Highlights
-
•
Fifteen cytokines were increased at ICU admission in Covid-19 patients.
-
•
A relatively moderate increase in cytokine concentrations was observed.
-
•
IL-1ra, IL-6 and IP-10 correlated with respiratory failure and acute kidney injury.
-
•
IL-8 may be a future biomarker due to its correlation with 30-day mortality.
Keywords: COVID-19, Intensive care, Acute kidney injury, Biomarkers, Cytokine storm, Inflammation
Abstract
Background
The infection caused by SARS CoV-2 has been postulated to induce a cytokine storm syndrome that results in organ failure and even death in a considerable number of patients. However, the inflammatory response in Corona virus disease-19 (Covid-19) and its potential to cause collateral organ damage has not been fully elucidated to date. This study aims to characterize the acute cytokine response in a cohort of critically ill Covid-19 patients.
Method
24 adults with PCR-confirmed Covid-19 were included at time of admission to intensive care a median of eleven days after initial symptoms. Eleven adult patients admitted for elective abdominal surgery with preoperative plasma samples served as controls. All patients were included after informed consent was obtained. 27 cytokines were quantified in plasma. The expression of inflammatory mediators was then related to routine inflammatory markers, SAPS3, SOFA score, organ failure and 30-day mortality.
Results
A general increase in cytokine expression was observed in all Covid-19 patients. A strong correlation between respiratory failure and IL-1ra, IL-4, IL-6, IL-8 and IP-10 expression was observed. Acute kidney injury development correlated well with increased levels of IL-1ra, IL-6, IL-8, IL-17a, IP-10 and MCP-1. Generally, the cohort demonstrated weaker correlations between cytokine expression and 30-day mortality out of which IL-8 showed the strongest signal in terms of mortality.
Conclusion
The present study found that respiratory failure, acute kidney injury and 30-day mortality in critically ill Covid-19 patients are associated with moderate increases of a broad range of inflammatory mediators at time of admission.
1. Introduction
Corona virus disease – 2019 (Covid-19) is an acute viral infection caused by severe acute respiratory syndrome virus 2 (SARS CoV-2), a novel β-coronavirus. Clinical presentation ranges from mild to severe illness associated with acute respiratory failure requiring intensive care and considerable mortality. No specific treatment exists to date thus intensive care measures are mainly supportive. ICU mortality has been reported between 16 and 78% [1]. Virally induced acute respiratory distress syndrome (ARDS) and systemic hyperinflammation appear common features of critically ill Covid-19 patients [2]. Elevated cytokine levels and infection-related biomarkers are associated with increasing severity of illness [3], [4]. The pronounced inflammatory response, often described as a cytokine storm syndrome, has become a point of interest for potential anti-inflammatory treatments such as interleukin-6 antagonists and glucocorticoids [5]. Inflammation is a complex and dynamic response that is a result of an intricate network of numerous interacting mediators that alters over time [6]. No immunomodulatory treatment has thus far become mainstay treatment in ARDS and glucocorticoids are only implemented in sepsis during refractory shock [7], [8]. Hence, the introduction, target and timing of immunomodulatory therapies remains elusive in Covid-19. Further understanding of the inflammatory response in Covid-19 is required to delineate targets and timing of possible immunomodulatory treatments. The present study aims to characterize the inflammatory profile at time of admission in a cohort of patients with PCR confirmed Covid-19 requiring intensive care treatment and to correlate plasma cytokine expression with routine infection-related biomarkers, physiological scoring, organ failure and 30-day mortality.
2. Materials and method
The study was approved by the National Ethical Review Agency (EPM) (No. 2020-01623). The protocol of the study was registered prior to initiation (Clinical Trials ID: NCT04316884). Informed consent was obtained from the patient or next of kin if the patient was unable to give consent. The Declaration of Helsinki and its subsequent revisions were followed. STROBE guidelines were followed for reporting. This prospective observational study was performed at the intensive care unit (ICU) of Uppsala University Hospital, Sweden, a tertiary hospital.
2.1. Data collection and patient groups
A total of 35 patients with plasma samples in the PRONMED Biobank (BbA-827-2018-009) were included in the study. The patients were 24 adults with PCR-confirmed Covid-19 admitted to the ICU. This was the initial Covid-19 cohort treated in the intensive care unit at Uppsala University Hospital between March 14th and April 8th, 2020. Controls were eleven adult patients admitted for elective abdominal surgery for peritoneal carcinosis with preoperative plasma samples in the PRONMED Biobank.
Clinical data were recorded prospectively daily. Simplified Acute Physiology Score 3 (SAPS3), Sequential Organ Failure Assessment (SOFA) score, renal function, circulatory support and respiratory support data were collected as reported in the results [9], [10]. Patients with acute kidney injury (AKI) were identified according to Kidney Disease: Improving Global Outcome (KDIGO) criteria [11]. PaO2/FiO2 ratio was estimated in the control cohort from registered oxygen saturation (SpO2) with a mean of 55.55 (±9.57). Blood samples were collected on the day of admission to the ICU. The most recent bloodsample preoperatively was used in the control group. Routine chemistry including hemoglobin, white blood cell count (WBC) and platelets, inflammatory markers including C-reactive protein (CRP), procalcitonin (PCT) and ferritin, and kidney function tests were performed in the hospital central laboratory.
