Abstract
Background
Epidemiological evidence suggests that anti-inflammatory and immunomodulatory properties of statins may reduce the risk of infections and infection-related complications.
Objective
We aimed to assess the impact of prior statin use on coronavirus disease (COVID-19) severity and mortality.
Methods
In this observational multicenter study, consecutive patients hospitalized for COVID-19 were enrolled. In-hospital mortality and severity of COVID-19 assessed with National Early Warning Score (NEWS) were deemed primary and secondary outcomes, respectively. Propensity score (PS) matching was used to obtain balanced cohorts.
Results
Among 842 patients enrolled, 179 (21%) were treated with statins before admission. Statin patients showed more comorbidities and more severe COVID-19 (NEWS 4 [IQR 2–6] vs 3 [IQR 2–5], p < 0.001). Despite having similar rates of intensive care unit admission, noninvasive ventilation, and mechanical ventilation, statin users appeared to show higher mortality rates. After balancing pre-existing relevant clinical conditions that could affect COVID-19 prognosis with PS matching, statin therapy confirmed its association with a more severe disease (NEWS ≥5 61% vs. 48%, p = 0.025) but not with in-hospital mortality (26% vs. 28%, p = 0.185). At univariate logistic regression analysis, statin use was confirmed not to be associated with mortality (OR 0.901; 95% CI: 0.537 to 1.51; p = 0.692) and to be associated with a more severe disease (NEWS≥5 OR 1.7; 95% CI 1.067–2.71; p = 0.026).
Conclusions
Our results did not confirm the supposed favorable effects of statin therapy on COVID-19 outcomes. Conversely, they suggest that statin use should be considered as a proxy of underlying comorbidities, which indeed expose to increased risks of more severe COVID-19.
Keywords: Coronavirus disease 2019 (COVID-19), Statin therapy, In-hospital mortality, Acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Introduction
Coronavirus disease 2019 (COVID-19) is a viral infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), potentially leading to acute respiratory distress syndrome (ARDS) requiring invasive ventilation and intensive care unit (ICU) admission. It has to be underlined though, that some patients may be asymptomatic or have mildly symptomatic COVID-19.1, 2, 3 Despite the exact pathophysiologic mechanisms of COVID-19 remain largely unknown, it has been suggested that, during the response to SARS-CoV-2 infection, the immune dysregulation and the high levels of pro-inflammatory cytokines might represent pivotal causes of tissue injury.4, 5, 6 The severity of COVID-19 seems to be related with older age and comorbidities. In particular, patients with pre-existing cardiovascular disease (CVD) have an elevated risk of adverse outcomes, and, on the other hand, SARS-CoV-2 infection is associated with vascular and arrhythmic complications.10, 11, 12, 7, 8, 9
Traditional cardiovascular (CV) risk factors, such as dyslipidemia, are recognized to have an impact on the immune system,13, 14, 15 and therefore it has been postulated that pre-existing CV risk factors may be related with the immune response to SARS-CoV-2 infection and with COVID-19 severity.7
Hydroxymethyl-glutaryl Coenzyme A (HMG-CoA) reductase inhibitors, or statins, which are widely used to prevent and treat CVD because of their lipid-lowering ability,16 have been proposed to have a role as an add-on treatment for COVID-19 patients.17 , 18 This role has been suggested not only in light of their proven lipid-lowering ability, but mostly because of their known pleiotropic effects on vascular balance, thrombogenic response,19 oxidative stress, and inflammation, at different levels.20 Indeed, some observational studies conducted among outpatients and hospitalized patients with sepsis and/or
Pneumonia, have demonstrated that statin therapy is associated with a reduced risk of pneumonia, a milder disease, and a reduced mortality,21, 22, 23, 24 while others25 , 26 did not show a beneficial relationship between statin use and clinical outcomes in the same settings. Despite mechanisms of actions and advantages of statins that have been proposed,17 little is known about their real world clinical impact in COVID-19 patients. Hence, we performed a retrospective study to assess the association of statin therapy at hospital admission with clinical outcomes in COVID-19 patients.
Methods
Study population
The study was approved by the institutional ethics board of the Italian hospitals involved (Luigi Sacco Hospital, Milan; Policlinico Umberto I Hospital, Rome; Spedali Civili Hospital, Brescia; Humanitas Gavazzeni Hospital; Bergamo). From February 23, 2020 to March 31, 2020, 987 consecutive patients presenting to the emergency department (ED) of the hospitals involved were screened. Confirmed cases of COVID-19 were defined with a positive result on a reverse-transcriptase-polymerase-chain-reaction assay performed on a nasopharyngeal swab, in accordance with World Health Organization (WHO) guidelines.27
Inclusion criteria for study enrollment were:
-
•
age ≥ 18 years;
-
•
an in-hospital stay of at least 24 h, defined as ED management and subsequent early discharge or hospital admission;
-
•
all patients included in the study had completed their hospital course (discharge or death) at the time of data analysis.
