matching (PSM) was designed. Primary outcomes included the severity and mortality rates. Secondary outcomes included the duration of fever, virus clearance time, length of hospital stay, and use of antibiotics.
Results
A total of 639 non-severe patients with COVID-19 were enrolled. Forty-five patients received IVIG therapy and 594 received non-IVIG therapy. After PSM (1:2 ratio), the baseline characteristics were well balanced between the IVIG group (n = 45) and control group (n = 90). No statistically significant difference was found between the IVIG group and control group in the duration of fever (median 3 vs 3 days, p = 0.667), virus clearance time (median 11 vs 10 days, p = 0.288), length of hospital stay (median 14 vs 13 days, p = 0.469), or use of antibiotics (40% vs 38.9%, p = 0.901). Meanwhile, compared to the IVIG group, no more patients in the control group progressed to severe disease (3.3% vs 6.6%, p = 0.376) or died (0 vs 2.2%, p = 0.156).
Conclusions
In non-severe patients with COVID-19, no benefit was observed with IVIG therapy beyond standard therapy.
Keywords: Severe acute respiratory syndrome coronavirus 2, Coronavirus disease 2019, Intravenous immunoglobulin, Efficacy evaluation, Mortality
Introduction
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused an outbreak of coronavirus disease 2019 (COVID-19) (Wu et al., 2020). Person-to-person transmission of SARS-CoV-2 has been confirmed, and individuals with asymptomatic infections have been identified as potential infection sources (Chan et al., 2020, Rothe et al., 2020). To date, SARS-CoV-2 has spread to almost all countries worldwide, and the number of confirmed cases and deaths has been growing rapidly due to the high rate of infectivity (R 0). As of September 25, 2020, there had been 32 730 945 confirmed cases of COVID-19, including 991 224 deaths, reported to the World Health Organization (WHO) globally (World Health Organization, 2020a).
So far, no antiviral drugs have been approved for the treatment of COVID-19. Given the rapid spread of SARS-CoV-2, there is an urgent need to explore pre-existing therapeutic options while novel therapies are being developed. Intravenous immunoglobulin (IVIG) is a blood product containing polyclonal immunoglobulin G isolated and pooled from healthy donors, which is usually used for the treatment of immunodeficiencies, autoimmune diseases, and inflammatory conditions (Galeotti et al., 2017). In addition, IVIG provides passive immune protection against a broad range of pathogens, and has been used in the treatment of severe infections (Borte et al., 2017). Moreover, IVIG has favorable clinical tolerability and safety for patients with viral infections. Therefore, IVIG does appear to be a promising candidate for the treatment of COVID-19.
Nguyen et al. reviewed the mechanism and utility of IVIG in viral infections, and consider that its use may be beneficial in patients with COVID-19 through immune modulation (Nguyen et al., 2020). Cao et al. reported three patients with COVID-19 who received high-dose IVIG at the time of initiation of respiratory distress, with satisfactory clinical and radiographic recovery (Cao et al., 2020). Mohtadi et al. enrolled five severely ill COVID-19 patients and found that treatment with IVIG could improve the clinical condition and prevent the progression of pulmonary lesions (Mohtadi et al., 2020). However, these two case reports enrolled only a very small number of patients. Clear demonstration of the therapeutic benefit of IVIG in COVID-19 patients will require more evidence. The aim of this study was to assess the efficacy of IVIG in non-severe patients with COVID-19 based on a large sample cohort study.
Methods
Patients
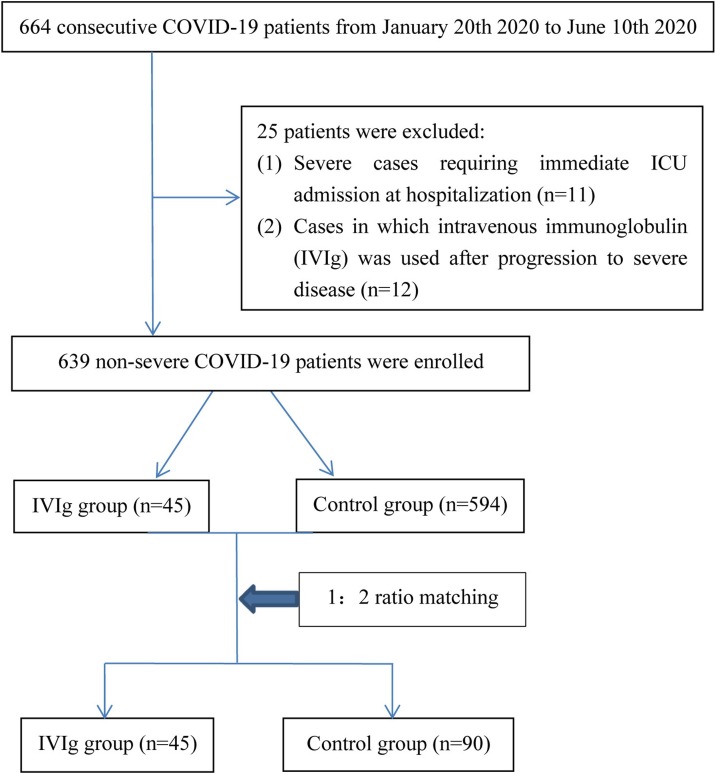
The records of 664 patients with COVID-19 admitted to Shanghai Public Health Clinical Center between January 20, 2020 and June 10, 2020 were reviewed retrospectively. Shanghai Public Health Clinical Center is a designated hospital for the treatment of patients with COVID-19 in Shanghai, China. Exclusion criteria were (1) severe cases requiring immediate intensive care unit (ICU) admission at hospitalization (n = 11); (2) cases in which IVIG was used after progression to severe disease (n = 14). Finally, 639 non-severe patients with COVID-19 were enrolled. A flow chart of the study population is shown in Figure 1 .
