Abstract
Background
Observational studies suggest that the risk and clinical prognosis of coronavirus disease 2019 (COVID-19) are related to low vitamin D status; however, the data are inconsistent.
Objectives
We conducted a systematic review and meta-analysis to assess the association between low vitamin D status and COVID-19.
Methods
A systematic search was conducted with PubMed, Embase, and the Cochrane Library from database inception to September 25, 2020. The standardized mean difference (SMD) or odds ratio (OR) and corresponding 95% confidence interval (CI) was applied to estimate pooled results. Random - or fixed-effect models based on heterogeneity were used for the meta-analysis. Funnel plots and Egger regression tests were used to assess publication bias.
Results
A total of ten articles with 361,934 participants were selected for meta-analysis. Overall, the pooled OR in the fixed-effect model showed that vitamin D deficiency or insufficiency was associated with an increased risk of COVID-19 (OR = 1.43, 95% CI 1.00–2.05). In addition, COVID-19-positive individuals had lower vitamin D levels than COVID-19-negative individuals (SMD = -0.37, 95% CI = -0.52 to -0.21). Significant heterogeneity existed in both endpoints. Funnel plots and Egger regression tests revealed significant publication bias.
Conclusions
This systematic review and meta-analysis indicated that low vitamin D status might be associated with an increased risk of COVID-19 infection. Further studies are needed to evaluate the impact of vitamin D supplementation on the clinical severity and prognosis in patients with COVID-19.
Systematic Review Registration
PROSPERO registration no: CRD42020216740.
Keywords: Coronavirus disease 2019, Vitamin D, Meta-analysis, Low vitamin D status, 25-hydroxyvitamin D
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had a catastrophic impact worldwide (Walker et al., 2020). Although it is difficult to compare national data, mortality from COVID-19 is significantly higher in some countries than in others (Li 2020). For example, Spain, Italy, and the United Kingdom have higher mortality rates than the United States and Germany. Multiple factors contribute to this difference, including differences in aging, general health, government decisions, accessibility and quality of healthcare, and socioeconomic status (Patel et al., 2020, Raifman and Raifman, 2020). Recent observational studies have linked the population’s relative vitamin D status to COVID-19 outcomes.
Vitamin D is also called cholecalciferol (vitamin D3) or ergocalciferol (vitamin D2), which are hormone precursors and play an important role in regulating the metabolism of calcium and phosphate (Kulda 2012). The vitamin D biosynthetic pathway begins with ultraviolet B radiation of 7-dehydrocholesterol on the bare skin exposed to strong sunlight, which is the primary source, as few foods contain vitamin D (Bouillon 2017). 125-dihydroxyvitamin D is responsible for the function of vitamin D, not 25-hydroxyvitamin D ([25(OH)D]), which requires CYP27B1 to transform into active vitamin D. A substantial body of evidence shows that local synthesis of active vitamin D is critical for the immunomodulatory role of vitamin D against inflammation and microbes beyond the systemic level of 25-hydroxyvitamin D and bone; however, extrarenal vitamin D metabolism and its regulatory loop are not yet fully understood (Xu et al., 2020). Various studies now support that vitamin D inhibits lymphocyte proliferation, antibody production, and cytokine synthesis through monocyte and cell-mediated immune stimulation (Kara et al., 2020). Low vitamin D status is also regarded as an epidemic and a global public health problem, especially in Europe. It is related to an increase in infectious and non-infectious diseases, especially upper respiratory tract infections. (De La Puente-Yague et al., 2018; Jagannath et al., 2018, Martineau et al., 2017). This association was confirmed by a meta-analysis, including 25 randomized controlled trials, which showed that vitamin D supplementation is beneficial for respiratory diseases. Recently, a substantial body of evidence has clearly linked COVID-19 outcomes with low vitamin D status, but the results from those published to date are conflicting: two retrospective studies reported independent associations between low pre-pandemic 25(OH)D levels and the subsequent incidence and severity of COVID-19(Meltzer et al., 2020) (Avolio et al. 2020), while an analogous study in the UK did not support the potential link between 25(OH)D concentration and the risk of severe COVID-19 infection and mortality(Hastie et al., 2020a).
Considering the impact of the COVID-19 risk that potentially results from low vitamin D status, several studies have explored this association. However, the results of these studies are conflicting. To clarify these contradictory results and more accurately assess the relationship between low vitamin D status and COVID-19 risk, we performed a meta-analysis of published studies to provide a clinical reference.
Methods
The preferred reporting item for systematic review and meta-analysis protocol (PRISMA) guidelines were followed for reporting the results of this review (Appendix S1)(Moher, 2009).