2.2. Cytokine assay
Citrated plasma samples were analyzed for 27 biomarkers with Bio-plex assay using a Luminex MagPix instrument (Bio-Rad Laboratories AB, Sundbyberg, Sweden) as listed: Interleukin (IL)-1β, IL-2, IL-4 to IL-10, IL-12, IL-13, IL-15, IL-17A, interferon gamma (IFN γ), interferon gamma-induced protein 10 (IP-10), monocyte chemotactic protein 1 (MCP-1), macrophage inflammatory protein 1 alpha (MIP-1α), macrophage inflammatory protein 1 beta (MIP-1β), tumor necrosis factor (TNF α), IL-1 receptor antagonist (IL-1ra), Regulated on Activation, Normal T Cell Expressed and Secreted (RANTES), Platelet-derived growth factor BB (PDGF-bb), Basic fibroblast growth factor (FGF), Granulocyte colony-stimulating factor (GM-CSF), Granulocyte-macrophage colony-stimulating factor (GM-CSF), Vascular Endothelial Growth Factor (VEGF) and eotaxin. The cytokine concentrations were measured in plasma as we consider this superior to serum. During the coagulation process needed to prepare serum, the blood cells are activated and cytokines may be released from platelets and white blood cells.
2.3. Statistical analysis
Continuous variables are presented as mean (SD) or median (IQR) and categorical variables as number of observations (percent of total number of observations). Mann-Whitney U test was used for group comparisons, Bonferroni adjusted. Correlation between variables was analyzed with Spearman Rank. A two-sided p < 0.05 was considered significant. The mean of routine chemistry values from the day of admission was calculated in the case of multiple analysis performed in the Covid-19 cohort. Cytokines with observed concentrations below limit of detection were treated as missing in the statistical analysis. All statistical analyses were performed using SPSS Statistics, Version 26.0 (IBM SPSS Statistics, IBM Corp). Figures were constructed using R version 3.5.0.
3. Results
3.1. Patient characteristics
The controls had an even distribution of men and women and the most common comorbidities were hypertension and diabetes in addition to their cancer diagnosis (Table 1 ). In the Covid-19 cohort the majority were male (75%). The most common comorbidities were hypertension, pulmonary disease and diabetes mellitus (54%, 29% and 25% respectively). One fifth (21%) of the Covid-19 cohort were treated with corticosteroids before admission to the ICU. The median ICU admission was eleven days after the initial symptoms associated with Covid-19. Median SAPS3 score and SOFA score at the day of admission were 53(13) and 6(6) respectively. During the first seven days of intensive care 18 (75%) patients required mechanical ventilation and 16 (67%) patients were treated with norepinephrine, no other vasopressor was administered. 24 (100%) patients developed AKI based on increased creatinine and/or loss of urine production. 17 (71%) developed AKI grade 2 and three (13%) patients progressed to grade 3. Seven (29%) patients died within 30 days after ICU admission.
Table 1.
Patient Characteristics.
| Control | Covid19 | |
|---|---|---|
| n (%) | 11 (100%) | 24 (100%) |
| Age mean, (SD) | 65.09 (4.09) | 56.29 (16.35) |
| Women, n (%) | 6 (55%) | 6 (25%) |
| BMI, mean (SD) | 28.46 (6.03) | 28.85 (4.50) |
| Covid day arrival, mean (SD) | NA | 10.74 (3.74) |
| Hypertension, n (%) | 4 (36%) | 13 (54%) |
| Heartfailure, n (%) | 1 (9%) | 0 (0%) |
| Ischemic heart disease, n (%) | 0 (0%) | 3 (13%) |
| Diabetes mellitus, n (%) | 2 (18%) | 6 (25%) |
| Lungdisease, n (%) | 1 (9%) | 7 (29%) |
| Leverfailure, n (%) | 0 (0%) | 0 (0%) |
| Malignancy, n (%) | 11 (100%) | 2 (8%) |
| Steroid treatment before admission, n (%) | 1 (9%) | 5 (21%) |
| 30-day mortality n (%) | 0 (0%) | 7 (29%) |
| SAPS3, mean (SD) | NA | 54 (9.05) |
| SOFA day of admission, mean (SD) | NA | 6 (2.93) |
| SOFA day 3, mean (SD) | NA | 6 (2.05) |
| SOFA day 7, mean (SD) | NA | 7 (1.98) |
| PaO2/FiO2 (kPa) lowest day 1, mean (SD) | NA | 15.8 (3.82) |
| PaO2/FiO2 (kPa) lowest during first week, mean (SD) | NA | 12.7 (2.57) |
| Mechanical ventilation, n (%) | NA | 18 (75%) |
| Vasopressor, n (%) | NA | 16 (67%) |
| Acute Kidney Injury n (%) | 0 (0%) | 24 (100.0%) |
| Grade 1 n(%) | NA | 4 (17%) |
| Grade 2 n(%) | NA | 17 (71%) |
| Grade 3 n(%) | NA | 3 (13%) |
3.2. Routine chemistry in Covid-19 cohort vs control group
Hemoglobin was slightly below normal range (130–170 g/L) both in the controls preoperatively (129 g/L) and in the Covid-19 cohort (123 g/L). Leukocytes were not significantly different in controls (7.3 × 109/L) than in the Covid-19 cohort (8.9 × 109/L) at admission, both groups presented with values within the normal range (3.5–9.0 × 109/L). In addition, lymphocytes (0.9 × 109/L) and platelets (227 × 109/L) were within normal range in the Covid-19 cohort (0.7–3.9 × 109//L and 150-350 × 109/L respectively). At admission, neutrophils were above normal range (5.9 × 109/L, 1.3–5.4 × 109/L). CRP (<5 mg/L) and ferritin (25–310 μg/L) were distinctly elevated at admission (196 mg/L and 1647 μg/L respectively). PCT (<0.05 μg/L) was slightly increased at admission (1.1 μg/L) (Table 2 ).