Figure 1.
Flow diagram of the study population. A total of 664 patients with COVID-19 were initially analyzed. Finally, 639 non-severe patients with COVID-19 were enrolled. Among these patients, 45 received IVIG therapy and 594 received control therapy.
Diagnostic criteria
Patients with COVID-19 were diagnosed following the WHO guidelines (World Health Organization, 2020b). Laboratory confirmation of SARS-CoV-2 infection was made by the Center for Disease Prevention and Control of China, using the reverse transcription polymerase chain reaction (RT-PCR) method. According to the novel coronavirus pneumonia prevention and control program published by the National Health Committee of China, non-severe patients were defined as those without any of the following (National Health Commision of China, 2020): (1) respiratory distress, with a respiratory rate ≥30/min; (2) pulse oxygen saturation ≤93% in the resting state; (3) oxygenation index ≤300 mmHg; (4) requirement for mechanical ventilation; (5) shock; (6) combined with other organ failure and the need for treatment in the ICU.
Data collection
The demographic characteristics, comorbidities, vital signs, laboratory parameters, chest computed tomography (CT) results, treatments, and clinical outcomes were extracted from the electronic medical records. Vital signs were monitored daily; laboratory tests were examined every 3–5 days and chest CT scans were performed every 3–7 days.
Outcomes and definitions
In this study, the primary outcomes included the severity rate and mortality rate. Secondary outcomes included the duration of fever, virus clearance time, length of hospital stay, and use of antibiotics. The virus clearance time was defined as the time from illness onset to two consecutive negative tests for SARS-CoV-2 with an interval of at least 24 h. The duration of fever was defined as the time from fever onset to a persistently normal temperature.
Statistical analysis
Variables with a normal distribution were recorded as the mean ± standard deviation (SD), those with a non-normal distribution as the median (interquartile range, IQR), and categorical variables as the count (percentage). The t-test, Mann–Whitney test, and Chi-square test were applied to variables with a normal distribution, continuous variables with a non-normal distribution, and categorical variables, respectively. Propensity score matching (PSM) is a powerful tool for comparing groups with similar observed characteristics without specifying the relationship between confounders and outcomes (Haukoos and Lewis, 2015). In this study, PSM was used to adjust for differences in the baseline characteristics of patients in the IVIG group and control group. Propensity scores for all patients were estimated according to the essential covariates that might have affected patient assignment to the IVIG group or control group, as well as clinical outcomes. The treatment effect of IVIG on clinical outcomes was analyzed with adjustment for variables associated with the clinical outcomes. A 1:2 exposed to unexposed matched analysis was performed based on the estimated propensity scores of each patient, and the caliper was set as 0.25 (Benedetto et al., 2018). All statistical analyses were conducted with SPSS software version 15.0 (SPSS Inc., Chicago, IL, USA), and statistical significance was set at a two-sided p-value of <0.05.
Results
Baseline characteristics of the patients with and without IVIG therapy
The baseline characteristics of the patients in the two study groups are shown in Table 1 . The median age was 37 years (IQR 25–53 years); 347 patients were male (54.3%) and 134 patients had comorbidities (21.0%). In this study, the median time between the first symptom and admission was 5 days (IQR 3–8 days). The median white blood cell (WBC) count, lymphocyte count, C-reactive protein (CRP), lactate dehydrogenase (LDH), and D-dimer levels were 5.2 × 109/l (IQR 4.2–6.5 × 109/l), 1.4 × 109/l (IQR 1.0–1.8 × 109/l), 0.8 mg/l (IQR 0.5–8.8 mg/l), 203 U/l (IQR 180–239 U/l), and 0.3 ng/mL (IQR 0.2–0.5 ng/mL), respectively. Among the 639 patients enrolled, 53 received corticosteroid therapy and 45 received IVIG therapy.
