Abstract
Scope
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has become pandemic, reaching almost one million death worldwide. At present standard treatment for coronavirus disease 2019 (COVID-19) is not well defined because the evidence, either from randomized or observational studies, with conflicting results, has led to rapid changes in treatment guidelines. Our aim was to narratively summarize the available literature on the management of COVID-19 in order to combine current evidence and interpretation of the data by experts who are treating patients in the frontline setting.
Methods
The panel conducted a detailed review of the literature and eventual press releases from randomized clinical trials for each possible available treatment. Inductive PubMed search waws performed for publications relevant to the topic, including all clinical trials conducted. The result was a flowchart with treatment indications for patients with COVID-19.
Implications
After 6 months of a pandemic situation and before a possible second coronavirus wave descends on Europe, it is important to evaluate which drugs proved to be effective while also considering that results from many randomized clinical trials are still awaited. Indeed, among treatments for COVID-19, only glucocorticoids have resulted in an association with a significant decrease in mortality in published randomized controlled trials. New therapeutic strategies are urgently needed.
Keywords: Coronavirus, COVID-19, Pneumonia, SARS-CoV-2, Therapy
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the new severe acute syndrome called coronavirus disease 2019 (COVID-19) [1]. Since the beginning of the epidemic, the treatment of the first cases relied on drugs showing some efficacy to treat other viruses responsible for epidemics, such as influenza, severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and Ebola, but during the following months, recommendations on treatment changed according to new studies. At present only limited therapeutic options have been approved for the treatment of COVID-19 by regulatory agencies on the basis of randomized controlled trials (RCTs). Moreover, clinicians who at present are facing either the first or the beginning of the second epidemic wave are influenced in their everyday clinical practice, not only by new published studies but also by press releases concerning RCTs.
On the basis of the evidence present in the literature, we, the Italian Society of Infectious and Tropical Diseases (SIMIT), aimed to determine which treatment should to be considered as the standard of care for COVID-19, with particular attention to severe cases.
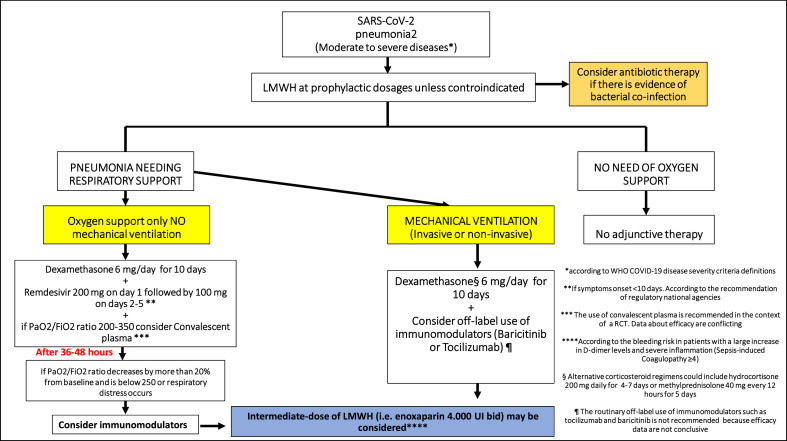
A critical literature review was performed to evaluate published evidence on the available therapeutic strategies for severe COVID-19. Members of the panel included 24 infectious disease specialists from SIMIT. Inductive PubMed search for publications relevant to the topic was conducted. Studies included in the review were not only RCTs but also observational studies with large sample sizes. Moreover, because a nonnegligible number of relevant RCTs have not yet been published, we have included data from press releases and preprint studies. The panel was divided into four groups, each evaluating the evidence for each therapeutic strategy and drafting the part of the document concerning that topic. Finally, a narrative review was conducted, and a final consensus on the whole document was obtained from the entire panel of expert in order finalize the report and draw the consensus flowchart for treatment of severe cases of COVID-19 (Fig. 1 ).
Fig. 1.
Flowchart for treatment of severe cases of coronavirus disease 2019 (COVID-19).
Treatment strategies
Antivirals
In the first weeks of the SARS-CoV-2 pandemic, local guidelines recommended the use of HIV protease inhibitors (lopinavir/ritonavir as the first choice, darunavir (DRV)/cobicistat as the second) [2]. Afterwards, the effect of these anti–COVID-19 treatments was evaluated in RCTs. The first was performed in China on 199 patients with laboratory-confirmed SARS-CoV-2 and demonstrated, even if underpowered, that no benefit was observed with lopinavir/ritonavir treatment beyond standard-of-care therapy in hospitalized adult patients [3]. These data were confirmed by the RECOVERY and SOLIDARITY trials, which concluded that there was no beneficial effect of lopinavir/ritonavir in patients hospitalized with COVID-19 [4,5]. As regards to DRV/cobicistat, no clear clinical evidence supports the use of DRV (boosted with either ritonavir or cobicistat) in viral diseases other than HIV, and DRV had no antiviral activity against SARS-CoV-2 at clinically relevant concentrations [6].