Data sources and searches
We conducted a systematic search of the PubMed, Embase, and Cochrane Library databases from database inception to September 25, 2020, using thesaurus terms and keywords; the following search terms were entered: ("coronavirus disease 2019″ OR “COVID-19″ OR “SARS−COV-2″ OR “Coronavirus”) AND (“vitamin D” OR “25(OH)D” OR “25-hydroxyvitamin D” OR “hydroxycholecalciferols” OR “hypovitaminosis D”). No language restrictions were applied. We contacted the authors of the articles if the data were not available. We also manually searched the references of included articles for the latest reviews. The search strategy is presented in Table S1.
Study selection
We first conducted a preliminary screening of titles and abstracts; the second screening involved a full-text review. Two researchers independently screened information at each stage. Disagreements were resolved through consensus and, if necessary, with a third independent reviewer. In this study, the population (P) included individuals with COVID-19 who had low vitamin D status, including vitamin D deficiency or insufficiency, (E), and were compared (C) to individuals without COVID-19. The primary outcome (O) was incident COVID-19. Observational studies (S) were included in this meta-analysis. Vitamin D deficiency or insufficiency is defined as a 25(OH)D below 20 ng/mL (50 nmol/L) or as a 25(OH)D of 21–29 ng/ml (52.5–72.5 nmol/L), respectively (Bolland et al., 2016). Binary variables report odds ratios (ORs) and corresponding 95% confidence intervals (CIs) (or the data used to calculate them). Continuous variables report the levels of vitamin D, expressed as the mean ± standard deviation. Case reports, case series, duplicate reports, commentaries, and author responses were excluded.
Data extraction
Standardized data collection tables were used for data extraction. We extracted the reported OR and the corresponding 95% CI or other data to calculate these indicators. We also extracted the characteristics of each trial and recorded the data as follows: first author, year of publication, country of publication, time of the study, characteristics of the study population, baseline age, total number of participants, number of vitamin D deficiency or insufficiency events, and average vitamin D level. Two reviewers independently performed research selection and data extraction. Any discrepancies were resolved through discussion.
Quality assessment
The methodological quality of the included study was assessed by the modified Newcastle-Ottawa scale (NOS)(Stang 2010), which consists of three factors: patient selection, comparability of the study groups, and assessment of outcome. Each study has a score of 0–9 (assigned as stars), and observational studies with six or more stars are considered high quality. Two researchers conducted a quality assessment and resolved any discrepancies through discussion or consensus.
Statistical analysis
Stata software (version 16.0, StataCorp, College Station, TX, USA) was used for pooled estimates. Dichotomous data were analyzed using the ORs computed by the Mantel Haenszel method (fixed or random models) and the corresponding 95% CIs. Continuous outcomes measured on the same scale are expressed as the mean value and standard deviation, and the standardized mean difference (SMD) was used for analysis. The I-square (I 2) test was performed to assess the impact of study heterogeneity on the meta-analysis results. According to the Cochrane review guidelines, if there is significant heterogeneity at I 2 > 50%, the random effect model is chosen; otherwise, the fixed-effect model is used (Higgins et al., 2003). Sensitivity analysis of the primary endpoint was conducted by sequential removal of each trial to assess the individual studies' impact on overall pooled estimates. Subgroup analysis was performed based on the 25(OH)D measurement units (ng/mL and nmol/L). We explored publication bias using funnel plots and Egger regression tests. Statistical assessment was two-tailed and was considered statistically significant at P < 0.05.