Table 2.
Routine Chemistry at Admission.
| Controls preoperatively, mean (SD) | Covid19 ICU admission, mean (SD) | |
|---|---|---|
| Hemoglobin (g/L) | 129 (12) | 123 (19) |
| Leukocytes (10^9/L) | 7.3 (1.6) | 8.9 (7.8) |
| Lymphocytes (10^9/L) | NA | 0.9 (0.26) |
| Neutrophils (10^9/L) | NA | 5.9 (4.1) |
| Platelets (10^9/L) | 301 (123) | 227 (104) |
| C-Reactive protein (mg/L) | 18 (24) | 196 (95)* |
| Procalcitonin (µg/L) | NA | 1.1 (1.6) |
| Ferritin (µg/L) | NA | 1647 (1402) |
Denotes significant difference between groups Bonferroni adjusted.
3.3. Cytokine concentration at admission in Covid-19 cohort vs control
IL-5, IL-15 and VEGF had observed plasma concentrations (ρg/mL) that were consistently below detectable range. These were excluded from further statistical analyses. Nine cytokines, IL-1β (n = 10, 91%), IL-2 (n = 7, 64%), IL-7 (n = 10, 91%), IL-10 (n = 7, 64%), 1L-12 (n = 6, 55%), GM-CSF (n = 4, 36%), IFN γ (n = 9, 82%), PDGF-bb (n = 9, 82%) and MIP-1a (n = 4, 36%) had values below the detectable range in the control group (n = 11, 100%). GM-CSF (n = 19, 79%) also had values below detectable range in the Covid-19 cohort. Out of the 24 cytokines with detectable plasma concentrations, Il-1β, IL-1ra, IL-2, IL-4, IL-6, IL-7, IL-8, IL-10, IL-13, IL-17a, G-CSF, IFN γ, IP-10, MCP-1 and TNF α were significantly higher in the Covid-19 cohort than in controls at the time of admission to the ICU (Table 3 ). The ratio of TNF α to IL-10, which has been used to grade anti-inflammatory activity, was significantly different in the two groups, the mean ratio (±SD) in controls and Covid-19 was 405 (±102) and 26 (±3) respectively.
Table 3.
Plasma Cytokine Concentrations Covid-19 vs Controls.
| Cytokine | Control, mean (SD) | Covid19 ICU Day 1, mean (SD) |
|---|---|---|
| IL-1b | 2.87 (1.70) | 8.82 (9.11)* |
| IL-1ra | 117.07 (73.68) | 1139.83 (766.61)* |
| IL-2 | 3.63 (2.45) | 7.90 (2.60)* |
| IL-4 | 1.51 (0.47) | 3.44 (1.06)* |
| IL-6 | 4.19 (4.30) | 59.93 (71.04)* |
| IL-7 | 14.83 (8.31) | 32.23 (6.09)* |
| IL-8 | 5.02 (2.79) | 22.15 (13.84)* |
| IL-9 | 223.28 (74.14) | 284.05 (29.02) |
| IL-10 | 0.74 (1.03) | 8.55 (6.83)* |
| IL-12 | 3.39 (1.72) | 4.75 (2.18) |
| IL-13 | 2.49 (1.26) | 6.90 (6.52)* |
| IL-17A | 12.60 (6.76) | 24.50 (3.99)* |
| Eotaxin | 37.46 (15.72) | 42.17 (19.43) |
| FGF basic | 37.76 (17.71) | 53.92 (5.92) |
| G-CSF | 32.94 (15.61) | 82.80 (49.80)* |
| GM-CSF | 0.96 (0.61) | 2.19 (2.64) |
| IFN-g | 4.53 (3.08) | 26.67 (18.56)* |
| IP-10 | 397.88 (167.56) | 3856.66 (2801.47)* |
| MCP-1 | 22.52 (3.73) | 178.12 (220.31)* |
| MIP-1a | 1.31 (1.50) | 6.28 (8.51) |
| PDGF-bb | 472.64 (614.86) | 753.39 (448.80) |
| MIP-1b | 191.84 (60.92) | 243.33 (24.72) |
| RANTES | 3431.39 (1982.90) | 4025.62 (1694.89) |
| TNF-a | 88.45 (29.10) | 158.57 (77.31)* |
Denotes significant difference vs control (Bonferroni corrected).
