Abstract
Objectives
The COVID-19 pandemic is a global health problem. We, as the EMERGE (EMErging RheumatoloGists and rEsearchers) group of PReS (Pediatric Rheumatology European Society) analyzed how the pandemic has affected pediatric rheumatology practice.
Methods
An online survey was developed to assess changes in pediatric rheumatology practice due to the pandemic. Results were analyzed using descriptive statistics.
Results
From 70 countries, 493 pediatric rheumatologists (80.3% in pediatric rheumatology practice for ≥5 years) responded to the survey. Around 70% disagreed that the pandemic led to reduced prescription of nonsteroidal anti-inflammatory drugs, conventional synthetic and biologic disease-modifying antirheumatic drugs. Almost half were more likely to taper corticosteroids faster. One-fifth hesitated to switch the major immunosuppressant during a flare. Patients encountering difficulties obtaining hydroxychloroquine and tocilizumab due to shortages were noted by 192 (38.9%) and 44 (8.9%), respectively. Twenty to 30% indicated that their patients had experienced a flare or delay in diagnosis/intervention due to postponed appointments.53% mentioned use of phone calls/smartphone applications while 47% shifted towards video consultations for patient care. Respondents indicated an increased number of patients with Kawasaki disease (30%), macrophage activation syndrome (15.6%), unusual vasculitic rashes (31.4%), and hyperinflammation (33.5%) during the pandemic.
Conclusion
This is the largest survey to date addressing changes in pediatric rheumatology practice due to the COVID-19 pandemic. Primary changes were due to delays in clinic appointments, increase in use of virtual technologies, and concerns about the use of immunosuppressive therapies. An increased number of patients with Kawasaki disease/hyperinflammation mentioned by the respondents is noteworthy.
Keywords: COVID-19, Pandemic, Pediatric rheumatology, Survey, Kawasaki disease, Macrophage activation syndrome
Abbreviations: ACE, angiotensin-converting enzyme; ACR, American College of Rheumatology; CARRA, Childhood Arthritis and Rheumatology Research Alliance; COVID-19, coronavirus disease 2019; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; EMERGE, EMErging RheumatoloGists and rEsearchers; EULAR, European League Against Rheumatism; HCQ, hydroxychloroquine; IL-6, interleukin 6; MAS, macrophage activation syndrome; NSAIDs, nonsteroidal anti-inflammatory drugs; PReS, Pediatric Rheumatology European Society; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SLE, systemic lupus erythematosus; WHO, World Health Organization
What was already known about the subject:
• COVID-19 pandemic threatens millions of lives worldwide
• As pediatric rheumatologists, we take care of a vulnerable population
What was learned from this study:
• The main changes in pediatric rheumatology practice during the COVID-19 pandemic were due to delays in clinic appointments, increase in use of virtual technologies to decrease in-person visits, use of anti-rheumatic drugs treatment/prophylaxis, and concerns about using immunosuppressive therapies
• An increase in the number of patients with Kawasaki disease/hyperinflammation was also mentioned by the respondents of the survey.
• Understanding the challenges imposed by the COVID-19 pandemic on the community of pediatric rheumatologists will help tailor future recommendations regarding the management of pediatric rheumatology patients during the pandemic according to the needs in daily clinical practice.
Alt-text: Unlabelled box
Introduction
A novel coronavirus, SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), caused a severe outbreak that emerged in China in December 2019 [1,2]. This infection, termed COVID-19 (coronavirus disease 2019) by the World Health Organization (WHO), quickly spread worldwide, and it was declared a pandemic on March 11, 2020. As of July 13, 2020, there were 12,768,307 confirmed cases and 566,654 deaths ascribed to the COVID-19 worldwide (WHO situation report 175) for a mortality rate of around 4.4%. Most affected individuals have a mild infection. However, it can also lead to a cytokine storm and acute respiratory distress syndrome, which are the major causes of mortality in severe cases [3,4]. COVID-19 affects elderly individuals, smokers, and patients with chronic diseases such as diabetes mellitus more severely [5,6]. On the other hand, children are infected less often and with a milder form of the disease [7]. However, we, in the pediatric rheumatology community started to recognize that children may display a serious hyperinflammatory syndrome often resembling Kawasaki disease shock syndrome [8], [9], [10].
