Abstract
COVID-19 has nowadays affected almost all our societies and global health systems. The latest deadly pandemic has heavily influenced both life and livelihood worldwide. SARS-CoV-2 is the causative organism of COVID-19, that is spreading and infecting significantly higher compared to other coronavirus, due to its constant mutation characteristics. At present although several extensive clinical trials are ongoing, neither approved drug therapy nor any vaccine are available to safely fight SARS-CoV-2. However, a progressive race among numerous research groups to discover a radical cure for the COVID-19 is under way. This review aims to provide an updated insight of the current research, development and trials on repurposing existing drugs and preventive intervention for COVID-19, along with the related issues, complexities and challenges, especially after the observed high transmissibility lately.
Keywords: SARS-CoV-2, COVID-19, Drug repurposing, Vaccine, Computational docking
Introduction
Covid-19 has already caused 32,925,668 positive cases and 995,352 deaths worldwide, as of 27th September, 2020 [1]. The World Health Organization (WHO) has confirmed this ongoing deadly disease as a global public health emergency. Covid-19 caused by SARS-CoV-2 is a single strand RNA beta coronavirus of the family Coronaviridae [1,2]. Genome sequencing data suggest that SARS-CoV-2 has nearly 79% similarity and 50% similarity with SARS-CoV and MARS-CoV, respectively. However, unlike other coronavirus SARS-CoV-2 has exhibited so far low mortality rate, but its spreading rate and infectivity is very high. High spreading rate of SARS-CoV-2 may be due to its mutation capability which makes the virus more contagious [3]. Three new central variants of SARS-CoV-2 have been already found and named as A, B, and C. From early 2020 several thousand mutation and 4400 amino acid substitution has occurred for the same virus, mostly occurred to the D614 G domain of the spike protein. As it is well known, this spike protein by whichinteracts and enters into the host cell have so far demonstrated 14 mutations, while parts of the genome having exhibited more mutations could become flexible, leading to tolerate changes without harming the virus. Other scientists have also suggested that this mutation probably can later affect the sensitivity of virus to neutralize antibodies.COVID-19 prevention and treatment have apparently recognized as great challenges, since SARS-CoV-2 has up to now demonstrated many natural, intermediate and final hosts [[4], [5], [6], [7], [8]].
Unfortunately, no clinically approved drug or vaccine which can effectively be working against SARS-CoV-2, has made the breakthrough. To develop a new drug and repurposing an existing one, (with known toxicity and pharmacokinetics), it would be very important to understand the full details of SARS-CoV-2 and the way how it hijacks the host cells. Needless to say, that it is also crucial to explore and identify all the host proteins which are targeted by COVID-19 virus. Furthermore the host-virus interface investigation can provide us long lasting and broad-spectrum therapy [9,10]. SARS-CoV-2 studies for treatment started practically from the day its genome became known, thanks to the full exploitation of computational approaches [10]. The most important targets found for drug targeting are some viral proteins and host cell proteins which are not limited to: viral spike glycoprotein, furin activation site, protease enzymes and RNA polymerase; host cell – ACE2 receptor, protease enzymes, etc. Therefore, therapies to treat COVID-19 can be divided into two categories – targeting SARS-CoV-2 and host cells proteins or improve human immune system. New drug development is a time consuming process, thus the use of existing drug database (broad spectrum antiviral, protease and RNA polymerase inhibitor etc.) may be one of the few solutions for the pandemic [11,12]. Jiang suggested that, they have to confirm first of all the safety and efficacy of a vaccine or a drug, before start using it for COVID -19 [13].
Present review is focused on the research, development and clinical trials that are going on for repurposing existing drugs and preventive intervention (vaccines) for COVID-19.
Diagnosis and challenges
Current assays require designing small pieces of DNA that match sections of the viral genome in the sputum or blood serum. However, there are still many uncertainties concerning the kinetics [14] of SARS-CoV-2 viral shedding, thus, the test timing may substantially affect the result. The WHO has appointed SARS-COV-2 referral laboratories for testing, though capabilities remain limited, due to the required sophisticated technologies.
The diagnostic tests are scheduled on a genome-based standard technology known as reverse-transcriptase polymerase chain reaction (RT-PCR) and laboratory results are at best available within 4 hours. To date, none of these tests have been fully standardized [14]. The problem with RT-PCR test is that it can identify viral genetic material, only if there is enough RNA in the sample. Thus in an early infection, there is not often adequate enough RNA material before someone starts to feel really sick. RT-PCR test could therefore deliver false negative results and less useful information for asymptomatic patients.
In the midst of the rapidly evolving disease outbreak, health care systems need to be able to carry out high-volume of testing with reliability to detect the virus during the incubation period. This may be imminent. The following step for monitoring as the spread of the virus will follow a different, more convenient and significantly more effective diagnostic [15] approach, such as a blood test that identifies antibodies against the SARS-CoV-2 virus, within minutes rather than hours. Many research [16] groups are now working on developing such tests, which still require validation with well characterized sera (blood samples from infected individuals) in order to be proved reliable for general medical use and epidemiological investigations (e.g. assessing the number of infections and immunity against the virus).