Results
Selected studies
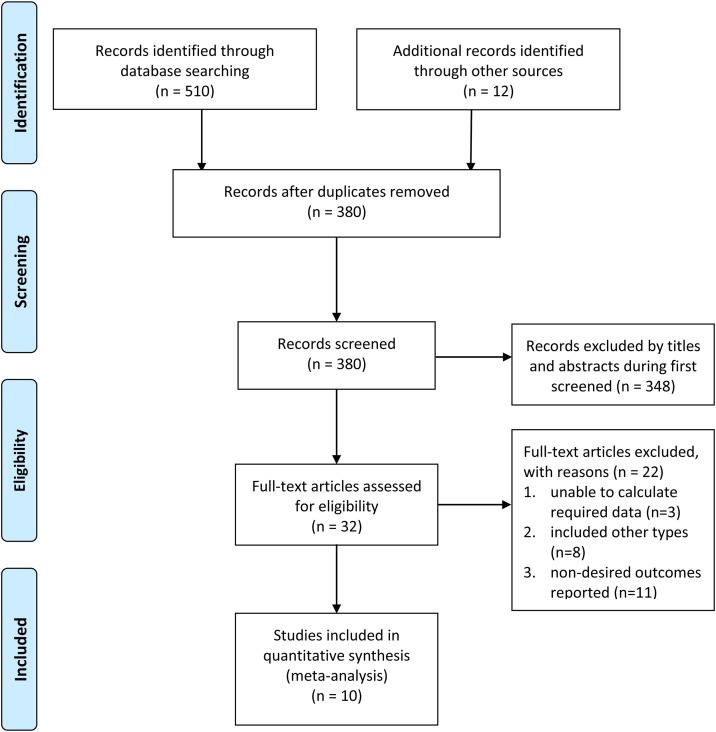
As shown in the PRISMA flow chart ( Figure 1 ), we searched 522 related records from all electronic databases, of which 142 were excluded as duplicates. The remaining 380 records were filtered according to the titles and abstracts; 348 were excluded due to unrelated topics. We reviewed the full text of the remaining 32 studies and identified ten that met the inclusion criteria of the meta-analysis (Baktash et al., 2020, Chodick et al., 2020, Avolio et al., 2020, Hastie et al., 2020b; Im et al., 2020; Mardani et al., 2020, Meltzer et al., 2020; Merzon et al., 2020; Raisi-Estabragh et al., 2020, Ye et al., 2020).
Figure 1.
Flow diagram of the literature search process.
Study characteristics
Ten case-control studies involving 376,596 participants were included in the meta-analysis, including 4178 COVID-19-positive participants and 372,418 COVID-19-negative participants. The sample size of the studies varied greatly, from 105 to 248,598. Most participants were at least 50 years old. Most studies were conducted in Asia (n = 5), followed by Europe (n = 4)(Baktash et al., 2020, Avolio et al., 2020, Hastie et al., 2020a, Raisi-Estabragh et al., 2020),(Chodick et al., 2020; Im et al., 2020; Mardani et al., 2020; Merzon et al., 2020; Ye et al., 2020), and the United States (n = 1) (Meltzer et al. 2020). Table 1 lists the main descriptive statistics for all included studies.
Table 1.
Summary characteristics of studies included in the meta-analysis.
| Study | Country | Study D esign |
Sample size | Age COVID-19 positive COVID-19 negative | Gender Male (%) | Definition of vitamin D status [25(OH)D] | 25(OH)D level | ||
|---|---|---|---|---|---|---|---|---|---|
| COVID-19 positive | COVID-19 negative NOS | ||||||||
| Raisi-Estabragh et al. (2020) | United Kingdom | case-control study | 4510 | 68.11 (9.23) a | 68.91 (8.72) | 2201 (48.8%) | NR | 33.88 ± 27.01 nmol/L | 35.45 ± 26.78 nmol/L 8 |
| Baktash et al. (2020) | United Kingdom | case-control study | 105 | mean age 81 years, range 65–102 | 57 (54.3%) | vitamin D-deficient (≤30 nmol/L) vitamin D-replete (>30 nmol/L) | 31.33 ± 20.44 nmol/L | 51.67 ± 30.92 nmol/L 5 | |
| Chodick et al. 2020 | Israel | case-control study | 14,520 | 40.6 (19.1) a 37.0 (19.1) | 6880 (47.4%) | NR | 23.6 ± 8.6 ng/mL | 24.1 ± 9.1 ng/mL 9 | |
| Hastie et al. (2020) | United Kingdom | case-control study | 348,598 | 49 (40−58) b | 49 (38−57) | 168,391 (48.3%) | vitamin D deficiency (< 25 nmol/L) vitamin D insufficiency (< 50 nmol/L) | 30.0 ± 27.6 nmol/L | 27.5 ± 25.1 nmol/L 8 |
| Avolio et al. (2020) | Switzerland. | case-control study | 102 | 74 (65−81) b | 73 (61−82) | NR | NR | 13.43 ± 10.01 ng/mL | 21.33 ± 16.31 ng/mL 6 |
| I’m et al. (2020) | Korea | case-control study | 200 | 52.2 (20.7) a | 52.4 (20.2) | NR | vitamin D deficiency (< 20 ng/dl) severe vitamin D deficiency (< 10 ng/dl) | 15.7 ± 7.9 ng/mL | 25.0 ± 13.2 ng/mL 9 |
| Merzon et al. (2020) | Israel | case-control study | 7807 | 35.58 (34.49−36.67) c | 47.35 (46.87−47.85) | 4573 (58.6%) | vitamin D deficiency (<30 ng/mL) | NR | 9 |
| Mardani et al. (2020) | Iran | case-control study | 123 | mean age 42 years, range 18−78 | 65 (52.8%) | vitamin D sufficient (>30 ng/mL) vitamin D insufficient (<30 ng/mL) | 18.54 ± 11.63 ng/mL | 30.17 ± 9.05 ng/mL 7 | |
| Meltzer et al. (2020) | United States | case-control study | 489 | 49.2 (18.4) a | 123 (25.2%) | vitamin D deficient (<20 ng/mL) not deficient (≥20 ng/mL) | NR | 8 | |
| Ye et al. (2020) | China | case-control study | 142 | 43 (32–59) b 42 (31–52) | 55 (38.7%) | vitamin D deficiency was defined as a 25(OH)D<50 nmol/L, vitamin D insufficiency as 50 nmol/L≤25(OH)D<75 nmol/L, and vitamin D sufficiency as 25(OH)D≥75 nmol/L | 54.5 ± 18.4 nmol/L | 71 ± 19.7 nmol/L 8 | |
COVID-19 = coronavirus disease 2019; a = mean (SD); b = median (IQR); c = mean age, (years, 95% CI); NR = not report.
