Abstract
Objective
To study the impact of a 60-day pilot of an innovative virtual-care model using general internal medicine physicians and nurses to respond rapidly to more than 1200 coronavirus disease-2019 (COVID-19)-positive nasopharyngeal polymerase chain reaction tests.
Patients and Methods
The current study was approved by the Mayo Clinic COVID-19 Research Committee and the Mayo Clinic Institutional Review Board. The data for all SARS-CoV-2–positive patients treated by our team were entered into a prospectively maintained internal research electronic data capture database. We searched this database retrospectively for the first 60 days of our program (March 23, 2020 to May 22, 2020). The data included basic deidentified demographics; symptoms at intake into the program; date of symptom onset; risk factors; location; and outcomes including hospitalization, admission to intensive care unit, and death.
Results
Patients were contacted, on average, 6.3 hours after their results became available. There was a total of 138 ED visits. Of these, 40% were admitted to the hospital, with 36% of those admitted requiring intensive care unit level of care. Of the 849 patients in this sample, there were only 2 deaths (0.23%) at 60 days.
Conclusion
Our innovative multidisciplinary COVID team provided excellent clinical care for patients with COVID, with a very low mortality rate compared with the national average. Although data are not available on a national scale for time to contact patient, our team was able to contact patients within the established recommendation for contact within 48 hours of testing, which is optimal.
Abbreviations and Acronyms: COVID-19, coronavirus disease 2019; ED, emergency department; EHR, electronic health record; GIM, general internal medicine; ICU, intensive care unit; ID, infectious diseases; OCPHD, Olmsted County Public Health Department; PCR, polymerase chain reaction; PPE, personal protective equipment; RMS, remote monitoring system; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization
In late December 2019, a cluster of viral pneumonia cases emerged in Wuhan, China, with these cases subsequently being linked to common exposure at a live animal market. Soon after, human to human transmission was reported, most notably among health care workers.1,2 Samples from respiratory epithelial cells of affected patients revealed a novel beta coronavirus, which was named severe acute respiratory syndrome coronavirus (SARS-CoV-2) because of its similar initial presentation to the outbreak of SARS-CoV in 2002, and the clinical syndrome was named coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO).3 COVID-19 rapidly spread across the globe within the next few months, resulting in the current pandemic being declared on March 11, 2019, by the WHO, and since has created unprecedented strain on the health care infrastructure of multiple countries. The majority of infected patients (approximately 80%) present with mild symptoms including fever, cough, myalgias, and diarrhea or are asymptomatic.4, 5, 6 Approximately 20% of patients may have more severe presentations, including dyspnea and acute hypoxic respiratory failure secondary to the insidious onset of acute respiratory distress syndrome (ARDS), and may need hospitalization.4 This has led to critical shortages of intensive care unit (ICU) beds, ventilators, and personal protective equipment (PPE).7
Although COVID-19 is certainly capable of causing severe disease in young, healthy patients, the bulk of those adversely affected belong to high-risk categories. Early clinical data have demonstrated that older age, smoking history, and history of cardiac disease predict worse outcomes.6,7 Although 80% of affected persons do not require hospitalization, many will seek ED care owing to a high level of global concern. Because of the risk of transmission to health care workers and necessary management of resources, it is imperative to preserve inpatient care for those who meet criteria and to minimize ED use. The low-risk and stable high-risk COVID-19 population can be managed in the outpatient setting, but the necessary infrastructure may become overwhelmed when faced with pandemic patient loads. In the setting of a critical shortage of hospital-based resources, including PPE, beds, and ventilators, a telemedicine or virtual initiative is aptly positioned to intervene and address several of these challenges.8 A centralized physician team is preferable to having hundreds of community-based physicians managing these patients to provide standardized, high-quality care that complies with the rapidly changing best-practice recommendations.
The use of physician-initiated, non–face-to-face patient encounters has the potential to triage patients effectively who are at increased risk of severe disease from the majority, who will recover without intervention.9 The use of telephone support and noninvasive telemonitoring devices to follow patients’ symptom and vital sign trends with centralized nursing may help to identify patients earlier and effectively triage those in need of escalating care to the emergency department or inpatient settings, while reassuring those who can safely continue in-home monitoring.10 This risk stratification should ideally occur as soon as possible after the positive results of SARS-CoV-2 testing are received.
