Abstract
A pneumonia outbreak of unknown aetiology emerged in Wuhan, China in December 2019. The causative organism was identified on 7th January 2020 as a novel coronavirus (nCoV or 2019-nCoV), later renamed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The resulting coronavirus disease (COVID-19) has infected over 88 million individuals, resulted in over 1.9 million deaths, and has led to an unprecedented impact on research activities worldwide. Extraordinary challenges have also been imposed on medical and surgical trainees following redeployment to full-time clinical duties. Moreover, the introduction of travel restrictions and strict lockdown measures have forced the closure of many institutions and laboratories working on research unrelated to the pandemic. The lockdown has similarly stifled supply chains and slowed research and development endeavours, whilst research charities have endured significant financial strains that have since reshaped the allocation and availability of funds. However, worldwide scientific adaptation to the COVID-19 pandemic has been observed through unprecedented levels of international collaboration alongside the uprise of remote telecommunication platforms. Although the long-term consequence of the COVID-19 pandemic on research and academic training is difficult to ascertain, the current crises will inevitably shape working and teaching patterns for years to come. To this end, we provide a comprehensive and critical evaluation of the impact of COVID-19 on scientific research and funding, as well as academic medical and surgical training.
Keywords: COVID-19, Surgery, Science, Academia
1. Introduction
The novel coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged as an unknown entity in Wuhan, China in December 2019 before being later identified on 7th January 2020 [1]. Similar to the virus having caused the 2003 SARS large-scale epidemic, leading to ~800 deaths and affecting ~8,000 individuals, SARS-CoV-2 has already infected more than 88 million people and caused over 1.9 million deaths worldwide. Evidence suggests that human-to-human transmission occurs via droplets, direct contact, and tears. These findings have led to the imposition of strict confinement, lockdown, and travel restriction measures in an attempt to reduce the spread of the contagion. Although effective in limiting transmission, such measures have resulted in a multitude of socio-economic implications alongside fears of an impending economic crisis and insurmountable recession [2].
The COVID-19 pandemic has affected research activities to a great extent including several challenges imposed on the clinical and surgical trainee. As a direct consequence of lockdown measures and the closure of most university research facilities, all research activities and clinical trials unrelated to COVID-19 came to a halt. Changes have also led to a substantial number of clinical academics returning to full-time clinical duties. In the UK, preliminary estimates suggest that over 1,500 academic trainees in England have been redeployed to clinical posts [3]. Similar responses have also been seen from those in out of programme surgical research, alongside changes to academic publishing, research funding, and scientific conferences. Rapid assessment of the consequences of the often-substantial disruptions to training is key. This will help guide the development of an action plan to achieve optimal overall career progression. To understand this impact, we provide a comprehensive review and critical evaluation of the COVID-19 pandemic on scientific research and funding, as well as academic clinical and surgical training.
2. The evolution and current status of COVID-19
The last ten months have necessitated unprecedented global efforts to understand SARS-CoV-2 and the disease that it is responsible for causing, COVID-19. When SARS-CoV-1 was first identified in humans in 2002, the genomic sequencing of the virus cost several months [4]. Technological advancements have since allowed SARS-CoV-2 to be mapped within weeks of the first recognised case [5]. Months later, this genetic information has enabled sophisticated tracing of transmission chains, allowing researchers to understand that a particularly infectious variant of the virus has now become globally dominant [6,7].
COVID-19 was initially promulgated as a respiratory disease, causing characteristic ground glass opacities on chest scans. It has since emerged that the disease also affects blood vessels, leading to distinctive vascular features such as endothelial injury, angiogenesis, and widespread thrombosis [8]. Thus, SARS-CoV-2 should be considered more than a respiratory virus. Further autopsy-based studies have indicated several other extrapulmonary manifestations impacting the gastrointestinal, neurological, renal, and cardiovascular systems [9]. The RECOVERY collaborative group demonstrated the use of dexamethasone to result in lower 28-day mortality rates compared to patients receiving invasive ventilation or oxygen alone [10].
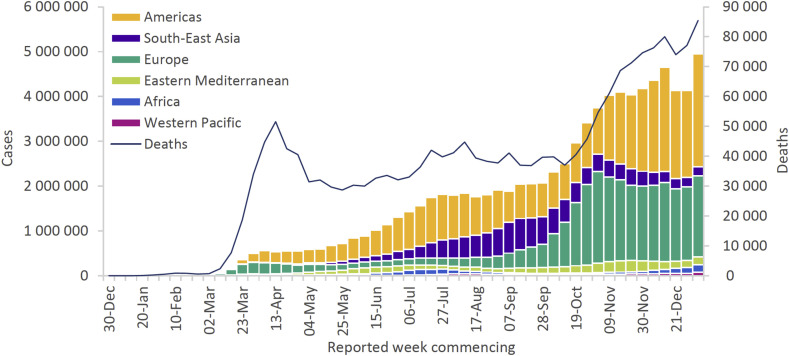
As of 12th January 2021, the World Health Organization (WHO) has reported over 88 million laboratory-confirmed cases of COVID-19 and over 1.8 million deaths [11]. Caution should be exercised when interpreting the number of COVID-19 cases reported by national authorities. As elucidated by Nicola et al., a multitude of factors contribute to the formulation of these figures [12]. Nevertheless, data collected by the WHO affirms that the Region of the Americas continues to account for the greatest proportion of cumulative cases and deaths (Fig. 1 ) [11].