As pediatric rheumatologists, we take care of children with rheumatic diseases who are treated primarily with immunomodulatory or immunosuppressive therapies. The immune dysregulation caused by both the rheumatic disease itself and the medications used to treat it, put our patients in a vulnerable group during the COVID-19 pandemic. We are also participating in the multidisciplinary management of COVID-19 patients, as several aspects of COVID-19 resemble rheumatic diseases [11]. Moreover, some drugs that are used by our patients are currently being used or tested for treatment of COVID-19 [11].
Data regarding the infection rate and course of COVID-19 in children with rheumatic diseases are lacking. The recent report from the COVID-19 Global Rheumatology Alliance Physician Reported Registry (n = 600), did not include any pediatric COVID-19 patients with a rheumatic disease [12]. In the global ongoing survey (The EULAR COVID-19 database -in collaboration with PReS), only 1% of the affected rheumatology patients with COVID-19 are below 18 years of age [13].The international rheumatology societies such as ACR (American College of Rheumatology), EULAR (European League Against Rheumatism), and PReS (Pediatric Rheumatology European Society) recommend continuing immunosuppressive therapies for effective disease control in patients with rheumatic diseases [14], [15], [16]. However, there may be concerns about the use of moderate-to-high doses of corticosteroid or several immunosuppressants, because these might interfere with the effective clearance of the SARS-CoV-2 in the initial phase of COVID-19.
In this study, we analyzed how the COVID-19 pandemic has affected pediatric rheumatology practice, especially regarding the use of antirheumatic drugs and changes in clinical care.
Methods
We, as the EMERGE (EMErging RheumatoloGists and rEsearchers) group of PReS (Pediatric Rheumatology European Society) conducted an online survey consisting of 18 questions (Supplementary File 1) using the SurveyMonkey online software. Pediatric rheumatologists, including fellows-in-training, were invited to complete the survey by e-mail. In May 2020, the survey was electronically sent to the members of PReS, and it was posted on the PReS website. It was also sent to the members of CARRA (Childhood Arthritis and Rheumatology Research Alliance) and the Pediatric Rheumatology Bulletin Board, a worldwide electronic listserv. The respondents completed the survey voluntarily and anonymously.
Majority of the questions had a multiple-choice answer with free text allowed for specific fields. Moreover, respondents could use free text when answering to the questions about the adjustments made to their clinical practice, while four questions regarding the influence of the COVID-19 pandemic to prescribing some of the medications had answers on a 5-point Likert scale, ranging from strongly agree to strongly disagree. The first five questions collected demographic information (age, sex, place of practice, country of practice) and years in pediatric rheumatology practice. Eight questions were about the changes in clinical practice with prescribing nonsteroidal anti-inflammatory drugs (NSAIDs), conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), and biologic DMARDs. Two questions were about the effect of COVID-19 on school activities of pediatric rheumatology patients, and one question was about the adjustments made in clinical practice due to COVID-19. There was one question about the difficulties that pediatric rheumatology patients experienced due to the pandemic and the other about the increased number of patients with certain conditions since it began.
Results were analyzed using descriptive statistics. Numbers and percentages were used to present the data.
Results
Worldwide, 493 pediatric rheumatologists (67% female) from 70 countries (Supplementary Table 1) responded to the survey. The responses to individual questions in the survey are presented in the Supplementary Table 2. Around 70% of the respondents were ≥40 years of age and were practicing in a university hospital (Table 1 ). Most respondents (n = 396; 80.3%) had been in pediatric rheumatology practice for at least five years.
Table 1.
Demographics of the 493 pediatric rheumatologists who responded to the COVID-19 Pediatric Rheumatology Survey.