SARS-CoV-2 was detected in specimens from multiple sites of 205 patients with COVID-19: 1. Lower respiratory tract samples, most often testing, found positive for the virus. 2. Importantly, live virus was detected in feces, implying that SARS-CoV-2 may be transmitted by the fecal route. 3. A small percentage of blood samples had positive PCR test results, suggesting that infection sometimes may be systemic. 4. Transmission of the virus by respiratory and extra respiratory routes may help to assess the rapid spread of the disease. 5. In addition, testing of specimens from multiple sites may improve the sensitivity and reduce the false-negative test results [17]. Two smaller studies have reported the presence of SARS-CoV-2 in anal or oral swabs and blood from 16 patients in Hubei Province [18], and viral load in throat swabs and sputum from 17 confirmed cases [19].
Existing drug therapy and recent clinical trials
A total of 21 different target proteins of SARS-COV-2 virus were found using in-silico approach and these targets were investigated against different synthetic and natural source of drugs. Computational docking studies suggest that no compound was found, that can inhibit the SARS-COV 2 viral infection by antagonizing ACE2–Spike complex, rather than only antagonize ACE2 enzyme activities. Interestingly, a blend of natural bioflavonoids, as. hesperetin, myricetin, and piperine demonstrated also promising antiviral efficacy. In very recent preliminary study these natural products bind with high affinity to the spike protein, helicase, and protease sites on the ACE2 receptor causing conformational change to inhibit viral entry of coronaviruses [10].
Computational docking studies suggest that antiviral drug remdesivir is not only active against SARS-CoV-2 pneumonia by inhibiting RNA polymerase, but also bind to TMPRSS2, a human protein which promote virus infection. Remdesivir showed potent antiviral activity in clinical trial against SARS-CoV-2 infection in the United States, Europe, and Japan [20].
Remdesivir has been short listed as one of the possible wonder drugs. Two clinical phase 3 trial of remdesivir therapy injection against COVID-19 is going on in China, and it is looking very promising as an effective antiviral therapy for COVID-19 [21]. On the contrary a recent clinical trial performed by Gilead Sciences Inc. (developer of the drug) on a group of 237 patients did not show any significant improvement; However preliminary clinical data found by Institutes of Health (NIH) indicates that remdesivir improves patient recovery with advanced COVID-19 infections. The randomized trial involved 1063 patients with advanced COVID-19 conditions, the patients treated with remdesivir had a 31% faster recovery time (from 15 days to 11 days) and lower mortality rate compared with those who received placebo.
In a separate placebo-controlled multicenter study performed by Wang et al. (2020) suggests that patients admitted to hospital for severe COVID-19 and treated with remdesivir did not demonstrate any significant effect. However, the study with larger sample size and using high dose of remdesivir is still ongoing [22].
Recently, FDA approved nucleotide polymerase inhibitor antivirals ribavirin and sofosbuvir used in the treatment of hepatitis C are also repurposing for the treatment of COVID-19. Ribavirin and sofosbuvir can bind to active site of RdRp. Since SARS-CoV-2 has 97% identical RdRp sequence of SARS-CoV, therefore, these drugs could probably work against COVID-19 [23].
The Japanese antiviral drug Flavirapir, a broad spectrum inhibitor of viral RNA polymerase has showed effectiveness in treating mild COVID-19 cases in China; Clinical trial is going on in Japan and Italy, however its clinical evaluation is not yet completed. [24]. Flavirapir has been used for treatment of human infection with Ebola virus in West Africa in 2014 [25,26].
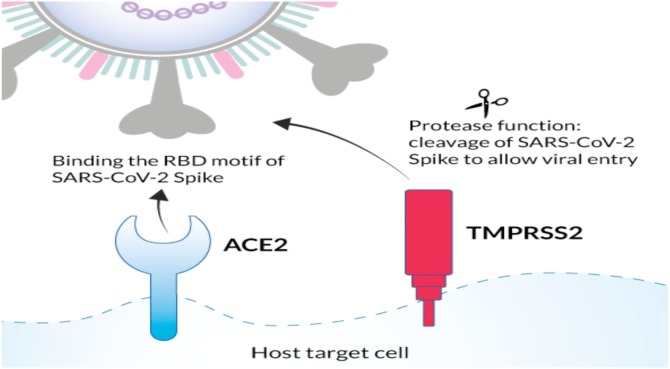
SARS-COV-2 infection (COVID-19) depends on host cell factors such as ACE2 and TMPRSS2 (Fig. 1 ), demonstrating that human cell entry of SARS-CoV-2 depends on binding of the viral spike proteins to ACE2 receptors and spike protein priming by host cell proteases TMPRSS2 [27].
Fig. 1.
COVID-19 host cell factors (receptors): ACE2 and TMPRSS2.