3.4. Cytokine concentration at admission and outcome
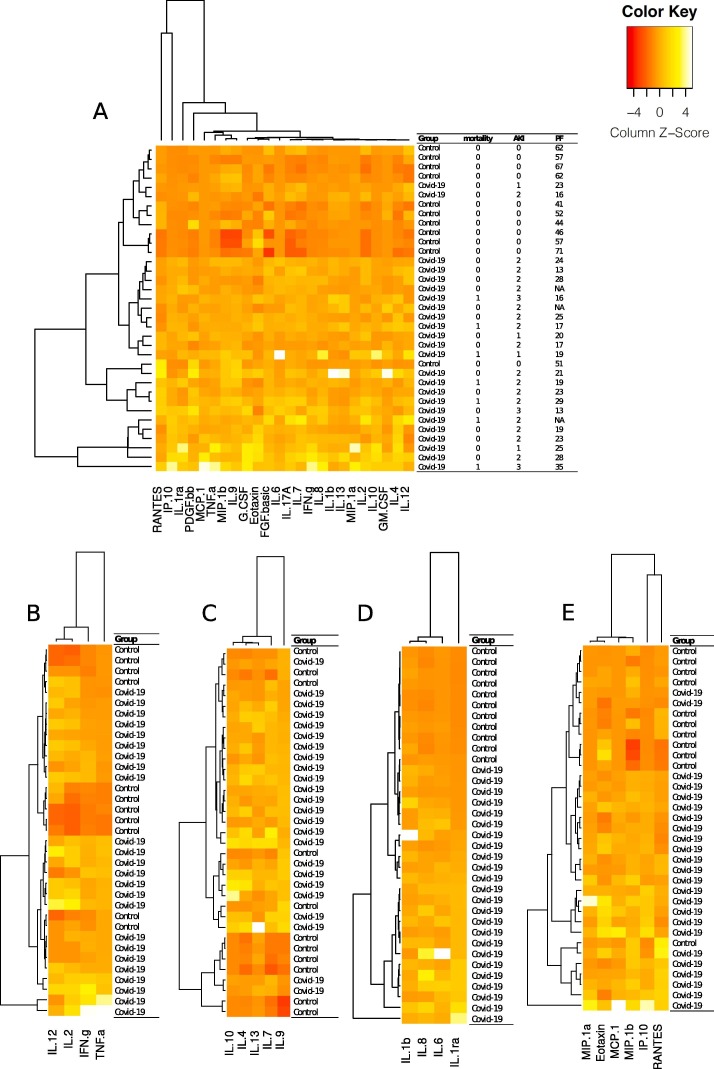
Plasma concentrations of IL-4, IL-6, IL-8, IL-10, FGF, IP-10, MCP-1 and TNF α correlated with 30-day mortality. Only a few cytokines, G-CSF, MIP-1a and TNF α correlated with SAPS score. The same cytokines with the addition of MCP-1 correlated weakly with SOFA score. Respiratory failure, presented as PaO2/FiO2 ratio, and AKI correlated with all detectable cytokines except IL-12, eotaxin, GM-CSF, MIP-1a, MIP-1b and RANTES (Fig. 1 ). To summarize, IL-12, eotaxin, GM-CSF and RANTES did not correlate with any of the outcome variables. The cytokines that correlated with respiratory failure, AKI and mortality were IL-4, IL-6, IL-8, IL-10, FGF, IP-10, MCP-1 and TNF α (Table 4 ). The ratio between TNF α and IL-10 correlated positively with respiratory failure (p = 0.000) and correlated negatively with AKI (p = 0.002) and 30-day mortality (p = 0.032).
Fig. 1.
A heatmap showing hierarchical clustering of individual patients according to the detectable plasma concentration of 24 out of 27 analyzed cytokines (A). The clustering of patients in the general heatmap is largely driven by the relatively high concentrations of IP-10 and RANTES (A). The cytokines were further separated into subgroups and shown in separate heatmaps; Th1-cytokines (B), Th2-cytokines (C), Nucleotide-binding oligomerization domain, Leucine rich Repeat and Pyrin domain containing Proteins (NLRP) associated cytokines (D) and chemokines (E). IL-12, IL-2, IFN γ and TNF α were categorized as Th1-cytokines. Th1 cytokines participate in cell mediated immunity, promotes inflammation and tissue damage [40]. IL-10, IL-4, IL-13, IL-7 and IL-9 were categorized as Th2-cytokines (C). Th2-cytokine expression plays a role in humoral immunity and may act anti-inflammatory [41]. NLPR-associated mediators (D) IL-1β, IL-6, IL-8 and IL-1ra take part in the innate immune response including promoting immune cell infiltration of infected tissues. Notably, these cytokines clustered the groups perfectly [42], [43]. MIP-1a, eotaxin, MCP-1, MIP-1b, IP-10 and RANTES were grouped as chemokines [44]. The color key of the calculated z-score represents a scale of −4 to 4 SD where the individual value departs from the group mean as shown in the figure.
Table 4.
Correlations between Cytokine Concentration and Outcome.