| Characteristics | Number of respondents, n (%) |
|---|---|
| Sex, female | 330 (66.9%) |
Age
|
|
Place of practice
|
|
Years in pediatric rheumatology practice
|
|
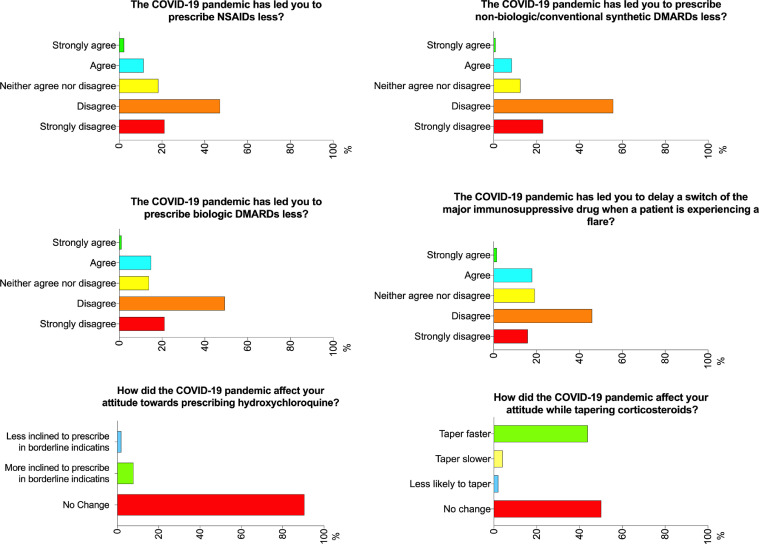
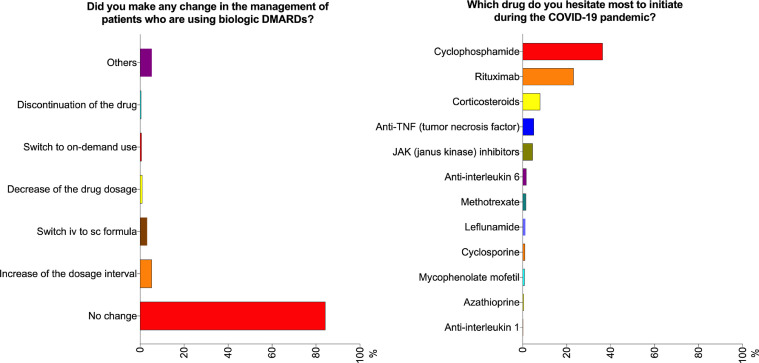
Slightly more than 70% of the respondents disagreed that the COVID-19 pandemic had led to a decrease in prescription of NSAIDs, csDMARDs, and biologic DMARDs (Fig. 1 ). In contrast, 13.5%, 9.1%, and 15.8% agreed that the prescription rate for NSAIDs, csDMARDs, and biologic DMARDs, respectively, was lower. The majority (84.2%) did not change their management of patients using biologic DMARDs (Fig. 2 ). 8% of the respondents were more inclined to prescribe hydroxychloroquine (HCQ), while most (90.5%) did not report any change in their attitude towards prescribing this drug. Interestingly, 216 (43.8%) were more likely to taper corticosteroids faster (Fig. 1). Around 20% of the respondents agreed that the COVID-19 pandemic had led them to delay changing a major immunosuppressant drug when a patient was experiencing a flare. The respondents reported that they hesitated most to initiate cyclophosphamide (36.3%), followed by rituximab (23.1%) during the pandemic (Fig. 2).
Fig. 1.
Opinions of the pediatric rheumatologists regarding prescription of anti-rheumatic therapies. COVID-19, coronavirus disease 2019; DMARD, disease modifying anti-rheumatic drugs.
Fig. 2.
Opinions of the pediatric rheumatologists regarding the management of patients using biologic disease modifying anti-rheumatic drugs (DMARDs) and initiating biologic DMARDs. COVID-19, coronavirus disease 2019.
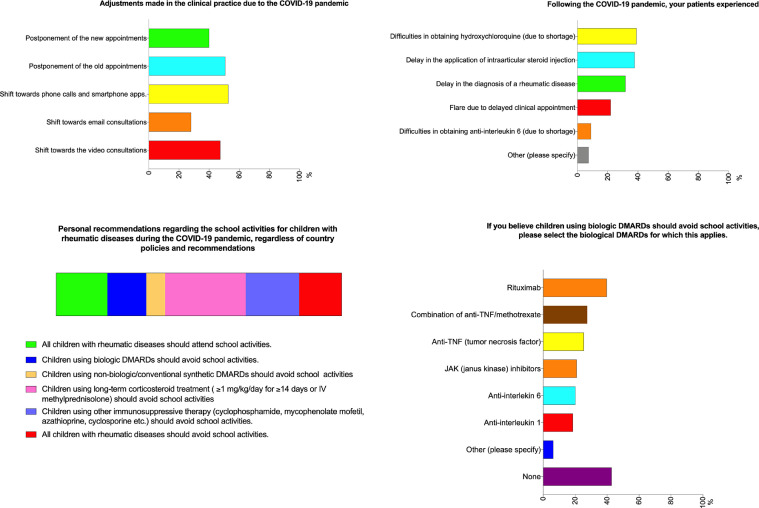
Among the respondents, 192 (38.9%) mentioned that their patients had difficulties obtaining HCQ due to shortages, and 44 (8.9%) noted the same problem with tocilizumab (Fig. 3 ). Around one-third of respondents indicated that their patients had experienced a delay in the diagnosis of a rheumatic disease or in receiving an intraarticular corticosteroid injection, while 108 (21.9%) stated that their patients experienced a flare due to delayed appointments (Fig. 3).