Study design
Patients were divided into 2 groups (statin vs. no-statin group): patients were included in the statin group if they were taking statins for at least 1 month before hospital admission for COVID-19. Data recorded at the time of ED admission/hospitalization included age, sex, medical history, symptoms reported to ED presentation, time from symptom onset, vital signs, laboratory values, arterial blood gas analysis, home medical therapy. Data regarding in-hospital management, medical treatment and clinical outcomes were evaluated during data collection. Chronic kidney disease (CKD) was defined by the presence of an estimated glomerular filtration rate (eGFR) ≤ 60 mL/min/m2, calculated with the CKD-EPI equation. Fever was defined as axillary temperature of at least 37.5 °C. Sepsis and septic shock were defined according to the 2016 Third International Consensus Definition.28 Myocardial injury was defined in accordance with the fourth universal definition of myocardial infarction (MI)29 (high sensitivity cardiac troponin [hs-cTn] above the 99th percentile of the upper reference limit). National Early Warning Score (NEWS)30 was retrospectively assessed to determine the severity of illness and clinical deterioration at the moment of hospital admission. The six parameters included in this scoring system are respiratory rate, oxygen saturation, temperature, systolic blood pressure, heart rate and level of consciousness (Table 1 - data supplement). Patients with moderate to severe disease were defined by a NEWS ≥ 5 while patients with mild disease were defined by a NEWS < 5, in order to establish a group of patients that in real-world multicenter registry may exhibit a more severe disease. The primary outcome was in-hospital mortality. The secondary outcome of interest was the COVID-19 severity at presentation, defined by a NEWS ≥ 5.
Table 1.
Balancing check of covariates before and after propensity score matching based on logit model.
| Unmatched or matched | mean |
ASMD |
t-test |
|||
|---|---|---|---|---|---|---|
| Statin | No-Statin | t | p | |||
| Male | U | 0.704 | 0.593 | −0.2293 | −2.836 | 0.005 |
| M | 0.690 | 0.669 | −0.042 | −0.376 | 0.707 | |
| Age > 65 | U | 0.737 | 0.416 | −0.6652 | −7.898 | <.001 |
| M | 0.731 | 0.690 | −0.091 | −0.775 | 0.439 | |
| Obesity | U | 0.134 | 0.0830 | −0.1756 | −1.846 | 0.037 |
| M | 0.124 | 0.117 | −0.021 | −0.180 | 0.858 | |
| Hypertension | U | 0.771 | 0.365 | −0.8644 | −10.262 | <0.001 |
| M | 0.731 | 0.697 | −0.076 | −0.648 | 0.517 | |
| Diabetes | U | 0.352 | 0.116 | −0.6550 | −6.222 | <.001 |
| M | 0.303 | 0.359 | 0.0915 | 0.997 | 0.319 | |
| Smoking | U | 0.185 | 0.0920 | −0.2950 | −2.964 | 0.003 |
| M | 0.138 | 0.124 | −0.040 | −0.347 | 0.729 | |
| CAD | U | 0.413 | 0.0573 | −1.1595 | −9.371 | <.001 |
| M | 0.284 | 0.248 | −0.077 | −0.663 | 0.507 | |
| AF | U | 0.168 | 0.0679 | −0.3533 | −3.363 | <.001 |
| M | 0.159 | 0.152 | −0.019 | −0.162 | 0.872 | |
| HF | U | 0.112 | 0.0271 | −0.4127 | −3.460 | <.001 |
| M | 0.0966 | 0.0966 | 0.000 | 0.000 | 1.000 | |
| CKD | U | 0.140 | 0.0558 | −0.3235 | −3.053 | 0.003 |
| M | 0.124 | 0.131 | 0.020 | 0.175 | 0.861 | |
| COPD | U | 0.0694 | 0.0838 | −0.0556 | −0.660 | 0.510 |
| M | 0.0828 | 0.103 | 0.071 | 0.605 | 0.546 | |
| Cerebrovascular disease | U | 0.0271 | 0.0782 | −0.2683 | −2.421 | 0.016 |
| M | 0.0552 | 0.0621 | 0.029 | 0.249 | 0.804 | |
| Malignancy | U | 0.112 | 0.0694 | −0.1577 | −1.655 | 0.062 |
| M | 0.0966 | 0.0552 | −0.0897 | −1.330 | 0.185 | |
ASMD = Absolute standardized mean difference; CAD = Coronary artery disease; AF = atrial fibrillation; HF = heart failure; CKD = Chronic kidney disease; COPD = Chronic obstructive pulmonary disease.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD) or median and interquartile range (IQR) if not normally distributed according to D'Agostino-Pearson test. Categorical data are expressed as absolute values and proportions. Student's t-test for independent samples with a confidence interval of 95% was used for continuous variables; otherwise, either logarithmic transformation of variables or nonparametric tests (Mann–Whitney U) were used. A chi-squared test or a Fisher exact test were performed to examine whether there was an association between categorical variables. Univariate linear regression was used to assess correlation between variables. Odds ratio (OR) and 95% confidence interval (CI) were reported. A two tailed P values < 0.05 were considered significant. All statistical analyses were performed using IBM SPSS Statistics (version 26.0).
Propensity score matching
We used the propensity score (PS) method to minimize the effect of potential confounders on selection bias. The PS for an individual was defined as the conditional probability of taking statin given the individual's clinical pre-existing conditions (covariates). To estimate the scores, we created a logistic regression model including twelve relevant covariates potentially related to COVID-19 clinical presentation and outcome. Variables considered for the PS matching were age ≥ 65 years, male gender, obesity, hypertension, diabetes, smoke, coronary artery disease (CAD), atrial fibrillation (AF), heart failure (HF), chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), cerebrovascular disease and malignancy. Patients with prior statin therapy use were matched 1:1 to no-statin therapy patients by PS, using the nearest neighbor method with a caliper of 0.1 and no replacement. Absolute standard difference and t-test were applied for balancing check of covariates both before and after matching. For good balancing, t-test for equality of means should be non-significant after matching, and the absolute standard difference should be < 0.1 after matching (Table 1).
