To the editor:
We read with great interest the article of Zhang et al 1 on “CD4+ T, CD8+ T counts and severe COVID-19: A meta-analysis”, which investigated the relationship between CD4+ T counts, CD8+ T counts, CD4/CD8 ratio and the severity of COVID-19 patients, showing that both CD4+ T and CD8+ T counts may serve as biomarkers for predicting COVID-19 severity, although CD8+ T may be a more sensitive predictor of clinical outcome than CD4+ T in COVID-19 patients. According to the updated numbers on the global confirmed cases/deaths from COVID-19, SARS-CoV-2 spreads much more rapidly and overwhelms most emergency departments, hospitals, and healthcare systems. Therefore, effective laboratory biomarkers for the estimation of clinical symptoms or for predicting the clinical outcome of COVID-19 are urgently needed 2.
SARS-CoV-2 predominantly causes respiratory and intestinal tract infections and induces a wide spectrum of clinical symptoms 3 , 4. Among the COVID-19 patients in Hong Kong, diarrhea occurred in approximately 20% of them, even earlier than pyrexia 4, which could be the initial symptoms, when SARS-CoV-2 RNA could be detected in the gastrointestinal tract using anal swabs and stool specimens 5. The relationship between gastrointestinal symptoms and host mucosal chemokine responses to coronavirus infection is poorly understood. The mucosa-associated epithelial chemokine (MEC/CCL28) is secreted from epithelial cells in the salivary glands, colon, trachea, lungs and mammary glands (Fig. 1 A), and is involved in lymphocyte trafficking as well as mucosal and memory immunity 6 , 7. In particular, high expression of CCL28 in the nasal tract is helpful for antigen presentation and defense against inhaled pathogens 8. In the lack of vaccines and specific drugs, it is tempting to understand the outcomes of SARS-CoV-2-infected patients by assessing blood-cell ratios, blood-gas analysis data, radiological characteristics and clinical symptoms after hospitalization, which may provide information for treatment 1 9, If a molecular marker can be found to indicate a systemic immunity or clinical outcomes in patients, particularly elderly individuals, it will help save medical resources during the pandemic 2.
Figure 1.
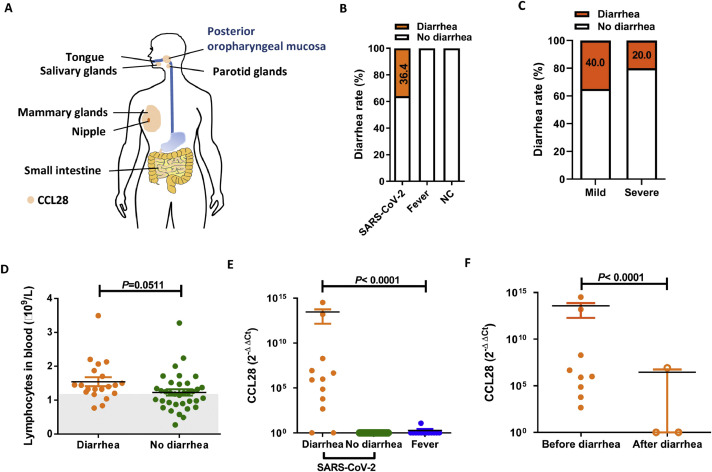
Diarrhea status and CCL28 mRNA expression levels in posterior oropharyngeal mucosa samples. A. Expression diagram of CCL28 mucosal chemokine in the human body. CCL28, in orange, is expressed in salivary, parotid gland, mammary glands, intestinal mucosa, posterior oropharyngeal mucosa and some other mucosa tissues. B. Comparison of diarrhea rates in three cohorts. C. Comparison of diarrhea rates in severe and mildly ill patients. D. Comparison of blood T lymphocyte counts in SARS-CoV-2-infected patients with and without diarrhea. E. Comparison of CCL28 mRNA expression levels in SARS-CoV-2-infected patients with diarrhea, SARS-CoV-2-infected patients without diarrhea and patient with fever. CCL28 and housekeeping gene detections were carried out by real-time RT-PCR in conjunction with 2 × SYBR Green PCR Master Mix. PCR primers for human CCL28 were designed as follows: 5′-CCA TAC TTC CCA TTG CCT CC-3′ (sense) and 5′-GAT TCT TCT GCG CTT GAC-3′ (antisense), yielding a 152 bp product. The housekeeping gene GAPDH was used as an internal control for the examination of human gene expression; the primer sequences used for GAPDH were 5′-AAG AAG GTG GTG AAG CAG G-3′ (sense) and 5′-GTC AAA GGT GGA GGA GTG G-3′ (antisense), yielding a 114 bp product. Fold changes in CCL28 mRNA expression levels in mucosa were determined and graphed as 2−ΔΔCt, where -ΔΔCt = - [(CtCCL28unknown-CtGAPDH unknown) - (CtCCL28 NC-CtGAPDH NC)]. F. The difference in CCL28 mRNA expression levels before and after diarrhea. For the calculation, a 2−ΔΔCt value equal to 1 means that the Ct value was over 40 and that the expression level of the gene was below the lower limit of detection. Statistical analyses were performed using the unpaired t-test to compare lymphocyte counts and CCL28 expression levels. Data are presented as the mean ± standard error of the mean (SEM). AP value <0.05 was considered statistically significant.