| Cytokine | 30-day Mortality | C-reactive protein | SAPS3 | SOFA ICU day 1 | Lowest PaO2/FiO2 ICU day 1 | Acute Kidney Injury |
|---|---|---|---|---|---|---|
| IL-1b | 0.212 | 0.344 | −0.031 | 0.144 | −0.625 * | 0.659 * |
| IL-1ra | 0.297 | 0.711* | 0.278 | 0.195 | −0.722 * | 0.715 * |
| IL-2 | 0.335 | 0.388* | 0.338 | −0.003 | −0.413* | 0.421* |
| IL-4 | 0.351* | 0.459* | 0.270 | 0.192 | −0.697 * | 0.667 * |
| IL-6 | 0.410* | 0.785* | 0.208 | 0.290 | −0.722* | 0.760 * |
| IL-7 | 0.258 | 0.250 | −0.044 | −0.058 | −0.567 * | 0.559 * |
| IL-8 | 0.503* | 0.715* | 0.239 | 0.344 | −0.681 * | 0.728* |
| IL-9 | 0.311 | 0.420* | 0.017 | −0.030 | −0.450* | 0.405 * |
| IL-10 | 0.463* | 0.484* | 0.346 | 0.223 | −0.667* | 0.614 * |
| IL-12 | 0.334 | 0.106 | −0.165 | −0.143 | −0.217 | 0.122 |
| IL-13 | 0.152 | 0.373* | 0.010 | 0.269 | −0.532 * | 0.637 * |
| IL-17a | 0.286 | 0.518* | 0.086 | −0.021 | −0.656* | 0.705 * |
| Eotaxin | 0.149 | −0.124 | 0.211 | 0.266 | −0.105 | 0.101 |
| FGF basic | 0.416* | 0.245 | 0.239 | −0.031 | −0.554 * | 0.520 * |
| G-CSF | 0.334 | 0.431* | 0.496 * | 0.437* | −0.586 * | 0.592 * |
| GM-CSF | 0.287 | 0.201 | −0.130 | 0.134 | −0.318 | 0.353 |
| IFN-g | 0.308 | 0.704* | 0.310 | 0.368 | −0.621* | 0.692 * |
| IP-10 | 0.410* | 0.695* | 0.361 | 0.357 | −0.704* | 0.826 * |
| MCP-1 | 0.439* | 0.619* | 0.280 | 0.476* | −0.635* | 0.762 * |
| MIP-1a | 0.281 | 0.422* | 0.504* | 0.460* | −0.114 | 0.274 |
| PDGF-bb | 0.132 | 0.348 | 0.282 | −0.163 | −0.426 * | 0.381 * |
| MIP-1b | 0.301 | 0.418* | 0.113 | 0.072 | −0.338 | 0.304 |
| RANTES | 0.226 | 0.177 | 0.078 | 0.25 | −0.186 | 0.219 |
| TNF-a | 0.410* | 0.590* | 0.546* | 0.432* | −0.568* | 0.577 * |
Denotes significant correlation (p < 0.05).
4. Discussion
The main finding of the present study is that organ failure in critically ill Covid-19 patients is associated with increases of a broad range of inflammatory mediators at time of admission. IL-1β, IL-1ra, IL-2, IL-4, IL-6, IL-7, IL-8, IL-10, IL-13, IL-17a, G-CSF, IFN γ, IP-10, MCP-1 and TNF α were all significantly raised at admission. At this timepoint all patients presented with respiratory failure. The cytokines that were markedly elevated compared to the controls were IL-1ra, IL-6, IL-10 and IP-10. A previous study by Huang et al showed elevations in IL-2, IL-7, IL-10, G-CSF, IP-10, MCP-1, MIP-1a and TNF α and observed higher levels of expression in critical patients with Covid-19 [12]. In line with this study, all but one of the mentioned mediators were significantly elevated in our critically ill cohort although some more moderately than others.
Dysregulated and exaggerated inflammatory processes are known to result in collateral organ damage in conditions such as sepsis and ARDS. In the present study, the actual concentrations of plasma cytokines, although raised in the Covid-19 cohort, are in some cases rather moderate in comparison to other inflammatory states with associated organ failure such as sepsis [13]. For example, IL-6 measured in plasma is often raised to the thousands in sepsis and septic shock while IL-6 ranged between 4 and 271 ρg/mL in our study [14]. A recent study noted differences in IL-6 levels at the moderate level between severe and critical Covid-19 and suggested its use as a biomarker [15]. Another study in patients admitted to the ICU due to septic shock had plasma IL-8 levels reaching several hundred ρg/mL yet we found a range of 6–62 ρg/mL [16].
The present result strengthens the argument that Covid-19 patients that progress to respiratory failure are in a state of inflammation with a general increase in inflammatory mediators. The term cytokine storm syndrome (CSS) is often mentioned as a propagator of severe Covid-19. CSS refers to a state of hypercytokinemia, hyperferritinemia and multi-organ-failure [17]. In our study, Covid-19 patients presented with increased levels of ferritin as well as at least dual organ failure involving lungs and kidneys. The cytokines raised in CSS including IL-1β, IL-2, IL-6, IL-17a, IL-8, TNF α and MCP-1 were significantly yet moderately elevated in our cohort. Other key characteristics involving the reticuloendothelial system such as bone marrow hemophagocytosis were lacking [3]. There remains a question regarding the dynamics of cytokine release in Covid-19 and if cytokine storm is the most apt description [18].
The TNF α and IL-10 ratio is commonly used to estimate pro-inflammatory vs anti-inflammatory activity in the inflammatory response [19]. In our study, this ratio was strongly correlated with respiratory failure (r = 0.731) and although significantly correlated with AKI and 30-day mortality these relationships were weaker (r = −0.543 and r = −0.388 respectively). This signals a warped balance between the gas and break of the inflammatory response related to organ failure and mortality. However, cytokines are small (<40 kDa) proteins produced in response to molecular patterns associated with pathogens or self-injury. Their effects are dynamic dependent on context and a simple pro- vs anti-inflammatory division does not always hold true [12], [20].
In our study IFN γ was raised at the time of admission. Coronaviruses, such as those causing Severe Acute Respiratory Syndrome (SARS) and Middle Eastern Respiratory Syndrome (MERS) in addition to SARS CoV-2, have previously been noted to demonstrate interferon suppression that in turn has been suggested to initially delay the innate immune response and later contribute to its dysregulation and collateral lung damage [21], [22]. The patients in this study were admitted at a median of eleven days after initial symptoms and respiratory failure was already present at admission and hence the IFN γ levels may reflect a later stage in the disease.
