ABSTRACT
Introduction
The recent global outbreak of coronavirus disease 2019 (COVID-19) has become a pandemic with a lot of sufferers. Excessive inflammation, exaggerated immune response, with ultimate apoptosis contribute to COVID-19 pathology that progress to acute lung acute respiratory distress.
Objective
To shed a light on the likely benefits of the oral phosphodiesterase 5 (PDE5) inhibitor adjuvant role in combating COVID-19 infection.
Methods
A literature review was performed in the PubMed/Medline database, Scopus, Cochrane Library, EMBASE, Academic Search Complete, Google Scholar, and CINAHL databases using the keywords COVID-19; phosphodiesterase-5 inhibitors; cytokine storm; respiratory distress.
Results
Despite the worsening trends of COVID-19, still no drugs are validated to have significant clinical efficacy in the treatment of patients with COVID-19 in large-scale studies. While the progress toward a curative agent and/or vaccine is certainly hopeful, the principal limiting factor in such public health emergencies is always the time. Therefore, a preexisting licensed therapeutic(s) might offer a reprieve to the healthcare systems operating at the edge of capacity. In this context, the innovation of oral PDE5 inhibitors with their valuable effects on erection have provided a breakthrough in the treatment of erectile dysfunction and opened new fields of clinical application for this class of drugs. Oral PDE5 inhibitors have been demonstrated to possess many beneficial useful additional implications with acknowledged anti-inflammatory, antioxidant, immune response regulation, and antiapoptotic properties. These properties have been elucidated through the nitric oxide/soluble guanylyl cyclase/cyclic guanylate monophosphate pathway in addition to the emerged hemeoxygenase-1 enzyme as well as hydrogen sulfide pathways. These properties could support repurposing oral PDE5 inhibitors’ potential adjuvant use in targeting different aspects of COVID-19 infection.
Conclusion
Oral PDE5 inhibitors retain several acknowledged off-labeled useful implications with anti-inflammatory, antioxidant, immune response regulation, and antiapoptotic properties. These properties may support repurposing oral PDE5 inhibitors’ potential adjuvant use in the protocols combating COVID-19 manifestations.
Keywords: COVID-19, PDE5 Inhibitors, Sildenafil, Tadalafil, Vardenafil, SARS-CoV-2
Introduction
Since December 2019, a series of global health concern pneumonia cases were reported in Wuhan, Hubei Province, China with coronavirus disease 2019 (COVID-19) with substantial mortalities. The etiological agent of COVID-19 has been confirmed as a novel coronavirus, now known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1,2 Coronaviruses have large (~30-kb) single-stranded, positive-sense RNA genomes that are divided into a 5′ two-thirds and a 3′ third. The first two-thirds code for 2 large polyproteins that are proteolytically cleaved into non-structural proteins essential for the production of new viral genetic material. The rest codes for its structural proteins and carry the accessory genes that produce virions and alter the host response.3,4 Asymptomatic or minimally symptomatic infection with COVID-19 can result in silent transmission to large numbers of people, resulting in an extension of the disease with an overall increase in its morbidity and mortality.
Currently, no treatment for SARS-CoV-2 is approved because of the lack of evidence, although several protocols have been presented such as antiviral therapy, corticosteroid therapy, vitamins, antimalarial, and so on. However, there is still a lack of precise treatment(s) for the harsh COVID-19 manifestations.5,6 Although the progress toward a curative agent and/or vaccine is certainly hopeful, the principal limiting factor in this public health emergency is always the time factor. Therefore a preexisting licensed molecule(s) might offer a reprieve to healthcare systems operating at the edge of capacity.
Currently, oral phosphodiesterase 5 (PDE5) inhibitors, acting on the nitric oxide/soluble guanylyl cyclase/cyclic guanylate monophosphate (NO/sGC/cGMP) pathway, represent the first-line therapy for erectile dysfunction with good responses.7,8 Specifically, PDE5 hydrolyzes cGMP into 5′GMP by blocking cGMP hydrolysis, potentiates the effects of cGMP, resulting in decreased intracellular calcium, penile smooth muscle relaxation, and vasodilatation with increased penile blood flow.9 Currently, 4 oral PDE5 inhibitors are approved by the U.S. Food and Drug Administration (sildenafil [Viagra], vardenafil [Levitra], tadalafil [Cialis], and avanafil [Stendra]) with good efficacy and tolerable adverse effects.10,11
Sildenafil citrate was released in 1998, has a maximal plasma concentration (Tmax) at 60 min on an empty stomach, and acts for 4–6 hours. Vardenafil hydrochloride was approved in 2003, has a Tmax of 60 min on an empty stomach, and acts for up to 7 hours. Tadalafil was approved in 2003, has a Tmax of 120 min with/without an empty stomach, and acts up to 36 hours. Later on, avanafil was approved in 2012 and has a Tmax of 30–45 min on an empty stomach.12 These drugs are available in different doses; sildenafil citrate 25, 50, 100 mg; tadalafil 2.5, 5, 10, 20 mg; vardenafil hydrochloride 2.5, 5, 10, 20 mg; and avanafil 50, 100, 200 mg.