Fig. 3.
Opinions of the pediatric rheumatologists regarding changes to clinical practice, difficulties experienced by the patients, and school activities.
About half of the respondents canceled scheduled appointments with established patients, and 40% postponed clinical appointments with new patients. Phone calls and/or smartphone applications were used by 53% of the respondents and 47% shifted towards video consultations for patient care (Fig. 3).
Almost half of the respondents (45%) believe that children on long term corticosteroid treatment (≥1 mg/kg/day prednisone equivalent for ≥14 days) should avoid attending school, while 20% believe that children using biologic DMARDs should avoid school activities, especially those taking rituximab (39.7%) (Fig. 3).
The respondents indicated that they had seen increases in the number of patients with Kawasaki disease (30%), macrophage activation syndrome (MAS) (15.6%), unusual vasculitic rashes (31.4%), and hyperinflammation (33.5%) since the beginning of the COVID-19 pandemic.
Discussion
The results of the current survey study reflect the perspectives of pediatric rheumatologists during the COVID-19 pandemic. At present, this is the largest survey study addressing the effects of the pandemic on pediatric rheumatology practice. Response rate of almost 500 pediatric rheumatologists is considered very high, and highlights the influence and the effect of the pandemic on the caring physicians. Almost half of the respondents were more likely to taper corticosteroids faster, and one-fifth hesitated to switch a patient's major immunosuppressant drug during a flare. In addition, 15% agreed that the COVID-19 pandemic had led to the reduced prescription of biologic DMARDs. Pediatric rheumatology patients experienced delays in the diagnosis of a rheumatic disease or in receiving an intraarticular corticosteroid injection. Some patients had difficulties obtaining HCQ and tocilizumab, mainly due to delayed clinical appointments and shortages of the drugs due to their use for COVID-19 treatment or prophylaxis. A shift towards the use of virtual technologies for routine clinical care was observed.
In the survey by Gupta et al. (221 respondents, 93% adult rheumatologists, 60% in rheumatology practice for >5 years), almost half indicated that they decreased use of biologic DMARDs, while 12.2% stated the same for csDMARDs [17]. In our study, these ratios were 15.8% and 9.1%, respectively. In another survey study among 271 rheumatologists from U.S.A. (9% pediatric rheumatologists and 3% were treating both adult and pediatric populations), 50% of the respondents disagreed that pandemic led them to reduce the use/dosage/frequency of biologic drugs [18]. However, it is noteworthy that pediatric rheumatologists had higher odds of reducing the use/dosage/frequency of biologic drugs compared to adult rheumatologists [18]. Gupta et al. reported that around two-third (66.5%) of the respondents were more inclined to initiate HCQ, and 64.2% were less inclined to change the major immunosuppressant drug during a flare (vs. 8% and 20% in our study, respectively) [17]. During the pandemic, rheumatologists were concerned most about the use of rituximab, followed by cyclophosphamide, as compared to cyclophosphamide followed by rituximab, in our study. In the study by Mehta et al., 56% of the respondents agreed that pandemic led them to reduce the use/dosage/frequency of corticosteroids [18]. Similarly, around 60% preferred earlier taper of corticosteroid treatment during inactive disease in the study by Gupta et al. [17] vs. 43.8% in our study. The ratios regarding the use of csDMARDs and corticosteroids were similar between the study by Gupta et al. and our study. However, their respondents were more inclined to initiate HCQ, reduce the use of biologic DMARDs, and less inclined to change the major immunosuppressant during a flare, as compared to our respondents. The survey by Gupta et al. was taken two months before ours. In this period, we gained more scientific evidence about the use of biologics, the prophylactic potential of HCQ, and the risks associated with immunosuppressant use during the pandemic, which may explain some of the differences. Another point is that adult patients probably have more comorbidities than their pediatric counterparts, and old age is an independent risk-factor for severe COVID-19. These points might have caused more hesitation in adult rheumatologists while changing major immunosuppressants or initiating biologic DMARDs, as compared to pediatric rheumatologists.
Previous studies have reported an association between immunosuppression and increased risk of serious infections in patients with rheumatic diseases [19,20]. On the other hand, active rheumatic disease is a significant risk factor for infection [21,22]. The scientific evidence from previous studies forms the basis for the concerns of pediatric rheumatologists while planning treatment for their patients during the COVID-19 pandemic.