The Covid-19 cohort presented with respiratory failure at the time of admission, with a mean PaO2/FiO2 of 15.8 (±3.82) kPa during the first day of intensive care. During the first week in the ICU 75% of patients required mechanical ventilation and these selected patients met the criteria of moderate to severe ARDS according to Berlin criteria [23]. The mean PaO2/FiO2 was lowered in the Covid-19 patients during the first week pointing towards a progression in respiratory failure. The inflammatory mediators that correlated with PaO2/FiO2 included the majority of cytokines in our assay which may be attributed to the lung as the focal point of the infection and inflammatory response. The development of and poor prognosis in ARDS of other etiologies has been associated with a span of inflammatory markers including IL1β, IL-2, IL-4, IL-6, IL-8 and TNF α [24], [25]. IL-2, IL-4, IP-10 and MCP-1 have specifically been associated with mortality in ARDS [26]. Our findings are in-line with these previous studies. The cytokines that displayed the strongest correlation with respiratory failure were IL-1ra, IL-6 and IP-10 (r > 0.700, p = 0.000, p = 0.000 and p = 0.000 respectively). IP-10 is a chemokine secreted by epithelial cells in the airways as well as several immune cells including lymphocytes and neutrophils in response to TNF α and IFN γ [27]. It promotes and maintains inflammation and has been designated a biomarker in airway infections, interestingly it has been suggested as protective in coronavirus infections [28].
All Covid-19 patients in our study developed AKI during the first week in the ICU. This incidence is much higher than previously reported in Covid-19 [29], [30], [31], [32]. In ARDS in general the reported incidence of AKI is close to 50% [33]. The reason for our high incidence is unclear. It may be attributed to the large number of mechanically ventilated patients or other factors such as a restrictive fluid regime [34]. Although 67% of patients were treated with norepinephrine, they remained relatively hemodynamically stable as reflected in the SOFA score. Nearly all of the cytokines in our study correlated with AKI development. However, those with the strongest correlation were IL-1ra, IL-6, IL-8, IL-17a, IP-10 and MCP-1 (r > 0.700 respectively). IL1β, IL-6, IL-8 and TNF α have been associated with AKI in ARDS and these were all increased at time of admission in our cohort. In addition, IL-6 and IL-8 were among those with the strongest correlation. AKI in Covid-19 has been associated with increased mortality [32], [35]. Furthermore, AKI development early in ARDS is independently associated with longer duration of mechanical ventilation and increased mortality [36]. The interaction between AKI and ARDS development is not completely understood to date although partly attributed to inflammation [37]. Inflammatory mediators that have been designated possible roles in this lung-kidney interaction are IL1β, IL-6, IL-8, TNF α and MIP-2 [38].
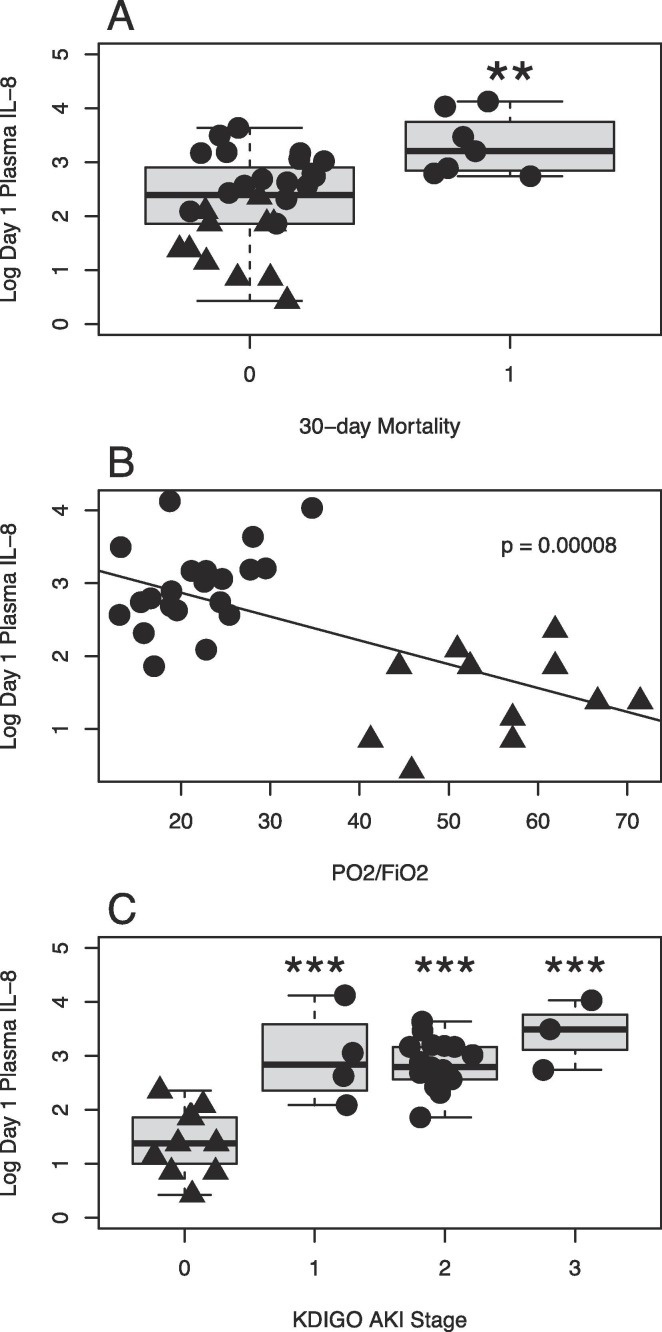
A small majority of the analyzed inflammatory mediators correlated with 30-day mortality. However, these correlations were generally weaker than those with respiratory failure and AKI. The strongest correlation could be seen for IL-8 (r = 0.503, p = 0.002). IL-8 also correlated strongly with respiratory failure and AKI (Fig. 2 ). IL-8 is a chemokine released by a variety of cell types including endothelial and epithelial cells as well as macrophages and monocytes. Its receptors are expressed on various leukocytes including neutrophils and natural killer cells (NK cells). Activation induces chemotaxis and in the case of neutrophils also degranulation and generation of reactive oxygen species [39]. The accumulation of activated neutrophils causing collateral damage to lung tissue has been suggested previously as a mechanism in ARDS.
