Abstract
Background
In December 2019, a novel coronavirus, SARS-CoV-2 caused a series of acute atypical respiratory diseases worldwide. However, there is still a lack of drugs with clear curative effects, and the clinical trial research of vaccines has not been completely finished.
Purpose
LH capsules are approved TCM patent medicine that are widely used for the treatment of respiratory tract infectious diseases caused by colds and flu. On April 12, 2020, LH capsules and granules were officially repurposed by the China Food and Drug Administration (CFDA) for patients with mild COVID-19 based on their safety and efficacy demonstrated through multicentre, randomized, controlled clinical trials. We hope to conduct a comprehensive review of it through modern pharmacy methods, and try to explain its possible mechanism.
Methods
Using the full names of LH capsules Lianhuaqingwen, Lianhua Qingwen andSARS-COV-2, COVID-19 as the keywords of the search terms, systemically search for existing related papers in various databases such as Web of Science and PubMed. And completed the collection of clinical data in ClinicalTrials.gov and Chinese Clinical Trial Registry. Last but not least, we have sorted out the anti-inflammatory and antiviral mechanisms of LH capsules through literature and Selleck.
Results
This review systematically sorted out the active ingredients in LH capsules. Furthermore, the related pharmacological and clinical trials of LH capsule on SARS-CoV-2, IAV and IBV were discussed in detail. Moreover, the present review provides the first summary of the potential molecular mechanism of specific substances in LH capsules involved in resistance to SARS-COV-2 infection and the inhibition of cytokine storm syndrome (CSS) caused by IL-6.
Conclusion
This review summarizes the available reports and evidence that support the use of LH capsules as potential drug candidates for the prevention and treatment of COVID-19. However, TCM exerts its effects through multiple targets and multiple pathways, and LH capsules are not an exception. Therefore, the relevant mechanisms need to be further improved and experimentally verified.
Keywords: Forsythia honeysuckle (Lianhuaqingwen,LH) capsules; COVID-19; SARS-CoV-2; Cytokine storm syndrome (CSS); 3C-like protease (3CLpro)
Abbreviations: 3CLpro, 3C-like protease; CC50, 50% Cytotoxic concentration; IC50, 50% Inhibition concentration; LD50, 50% Lethal dose; MLD50, 50% Minimum lethal dose; TCID50, 50% Tissue culture infective dose; AIDS, Acquired immune deficiency syndrome; AECOPD, Acute exacerbation of chronic obstructive pulmonary disease; ARDS, Acute respiratory distress syndrome; Vero E6, African Green Monkey Kidney Epithelial-6; ACE2, Angiotensin-converting enzyme 2; AQP3, Aquaporins 3; CCL-2/MCP-1, C—C motif ligand 2/monocyte chemoattractant protein-1; CFDA, China Food and Drug Administration; COPD, Chronic obstructive pulmonary disease; CT, Computed tomography; CAT, COPD assessment test; COVID-19, Coronavirus disease 2019; CXCL-10/IP-10, C-X-C Motif Chemokine Ligand 10/ Interferon Gamma-induced Protein 10; CSS, Cytokine storm syndrome; CPE, Cytopathic effect; DMSO, Dimethyl sulfoxide; E protein, Envelope protein; ERK, Extracellular signal-regulated kinase; FBS, Fatal bovine serum; LH capsules, Forsythia honeysuckle (Lianhuaqingwen) capsules; gp-130, Glycoprotein 130; Grb2, Growth factor receptor-bound protein 2; TD50, Half-toxic dose; HVJ, Hemagglutinating virus of Japan; HSV-1, Herpes simplex virus type 1; HPLC, High-performance liquid chromatography; Hep-2, Human epithelial type 2; Huh-7, Human Hepatocellular Carcinoma-7; HIV, Human immunodeficiency virus; IL-6R, IL-6 Receptor; IAV, Influenza A virus; IBV, Influenza B virus; IP-10, Interferon-inducible protein-10; IFN-λ1, Interferon-λ1; IL-6, Interleukin-6; IL-8, Interleukin-8; JAK/STAT, Janus kinase/signal transducers and activators of transcription; JAK1/2, Janus kinase1/2; MIP-1β, Macrophage Inflammatory Protein-1β; MDCK, Madin-darby canine kidney; mTOR, Mammalian target of rapamycin; M protein, Membrane protein; mIL-6R, Membrane-bound form IL-6 Receptor; MTT, Methyl Thiazolyl Tetrazolium; MERS, Middle east respiratory syndrome; MAPK, Mitogen-activated protein kinase; MEK, Mitogen-activated protein kinase kinase; MCP-1, Monocyte chemotactic protein 1; MOF, Multifunctional organ damage; MOI, Multiplicity of infection; NHC, National Health Commission; nsps, Non-structural proteins; TD0, Non-toxic Dose; NF-kB, Nuclear transcription factor kappa-B; ORFs, Open reading frames; PLpro, Papain-like proteases; PRC, People's Republic of China; PHN, Phillyrin; PBS, Phosphate buffered saline; PI3K, Phosphoinositide 3-kinases; PKA/p-CREB, Protein kinase A /phosphorylated cAMP response element-binding protein; PKB, Akt, Protein kinase B; QC, Quality control; qPCR, Quantitative PCR; Ras, Ras GTPase; RANTES, Regulated on activation normal T cell expressed and secreted; RSV, Respiratory syncytial virus; RT-PCR, Reverse transcription PCR
Graphical abstract

1. Introduction
In December 2019, a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), caused a series of acute atypical respiratory diseases in Wuhan, Hubei Province, China (Chan et al., 2020). The disease was termed coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO). The clinical symptoms of COVID-19 include fever, fatigue, and dry cough, and some patients also experience nasal congestion, runny nose, inappetence, diarrhoea and pneumonia on computed tomography (CT) (Huang et al., 2020; Wang et al., 2020a). Some severe cases can rapidly develop into acute respiratory distress syndrome (ARDS), refractory metabolic acidosis, septic shock, and coagulation dysfunction (clinical features of patients infected with 2019 novel coronavirus in Wuhan). COVID-19 has spread more rapidly than severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) (Park et al., 2020; Vellingiri et al., 2020). Specifically, COVID-19 has spread to more than 200 countries across the world and has claimed more than 1014,152 human lives as of October 1, 2020. Unfortunately, there is currently no vaccine or antiviral treatment for patients with suspected or confirmed COVID-19 (Amin and Jha, 2020; Kouznetsov, 2020; Shang et al., 2020; Shi et al., 2020). The drugs that are clinically used include remdesivir, abidol, and RNA synthesis hormones (Chu et al., 2004; Liu et al., 2020a; Lu, 2020; Zheng et al., 2020), but the efficacy and safety of these drugs still need further clinical confirmation. With respect to vaccines, 30 vaccine candidates have been reported to the WHO and are in the clinical phase, but only six vaccines have entered the III clinical phase as of August 20, 2020 (Florindo et al., 2020). However, the in vivo effectiveness and side effects of these vaccines and chemical candidates need further evaluation (Ejemel et al., 2020).
Based on experience regarding the prevention and treatment of SARS obtained during the related epidemic, the National Health Commission (NHC) of the People's Republic of China (PRC) recommended the TCM LH capsules as an eligible pioneer treatment for COVID-19 (Del Valle et al., 2020; Xu and Zhang, 2020). Retrospectively, the utilization of this TCM alone or in combination with conventional treatment has exhibited clinical effectiveness without increasing adverse drug reactions (Liu et al., 2020b; Ye et al., 2020).
LH is a widely used herbal medicine formulation for treating colds and flu in China that consists of 13 medicinal materials (Wang et al., 2016; Wu et al., 2020b). LH can be administered as three different forms, namely, tablets, capsules, and granules, and among these, capsules are the most widely used form in clinical practice. These capsules combine the medical erudition and philosophy of three well-known traditional Chinese epidemic physicians spanning 1500 years of three dynasties from Eastern Han to Ming and Qing. Ma Xing Shi Gan decoction, recorded in the Treatise on Febrile and Miscellaneous Disease by Zhang Zhongjing, a medical sage during the Eastern Han Dynasty (Wang et al., 2020c), is a famous prescription that has been particularly used for the treatment of febrile infectious diseases over two thousand years. The LH components ephedra, apricot, plaster and liquorice originate from the Ma Xing Shi Gan decoction. Additionally, another four herbs in the prescription, namely, forsythia, liquorice, honeysuckle and mint, are derived the Yin Qiao San formula, which is included in the Treatise on Epidemic Febrile Disease written by Wu Tang, an outstanding Chinese medicine expert during the Qing Dynasty. Moreover, according to the famous literary work General Treatise on Plague written by the well-known physician Wu Youxing during the Ming Dynasty, LH also includes rhubarb for the treatment of epidemic febrile plaques. According to TCM theory, the clinical symptoms of COVID-19 are linked to epidemic febrile diseases. Rhodiola, Radix isatidis, Dryopteris crassirhizoma, Houttuynia cordata and patchouli have been added to the LH formula based on evidence-based pharmacological and medical studies (Fig. 1).
Fig 1.
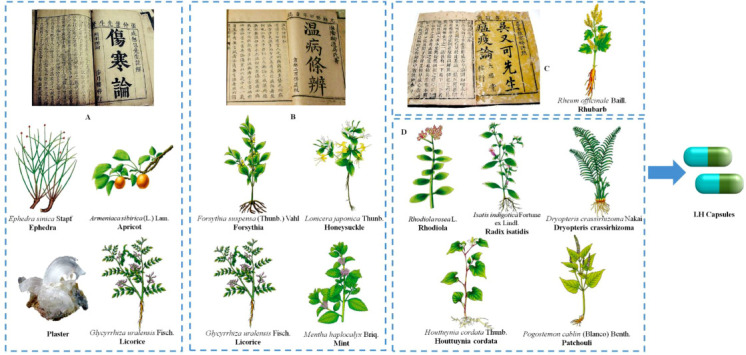
Provenance of LH capsule prescriptions derived from the Treatise on Febrile and Miscellaneous Disease (A), China's first clinical medicine monograph written by the medical sage Zhang Zhongjing in the Eastern Han Dynasty (AD 25–220), the Treatise on Epidemic Febrile Disease (B) written by Wu Tang during the Qing Dynasty (AD 1798), and the General Treatise on Plague (C) written by Wu Youxing during the Ming Dynasty (1642 AD).
Before the COVID-19 pandemic, LH capsules have been approved for the repurposed treatment of many public health emergencies, such as SARS, H1N1 and H3N2 influenza, and have exhibited significant effectiveness according to clinical statistics (Gao et al., 2020; Tao et al., 2013). Recently, an analysis of the treatment of COVID-19 using LH capsules indicated that these capsules can significantly relieve clinical symptoms in patients with fever, weakness, and cough and reduce the course of COVID-19 (Duan et al., 2011; Li et al., 2020a; Runfeng et al., 2020). Based on their good clinical efficacy, LH capsules have been included in the Diagnosis and Treatment Programs of 2019 New Coronavirus Pneumonia (from fourth to eighth editions) formulated by the NHC of China. On April 12, 2020, LH capsules and granules were approved for the treatment of patients with mild and moderate COVID-19 (Khan et al., 2020).
2. Composition and manufacturing procedure
LH capsules are composed of 13 TCM, and thecomposition and preparation process have been included in the Chinese Pharmacopoeia (2015 edition). The formulation of every batch of 1000 capsules is shown in Table 1 , and the specific manufacturing process is shown in Fig 2 .
Table 1.
Chinese herbal medicines in LH capsules.
| No. | Medicine source | Part | Weight (g) |
|---|---|---|---|
| 1 | Forsythia suspensa (Thunb.) Vahl. | Fruit | 255 |
| 2 | Lonicera japonica Thunb. | Flower | 255 |
| 3 | Plaster | / | 255 |
| 4 | Isatis indigotica Fortune ex Lindl. | Root | 255 |
| 5 | Dryopteris crassirhizoma Nakai | Rhizome and petiole residues | 255 |
| 6 | Houttuynia cordata Thunb. | Aboveground parts | 255 |
| 7 | Ephedra sinica Stapf | Stem | 85 |
| 8 | Armeniaca sibirica (L.) Lam. | Seed | 85 |
| 9 | Pogostemon cablin (Blanco) Benth. | Aboveground parts | 85 |
| 10 | Rhodiola rosea L. | Whole herb | 85 |
| 11 | Glycyrrhiza uralensis Fisch. | Root | 85 |
| 12 | Rheum officinale Baill. | Root and rhizome | 51 |
| 13 | Mentha haplocalyx Briq. | Leaf and stem (menthol) | 7.5 |
Fig 2.
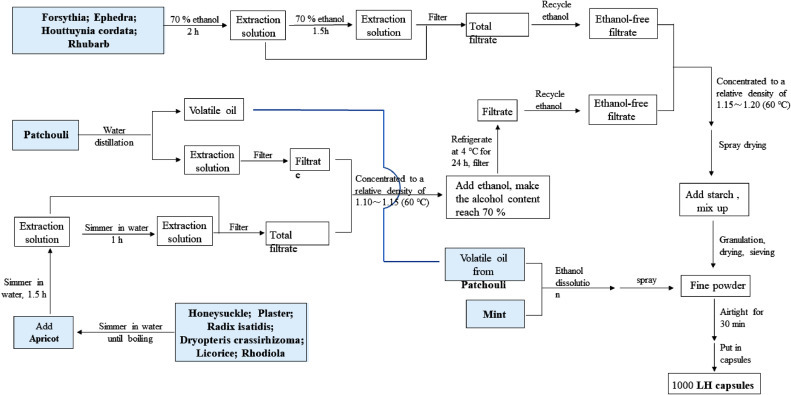
Procedure used for the manufacturing of LH capsules.
According to the above schematic diagram showing the manufacturing flowchart of 13 medicinal materials, patchouli is added to boiled water, the volatile oil is distilled and collected, and the water extract is filtered and set aside. Forsythia, ephedra, Houttuynia cordata and rhubarb are extracted twice with 70% ethanol, and the first and second extractions are performed for 2 and 1.5 h, respectively. The extracts are filtered and combined, and the ethanol extract is recovered and set aside. Honeysuckle plaster, Radix isatidis, Dryopteris crassirhizoma, liquorice, and Rhodiola are boiled in water, and apricot is then added. The apricot is decocted twice, and the first and second decoctions are performed for 1.5 and 1 h, respectively. The decoction is filtered, and the filtrates are combined. The aqueous solution obtained after patchouli oil extraction was added, and the resulting mixture was concentrated to a relative density of 1.10~1.15 (60 °C). Ethanol was added to obtain an alcohol content of 70%, and the mixture was refrigerated at 4 °C for 2 to 4 h and filtered to recover the ethanol. Combined with the abovementioned alcohol extracts of the four flavours, forsythia concentrated to a relative density of 1.15~1.20 (60 °C) is spray dried and mixed with an appropriate amount of starch into granules, and the granules are then dried and sieved. Subsequently, the sieved fine powder is separated from the granules. The dissolved mint and patchouli volatile oil with an appropriate amount of ethanol are sprayed into a fine powder and mixed with the abovementioned granules, and the capsule shells are then filled with the mixture and sealed to obtain LH capsules.