The high tolerability of oral PDE5 inhibitors, ease of administration, and its safety margin (except if combined with nitrates) have made these molecules an attractive tool to explore their physiological functions, beyond its immediate prescribed indications, with collateral benefits for a multitude of useful implications beyond erection.13–16
This article sheds a light on the likely benefits of the adjuvant use of oral PDE5 inhibitors in the protocols combating COVID-19 virus infection.
Methods
A literature review has been performed in the PubMed/Medline database, Scopus, Cochrane Library, EMBASE, Academic Search Complete, Google Scholar, and CINAHL databases to search articles published from inception to August 2020 using the terms COVID-19; phosphodiesterase-5 inhibitors; sildenafil; tadalafil; vardenafil; avanafil; cytokine storm; respiratory distress.
COVID-19 Pathophysiology
Many researchers focused their attention on the specific protein that allows the COVID-19 virus to infect human cells, namely the angiotensin-converting enzyme 2 (ACE2) receptor. This receptor provides the entry point for the virus to hook into a wide range of non-immune cells, such as respiratory and intestinal epithelial cells, endothelial cells, renal tubules, cerebral neurons, and immune cells, such as alveolar monocytes/macrophages.17 In this context, oral epithelial cells and other respiratory tract areas have an extensive expression of ACE2, explaining their susceptibility to viral entry.18–20 Then, the virus spreads into the lower respiratory tract (the lungs) where epithelial cells, in particular type II pneumocytes, express ACE2.20 Infection in the lungs, and especially damage of the alveoli, has been raised to be the primary cause of morbidity in COVID-19.21
Common reported symptoms of COVID-19 are fever, cough, myalgia, headache, diarrhea, dyspnea, pneumonia, and in some cases acute respiratory distress syndrome. Besides, most of the infected cases experience significant decreases in hemoglobin, neutrophil counts, and significant increases in serum ferritin, erythrocyte sedimentation rate, C-reactive protein, albumin, and lactate dehydrogenase. The oxygen-carrying capacity of the erythrocytes would, therefore, be compromised exacerbating the difficulties experienced by these patients in maintaining a sufficient partial pressure of oxygen in their alveoli.22,23
In this framework, in susceptible patients, the lung cells exhibit intense inflammation because of its inability to exchange carbon dioxide and oxygen frequently resulting in ground-glass–like images and respiratory distress.24 In addition, apoptosis of the endothelial cells damages the pulmonary microvascular and alveolar epithelial cells causing vascular leakage and alveolar edema that ultimately leads to hypoxia and multiple organ failure.25
Certain patients experience severe inflammation and cytokine storm, with overwhelming immune activation that attacks the host. This cytokine storm includes several proinflammatory cytokines (Interleukin [IL]-1β, IL-2, IL-6, IL 7, IL-8, IL-10, granulocyte-macrophage colony-stimulating factor, and reactive oxygen species) and chemokines (C-C Motif Chemokine Ligand (CCL) 2, CCL-5, interferon gamma–induced protein 10, macrophage inflammatory protein (MCP1), MIP1A (monocyte chemoattractant protein), and CCL3 contribute to the occurrence of acute respiratory distress syndrome).25–28 The accumulated mononuclear macrophages receive activating signals through the interferon alfa/beta receptors on their surface to produce more monocyte chemoattractants (CCL2, CCL7, and CCL12) with additional accumulation of mononuclear macrophages. In turn, these macrophages produce higher levels of the proinflammatory cytokines (tumor necrosis factor [TNF], -6, IL1-β, and inducible NOS), that induce T cell apoptosis, which hinders viral clearance.29 Such rapid viral replication and vigorous proinflammatory cytokine/chemokine response induces apoptosis in the lung epithelial and endothelial cells through mechanisms involving Fas-Fas ligand or TNF-related apoptosis-inducing ligand death receptor 5.30
Rational of Oral PDE5 Inhibitor Role
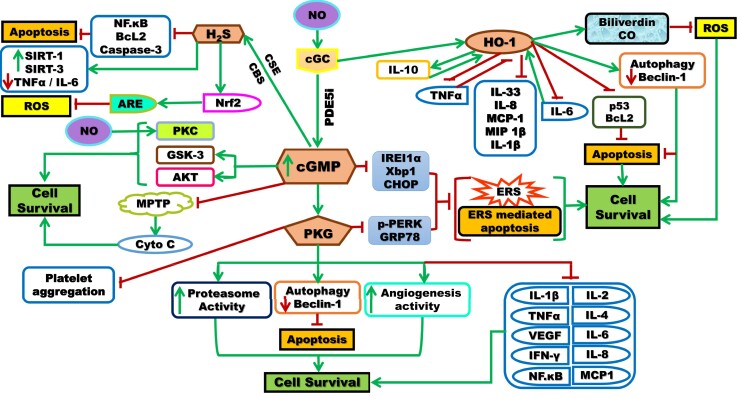
Oral PDE5 inhibitors have been demonstrated to exhibit several favorable effects and implications that might justify their ability for targeting multiple aspects in combating COVID-19 pathologic manifestations (Figure 1).
Figure 1.