Quality assessment
Of the included studies, eight (Chodick et al., 2020, Avolio et al., 2020, Hastie et al., 2020b, Mardani et al., 2020, Meltzer et al., 2020; Merzon et al., 2020; Raisi-Estabragh et al., 2020, Ye et al., 2020) were classified as high-quality, and two studies (Baktash et al., 2020; Im et al., 2020) were classified as medium-quality, with an average score of 7.7 ( Table 1 ). Overall, the evidence contributing to these analyses was assessed as being of high quality.
Results of the meta-analysis
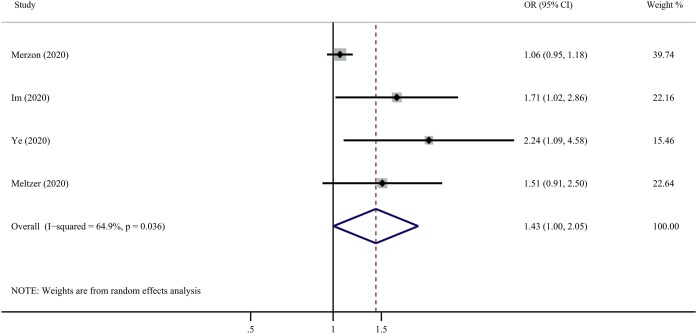
Four of the ten studies reported the association between vitamin D deficiency or insufficiency and COVID-19 infection (Im et al., 2020; Meltzer et al., 2020; Merzon et al., 2020; Ye et al., 2020). Overall, the pooled OR in a fixed-effect model showed that vitamin D deficiency or insufficiency was associated with an increased risk of COVID-19 infection (OR = 1.43, 95% CI 1.00–2.05). However, high heterogeneity was observed in the studies (I2 = 64.9%, p = 0.036) ( Figure 2 ).
Figure 2.
Results from the random-effect model that compared the odds of low vitamin D status among individuals with COVID-19 positivity and negativity. COVID-19 = coronavirus disease 2019.
