Abstract
The first confirmed case of novel Coronavirus Disease 2019 (COVID-19) in the United States was reported on January 20, 2020. As of November 24, 2020, close to 12.2 million cases of COVID-19 was confirmed in the US, with over 255,958 deaths. The rapid transmission of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), its unusual and divergent presentation has strengthened the status of COVID-19 as a major public health threat. In this review, we aim to 1- discuss the epidemiological data from various COVID-19 patient cohorts around the world and the USA as well the associated risk factors; 2- summarize the pathophysiology of SARS-CoV-2 infection and the underlying molecular mechanisms for the respiratory and cardiovascular manifestations; 3- highlight the potential treatments and vaccines as well as current clinical trials for COVID-19.
Keywords: Physiopathology, COVID-19, Clinical features, Treatment
Abbreviations and acronyms: AC, Anticoagulation; ACE2, Angiotensin-converting enzyme 2; AngI, Angiotensin I; ARB, AngII-type I receptor blockers; ARDS, Acute respiratory distress syndrome; AT1R, angiotensin II type 1 receptor; BNP, B-type natriuretic peptide; CMRI, Cardiovascular magnetic resonance; COVID-19, Coronavirus disease 2019; CRP, C-reactive protein; CT, Computed tomography; CTD, C-terminal domain; DENV, Dengue virus; DIC, Disseminated intravascular coagulation; DPP4, Dipeptidyl peptidase IV; EC, Endothelial cells; ECG, Electrocardiogram; ECMO, Extracorporeal membrane oxygenation; FDA, Food and drug administration; GGO, Ground-glass opacities; HIV, Human immunodeficiency virus infection; HCoV, Human coronavirus; ICU, Intensive care Unit; IFN, Interferon; IgG, Immunoglobulin G; IL, Interleukin; JAK2, Janus kinase 2; JEV, Japanese encephalitis virus; LV, Left ventricle; LVEF, Left ventricular ejection fraction; MERS-CoV, Middle east respiratory syndrome-coronavirus; MRA, Mineralocorticoid receptor antagonists; MRI, Magnetic resonance imaging; NTD, N-terminal domain; PAR-1, Proteinase-activated receptor 1; PE, Pulmonary embolism; PEEP, Positive end-expiratory pressure; PKC, Protein kinase C; RAS, Renin-angiotensin system; RBD, Receptor-binding domain; RBM, Receptor-binding motif; RDV, Remdesivir; RV, Right ventricle; SARS-CoV, Severe acute respiratory syndrome coronavirus; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; S-protein, Spike protein; STAT3, Signal transducer and activators of transcription 3; STEMI, ST-segment elevated myocardial infarction; TMPRSS2, Transmembrane serine protease 2; TNF, Tumor necrosis factor; TTC, Takotsubo cardiomyopathy; TTE, Transthoracic echocardiography; vWF, Von Willebrand factor; WHO, World Health Organization
Graphical abstract
1. Introduction
In December 2019, many cases of pneumonia-like disease of unknown etiology were reported in the Wuhan/Hubei providence of China. The novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was identified as the cause of the disease named Coronavirus Disease 2019 (COVID-19) [1,2]. COVID-19 was declared a pandemic by the World Health Organization in March 2020. As of November 24 2020, the number of confirmed cases had passed 59.8 million worldwide, and the death toll had risen to more than 1,408,271. While SARS-CoV-2 is not the first coronavirus to infect humans or to become a global health concern, many questions have arisen regarding factors influencing one’s individual risk and clinical outcomes since the emergence of the COVID-19 pandemic.
The COVID-19 Associated Hospitalization Surveillance Network (COVID-NET, including 14 US States) was created to conduct population-based surveillance for RT-PCR confirmed COVID-19 hospitalizations from March 1-30, 2020. The COVID-NET reported that among a cohort of 1,482 hospitalized patients, 74.5% were greater than 50 years old, with higher rates of hospitalization in adults greater than 65 years of age, and 54.4% were male [3]. The majority (90%) of these patients had at least one or more of these underlying chronic conditions: obesity, hypertension, chronic lung disease, diabetes, and other cardiovascular diseases [3]. Alongside age-dependent susceptibility, prior studies have also confirmed male predominance in the incidence of COVID-19 [4]. Epidemiology, pathophysiology, and underlying molecular mechanisms of respiratory and cardiovascular manifestations, as well as available treatments, current clinical trials, and the development of new vaccines for COVID-19, are the focus of this review.
2. Pathogenesis of SARS-CoV-2
2.1. Viral structure and cell entry
Human coronaviruses (HCoVs) belong to the genus Alphacoronavirus or Betacoronavirus of the Coronavirinae subfamily, a large group of positive-stranded RNA viruses [5,6]. Most HCoVs are relatively harmless pathogens and may induce mild respiratory symptoms. The Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) are two exceptions as they are highly pathogenic and are responsible for the 2002-2004 and 2012 epidemics, respectively [7]. It is believed that MERS-CoV and SARS-CoV originated from bats with dromedary camels and palm civets as an intermediary, respectively [8]. However, the origin of SARS-CoV-2 interspecies transfer is not fully elucidated. It is believed that it may be through bats with pangolins as the potential intermediary [1,9].
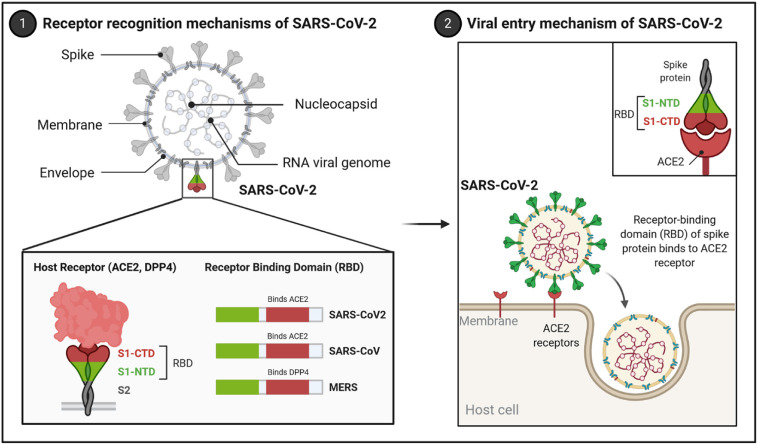
Unlike MERS-CoV2, whose host entry receptor is dipeptidyl peptidase IV (DPP4), both SARS and SARS-CoV-2 utilize angiotensin-converting enzyme 2 (ACE2) as the cell entry receptor [10,11]. ACE2 is the first homolog of human ACE and a crucial regulator of the renin-angiotensin system (RAS), a signaling pathway involved in hemodynamic regulation such as systemic vascular resistance, as well as fluid and electrolyte balance. ACE2 exists as membrane-bound and soluble receptors. The spike (S) protein on the coronavirus envelope is directly involved in the viral cell entry by attachment and fusion [6]. The membrane-bound form of ACE2 mediates the CoV-2 S-protein binding [1,10,11]. S-protein binding to ACE2 initiates the cleavage of the protein into the S1 and S2 subunits. The S1 subunit containing the RBD mediates binding to ACE2’s peptidase domain (Fig. 1 ). This initiates the priming of the coronavirus by transmembrane serine protease 2 (TMPRSS2), resulting in the cleavage of S2’ site [11].
Fig. 1.
Receptor recognition and cell entry mechanisms of SARS-CoV-2. The receptor recognition mechanisms of SARS-CoV-2 is mediated by the receptor-binding domain (RBD) of the surface spike glycoprotein (S protein) of SARS-CoV-2. The S protein is cleaved by proteases expressed in host cells into the S1 and S2 subunits. S1 contains an N-terminal domain (NTD) and a C-terminal domain (CTD). The S1-CTD domain in SARS-CoV and SARS-CoV-2 recognizes the angiotensin-converting enzyme II (ACE2) receptor, while the S1-CTD domain in the MERS virus recognizes the DPP4 protein. After the binding of S protein to ACE2, the virus is internalized by endocytosis. Created with BioRender.com
2.2. ACE2 receptor function and its role in SARS-CoV-2 infection and pathogenesis
Through a complex cascade, angiotensinogen is first converted to Angiotensin I (Ang I) by renin and next converted to Angiotensin II (Ang II) via the ACE. Ang II regulates various pathways involved in cardiovascular diseases and pulmonary fibrosis. Given the vascular, cardiac, and pulmonary dysfunction, the use of RAS inhibitors has been significant in the management of cardiopulmonary diseases.
ACE2, a monocarboxypeptidase, converts Ang I to Ang 1-7. Unlike Ang II, Ang 1-7 mediates several anti-inflammatory, anti-fibrotic, anti-arrhythmogenic, and anti-proliferative effects [12]. ADAM metalloproteinase 17 (ADAM17), also known as tumor necrosis factor-α converting enzyme (TACE), is a metallopeptidase and disintegrin that mediates the ectodomain shedding of ACE2 and leads to the formation of a soluble enzyme. Although the membrane-bound form of ACE2 regulates the ACE2/Ang1-7 axis, the role of soluble ACE2 remains largely unclear. ACE2 is expressed in the lungs, cardiovascular, renal, testes, and gastrointestinal tissues. It is also highly expressed in the oral cavity, especially on the tongue, suggesting that oral mucosa may serve as a high-risk route of SARS-CoV-2 transmission [13].
In order to gain further insights into the role of ACE2 and expression heterogeneity in human tissue, nine publicly available single-cell RNA-seq (scRNA-seq) datasets were re-analyzed to define the single-cell transcriptomic profiling of ACE2 expression in ileum [14], kidney [15], testis [16], lung [[17], [18], [19]], bronchus [18,20], and nasal mucosa [18]. The highest expression was observed in the intestinal tract, kidney, testis, gallbladder, and heart. Lower expression was found in thyroid gland and adipose tissue. These results were overall consistent with the previously published transcriptomics datasets generated from the HPA, GTEx, and FANTOM5 initiatives [[21], [22], [23]]. At the cell type-specific level, scRNA-seq datasets confirmed higher expression levels in > 60% of ileal enterocytes in the small intestine and > 6% of renal proximal tubules in the kidney. Using three different datasets, analysis of the human lungs suggested enrichment in ACE2 expression in less than 1% of alveolar cells type 2 (AT2) [[17], [18], [19]]. Interestingly, a lower expression level of ACE2 was also detected in 2–3% and 7% of the cells in bronchus and nasal mucosa, respectively, with higher expression found in ciliated cells and goblet cells. Additional studies performed by Hikmet and colleagues investigated the expression pattern of ACE2 in more than 150 different cell types corresponding to all major human tissues and organs based on stringent immunohistochemical analysis [24]. The authors confirmed the previous results obtained from datasets at the mRNA and protein level. ACE2 expression was mainly observed in enterocytes, renal tubules, gallbladder, cardiomyocytes, male reproductive cells, placental trophoblasts, ductal cells, eyes, and vasculature. Another study showed that ACE2 expression was increased with age in the pulmonary alveolar epithelial barrier, cardiomyocytes, and vascular endothelial cells, which may explain age-linked susceptibility to SARS-CoV-2 [25].
The virus’s higher binding affinity to ACE2 may partially explain the higher transmission rate of SARS-CoV-2 compared to SARS-CoV [26]. However, greater affinity is unlikely to be the sole reason for the novel coronavirus’ rapid transmission compared to SARS-CoV infections as other determinates, such as the unique furin cleavage site at the S1-S2 boundary of the SARS-CoV2 S-protein, may be at play [27].
3. Pulmonary pathological features of COVID-19
3.1. Clinical, radiological, and histopathological features
Despite a broad clinical course, the predominant respiratory involvement of COVID-19 infection may be attributed to the large surface area of the pulmonary tissue, which increases the susceptibility of the virus infection. The clinical manifestations of COVID-19 range from mild to critical, with most patients developing only mild or no symptoms [28]. Approximately 5% of cases are described as severe and are associated with septic shock, multi-organ failure, acute kidney injury, and cardiac injury. Among the severe illness, there are severe pneumonia, ARDS, sepsis, and septic shock [29]. Moderate pneumonia is characterized by respiratory symptoms such as cough and shortness of breath. Severe pneumonia is associated with extreme dyspnea, respiratory distress, tachypnea, and hypoxia. ARDS usually suggests a new-onset of respiratory failure and reveals deterioration of the respiratory capacity. Different forms of ARDS have been identified and classified based on the severity of hypoxemia. One of the primary cell types affected by SARS-CoV-2 is the type II alveolar cells. Alveolar cells are predominantly localized in the peripheral and subpleural lung regions. Analysis of lung biopsies from COVID-19 patients showed the desquamation of pneumocytes, hyaline membrane formation, multinucleated syncytial cells, and interstitial lymphocyte infiltration. These clinical features are usually associated with a viral infection and ARDS [30,31].
