Abstract
Background
Low back pain (LBP) and decreased mobility function are common problem among older people. Muscle weakness has been reported as a risk factor for these conditions, and exercise therapy can improve them. We created a novel exercise device that also measures abdominal trunk muscle strength. Malnutrition has also emerged as a major problem among older people. Muscle is a direct key linking decreased mobility function and malnutrition. This study aimed to examine the associations of LBP with not only decreased physical function and muscle weakness but also nutritional status of older people.
Methods
We examined the associations of LBP with muscle weakness, decreased mobility function (locomotive syndrome [LS]), and malnutrition among older women. The study included 101 female patients aged 60 years or older scheduled to undergo surgery for degenerative lower extremity diseases. Preoperatively, physical tests including abdominal trunk muscle strength assessment using the device and laboratory tests were conducted. Subjects with LBP (numerical rating scale ≥2; range, 0–4) during the preceding month were allocated to the LBP group (n = 36). Other subjects were allocated to the non-LBP group (n = 65).
Results
The LBP group had lower abdominal trunk and knee extensor muscle strength, lower serum albumin, and hemoglobin levels as blood biomarkers associated with malnutrition risk, and higher LS test scores than the non-LBP group. A multivariate analysis showed that abdominal trunk muscle weakness and advanced LS were associated with LBP. LBP intensity was negatively correlated with abdominal trunk and knee extensor muscle strength and positively correlated with the LS test score. The serum hemoglobin level was negatively correlated with the LS test score.
Conclusion
Abdominal trunk muscle weakness and decreased mobility function were associated with LBP among older women.
Introduction
Low back pain (LBP) is a common problem globally [1]. LBP affects approximately 80% of people at some time in life [2, 3]. The prevalence of LBP is related to aging and is the main cause of disability for older people in developing and developed countries [4, 5]. Trunk muscle weakness has been reported as a risk factor for LBP [6, 7]. In a systemic review, Hayden et al. [8] reported that among various types of exercise therapies for chronic LBP, strengthening exercises were the most effective for improving physical functions. For older patients with chronic LBP, compliance with performing necessary exercise and the motivation to exercise are generally limited [9, 10]. Unfortunately, research of exercise for chronic LBP has frequently focused on the working population and has excluded the older population due to age-related co-morbidities [10–12]. A significant number of older patients with chronic LBP are unable to continue their recommended exercise regimen because they have pain, deformities, and/or loss of flexibility in the spine and extremities and because of muscle weakness [12, 13].
Decreased mobility function is an inevitable outcome of aging, and an increasing proportion of older individuals require care because of impaired mobility [14, 15]. Because increasing health care costs associated with aging societies impose a growing economic burden, there is an urgent need to extend the healthy life expectancy and reduce health disparities. Locomotive syndrome (LS), proposed by the Japanese Orthopaedic Association, is a condition characterized by reduced mobility due to impairment of the locomotive organs [16]. The progression of this syndrome limits the ability of individuals to carry out activities of daily living. LS is associated with multiple factors, including osteoporosis, osteoarthritis, and sarcopenia, which can cause LBP [17, 18]. Exercise therapy can improve the mobility function of patients with LS. However, because a significant proportion of patients are older adults with degenerative locomotive organ diseases, careful selection of the type and intensity of exercise is necessary [18].
We created a novel exercise device for abdominal trunk muscles (Fig 1) (trunk muscle exercise device; Nippon Sigmax Co., Ltd., Shinjuku-ku, Tokyo, Japan) [19]. Using this device, subjects can strengthen abdominal trunk muscles and measure their muscle strength while sitting, without moving the trunk or lumbar spine. We have previously demonstrated that this device reliably measures the strength of abdominal trunk muscles [20].
Fig 1. Novel exercise device for abdominal trunk muscles.
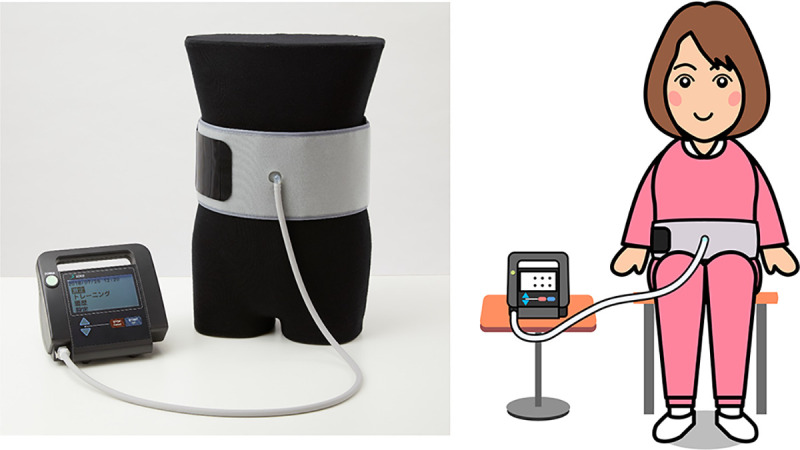
(Left) Photograph of the device. (Right) Artistic rendering of a patient wearing the device.