Remdesivir (GS-5734) is a nucleoside analogue prodrug displaying in vitro inhibitory effects on pathogenic animal and human coronaviruses, including SARS-CoV-2 [7]. Preliminary data showed that remdesivir was superior to placebo in shortening the time to recovery but did not show a significant difference in mortality [8]. In a subsequent retrospective cohort study comparing 312 patients with severe COVID-19 who received remdesivir with 818 matched patients, remdesivir was associated with significantly greater recovery and 62% reduced odds of death versus standard-of-care treatment [9]. A RCT involving hospitalized patients with confirmed SARS-CoV-2 infection did not show a significant difference between a 5-day course and a 10-day course of remdesivir, and a clinical improvement of 2 points or more on the ordinal scale occurred in 64% of patients in the 5-day group and in 54% in the 10-day group [10]. Finally, data from the ACTT-1 double-blind RCT has been recently published [11]. The study, conducted on 1062 patients, showed that patients who received a 10-day course of remdesivir had a shorter time to recovery than those receiving placebo (10 vs. 15 days, p < 0.001) and were more likely to have clinical improvement at day 15. No significant difference in term of mortality was detected at day 29. The magnitude of effect was significantly greater among patients receiving oxygen via a mask compared to those not receiving oxygen or needing noninvasive or invasive mechanical ventilation [11]. Of importance, the interim results from the Solidarity Trial endorsed by World Health Organization (WHO) found that remdesivir had little or no effect on overall mortality, initiation of ventilation and duration of hospital stay in hospitalized patients with COVID-19 [5]. Although the magnitude of benefit on mortality is still to be defined, the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) issued an emergency use authorization of the drug for COVID-19 treatment. At present remdesivir is approved by the Italian Medicines Agency (AIFA) for COVID-19 patients with pneumonia needing oxygen supplementation but not for patients needing high-flow oxygen, noninvasive or invasive mechanical ventilation or extracorporeal membrane oxygenation [12].
Data about favipiravir and ribavirin are limited [13].
Hydroxychloroquine therapy
Hydroxychloroquine (HCQ) was one of the first drugs used worldwide to treat COVID-19. The first observational French study, which was strongly criticized, reported enthusiastic results, with HCQ associated with azithromycin for the treatment of COVID-19 [14]. Since then, no clear evidence of the efficacy of this drug against SARS-CoV-2 has been published. At present we know that HCQ does not substantially reduce symptom severity in outpatients with early mild COVID-19 [15], and there is a dearth of evidence to support the efficacy of HCQ in preventing COVID-19 [16]. A RCT conducted in patients with mild to moderate COVID-19 pneumonia showed no impact on 15-day clinical improvement of HCQ, either alone or in combination with azithromycin [17]. Moreover, both the RECOVERY and SOLIDARITY trials discontinued the HCQ arm because no benefits of this drug over the standard of care was detected [4,5,18].
International guidelines such as the US National Institutes of Health and the Infectious Diseases Society of America COVID-19 treatment guidelines recommend against the use of chloroquine or HCQ for the treatment of COVID-19 [19,20].
Anticoagulant therapy
COVID-19 has been associated with vascular inflammation, endothelial dysfunction and a hypercoagulable state that may predispose the subject to haemostatic abnormalities such as arterial thrombosis or thromboembolic disease [21]. This condition has been associated with poor outcomes, especially in patients with a severe disease [22]. This has fueled interest in the use of heparin for its anticoagulant and anti-inflammatory activity.
Data from a retrospective study in China showed that anticoagulant therapy with low-molecular-weight heparin (LMWH) seems to be associated with a better outcome, reducing 28-day mortality of patients with sepsis-induced coagulopathy score of ≥4 or d-dimer more than sixfold of the upper limit of normal [23]. Results from a large US cohort of COVID-19–hospitalized patients confirmed that systemic anticoagulant treatment could be associated with improved outcomes [24]. A study conducted on 4389 hospitalized COVID-19 patients found an approximately 50% reduced hazard of in-hospital mortality and a 30% reduced hazard of intubation compared to patients without anticoagulant therapy [25].
At present several RCTs are ongoing in order to assess doses, risk and benefits of anticoagulant therapy (ClinicalTrials.gov). In the meantime, many scientific societies have released interim guidance for the management of thromboembolism and anticoagulation therapy [[26], [27], [28], [29]]. This is a summary of current recommendations.
