Sir,
In early 2020, COVID-19 (coronavirus disease 2019), caused by the novel coronavirus SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), spread worldwide causing more than 4,500,000 cases and 300,000 deaths as of May 2020. Several molecules have shown promising antiviral activity against SARS-CoV-2, but no specific therapy has proven effective for COVID-19 [1]. Therapy with β-interferons has shown in vitro activity against SARS-CoV-2 [2]. At the same time, triple antiviral therapy with interferon β-1b (IFN-β1b), lopinavir/ritonavir (LPV/r) and ribavirin proved superior to LPV/r alone for the treatment of mild-to-moderate COVID-19 [3]. Recently, Hong et al. showed that with triple combination therapy with LPV/r, hydroxychloroquine (HCQ) and IFN-β1b demonstrated improvement of severe pneumonia in five patients with COVID-19 pneumonia [4].
Here we evaluated the effect of an interferon-based combination therapy in critically ill patients with COVID-19.
Eight patients admitted to the intensive care unit (ICU) of Sant’Orsola University Hospital (Bologna, Italy) for COVID-19-related severe acute respiratory distress syndrome between 10 March 2020 and 10 April 2020 were included in this study. Diagnosis of SARS-CoV-2 infection was confirmed by RT-PCR on nasopharyngeal swabs. All patients were intubated within 12 h from ICU admission. All patients in the interferon group (n = 5) were treated according to local clinical protocols with interferon β-1a (IFN-β1a; Rebif®) in combination with HCQ (Plaquenil®) and LPV/r (Kaletra®). Patients in the control group did not received antivirals or immunomodulators, whereas two patients received HCQ (Plaquenil®) prior to hospitalisation. Baseline demographic and clinical characteristics of the patients are given in Supplementary Table S1. Bronchoalveolar lavage samples were collected by bronchoscopy at intubation and 7 days thereafter. Viral load was assessed in triplicate by the ΔCt method (Ctgene–Cthousekeeping). The effect on viral load was assessed with 95% confidence interval (CI) of the mean of the differences in ΔCt between Day 0 and Day 7 (ΔΔCt).
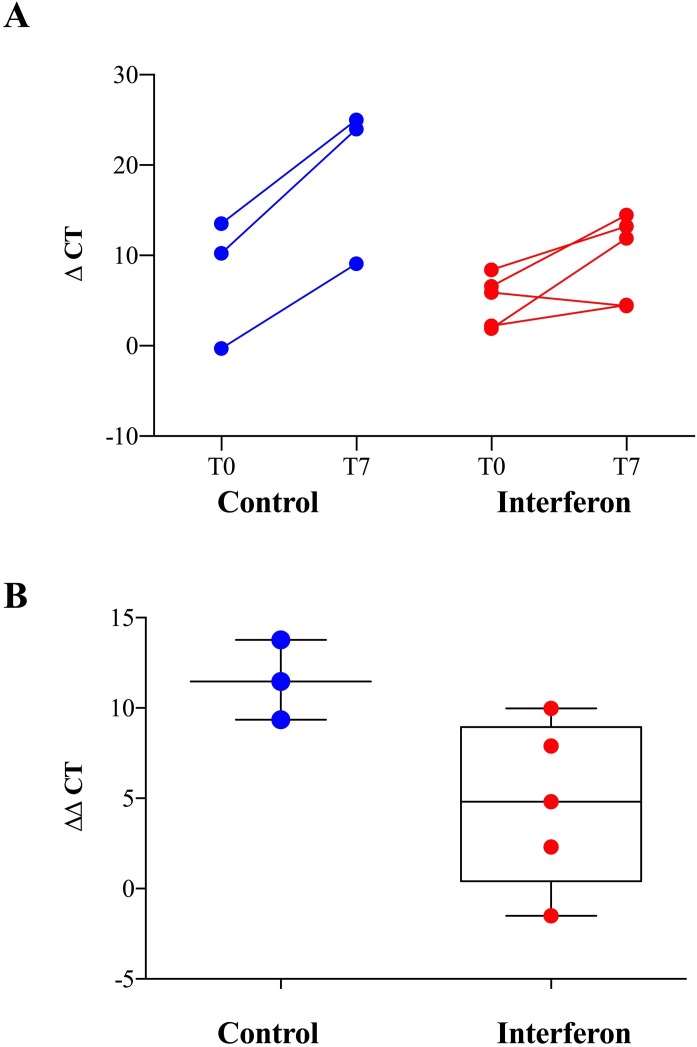
Compared with the control group, the interferon group showed no significant ΔCt reduction from Day 0 through Day 7 in the lower respiratory tract of critically ill patients (Fig. 1 A). Compared with baseline, the mean viral load reduction after 7 days of intubation in patients treated with interferon was 4.7 ΔCt (95% CI −0.09 to 10.33), whereas the viral load reduction in patients in the control group was 11.54 ΔCt (95% CI 6.05–17.01) (Fig. 1B).