Fig. 1.
Number of COVID-19 cases reported weekly by WHO Region and global deaths, 30th December 2019 through 10th January 2021 [11].
3. Impact on basic science
Amid the rising number of COVID-19 cases, most countries have resorted to imposing nationwide lockdown measures to curb viral transmission and spread. These measures have included closing schools, universities, and research institutions, alongside borders and businesses, and have led to the imposition of strict restrictions on population movement [2]. As a result, universities and research institutions have had to carefully define and allocate essential research activities, and all non-essential research inevitably came to a halt. For example, Florida State University have outlined the following as essential activities [13]:
-
1.
Those aimed at fighting COVID-19.
-
2.
Clinical research activities which, if halted, could harm participants.
-
3.
Those involved with the maintenance of the viability of living organisms and samples.
-
4.
Activities requiring safe maintenance of critical equipment and infrastructure.
-
5.
Those where discontinuation could threaten safety or lead to a severe loss of equipment, critical samples, or data.
Much of research within the basic sciences involves wet laboratory work, making it difficult for research scientists to transition toward working from home. In comparison, computational dry laboratory research can be performed remotely [14,15]. In a survey conducted between 15th and 23rd April 2020, 25% of life scientists reported between 1 and 6 months worth of lost work due to the closure of universities and research institutions, with the majority of these being from wet lab (73%) as opposed to dry lab (31%) researchers. Additionally, only 10% of wet lab researchers reported “at least 80% productivity”, in comparison to 29% of dry lab researchers [16]. In another survey conducted by Research Australia in May 2020, an inability to perform remote research was identified as a major issue affecting over 50% of participants. It was also highlighted that research outcomes among basic science researchers were more likely to be affected after the year 2020, in comparison to clinical researchers [17].
The COVID-19 crisis has impacted scientific research involving laboratory animals. Due to staff shortages (resulting from sickness and quarantine), and disruptions in the supply chain of essential goods such as bedding, food, and veterinary drugs, concerns regarding the care of laboratory animals have been raised [18]. In a microbial immunology lab based at the University of Pennsylvania Perelman School of Medicine, over three-quarters of rodents were culled (bred to yield genotypes capable of facilitating study of the immune system response to bacterial invaders). Such scientifically valued resources could take years to re-build to pre-COVID-19 levels [18]. Over a thousand mice and/or rats were similarly culled at The Max Delbrück Center for Molecular Medicine in Germany, and hundreds of transgenic fruit flies were discarded at Tsinghua University in China. In both cases, disruption caused by the pandemic and elapsed time would have led to these organisms being unusable for experimentation [19,20]. Numerous universities and research institutions have urged laboratories to reduce the number of live animals in their care. Inevitably, this has raised ethical questions [18].
Similar disruptions have been felt by researchers involved with cell culture. A team of stem-cell scientists based at the Allen Institute for Cell Science in Seattle reported to having frozen hundreds of cell lines due to the closure of research laboratories. Once thawed, experiments are conducted to confirm the integrity of the experimental cell line. However, there is the possibility that issues regarding a compromise in integrity may arise, affecting all future and downstream applications [20]. Post-thawing of samples can also be time-consuming to researchers. As a result, laboratories may require many months to re-build their research back to pre-COVID-19 levels, and perhaps even years in the event of compromised samples.
Supply shortages have also affected basic scientific research. A sizeable proportion of researchers are required to wear personal protective equipment (PPE) to protect themselves from the risks of laboratory work. Considering marmosets, macaques, and certain strains of cats, mice, and ferrets have been suggested to be susceptible to coronavirus infection, adequate PPE is necessary to provide user protection and to avoid cross-infection [18]. However, the COVID-19 crisis saw severe PPE shortages worldwide, alongside the prioritisation and allocation of available stocks to frontline healthcare workers [21]. In addition, researchers faced shortages in other laboratory resources including cell culture media and RNA extraction reagents – materials utilised for the purposes of COVID-19 testing [20]. In response to institution closures, many laboratories donated PPE stocks to hospital staff, alongside other laboratory materials to aid the COVID-19 testing effort.
Inevitably, the current pandemic saw a shift in research towards those focused on COVID-19 [22]. For example, scientists involved in research related to ophthalmology at the Institut de la Vision in Paris turned to supporting laboratories involved in activities pertaining to COVID-19 [23]. Several cancer research laboratories have also switched to exploring the therapeutic potential of anti-neoplastic agents in COVID-19 [24]. Candidates such as hydroxychloroquine, lopinavir/ritonavir, and remdesivir are repurposed therapeutics having originally emerged from studies on malaria, HIV and Ebola, respectively [25]. With new and ongoing non-COVID-19 research paused as well as a growing number of scientists switching to COVID-19 research, there is increasing concern that basic science research on other leading diseases including cardiometabolic diseases and cancer, which contribute to significant morbidity and mortality worldwide, could become irrevocably stifled [26]. Should scientists embrace this change, a vast shift in the scientific landscape may ensue. The crisis may also have a strong influence on incoming students, leading to an increase in interest in infectious diseases and statistical modelling programmes.