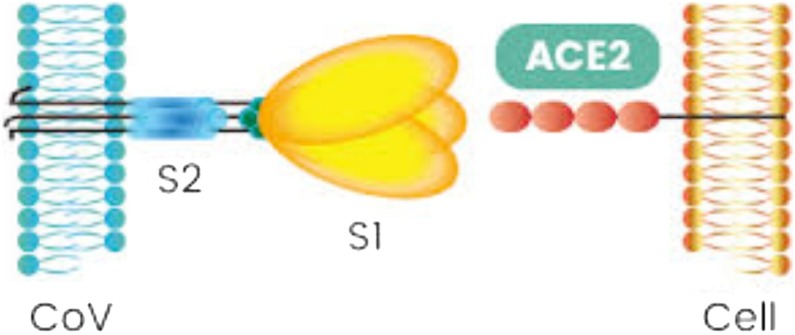
Therefore, a protease inhibitor (e.g.-ritonavir, lopinavir etc.) can be used to block the entry of SARS-CoV-2. Although preclinical data suggested potential benefit, other study suggests that there is no yet clear role of ritonavir and lopinavir for treating COVID-19, [27]. This phenomenon is also observed by Inoue research group at the University of Tokyo that the drug nafamostat mesylate (Fusan®), used to treat acute pancreatitis, could be a strong candidate against COVID-19 by inhibiting the protease enzyme. The spike protein (S) is cleaved into S1 and S2 by a human cell (Fig. 2 ) derived proteolytic enzyme, probably Furin. S1 then binds to ACE2 receptor and S2, is cleaved by TMPRSS2 a human cell surface serine protease, resulting in membrane fusion. Nafamostat can possibly inhibit the human cell surface protease enzyme TMPRSS2 [28].
Fig. 2.
COVID-19 spike protein (S1, S2 subunits).
Nafamostat is also found to inhibit MERS-CoV protein-initiated membrane fusion effectively [29], in less than one-tenth of the of camostat mesylate (Foypan®) concentration. A German group recently identified camostat as an inhibitor of SARS-CoV-2 infection [27]. Li Hua, from Huazhong University of Science and Technology in Wuhan, China reported that Spike protein of SARS-CoV-2 differs from other coronaviruses, having a site which is activated by a host-cell enzyme called Furin. Li’s Same team is also searching for new molecules that could block Furin, and can be used as a therapy [30].
Some part of the viral genome exhibiting less mutation are the best targets for antiviral drugs [31]. Spike protein mutations suggest that it may transmit, interact and fuse into host cell differently, therefore, antiviral drug selection and targeting should be based on the new genome data [8].
Recent experimental results suggest that ACE2 expression is increased in patient with diabetes and hypertension treated with ACE inhibitors and angiotensin II type-I receptor blockers (ARBs). Therefore, increased expression of ACE2 would expedite infection with COVID-19. Thus, diabetes and hypertension treatment with ACE2-stimulating drugs may increase the risk of developing severe and fatal COVID-19. ACE2 expression can also be increased by thiazolidinediones and ibuprofen [32,33].
AstraZeneca and Saint Luke’s Mid America Heart Institute have started Phase III clinical trial with SGLT2 inhibitor dapagliflozin, for managing diabetes mellitus type 2, in COVID-19 patients. The goal of the trial (called DARE-19) is to investigate whether dapagliflozin (Farxiga®) can be used for adult patients with COVID-19, who are at risk for developing serious complications, such as an organ failure (https://ClinicalTrials.gov. Identifier: NCT04350593) [34].
A group of Quantitative Bioscience Institute, University of California, San Francisco (UCSF) has mapped systematically the interaction landscape between SARS-CoV-2 proteins and human proteins. They have focused on 26 SARS-CoV-2 viral proteins to figure out which human cell interact founding that around 332 human proteins interact with SARS-CoV-2 proteins, having identified 69 compounds that can target 66 human proteins or host factors. 29 of these drugs are already FDA approved (Selinexor, Ruxolitinib, Captopril, Chloramphenicol, Rapamycin, etc.) 28 preclinical compounds and 12 drugs are in clinical trials, [9].
Selinexor (Xpovio®), an FDA approved medicine for the treatment of the disease refractory multiple myeloma, is assessed in a randomized clinical trial for hospitalized patients with severe COVID-19. Hyper-inflammation with high levels of cytokines such as IL6, IL1, IFNg etc. is an important feature of COVID-19 symptomatology. Selinexor and other selective inhibitors of nuclear export (SINE) compounds have potent anti-inflammatory activity working via the inhibition of NF-kB, which can reduce the cytokine level, and may be favorable to critical patients with COVID-19. (Karyopharm Therapeutics Inc), (https://www.onclive.com/web-exclusives/selinexor-covid19-global-trial-launching).
As, it is known, hyper-inflammation can also be treated using steroids, intravenous immunoglobulin, selective cytokine blockade (eg, sarilumab, tocilizumab) and JAK inhibition. Ruxolitinib (Jakafi) a JAK1/JAK2 inhibitor is currently approved by the FDA for the treatment of polycythemia vera in adults. RUXCOVID, (Incyte, US and Novartis outside of the US), a global collaborative study started a phase III clinical trial to evaluate the efficacy and safety of ruxolitinib (Jakafi), in COVID-19 patients with induced cytokine storm (https://investor.incyte.com/news-releases/news-release-details/incyte-announces-plans-initiate-phase-3-clinical-trial). Based on evidence researchers suggested that a subgroup of patients with severe COVID-19 may have a cytokine storm syndrome. Authors also mentioned that JAK inhibition, similar to ruxolitinib, could potentially inhibit both inflammation and cellular entry of virus in COVID-19 [35].
