Abstract
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the causing agent of Coronavirus Disease-2019 (COVID-19), is likely to be originated from bat and transmitted through intermediate hosts. However, the immediate source species of SARS-CoV-2 have not yet been confirmed. Here, we used diversity analysis of the angiotensin I converting enzyme 2 (ACE2) that serves as cellular receptor for SARS-CoV-2 and transmembrane protease serine 2 (TMPRSS2), which has been proved to be utilized by SARS-CoV-2 for spike protein priming. We also simulated the structure of receptor-binding domain of SARS-CoV-2 spike protein (SARS-CoV-2S RBD) with the ACE2s to investigate their binding affinity to determine the potential intermediate animal hosts that could spread the SARS-CoV-2 to humans in South Asia. We identified cow, buffalo, goat and sheep, which are predominant species in the household farming system in South Asia that can potentially be infected by SARS-CoV-2. All the bird species studied along with rat and mouse were considered less potential to interact with SARS-CoV-2. The interaction interfaces of SARS-CoV-2S RBD and ACE2 protein complex suggests pangolin as a potential intermediate host in SARS-CoV-2. Our results provide a valuable resource for the identification of potential hosts for SARS-CoV-2 in South Asia and henceforth reduce the opportunity for a future outbreak of COVID-19.
Keywords: COVID-19, SARS-CoV-2, Host range, ACE2, TMPRSS2
Highlights
-
•
Host range of SARS-CoV-2 was predicted based on ACE2 and TMPRSS2 protein sequences of the hosts.
-
•
Cow, buffalo, goat and sheep were identified as highly potential for SARS-CoV-2 infection in South Asia.
-
•
Pangolin was suggested as the intermediate host of SARS-CoV-2.
1. Introduction
Coronavirus Disease 2019 (COVID-19) caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) (Zhu et al., 2020; Wu et al., 2020) has become a pandemic and spread ~213 countries. The disease has already claimed more than 1 million lives and around 33 million infections worldwide as of 28 September 2020 (https://www.worldometers.info/coronavirus). These figures are still growing in an alarming rate. The pandemic creates an overwhelming situation which frightens the public health and economy worldwide with an enormous effect.
The comparative analysis of SARS-CoV-2 sequence with other related viruses showed that bats are likely to be the originating species of this virus (Zhou et al., 2020). The zoonotic origin of SARS-CoV-2, predicted by some early cases which were linked to the Huanan seafood and wild animal market in Wuhan city of China, also raised the possibility of the transmission of SARS-CoV-2 through an intermediate host (Lu et al., 2020). Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and Middle East Respiratory Syndrome Coronavirus (MERS-CoV) were also originated from bat species and considered to be transmitted to human via intermediate host palm civets (Guan, 2003) and dromedary camels (Drosten et al., 2014) respectively. However, the intermediate host or immediate source species of SARS-CoV-2 has not yet been confirmed. In the late 19th century, there was a pandemic caused by a human coronavirus (Vijgen et al., 2005) which might have originated from cattle or swine (Chen et al., 2005). In addition, cross species transmission was reported in some studies where it was shown that coronaviruses can be transmitted from bats to human and other wildlife species (Lam et al., 2020) and also from human to tiger (United States Department of Agriculture Animal and Plant Health Inspection Service, 2020). Therefore, it is important to know the possible host range of SARS-CoV-2 for taking effective measures to control the pandemic and further cross species transmission. Detection of viruses from individuals of all the species present within a certain region in a pandemic situation is very difficult in terms of time and labor required for the lab work. Prediction of potential host species with in silico approaches based on the known factors of host-pathogen interaction could make the task feasible.
Host range determination and pathogenesis of coronaviruses largely depend on the receptor recognition by viral spike (S) protein (Li, 2013). Receptor-binding domain (RBD) of SARS-CoV-2 spike protein (SARS-CoV-2S RBD) utilizes angiotensin-converting enzyme 2 (ACE2) as the functional receptor of the host to enter the cell (Lan et al., 2020). Human ACE2 was identified as the cellular receptor for SARS-CoV also (Li et al., 2003). ACE2 is primarily associated to work in controlling blood pressure and to regulate the function of kidney and heart (Anguiano et al., 2017). A recent study showed that ACE2 of civet, swine and Chinese horseshoe bat could be utilized by SARS-CoV-2 as entry receptor (Zhou et al., 2020). Binding of the surface unit, S1, of the S protein with cellular receptor facilitates viral attachment to the surface of target cells and the transmembrane unit, S2, is required to allow fusion of viral and cellular membranes (Hofmann and Pohlmann, 2004). However, coronaviruses require S protein priming, cleavage at the S1/S2 and the S2, to facilitate the binding with receptor for viral entry to host cell and so does the need of cellular protease (Gallagher and Buchmeier, 2001; Shulla et al., 2011).