4. Impact on clinical science
Since March 2020, the challenge of performing clinical research has been immense due to the suspension of many healthcare services. The impact of COVID-19 on clinical studies was immediate and will have long-standing consequences. Many clinical trials have been paused with the exception of those focused on COVID-19. Enrolment into new studies has also been suspended due to the risk of spreading COVID-19. Where possible, on-going and future clinical trials have been revised to allow for the implementation of virtual monitoring systems to minimize participants' risk of infection [27].
The US Food and Drug Administration (FDA) has since provided guidance on the conduct of clinical trials of medical products during the COVID-19 pandemic [28]. This was performed to assist sponsors in assuring the safety of trial participants, to maintain trial integrity, and to ensure compliance with good clinical practice (GCP). For current trials, considerations have included the use of electronic data capture via telemedicine, phone interview, or alternative locations for assessment, ensuring that participants are continually informed of any changes to the study protocol, decisions on whether alterations to the original statistical analysis plan should be made in light of potential missing data (e.g. from missed study visits or study discontinuation) and the potential need to stop ongoing recruitment [28,29].
In addition to ongoing clinical trials, COVID-19 has affected trainees in all areas of research. The integrated clinical academic training pathway provides dedicated time to undertake clinical research, research training, and professional development whilst maintaining exposure to clinical practice. In the early phases of the pandemic, the authorities of Health Education England (HEE) contacted those in such placements to be seconded back into their clinical programme of training [30]. Appropriate pauses or deadline extensions for the submission of academic work were granted to those returning early. The HEE has since indicated the provision of adequate support to guarantee a safe transition. Due to the shift in focus from research to patient care, the suspension of research activity has also been seen amongst the senior physician-scientist. Sponsors and funding bodies have since indicated extensions to grants to protect the careers of many. Evidently, the COVID-19 pandemic will have a significant and long-lasting impact on the productivity of the scientific workforce worldwide [27].
5. Impact on researchers
Early-career researchers (ECR) and PhD students have been particularly hard struck by the COVID-19 pandemic [31]. The income of both are vulnerable to economic change and research programme time is often limited. PhD students typically rely on fellowships spanning one to four years in length. Added time due to lost productivity owing to COVID-19 may result in the incurrence of additional tuition and research consumable fees. ECRs are expected to yield high outputs during their short-term contracts, spanning one to two years in length. An inability to complete the originally proposed research programme objectives within a pre-defined timeline could impact future job applications [31].
A survey conducted by Nature between mid-June and end of July 2020 indicated that almost two-thirds of postdoctoral researchers had felt that the COVID-19 pandemic could dampen future career prospects [[32], [33], [34]]. Several other factors have fuelled concerns regarding career progression. For example:
-
1.
Publications: Due to the closure of universities and research institutions, researchers may be unable to complete essential experiments required for the submission of scientific work. Of course, they may still be able to catch up on unfinished work.
-
2.
Funding: Since research output is often used to make funding decisions, researchers may struggle to obtain the necessary funding to complete their planned research programmes, leading many academic contributions to go unrecognized.
-
3.
Networking: With the majority of worldwide scientific events transitioning to a virtual platform, many researchers may lose important career development opportunities in the form of building their reputation within the scientific community via networking or research dissemination. Additionally, junior researchers may feel particularly vulnerable when sharing sensitive information and novel ideas digitally.
-
4.
Job cuts and hiring freeze: Due to an unprecedented decline in international student enrolment and the resulting fall in university revenue, several universities are under financial pressure. Many have responded with job cuts and the imposition of hiring slowdowns and freezes.
Inevitably, navigating the academic job market in the COVID-19-era has become increasingly more complex [35]. As pressure mounts on existing staff to prioritise teaching over research, concerns have also been raised regarding a reduction in laboratory personnel as well as lost collaborative research and informal training opportunities offered by traditional laboratories to junior researchers [36].
For international researchers, the inflexible nature of visa time limits could result in many having to leave their country of study or work prior to research programme completion [32,33]. Moreover, this particular group of researchers may experience additional pressures in terms of finding a suitable job on the next rung of the academic career ladder due to strict visa time limits, especially if planning to continue at their current country of study or work. Due to the resultant international travel bans, several international researchers could not take up overseas jobs that they had previously secured [32,33].
6. Gender inequality
Lockdown orders have also imposed new challenges for mothers in academia, highlighting the persistence of gender inequality in science alongside the need for equity for all [37,38]. Several previous studies have indicated that female academics are more likely to assume parenting or domestic responsibilities in comparison to their male counterparts. This could be particularly true for women from countries with high levels of gender inequality [39]. With the closure of universities and research institutions, researchers have been compelled to work from home, however female researchers are more likely to shoulder the added responsibilities of childcare and home-schooling. Female researchers have also continued to battle underrepresentation in academia and the aforementioned challenges brought by COVID-19, which may have contributed to widening of the pre-existing gender gap in research. It has also been noted that women make up only a third of all authors named on COVID-19 related publications, and that this female underrepresentation is particularly marked among first and last authorship positions [39]. In addition, male authors on popular preprint repositories including arXiv and bioRxiv increased at a greater rate than their female counterparts [40]. Female researchers are also more likely to hold casual or short-term job contracts and are therefore more susceptible to job loss or lost research opportunities [41].
