Abstract
Background
Coronavirus disease-2019 (COVID-19) is thought to predispose patients to thrombotic disease. To date there are few reports of ST-segment elevation myocardial infarction (STEMI) caused by type 1 myocardial infarction in patients with COVID-19.
Objectives
The aim of this study was to describe the demographic, angiographic, and procedural characteristics alongside clinical outcomes of consecutive cases of COVID-19–positive patients with STEMI compared with COVID-19–negative patients.
Methods
This was a single-center, observational study of 115 consecutive patients admitted with confirmed STEMI treated with primary percutaneous coronary intervention at Barts Heart Centre between March 1, 2020, and May 20, 2020.
Results
Patients with STEMI presenting with concurrent COVID-19 infection had higher levels of troponin T and lower lymphocyte count, but elevated D-dimer and C-reactive protein. There were significantly higher rates of multivessel thrombosis, stent thrombosis, higher modified thrombus grade post first device with consequently higher use of glycoprotein IIb/IIIa inhibitors and thrombus aspiration. Myocardial blush grade and left ventricular function were significantly lower in patients with COVID-19 with STEMI. Higher doses of heparin to achieve therapeutic activated clotting times were also noted. Importantly, patients with STEMI presenting with COVID-19 infection had a longer in-patient admission and higher rates of intensive care admission.
Conclusions
In patients presenting with STEMI and concurrent COVID-19 infection, there is a strong signal toward higher thrombus burden and poorer outcomes. This supports the need for establishing COVID-19 status in all STEMI cases. Further work is required to understand the mechanism of increased thrombosis and the benefit of aggressive antithrombotic therapy in selected cases.
Key Words: COVID-19, primary percutaneous coronary intervention, ST-segment elevation myocardial infarction, thrombosis
Abbreviations and Acronyms: ACT, activated clotting time; COVID-19, Coronavirus disease-2019; ECG, electrocardiogram; GP, glycoprotein; PCI, percutaneous coronary intervention; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis In Myocardial Infarction
Central Illustration
Coronavirus disease-2019 (COVID-19) caused by the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) has been shown to result in coagulation abnormalities and predisposes patients to thrombotic disease, both in the venous and arterial circulations (1). This is believed to be secondary to inflammation, platelet activation, endothelial dysfunction, and stasis (2). Despite this exaggerated risk, reduced presentations with ST-segment elevation myocardial infarction (STEMI) and other thrombotic disorders such as cerebrovascular accident have been seen during the pandemic, mainly as a result of social isolation and behavioral changes. Furthermore, emergency cardiac catheterization reveals a variety of findings in patients with COVID-19 with ST-segment elevation, including classic type 1 myocardial infarction (obstructive coronary artery disease), angiographically normal epicardial coronary arteries, and/or left ventricular dysfunction due to myocarditis or stress-induced cardiomyopathy (3, 4, 5). Some institutions and opinion leaders have suggested thrombolytic therapy as the preferred initial mode of reperfusion to protect health care providers (6), although clinical bodies have advocated continuation of primary percutaneous coronary intervention (PCI) as the default strategy (7,8). There are limited reports of treating COVID-19–positive patients presenting with type I myocardial infarction leading to STEMI who receive primary PCI, highlighted by recent studies finding only 10 of 18 patients with STEMI on electrocardiogram having obstructive disease (4).
Understanding the natural history and treatment responses of patients with COVID-19 presenting with STEMI is essential to inform patient management decisions and protect health care workers. As the largest coronary intervention center in the United Kingdom, Barts Heart Centre is well placed to provide contemporary analysis of STEMI cases during the COVID-19 era. We describe the demographic, angiographic, and procedural characteristics as well as clinical outcomes in consecutive cases of COVID-19–positive patients with STEMI comparing outcomes with COVID-19–negative patients.
Methods
Study design and patient population
This was a single-center, observational study of 115 consecutive patients admitted with confirmed STEMI at Barts Heart Centre between March 1, 2020, and May 20, 2020. This study was designed to assess the incidence of COVID-19 among patients admitted with STEMI and to compare their baseline characteristics, and angiographic, procedural, and clinical outcomes with patients with STEMI who were COVID-19-negative. Patients were included if they were admitted to Barts Heart Centre via the London Ambulance Service either directly from home or via partner district hospitals with cardiac chest pain and STEMI (ST-segment elevation in 2 or more contiguous leads ≥0.2 mV) on their electrocardiogram (ECG) or patients admitted with an out-of-hospital cardiac arrest and ECG meeting diagnostic criteria for STEMI after return of spontaneous circulation. Angiographic confirmation of occlusive coronary disease was performed in all cases. Patients with out-of-hospital cardiac arrest who did not achieve return of spontaneous circulation, and those with non-STEMI were excluded from the study.