Similar to decreased mobility function, malnutrition is a highly prevalent condition among older people and is linked to higher morbidity, higher mortality, and lower quality of life [21, 22]. Malnutrition can also contribute to musculoskeletal health and is associated with chronic musculoskeletal pain among older adults [23, 24]. There are different mechanisms in the development of malnutrition in older adults and others. Chronic musculoskeletal pain including LBP and decreased physical function reduce the mobility of the elderly. Immobility attributable to musculoskeletal conditions is associated with sarcopenia and decreased oral food intake [25]. Decreased mobility function and malnutrition are two conditions that are prevalent in older people. These two conditions may be correlated and may potentiate each other, resulting in poor health-related outcomes [21, 26]. Muscle is a direct key linking decreased mobility function and malnutrition. Decreased muscle strength has been reported as an indicator of decreased mobility function [27], and decreased muscle volume has been presented as an outcome of malnutrition [28].
A previous study involving a small sample size demonstrated the links among LBP, muscle strength, and decreased mobility function of adults during the seventh and eighth decades of life [29]. To date, the association of LBP with muscle strength-related physical function and nutritional status has not been well-examined; however, it is an important issue worth considering in more detail. In the present study, we therefore investigated the associations of LBP with muscle strength, mobility function, and nutritional status among more than 100 older women.
Materials and methods
Ethics statement
This study was approved by the Kanazawa University ethics committee (ethic approval code: 2015–063), and written informed consent was obtained from each subject.
Subjects
Between January 2016 and December 2017, we recruited 119 consecutive patients 60 years or older who were slated to undergo surgery because of degenerative lower extremity diseases at Kanazawa University hospital and who agreed to participate in preoperative physical tests. Male patients were excluded from the analysis because there were only 13 which was not sufficient, and because the analyses of men and women needed to be separate owing to the large differences in muscle strength between men and women. Patients who had previously undergone spine surgery or had been diagnosed with rheumatological diseases were excluded from the study. Patients whose knee extensor muscle strength could not be assessed adequately owing to pain from degenerative diseases of the lower extremities and patients whose physical or laboratory data were missing were also excluded from the analysis. Finally, a total of 101 female patients 60 years or older were evaluated duringthe study (Table 1).
Table 1. Patient characteristics and inclusion/exclusion criteria of the study.
| Characteristic | |
| No. of subjects | 101 |
| Age (years), mean ± SD [range] | 69.7 ± 6.4 [60–86] |
| Height (cm), mean ± SD [range] | 151.3 ± 6.1 [129.8–165.4] |
| Weight (kg), mean ± SD [range] | 54.1 ± 8.8 [35.0–81.4] |
| BMI (kg/cm2), mean ± SD [range] | 23.7 ± 3.8 [16.7–36.7] |
| Grip power (kg), mean ± SD [range] | 20.7 ± 5.7 [8.0–39.5] |
| Degenerative diseases of the lower extremities treated with surgeries | Hip joint diseases (68) |
| Knee joint diseases (15) | |
| Foot & Ankle joint diseases (18) | |
| Inclusion criteria of the study | |
| • 60 years-old or older female | |
| • Capable of understanding the content of the study, and giving the informed consent | |
| • Capable of performing preoperative physical tests without any difficulties in this study | |
| • Capable of obtaining preoperative laboratory data in this study | |
| Exclusion criteria of the study | |
| • Male or younger than 60 years | |
| • History of spinal surgery | |
| • Comorbidity of rheumatological diseases | |
| • Missing data in the physical or laboratory tests | |
BMI, body mass index; SD, standard deviation
Exercise device
The exercise device used during this study has a design similar to that of a sphygmomanometer, with an inflatable cuff and a mechanical manometer to measure pressure (Fig 1). The procedure for measuring abdominal trunk muscle strength (ATMS) of the study participants has been described in detail elsewhere [19, 20]. When measuring strength or performing strengthening exercises using the device, subjects were able to contract the abdominal trunk muscles against the pressure from the inflated cuff while sitting, without the need to move the trunk. The isometric muscle contraction does not strain the spine or extremities. Previous studies have reported that no participants experienced adverse events, including exacerbation of back pain or onset of pain or discomfort in the abdomen, during exercise or strength assessment using the device [19, 20, 29, 30].