In addition to early risk stratification of SARS-CoV-2–positive patients and subsequent outpatient monitoring, patient quarantine and contact tracing should start as soon as possible. Virtual patient education on the importance of quarantine should be discussed with every SARS-CoV-2–positive patient and will minimize the number of secondary infections. All of these essential tasks, including risk stratification, monitoring, tracing, and quarantine education, can be done effectively using telemedicine. We describe our comprehensive, multidisciplinary telehealth surveillance program to address the population-based health concerns of the COVID-19 pandemic. We also report the first 60 days of this program in which we treated more than 1200 SARS-CoV-2–positive patients.
Patients and Methods
The current study was approved by the Mayo Clinic COVID-19 Research Committee and the Mayo Clinic Institutional Review Board. The data for all SARS-CoV-2 positive patients treated by our team were entered into a prospectively maintained internal Research Electronic Data Capture (REDCap) database. Some patients declined to be followed by the COVID Frontline Care Team (CFCT), and we did not follow patients already in clinical facilities such as group homes and skilled nursing facilities. We retrospectively searched this database for 60 days, starting with the first patient seen virtually by our team on March 23, 2020, to May 22, 2020. The data included basic deidentified demographics, symptoms at intake into the program, date of symptom onset, risk factors, and location.
Care-Team Design
At our institution, the process of identifying, triaging, and subsequently following up care of SARS-CoV-2–positive patients was initially handled by our colleagues in the infectious disease (ID) division. However, given the limited ID personnel resources and expanding duties during the COVID-19 pandemic, a joint relationship with general internal medicine (GIM) was established. In this model, the GIM division assumed the outpatient management of patients with positive polymerase chain reaction (PCR) results for SARS-CoV-2, with ongoing collaboration with ID. In turn, ID physicians focused more on inpatient consults on COVID-19 infections, development of institutional COVID-19 protocols, and infection-control measures. The overarching goal was to develop a centralized team that would respond rapidly to SARS-CoV-2– positive test results from our institution’s various testing centers' electronic health records (EHRs [Epic Verona, Wisconsin]) and coordinate the level of outpatient care.
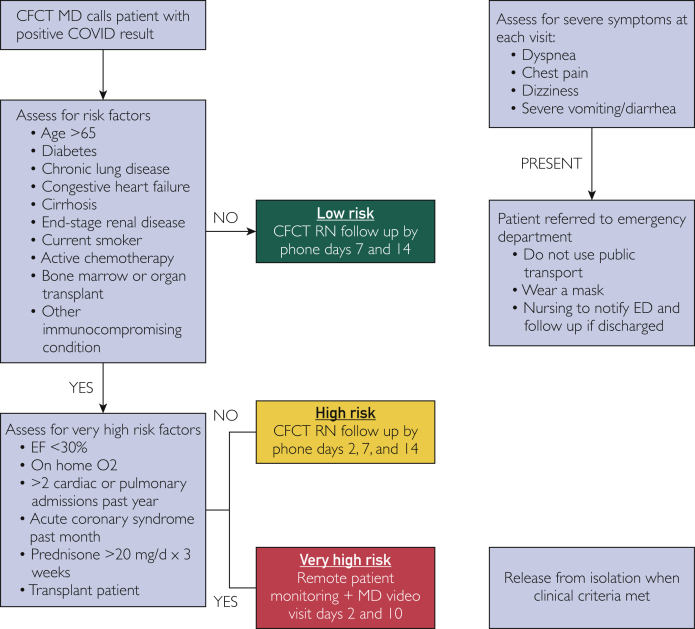
Before forming the GIM CFCT, the specific workflow was developed between the ID and GIM divisions, identifying a number of different clinical scenarios in which the positive cases would need to be assessed and followed. The GIM CFCT team would respond to positive SARS-CoV-2 results from all the testing sites at Mayo Clinic Rochester, as well as the affiliated Mayo Clinic Health System community-based practices serving southern Minnesota, northern Iowa, and western Wisconsin. After testing was performed, patients were instructed to isolate at home until contacted by a community medicine nurse (if test results were negative) or CFCT physician (if test results were positive). In addition, patients who had been dismissed from the hospital because of COVID-19–related illness were followed by the CFCT. Identified COVID-19 disease risk factors were used to stratify patients further into low-, high-, and very high-risk groups (Figure 1). The very high-risk group was eventually combined with the high-risk group. These risk factors were based on available literature, which has identified that those patients who are male, above the age of 65, have structural heart or lung disease, are immunocompromised, have malignancies, have end-stage renal disease, are current smokers, or have diabetes mellitus have worse prognoses in COVID-19.4,5,11, 12, 13 The initial physician phone call to the patient for risk stratification would occur within hours of the results being available in the EHR, and nursing follow-up notification would occur within 24 hours. Patients with severe symptoms on the initial phone call were instructed to go to the emergency department for further evaluation.