Fig. 1.
(A) Viral load in bronchoalveolar lavage samples collected from critically ill COVID-19 patients treated with interferon-based therapy (interferon β-1a in combination with hydroxychloroquine and lopinavir/ritonavir) versus the control group at intubation (T0) and 7 days thereafter (T7). Relative quantification of SARS-CoV-2 was determined by calculating the difference in the Ct value of the nucleocapsid N1 gene to the Ct value of the reference ribonuclease P (RNAse P) gene. (B) Differences in ΔCt between Day 0 and Day 7 (ΔΔCt) in patients treated with interferon-based therapy versus the control group.
Clinical variables were not significantly different between the groups. Two of five patients in the interferon group and two of three patients in the control group died within 28 days after ICU admission; time to ICU discharge in surviving patients was 12 ± 2 days and 15 ± 2 days in the interferon and control groups, respectively. Clearance of viral RNA of SARS-CoV-2 in nasopharyngeal swabs was not significantly different between the groups and occurred in 13 ± 3.9 days and 11 ± 3.9 days in the interferon and control groups, respectively.
Overall, our findings show only a slight reduction in SARS-CoV-2 viral load in lower respiratory specimens of critically ill patients with COVID-19 treated with IFN-β1a in combination with HCQ and LPV/r. These findings are in accordance with a previous study showing that treatment with interferon plus ribavirin was not associated with a significant reduction in 90-day mortality or faster RNA clearance in critically ill patients with Middle East respiratory syndrome coronavirus (MERS-CoV) infection [5]. Moreover, we started IFN-β1a therapy at ICU admission, on average 7 days after symptom onset, so the long interval between infection and IFN administration may also have played a role in reducing antiviral efficacy.
We believe that our preliminary results—although limited to viral load clearance—may be helpful in better characterising treatment options for critically ill COVID-19 patients.
Funding
None.
Conflict of interest
None declared.
Ethical approval
This study was approved by the local institutional review board (Comitato Etico AVEC) [approval no. n.283/2020/Oss/AOUBo].
References
- 1.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 2.Mantlo E., Bukreyeva N., Maruyama J., Paessler S., Huang C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antiviral Res. 2020;179 doi: 10.1016/j.antiviral.2020.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung I.F., Lung K.C., Tso E.Y., Liu R., Chung T.W., Chu M.Y. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong S.I., Ryu B.H., Chong Y.P., Lee S., Kim S., Kim H.C. Five severe COVID-19 pneumonia patients treated with triple combination therapy with lopinavir/ritonavir, hydroxychloroquine, and interferon β-1b. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.106052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arabi Y.M., Shalhoub S., Mandourah Y., Al-Hameed F., Al-Omari A., Al Qasim E. Ribavirin and interferon therapy for critically ill patients with Middle East respiratory syndrome: a multicenter observational study. Clin Infect Dis. 2020;70:1837–1844. doi: 10.1093/cid/ciz544. [DOI] [PMC free article] [PubMed] [Google Scholar]