Schematic representation of the signaling pathways involved in the mechanism of actions of PDE5i that act by inhibiting PDE5, the cGMP degrading enzyme, leading to increased intracellular cGMP that acts by activating many kinases, customarily PKG, but it also triggers the activation of PKC/Akt and GSK-3, PKC can be activated by NO, in a cGMP-independent manner. cGMP inhibits MPTP formation and Cyto C release. cGMP activation can, alone/through PKG, suppress the function of molecules associated with ERS and thus prevents ERS and ERS-mediated apoptosis. Besides, PKG stimulates proteasome activity, autophagy and increases angiogenesis resulting in the inhibition of cellular apoptosis and promotion of cell survival. The PKG pathway inhibits many cytokines, and inflammatory mediators leading to cell survival. PDE5i could induce HO-1 expression through Nrf2. HO-1 enzyme has the affinity to inhibit cytokine and chemokine responses such as IL-1β, IL-8, IL-33, MCP-1, MIP-1β, and apoptotic markers. The pro-inflammatory chemokine IL-6 upregulates HO-1, which in turn inhibits IL-6 to limit the inflammatory responses. There is a positive feedback loop between HO-1 and IL-10 (anti-inflammatory) and a negative feedback loop between HO-1 and TNF-α (pro-inflammatory). HO-1 enzyme also produces biliverdin, CO that antagonizes ROS and stimulates autophagy that antagonizes apoptosis with the ultimate cell survival. In addition, PDE5i induces H2S production, stimulated by CSE and CBS enzymes, with an affinity to inhibit inflammatory mediators (TNF-α, IL-6), apoptotic markers (NF.kB, Bcl2, caspase-3) and antagonizes ROS with the ultimate cell survival. Akt = protein kinase B; ARE = antioxidant responsive element; Bcl2 = B-cell lymphoma 2; cGMP = cyclic guanosine monophosphate; CHOP = C/EBP homologous protein; Co = carbon monoxide; CSE = cystathionine gamma-lyase; ERS = endoplasmic reticulum stress; GRP78 = glucose-regulated protein 78; GSK-3 = glycogen synthase kinase-3; H2S = hydrogen sulfide; HO-1 = hemeoxygenase-1; IFN-γ = interferon gamma; IL = interleukin; IREI 1α = inositol-requiring enzyme 1α; MCP-1 = monocyte chemoattractant protein-1; MIP 1β = macrophage inflammatory protein 1-β; MPTP = mitochondrial permeability transition pore; NF-κB = nuclear factor-kappa B; NO = nitric oxide; Nrf2 = nuclear factor (erythrocyte-derived 2)-like 2; PDE5i = phosphodiesterase-5 inhibitors; PKC = protein kinase C; PKG = protein kinase G; p-PERK = phosphoprotein kinase-like ER kinase; ROS = reactive oxygen species; Sirt = sirtuin; TNFα = tumor necrosis factor-alpha; VEGF = vascular endothelial growth factor; Xbp1 = X-box binding protein 1. Figure 1 is available in color online at www.smr.jsexmed.org.
Counteracting the Angiotensin II–Mediated Downregulation of Angiotensin II Type I Receptor
Angiotensin II (Ang II) and NO signaling pathways have been reported to mutually regulate each other by multiple mechanisms. Ang II is recognized to regulate the expression of NO synthase and NO production, where NO downregulates the Ang II type I (AT1) receptor. Besides, the downstream effectors of both Ang II and NO signaling pathways also interact with each other with a feedback mechanism.31 PDE5 inhibitors were suggested to inhibit the intrapulmonary vasoconstriction caused by AT1 receptor downregulation owing to SARS-CoV-2-ACE2 binding alveolar cells, bronchial epithelium, and vascular endothelium through the NO/sGC/cGMP pathway.32,33
The endogenous mammalian peptide AngII is hypothesized to prevent infection from SARS-CoV-2 in multiple ways: (i) it normally binds to ACE2 during its degradation and hydrolysis into angiotensin-(1–7)34 competing with the SARS-CoV-2 for the ACE2 receptor; (ii) the binding of Ang II to the AT1 receptor has been revealed to cause internalization and downregulation of ACE2 through an extracellular signal–regulated kinase 1/2 and p38 MAP kinase pathway;35,36 and (iii) Ang II has been shown to cause AT1 receptor-dependent destruction of ACE2 through ubiquitination and transport into lysosomes. The competitive inhibition, downregulation, internalization, and then degradation of ACE2 decrease the intensity of viral infection by interfering with host cell entry of the virus.37
Lately, Qiao et al38 reported their calculations on the inhibitors for the SARS-CoV-2 3CL protease and the spike protein for the potential treatment of COVID-19. These authors showed that the most potent promising inhibitors of the SARS-CoV-2 3CL protease include saquinavir, tadalafil, rivaroxaban, sildenafil, dasatinib, vardenafil, and montelukast owing to their high docking scores (<−8.5 kcal/mol).