In this retrospective study, we included 55 hospitalized COVID-19 patients between January 25 and March 31, 2020 (Ethics No. 2020-010-1) in a regional business hub Wuxi of China, during the lockdown period, and analyzed the demographic and medical data. We investigated the diarrhea symptom and CCL28 mucosal expression from three cohorts: COVID-19 patients, patients with fever, and negative control (NC) patients. Twenty (36.4%) of 55 COVID-19 patients developed diarrhea (Fig. 1B, Table 1 ), and the incidence of diarrhea in mild illness group (40%, 18/45) was two-fold higher than that of severe illness group (20%, 2/10) (Fig. 1C, Table 1). There were no statistically significant differences in blood lymphocyte counts (the lowest counts during hospitalization) between individuals with and without diarrhea (p ≥ 0.05). However, some individuals with lower lymphocyte counts were found in the group without diarrhea (<1.1 × 109/L) (Fig. 1D) and those patients accompanied with severe clinical manifestations.
Table 1.
Demographics, basic features and clinical outcomes of SARS-CoV-2-infected individuals.
| All patients (n=55) | Diarrhea (n=20) | No Diarrhea (n=35) | P value | |
|---|---|---|---|---|
| Age (years) | ||||
| 0-19 | 10 (18.2) | 2 (10.0) | 8 (22.9) | 0.73 |
| 20-44 | 17 (30.9) | 5 (25.0) | 12 (34.3) | 0.74 |
| 45-54 | 10 (18.2) | 5 (25.0) | 5 (14.3) | 0.62 |
| 55-64 | 11 (20.0) | 6 (30.0) | 5 (14.3) | 0.52 |
| 65-74 | 7 (12.7) | 2 (10.0) | 5 (14.3) | 0.72 |
| Mean (SEM) age (years)# | 41.1 (2.6) | 44.3 (4.1) | 40.6 (3.1) | 0.49 |
| Severe illness mean (SEM) age (years)# | 53.4 (6.6) | 45.5 (9.5) | 51.9 (7.6) | 0.73 |
| Comorbidities | 18 (32.7) | 9 (45.0) | 9 (25.7) | 0.23* |
| Male | 32 (58.2) | 11 (55.0) | 21 (60.0) | 0.78* |
| Severe illness | 10 (18.2) | 2 (10.0) | 8 (22.9) | 0.30* |
| Male | 7 (70.0) | 1 (50.0) | 6 (75.0) | >0.99* |
| Comorbidities | 8 (80.0) | 2 (100.0) | 6 (75.0) | >0.99* |
| Mild illness | 45(81.8) | 18 (90.0) | 27 (77.1) | 0.30* |
| Comorbidities | 15 (18.1) | 6 (33.3) | 9 (33.3) | >0.99* |
| First-generation (G1) patients | 35 (63.6) | 15 (70.0) | 20 (57.1) | 0.25* |
| Second-generation (G2) patients | 15 (27.3) | 5 (30.0) | 10 (28.6) | >0.99* |
| Third-generation (G3) patients | 5 (9.1) | 0 | 5 (14.3) | 0.16* |
Most data are presented as n (%) and n/N (%), and N is the total number of patients with available data; Age data (#) are presented as the mean (standard errors of the mean, SEM); SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; First-generation patients mean infection import from other cities or countries; Second-generation patients mean infection due to contacting with G1 patients; Third-generation patients mean infection due to contacting with G2 patients. Statistical analyses were performed using the unpaired t-test and Fisher's exact test (*) to compare diarrhea and no diarrhea patients (P value). A P value <0.05 was considered statistically significant.
CCL28 has been used as a novel biomarker to provide a better and more comprehensive prognostic evaluation for cancer patients along with the current practical prognostic factors 7 , 10, showing that the expression of CCL28 mRNA is usually well correlated with protein expression in mucosa samples 10. CCL28 can represent the responsiveness of respiratory and gastrointestinal mucosal immunity. We therefore used real-time RT-PCR to examine the epithelial expression level of CCL28 mRNA in the posterior oropharyngeal mucosa of SARS-CoV-2-infected individuals and suspected respiratory outpatient paired individuals with fever. Since some SARS-CoV-2 positive samples were discarded in the early SARS-CoV-2 epidemic, we reserved 32 samples (including 12 diarrhea patients). Ten out of 12 (83.3%) diarrhea patients developed high expression level of CCL28 compared with those without diarrhea and fever individuals (p<0.0001) (Fig. 1E). We also found that diarrhea as one of the gastrointestinal symptoms appeared at onset in some individuals, while others had diarrhea after hospitalization. To address the relationship between CCL28 expression and diarrhea, we chose the samples at the time of COVID-19 confirmation and as a date benchmark. In the COVID-19 diarrhea patients, we noted that 100% (9/9) individuals developed relatively high expression level of CCL28 before the onset of diarrhea, while 33.3% (1/3) individual developed relatively high level of CCL28 mRNA expression after diarrhea (p<0.0001) (Fig. 1F). These results suggest that CCL28 mucosal expression appears to associate with the appearance of diarrhea.