Abnormalities in chest radiography were reported in most of the COVID-19 patients. After multiple imaging studies using the chest CT scans, it is now well accepted that diffuse bilateral ground-glass opacities (GGOs) is the most common finding at all stages of the COVID-19 disease, followed by consolidations [32,33]. Venugopal et al. have recently published a systematic analysis and meta-review of reports presenting CT features that provide a comprehensive view of the disease pattern and progression in different clinical stages. After an extensive literature search, they short-listed and reviewed 49 studies, including over 4145 patients with 3615 RT-PCR positive cases of COVID-19 disease. The following were reported: 1) Diffuse bilateral GGOs as the most common finding at all disease stages; 2) A clear correlation of CT findings correlating with the clinical outcomes. Initial patchy GGO’s and consolidations progress to diffuse lesions with septal thickening. The further course can be either diffuse “white-out” lungs needing ICU admissions or complete resolution with or without residual fibrotic strips; 3) There is evidence that CT features such as juxtalesional pulmonary vascular prominence, pleural effusion, and lymphadenopathy can be used as prognostic markers of COVID-19 disease; 4) no evidence to favor the use of CT as an initial screening modality for COVID-19 was reported. In summary, the wide variation in the lesion descriptors supports the arguments in the statements made by radiology societies urging for the use of standardized lexicons and structured reports. Importantly, the authors strongly recommended sharing all imaging and report data that are the backbone of such research papers. This is particularly important during pandemics like COVID-19 since additional studies can be performed by researchers around the world to develop tools and insights in fighting COVID-9 disease quickly. Moreover, future prospective studies are needed to evaluate imaging findings in chronic COVID-19 patients.
3.2. Acute respiratory distress syndrome (ARDS)
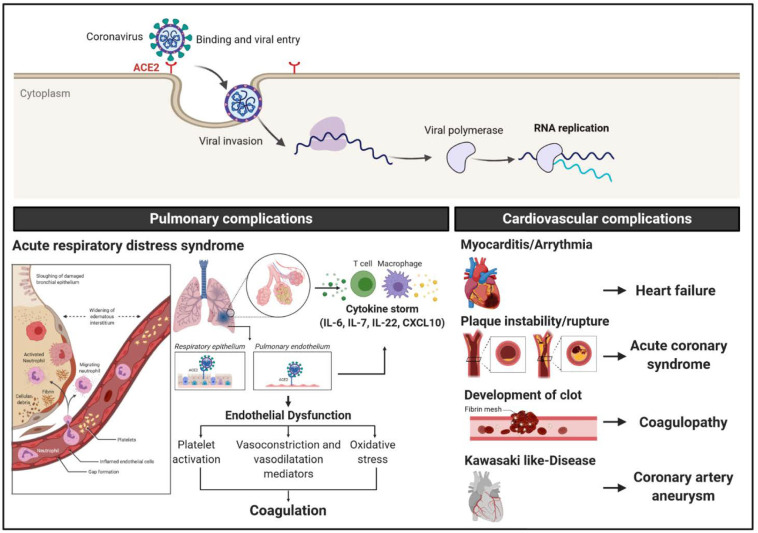
Patients with ARDS showed compromised gas exchange due to acute inflammation, fluid accumulation, and progressive fibrosis (Fig. 2 ). Dysfunction of type-I and type-II alveolar cells reduces the production of pulmonary surfactant and increases surface tension, subsequently diminishing the capacity of the lungs to expand and leading to acute hypoxemic respiratory failure.
Fig. 2.
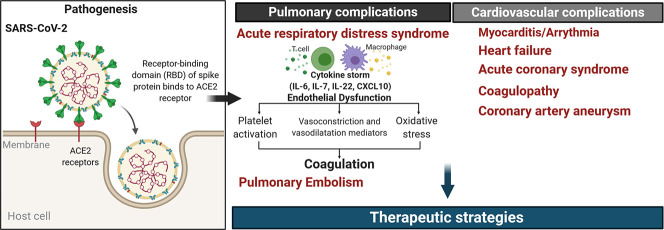
Pulmonary and cardiovascular complications of SARS-CoV-2. The clinical manifestations of COVID-19 include, in part, pulmonary and cardiovascular complications. The pulmonary pathological features of COVID-19 is associated with the development of acute respiratory distress syndrome (ARDS). The infection of the respiratory epithelium and pulmonary endothelium exacerbate the inflammation response and lead to the cytokine storm. Besides, the endothelial dysfunction potentiates platelet activation, alters the homeostasis between vasoconstrictors/vasodilators, and increases the oxidative stress in vascular cells. Severe cases of COVID-19 have been associated with various cardiovascular complications such as myocarditis, arrhythmia, and atherosclerotic plaque instability, and rupture, coagulopathy as well as coronary artery aneurysms in Kawasaki-like disease in children. Created with BioRender.com
Alongside the production of Ang II, ACE degrades bradykinin, a potent endothelial vasodilator [34]. Ang II, via its AT1 receptor, also regulates several pathways associated with tissue injury, inflammation including free radical generation, recruitment of inflammatory cells, adhesion of monocytes and neutrophils to endothelial and mesangial cells. Altogether, Ang II promotes thrombosis as well as the synthesis and release of cytokines [12], which are directly related to the pathogenesis of ARDS. The Berlin definition classifies the severity of ARDS based on the oxygenation index (PaO2/FiO2) and positive end-expiratory pressure (PEEP) of ≥ 5 cmH2O. At this time, the severity of ARDS in COVID-19 patients is assessed based on the following oxygenation index parameters: 1) 200mmHg ≤PaO2/FiO2< 300mmHg is considered mild, 2) 50mmHg ≤PaO2/FiO2< 200mmHg is classified as mild-moderate, and 3) PaO2/FiO2 <150mmHg is considered as moderate-severe [35]. In COVID-19 patients with ARDS, prone positioning and the use of neuromuscular blockers are recommended for patients with PaO2/FiO2<150mmHg. This differs from standard ARDS protocols of <100mmHg [35] and suggests that a more suitable classification is required for COVID-19-related ARDS.
3.3. Lung inflammation
The host’s immune responses are, in part, responsible for ARDS. COVID-19 infection is characterized by a systemic inflammation associated with complications. SARS-CoV-2 induces a “cytokine storm,” which is triggered by an inappropriate and excessive response of type 1/type 2 T helper (Th) cells and Th17/T regulatory lymphocytes, significant regulators of local inflammation. This abnormal response results in the aberrant release of a series of pro-inflammatory cytokines including various interleukins, G-CSF, GM-CSF, interferon-γ (IFN-γ), tumor necrosis factor-α (TNFα), IP10, MCP1, MIP1A, and MIP1B [36,37]. This type of immune-pathogenic response significantly increases the risk of developing a multi-organ failure and even death [38,39]. The cytokine profile of COVID-19 patients has shown elevated levels of pro-inflammatory cytokines, including IL-2, IL-6, Il-7, interferon-γ, G-CSF, and TNFα [40]. Analysis of the cytokine profiles in 53 COVID-19 patients from Wuhan, China, revealed that IP-10, MCP-3, and IL-1 receptor antagonist (IL-1RA) were significantly higher in the severe cases and were associated with PaO2/FiO2 and Murray score compared to the mild cases [41]. The Murray scoring system is based on four different pulmonary variables: hypoxemia, pulmonary compliance, chest radiographic findings, and level of positive end-expiratory pressure. Each variable is scored from 0 to 4 according to the severity of the condition. A score of ≥3 (or 2.5 if rapid deterioration) indicates that the patient should be referred for extracorporeal membrane oxygenation (ECMO) rather than conventional ventilation.
In the lungs, ACE2 is primarily produced in Clara and type II alveolar epithelial cells [42]. Prior studies have demonstrated that the downregulation of ACE2 in different models of acute lung injury [43]. ACE2 modulates the expression of pro-inflammatory cytokines by decreasing the expression of TNF-α, IL-1β, IL-6, MCP-1, and TGF-β while increasing the expression of the anti-inflammatory cytokine IL-10 [44,45]. Ang 1-7 showed vasodilatory, natriuretic, anti-inflammatory, anti-proliferative, and endothelium protective properties. Hence the ACE2/Ang1-7 axis may protect the lungs and heart against potential injuries [46,47]. Previous studies in wild-type mice have demonstrated that in vivo administration of SARS-CoV S protein worsens acute lung failure and reduces the expression of ACE2 in the lungs. After SARS-CoV-2 infection, the downregulation of ACE2 expression in the lungs increased the pulmonary vascular permeability and led to pulmonary edema [10]. Interestingly, blocking the AngII receptor type 1 (AT1R) attenuated these effects [10]. With regards to SARS-CoV-2, recent studies have demonstrated that the binding of the S-protein to ACE2 receptors leads to its downregulation, creating an ACE/ACE2 imbalance which promotes rapid vasoconstriction/low blood flow in the pulmonary circulation, followed by ventilation/perfusion mismatch and consequently respiratory failure [48].
3.4. Endothelial dysfunction
The pulmonary vascular endothelium is a major component of the alveolar-capillary unit located at the interface between the bloodstream and lung tissue and regulates various physiological and immunological functions [49]. The remarkable surface area of the pulmonary endothelium plays a key role in gas exchange, as well as the regulation of the barrier integrity and function. It regulates the pulmonary vascular tone by secreting vasoactive mediators such as nitric oxide, prostacyclin, endothelin, and serotonin. Respiratory lung diseases are often associated with endothelial dysfunction, characterized by a shifting of the vascular equilibrium towards aberrant and excessive vasoconstriction, inflammation, and pro-coagulant phenotype [50,51].
In patients with COVID-19, several studies have revealed the presence of systemic arterial events associated with capillary endothelial cells (EC) injury and microvascular dysfunction in different vascular beds [52]. Interestingly, Varga et al. have identified the presence of viral bodies within ECs and an accumulation of inflammatory cells, with evidence of both apoptosis and pyroptosis that might have an essential role in EC injury in patients with COVID-19. The authors suggested that SARS-CoV-2 infection may mediate the development of endotheliitis in several organs [53]. In the lung, ACE2 is abundantly expressed at the surface of pulmonary ECs and vascular smooth muscle cells, type I and II alveolar epithelial cells, and bronchial epithelial cells [54]. The early computed tomography (CT) studies showing the presence of vascular thickening in COVID-19 patients who develop pneumonia compared to non-COVID-19 pneumonia, suggesting a possible tissue tropism of SARS-CoV-2 toward pulmonary vascular cells [55].
The expression level of ACE2 in the pulmonary endothelium might predispose patients to SARS-CoV-2 infection and influence the severity of COVID-19. Li et al. have characterized the ACE2 receptor expression pattern in the lungs of healthy populations, patients with underlying diseases, and cigarette smokers using a bioinformatics approach based on previously published datasets extracted from six independent studies [40]. Surprisingly, the expression levels of ACE2 receptor in lung tissues and epithelial cells were not significantly different between healthy populations and patients with asthma or chronic obstructive pulmonary diseases [40]. Similarly, the expression level of ACE2 was not significantly different between males and females, younger and older population in any tissue [40]. A significant increase in ACE2 receptor expression was identified only in long-term smokers [40].