Immunomodulating agents (sarilumab, eculizumab, and tocilizumab etc.) can also be used as an adjunct therapy for Covid-19 patients as it were evident in different trials and case studies. Regeneron and Sanofi’s sarilumab (Kevzara) was safe and effective against Covid-19, thus areready to run phase III trial including more than 600 patients for evaluating sarilumab (Kevzara) in hospitalized “critical” COVID-19 patients. Regeneron reported that Sarilumab used for rheumatoid arthritis, an interleukin-6 (IL-6) receptor antibody, is currently under investigated, due to its ability to reduce the overactive inflammatory immune response associated with COVID-19. A large number of patients with SARS-CoV2 infection and severe pneumonia or acute respiratory distress syndrome (ARDS) treated with another immunomodulating agent eculizumab as an off-label drug. Results suggest that eculizumab may play a key role in the treatment of severe cases of COVID-19, which can be confirmed by the end of ongoing SOLID-C19 trial [36].
A commonly used arthritis drug such as tocilizumab, has shown “promising results” in two coronavirus patients in Italy (P. Ascierto), being effective against pneumonia caused by COVID-19,”<-- --> [37]. Another study performed on a total of 21 patients treated with tocilizumab from February 5 to February 14, 2020. One third of the patients were admitted in The First Affiliated Hospital of University of Science and Technology of China (Anhui Provincial Hospital) and two third in Anhui Fuyang Second People’s Hospital, where tocilizumab found an effective drug for the treatment in severe patients of COVID-19 [38]. FDA has approved a randomized, double-blind, placebo-controlled phase III clinical trial on 330 patients to investigate the safety and efficacy of intravenous (IV) tocilizumab (Actemra) named COVACTA plus standards of care in hospitalized adult patients with severe COVID-19 pneumonia (https://ClinicalTrials.gov; Identifier: NCT04320615). Guiqiang Wang, at Peking University First Hospital is running a clinical trial on 150 patients to assess the efficacy and safety of favipiravir in combination with tocilizumab in the treatment of COVID-19. (https://clinicaltrials.gov/ct2/show/NCT04310228).
Silmitasertib (CX-4945), by Senhwa Biosciences, Inc is found potential towards COVID-19 improved treatment. Quantitative Biosciences Institute (UCSF-QBI), found that by inhibiting CK2, particularly via the disruption of CK2, promotes the formation of Stress Granules (SGs), resulting in the inhibition of COVID-19 proliferation. Silmitasertib is also a targeted inhibitor of Casein kinase 2 (CK2), the only CK2 inhibitor currently, under clinical trials [9].
The fact that chloramphenicol an antibiotic that binds the 50S subunit of the mitochondrial ribosome, suppressing mitochondrial protein translation for replication, and inhibits peptidyl transferase, preventing translocation, could probably exhibit anti- COVID-19 actions [10]. ICGEB N. Delhi explore the potential repurposing of the antiepileptic drug valproic acid against SARS-CoV2. This team has carried out a broad computational study over 1.2 million small molecules. The results revealed that valproic acid Coenzyme-A, a metabolite of the prodrug valproic acid, binds effectively to the SARS-CoV2 RNA-dependent RNA polymerase (RdRp). (https://www.icgeb.org/covid-19-sars-cov-2-updates-from-icgeb-new-delhi-and-cape-town/)
Clinical trials perspective by Mullard mentioned that over 180 clinical trials on potential COVID-19 therapy are going on, worldwide, whereas other 150 trial are starting soon (Table 1 ). It is obvious that, multiple small trials may not produce the strong (solid) evidence that required to construct the relative effectiveness of possible treatments [39]. To address this issue, World Health Organization has launched the solidarity Master Protocol to coordinate the COVID-19 clinical trials. The first four solidarity trials according to WHO officials to fight COVID-19 are the anti-malaria chloroquine and hydroxychloroquine, the antiviral remdesivir, HIV drugs lopinavir and ritonavir and a combination of lopinavir, ritonavir with interferon-beta. The inhaler form of interferon (code named SNG001) found to improve the recovery of asthma and chronic obstructive pulmonary disease in patients with lung infections. Thus clinical trial of SNG001 is starting in U.K. critically ill Covid-19 patients (www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments).
Table 1.
