Abstract
Introduction
The Republic of the Congo detected its first case of coronavirus disease 2019 (COVID-19) on March 14, 2020, and within several weeks, the country had introduced protective measures that were still in force in July 2020. Over the course of time, the progression in the number of clinical cases has appeared to be lower than expected, although reverse transcription polymerase chain reaction (RT-PCR) testing has been somewhat limited. In order to evaluate the incidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) within the Congolese population, a seroprevalence study was conducted on healthy individuals from different districts of Brazzaville who were willing to know their COVID-19 infection status.
Methods
Oropharyngeal swab and blood samples were collected from 754 healthy volunteers between April 2020 and July 2020. The samples were analyzed for SARS-CoV-2 using a qualitative RT-PCR assay, and Immunoglobulin G (IgG) and Immunoglobulin M (IgM) antibodies were detected using two different rapid tests.
Results
A total of 56 participants (7.4%) tested positive for SARS-CoV-2. The remaining 698 participants (92.6%) had negative RT-PCR results; of these, 117 were found to have anti-SARS-CoV-2 antibodies using serological tests. For these RT-PCR-negative subjects, the seroprevalence of IgG and IgM was found to increase over time: from 1.7% and 2.5% in April, up to 14.2% and 17.6% in July, respectively. In April 2020, 5% of the women were found to have IgG or IgM antibodies, whereas the antibodies were not detected in any of the men. The seroprevalence in RT-PCR negative subjects was significantly higher in women within IgG (P = 0.012) and IgM (P = 0.045) over the first three months.
Conclusion
The proportion of the population who seroconvert over the course of the first wave is an important data to predict the risk of future COVID-19 waves and this will facilitate the efficient use of limited resources in a low income country like the Republic of the Congo.
Keywords: COVID-19, The Republic of the Congo, Asymptomatic, IgG, IgM, Adults
Introduction
The occurrence of asymptomatic individuals with coronaviruses presents a significant public health issue (Nikolai et al., 2020). As of December 15, 2020, there had been more than 73,557,491 coronavirus disease 2019 (COVID-19) infections world-wide and 1,637,053 deaths (University JH, 2020). The United States had the highest number of infections in the world, with 16,724,753 cases and 303,849 deaths. In Africa, South Africa had the highest number of cases (873,678) and deaths (23,661). The Republic of the Congo, with an estimated population of 5,300,000, had recorded 6200 cases and 100 deaths, as of December 12, 2020 (SITREP 115, Congo).
While most countries, including those in Central Africa, have set-up virus-detection procedures for screening symptomatic individuals and close contacts of COVID-19 cases, systems are lacking that would enable the systematic detection of mild and asymptomatic cases. It is clear that more testing would enable the identification of more asymptomatic individuals, but this would be particularly challenging for low-resource countries, like many in sub-Saharan Africa (https://ourworldindata.org/coronavirus-testing).
In the Republic of the Congo, the testing capacity has increased since the beginning of the outbreak on March 14, 2020, when the first case was detected. However, testing remains limited (Velavan and Meyer, 2020), thus rendering it difficult to evaluate the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) within the population. When testing is restricted to symptomatic cases, an accurate estimate of the incidence cannot be obtained. In the Republic of the Congo, Brazzaville and Pointe-Noire are the largest cities, and they have the highest number of COVID-19 cases (4087 and 1600, respectively; SITREP 115, Congo, December, 12th 2020).
The World Health Organization (WHO) has recommended the molecular diagnosis of SARS-CoV-2 using reverse transcription polymerase chain reaction (RT-PCR) (World Health Organization, 2020). However, the technique is expensive and requires specialized equipment, trained personnel, and a reliable electricity supply. Rapid diagnostic tests (RDT) have been developed that detect the antibodies produced against the virus, and they could be considered as an alternative for identifying individuals who have been exposed to the infection a minimum of 7–14 days beforehand. The tests detect the presence of Immunoglobulin G (IgG) and Immunoglobulin M (IgM) antibodies, which reflect the course of the infection, and can demonstrate seroconversion at a time when the RT-PCR sensitivity has decreased (Hains et al., 2020, Wang et al., 2020). This tool could therefore be used to inform public health authorities concerning the development of herd immunity within the population (Nikolai et al., 2020). The aim of this study was to determine the seroprevalence of SARS-CoV-2 in the Congolese population residing in Brazzaville.