-
•
Deep venous thrombosis (DVT) prophylaxis should be prescribed for all hospitalized nonpregnant adults with confirmed or highly suspected COVID-19, regardless of DVT risk assessment score, as per guidelines for non–COVID-19 hospitalized patient, unless contraindicated (e.g. severe thrombocytopenia, active bleeding). LMWH is preferred over oral anticoagulant therapy in hospitalized patients with critical disease because of the shorter half-life, best route of administration and fewer drug–drug interactions.
-
•
For non–critically hospitalized patients, a standard dose of LMWH is recommended.
-
•
For severe critical patients, intermediate-dose LMWH (i.e., enoxaparin 40 mg provided subcutaneously (sc) twice daily, enoxaparin 0.5 mg/kg sc twice daily, heparin 7500 U sc three times daily or low-intensity heparin infusion) may be considered on an individual basis in patients with multiple risk factors for DVT (i.e. body mass index >30 kg/m2, previous DVT, active cancer, large increase in d-dimers, severe inflammation or signs of imminent respiratory failure).
-
•
The therapeutic dose of LMWH is not supported by evidence outside of proven DVT.
-
•
Treatment duration recommended is until hospital discharge and for at least 7 to 14 days.
-
•
Standard risk for bleeding should be considered before prescribing anticoagulant therapy by assessing individual potential advantages.
Anti-inflammatory drugs
Patients with SARS-CoV-2 infection may develop a so-called cytokine storm, characterized by the increase of many cytokines, mainly interleukin (IL)-1 and IL-6, which promotes the development of an acute respiratory distress syndrome [30,31].
Tocilizumab is a recombinant humanized monoclonal antibody of the IgG1 class directed against both the soluble and the membrane-bound IL-6 receptor [32]. Several observational studies have shown promising results [[33], [34], [35]]. In particular, a large retrospective Italian cohort study evaluated the effect of two doses of tocilizumab (administered as intravenous or sc formulation) on the outcome of patients with severe SARS-CoV-2 pneumonia (oxygen saturation <92% in room air and a PaO2/FiO2 (mm Hg) ratio of <250 or a decrease in PaO2/FiO2 greater than 30% in the last 24 hours). The risk of invasive mechanical ventilation/death was reduced for participants treated with tocilizumab from fitting a Cox regression analysis adjusted for gender, age and Sequential Organ Failure Assessment (SOFA) score (adjusted hazard ratio 0.61; 95% confidence interval (CI) 0.40–0.92; p 0.02). The 14-day mortality rate was 20% in the standard-care group compared to 7% (p < 0.001) in the tocilizumab group [34]. Similar results were observed in a large observational study conducted on intensive care unit (ICU) patients across the United States [35]. A recently published RCT (CORIMUNO-19-TOCI-1) conducted on COVID-19 patients with moderate to severe SARS-CoV-2 pneumonia confirmed a positive effect of tocilizumab on the composite endpoint of mechanical ventilation/death, but not on 28-day mortality [36]. Instead, the scenario could be completely different in patients whose disease is not severe. A multicentre RCT by Salvarani et al. did not confirm the efficacy of early administration of tocilizumab in COVID-19 patients with a PaO2/FiO2 ratio between 200 and 300, and tocilizumab use had no benefit on disease progression, defined as need of ICU admission for invasive mechanical ventilation, death from all causes or clinical deterioration expressed by PaO2/FiO2 ratio <150 [37]. Another RCT on 243 patients showed no effect for preventing intubation or death in moderately ill hospitalized patients with COVID-19 [38]. Concerning patients with more severe disease, final results from EMPACTA trials conducted on ethnical minorities have been published recently showing that tocilizumab reduced the likelihood of progression to the composite outcome of mechanical ventilation or death but did not improve survival [39]. Moreover, these favorable results were confirmed by the REMAP-CAP trial that compared tocilizumab and sarilumab to no-immunomodulators [40]. Data from other RCTs as REMDACTA and COVACTA aimed to assess the safety and efficacy of tocilizumab in patients with SARS-Cov2 pneumonia will be available in the next future [41], [42]. A recent metanalysis including preliminary results from these 2 last trials together with previous RCTs and results from observational studies demonstrated moderate-certainty evidence that tocilizumab reduces mechanical ventilation in hospitalized COVID-19 patients, while the effect on mortality needs to be assessed in further studies [43]. Concerning sarilumab, another IL-6 antagonist, a recent press release from Sanofi stated that it failed the primary endpoint in a RCT [44], but the still unpublished results of REMAP-CAP showed a similar efficacy of tocilizumab and sarilumab [40].