Figure 1.
Risk stratification for COVID patients.
We initially recruited a small force of GIM physicians and GIM nurses to accomplish the initial goals of the CFCT. The physicians rapidly became knowledgeable about COVID-19 clinical presentations and complications and created education modules to enable further expedient recruitment and coverage of physicians as needed. Most of the GIM physicians had recent or current hospital experience, so they were familiar with triage and management of the decompensating patient. The GIM nurses were trained by our home parenteral and enteral nursing (HPEN) and complex-care nursing teams, which have experience working with the virtual care of patients with complex cases. Our nursing teams made follow-up phone calls on days 2, 7, and 14 to assess for changes in symptoms. In addition, they developed and delivered education modules centered on the principles of the importance of self-quarantine and social distancing. They also staffed a nurse line for patients to call in with worsening symptoms. If symptoms warranted escalation, the nursing pool routed these concerns to the physicians on call. This phone line was covered after hours by on-call physicians, who would provide triage for patient concerns. This ensured that patients had a single contact number that was staffed 24 hours a day to call regarding COVID-19–related symptoms.
In those patients deemed high risk on initial assessment or following COVID-19 hospital discharge, remote monitoring systems (RMS) were delivered to patients' homes. Patient symptoms and vital signs were reported at least twice daily and continuously monitored by a remote patient monitoring (RPM) team of nurse coordinators and support staff. The COVID-19 RPM program comprised 2 care pathways and RMS: a complex care monitoring plan, by which a cellular-enabled tablet collected patient-reported symptom assessments and connected with a Bluetooth-enabled scale, blood pressure monitor, pulse oximeter, and thermometer, and an interactive care plan (ICP), by which patients were given pulse oximeters and thermometers and used the Mayo mobile app (EHR portal) on their smartphones or tablets to self-report symptoms, temperature, and oxygen saturation. In both care pathways, any abnormal symptoms or deviation in vital signs beyond predefined parameters established by a multidisciplinary team (CFCT, ID, RPM) created alerts to the RPM dashboards and nursing team. The RPM nurse would then contact patients; evaluate their symptoms; troubleshoot any technological errors; and escalate as necessary to the CFCT physician for further assessment, with the provision of direct escalation to a higher-acuity center in cases of emergency.
The CFCT worked with other COVID-19 stakeholders, including occupational health, the Olmsted County Public Health Department (OCPHD) department, and Mayo Clinic Infection Prevention and Control. As the primary team responding to COVID-19 diagnoses, the CFCT is able to identify high-risk contacts and emerging trends in the community. We modified our process to collect data surrounding possible contacts; recent work exposure; and home address to identify any potential clusters of cases, which were then communicated to these stakeholders. In addition, we established daily communication strategies to communicate effectively with OCPHD to help facilitate the delivery of critical information on COVID-19–positive patients.
Statistical Design
All data are presented as mean ± standard deviation for normally distributed data and median for nonparametric data. All statistical analysis and graphical figures was performed using R version 3.6.3. (Microsoft, Redmond, California).