Data regarding the rate and course of COVID-19 in patients with rheumatic diseases are limited. A systematic literature search (from November 1, 2019 to May 11, 2020) revealed 17 articles describing 231 COVID-19 patients with rheumatic diseases [11]. The most common underlying disease was systemic lupus erythematosus (SLE) (n = 117), followed by rheumatoid arthritis (n = 45). Among them, only one was a pediatric patient: a 6-month-old infant with Kawasaki disease [23]. Nine of the 231 patients (3.9%) died.
Recently, Gianfrancesco et al. reported the characteristics of 600 COVID-19 patients with rheumatic diseases submitted to the COVID-19 Global Rheumatology Alliance Physician Reported Registry [12]. None of these patients was younger than 18 years of age, and only 5% were between 18 and 29 years of age [12]. Most of these patients (91%) recovered from COVID-19. Among these patients with rheumatic disease, older age (>65 years), the presence of other comorbidities such as diabetes mellitus, and moderate-to-high doses of glucocorticoids (≥10 mg/day) were all associated with higher odds of hospitalization [12]. On the other hand, using NSAIDs, csDMARDs, or biologic DMARDs was not associated with a higher risk of hospitalization. Moreover, anti-TNF use was significantly associated with fewer hospitalizations. No significant association was observed between the use of antimalarial drugs and hospitalization [12]. The results presented by Gianfrancesco et al. could be reassuring for the 10–15% of our respondents who agreed that COVID-19 had led to decreased prescription of NSAIDs, csDMARDs, and biologic drugs. Currently, there is no confirmed report of a pediatric rheumatology patient treated with biologic DMARDs contracting COVID-19. Filocamo et al. reported that there was no confirmed case of COVID-19 among 123 pediatric rheumatology patients treated with biologic drugs [24]. Similarly, no COVID-19 case was observed among 173 Turkish pediatric rheumatology patients treated with biologics [25].
The concerns about systemic corticosteroids have led to faster tapering in almost half of our respondents; this practice is supported by the results reported by Gianfrancesco et al. [12]. Another area of concern in our survey study was initiating a major immunosuppressive drug such as cyclophosphamide or rituximab. In the cohort of COVID-19 Global Rheumatology Alliance, cyclophosphamide was included in the csDMARDs and rituximab in the biologic DMARDs: they did not observe a significant association between hospitalization rate and use of these drugs [12]. However, the exact number of patients using cyclophosphamide or rituximab is not mentioned. Thus, it is difficult to draw solid conclusions about the risk of severe COVID-19 complications in patients using these drugs.
Based on anecdotal reports of severe COVID-19 in patients using NSAIDs and the possible effect of ibuprofen on ACE (angiotensin-converting enzyme) expression, which is a receptor for SARS-CoV-2 [26,27], there were concerns about NSAID use during the COVID-19 pandemic, among almost 15% of our respondents. However, no specific causal association between NSAID use and severe COVID-19 course has been verified. Moreover, NSAID use was not associated with an increased rate of hospitalization in the largest cohort of COVID-19 patients with rheumatic diseases [12]. Still, in another recent survey study among rheumatologists, 11% of the respondents stated that they were recommending to their patients to decrease or avoid NSAIDs even when they had no COVID-19 symptoms [18].
Hydroxychloroquine is an antimalarial drug that is mainly used in pediatric rheumatology practice for treating SLE [28]. As it may interfere with SARS-CoV-2-host interaction and the maturation process of viral proteins, it was initially suggested as a potential agent for prophylaxis and treatment of COVID-19 [29,30]. This may be the main reason why 8% of our respondents were more inclined to prescribe HCQ during the COVID-19 pandemic. While there is ongoing debate about its efficacy for COVID-19 treatment, most studies have not supported a prophylactic effect of HCQ for COVID-19 [12,[31], [32], [33]]. Moreover, several researchers have noted the risk of insufficient availability for patients who require HCQ for their underlying disease in case it is prescribed prophylactically for COVID-19 [34], [35], [36]. In our study, more than one-third (38.9%) of the respondents stated that their patients experienced difficulties obtaining HCQ due to shortages. In a recent worldwide online survey study on HCQ/CQ use by rheumatologists, 71% of the respondents reported that at least some of their patients experienced HCQ/CQ shortages during the pandemic [37].