At the time of writing, the global case fatality rate was ∼6.5% of laboratory-confirmed SARS-CoV-2-infected patients. The majority of those with severe disease and poor outcomes tended to be patients with comorbidities, including hypertension, diabetes, obesity, asthma, chronic obstructive pulmonary disease, or advanced age 2 , 3. As shown in Table 1, we found that 45 (81.8%) of 55 patients were mild illness and 10 (18.2%) were severe illness; the mean age of severely ill individuals was 53.4 ± 6.6 years; 18 (32.7%) of 55 patients had underlying diseases, especially in diarrhea individual (9/20, 45%); 32 (58.2%) of 55 patients were male, and 7 (70%) of 10 male patients developed severe illness but only one developed diarrhea. During the course of illness, our data showed that 2 (10.0%) severe patients and 18 (90.0%) mild patients of these 20 patients had diarrhea, and the mild patients were easier to recover during hospitalization. More individuals with diarrhea constituted first-generation patients (70.0%). Since the COVID-19 outbreak, only two severe illness patients developed pulmonary failure and received lung transplantation, and neither had diarrhea. There was no death of COVID-19 case in Wuxi city. Taken together, these results suggest that diarrhea is likely to occur in patients with mild symptoms of SARS-CoV-2 infection.
In conclusion, we found that CCL28 expression levels increased in patients both before and after having diarrhea symptoms and those patients displayed less severe respiratory syndrome symptoms, indicating a good mucosal immune response which may reduce disease severity. Our results provide a basis for studying the role of CCL28 in mucosal immunity related to SARS-CoV-2 infection and its relationship with clinical outcomes. As SARS-CoV-2 replicates in the upper and lower respiratory and gastrointestinal tracts of patients, CCL28 expression levels may be used as an indicator to investigate mucosal immune responses, providing information for the development of an early warning model for predicting the outcomes of COVID-19.
Declaration of Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Financial support
This work was funded by the Wuxi Top Talents Program (BJRC2020), the Wuxi Key Medical Talents Program (ZDRC024), the Wuxi Medical Development Discipline (FZXK006), the National Natural Science Foundation of China (81701550), the National Mega-Projects against Infectious Diseases (2018ZX10301406-002), the Emergency Prevention and Control Capacity Program for New Severe Infectious diseases of National Institute for Viral Disease Control and Prevention, the 135 Strategic Program of Chinese Academy of Sciences, and the Open Research Fund Program of the State Key Laboratory of Virology of China (2019IOV005).
Acknowledgements
We thank Xike Zhou for providing technical support.
References
- 1.Zhang H, Wu T. CD4+T, CD8+T counts and severe COVID-19: A meta-analysis. The Journal of Infection. 2020;81(3):e82–e84. doi: 10.1016/j.jinf.2020.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou H, Zhang B, Huang H. Using IL-2R/lymphocytes for predicting the clinical progression of patients with COVID-19. Clinical and Experimental Immunology. 2020;201(1):76–84. doi: 10.1111/cei.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vena A, Giacobbe DR, Di Biagio A. Clinical characteristics, management and in-hospital mortality of patients with COVID-19 In Genoa, Italy. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2020 Aug 15 doi: 10.1016/j.cmi.2020.07.049. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee N, Hui D, Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. The New England jouranl of Medicine. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 5.Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. Journal of Gastroenterology Hepatology. 2020;35(5):744–748. doi: 10.1111/jgh.15047. [DOI] [PubMed] [Google Scholar]
- 6.Hernández-Ruiz M, Zlotnik A. Mucosal chemokines. Journal of Interferon & Cytokine Research: the Official Journal of the International Society for Interferon and Cytokine Research. 2017;37(2):62–70. doi: 10.1089/jir.2016.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang XL, Liu KY, Lin FJ, Shi HM, Ou ZL. CCL28 promotes breast cancer growth and metastasis through MAPK-mediated cellular anti-apoptosis and pro-metastasis. Oncology Report. 2017;38(3):1393–1401. doi: 10.3892/or.2017.5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohan T, Kim J, Berman Z. Co-delivery of GPI-anchored CCL28 and influenza HA in chimeric virus-like particles induces cross-protective immunity against H3N2 viruses. Journal of Controlled Release: Official Journal of the Controlled Release Society. 2016;233:208–219. doi: 10.1016/j.jconrel.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang F, Nie J, Wang H. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. Journal of Infectious Disease. 2020;221(11):1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu GX, Lan J, Sun Y, Hu Y-J, Jiang G-S. Expression of the chemokine CCL28 in pleomorphic adenoma and adenolymphoma of the human salivary glands. Experimental and Therapeutic Medicine. 2012;4(1):65–69. doi: 10.3892/etm.2012.544. [DOI] [PMC free article] [PubMed] [Google Scholar]