Materials and methods
Study area
The study was conducted in Brazzaville, capital of the republic of the Congo. Individuals were recruited at the Health center of the Fondation Congolaise pour la Recherche Médicale located in Massissia (diatsrict of Madibou), southern part of Brazzaville, from April to July 2020. The municipality of Madibou is located in the south of the city of Brazzaville with an area of 80 km2. The population of Madibou is 100,000 (District 8 Madibou).
Study participants and sample collection
We performed a cross-sectional study by proceeding to an open-invitation screening in Brazzaville using mouth to mouth spread of information and also by contacting most of the biggest companies and societies established in the capital and encouraging their workers to be tested. Only individuals without any disease symptom, aged more than 18 years old and giving a written consent were enrolled. During the interview before enrolment, some of the participants reported to have been in contact with a diagnosed COVID-19 positive patient. The participants were resident of all the districts of Brazzaville. Personal data (age, sex, occupation, place of residence) and oropharyngeal swab and blood samples were collected and stored at −80 °C until analysis performance.
SARS-CoV-2 detection
RNA was extracted using QIAamp Viral RNA Mini kit (Qiagen, Valencia, CA) according to instructions. Qualitative RT-PCR assays to detect SARS-CoV-2 were performed using a clinically validated kit approved by the Chinese National Medical Products Administration (Liferiver, Shanghai, China) on a a high-performance, high-throughput PCR platform (96 well plates) LightCycler® 480 RealTime PCR System (Roche Diagnostics) according to the instructions, with Ct values below 40 considered positive.
IgG/IgM detection
Samples were tested using two COVID-19 IgG/IgM rapid test cassettes: Sienna™ (Salofa Oy, Salo, Finland) and UNscience® (UNscience Biotechnology, Wuhan, China,). The Sienna™ COVID-19 IgG/IgM rapid test cassettes have a sensitivity of 88.24% for IgG and 91.76% for IgM; the specificity is 99.46% for IgG and 99.19% for IgM. The sensitivity of the UNscience® COVID-19 IgG/IgM rapid test is 98.81%, and the specificity is 98.02%. Both of the tests are immunochromatographic assays and use a combination of particles coated with SARS-CoV-2 antigens for the qualitative detection of IgG and IgM antibodies. To perform the tests, a small amount of the sample is applied to the cassette (10 μl for Sienna™; 20 μl for UNscience®), and the buffer is then added (two drops for Sienna™; one drop for UNscience®). The results are read 10–20 min later. The result is positive when one or two colored lines appear in the test regions for IgG and IgM, as well as a colored line in the control region. The result is negative when there is a colored line in the control region, but no line appears in the test regions for IgG and IgM. The result is invalid when the control line fails to appear. In this study, a sample was considered to be positive when there were positive test results for both RDTs.
Ethics statement
The study was approved by the Institutional Ethics Committee of the Congolese Foundation for Medical Research, Brazzaville, the Republic of the Congo. Before enrollment, written informed consent was obtained from the participants, and the confidentiality of the data was ensured.
Statistical analysis
The data were entered into CSpro (version 7.4.0) and analyzed using SPSS version 24. GraphPad (version 8.0.4) was used to generate the figures, and maps were created using QGIS. Descriptive statistics were calculated for all of the variables, including the prevalence rates, and adjustments were made for clustering effects in the RT-PCR results. Chi-square tests and Fisher’s exact tests were used to analyze the associations between the following variables: RT-PCR (+/−), IgG(+/−), IgM(+/−), sex, age, month of enrollment, and place of residence. P values less than 0.05 were considered to be statistically significant.
Results
Sociodemographic findings
A total of 754 participants (463 men; 291 women) were recruited between April 1, 2020 and July 31, 2020, all of whom resided in the districts of Brazzaville. The average age was 39.69 years (SD = 13.07; range = 18–90 years). Of the 754 samples taken from asymptomatic subjects, 56 were found to have an active SARS-CoV-2 infection (7.4%). For the samples taken from the men, 9.3% (43/463) were found to have the infection, compared to 4.5% (13/291) for the women. This sex difference was statistically significant (p = 0.014). The infection was not found to be associated with age. The most affected district of Brazzaville was Moungali, which had 17 cases (18.1%). There was significant difference (p = 0.004). Participants with a positive RT-PCR test result were referred to the health care program of the COVID-19 National Response Committee.