3. Quality control (QC) and ingredient profile
The QC of the LH capsules is recorded in the Chinese Pharmacopoeia (2015 edition), and this process includes thin-layer chromatography (TLC) for characteristic identifications and high-performance liquid chromatography (HPLC) for assessing the representative natural product content. With respect to characteristic identification, LH capsules are hard capsules filled with contain brownish yellow to yellowish brown granules and powder. The capsules exhibit a slight fragrance and bitterness. In the TLC detection, houttuynia cordata, rhubarb, and liquorice are identified with control medicinal herbs. And honeysuckle, ephedra, and mint are identified with chlorogenic acid, menthol and ephedrine hydrochloride, respectively. To avoid the adulteration of wild honeysuckle, macranthoidin B (CAS:136849-88-2) cannot be checked by TLC. Moreover, the Chinese Pharmacopoeia stipulates that the content of phillyrin (12) in each capsule should not be less than 0.17 mg. Finally, QC requires routine testing for capsules, which includes their moisture, disintegration time limit, and difference in capacity.
LH capsules are composed of a variety of Chinese medicinal materials, and nearly 400 biologically active ingredients have been reported. Weina Jia et al. established a fast ultrahigh-performance liquid chromatography method that combines a diode-array detector and quadrupole time-of-flight mass spectrometry (UPLC-DAD-QTOF-MS), and 61 activities, including forsythoside A have been detected in LH capsules(Jia et al., 2015). To clarify the chemical substance basis of LH capsules, the manufacturer Beijing Yiling Pharmaceutical Co., Ltd. tested the compounds in the total extract of LH capsules obtained with different proportions of ethanol aqueous solution. Among these, 18, 8, 20 compounds were sequentially separated and identified from 30%, 50%, and 70% ethanol aqueous solution (Bi.D, 2018; Shen, 2019). Herein, we detail the abovementioned compounds that have been separated and structurally confirmed and provide a list of the medicinal weights in the capsules in Table 2 in descending order of weight. Their structures are shown in Fig 3 , and the active ingredients Forsythia, honeysuckle, liquorice and rhubarb are fully described.
Table 2.
Active substances of LH capsules.
| No. | Compound name | CAS code | Chemical formula | Source |
|---|---|---|---|---|
| 1 | Cornoside | 40,661–45–8 | C14H20O8 | Forsythia suspensa (Thunb.) Vahl |
| 2 | Rengynic acid-1′-O-β-d-glucoside | / | C14H23O9 | Forsythia suspensa (Thunb.) Vahl |
| 3 | Quercetin | 117–39–5 | C15H10O7 |
Forsythia suspensa (Thunb.) Vahl; Lonicera japonica Thunb.; Houttuynia cordata Thunb.; Ephedra sinica Stapf; Pogostemon cablin (Blanco) Benth.; Glycyrrhiza uralensis Fisch. |
| 4 | (+)-Pinoresinol | 487–36–5 | C20H22O6 | Forsythia suspensa (Thunb.) Vahl |
| 5 | (+)-Epipinoresinol | 24,404–50–0 | C20H22O6 | Forsythia suspensa (Thunb.) Vahl |
| 6 | Forsythoside E | 93,675–88–8 | C20H30O12 | Forsythia suspensa (Thunb.) Vahl |
| 7 | Quercitrin | 522–12–3 | C21H20O11 | Forsythia suspensa (Thunb.) Vahl; Houttuynia cordata Thunb. |
| 8 | Hyperoside | 482–36–0 | C21H20O12 | Forsythia suspensa (Thunb.) Vahl; Houttuynia cordata Thunb. |
| 9 | (+)-Pinoresinol-β-d-glucoside | 69,251–96–3 | C26H32O11 | Forsythia suspensa (Thunb.) Vahl |
| 10 | Rutin | 153–18–4 | C27H30O16 | Forsythia suspensa (Thunb.) Vahl |
| 11 | Arctiin | 20,362–31–6 | C27H34O11 | Forsythia suspensa (Thunb.) Vahl |
| 12 | Phillyrin | 487–41–2 | C27H34O11 | Forsythia suspensa (Thunb.) Vahl |
| 13 | Forsythoside A | 79,916–77–1 | C29H36O15 | Forsythia suspensa (Thunb.) Vahl |
| 14 | Forsythoside I | 1,177,581–50–8 | C29H36O15 | Forsythia suspensa (Thunb.) Vahl |
| 15 | Forsythoside H | 1,178,974–85–0 | C29H36O15 | Forsythia suspensa (Thunb.) Vahl |
| 16 | Suspensaside | 84,213–44–5 | C29H36O16 | Forsythia suspensa (Thunb.) Vahl |
| 17 | Protocatechualdehyde | 139–85–5 | C7H6O3 | Lonicera japonica Thunb. |
| 18 | Quinic acid | 77–95–2 | C7H12O6 | Lonicera japonica Thunb. |
| 19 | Ethyl caffeate | 102–37–4 | C11H12O4 | Lonicera japonica Thunb. |
| 20 | Kaempferol | 520–18–3 | C15H10O6 |
Lonicera japonica Thunb.; Dryopteris crassirhizoma Nakai; Houttuynia cordata Thunb.; Ephedra sinica Stapf; Glycyrrhiza uralensis Fisch. |
| 21 | Chlorogenic acid | 327–97–9 | C16H18O9 | Lonicera japonica Thunb. |
| 22 | Neochlorogenic acid | 906–33–2 | C16H18O9 | Lonicera japonica Thunb. |
| 23 | Cryptochlorogenic acid | 905–99–7 | C16H18O9 | Lonicera japonica Thunb. |
| 24 | Loganic acid | 22,255–40–9 | C16H24O10 | Lonicera japonica Thunb. |
| 25 | Sweroside | 14,215–86–2 | C16H22O9 | Lonicera japonica Thunb. |
| 26 | Tricin | 520–32–1 | C17H14O7 | Lonicera japonica Thunb. |
| 27 | 7-Epi-vogeloside | 118,627–52–4 | C17H24O10 | Lonicera japonica Thunb. |
| 28 | Secoxyloganin | 58,822–47–2 | C17H24O11 | Lonicera japonica Thunb. |
| 29 | Vogeloside | 60,077–47–6 | C17H24O10 | Lonicera japonica Thunb. |
| 30 | Loganin | 18,524–94–2 | C17H26O10 | Lonicera japonica Thunb. |
| 31 | 3,5-Dicaffeoylquinic acid | 2450–53–5 | C25H24O12 | Lonicera japonica Thunb. |
| 32 | 3,4-Dicaffeoylquinic acid | 14,534–61–3 | C25H24O12 | Lonicera japonica Thunb. |
| 33 | Kaempferol-3-O-rutinoside | 17,650–84–9 | C27H30O15 | Lonicera japonica Thunb. |
| 34 | Hesperidin | 520–26–3 | C28H34O15 | Isatis indigotica Fortune ex Lindl. |
| 35 | Liriodendrin | 96,038–87–8 | C34H46O18 | Isatis indigotica Fortune ex Lindl. |
| 36 | Gallic acid | 149–91–7 | C7H6O5 | Houttuynia cordata Thunb. |
| 37 | Cepharanone B | 53,948–09–7 | C17H13NO3 | Houttuynia cordata Thunb. |
| 38 | 4,5-Dioxodehydroasimilobine | 82,644–81–3 | C17H11NO4 | Houttuynia cordata Thunb. |
| 39 | Ephedrine hydrochloride | 50–98–6 | C10H16ClNO | Ephedra sinica Stapf |
| 40 | Citric acid | 77–92–9 | C6H8O7 | Armeniaca sibirica (L.) Lam. |
| 41 | (2S)-Sambunigrin | 99–19–4 | C14H17NO6 | Armeniaca sibirica (L.) Lam. |
| 42 | Amygdalin | 29,883–15–6 | C20H27NO11 | Armeniaca sibirica (L.) Lam. |
| 43 | (E)-Ethyl 3-(4-hydroxyphenyl) acrylate | 7362–39–2 | C11H12O3 | Rhodiola rosea L. |
| 44 | Salidroside | 10,338–51–9 | C14H20O7 | Rhodiola rosea L. |
| 45 | Rhodiosin | 86,831–54–1 | C27H30O16 | Rhodiola rosea L. |
| 46 | Isoliquiritigenin | 961–29–5 | C15H12O4 | Glycyrrhiza uralensis Fisch. |
| 47 | Liquiritigenin | 578–86–9 | C15H12O4 | Glycyrrhiza uralensis Fisch. |
| 48 | Naringenin | 480–41–1 | C15H12O5 | Glycyrrhiza uralensis Fisch. |
| 49 | Formononetin | 485–72–3 | C16H12O4 | Glycyrrhiza uralensis Fisch. |
| 50 | Glycycoumarin | 94,805–82–0 | C21H20O6 | Glycyrrhiza uralensis Fisch. |
| 51 | Liquiritin | 551–15–5 | C21H22O9 | Glycyrrhiza uralensis Fisch. |
| 52 | Isoliquiritin | 5041–81–6 | C21H22O9 | Glycyrrhiza uralensis Fisch. |
| 53 | Ononin | 486–62–4 | C22H22O9 | Glycyrrhiza uralensis Fisch. |
| 54 | Liquiritin apioside | 199,796–12–8 | C26H30O13 | Glycyrrhiza uralensis Fisch. |
| 55 | Isoliquiritin apioside | 120,926–46–7 | C26H30O13 | Glycyrrhiza uralensis Fisch. |
| 56 | Liguiritigenin-7-O-D-apiosyl-4′-O-D-glucoside | 199,796–12–8 | C26H30O13 | Glycyrrhiza uralensis Fisch. |
| 57 | Liquorice saponin E2 | 119,417–96–8 | C42H60O16 | Glycyrrhiza uralensis Fisch. |
| 58 | Glycyrrhizic acid | 1405–86–3 | C42H62O16 | Glycyrrhiza uralensis Fisch. |
| 59 | Liquorice saponin B2 | 118,536–86–0 | C42H64O15 | Glycyrrhiza uralensis Fisch. |
| 60 | Liquorice saponin H2 | 118,441–85–3 | C42H62O16 | Glycyrrhiza uralensis Fisch. |
| 61 | Liquorice saponin K2 | 135,815–61–1 | C42H62O16 | Glycyrrhiza uralensis Fisch. |
| 62 | Liquorice saponin G2 | 118,441–84–2 | C42H62O17 | Glycyrrhiza uralensis Fisch. |
| 63 | 22β-Acetoxyglycyrrhizin | 938,042–17–2 | C44H64O18 | Glycyrrhiza uralensis Fisch. |
| 64 | Chrysophanol | 481–74–3 | C15H10O4 | Rheum officinale Baill. |
| 65 | Emodin | 518–82–1 | C15H10O5 | Rheum officinale Baill. |
| 66 | Aloe emodin | 481–72–1 | C15H10O5 | Rheum officinale Baill. |
| 67 | Rhein | 478–43–3 | C15H8O6 | Rheum officinale Baill. |
| 68 | Citreorosein | 481–73–2 | C15H10O6 | Rheum officinale Baill. |
| 69 | Polydatin | 65,914–17–2 | C20H23O9 | Rheum officinale Baill. |
| 70 | Chrysophanol 1-glucoside | 4839–60–5 | C21H20O9 | Rheum officinale Baill. |
| 71 | Emodin-8-glucoside | 23,313–21–5 | C21H20O10 | Rheum officinale Baill. |
| 72 | Aloe-emodin-8-O-β-D-glucopyranoside | 33,037–46–6 | C21H20O10 | Rheum officinale Baill. |
| 73 | Physcion-8-O-β-D-glucopyranoside | 26,296–54–8 | C22H22O10 | Rheum officinale Baill. |
| 74 | Menthol | 1,181,819–05–5 | C10H20O | Mentha haplocalyx Briq. |
Fig 3.
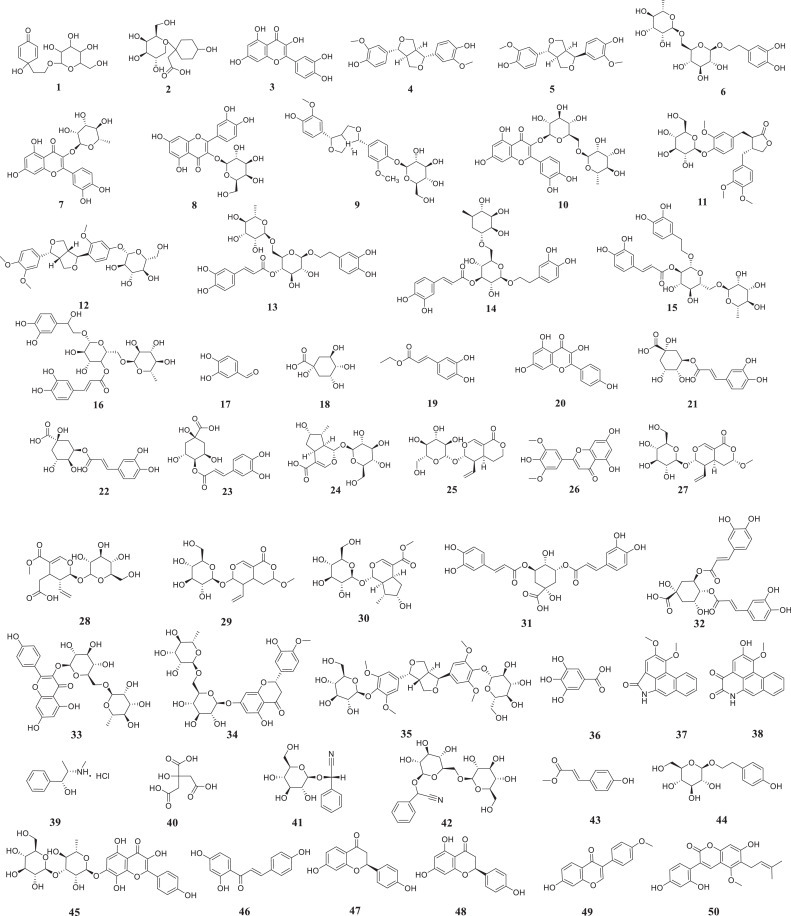
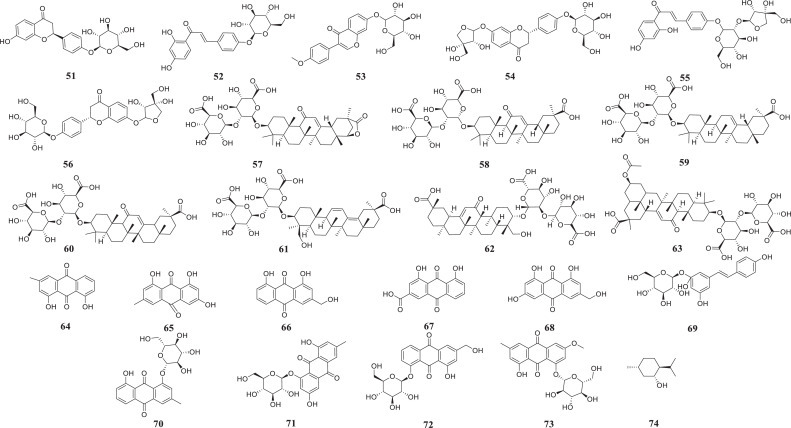
Structure of the active substance of LH capsules (shown in Table 2).