Results
Patient Characteristics
A total of 1291 patients had positive SARS-CoV-2 PCR test results and were referred for management by CFCT. Research authorization consent was provided by the 849 patients who were included in the study. Twenty-one patients were unable to be contacted by the CFCT team physician and were thus excluded from final analysis. Table 1 shows demographic characteristics of our patients. The median age of patients was 40 years (interquartile range [IQR]: 29 to 54), and there was a slight female majority (n=444; 54%), with most patients residing in the state of Minnesota (n=793; 96%). The majority of patients were determined to be at low risk for COVID-19–related complications (n=653: 79%), with 60 (7%) at medium risk, and 107 (13%) at high risk (Figure 2). Table 2 shows risk factors for severe COVID infection with most patients having no risk factors (61%), and diabetes (11%), asthma (7.7%), age >65 years (7.5%), and being a current smoker (6.0%) the most commonly identified. Reported symptoms are shown in Table 3, with the 4 most commonly reported symptoms being cough (n=392; 47%), headache (n=315; 38%), myalgia (n=311; 38%), and fever (n=267; 32%).
Table 1.
Baseline Demographics
| Characteristic | N=828 |
|---|---|
| Age, median (IQR) | 40 (29, 54) |
| Gender, n (%) | |
| Female | 444 (54%) |
| Male | 384 (46%) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 162 (20%) |
| Not Hispanic or Latino | 635 (77%) |
| Other | 31 (3.7%) |
| Race, n (%) | |
| American Indian/Alaskan Native | 3 (0.4%) |
| Asian | 50 (6.0%) |
| Black or African American | 209 (25%) |
| Other | 141 (17%) |
| White | 425 (51%) |
| State, n (%) | |
| Iowa | 11 (1.3%) |
| Minnesota | 793 (96%) |
| Nebraska | 1 (0.1%) |
| New York | 1 (0.1%) |
| North Dakota | 1 (0.1%) |
| Texas | 1 (0.1%) |
| Wisconsin | 20 (2.4%) |
IQR, interquartile range.
Figure 2.
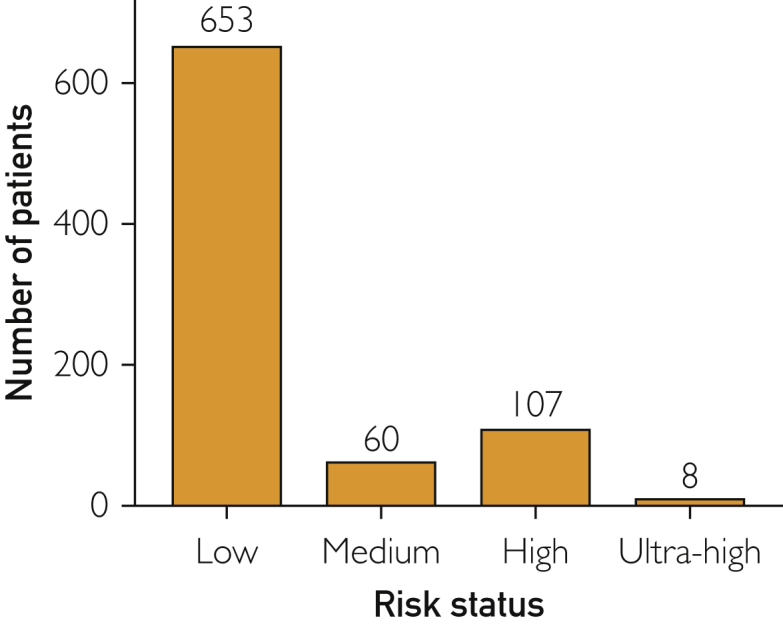
Risk status.
Table 2.
Risk Factors
| Characteristic | Female, N=444 | Male, N=384 | Overall, N=828 |
|---|---|---|---|
| Age >65 | 29 (6.5%) | 33 (8.6%) | 62 (7.5%) |
| Diabetes | 56 (13%) | 34 (8.9%) | 90 (11%) |
| COPD/emphysema | 6 (1.4%) | 5 (1.3%) | 11 (1.3%) |
| Asthma | 41 (9.2%) | 23 (6.0%) | 64 (7.7%) |
| Chronic lung disease | 16 (3.6%) | 11 (2.9%) | 27 (3.3%) |
| Congestive heart failure | 10 (2.3%) | 4 (1.0%) | 14 (1.7%) |
| Coronary artery disease | 12 (2.7%) | 14 (3.6%) | 26 (3.1%) |
| Current smoker | 21 (4.7%) | 29 (7.6%) | 50 (6.0%) |
| Active chemotherapy | 3 (0.7%) | 4 (1.0%) | 7 (0.8%) |
| Bone marrow transplant | 1 (0.2%) | 0 (0%) | 1 (0.1%) |
| Solid organ transplant | 1 (0.2%) | 2 (0.5%) | 3 (0.4%) |
| Other immune compromised condition | 9 (2.0%) | 9 (2.3%) | 18 (2.2%) |
| End-stage liver disease | 3 (0.7%) | 2 (0.5%) | 5 (0.6%) |
| Obesity | 24 (5.4%) | 7 (1.8%) | 31 (3.7%) |
| Other | 57 (13%) | 35 (9.1%) | 92 (11%) |
| None | 267 (60%) | 241 (63%) | 508 (61%) |
Statistics presented: n (%).