Serology testing
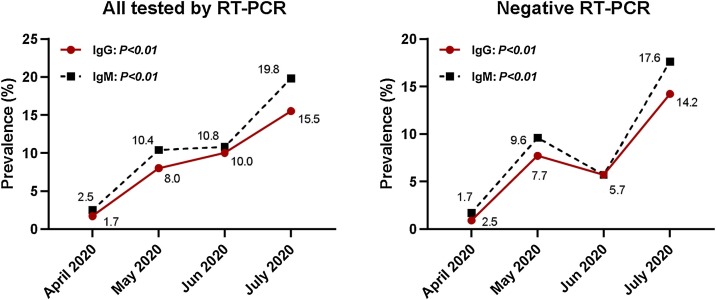
The overall seroprevalence of IgM and/or IgG was 19.8% (n = 149/754). It was found that 9.28% (n = 70/754) of the samples were positive for both IgM and IgG (IgM+IgG+). IgG was detected in 10.7% (n = 81/754) of the total samples, while IgM was detected in 13.7% (n = 103/754) of the samples (Table 1). In April 2020, the overall seroprevalence was 1.7% for IgG and 2.5% for IgM; this increased over time, and in July it reached 15.5% and 19.8% for IgG and IgM, respectively (Figure 1). Of the 754 study participants, a total of 698 individuals were RT-PCR negative (92.6%). Of these, 117 had detectable anti-SARS-CoV-2 antibodies; there were 9.2% (n = 64/698) with IgG+; 11.5% (n = 80/698) with IgM+; and 7.7% (n = 54/698) with both IgG+ and IgM+ (Table 1). The seroprevalence increased over time, from 1.7 % (IgG+) and 2.5% (IgM+) in April 2020, up to 14.2% (IgG+) and 17.6% (IgM+) in July 2020 (Figure 1).
Table 1.
Number of Congolese participants with IgG and IgM according to the presence or absence of the SARS-CoV-2 virus.
| Immunoglobulin type | All participants N = 754 |
SARS-CoV-2 RT-PCR |
||
|---|---|---|---|---|
| Negative N = 698 |
Positive N = 56 |
p Value | ||
| IgG+ | 81 (10.7) | 64 (9.2) | 17 (30.4) | <0.0001 |
| IgG− | 673 (89.3) | 634 (90.8) | 39 (69.6) | |
| IgM+ | 103 (13.7) | 80 (11.5) | 23 (41.1) | <0.0001 |
| IgM− | 651 (86.3) | 618 (88.5) | 33 (58.9 | |
Figure 1.
The prevalence of IgM and IgG antibodies against SARS-CoV-2 in asymptomatic Congolese individuals from April 2020 to July 2020.
IgG/IgM and gender
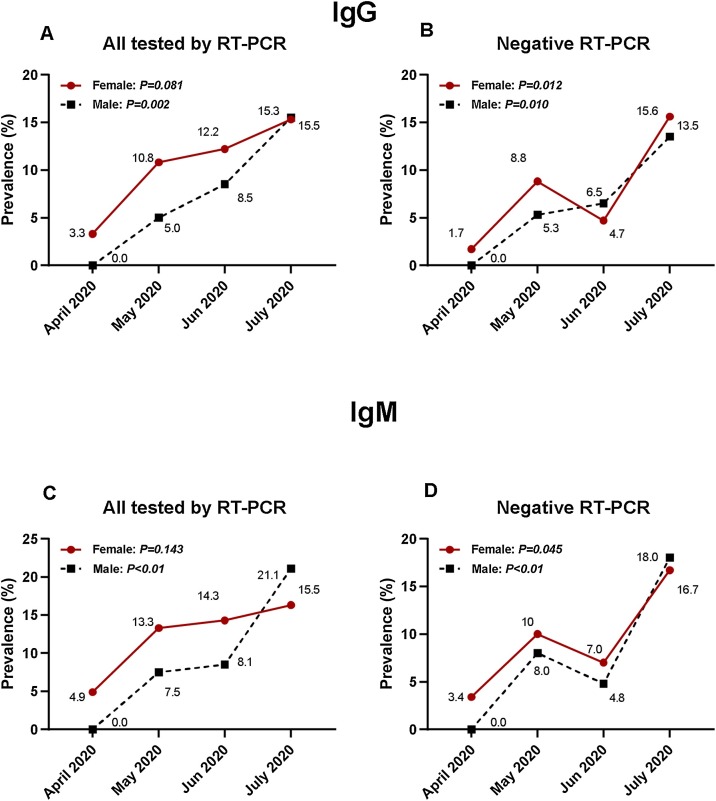
In April 2020, IgG was detected in 3.3% of the women, and IgM was detected in 4.9% of the women. In contrast, there were no detectable IgG or IgM antibodies for the men. In April, May, and June, the seroprevalence in IgG was higher in the women, and this sex difference was statistically significant RT-PCR negative individuals (p = 0.012) (Figure 2).