Pulmonary Implications
PDE5 inhibitors appear to be particularly appropriate in treating pulmonary diseases because PDE5 is expressed in high levels in the lung tissue and is highly specific for hydrolysis of cGMP.39
Sildenafil citrate and tadalafil received approval by the U.S. Food Drug Administration and the European Medicines Agency in 2005 and 2009, respectively, for treating pulmonary arterial hypertension functional classes (FC) II and III indication of patients with WHO-functional class and a class IIa indication in patients with WHO-functional class IV (men, women, newborn).40 Improved endothelial function, as well as prevention of impaired arterial relaxation, is the mechanism that explain the favorable effects of PDE5 inhibitors in these patients.41,42 Sildenafil citrate use has been verified to exhibit protective effects in pulmonary arterial hypertension cases by suppressing multiple cytokines involved in the neutrophil and mononuclear cell recruitment including cytokine-induced neutrophil chemoattractant-1, cytokine-induced neutrophil chemoattractant-2α/β, tissue inhibitor of metalloproteinase-1, IL-1α, lipopolysaccharide-induced CXC chemokine, monokine induced by interferon gamma, macrophage inflammatory protein-1α, and macrophage inflammatory protein-3α. Besides, sildenafil use has been demonstrated to reduce extracellular signal–regulated kinase 1/2 and p38 MAPK activation with enhanced activation of the cytoprotective Akt pathway.43,44
In cases of acute lung injury, the positive effects of sildenafil use result from inhibiting the proliferation of regulatory T cells and the production of proinflammatory cytokines, autoantibodies, and modulating platelet activation, angiogenesis, pulmonary vasoreactivity.45
Sildenafil use has been validated to reduce the leak of neutrophils into the lung, the release of proinflammatory mediators TNF-α, IL-8 and IL-6, level of nitrite/nitrate, markers of oxidative stress (3-nitrotyrosine and malondialdehyde), lung edema, protein content in the bronchoalveolar lavage fluid, apoptosis of epithelial cells with ultimate improve in the respiratory parameters.46
In chronic obstructive pulmonary disease, sildenafil use has been reported to improve pulmonary hemodynamics by inhibiting hypoxic vasoconstriction and facilitating the weaning of these patients from the ventilators owing to improved respiratory parameters.47
In hypoxic conditions, sildenafil use was shown to increase the exercise capacity in acute normobaric hypoxia by improving arterial oxygenation.48 In their work, Gibbs49 associated sildenafil use with ameliorated hypoxic pulmonary vasoconstriction with increased exercise capacity and stroke volume. In addition, Watanabe et al50 pointed out that sildenafil use decreases pulmonary vascular resistance and improves dyspnea in patients with interstitial pneumonia. In their work, Gammella et al51 revealed that sildenafil and erythropoietin treatments protect endothelial cells in hypoxic states, whereas Czövek et al52 reported that sildenafil use prevents the hyperoxia-induced development of bronchial hyperreactivity by preserving the normal end-expiratory lung volume and inhibiting airway inflammation.
Although hydrogen sulfide (H2S) was reported as an endogenous inhibitor of PDE activity,53 sildenafil use has been demonstrated to promote remarkably intracellular H2S production that controls proliferation in the pulmonary arterial smooth muscle cells by inducing vasodilation and reducing oxidative stress and inflammation.54,55 Besides, the protein levels of the enzymes cystathionine γ-lyase and cystathionine-β-synthase and the intracellular concentration of calcium were also increased.
Prophylactic treatment with an optimal dose of sildenafil citrate was demonstrated to significantly increase lung cGMP levels, prolongs median survival, and reduces fibrin deposition, total protein content in bronchoalveolar lavage fluid, inflammation, and septum thickness.56
Anti-inflammatory and Cellular Implications
PDE5 inhibitors target the enzyme PDE5 responsible for the selective degradation of cGMP leading to increased intracellular cGMP. cGMP possesses intense anti-inflammatory effects by reducing the expression of the proinflammatory cytokines IL-1β and TNF-α and increasing the expression of the anti-inflammatory cytokine IL-10.57 In this context, Dalamaga et al58 raised the role of PDE4 inhibition and cyclic adenosine monophosphate in attenuating the cytokine storm in COVID-19, through the upstream inhibition of proinflammatory molecules, particularly TNF-α, and in regulating the proinflammatory/anti-inflammatory balance. These authors believed that selective PDE4 inhibitors may represent a promising option for the early phase of COVID-19 pneumonia before the cytokine storm and severe multiorgan dysfunction take place. Besides, Seirafianpour et al59 raised many confirmatory data on proper efficacy of pentoxifylline, a methyl-xanthine derivative that inhibits PDE4, on controlling COVID-19 and its consequences with antiviral, anti-inflammatory, antioxidative, immune-modulatory, bronchodilator, and respiratory supportive effects.
PDE5 inhibitors have been demonstrated to be a highly protective agent in preventing lung and kidney damage owing to induced sepsis by the maintenance of the oxidant-antioxidant status and decreased TNF-α.60 It has been reported that increased bioavailability of cGMP is beneficial in ameliorating the inflammation associated with intense sepsis.61
The PDE5 inhibitor sildenafil has been observed to produce a significant sustained reduction of fibrinogen, high-sensitivity C-reactive protein, high-sensitivity IL-6, TNF-α independent of their baseline values.62
Pieces of evidence pointed to the participation of cGMP-dependent protein kinase in the complex cellular signaling pathways related to cell survival/apoptosis.63 In their work, Choi et al64 advocated that the PDE5 inhibitor sildenafil citrate has antiapoptotic effects by induction of iNOS, eNOS, and decreased BCL2-associated X protein/Bcl2 ratio. In addition, Puzzo et al65 showed that sildenafil citrate use can inhibit the expression of apoptotic molecules such as; caspase-3 (proapoptotic factor), BCL2-associated X protein (an apoptotic factor), and p38 mitogen-activated protein kinases. Collectively, Duarte-Silva and Peixoto66 pointed out that sildenafil citrate use inhibits apoptosis by 2 interconnected mechanisms directly by modulating caspase expression (through extrinsic and intrinsic pathways) and indirectly by modulating the expression of molecules elaborated in cell death and/or cell survival.