Anakinra is an IL-1 receptor antagonist that blocks activity of the proinflammatory cytokines IL-1α and IL-1β and is used to treat autoinflammatory disorders at a daily dose of 100 mg sc in adult patients [45]. At present data from RCTs are not available; nevertheless, small clinical studies have been published showing an advantage of high doses of anakinra [[46], [47], [48]].
Concerning possible side effects of immunomodulatory drugs, despite previous cohort studies suggested a possible increased risk of infectious complications compared to standard of care including life-threatening herpes simplex reactivation [34], [49], these evidences was not confirmed in subsequent randomised studies [43].
Janus kinase (JAK) 1/2 inhibitors have been also proposed as attractive candidates to treat COVID-19 as a result of their properties as anti-inflammatory agents and their hypothesized off-target antiviral effect against SARS-CoV-2 [50]. The most interesting drug of this group is baricitinib, and preliminary experience has demonstrated a high rate of recovery in COVID-19 patients receiving this drug [51,52]. Baricitinib has been recently approved by the FDA for the treatment of COVID-19 in combination with remdesivir on the basis of the ACTT2 trial which showed a better outcome than remdesivir alone in patients with high-flow oxygen and non-invasive mechanical ventilation [53], [54]. RCTs on the use of baricitinib for patients with COVID-19 are ongoing. Other immunomodulators currently being studied are interferon beta and Bruton tyrosine kinase inhibitors [55], [56].
Concerning immunomodulatory drugs, all international guidelines recommend their use only in the context of a RCT, but it is not easy to randomize a study during the pandemic's peak, and not all centres have access to trials [19,20].
Finally, an important role among anti-inflammatory drugs should be played by glucocorticoids. Glucocorticosteroids act as a wider immune modulator than the single cytokine blockers [57], [58]. At beginning of the epidemic, the use of these drugs was somehow discouraged, and the WHO recommended against the routine use of corticosteroids in patients with COVID-19 unless indicated for another reason [59]. The RECOVERY trial showed that dexamethasone at a dosage of 6 mg provided once daily for up to 10 days compared to standard of care reduced deaths by one third in patients receiving invasive mechanical ventilation (29.0% vs. 40.7%; relative risk 0.65 (95% CI 0.51–0.82); p < 0.001) and by one fifth in patients receiving oxygen without invasive mechanical ventilation (21.5% vs. 25.0%; relative risk 0.80 (95% CI 0.70–0.92); p 0.002), but it did not reduce mortality in patients not receiving respiratory support at randomization (17.0% vs. 13.2%; relative risk 1.22 (95% CI 0.93–1.61); p 0.14) [60]. To date, all international guidelines strongly suggest the use of systemic (i.e. intravenous or oral) corticosteroid therapy (e.g. 6 mg of dexamethasone orally or intravenously daily or 50 mg of hydrocortisone intravenously every 8 hours) for 7 to 10 days in patients with severe and critical COVID-19 while conditionally recommending that corticosteroid therapy be withheld from patients with nonsevere COVID-19 not receiving respiratory support [61], [62].
Convalescent plasma
Another potential therapeutic strategy for patients with SARS-CoV-2 infection is passive transfer of neutralizing antibodies using plasma from patients who recovered from COVID-19. There is evidence that over 99% of patients with laboratory-confirmed SARS-CoV-2 infection develop a detectable antibody response, and 88% of them present neutralizing antibodies [63]. The safety and tolerability of the administration of convalescent plasma (CP) have been evaluated in an observational study on more than 5000 patients and showed an incidence of serious adverse events in the first 4 hours of infusion of <1% [64]. Concerning efficacy, an observational study conducted on 138 patients from China showed that 70% of patients with severe respiratory impairment improved and did not need oxygen support within 7 days from infusion [65]. Promising results were also reported from a single-arm Italian study showing a beneficial effect of CP on 7-day hospital mortality compared to expected mortality from the National Statistics in Italy [66], and a preprint (not peer reviewed) large observational study of 35 322 hospitalized COVID-19 patients found that the early infusion of CP was associated with improved 7- and 30-day mortality [67].
However, results from an underpowered RCT from China did not show a significant shortening in time to clinical improvement [68]. More recently, data from an open-label multicentre RCT from India have been published. The main outcome measure was a composite endpoint of progression to severe disease or all- cause mortality at 28 days post-enrolment. The administration of CP was not associated with improved outcomes [69]. The major drawback of this study is that the presence and levels of neutralizing antibodies were not measured a priori, and a low titre (less than 1:160) of neutralizing antibodies may be associated with reduced efficacy of CP. A further RCT, the PLACID study, did not detect significant difference between the CP and the placebo group in the distribution of clinical outcomes, and the overall mortality was 10.96% in the convalescent plasma group and 11.43% in the placebo group, for a risk difference of −0.46 percentage points (95% CI, −7.8 to 6.8) [70]. Instead, a recently published RCT found that early administration (within 72 hours after the onset of COVID-19 symptoms) was effective in reducing the risk of progression to severe diseases in a population of elederly patients with COVID-19 [71]. The TSUNAMI trial (NCT04393727) is currently investigating the efficacy and safety of CP units with a titre of ≥1:160 for the treatment of patients with moderately severe disease, but data are not yet available. The use of CP is approved by the FDA for the treatment of COVID-19 [72].