7. Funding
The COVID-19 lockdown has led to monumental implications for charities and funding for scientific research. The increasingly likely prospect of a long-term economic downturn could lead to longer-term impacts on development budgets, funding from direct donations, as well as government and charitable grants. Moreover, a reduction in fundraising income due to the cancellation of events and nationwide lockdowns have led to concerns regarding charity-funded research, the premature closure of sites working overseas, and the furlough of charity staff [42].
Medical research charities such as Cancer Research UK announced the decision to cut £44 million in funding across its research portfolio in April 2020 [43]. The British Heart Foundation has also predicted a 50% decline in net income, alongside the possibility of halving investment into new scientific endeavours (from £100 million to £50 million the following year) [44,45]. Research investigating the impact of the COVID-19 crisis has also revealed a 24% reduction in total income, equating to a £12.4 billion total loss [46]. In response to the potential decline of overseas student fee income, many universities have requested governmental support to temporarily boost their research funds [47]. To mitigate both short and long term damage, many are calling upon action from governments to strengthen their commitment to funding innovation ecosystems and to protect decades of research investment [3].
To compound this, many organisations are assisting researchers by repurposing their grant funds to facilitate a switch to COVID-19 research. A multitude of funding opportunities have since been launched including the National Institute for Health Research (NIHR) and Global Effort on COVID-19 programme (BECO; for long-term research into the COVID-19 pandemic), Microsoft AI for Health COVID-19, EUREKA Funding authorities (to yield solutions for the next human high-impact pandemic), the British Endodontic Society (BES; to address the impact of aerosol generating procedures in dentistry), the British Society for Antimicrobial Chemotherapy (BSAC; to better understand and address the COVID-19 outbreak), and the US National Institutes of Health (NIH; emergency awards for rapid investigation of SARS-CoV-2 and COVID-19).
8. Academic publishing
The COVID-19 crisis has similarly imposed challenges on the longstanding model of research publication, which has proven inadequate in situations requiring the rapid dissemination of data [48]. The prevailing model is notably dominated by for-profit academic publishing houses, although many researchers have long voiced support for an open access model. In response to COVID-19, commercial publishers have temporarily halted paywalls on coronavirus-related research to support research efforts and communications. Many traditional journals have also devised initiatives to help enhance the peer review process such as recruiting a pool of rapid review scientists and permitting extended or flexible revision timelines, all whilst ensuring that rigour and reproducibility remain paramount. Many researchers have also turned to non-peer reviewed preprint servers (e.g. medRxiv and bioRxiv servers) to facilitate the rapid dissemination of information. Moreover, the European COVID-19 Data Portal, announced by the European Commission President, aims to help scientists coordinate the sharing of research data pertaining to pathogenesis, epidemiology, genetics, and potential therapies [49]. Others have utilised existing cohort data to dissect the role of host genetics [50]. The resulting degree of unparalleled data sharing has fostered an unprecedented level of global scientific collaboration. Although fruitful, such endeavours have raised numerous issues including how to uphold the basic standards of scientific conduct and integrity whilst preventing the risk of data misinterpretation. More recently, a number of high-profile retractions have been made in journals such as New England Journal of Medicine and The Lancet [51], leading to a reform in editorial policies [52].
9. Conferences
The COVID-19 pandemic hit the business events industry with unprecedented force leading to the cancellation of many large-scale annual conferences and small society meetings [53]. These decisions were enacted in light of the logistical difficulties that would be faced, including an inability to guarantee the safety of participants or to mitigate the negative effects of travel restrictions. Several events were alternatively postponed; however, this option is non-viable for events in which there is a focus on the delivery of cutting-edge information or time-sensitive data. The evolving situation is also difficult to predict.
In contrast, a significant number of meetings have been redesigned as virtual events including the International Associations of Surgeons of Great Britain & Ireland (ASGBI) Congress (Glasgow, UK), European Molecular Biology Organization (EMBO) Symposium “The four dimensional genome – Virtual” (Heidelberg, Germany) and the 2020 edition of “The Biology of Genomes” (Cold Spring Harbor Laboratory, New York, US). This new format brings several unique opportunities but also numerous technical and organizational challenges. Virtual conferences allow attendance irrespective of travel restrictions and geographic travel constraints (reducing the associated carbon footprint), remove logistical and financial barriers to attendance, offer the advantage of accommodating thousands of attendees as opposed to hundreds, enable moderators to better control the flow of discussions, and are compatible with event marketing and sponsorship strategies. Virtual conference-based bioinformatics and programming courses also provide users with the opportunity to expand their scientific skill set, developing into more interdisciplinary hybrid data scientists. Conversely, virtual conferences do not completely replicate the in-person conference experience, scientific discussions, ‘blue sky’ thinking over a cup of coffee, nor do they provide an opportunity for social impromptu gatherings. Although use of a virtual conference format may lose appeal once lockdown measures loosen, it is nevertheless cromulent to consider that such platforms will complement in-person events post-COVID-19.
10. Outlook and future considerations
As with the rest of ‘normal’ life, the research landscape has been significantly and detrimentally altered by COVID-19. The changes having come into effect over the past several months are unlikely to revert to the old status quo in the near future and so a ‘new normal’ may have to be reached [54].