Results
A total of 842 patients met the inclusion criteria and were therefore enrolled as study cohort. Propensity score matching (PS) identified 145 patients in each group according to prior statin use.
Baseline and characteristic on admission before and after propensity score matching
Patient demographic and clinical characteristics for the unadjusted and PS matched patients are shown in Table 2 . As many as thirteen covariates, identifying demographic and pre-existing clinical conditions (age, elderly patients [≥65 years], gender, obesity, hypertension, diabetes, dyslipidemia, smoke, CAD, AF, HF, CKD and cerebrovascular disease), were significantly different (p < 0.05) between the two groups before matching. However, all covariates were comparable and well balanced (p > 0.05) between both groups after PS matching. Before PS matching of demographic and pre-existing clinical variables, patients in statin therapy showed lower oxygen saturation, PaO2, PaO2/FiO2 ratio, and a higher NEWS (4 [IQR 2–6) vs. 3 [IQR 2–5] p < 0.001) with a higher incidence of patients on statin therapy presenting moderate/severe disease (as meant as a NEWS≥5) at the moment of hospital admission (58% vs. 44%, p < 0.001). After PS matching, only the presence of moderate/severe disease remained significantly more represented in the group of patients on statin therapy respect to patients in non-statin group (61% vs. 48%, p = 0.025). This secondary outcome remained significantly associated with statin use also to univariate logistic regression (OR 1.7; 95% CI 1.067–2.71; p = 0.026).
Table 2.
Demographic and clinical variables by group among unadjusted and propensity-matched cohorts, stratified by statin therapy.
| Variables | Overall population (N = 842) | Unadjusted data |
Propensity score matched data |
||||
|---|---|---|---|---|---|---|---|
| Statin group (N = 179) | No-statin group (N = 663) | P-value | Statin group (N = 145) | No-statin group (N = 145) | P-value | ||
| Demographics | |||||||
| Age, years | 64 (61–77) | 73 (65–81) | 61 (49–74) | < .001 | 71 (64–79) | 72 (61–80) | 0.707 |
| Age ≥ 65, N (%) | 123 | 276 | < .001 | 106 (73) | 100 (69) | 0.439 | |
| Male gender, N (%) | 519 (62) | 126 (70) | 393 (59) | 0.006 | 100 (69) | 97 (67) | 0.707 |
| BMI (kg/m2) ≥ 30 kg/m, N (%) | 79 (9) | 24 (13) | 55 (8) | 0.037 | 18 (12) | 17 (12) | 0.797 |
| Comorbidities, N (%) | |||||||
| Hypertension | 380 (45) | 138 (77) | 242 (37) | < .001 | 106 (73) | 101 (70) | 0.517 |
| Diabetes | 140 (17) | 63 (35) | 77 (12) | < .001 | 44 (30) | 52 (36) | 0.319 |
| Dyslipidemia | 201 (24) | 177 (98) | 24 (4) | < .001 | |||
| Smoking | 94 (11) | 33 (18) | 61 (9) | < .001 | 20 (14) | 18 (12) | 0.729 |
| CAD | 112 (13) | 74 (41) | 38 (6) | < .001 | 41 (28) | 36 (25) | 0.507 |
| Atrial fibrillation | 75 (9) | 30 (17) | 45 (7) | < .001 | 23 (17) | 22 (15) | 0.872 |
| Heart failure | 38 (5) | 20 (11) | 18 (3) | < .001 | 14 (10) | 14 (10) | 1.000 |
| CKD | 62 (7) | 25 (14) | 37 (6) | < .001 | 18 (13) | 19 (13) | 0.861 |
| COPD | 61 (7) | 15 (8) | 46 (7) | 0.510 | 12 (8) | 15 (10) | 0.546 |
| Cerebrovascular disease | 32 (4) | 14 (8) | 18 (3) | 0.001 | 8 (6) | 9 (6) | 0.804 |
| Malignancy | 66 (8) | 20 (11) | 46 (7) | 0.062 | 14 (10) | 8 (6) | 0.185 |
| Statins therapy, N (%) | |||||||
| Atorvastatin | 87 (49) | – | |||||
| Simvastatin | 54 (30) | – | |||||
| Rosuvastatin | 29 (16) | – | |||||
| Other Statins | 9 (5) | – | |||||
| Symptoms on admission, N (%) | |||||||
| Cough | 594 (71) | 118 (66) | 476 (72) | 0.126 | 100 (69) | 107 (74) | 0.364 |
| Shortness of breath | 402 (48) | 96 (54) | 306 (46) | 0.076 | 76 (52) | 74 (51) | 0.815 |
| Sore throat | 149 (18) | 18 (10) | 131 (20) | 0.003 | 16 (11) | 29 (20) | 0.035 |
| Sputum production | 39 (5) | 10 (6) | 29 (4) | 0.494 | 9 (6) | 7 (5) | 0.608 |