Forsythia is one of the main components of LH capsules. Forsythia fruit is widely used as a TCM and contains a wide range of active ingredients that exert anti-inflammatory, antibacterial, antiviral, anticancer and other pharmacological effects (Chen et al., 1999; Han et al., 2012; Hao et al., 2010; Ko et al., 2005). As detailed in the Chinese Pharmacopoeia (2015 edition), TCM preparations containing forsythia are mainly used for reducing fever and detoxification (Wang et al., 2018). Compounds 1–16 originate from forsythia. The characteristic components derived from forsythia that have been identified in LH capsules mainly include phenylethanol glycosides, lignans and flavonoids. Lignans are typical representatives of forsythia. (+)-Pinoresinol (4) and phillyrin (PHN,12), two furan lignans, were isolated in 1977, and 12 is one of the specific components of forsythia (Shi et al., 2009). More recently, (+)-pinoresinol (5), (+)-pinoresinol-β-D-glucoside (9), and arctiin (11) were also isolated from forsythia. Forsythoside E (6), forsythoside A (13) and their isomers forsythoside I (14) and forsythoside H (15) are all phenethanol glycosides; 13 was the first of these compounds isolated from forsythia in 1982 (Wang et al., 2018). The other four flavonoids from forsythia are quercetin (3), quercitrin (7), hyperoside (8), and rutin (10). Compound 10 can also be isolated from forsythia flowers, and 3 in LH capsules can additionally be derived from honeysuckle, Houttuynia cordata, ephedra, liquorice and patchouli (Jiang et al., 2011; Zhang et al., 2015a).
Compounds 17–33 originate from honeysuckle, which is often clinically used to fight inflammation (Yang et al., 2019). Its main active ingredients are phenolic acids and flavonoids (Peng et al., 2005). Chlorogenic acid (21), a bitter substance formed by caffeic acid and quinic acid, is a representative substance of honeysuckle and can also be found in Eucommia, tea and coffee (Jung et al., 2014; Yang et al., 2020b; Zhang et al., 2008). Due to its anti-inflammatory, antioxidant, and antiviral activities, 21 exerts protective effects on myocarditis, cerebral ischaemia, hypertension, and liver damage (Lee et al., 2012; Zhao et al., 2012), and its anticancer and gastric protective effects have also been confirmed in recent years (Cristians et al., 2013; Hou et al., 2017; Lee et al., 2017; Liu et al., 2013; Xu et al., 2013; Xue et al., 2017).
Compounds 46–63 were isolated from liquorice. Structurally, these compounds can be divided into triterpene saponins (57–62), flavonoids (46–56, 63) and glycycoumarin (50). The most representative substance is glycyrrhizic acid (58) (Fiore et al., 2004), which can prevent and treat viral infections, immune deficiencies and malignant tumours induced by various causes (Baltina et al., 2009; Bentz et al., 2019; Matsumoto et al., 2013). Studies have shown that 58 and its monoammonium salt-glycyrrhizin amide can inhibit the replication of several DNA and RNA viruses, including vaccinia virus (Baba and Shigeta, 1987; Pompei et al., 1979), and these compounds are also effective inhibitors of herpes simplex virus type 1 (HSV-1) (Utsunomiya et al., 1995). 58 and its monoammonium salt have been used externally to treat skin and oral infections caused by HSV-1, and this compound is also effective against hepatitis A, B and C viruses (Arase et al., 1997; Crance et al., 1994; Sato et al., 1996). In addition, 58 is the main natural product used for the treatment of patients with human immunodeficiency virus (HIV) (De Clercq, 2000). Preliminary clinical studies have shown that the daily administration of 800–1600 mg of this compound to patients with acquired immune deficiency syndrome (AIDS) will increase the number of T4 lymphocytes in the patients’ body and reduce the number of pathogens (Ito et al., 1988).
Fever is the main symptom of COVID-19 and an important indicator for early screening. Rhubarb can promote the excretion of faeces and thereby removes heat from the body. It has been reported that Aloe emodin (66) and Rhein (67) might exert a laxative effect by directly acting on the colon (Gong et al., 2015; Takayama et al., 2012). According to published reports, 67 plays a role in stimulating intestinal smooth muscle M receptors and inhibiting the activity of Na+-K+-ATPase in the intestinal cell membrane to prevent Na transport and thereby promote defecation (Takayama et al., 2012). Additionally, 66 can increase both the intestinal water content and mucus secretion. Each of these two compounds can promote the other's effects (Yagi and Yamauchi, 1999). The laxative effect of 65 is related to the nervous system and induced indirectly (Chen et al., 2012; Xu et al., 2012). A previous study showed that the laxative effect of 65 is related to upregulation of the protein kinase A/phosphorylated cAMP response element-binding protein (PKA/p-CREB) signalling pathway and increased expression of aquaporin 3 (AQP3) (Zheng et al., 2014).
4. Safety and effectiveness of LH capsules
LH capsules have re-attracted attention due to their effectiveness against COVID-19; specifically, these capsules can inhibit SARS-COV-2 replication and regulate the secretion of inflammatory cytokines (Li et al., 2020b). Experiments using chicken embryos and cytotoxicity assays have shown that LH capsules are safe, and modern pharmacological studies have demonstrated that LH capsules exert a good inhibitory effect on influenza A virus (IAV) and influenza B virus (IBV) (Ding et al., 2017; Zhao et al., 2014). In addition, LH capsules can antagonize PM2.5-induced lung oxidative damage in rats (Ping et al., 2016). Here, we compile the anti-inflammatory and antiviral pharmacological studies related to LH capsules.
4.1. Safety
4.1.1. Cytotoxicity assays
MDCK cells: Originator Beijing Yiling Pharmaceutical Co., Ltd., assessed the cytotoxicity of LH capsules on Madin-Darby canine kidney (MDCK) cells and human epithelial type 2 (Hep-2) cells. Using the micro-cell culture method, a monolayer of MDCK cells was cultured in a 96-well plate. In this experiment, the two-fold gradient dilution method was used to dilute the LH capsules solution (11.48 mg/mL) with cell maintenance solution, and the diluted solutions are added to the cell culture wells. Each dilution was included in four wells, and each well contained 100 μL. The cells were incubated for 3 to 4 days at 37 °C under 5% CO2, and the results were observed. This analysis revealed that the maximum nontoxic dose (TD0) of LH capsules to MDCK cells was 0.72 mg/mL and that the half-toxic dose (TD50) was 1.02 mg/mL.
In another cytotoxicity assay, Ding et al. seeded MDCK cells on 96-well plates and cultured them at 37 °C in the presence of 5% CO2 for 24 h(Ding et al., 2017). After the cells reached a confluence of 80–90%, the medium containing different concentrations of LH capsules (0.625–20 mg/mL, 100 μL/well) was aspirated, and the cells were incubated for an additional 48 h at 37 °C. Approximately 20 μL of methyl thiazolyl tetrazolium (MTT) at a concentration of 5 mg/mL was then added to each well, and the cells were incubated for an additional 4 h at 37 °C. The medium was subsequently removed, and the formazan crystals were dissolved with dimethyl sulfoxide (DMSO) (100 μL/well). An analysis of the results calculated the TD50 of LH capsules as 3.17 mg/mL. The research group conducted similar experiments in related studies on IBV and found that the TD50 of LH capsules to MDCK cells was 4.0221±0.0471 mg/mL(Yang et al., 2020a). The coincubation of the cells with the drug for 48 h at 37 °C in a humid atmosphere of 5% CO2 revealed that the LH capsules exerted no obvious cytotoxic effect on MDCK cells over a concentration range of 0.075–1.0 mg/mL.
Hep-2 cells: Beijing Yiling Pharmaceutical Co., Ltd., also assessed the cytotoxicity of LH capsules to Hep-2 cells. In this experiment, a culture of monolayer cells was first conducted. The culture solution was then poured out in the culture plate, and 100 μL of maintenance solution was added to each well. Subsequently, 100 μL of each drug solution was added to three wells in the first row, and the mixture was mixed well. Subsequently, 100 μL was moved from the first row to the next row, and this step was repeated until row 7. The wells in row 8 were maintained as normal cell controls without drugs, and 100 μL of culture medium was added to each well to obtain a total volume per well of 200 μL. The MDCK cells were then cultured under the same culture conditions, and the results showed that the TD0 and TD50 for Hep-2 cells were 1.56 mg/mL and 2.24 mg/mL, respectively.
Vero E6 and Huh-7 cells: Runfeng et al. evaluated the cytotoxic effect of LH capsules on African green monkey kidney epithelial-6 (Vero E6) and human hepatocellular carcinoma-7 (Huh-7) cells using the MTT assay (Runfeng et al., 2020). The Vero E6 and Huh-7 cells in a 96-well plate were washed with phosphate buffered saline (PBS), incubated with LH capsules at a concentration of 24 mg/mL for 72 h and stained with 0.5 mg/mL MTT solution for 4 h. The supernatant was removed, and the formed formazan crystals were dissolved in 200 μL of DMSO. The experiments were performed in triplicate. The researchers then measured the absorbance at 490 nm and calculated the 50% cytotoxic concentration (CC50). At a high concentration of 600 μg/mL, LH capsules exhibited no obvious cytotoxicity to these two cells.
4.1.2. Chicken embryo experiment
The maximum nontoxic dose of chicken embryos for LH capsules was investigated (Li et al., 2014). Test drug solutions containing 1.00, 0.500, 0.250, 0.125, 0.0625, 0.0313, 0.0156, 0.00781, or 0.00390 g of drug per millilitre were inoculated into 10-day-old SPF chicken embryos, and each concentration was inoculated into five chicken embryo (0.1 mL/embryo). The inoculated chicken embryos were then placed in a 37 °C incubator for 96 h; those that died within 24 h were removed, and the death of the chicken embryos was recorded. The test results showed that the LH capsules at the above-listed concentrations exerted no visually harmful effects on chicken embryos.
4.2. Antiviral effects
4.2.1. SARS-COV-2
Li et al. found that LH capsules can inhibit the replication of SARS-COV-2 in Vero E6 cells (Li et al., 2020c). Vero E6 cells were infected with the 50% tissue culture infective dose (TCID50) of the virus and incubated with LH capsules at a concentration of 150 μg/mL, 300 μg/mL, or 600 μg/mL for 72 h. Through the cytopathic effect (CPE), LH capsules exhibited the ability to inhibit the replication of SARS-CoV-2 virus with an 50% Inhibition Concentration (IC50) value of 411.2 µg/mL. In addition, treatment with LH capsules after infection can also inhibit virus plaque formation in a dose-dependent manner. In the experiment, remdesivir was used as a positive control, and its IC50 value and total plaque formation inhibition were 0.651 µM and 5 µM, respectively. To further confirm the effectiveness of LH capsules in inhibiting SARS-CoV-2 virus replication, these researchers observed virus particles in ultrathin sections of infected cells under an electron microscope. The results showed that the virus spindle treated with LH capsules (600 µg/mL) was sharp, which is significantly different from the typical spherical shape of the viral particles.
4.2.2. IAV
4.2.2.4. 4.2.2.1 In vitro study
Yuewen Ding et al. used CPE and MTT assays to assess the ability of LH capsules to inhibit different virus strains of H7N9, H6N2, H9N2, and H3N2 (Ding et al., 2017). The IC50 values ranged from 0.2 to 2 mg/mL, and the selection index (SI) ranged from 1.59 to 15.85. Among these viruses, LH capsules exerted their best inhibitory effect on H3N2. In the plaque reduction experiment using H1N1 and H3N2, LH capsules also exerted a dose-dependent effect on the formation of viral plaques. Subsequently, to determine the specific steps through which LH capsules inhibit virus replication, the research group infected MDCK cells with H1N1. LH capsules (2 mg/mL) were added to the cells at 0, 2, 4, 6, 8 and 10 h after infection. Twelve hours after infection, the content of progeny virus in the cell supernatant was measured. Interestingly, the virus titre decreased significantly during 2 h of incubation with LH capsules, and the effect was not obvious during the rest of the incubation time. This study shows that LH capsules mainly inhibit virus replication at the early stage. The research group further studied the effect of LH capsules on the nuclear export of ribonucleoprotein (RNP) in human alveolar epithelial (A549) cells infected with H1N1. The results showed that LH capsules can inhibit virus-induced nuclear transcription factor kappa-B (NF-kB) activation within 24 h such that the influenza virus RNP complex is effectively retained in the nucleus of infected cells.
As detailed in the LH capsules patent (CN101862391B), the Klein-Defors suspension killing and infection experiment was used to determine the effect of the drug on virus killing (Li et al., 2014). Different concentrations of LH capsules were reacted with avian influenza H5N1 and H9N2 subtype viruses (9:1) for 10 min, and the results showed that the LH capsules at a concentration of 0.01 g/mL resulted in virus killing rates of 55.32% and 68.37%, respectively. At a concentration of 0.1–0.25 g/mL, the virus killing rate reached 99.9%, which indicates a significant virus killing effect. At 0.5–1 g/mL, the virus killing rate obtained with the LH capsules reached 100%, indicating a complete virus killing effect.
In vivo study: To determine the antiviral effect in vivo, mice were infected intranasally with 50 μL of H1N1 virus at 2 × 50% minimum lethal dose (MLD50). Each group of mice was orally administered 1300 and 650 mg/kg/day LH capsule solutions, and the control animals were treated with blank solvent only. The drug was administered twice a day (12 h apart) for 5 days, and each group consisted of three mice. At 2, 4, 6, and 8 days after infection, the lung virus titre in the infected mice was detected. Among the time points, the results showed that the lung titres of the mice treated with the two above-mentioned concentrations were decreased after 6 and 8 days of infection, and the effect obtained with 1300 mg/kg/day was more obvious.
As detailed in the patent application from Beijing Yiling Pharmaceutical Co., Ltd. (CN 104,825,622 A), an in vivo study of LH capsules was also completed (Zhang et al., 2015b). The mice were randomly divided into two groups administered LH capsules at a dose of 1300 mg/kg/day and 650 mg/kg/day, and the positive control group (ribavirin) was administered 75 mg/kg/day; some mice were also assigned to the Ma Xing Shi Gan decoction, Yinqiao Powder medicinal mixed dry powder, rhubarb, and negative groups. As a result, a total of eight groups were included in the experiment, and each group consisted of 10 female animals. Under mild ether anaesthesia, the mice in each group were infected with 50 μL of 50% lethal dose (LD50) H3N2 influenza virus H3N2 allantoic fluid. Two hours after the mice were infected with the virus, each mouse in the drug group was given 0.2 mL intragastrically, and the normal control group and the virus control group were given 0.2 mL of normal saline once a day for 5 consecutive days. On the 5th day after infection, three mice in each group were anaesthetized and killed, their lungs were dissected, the virus titre was measured, and the TCID50 value was calculated. The remaining mice in each group were sacrificed by anaesthesia on the 7th day after infection. The lung lesions were dissected, and the body and lung weights were measured. The experimental results showed that the two doses of LH capsules can reduce the increase in the lung index caused by virus infection in mice, and the difference was very significant (p< 0.01). The lung index of the other drug groups was not significantly different from that of the virus control group. In addition, the virus titre in the lung tissue of the mice administered LH capsules at doses of 1300 and 650 mg/kg/day was also significantly reduced (p< 0.05), whereas the other drugs exerted no significant inhibitory effect. In addition, the protective effect of LH capsules on the lethality of virus-infected mice was further studied. The mice were subjected to the same treatments and observed for 15 consecutive days. Among the various groups, the groups administered LH capsules at doses of 1300 and 650 mg/kg/day significantly prolonged the survival of the mice (p< 0.05), and the dose of 1300 mg/kg/day also significantly reduced the risk of death in mice (p< 0.05).