Table 1.
Baseline characteristics of patients treated with or without IVIG; results are n (%) or median (interquartile range).
| All (n = 639) | IVIG group (n = 45) | Control group (n = 594) | p-Value | |
|---|---|---|---|---|
| Age (years) | 37 (25–53) | 56 (42–67) | 36 (24–51) | <0.001 |
| Male | 347 (54.3%) | 23 (51.1%) | 324 (54.5%) | 0.656 |
| Comorbidity | 134 (21.0%) | 23 (51.1%) | 111 (18.7%) | <0.001 |
| Hypertension | 84 (13.1%) | 16 (35.6%) | 68 (11.5%) | <0.001 |
| Diabetes | 38 (6.0%) | 5 (11.1%) | 33 (5.6%) | 0.129 |
| Chronic heart disease | 16 (2.9%) | 3 (6.7%) | 13 (2.2%) | 0.064 |
| Chronic liver disease | 12 (2.5%) | 2 (4.4%) | 10 (1.7%) | 0.188 |
| Chronic pulmonary disease | 12 (2.5%) | 1 (2.2%) | 11 (1.9%) | 0.860 |
| Laboratory parameters at admission | ||||
| WBC count (109/l) | 5.2 (4.2–6.5) | 4.7 (3.9–5.8) | 5.2 (4.2–6.5) | 0.118 |
| Lymphocyte count (109/l) | 1.4 (1.0–1.8) | 0.9 (0.7–1.2) | 1.4 (1.1–1.8) | <0.001 |
| C-reactive protein (mg/l) | 0.8 (0.5–8.8) | 19.8 (7.6–41.8) | 0.5 (0.5–7.6) | <0.001 |
| LDH (U/l) | 203 (180–239) | 272 (207–357) | 201 (178–233) | <0.001 |
| D-dimer (ng/mL) | 0.3 (0.2–0.5) | 0.5 (0.3–0.9) | 0.3 (0.2–0.5) | <0.001 |
| Treatments | ||||
| Corticosteroids | 53 (8.3%) | 9 (20%) | 44 (7.4%) | 0.003 |
| Chinese medicine | 487 (76.2%) | 31 (68.9%) | 456 (76.8%) | 0.231 |
| Hydroxychloroquine | 266 (41.6%) | 1 (2.2%) | 265 (44.6%) | <0.001 |
| Thymosin α | 184 (28.8%) | 40 (88.9%) | 144 (24.2%) | <0.001 |
| Arbidol | 137 (21.4%) | 29 (64.4%) | 108 (18.2%) | <0.001 |
| Lopinavir/ritonavir | 113 (17.7%) | 21 (46.7%) | 92 (15.5%) | <0.001 |
| Possible adverse event | ||||
| Acute kidney injury | 45 (7.0%) | 4 (8.9%) | 41 (6.9%) | 0.616 |
IVIG, intravenous immunoglobulin; LDH, lactate dehydrogenase; WBC, white blood cell. p-Values indicate differences between IVIG and control, with p < 0.05 being considered statistically significant.
Most patients received antiviral agents including Chinese medicine (n = 487, 76.2%), hydroxychloroquine (n = 266, 41.6%), thymosin α (n = 184, 28.8%), arbidol (n = 137, 21.4%), and lopinavir/ritonavir (n = 113, 17.7%).
As shown in Table 1, patients who were older (56 vs 36 years, p < 0.001), more commonly had a comorbidity (51.1% vs 18.7%, p < 0.001), and those who had higher CRP (19.8 vs 0.5 mg/l, p < 0.001), LDH (272 vs 201 U/l, p < 0.001), and D-dimer (0.5 vs 0.3 ng/mL, p < 0.001) were more likely to be treated with IVIG. Moreover, corticosteroids (20% vs 7.4%, p = 0.003), thymosin α (88.9% vs 24.2%, p < 0.001), arbidol (64.4% vs 18.2%, p < 0.001), and lopinavir/ritonavir (46.7% vs 15.5%, p < 0.001) were more frequently used in the IVIG group, while hydroxychloroquine (2.2% vs 44.6%, p < 0.001) was less common in the IVIG group compared with the control group. No statistically significant difference was found between the IVIG group and control group in the rate of acute kidney injury during the hospitalization (8.9% vs 6.9%, p = 0.616).
Details of administration of IVIG
In this study, 45 patients received IVIG therapy and 594 patients received standard therapy. The use of IVIG was decided by joint discussion of five experts from the Shanghai Medical Expert Group for the Treatment of COVID-19, based on patient demographics, laboratory parameters, and chest CT scans. The doses of IVIG and durations of administration were as follows: (1) 10 g/day for 3 days, 8 patients; (2) 10 g/day for 5 days, 13 patients; (3) 20 g/day for 3 days, 16 patients; (4) 20 g/day for 5 days, 8 patients. IVIG therapy was initiated within a median of 2 days (IQR 1–3 days) of hospital admission.