SARS-CoV was reported to use transmembrane protease serine 2 (TMPRSS2) for S protein priming (Shulla et al., 2011; Glowacka et al., 2011; Matsuyama et al., 2010). A recent study showed that beside ACE2 as the receptor, SARS-CoV-2 also utilizes TMPRSS2 as cellular protease for viral entry into primary target cells and for viral spread in the infected host (Hoffmann et al., 2020). Therefore, comparative analysis of ACE2 and TMPRSS2 of target species could be served as the method for quick screening of potential host range.
In this study, we considered the subsequent analysis of ACE2 and TMPRSS2 to predict the potential as well as the intermediate host of SARS-CoV-2 among the selected species, which are mostly common in human habitation of South Asia (Bangladesh, Bhutan, India, Maldives, Nepal, Pakistan, and Sri Lanka). More than 1.8 billion people, about 24% of the world population, are living in South Asia (Country Comparison: Population. CIA. July, 2020) and more than 0.1 million deaths (~10% of global deaths) and around 7 million cases (~ 20% of global cases) were reported in this part of the world to COVID-19 pandemic as of 28 September 2020 (https://www.worldometers.info/coronavirus). However, recent works have only considered ACE2 and did not highlight the species commonly present in South Asia to predict the host range of SARS-CoV-2 (Liu et al., 2020; Qiu et al., 2020; Luan et al., 2020a, Luan et al., 2020b) in this region. Here, we made use of the ACE2 protein sequences of 35 species and TMPRSS2 of 30 species, subjected to be categorized in terms of their utilization by SARS-CoV-2S protein through comparative analysis.
2. Materials and methods
2.1. Sequences used in the study
Protein sequences of ACE2 and TMPRSS2 were retrieved from either UniProt Knowledgebase or the NCBI GenBank Database. The amino acid (aa) sequences of ACE2 used in this study were from 35 species whereas 30 TMPRSS2 sequences were considered as the TMPRSS2 sequences of palm civet, mink, intermediate horseshoe bat, Chinese rufous horseshoe bat and Pearson's horseshoe bat were not available in the databases. We only considered those species which are commonly seen in South Asia region. As for example, in case of bat there are so many species in the world but we only considered the bat species which are mostly available at South Asia. The species names along with the sequence IDs are given in Table 1 .
Table 1.
List of the species with sequence IDs.
| English Name | Scientific Name | ACE2 Sequence ID | TMPRSS2 Sequence ID |
|---|---|---|---|
| Human | Homo sapiens | Q9BYF1 | O15393 |
| Palm civet | Paguma larvata | Q56NL1 | – |
| Mink | Mustela lutreola biedermanni | QNC68911.1 | – |
| Elephant | Loxodonta africana | G3T6Q2 | G3UD10 |
| Rhesus macaque | Macaca mulatta | F7AH40 | F6SVR2 |
| Squirrel | Ictidomys tridecemlineatus | I3M887 | I3MJY8 |
| Mouse | Mus musculus | Q8R0I0 | Q9JIQ8 |
| Rat | Rattus norvegicus | Q5EGZ1 | Q920K3 |
| Rabbit | Oryctolagus cuniculus | G1TEF4 | XP_008250697.1 |
| Intermediate horseshoe bat | Rhinolophus affinis | QMQ39244.1 | – |
| Chinese rufous horseshoe bat | Rhinolophus sinicus | U5WHY8 | – |
| Pearson's horseshoe bat | Rhinolophus pearsonii | ABU54053.1 | – |
| Large flying fox | Pteropus vampyrus | XP_011361275.1 | XP_023393967.1 |
| Greater horseshoe bat | Rhinolophus ferrumequinum | E2DHI2 | XP_032944708.1 |
| Pangolin | Manis javanica | XP_017505752.1 | XP_017508127.1 |
| Tiger | Panthera tigris altaica | XP_007090142.1 | XP_015396688.1 |
| Cat | Felis catus | Q56H28 | M3W8X2 |
| Dog | Canis lupus familiaris | J9P7Y2 | A0A455R0B8 |