| Myalgia | 97 (12) | 26 (15) | 71 (11) | 0.156 | 22 (15) | 20 (14) | 0.740 |
| Diarrhea | 87 (10) | 20 (11) | 67 (10) | 0.678 | 18 (12) | 11 (8) | 0.172 |
| Nausea/Vomiting | 39 (5) | 12 (7) | 27 (4) | 0.138 | 12 (8) | 5 (3) | 0.081 |
| Symptom onset, days | 6 (3–9) | 7 (3–10) | 0.346 | 6 (3–8) | 7 (3–10) | 0.438 | |
| Hospital admission | |||||||
| Fever, N (%) | 736 (87) | 149 (83) | 587 (89) | 0.058 | 121 (83) | 129 (89) | 0.174 |
| Temperature, °C | 38 (37.5–38.3) | 38 (37.5–34.4) | 38 (37.6–38.3) | 0.111 | 38 (37.5–38.4) | 38 (37.5–38.2) | 0.628 |
| Heart rate, beat x min | 38 (75–99) | 82 (73–96) | 86 (75–99) | 0.061 | 82 (75–95) | 85 (74–100) | 0.698 |
| Respiratory Rate,min. | 20 (18–21) | 20 (18–20) | 20 (18–21) | 0.129 | 20 (18–20) | 20 (20–22) | 0.075 |
| Oxygen saturation, % | 96 (93–98) | 95 (91–97) | 96 (93–98) | < .001 | 95 (91–97) | 96 (92–98) | 0.137 |
| PaO2/FiO2, mmHg | 310 (190–386) | 265 (133–338) | 324 (214–395) | < .001 | 274 (149–348) | 305 (184–367) | 0.082 |
| PaO2, median | 78 (67–89) | 76 (63–88) | 78 (68–90) | 0.048 | 74.5 (62.3–87) | 74 (63–86.5) | 0.710 |
| MAP, mmHg | 97 (90–105) | 99 (90–107) | 97 (90–105) | 0.222 | 100 (90–110) | 95 (87–103) | 0.064 |
| NEWS | 3 (2–5) | 4 (2–6) | 3 (2–5) | < .001 | 4 (2–6) | 3 (2–5) | 0.054 |
| Moderate/severe disease (NEWS ≥ 5) | 104 (58) | 291 (44) | < .001 | 88 (61) | 69 (48) | 0.025 | |
Continuous variables are reported as median (interquartile range).
BMI=Body mass index; CAD= Coronary artery disease; CKD = Chronic kidney disease; COPD= Chronic obstructive pulmonary disease; PaO2 = oxygen partial pressure at arterial gas analysis; FiO2 = fraction of inspired oxygen; MAP = mean aortic pressure; NEWS = National Early Warning Score.
In-hospital management and outcomes before and after propensity score matching
Data regarding in-hospital management and clinical outcomes are summarized in Table 3 . In the overall population, patients in the statin group were more likely to receive oxygen support (86% vs. 75% p = 0.002) and to develop myocardial injury (20% vs. 9%, p < 0.001) than patients in non-statin group, as well as showing higher all-cause mortality rate (29% vs. 20%, p = 0.006) and a longer in-hospital stay. With regard to laboratory and radiological findings (Table 4 ), in the non-matched population, patients in the statin group showed a higher white blood cell count, lactate dehydrogenase, creatine phosphokinase, creatinine, and hs-cTn, with lower levels of hemoglobin compared with patients in non-statin group patients. Based on radiological findings, pneumonia was diagnosed in the majority of patients (89%) on admission, with a higher incidence among patients in statin therapy (94 vs. 87%; p = 0.013) and also showed worse radiological features. After PS matching, only hemoglobin level remained significantly lower in the statin group respect to no-statin group (13.2; IQR 12–14.4 vs. 13.6; IQR 12.4–15; p = 0.037). Of note, in the statin patient cohort, a significantly lower level of C-reactive protein was noted with respect to the control group (20.2; IQR 8.13–99.7 vs. 36; IQR 11.6–99.7; p = 0.021). Moreover, both groups showed a similar prevalence of pneumonia on admission, while worse radiological features were confirmed after PS matching in the statin group. While mortality rates significantly differed among the two unmatched groups (29% vs. 20%, p = 0.006), after PS matching no significant differences were found (26% vs. 28%, p = 0.185). At univariate logistic regression analysis for mortality, statin use was confirmed not to be associated with the primary outcome (OR 0.901; 95% CI: 0.537 to 1.51; p = 0.692).
Table 3.
In-hospital management and outcomes by group among unadjusted and propensity-matched cohorts, stratified by statin therapy.