Antibiotic therapy in patients with SARS-CoV-2 pneumonia
During the first phase of the coronavirus pandemic, many national and international guidelines on COVID-19 management recommended that empirical antibiotic treatment be considered in all critically ill patients with COVID-19 pneumonia, according to previous data from severe influenza A infection [19,20]. As a consequence, a recently published international survey collecting data on antibiotic use in patients with COVID-19 among 166 participants from 23 countries and 82 different hospitals showed that antibiotic therapy was prescribed in the majority of cases (61.8%). The authors reported a median duration of antibiotic therapy in COVID-19 patients of 5 days in the United Kingdom and North America, and 8 days in Italy [73]. However, the prevalence of bacterial coinfections in COVID-19 seems to be low [74]. A recent systematic review showed that rates of bacterial coinfections reported in patients with COVID-19 appear to be 7%, increasing to 14% in studies that include only ICU patients [75]. These data suggest a significantly lower frequency of COVID-19 coinfections compared to severe cases of 2009's influenza A H1N1 but similar rates to patients with Middle East respiratory syndrome–CoV and SARS-CoV disease [76]. Of interest, Staphylococcus aureus, Streptococcus pneumoniae and Streptococcus pyogenes appear to be uncommon in patients with COVID-19, while the most common isolated bacteria are Mycoplasma pneumoniae, Pseudomonas aeruginosa and Haemophilus influenzae [77]. A recent study from the United Kingdom showed that the incidence of early confirmed bacterial coinfections (0–5 days after admission) is lower than that superinfections detected during the hospital course [78]. Most superinfections diagnosed in this study were caused by Gram-negative bacilli, including Enterobacter spp., Pseudomonas spp. and Serratia spp [75]. Moreover, data on the prevalence of multidrug-resistant organisms among COVID-19 patients are scarce. Future studies will be crucial to produce robust data about attributable mortality in COVID-19 patients with bacterial coinfections in order to solve the dilemma about the best timing for starting antibiotic therapy in these patients. It important to underline that without a strong rationale, the indiscriminate use of antibiotics in patients with COVID-19 increases the risk of side effects, drug interactions and selection of multidrug-resistant organisms [79,80].
Interpretation of data
Recent months have seen a rollercoaster of treatment guidelines for COVID-19 based on published papers and press releases. Indeed, randomized clinical trials and observational studies often gave contradicting results, resulting in sudden changes in the prescribing habits of clinicians and regulatory agencies. A major problem is that the results of important randomized trials have not yet been published, so results are difficult to interpret. Indeed, each one of us has experienced the difficulty of selecting patients to enroll onto randomized trials vs. placebo or standard of care during epidemic waves, and some differences among studies could be due to the characteristics of the selected patients or to the different time points within an epidemic wave.
Because severe COVID-19 has resulted in a mortality of around 20% among hospitalized patients in European countries, it is important to understand which drugs could actually decrease this level of mortality. At present no drug has reached this goal in randomized clinical trials because remdesivir, tocilizumab and sarilumab did not meet the mortality endpoint despite large sample sizes, and the effect on mortality of dexamethasone varied widely according to the level of respiratory support at randomization. For example, patients allocated to dexamethasone receiving oxygen without invasive mechanical ventilation had a mortality rate of 21.5% compared to 25% in the control arm. What it is really needed is information about the exact timing and dose of drug delivery as well as knowledge of the characteristics of the population that could most benefit from each therapeutic intervention. Subanalyses of large randomized trials could help fill some of these gaps.
Conclusions
On the bases of the evidence, press releases from pharmaceutical companies concerning randomized clinical trials and our everyday clinical experiences (which may differ from trial results), it is difficult to decide what the standard of care should be for patients with COVID-19 severe pneumonia, particularly because of differing inclusion criteria and differing time points within the pandemic. In general we do not suggest the use of protease inhibitors and HCQ in patients with COVID-19 pneumonia. Moreover, the available evidence does not support the systematic prescription of broad-spectrum empirical antimicrobials, which emphasizes the need to develop antimicrobial policies and appropriate stewardship interventions specifically designed for the COVID-19 pandemic.