| Fox | Vulpes vulpes | A0A3Q7RAT9 | A0A3Q7SCF4 |
| Pig | Sus scrofa | K7GLM4 | A7VK02 |
| Cow | Bos taurus | XP_019811720.1 | XP_019818444.1 |
| Buffalo | Bubalus bubalis | XP_006041602.1 | XP_025147919.1 |
| Goat | Capra hircus | A0A452EVJ5 | A0A452FYA6 |
| Camel | Camelus dromedarius | XP_031301717.1 | XP_031315750.1 |
| Horse | Equus caballus | F6V9L3 | A0A3Q2KVY5 |
| Donkey | Equus asinus | XP_014713133.1 | XP_014713133.1 |
| Sheep | Ovis aries | W5PSB6 | W5PKW5 |
| Chicken | Gallus gallus | F1NHR4 | F1NY88 |
| Duck | Anas platyrhynchos | XP_012949915.2 | A0A493STU7 |
| Pigeon | Columba livia | XP_021154486.1 | XP_021154486.1 |
| Crow | Corvus moneduloides | XP_031956594.1 | XP_031956971.1 |
| Sparrow | Zonotrichia albicollis | XP_005491832.2 | XP_005489082.1 |
| Turkey | Meleagris gallopavo | G1NPB8 | G1NPB8 |
| Quail | Coturnix japonica | XP_015742063.1 | XP_015738397.1 |
| Frog | Nanorana parkeri | XP_018418558.1 | XP_018412946.1 |
2.2. Phylogenetic analysis
Evolutionary analysis was performed using molecular evolutionary genetics analysis version X (MEGA-X) (Kumar et al., 2018). Multiple comparisons were conducted using ClustalW multiple sequence alignment program incorporated in MEGA-X. Phylogenetic trees were generated with Jones-Taylor-Thornton evolutionary model using a maximum-likelihood method with 1000 bootstrap replicates.
2.3. SARS-CoV-2S RBD and ACE2 binding capacity prediction
The critical aa sites for the utilization by SARS-CoV-2 were selected based on the known interactions of ACE2 and SARS-CoV-2 residues. A total of 20 aa sites were selected as important according to two recently solved crystal structures of the SARS-CoV-2S RBD in complex with human ACE2 (Lan et al., 2020; Shang et al., 2020). We developed a scoring system for predicting the likelihood of the SARS-CoV-2 binding to ACE2s of 35 species. The initial score for every aa in the 20 selected residue sites which are identical with human ACE2 was set to 5. The substitution on each critical site produced a score of 0. The aa substitutions reported to abolish or strongly inhibit the receptor binding with SARS-CoV resulted in a reduction of its mark to −5. The final score was calculated by summation of the value of each of the 20 critical aa residues. The binding capacity of ACE2s of different species was ranked based on the final score. The ACE2s of the species scored below 50 were considered less potential to be utilized by SARS-CoV-2S RBD.
2.4. Homology-based structure simulation of ACE2-RBD complex
The recently solved crystal structure of SARS-CoV-2S RBD (Lan et al., 2020) bound to the cell receptor ACE2 was used as template (PDB: 6M0J) for homology modeling to simulate the interaction interfaces of SARS-COV-2S RBD and ACE2 of human, pangolin, intermediate horseshoe bat, cow, goat, cat and dog using MODELLER (Sali and Blundell, 1993) incorporated in Chimera software version 1.14. The structures were visualized and analyzed with Chimera software version 1.14 (Pettersen et al., 2004).
3. Results
3.1. Phylogenetic analysis of ACE2 and TMPRSS2
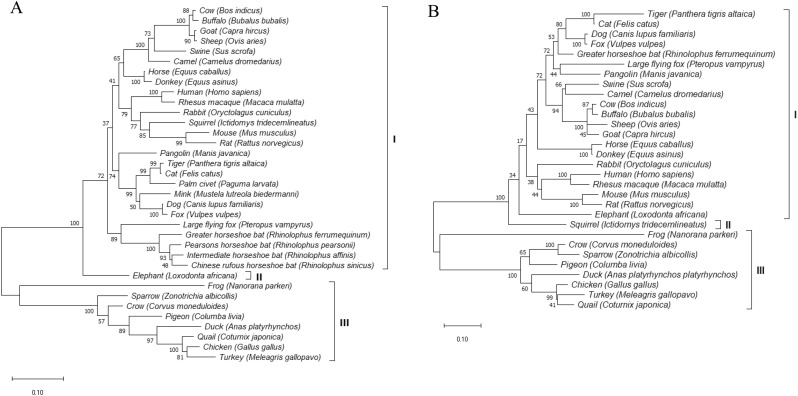
To investigate the molecular basis of evolution we studied overall sequence variation of ACE2 and TMPRSS2 among the selected species. For this purpose, phylogenetic trees were constructed based on the aa sequences of ACE2 and TMPRSS2 (Fig. 1 ). Results showed that human ACE2 was clustered with rhesus macaque but separated from mouse and rat ACE2 in both of the trees. The members of Bovidae family such as cow, buffalo, goat and sheep formed a cluster and all the bird species were clustered with chicken in ACE2 and TMPRSS2 trees.