Interventional procedures
All health care professionals involved in each of the procedures wore full personal protective equipment; this was hospital policy for all STEMI cases during the study period. The interventional strategy was at the discretion of the operator, including the use of direct stenting, pre-/post-dilatation, aspiration thrombectomy, and treatment of bystander noninfarct-related artery stenoses. All patients received a loading dose of aspirin 300 mg and either clopidogrel 600 mg or ticagrelor 180 mg before the procedure. All patients then received 75 mg aspirin per day plus either 75 mg clopidogrel per day or 90 mg ticagrelor twice-daily maintenance therapy. During primary PCI, unfractionated heparin was administered in a loading dose of 70 to 100 U/kg with the activated clotting time (ACT) maintained >250 s. ACTs were recorded at 10- to 15-min intervals after the initial dose of heparin. Glycoprotein (GP) IIb/IIIa inhibitors were used at the operator’s discretion and according to local guidelines.
Investigations
All patients with STEMI had baseline serological samples before cardiac catheterization for full blood count, renal and liver function tests, C-reactive protein, D-dimer, fibrinogen, clotting, ferritin, lactate dehydrogenase, ferritin, creatine kinase, and high sensitivity (hs)-troponin T. Post-catheterization, all patients underwent routine nasal/pharyngeal swab for the SARS-CoV-2 virus using real-time reverse transcriptase--polymerase chain reaction (RT-PCR) irrespective of symptoms.
Patients with COVID-19 had a confirmed diagnosis based on the identification of SARS-CoV-2 on nasal/pharyngeal swab or, preceding COVID-19 symptoms alongside diagnostic radiological chest imaging. All of these patients were managed as COVID-19 positive as per center policy.
Data collection
The following data fields were collected as part of our center’s routine practice for the British Cardiovascular Intervention Society audit submissions, including patient age, sex, ethnicity, height, weight, cardiovascular risk factors, time of symptom onset, and time of arrival at primary PCI hospital. In addition, the following procedure-related data were collected prospectively: target vessel; number of diseased vessels; use of diagnostic devices such as intravascular ultrasound, optical coherence tomography, or pressure wire; use of aspiration thrombectomy; post-dilatation; and use of GP IIb/IIIa inhibitor. Data regarding intraprocedural anticoagulant use and ACTs were collected from patient records with data collectors blinded to patients’ COVID-19 status.
Endpoints
The primary endpoint was all-cause in-hospital mortality. Secondary endpoints included thrombus burden, Thrombolysis In Myocardial Infarction (TIMI) flow, myocardial blush grade, length of hospitalization, and the need for intensive care unit admission. A panel of 3 interventional cardiologists, blinded to patient COVID-19 status, retrospectively reviewed cine-angiographic images of all patients and scored pre- and post-PCI TIMI flow in the infarct-related artery, thrombus burden pre- and post-PCI (modified thrombus grade for grade 5 thrombus post initial balloon inflation) (9), and myocardial blush grade (10).
All patient data were anonymized before analysis. The local ethics committee advised that formal ethical approval was not required.
Statistical analysis
Descriptive statistical analyses were performed using SPSS Statistics version 25.0 (IBM, Armonk, New York). A 2-sided p value <0.05 defined statistical significance. Variables are expressed as counts (percentages), mean ± SD, and median (interquartile range) as appropriate. Chi-square analysis or Fisher exact test was used to compare categorical data between groups. The independent samples Student’s t-test or analysis of variance test was used to compare normally distributed continuous data between groups, and the Mann-Whitney U test was used to compare the distribution of skewed continuous data between groups. Correlation was performed using Pearson’s correlation analysis and Spearman’s correlation analysis in the case of skewed variables.
Results
The study population consisted of 115 consecutive patients with confirmed STEMI who were admitted during a 12-week period. The median age was 62 years, 78% were male, and 48.7% were from Black, Asian, or minority ethnic groups. Of the 115 patients, 39 (33.9%) were diagnosed with concurrent COVID-19 infection, and 76 (66.1%) patients showed no clinical evidence of COVID-19 infection (non-COVID-19 group).
Patient characteristics
There were no significant differences in age, sex, ethnic background, or body mass index between the 2 groups (Table 1 ). Patients presenting with concurrent COVID-19 infection were more likely than non-COVID-19 patients to be diabetic (46% vs. 26%, p = 0.038), hypertensive (72% vs. 42%, p = 0.003), hyperlipidemic (62% vs. 37%, p = 0.038) and were more likely to have a history of previous PCI (23% vs. 7%, p = 0.016). Time from symptoms to reperfusion, ECG presentations, rates of cardiogenic shock, and requirement for pre-hospital intubation were similar in both groups. However, there was a higher incidence of cardiac arrest in patients who were COVID-19 positive compared with the non-COVID-19 group (28% vs. 9%, p = 0.0013).
Table 1.