Variables associated with the primary outcomes
Variables associated with the primary outcomes are shown in Table 2 . Univariate analysis showed that age, comorbidity, lymphocyte count, CRP, LDH, and use of corticosteroids were associated with the primary outcomes (all p < 0.05). Multivariable analysis identified age (odds ratio (OR) 1.058, 95% confidence interval (CI) 1.007–1.140, p = 0.015), comorbidities (OR 2.080, 95% CI 1.015–14.798, p = 0.048), lymphocyte count (OR 0.765, 95% CI 0.074–0.956, p = 0.022), and use of corticosteroids (OR 3.376, 95% CI 1.495–23.011, p = 0.015) as the independent variables associated with the primary outcomes.
Table 2.
Variables associated with the primary outcomesa.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age (years) | 1.097 (1.028–1.169) | 0.005 | 1.058 (1.007–1.140) | 0.015 |
| Male sex | 1.691 (0.308–9.299) | 0.546 | ||
| Comorbidity | 7.738 (1.402–42.713) | 0.019 | 2.080 (1.015–14.798) | 0.048 |
| WBC count (109/l) | 1.103 (0.081–1.502) | 0.534 | ||
| Lymphocyte count (109/l) | 0.124 (0.016–0.571) | 0.007 | 0.765 (0.074–0.956) | 0.022 |
| Platelet count (109/l) | 0.988 (0.974–1.002) | 0.093 | ||
| CRP (mg/l) | 1.033 (1.012–1.053) | 0.002 | 1.010 (0.981–1.040) | 0.493 |
| LDH (U/l) | 1.006 (1.000–1.011) | 0.040 | 1.01 (0.990–1.011) | 0.949 |
| D-dimer (ng/mL) | 1.015 (0.793–1.300) | 0.903 | ||
| Corticosteroids | 12.187 (2.395–62.062) | 0.003 | 3.376 (1.495–23.011) | 0.015 |
| Hydroxychloroquine | 4.357 (0.134–8.947) | 0.626 | ||
| Lopinavir/ritonavir | 2.351 (0.425–12.997) | 0.327 | ||
OR, odds ratio; CI, confidence interval; WBC, white blood cell; CRP, C-reactive protein; LDH, lactate dehydrogenase. The multivariate analysis was fitted by including the factors associated with the primary outcomes in the univariate analysis (p < 0.05).
Primary outcomes included the severity rate and mortality rate.
Variables associated with the secondary outcomes
Variables associated with the secondary outcomes are shown in Table 3 . On multivariate analysis, age (OR 0.73, p = 0.047), LDH level at hospital admission (OR 3.27, p = 0.038), and the use of corticosteroids during the hospital stay (OR 3.78, p < 0.001) were associated with the duration of fever (<7 vs ≥7 days). On multivariate analysis, age (OR 1.48, p = 0.041), comorbidity (OR 1.42, p = 0.017), LDH level on hospital admission (OR 2.73, p = 0.019), and the use of corticosteroids during the hospital stay (OR 6.68, p < 0.001) were associated with the time to virus clearance (<14 vs ≥14 days). On multivariate analysis, age (OR 2.74, p = 0.023), comorbidity (OR 2.31, p = 0.028), and the use of corticosteroids during the hospital stay (OR 7.09, p < 0.001) were associated with the use of antibiotics during the hospitalization.
Table 3.
Variables associated with the secondary outcomes (multivariate analysis model).
| Duration of fever (<7 vs ≥7 days) | Virus clearance time (<14 vs ≥14 days) | Use of antibiotics (no vs yes) | ||||
|---|---|---|---|---|---|---|
| OR | p-Value | OR | p-Value | OR | p-Value | |
| Age (years) | 0.73 | 0.047 | 1.48 | 0.041 | 2.74 | 0.023 |
| Male | 1.62 | 0.386 | 0.76 | 0.783 | 0.73 | 0.241 |
| Comorbidity | 0.64 | 0.426 | 1.42 | 0.017 | 2.31 | 0.028 |
| WBC count (109/l) | 1.03 | 0.311 | 1.81 | 0.178 | 2.69 | 0.517 |
| Lymphocyte count (109/l) | 0.14 | 0.747 | 0.62 | 0.430 | 2.63 | 0.195 |
| Platelet count (109/l) | 3.36 | 0.067 | 3.22 | 0.073 | 2.17 | 1.160 |
| CRP (mg/l) | 1.69 | 0.793 | 0.97 | 0.324 | 4.14 | 0.525 |
| LDH (U/l) | 3.27 | 0.038 | 2.73 | 0.019 | 1.38 | 0.544 |
| D-dimer (ng/mL) | 1.93 | 0.334 | 0.83 | 0.363 | 1.81 | 0.178 |
| Corticosteroids | 3.78 | <0.001 | 6.68 | <0.001 | 7.09 | <0.001 |
| Hydroxychloroquine | 2.45 | 0.808 | 1.21 | 0.260 | 1.29 | 0.265 |
| Lopinavir/ritonavir | 2.28 | 0.915 | 1.46 | 0.649 | 1.42 | 0.214 |
OR, odds ratio; WBC, white blood cell; CRP, C-reactive protein; LDH, lactate dehydrogenase. A p-value of <0.05 was considered statistically significant.