PDE5 inhibition by sildenafil was reported to suppress both the endoplasmic reticulum stress (ERS) and ERS-induced apoptosis by decreasing X-Box binding protein 1 expression, phosphoprotein kinase-like ER kinase, and 78-kDa glucose-regulated protein in a protein kinase–dependent manner.67
PDE5 inhibitors had been reported to induce hemeoxygenase-1 (HO-1) enzyme expression through the sGC-cGMP pathway.68,69 HO-1 enzyme catalyzes the rate-limiting step in the oxidative degradation of heme to biliverdin and CO, which share many properties with NO, including activation of sGC signal transduction, gene regulation.70,71 HO-1 enzyme or its reaction products as CO are effective inducible antioxidants and antiapoptotic molecules that protect against inflammation, cleavage of adhesion proteins E-cadherin, and apoptosis by constraining both p53 and Bcl2.72 Besides, HO-1 has been reported to have antiviral activity by inhibiting viral growth.73,74
PDE5 inhibitors behave as antioxidants by inhibiting the free radical formation supporting antioxidant redox systems.75 It was demonstrated that the protective effect of sildenafil intake on the epithelial cells is a consequence of xanthine oxidase inhibition with decreased free oxygen radical production.76
NO inhibits platelet aggregation primarily via a cGMP-dependent process.77,78 In their study, Gudmundsdóttir et al79 determined that sildenafil citrate use potentiates NO-mediated inhibition of platelet aggregation through blockade of cGMP metabolism and that PDE5 inhibitors may have imperative antiplatelet actions.
Discussion
Since the outbreak of the novel COVID-19, the medical research community is in a race to find a cure for that pandemic with unprecedented worldwide respectful research efforts to control the infection and save the lives of severely infected patients. Therefore, in these urgent circumstances, exploring the beneficial repurposing effects of some drugs even being off label (for uses other than what it was approved for) might be of potential help worldwide and a realistic solution.80 Therefore, the selection of a molecule(s) backed by years of safety margin, widespread use, and affordability may offer the opportunity to prevent/treat infection and its disabling complications that ensue. Several options, such as tocilizumab,81 hydroxychloroquine and ivermectin,82 PDE-4 inhibition,58,59 combined interferon beta-1b, lopinavir-ritonavir, and ribavirin,83 were raised to manage such pandemic. In addition, camostat mesylate, remdesivir, favipiravir, baricitinib, convalescent plasma, and humanized monoclonal antibodies were suggested as therapeutics for the potential treatment of SARS-CoV-2.84
In this context, oral selective PDE5 inhibitors drugs possess extensive clinical support backed by a solid mechanistic scientific rationale based on their ability to target multiple aspects of the underlying disease processes that make COVID-19 so deadly. Therefore, PDE5 inhibitors are worthy repurposing candidates to be thought of on the likely benefits of their adjuvant use in the protocols aimed to combat COVID-19 infection based on the aforementioned rationales. Fortunately, the available PDE5 inhibitors have many existing flexible doses, forms (oral tablets, oral dispersible tablets, and so on), and regimens (daily or on demand) that could be assessed by the health authorities.
Conclusion
Optimal real-world repurposing requires a track record of safety, affordability, and access for drug candidates. Oral PDE5 inhibitors retain many beneficial acknowledged useful off-labeled implications with its anti-inflammatory, antioxidant, immune response regulation, as well as antiapoptotic properties. These aforementioned properties may support repurposing oral PDE5 inhibitors’ potential adjuvant use in the protocols combating COVID-19 manifestation especially along with the respiratory affection. Besides, it should be highlighted that the main stakeholder in the management of any illness is the patient and not any particular special interest group, political party, or pharmaceutical manufacturer.
Statement of authorship
Taymour Mostafa: Conceptualization, Methodology, Investigation, Resources, Writing - Review & Editing.
Funding
None.
Acknowledgments
Thanks to Dr Mina Onsy for his help in designing the illustrative figure.