Fig. 1.
Phylogenetic analysis of protein sequences from the selected species. The trees were constructed on the whole aa sequences using maximum-likelihood method by MEGAX with 1000 bootstrap replicates. A, phylogenetic tree of ACE2 protein sequences of different species. B, phylogenetic tree of TMPRSS2 protein sequences of different species.
3.2. ACE2 utilizing capability by SARS-CoV-2S RBD
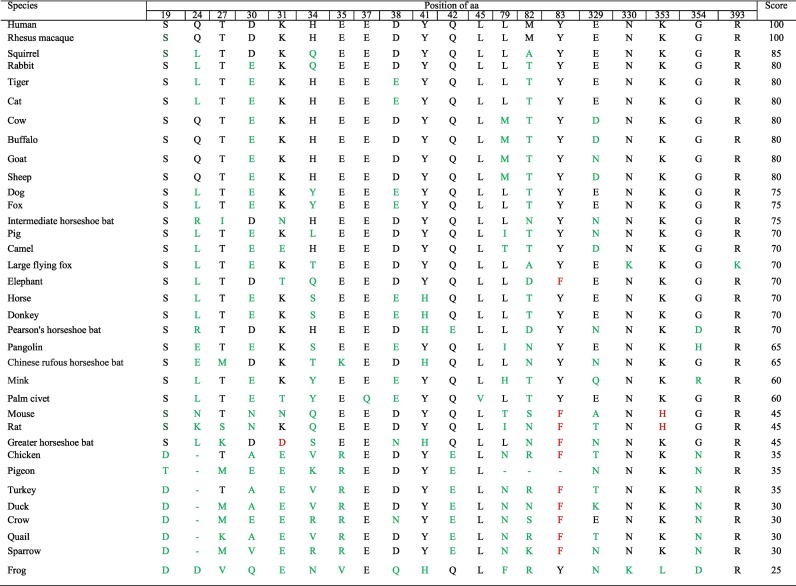
We determined 20 potential aa residues of ACE2 important in interacting with SARS-CoV-2 based on the recently solved crystal structure of the SARS-CoV-2S RBD in complex with human ACE2 (Lan et al., 2020; Shang et al., 2020). These aa residues were S19, Q24, T27, D30, K31, H34, E35, E37, D38, Y41, Q42, L45, L79, M82, Y83, E329, N330, K353, G354 and R393. The substitutions such as K31D, Y83F and K353H/A/D were reported to strongly inhibit or abolish the binding with SARS-CoV (Li et al., 2005; Wan et al., 2020). According to our scoring criteria where we set 5 marks for identical residue, 0 for substitution and − 5 for substitution responsible for strong inhibition to bind with ACE2 compared to human ACE2 in corresponding site, we listed 34 species chronologically from highest to lowest score (Table 2 ). The utilization capacity of SARS-CoV-2S RBD was predicted based on the cumulative final score achieved through summation of 20 individual scores. As shown in Table 2, only rhesus macaque achieved full score of 100 while squirrel got second highest score of 85. Species from the family Bovidae (cow, buffalo, goat and sheep) scored third highest of 80 along with cat, tiger and rabbit. Intermediate horseshoe bat scored 75 while other bats except greater horseshoe bat; pangolin, probable reservoir of SARS-CoV-2 (Zhang et al., 2020); palm civet, considered as intermediate host of SARS-CoV (Guan, 2003); and camel, intermediate host of MERS-CoV (Lam et al., 2020) scored between 60 and 70. According to our scoring system, the ACE2s of the species scored below 50 were considered less potential to be utilized by SARS-CoV-2S RBD. All the bird species along with rat and mouse scored less than 50.
Table 2.
Key aa comparison of ACE2 among different species interacting with SARS-CoV-2 S RBD.
Note: Substituted residues compared to human ACE2 are colored with Green and substituted residues that were reported to strongly inhibit or abolish the binding with.
SARS-CoV-2 are colored with Red.