Baseline Patient Characteristics
| Non-COVID-19 (n = 76) | COVID-19 (n = 39) | p Value | |
|---|---|---|---|
| Age, yrs | 61.7 ± 12.6 | 61.7 ± 11.0 | 0.633 |
| Male | 57 (75.0) | 33 (84.6) | 0.340 |
| Black, Asian, minority ethnicity | 34 (44.7) | 22 (56.4) | 0.246 |
| Body mass index, kg/m2 | 26.87 (22.6–29.4) | 26.7 (24.8–30.7) | 0.363 |
| Past medical history | |||
| Hypertension | 32 (42.1) | 28 (71.8) | 0.003∗ |
| Hypercholesterolemia | 28 (36.8) | 24 (61.6) | 0.017† |
| Diabetes mellitus | 20 (26.3) | 18 (46.2) | 0.038† |
| Smoking history | 35 (46.1) | 24 (61.6) | 0.167 |
| Previous myocardial infarction | 3 (3.9) | 6 (15.4) | 0.060 |
| Previous PCI | 5 (6.6) | 9 (23.1) | 0.016† |
| STEMI presentation | |||
| Time chest pain to reperfusion, h | 4 (2–7) | 4 (2–6) | 0.845 |
| ECG presentation | 0.707 | ||
| Anterior/LBBB | 43 (56.7) | 24 (61.6) | - |
| Inferior | 27 (35.5) | 11 (28.2) | - |
| Lateral | 3 (3.9) | 3 (7.7) | - |
| Posterior | 3 (3.9) | 1 (2.6) | |
| Cardiac arrest | 7 (9.2) | 11 (28.2) | 0.0133† |
| Cardiogenic shock | 8 (10.5) | 6 (15.4) | 0.549 |
| Intubated | 5 (6.6) | 5 (12.8) | 0.303 |
| Laboratory values | |||
| Troponin T, ng/l | 369 (78.5–1,109) | 1,221 (179–4,143) | 0.0028∗ |
| White cell count, 109/l | 12.2 (9.7–14.9) | 12.9 (10.6–16.4) | 0.596 |
| Lymphocyte count, 109/l | 1.7 (1.3–2.2) | 1.3 (0.7–2.0) | 0.0002‡ |
| Lactate dehydrogenase, unit/l | 374.5 (268–707) | 553 (340–935) | 0.1489 |
| D-dimer, mg/l | 0.52 (0.40–1.0) | 1.86 (0.98–6.6) | 0.0012∗ |
| Fibrinogen, g/l | 3.88 (3.2–4.6) | 4.26 (3.2–7.3) | 0.0842 |
| Ferritin, μg/l | 194 (121–308) | 323 (174–859) | 0.0976 |
| Creatinine, μmol/l | 81 (72–98) | 80 (71–118) | 0.827 |
| Creatine kinase, μ/l | 641.5 (163.5–1,376) | 493 (165–1,613) | 0.822 |
| C-reactive protein, mg/l | 12 (5–50) | 50 (8–185) | 0.010† |
Values are mean ± SD, n (%), or median (interquartile range).
COVID = coronavirus disease; ECG = electrocardiogram; LBBB = left bundle branch block; PCI = percutaneous coronary intervention; STEMI = ST-segment elevation myocardial infarction.
p < 0.01,
p < 0.05,
p<0.001.
Patients with concurrent COVID-19 infection had higher levels of hs-troponin (1,221 ng/l vs. 369 ng/l, p = 0.0028), D-dimer (1.86 mg/l vs. 0.52 mg/l, p = 0.0012), and C-reactive protein (12 mg/l vs. 50 mg/l, p = 0.01) but lower lymphocyte counts (1.3 109/l vs. 1.7 109/l, p = 0.0002) than non-COVID-19 patients. Similar levels of lactate dehydrogenase, fibrinogen, and ferritin were seen in the 2 groups, although trends to higher levels in the COVID-19 group were seen in all 3 measurements (p = 0.14, p = 0.08, and p = 0.09, respectively).
Procedural characteristics
All patients underwent a primary PCI procedure in both groups (Table 2 ). Median door-to-balloon times were within 60 min and similar for both groups. There was evidence of higher thrombogenicity in the COVID-19 group with significantly higher rates of multivessel thrombosis (p = 0.0003) and stent thrombosis (p = 0.04). Despite similar levels of baseline TIMI flow grade 0/1 and thrombus grade 4/5, the modified thrombus grade post first device for cases with thrombus grade 5 was significantly higher in the COVID-19 group (modified thrombus grade 4/5 in 75% vs. 31%, p = 0.0006). In keeping with this, there was significantly greater use of GP IIb/IIIa inhibitors (p < 0.0001) and aspiration thrombectomy (p = 0.0021) in patients with COVID-19. Although TIMI flow grade 3 was achieved at similar high levels in both groups, myocardial blush grade was significantly lower in the COVID-19 group (myocardial blush grade of 2 to 3 in 54% vs. 93%, p < 0.0001), median peak plasma hs-troponin concentration was greater and left ventricular ejection fraction was significantly lower in the COVID-19 group compared with the non-COVID-19 group (42.5% vs. 45%, p = 0.019).