Prospective Drug candidates in clinical trials for COVID-19 (https://ClinicalTrials.gov, ChiCTR.org.cn; [46]).
| Drug candidate | Description & FDA approval | Developer | Current stage of clinical evaluation for Covid 19 | Regulatory status of the candidate |
|---|---|---|---|---|
| Remdesivir | FDA approved in case of emergency for COVID 19; antiviral; inhibiting RNA synthesis in coronaviruses | Gilead, results released in April 2020 (severe disease) and expected in May 2020 (moderate disease); NIAID trial preliminary data released in April 2020, full results expected in May, 2020 | Phase III | NCT04292730 |
| Hydroxychloroquine or chloroquine | FDA approved as anti-malarial drugs | Peking University Third Hospital, Mayo Hospital and Services Hospital, Lahore, Pakistan Kidney and Liver Institute, WHO | Phase IV | ChiCTR2000029898 NCT04351191 |
| Hydroxychloroquine or chloroquine with Azithromycin | FDA approved; anti-malarial drugs and antibiotic | Washington University School of Medicine | Phase III | NCT04341727 |
| Favipiravir | antiviral against influenza; Not FDA approved | Giuliano Rizzardini, FUJIFILM Toyama Chemical Co., Ltd., Tokyo, Japan | Phase III | NCT04336904 |
| Favipiravir with Tocilizumab | FDA approved; antiviral and monoclonal antibody | Peking University First Hospital | Phase III | NCT04310228 |
| Lopinavir/Ritonavir | FDA approved; antiviral | Darrell Tan, St. Michael's Hospital, Toronto | Phase III | NCT04321174 |
| Danoprevir; Ritonavir; Interferon | FDA approved antiviral, immune suppression | The Ninth Hospital of Nanchang, Ascletis Pharmaceuticals Co., Ltd. | Phase IV | NCT04291729 |
| Sarilumab | FDA approved; human monoclonal antibody against interleukin-6 receptor and rheumatoid arthritis | Regeneron-Sanofi | Phase III | NCT04315298 |
| Tocilizumab | FDA approved; human monoclonal antibody against interleukin-6 receptor and rheumatoid arthritis | Hoffmann-La Roche and Genentech | Phase III | NCT04320615 |
| Ruxcovid | FDA approved a JAK1/JAK2 inhibitor used for polycythaemia vera | Incyte Corporation, Wilmington, DE, USA | Phase III | NCT04331665 |
| Camostat Mesylate | FDA approved used to treat acute pancreatitis | Yale University | Phase II a | NCT04353284 |
| Selinoxor | FDA approved patients with relapsed or refractory multiple myeloma | Karyopharm Therapeutics Inc | Phase II | NCT04355676 |
| Ivermactin | FDA-approved; anti-parasitic drug | Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins | Phase II | NCT04374279 |
| Famotidine | FDA approved; OTC drugs used for heart burn patients | Northwell Health | Phase III | NCT04370262 |
| Dapagliflozin | FDA approved; Dapagliflozin is a sodium-glucose cotransporter 2 (SGLT2) inhibitor indicated for managing diabetes mellitus type 2 | Saint Luke's Health System with AstraZeneca and George Clinical Pty Ltd | Phase III | NCT04350593 |
| Ascorbic acid (Vitamin-C) | Vitamin C is recommended for the prevention and treatment of scurvy and vitamin deficiency | Thomas Jefferson University | Phase II | NCT04363216 |
Chloroquine and hydroxychloroquine, with no clear yet mode of action, have shown activity against SARS-CoV-2 in-vitro and during ongoing preclinical study, though a number of severe adverse drug reactions detected, such as cardiac arrhythmias, less efficacy and more mortality rate [40]. Above clinical trial conducted in France over 20 patients suggested that hydroxychloroquine is associated with significant viral load reduction in COVID-19 patients, while its effect is reinforced by the anti-bacterial agent azithromycin. However, a group of experts from the National Institute of Allergy and Infectious Diseases, USA, has not recommended the combination of hydroxychloroquine/ azithromycin for COVID-19 treatment, since their possible toxicities could increase the risk of sudden cardiac death [41]. In addition, another group [42] recommended the therapeutic ban of lopinavir/ritonavir or other HIV protease inhibitors, and interferon because of negative clinical trial data and due to no effect for patients with SARS and MERS. Above serious recommendations could change many clinical trials presently in progress. “It’s all based on the data,” said group member prof. Susan Swindells, at the University of Nebraska College of Medicine. Furthermore, FDA warns that COVID-19 patient treatment using hydroxychloroquine and chloroquine are associated with significant side effects, related to patient’s cardiac health [43].
On the other side, FDA-approved anti-parasitic drug ivermactin showed broad-spectrum anti-viral in-vitro activity against the causative virus SARS-CoV-2. A collaborative study between Monash University’s Biomedicine Discovery Institute (BDI) and the P. Doherty Institute of Infection and Immunity confirmed that ivermectin can stop SARS-CoV-2 growth in cell cultures, by wiping out all genetic information within two days [44].
M. Callahan et al, from the Massachusetts General Hospital found that Covid-19 patients in Wuhan, China, treated with famotidine was dying at a rate about 14% compared to 27% for those who are not on the drug. A group from US conducts a molecular modeling study using 2600 different compounds which can interact with new papain like viral protease enzyme [45]. Finally they have selected three best hit and famotidine was one of them. Famotidine an active OTC low cost safe drug used for heart burn patients was first time used for Covid-19 patient through intravenous route at nine times the heartburn dose at Northwell Health in New York. 187 critically diagnosed COVID-19 patients, including many on ventilators, have been included in the trial, which aims for a total of 1174 people. The director of the Cold Spring Harbor Laboratory Cancer Center, David Tuveson recommended famotidine to his 44-years old sister (tested positive for COVID-19). She with her five sick co-workers, including three with COVID-19 positive, showed significant improvements after taking the drug. Tuveson shared this with Science and credits the heartburn drug famotidine. “I would say that was a penicillin effect,” he says. Timothy Wang, head of gastroenterology at Columbia University Medical Center, saw more evidence of famotidine’s activity in his records from 1620 hospitalized COVID-19 patients. Current COVID-19 drug candidates in clinical trials are presented in Table 1.