References
- 1. Ahn D., Shin H., Kim M.et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19). J Microbiol Biotechnol 2020;30: 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dhama K., Khan S., Tiwari R.et al. Coronavirus disease 2019–COVID-19. Clin Microbiol Rev 2020;33: e00028-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lai C.C., Shih T.P., Ko W.C.et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents 2020;55: 105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ge H., Wang X., Yuan X.et al. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis 2020;39: 1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang C., Wang Y., Li X.et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahsan W., Javed S., Al Bratty M.et al. Treatment of SARS-CoV-2: how far have we reached? Drug Discov Ther 2020;14: 67–72. [DOI] [PubMed] [Google Scholar]
- 7. Hassan A., El-Hadidy M., El-Deeck B.S.et al. Couple satisfaction to different therapeutic modalities for organic erectile dysfunction. J Sex Med 2008;5: 2381–2391. [DOI] [PubMed] [Google Scholar]
- 8. Akhvlediani N.D., Matyukhov I.P.. Current role of sildenafil in the management of erectile dysfunction. Urologiia 2018;2: 142–146. [PubMed] [Google Scholar]
- 9. Goldstein I., Burnett A.L., Rosen R.C.et al. The serendipitous story of sildenafil: an unexpected oral therapy for erectile dysfunction. Sex Med Rev 2019;7: 115–128. [DOI] [PubMed] [Google Scholar]
- 10. Goldstein I., Tseng L.J., Creanga D.et al. Efficacy and safety of sildenafil by age in men with erectile dysfunction. J Sex Med 2016;13: 852–985. [DOI] [PubMed] [Google Scholar]
- 11. Yafi F.A., Sharlip I.D., Becher E.F.. Update on the safety of phosphodiesterase type 5 inhibitors for the treatment of erectile dysfunction. Sex Med Rev 2018;6: 242–252. [DOI] [PubMed] [Google Scholar]
- 12. Andersson K.E. PDE5 inhibitors-pharmacology and clinical applications 20 years after sildenafil discovery. Br J Pharmacol 2018;175: 2554–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mostafa T. Oral phosphodiesterase type 5 inhibitors: nonerectogenic beneficial uses. J Sex Med 2008;5: 2502–2518. [DOI] [PubMed] [Google Scholar]
- 14. Mostafa T. Useful implications of low dose long-term use of PDE-5 inhibitors. Sex Med Rev 2016;4: 270–284. [DOI] [PubMed] [Google Scholar]
- 15. Hong J.H., Kwon Y.S., Kim I.Y.. Pharmacodynamics, pharmacokinetics and clinical efficacy of phosphodiesterase-5 inhibitors. Expert Opin Drug Metab Toxicol 2017;13: 183–192. [DOI] [PubMed] [Google Scholar]
- 16. Mostafa T. Non-sexual implications of phosphodiesterase type 5 inhibitors. Sex Med Rev 2017;5: 170–199. [Google Scholar]
- 17. Li Y., Zhou W., Yang L.et al. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol Res 2020;157: 104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xu H., Zhong L., Deng J.et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 2020;12: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou F., Yu T., Du R.et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang H., Penninger J.M., Li Y.et al. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 2020;46: 586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. He F., Deng Y., Li W.. (2020). Coronavirus disease 2019 (COVID-19): what we know. J Med Virol 2020;92: 719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu Z., Shi L., Wang Y.et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8: 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baj J., Karakuła-Juchnowicz H., Teresiński G.et al. COVID-19: specific and non-specific clinical manifestations and symptoms: the current state of knowledge. J Clin Med 2020;9: 1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pan F., Ye T., Sun P.et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology 2020;295: 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ye Q., Wang B., Mao J.. The pathogenesis and treatment of the ’Cytokine Storm’ in COVID-19. J Infect 2020;80: 607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen N., Zhou M., Dong X.et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pedersen S.F., Ho Y.C.. SARS-CoV-2: a storm is raging. J Clin Invest 2020;130: 2202–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mehta P., Mcauley D.F., Brown M.et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395: 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Channappanavar R., Fehr A.R., Vijay R.et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe 2016;19: 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodrigue-Gervais I.G., Labbé K., Dagenais M.et al. Cellular inhibitor of apoptosis protein cIAP2 protects against pulmonary tissue necrosis during influenza virus infection to promotehost survival. Cell Host Microbe 2014;15: 23–35. [DOI] [PubMed] [Google Scholar]
- 31. Yan C., Kim D., Aizawa T.et al. Functional interplay between angiotensin II and nitric oxide cyclic GMP as a key mediator. Arterioscler Thromb Vasc Biol 2003;23: 26–36. [DOI] [PubMed] [Google Scholar]