Table 2.
Procedural Characteristics
| Non-COVID-19 (n = 76) | COVID-19 (n = 39) | p Value | |
|---|---|---|---|
| Coronary intervention | 74 (97.3) | 38 (97.4) | 1.000 |
| Door-to-balloon time, min | 50 (34.8–57.5) | 52 (39–70) | 0.248 |
| Duration of case, min | 50 (39–70) | 55 (44–90) | 0.054 |
| Culprit vessel | 0.621 | ||
| LMS | 1 (1.4) | 2 (5.1) | - |
| LAD | 41 (55.4) | 22 (56.4) | - |
| Cx | 1 (1.4) | 4 (10.3) | - |
| RCA | 24 (32.4) | 10 (25.6) | - |
| Multivessel thrombosis | 0 (0.0) | 7 (17.9) | 0.0003‡ |
| Stent thrombosis | 1 (1.2) | 4 (10.3) | 0.0445† |
| Baseline TIMI flow grade 0–1 | 60 (80.0) | 32 (82.1) | 0.798 |
| Baseline thrombus grade (4–5) | 59 (77.3) | 33 (84.6) | 0.440 |
| Modified thrombus grade post first device | 51 | 28 | 0.005∗ |
| 0 | 2 (3.9) | 0 (0.0) | |
| 1 | 9 (17.6) | 0 (0.0) | |
| 2 | 11 (21.6) | 1 (3.6) | |
| 3 | 13 (25.5) | 7 (25.0) | |
| 4 | 12 (23.5) | 12 (42.9) | |
| 5 | 4 (7.8) | 8 (28.6) | |
| Modified thrombus grade (4–5) | 16 (31.4) | 21 (75) | 0.0006‡ |
| GP IIb/IIIa inhibitor use | 7 (9.2) | 23 (59.0) | <0.0001‡ |
| Aspiration thrombectomy use | 1 (1.3) | 7 (17.9) | 0.0021∗ |
| Total heparin dose, U | 10,066 ± 3,176 | 11,125 ± 3,875 | 0.151 |
| Average ACT | 287.8 ± 66.9 | 270.6 ± 69.5 | 0.261 |
| Total heparin dose per weight, U/kg | 134.1 ± 35.9 | 146.2 ± 43.5 | 0.151 |
| Total heparin dose per kg per min of case, units/kg/min | 2.6 ± 0.9 | 2.3 ± 0.8 | 0.144 |
| Multivessel PCI | 5 (6.8) | 8 (20.5) | 0.033† |
| Procedural success | |||
| Post-PCI TIMI flow grade 3 | 70 (93.3) | 35 (89.7) | 0.687 |
| Myocardial blush grade (end) 2–3 | 70 (93.3) | 21 (53.8) | <0.0001‡ |
| LV ejection fraction, % | 45 (40–55) | 42.5 (30–50) | 0.019† |
Values are n (%), median (interquartile range), mean ± SD, or n.
ACT = active clotting time; Cx = circumflex; GP = glycoprotein; LAD = left anterior descending; LMS = left main stem; LV = left ventricular; RCA = right coronary artery; TIMI = Thrombolysis In Myocardial Infarction. Other abbreviations as in Table 1.