COVID-19 has a variety of features, some of which are similar to the characteristics of rheumatic diseases. The severe end of the COVID-19 clinical spectrum is a cytokine release syndrome that resembles MAS, which is associated with systemic juvenile idiopathic arthritis, SLE, or Kawasaki disease in children [11,30]. Around 15% of our respondents mentioned an increased number of MAS cases during the pandemic. Biologic DMARDs, especially anti-interleukin 6 (anti-IL-6) and anti-IL-1 drugs, are currently used for the treatment of COVID-19 associated cytokine storm [38], [39], [40]. Approximately 10% of our respondents indicated that their patients had difficulties obtaining tocilizumab (anti-IL-6) due to shortages.
Reports of skin lesions associated with COVID-19 infection, such as violaceous macules, livedoid rash, purpura, and chilblain-like lesions are increasing [41], [42], [43]. Especially chilblain like lesions were mainly observed in children and adolescents with COVID-19 who were otherwise asymptomatic [41]. One-third of our respondents stated that they observed an increase in the number of patients with unusual vasculitic rashes.
Hyperinflammatory syndrome is a relatively new association of COVID-19, which has been mainly observed in children [8], [9], [10]. Some patients present with features such as conjunctivitis, rash, oral mucocutaneous changes, and coronary dilatation, resembling Kawasaki disease. These patients may have cardiac abnormalities and severe cases may progress to toxic shock syndrome. The COVID-19 associated hyperinflammatory syndrome in children is of critical importance because it emphasizes that children do not always experience a mild form of the disease. In fact, over 30% of our respondents mentioned an increased number of patients with Kawasaki disease or hyperinflammation since the beginning of the COVID-19 pandemic, which is in agreement with the relevant literature.
Our respondents mentioned that they needed to change their routine patients care by canceling scheduled appointments with established patients or postponing clinical appointments with new patients. Although, 20–30% of the respondents indicated that their patients experienced a flare or delay in the diagnosis or intervention, due to postponed clinical appointments. Of the respondents, 53% mentioned use of phone calls and/or smartphone applications while 47% shifted towards video consultations for patient care. Before the pandemic, the use of virtual technology in most healthcare systems was minimal [44]. During the COVID-19 pandemic, virtual technologies were rapidly implemented to maintain access and continuity of medical care by minimizing in-person visits [45]. This rapid implementation may have caused some adaptation problems for both physicians and patients or their parents. This may be the main reason for delays in patient care. Shenoy et al. recently reported their experience with implementing teleconsultations (mainly through WhatsApp video conferences) for the care of patients with rheumatic diseases in India [46]. Teleconsultations was accepted by 74% of the patients at the beginning, but increased with time. The median satisfaction of the patients from teleconsultations was nine on a 0–10 scale (10, the highest). And, three-fourths of respondents mentioned that they would have stopped drugs or self-medicated without teleconsultation [46]. Thus, during the restrictions dictated by the pandemic, virtual technologies were a good source for continuing patient care, and the problems with adaptation can be overcome with time and experience.
The most important limitation of our study was the heterogeneity regarding the stage of the pandemic in the countries where the responding pediatric rheumatologists practice, which might have affected their perspectives. Also, the results are time-sensitive and would be different if the survey was to be given again today. Another limitation was the respondent bias and this group may not represent all pediatric rheumatologists although the number of respondents were high. On the other hand, the main strength of the study was the very high number of respondents from different countries. The majority of respondents were experienced pediatric rheumatologists, as well. Lastly, some countries with a severe COVID-19 burden such as China were underrepresented. And, a few other countries with 1–2 respondent(s) may not be fully represented.
Conclusion
The results of the survey suggested that the COVID-19 pandemic had indeed affected pediatric rheumatology practice. Most changes arose from delays in clinic appointments, concerns about the immunosuppressive effects of antirheumatic therapies, the use of antirheumatic drugs for COVID-19 treatment/prophylaxis, and increased use of virtual technologies to minimize face to face visits. In addition, an increase in the number of patients with Kawasaki disease or hyperinflammation syndrome was mentioned by a substantial number of respondents was noteworthy and consistent with the increased reports in the literature. Better understanding of the challenges imposed by the COVID-19 pandemic on the community of pediatric rheumatologists will help tailor future updated recommendations regarding the management of our patients during the pandemic, school and social attendance according to the needs of routine clinical practice [16].
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
Acknowledgments
We wish to thank PReS, EMERGE group of PReS, and CARRA for disseminating the survey. We are grateful for the contribution of the pediatric rheumatologists worldwide, who completed the survey.