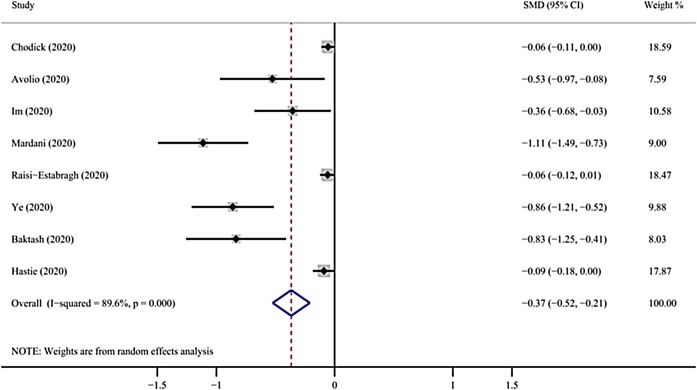
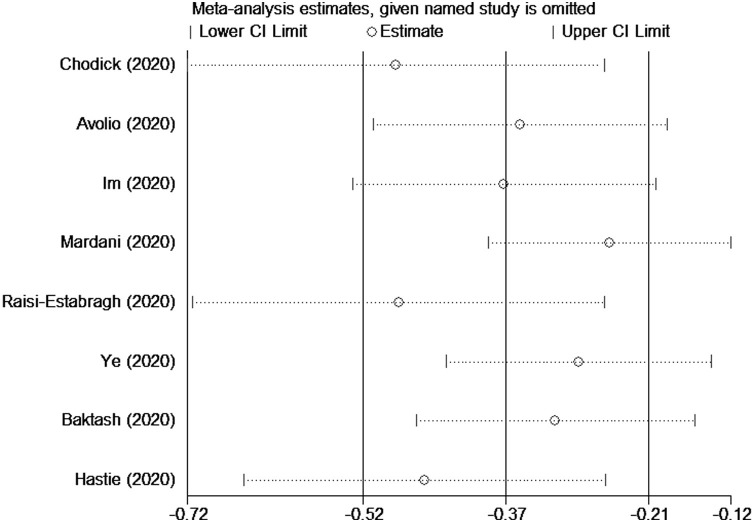
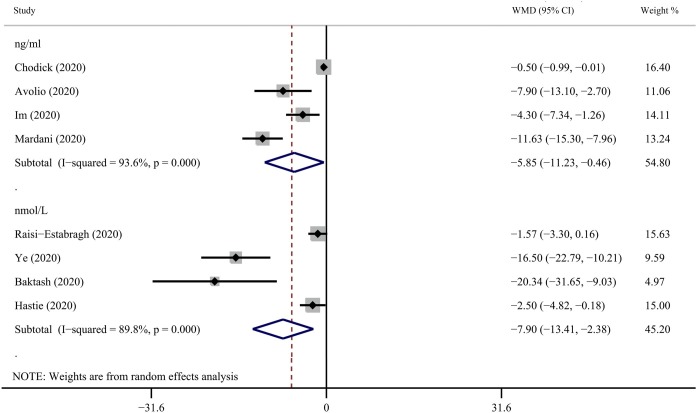
Seven studies evaluated vitamin D levels in COVID-19-positive and-negative participants (Baktash et al., 2020, Chodick et al., 2020, Avolio et al., 2020, Hastie et al., 2020a; Im et al., 2020; Mardani et al., 2020, Raisi-Estabragh et al., 2020, Ye et al., 2020). Overall, we found that the average vitamin D level of the COVID-19-positive group was lower than the COVID-19-negative group (SMD = -0.37, 95% CI = -0.52 to -0.21, I 2 = 89.6%) ( Figure 3 ). The robustness of the results was evaluated by deleting each study in turn and reanalyzing the data sets, which did not lead to significant changes in the pooled OR estimate ( Figure 4 ); however, there was still serious heterogeneity. We conducted a subgroup analysis based on the 25(OH)D measurement units (ng/mL and nmol/L) and found positive results (nmol/L: WMD = -7.90, 95% CI = -13.41 to -2.38, I 2 = 89.8%; ng/mL: WMD = -5.85, 95% CI = -11.23 to -0.46, I 2 = 93.6%) ( Figure 5 ).
Figure 3.
Results from the random-effect model that compared the serum 25(OH)D levels among individuals with COVID-19 positivity and negativity. 25(OH)D = 25-hydroxyvitamin D; COVID-19 = coronavirus disease 2019.
Figure 4.
Sensitivity analysis was performed by excluding each study in turn.
Figure 5.
Subgroup analysis based on the 25(OH)D measurement units (ng/mL and nmol/L) comparing the serum vitamin D levels among individuals with COVID-19 positivity and negativity. 25(OH)D = 25-hydroxyvitamin D; COVID-19 = coronavirus disease 2019.
Publication bias
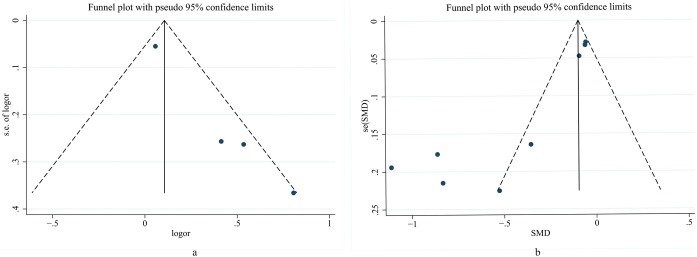
Visual inspection of the funnel plot identified substantial asymmetry ( Figure 6 ). Additionally, Egger’s regression asymmetry test also indicated publication bias (p = 0.001; p = 0.009).
Figure 6.
The visual forest plots were performed to assess publication bias. (a) represents binary variable; (b) represents continuous variable.