Concerning the other treatments, because there are conflicting results from clinical trials (such as with tocilizumab and remdesivir), we think that a therapeutic strategy based on a specific sequencing and/or combination of different treatments should be prioritized in future clinical trials. The SIMIT recommendations are a stepwise approach that permits distinguishing standard of care on the basis of respiratory impairment. We suggest that LMWH be provided at a prophylactic dosage in all hospitalized patients with COVID-19. In case of oxygen supplementation, glucocorticoids (preferably dexamethasone) should be started. The use of remdesivir for 5 days and the addition of CP (the latter particularly in patients with mild to moderate illness and PaO2/FiO2 ratio of 200–350) should be considered with caution and according to the recommendations of regulatory agencies because data suggesting benefit from these strategies are limited, and conclusive RCTs are ongoing. In the absence of clinical and respiratory response (stabilization or increase in PaO2/FiO2 on day 2 or continuous deterioration) tocilizumab or other immunomodulatory agents such as baricitinib could be taken into consideration. A description of the suggested therapeutic approach is presented in Fig. 1. Finally, as a result of the incomplete evidence in treatment approach of COVID-19, we suggest that all these drugs be considered for use in clinical trials.
Transparency declaration
The authors declare that they have no conflicts of interest.
Editor: L. Leibovici
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel Coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lombardy section Italian Society Infectious and Tropical Diseases Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Infez Med. 2020;28:143–152. [PubMed] [Google Scholar]
- 3.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of Lopinavir–Ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020 7;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Recovery: randomised evaluation of COVID-19 therapy. Available at: https://www.recoverytrial.net/.
- 5.Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V., Karim Q.A. 2020. Repurposed antiviral drugs for COVID-19 –interim WHO SOLIDARITY trial results. NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Meyer S., Bojkova D., Cinatl J., Van Damme E., Buyck C., Van Loock M. Lack of antiviral activity of darunavir against SARS-CoV-2. Int J Infect Dis. 2020;97:7–10. doi: 10.1016/j.ijid.2020.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown A.J., Won J.J., Graham R.L. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 2019;169:104541. doi: 10.1016/j.antiviral.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., for the ACTT-1 Study Group Members Remdesivir for the Treatment of COVID-19 - Preliminary Report. N Engl J Med. 2020;383:994. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 9.Olender S.A., Perez K.K., Go A.S., Balani B., Price-Haywood E.G., Shah N.S. Remdesivir for severe COVID-19 versus a cohort receiving standard of care. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1041. ciaa1041 Jul 24;ciaa1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman J.D., Lye D., Hui D.S., Marks K.M., Bruno R., Montejano R. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med. 2020;383:1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agenzia Italiana del Farmaco (AIFA). Remdesivir. Available at: https://www.aifa.gov.it/documents/20142/1123276/remdesivir_18.09.2020.pdf/022f3b77-7830-da72-18b8-66005d169558.
- 13.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19) JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 14.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skipper C.P., Pastick K.A., Engen N.W., Bangdiwala A.S., Abassi M., Lofgren S.M. Hydroxychloroquine in non-hospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2020;173:623–631. doi: 10.7326/M20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah S., Das S., Jain A., Misra D.P., Negi V.S. A systematic review of the prophylactic role of chloroquine and hydroxychloroquine in coronavirus disease-19 (COVID-19) Int J Rheum Dis. 2020;23:613–619. doi: 10.1111/1756-185X.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate COVID-19. N Engl J Med. 2020;383:2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiolet T., Guihur A., Rebeaud M.E., Mulot M., Peiffer-Smadja N., Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:138–140. doi: 10.1016/j.cmi.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/. [PubMed]
- 20.Bhimraj A., Morgan R.L., Shumaker A.H., Lavergne V., Baden L., Chi-Chung Cheng V. Infectious Diseases Society of America Guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020:ciaa478. doi: 10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marietta M., Coluccio V., Luppi M. COVID-19, coagulopathy and venous thromboembolism: more questions than answers. Intern Emerg Med. 2020:1–13. doi: 10.1007/s11739-020-02432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paranjpe I., Fuster V., Lala A., Russak A.J., Glicksberg B.S., Levin M.A. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nadkarni G.N., Lala A., Bagiella E., Chang H.L., Moreno P.R., Pujadas E. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with Covid-19: a single health system study. J Am Coll Cardiol. 2020;76:1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., Clark C., Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. From NIH website (https://www.COVID19treatmentguidelines.nih.gov/). Accessed 2020 May 18. [PubMed]