3.5. Pulmonary embolism
In COVID-19 disease, excessive inflammation, oxygen deprivation, immobilization, and disseminated intravascular coagulation are all well-known mediators of a prothrombotic state (Fig. 2). In a retrospective study, Poyiadji and colleagues evaluated the clinical characteristics of COVID-19 patients with pulmonary embolism (PE) by comparing their inflammatory markers and D-dimer with data extracted from CT pulmonary angiogram [56]. In a cohort of 328 COVID-19 patients, 22% were diagnosed with PE in the Henry Ford Health System, Detroit, MI (retrieved from Picture Archiving and Communication System database). The authors identified obesity, elevated D-dimer, elevated C-reactive protein (CRP) as risk factors for PE in COVID-19 patients [56]. Consistently, studies have reported an incidence of PE between 20-30% in COVID-19 patients [57,58]. Patients with a BMI >30 kg/m2 were three times more at risk for developing a PE. However, the lack of specific studies in this population, the recommendations to use of heparin may have beneficial effects in COVID-19 patients with effective doses and monitoring, in particular, those with high BMI [59]. A higher risk of vessel thrombosis has been correlated with the severity of the disease and multi-organ involvement, thus lending support to the argument for therapeutic anticoagulation (AC) in patients with COVID-19 who have elevated D-dimer levels [60]. Another multicenter cohort study that included 1,240 hospitalized COVID-19 patients showed that 103 out of 1240 (8.3%) were diagnosed with PE by CT pulmonary angiography [61]. This is a relatively low prevalence as compared with previously reported studies, probably due to the exclusion of the most severely ill ICU-COVID-19 patients. In another large retrospective study, among 2,773 COVID-19 patients hospitalized at Mount Sinai Hospital, 786 (28%) received systemic AC, and 14% were intubated. In-hospital mortality was 22.5% in patients treated with systemic AC and 22.8% in non-AC patients. Only intubated patients (n = 395) benefited from AC, with a mortality of 29% compared with 62.7% in those who did not receive AC treatment [62]. A suspicion for PE should be raised if there is a sudden deterioration in respiratory status that is not explained by significant radiological changes in the lung in conjunction with high titers of D-dimers [63]. Recommendation from the 2019 ESC Guidelines issued before COVID-19 hit the global community: ‘Initiation of anticoagulation is recommended without delay in patients with a high or intermediate clinical probability of PE while a diagnostic workup is in progress’ [64] that could be added while COVID-19 preventive measures are being implemented [65]. A recent study by Biere-Rafi et al. added to the growing evidence that statin therapy may have a protective effect on thromboembolic disease development in general and PE, specifically [66].
4. Cardiovascular complications in COVID-19
Although respiratory symptoms have been initially considered as the primary clinical manifestations of COVID-19, patients that develop severe cardiovascular events. Patients with COVID-19 and cardiovascular metabolic conditions showed higher risks of complications and mortality rates (Fig. 2) [67].
4.1. Myocardial injury
Early reports from Wuhan, China indicated that approximately 7% of their 138 patients had elevated levels of troponin suggestive of acute myocardial injury (MI) [68]. Additional studies demonstrated that patients with MI had elevated CRP, NT-proBNP, and procalcitonin, which was associated with increased risk of in-hospital mortality [69]. Although the mechanism of MI in COVID-19 remains to be elucidated, it may involve a combination of direct myocyte involvement, secondary injury from significant inflammation provoked by cytokine dysregulation, and/or direct viral infection [70]. Recent endomyocardial biopsies from affected patients demonstrate the presence of both inflammatory infiltrates and viral matter [71]. Additional cardiac autopsies have shown the presence of lymphocytic infiltrates in the RV myocardium [72] as well as cardiomyocyte hypertrophy, degeneration, and necrosis in addition to macrophage infiltration in the myocardium [73]. Potential etiologies of acute MI in the setting of infection with the novel coronavirus include myocarditis [74], ischemic injury as a result of cardiac microvascular or coronary disease [69], Takotsubo’s cardiomyopathy [75], septic cardiomyopathy [76], or secondary to hypoxic injury, RV strain (acute cor pulmonale) [77].
Acute MI poses immediate concern for compromised myocardial perfusion. While rates of acute hospitalization for ST-segment elevated myocardial infarction (STEMI) have decreased during the COVID-19 pandemic, there is an incidence of delayed presentation and subsequently delayed coronary intervention [78]. Considering the type and location of the culprit coronary lesion, delayed intervention poses a risk for associated non-reversible complications including but not limited to complete atrioventricular heart block, severe ischemic cardiomyopathy, left ventricular aneurysm formation, or apical thrombosis [71]. Additionally, there are cases of STEMI in younger patients in the setting of COVID-19 infection [79]. Although ruling out acute coronary syndrome is appropriate in the correct clinical context, we must remain aware that during the COVID-19 pandemic, ST-elevation and troponin release may be a result of several STEMI mimics.
In the case of COVID-19 related myocarditis, aside from the clinical presentation and assessment of cardiac and inflammatory biomarkers, an additional diagnostic evaluation is required. ECG abnormalities, including those typical for pericarditis such as ST elevation or PR depression, may be observed in myocarditis, but these are not conclusive. Endomyocardial biopsies are a definitive diagnostic tool for myocarditis. There are limitations, including the expertise required, risk of contagious spread, and associated false-negative results [80]. One of the largest limitations in regards to evidence of potential myocarditis or myopericarditis is the limited and mixed evidence reported in single-patient case studies [81,82]. Cardiac imaging, including echocardiography, MRI, and CT imaging, are vital non-invasive diagnostic tools that aid in determining the extent of cardiac structural and functional dysfunction in the setting of COVID-19 infection.
Several reports have noted patients presenting with confirmed COVID-19 and Takotsubo’s cardiomyopathy (TTC), as confirmed by TTE [75,83]. Briefly, TTC, also known as stress cardiomyopathy, is acute and transient cardiomyopathy predominantly seen in women, which mimics acute coronary syndrome and is typically caused by acute emotional stress [84] but may also be observed in the setting of respiratory failure and infection [85]. Typical echocardiographic presentation of TTC is the presence of reversible left ventricular (LV) apical ballooning in the absence of occlusive coronary disease [84]. There are reports of increased incidence of TCC during the COVID-19 pandemic (March-April 2020) than in years prior [83]. In a study of 118 COVID-19 confirmed patients who entered Mount Sinai Hospital in New York between March and April 2020 with a clinical indication for TTE, 5 (4.2%) had features consistent with TTC with 4 having typical LV apical ballooning and 1 having circumferential hypokinesis of the basal walls, which is consistent with reversed TTC [75]. Overall, TTC should be a possible differential to consider for MI in patients presenting with COVID-19.
Sepsis-related cardiomyopathy should also be considered in the setting of COVID-19. In a cohort of 21 confirmed COVID-19 patients admitted to the ICU in Washington State, 14 patients (67%) required vasopressors, and 7 (33%) developed cardiomyopathies [76]. Again, in patients with septic shock, TTE evidence of septic cardiomyopathy includes depression in LV systolic function with low-normal filling pressure accompanied by LV dilatation [86]. Although limited case reports have demonstrated the presence of COVID-19 related septic cardiomyopathy, the possibility of occurrence should not be excluded in patients with MI, considering sepsis is also associated with troponin release.
Other secondary causes of MI should also be considered in confirmed COVID-19 patients. In a retrospective study of 110 confirmed cases with TTC, RV dilatation was present in 32 patients (31%), of which 66% had RV hypokinesis, and 21% had moderate to severe tricuspid regurgitation [87]. This study provides us with two potential etiologies of RV strain, including acute cor pulmonale as well as implications of mechanical ventilation. Considering myocardial dysfunction and coagulopathy observed in COVID-19, acute cor pulmonale should be considered as a putative cause of MI as well. With regards to mechanical ventilation, increased levels of positive end-expiratory pressure (PEEP) also contributes to increasing the pulmonary vascular resistance (PVR) through the induction of dead space ventilation and compression of the pulmonary vasculature. Therefore, mechanical ventilation may also be a major factor impacting RV physiology, and one should be cautious of the implications of adjusting ventilation settings appropriately.
Aside from potential etiologies of MI, prolonged implications of MI in the setting of COVID-19 infection remain unclear. In a retrospective observational study of 26 patients who recovered from COVID-19 and underwent cMRI, 58% had noted abnormalities, including myocardial edema (54%) with predominant involvement of the left ventricular wall segment and other late gadolinium enhancement abnormalities suggestive of fibrosis (8%) [88]. Patients with positive findings also had noted RV function impairment, including ejection fraction, cardiac index, and stroke volume. Therefore, cardiac imaging is a vital non-invasive diagnostic tool in determining the extent of cardiac structural and functional dysfunction as a result of COVID-19 infection.
4.2. Arrhythmia
A study performed in 187 patients with COVID-19 have reported an increased incidence in overall ventricular tachycardia (VT)/ventricular fibrillation (VF) [89], cardiac arrest (pulseless electrical activity (PEA), asystole events, Torsades de pointes) [90], and other arrhythmias including atrial fibrillation (AF), bradyarrhythmias, and non-sustained ventricular tachycardia (NSVT) [91,92]. In a study of 700 COVID-19 hospitalized patients, 9 had a cardiac arrest, 25 had incidental atrial fibrillation requiring medical management with amiodarone, 9 had clinically significant bradyarrhythmias, and 10 had NSVT events. Prevalence of these events was highest amongst ICU patients, suggesting that the incidence of cardiac arrest and arrhythmias may correspond with a more severe clinical course [91]. In a study of 136 patients hospitalized with severe COVID-19 pneumonia in Wuhan, China, and had an in-hospital cardiac arrest, 119 (87.5%) had a respiratory cause of arrest [93]. Studies also reported an increased incidence of VT/VF in patients with elevated troponin, suggesting the development of arrhythmias may be a result of MI [89]. The incidence of arrhythmia in COVID-19 may be in the setting of respiratory distress, myocarditis, electrolyte derangements, intravascular volume imbalance, or drug side effects, as reviewed by Dherange et al. [94]. However, the mechanisms resulting in their development remain to be elucidated.
4.3. Coagulopathy in COVID-19
The immune response to SARS-CoV-2 infection is characterized by an overproduction of pro-inflammatory cytokines that activate the coagulation cascade. Components of the cytokine storm facilitate the adhesion of platelets by increasing the production of the Von Willebrand factor (vWF). Moreover, high levels of IL-6, IL-1, TNF-α, and thrombin in COVID-19 patients can promote clot formation by activating platelets. Thrombin also activates the proteinase-activated receptor 1 (PAR-1), expressed on monocytes, microparticles, and ECs upregulating the expression of tissue factor that contributes to inflammatory responses at mucosal surfaces. In COVID-19 patients, an imbalance between coagulants and anticoagulants promotes a pro-coagulation state. For example, tissue factor activates the extrinsic coagulation pathway, downregulates activated protein C (a clotting inhibitor), and inhibits the fibrinolytic processes. Furthermore, the upregulation of plasminogen activator inhibitor-1 blocks the activation of plasminogen, thus resulting in a decrease in the breakdown of fibrin clots. The levels of clotting factors could be predictive of major adverse cardiac event (MACE) risks in COVID-19 survivors. This is consistent with the presence of pulmonary microthrombi reported in critically ill COVID-19 patients and DIC diagnosed in 71% of the non-survivors of COVID-19.
4.4. Stroke
Aside from the formation of microthrombi, an increased incidence of venous thromboembolism and stroke have been reported. From March 23 to April 7 2020, five COVID-19 patients in the Mount Sinai Health System younger than 50 years old presented with new-onset symptoms of large-vessel ischemic stroke. Of these five patients, two had no underlying risk factors for stroke, and four were not on anticoagulants or statins [95]. An epidemiological study of a cohort of 217 patients from Wuhan, China, showed a 5.7% incidence of stroke amongst inpatients with severe COVID-19 infection [96]. The etiology of these cerebrovascular events can possibly be attributed to the coagulopathy and EC observed in COVID-19 infection. However, this association with regards to large-vessel stroke observed in younger cohorts of patients requires further investigation.
4.5. Kawasaki-like disease
Epidemiological data confirmed that children younger than 18 years represent only 1.7% of national cases of COVID-19 in the USA [97]. Dong and collaborators performed a retrospective study at the Shanghai Children's Medical Center, Shanghai, China, on the epidemiological characteristics of 2135 pediatric patients exposed to a COVID-19 patient or from an epidemic area (i.e., Hubei province, China). Of note, only 728 pediatric cases were confirmed for COVID-19. The authors found that 21% of the patients were asymptomatic, 58% had mild disease, 19% had moderate disease, 1% had severe disease, 1% were in critical condition, and no deaths were reported [98]. However, increasing concerns about a novel severe Kawasaki-like disease in children related to COVID-19 infection were reported.