Assessment
We measured the body height, body weight, and body mass index (BMI) of the participants. Grip power was measured using a dynamometer (TTM Dynamometer; Tsutsumi, Tokyo, Japan). Knee extensor muscle strength (KEMS) was measured using a hand-held dynamometer (μTas F-1; ANIMA Corp., Tokyo, Japan). The KEMS values were divided by body weight (N/kg). To measure KEMS, subjects were seated in an elevated chair with the knees flexed 90°, feet above the floor, and arms crossed in front of the body. The dynamometer was placed on the anterior surface of the leg, 10 cm proximal to the malleoli. Subjects were instructed to push against the dynamometer by attempting to straighten the knees [31]. The right and left grip power and KEMS were measured once, and the higher strength values of each were recorded. We measured the ATMS twice using the device and recorded the higher measurement. We obtained responses to the 25-Question Geriatric Locomotive Function Scale (GLFS-25), which is a tool used to assess LS [32]. A high GLFS-25 score indicated advanced LS (decreased mobility function). We also obtained each patient’s 5-point numerical rating scale (NRS) score for the back, LBP, or buttocks (0 = no pain to 4 = severe pain) from the response to question 2 of the GLFS-25. Subjects with LBP (NRS score ≥2; range 0–4) during the past 1 month were allocated to the LBP group (n = 36). The other subjects were allocated to the non-LBP group (n = 65). Decreased mobility function (i.e., LS) was assessed using the total score of 24 questions (the GLFS-24) of the GLFS-25 after eliminating the score of question 2 (which assesses the presence and intensity of LBP). To evaluate the nutritional status of the subjects, BMI and data regarding the preoperative serum levels of total protein (g/dL), albumin (g/dL) and hemoglobin (g/dL) and the total lymphocyte count (102/μL) were collected via chart review.
Statistical analysis
All data are presented as means and standard deviations. The Shapiro–Wilk test was used to assess the normality of the distribution of the data. Differences in continuous variables between two groups were examined using the Student’s t-test for parametric data and the Mann–Whitney U test for nonparametric data. Multivariate logistic regression analyses were performed to determine the factors significantly associated with LBP. Spearman’s correlation analysis was used to evaluate the correlations of measures with the degree of LBP (5-point NRS score), and Pearson’s correlation analysis was used to evaluate the correlations of measures with ATMS, and the GLFS-24 score. Cohen d effect sizes were also calculated to determine the magnitude of the group differences under the two conditions; they were considered trivial (<0.20), small (0.20–0.50), moderate (0.50–0.80), or large (≥0.80) [33]. All statistical analyses were performed using SPSS version 25 (IBM Corp., Armonk, NY, USA). Analysis items with P value <0.05 were considered statistically significant.
Results
None of the subjects involved in the present study reported pain during the muscle strength measurements, including ATMS measurements using the device. According to the univariate analysis, there were no differences between the LBP and non-LBP groups regarding age, BMI, grip power, and serum levels of total protein and total lymphocyte count. However, the ATMS, KEMS, and serum albumin and hemoglobin levels were significantly lower in the LBP group than in the non-LBP group. GLFS-24 scores were significantly higher in the LBP group (Table 2). They corresponded to effect sizes of d = 0.91, d = 0.60, d = 0.98, d = 0.61, and d = 0.59, which are considered large or moderate effect sizes. The multivariate logistic regression analysis showed that ATMS (odds ratio, 0.82; P = 0.03) and GLFS-24 scores (odds ratio, 1.04; P <0.01) were independently associated with LBP (Table 3).
Table 2. Univariate analysis of differences between the LBP and non-LBP groups.