As previously mentioned, virtually all non-COVID-19 research was halted during the peak of the pandemic with the closure of laboratories and the secondment of clinician-researchers back to the front lines [55,56]. As research facilities begin to reopen, the “safety of research participants and personnel is of paramount importance”, and studies must be conducted in line with guidance such as the NIHR Restart Framework [57]. Given current social distancing requirements and resource availability, prioritisation of research, naturally favouring COVID-19-related work, is likely to continue for some time: Level 1 – essential studies providing evidence for pandemic management; Level 2 – studies where the research protocol includes an urgent treatment or intervention without which patients could come to harm; and Level 3 – all other studies. Institutions and research organisations should therefore work to ensure they provide appropriate physical space for work to be conducted. Space efficiency should be maximised by restructuring laboratories and establishing working partitions.
Research funding is a further point of concern. With many charities and organisations facing a reduction in funds to support research, there is the potential that a decline in opportunities to conduct studies will soon ensue [44]. Grant prospects may become more infrequent and applications more competitive. Financial stimulation needs to be ensured, and whilst government-funded research bodies have committed to upholding the funding of research [58], government spending in a time of recession is likely to have a toll on research funding. Whilst a number of funders are offering administrative relief to help researchers affected by COVID-19, there is significant concern regarding funding after the pandemic [59]. Moving forwards, consideration should be given to support non-COVID-19 research and funding bodies should seek to catalyse the resurgence of normal research activities through specific funding opportunities and stimulus packages.
With elements of social distancing and the need to protect vulnerable members of society likely to continue for some time, researchers must also consider the ongoing implications on research using human participants [60]. Indeed, suspension of enrolment was responsible for the majority of disruption in clinical trials during the pandemic [61]. As human participation in studies resumes, these obstacles must be overcome by assessing the need for face-to-face interactions and the potential use of remote recruitment and monitoring of research participants [62]. Surgical clinical research is also called into question, for similar reasons, given the drive to minimize unnecessary patient contact alongside the dramatic decline in elective surgeries performed over the course of the pandemic [[63], [64], [65]] leading to potential biases in surgical study outcomes [66]. As the backlog of surgical procedures begins to be tackled, surgical research is unlikely to be at the forefront of healthcare system providers’ minds. However, if we are to avoid a drought in scientific discovery and advancement academic surgical authorities should encourage the restart of clinical research, perhaps via modified protocols and processes to ensure safe, efficient, and effective studies.
Those seconded back to the hospital front lines from their surgical academic training posts will now be many months behind on their research schedules. Appropriate allowances and support mechanisms must therefore be implemented in order to support a gradual transition back to academic work. The Clinical Academic Training Forum has developed a series of principles in order to support this [3], with the aim of “supporting and progressing the careers of trainee clinical academics”. It suggests actions for postgraduate deans and training programme directors, universities and research institutes, funders, and indeed trainees in attempting to address the major challenges in restarting research effectively and delivering effective re-entry mechanisms. Moreover, it is not atypical for trainee surgeons to undertake an international fellowship in order to broaden their clinical experience and research exposure [67]. Given the disruption that the current pandemic has had on surgical trainees' experiences of overseas fellowship programmes [68], and in light of ongoing border closures, travel restrictions and mandatory arrival quarantines, it seems unlikely that these fellowship programmes will be able to fulfil their full purpose in the near future. Academic trainees should investigate the possibility of domestic fellowships or other local prospects for training opportunities.
There are a number of positive lessons which can be drawn from researchers’ experiences of academia during the COVID-19 pandemic. The most pertinent of these can be summarised in two words - collaboration and speed. International research efforts, built on collaboration, have allowed for significant breakthroughs to be made regarding our understanding of the pandemic [69]. The open sharing of knowledge and research efforts has stimulated global collaborative bonds with common purpose. It is our hope that these will continue beyond the pandemic, for the benefit of both education and research [70]. Furthermore, all facets of research (proposals, funding, basic research, clinical research, publication and translation into practice) have typically followed a slow and archaic roadmap [71]. The COVID-19 pandemic has demonstrated that all areas of research can be performed much more swiftly and efficiently [72]. Perhaps this too will be a positive element that can be taken forward as we begin to consider the future outlook for scientific research post-COVID-19.
11. Conclusion
COVID-19 has led to the imposition of numerous scientific limitations on clinical and surgical trainees worldwide. Basic scientific research has been particularly hard hit in light of nationwide lockdown measures, whilst clinical scientists have witnessed similar hardships in response to the suspension of healthcare services and an abrupt return to full-time clinical duties. Although the scientific infrastructure used to promote research preparedness in response to worldwide crises differs across continents, research scientists have mobilised swiftly with an extraordinary show of solidarity. The resulting level of international collaboration is greatly anticipated to shape the future of scientific research, coinciding with the uprise of virtual and telecommunication modalities for data dissemination and communication. The long-term consequence of the COVID-19 pandemic on research and training programmes is difficult to ascertain, however it is anticipated that the current crises will shape working and teaching patterns within the scientific world for years to come. Although the overwhelming effects of the COVID-19 pandemic on the medical and surgical academic workforce have been undeniable, major global events often leave dramatic imprints on science, and overcoming these academic hurdles requires a concerted research effort.
Data statement
The data in this review is not sensitive in nature and is accessible in the public domain. The data is therefore available and not of a confidential nature.