- 28.Barnes G.D., Burnett A., Allen A., Blumenstein M., Clark N.P., Cuker A. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50:72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marietta M., Ageno W., Artoni A., De Candia E., Gresele P., Marchetti M. COVID-19 and haemostasis: a position paper from Italian Society on Thrombosis and Haemostasis (SISET) Blood Transfus. 2020;18:167–169. doi: 10.2450/2020.0083-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giavridis T van der Stegen S.J.C., Eyquem J., Hamieh M., Piersigilli A., Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24:731–738. doi: 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedersen S.F., Ho Y.-C. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hennigan S., Kavanaugh A. Interleukin-6 inhibitors in the treatment of rheumatoid arthritis. Ther Clin Risk Manag. 2008;4:767–775. doi: 10.2147/tcrm.s3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatology. 2020:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biran N., Ip A., Ahn J., Go R.C., Wang S., Mathura S. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2:e603–e612. doi: 10.1016/S2665-9913(20)30277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hermine O., Mariette X., Tharaux P.-L., Resche-Rigon M., Porcher R., Ravaud P. Effect of tocilizumab vs usual care in adults hospitalized with Covid-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salvarani C., Dolci G., Massari M., erlo D.F., Cavuto S., Savoldi L. for the RCT-TCZ-COVID-19 Study Group. Effect of tocilizumab vs standard of care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salama C., Han J., Yau L., Reiss W.G., Kramer B., Neidhart J.D. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The REMAP-CAP Investigators. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19–Preliminary report medRxiv preprint. 10.1101/2021.01.07.21249390; this version posted January 7, 2021. [DOI]
- 41.Roche initiates phase III clinical trial of Actemra/RoActemra plus remdesivir in hospitalised patients with severe COVID-19 pneumonia [Internet]. [cited 2020 Oct 23]; Available from: https://www.roche.com/media/releases/med-cor-2020-05-28.htm.
- 42.Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 associated pneumonia [Internet]. [cited 2020 Oct 23]; Available from: https://www.roche.com/investors/updates/inv-update-2020-07-29.htm.
- 43.Tleyjeh I.M., Kashour Z., Damlaj M., Riaz M., Tlayjeh H., Altannir M. Efficacy and safety of tocilizumab in COVID-19 patients: A living systematic review and meta-analysis. Clin Microbiol Infect. 2021;27(2):215–227. doi: 10.1016/j.cmi.2020.10.036. PMCID: PMC7644182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanofi and Regeneron provide update on Sarilumab Phase 3 U.S. trial in COVID-19 patients PARIS and TARRYTOWN, N.Y. [Internet]. [cited 2020 July 2]; Available from: https://www.sanofi.com/en/media-room/press-releases/2020/2020-07-02-22-30-00.
- 45.Cavalli G., Dinarello C.A. Treating rheumatological diseases and co-morbidities with interleukin-1 blocking therapies. Rheumatology. 2015;54:2134–2144. doi: 10.1093/rheumatology/kev269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D., Oltolini C. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Navarro-Millán I., Sattui S.E., Lakhanpal A., Zisa D., Siegel C.H., Crow M.K. Use of Anakinra to prevent mechanical ventilation in severe COVID-19: a case series. Arthritis Rheumatol. 2020;72:1990–1997. doi: 10.1002/art.41422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huet T., Beaussier H., Voisin O., Jouveshomme S., Dauriat G., Lazareth I. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2:e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Busani S., Bedini A., Biagioni E., Busani S., Bedini A., Biagioni E. Two fatal cases of acute liver failure due to HSV-1 infection in COVID-19 patients following immunomodulatory therapies. Clin Infect Dis. 2020:ciaa1246. doi: 10.1093/cid/ciaa1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A. Baricitinib as a potential treatment for 2019-nCov acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Titanji B.K., Farley M.M., Mehta A., Connor-Schuler R., Moanna A., Cribbs S.K. Use of baricitinib in patients with moderate and severe COVID-19. Clin Infect Dis. 2020:ciaa879. doi: 10.1093/cid/ciaa879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stebbing J., Krishnan V., de Bono S., Ottaviani S., Casalini G., Richardson P.J. Sacco Baricitinib Study Group. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol Med. 2020;12:e12697. doi: 10.15252/emmm.202012697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.US Food and Drug Administration (FDA). Coronavirus (COVID-19) Update: FDA authorizes drug combination for treatment of COVID-19 [news release]. 19 November. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-drug-combination-treatment-covid-19, 2020.