| Measure | LBP group | Non-LBP group | p value |
|---|---|---|---|
| No. of patients | 36 | 65 | |
| Physical measurements | |||
| Age (years), mean ± SD | 70.9 ± 6.9 | 69.0 ± 6.0 | 0.17 |
| BMI (kg/cm2), mean ± SD | 23.6 ± 3.6 | 23.7 ± 4.0 | 0.92 |
| Grip power (kg), mean ± SD | 19.7 ± 5.8 | 21.2 ± 5.6 | 0.20 |
| ATMS (kPa), mean ± SD | 4.0 ± 2.4 | 6.0 ± 3.4 | <0.01 |
| KEMS (N/kg), mean ± SD | 3.2 ± 1.2 | 3.8 ± 1.3 | 0.03 |
| GLFS-24 Scores, mean ± SD | 52.1 ± 19.9 | 35.1 ± 19.9 | <0.01 |
| Blood biomarkers associated with risk of malnutrition | |||
| Total protein (g/dL), mean ± SD | 7.2 ± 0.4 | 7.1 ± 0.4 | 0.57 |
| Albumin (g/dL), mean ± SD | 4.1 ± 0.3 | 4.3 ± 0.2 | 0.02 |
| Hemoglobin (g/dL), mean ± SD | 12.6 ± 1.1 | 13.1 ± 1.1 | 0.02 |
| Total lymphocyte (102/μL), mean ± SD | 16.8 ± 5.5 | 17.7 ± 6.5 | 0.52 |
ATMS, abdominal trunk muscle strength; BMI, body mass index
GLFS-24, 24-Question Geriatric Locomotive Function Scale
KEMS, knee extensor muscle strength; LBP, low back pain; SD, standard deviation
Table 3. Multivariate logistic regression analysis of the factors associated with LBP.
| Factors | OR | 95% CI | p value |
|---|---|---|---|
| ATMS (kPa) | 0.823 | 0.688–0.985 | 0.03 |
| KEMS (N/kg) | 0.883 | 0.592–1.317 | 0.54 |
| GLFS-24 Scores | 1.035 | 1.010–1.062 | <0.01 |
| Albumin (g/dL) | 0.227 | 0.026–1.944 | 0.18 |
| Hemoglobin (g/dL) | 0.871 | 0.539–1.408 | 0.57 |
ATMS, abdominal trunk muscle strength; CI, confidence interval; GLFS-24, 24-Question Geriatric Locomotive Function Scale; LBP, low back pain; OR, odds ratio
Table 4 shows the correlations of measures with the degree of LBP, ATMS, and GLFS-24 scores. The intensity of LBP had a mildly negative correlation with ATMS (simple regression coefficients: b = -0.26; r2 = 0.07; P = 0.01) and KEMS (b = -0.23; r2 = 0.05; P = 0.02), and a mildly positive correlation with the GLFS-24 score (b = 0.39; r2 = 0.15; P < 0.01). They corresponded to effect sizes of d = 0.66, d = 0.55, and d = 0.95, which are considered large or moderate effect sizes. However, there was no correlation between the intensity of LBP and the other items evaluated. The serum hemoglobin level showed a mildly negative correlation with the GLFS-24 score (b = -0.35; r2 = 0.126; P < 0.01) and large effect size (d = 0.95) (Table 4).
Table 4. Correlation with the degrees of LBP, abdominal trunk muscle strength, and locomotive syndrome among all 101 participants.
| 5-point NRS score for LBP | ATMS (kPa) | GLFS-24 score | ||||
|---|---|---|---|---|---|---|
| R value | P value | R value | P value | R value | P value | |
| Age (years) | 0.13 | 0.21 | -0.14 | 0.16 | 0.15 | 0.15 |
| BMI (kg/cm2) | 0.14 | 0.16 | 0.02 | 0.82 | -0.03 | 0.75 |
| Grip power (kg) | -0.09 | 0.37 | 0.38 | <0.01 | -0.23 | 0.02 |
| ATMS (kPa) | -0.23 | 0.01 | — | — | -0.19 | 0.06 |
| KEMS (N/kg) | -0.21 | 0.02 | 0.41 | <0.01 | -0.24 | 0.02 |
| GLFS-24 Scores | 0.35 | <0.01 | -0.19 | 0.06 | — | — |
| Total protein (g/dL) | 0.14 | 0.15 | -0.11 | 0.29 | 0.03 | 0.79 |
| Albumin (g/dL) | -0.06 | 0.57 | 0.11 | 0.27 | -0.17 | 0.09 |
| Hemoglobin (g/dL) | -0.16 | 0.12 | 0.14 | 0.17 | -0.35 | <0.01 |
| Total lymphocyte (103/μL) | -0.01 | 0.91 | 0.05 | 0.61 | 0.04 | 0.70 |
ATMS, abdominal trunk muscle strength; BMI, body mass index; GLFS-24, 24-Question Geriatric Locomotive Function Scale; KEMS, knee extensor muscle strength; LBP, low back pain; NRS, numerical rating scale
Discussion
The current study examined whether muscle strength, including ATMS measured using an innovative device, mobility dysfunction assessed using the LS assessment (the GLFS-24 score), and nutrition status assessed using preoperative non-acute laboratory data and BMI were associated with LBP among older women. Our results demonstrated that the ATMS and KEMS of the subjects with considerable LBP (NRS score ≥2) were significantly lower than those of subjects without LBP. The GLFS-24 scores were higher among subjects with considerable LBP than among subjects without LBP. Multivariate analyses revealed that low ATMS and advanced LS were associated with LBP. However, grip power and KEMS were not associated with LBP. According to the univariate analyses, subjects with considerable LBP had lower serum albumin and hemoglobin levels compared with subjects without LBP. Furthermore, the serum hemoglobin level was correlated with mobility function.