p < 0.01,
p < 0.05,
p<0.001
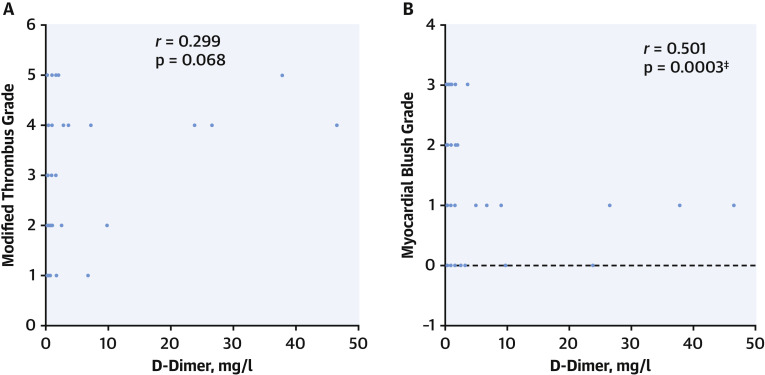
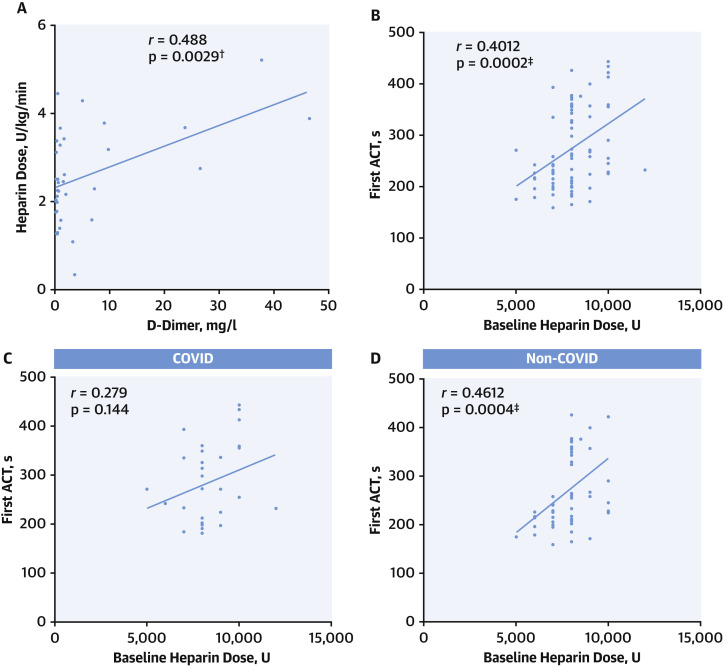
Levels of blood D-dimer correlated with modified thrombus grade (r = 0.299, p = 0.068), myocardial blush grade (r = 0.501, p = 0.0003) (Figure 1 ) and level of heparinization required to maintain ACT >250 s during primary PCI (r = 0.488, p = 0.0029) (Figure 2 ).
Figure 1.
Increased Arterial Thrombogenicity Associated With D-Dimer Levels
(A) Correlation between D-dimer and thrombus grade in all patients (p = 0.068). (B) Correlation between D-dimer and myocardial blush grade for all patients (p = 0.0003). ∗p < 0.01, †p < 0.05, ‡p<0.001.
Figure 2.
Requirement for Heparin During Primary PCI
(A) Correlation between D-dimer and total heparin dose U/kg/min required to maintain activated clotting time (ACT) >250 s during primary percutaneous coronary intervention (PCI) procedure for all patients (p = 0.0029). (B) Correlation between baseline heparin dose and first ACT measured after a 10- to 15-min interval in all patients (p = 0.0002). (C) Correlation between baseline heparin dose and first ACT measured after a 10- to 15-min interval in coronavirus disease-2019 (COVID-19) group (p = 0.144). (D) Correlation between baseline heparin dose and first ACT measured after a 10- to 15-min interval in non-COVID-19 group (p = 0.0004). ∗p < 0.01, †p < 0.05, ‡p<0.001.
There were no significant differences between groups in the total dose of heparin administered (COVID-19 11,125 U vs. non-COVID-19 10,066 U, p = 0.15), and similar average ACTs were achieved during the procedures (p = 0.261). Although first measured ACTs correlated with administered baseline heparin dose in the whole cohort (r = 0.401, p = 0.0002), and in the non-COVID group (r = 0.461, p = 0.0004), this correlation was not seen in the COVID group (r = 0.279, p = 0.144) suggesting that more heparin was required in the COVID group to achieve similar ACTs. A trend to higher heparin dose per kg (p = 0.15) was observed in the COVID group; however, this was not statistically significant and no difference was seen when adjusting for procedural time (p = 0.14).
In-hospital outcomes
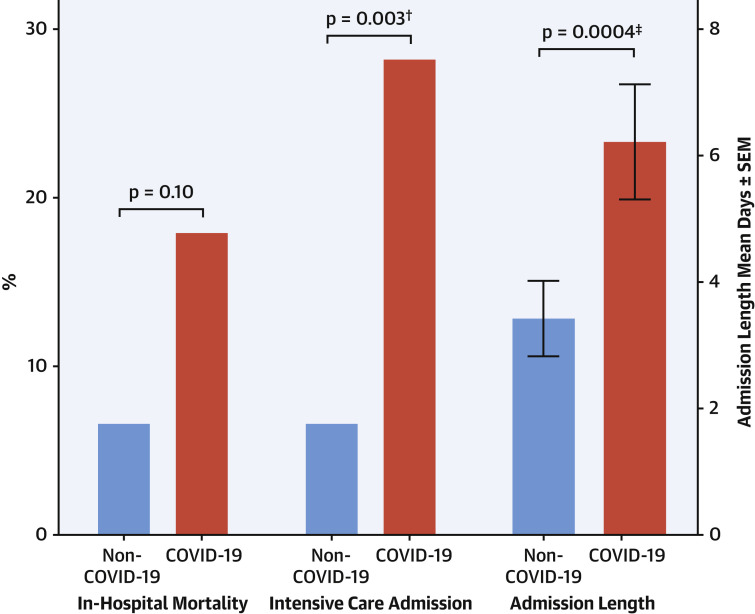
The COVID-19 group had significantly longer in-patient stays (p = 0.0004) and were more likely to require intensive care (p = 0.003), reflecting a greater level of morbidity in this STEMI cohort. However, despite numerically higher rates of in-hospital mortality among the COVID-19 STEMI patients, this did not reach significance (17.9% vs. 6.5%, p = 0.10) (Figure 3 ).