Blood plasma from the COVID-19 survivors seems that it can be an effective short-term treatment for patients. The plasma from a recovered patient, which obviously contains antibodies can be given (the injection suitable time is still unknown) to a new patient suffering from COVID-19. FDA has begun permitting convalescent plasma to be used in seriously ill or immediately life-threatening COVID-19 patients, from March 24th, 2020. Currently 12 registered clinical trials are going on to investigate the effect of convalescent plasma or immunoglobulins (IgM and IgG) in COVID-19 [46].
Corticosteroid therapy for viral pneumonia and the use of NSAIDs in patients with COVID-19 symptoms is still under FDA careful screening, since a very recent paper suggests a correlation between increased ACE2 expression and ibuprofen may finally lead to a worse outcome for COVID-19 patients. However acetaminophen may be used for temperature control [47].
COVID-19 patients have been also treated with high doses of intravenous (IV) vitamin C. However, no scientific evidence that high dose vitamin C works for COVID-19 infections was observed. An ongoing study in China is focused to figure out, whether this treatment is useful for patients with severe COVID-19. Thomas Jefferson University is running a phase II clinical trial on 66 COVID-19 patients in order, to evaluate the safety and efficacy of ascorbic acid in the form of sequential I.V. infusions (Ascor®). Previous studies suggest that treatment with high-dose of vitamin C (along with corticosteroids and thiamine) prevent deaths among people suffering from sepsis and acute respiratory distress syndrome (ARDS), in which the lungs fill with fluid. However high doses of vitamin C can cause a number of side effects, including cramps, nausea, and an increased risk of kidney stones [48,49].
Vaccine therapy and their clinical trials
Globally 115 candidate vaccines development is underway, 78 of which are confirmed as active while 37 are unconfirmed [50]. According to WHO, 10 candidate vaccines are approved for clinical trials (Table 2 ) and 123 candidate vaccines are in preclinical stage (Draft landscape of COVID-19 candidate vaccines – 2nd June 2020). However, Dr. A. Fauci, Director of the National Institute of Allergy and Infectious Diseases, USA, told the media in March that a vaccine won’t be available for widespread use for at least another 12 to 18 months, due to complete the phase III clinical studies. Most of these vaccines developed in clinical trials, or are candidate drugs focused on spike protein. However recent research showed that spike protein of SARS-CoV-2 is exhibiting mutation and two the most important mutations observed are D614G and S943P. Scientists are very concerned about D614G mutation, which can increase transmissibility (observed already during the second virus wave) and severity of the disease. The second mutation S943P is associated with a cluster of mutation near to the fusion region and occurring by recombination of different strains [8]. Therefore, scientists now working on vaccine development and trials should obviously be well aware about the recent data of viral mutation.
Table 2.
COVID-19: candidate vaccines in Phase I-II trials (Draft landscape of COVID-19 candidate vaccines – 2nd June, 2020).
| Platform | Type of candidate vaccine | Developer | Coronavirus target | Coronavirus candidate: Current stage of clinical evaluation/regulatory status |
|---|---|---|---|---|
| Non-Replicating Viral Vector | ChAdOx1-S | University of Oxford/AstraZeneca | SARS-CoV2 | Phase2b/3, 2020-001228-32, Phase ½, 2020-001072-15 |
| Non-Replicating Viral Vector | Adenovirus Type 5 Vector | CanSino Biological Inc./Beijing Institute of Biotechnology | SARS-CoV2 | Phase 2, ChiCTR2000031781, Phase 1, ChiCTR2000030906 |
| RNA | LNP-encapsulated mRNA | Moderna/NIAID | SARS-CoV2 | Phase 2, NCT04405076, Phase 1, NCT04283461 |
| Inactivated | Inactivated | Wuhan Institute of Biological Products/Sinopharm | SARS-CoV2 | Phase ½, ChiCTR2000031809 |
| Inactivated | Inactivated | Beijing Institute of Biological Products/Sinopharm | SARS-CoV2 | Phase 1/2 ChiCTR2000032459 |
| Inactivated | Inactivated + alum | Sinovac | SARS-CoV2 | Phase ½, NCT04383574, NCT04352608 |
| Protein Subunit | Full length SARS CoV-2 recombinant glycoprotein nanoparticle vaccine adjuvanted with Matrix M | Novavax | SARS-CoV2 | Phase ½, NCT04368988, |
| RNA | 3 LNP-mRNAs | BioNTech/Fosun Pharma/Pfizer | SARS-CoV2 | Phase 1/2 2020-001038-36 NCT04368728 |
| Inactivated | Inactivated | Institute of Medical Biology, Chinese Academy of Medical Sciences | SARS-CoV2 | Phase 1 |
| DNA | DNA plasmid vaccine with electroporation | Inovio Pharmaceuticals | SARS-CoV2 | Phase 1, NCT04336410 |
Miller et al. (2020) detected an interesting similarity between universal BCG vaccination policy and reduced fatality for COVID-19. They were suggesting that countries (i.e., Italy, Nederland, USA etc.) without universal policies of BCG vaccination have been more severely affected, compared to countries with universal and long-standing BCG policies [51]. Some clinical trials are on truck in the Australia and Netherlands to find out whether existing tuberculosis vaccines might also protect against SARS-CoV-2. Several scientists think that Polio vaccine might boost the immune system just enough to fight off the new coronavirus, although there is no solid evidence yet.