Discussion
This meta-analysis was conducted based on ten studies that assessed the impact of vitamin D deficiency or insufficiency on COVID-19 outcomes. According to the available evidence, we found that low vitamin D levels are associated with an increased risk of COVID-19 infection (OR = 1.43, 95% CI 1.00–2.05). The findings also suggest that COVID-19 infected individuals have lower vitamin D levels than those who are not infected (SMD = -0.37, 95% CI = -0.52 to -0.21). This study clearly links the outcomes of COVID-19 with low vitamin D status.
The study by Hastie et al. does not support a potential link between 25(OH)D concentrations and the risk of severe COVID-19 infection because they collected data on vitamin D levels between 2006–2010 and linked them to COVID-19 mortality today, more than a decade later. We question the validity of their results and such a comparison because vitamin D levels vary with age and season. Therefore, we conducted a sensitivity analysis by excluding this study to observe the impact on the overall effect estimate and found that the results did not substantially change (SMD=-0.46, 95% CI: -0.65 to -0.26).
The association between low vitamin D status and metabolism, autoimmunity, and infectious diseases has received widespread attention (Holick, 2017). In particular, some studies have highlighted that low vitamin D status may lead to an increased risk of respiratory infections. Chalmers et al. found that bronchitis dilated patients with vitamin D deficiency were more likely to be colonized by bacteria and have increased respiratory tract inflammation (Chalmers et al., 2013). Mamani et al. indicated that low levels of serum 25-hydroxyvitamin D (25(OH)D) were associated with a high incidence of community-acquired pneumonia and the severity of the disease (Mamani et al., 2017). Also, Dancer et al. demonstrated that survivors with acute respiratory distress syndrome (ARDS) have higher vitamin D levels than nonsurvivors, suggesting that vitamin D supplementation may have a therapeutic effect (Dancer et al., 2015). This led to the hypothesis that low vitamin D status might also be associated with an increased risk of COVID-19. Indeed, from clinical observations to randomized controlled trials, researchers worldwide are focusing on this issue. Based on the available evidence, we conducted this systematic review and found that vitamin D deficiency is associated with an increased risk of COVID-19.
There may be multiple roles of vitamin D in COVID-19 infection. First, vitamin D deficiency can reduce innate cellular immunity and stimulate cytokine storms, which are related to the worsening of ARDS associated with COVID-19. s, vitamin D supports the antimicrobial peptides produced in the epithelium of the respiratory tract, which makes viral infections and COVID-19 symptoms unlikely. Third, vitamin D may help reduce the inflammatory response to SARS-CoV-2 infection (Daneshkhah et al., 2020, Mitchell, 2020). Dysregulation of this response, especially of the renin-angiotensin system, is characteristic of COVID-19, and the degree of overactivation is associated with a poorer prognosis.
Several possibilities exist for the reduced vitamin D levels in COVID-19 patients. Many factors affect vitamin D levels, such as age, region, season, and race. Vitamin D is a fat-soluble vitamin produced by 7-dehydrocholesterol due to the action of ultraviolet B radiation; it is subsequently converted to 25(OH)D in the liver and then to the active form in the kidneys or other organs (Carpagnano et al., 2020). COVID-19 broke out in the winter with low sunlight exposure in the Northern Hemisphere, when levels of 25-hydroxyvitamin D are at their nadir. Patients with COVID-19 are required to be isolated or hospitalized after infection, during which time the skin cannot get enough sunlight. Most participants were over 50 years old, which may be one reason for low vitamin D. Also, an imbalanced diet during hospitalization cannot obtain sufficient vitamins from food, leading to vitamin D deficiency.
In previous studies, intervention trials have rarely shown the benefits of vitamin D supplementation as a treatment or preventive measure. For example, several meta-analyses of vitamin D supplementation trials failed to show significant improvement in blood pressure, insulin sensitivity, or lipid parameters, failing to show a benefit even in the prevention of fracture events (Al Mheid and Quyyumi, 2017, Beveridge et al., 2015, Moyer, 2013). This makes it challenging to investigate the benefits of vitamin D supplementation for COVID-19. However, a significant exception to this general trend is upper respiratory tract infections: a meta-analysis of 25 randomized controlled trials showed that vitamin D supplementation protected against acute respiratory tract infections and that patients with serum 25(OH)D levels < 25 nmol/L gained the most benefit (Martineau et al., 2017). To date, we found two studies evaluating the impact of vitamin D supplementation on the clinical outcome of COVID-19. One reported four vitamin D-deficient patients diagnosed with COVID-19 who were provided either cholecalciferol of 1000 IU daily (standard dose) or ergocalciferol 50,000 IU daily for five days (high dose) as part of supplementation. The results show that patients receiving high-dose vitamin D supplements exhibited improved clinical rehabilitation, reflected in shorter hospital stay, lower oxygen demand, and a reduction in inflammatory marker status (Ohaegbulam et al., 2020). Another study evaluated the effect of calcifediol treatment on intensive care unit admission and mortality rate among patients hospitalized for COVID-19, demonstrating that high-dose calcifediol administration significantly reduced the need for ICU treatment in COVID-19-admitted patients (Entrenas et al., 2020). Pending the results of such trials, we recommend vitamin D supplementation to reach the reference nutritional intake, ranging from 400 IU/day in the UK to 600–800 IU/day in the United States. These levels are based on the benefits of vitamin D for bone and muscle health. Still, there is a chance that their implementation might also reduce the impact of COVID-19 in populations with vitamin D deficiency (Martineau and Forouhi, 2020).