Figure 3.
In-Hospital Outcomes for Non-COVID-19 and COVID-19 Groups
In-hospital outcomes including intensive care admission, in-hospital mortality, and admission length. COVID-19 = coronavirus disease-2019. ∗p < 0.01, †p < 0.05, ‡p<0.001.
Discussion
This observational study represents the first comparative data to describe the impact of COVID-19 infection on patients presenting with STEMI to a primary PCI center (Central Illustration ). This analysis demonstrates a clear signal of increased thrombus burden in STEMI patients who are infected with COVID-19 compared with STEMI patients who are not infected. This is evidenced by a higher incidence of multiple thrombotic culprit lesions as well as stent thrombosis, higher thrombus grade, lower resultant myocardial blush grade, and associated increased use of GP IIb/IIIa inhibition and thrombus aspiration. Consistent with this, COVID-19 infection was associated with greater myocardial damage with lower left ventricular systolic function and increased troponin levels, despite similar median ischemia times in the 2 groups. Higher rates of pre-hospital cardiac arrest, intensive care admission, longer in-patient stays, and a trend toward increased in-hospital mortality were also seen in this patient group.
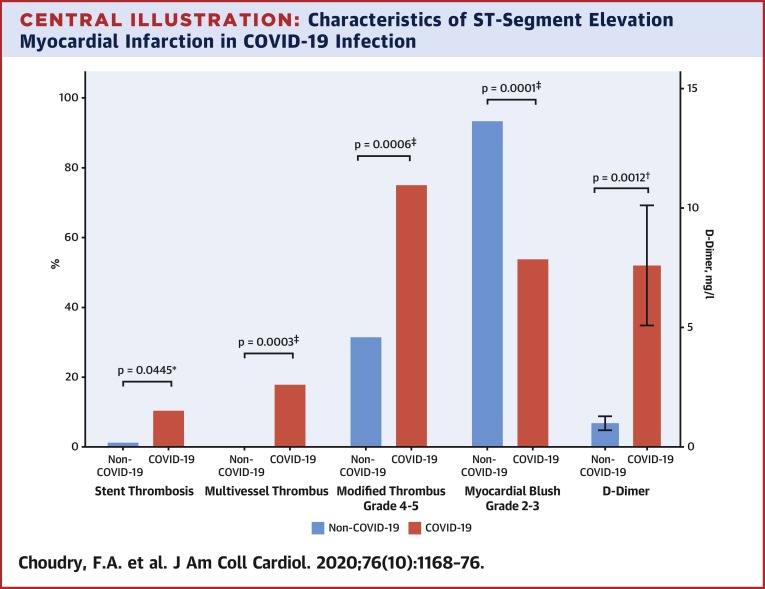
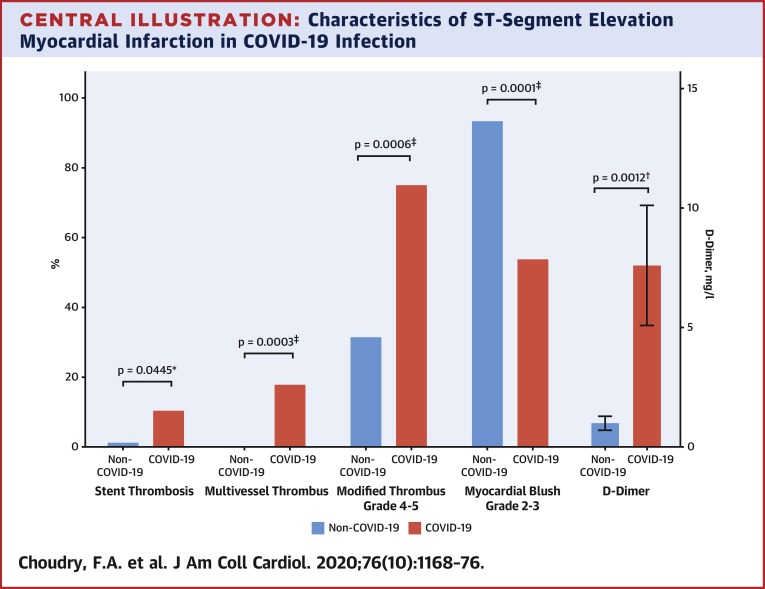
Central Illustration.