Characteristics of patients after PSM
As there were significant differences in baseline characteristics, patients were selected by PSM method according to a 1:2 ratio. The covariates that might have affected the primary and secondary outcomes (age, comorbidity, lymphocyte count, LDH, and the use of corticosteroids) were matched between the IVIG patients and control patients. The quality of matching is expressed as the Euclidean distance, the distance in multi-dimensional space. The Euclidean distance between the IVIG group and the control group was normalized to 0–1, such that the smaller Euclidean distance gives the higher similarity. After PSM, the mean Euclidean distance was 0.12 mm (SD 0.08 mm, p < 0.001) for age, 0.14 mm (SD 0.12 mm, p < 0.001) for comorbidity, 0.16 mm (SD 0.13 mm, p < 0.001) for lymphocyte count, 0.13 mm (SD 0.10 mm, p < 0.001) for LDH, and 0.18 mm (SD 0.16 mm, p < 0.001) for the use of corticosteroids. The low Euclidean distance value (all <0.2 mm) suggested that all variables associated with the primary and secondary outcomes were well balanced between the IVIG and control groups (Table 4 ).
Table 4.
Characteristics of patients after propensity score matching; results are n (%) or median (interquartile range).
| IVIG group (n = 45) | Control group (n = 90) | p-Value | |
|---|---|---|---|
| Age (years) | 56 (42–67) | 55 (42–65) | 0.874 |
| Male | 23 (51.1%) | 50 (55.6%) | 0.625 |
| Comorbidities | 23 (51.1%) | 48 (53.3%) | 0.807 |
| Hypertension | 16 (35.6%) | 30 (33.3%) | 0.797 |
| Diabetes | 5 (11.1%) | 12 (13.3%) | 0.714 |
| Chronic heart disease | 3 (6.7%) | 7 (7.8%) | 0.816 |
| Chronic liver disease | 2 (4.4%) | 3 (3.3%) | 0.747 |
| Chronic pulmonary disease | 1 (2.2%) | 1 (1.1%) | 0.614 |
| Laboratory parameters at admission | |||
| White blood cell count (109/l) | 4.7 (3.9–5.8) | 4.5 (3.7–5.6) | 0.289 |
| Lymphocyte count (109/l) | 0.9 (0.7–1.2) | 1.0 (0.6–1.3) | 0.597 |
| C-reactive protein (mg/l) | 19.8 (7.6–41.8) | 22.5 (9.4–40.6) | 0.684 |
| Lactate dehydrogenase (U/l) | 272 (207–357) | 266 (205–321) | 0.663 |
| D-dimer (ng/mL) | 0.5 (0.3–0.9) | 0.5 (0.3–0.8) | 0.772 |
| Treatments | |||
| Corticosteroids | 9 (20%) | 16 (17.8%) | 0.754 |
| Chinese medicine | 31 (68.9%) | 65 (72.2%) | 0.687 |
| Hydroxychloroquine | 1 (2.2%) | 5 (5.6%) | 0.376 |
| Thymosin α | 40 (88.9%) | 70 (77.8%) | 0.117 |
| Arbidol | 29 (64.4%) | 50 (56%) | 0.323 |
| Lopinavir/ritonavir | 21 (46.7%) | 37 (41.1%) | 0.505 |
IVIG, intravenous immunoglobulin. p-Values indicate differences between the IVIG group and control group, with p < 0.05 considered statistically significant.
Evaluation of the efficacy of IVIG in the propensity-matched groups
Comparisons between the patients treated with and without IVIG in the propensity-matched groups are shown in Table 5 . No statistically significant difference was found between the IVIG group and the control group in the duration of fever (median 3 vs 3 days, p = 0.667), time to virus clearance (median 11 vs 10 days, p = 0.288), length of hospital stay (median 14 vs 13 days, p = 0.469), or the use of antibiotics (40% vs 38.9%, p = 0.901). Meanwhile, compared to the IVIG group, no more patients in the control group progressed to severe disease (3.3% vs 6.6%, p = 0.376) or died (0 vs 2.2%, p = 0.156).
Table 5.