3.3. Structure simulation by homology modeling of ACE2-RBD complex
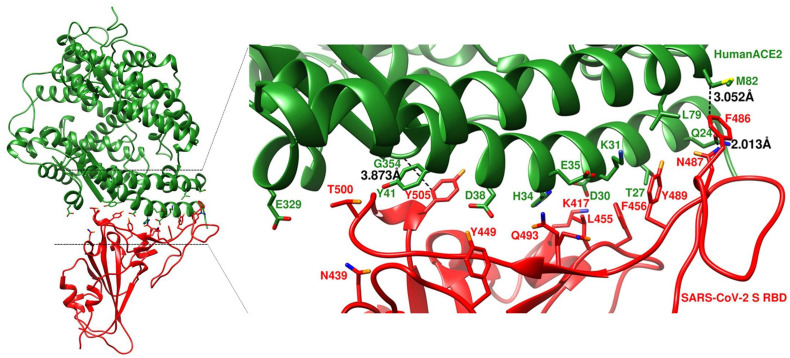
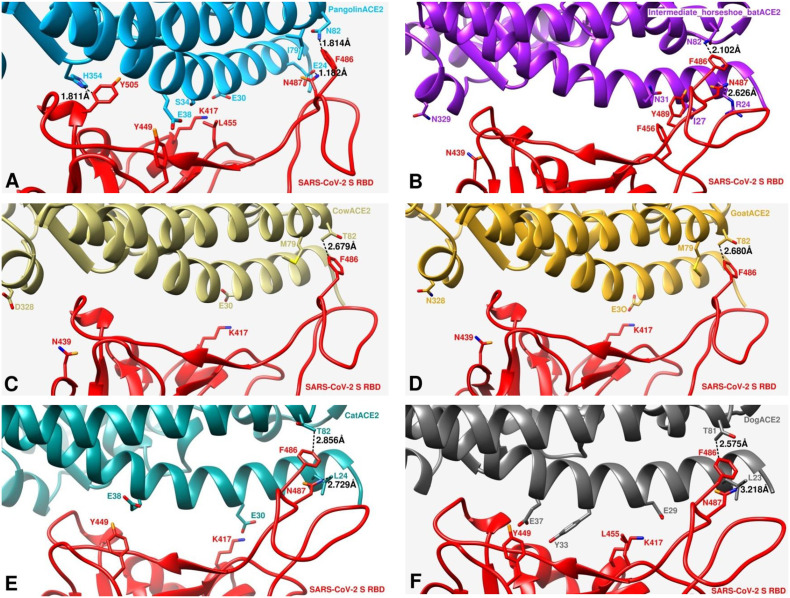
The effect of residue substitutions at the atomic level is crucial for explaining the various receptor activities. To investigate such effects, we simulated the potential structures of SARS-COV-2S RBD and ACE2 protein complex of human, pangolin, intermediate horseshoe bat, cow, goat, cat and dog using homology-based modeling based on the crystal structure of SARS-CoV-2S RBD bound to the cell receptor ACE2 (PDB: 6M0J). Atoms of the substituted residues of ACE2s compared to corresponding residue of human ACE2 and atoms of the respective interacting residues of SARS-COV-2S RBD in the binding interface were shown (Fig. 2, Fig. 3 ). We noticed some reduced or increased distances between the atoms of the interacting residues due to the substitutions. For instance, the atomic distances between N487 of SARS-COV-2S RBD and corresponding substituted residues of ACE2 were 1.182 Å, 2.626 Å, 2.729 Å and 3.218 Å for pangolin (Fig. 3A), intermediate horseshoe bat (Fig. 3B), cat (Fig. 3E) and dog (Fig. 3F) respectively which was 2.013 Å in case of human (Fig. 2). Human ACE2 showed M as the aa in 82 position, while N82, N82, T82, T82, T82 and T81 was the corresponding aa in ACE2 of pangolin, intermediate horseshoe bat, cow, goat, cat and dog, respectively (Table 2). The atomic distance between the respective aa in 82 position of ACE2 and F486 of SARS-COV-2S RBD was 3.052 Å in human (Fig. 2), 1.814 Å in pangolin (Fig. 3A), 2.102 Å in intermediate horseshoe bat (Fig. 3B), 2.679 Å in cow (Fig. 3C), 2.680 Å in goat (Fig. 3D), 2.856 Å in cat (Fig. 3E) and 2.575 Å in dog (Fig. 3F). In addition, the corresponding residue of G354 in pangolin was changed to H and this substitution produced more close contact with SARS-COV-2 (Fig. 3A). Despite the aa changes, the interaction interfaces of these species ACE2s with SARS-COV-2S RBD confirmed their potential interaction with this virus.
Fig. 2.
Overall structure simulation and structural details at the binding interface of SARS-COV-2S RBD and human ACE2. HumanACE2 and SARS-COV-2 S RBD are in forest green and red, respectively.
Fig. 3.