Finally, and within the scope of the existing complexity of COVID-19 drug and vaccine candidates, the UK trial of the leading coronavirus vaccine (AZD1222), was abruptly halted on 6 September 2020, because of safety concerns. The trial was eventually restarted within a week. So far, some 18000 people globally have received the vaccine, according to the University of Oxford (doi:https://doi.org/10.1038/d411586-020=02633-6). Press reports indicate that the clinical trial inhibition is due to an inflammatory reaction in the spinal cord (transverse myelitis) in one of the volunteers participating in the clinical study.
Conclusions
SARS-CoV-2 is constantly mutating a fact that makes the virus more transmissible and contagious compared to other coronavirus. Recent research report suggests that multiple strains of the virus are present in the same region, which could make the virus more pathogenic and can lead to conformational changes in the region. Two important mutations of viral spike protein may guide the virus to interact differently with the host cell. This will not only affect the selection of an effective drug which can inhibit spike protein and ACE 2 interactions, but also different type of vaccine may be suitable at different region. Selecting a drug therapy is out-most important, since different drugs may be effective at different stages of infection. For example, adjunct therapy like immunomodulator may be effective at early stage of the infection, while antiviral drug like remdesivir can be effective for critical COVID-19 patients. No side-effect/toxicity dose selection is another critical factor which is associated with the safety and efficacy of the drug. Combination therapy might be an option which could work more efficiently against SARS-CoV-2 like a broad-spectrum therapy. Therefore, genomic sequence of the SARS-CoV-2 virus should be conducted in every country and region to elucidate its molecular details. Based on genomic sequence and using computation docking approaches, we could approach the best possible hit from the prospective drugs being in clinical trials nowadays for COVID-19. As a final conclusion, universities, research institutes and companies involved with the development of new vaccine and therapy armamentarium must take in serious account he most and early current data of mutation for better prevention, treatment and control of the pandemic COVID-19.
Funding
No funding sources.
Competing interests
None declared.
Ethical approval
Not required.
References
- 1.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. The Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. Journal of travel medicine. 2020 doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gates B. Responding to Covid-19—a once-in-a-century pandemic? New England Journal of Medicine. 2020;382(18):1677–1679. doi: 10.1056/NEJMp2003762. [DOI] [PubMed] [Google Scholar]
- 5.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell host & microbe. 2020 doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angeletti S., Benvenuto D., Bianchi M., Giovanetti M., Pascarella S., Ciccozzi M. COVID‐2019: the role of the nsp2 and nsp3 in its pathogenesis. Journal of medical virology. 2020 doi: 10.1002/jmv.25719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korber B., Fischer W., Gnanakaran S.G., Yoon H., Theiler J., Abfalterer W., Foley B., Giorgi E.E., Bhattacharya T., Parker M.D. Spike mutation pipeline reveals the emergence of a more transmissible form of SARS-CoV-2. bioRxiv. 2020 [Google Scholar]
- 9.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O’Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020:1–13. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharmaceutica Sinica B. 2020 doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., van Nieuwkoop S., Bestebroer T.M., van den Hoogen B.G., Neyts J., Snijder E.J. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrobial agents and chemotherapy. 2014;58(8):4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyall J., Coleman C.M., Hart B.J., Venkataraman T., Holbrook M.R., Kindrachuk J., Johnson R.F., Olinger G.G., Jahrling P.B., Laidlaw M. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrobial agents and chemotherapy. 2014;58(8):4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang S. World view. Nature. 2020;579:321. doi: 10.1038/d41586-020-00751-9. [DOI] [PubMed] [Google Scholar]
- 14.First CRISPR therapy dosed. Nature Biotechnology 2020, 38(4):382-382. [DOI] [PubMed]
- 15.Nogrady B: How SARS-CoV-2 Tests Work and What’s Next in COVID-19 Diagnostics. In.: TheScientists; 2020.
- 16.Singapore First to Test Out COVID-19 Serological Assay in Outbreak Contact Tracing. In: Global Biodefense.https://globalbiodefense.com/headlines/singapore-first-to-test-out-covid-19-serological-assay-in-outbreak-contact-tracing/; 28 Feb 2020.