The present study has some limitations. First, correlation does not equal causation, and whether low vitamin D levels are a cause or consequence of COVID-19 remains uncertain. Caution should be exercised when interpreting these results. Second, there are discrepancies in the number and sample size of the included studies, leading to some large variances in effect size estimates. Third, significant heterogeneity was found. The source of heterogeneity was not explored because too few studies were available for each endpoint. We only used random-effects models to address heterogeneity, which may affect the strength and extrapolation of conclusions. Fourth, publication bias may affect our results because negative studies are less likely to be published.
Conclusion
In conclusion, low serum vitamin D status may be related to the increased risk of COVID-19. Individuals with vitamin D deficiency should receive special attention, and future research should focus on the benefits of vitamin D supplementation.
Funding
This work was supported by the China National Science and Technology Major Project for “Essential new drug research and development” (No.2018ZX09301038-003). The funding source had no role in the study.
Declaration of interest
The authors have no relevant interests to declare.
Ethics
This study did not require ethical approval because the meta-analysis was based on published research and the original data are anonymous.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.12.077.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Al Mheid I., Quyyumi A.A. Vitamin D and Cardiovascular Disease: Controversy Unresolved. J Am Coll Cardiol. 2017;70(1):89–100. doi: 10.1016/j.jacc.2017.05.031. [DOI] [PubMed] [Google Scholar]
- Avolio A., Avataneo V., Manca A. 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients. 2020;12:1359. doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baktash V., Hosack T., Patel N. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad Med J. 2020 doi: 10.1136/postgradmedj-2020-138712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge La, Struthers Ad, Khan F., Jorde R., Scragg R., Macdonald Hm. Effect of Vitamin D Supplementation on Blood Pressure: a Systematic Review and Meta-analysis Incorporating Individual Patient Data. JAMA Intern Med. 2015;175(5):745–754. doi: 10.1001/jamainternmed.2015.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13:466–479. doi: 10.1038/nrendo.2017.31. [DOI] [PubMed] [Google Scholar]
- Bolland M.J., Avenell A., Grey A. Should adults take vitamin D supplements to prevent disease? BMJ. 2016;355:i6201. doi: 10.1136/bmj.i6201. [DOI] [PubMed] [Google Scholar]
- Carpagnano G.E., Di Lecce V., Quaranta V.N., Zito A., Buonamico E., Capozza E. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J Endocrinol Invest. 2020:1–7. doi: 10.1007/s40618-020-01370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers J.D., McHugh B.J., Docherty C., Govan J.R., Hill A.T. Vitamin-D deficiency is associated with chronic bacterial colonisation and disease severity in bronchiectasis. Thorax. 2013;68:39–47. doi: 10.1136/thoraxjnl-2012-202125. [DOI] [PubMed] [Google Scholar]
- Chodick G., Nutman A., Yiekutiel N., Shalev V. Angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers are not associated with increased risk of SARS-CoV-2 infection. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancer R.C., Parekh D., Lax S., D’Souza V., Zheng S., Bassford C.R. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS) Thorax. 2015;70:617–624. doi: 10.1136/thoraxjnl-2014-206680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshkhah A., Agrawal V., Eshein A., Subramanian H., Roy H.K., Backman V. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients. Aging Clin Exp Res. 2020:1–18. doi: 10.1007/s40520-020-01677-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Puente-Yague M., Cuadrado-Cenzual M.A., Ciudad-Cabanas M.J. Vitamin D: And its role in breast cancer. Kaohsiung J Med Sci. 2018;34:423–427. doi: 10.1016/j.kjms.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entrenas C.M., Entrenas C.L., Vaquero B.J., Alcalá D.J., López M.J. “Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study”. J Steroid Biochem Mol Biol. 2020;203 doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie C.E., Mackay D.F., Ho F., Celis-Morales C.A., Katikireddi S.V., Niedzwiedz C.L. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab Syndr. 