| Variables | Overall population (N = 842) | Unadjusted data |
Propensity score matched data |
||||
|---|---|---|---|---|---|---|---|
| Statin group (N = 179) | No-statin group (N = 663) | P-value | Statin group (N = 145) | No-statin group (N = 145) | P-value | ||
| In-hospital treatment, N (%) | |||||||
| Non-invasive ventilation | 286 (34) | 66 (37) | 220 (33) | 0.355 | 49 (34) | 50 (34) | 0.902 |
| Oxygen suypport | 651 (77) | 154 (86) | 497 (75) | 0.002 | 122 (84) | 114 (79) | 0.229 |
| Intubation | 42 (5) | 6 (3) | 36 (5) | 0.258 | 6 (4) | 10 (7) | 0.305 |
| Antibiotics | 371 (44) | 84 (47) | 287 (43) | 0.385 | 65 (45) | 85 (59) | 0.019 |
| Corticosteroids | 93 (11) | 23 (13) | 70 (11) | 0.386 | 17 (12) | 18 (12) | 0.858 |
| Hydroxychloroquine | 680 (81) | 152 (85) | 528 (80) | 0.112 | 121 (83) | 120 (83) | 0.876 |
| Antiviral therapy | 466 (55) | 93 (52) | 373 (56) | 0.304 | 72 (50) | 96 (66) | 0.040 |
| Heparin | 382 (47) | 94 (53) | 298 (45) | 0.072 | 70 (48) | 63 (43) | 0.411 |
| Tocilizumab | 126 (15) | 24 (13) | 102 (15) | 0.511 | 19 (13) | 12 (8) | 0.185 |
| Outcomes, N (%) | |||||||
| ICU admission | 46 (5) | 6 (3) | 40 (6) | 0.162 | 6 (4) | 11 (8) | 0.213 |
| ARDS | 155 (18) | 41 (23) | 114 (17) | 0.080 | 24 (17) | 32 (22) | 0.235 |
| Myocardial Injury | 96 (11) | 35 (20) | 61 (9) | < .001 | 25 (17) | 28 (19) | 0.670 |
| Acute kidney injury | 26 (3) | 5 (3) | 21 (3) | 0.798 | 4 (3) | 9 (6) | 0.157 |
| Septic Shock/Sepsis | 19 (2) | 5 (3) | 14 (2) | 0.586 | 4 (3) | 4 (3) | 1.000 |
| Death | 182 (22) | 52 (29) | 130 (20) | 0.006 | 38 (26) | 41 (28) | 0.185 |
| Hospital stay, days | 9 (5–15) | 11 (6–16) | 9 (5–14) | 0.026 | 11 (6–16) | 11 (7–16) | 0.920 |
ICU = intensive care unit; ARDS = acute respiratory distress syndrome.
Table 4.
Laboratory findings by group among unadjusted and propensity-matched cohorts, stratified by statin therapy.
| Variables | Reference range | Unadjusted data |
P-value | Propensity score matched data |
P-value | ||
|---|---|---|---|---|---|---|---|
| Statin group (N = 179) | No-statin group (N = 663) | Statin group (N = 145) | No-statin group (N = 145) | ||||
| Laboratory findings | |||||||
| White blood cell count, per 109/L | 4.19–9.35 | 6.4 (4.8–9.53) | 5.9 (4.6–7.99) | 0.029 | 6.22 (4.8–9.14) | 6.0 (4.97–8.22) | 0.678 |
| Lymphocytes, per 109/L | 1.13–3.37 | 0.93 (0.66–1.3) | 0.98 (0.7–1.4) | 0.183 | 0.98 (0.695–1.3) | 0.98 (0.68–1.36) | 0.635 |
| Neutrophils, per 109/L | 1.91–6.23 | 4.69 (3.13–7.3) | 4.14 (3–6) | 0.013 | 4.64 (3.17–6.39) | 4.4 (3.36–6.39) | 0.621 |
| Monocytes, per 109/L | 0.29–0.81 | 0.45 (0.3–0.7) | 0.43 (0.3–0.6) | 0.124 | 0.43 (0.3–0.7) | 0.46 (0.33–0.62) | 0.870 |
| Platelet count, per 109/L | 155–360 | 201 (143–282) | 193 (153–252) | 0.401 | 207 (148–277) | 196 (154–241) | 0.355 |
| Haemoglobin, g/dL | 12.0–6.0 | 13.2 (12–14.4) | 13.8 (12.7–14.8) | < .001 | 13.2 (12–14.4) | 13.6 (12.4–15) | 0.037 |
| C-Reactive protein, mg/L | <10 | 20.6 (8.72–67.4) | 18.4 (6.01–67.5) | 0.303 | 20.2 (8.13–99.7) | 36 (11.6–99.7) | 0.021 |
| Lactate dehydrogenase, U/L | 125–360 | 360 (271–492) | 310 (235–404) | 0.001 | 352 (261–473) | 335 (269–403) | 0.328 |
| Creatine phosphokinase, U/L | 29–322 | 134 (73.8–283) | 98 (58–181) | 0.004 | 113 (73.8–289) | 134 (65–229) | 0.156 |
| Creatinine, mg/dL | 0.5–0.95 | 1.04 (0.84–1.4) | 0.92 (0.77–1.1) | < .001 | 1 (0.827–1.33) | 1.01 (0.83–1.3) | 0.728 |
| High-sensitivity cardiac troponin T, ng/L | <14 | 31 (13–50) | 16 (7–40) | < .001 | 31 (13–43) | 32 (17.5–64) | 0.088 |
| D-Dimer, μg/L | <500 | 806 (356–2901) | 918 (490–2228) | 0.739 | 772 (351–3171) | 1566 (572–3171) | 0.404 |
| Chest X-Ray findings, N (%) | |||||||
| Pneumonia on admission | 168 (94) | 578 (87) | 0.013 | 137 (95) | 129 (89) | 0.089 | |
| Pulmonary infiltrations | 131 (73) | 425 (64) | 0.023 | 98 (68) | 106 (73) | 0.305 | |
| Ground-glass opacity | 104 (58) | 330 (50) | 0.048 | 84 (58) | 55 (38) | <.001 | |
| Consolidation lesions | 113 (63) | 313 (47) | < .001 | 88 (61) | 61 (42) | 0.002 | |
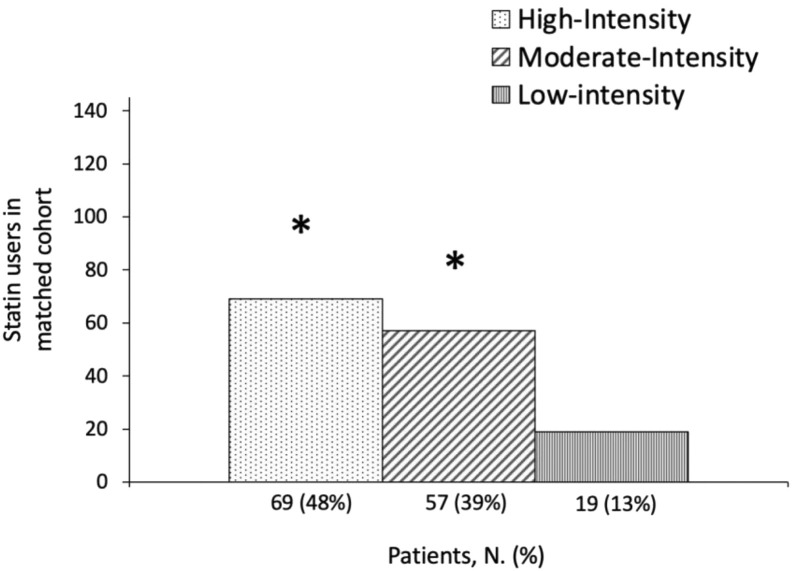
Statin intensity analysis in propensity score matched population