Previous studies have reported that patients with LBP have weaker trunk muscles than asymptomatic individuals [6, 7, 34, 35]. However, most of the strength measurements used during these studies (including sit-ups [34], hyperextension of the trunk [35], and isometric and isokinetic tests using the Cybex [6] and Biodex [7] Systems) imposed strain or stress on the lumbar spine that could induce LBP. LBP induced during strength measurements can decrease the muscle strength to be measured. Recently, in the literature, lumbar stabilization exercises, including the plank, side bridge, and pelvic tilt, have been recommended to improve LBP and physical function [36, 37]. However, many older patients with LBP, other locomotive organ disorders, and/or fragility are unable to perform these exercises because they induce pain in the extremities and spine with significant strain or stress. During strength measurements or exercises using the device, subjects are able to contract the abdominal trunk muscles while sitting without moving the trunk. This isometric muscle contraction does not strain the spine or extremities. Therefore, it may be feasible for older patients to use the device. We previously demonstrated that exercises performed using the device significantly increased strength and activated the abdominals, diaphragm, and pelvic floor muscles [20]. ATMS, as measured by the device, is generated by the contraction of these abdominal core muscles to increase the intra-abdominal pressure and lumbar stability. The results of the present study indicated that ATMS weakness measured using the device was associated with LBP among older women. A pilot study involving older patients with chronic LBP and deteriorated physical ability demonstrated that strengthening exercises performed using the device effectively improved chronic LBP and mobility function [30].
Previous studies have reported that chronic LBP was associated with decreased mobility function in older populations [29, 38]. The results of the current study showed that severe LS was associated with LBP among older women who were scheduled to undergo surgery because of degenerative lower extremity diseases. Because of advanced diseases of the lower extremities requiring surgery, participants involved in the present study had advanced LS and higher LS test scores compared with the older general population [39]. Even with the advanced LS condition of the lower extremity, the presence of LBP was significantly related to the severity of the decreased mobility function assessed using the LS test (the GLFS-24 score eliminated the score for LBP from the GLFS-25 score). Chronic pain including LBP in the older population reduces mobility function [40]. Therefore, exercise and physical activity are recommended to decrease LBP and disability [10, 11, 18, 40]. However, not all treatments options normally indicated for the young population can be applied to the older population, because they have muscle weakness, osteoporosis, and other musculoskeletal pain and dysfunction in the extremities [10–13, 18]. Adherence and motivation are also important for exercise therapy for older patients [9]. Further studies and efforts are required to resolve these problems.
The present study examined the association between the presence of LBP and nutritional status. Most of the nutritional assessment tools included BMI as a criterion for defining malnutrition. In the present study, we were able to collect the non-acute phase laboratory data of all subjects, including data regarding serum levels of total protein, albumin, and hemoglobin, total lymphocyte count; and BMI, because the laboratory data were obtained routinely under stable preoperative and non-acute conditions, which made patients eligible for surgery for degenerative musculoskeletal diseases. Furthermore, patients with inflammatory diseases were excluded from the analyses performed during the study. These biomarkers were also proven to be associated with the risk of malnutrition among older adults [41, 42]. In the present study, serum albumin and hemoglobin levels were significantly lower in the LBP group than in the non-LBP group, but they were not associated with LBP in the multivariate analysis. Several inflammatory biomarkers have been reported to be associated with acute or chronic LBP [43, 44]. However, no studies have reported an association between hypoalbuminemia or anemia and LBP. Interestingly, the serum hemoglobin level was negatively correlated with decreased mobility function (GLFS-24 score). A systematic review and meta-analysis concluded that BMI, hemoglobin, and total cholesterol were useful biomarkers of malnutrition in older adults [41]. The malnutrition risk that was represented by lower serum hemoglobin levels in older adults was related to decreased mobility function. A systematic review highlighted that physical activity can decrease chronic LBP and improve disability in older patients [10]. The relationship of malnutrition with decreased mobility may also affect the presence of LBP in older adults. However, future studies involving a larger cohort of older individuals and detailed nutritional risk screening should aim to identify the malnutrition risk factors that can be associated with LBP and muscle strength as well as decreased mobility function.