Ethical approval
None required.
Funding
None received.
Author contribution
CS: Conceptualization, data curation, resources, writing original draft, editing drafts, approval of final article.
GM, TF, AK, MG, MA, RA: Data curation, resources, writing original draft, editing drafts, approval of final article.
Guarantor
Catrin Sohrabi, Corresponding Author, csohrabi42@gmail.com
Riaz Agha, Senior Author, mail@riazagha.com.
Research registration number
None.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
None.
Acknowledgements
None.
References
- 1.Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A., et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicola M., Alsafi Z., Sohrabi C., Kerwan A., Al-Jabir A., Iosifidis C., et al. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int. J. Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute for Health Research Progressing UK clinical academic training in 2020: addressing the challenges of COVID-19. 2020. https://www.nihr.ac.uk/documents/progressing-uk-clinical-academic-training-in-2020-addressing-the-challenges-of-covid-19/24958
- 4.Bi S., Qin Ed, Xu Z., Li W., Wang J., Hu Y., et al. Complete genome sequences of the SARS-CoV: the BJ group (isolates BJ01-BJ04) Dev. Reprod. Biol. 2003;1(3):180–192. doi: 10.1016/S1672-0229(03)01023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4):812–827 e19. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicola M., O'Neill N., Sohrabi C., Khan M., Agha M., Agha R. Evidence based management guideline for the COVID-19 pandemic - review article. Int. J. Surg. 2020;77:206–216. doi: 10.1016/j.ijsu.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 10.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., et al. RECOVERY Collaborative Group Dexamethasone in hospitalized patients with covid-19 - preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization . WHO; 2020. Coronavirus Disease (COVID-19) - Weekly Epidemiological Update. [Google Scholar]
- 12.Nicola M., Sohrabi C., Mathew G., Kerwan A., Al-Jabir A., Griffin M., et al. Health policy and leadership models during the COVID-19 pandemic: a review. Int. J. Surg. 2020;81:122–129. doi: 10.1016/j.ijsu.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Florida State University News Requesting information on essential research activities. 2020. https://news.fsu.edu/announcements/2020/03/25/requesting-information-on-essential-research-activities/
- 14.Rohrig B., du Prel J.B., Wachtlin D., Blettner M. Types of study in medical research: part 3 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2009;106(15):262–268. doi: 10.3238/arztebl.2009.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omary M.B., Hassan M. Opinion: here's how we restore productivity and vigor to the biomedical research workforce in the midst of COVID-19. Proc. Natl. Acad. Sci. U. S. A. 2020;117(33):19612–19614. doi: 10.1073/pnas.2014730117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korbel J.O., Stegle O. Effects of the COVID-19 pandemic on life scientists. Genome Biol. 2020;21(1):113. doi: 10.1186/s13059-020-02031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peeters A., Mullins G., Becker D., Orellana L., Livingston P. COVID-19’s impact on Australia’s health research workforce. Lancet. 2020;396:461. doi: 10.1016/S0140-6736(20)31533-6. 10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimm D. Respirators, quarantines, and worst-case scenarios: lab animal facilities grapple with the pandemic. Science. 2020 [Google Scholar]
- 19.Johri N. How lab animals have fared in the coronavirus crisis. 2020. https://www.dw.com/en/how-lab-animals-have-fared-in-the-coronavirus-crisis/a-54002319
- 20.Madhusoodanan J. Frozen cells and empty cages: researchers struggle to revive stalled experiments after the lockdown. Nature. 2020 doi: 10.1038/d41586-020-01704-y. [DOI] [PubMed] [Google Scholar]
- 21.Emanuel E.J., Persad G., Upshur R., Thome B., Parker M., Glickman A., et al. Fair allocation of scarce medical resources in the time of covid-19. N. Engl. J. Med. 2020;382(21):2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 22.Ranganathan S. Slow research” in the time of Covid-19. Indian J. Med. Ethics. 2020;5(3):212–214. doi: 10.20529/IJME.2020.072. [DOI] [PubMed] [Google Scholar]
- 23.Nassisi M., Audo I., Zeitz C., Varin J., Wohlschlegel J., Smirnov V., et al. Impact of the COVID-19 lockdown on basic science research in ophthalmology: the experience of a highly specialized research facility in France. Eye. 2020;34(7):1187–1188. doi: 10.1038/s41433-020-0944-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moujaess E., Kourie H.R., Ghosn M. Cancer patients and research during COVID-19 pandemic: a systematic review of current evidence. Crit. Rev. Oncol. Hematol. 2020;150:102972. doi: 10.1016/j.critrevonc.2020.102972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanow S.K., Good M.F. Nonessential research in the new normal: the impact of COVID-19. Am. J. Trop. Med. Hyg. 2020;102(6):1164–1165. doi: 10.4269/ajtmh.20-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeggini E., Baumann M., Gotz M., Herzig S., Hrabe de Angelis M., Tschop M.H. Biomedical research goes viral: dangers and opportunities. Cell. 2020;181(6):1189–1193. doi: 10.1016/j.cell.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiner D.L., Balasubramaniam V., Shah S.I., Javier J.R. Pediatric Policy C. COVID-19 impact on research, lessons learned from COVID-19 research, implications for pediatric research. Pediatr. Res. 2020;88(2):148–150. doi: 10.1038/s41390-020-1006-3. [DOI] [PubMed] [Google Scholar]