We thank Faye Schreiber for editing the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.semarthrit.2020.09.008.
Appendix. Supplementary materials
References
- 1.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Misra D.P., Agarwal V., Gasparyan A.Y., Zimba O. Rheumatologists' perspective on coronavirus disease 19 (COVID-19) and potential therapeutic targets. Clin Rheumatol. 2020 doi: 10.1007/s10067-020-05073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutierrez-Ocampo E., Villamizar-Pena R., Holguin-Rivera Y., Escalera-Antezana J.P. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vardavas C.I., Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob Induc Dis. 2020;18:20. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Licciardi F., Pruccoli G., Denina M., Parodi E., Taglietto M., Rosati S. SARS-CoV-2-induced Kawasaki-like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics. 2020 doi: 10.1542/peds.2020-1711. [DOI] [PubMed] [Google Scholar]
- 9.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toubiana J., Poirault C., Corsia A., Bajolle F., Fourgeaud J., Angoulvant F. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batu E.D., Ozen S. Implications of COVID-19 in pediatric rheumatology. Rheumatol Int. 2020;40:1193–1213. doi: 10.1007/s00296-020-04612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gianfrancesco M., Hyrich K.L., Al-Adely S., Carmona L., Danila M.I., Gossec L. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.https://www.eular.org/eular_covid19_database.cfm. Last accessed on September 14, 2020.
- 14.Landewe R.B., Machado P.M., Kroon F., Bijlsma H.W., Burmester G.R., Carmona L. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Ann Rheum Dis. 2020;79:851–858. doi: 10.1136/annrheumdis-2020-217877. [DOI] [PubMed] [Google Scholar]
- 15.Mikuls T.R., Johnson S.R., Fraenkel L., Arasaratnam R.J., Baden L.R., Bermas B.L. American College of Rheumatology Guidance for the management of rheumatic disease in adult patients during the COVID-19 pandemic: version 1. Arthritis Rheumatol. 2020 doi: 10.1002/art.41301. [DOI] [PubMed] [Google Scholar]
- 16.https://www.pres.eu/news/newsstory.html?id=29. Last accessed on September 14, 2020.
- 17.Gupta L., Misra D.P., Agarwal V., Balan S., Agarwal V. Management of rheumatic diseases in the time of covid-19 pandemic: perspectives of rheumatology practitioners from India. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217509. [DOI] [PubMed] [Google Scholar]
- 18.Mehta B J.-K.D., Mancuso C.A., Bass A.R., Moezinia C.J., Gibofsky A., Goodman S.M., Ibrahim S. Geographical variations in COVID-19 perceptions and patient management: a national survey of rheumatologists. Semin Arthritis Rheum. 2020 doi: 10.1016/j.semarthrit.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leuvenink R., Aeschlimann F., Baer W., Berthet G., Cannizzaro E., Hofer M. Clinical course and therapeutic approach to varicella zoster virus infection in children with rheumatic autoimmune diseases under immunosuppression. Pediatr Rheumatol Online J. 2016;14(1):34. doi: 10.1186/s12969-016-0095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Listing J., Gerhold K., Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology. 2013;52(1):53–61. doi: 10.1093/rheumatology/kes305. [DOI] [PubMed] [Google Scholar]
- 21.Au K., Reed G., Curtis J.R., Kremer J.M., Greenberg J.D., Strand V. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785–791. doi: 10.1136/ard.2010.128637. [DOI] [PubMed] [Google Scholar]
- 22.Danza A., Ruiz-Irastorza G. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus. 2013;22(12):1286–1294. doi: 10.1177/0961203313493032. [DOI] [PubMed] [Google Scholar]
- 23.Jones V.G., Mills M., Suarez D., Hogan C.A., Yeh D., Bradley Segal J. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020;10:537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 24.Filocamo G., Minoia F., Carbogno S., Costi S., Romano M., Cimaz R. Absence of severe complications from SARS-CoV-2 infection in children with rheumatic diseases treated with biologic drugs. J Rheumatol. 2020 doi: 10.3899/jrheum.200483. [DOI] [PubMed] [Google Scholar]
- 25.Kasap Cuceoglu M., Batu E.D., Bilginer Y., Ozen S. COVID-19 in paediatric rheumatology patients treated with b/tsDMARDs: a cross-sectional patient survey study. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218341. [DOI] [PubMed] [Google Scholar]