Characteristics of ST-Segment Elevation Myocardial Infarction in COVID-19 Infection
ST-segment elevation myocardial infarction (STEMI) in coronavirus disease-2019 (COVID-19) infection is associated with significantly higher rates of stent thrombosis (p = 0.0445), multivessel thrombus (p = 0.0003), modified thrombus grade 4 to 5 (p = 0.0006), lower rates of myocardial blush grade 2 to 3 (p = 0.0001), and raised D-dimer levels (p = 0.012). ∗p < 0.01, †p < 0.05, ‡p < 0.001.
Patients with STEMI who were COVID positive more often had diabetes, hypertension, and blood abnormalities, reflecting a systemic inflammatory response (lymphopenia, elevated D-dimers, and C-reactive protein levels) compared with COVID-19-negative patients. These features are those previously reported to be associated with more aggressive infection and poorer outcomes in COVID cohorts without STEMI (11, 12, 13, 14). Interestingly, levels of D-dimer were found to correlate with thrombus grade, myocardial blush grade, and levels of heparin requirement during the primary PCI procedure, with a suggestion of higher heparin doses required to achieve therapeutic ACTs in this cohort. Taken together, these data support the role of early assessment of COVID-19 status in all STEMI patients, as it may have major implications in patient management.
COVID-19 infection is associated with a pro-thrombotic state. The occurrence of venous thrombo-embolic complications, both clinically apparent and subclinical, appears to be an important manifestation of the disease and one that is related to disease severity and outcome (1,15, 16, 17). Increased thrombogenicity in acute ischemic stroke also has been described (18). Moreover, emerging data from large COVID-19 cohorts without STEMI (19) suggest that the use of anticoagulation confers mortality benefit in this patient group. However, to date there have been no reports of increased coronary artery thrombus burden, rather the number of patients presenting with STEMI has reduced with an increased incidence of nonobstructed coronary arteries in the published small case series of patients with STEMI (3,4). Here we describe features apparent on angiographic assessment such as multivessel thrombosis, stent thrombosis, and high thrombus burden that should raise the suspicion of COVID-19 infection. STEMI was the first manifestation of the disease in this cohort, and our data suggest that presentation of STEMI might itself be considered a thrombotic complication of COVID-19 infection. Of note, the median duration of symptoms to reperfusion was 4 h in both COVID-19-positive and COVID-19-negative patients. Therefore, delayed presentation in COVID-positive patients could not account for the observed differences between groups.
Mechanisms that trigger presentation with STEMI and its associated higher arterial thrombus burden in patients with COVID-19 are as yet unknown. Relative to venous thromboembolism, arterial thrombus formation is more likely to be due to platelet activation and endothelial dysfunction. Pre-COVID-19 data regarding the influenza virus suggest that patients with acute respiratory infections are at significantly elevated risk for developing atherosclerotic plaque rupture leading to myocardial infarction as a result of the profound inflammatory response and hemodynamic changes (20). SARS-CoV-2 infection causes a systemic inflammatory response that leads to endothelial and hemostatic activation, including activation of platelets and the coagulation cascade (21). Patients in the coronary risk groups of diabetes and hypertension have shown increased incidence of COVID-19 and subsequent case fatality (11). In keeping with this, the data presented here show significantly higher rates of diabetes, hyperlipidemia, and hypertension in the COVID-19 group, suggesting that these conditions may also confer an increased risk of STEMI in COVID-19-infected patients. Mechanisms for this might include increased endothelial dysfunction or their effects on the immune system (21, 22, 23, 24, 25).
Although our data point toward a higher morbidity and mortality in the COVID-19 group compared with the non-COVID-19 group, the rate of in-hospital mortality was lower than that previously published in a smaller cohort of 18 COVID-19-positive patients with STEMI (4) (18% compared with 72% in-hospital mortality). This is likely to be related to the difference in the cohorts studied. Although our study focused on patients presenting through the primary PCI pathway, with STEMI as their first presentation of COVID-19, all receiving PCI, the previous case series is a more heterogeneous cohort, of which only 6 individuals presented through this pathway and received emergent coronary assessment, with the remaining patients developing ECG changes while admitted with advanced COVID-19 infection. Therefore, it is likely that the severity of COVID-19 infection may have been less in our study cohort in which 12.8% of patients were ventilated compared with 70% in other series.
The COVID-19 era has presented numerous challenges for emergency intervention services, which have required restructuring to maintain health care delivery (26). COVID-19 status is invariably not known at the time of presentation. Most primary PCI units manage STEMI patients as if they are COVID-19 positive because of the risk of peri-procedure cardiac arrest and the potential for aerosol generation. Changes have been required in the management of access to the unit, the designation of COVID-19 catheter laboratories, the use of personal protective equipment, deep laboratory cleaning after cases, ward reorganization, and patient COVID-19 testing protocols. Despite these challenges, primary PCI was delivered within existing guidelines (median door-to-balloon times 50 min).