Comparisons of interactions at the SARS-CoV-2S RBD and ACE2 interfaces. A, simulation of structural details at the binding interface of SARS-COV-2 S RBD and PangolinACE2. PangolinACE2 and SARS-CoV-2S RBD are in deep skyblue and red, respectively. B, simulation of structural details at the binding interface of SARS-CoV-2S RBD and Intermediate horseshoe batACE2. Intermediate horseshoe batACE2 and SARS-CoV-2S RBD are in purple and red, respectively. C, simulation of structural details at the binding interface of SARS-CoV-2S RBD and CowACE2. CowACE2 and SARS-CoV-2S RBD are in dark khaki and red, respectively. D, simulation of structural details at the binding interface of SARS-CoV-2S RBD and GoatACE2. GoatACE2 and SARS-CoV-2S RBD are in goldenrod and red, respectively. E, simulation of structural details at the binding interface of SARS-CoV-2S RBD and CatACE2. CatACE2 and SARS-CoV-2S RBD are in dark cyan and red, respectively. F, simulation of structural details at the binding interface of SARS-CoV-2S RBD and DogACE2. DogACE2 and SARS-CoV-2S RBD are in dim gray and red, respectively.
4. Discussion
It is very important to determine the species range of a zoonotic disease like COVID-19 to identify the potential intermediate host and natural reservoir of the causing agent for combating the disease in effective ways. Although the bat species, detected previously as the source of SARS-CoV (Guan, 2003) and MERS-CoV (Drosten et al., 2014), was also identified as the probable origin of novel coronavirus SARS-CoV-2, the intermediate host of this virus is still not clear. We sought to identify the species, which have the probability to be infected with SARS-CoV-2 within the surroundings of human habitation in South Asia. For this purpose, we performed the diversity analysis of the selected species in terms on the aa composition of two proteins; ACE2, the host cell receptor of SARS-CoV-2 (Li et al., 2003) and TMPRSS2, which has been proved to be utilized by SARS-CoV-2 for S protein priming (Shulla et al., 2011; Glowacka et al., 2011; Matsuyama et al., 2010).
We generated phylogenetic trees to cluster the ACE2 and TMPRSS2 sequences of different species based on the evolutionary distance (Fig. 1). Then, we looked for the residue level interaction of SARS-CoV-2 with these two proteins by analyzing protein complex structure. Unfortunately, the structure of TMPRSS2 or its interacting complex with SARS-CoV-2 is not reported yet. So, we took only ACE2 in consideration for further analysis. We intended to predict the capability of SARS-CoV-2S RBD to utilize host cell receptor by investigating the substitution of critical aa in ACE2 of different species compared to human ACE2. We determined 20 aa critical in the binding interface of SARS-COV-2S RBD and ACE2 protein complex according to two solved crystal structures (Wu et al., 2020; Zhou et al., 2020). We listed the species in descending order of respective score regarding the binding capacity of ACE2 with SARS-CoV-2S RBD based on our scoring system (Table 2). Next, we tried to interpret the result of these two strategies to predict the likeliness of those species to interact with SARS-CoV-2.
Only the rhesus macaque which was reported to be as susceptible as human to SARS-CoV-2 (Munster et al., 2020), showed 100% similarity with human ACE2 (Table 2). Among the species which are very common in household farming system in South Asia- cow, buffalo, goat and sheep were predicted to be highly susceptible to SARS-CoV-2 by achieving the high score of 80. Special care should be taken to handle the management of these animals. Beside the bearing status as pet animal, a major population of cats and dogs are ownerless and seen frequently in the habitat of this part of Asia. According to the Table 2, cat gathered 5 points more than dog regarding the utilizing capability of ACE2 by SARS-CoV-2. This result is consistent with the study where SARS-CoV-2 was replicated efficiently in cat but poorly in dog (Shi et al., 2020). Tiger ACE2 was clustered with cat (Fig. 1A) and produced same score (Table 2). This finding is in agreement with the incident of a captive Malayan tiger which was reported to be positive with SARS-CoV-2 infection (United States Department of Agriculture Animal and Plant Health Inspection Service, 2020). According to our findings, ACE2 of squirrel is an efficient (Table 2) receptor with second highest score of 85 suggesting its more susceptibility to SARS-CoV-2 infection than cat. Interestingly the intermediate horseshoe bat, whose affiliated coronavirus RaTG13 was reported to be most closely related with SARS-CoV-2 in terms of evolution, scored 75 which is the highest among the studied bat species. A range of the moderate score 60–70 was gained by other bat species, mink and probable reservoir or intermediate host of SARS-CoV-2 (pangolin) (Zhang et al., 2020), SARS-CoV (palm civet) (Guan, 2003) and MERS-CoV (camel) (Drosten et al., 2014). Our results differ with the findings where the animals such as civet, Chinese rufous horseshoe bat (Wan et al., 2020), pangolin and horse (Qiu et al., 2020) were predicted as the potential hosts with high risk for SARS-CoV-2 infection. Only the greater horseshoe bat within the bat species was found less potential. In addition, palm civet showing unique substitutions 37EQ and L45V and the large flying fox showing rare substitutions N330K and R393K (Table 2), could be the subject of further study to find the consequences of these alterations. Interestingly mink showed the highest number (3) of rare mutations of L79H, E329Q and G354R among the potential hosts. Considering the recent SARS-CoV-2 infection in mink on two farms in the Netherlands (Oreshkova et al., 2020), these mutations could also be the subject of laboratory experiment.