Evaluation of the efficacy of IVIG in propensity-matched groups; results are n (%) or median (interquartile range).
| IVIG group (n = 45) | Control group (n = 90) | p-Value | |
|---|---|---|---|
| Primary outcomes | |||
| Developed to severe case | 3 (6.6%) | 3 (3.3%) | 0.376 |
| Died | 1 (2.2%) | 0 | 0.156 |
| Secondary outcomes | |||
| Duration of fever (days)a | 3 (1–5) | 3 (1–5) | 0.667 |
| Virus clearance time (days) | 11 (8–15) | 10 (8–15) | 0.288 |
| Length of hospital stay (days) | 14 (12–17) | 13 (11–18) | 0.469 |
| Antibiotics therapy | 18 (40%) | 35 (38.9%) | 0.901 |
| Use of ≥2 antibiotics | 5 (11.1%) | 9 (10%) | 0.842 |
IVIG, intravenous immunoglobulin.
The duration of fever was defined as the time from fever onset to a persistently normal temperature.
Cox regression analysis for the comparison of time variables between the groups
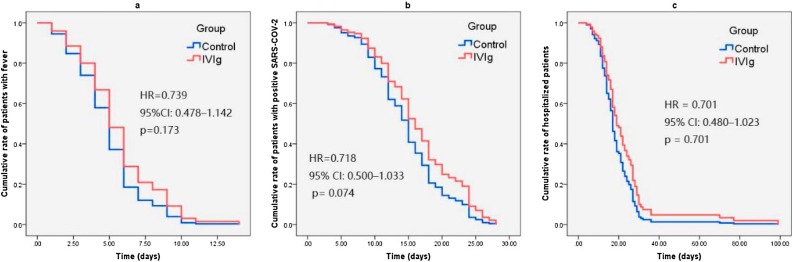
Cox regression analysis showed that there was no significant difference in the duration of fever (hazard ratio (HR) 0.739, 95% CI 0.478–1.142, p = 0.173) (Figure 2a), time to virus clearance (HR 0.718, 95% CI 0.500–1.033, p = 0.074) (Figure 2 b), or length of hospital stay (HR 0.701, 95% CI 0.480–1.023, p = 0.701) (Figure 2c) between the IVIG group and control group during the follow-up period. In this study, only one death was observed in the IVIG group and no deaths were observed in the control group. Therefore, a survival analysis was not performed.
Figure 2.
Cox regression analysis for the comparison of time variables between the groups. Cox regression analysis showed no significant difference between the IVIG group and control group in (a) the duration of fever (HR 0.739, 95% CI 0.478–1.142, p = 0.173), (b) the time to virus clearance (HR 0.718, 95% CI 0.500–1.033, p = 0.074), or (c) the length of hospital stay (HR 0.701, 95% CI 0.480–1.023, p = 0.701) during the follow-up period.
Discussion
Recently, the COVID-19 pandemic has represented the most widespread infectious challenge. IVIG from healthy donors has been used safely for decades, not only to treat autoimmune diseases but also in infectious diseases. Given the urgent need for therapies to alleviate the global burden of SARS-CoV-2 infection and death while awaiting a vaccine, Scoppetta et al. believed that it is imperative to widen the therapeutic opportunities of IVIG (Scoppetta et al., 2020). The rationale for the use of IVIG in COVID-19 has been explained in previous review articles (Nguyen et al., 2020, Jawhara, 2020). IVIG may modulate the immune response via multiple mechanisms, including blocking a wide array of proinflammatory cytokines, Fc-gamma receptors (FcRs), and leukocyte adhesion molecules, suppressing pathogenic Th1 and Th17 subsets, and neutralizing pathogenic autoantibodies (Jawhara, 2020).
The use of IVIG has been reported in the treatment of COVID-19. Cao et al. reported the cases of three patients with severe COVID-19 who received high-dose IVIG with satisfactory recoveries (Cao et al., 2020). Sheianov et al. presented the cases of three patients with severe COVID-19 who had failed to achieve substantial improvement on initial treatment (Sheianov et al., 2020). They subsequently received pulse therapy with methylprednisolone and IVIG (20 g/day), which was associated with a prompt resolution of respiratory failure, elimination of clinical manifestations, and reversal of the pulmonary CT changes (Sheianov et al., 2020). However, confounding factors in the two studies included the lack of case-matched control patients. Thus, the field lacks strong evidence to better understand the efficacy of IVIG. In this retrospective cohort study, it was found that IVIG therapy did not show a benefit in the treatment of non-severe patients with COVID-19. The results are consistent with those of a review, which concluded that the evidence is insufficient to support the efficacy of IVIG in the treatment of COVID-19 (Zhang et al., 2020).