The strength of this study is that it presents real-world consecutive data from all patients with STEMI admitted to a single center during the COVID-19 outbreak in the United Kingdom, both with and without the infection. The compared COVID-19 and non-COVID-19 group faced the same health care restrictions and were managed according to the same, modified, COVID-19 primary PCI pathway and protocol. Moreover, our center had an early adoption of COVID-19 testing for all patients with STEMI. All patients with STEMI received a nasal/pharyngeal swab as well as chest imaging and laboratory testing for markers of severe COVID-19 infection to assist in diagnosis in the event of a negative nasal/pharyngeal swab.
Study limitations
Despite this being the largest series to date, it is a relatively small retrospective observational study in a single center and therefore has all the limitations of this type of analysis including bias and the potential for confounding. Furthermore, as has been universally accepted, the sensitivity of diagnostic testing for SARS-CoV-2 is modest at approximately 60% to 70% with nasal/pharyngeal swab (27).
Conclusions
We present the largest comparative dataset of COVID-19 versus non-COVID-19 patients presenting with STEMI. Concurrent infection with COVID-19 presents new procedural challenges and is associated with poorer outcomes. The strong signal toward significantly higher thrombus burden is a novel finding that raises the question of more aggressive antithrombotic therapy in selected COVID-19 STEMI cases and provides a rationale for establishing COVID-19 status in all STEMI cases. Further work is required to unravel the underlying mechanism of coronary thrombosis in patients with COVID-19 infection.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: Patients presenting with STEMI who test positive for COVID-19 have a higher thrombus burden than those without COVID-19.
TRANSLATIONAL OUTLOOK: Further work is necessary to understand the mechanisms responsible for increased thrombus burden in patients with concurrent STEMI and COVID-19 infection and establish optimum antithrombotic management.
Acknowledgment:
The authors thank all the 3rd floor and cath lab staff of Barts Heart Centre for their hard work and professionalism during the COVID-19 outbreak, and the NHS patients who have been very supportive throughout this difficult time.
Footnotes
Dr. Jones is funded from The Barts Charity. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
References
- 1.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stefanini G.G., Montorfano M., Trabattoni D. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation. 2020;141:2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bangalore S., Sharma A., Slotwiner A. ST-segment elevation in patients with Covid-19 — a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1096. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jing Z.C., Zhu H.D., Yan X.W., Chai W.Z., Zhang S. Recommendations from the Peking Union Medical College Hospital for the management of acute myocardial infarction during the COVID-19 outbreak. Eur Heart J. 2020;41:1791–1794. doi: 10.1093/eurheartj/ehaa258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curzen N. An extended statement by the British Cardiovascular Intervention Society president regarding the COVID-19 pandemic. Interv Cardiol Rev. 2020;15:e01. doi: 10.15420/icr.2020.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chieffo A., Stefanini G.G., Price S. EAPCI position statement on invasive management of acute coronary syndromes during the COVID-19 pandemic. Eur Heart J. 2020;41:1839–1851. doi: 10.1093/eurheartj/ehaa381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sianos G., Papafaklis M.I., Serruys P.W. Angiographic thrombus burden classification in patients with ST-segment elevation myocardial infarction treated with percutaneous coronary intervention. J Invasive Cardiol. 2010;22:6B–14B. [PubMed] [Google Scholar]
- 10.Van’t Hof A.W.J., Liem A., Suryapranata H., Hoorntje J.C.A., De Boer M.J., Zijlstra F. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: Myocardial blush grade. Circulation. 1998;97:2302–2306. doi: 10.1161/01.cir.97.23.2302. [DOI] [PubMed] [Google Scholar]
- 11.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan B.E., Chong V.C.L., Chan S.S.W. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020;95:e131–e134. doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 16.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beyrouti R., Adams M.E., Benjamin L. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020 Apr 30 doi: 10.1136/jnnp-2020-323586. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paranjpe I., Fuster V., Lala A. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 May 5 doi: 10.1016/j.jacc.2020.05.001. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwong J.C., Schwartz K.L., Campitelli M.A. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378:345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 21.Masi P., Hekimian G., Lejeune M. Systemic inflammatory response syndrome is a major contributor to COVID-19-associated coagulopathy: insights from a prospective single center cohort study. Circulation. 2020;142:611–614. doi: 10.1161/CIRCULATIONAHA.120.048925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Libby P., Ridker P.M., Hansson G.K. Inflammation in atherosclerosis. From pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tall A.R., Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saltiel A.R., Olefsky J.M. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zidar D.A., Al-Kindi S.G., Liu Y. Association of lymphopenia with risk of mortality among adults in the US general population. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Driggin E., Madhavan M.V., Bikdeli B. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W., Xu Y., Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. J Am Med Assoc. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]