Kawasaki disease (KD) is a rare acute pediatric vasculitis of the medium caliber vessels, of unknown etiology. Inflammation of the coronary arteries can lead to the development of coronary artery aneurysms, one of the main complications of Kawasaki disease [99]. Because the clinical manifestation and biochemical test results of the cases diagnosed during the COVID-19 pandemic are slightly different from the Kawasaki disease, these cases have been classified as Kawasaki-like disease. The first pediatric cases of Kawasaki-like disease were reported in the Bergamo province, Italy [100]. Verdoni et al. evaluated the incidence and features of patients with Kawasaki-like disease diagnosed during the COVID-19 pandemic [100]. The authors found a monthly rate during the COVID-19 outbreak 30 times greater compared to the monthly incidence in the previous five years. Children diagnosed with Kawasaki-like disease during the SARS-CoV-2 pandemic were more likely to show severe symptoms and cardiovascular complications due to inflammation [100]. Clinical features of toxic shock syndrome were reported in 50% of children with Kawasaki-like disease.
5. Potential therapies for COVID-19
Given the high mortality rate associated with the COVID-19 pandemic, the high transmission rate, and the socioeconomic implications of the pandemic, there is an urgent need for identifying new therapies and treatments. An in vitro study has assessed the antiviral efficacy of previously FDA-approved drugs (ribavirin, penciclovir, nitazoxanide, nafamostat, chloroquine, remdesivir, and favipiravir) on a clinical isolate of
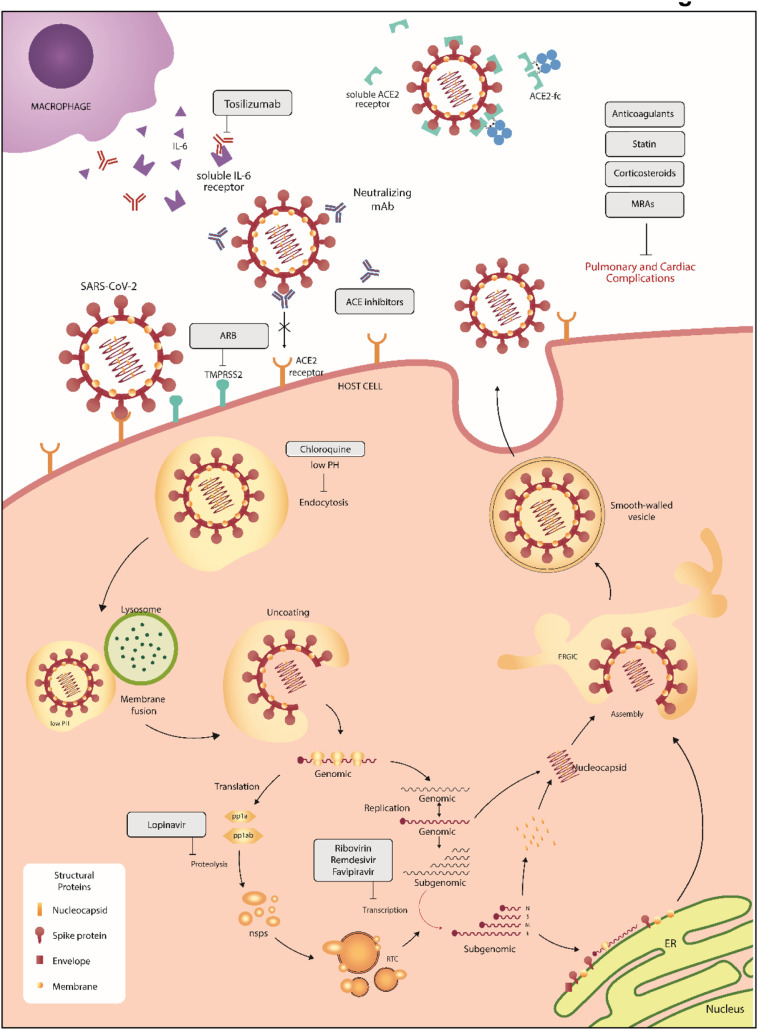
SARS-CoV-2 [101]. In response to these repurposed drugs, the antiviral efficacy was defined by the degree of cytotoxicity, virus yield, and infection rate [101]. Of these agents, chloroquine and remdesivir were more efficient in reducing the SARS-CoV-2 transmission [101]. However, while in vitro studies initially provided promising results for chloroquine and its analog hydroxychloroquine [102], these drugs are no longer a standard for therapy for SARS-CoV-2 infections due to their questionable clinical efficacy and associated adverse cardiovascular effects with regards to prolongation of QT intervals [103,104]. Our goal in this review is to highlight current standards of treatment for COVID-19. These include the antiviral drug remdesivir, the use of convalescent plasma, dexamethasone, IL-6 inhibitors, and prophylactic AC in hospitalized patients. Other investigative therapies include recombinant human ACE2 (rhACE2), regulatory T cells, and TPRSS2 inhibitors. The DISCOVERY trial [NCT04315948] is an open clinical trial assessing the efficacy and safety of several treatments for COVID-19 in hospitalized adults, including remdesivir, lopinavir/ritonavir, interferon beta1-a, and hydroxychloroquine (Fig. 3 ). The RECOVERY trial [NCT04381936] is another open clinical trial investigating the effects of lopinavir-ritonavir, hydroxychloroquine, corticosteroids, azithromycin, convalescent plasma, or tocilizumab on reducing mortality rates amongst patients hospitalized with severe COVID-19 infection.
Fig. 3.
SARS-CoV-2 life cycle in host cells and potential therapies. The interaction between the surface spike glycoprotein (S) and ACE2 mediates the cell entry into the host cells, and the coronavirus life cycle is initiated. After the viral invasion, the viral genome is replicated and translated into viral structural proteins. The S, E, and M proteins are assembled with nucleocapsid to encapsulate the replicated genome and form mature virions. Finally, the virus is exported out of the cell through exocytosis. Several treatments are being investigated for COVID-19 and include chloroquine and its analogs, angiotensin II receptor blockers, ACE2 inhibitors, remdesivir, convalescent plasma, dexamethasone, IL-6 inhibitors, lopinavir, darunavir, and azithromycin. In addition, multiple therapeutic options aim to circumvent the pulmonary and cardiac complications, such as anticoagulants, statin, corticosteroids, and mineralocorticoid receptor antagonists. MRAs: mineralocorticoid receptor antagonist; ARB: angiotensin II receptor blockers; ACE: angiotensin-converting enzyme 2; mAb: monoclonal antibody; IL-6: Interleukin-6; ER: endoplasmic reticulum.
5.1. Anticoagulant therapy
Previous studies reported increased thromboembolic events and improved outcomes with AC therapy in COVID-19 patients [62,105]. The specific role of AC therapy in disease management remains unclear, though Ying at al. have compared CoV-2 to non-CoV-2 pneumonia in a retrospective study of ~550 patients and found that CoV-2 inpatients had higher platelet counts and markedly elevated D-dimer, which progressed throughout the course of the disease [106,107]. The one-month mortality of patients receiving heparin was lower than non-CoV2 pneumonia patients. Taken together, these findings suggested that coagulopathy is a major clinical feature in severe cases of COVID-19.
In a recent study with a cohort of ~ 2,773 hospitalized patients with laboratory-confirmed COVID-19, the in-hospital mortality was 22.5% with a median survival of 21 days when patients were receiving AC [62], while 22.8% mortality and a median survival of 14 days were reported in patients who did not receive AC. However, patients receiving AC were more likely to need mechanical ventilation. Prospective randomized trials are required to further investigate the roles of hemostasis and the potential of AC to improve COVID-19 outcomes. Given the cardiopulmonary implications of COVID-19, further epidemiological studies are needed to define the appropriate medical management of these patients.
5.2. Remdesivir
Remdesivir (RDV) is an investigational broad-spectrum antiviral medication that has been clinically developed for the treatment of the Ebola virus disease. An adenosine nucleotide analog, the active triphosphate form of remdesivir (RDV-TP), acts as a substrate for several viral RNA-dependent RNA polymerases (RdRp) and competes for ATP incorporation during RNA synthesis [108]. Using in vitro and in vivo animal models, the effectiveness of remdesivir (GS-5734) in the setting of SARS-CoV and MERS-CoV has been demonstrated [109,110]. Similar results were found with SARS-CoV, MERS-CoV, and SARS-CoV-2 [111]. Although in vitro studies have demonstrated the efficacy of RDV on the replication of SARS-CoV-2 [101], further translational studies are required to assess the effectiveness and safety of RDV in the setting of COVID-19.
A recent study assessed the efficacy of compassionate-use of RDV in 53 patients hospitalized with COVID-19, where 60% of patients showed reduced oxygen requirements, and 13% of patients showed improvements in overall mortality [112]. While this study reports promising results, it is essential to note the limitations, such as the lack of a control group, the short duration of follow-up, and the limited cohort size. Several ongoing clinical studies are currently investigating the use of RDV in COVID-19 patients. These randomized trials aim to 1) assess the safety and efficacy of a five- and ten-day course treatment of RDV in hospitalized patients with severe COVID-19 [NCT04292899]; 2) compare the RDV efficacy to the standard of care treatments in hospitalized patients with moderate COVID-19 [NCT04292730], and 3) Expand the use of RDV in patients with COVID-19 requiring invasive mechanical ventilation [NCT04323761].
5.3. Convalescent plasma
The use of convalescent plasma for prophylactic and therapeutic purposes is used in several diseases, including rabies, hepatitis B, polio, measles, Ebola virus disease, and influenza [113]. Convalescent plasma relies on the principles of passive immunity and aims to provide a short-medium term humoral immunity. With regards to COVID-19, plasma is harvested from patients who had recovered from COVID-19 and have detectable SARS-CoV-2 IgG products by ELISA or other immunoquantitative assays. Of note, the use of convalescent plasma consists of the same risk of transfusion-transmitted infection. Therefore, donors must satisfy all the blood donor criteria that are mandated by the FDA. Since plasma contains several coagulation factors, including the von Willebrand factor, its use is also indicated in patients with underlying bleeding disorders, coagulopathy, and risk of thrombosis. Currently, a clinical trial is assessing the safety of such treatments in COVID-19 patients [NCT04333355]. Several ongoing studies are actively investigating the use of convalescent plasma to -1) Determine its safety and efficacy in treating and preventing pulmonary complications associated with COVID-19 when administered during the early phases of the disease [NCT04358211]; -2) Evaluate its effectiveness as a treatment for hospitalized patients with severe COVID-19 or considered at risk [NCT04347681]. These clinical trials should further improve our understanding by assessing the therapeutic potential of convalescent plasma in COVID-19 patients.
5.4. Dexamethasone and other corticosteroids
Although corticosteroids showed anti-inflammatory benefits, the use of corticosteroids in COVID-19-related ARDS was initially controversial, and the World Health Organization (WHO) advised limiting the use of systemic corticosteroids in COVID-19-associated ARDS [114]. Since a clinical study from the 2002 SARS-CoV epidemic demonstrated that patients receiving early high-dose steroids had reduced mortality (112), several clinical studies have investigated the role of corticosteroids in the clinical management of ARDS. Strong pro-inflammatory response and decreased ICU mortality were reported in these cases [115]. However, these findings were considered controversial, as no mortality benefits were found [115]. Further studies investigating the effects of glucocorticoid therapy in a SARS porcine model showed that treatment with dexamethasone aided with a reduction in IL-6, T-cell frequency, and Th1 gamma interferon-secreting cells in the spleen, tracheobronchial lymph nodes, and serum [116].
Results of the RECOVERY trial [NCT04381936] consisting of a cohort of 2,104 hospitalized patients with moderate-severe COVID-19 showed a ten-day course of dexamethasone 6 mg daily reduced 28-day mortality by 35% in patients receiving invasive mechanical ventilation compared to patients receiving standard care and by 20% in patients receiving supplemental oxygen without invasive mechanical ventilation [55]. No mortality benefit was observed amongst patients who were not receiving respiratory support. No adverse effects were reported in the treated patients [55].
Dexamethasone therapies showed beneficial therapeutic effects in severe cases of
COVID-19 with a hyperactive immune response. Its anti-inflammatory properties efficiently reduced inflammation-mediated lung injury and the associated mortality. However, the immunosuppressive properties of steroids such as dexamethasone have been of concern for treating viral respiratory infections such as COVID-19. The initial findings of RECOVERY clinical trial [NCT04381936] are considered extremely hopeful, with the WHO welcoming these results, and the UK government has already authorized the use of dexamethasone for patients receiving oxygen therapy and ventilation [117].