- 28.U.S. Department of Health and Human Services Food and Drug Administration. FDA guidance on conduct of clinical trials of medical products during COVID-19 public health emergency: guidance for industry, investigators, and institutional review boards. 2020. https://www.fda.gov/media/136238/download
- 29.Fleming T.R., Labriola D., Wittes J. Conducting clinical research during the COVID-19 pandemic: protecting scientific integrity. J. Am. Med. Assoc. 2020;324(1):33–34. doi: 10.1001/jama.2020.9286. [DOI] [PubMed] [Google Scholar]
- 30.The Royal College of Emergency Medicine COVID-19 FAQs from trainees. 2020. https://www.rcem.ac.uk/docs/Coronavirus/COVID-19%20Trainees%20FAQs%207%20April%202020.pdf
- 31.Paula J.R. Lockdowns due to COVID-19 threaten PhD students' and early-career researchers' careers. Nat. Ecol. Evol. 2020;4(8):999. doi: 10.1038/s41559-020-1231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woolston C. Pandemic darkens postdocs' work and career hopes. Nature. 2020;585(7824):309–312. doi: 10.1038/d41586-020-02548-2. [DOI] [PubMed] [Google Scholar]
- 33.Termini C.M., Traver D. Impact of COVID-19 on early career scientists: an optimistic guide for the future. BMC Biol. 2020;18(1):95. doi: 10.1186/s12915-020-00821-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woolston C. Junior researchers hit by coronavirus-triggered hiring freezes. Nature. 2020;582(7812):449–450. doi: 10.1038/d41586-020-01656-3. [DOI] [PubMed] [Google Scholar]
- 35.Bodin M. University redundancies, furloughs and pay cuts might loom amid the pandemic, survey finds. Nature. 2020 doi: 10.1038/d41586-020-02265-w. [DOI] [PubMed] [Google Scholar]
- 36.Marshman I., Baré E., Beard J. The Conversation; 2020. As universities face losing 1 in 10 staff, COVID-driven cuts create 4 key risks. [Google Scholar]
- 37.Staniscuaski F., Reichert F., Werneck F.P., de Oliveira L., Mello-Carpes P.B., Soletti R.C., et al. Impact of COVID-19 on academic mothers. Science. 2020;368(6492):724. doi: 10.1126/science.abc2740. [DOI] [PubMed] [Google Scholar]
- 38.Malisch J.L., Harris B.N., Sherrer S.M., Lewis K.A., Shepherd S.L., McCarthy P.C., et al. Opinion: in the wake of COVID-19, academia needs new solutions to ensure gender equity. Proc. Natl. Acad. Sci. U. S. A. 2020;117(27):15378–15381. doi: 10.1073/pnas.2010636117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinho-Gomes A.C., Peters S., Thompson K., Hockham C., Ripullone K., Woodward M., et al. Where are the women? Gender inequalities in COVID-19 research authorship. BMJ Glob. Health. 2020;5(7) doi: 10.1136/bmjgh-2020-002922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vincent-Lamarre P., Sugimoto C.R., Vincent L. The decline of women’s research production during the coronavirus pandemic. Nat. Index. 2020 [Google Scholar]
- 41.Gewin V. The career cost of COVID-19 to female researchers, and how science should respond. Nature. 2020;583(7818):867–869. doi: 10.1038/d41586-020-02183-x. [DOI] [PubMed] [Google Scholar]
- 42.Stoye E. How research funders are tackling coronavirus disruption. Nature. 2020 doi: 10.1038/d41586-020-01120-2. [DOI] [PubMed] [Google Scholar]
- 43.Cancer Research UK COVID-19: why we’re making cuts to our research funding. 2020. https://scienceblog.cancerresearchuk.org/2020/04/16/protecting-our-future-by-taking-action-now-why-were-making-cuts-to-our-research-funding/
- 44.Webster P. How is biomedical research funding faring during the COVID-19 lockdown? Nat. Med. 2020 doi: 10.1038/d41591-020-00010-4. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell J. BHF funding more than half of all UK heart and circulatory disease research. 2020. https://www.bhf.org.uk/what-we-do/news-from-the-bhf/news-archive/2020/january/bhf-funding-more-than-half-of-all-uk-heart-and-circulatory-disease-research#:~:text=BHF%20funding%20more%20than%20half%20of%20all%20UK%20heart%20and%20circulatory%20disease%20research,-28%20January%202020&text=We%20fund%20more%20than%20half,Medical%20Research%20Council%20(MRC)
- 46.Institute of Fundraising Round-up: coronavirus impact on charities. 2020. https://www.institute-of-fundraising.org.uk/guidance/coronavirus/round-up-coronavirus-impact-on-charities/
- 47.Dolton P. The COVID-19 pandemic is causing a crisis in the UK universities. 2020. https://voxeu.org/article/covid-19-pandemic-causing-crisis-uk-universities