The current study had several limitations, including the relatively small sample size and the inclusion of many participants with locomotive organ disorders of the lower extremities, which may have impacted the results. The absence of a correlation between ATMS and decreased mobility function (GLFS-24 scores) may have occurred because of the presence of other musculoskeletal diseases. In this study, the malnutrition risk was evaluated only using blood biomarkers and BMI. The evaluation did not include the preoperative dietary status. The cause of LBP among the subjects might be multifactorial; however, it was not evaluated. Both acute and chronic LBP were assessed by question 2 of the GLFS-25 during the present study. Back muscle strength, which has been reported to be associated with LBP and physical function among older people [45, 46], was not measured in the present study. Despite its limitations, the present study showed that older patients with LBP had lower ATMS and decreased mobility function than those without LBP. Our device is a viable option for measuring ATMS as a factor associated with LBP.
Conclusion
Abdominal trunk muscle weakness and advanced LS were associated with LBP in older women. Further studies are needed to validate the association of malnutrition with LBP and muscle strength as well as decreased mobility function.
Supporting information
(DOCX)
Acknowledgments
We would like to thank Editage (http://www.editage.jp/) for English language editing.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This research was supported by a research grant from the Japanese Society for Musculoskeletal Medicine. S.K. (the corresponding author) received the grant. The grand did not have a grant number. URL: http://www.jsmr.org/ The funder did not play any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Airaksinen O, Brox JI, Cedraschi C, Hildebrandt J, Klaber-Moffett J, Kovacs F, et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J. 2006;15(Suppl 2):s192–s300. 10.1007/s00586-006-1072-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujii T, Matsudaira K. Prevalence of low back pain and factors associated with chronic disabling back pain in Japan. Eur Spine J. 2013;22(2):432–438. 10.1007/s00586-012-2439-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354(9178):581–585. 10.1016/S0140-6736(99)01312-4 [DOI] [PubMed] [Google Scholar]
- 4.Wu A, March L, Zheng X, Huang J, Wang X, Zhao X, et al. Global low back pain prevalence and years lived with disability from 1990 to 2017: estimates from the Global Burden of Disease Study 2017. Ann Transl Med. 2020;8(6):299 10.21037/atm.2020.02.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, et al. What low back pain is and why we need to pay attention. Lancet. 2018;391(10137):2356–2367. 10.1016/S0140-6736(18)30480-X [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, Hoshino Y, Nakamura K, Kariya Y, Saita K, Ito K. Trunk muscle weakness as a risk factor for low back pain. A 5-year prospective study. Spine (Phila Pa 1976). 1999;24(1):54–57. 10.1097/00007632-199901010-00013 [DOI] [PubMed] [Google Scholar]
- 7.Cho KH, Beom JW, Lee TS, Lim JH, Lee TH, Yuk JH. Trunk muscles strength as a risk factor for nonspecific low back pain: a pilot study. Ann Rehabil Med. 2014;38(2):234–240. 10.5535/arm.2014.38.2.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayden JA, van Tulder MW, Tomlinson G. Systematic review: strategies for using exercise therapy to improve outcomes in chronic low back pain. Ann Intern Med. 2005;142(9):776–785. 10.7326/0003-4819-142-9-200505030-00014 [DOI] [PubMed] [Google Scholar]
- 9.Phillips EM, Schneider JC, Mercer GR. Motivating elder to initiate and maintain exercise. Arch Phys Med Rehabil. 2004;85(7 Suppl 3):S52–57. 10.1016/j.apmr.2004.03.012 [DOI] [PubMed] [Google Scholar]
- 10.Vadalà G, Russo F, De Salvatore S, Cortina G, Albo E, Papalia R, et al. Physical activity for the treatment of chronic low back pain in elderly patients: a systematic review. J Clin Med. 2020;9(4):1023 10.3390/jcm9041023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia WJ, Johnson A, Keldermans D, Tang B. Exercise and low back pain in the older adult: current recommendations. J Allied Health. 2019;48(4):302–307. [PubMed] [Google Scholar]