- 32. Busse L.W., Chow J.H., McCurdy M.T.et al. COVID-19 and the RAAS-a potential role for angiotensin II? Crit Care 2020;24: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Isidori A.M., Giannetta E., Pofi R.et al. Targeting the NO-cGMP-PDE5 pathway in COVID-19 infection. Andrology 2020. 10.1111/andr.12837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chawla L.S., Chen S., Bellomo R.et al. Angiotensin converting enzyme defects in shock: implications for future therapy. Crit Care 2018;22: 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koka V., Huang X.R., Chung A.C.et al. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am J Pathol 2008;172: 1174–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fernandes T., Hashimoto N.Y., Magalhaes F.C.et al. Aerobic exercise training-induced left ventricular hypertrophy involves regulatory MicroRNAs, decreased angiotensin-converting enzyme-angiotensin ii, and synergistic regulation of angiotensin-converting enzyme 2-angiotensin (1-7). Hypertension 2011;58: 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ishiyama Y., Gallagher P.E., Averill D.B.et al. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension 2004;43: 970–976. [DOI] [PubMed] [Google Scholar]
- 38. Qiao Z., Zhang H., Ji H.F.et al. Computational view toward the inhibition of SARS-CoV-2 spike glycoprotein and the 3CL protease. Computation (Basel) 2020;8: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Corbin J.D., Beasley A., Blount M.A.et al. High lung PDE5: a strong basis for treating pulmonary hypertension with PDE5 inhibitors. Biochem Biophys Res Commun 2005;334: 930–938. [DOI] [PubMed] [Google Scholar]
- 40. Galie N., Corris P.A., Frost A.et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol 2013;62: D60–D72. [DOI] [PubMed] [Google Scholar]
- 41. Beltrán-Gámez M.E., Sandoval-Zárate J., Pulido T.. Phosphodiesterase-5 inhibitors for the treatment of pulmonary arterial hypertension. Arch Cardiol Mex 2015;85: 215–224. [DOI] [PubMed] [Google Scholar]
- 42. Karasu-Minareci E., Ozbudak I.H., Ozbilim G.et al. Acute effects of vardenafil on pulmonary artery responsiveness in pulmonary hypertension. Sci World J 2012;2012: 718279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kiss T., Kovacs K., Komocsi A.et al. Novel mechanisms of sildenafil in pulmonary hypertension involving cytokines/chemokines, MAP kinases and Akt. PLoS One 2014;9: e104890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barnes H., Brown Z., Burns A.et al. Phosphodiesterase 5 inhibitors for pulmonary hypertension. Cochrane Database Syst Rev 2019;1: CD012621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kosutova P., Mikolka P., Balentova S.et al. Effects of phosphodiesterase 5 inhibitor sildenafil on the respiratory parameters, inflammation and apoptosis in a saline lavage-induced model of acute lung injury. J Physiol Pharmacol 2018;69: [DOI] [PubMed] [Google Scholar]
- 46. Blanco I., Gimeno E., Munoz P.A.et al. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Respir Crit Care Med 2010;181: 270–278. [DOI] [PubMed] [Google Scholar]
- 47. Vitulo P., Stanziola A., Confalonieri M.et al. Sildenafil in severe pulmonary hypertension associated with chronic obstructive pulmonary disease: a randomized controlled multicenter clinical trial. J Heart Lung Transplant 2017;36: 166–174. [DOI] [PubMed] [Google Scholar]
- 48. Faoro V., Lamotte M., Deboeck G.et al. Effects of sildenafil on exercise capacity in hypoxic normal subjects. High Alt Med Biol 2007;8: 155–163. [DOI] [PubMed] [Google Scholar]
- 49. Gibbs J.S. Biventricular function at high altitude: implications for regulation of stroke volume in chronic hypoxia. Adv Exp Med Biol 2007;618: 13–24. [DOI] [PubMed] [Google Scholar]
- 50. Watanabe N., Taniguchi H., Kondoh Y.et al. Clinical efficacy of sildenafil in interstitial pneumonia with pulmonary hypertension. Nihon Kokyuki Gakkai Zasshi 2011;49: 151–155. [PubMed] [Google Scholar]
- 51. Gammella E., Leuenberger C., Gassmann M.et al. Evidence of synergistic/additive effects of sildenafil and erythropoietin in enhancing survival and migration of hypoxic endothelial cells. Am J Physiol Lung Cell Mol Physiol 2013;304: L230–L239. [DOI] [PubMed] [Google Scholar]
- 52. Czövek D., Peták F., Donati Y.et al. Prevention of hyperoxiainduced bronchial hyperreactivity by sildenafil and vasoactive intestinal peptide: impact of preserved lung function and structure. Respir Res 2014;15: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bucci M., Papapetropoulos A., Vellecco V.et al. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol 2010;30: 1998–2004. [DOI] [PubMed] [Google Scholar]
- 54. Zhang H.X., Liu S.J., Tang X.L.et al. H2S attenuates LPS-induced acute lung injury by reducing oxidative/nitrative stress and inflammation. Cell Physiol Biochem 2016;40: 1603–1612. [DOI] [PubMed] [Google Scholar]
- 55. Yao Z., Wang C.. A novel mechanism of sildenafil improving the excessive proliferation and H2S production in pulmonary arterial smooth muscle cells. J Cardiovasc Pharmacol 2019;74: 355–363. [DOI] [PubMed] [Google Scholar]
- 56. de Visser Y.P., Walther F.J., Laghmani E.et al. Sildenafil attenuates pulmonary inflammation and fibrin deposition, mortality and right ventricular hypertrophy in neonatal hyperoxic lung injury. Respir Res 2009;10: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rapôso C., Luna R.L., Nunes A.K.et al. Role of iNOS-NO-cGMP signaling in modulation of inflammatory and myelination processes. Brain Res Bull 2014;104: 60–73. [DOI] [PubMed] [Google Scholar]