- 48.Callaway E. Will the pandemic permanently alter scientific publishing? Nature. 2020;582(7811):167–168. doi: 10.1038/d41586-020-01520-4. [DOI] [PubMed] [Google Scholar]
- 49.COVID-19 Data Portal The COVID-19 data portal. 2020. https://www.covid19dataportal.org/
- 50.Bycroft C., Freeman C., Petkova D., Band G., Elliott L.T., Sharp K., et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.RetractionWatch Retracted coronavirus (COVID-19) papers. 2020. https://retractionwatch.com/retracted-coronavirus-covid-19-papers/
- 52.Davey M. The Guardian; 2020. The Lancet Changes Editorial Policy after Hydroxychloroquine Covid Study Retraction.https://www.theguardian.com/world/2020/sep/22/the-lancet-reforms-editorial-policy-after-hydroxychloroquine-covid-study-retraction [Google Scholar]
- 53.Delacrétaz E., Delacrétaz R., Rowe N. Sustainable medical conferences in the post-COVID-19 world. 2020. https://blogs.bmj.com/bmj/2020/08/27/sustainable-medical-conferences-in-the-post-covid-19-world/
- 54.Gibney E. The pandemic mixed up what scientists study - and some won't go back. Nature. 2020;582(7811):173–174. doi: 10.1038/d41586-020-01525-z. [DOI] [PubMed] [Google Scholar]
- 55.Thornton J. Clinical trials suspended in UK to prioritise covid-19 studies and free up staff. BMJ. 2020;368:m1172. doi: 10.1136/bmj.m1172. [DOI] [PubMed] [Google Scholar]
- 56.Servick K. Updated: labs go quiet as researchers brace for long-term coronavirus disruptions. Science. 2020 [Google Scholar]
- 57.National Institute for Health Research Restart framework. 2020. https://www.nihr.ac.uk/documents/restart-framework/24886
- 58.Harper L., Kalfa N., Beckers G.M.A., Kaefer M., Nieuwhof-Leppink A.J., Fossum M., et al. The impact of COVID-19 on research. J. Pediatr. Urol. 2020;16(5):715–716. doi: 10.1016/j.jpurol.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prudencio M., Costa J.C. Research funding after COVID-19. Nat. Microbiol. 2020;5(8):986. doi: 10.1038/s41564-020-0768-z. [DOI] [PubMed] [Google Scholar]
- 60.Medicines and Healthcare products Regulatory Agency . UK Government; 2020. Managing Clinical Trials during Coronavirus (COVID-19) [Google Scholar]
- 61.GlobalData Healthcare Clinical trial disruption due to Covid-19 has begun to slow. 2020. https://www.clinicaltrialsarena.com/comment/clinical-trial-disruption-slowing/
- 62.Goldsack J.C., Izmailova E.S., Menetski J.P., Hoffmann S.C., Groenen P.M.A., Wagner J.A. Remote digital monitoring in clinical trials in the time of COVID-19. Nat. Rev. Drug Discov. 2020;19(6):378–379. doi: 10.1038/d41573-020-00094-0. [DOI] [PubMed] [Google Scholar]
- 63.Diaz A., Sarac B.A., Schoenbrunner A.R., Janis J.E., Pawlik T.M. Elective surgery in the time of COVID-19. Am. J. Surg. 2020;219(6):900–902. doi: 10.1016/j.amjsurg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Al-Jabir A., Kerwan A., Nicola M., Alsafi Z., Khan M., Sohrabi C., et al. Impact of the Coronavirus (COVID-19) pandemic on surgical practice - Part 1. Int. J. Surg. 2020;79:168–179. doi: 10.1016/j.ijsu.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al-Jabir A., Kerwan A., Nicola M., Alsafi Z., Khan M., Sohrabi C., et al. Impact of the Coronavirus (COVID-19) pandemic on surgical practice - Part 2 (surgical prioritisation) Int. J. Surg. 2020;79:233–248. doi: 10.1016/j.ijsu.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McBride K.E., Brown K.G.M., Fisher O.M., Steffens D., Yeo D.A., Koh C.E. Impact of the COVID-19 pandemic on surgical services: early experiences at a nominated COVID-19 centre. ANZ J. Surg. 2020;90(5):663–665. doi: 10.1111/ans.15900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fitzgerald J.E., Milburn J.A., Khera G., Davies R.S., Hornby S.T., Giddings C.E. Clinical fellowships in surgical training: analysis of a national pan-specialty workforce survey. World J. Surg. 2013;37(5):945–952. doi: 10.1007/s00268-013-1949-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nicholas C., Hatchell A., Webb C., Temple-Oberle C. COVID-19 and the impact on surgical fellows: a uniquely vulnerable learner. J. Surg. Educ. 2020;S1931-7204(20):30314–30317. doi: 10.1016/j.jsurg.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fry C.V., Cai X., Zhang Y., Wagner C.S. Consolidation in a crisis: patterns of international collaboration in early COVID-19 research. PloS One. 2020;15(7) doi: 10.1371/journal.pone.0236307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buitendijk S., Ward H., Shimshon G., Sam A.H., Sharma D., Harris M. COVID-19: an opportunity to rethink global cooperation in higher education and research. BMJ Glob. Health. 2020;5(7) doi: 10.1136/bmjgh-2020-002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morris Z.S., Wooding S., Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J. R. Soc. Med. 2011;104(12):510–520. doi: 10.1258/jrsm.2011.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lurie N., Saville M., Hatchett R., Halton J. Developing covid-19 vaccines at pandemic speed. N. Engl. J. Med. 2020;382(21):1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]