4.2.3. IBV
In addition, SARS-COV-2 and IAV were inhibited by the LH capsules. The study conducted by Zhang Huixin and colleagues confirmed that LH capsules also exerted a good inhibitory effect on IBV. MDCK monolayer cells on a 96-well plate were added to each well with 100 μL of different concentrations of the drugs and 50 μL of IBV strain (100 TCID50). After adsorption for 1 h, the medium was poured out, and 100 μL of maintenance solution was added. Each group was incubated in a 5% CO2 incubator at 37 °C for 4 to 5 days, and the experiment was terminated when the CPE of the virus control well reached 4+. The experiments showed that the LH capsules exert a strong inhibitory effect on IBV, with an IC50 of 0.056 mg/mL. Moreover, in the antiviral experiments performed with Hep-2 cells, LH capsules also exerted a constant effect on five other viruses, such as parainfluenza virus type 1 (HVJ) and respiratory syncytial virus (RSV).
The research conducted by Chunguang Yang et al. on the anti-IBV activities of LH capsules provides further details (Yang et al., 2020a). First, five different IBVs (multiplicity of infection (MOI) = 0.01) were seeded into a monolayer of confluent MDCK cells in a 96-well plate. After 2 h, the inoculum was replaced with a test medium containing a series of LH capsules or DMSO (less than 1%). The plates were incubated at 34 °C with 5% CO2 for 72 h. The inhibition rate was determined by the MTT method. As a result, the IC50 ranged from 0.228±0.150 to 0.754±0.161 mg/mL, and the SI ranged from 5.3 to 17.6. Subsequently, in the time course assay, LH capsules also inhibited early virus replication. Treatment with LH capsules at a dose of 0.6 mg/mL 0–4 h after infection can reduce IBV production, but the effects of the LH capsules was not sufficient after more than 6 h of treatment. To further identify the effect of LH capsules on IBV replication, the synthesis of viral nucleoprotein in infected cells was examined by immunofluorescence. The virus-infected cells were treated with 0.6 mg/mL LH capsules for 8 h. Compared with the DMSO treatment, LH capsules significantly reduced the synthesis of nucleoproteins. Through immunofluorescence experiments, the research groups also found that LH capsules can reduce the binding of the virus to MDCK cells.
4.3. Anti-inflammatory effect
4.3.1. SARS-COV-2
To determine the effect of LH capsules on the expression of cytokines and chemokines induced by SARS-CoV-2, Li Runfeng et al. measured the relative mRNA expression levels in Huh-7 monolayer cells. Huh-7 cells in a 12-well plate were washed with PBS and then exposed to SARS-CoV-2 at an MOI of 1 for 2 h at 37 °C. The inoculum was removed and replaced with the indicated concentrations of LH capsules, and the controls were subjected to mock treatment with DMEM supplemented with 2% foetal bovine serum (FBS). The cells were then harvested for RNA isolation and quantitative PCR (qPCR). The results showed that LH capsules inhibited the increase in the expression of tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6), C—C motif ligand 2/monocyte chemoattractant protein-1 (CCL-2/MCP-1), and C-X-C motif chemokine ligand 10/interferon gamma-induced protein 10 (CXCL-10/IP-10) in a concentration-dependent manner.
4.3.2. H1N1
The study conducted by Yuewen Ding et al. confirmed that LH capsules can also regulate cytokine production during H1N1 virus infection (Ding et al., 2017). A549 cells infected with H1N1 (MOI = 0.1) for 24 h were treated with LH capsules (1.5–3 mg/mL), and the mRNA levels of the cytokines/chemokines IL-6, interleukin-8 (IL-8), IP-10, TNF-α, MCP-1 were detected by qRT-PCR. Experimental data show that LH capsules treatment can significantly reduce the expression of the above-mentioned substances. Moreover, the analysis of lung inflammation in mice revealed that LH capsules can also strongly reduce the expression of proinflammatory cytokines (TNF-α and IL-6) and chemokines. The levels of IL-1β and IP-10 were also inhibited by LH capsules in a dose-dependent manner. Further histopathological research revealed that LH capsules treatment can improve vascular inflammation and cell exudate secretion caused by viral infection.
4.3.3. IBV
LH capsules also exerted better anti-inflammatory effects in IBV(Yang et al., 2020a). In the study conducted by Chunguang Yang, A549 cells were cultured in a 96-well plate at 37 °C with 5% CO2 and then infected with IBV (0.1 MOI) for 2 h. The inoculum was removed, and the cells were treated with 0.15, 0.3, and 0.6 mg/mL LH capsules. After 24 h of infection, total RNA was extracted, and cDNA was synthesized. A real-time PCR system was then used for the detection of inflammatory factor gene expression. The experimental results showed that the normal T cell expressed and secreted (RANTES), IL-6, IL-8, IP-10, TNF-α and MCP-1, macrophage inflammatory protein-1β (MIP-1β) and interferon-λ1 (IFN-λ1) mRNA levels were decreased in the cells treated with ≥0.15 mg/mL LH capsules. The mRNA levels of most inflammatory factors showed a dose-dependent decrease after treatment with LH capsules. Further pathological studies revealed that LH capsules at doses of 100–400 mg/kg/day can reduce pathological changes in the lungs. LH capsules alone do not exert a strong effect on IBV. The research group further studied the combination of LH capsules and other drugs. The results confirmed that the combination of LH capsules (200 mg/kg/day) and oseltamivir (2 mg/kg/day) significantly reduced the level of inflammation compared with the decreases obtained with either drug alone. In addition, the virus titres in lung tissue of mice was also decreased by this combination treatment.
5. LH capsules currently under clinical studies
LH capsules were developed during the SARS epidemic in 2003 and have been used for nearly 20 years. Currently, LH capsules have been or are being used in 14 registered clinical trials globally (Table 3 ), including clinical trials against influenza, COVID-19, non-influenza viral pneumonia, and acute exacerbation of chronic obstructive pulmonary disease (AECOPD). Here, we summarize the Phase III clinical studies of COVID-19 and the phase II clinical studies of uncomplicated influenza that have been or are being conducted abroad. In addition, Among the 12 domestic clinical trials, we detailed the two clinical trials related to COVID-19 and the results from two experiments, resulting in a total of four trials.
Table 3.
Clinical studies.
| No. | Registration number | Registration date | Researcher unit | Registration topic |
|---|---|---|---|---|
| Clinical trials conducted outside of China | ||||
| 1 | NCT04433013 | June 16, 2020 | Nanyang Technological University, Ministry of Health, Singapore | A Randomized Controlled Trial Assessing the Efficacy of Lianhua Qingwen as an Adjuvant Treatment for Patients with Mild Symptoms of COVID-19 |
| 2 | NCT02867358 | August 15, 2016 | Yiling Pharmaceutical Inc. | A Multi-centre, Randomized, Double-blind, and Placebo-controlled Phase II Clinical Study to Investigate the Safety and Efficacy of Two Doses of KT07 Compared to Placebo in Subjects with Acute Uncomplicated Influenza |
| Clinical research conducted in China | ||||
| 1 | ChiCTR2000035046 | July 29, 2020 | 920th Hospital of the PLA Joint Logistics Support Force | Efficacy and Outcomes of Lian-Hua Qing-Wen Capsules in the Treatment of Novel Coronavirus Pneumonia (COVID-19): A Medical Record-Based Retrospective Study |
| 2 | ChiCTR2000029434 | February 01, 2020 | Hebei Yiling Hospital, Renmin Hospital of Wuhan University | A Randomized, Open-Label, Blank-Controlled Trial of Lian-Hua Qing-Wen Capsules/Granules in the Treatment Of Novel Coronavirus Pneumonia (COVID-19) |
| 3 | ChiCTR2000029433 | February 01, 2020 | Hebei Yiling Hospital, Renmin Hospital of Wuhan University | A Randomized, Open-label, Blank-controlled Trial of Lian-Hua Qing-Wen Capsules/Granules in the Treatment of Suspected Novel Coronavirus Pneumonia (COVID-19) |
| 4 | ChiCTR1900028390 | December 20, 2019 | First Teaching Hospital of Tianjin University of TCM | A Multi-centre, Randomized, Double-blind, Parallel-controlled Trial of the Efficacy and Safety of Lian-Hua Qing-Wen Granules in the Treatment of Influenza in Children |
| 5 | ChiCTR1900024082 | June 24, 2019 | Hebei Yiling Hospital | Study of the Pharmacokinetics of Lian-Hua Qing-Wen Capsules in Healthy Human Beings |
| 6 | ChiCTR1900021460 | February 22, 2019 | Hebei Yiling Hospital | Pharmacokinetics of Lianhua Qingwen Capsules in the Healthy Human Body |
| 7 | ChiCTR1900020551 | January 08, 2019 | Guangdong Provincial Hospital of TCM (Second Affiliated Hospital of Guangzhou University of Chinese Medicine) | Lianhuaqingwen Capsule in the Treatment of Senile Mild Influenza: A Prospective, Randomized, Double-blind, Positive Drug Controlled Trial |
| 8 | ChiCTR-IPR-16,007,773 | January 15, 2016 | Beijing Ditan Hospital Capital Medical University | Randomized, Double-blind, Placebo - controlled, Multi-centre Clinical Study of Lianhuaqinwen Grain for the Treatment of Viral Pneumonia without Influenza |
| 9 | ChiCTR-IOR-15,006,144 | March 13, 2015 | Guangdong Provincial Hospital of TCM | The Influence of Lianhuaqingwen Capsule after Bone Marrow Transplantation in Patients with Respiratory Viral Infections Evaluation |
| 10 | ChiCTR-TRC-13,003,240 | April 09, 2013 | Huashan Hospital, Fudan University | The Auxiliary Effect of Lianhuaqingwen (LHQW) in Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD): A Randomized, Controlled Clinical Trial |
| 11 | ChiCTR-TRC-09,000,590 | October 31, 2009 | Beijing Youan Hospital, Capital Medical University | Lianhua Qingwen Capsule for the Treatment of H1N1 Flu: A Double Blinding and Multi-centre, Randomized Placebo-Controlled Trial |
| 12 | ChiCTR-TRC-09,000,589 | October 31, 2009 | Beijing Youan Hospital, Capital Medical University | Lianhua Qingwen Capsule for the Treatment of H1N1 Flu: A Double Blinding, Double Dummy and Multi-centre Randomized Control Trial |
5.1. Clinical trials conducted in China
5.1.1. COVID-19 (Singapore)
To evaluate the efficacy of LH capsules on patients with mild COVID-19, Nanyang Technological University and the Ministry of Health of Singapore jointly launched a Phase III clinical trial of Lianhua Qingwen (NCT04433013). This a randomized, parallel interventional study recruited a total of 300 men and women aged older than 21 years, who were divided into the treatment and control groups and treated for 8 days. The treatment group will take four LH capsules three times a day after meals, whereas the control group will take placebo capsules using the same schedule. The main outcome measure of the experiment will be the proportion of COVID-19 patients who became negative. The secondary measures will be the number of days required for the drug to relieve clinical symptoms, the proportion of patients with mild disease that progresses to moderate or severe and the proportion of positive patients with a Ct value>30. The study is expected to be completed in February 2021.
5.1.2. Acute uncomplicated influenza (United States)
As early as 2016, the FDA launched a multicentre, randomized, double-blind, and placebo-controlled phase II clinical trial of LH capsules (NCT02867358). The purpose of this study was to investigate the clinical efficacy of the drug in alleviating fever and other symptoms (including nasal congestion, sore throat, cough, aches and pains, fatigue, headache, chills or sweating) in patients with acute uncomplicated influenza. The trial recruited a total of 420 male and female patients between the ages of 18 and 65, and these patients were equally divided into the high-dose LH capsule-treated group, the low-dose LH capsule-treated group, and the control group. The patients in the high-dose group took six LH capsules twice a day, and the patients in the low-dose group took four LH capsules and two placebos twice a day. The control patients took the same dose of placebo. All the groups were treated for 19 days. Finally, the improvement in reducing the duration of all disease symptoms compared with the placebo was the primary outcome measure, and the reduction of the duration of individual symptoms and the quality of life of the subjects were secondary measures. The shedding of influenza virus was observed on days 1, 3 and 6. At present, no results have been disclosed.
5.2. Clinical research conducted in China
5.2.1. COVID-19
Two clinical trials of LH capsules for the treatment of COVID-19 are being conducted in China. One of these trials aims to explore the clinical efficacy of LH capsules in the treatment of COVID-19 and the resulting prognosis (ChiCTR2000035046). Unfortunately, the trial has not yet been approved by the ethics committee. Another study aims to determine the safety and effectiveness of LH capsules in patients with COVID-19. This prospective multicentre open-label randomized controlled trial is being performed with patients with confirmed COVID-19 (Hu et al., 2020). A total of 284 patients aged older than 18 years were randomly assigned to receive the conventional treatment alone or in combination with LH capsules (four capsules, three times a day) for 14 days. The conventional treatment usually included adjuvant therapy, such as oxygen therapy, antiviral drugs and symptomatic treatment. The main endpoint was the recovery rate of the patient's symptoms (fever, fatigue, and cough). The recovery rate of the treatment group was significantly higher than that of the conventional group (91.5% vs. 82.4%, p = 0.022), and the median symptom recovery time of the treatment group was clearly shorter (7 days vs. 10 days, p< 0.001). The times to the recovery of fever (2 vs. 3 days), fatigue (3 vs. 6 days) and cough (7 vs. 10 days) in the treatment group were also distinctly reduced compared with those found with the control group (all p< 0.001). The improvement of chest computed tomographic manifestations (83.8% vs. 64.1%, p< 0.001) and clinical cure rate (78.9% vs. 66.2%, p = 0.017) obtained with the treatment group were also higher than those obtained with the control group. However, no difference in the rate of severe cases or the viral RNA conversion rate was found between the two groups (both p>0.05). In addition, no serious adverse reactions were observed during the experiment.
5.2.2. AECOPD
Liang Dong et al. conducted a clinical trial (ChiCTR-TRC-13,003,240) to explore the clinical efficacy of LH capsules on patients with AECOPD and the effects on the IL-8 and TNF-α levels (Dong et al., 2014). One hundred subjects were randomly assigned to the LH capsule group and the routine group at a ratio of 1:1, and these two groups were administered LH capsules (1.4 g per dose, three times per day, treatment duration of 7 days) combined with conventional Western medicine and conventional Western medicine treatment alone. Each group was separated into low-risk and high-risk subgroups. The conventional treatments included the following: inhaled medications, salmeterol/fluticasone (250/50 μg); bronchodilators, theophylline; expectorant antispasmodic agent, ambroxol; antibacterial agents, β-lactams; if allergic to β-lactams, fluoroquinolones were administered; and controlled therapy oxygen. The chronic obstructive pulmonary disease (COPD) assessment test (CAT) scores of the LH capsule group and the high-risk subgroup improved significantly starting on day 5, but the other groups only showed improvement after the treatment was completed. After treatment, the expression levels of IL-8, TNF-α, IL-17 and IL-23 in the sputum and the expression levels of IL-8 and IL-17 in the blood were significantly reduced. Similar results were also found in the subgroups. These data indicated that LH capsules can accelerate the improvement of patients with AECOPD, particularly the high-risk patients, and the underlying mechanism of action might be related to reductions in inflammatory mediator release.
5.2.3. H1N1
H1N1 virus infection with severe complications is associated with a high risk. To evaluate the efficacy and safety of LH capsules for the treatment of patients with H1N1, China has performed related clinical studies (ChiCTR-TRC-09000589 (Duan et al., 2011)). A total of 244 patients diagnosed with H1N1 virus infection by real-time reverse transcription PCR (RT-PCR) and aged between 16 and 65 years were randomly divided into two groups, each with 122 cases. The two groups received LH capsule or oseltamivir treatment for 5 days and were observed for 7 days. The primary endpoint was the end of the disease course. Two hundred forty (98.36%) of the 244 patients completed the study, and their median age was 21 years. The median duration (69 h vs. 85 h, p> 0.05) and the virus clearance time (103 h vs. 96 h, p> 0.05) did not show significant differences between the LH capsule- and oseltamivir-treated groups. However, it is worth noting that the LH capsules significantly reduced the severity of the disease and the duration of symptoms, including fever, cough, sore throat, and fatigue (p< 0.05). Compared with oseltamivir, LH capsules achieved similar therapeutic effects, including a reduced disease duration and a shorter virus clearance time, and no serious adverse reactions were detected. Therefore, LH capsules might be an alternative treatment for H1N1 virus infection.