In-hospital mortality and severity of disease within the balanced cohort of statin users, stratified by statin intensity,31 are shown in Table 5 . The majority of patients were treated with high intensity (48%) and moderate-intensity statins (39%) respect to low-intensity (13%) (p < 0.05) (Fig. 1 ). At univariate logistic regression, patients using high intensity and moderate intensity statins showed a worse clinical presentation of COVID-19 (NEWS ≥ 5) at hospital presentation respect to patients taking low-intensity statins (OR 3.88; 95% CI: 1.356–11.10 p = 0.011 and OR 2.96; 95% CI 1.022–8.55; p = 0.046, respectively). Of note, these data did not correlate with the in-hospital mortality that remained similar among groups.
Table 5.
Multinomial univariate logistic regression of in-hospital death and moderate/severe disease (NEWS ≥ 5) according to statin intensity.
| Statin Intensity | Predictor | Estimate | SE | Z | p | OR | 95% Confidence Interval |
|
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Moderate Intensity vs. Low Intensity | Death | 0.357 | 0.635 | 0.562 | 0.574 | 1.43 | 0.412 | 4.96 |
| NEWS ≥ 5 | 1.084 | 0.542 | 2.00 | 0.046 | 2.96 | 1.022 | 8.55 | |
| High Intensity vs. Low Intensity | Death | 0.439 | 0.621 | 0.707 | 0.480 | 1.55 | 0.459 | 5.24 |
| NEWS ≥ 5 | 1.357 | 0.536 | 2.52 | 0.011 | 3.88 | 1.356 | 11.10 | |
| High Intensity vs. Moderate Intensity | Death | 0.0823 | 0.404 | 0.203 | 0.839 | 1.167 | 0.773 | 1.762 |
| NEWS ≥ 5 | 0.273 | 0.376 | 0.728 | 0.467 | 1.314 | 0.629 | 2.745 | |
OR = odds ratio; NEWS = National Early Warning Score.
Fig. 1.
Schematic representation of statin intensity distribution in the matched cohort of statin users (N = 145).∗p < 0.05 vs. Low-Intensity group.
Discussion
To the best of our knowledge, this is the first study specifically focused on the role of statins in COVID-19 patients. Hereby, we report data demonstrating that patients on prior statin therapy had a worse disease severity at the time of hospital admission for COVID-19 with respect to non-statin users, but nevertheless, statin therapy was not associated with an increased risk of in-hospital death. The proportion of our patients receiving statins (21%) was similar to that reported in other studies.32 In the statin group, 46% of patients took statins for secondary prevention, whereas 54% took statins for dyslipidemia and primary prevention. The overall mortality rate reported in our cohort is higher than reported in similar series from China3 , 9 , 33 , 34 but is consistent with other cohorts from Italy and USA.35, 36, 37 Our findings in the overall population confirmed that patients with CVD, and therefore receiving statins, have a higher mortality rate (29%), similar to what reported in other cohorts (Inciardi et al.,37 mortality in cardiac patients: 35.8%). Indeed, statin use underlies the presence of CV risk factors as dyslipidemia or CAD, taking into account that patients with CVD are supposed to exhibit worse outcomes.34 , 38 This finding can explain why patients with COVID-19 taking domiciliary statin therapy showed an increased in-hospital mortality. This negative relation is further confirmed by the reduction in this mortality rate after balancing pre-existing CV risk factors with PS matching.
The higher COVID-19 severity in the statin group, assessed with NEWS, is not matched with a higher mortality rate in these patients. In our series, COVID-19 severity was evaluated with NEWS to avoid confounding factors related with in-hospital treatment and ICU admission, also because, due to the Italian critical care increasing demand during the COVID-19 outbreak,39 , 40 we could not rely on ICU admission. Indeed, mechanical ventilation and ICU admission were sometimes reserved to younger patients with less comorbidities that were more likely to recover from severe COVID-19. Indeed, while SOFA score showed its ability to predict the in-hospital mortality in sepsis patients already admitted in ICU, NEWS showed a greater ability to predict the risk of ICU admission among patients evaluated in the ED.41 For these reasons, in contrast with other similar studies,2 , 37 we preferred NEWS over SOFA score or other scores useful to track patients’ status during the stay in ICU.