- 17.Wenling Wang Y.X., Gao Ruqin. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. Jama. 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W., Du RH Li B, Zheng X.S., Yang X.L., Hu B., Wang Y.Y., Xiao G.F., Yan B., Shi Z.L. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9(1):386–389. doi: 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. The Lancet Infectious diseases. 2020;20(4):411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A. First case of 2019 novel coronavirus in the United States. New England Journal of Medicine. 2020 doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko W.-C., Rolain J.-M., Lee N.-Y., Chen P.-L., Huang C.-T., Lee P.-I., Hsueh P.-R. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. International journal of antimicrobial agents. 2020 doi: 10.1016/j.ijantimicag.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. The Lancet. 2020 doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life sciences. 2020 doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ, Smoot J, Gregg AC, Daniels AD, Jervey S: Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. In.: ACS Publications; 2020. [DOI] [PMC free article] [PubMed]
- 25.Shiraki K., Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacology & therapeutics. 2020 doi: 10.1016/j.pharmthera.2020.107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proceedings of the Japan Academy, Series B. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y., Yang C., Xu X.-F., Xu W., Liu S.-W. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacologica Sinica. 2020;41(9):1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto M., Kiso M., Sakai-Tagawa Y., Iwatsuki-Horimoto K., Imai M., Takeda M., Kinoshita N., Ohmagari N., Gohda J., Semba K. The anticoagulant nafamostat potently inhibits SARS-CoV-2 infection in vitro: an existing drug with multiple possible therapeutic effects. bioRxiv. 2020 doi: 10.3390/v12060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mallapaty S. Why does the coronavirus spread so easily between people? Nature. 2020;579(7798):183. doi: 10.1038/d41586-020-00660-x. [DOI] [PubMed] [Google Scholar]
- 31.Lauring A.S., Frydman J., Andino R. The role of mutational robustness in RNA virus evolution. Nat Rev Microbiol. 2013;11(5):327–336. doi: 10.1038/nrmicro3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? The Lancet Respiratory Medicine. 2020 doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., Lee M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. Jama. 2020 doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., Lee M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. Jama. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diurno F., Numis F., Porta G., Cirillo F., Maddaluno S., Ragozzino A., De Negri P., Di Gennaro C., Pagano A., Allegorico E. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. European Review for Medical and Pharmacological Sciences. 2020;24(7):4040–4047. doi: 10.26355/eurrev_202004_20875. [DOI] [PubMed] [Google Scholar]
- 37.Toniati P., Piva S., Cattalini M., Garrafa E., Regola F., Castelli F., Franceschini F., Focà E., Andreoli L., Latronico N. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmunity Reviews. 2020;19 doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID‐19: a single center experience. Journal of Medical Virology. 2020 doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mullard A. Coordinating the COVID-19 pipeline. Nature reviews Drug discovery. 2020 doi: 10.1038/d41573-020-00068-2. [DOI] [PubMed] [Google Scholar]
- 40.Gautret P., Lagier J.-C., Parola P., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. International journal of antimicrobial agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Rosenberg E.S., Dufort E.M., Udo T., Wilberschied L.A., Kumar J., Tesoriero J., Weinberg P., Kirkwood J., Muse A., DeHovitz J. Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. Jama. 2020;323(24):2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meini S., Pagotto A., Longo B., Vendramin I., Pecori D., Tascini C. Role of Lopinavir/Ritonavir in the Treatment of Covid-19: A Review of Current Evidence, Guideline Recommendations, and Perspectives. J Clin Med. 2020;9(7):2050. doi: 10.3390/jcm9072050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M., Mourão M.P.G., Brito-Sousa J.D., Baía-da-Silva D., Guerra M.V.F. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: A randomized clinical trial. JAMA network open. 2020;3(4) doi: 10.1001/jamanetworkopen.2020.8857. e208857-e208857. [DOI] [PubMed] [Google Scholar]
- 44.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral research. 2020 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borrell B. New York clinical trial quietly tests heartburn remedy against coronavirus. Science. 2020 [Google Scholar]
- 46.Lythgoe M.P., Middleton P. Ongoing clinical trials for the management of the COVID-19 pandemic. Trends in Pharmacological Sciences. 2020 doi: 10.1016/j.tips.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell B., Moss C., Rigg A., Van Hemelrijck M. COVID-19 and treatment with NSAIDs and corticosteroids: should we be limiting their use in the clinical setting? ecancermedicalscience. 2020:14. doi: 10.3332/ecancer.2020.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Truwit J.D., Hite R.D., Morris P.E., DeWilde C., Priday A., Fisher B., Thacker L.R., Natarajan R., Brophy D.F., Sculthorpe R. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. Jama. 2019;322(13):1261–1270. doi: 10.1001/jama.2019.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marik P.E., Khangoora V., Rivera R., Hooper M.H., Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 2017;151(6):1229–1238. doi: 10.1016/j.chest.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 50.Le T.T., Andreadakis Z., Kumar A., Roman R.G., Tollefsen S., Saville M., Mayhew S. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020 doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 51.Miller A., Reandelar M.J., Fasciglione K., Roumenova V., Li Y., Otazu G.H. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. medRxiv. 2020 [Google Scholar]