6. Molecular mechanism
6.1. Antiviral effect
SARS-COV-2 is a (+) single-stranded RNA virus (Chen et al., 2020a). The virus particles are round or elliptical with a diameter of approximately 80–120 nm (Chen et al., 2020b) and contain three main proteins, namely, the envelope (E), membrane (M) and spike (S) proteins (He et al., 2005; Jiang et al., 2005; Leung et al., 2004; Schoeman and Fielding, 2019; Shang et al., 2020). The S protein binds to the recognized receptor angiotensin-converting enzyme 2 (ACE2) of SARS-CoV-2 in host cells to allow the virus to be absorbed and internalized into the cytoplasm (Ali and Vijayan, 2020; Wu et al., 2020a; Yi et al., 2020; Zost et al., 2020). Cell lines stably expressing ACE2 are highly transduced by the SARS-CoV-2 virus, and treatment with anti-ACE2 antibodies can reduce virus entry. These findings make the ACE2-S protein interaction an ideal target for antiviral therapy (McCallum et al., 2020). The Chinese scientific research team uses Cytoscape software to construct a model of the interactions between the “drug-component-target-disease” network and potential targets and predicts the mechanism of action through enrichment analysis. This model combines the main active components of LH capsules with ACE2 and was tested by molecular docking, and the results showed that compounds 3 and 48 exhibit a good binding ability to ACE2. Surprisingly, the binding capacity of these natural compounds is better than those of lopinavir, ritonavir, ribavirin and other small-molecule drugs (Ling et al., 2020), which suggests that LH capsules might exert their antiviral effects by acting on the ACE2 receptor (Fig 4 ).
Fig 4.
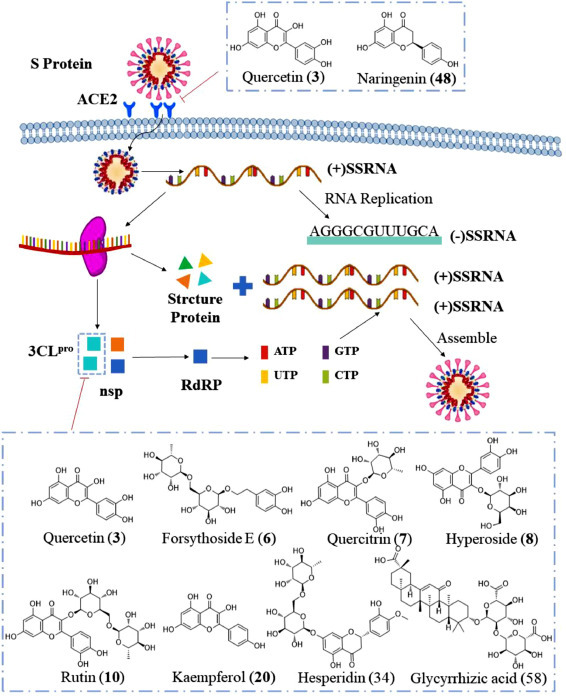
Antiviral mechanism of LH capsule.
After SARS-COV-2 enters the cell, the replicase gene is translated from the virion genomic RNA. The replicase gene encodes two large open reading frames (ORFs), which can directly translate two polyproteins, pp1a and pp1ab (Florindo et al., 2020; Perlman and Netland, 2009). These two polyproteins are the precursors of non-structural proteins (nsps), which release 16 nsps (nsp1-nsp16) with different activities after autohydrolysis and shearing. Among these nsps, nsp3 encodes papain-like proteases (PLpro) (Báez-Santos et al., 2015), whereas nsp5 encodes 3CLpro (Marra et al., 2003; Vuong et al., 2020; Ziebuhr et al., 2000). PLpro cleaves the nsp1/2, nsp2/3, and nsp3/4 boundaries, whereas 3CLpro is responsible for the remaining 11 cleavage events (nsp4-nsp16) (Wang et al., 2020b). nsp4-nsp16 are responsible for the important life processes of protein translation, cleavage, modification and nucleic acid synthesis. For example, nsp12 can encode the RNA-dependent RNA polymerase (RdRp), which is responsible for the genome replication process of RNA viruses (Li et al., 2020a; Liang et al., 2020; Wang et al., 2020b). Therefore, the inhibition of 3CLpro can prevent the processes of RNA replication and transcription, which can effectively block the proliferation of the virus (Liu et al., 2009, 2020d).
The molecular docking studies performed by Ling Xiaoying and Cheng-hao Ye showed that compounds 3, 6, 8, 10, and 20 in the LH capsules exhibit a good binding ability to 3CLpro (PDB: 6LU7) (Ling et al., 2020; Ye. et al., 2020b), and the binding energies are −7.6, −8.2, −8.4, −8.9, and −7.8 kcal∙mol−1, respectively. According to published reports, the binding energy of lopinavir against SARS-CoV-2 can only reach −7.3 kcal∙mol−1. Among these compounds, 3 directly interacts with residues LEU141 and SER144, as shown in Fig 5 A. Compound 6 and residues LEU141, SER144, CYS145, HIS 163, GLU166, and THR190 formed an excellent hydrogen bonding force (Fig 5 B). The interaction between 8 and 3CLpro is achieved through TYR54, ASN142, CLY143, GLU166 and other residues, as shown in Fig 5 C. Compound 10 was found to be superior because it binds most closely to GLY143, SER144 and GLU166, with a binding affinity of −8.9 kcal∙mol−1(Fig 5 D). It is worth noting that although the molecular docking results show that 20 only binds to residues LEU141 and TYP54, it still exhibits a high binding energy (Fig 5 E).
Fig 5.
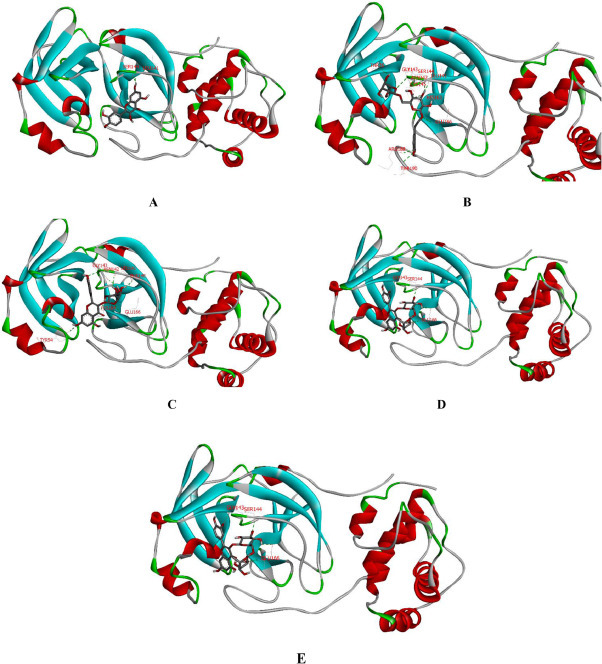
Binding patterns of ligands, including 3 (A), 6 (B), 8 (C), 10 (D), and 20 (E), with 3CLpro determined by molecular docking.
In addition, the Atanu Singha Roy team docked 17 natural compounds with the 3CLpro (PDB: 6Y84) (Das et al., 2020). The detailed results are shown in Fig 6 A- 6 C. Compound 7 is stabilized by hydrogen bonding with THR25, GLY143 and GLU166 and amide-π stacking with THR45 along with other interactions, as depicted in Fig 6 A. Hesperidin (34) interacts through hydrogen bonding with THR24, THR25, THR45, HIS4, SER46, and CYS145, amide-π stacked interactions with THR45, and π-alkyl interactions with MET49 and CYS145 (Fig 6 B). Compound 58 forms a hydrogen bond with THR190, an alkyl hydrophobic bond with MET49, CYS145 and MET165, and π-alkyl interactions with HIS41 (Fig 6 C). Therefore, according to above results, LH capsules may indeed have the ability to inhibit SARS-COV-2 replication.
Fig 6.
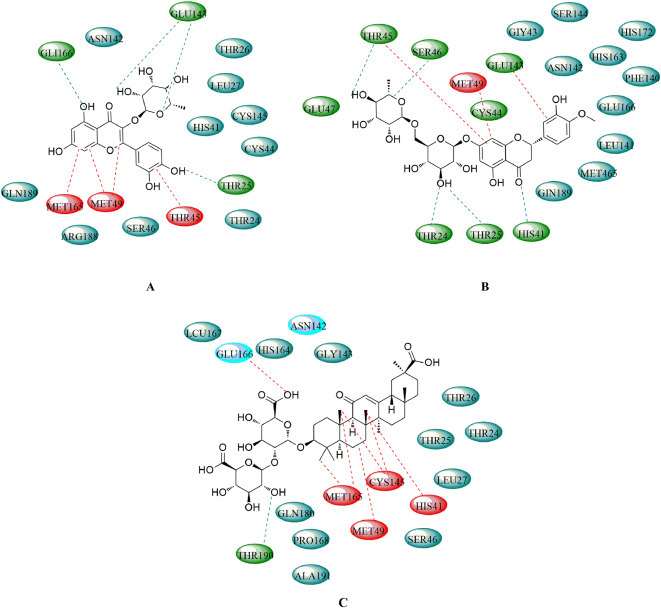
Schematic diagram of the interactions between SARS-CoV-2 3CLpro and compounds 7 (A), 34 (B), and 58 (C).
6.2. Inhibition of cytokine storm syndrome (CSS) through the JAK-STAT, PI3K and MAPK pathways
Compared with other coronaviruses, SARS-CoV-2 has a relatively higher mortality rate, which is related to the existence of a cytokine storm, and this finding might indicate that excessive inflammation plays a role in the pathogenesis of COVID-19. A cytokine storm is an excessive defence response of the host that involves the secretion of vascular endothelial growth factor (VEGF), MCP-1 and IL-8 (Tanaka et al., 2016), can easily cause multifunctional organ damage (MOF) and ARDS (Hu et al., 2020) and is also an important factor for the rapid development of COVID-19. Therefore, inhibiting the occurrence of cytokine storms is one of the important measures for rescuing critically ill patients.
IL-6 is one of the key cytokines involved in cytokine storms induced by infection (Hu et al., 2020). In the “Diagnosis and Treatment Plan for New Coronavirus Pneumonia’’, tocilizumab was suggested as an immunotherapy drug for critically ill patients, and this drug has been approved by the US FDA for the treatment of CSSs (Magro, 2020). IL-6 signals through the cell surface type I cytokine receptor complex and triggers downstream signal transduction and gene expression. The complex is composed of IL-6 receptor (IL-6R) and signal transduction glycoprotein 130 (gp-130). IL-6R exists not only in a membrane-bound form (mIL-6R) but also in a soluble form (sIL-6R). According to different receptors, IL-6 signal conduction can be divided into classic cis signalling and trans signalling (Kang et al., 2019). In classical signal transduction, IL-6 forms a complex with mIL-6R and gp-130 to induce downstream reactions. Because the expression of mIL-6R is limited to immune cells (Moore and June 2020), it mainly plays a leading anti-inflammatory effect at low concentrations of IL-6. When IL-6 levels increase, IL-6 is attracted to cells that do not express mIL-6R because gp-130 is almost universally expressed in most tissues. Therefore, sIL-6R can activate the proinflammatory response in the body (Braun et al., 2016; Zhang et al., 2020c). The pathways downstream of IL-6 are mainly Janus kinase/signal transducers and activators of transcription (JAK/STAT) and the interconnected phosphoinositide 3-kinases (PI3K) and mitogen-activated protein kinase (MAPK) pathways.
Many pharmacological studies have shown that LH capsules efficiently inhibit the expression of IL-6 (Ding et al., 2017; Hu et al., 2020). Compound 12 is a representative component of the main herb forsythia in LH capsules (Yang et al., 2017) and exerts powerful anti-inflammatory, antibacterial and antiviral effects. In addition, as the only control ingredient in the pharmacopoeia, the presence of 12 in LH capsules can be guaranteed. Here, we elucidate the molecular mechanisms of 12 that inhibit IL-6-induced CSS through the JAK-STAT, PI3K and MAPK pathways (Fig 7 ).
Fig 7.
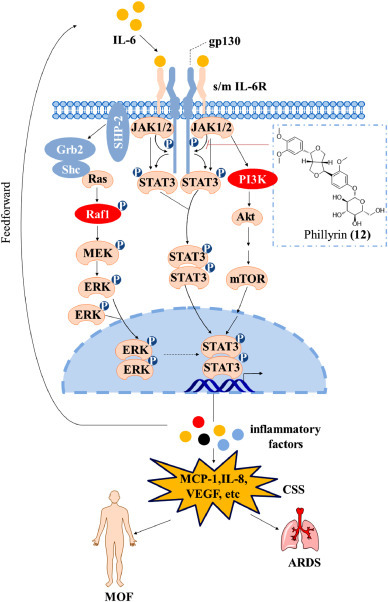
Mechanism through which LH capsules inhibit CSS.
The JAK/STAT cascade provides a direct mechanism for converting extracellular signals into transcriptional responses and is one of the few multi-effect cascade signals (Rawlings et al., 2004). This pathway is important for a series of cytokines and growth factors (including IL-6) in mammals. When the IL-6 complex is exposed, Janus kinase 1/2 (JAK1/2) comes in close proximity, which allows dephosphorylation. JAK1/2 then phosphorylate each other on tyrosine residues located in regions called activation loops through a process called transphosphorylation, which increases the activity of their kinase domains (Jatiani et al., 2010). Activated JAK1/2 then phosphorylates tyrosine residues on the receptor to form a binding site for proteins with Src homology 2 (SH2) domains (Aaronson and Horvath, 2002; Jatiani et al., 2010). In addition, signal transducer and activator of transcription 3 (STAT3) uses its SH2 domain to bind to the phosphorylated tyrosine on the receptor and is then phosphorylated by JAK1/2 tyrosine, which results in the dissociation of STAT3 from the receptor (Sansone and Bromberg, 2012; Schindler et al., 2007). Activated STAT3 form dimers, and these dimers are transported to the nucleus to induce the expression of genes encoding IL-6 and other cytokines(Rawlings et al., 2004). Therefore, a large number of cytokines can be produced through the JAK-STAT pathway. These cytokines can also further activate STAT3, which results in the triggering of an inflammatory cytokine storm (Yu et al., 2009).