Fig. 2.
Plasma concentrations of IL-8 at admission to intensive care in Covid-19 patients is associated with 30-day mortality (A), respiratory failure presented as PaO2/FiO2 (B) and acute kidney injury (AKI) stratified by Kidney Disease: Improved Global Outcome (C). Plasma concentrations of IL-8 demonstrated the strongest correlation with 30-day mortality (r = 0.503, p = 0.002) among the 27 cytokines analyzed in our panel. Increased plasma expression of IL-8 in Covid-19 correlated well with both respiratory failure (r = −0.681) as well as AKI development (r = 0.728, p = 0.000). Data shown as logarithmically transformed plasma concentration preoperatively in controls (filled triangles) and at admission to intensive care in Covid-19 patients (filled circles).
There are several limitations to this study. First of all, the controls although not displaying any deterioration in organ functions preoperatively had a malignant diagnosis and may therefore have an affected inflammatory state at baseline. Furthermore, we do not study the inflammatory mediators over time. Many of these patients require long periods of intensive care before clinical improvement. Hence, it would be of interest to follow the inflammatory response during the entire ICU stay and elucidate any inflammatory profile differences in organ failure resolution compared to organ failure progression and mortality. Lastly, the small number of patients constitutes an additional limitation.
5. Conclusion
Organ failure in critically ill Covid-19 patients is associated with increases of a broad range of inflammatory mediators at time of admission to the ICU. However, some appear only moderately increased in comparison to other inflammatory conditions such as sepsis. A strong correlation was found between respiratory failure and IL-1ra, IL-4, IL-8 and IP-10 expression. Acute kidney injury development was frequent and correlated well with increased levels of IL-1ra, IL-6, IL-8, IL-17a, IP-10 and MCP-1. Weaker correlations were generally observed between cytokine expression and 30-day mortality. IL-8 demonstrated the strongest signal in terms of mortality. The dynamics of cytokine release during the course of severe Covid-19 needs further investigation, as do the question whether cytokine storm syndrome is the most suitable description of the pathogenesis.
CRediT authorship contribution statement
Sara Bülow Anderberg: Resources, Formal analysis, Visualization, Writing - original draft, Writing - review & editing. Tomas Luther: Investigation, Resources, Writing - review & editing. Malin Berglund: Investigation, Writing - review & editing. Rolf Larsson: Investigation, Resources, Writing - review & editing. Sten Rubertsson: Conceptualization, Supervision, Writing - review & editing. Miklos Lipcsey: Conceptualization, Methodology, Resources, Supervision, Project administration, Writing - review & editing. Anders Larsson: Conceptualization, Resources, Writing - review & editing. Robert Frithiof: Conceptualization, Methodology, Resources, Supervision, Project administration, Funding acquisition, Writing - review & editing. Michael Hultström: Conceptualization, Methodology, Resources, Supervision, Project administration, Funding acquisition, Visualization, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement and Funding
The study was funded by SciLifeLab/KAW national COVID-19 research program project grant to Michael Hultström (KAW 2020.018). The study was also in part supported by funds from the Swedish Research Council granted to Robert Frithiof (grant no 2014-02569 and 2014-07606).
We thank the study nurses Joanna Wessbergh and Elin Söderman and the biobank research assistants Philip Karlsson and Erik Danielsson for their expert help with compiling study data and organizing sample analysis.