5.5. IL-6 inhibitors
IL-6 is a pro-inflammatory cytokine produced predominantly by macrophages in response to pathogen-associated molecular patterns or damage-associated molecular patterns. Its protective function involves the removal of pathogens by promoting both an innate and adaptive immune response [118]. Overproduction of IL-6 is associated with the pathogenesis of several chronic inflammatory diseases such as rheumatoid arthritis, juvenile idiopathic arthritis, and Castleman disease [118]. Tocilizumab is a monoclonal antibody that competitively blocks the binding of IL-6 to both the soluble and membrane-bound IL-6 receptor. Tocilizumab has been used as a possible treatment for treating rheumatoid arthritis and cytokine release syndrome.
At this time, several ongoing clinical trials are assessing the efficacy of tocilizumab in hospitalized patients with COVID-19-related pneumonia [NCT04317092] and severe cases of COVID-19 in a cohort of intubated and non-intubated patients [NCT04377659]. Additional studies are currently investigating the potential role of IL-6 and soluble IL-6 receptor as predictors of efficacy and safety in patients with severe COVID-19 pneumonia treated with Tocilizumab [NCT04359667]. Unlike tocilizumab, which competitively inhibits the binding of IL-6 to its receptor, siltuximab directly targets and interacts with IL-6 to reduce its bioavailability [119]. With regards to COVID-19, two ongoing clinical studies investigate the efficacy of compassionate use of siltuximab in COVID-19 ARDS patients [NCT04322188] and the use of siltuximab versus methylprednisolone in hospitalized patients with COVID-19 [NCT04329650].
5.6. ACE inhibitors and angiotensin II receptor blockers
During the early phase of the COVID-19 pandemic, many questions were raised regarding the use of RAS inhibitors in cardiovascular patients with COVID-19. Several studies have shown that ACE inhibitors, Ang II type I receptor blockers (ARBs), and mineralocorticoid receptor antagonists (MRAs) upregulate the expression of ACE2 [120,121]. Given the unclear link between increased ACE2 expression and COVID-19 susceptibility/virulence, several societies, including the American College of Cardiology, American Heart Association, Heart Failure Society of America, European Society of Cardiology, have recommended using these RAS antagonists [122,123]. Further reviews have suggested that the anti-inflammatory, anti-fibrotic, vasodilatory effects of ACE2/Ang 1-7 axis of RAS inhibitors may attenuate the severity of clinical outcomes in patients with COVID-19 [124]. In patients with underlying cardiovascular comorbidities, the use of RAS inhibitors such as ACE inhibitors, ARBs, and MRAs reduced the Ang II-mediated pro-inflammatory effects. Concomitantly, recent studies have suggested that the clinical benefits associated with these drugs may be attributed to their role in the upregulation of ACE2 expression and activity [120,125]. Clinical studies using a large cohort of patients have shown that patients treated with ACE inhibitors and ARBs had a lower risk of developing pneumonia [126] and ARDS [34]. From a cardiopulmonary perspective, no clear indication or data is supporting the benefits of discontinuing or holding the use of RAS antagonists in these patients. Several clinical studies aim to evaluate the effect of ACE inhibitors, Angiotensin II receptor blockers (ARBs), and mineralocorticoid receptor antagonists (MRAs) in patients with COVID-19 and pre-existing cardiovascular disease history [NCT04318301, NCT04318418, NCT04312009, NCT04311177, NCT04330300, NCT04329195].
5.7. Statin
Based on our understanding of the inhibitory effect of statins on coagulopathy, endothelial dysfunction, and inflammation, it is believed that statin therapy may lessen the impact of COVID-19 infection and prevent the development of PE. The current empirical guidance for COVID-19 patients who are already on statin therapy is to continue the treatment if not contraindicated [127], such as an ongoing reduction of total and LDL cholesterol [128], upregulation of expression of ACE2, hence, the potential to increase SARS-CoV-2 entry into cells or myositis and liver dysfunction [96,129]. In a retrospective cohort study of 717 patients with confirmed COVID-19 by PCR admitted and hospitalized from January 22 to April 15, 2020, at the National Centre of Infectious Diseases (NCID), Singapore, Tan et al. have shown that statin use is associated with lower ICU admission and lower disease severity in COVID-19 infection [130]. Similar results have been reported from a retrospective study on 13,981 patients with COVID-19 in Hubei Province, China, among which 1,219 received statins [131]. The authors found that the in-Hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Zhang and collaborators showed that the risk for 28-day all-cause mortality was 5.2% in the statin group against 9.4% in the non-statin group, with an adjusted hazard ratio of 0.58 [131]. Similarly, another retrospective report in a cohort of 154 COVID-19 cases reported that statin intake was significantly associated with the asymptomatic status of COVID-19 [132].
6. COVID-19 vaccine
Currently, there is no FDA-approved or authorized vaccine for the prevention of COVID-19. Due to the rapid transmission of SARS-CoV-2, increasing incidence of disease around the world, and the urgent need to address the global health crisis, 234 vaccine candidates have been developed in a record time by more than two hundred companies and universities worldwide, according to the WHO and Milken Institute [133]. Within a few months, tremendous efforts have been undertaken to further investigate their safety and therapeutic efficacy in preventing COVID-19 to ultimately decrease rates of infection and death worldwide. The new investigational vaccines include inactivated viruses, protein subunits and peptides, viral vector-based delivery, synthetic DNA, and RNA-based immunization approaches (Table 1 ) [134].
Table 1.
Overview of the main vaccine candidates for COVID-19. The table recapitulates the main candidate vaccines that are being developed and currently under clinical investigation. Name, type of vaccine, sponsor, trial phase, and clinical trial registry are indicated.
| Candidate | Type | Sponsor | Trial Phase | Clinical trial registry |
|---|---|---|---|---|
| BNT162 | mRNA-based vaccine | Pfizer, BioNTech | Phase 3 | ChiCTR2000034825, EudraCT 2020-001038-36, NCT04368728, NCT04380701, NCT04523571, NCT04537949 |
| mRNA-1273 | mRNA-based vaccine | Moderna | Phase 3 | NCT04283461, NCT04405076, NCT04470427 |
| JNJ-78436735 (formerly Ad26.COV2.S) |
Non-replicating viral vector | Janssen Pharmaceutical | Phase 3 | NCT04436276, NCT04505722 |
| Sputnik V | Non-replicating viral vector | Gamaleya Research Institute, Acellena Contract Drug Research and Development | Phase 3 | NCT04437875, NCT04436471, NCT04530396, NCT04564716, NCT04587219 |
| Ad5-nCoV | Recombinant adenovirus vaccine | CanSino Biologics | Phase 3 | ChiCTR2000030906, ChiCTR2000031781, NCT04313127, NCT04341389, NCT04398147, NCT04526990, NCT04540419, NCT04566770, NCT04568811 |
| AZD1222 | Adenoviral vector vaccine | The University of Oxford; AstraZeneca; IQVIA; Serum Institute of India | Phase 3 | CTRI/2020/08/027170, EudraCT 2020-001072-15, EudraCT 2020-001228-32, ISRCTN89951424, NCT04324606, NCT04400838, NCT04444674, NCT04516746, NCT04540393, NCT04568031, PACTR202005681895696, PACTR202006922165132" |
| CoronaVac | Inactivated vaccine | Sinovac | Phase 3 | NCT04352608, NCT04383574, NCT04456595, NCT04508075, NCT04551547, NCT04582344, 669/UN6.KEP/EC/2020 |
| Covaxin | Inactivated vaccine | Bharat Biotech; National Institute of Virology | Phase 3 | CTRI/2020/07/026300, NCT04471519 |
| No name announced | Inactivated vaccine | Wuhan Institute of Biological Products; China National Pharmaceutical Group (Sinopharm) | Phase 3 | ChiCTR2000031809, ChiCTR2000034780, ChiCTR2000039000 |
| NVX-CoV2373 | Nanoparticle vaccine | Novavax | Phase 3 | NCT04368988, NCT04533399, EudraCT 2020-004123-16, NCT04583995 |
In the fight against COVID-19, an innovative nanotechnology approach has rapidly reached clinical testing and demonstrated encouraging early results. Both frontrunner companies, Moderna and Pfizer/BioNTech, encapsulated their mRNA vaccines using lipid nanoparticles to protect the mRNA from nuclease degradation [135,136]. mRNA-based vaccines allow the rapid translation of antigens in the cytoplasm and, therefore, an immediate immune response to prevent COVID-19 disease [137]. Following the first genome sequencing results of SARS-CoV-2, Moderna and Pfizer/BioNTech have generated new experimental vaccines using the same technology. Their vaccine candidates rely on a specific mRNA that encodes the trimerized SARS-CoV-2 spike glycoprotein RBD antigen [135,136]. As previously described, the coronavirus spike protein plays a critical role in the virus entry into the host cells and trigger someone's immune response to produce antibodies against the virus without causing infection and inducing the detrimental outcomes that are associated with SARS-CoV-2.
Pfizer/BioNTech has developed an RNA-based vaccine that is based on a platform of nucleoside-modified messenger RNA (modRNA). The vaccine candidate (BNT162b, variant RBP020.2) includes a T4 fibritin-derived trimerization domain and encodes an optimized SARS-CoV-2 full-length spike protein antigen to enhance the immune response. Pfizer and BioNTech have recently announced in a press release that their investigational mRNA-based vaccine, BNT162b2, against SARS-CoV-2 achieved success based on the first interim analysis from Phase 3 clinical study [138]. The Phase 3 clinical trial of BNT162b2 began on July 27, 2020, and has enrolled more than 43,661 people worldwide in the Pfizer vaccine trials, with 42% having diverse racially and ethnically backgrounds, as of November 16, 2020 [138]. Of note, 41,135 have received a second dose of the vaccine candidate as of November 16, 2020. Of note, half of whom have received a placebo. The pharmaceutical companies have reported that no serious safety concerns have been observed. Importantly, 170 cases of COVID-19 infection were confirmed in trial participants. 162 infections were reported from the placebo group, and 8 in the group treated with the Pfizer’s vaccine [138]. Collectively, the first interim efficacy analysis conducted by an external and independent Data Monitoring Committee revealed that the vaccine candidate BNT162b2 was found to be around 95% effective in preventing COVID-19 in participants, without evidence of prior SARS-CoV-2 infection .
Moderna, a biotechnology company pioneering messenger RNA (mRNA) therapeutics, co-developed a new mRNA vaccine candidate (mRNA-1273) against COVID-19 using the same technology in partnership with the National Institute of Allergy and Infectious Diseases’s Vaccine Research Center [135]. The mRNA-1273 encodes the prefusion stabilized form of the S antigen (named S-2P) that includes a transmembrane anchor and an intact S1–S2 cleavage site [135]. On November 17, the U.S. NIH-appointed Data Safety Monitoring Board (DSMB) for the Phase 3 study of mRNA-1273 has concluded that the trial has met the statistical criteria pre-specified in the study protocol for efficacy, with a vaccine efficacy of 94.5%, and confirmed the preliminary success of this new investigational vaccine [139]. In this study, known as the COVE study, more than 30,000 participants were enrolled across 100 clinical research sites in the United States, and 37% of trial volunteers were from racial and ethnic minorities. About 50 % received the vaccines, and the other half received a placebo. Notably, only 95 participants with confirmed cases of COVID-19 were reported [139]. Out of the 95 positive COVID-19 cases reported, only five received the actual mRNA-1273 vaccine, against 90 who received the placebo. This first interim analysis from the Phase 3 trial provided the first clinical validation of Moderna’s vaccine efficiency in preventing COVID-19.
The race for a COVID-19 vaccine has led to the development of several vaccines, currently under clinical investigation to further evaluate their therapeutic efficacy. Several mRNA-based vaccines are now entering the late-stage trials and have demonstrated encouraging early results. Given the new vaccine format, their safety, effectiveness, and immunogenicity should be carefully investigated. Furthermore, demographic factors such as race, ethnicity, age, sex, and comorbidities should be considered in the design of the clinical trials. Finally, it is of great importance that pharmacovigilance and epidemiological studies be conducted post-vaccine deployment to thoroughly confirm vaccine safety and clinical significance.