- 26.Day M. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:m1086. doi: 10.1136/bmj.m1086. [DOI] [PubMed] [Google Scholar]
- 27.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arici Z.S., Batu E.D., Ozen S. Reviewing the recommendations for lupus in children. Curr Rheumatol Rep. 2015;17(3):17. doi: 10.1007/s11926-014-0489-5. [DOI] [PubMed] [Google Scholar]
- 29.Scuccimarri R., Sutton E., Fitzcharles M.A. Hydroxychloroquine: a potential ethical dilemma for rheumatologists during the COVID-19 pandemic. J Rheumatol. 2020;47:783–786. doi: 10.3899/jrheum.200369. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gendelman O., Amital H., Bragazzi N.L., Watad A., Chodick G. Continuous hydroxychloroquine or colchicine therapy does not prevent infection with SARS-CoV-2: insights from a large healthcare database analysis. Autoimmun Rev. 2020 doi: 10.1016/j.autrev.2020.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah S., Das S., Jain A., Misra D.P., Negi V.S. A systematic review of the prophylactic role of chloroquine and hydroxychloroquine in coronavirus disease-19 (COVID-19) Int J Rheum Dis. 2020;23:613–619. doi: 10.1111/1756-185X.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monti S., Balduzzi S., Delvino P., Bellis E., Quadrelli V.S., Montecucco C. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis. 2020;79(5):667–668. doi: 10.1136/annrheumdis-2020-217424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jakhar D., Kaur I. Potential of chloroquine and hydroxychloroquine to treat COVID-19 causes fears of shortages among people with systemic lupus erythematosus. Nat Med. 2020;26(5):632. doi: 10.1038/s41591-020-0853-0. [DOI] [PubMed] [Google Scholar]
- 35.Kim A.H.J., Sparks J.A., Liew J.W., Putman M.S., Berenbaum F., Duarte-Garcia A. A rush to judgment? Rapid reporting and dissemination of results and its consequences regarding the use of hydroxychloroquine for COVID-19. Ann Intern Med. 2020;172:819–821. doi: 10.7326/M20-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moiseev S., Avdeev S., Brovko M., Novikov P., Fomin V. Is there a future for hydroxychloroquine/chloroquine in prevention of SARS-CoV-2 infection (COVID-19)? Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217570. [DOI] [PubMed] [Google Scholar]
- 37.Mehta B., Moezinia C.J., Jannat-Khah D., Gibofsky A., Tornberg H., Pearce-Fisher D. Hydroxychloroquine and chloroquine in COVID-19: a survey of prescription patterns among rheumatologists. J Clin Rheumatol. 2020 doi: 10.1097/RHU.0000000000001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alattar R., Ibrahim T.B.H., Shaar S.H., Abdalla S., Shukri K., Daghfal J.N. Tocilizumab for the treatment of severe coronavirus disease 2019. J Med Virol. 2020;127 doi: 10.1002/jmv.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alzghari S.K., Acuna V.S. Supportive treatment with tocilizumab for COVID-19: a systematic review. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325–ee31. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basatneh R., Vlahovic T.C. Addressing the question of dermatologic manifestations of SARS-CoV-2 infection in the lower extremities: a closer look at the available data and its implications. J Am Podiatr Med Assoc. 2020 doi: 10.7547/20-074. [DOI] [PubMed] [Google Scholar]
- 42.Bouaziz J.D., Duong T., Jachiet M., Velter C., Lestang P., Cassius C. Vascular skin symptoms in COVID-19: a french observational study. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandez-Nieto D., Jimenez-Cauhe J., Suarez-Valle A., Moreno-Arrones O.M., Saceda-Corralo D., Arana-Raja A. Characterization of acute acro-ischemic lesions in non-hospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.05.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harvey J.B., Valenta S., Simpson K., Lyles M., McElligott J. Utilization of outpatient telehealth services in parity and nonparity states 2010-2015. Telemed J E Health. 2019;25(2):132–136. doi: 10.1089/tmj.2017.0265. [DOI] [PubMed] [Google Scholar]
- 45.Wosik J., Fudim M., Cameron B., Gellad Z.F., Cho A., Phinney D. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc. 2020;27(6):957–962. doi: 10.1093/jamia/ocaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shenoy P., Ahmed S., Paul A., Skaria T.G., Joby J., Alias B. Switching to teleconsultation for rheumatology in the wake of the COVID-19 pandemic: feasibility and patient response in India. Clin Rheumatol. 2020 doi: 10.1007/s10067-020-05200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.