Figure 2.
A: Prevalence of SARS-CoV-2 IgG according to sex from April 2020 to June 2020 in Congolese participants.
B: Prevalence of SARS-CoV-2 IgG according to sex from April 2020 to June 2020 in Congolese participants with a negative RT-PCR.
C: Prevalence of SARS-CoV-2 IgM according to sex from April 2020 to June 2020 in Congolese participants.
D: Prevalence of SARS-CoV-2 IgM according to sex from April 2020 to June 2020 in Congolese participants with a negative RT-PCR.
Discussion
This cross-sectional study is the first to report the seroprevalence of SARS-CoV-2 in the Congolese population and, to the best of our knowledge, in the Central African population. It was a pilot investigation that only used two serological RDTs, as the study was conducted during the lockdown with limited additional resources, such as reagents. The study was carried out as part of a massive screening campaign to detect SARS-CoV-2 within the general Congolese population residing in Brazzaville, the capital, which is the city with the highest incidence of COVID-19 (about 65%; SITREP 115, Congo).
In sub-Saharan Africa, the seroprevalence of SARS-CoV-2 antibodies has been reported to be 5.6% in Kenya, based on the results of blood donors (Uyoga et al., 2020), and 12.3% in Malawi, based on the results of healthcare workers (Chibwana et al., 2020). We found a seroprevalence of 19.8% in the Republic of the Congo, based on the results of all of the study participants; for the RT-PCR negative subjects, the prevalence stood at 16.8%. This is around half of that found in the United States, the most affected country so far, where a seroprevalence of 31.5% has been reported at the epicenter of the country’s COVID-19 outbreak (Naranbhai et al., 2020). In Switzerland, the prevalence of antibodies has been reported to be somewhat lower, standing at 1.6%, based on the results of enzyme-linked immunosorbent assays (ELISA) in healthcare workers (Korth et al., 2020). Our study found that 7.4% of adults without any symptoms had an active SARS-CoV-2 infection. While there is evidence from some countries that lockdowns may have reduced the incidence of the disease (Ogunleye et al., 2020, Alfano and Ercolano, 2020), the present study was not able to determine whether there was an effect on the number of asymptomatic infections. This is due to the sample size as well as limited data; for instance, the number of cases (confirmed or suspected) before the lockdown was not available. Our results revealed a high prevalence of SARS-CoV-2 antibodies, much higher than the 3.35% reported in Wuhan, the epicenter of the COVID-19 outbreak in China (Ling et al., 2020). This implies that the population of Brazzaville was still exposed to the virus at the end of the study period. With an estimated population of 1,838,348, an overall seroprevalence of 19.8% would mean that around 363,993 inhabitants would be susceptible to seroconvert to SARS-CoV-2, that is around one-fifth of the inhabitants of Brazzaville.