Although JAK/STAT signalling is the main pathway mediated by IL-6R, this pathway can also be integrated with other pathways. The most typical interaction is related to the receptor tyrosine kinase mitogen-activated protein kinase (MAPK) pathway (Rawlings et al., 2004). Activated JAK1/2 can phosphorylate tyrosine kinase on the receptor. The activated receptor can be used as a docking site for SH2 adaptor proteins from other signalling pathways, including src homology region 2 domain-containing phosphatase-2 (SHP-2) and SHC-transforming protein 1 (Shc). The adaptor protein stimulates Ras GTPase (Ras) by recruiting growth factor receptor-bound protein 2 (Grb2) adaptors. Ras further activates the protein kinase activity of Raf kinase, and Raf kinase then phosphorylates and activates mitogen-activated protein kinase (MEK) and extracellular signal-regulated kinase (ERK) to complete the MAPK cascade reaction. Activated ERK forms a dimer and enters the nucleus, which can increase the transcription of STAT genes and thereby induce the secretion of inflammatory cytokines. JAK-STAT signal transduction can also be integrated with the PI3K pathway (Foster et al., 2003). When the receptor is activated by JAK, the PI3K protein with the SH2 domain binds to the phosphorylated receptor (Fry, 1994; Rawlings et al., 2004). Activated PI3K activates protein kinase B (PKB, Akt), which results in its localization on the plasma membrane. Akt stimulates the downstream effect of mTOR through multiple levels (Liu et al., 2009). The translocation of mammalian target of rapamycin (mTOR) into the nucleus can also stimulate STAT3 to produce cytokines. In a lipopolysaccharide-induced inflammation model, Xiaolong Pan et al. found that 12 can inhibit the activation of STAT3 and the secretion of inflammatory factors, including IL-6, by weakening the phosphorylation of JAK1/2 (Pan et al., 2014). This finding indicates that LH capsules can potentially inhibit CSSs through three pathways, namely, the JAK-STAT pathway and its related PI3K and MAPK pathways.
7. Conclusion
TCM has a long history and involves holistic concepts, including the balance of yin and yang and syndrome differentiation and treatment, and is thus quite different from Western medicine (Ren et al., 2020; Zhang et al., 2017; Zhao et al., 2020). Due to the unique knowledge and experience regarding TCM in the prevention and control of diseases, TCM has played an indispensable role in the prevention and treatment of several epidemics (Ji et al., 2020). During the SARS epidemic in 2003, TCM intervention achieved remarkable results (Chen and Nakamura, 2004). COVID-19 was rapidly controlled in China, and the use of TCM has also played a vital role (Panyod et al., 2020; Ren et al., 2020; Ye et al., 2020; Zhang et al., 2020b; Zhuang et al., 2020). According to statistics, the proportion of TCM in the treatment of confirmed cases in China has reached 92%, and the usage and total effective rate of TCM in confirmed cases in Hubei Province have exceeded 90%. Moreover, during the treatment of COVID-19, China has also provided experience regarding the use of traditional medicine to Italy, Germany, Japan, South Korea, Pakistan and other countries. In addition to China, the Japanese Traditional Chinese Medicine Association advised that COVID-19 patients take Kampo medicines, including Maxing Shigan Tang, depending on their symptoms, and India's AYUSH Ministry also recommends the use of turmeric, knotweed, liquorice and other botanicals to prevent and treat COVID-19 by reducing the risk of infection and inducing anti-viral effects (Kumar et al., 2020). The experience regarding the treatment of COVID-19 has also proven that the use of TCM exerts significant effects on the time of nucleic acid conversion and CT image improvement rate. It is an important way for improving the cure rate, shorten the course of the disease, delay the progression of the disease, and reduce the mortality rate (Ni et al., 2020). It is worth recognizing that LH capsules are the most widely used TCM for the treatment of COVID-19. In the Diagnosis and Treatment Guidelines of 2019 New Coronavirus Pneumonia promulgated by the General Office of the NHC and the State Administration of TCM, LH capsules were recommended for individuals in the medical observation period.
Based on the results from pharmacological trials and clinical data, LH capsules were approved for the treatment of patients with mild and common COVID-19 in on April 12, 2020. Currently, LH capsules have been approved for marketing in the Philippines, Brazil, Indonesia, Canada, Mozambique, Romania, Thailand, Ecuador, Singapore, and Laos. Registration began in more than 30 countries around the world. In this article, we reviewed the composition and manufacturing procedure, QC, active ingredients, pharmacology and clinical trials of LH capsules. At present, the material basis and quality standards of LH capsules are lacking, but these capsules contain more than 300 compounds from 13 types of medicinal materials. However, only 74 have been isolated and identified so far; thus, the material basis of these capsules still needs further study. In terms of quality standards, LH capsules are similar to other TCMs. Under the content determination item in the 2015 Chinese Pharmacopoeia, only the main active substance PHN is studied. The quality of the medicine cannot be well controlled. Second, research on fingerprints has not been performed with respect to quality standards. The fingerprint spectrum can reflect the type and quantity of the chemical components contained in the capsules, the overall description and the evaluation of the drug quality. Therefore, follow-up studies are needed to further strengthen and improve the research.
The pharmacological results show that LH capsules have no obvious side effects and exert good anti-inflammatory and antiviral effects on SARS-COV-2, IAV and IBV (Wei et al., 2020). Research on SARS-COV-2 has shown that LH capsules can inhibit virus replication and change the virus shape. Moreover, these capsules can exert an anti-inflammatory effect by inhibiting the expression of TNF-α, IL-6, CCL-2/MCP-1 and other inflammatory factors in a dose-dependent manner. To date, four clinical trials related to COVID-19 are registered, but the results from only one trial have been disclosed. The results show that the use of LH capsules in combination with conventional treatment methods, such as antiviral drugs and oxygen therapy, can effectively improve the clinical cure rate and exert obvious therapeutic effects on symptoms such as fever, fatigue, and cough (Liu et al., 2020b, 2020c; Ping et al., 2016; Xiao et al., 2020; Zhang et al., 2020d). This finding might be related to the antiviral activity of LH capsules and their anti-inflammatory effects (Li et al., 2020d). In addition, the experimental results show that LH capsules have a good safety profile. It is worth noting that no significant differences in the conversion rate of severe cases and the negative rate of virus detection were found between the two groups of patients. However, whether longer administration times translate into greater efficacy is worth further research. Due to the urgency regarding the prevention and control of the SARS-CoV-2 epidemic, this study cannot be double-blind. Under limited conditions, an objective, randomized parallel controlled trial design was adopted. In the future, a double-blind, prospective randomized controlled trial is still needed, and this trial should be promoted as a global multicentre clinical trial that includes as many overseas regions as much as possible to fully evaluate the efficacy of LH capsules in a larger patient population.
In addition, LH capsules might exert an anti-SASR-CoV-2 effect by inducing anti-viral effects and inhibiting inflammatory cytokine storms. Specifically, quercetin and naringenin in LH capsules can be docked with ACE2 to prevent its binding to the S protein. As demonstrated by molecular docking, forsythoside E, rutin and six other active substances can be well docked with SARS-COV-2 3CLpro to inhibit virus replication (Zhang et al., 2020a). A study revealed that the force for the binding between rutin and 3CLpro is markedly higher than that for the binding between chloroquine and hydroxychloroquine(Mt et al., 2020). As part of the anti-inflammatory mechanisms, compound 12 in LH capsules can inhibit the inflammatory cytokine storm by inhibiting the phosphorylation of JAK1/2, which is downstream of IL-6 (Billing et al., 2019). IL-6 can be used as a biomarker of the severity and prognosis related to cytokine storms (Del Valle et al., 2020; Guo et al., 2020; Liu et al., 2020a), and its expression is higher than those of TNF-α and IL-1. High concentrations of IL-6 can induce various pathological functions related to thrombosis, vascular leakage and myocardial dysfunction, and these effects lead to tissue hypoxia, hypotension, multiple organ dysfunction, and diffuse intravascular coagulation. JAK-STAT is an important pathway for the release of a series of inflammatory factors, including IL-6. In addition, JAK can affect the MAPK and PI3K pathways, which are also downstream of IL-6 (Tong et al., 2020). However, due to its large number of compounds, a TCM exerts its effects through multiple targets and multiple pathways, and LH capsules are not an exception. Therefore, the relevant mechanisms need to be further improved and experimentally verified. For example, studies have shown that quercetin can inhibit the invasion of viruses by inhibiting Ca2+ channels.
Last but not least, although previous studies have proven that LH capsules exert a certain effect on COVID-19, these capsules should be taken under the guidance of a pharmacist. According to the theory of Chinese medicine and the drug property of LH capsules, individuals with colds or with a weak spleen or stomach should pay attention to the use of these drugs.
CRediT authorship contribution statement
Chengyuan Liang: Conceptualization, Writing - review & editing, Supervision, Project administration, Funding acquisition. Nan Hui: Conceptualization, Writing - original draft, Visualization. Yuzhi Liu: Writing - original draft, Writing - review & editing. Guaiping Qiao: Conceptualization, Investigation. Juan Li: Conceptualization, Resources. Lei Tian: Visualization. Xingke Ju: Writing - review & editing. Minyi Jia: Writing - original draft. Hong Liu: Project administration. Wenqiang Cao: Project administration. Pengcheng Yu: Supervision. Han Li: Project administration. Xiaodong Ren: Visualization, Project administration.
Declaration of Competing Interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81602967, 81803784), the China Postdoctoral Science Foundation (Grant No. 2016M592898XB, 2019M663921XB), the Basic Research Program of Natural Science of Shaanxi Province (Grant No. 2019JQ-779), the Basic Research Plan of the Education Department of Shaanxi Province (Grant No.19JC006), and the College Students’ Innovative Entrepreneurial Training Program (Grant No. 201510708172, 201610708019,2019107080827).
References
- Aaronson D.S., Horvath C.M. A road map for those who don't know JAK-STAT. Science. 2002;296:1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- Ali A., Vijayan R. Dynamics of the ACE2-SARS-CoV-2/SARS-CoV spike protein interface reveal unique mechanisms. Sci. Rep. 2020;10:14214. doi: 10.1038/s41598-020-71188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin S.A., Jha T. Fight against novel coronavirus: a perspective of medicinal chemists. Eur. J. Med. Chem. 2020;201 doi: 10.1016/j.ejmech.2020.112559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arase Y., Ikeda K., Murashima N., Chayama K., Tsubota A., Koida I., Suzuki Y., Saitoh S., Kobayashi M., Kumada H. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 1997;79:1494–1500. doi: 10.1002/(sici)1097-0142(19970415)79:8<1494::aid-cncr8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Baba M., Shigeta S. Antiviral activity of glycyrrhizin against varicella-zoster virus in vitro. Antiviral Res. 1987;7:99–107. doi: 10.1016/0166-3542(87)90025-8. [DOI] [PubMed] [Google Scholar]
- Báez-Santos Y.M., St John S.E., Mesecar A.D. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltina L.A., Kondratenko R.M., Baltina L.A., Jr., Plyasunova O.A., Pokrovskii A.G., Tolstikov G.A. Prospects for the creation of new antiviral drugs based on glycyrrhizic acid and its derivatives (a review) Pharm. Chem. J. 2009;43:539–548. doi: 10.1007/s11094-010-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz G.L., Lowrey A.J., Horne D.C., Nguyen V., Satterfield A.R., Ross T.D., Harrod A.E., Uchakina O.N., McKallip R.J. Using glycyrrhizic acid to target sumoylation processes during Epstein-Barr virus latency. PLoS One. 2019;14 doi: 10.1371/journal.pone.0217578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi D. Study on chemical constituents of Lianhua Qingwen Capsules (I) Chin. Herb. Med. 2018;49:795–800. [Google Scholar]
- Billing U., Jetka T., Nortmann L., Wundrack N., Komorowski M., Waldherr S., Schaper F., Dittrich A. Robustness and information transfer within IL-6-induced JAK/STAT signalling. Comun. Biol. 2019;2:27. doi: 10.1038/s42003-018-0259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun G.S., Nagayama Y., Maruta Y., Heymann F., van Roeyen C.R., Klinkhammer B.M., Boor P., Villa L., Salant D.J., Raffetseder U., Rose-John S., Ostendorf T., Floege J. IL-6 trans-signaling drives murine crescentic GN. J. Am. Soc. Nephrol. 2016;27:132–142. doi: 10.1681/ASN.2014111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., Xing F., Liu J., Yip C.C., Poon R.W., Tsoi H.W., Lo S.K., Chan K.H., Poon V.K., Chan W.M., Ip J.D., Cai J.P., Cheng V.C., Chen H., Hui C.K., Yuen K.Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Tian E.K., He B., Tian L., Han R., Wang S., Xiang Q., Zhang S., El Arnaout T., Cheng W. Overview of lethal human coronaviruses. Signal Transduct. Target. Ther. 2020;5:89. doi: 10.1038/s41392-020-0190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.C., Chen H.Y., Shiao M.S., Lin Y.L., Kuo Y.H., Ou J.C. Inhibition of low density lipoprotein oxidation by tetrahydrofurofuran lignans from Forsythia suspensa and Magnolia coco. Planta Med. 1999;65:709–711. doi: 10.1055/s-1999-14093. [DOI] [PubMed] [Google Scholar]
- Chen D., Xiong Y., Wang L., Lv B., Lin Y. Characteristics of emodin on modulating the contractility of jejunal smooth muscle. Can. J. Physiol. Pharmacol. 2012;90:455–462. doi: 10.1139/y2012-004. [DOI] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Nakamura T. Statistical evidence for the usefulness of Chinese medicine in the treatment of SARS. Phytother. Res. 2004;18:592–594. doi: 10.1002/ptr.1485. [DOI] [PubMed] [Google Scholar]
- Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S., Kao R.Y., Poon L.L., Wong C.L., Guan Y., Peiris J.S., Yuen K.Y. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crance J.M., Lévêque F., Biziagos E., van Cuyck-Gandré H., Jouan A., Deloince R. Studies on mechanism of action of glycyrrhizin against hepatitis A virus replication in vitro. Antiviral Res. 1994;23:63–76. doi: 10.1016/0166-3542(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Cristians S., Bye R., Navarrete A., Mata R. Gastroprotective effect of Hintonia latiflora and Hintonia standleyana aqueous extracts and compounds. J. Ethnopharmacol. 2013;145:530–535. doi: 10.1016/j.jep.2012.11.025. [DOI] [PubMed] [Google Scholar]