The correlation between genetic distances depicted by Fig. 1 and scoring based ranking for interaction with SARS-CoV-2 (Table 2) was found interesting. The bird species along with frog, with scores less than 50% compared to human ACE2 (Table 2), could be ruled out from probable host range of SARS-CoV-2 and their clustering under group III in ACE2 tree (Fig. 1A) further supported the idea. Despite achieving low score of 45, mouse, rat and greater horseshoe bat were clustered in group I where all the other studied species which produced substantial scores were clustered. Looking at the Table 2 we can see two changes; K31D in greater horseshoe bat and K353H in rat and mouse ACE2s. In addition, Y83F substitution was observed in these three species. All these alterations inhibit or abolish the binding of ACE2 with SARS-CoV (Li et al., 2005; Wan et al., 2020) and might be the reason of their inconsistent positioning in phylogenetic tree and scoring based ranking table. Our finding is also in agreement with the infectivity studies of rat and mouse (Zhou et al., 2020; Hoffmann et al., 2020). Elephant was the only member of group II. It should be noted that, among the species with substantial scores only elephant bears inhibiting mutations in 83 AA position (Table 2). The overall clustering pattern of the species in TMPRSS2 tree (Fig. 1B) is found almost similar to that of ACE tree (Fig. 1A). Only exception is the position of squirrel and elephant. Compared to ACE tree, elephant was included to group I and squirrel was placed in group II as the only member of this group in TMPRSS2 tree. Although we could not predict the residue level utilizing capacity of TMPRSS2 by SARS-CoV-2 due to the unavailability of TMPRSS2 structure, from the above results we could say that the interaction pattern of TMPRSS2 with SARS-CoV-2 for the studied species would follow same prediction we inferred for ACE2 by analyzing Table 2 and Fig. 1A. Beside in-depth analysis of ACE2, TMPRSS2 tree thus brought us extra confidence to our effort to predict potential hosts.
We further tried to investigate the binding affinity of ACE2 of six species (pangolin, intermediate horseshoe bat, cow, goat, cat and dog) along with human ACE2 with SARS-CoV-2 by simulating the potential structure of ACE2 and SARS-CoV-2S RBD protein complex (Fig. 2 and Fig. 3) based on the recently solved crystal structure (PDB: 6M0J) (Lan et al., 2020). We noticed that some residue changes in different ACE2 compared to human ACE2 may affect their interaction with SARS-CoV-2S RBD in terms of the atomic distances within the interacting residues in the binding interface. Only the substitution in the 82 aa position is commonly seen to all the six species- substituted by N in pangolin and bat and by T in other four species (Table 2). Although all these substitutions showed reduction in the atomic distances in contacting F486 of SARS-CoV-2S RBD (Fig. 3), pangolin and bat produced closer contact than other four species. Our result agrees with the study which also claimed that N82 in ACE2 is closer to SARS-CoV-2S RBD than M82 (Luan et al., 2020a, Luan et al., 2020b). In case of pangolin Q24E showed less distant contact with N487 of SARS-CoV-2S RBD (Fig. 3A), while substitution of aa in 24 position by other than E resulted more distant contact than human ACE2 (Fig. 2 and Fig. 3). In addition, G354H which is unique to pangolin produced more than 50% closer contact with Y505 of SARS-CoV-2S RBD. The hypothesis that pangolin was involved in the evolution of SARS-CoV-2 is further supported by these results.
In a nutshell, we sought to combine phylogenetic analysis, identification and marking of key amino acids of host proteins utilized by SARS-CoV-2 to predict the potential host range among the species which are closely associated with daily activities of the people of South Asia region. Although this is plausible to predict the susceptibility to SARS-CoV-2 infection based on overall similarity of receptor residues, a single aa polymorphism or the glycosylated residue can disrupt the binding of the receptor to the virus (Lu et al., 2015). Moreover, this might not be straight forward to identify individual reservoir within the same species because of intraspecies variation. Therefore, direct laboratory data and epidemiological investigation are required to make confirmed call. Nonetheless, our predictions provide quick assessment ahead of time-consuming individual laboratory experiments to detect risk associated species and so to develop proactive measure accordingly in controlling further cross species transmission. Our findings from structure simulation suggested pangolin as a potential intermediate host in SARS-CoV-2 evolution. Results of our analysis may also help to design optimized ACE2 for host mediated research to study SARS-CoV-2 infection.