Several studies have evaluated the role of statins as an add-on treatment during infections. In a metanalysis, Tleyeh et al.42 examined nine cohorts addressing the role of statins in treating bacteremia, pneumonia, and sepsis, and they showed that statin use could be associated with a beneficial effect in treating and preventing various infections, with an adjusted effect estimate of 0.55 (95% confidence interval, 0.36–0.83; I2 = 76.5%) in favor of statins.42 Conversely, Yende et al.,25 among 1895 inpatients with community-acquired pneumonia did not found evidence of a protective effect of statin use on meaningful clinical outcomes. Similarly, in a population-based case-control study on 3360 patients, Dublin et al.26 showed that statin use was not associated with decreased risk of pneumonia among immunocompetent community-dwelling older people. It has been postulated that statins may have beneficial effects in influencing the clinical course of COVID-19 by mitigating the impact of viral infection through their immunomodulatory and anti-inflammatory properties.43 In an animal model, statins have shown to stabilize MYD88 levels and attenuate myocardial remodeling after a proinflammatory trigger such as hypoxia44 that is a crucial feature of SARS-CoV-2 infection. This supposed beneficial effect was only partially noticed in our cohort, as patients receiving domiciliary statin therapy showed a lower C-reactive protein level, although a worse PaO2 and PaO2/FiO2 ratio on admission.
Moreover, COVID-19 patients with CV comorbidities exhibit direct cardiac involvement and worse outcomes.34 , 38 In this setting, COVID-19 patients may theoretically take advantage of statin therapy also for their cardioprotective actions and for the upregulation of Angiotensin-Converting Enzyme (ACE) 2 via epigenetic modifications,45 that SARS-CoV-2 employs for cellular entry.46
Our results did not confirm these supposed favorable effects of statin therapy, even after propensity score matching, so that our different results can mainly be explained by a “healthy user effect” and with indication bias noticed in other observational studies on statin use in similar clinical settings.42 The higher severity of COVID-19, exhibited in patients on high-intensity statin treatment, could be partially explained with the enhanced severity of underlying CV risk factors and CVD in those patients.
Limitations
Because of the retrospective nature of our study, whether our patients received statins was extracted from our in-hospital medical records, and specific data on the in-hospital drug regimen of statins (continuation vs. discontinuation) are lacking, though generally these drugs were continued during hospitalization, at least in the non-ICU setting. Indeed, precise data on drug administration in the ICU setting are lacking. Another potential limitation is represented by the struggle to assess compliance with statin therapy up to the patient's admission to the hospital. Given the logistical limitations during the COVID-19 outbreak, some laboratory data (i.e. lipid profile) were not collected in all patients. Moreover, our in-hospital based study cannot be considered representative of the overall “statin population” affected by COVID-19. Thus, since this was not a population-based study, it is still possible that the use of statin therapy could be associated with improved outcome in mild disease where patients do not seek hospital care. Moreover, patients who came to the ED but were discharged early (<24 h from admission) were not included. Therefore, the potential beneficial effects of statin therapy in COVID-19 patients treated outside of the hospital setting and its impact on mild disease need to be further investigated.
Conclusions
Our data suggest that statin therapy does not affect in-hospital mortality in COVID-19 patients. Therefore, caution should be warranted in attributing benefits to statin therapy as an adjuvant or independent therapy and a part of the standard care for COVID-19 patients. These effects may instead reflect unmeasured baseline differences between users and non-users, that in our study were limited by using propensity score matching.
Footnotes
Founding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest: None.
Contribution statements: Gianfranco Mitacchione and Giovanni B. Forleo conceived and direct the study. Gianfranco Mitacchione and Marco Schiavone wrote the manuscript in consultation with Antonio Curnis. Giosuè Mascioli, Paolo Severino, Federica Sabato, Maria M. Caracciolo, Gianmarco Arabia collected the data. Alessio Gasperetti and Laura D'Erasmo performed the statistical analysis, Spinello Antinori drafts tables and figures. Maurizio Viecca, Marcello Arca and Massimo Galli contributed to the interpretation of the result. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Supplementary data related to this article can be found online at https://doi.org/10.1016/j.jacl.2020.12.008.