Based on previous cases reports (Cao et al., 2020, Sheianov et al., 2020), a high dose of IVIG administered at the appropriate point could successfully block the progression of the disease cascade and improve the outcomes of severely or critically ill COVID-19 patients. Mohtadi et al. also reported the effects of IVIG administration in severely ill COVID-19 patients (Mohtadi et al., 2020). Five severely ill COVID-19 patients in whom standard treatments had failed were administered IVIG, and all of these patients showed a good therapeutic response and were discharged from the hospital in a stable clinical condition (Mohtadi et al., 2020). Lanza et al. described a 42-year-old woman, admitted to Monaldi Hospital after 15 days of persistent respiratory failure, who was treated with an IVIG infusion with a successful outcome (Lanza et al., 2020). Xie et al. studied 58 patients diagnosed with severe COVID-19, and found that the use of IVIG could reduce the use of mechanical ventilation and shorten the hospital length of stay (Xie et al., 2020). Based on the above studies, although the present study results do not support the use of IVIG in non-severe patients with COVID-19, IVIG could be considered for use in severely and critically ill patients.
Besides COVID-19, there is a debate on the use of IVIG in the treatment of severe acute respiratory syndrome coronavirus (SARS-CoV). Stockman et al. performed a review of treatment options in severe acute respiratory syndrome (SARS) patients, including IVIG (Stockman et al., 2006). Despite five studies on the use of IVIG or convalescent plasma being evaluated, these studies were deemed inconclusive since the effects of IVIG could not be distinguished from those of other factors, including comorbidities, stage of illness, and the effect of other treatments (Stockman et al., 2006). Therefore, it was not possible to determine whether IVIG benefited patients during the SARS outbreak. Wang et al. conducted a prospective study on SARS patients with pneumonia, in whom IVIG was administered for leukopenia or thrombocytopenia, or if there was rapid progression of disease on radiography (Wang et al., 2004). The study found that IVIG led to significant improvements in leukocyte and platelet counts, but acknowledged that there was no control group to objectively evaluate the responses. In the present study, we analyzed the effect of IVIG in non-severe patients with COVID-19 with adjustment for variables associated with the primary and secondary outcomes and comparison with a control group.
In this study, lopinavir/ritonavir (46.7% vs 15.5%, p < 0.001) was more commonly used in the IVIG group, while hydroxychloroquine (2.2% vs 44.6%, p < 0.001) was less commonly used in the IVIG group, compared with the control group. We have reason to believe that hydroxychloroquine and lopinavir/ritonavir had no effect on hospitalized patients with COVID-19. The Solidarity Trial is an international clinical trial launched by the WHO and partners. The results of the Solidarity Trial showed that remdesivir, hydroxychloroquine, lopinavir, and interferon regimens had little or no effect on hospitalized patients with COVID-19, as indicated by overall mortality, initiation of ventilation, and duration of hospital stay (WHO Solidarity Trial Consortium et al., 2020).
This study has limitations. First, the study was a retrospective research, and the dose and duration of IVIG was not randomized. As a tentative therapy, and considering the side effects and high price, IVIG was used selectively for patients with more risk factors for a worse evolution of COVID-19 according to the joint discussions of at least five experts from the Shanghai Medical Expert Group for the Treatment of COVID-19. The selective biases in dose and duration of IVIG might have affected the efficacy of IVIG in non-severe COVID-19 patients. Therefore, prospective randomized controlled trials are needed to validate the results. Second, the study did not evaluate the efficacy of IVIG in severe patients with COVID-19. As almost all severe cases in our hospital received IVIG treatment, no control group could be used to evaluate the efficacy of IVIG in severe cases. Controlled clinical trials are needed to evaluate the efficacy of IVIG in severe COVID-19. Third, in this study, the PSM method was used to balance the differences between the IVIG group and control group. However, the PSM method is limited by adjusting for observed variables only; it cannot account for residual confounding of many variables. Thus, the PSM method used is still subject to bias. Therefore, the results need confirmation in a prospective randomized clinical trial. Fourth, no children were enrolled in this study, because children with COVID-19 were hospitalized at another designated hospital in Shanghai.
In conclusion, in non-severe patients with COVID-19, no benefit was observed with IVIG treatment beyond standard therapy. The results of this study do not support the use of IVIG in the treatment of non-severe patients with COVID-19. Randomized controlled trials or at least non-randomized prospective trials with a control group are needed to confirm the findings of this study.
Financial support
This study was supported by grant No. 17,411,969,700 from the Shanghai Association for Science and Technology and grant No. 19YF1441200 from the Shanghai Sailing Plan Program.
Role of the sponsor
The funding organizations are public institutions and had no role in the design and conduct of the study, collection, management, and analysis of the data, or the preparation, review, and approval of the manuscript.
Availability of data and materials
We declare that the materials described in this article, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality. The supporting data can be accessed from Qiang Li (corresponding author), e-mail: liqiang66601@163.com.