- Das S., Sarmah S., Lyndem S., Singha Roy A. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J. Biomol. Struct. Dyn. 2020:1–11. doi: 10.1080/07391102.2020.1763201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. Current lead natural products for the chemotherapy of human immunodeficiency virus (HIV) infection. Med. Res. Rev. 2000;20:323–349. doi: 10.1002/1098-1128(200009)20:5<323::aid-med1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Del Valle D.M., Kim-Schulze S., Huang H.-.H., Beckmann N.D., Nirenberg S., Wang B., Lavin Y., Swartz T.H., Madduri D., Stock A., Marron T.U., Xie H., Patel M., Tuballes K., Van Oekelen O., Rahman A., Kovatch P., Aberg J.A., Schadt E., Jagannath S., Mazumdar M., Charney A.W., Firpo-Betancourt A., Mendu D.R., Jhang J., Reich D., Sigel K., Cordon-Cardo C., Feldmann M., Parekh S., Merad M., Gnjatic S. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020 doi: 10.1038/s41591-41020-41051-41599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Zeng L., Li R., Chen Q., Zhou B., Chen Q., Cheng P.L., Yutao W., Zheng J., Yang Z., Zhang F. The Chinese prescription lianhuaqingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function. BMC Complement. Altern. Med. 2017;17:130. doi: 10.1186/s12906-017-1585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Xia J.W., Gong Y., Chen Z., Yang H.H., Zhang J., He J., Chen X.D. Effect of lianhuaqingwen capsules on airway inflammation in patients with acute exacerbation of chronic obstructive pulmonary disease. Evid. Based Complement. Altern. Med. 2014;2014 doi: 10.1155/2014/637969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z.P., Jia Z.H., Zhang J., Liu S., Chen Y., Liang L.C., Zhang C.Q., Zhang Z., Sun Y., Zhang S.Q., Wang Y.Y., Wu Y.L. Natural herbal medicine Lianhuaqingwen capsule anti-influenza A (H1N1) trial: a randomized, double blind, positive controlled clinical trial. Chin. Med. J. 2011;124:2925–2933. [PubMed] [Google Scholar]
- Ejemel M., Li Q., Hou S., Schiller Z.A., Tree J.A., Wallace A., Amcheslavsky A., Kurt Yilmaz N., Buttigieg K.R., Elmore M.J., Godwin K., Coombes N., Toomey J.R., Schneider R., Ramchetty A.S., Close B.J., Chen D.-.Y., Conway H.L., Saeed M., Ganesa C., Carroll M.W., Cavacini L.A., Klempner M.S., Schiffer C.A., Wang Y. A cross-reactive human IgA monoclonal antibody blocks SARS-CoV-2 spike-ACE2 interaction. Nat. Commun. 2020;11:4198. doi: 10.1038/s41467-020-18058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore C., Salvi M., Palermo M., Sinigaglia G., Armanini D., Toninello A. On the mechanism of mitochondrial permeability transition induction by glycyrrhetinic acid. Biochim. Biophys. Acta. 2004;1658:195–201. doi: 10.1016/j.bbabio.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Florindo H.F., Kleiner R., Vaskovich-Koubi D., Acúrcio R.C., Carreira B., Yeini E., Tiram G., Liubomirski Y., Satchi-Fainaro R. Immune-mediated approaches against COVID-19. Nat. Nanotechnol. 2020;15:630–645. doi: 10.1038/s41565-020-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster F.M., Traer C.J., Abraham S.M., Fry M.J. The phosphoinositide (PI) 3-kinase family. J. Cell Sci. 2003;116:3037–3040. doi: 10.1242/jcs.00609. [DOI] [PubMed] [Google Scholar]
- Fry M.J. Structure, regulation and function of phosphoinositide 3-kinases. Biochim. Biophys. Acta. 1994;1226:237–268. doi: 10.1016/0925-4439(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Gao D., Niu M., Wei S.Z., Zhang C.E., Zhou Y.F., Yang Z.W., Li L., Wang J.B., Zhang H.Z., Zhang L., Xiao X.H. Identification of a pharmacological biomarker for the bioassay-based quality control of a thirteen-component TCM formula (Lianhua Qingwen) used in treating influenza a virus (H1N1) infection. Front. Pharmacol. 2020;11:746. doi: 10.3389/fphar.2020.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X.H., Li Y., Zhang R.Q., Xie X.F., Peng C., Li Y.X. The synergism mechanism of Rhubarb Anthraquinones on constipation elucidated by comparative pharmacokinetics of Rhubarb extract between normal and diseased rats. Eur. J. Drug Metab. Pharmacokinet. 2015;40:379–388. doi: 10.1007/s13318-014-0216-7. [DOI] [PubMed] [Google Scholar]
- Guo C., Li B., Ma H., Wang X., Cai P., Yu Q., Zhu L., Jin L., Jiang C., Fang J., Liu Q., Zong D., Zhang W., Lu Y., Li K., Gao X., Fu B., Liu L., Ma X., Weng J., Wei H., Jin T., Lin J., Qu K. Single-cell analysis of two severe COVID-19 patients reveals a monocyte-associated and tocilizumab-responding cytokine storm. Nat. Commun. 2020;11:3924. doi: 10.1038/s41467-020-17834-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Piao X.S., Zhang H.Y., Li P.F., Yi J.Q., Zhang Q., Li P. Forsythia suspensa extract has the potential to substitute antibiotic in broiler chicken. Asian Australas. J. Anim. Sci. 2012;25:569–576. doi: 10.5713/ajas.2011.11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y., Li D., Piao X., Piao X. Forsythia suspensa extract alleviates hypersensitivity induced by soybean beta-conglycinin in weaned piglets. J. Ethnopharmacol. 2010;128:412–418. doi: 10.1016/j.jep.2010.01.035. [DOI] [PubMed] [Google Scholar]
- He Y., Zhou Y., Siddiqui P., Niu J., Jiang S. Identification of immunodominant epitopes on the membrane protein of the severe acute respiratory syndrome-associated coronavirus. J. Clin. Microbiol. 2005;43:3718–3726. doi: 10.1128/JCM.43.8.3718-3726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou N., Liu N., Han J., Yan Y., Li J. Chlorogenic acid induces reactive oxygen species generation and inhibits the viability of human colon cancer cells. Anticancer Drugs. 2017;28:59–65. doi: 10.1097/CAD.0000000000000430. [DOI] [PubMed] [Google Scholar]
- Hu K., Guan W.J., Bi Y., Zhang W., Li L., Zhang B., Liu Q., Song Y., Li X., Duan Z., Zheng Q., Yang Z., Liang J., Han M., Ruan L., Wu C., Zhang Y., Jia Z.H., Zhong N.S. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Sato A., Hirabayashi K., Tanabe F., Shigeta S., Baba M., De Clercq E., Nakashima H., Yamamoto N. Mechanism of inhibitory effect of glycyrrhizin on replication of human immunodeficiency virus (HIV) Antiviral Res. 1988;10:289–298. doi: 10.1016/0166-3542(88)90047-2. [DOI] [PubMed] [Google Scholar]
- Jatiani S.S., Baker S.J., Silverman L.R., Reddy E.P. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979–993. doi: 10.1177/1947601910397187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S., Bai Q., Wu X., Zhang D.W., Wang S., Shen J.L., Fei G.H. Unique synergistic antiviral effects of Shufeng Jiedu Capsule and oseltamivir in influenza A viral-induced acute exacerbation of chronic obstructive pulmonary disease. Biomed. Pharmacother. 2020;121 doi: 10.1016/j.biopha.2019.109652. [DOI] [PubMed] [Google Scholar]
- Jia W., Wang C., Wang Y., Pan G., Jiang M., Li Z., Zhu Y. Qualitative and quantitative analysis of the major constituents in Chinese medical preparation Lianhua-Qingwen capsule by UPLC-DAD-QTOF-MS. Sci.World J. 2015;2015 doi: 10.1155/2015/731765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Zhou G., Li Y. Micelle-mediated extraction for the analysis of chlorogenic acid, rutin, and quercetinin honeysuckle by HPLC-UV. J. Liq. Chromatogr. Related Technol. 2011;34:1473–1487. [Google Scholar]
- Jiang S., He Y., Liu S. SARS vaccine development. Emerg. Infect. Dis. 2005;11:1016–1020. doi: 10.3201/eid1107.050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J.W., Kim J.M., Jeong J.S., Son M., Lee H.S., Lee M.G., Kang H.E. Pharmacokinetics of chlorogenic acid and corydaline in DA-9701, a new botanical gastroprokinetic agent, in rats. Xenobiotica. 2014;44:635–643. doi: 10.3109/00498254.2013.874610. [DOI] [PubMed] [Google Scholar]
- Kang S., Tanaka T., Narazaki M., Kishimoto T. Targeting interleukin-6 signaling in Clinic. Immunity. 2019;50:1007–1023. doi: 10.1016/j.immuni.2019.03.026. [DOI] [PubMed] [Google Scholar]
- Khan S., Ali A., Shi H., Siddique R., Shabana, Nabi G., Hu J., Wang T., Dong M., Zaman W., Han G. COVID-19: clinical aspects and therapeutics responses. Saudi Pharm. J. 2020;28:1004–1008. doi: 10.1016/j.jsps.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H.C., Wei B.L., Chiou W.F. Dual regulatory effect of plant extracts of Forsythia suspense on RANTES and MCP-1 secretion in influenza A virus-infected human bronchial epithelial cells. J. Ethnopharmacol. 2005;102:418–423. doi: 10.1016/j.jep.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Kouznetsov V.V. COVID-19 treatment: much research and testing, but far, few magic bullets against SARS-CoV-2 coronavirus. Eur. J. Med. Chem. 2020;203 doi: 10.1016/j.ejmech.2020.112647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Singh S.B., Singh S. COVID-19: environment concern and impact of Indian medicinal system. J. Environ. Chem. Eng. 2020;8 doi: 10.1016/j.jece.2020.104144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Lee J.S., Jang H.J., Kim S.M., Chang M.S., Park S.H., Kim K.S., Bae J., Park J.W., Lee B., Choi H.Y., Jeong C.H., Bu Y. Chlorogenic acid ameliorates brain damage and edema by inhibiting matrix metalloproteinase-2 and 9 in a rat model of focal cerebral ischemia. Eur. J. Pharmacol. 2012;689:89–95. doi: 10.1016/j.ejphar.2012.05.028. [DOI] [PubMed] [Google Scholar]
- Lee M.S., Lee S.O., Kim K.R., Lee H.J. Sphingosine kinase-1 involves the inhibitory action of HIF-1α by chlorogenic acid in hypoxic DU145 cells. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18020325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D.T., Tam F.C., Ma C.H., Chan P.K., Cheung J.L., Niu H., Tam J.S., Lim P.L. Antibody response of patients with severe acute respiratory syndrome (SARS) targets the viral nucleocapsid. J. Infect. Dis. 2004;190:379–386. doi: 10.1086/422040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Yang L., Liu F.F., Ma X.N., He P.L., Tang W., Tong X.K., Zuo J.P. Overview of therapeutic drug research for COVID-19 in China. Acta Pharmacol. Sin. 2020;41:1133–1140. doi: 10.1038/s41401-020-0438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.C., Zhang Z.H., Zhou W.C., Chen J., Jin H.Q., Fang H.M., Chen Q., Jin Y.C., Qu J., Kan L.D. Lianhua Qingwen prescription for coronavirus disease 2019 (COVID-19) treatment: advances and prospects. Biomed. Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R.F., Hou Y.L., Huang J.C., Pan W.Q., Ma Q.H., Shi Y.X., Li C.F., Zhao J., Jia Z.H., Jiang H.M., Zheng K., Huang S.X., Dai J., Li X.B., Hou X.T., Wang L., Zhong N.S., Yang Z.F. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020;156 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Wang, C., Zheng, L., An, J., 2014. Application of traditional Chinese medicine composition in preparing medicine for treating human avian influenza, CN101862391B.
- Li X., Yang Y., Liu L., Yang X., Zhao X., Li Y., Ge Y., Shi Y., Lv P., Zhang J., Bai T., Zhou H., Luo P., Huang S. Effect of combination antiviral therapy on hematological profiles in 151 adults hospitalized with severe coronavirus disease 2019. Pharmacol. Res. 2020;160 doi: 10.1016/j.phrs.2020.105036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C., Tian L., Liu Y., Hui N., Qiao G., Li H., Shi Z., Tang Y., Zhang D., Xie X., Zhao X. A promising antiviral candidate drug for the COVID-19 pandemic: a mini-review of remdesivir. Eur. J. Med. Chem. 2020;201 doi: 10.1016/j.ejmech.2020.112527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling X., Tao J., Sun X., Yuan B. Study on the material basis and mechanism of Lianhua Qingwen decoction against coronavirus based on network pharmacology. Chin. Herb. Med. 2020;51:1723–1730. [Google Scholar]
- Liu F., Ji C., Luo J., Wu W., Zhang J., Zhong Z., Lankford S., Huang H., Lin F., Wang Y., Mo G., Hu X., Jiang T., Shao Y., Ji S., Zhang Y., Qin E., Mu J. Clinical characteristics and corticosteroids application of different clinical types in patients with corona virus disease 2019. Sci. Rep. 2020;10:13689. doi: 10.1038/s41598-020-70387-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Gao Y., Yuan Y., Yang K., Shi S., Zhang J., Tian J. Efficacy and Safety of Integrated Traditional Chinese and Western Medicine for Corona Virus Disease 2019 (COVID-19): a systematic review and meta-analysis. Pharmacol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Zhang T., Ma L., Wang H., Cao Y., Yang Y., Pei H., Li H. Efficacy and safety of Lianhua Qingwen in the treatment of patients with moderate COVID-19 infection: a protocol for systematic review and meta analysis. Med. (Baltimore). 2020;99:e21614. doi: 10.1097/MD.0000000000021614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Cheng H., Roberts T.M., Zhao J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Liu H., Sun Q., Liang H., Li C., Deng X., Liu Y., Lai L. Potent inhibitors of SARS-CoV-2 3C-like protease derived from N-substituted isatin compounds. Eur. J. Med. Chem. 2020;206 doi: 10.1016/j.ejmech.2020.112702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.J., Zhou C.Y., Qiu C.H., Lu X.M., Wang Y.T. Chlorogenic acid induced apoptosis and inhibition of proliferation in human acute promyelocytic leukemia HL-60 cells. Mol. Med. Rep. 2013;8:1106–1110. doi: 10.3892/mmr.2013.1652. [DOI] [PubMed] [Google Scholar]
- Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- Magro G. SARS-CoV-2 and COVID-19: is interleukin-6 (IL-6) the 'culprit lesion' of ARDS onset? What is there besides Tocilizumab? SGP130Fc. Cytokine X. 2020;2 doi: 10.1016/j.cytox.2020.100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C., Roper R.L. The Genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Matsuura T., Aoyagi H., Matsuda M., Hmwe S.S., Date T., Watanabe N., Watashi K., Suzuki R., Ichinose S., Wake K., Suzuki T., Miyamura T., Wakita T., Aizaki H. Antiviral activity of glycyrrhizin against hepatitis C virus in vitro. PLoS One. 2013;8:e68992. doi: 10.1371/journal.pone.0068992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum M., Walls A.C., Bowen J.E., Corti D., Veesler D. Structure-guided covalent stabilization of coronavirus spike glycoprotein trimers in the closed conformation. Nat. Struct. Mol. Biol. 2020 doi: 10.1038/s41594-41020-40483-41598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- Mt R., Alajmi M., Hussain A. Natural compounds as inhibitors of SARS-CoV-2 main protease (3CLpro): a molecular docking and simulation approach to combat COVID-19. ChemRxiv. 2020 doi: 10.26434/chemrxiv.12362333.v2. [DOI] [PubMed] [Google Scholar]
- Ni L., Chen L., Huang X., Han C., Xu J., Zhang H., Luan X., Zhao Y., Xu J., Yuan W., Chen H. Combating COVID-19 with integrated traditional Chinese and western medicine in China. Acta Pharm. Sin. B. 2020;10:1149–1162. doi: 10.1016/j.apsb.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Cao X., Li N., Xu Y., Wu Q., Bai J., Yin Z., Luo L., Lan L. Forsythin inhibits lipopolysaccharide-induced inflammation by suppressing JAK-STAT and p38 MAPK signalings and ROS production. Inflamm. Res. 2014;63:597–608. doi: 10.1007/s00011-014-0731-7. [DOI] [PubMed] [Google Scholar]