7. Future perspective
Details on the epidemiology of SARS-CoV-2, its pathogenesis, and efficient treatments of COVID-19 are still evolving and controversial. Insights from the previous SARS-CoV and MERS-CoV epidemics and adaptation of clinical management from similar syndromes such as ARDS and cytokine release syndrome have provided the medical communities with promising pharmacological tools for the management of patients with COVID-19. Further epidemiological studies using all available and reliable databases around the world are required to clarify and define the nature of the disease. Besides ongoing efforts to diagnose COVID-19 in the early stages, it is of great importance for early therapeutic interventions to improve the quality of life and increase life expectancy. Finally, further investigation is imperative to evaluate the therapeutic potential of repurposed drugs for the clinical management of COVID-19 patients. Data from larger cohorts with regards to the efficacy and safety of currently in use investigational drugs (ex: remdesivir, tocilizumab) and adjuvant therapies (e.g., convalescent plasma, anticoagulants, steroids) will be critical for the standardization of health care protocols and procedures of COVID-19 infection.
Disclosure statement
None of the authors have any disclosures.
Ethics approval and consent to participate
Not applicable.
Authors’ contributions
WH, MB, AB, AE, KF, DAG, and LH contributed to writing and editing the manuscript. All authors have approved the final version.
Funding
This study was supported by the NIH R01 HL133554 (to LH) and AHA Innovative Project Award 18IPA34170321 (to LH), and the NIH 5T32HL007824-22 (to MB).
Acknowledgments
Only the authors listed on the manuscript contributed to the article.
References
- 1.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg S., Kim L., Whitaker M., O'Halloran A., Cummings C., Holstein R., Prill M., Chai S.J., Kirley P.D., Alden N.B., Kawasaki B., Yousey-Hindes K., Niccolai L., Anderson E.J., Openo K.P., Weigel A., Monroe M.L., Ryan P., Henderson J., Kim S., Como-Sabetti K., Lynfield R., Sosin D., Torres S., Muse A., Bennett N.M., Billing L., Sutton M., West N., Schaffner W., Talbot H.K., Aquino C., George A., Budd A., Brammer L., Langley G., Hall A.J., Fry A. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Ann. Rev. Virol. 2016;3(1):237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23(2):130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azhar E.I., El-Kafrawy S.A., Farraj S.A., Hassan A.M., Al-Saeed M.S., Hashem A.M., Madani T.A. Evidence for camel-to-human transmission of MERS coronavirus. N. Engl. J. Med. 2014;370(26):2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- 9.Zhang T., Wu Q., Zhang Z. Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Curr. Biol. 2020;30(7) doi: 10.1016/j.cub.2020.03.022. 1346-1351 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.052. 271-280 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.A.C. Simoes e Silva, Silveira K.D., Ferreira A.J., Teixeira M.M. ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis. Br. J. Pharmacol. 2013;169(3):477–492. doi: 10.1111/bph.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S., Zhou X., Zhang T., Wang Z. The need for urogenital tract monitoring in COVID-19. Nat. Rev. 2020;17(6):314–315. doi: 10.1038/s41585-020-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao J., Yu Z., Chen Y., Bao M., Zou C., Zhang H., Liu D., Li T., Zhang Q., Li J., Cheng J., Mo Z. Single-cell RNA sequencing of human kidney. Scientific data. 2020;7(1):4. doi: 10.1038/s41597-019-0351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo J., Grow E.J., Mlcochova H., Maher G.J., Lindskog C., Nie X., Guo Y., Takei Y., Yun J., Cai L., Kim R., Carrell D.T., Goriely A., Hotaling J.M., Cairns B.R. The adult human testis transcriptional cell atlas. Cell Res. 2018;28(12):1141–1157. doi: 10.1038/s41422-018-0099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reyfman P.A., Walter J.M., Joshi N., Anekalla K.R., McQuattie-Pimentel A.C., Chiu S., Fernandez R., Akbarpour M., Chen C.I., Ren Z., Verma R., Abdala-Valencia H., Nam K., Chi M., Han S., Gonzalez-Gonzalez F.J., Soberanes S., Watanabe S., Williams K.J.N., Flozak A.S., Nicholson T.T., Morgan V.K., Winter D.R., Hinchcliff M., Hrusch C.L., Guzy R.D., Bonham C.A., Sperling A.I., Bag R., Hamanaka R.B., Mutlu G.M., Yeldandi A.V., Marshall S.A., Shilatifard A., Amaral L.A.N., Perlman H., Sznajder J.I., Argento A.C., Gillespie C.T., Dematte J., Jain M., Singer B.D., Ridge K.M., Lam A.P., Bharat A., Bhorade S.M., Gottardi C.J., Budinger G.R.S., Misharin A.V. Single-Cell Transcriptomic Analysis of Human Lung Provides Insights into the Pathobiology of Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019;199(12):1517–1536. doi: 10.1164/rccm.201712-2410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braga F.A. Vieira, Kar G., Berg M., Carpaij O.A., Polanski K., Simon L.M., Brouwer S., Gomes T., Hesse L., Jiang J., Fasouli E.S., Efremova M., Vento-Tormo R., Talavera-López C., Jonker M.R., Affleck K., Palit S., Strzelecka P.M., Firth H.V., Mahbubani K.T., Cvejic A., Meyer K.B., Saeb-Parsy K., Luinge M., Brandsma C.A., Timens W., Angelidis I., Strunz M., Koppelman G.H., van Oosterhout A.J., Schiller H.B., Theis F.J., van den Berge M., Nawijn M.C., Teichmann S.A. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat. Med. 2019;25(7):1153–1163. doi: 10.1038/s41591-019-0468-5. [DOI] [PubMed] [Google Scholar]
- 19.Han X., Zhou Z., Fei L., Sun H., Wang R., Chen Y., Chen H., Wang J., Tang H., Ge W., Zhou Y., Ye F., Jiang M., Wu J., Xiao Y., Jia X., Zhang T., Ma X., Zhang Q., Bai X., Lai S., Yu C., Zhu L., Lin R., Gao Y., Wang M., Wu Y., Zhang J., Zhan R., Zhu S., Hu H., Wang C., Chen M., Huang H., Liang T., Chen J., Wang W., Zhang D., Guo G. Construction of a human cell landscape at single-cell level. Nature. 2020;581(7808):303–309. doi: 10.1038/s41586-020-2157-4. [DOI] [PubMed] [Google Scholar]
- 20.Lukassen S., Chua R.L., Trefzer T., Kahn N.C., Schneider M.A., Muley T., Winter H., Meister M., Veith C., Boots A.W., Hennig B.P., Kreuter M., Conrad C., Eils R. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020;39(10) doi: 10.15252/embj.20105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uhlén M., Fagerberg L., Hallström B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson Å., Kampf C., Sjöstedt E., Asplund A., Olsson I., Edlund K., Lundberg E., Navani S., Szigyarto C.A., Odeberg J., Djureinovic D., Takanen J.O., Hober S., Alm T., Edqvist P.H., Berling H., Tegel H., Mulder J., Rockberg J., Nilsson P., Schwenk J.M., Hamsten M., von Feilitzen K., Forsberg M., Persson L., Johansson F., Zwahlen M., von Heijne G., Nielsen J., Pontén F. 2015. Proteomics. Tissue-based map of the human proteome, Science (New York, N.Y.) 347(6220) 1260419. [DOI] [PubMed] [Google Scholar]
- 22.Keen J.C., Moore H.M. The Genotype-Tissue Expression (GTEx) Project: Linking Clinical Data with Molecular Analysis to Advance Personalized Medicine. J. Personal. Med. 2015;5(1):22–29. doi: 10.3390/jpm5010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu N.Y., Hallström B.M., Fagerberg L., Ponten F., Kawaji H., Carninci P., Forrest A.R., Hayashizaki Y., Uhlén M., Daub C.O. Complementing tissue characterization by integrating transcriptome profiling from the Human Protein Atlas and from the FANTOM5 consortium. Nucleic Acids Res. 2015;43(14):6787–6798. doi: 10.1093/nar/gkv608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hikmet F., Méar L., Edvinsson Å., Micke P., Uhlén M., Lindskog C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020;16(7) doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma S., Sun S., Li J., Fan Y., Qu J., Sun L., Wang S., Zhang Y., Yang S., Liu Z., Wu Z., Zhang S., Wang Q., Zheng A., Duo S., Yu Y., Belmonte J.C.I., Chan P., Zhou Q., Song M., Zhang W., Liu G.H. Single-cell transcriptomic atlas of primate cardiopulmonary aging. Cell Res. 2020:1–18. doi: 10.1038/s41422-020-00412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortega J.T., Serrano M.L., Pujol F.H., Rangel H.R. Role of changes in SARS-CoV-2 spike protein in the interaction with the human ACE2 receptor: An in silico analysis. EXCLI J. 2020;19:410–417. doi: 10.17179/excli2020-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia S., Lan Q., Su S., Wang X., Xu W., Liu Z., Zhu Y., Wang Q., Lu L., Jiang S. The role of furin cleavage site in SARS-CoV-2 spike protein-mediated membrane fusion in the presence or absence of trypsin. Signal transduction and targeted therapy. 2020;5(1):92. doi: 10.1038/s41392-020-0184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y.C., Liao C.H., Chang C.F., Chou C.C., Lin Y.R. A Locally Transmitted Case of SARS-CoV-2 Infection in Taiwan. N. Engl. J. Med. 2020;382(11):1070–1072. doi: 10.1056/NEJMc2001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 30.Goh K.J., Choong M.C., Cheong E.H., Kalimuddin S., Duu Wen S., Phua G.C., Chan K.S., Haja Mohideen S. Rapid Progression to Acute Respiratory Distress Syndrome: Review of Current Understanding of Critical Illness from COVID-19 Infection. Ann. Acad. Med. Singap. 2020;49(3):108–118. [PubMed] [Google Scholar]
- 31.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N., Diao K., Lin B., Zhu X., Li K., Li S., Shan H., Jacobi A., Chung M. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology. 2020;295(3):200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venugopal V.K., Mahajan V., Rajan S., Agarwal V.K., Rajan R., Syed S., Mahajan H. 2020. A Systematic Meta-Analysis of CT Features of COVID-19: Lessons from Radiology, medRxiv : the preprint server for health sciences. 2020.04.04.20052241. [Google Scholar]
- 34.Marshall R.P., Webb S., Bellingan G.J., Montgomery H.E., Chaudhari B., McAnulty R.J., Humphries S.E., Hill M.R., Laurent G.J. Angiotensin converting enzyme insertion/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2002;166(5):646–650. doi: 10.1164/rccm.2108086. [DOI] [PubMed] [Google Scholar]
- 35.Li X., Ma X. Acute respiratory failure in COVID-19: is it "typical" ARDS? Crit. Care. 2020;24(1):198. doi: 10.1186/s13054-020-02911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rizzo P., Vieceli Dalla F., Sega, Fortini F., Marracino L., Rapezzi C., Ferrari R. COVID-19 in the heart and the lungs: could we "Notch" the inflammatory storm? Basic Res. Cardiol. 2020;115(3):31. doi: 10.1007/s00395-020-0791-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53(3):368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yi Y., Lagniton P.N.P., Ye S., Li E., Xu R.H. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int. J. Biol. Sci. 2020;16(10):1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kong S.Y., Bostick R.M., Flanders W.D., McClellan W.M., Thyagarajan B., Gross M.D., Judd S., Goodman M. Oxidative balance score, colorectal adenoma, and markers of oxidative stress and inflammation. Cancer Epidemiol. Biomark. Prev. 2014;23(3):545–554. doi: 10.1158/1055-9965.EPI-13-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China, Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y., Shen C., Li J., Yuan J., Yang M., Wang F., Li G., Li Y., Xing L., Peng L., Wei J., Cao M., Zheng H., Wu W., Zou R., Li D., Xu Z., Wang H., Zhang M., Zhang Z., Liu L., Liu Y. 2020. Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome, medRxiv : the preprint server for health sciences. 2020.03.02.20029975. [Google Scholar]