Compliance with ethics guidelines
Verbal informed consent was obtained from all participants. The Ethics Committee of Shanghai Public Health Clinical Center approved this study (JY-2020-S095−02). This study was performed in accordance with the Declaration of Helsinki.
Consent for publication
All authors read and approved the manuscript.
Conflict of interest
None.
Author contributions
Study concept and design: Qiang Li and Liang Chen. Data collection: Chenlu Huang, Ling Fei, Weixia Li, Wei Xu, and Xudong Xie. Analysis and interpretation of data: Chenlu Huang, Ling Fei, Weixia Li, and Qiang Li. Drafting of the manuscript: Qiang Li. Critical revision of the manuscript: Liang Chen.
Acknowledgements
We thank all of the doctors who work at Shanghai Public Health Clinical Center for their efforts in the diagnosis and treatment of patients with COVID-19.
References
- Benedetto U., Head S.J., Angelini G.D. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg. 2018;53:1112–1117. doi: 10.1093/ejcts/ezy167. [DOI] [PubMed] [Google Scholar]
- Borte M., Melamed I.R., Pulka G. Efficacy and Safety of Human Intravenous Immunoglobulin 10% (Panzyga(R)) in Patients with Primary Immunodeficiency Diseases: a Two-Stage, Multicenter, Prospective, Open-Label Study. J Clin Immunol. 2017;37:603–612. doi: 10.1007/s10875-017-0424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Liu X., Bai T. High-Dose Intravenous Immunoglobulin as a Therapeutic Option for Deteriorating Patients With Coronavirus Disease 2019. Open Forum Infect Dis. 2020;7:a102. doi: 10.1093/ofid/ofaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti C., Kaveri S.V., Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29:491–498. doi: 10.1093/intimm/dxx039. [DOI] [PubMed] [Google Scholar]
- Haukoos J.S., Lewis R.J. The Propensity Score. JAMA. 2015;314:1637–1638. doi: 10.1001/jama.2015.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawhara S. Could Intravenous Immunoglobulin Collected from Recovered Coronavirus Patients Protect against COVID-19 and Strengthen the Immune System of New Patients? Int J Mol Sci. 2020;21:7. doi: 10.3390/ijms21072272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza M., Polistina G.E., Imitazione P. Successful intravenous immunoglobulin treatment in severe COVID-19 pneumonia. IDCases. 2020:e794. doi: 10.1016/j.idcr.2020.e00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohtadi N., Ghaysouri A., Shirazi S. Recovery of severely ill COVID-19 patients by intravenous immunoglobulin (IVIG) treatment: A case series. Virology. 2020;548:1–5. doi: 10.1016/j.virol.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Commision of China . 2020. New coronavirus pneumonia revention and control program (the fifth edition)http://www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440 [Google Scholar]
- Nguyen A.A., Habiballah S.B., Platt C.D. Immunoglobulins in the treatment of COVID-19 infection: Proceed with caution! Clin Immunol. 2020;216 doi: 10.1016/j.clim.2020.108459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe C., Schunk M., Sothmann P. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoppetta C., Di Gennaro G., Polverino F. Editorial - High dose intravenous immunoglobulins as a therapeutic option for COVID-19 patients. Eur Rev Med Pharmacol Sci. 2020;24:5178–5179. doi: 10.26355/eurrev_202005_21214. [DOI] [PubMed] [Google Scholar]
- Sheianov M.V., Udalov Y.D., Ochkin S.S. Pulse Therapy With Corticosteroids and Intravenous Immunoglobulin in the Management of Severe Tocilizumab-Resistant COVID-19: A Report of Three Clinical Cases. Cureus. 2020;12:e9038. doi: 10.7759/cureus.9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.T., Sheng W.H., Fang C.T. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg Infect Dis. 2004;10:818–824. doi: 10.3201/eid1005.030640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Solidarity Trial Consortium, Pan H., Peto R. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med. 2020 doi: 10.1056/NEJMoa2023184. Dec 2; NEJMoa2023184. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. Coronavirus disease situation reports-190. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200728-covid-19-sitrep-190. Accessed 28 July 2020. [Google Scholar]
- World Health Organization . 2020. Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance. https://www.who.int/internal-publicationsdetail/clinical-management- of-severe- acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed 5 February 2020. [Google Scholar]
- Wu F., Zhao S., Yu B. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Cao S., Dong H. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.044. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Yang Y., Yang N. Effectiveness of intravenous immunoglobulin for children with severe COVID-19: a rapid review. Ann Transl Med. 2020;8:625. doi: 10.21037/atm-20-3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We declare that the materials described in this article, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality. The supporting data can be accessed from Qiang Li (corresponding author), e-mail: liqiang66601@163.com.