- 12.Rudy TE, Weiner DK, Lieber SJ, Slaboda J, Boston JR. The impact of chronic low back pain on older adults: a comparative study of patients and controls. Pain. 2007;131(3):293–301. 10.1016/j.pain.2007.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coyle PC, Velasco T, Sions JM, Hicks GE. Lumbar mobility and performance-based function: an investigation in older adults with and without chronic low back pain. Pain Med. 2017;18(1):161–168. 10.1093/pm/pnw136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshimura N, Muraki S, Oka H, Mabuchi A, En-Yo Y, Yoshida M, et al. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metab. 2009;27(5):620–628. 10.1007/s00774-009-0080-8 [DOI] [PubMed] [Google Scholar]
- 15.Kadono Y, Yasunaga H, Horiguchi H, Hashimoto H, Matsuda S, Tanaka S, et al. Statistics for orthopedic surgery 2006–2007: data from the Japanese Diagnosis Procedure Combination database. J Orthop Sci. 2010;15(2):162–170. 10.1007/s00776-009-1448-2 [DOI] [PubMed] [Google Scholar]
- 16.Nakamura K. A “super-aged” society and the “locomotive syndrome”. J Orthop Sci. 2008;13(1):1–2. 10.1007/s00776-007-1202-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishibashi H. Locomotive syndrome in Japan. Osteoporos Sarcopenia. 2018;4(3): 86–94. 10.1016/j.afos.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura K, Ogata T. Locomotive Syndrome: Definition and Management. Clin Rev Bone Miner Metab. 2016;14(2):56–67. 10.1007/s12018-016-9208-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato S, Murakami H, Inaki A, Mochizuki T, Demura S, Nakase J, et al. Innovative exercise device for the abdominal trunk muscle: an early validation study. PLoS One 2017;12(2):e0172934 10.1371/journal.pone.0172934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato S, Inaki A, Murakami H, Kurokawa Y, Mochizuki T, Demura S, et al. Reliability of the muscle-strength measurement and effects of the strengthening by an innovative exercise device for the abdominal trunk muscles. J Back Musculoskelet Rehabil. 2020;33(4):677–684. 10.3233/BMR-181419 [DOI] [PubMed] [Google Scholar]
- 21.Saka B, Kaya O, Ozturk GB, Erten N, Karan MA. Malnutrition in the elderly and its relationship with other geriatric syndromes. Clin Nutr. 2010;29(6):745–748. 10.1016/j.clnu.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 22.Jiménez-Redondo S, Beltrán de Miguel B, Gavidia Banegas J, Guzmán Mercedes L, Gómez-Pavón J, Cuadrado Vives C. Influence of nutritional status on health-related quality of life of non-institutionalized older people. J Nutr Health Aging. 2014;18(4):359–364. 10.1007/s12603-013-0416-x [DOI] [PubMed] [Google Scholar]
- 23.Ramsey KA, Meskers CGM, Trappenburg MC, Verlaan S, Reijnierse EM, Whittaker AC, et al. Malnutrition is associated with dynamic physical performance. Aging Clin Exp Res. 2020;32(6):1085–1092. 10.1007/s40520-019-01295-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bárbara Pereira Costa A, Andrade Carneiro Machado L, Marcos Domingues Dias J, Keller Coelho de Oliveira A, Ude Viana J, da Silva SL, et al. Nutritional risk is associated with chronic musculoskeletal pain in community-dwelling older persons: the PAINEL Study. J Nutr Gerontol Geriatr. 2016;35(1):43–51. 10.1080/21551197.2015.1125325 [DOI] [PubMed] [Google Scholar]
- 25.Visvanathan R. Under-nutrition in older people: a serious and growing global problem. J Postgrad Med. 2003;49(4):352–260. [PubMed] [Google Scholar]
- 26.Chen CC, Schilling LS, Lyder CH. A concept analysis of malnutrition in the elderly. J Adv Nurs. 2001;36(1):131–142. 10.1046/j.1365-2648.2001.01950.x [DOI] [PubMed] [Google Scholar]
- 27.Reid KF, Fielding PA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40(1):4–12. 10.1097/JES.0b013e31823b5f13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen GL, Cederholm T, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition: a consensus report from the global clinical nutrition community. J Parenter Enter Nutr. 2019;43(1):32–40. 10.1002/jpen.1440 [DOI] [PubMed] [Google Scholar]
- 29.Kato S, Murakami H, Demura S, Yoshioka K, Shinmura K, Yokogawa N, et al. Abdominal trunk muscle weakness and its association with chronic low back pain and risk of falling in older women. BMC Musculoskelet Disord. 2019;20(1):273 10.1186/s12891-019-2655-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato S, Demura S, Kurokawa Y, Takahashi N, Shinmura K, Yokogawa N, et al. Efficacy and safety of abdominal trunk muscle strengthening using an innovative device in elderly patients with chronic low back pain: a pilot study. Ann Rehabil Med. 2020;44(3):246–255. 10.5535/arm.19100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki T. Reliability of measurements of knee extensor muscle strength using a pull-type hand-held dynamometer. J Phys Ther Sci. 2015;27(3):967–971. 10.1589/jpts.27.967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seichi A, Hoshino Y, Doi T, Akai M, Tobimatsu Y, Iwaya T. Development of a screening tool for risk of locomotive syndrome in the elderly: the 25-question Geriatric Locomotive Function Scale. J Orthop Sci. 2012;17(2):163–172. 10.1007/s00776-011-0193-5 [DOI] [PubMed] [Google Scholar]