COPD, chronic obstructive pulmonary disease.
Table 3.
Symptoms by Age Group
| Characteristic | <20 (N=40) | 20-39 (N=373) | 40-59 (N=289) | 60-79 (N=111) | >80 (N=15) | Overall (N=828) |
|---|---|---|---|---|---|---|
| Dyspnea | 6 (15%) | 54 (14%) | 51 (18%) | 29 (26%) | 2 (13%) | 142 (17%) |
| Chest pain or tightness | 4 (10%) | 47 (13%) | 37 (13%) | 5 (4.5%) | 1 (6.7%) | 94 (11%) |
| Cough | 19 (48%) | 162 (43%) | 146 (51%) | 53 (48%) | 12 (80%) | 392 (47%) |
| Fever | 11 (28%) | 115 (31%) | 102 (35%) | 37 (33%) | 2 (13%) | 267 (32%) |
| Chills | 7 (18%) | 85 (23%) | 67 (23%) | 27 (24%) | 1 (6.7%) | 187 (23%) |
| Myalgia | 10 (25%) | 134 (36%) | 123 (43%) | 43 (39%) | 1 (6.7%) | 311 (38%) |
| Sore throat | 10 (25%) | 87 (23%) | 51 (18%) | 19 (17%) | 1 (6.7%) | 168 (20%) |
| Headache | 15 (38%) | 157 (42%) | 113 (39%) | 28 (25%) | 2 (13%) | 315 (38%) |
| Anosmia/dysguesia | 9 (22%) | 119 (32%) | 72 (25%) | 20 (18%) | 1 (6.7%) | 221 (27%) |
| Congestion/rhinorrhea | 11 (28%) | 118 (32%) | 61 (21%) | 25 (23%) | 4 (27%) | 219 (26%) |
| Nausea/vomiting/abdominal pain | 3 (7.5%) | 37 (9.9%) | 37 (13%) | 12 (11%) | 2 (13%) | 91 (11%) |
| Diarrhea | 7 (18%) | 49 (13%) | 46 (16%) | 21 (19%) | 4 (27%) | 127 (15%) |
| Lightheadedness and/or dizziness | 7 (18%) | 59 (16%) | 45 (16%) | 20 (18%) | 3 (20%) | 134 (16%) |
| Fatigue | 1 (2.5%) | 18 (4.8%) | 24 (8.3%) | 13 (12%) | 3 (20%) | 59 (7.1%) |
| Other | 2 (5.0%) | 25 (6.7%) | 20 (6.9%) | 13 (12%) | 2 (13%) | 62 (7.5%) |
| None | 3 (7.5%) | 47 (13%) | 35 (12%) | 15 (14%) | 1 (6.7%) | 101 (12%) |
Statistics presented: n (%).
Time to Result Notification
The majority of laboratory results for PCR testing was available within 24 hours of sample collection. The overall time from positive test result to first contact for the cohort when data were available (n=767) was 6.3 hours, with 75% being less than 12 hours. For those patients with limited English proficiency, the average time from the test positive being resulted in the EHR until first contact with the patient (positive to first contact) was significantly increased compared with those whose primary language was English (P=<.001).
Patient Outcomes
One hundred fifteen patients (8.9%) were sent to the emergency department, based on the CFCT physician recommendations, for a total of 138 ED visits (Table 4). Of these, 40% were admitted to the hospital, with 36% of those admitted requiring ICU level of care (Table 5). For those patients requiring ICU levels of care, 9 (45%) were initially triaged as high to medium risk, and 11 (55%) were initially triaged as low risk. Of the 849 patients in this sample, there were only 2 deaths (0.23%) at 60 days.