The highest number of RT-PCR positive individuals was in the district of Moungali. This is located next to Talangaï, which has previously been found to have the highest number of symptomatic and contact cases (results from national screening; SITREP, Congo). The high prevalence of SARS-CoV-2 in Moungali could be attributed to non-compliance with containment measures, which may relate to the intensive business activity of this area. Similar observations have been made in Brazil (Borges et al., 2020). Seroprevalence studies provide good indicators of the scale and spread of the pandemic, and they can be used to predict the probability and timing of future recrudescent waves (Bryant et al., 2020). They can inform public health decisions concerning city lockdowns, church and school closures, travel restrictions, social distancing (Bryant et al., 2020), and interventions, such as vaccines and therapeutic initiatives (Mayne et al., 2020). If diagnostic tests are only performed when there is clinical suspicion, there can be a selection bias. Indeed, some authors have shown the importance of considering asymptomatic patients in order to assess with more certainty the prevalence of a disease (Sutton et al., 2020, Sood et al., 2020). It is known that bias due to false positives and false negatives can be common when serological testing is considered at the individual level (Mallapaty, 2020, Vogel, 2020). However, at the population level, it is possible to obtain a reliable estimate of the average seroprevalence, even when there is only moderate sensitivity and specificity (Bryant et al., 2020). In any case, in order to minimize the risk of any bias, we employed two different RDTs. Only samples that tested positive for both RDTs were considered to be positive. However, a consequence of this approach is that the reported seroprevalence may actually be an under-estimate. By using ELISA assays in future studies, the results may be found to differ. Since the start of the COVID-19 pandemic, the infection has been under-diagnosed because of limited testing capacity and also, most probably, because of the spontaneous resolution of untreated cases. The present study is important because it enabled cases to be detected that would otherwise have been missed, specifically around 15–19% of asymptomatic individuals. This seroprevalence is high compared to a study carried out in Korea, where a rate of just 7.6% was found (Song et al., 2020). The results of this study are in line with the findings of the national screening in the Republic of the Congo, which reported that 75% of infections are carried by men. The results are also similar to those reported by Shields et al. (2020).
Countries throughout Africa are currently developing vaccine strategies in order to be able to protect their populations (Graham, 2020, Nkengasong et al., 2020). In this context, it is important to have an idea of the local herd immunity so that it is possible to estimate the number of those needing to be vaccinated. The sero-epidemiological data will form a crucial part of this.
Conclusion
This study shows that there have been a significant number of undiagnosed SARS-CoV-2 infections in Brazzaville. By determining the proportion of the population who seroconvert over the course of the first wave of COVID-19 in the Republic of the Congo, it is possible to evaluate the risk of future waves more accurately, which will in turn facilitate the efficient use of limited resources.
Conflict of interest
None.
Author contributions
All authors have read and agreed to the published version of the manuscript.
Acknowledgements
We thank all the volunteers who accepted to participate in this study. We are grateful for the technical support of Mr Michael Kombo, Mr Mayassi Kelly and Mrs Brigitte Tumamo Fotso. This project was funded by PANDORA-ID-Net(EDCTP Grant Agreement RIA2016E-1609) and ITAIL-COVID-19. Francine Ntoumi is member of CANTAM (EDCTP-RegNet2015-1045) and PANDORA-ID-Net networks.
References
- Alfano V., Ercolano S. The efficacy of lockdown against COVID-19: a cross-country panel analysis. Appl Health Econ Health Policy. 2020;18(4):509–517. doi: 10.1007/s40258-020-00596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges L.P., Martins A.F., Melo M.S., Oliveira M.G.B., Neto J.M.R., Dósea M.B. Seroprevalence of SARS-CoV-2 IgM and IgG anti- bodies in an asymptomatic population in Sergipe, Brazil. Rev Panam Salud Publica. 2020;44:e108. doi: 10.26633/RPSP.2020.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant J.E., Azman A.S., Ferrari M.J., Arnold B.F., Boni M.F., Boum Y. Serology for SARS-CoV-2: apprehensions, opportunities, and the path forward. Sci Immunol. 2020;5(47) doi: 10.1126/sciimmunol.abc6347. [DOI] [PubMed] [Google Scholar]