Author statement
Rasel Ahmed conceived the work, analyzed the data and wrote the manuscript. Rasel Ahmed and Rajnee Hasan contributed to graphics processing. Rajnee Hasan, AMAM Zonaed Siddiki and M Shahidul Islam contributed to critical reviewing and editing the manuscript.
Declaration of Competing Interest
All authors have declared that no competing interests exist.
References
- Anguiano L., Riera M., Pascual J., Soler M.J. Circulating ACE2 in cardiovascular and kidney diseases. Curr. Med. Chem. 2017;24:3231–3241. doi: 10.2174/0929867324666170414162841. [DOI] [PubMed] [Google Scholar]
- Chen W., Yan M., Yang L. SARS-associated coronavirus transmitted from human to pig. Emerg. Infect. Dis. 2005;11(3):446–448. doi: 10.3201/eid1103.040824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Country Comparison . CIA; 2020. Population.https://www.cia.gov/library/publications/the-world-factbook/fields/335rank.html July. [Google Scholar]
- Drosten C., Kellam P., Memish Z.A. Evidence for camel-to-human transmission of MERS coronavirus. N. Engl. J. Med. 2014;371(14):1359–1360. doi: 10.1056/NEJMc1409847. [DOI] [PubMed] [Google Scholar]
- Gallagher T.M., Buchmeier M.J. Coronavirus spike proteins in viral entry and pathogenesis. Virology. 2001;279:371–374. doi: 10.1006/viro.2000.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowacka I., Bertram S., MA Mu¨ller. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011;85:4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302(5643):276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann H., Pohlmann S. Cellular entry of the SARS coronavirus. Trends Microbiol. 2004;12:466–472. doi: 10.1016/j.tim.2004.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T.T., Jia N., Zhang Y. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020;583:282–285. doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- Lan J., Ge J., Yu J. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- Li F. Receptor recognition and cross-species infections of SARS coronavirus. Antivir. Res. 2013;100:246–254. doi: 10.1016/j.antiviral.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Zhang C., Sui J., Kuhn J.H., Moore M.J., Luo S. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 2005;24:1634–1643. doi: 10.1038/sj.emboj.7600640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Xiao X., Wei X. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J. Med. Virol. 2020;92(6):595–601. doi: 10.1002/jmv.25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G., Wang Q., Gao G.F. Bat-to-human: spike features determining ‘host jump’ of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. 2015;23:468–478. doi: 10.1016/j.tim.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan J., Jin X., Lu Y., Zhang L. SARS-CoV-2 spike protein favors ACE2 from Bovidae and Cricetidae. J. Med. Virol. 2020:1–8. doi: 10.1002/jmv.25817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan J., Lu Y., Jin X., Zhang L. Spike protein recognition of mammalian ACE2 predicts the host range and an optimized ACE2 for SARS-CoV-2 infection. Biochem. Biophys. Res. Commun. 2020;526(1):165–169. doi: 10.1016/j.bbrc.2020.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Nagata N., Shirato K., Kawase M., Takeda M., Taguchi F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J. Virol. 2010;84:12658–12664. doi: 10.1128/JVI.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster V.J., Feldmann F., Williamson B.N. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature. 2020;585:268–272. doi: 10.1038/s41586-020-2324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreshkova N., Molenaar R.J., Vreman S. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Euro Surveill. 2020;25(23) doi: 10.2807/1560-7917.ES.2020.25.23.2001005. pii=2001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C. UCSF chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Zhao Y., Wang Q. Predicting the angiotensin converting enzyme 2 (ACE2) utilizing capability as the receptor of SARS-CoV-2. Microbes Infect. 2020;22(4–5):221–225. doi: 10.1016/j.micinf.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sali A., Blundell T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234(3):779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- Shang J., Ye G., Shi K. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Wen Z., Zhong G. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020;368(6494):1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulla A., Heald-Sargent T., Subramanya G., Zhao J., Perlman S., Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011;85:873–882. doi: 10.1128/JVI.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Agriculture Animal and Plant Health Inspection Service . 2020. USDA APHIS|USDA Statement on the Confirmation of COVID-19 in a Tiger in New York (April 13, 2020) [Google Scholar]
- Vijgen L., Keyaerts E., Moës E. Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J. Virol. 2005;79:1595–1604. doi: 10.1128/JVI.79.3.1595-1604.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. (e00127-20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Wu Q., Zhang Z. Probable Pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 2020;30:1346–1351. doi: 10.1016/j.cub.2020.03.022. (e2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]