References
- 1.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGonagle D., Sharif K., O'Regan A. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;102537 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W., Zhao Y., Zhang F. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin. Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reiss L.K., Schuppert A., Uhlig S. Inflammatory processes during acute respiratory distress syndrome: a complex system. Curr. Opin. Crit. Care. 2018;24(1):1–9. doi: 10.1097/MCC.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg K.P., Hudson L.D., Goodman R.B. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N. Engl. J. Med. 2006;354(16):1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 8.Rhodes A., Evans L.E., Alhazzani W. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 9.Moreno R.P., Metnitz P.G., Almeida E. SAPS 3–From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31(10):1345–1355. doi: 10.1007/s00134-005-2763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vincent J.L., Moreno R., Takala J. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 11.Section 2: AKI Definition. Kidney Int. Suppl. (2011) 2012; 2(1):19-36. [DOI] [PMC free article] [PubMed]
- 12.Kany S., Vollrath J.T., Relja B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019;20(23):6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumoto H., Ogura H., Shimizu K. The clinical importance of a cytokine network in the acute phase of sepsis. Sci. Rep. 2018;8(1):13995. doi: 10.1038/s41598-018-32275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozza F.A., Salluh J.I., Japiassu A.M. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit. Care. 2007;11(2):R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C., Fei D., Li X. IL-6 may be a good biomarker for earlier detection of COVID-19 progression. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chishti A.D., Shenton B.K., Kirby J.A. Neutrophil chemotaxis and receptor expression in clinical septic shock. Intensive Care Med. 2004;30(4):605–611. doi: 10.1007/s00134-004-2175-y. [DOI] [PubMed] [Google Scholar]
- 17.Behrens E.M., Koretzky G.A. Review: cytokine storm syndrome: looking toward the precision medicine era. Arthrit. Rheumatol. 2017;69(6):1135–1143. doi: 10.1002/art.40071. [DOI] [PubMed] [Google Scholar]
- 18.Kox M., Waalders N.J.B., Kooistra E.J. Cytokine levels in critically Ill patients with COVID-19 and other conditions. JAMA. 2020 doi: 10.1001/jama.2020.17052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsurumi A., Que Y.-A., Ryan C.M. TNF-α/IL-10 ratio correlates with burn severity and may serve as a risk predictor of increased susceptibility to infections. Front. Public Health. 2016;4(216) doi: 10.3389/fpubh.2016.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavaillon J.M. Pro- versus anti-inflammatory cytokines: myth or reality. Cell Mol. Biol. (Noisy-le-grand) 2001;47(4):695–702. [PubMed] [Google Scholar]
- 21.S. Wan, Q. Yi, S. Fan et al., Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv 2020:2020.2002.2010.20021832.
- 22.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Seminars Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Force* TADT: Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 307(23) (2012) 2526-2533. [DOI] [PubMed]
- 24.Fujishima S. Pathophysiology and biomarkers of acute respiratory distress syndrome. J. Intensive Care. 2014;2(1):32. doi: 10.1186/2052-0492-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terpstra M.L., Aman J., van Nieuw Amerongen G.P. Plasma biomarkers for acute respiratory distress syndrome: a systematic review and meta-analysis*. Crit. Care Med. 2014;42(3):691–700. doi: 10.1097/01.ccm.0000435669.60811.24. [DOI] [PubMed] [Google Scholar]
- 26.Bautista E., Arcos M., Jimenez-Alvarez L. Angiogenic and inflammatory markers in acute respiratory distress syndrome and renal injury associated to A/H1N1 virus infection. Exp. Mol. Pathol. 2013;94(3):486–492. doi: 10.1016/j.yexmp.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Quint J.K., Donaldson G.C., Goldring J.J.P. Serum IP-10 as a biomarker of human rhinovirus infection at exacerbation of COPD. Chest. 2010;137(4):812–822. doi: 10.1378/chest.09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu M., Guo S., Hibbert J.M. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011;22(3):121–130. doi: 10.1016/j.cytogfr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu Y., Sun J., Dai Z. Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Clin. Virol. 2020;127:104371. doi: 10.1016/j.jcv.2020.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L., Li X., Chen H. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am. J. Nephrol. 2020:1–6. doi: 10.1159/000507471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ronco C., Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat. Rev. Nephrol. 2020:1–3. doi: 10.1038/s41581-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pei G., Zhang Z., Peng J. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J. Am. Soc. Nephrol. 2020 doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panitchote A., Mehkri O., Hastings A. Factors associated with acute kidney injury in acute respiratory distress syndrome. Ann. Intensive Care. 2019;9(1):74. doi: 10.1186/s13613-019-0552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hultström M., von Seth M., Frithiof R. Hyperreninemia and low total body water may contribute to acute kidney injury in COVID-19 patients in intensive care. J. Hypertens. 2020;38(8):1613–1614. doi: 10.1097/HJH.0000000000002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng Y., Luo R., Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNicholas B.A., Rezoagli E., Pham T. Impact of early acute kidney injury on management and outcome in patients with acute respiratory distress syndrome: a secondary analysis of a multicenter observational study. Crit. Care Med. 2019;47(9):1216–1225. doi: 10.1097/CCM.0000000000003832. [DOI] [PubMed] [Google Scholar]
- 37.Darmon M., Clec'h C., Adrie C. Acute respiratory distress syndrome and risk of AKI among critically ill patients. Clin. J. Am. Soc. Nephrol. 2014;9(8):1347–1353. doi: 10.2215/CJN.08300813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malek M., Hassanshahi J., Fartootzadeh R. Nephrogenic acute respiratory distress syndrome: A narrative review on pathophysiology and treatment. Chin. J. Traumatol. 2018;21(1):4–10. doi: 10.1016/j.cjtee.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russo R.C., Garcia C.C., Teixeira M.M. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev. Clin. Immunol. 2014;10(5):593–619. doi: 10.1586/1744666X.2014.894886. [DOI] [PubMed] [Google Scholar]
- 40.Raphael I., Nalawade S., Eagar T.N. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74(1):5–17. doi: 10.1016/j.cyto.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torre D., Speranza F., Giola M. Role of Th1 and Th2 Cytokines in immune response to uncomplicated <em>Plasmodium falciparum</em> Malaria. Clin. Diagn. Lab. Immunol. 2002;9(2):348–351. doi: 10.1128/CDLI.9.2.348-351.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelley N., Jeltema D., Duan Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019;20(13) doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamkanfi M., Dixit V.M. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Zlotnik A., Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36(5):705–716. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]