Supplementary data
References
- 1.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Wan S., Yi Q., Fan S. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) medRxiv. 2020 [Google Scholar]
- 6.Schiavone M., Gobbi C., Biondi-Zoccai G. Acute coronary syndromes and covid-19: exploring the uncertainties. J Clin Med. 2020;9(6):1683. doi: 10.3390/jcm9061683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li B., Yang J., Zhao F. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitacchione G., Schiavone M., Gasperetti A., Forleo G.B. Ventricular tachycardia storm management in a COVID-19 patient: a case report. Eur Heart J Case Rep. 2020;4(FI1):1–6. doi: 10.1093/ehjcr/ytaa217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiavone M., Gasperetti A., Mancone M. Oral anticoagulation and clinical outcomes in COVID-19: an Italian multicenter experience. Int J Cardiol. 2020:1–5. doi: 10.1016/j.ijcard.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiavone M., Gasperetti A., Mancone M. Redefining the prognostic value of high-sensitivity troponin in COVID-19 patients: the importance of concomitant coronary artery disease. J Clin Med. 2020;9(10):3263. doi: 10.3390/jcm9103263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Libby P., Ridker P.M., Hansson G.K. Inflammation in atherosclerosis. From pathophysiology to practice. J Am Coll Cardiol. 2009;54(23):2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tall A.R., Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15(2):104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saltiel A.R., Olefsky J.M. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127(1):1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mach F., Baigent C., Catapano A.L. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 17.Castiglione V., Chiriacò M., Emdin M., Taddei S., Vergaro G. Statin therapy in COVID-19 infection. Eur Heart J Cardiovasc Pharmacother. 2020;6(4):258–259. doi: 10.1093/ehjcvp/pvaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu-Farha M., Thanaraj T.A., Qaddoumi M.G., Hashem A., Abubaker J., Al-Mulla F. The role of lipid metabolism in COVID-19 virus infection and as a drug target. Int J Mol Sci. 2020;21(10) doi: 10.3390/ijms21103544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao J.K., Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45(8):89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakashima B.I., Restrepo M., Anzueto A.M., Mortensen E. The potential role of statins in pneumonia. Curr Respir Med Rev. 2010;6(3):155–161. [Google Scholar]
- 21.Schlienger R.G., Fedson D.S., Jick S.S., Jick H., Meier C.R. Statins and the risk of pneumonia: a population-based, nested case-control study. Pharmacotherapy. 2007;27(3):325–332. doi: 10.1592/phco.27.3.325. [DOI] [PubMed] [Google Scholar]
- 22.Van De Garde E.M.W., Hak E., Souverain P.C., Hoes A.W., Van Den Bosch J.M.M., Leufkens H.G.M. Statin treatment and reduced risk of pneumonia in patients with diabetes. Thorax. 2006;61(11):957–961. doi: 10.1136/thx.2006.062885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myles P.R., Hubbard R.B., McKeever T.M., Pogson Z., Smith C.J.P., Gibson J.E. Risk of community-acquired pneumonia and the use of statins, ace inhibitors and gastric acid suppressants: a population-based case-control study. Pharmacoepidemiol Drug Saf. 2009;18(4):269–275. doi: 10.1002/pds.1715. [DOI] [PubMed] [Google Scholar]
- 24.Liappis A.P., Kan V.L., Rochester C.G., Simon G.L. The effect of statins on mortality in patients with bacteremia. Clin Infect Dis. 2001;33(8):1352–1357. doi: 10.1086/323334. [DOI] [PubMed] [Google Scholar]
- 25.Yende S., Milbrandt E.B., Kellum J.A. Understanding the potential role of statins in pneumonia and sepsis. Crit Care Med. 2011;39(8):1871–1878. doi: 10.1097/CCM.0b013e31821b8290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dublin S., Jackson M.L., Nelson J.C., Weiss N.S., Larson E.B., Jackson L.A. Statin use and risk of community acquired pneumonia in older people: population based case-control study. BMJ. 2009;338(7709):1486. doi: 10.1136/bmj.b2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO Laboratory Testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. WHO - Interim Guid. 2020;2019(January):1-7. https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117
- 28.Seymour C.W., Liu V.X., Iwashyna T.J. Assessment of clinical criteria for sepsis for the third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(8):762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thygesen K., Alpert J.S., Jaffe A.S. Fourth universal definition of myocardial infarction (2018) Eur Heart J. 2019;40(3):237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 30.Smith G.B., Prytherch D.R., Meredith P., Schmidt P.E., Featherstone P.I. The ability of the National Early Warning Score (NEWS) to discriminate patients at risk of early cardiac arrest, unanticipated intensive care unit admission, and death. Resuscitation. 2013;84(4):465–470. doi: 10.1016/j.resuscitation.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Grundy S.M., Stone N.J., Bailey A.L. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73(24):e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Almog Y., Shefer A., Novack V. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110(7):880–885. doi: 10.1161/01.CIR.0000138932.17956.F1. [DOI] [PubMed] [Google Scholar]
- 33.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA, J Am Med Assoc. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically ill patients in the seattle region — case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pellicori P. At the heart of COVID-19. Eur Heart J. 2020;41(19):1830–1832. doi: 10.1093/eurheartj/ehaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lala A., Johnson K., Januzzi J. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 40.Schiavone M., Forleo G.B., Mitacchione G., Gasperetti A., Viecca M., Tondo C. Quis custodiet ipsos custodes: are we taking care of healthcare workers in the Italian COVID-19 outbreak? J Hosp Infect. 2020;105(3):580–581. doi: 10.1016/j.jhin.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khwannimit B., Bhurayanontachai R., Vattanavanit V. Comparison of the accuracy of three early warning scores with SOFA score for predicting mortality in adult sepsis and septic shock patients admitted to intensive care unit. Heart Lung. 2019;48(3):240–244. doi: 10.1016/j.hrtlng.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Tleyjeh I.M., Kashour T., Hakim F.A. Statins for the prevention and treatment of infections: a systematic review and meta-analysis. Arch Intern Med. 2009;169(18):1658–1667. doi: 10.1001/archinternmed.2009.286. [DOI] [PubMed] [Google Scholar]
- 43.Zeiser R. Immune modulatory effects of statins. Immunology. 2018;154(1):69–75. doi: 10.1111/imm.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan X., Deng Y., Guo X., Shang J., Zhu D., Liu H. Atorvastatin attenuates myocardial remodeling induced by chronic intermittent hypoxia in rats: partly involvement of TLR-4/MYD88 pathway. Biochem Biophys Res Commun. 2014;446(1):292–297. doi: 10.1016/j.bbrc.2014.02.091. [DOI] [PubMed] [Google Scholar]
- 45.Fedson D.S., Opal S.M., Rordam O.M. Hiding in plain sight: an approach to treating patients with severe covid-19 infection. MBio. 2020;11(2) doi: 10.1128/mBio.00398-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin–angiotensin–aldosterone system inhibitors in patients with covid-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.