- 58. Dalamaga M., Karampela I., Mantzorosc C.S.. Commentary: phosphodiesterase 4 inhibitors as potential adjunct treatment targeting the cytokine storm in COVID-19. Metabolism 2020;109: 154282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Seirafianpour F., Mozafarpoor S., Fattahi N.et al. Treatment of COVID-19 with pentoxifylline: could it be a potential adjuvant therapy? Dermatol Ther 2020. e13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cadirci E., Halici Z., Odabasoglu F.et al. Sildenafil treatment attenuates lung and kidney injury due to overproduction of oxidant activity in a rat model of sepsis: a biochemical and histopathological study. Clin Exp Immunol 2011;166: 374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Deng M., Loughran P.A., Zhang L.et al. Shedding of the tumor necrosis factor (TNF) receptor from the surface of hepatocytes during sepsis limits inflammation through cGMP signaling. Sci Signal 2015;8: ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vlachopoulos C., Ioakeimidis N., Rokkas K.et al. Acute effect of sildenafil on inflammatory markers/mediators in patients with vasculogenic erectile dysfunction. Int J Cardiol 2015;182: 98–101. [DOI] [PubMed] [Google Scholar]
- 63. Wolter S., Dittmar F., Seifert R.. cCMP and cUMP in apoptosis: concepts and methods. Handb Exp Pharmacol 2017;238: 25–47. [DOI] [PubMed] [Google Scholar]
- 64. Choi D.E., Jeong J.Y., Lim B.J.et al. Pretreatment of sildenafil attenuates ischemia-reperfusion renal injury in rats. Am J Physiol Renal Physiol 2009;297: F362–F370. [DOI] [PubMed] [Google Scholar]
- 65. Puzzo D., Loreto C., Giunta S.et al. Effect of phosphodiesterase-5 inhibition on apoptosis and beta amyloid load in aged mice. Neurobiol Aging 2014;35: 520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Duarte-Silva E., Peixoto C.A.. Molecular mechanisms of phosphodiesterase-5 inhibitors on neuronal apoptosis. DNA Cell Biol 2018;37: 861–865. [DOI] [PubMed] [Google Scholar]
- 67. Gong W., Duan Q., Cai Z.et al. Chronic inhibition of cGMP-specific phosphodiesterase 5 suppresses endoplasmic reticulum stress in heart failure. Br J Pharmacol 2013;170: 1396–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liu X., Peyton K.J., Wang X.et al. Sildenafil stimulates the expression of gaseous monoxide-generating enzymes in vascular smooth muscle cells via distinct signaling pathways. Biochem Pharmacol 2012;84: 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Abdel Aziz M.T., El-Asmer M.F., Mostafa T.et al. Heme oxygenase vs. nitric oxide synthase in signaling mediating sildenafil citrate action. J Sex Med 2007;4: 4 Pt 2: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 70. Aziz M.T., Al-Asmar M.F., Mostafa T.et al. Assessment of heme oxygenase-1 (HO-1) activity in the cavernous tissues of sildenafil citrate-treated rats. Asian J Androl 2007;9: 377–381. [DOI] [PubMed] [Google Scholar]
- 71. Abdel Aziz M.T., Mostafa T., Atta H.et al. Putative role of carbon monoxide signaling pathway in penile erectile function. J Sex Med 2009;6: 49–60. [DOI] [PubMed] [Google Scholar]
- 72. Li F.J., Duggal R.N., Oliva O.M.et al. Heme oxygenase-1 protects corexit 9500A-induced respiratory epithelial injury across species. PLoS One 2015;10: e0122275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Espinoza J.A., León M.A., Céspedes P.F.et al. Heme oxygenase-1 modulates human respiratory syncytial virus replication and lung pathogenesis during infection. J Immunol 2017;199: 212–223. [DOI] [PubMed] [Google Scholar]
- 74. Kalamouni C., Frumence E., Bos S.et al. Subversion of the heme oxygenase-1 antiviral activity by Zika virus. Viruses 2018;11: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Perk H., Armagan A., Naziroğlu M.et al. Sildenafil citrate as a phosphodiesterase inhibitor has an antioxidant effect in the blood of men. J Clin Pharm Ther 2008;33: 635–640. [DOI] [PubMed] [Google Scholar]
- 76. Taibi G., Carruba G., Miceli V.et al. Sildenafil protects epithelial cell through the inhibition of xanthine oxidase and the impairment of ROS production. Free Radic Res 2010;44: 232–239. [DOI] [PubMed] [Google Scholar]
- 77. Yang H.M., Jin S., Jang H.et al. Sildenafil reduces neointimal hyperplasia after angioplasty and inhibits platelet aggregation via activation of cGMP-dependent protein kinase. Sci Rep 2019;9: 7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rondina M.T., Weyrich A.S.. Targeting phosphodiesterases in anti-platelet therapy. Handb Exp Pharmacol 2012;210: 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gudmundsdóttir I.J., McRobbie S.J., Robinson S.D.et al. Sildenafil potentiates nitric oxide mediated inhibition of human platelet aggregation. Biochem Biophys Res Commun 2005;337: 382–385. [DOI] [PubMed] [Google Scholar]
- 80. Rogosnitzky M., Berkowitz E., Jadad A.R.. Delivering benefits at speed through real-world repurposing of off-patent drugs: the COVID-19 pandemic as a case in point. JMIR Public Health Surveill 2020;6: e19199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Luo P., Liu Y., Qiu L.et al. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol 2020;92: 814–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Patrì A., Fabbrocini G.. Hydroxychloroquine and ivermectin: a synergistic combination for COVID-19 chemoprophylaxis and treatment? J Am Acad Dermatol 2020;82: e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hung I.F., Lung K.C., Tso E.Y.et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 2020;395: 1695–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Santos J., Brierley S., Gandhi M.J.et al. Repurposing therapeutics for potential treatment of SARS-CoV-2: a review. Viruses 2020;12: E705. [DOI] [PMC free article] [PubMed] [Google Scholar]