- 33.Sarafadeen R, Ganiyu S, Ibrahim AA. Effects of spinal stabilization exercise with real-time ultrasound imaging biofeedback in individuals with chronic nonspecific low back pain: a pilot study. J Exerc Rehabil. 2020;16(3):293–299. 10.12965/jer.2040380.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helewa A, Goldsmith C, Smythe H, Gibson E. An evaluation of four different measures of abdominal muscle strength: Patient, order and instrument variation. J Rheumatol. 1990;17(7):965–969. [PubMed] [Google Scholar]
- 35.Nourbakhsh MR, Arab AM. Relationship between mechanical factors and incidence of low back pain. J Orthop Sports Phys Ther. 2002;32(9):447–460. 10.2519/jospt.2002.32.9.447 [DOI] [PubMed] [Google Scholar]
- 36.Suh JH, Kim H, Jung GP, Ko JY, Ryu JS. The effect of lumbar stabilization and walking exercises on chronic low back pain: a randomized controlled trial. Medicine (Baltimore). 2019;98(26):e16173 10.1097/MD.0000000000016173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong YS, Jang GU, Park S. The effects of prone bridge exercise on the Oswestry disability index and proprioception of patients with chronic low back pain. J Phys Ther Sci. 2015;27(9):2749–2752. 10.1589/jpts.27.2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimachi K, Kimachi M, Takegami M, Ono R, Yamazaki S, Goto Y, et al. Level of low back pain-related disability is associated with risk of subsequent falls in an older population: locomotive syndrome and health outcomes in Aizu cohort study (LOHAS). Pain Med. 2019;20(12):2377–2384. 10.1093/pm/pny313 [DOI] [PubMed] [Google Scholar]
- 39.Yoshimura N, Muraki S, Iidaka T, Oka H, Horii C, Kawaguchi H, et al. Prevalence and co-existence of locomotive syndrome, sarcopenia, and frailty: the third survey of Research on Osteoarthritis/Osteoporosis Against Disability (ROAD) study. J Bone Miner Metab. 2019;37(6):1058–1066. 10.1007/s00774-019-01012-0 [DOI] [PubMed] [Google Scholar]
- 40.Schwan J, Sclafani J, Tawfik VL. Chronic pain management in the Elderly. Anesthesiol Clin. 2019;37(3):547–560. 10.1016/j.anclin.2019.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z, Pereira SL, Luo M, Matheson EM. Evaluation of blood biomarkers associated with risk of malnutrition in older adults: a systematic review and meta-analysis. Nutrients. 2017;9(8): 829 10.3390/nu9080829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cascio BL, Logomarsino JV. Evaluating the effectiveness of five screening tools used to identify malnutrition risk in hospitalized elderly: a systematic review. Geriatr Nurs. 2018;39(1):95–102. 10.1016/j.gerinurse.2017.07.006 [DOI] [PubMed] [Google Scholar]
- 43.Morris P, Ali K, Merritt M, Pelletier J, Macedo LG. A systematic review of the role of inflammatory biomarkers in acute, subacute and chronic non-specific low back pain. BMC Musculoskelet Disord. 2020;21(1):142 10.1186/s12891-020-3154-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van den Berg R, Jongbloed EM, de Schepper EIT, Bierma-Zeinstra SMA, Koes BW, Luijsterburg PAJ. The association between pro-inflammatory biomarkers and nonspecific low back pain: a systematic review. Spine J. 2018;18(11):2140–2151. 10.1016/j.spinee.2018.06.349 [DOI] [PubMed] [Google Scholar]
- 45.Kudo D, Miyakoshi N, Hongo M, Kasukawa Y, Ishikawa Y, Shimada Y. Impact of sagittal spine-pelvis-leg alignment and muscle strength on quality of life and low back pain in rural Japanese community- dwelling middle-aged and elderly persons. J Back Musculoskelet Rehabil. 2020;33(2):263–268. 10.3233/BMR-160618 [DOI] [PubMed] [Google Scholar]
- 46.Kasukawa Y, Miyakoshi N, Hongo M, Ishikawa Y, Noguchi H, Kamo K, et al. Relationships between falls, spinal curvature, spinal mobility and back extensor strength in elderly people. J Bone Miner Metab. 2010;28(1):82–87. 10.1007/s00774-009-0107-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.