2020;14:561–565. doi: 10.1016/j.dsx.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie C.E., Pell J.P., Sattar N. Vitamin D and COVID-19 infection and mortality in UK Biobank. Eur J Nutr. 2020:1–4. doi: 10.1007/s00394-020-02372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18:153–165. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- I’m J.H., Je Y.S., Baek J. Nutritional status of patients with coronavirus disease 2019 (COVID-19) Int J Infect Dis. 2020;100:390–393. doi: 10.1016/j.ijid.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannath V.A., Filippini G., Di Pietrantonj C., Asokan G.V., Robak E.W., Whamond L. Vitamin D for the management of multiple sclerosis. Cochrane D Syst Rev. 2018;9 doi: 10.1002/14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara M., Ekiz T., Ricci V., Kara Ö, Chang K.V., Özçakar L. Scientific Strabismus or Two Related Pandemics: COVID-19 & Vitamin D Deficiency. Brit J Nutr. 2020;124:736–741. doi: 10.1017/S0007114520001749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulda V. Vitamin D metabolism. Vnitr Lek. 2012;58:400–404. [PubMed] [Google Scholar]
- Li W.X. Worldwide inverse correlation between Bacille Calmette-Guerin immunization and COVID-19 morbidity and mortality. Res Sq [Preprint] 2020 doi: 10.21203/rs.3.rs-42927/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamani M., Miceli N., Ghasemi B.H., Vasheghani M., Poorolajal J. Association between serum concentration of 25-hydroxyvitamin D and community-acquired pneumonia: a case-control study. Int J Gen Med. 2017;10:423–429. doi: 10.2147/IJGM.S149049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardani R., Alamdary A., Mousavi N.S. Association of vitamin D with the modulation of the disease severity in COVID-19. Virus Res. 2020;289 doi: 10.1016/j.virusres.2020.198148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau A.R., Forouhi N.G. Vitamin D for COVID-19: a case to answer? Lancet Diabetes Endocrinol. 2020;8(9):735–736. doi: 10.1016/S2213-8587(20)30268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer D.O., Best T.J., Zhang H. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzon E., Tworowski D., Gorohovski A. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. Febs J. 2020;287:3693–3702. doi: 10.1111/febs.15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell F. Vitamin-D and COVID-19: do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. 2020;8:570. doi: 10.1016/S2213-8587(20)30183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer V.A. U.S. Preventive Services Task Force. Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;158(9):691–696. doi: 10.7326/0003-4819-158-9-201305070-00603. [DOI] [PubMed] [Google Scholar]
- Ohaegbulam K.C., Swalih M., Patel P., Smith M.A., Perrin R. Vitamin D Supplementation in COVID-19 Patients: A Clinical Case Series. Am J Ther. 2020;27:e485–e490. doi: 10.1097/MJT.0000000000001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J.A., Nielsen F.B.H., Badiani A.A., Assi S., Unadkat V., Patel B. Poverty, Inequality & COVID-19: The Forgotten Vulnerable. Public Health. 2020;183:110–111. doi: 10.1016/j.puhe.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raifman M.A., Raifman J.R. Disparities in the Population at Risk of Severe Illness From COVID-19 by Race/Ethnicity and Income. Am J Prev Med. 2020;59:137–139. doi: 10.1016/j.amepre.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisi-Estabragh Z., McCracken C., Bethell M.S. Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: study of 1326 cases from the UK Biobank. J Public Health (Oxf) 2020;42:451–460. doi: 10.1093/pubmed/fdaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- Walker P.G.T., Whittaker C., Watson O.J., Baguelin M., Winskill P., Hamlet The impact of COVID-19 and strategies for mitigation and suppression in low- and middle-income countries. Science. 2020;369:413. doi: 10.1126/science.abc0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Baylink D.J., Chen C.S., Reeves M.E., Xiao J., Lacy C. The importance of vitamin d metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19. J Transl Med. 2020;18(1):322. doi: 10.1186/s12967-020-02488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye K., Tang F., Liao X., Shaw B.A., Deng M., Huang Y. Does Serum Vitamin D Level Affect COVID-19 Infection and Its Severity? -A Case-Control Study. J Am Coll Nutr. 2020:1–8. doi: 10.1080/07315724.2020.1826005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.