- 54.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2031994. NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shalhoub S. Interferon beta-1b for COVID-19. Lancet. 2020;395:1670–1671. doi: 10.1016/S0140-6736(20)31101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Treon S.P., Castillo J.J., Skarbnik A.P., Soumerai J.D., Ghobrial I.M., Guerrera M.L. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood. 2020;135:1912–1915. doi: 10.1182/blood.2020006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rhen T., Cidlowski J.A. Antiinflammatory action of glucocorticoids— new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 58.Vandewalle J., Luypaert A., De Bosscher K., Libert C. Therapeutic mechanisms of glucocorticoids. Trends Endocrinol Metab. 2018;29:42–54. doi: 10.1016/j.tem.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 59.World Health Organization. 27 May 2020. Clinical management of COVID-19. Interim guidance. WHO/2019-nCoV/clinical/2020.5.
- 60.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.World Health Organization. Corticosteroids for COVID-19. Document WHO/2019-nCoV/Corticosteroids/2020.1. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1.
- 62.Siemieniuk R., Rochwerg B., Agoritsas T., Lamontagne F., Leo Y.S., Macdonald H. A living WHO guideline on drugs for COVID-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379. Update in: BMJ. 2020 Nov 19;371:m4475. [DOI] [PubMed] [Google Scholar]
- 63.Harvala H., Mehew J., Robb M.L., Ijaz S., Dicks S., Patel M. Convalescent plasma treatment for SARS-Cov-2 infection: analysis of the first 436 donors in England, 22 April to 12 May 2020. Euro Surveill. 2020;25:2001260. doi: 10.2807/1560-7917.ES.2020.25.28.2001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bloch E.M., Shoham S., Casadevall A., Sachais B.S., Shaz B., Winters J.L. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130:2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xia X., Li K., Wu L., Wang Z., Zhu M., Huang B. Improved clinical symptoms and mortality on severe/critical COVID-19 patients utilizing plasma transfusion. Blood. 2020;136:755–759. doi: 10.1182/blood.2020007079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perotti C., Baldanti F., Bruno R., Del Fante C., Seminari E., Casari S. Mortality reduction in 46 severe COVID-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial. Haematologica. 2020;105:2834–2840. doi: 10.3324/haematol.2020.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joyner M.J., Bruno K.A., Klassen S.A., Kunze K.L., Johnson P.W., Lesser E.R. Safety Update: COVID-19 Convalescent Plasma in 20,000 Hospitalized Patients. Mayo Clin Proc. 2020;95:1888–1897. doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening Covid-19: a randomized clinical trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agarwal A., Mukherjee A., Kumar G., Chatterjee P., Bhatnagar T., Malhotra P., PLACID Trial Collaborators Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V., Vallone M.G., Vázquez C. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2031304. NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Libster R., Pérez Marc G., Wappner D., Coviello S., Bianchi A., Braem V., Esteban I. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med. 2021 doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.US Food and Drug Administration (FDA). Recommendations for investigational COVID-19 convalescent plasma. 16 November. Available at: https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-COVID-19-convalescent-plasma, 2020.
- 73.Beović B., Doušak M., Ferreira-Coimbra J., Nadrah K., Rubulotta F., Belliato M. Antibiotic use in patients with COVID-19: a 'snapshot' Infectious Diseases International Research Initiative (ID-IRI) survey. J Antimicrob Chemother. 2020;75:3386–3390. doi: 10.1093/jac/dkaa326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sieswerda E., de Boer M.G.J., Bonten M.M.J., Boersma W.G., Jonkers R.E., Aleva R.M. Recommendations for antibacterial therapy in adults with COVID-19 - an evidence-based guideline. Clin Microbiol Infect. 2021;27:61–66. doi: 10.1016/j.cmi.2020.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.MacIntyre C.R., Chughtai A.A., Barnes M., Ridda I., Seale H., Toms R. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1) pdm09. BMC Infect Dis. 2018;18:637. doi: 10.1186/s12879-018-3548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clancy C.J., Nguyen M.H. COVID-19, superinfections and antimicrobial development: What can we expect? Clin Infect Dis. 2020:ciaa524. doi: 10.1093/cid/ciaa524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hughes S., Troise O., Donaldson H., Mughal N., Moore L.S.P. Bacterial and fungal coinfection among hospitalised patients with COVID-19: a retrospective cohort study in a UK secondary care setting. Clin Microbiol Infect. 2020;26:1395–1399. doi: 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huttner B.D., Catho G., Pano-Pardo J.R., Pulcini C., Schouten J. COVID-19: don't neglect antimicrobial stewardship principles! Clin Microbiol Infect. 2020;26:808–810. doi: 10.1016/j.cmi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rawson T.M., Moore L.S.P., Castro-Sanchez E., Charani E., Davies F., Satta G. COVID-19 and the potential long-term impact on antimicrobial resistance. J Antimicrob Chemother. 2020;75:1681–1684. doi: 10.1093/jac/dkaa194. [DOI] [PMC free article] [PubMed] [Google Scholar]