- 42.Wiener R.S., Cao Y.X., Hinds A., Ramirez M.I., Williams M.C. Angiotensin converting enzyme 2 is primarily epithelial and is developmentally regulated in the mouse lung. J. Cell. Biochem. 2007;101(5):1278–1291. doi: 10.1002/jcb.21248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rey-Parra G.J., Vadivel A., Coltan L., Hall A., Eaton F., Schuster M., Loibner H., Penninger J.M., Kassiri Z., Oudit G.Y., Thebaud B. Angiotensin converting enzyme 2 abrogates bleomycin-induced lung injury. J. Mol. Med. (Berl.) 2012;90(6):637–647. doi: 10.1007/s00109-012-0859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grobe J.L., Mecca A.P., Lingis M., Shenoy V., Bolton T.A., Machado J.M., Speth R.C., Raizada M.K., Katovich M.J. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1-7) Am. J. Physiol. Heart Circ. Physiol. 2007;292(2):H736–H742. doi: 10.1152/ajpheart.00937.2006. [DOI] [PubMed] [Google Scholar]
- 45.Guo Y.J., Li W.H., Wu R., Xie Q., Cui L.Q. ACE2 overexpression inhibits angiotensin II-induced monocyte chemoattractant protein-1 expression in macrophages. Arch. Med. Res. 2008;39(2):149–154. doi: 10.1016/j.arcmed.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 46.Yu L., Yuan K., Phuong H.T., Park B.M., Kim S.H. Angiotensin-(1-5), an active mediator of renin-angiotensin system, stimulates ANP secretion via Mas receptor. Peptides. 2016;86:33–41. doi: 10.1016/j.peptides.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 47.Ocaranza M.P., Rivera P., Novoa U., Pinto M., Gonzalez L., Chiong M., Lavandero S., Jalil J.E. Rho kinase inhibition activates the homologous angiotensin-converting enzyme-angiotensin-(1-9) axis in experimental hypertension. J. Hypertens. 2011;29(4):706–715. doi: 10.1097/HJH.0b013e3283440665. [DOI] [PubMed] [Google Scholar]
- 48.Wosten-van Asperen R.M., Lutter R., Specht P.A., Moll G.N., van Woensel J.B., van der Loos C.M., van Goor H., Kamilic J., Florquin S., Bos A.P. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J. Pathol. 2011;225(4):618–627. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- 49.Orfanos S.E., Mavrommati I., Korovesi I., Roussos C. Pulmonary endothelium in acute lung injury: from basic science to the critically ill. Intensive Care Med. 2004;30(9):1702–1714. doi: 10.1007/s00134-004-2370-x. [DOI] [PubMed] [Google Scholar]
- 50.Huertas A., Guignabert C., Barbera J.A., Bartsch P., Bhattacharya J., Bhattacharya S., Bonsignore M.R., Dewachter L., Dinh-Xuan A.T., Dorfmuller P., Gladwin M.T., Humbert M., Kotsimbos T., Vassilakopoulos T., Sanchez O., Savale L., Testa U., Wilkins M.R. Pulmonary vascular endothelium: the orchestra conductor in respiratory diseases: Highlights from basic research to therapy. Eur. Respir. J. 2018;51(4) doi: 10.1183/13993003.00745-2017. [DOI] [PubMed] [Google Scholar]
- 51.Rajendran P., Rengarajan T., Thangavel J., Nishigaki Y., Sakthisekaran D., Sethi G., Nishigaki I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013;9(10):1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20(7):389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poyiadji N., Cormier P., Patel P.Y., Hadied M.O., Bhargava P., Khanna K., Nadig J., Keimig T., Spizarny D., Reeser N., Klochko C., Peterson E.L., Song T. Acute pulmonary embolism and COVID-19. Radiology. 2020;201955 doi: 10.1148/radiol.2020201955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020;201544 doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leonard-Lorant I., Delabranche X., Severac F., Helms J., Pauzet C., Collange O., Schneider F., Labani A., Bilbault P., Moliere S., Leyendecker P., Roy C., Ohana M. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020;201561 doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F., Jeanpierre E., Rauch A., Labreuche J., Susen S. Pulmonary Embolism in Patients With COVID-19: Awareness of an Increased Prevalence. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 60.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fauvel C., Weizman O., Trimaille A., Mika D., Pommier T., Pace N., Douair A., Barbin E., Fraix A., Bouchot O., Benmansour O., Godeau G., Mecheri Y., Lebourdon R., Yvorel C., Massin M., Leblon T., Chabbi C., Cugney E., Benabou L., Aubry M., Chan C., Boufoula I., Barnaud C., Bothorel L., Duceau B., Sutter W., Waldmann V., Bonnet G., Cohen A., Pezel T. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur. Heart J. 2020;41(32):3058–3068. doi: 10.1093/eurheartj/ehaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paranjpe I., Fuster V., Lala A., Russak A., Glicksberg B.S., Levin M.A., Charney A.W., Narula J., Fayad Z.A., Bagiella E., Zhao S., Nadkarni G.N. Association of Treatment Dose Anticoagulation with In-Hospital Survival Among Hospitalized Patients with COVID-19. J. Am. Coll. Cardiol. 2020;76(1):122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zafar Y., Sattar Y., Ullah W., Roomi S., Rashid M.U., Khan M.S., Schmidt L. Proprotein convertase subtilisin/Kexin type-9 (PCSK-9) inhibitors induced liver injury - a retrospective analysis. J. Commun. Hospital Internal Med. Perspect. 2020;10(1):32–37. doi: 10.1080/20009666.2019.1710952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.M.J. Claeys, Y. Vandekerckhove, B. Cosyns, P. Van de Borne, P. Lancellotti, Summary of 2019 ESC Guidelines on chronic coronary syndromes, acute pulmonary embolism, supraventricular tachycardia and dislipidaemias, Acta Cardiol. (2020) 1-8.1. [DOI] [PubMed]
- 65.Torbicki A. COVID-19 and pulmonary embolism: an unwanted alliance. Eur. Heart J. 2020;41(32):3069–3071. doi: 10.1093/eurheartj/ehaa553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Biere-Rafi S., Hutten B.A., Squizzato A., Ageno W., Souverein P.C., de Boer A., Gerdes V.E., Büller H.R., Kamphuisen P.W. Statin treatment and the risk of recurrent pulmonary embolism. Eur. Heart J. 2013;34(24):1800–1806. doi: 10.1093/eurheartj/eht046. [DOI] [PubMed] [Google Scholar]
- 67.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L., Bi Z., Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., Huang H., Yang B., Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siripanthong B., Nazarian S., Muser D., Deo R., Santangeli P., Khanji M.Y., Cooper L.T., Jr., Chahal C.A.A. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walsh M.N., Sorgente A., Fischman D.L., Bates E.R., Grapsa J. The COVID-19 Pandemic and Cardiovascular Complications: What Have We Learned So Far? JACC Case Rep. 2020;2(9):1235–1239. doi: 10.1016/j.jaccas.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., Heinrich F., Mushumba H., Kniep I., Schröder A.S., Burdelski C., de Heer G., Nierhaus A., Frings D., Pfefferle S., Becker H., Bredereke-Wiedling H., de Weerth A., Paschen H.R., Sheikhzadeh-Eggers S., Stang A., Schmiedel S., Bokemeyer C., Addo M.M., Aepfelbacher M., Püschel K., Kluge S. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann. Intern. Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C., Mou H.M., Wang L.H., Zhang H.R., Fu W.J., Luo T., Liu F., Guo Q.N., Chen C., Xiao H.L., Guo H.T., Lin S., Xiang D.F., Shi Y., Pan G.Q., Li Q.R., Huang X., Cui Y., Liu X.Z., Tang W., Pan P.F., Huang X.Q., Ding Y.Q., Bian X.W. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua bing li xue za zhi = Chinese journal of pathology. 2020;49(5):411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 74.Kariyanna P.T., Sutarjono B., Grewal E., Singh K.P., Aurora L., Smith L., Chandrakumar H.P., Jayarangaiah A., Goldman S.A., Salifu M.O., McFarlane I.M. A Systematic Review of COVID-19 and Myocarditis. Am. J. Med. Case Rep. 2020;8(9):299–305. [Google Scholar]
- 75.Giustino G., Croft L.B., Oates C.P., Rahman K., Lerakis S., Reddy V.Y., Goldman M. Takotsubo Cardiomyopathy in COVID-19. J. Am. Coll. Cardiol. 2020;76(5):628–629. doi: 10.1016/j.jacc.2020.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., Lee M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Creel-Bulos C., Hockstein M., Amin N., Melhem S., Truong A., Sharifpour M. Acute Cor Pulmonale in Critically Ill Patients with Covid-19. N. Engl. J. Med. 2020;382(21) doi: 10.1056/NEJMc2010459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mafham M.M., Spata E., Goldacre R., Gair D., Curnow P., Bray M., Hollings S., Roebuck C., Gale C.P., Mamas M.A., Deanfield J.E., de Belder M.A., Luescher T.F., Denwood T., Landray M.J., Emberson J.R., Collins R., Morris E.J.A., Casadei B., Baigent C. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396(10248):381–389. doi: 10.1016/S0140-6736(20)31356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zendjebil S., Zeitouni M., Batonga M., El Bèze N., Guedeney P., Collet J.-P., Choussat R., Silvain J., Montalescot G. Acute Multivessel Coronary Occlusion Revealing COVID-19 in a Young Adult. JACC Case Rep. 2020;2(9):1297–1301. doi: 10.1016/j.jaccas.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kociol R.D., Cooper L.T., Fang J.C., Moslehi J.J., Pang P.S., Sabe M.A., Shah R.V., Sims D.B., Thiene G., Vardeny O. Recognition and Initial Management of Fulminant Myocarditis: A Scientific Statement From the American Heart Association. Circulation. 2020;141(6):e69–e92. doi: 10.1161/CIR.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 81.Inciardi R.M., Lupi L., Zaccone G., Italia L., Raffo M., Tomasoni D., Cani D.S., Cerini M., Farina D., Gavazzi E., Maroldi R., Adamo M., Ammirati E., Sinagra G., Lombardi C.M., Metra M. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):1–6. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Doyen D., Moceri P., Ducreux D., Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;395(10235):1516. doi: 10.1016/S0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jabri A., Kalra A., Kumar A., Alameh A., Adroja S., Bashir H., Nowacki A.S., Shah R., Khubber S., Kanaa N.A., Hedrick D.P., Sleik K.M., Mehta N., Chung M.K., Khot U.N., Kapadia S.R., Puri R., Reed G.W. Incidence of Stress Cardiomyopathy During the Coronavirus Disease 2019 Pandemic. JAMA Netw. Open. 2020;3(7) doi: 10.1001/jamanetworkopen.2020.14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Virani S.S., Khan A.N., Mendoza C.E., Ferreira A.C., de Marchena E. Takotsubo cardiomyopathy, or broken-heart syndrome. Tex. Heart Inst. J. 2007;34(1):76–79. [PMC free article] [PubMed] [Google Scholar]
- 85.Inouye M., Abraham G., Nelson C.P., Wood A.M., Sweeting M.J., Dudbridge F., Lai F.Y., Kaptoge S., Brozynska M., Wang T., Ye S., Webb T.R., Rutter M.K., Tzoulaki I., Patel R.S., Loos R.J.F., Keavney B., Hemingway H., Thompson J., Watkins H., Deloukas P., Di Angelantonio E., Butterworth A.S., Danesh J., Samani N.J. Genomic Risk Prediction of Coronary Artery Disease in 480,000 Adults: Implications for Primary Prevention. J. Am. Coll. Cardiol. 2018;72(16):1883–1893. doi: 10.1016/j.jacc.2018.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vieillard-Baron A. Septic cardiomyopathy. Ann. Intensive Care. 2011;1(1):6. doi: 10.1186/2110-5820-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Argulian E., Vogel B., Bohra C., Garg V.P., Talebi S., Lerakis S., Narula J. Right Ventricular Dilation in Hospitalized Patients with COVID-19 Infection, JACC. Cardiovasc. Imag. 2020;13(11):2459–2461. doi: 10.1016/j.jcmg.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang L., Zhao P., Tang D., Zhu T., Han R., Zhan C., Liu W., Zeng H., Tao Q., Xia L. Cardiac Involvement in Patients Recovered From COVID-2019 Identified Using Magnetic Resonance Imaging, JACC. Cardiovasc. Imag. 2020;13(11):2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baldi E., Sechi G.M., Mare C., Canevari F., Brancaglione A., Primi R., Klersy C., Palo A., Contri E., Ronchi V., Beretta G., Reali F., Parogni P., Facchin F., Bua D., Rizzi U., Bussi D., Ruggeri S., Oltrona Visconti L., Savastano S. Out-of-Hospital Cardiac Arrest during the Covid-19 Outbreak in Italy. N. Engl. J. Med. 2020;383(5):496–498. doi: 10.1056/NEJMc2010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bhatla A., Mayer M.M., Adusumalli S., Hyman M.C., Oh E., Tierney A., Moss J., Chahal A.A., Anesi G., Denduluri S., Domenico C.M., Arkles J., Abella B.S., Bullinga J.R., Callans D.J., Dixit S., Epstein A.E., Frankel D.S., Garcia F.C., Kumareswaram R., Nazarian S., Riley M.P., Santangeli P., Schaller R.D., Supple G.E., Lin D., Marchlinski F., Deo R. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17(9):1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kochav S.M., Coromilas E., Nalbandian A., Ranard L.S., Gupta A., Chung M.K., Gopinathannair R., Biviano A.B., Garan H., Wan E.Y. Cardiac Arrhythmias in COVID-19 Infection. Circ. Arrhythm. Electrophysiol. 2020;13(6) doi: 10.1161/CIRCEP.120.008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shao F., Xu S., Ma X., Xu Z., Lyu J., Ng M., Cui H., Yu C., Zhang Q., Sun P., Tang Z. In-hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan. China, Resuscitation. 2020;151:18–23. doi: 10.1016/j.resuscitation.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dherange P., Lang J., Qian P., Oberfeld B., Sauer W.H., Koplan B., Tedrow U. Arrhythmias and COVID-19: A review. JACC Clin. Electrophysiol. 2020;6(9):1193–1204. doi: 10.1016/j.jacep.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., De Leacy R.A., Shigematsu T., Ladner T.R., Yaeger K.A., Skliut M., Weinberger J., Dangayach N.S., Bederson J.B., Tuhrim S., Fifi J.T. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N. Engl. J. Med. 2020;382(20) doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Team C.C.-R. Coronavirus Disease 2019 in Children - United States, February 12-April 2, 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69(14):422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z., Tong S. Epidemiology of COVID-19 Among Children in China. Pediatrics. 2020;145(6) doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 99.Abou Sherif S., Ozden Tok O., Taskoylu O., Goktekin O., Kilic I.D. Coronary Artery Aneurysms: A Review of the Epidemiology, Pathophysiology, Diagnosis, and Treatment. Front. Cardiovasc. Med. 2017;4:24. doi: 10.3389/fcvm.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., Bonanomi E., D'Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P., Xiao W., Wang Y.N., Zhong M.H., Li C.H., Li G.C., Liu H.G. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. 2020;133(9):1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ooi E.E., Chew J.S., Loh J.P., Chua R.C. In vitro inhibition of human influenza A virus replication by chloroquine. Virol. J. 2006;3:39. doi: 10.1186/1743-422X-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chorin E., Wadhwani L., Magnani S., Dai M., Shulman E., Nadeau-Routhier C., Knotts R., Bar-Cohen R., Kogan E., Barbhaiya C., Aizer A., Holmes D., Bernstein S., Spinelli M., Park D.S., Stefano C., Chinitz L.A., Jankelson L. QT Interval Prolongation and Torsade De Pointes in Patients with COVID-19 treated with Hydroxychloroquine/Azithromycin. Heart Rhythm. 2020;17(9):1425–1433. doi: 10.1016/j.hrthm.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mercuro N.J., Yen C.F., Shim D.J., Maher T.R., McCoy C.M., Zimetbaum P.J., Gold H.S. Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(9):1036–1041. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lillicrap D. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J. Thromb. Haemost. 2020;18(4):786–787. doi: 10.1111/jth.14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J. Thromb. Thrombolysis. 2020;3:1–4. doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tchesnokov E.P., Feng J.Y., Porter D.P., Gotte M. Mechanism of Inhibition of Ebola Virus RNA-Dependent RNA Polymerase by Remdesivir. Viruses. 2019;11(4) doi: 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., Smith E.C., Case J.B., Feng J.Y., Jordan R., Ray A.S., Cihlar T., Siegel D., Mackman R.L., Clarke M.O., Baric R.S., Denison M.R. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio. 2018;9(2) doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brown A.J., Won J.J., Graham R.L., Dinnon K.H., 3rd, Sims A.C., Feng J.Y., Cihlar T., Denison M.R., Baric R.S., Sheahan T.P. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antivir. Res. 2019;169:104541. doi: 10.1016/j.antiviral.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gordon C.J., Tchesnokov E.P., Woolner E., Perry J.K., Feng J.Y., Porter D.P., Gotte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020;295(20):6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S.H., Cunningham J., D’Arminio Monforte A., Ismail S., Kato H., Lapadula G., L’Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A.O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S.K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R.P., Brainard D.M., Childs R., Flanigan T. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bloch E.M., Shoham S., Casadevall A., Sachais B.S., Shaz B., Winters J.L., van Buskirk C., Grossman B.J., Joyner M., Henderson J.P., Pekosz A., Lau B., Wesolowski A., Katz L., Shan H., Auwaerter P.G., Thomas D., Sullivan D.J., Paneth N., Gehrie E., Spitalnik S., Hod E., Pollack L., Nicholson W.T., Pirofski L.A., Bailey J.A., Tobian A.A. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Invest. 2020;130(6):2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.O. World Health . 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020, World Health Organization, Geneva. [Google Scholar]
- 115.Meduri G.U., Golden E., Freire A.X., Taylor E., Zaman M., Carson S.J., Gibson M., Umberger R. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131(4):954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 116.Zhang X., Alekseev K., Jung K., Vlasova A., Hadya N., Saif L.J. Cytokine responses in porcine respiratory coronavirus-infected pigs treated with corticosteroids as a model for severe acute respiratory syndrome. J. Virol. 2008;82(9):4420–4428. doi: 10.1128/JVI.02190-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020;582(7813):469. doi: 10.1038/d41586-020-01824-5. [DOI] [PubMed] [Google Scholar]
- 118.Rose-John S., Winthrop K., Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat. Rev. Rheumatol. 2017;13(7):399–409. doi: 10.1038/nrrheum.2017.83. [DOI] [PubMed] [Google Scholar]
- 119.Deisseroth A., Ko C.W., Nie L., Zirkelbach J.F., Zhao L., Bullock J., Mehrotra N., Del Valle P., Saber H., Sheth C., Gehrke B., Justice R., Farrell A., Pazdur R. FDA approval: siltuximab for the treatment of patients with multicentric Castleman disease. Clin. Cancer Res. 2015;21(5):950–954. doi: 10.1158/1078-0432.CCR-14-1678. [DOI] [PubMed] [Google Scholar]
- 120.Keidar S., Gamliel-Lazarovich A., Kaplan M., Pavlotzky E., Hamoud S., Hayek T., Karry R., Abassi Z. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ. Res. 2005;97(9):946–953. doi: 10.1161/01.RES.0000187500.24964.7A. [DOI] [PubMed] [Google Scholar]
- 121.Ishiyama Y., Gallagher P.E., Averill D.B., Tallant E.A., Brosnihan K.B., Ferrario C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43(5):970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 122.Kuster G.M., Pfister O., Burkard T., Zhou Q., Twerenbold R., Haaf P., Widmer A.F., Osswald S. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur. Heart J. 2020;41(19):1801–1803. doi: 10.1093/eurheartj/ehaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bozkurt B., Kovacs R., Harrington B. Joint HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19. J. Card. Fail. 2020;26(5):370. doi: 10.1016/j.cardfail.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brojakowska A., Narula J., Shimony R., Bander J. Clinical Implications of SARS-Cov2 Interaction with Renin Angiotensin System. J. Am. Coll. Cardiol. 2020;314:58–62. doi: 10.1016/j.jacc.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mazak I., Fiebeler A., Muller D.N., Park J.K., Shagdarsuren E., Lindschau C., Dechend R., Viedt C., Pilz B., Haller H., Luft F.C. Aldosterone potentiates angiotensin II-induced signaling in vascular smooth muscle cells. Circulation. 2004;109(22):2792–2800. doi: 10.1161/01.CIR.0000131860.80444.AB. [DOI] [PubMed] [Google Scholar]
- 126.Kang J.H., Kao L.T., Lin H.C., Wang T.J., Yang T.Y. Do outpatient statins and ACEIs/ARBs have synergistic effects in reducing the risk of pneumonia? A population-based case-control study. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0199981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.E.S.o. Cardiology ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. 2020. https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance (Accessed 09/01/2020)
- 128.Ravnskov U. Cholesterol-lowering treatment may worsen the outcome of a Covid-19 infection. 2020. https://www.bmj.com/content/368/bmj.m1182/rr-10 (Accessed 09/01/2020)
- 129.Ridruejo E., Soza A. The liver in times of COVID-19: What hepatologists should know. Ann. Hepatol. 2020;19(4):353–358. doi: 10.1016/j.aohep.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tan W.Y.T., Young B.E., Lye D.C., Chew D.E.K., Dalan R. Statin use is associated with lower disease severity in COVID-19 infection. Sci. Rep. 2020;10(1):17458. doi: 10.1038/s41598-020-74492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang X.J., Qin J.J., Cheng X., Shen L., Zhao Y.C., Yuan Y., Lei F., Chen M.M., Yang H., Bai L., Song X., Lin L., Xia M., Zhou F., Zhou J., She Z.G., Zhu L., Ma X., Xu Q., Ye P., Chen G., Liu L., Mao W., Yan Y., Xiao B., Lu Z., Peng G., Liu M., Yang J., Yang L., Zhang C., Lu H., Xia X., Wang D., Liao X., Wei X., Zhang B.H., Zhang X., Yang J., Zhao G.N., Zhang P., Liu P.P., Loomba R., Ji Y.X., Xia J., Wang Y., Cai J., Guo J., Li H. In-Hospital Use of Statins Is Associated with a Reduced Risk of Mortality among Individuals with COVID-19. Cell Metab. 2020;32(2) doi: 10.1016/j.cmet.2020.06.015. 176-187.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.De Spiegeleer A., Bronselaer A., Teo J.T., Byttebier G., De Tré G., Belmans L., Dobson R., Wynendaele E., Van De Wiele C., Vandaele F., Van Dijck D., Bean D., Fedson D., De Spiegeleer B. The Effects of ARBs, ACEis, and Statins on Clinical Outcomes of COVID-19 Infection Among Nursing Home Residents. J. Am. Med. Dir. Assoc. 2020;21(7) doi: 10.1016/j.jamda.2020.06.018. 909-914.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chung Y.H., Beiss V., Fiering S.N., Steinmetz N.F. COVID-19 Vaccine Frontrunners and Their Nanotechnology Design. ACS Nano. 2020;14(10):12522–12537. doi: 10.1021/acsnano.0c07197. [DOI] [PubMed] [Google Scholar]
- 134.Koirala A., Joo Y.J., Khatami A., Chiu C., Britton P.N. Vaccines for COVID-19: The current state of play. Paediatr. Respir. Rev. 2020;35:43–49. doi: 10.1016/j.prrv.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A., Flach B., Doria-Rose N.A., Corbett K.S., Morabito K.M., O'Dell S., Schmidt S.D., Swanson P.A., 2nd, Padilla M., Mascola J.R., Neuzil K.M., Bennett H., Sun W., Peters E., Makowski M., Albert J., Cross K., Buchanan W., Pikaart-Tautges R., Ledgerwood J.E., Graham B.S., Beigel J.H. An mRNA Vaccine against SARS-CoV-2 - Preliminary Report. N. Engl. J. Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.Y., Türeci Ö., Tompkins K.R., Lyke K.E., Raabe V., Dormitzer P.R., Jansen K.U., Şahin U., Gruber W.C. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Florindo H.F., Kleiner R., Vaskovich-Koubi D., Acúrcio R.C., Carreira B., Yeini E., Tiram G., Liubomirski Y., Satchi-Fainaro R. Immune-mediated approaches against COVID-19. Nat. Nanotechnol. 2020;15(8):630–645. doi: 10.1038/s41565-020-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pfizer Press Release: Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints. https://www.businesswire.com/news/home/20201118005595/en/ (Accessed November 18, 2020)
- 139.Moderna Press Release: Moderna’s COVID-19 Vaccine Candidate Meets its Primary Efficacy Endpoint in the First Interim Analysis of the Phase 3 COVE Study. https://investors.modernatx.com/news-releases/news-release-details/modernas-covid-19-vaccine-candidate-meets-its-primary-efficacy (Accessed November 17, 2020)