- Panyod S., Ho C.T., Sheen L.Y. Dietary therapy and herbal medicine for COVID-19 prevention: a review and perspective. J. Tradit. Complement. Med. 2020;10:420–427. doi: 10.1016/j.jtcme.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M., Won J., Choi B.Y., Lee C.J. Optimization of primer sets and detection protocols for SARS-CoV-2 of coronavirus disease 2019 (COVID-19) using PCR and real-time PCR. Exp. Mol. Med. 2020;52:963–977. doi: 10.1038/s12276-020-0452-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Liu F., Ye J. Determination of phenolic acids and flavones in Lonicera Japonica Thumb. By capillary electrophoresis with electrochemical detection. Electroanalysis. 2005;17:356–362. [Google Scholar]
- Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping F., Li Z., Zhang F., Li D., Han S. Effects of Lianhua Qingwen on pulmonary oxidative lesions induced by fine particulates (PM2.5) in rats. Chin. Med. Sci. J. 2016;31:233–238. doi: 10.1016/s1001-9294(17)30006-8. [DOI] [PubMed] [Google Scholar]
- Pompei R., Flore O., Marccialis M.A., Pani A., Loddo B. Glycyrrhizic acid inhibits virus growth and inactivates virus particles. Nature. 1979;281:689–690. doi: 10.1038/281689a0. [DOI] [PubMed] [Google Scholar]
- Rawlings J.S., Rosler K.M., Harrison D.A. The JAK/STAT signaling pathway. J. Cell Sci. 2004;117:1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- Ren J.L., Zhang A.H., Wang X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol. Res. 2020;155 doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runfeng L., Yunlong H., Jicheng H., Weiqi P., Qinhai M., Yongxia S., Chufang L., Jin Z., Zhenhua J., Haiming J., Kui Z., Shuxiang H., Jun D., Xiaobo L., Xiaotao H., Lin W., Nanshan Z., Zifeng Y. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020;156 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone P., Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J. Clin. Oncol. 2012;30:1005–1014. doi: 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Goto W., Yamamura J., Kurokawa M., Kageyama S., Takahara T., Watanabe A., Shiraki K. Therapeutic basis of glycyrrhizin on chronic hepatitis B. Antiviral Res. 1996;30:171–177. doi: 10.1016/0166-3542(96)00942-4. [DOI] [PubMed] [Google Scholar]
- Schindler C., Levy D.E., Decker T. JAK-STAT signaling: from interferons to cytokines. J. Biol. Chem. 2007;282:20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol. J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S. Study on the chemical constituents of Lianhua Qingwen capsules (III) Chin. Herb. Med. 2019;50:814–820. [Google Scholar]
- Shi R., Shan C., Duan X., Chen Z., Liu P., Song J., Song T., Bi X., Han C., Wu L., Gao G., Hu X., Zhang Y., Tong Z., Huang W., Liu W.J., Wu G., Zhang B., Wang L., Qi J., Feng H., Wang F.-.S., Wang Q., Gao G.F., Yuan Z., Yan J. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature. 2020;584:120–124. doi: 10.1038/s41586-020-2381-y. [DOI] [PubMed] [Google Scholar]
- Shi R., Xuan Z., Ma Y., Liu Y., Lu H., Sun T. Pharmacokinetics of forsythoside after intravenous administration in beagle dogs. Eur. J. Drug Metab. Pharmacokinet. 2009;34:101–105. doi: 10.1007/BF03191158. [DOI] [PubMed] [Google Scholar]
- Takayama K., Tsutsumi H., Ishizu T., Okamura N. The influence of rhein 8-O-β-D-glucopyranoside on the purgative action of sennoside A from rhubarb in mice. Biol. Pharm. Bull. 2012;35:2204–2208. doi: 10.1248/bpb.b12-00632. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Narazaki M., Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8:959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- Tao Z., Yang Y., Shi W., Xue M., Yang W., Song Z., Yao C., Yin J., Shi D., Zhang Y., Cai Y., Tong C., Yuan Y. Complementary and alternative medicine is expected to make greater contribution in controlling the prevalence of influenza. Biosci. Trends. 2013;7:253–256. [PubMed] [Google Scholar]
- Tong T., Wu Y.Q., Ni W.J., Shen A.Z., Liu S. The potential insights of traditional Chinese medicine on treatment of COVID-19. Chin. Med. 2020;15:51. doi: 10.1186/s13020-020-00326-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsunomiya T., Kobayashi M., Herndon D.N., Pollard R.B., Suzuki F. Glycyrrhizin (20 beta-carboxy-11-oxo-30-norolean-12-en-3 beta-yl-2-O-beta-D-glucopyranuronosyl-alpha-D-glucopyranosiduronic acid) improves the resistance of thermally injured mice to opportunistic infection of herpes simplex virus type 1. Immunol. Lett. 1995;44:59–66. doi: 10.1016/0165-2478(94)00183-r. [DOI] [PubMed] [Google Scholar]
- Vellingiri B., Jayaramayya K., Iyer M., Narayanasamy A., Govindasamy V., Giridharan B., Ganesan S., Venugopal A., Venkatesan D., Ganesan H., Rajagopalan K., Rahman P., Cho S.G., Kumar N.S., Subramaniam M.D. COVID-19: a promising cure for the global panic. Sci. Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong W., Khan M.B., Fischer C., Arutyunova E., Lamer T., Shields J., Saffran H.A., McKay R.T., van Belkum M.J., Joyce M.A., Young H.S., Tyrrell D.L., Vederas J.C., Lemieux M.J. Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication. Nat. Commun. 2020;11:4282. doi: 10.1038/s41467-020-18096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.H., Zhong Y., Zhang Y., Liu J.P., Wang Y.F., Jia W.N., Wang G.C., Li Z., Zhu Y., Gao X.M. A network analysis of the Chinese medicine Lianhua-Qingwen formula to identify its main effective components. Mol. Biosyst. 2016;12:606–613. doi: 10.1039/c5mb00448a. [DOI] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Grunewald M., Perlman S. Coronaviruses: an updated overview of their replication and pathogenesis. Methods Mol. Biol. 2020;2203:1–29. doi: 10.1007/978-1-0716-0900-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.X., Ma J.R., Wang S.Q., Zeng Y.Q., Zhou C.Y., Ru Y.H., Zhang L., Lu Z.G., Wu M.H., Li H. Utilizing integrating network pharmacological approaches to investigate the potential mechanism of Ma Xing Shi Gan decoction in treating COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2020;24:3360–3384. doi: 10.26355/eurrev_202003_20704. [DOI] [PubMed] [Google Scholar]
- Wang Z., Xia Q., Liu X., Liu W., Huang W., Mei X., Luo J., Shan M., Lin R., Zou D., Ma Z. Phytochemistry, pharmacology, quality control and future research of Forsythia suspensa (Thunb.) Vahl: a review. J. Ethnopharmacol. 2018;210:318–339. doi: 10.1016/j.jep.2017.08.040. [DOI] [PubMed] [Google Scholar]
- Wei T.Z., Wang H., Wu X.Q., Lu Y., Guan S.H., Dong F.Q., Dong C.L., Zhu G.L., Bao Y.Z., Zhang J., Wang G.Y., Li H.Y. In silico screening of potential spike glycoprotein inhibitors of SARS-CoV-2 with drug repurposing strategy. Chin. J. Integr. Med. 2020;26:663–669. doi: 10.1007/s11655-020-3427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M., Chen L., Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Chen Y., Ma Y., Yang Z., Yang N., Deng W., Chen Y., Sun Y., Li Y., Lin L. Clinical practice guideline on treating influenza in adult patients with Chinese patent medicines. Pharmacol. Res. 2020;160 doi: 10.1016/j.phrs.2020.105101. [DOI] [PubMed] [Google Scholar]
- Xiao M., Tian J., Zhou Y., Xu X., Min X., Lv Y., Peng M., Zhang Y., Yan D., Lang S., Zhang Q., Fan A., Ke J., Li X., Liu B., Jiang M., Liu Q., Zhu J., Yang L., Zhu Z., Zeng K., Li C., Zheng Y., Wu H., Lin J., Lian F., Li X., Tong X. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: a randomized controlled trial. Pharmacol. Res. 2020;161 doi: 10.1016/j.phrs.2020.105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zhang Y. Traditional Chinese Medicine treatment of COVID-19. Complement. Ther. Clin. Pract. 2020;39 doi: 10.1016/j.ctcp.2020.101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J.D., Liu S., Wang W., Li L.S., Li X.F., Li Y., Guo H., Ji T., Feng X.Y., Hou X.L., Zhang Y., Zhu J.X. Emodin induces chloride secretion in rat distal colon through activation of mast cells and enteric neurons. Br. J. Pharmacol. 2012;165:197–207. doi: 10.1111/j.1476-5381.2011.01573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R., Kang Q., Ren J., Li Z., Xu X. Antitumor molecular mechanism of chlorogenic acid on inducting genes GSK-3 β and APC and inhibiting gene β -catenin. J. Anal. Methods Chem. 2013;2013 doi: 10.1155/2013/951319. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Xue N., Zhou Q., Ji M., Jin J., Lai F., Chen J., Zhang M., Jia J., Yang H., Zhang J., Li W., Jiang J., Chen X. Chlorogenic acid inhibits glioblastoma growth through repolarizating macrophage from M2 to M1 phenotype. Sci. Rep. 2017;7:39011. doi: 10.1038/srep39011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T., Yamauchi K. Synergistic effects of anthraquinones on the purgative activity of rhein anthrone in mice. J. Pharm. Pharmacol. 1999;51:93–95. [PubMed] [Google Scholar]
- Yang C., Wang Y., He J., Yan W., Jiang H., Chen Q., Li L., Yang Z. Lianhua-Qingwen displays antiviral and anti-inflammatory activity and synergistic effects with oseltamivir against influenza B virus infection in the mouse model. Evid. Based Complement. Altern. Med. 2020;2020 doi: 10.1155/2020/3196375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Gong J.F., Shen L., Zhang C., Kou F.R., Gao J., Li Y., Bing Xu G. Development of an LC-MS/MS method for quantitative analysis of chlorogenic acid in human plasma and its application to a pharmacokinetic study in Chinese patients with advanced solid tumor. J. Pharm. Biomed. Anal. 2020;177 doi: 10.1016/j.jpba.2019.112809. [DOI] [PubMed] [Google Scholar]
- Yang L., Jiang H., Xing X., Yan M., Guo X., Man W., Hou A., Yang L. A biosensor-based quantitative analysis system of major active ingredients in Lonicera japonica Thunb. Using UPLC-QDa and chemometric analysis. Molecules. 2019;24 doi: 10.3390/molecules24091787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Zhou X., Huang W., Fang Q., Hu J., Yu L., Ma N., Zhang W. Protective effect of phillyrin on lethal LPS-induced neutrophil inflammation in zebrafish. Cell. Physiol. Biochem. 2017;43:2074–2087. doi: 10.1159/000484192. [DOI] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J., Fu J., Shang S., Shu Q., Zhang T. Epidemiological analysis of COVID-19 and practical experience from China. J. Med. Virol. 2020;92:755–769. doi: 10.1002/jmv.25813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye C., M. G., Lin W., Yu K., Li P., Chen G. Theoretical study of the anti-NCP molecular mechanism of traditional Chinese medicine Lianhua-Qingwen Formula (LQF) ChemRxiv. 2020 doi: 10.26434/chemrxiv.12016236.v12016231. [DOI] [Google Scholar]
- Yi C., Sun X., Ye J., Ding L., Liu M., Yang Z., Lu X., Zhang Y., Ma L., Gu W., Qu A., Xu J., Shi Z., Ling Z., Sun B. Key residues of the receptor binding motif in the spike protein of SARS-CoV-2 that interact with ACE2 and neutralizing antibodies. Cell. Mol.1 Immunol. 2020;17:621–630. doi: 10.1038/s41423-020-0458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Pardoll D., Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Yang R., Liu C.-.Z. Microwave-assisted extraction of chlorogenic acid from flower buds of Lonicera japonica Thunb. Sep. Purif. Technol. 2008;62:480–483. [Google Scholar]
- Zhang D.H., Wu K.L., Zhang X., Deng S.Q., Peng B. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J. Integr. Med. 2020;18:152–158. doi: 10.1016/j.joim.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.H., Zhang X., Peng B., Deng S.Q., Wang Y.F., Yang L., Zhang K.Z., Ling C.Q., Wu K.L. Network pharmacology suggests biochemical rationale for treating COVID-19 symptoms with a traditional Chinese medicine. Commun. Biol. 2020;3:466. doi: 10.1038/s42003-020-01190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Wang L., Liu S., Yang G.-.J. Separation and detection of electrochemical active compounds in Houttuynia cordata Thunb. by reversed-phase high-performance liquid chromatography with electrochemical detection. J. Liq. Chromatogr. Related Technol. 2015;38 [Google Scholar]
- Zhang R.Z., Yu S.J., Bai H., Ning K. TCM-Mesh: the database and analytical system for network pharmacology analysis for TCM preparations. Sci. Rep. 2017;7:2821. doi: 10.1038/s41598-017-03039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Li L., Shen A., Chen Y., Qi Z. Rational use of tocilizumab in the treatment of novel coronavirus pneumonia. Clin Drug Investig. 2020;40:511–518. doi: 10.1007/s40261-020-00917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Cao D., Liu J., Zhang Q., Liu M. Efficacy and safety of Lianhua Qingwen combined with conventional antiviral Western Medicine in the treatment of coronavirus disease (covid-19) in 2019: protocol for a systematic review and meta-analysis. Med. (Baltimore). 2020;99:e21404. doi: 10.1097/MD.0000000000021404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Liu, L., Tang, S., Wang, H., 2015b. Application of a traditional Chinese medicine composition in preparing medicines against influenza A H3N2 virus, CN104825622A.
- Zhao P., Yang H.Z., Lv H.Y., Wei Z.M. Efficacy of Lianhuaqingwen capsule compared with oseltamivir for influenza A virus infection: a meta-analysis of randomized, controlled trials. Altern. Ther. Health Med. 2014;20:25–30. [PubMed] [Google Scholar]
- Zhao X., Tan X., Shi H., Xia D. Nutrition and traditional Chinese medicine (TCM): a system's theoretical perspective. Eur. J. Clin. Nutr. 2020 doi: 10.1038/s41430-41020-00737-w. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Wang J., Ballevre O., Luo H., Zhang W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens. Res. 2012;35:370–374. doi: 10.1038/hr.2011.195. [DOI] [PubMed] [Google Scholar]
- Zheng L., Zhang L., Huang J., Nandakumar K.S., Liu S., Cheng K. Potential treatment methods targeting 2019-nCoV infection. Eur. J. Med. Chem. 2020;205 doi: 10.1016/j.ejmech.2020.112687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.F., Liu C.F., Lai W.F., Xiang Q., Li Z.F., Wang H., Lin N. The laxative effect of emodin is attributable to increased aquaporin 3 expression in the colon of mice and HT-29 cells. Fitoterapia. 2014;96:25–32. doi: 10.1016/j.fitote.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Zhuang W., Fan Z., Chu Y., Wang H., Yang Y., Wu L., Sun N., Sun G., Shen Y., Lin X., Guo G., Xi S. Chinese patent medicines in the treatment of coronavirus disease 2019 (COVID-19) in China. Front. Pharmacol. 2020;11:1066. doi: 10.3389/fphar.2020.01066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebuhr J., Snijder E.J., Gorbalenya A.E. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J. Gen. Virol. 2000;81:853–879. doi: 10.1099/0022-1317-81-4-853. [DOI] [PubMed] [Google Scholar]
- Zost S.J., Gilchuk P., Case J.B., Binshtein E., Chen R.E., Nkolola J.P., Schäfer A., Reidy J.X., Trivette A., Nargi R.S., Sutton R.E., Suryadevara N., Martinez D.R., Williamson L.E., Chen E.C., Jones T., Day S., Myers L., Hassan A.O., Kafai N.M., Winkler E.S., Fox J.M., Shrihari S., Mueller B.K., Meiler J., Chandrashekar A., Mercado N.B., Steinhardt J.J., Ren K., Loo Y.-.M., Kallewaard N.L., McCune B.T., Keeler S.P., Holtzman M.J., Barouch D.H., Gralinski L.E., Baric R.S., Thackray L.B., Diamond M.S., Carnahan R.H., Crowe J.E. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. 2020;584:443–449. doi: 10.1038/s41586-020-2548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