- Chibwana M.G., Jere K.C., Kamng’ona R., Mandolo J., Katunga-Phiri V., Tembo D. High SARS-CoV-2 seroprevalence in Health Care Workers but relatively low numbers of deaths in urban Malawi. medRxiv. 2020;2020 doi: 10.1101/2020.07.30.20164970. 07.30.20164970. [DOI] [Google Scholar]
- Graham B.S. Rapid COVID-19 vaccine development. Science. 2020;368(6494):945–946. doi: 10.1126/science.abb8923. [DOI] [PubMed] [Google Scholar]
- Hains D.S., Schwaderer A.L., Carroll A.E., Starr M.C., Wilson A.C., Amanat F. Asymptomatic seroconversion of immunoglobulins to SARS-CoV-2 in a pediatric dialysis unit. JAMA. 2020;323(23):2424–2425. doi: 10.1001/jama.2020.8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth J., Wilde B., Dolff S., Anastasiou O.E., Krawczyk A., Jahn M. SARS-CoV-2- specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128(July):104437. doi: 10.1016/j.jcv.2020.104437. Epub 2020 May 13. PMID: 32434708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling R., Yu Y., He J., Zhang J., Xu S., Sun R. Seroprevalence and epidemiological characteristics of immunoglobulin M and G antibodies against SARS-CoV-2 in asymptomatic people in Wuhan, China [preprint] Cold Spring Harbor: medRxiv. 2020;16 doi: 10.1101/2020.06.16.20132423. 06.16.20132423. [DOI] [Google Scholar]
- Mallapaty S. Will antibody tests for the coronavirus really change everything? Nature. 2020;580(7805):571–572. doi: 10.1038/d41586-020-01115-z. [DOI] [PubMed] [Google Scholar]
- Mayne E.S., Scott L., Semete B., Julsing A., Jugwanth S., Mampeule N. The role of serological testing in the SARS-CoV-2 outbreak. S Afr Med J. 2020;110(July (9)):842–845. [PubMed] [Google Scholar]
- Naranbhai V., Chang C.C., Beltran W.F.G., Miller T.E., Astudillo M.G., Villalba J.A. High seroprevalence of anti-SARS-CoV-2 antibodies in Chelsea, Massachusetts. J Infect Dis. 2020:jiaa579. doi: 10.1093/infdis/jiaa579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolai L.A., Meyer C.G., Kremsner P.G., Velavan T.P. Asymptomatic SARS Coronavirus 2 infection: Invisible yet invincible. Int J Infect Dis. 2020;100:112–116. doi: 10.1016/j.ijid.2020.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkengasong J.N., Ndembi N., Tshangela A., Raji T. COVID-19 vaccines: how to ensure Africa has access. Nature. 2020;586(October):198. doi: 10.1038/d41586-020-02774-8. [DOI] [PubMed] [Google Scholar]
- Ogunleye O.O., Basu D., Mueller D., Sneddon J., Seaton R.A., Yinka-Ogunleye A.F. Response to the novel corona virus (COVID-19) pandemic across Africa: successes, challenges and implications for the future. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.01205. [1205] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields A., Faustini S.E., Perez-Toledo M., Jossi S., Aldera E., Allen J.D. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020 doi: 10.1136/thoraxjnl-2020-215414. thoraxjnl-2020-215414. Epub ahead of print. PMID: 32917840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.K., Lee D.H., Nam J.H., Kim K.T., Do J.S., Kang D.W. IgG seroprevalence of COVID-19 among individuals without a history of the coronavirus disease infection in Daegu, Korea. J Korean Med Sci. 2020;35(July (29)) doi: 10.3346/jkms.2020.35.e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood N., Simon P., Ebner P., Eichner D., Reynolds J., Bendavid E. Seroprevalence of SARS-CoV-2–specific antibodies among adults in Los Angeles County, California, on April 10-11, 2020. JAMA. 2020;323(23):2425–2427. doi: 10.1001/jama.2020.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton D., Fuchs K., D’Alton M., Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382:2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University JH . 2020. COVID-19 case tracker.https://coronavirus.jhu.edu/ . [Accessed 15 December 2020] [Google Scholar]
- Uyoga S., Adetifa I.M.O., Karanja H.K., Nyagwange J., Tuju J., Wanjiku P. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Kenyan blood donors. Science. 2021;371(6524):79–82. doi: 10.1126/science.abe1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velavan T.P., Meyer C.G. The COVID-19 epidemic. Trop Med Int Health. 2020;25(3):278–280. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel G. First antibody surveys draw fire for quality, bias. Science. 2020;368(6489):350–351. doi: 10.1126/science.368.6489.350. [DOI] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. PMID: 32159775; PMCID: PMC7066521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization; 2020. Clinical management of COVID-19: interim guidance. 27 May 2020, https://apps.who.int/iris/handle/10665/332196. Licence: CC BY-NC-SA 3.0 IGO. [PubMed] [Google Scholar]