Table 4.
Emergency Department Visits
| Characteristic | Admit from emergency department |
Overall ED visits (N=138) | |
|---|---|---|---|
| No (N=83) | Yes (N= 55) | ||
| Age Group | |||
| <20 | 5 (6.0%) | 0 (0%) | 5 (3.6%) |
| 20-39 | 30 (36%) | 8 (15%) | 38 (28%) |
| 40-59 | 37 (45%) | 29 (53%) | 66 (48%) |
| 60-79 | 9 (11%) | 14 (25%) | 23 (17%) |
| >80 | 2 (2.4%) | 4 (7.3%) | 6 (4.3%) |
Statistics presented: n (%)
ED, emergency department.
Table 5.
Hospitalization and ICU Stays
| Characteristic | ICU stay |
Overall hospitalizations (N=55) | Length of stay (days, IQR) | |
|---|---|---|---|---|
| No (N=35) | Yes (N=20) | |||
| Age group | ||||
| 20-39 | 7 (20%) | 1 (5.0%) | 8 (15%) | 3 (2,3) |
| 40-59 | 17 (49%) | 12 (60%) | 29 (53%) | 7 (2,11) |
| 60-79 | 7 (20%) | 7 (35%) | 14 (25%) | 11 (8,18) |
| >80 | 4 (11%) | 0 (0%) | 4 (7.3%) | 4 (2,8) |
Statistics presented: n (%).
ICU, intensive care unit; IQR, interquartile range.
Discussion
The main objective of the CFCT model was to create a centralized virtual multidisciplinary group that would rapidly respond to COVID-19–positive tests from a large health care organization caring for patients across 3 states.14 This novel approach helped decrease the time the positive test result was delivered in the EHR to the time the patient was first contacted and risk stratification occurred. This coordination was performed using an entirely virtual platform, which greatly decreased potential exposure to health care workers and conserved precious PPE supplies.
The RPM team, combined with the CFCT physician and nurses, provided a centralized method of managing COVID-19–positive patients. This model fosters collaboration among several physician teams and used nurses as physician extenders by the establishment of well-defined decision trees and treatment algorithms. Early detection of patient decompensation, by using the remote-monitoring technology, allowed for establishment of pathways for direct admission of patients to the COVID-19 inpatient service or the emergency department, depending on patient stability. Further evaluation will be needed to determine if this virtual-care model and early detection of adverse trends reduced the need for hospital observation or admission, shortened length of stay, or reduced the need for ICU-level care. The ability to contact and risk stratify patients rapidly and then deliver these specifics to OCPHD can decrease lag time, thereby improving the efficacy of contact tracing.
Countries such as Iceland and South Korea have avoided the large health care burden associated with COVID-19.15 South Korea had cases as early as early January, following which a concerted nationwide containment strategy was implemented.15 The cornerstones of this containment strategy were aggressive widespread testing, prompt contact tracing, and quarantine of COVID-19–positive patients as well as those who had been exposed to SARS-CoV-2. During the South Korea COVID-19 peak in late February, they were performing more than 10,000 PCR tests a day. By June 1, 2020, they had reduced reported daily cases to 35. Rapid expansion of testing is the first critical step and should be offered initially to those for whom there was a high suspicion of COVID-19 and later to asymptomatic persons, as testing capacity increases. A critical component to any screening program is the actions taken immediately following the return of a positive test result. A recent article emphasized that a patient testing positive for COVID-19 needs to be notified immediately, educated, isolated, and their contacts identified.15 The importance of a rapid response to the positive test is highlighted by modeling data that suggest that, if contact tracing is to be effective, patients and contacts should be quarantined within 24 hours of testing.15,16 This may explain the successful response to COVID-19 in countries with centralized health care systems and robust public health infrastructures. The United States health care system is in dire need of multidisciplinary centralized care-team models that are capable of responding rapidly to positive test results.
Study Limitations
This was a single health care system retrospective review of our experience with COVID-19– positive patients. Despite the good clinical outcomes we reported, the lack of a comparison group makes it difficult to assess quantitatively the magnitude of our team’s impact. The centralized model may be challenging to reproduce in health systems that are less tightly integrated or rely on outside laboratories for testing. Because our testing was performed in a high-volume, centralized location with rapid turnaround time, and results were made available in a unified EHR, our CFCT team was able to respond rapidly (<6 hours), thus potentially improving outcomes. There is a need for prospective cohort trials to confirm the findings of our retrospective study.
Conclusion
Rapidly responding to positive tests (<24 hours) may lead to more effective resource allocation, isolation of infected patients, and contact tracing to initiate quarantine of exposed persons. Other countries with universal health care and robust public health systems have successfully employed these strategies. We successfully developed and rapidly implemented a similar type of care-team model in our health care system while incorporating outpatient risk stratification and remote patient-monitoring technology. This model can be adapted and extrapolated to other health care networks. Because of the noncentralized nature of the US health care system, this type of care-team model may provide an option to address public health concerns meticulously while triaging patient care with adequate risk stratification and managing COVID-19 disease and recovery in a virtual setting.
Acknowledgments
Drs Ganesh, Salonen, Bierle, and Hurt made substantial contributions to the concept and design of the study, the interpretation of data, and the critical revision of the manuscript for important intellectual content. They made substantial contributions to the acquisition of data, the analysis and interpretation of data, and the drafting of the manuscript. Drs Bhuiyan, Moehnke, Haddad, Tande, and Wilson made substantial contributions to critical revisions of the manuscript for important intellectual content.
Footnotes
Grant Support: The authors report no grant support for this article.
Potential Competing Interests: R.T.H. is a consultant for Nestle, with no competing interest to report. The other authors report no competing interests.
References
- 1.Shah A., Kashyap R., Tosh P., Sampathkumar P., O’Horo J.C. Guide to understanding the 2019 novel coronavirus. Mayo Clin Proc. 2020;95(4):646–652. doi: 10.1016/j.mayocp.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhuiyan M.N., Ganesh R., Ghosh A.K. COVID-19: a 2020 update. Indian J Med Sci. 2020;72(2):88–94. [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Z., Peng F., Xu B., et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W.-J., Ni Z.-Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranney M.L., Griffeth V., Jha A.K. Critical supply shortages: the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. 2020;382(18):e41. doi: 10.1056/NEJMp2006141. [DOI] [PubMed] [Google Scholar]
- 8.Greenhalgh T., Koh G.C.H., Car J. Covid-19: a remote assessment in primary care. BMJ. 2020;368:m1182. doi: 10.1136/bmj.m1182. [DOI] [PubMed] [Google Scholar]
- 9.Hollander J.E., Carr B.G. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 10.Bashi N., Karunanithi M., Fatehi F., Ding H., Walters D. Remote monitoring of patients with heart failure: an overview of systematic reviews. J Med Internet Res. 2017;19(1):e18. doi: 10.2196/jmir.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain V., Yuan J.-M. Systematic review and meta-analysis of predictive symptoms and comorbidities for severe COVID-19 infection. Public Global Health. 2020 doi: 10.1101/2020.03.15.20035360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patanavanich R., Glantz S.A. Smoking is associated with COVID-19 progression: a meta-analysis. Nicotine Tob Res. 2020;22(9):1653–1656. doi: 10.1093/ntr/ntaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang B., Li R., Lu Z., Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging. 2020;12(7):6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crane S.J., Ganesh R., Post J.A., Jacobson N.A. Telemedicine consultations and follow-up of patients with COVID-19. Mayo Clin Proc. 2020;95(suppl 9):S33–S34. doi: 10.1016/j.mayocp.2020.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walensky R.P., Del Rio C. From mitigation to containment of the COVID-19 pandemic: putting the SARS-CoV-2 genie back in the bottle. JAMA. 2020;323(19):1889–1890. doi: 10.1001/jama.2020.6572. [DOI] [PubMed] [Google Scholar]
- 16.Ferretti L., Wymant C., Kendall M., et al. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020;368(6491):eabb6936. doi: 10.1126/science.abb6936. [DOI] [PMC free article] [PubMed] [Google Scholar]