Background
Between March and April 2020, 84 elderly patients with suspected COVID-19 living in two nursing homes of Yepes, Toledo (Spain) were treated early with antihistamines (dexchlorpheniramine, cetirizine or loratadine), adding azithromycin in the 25 symptomatic cases. The outcomes are retrospectively reported. The primary endpoint is the fatality rate of COVID-19. The secondary endpoints are the hospital and ICU admission rates. Endpoints were compared with the official Spanish rates for the elderly. The mean age of our population was 85 and 48% were over 80 years old. No hospital admissions, deaths, nor adverse drug effects were reported in our patient population. By the end of June, 100% of the residents had positive serology for COVID-19. Although clinical trials are needed to determine the efficacy of both drugs in the treatment of COVID-19, this analysis suggests that primary care diagnosis and treatment with antihistamines, plus azithromycin in selected cases, may treat COVID-19 and prevent progression to severe disease in elderly patients.
Keywords: SARS-CoV-2, Coronavirus, Azithromycin, Antihistamines, Elderly
1. Introduction
COVID-19 disease is a highly transmissible viral infection caused by the SARS-CoV-2 coronavirus. This virus is closely related to the SARS-CoV and MERS-CoV coronaviruses. All three viruses can cause lung damage, acute respiratory distress syndrome and death [1].
The pandemic began in Toledo at the beginning of March and increased rapidly in intensity in the following weeks. To avoid contagion, the Service of Health of Castilla-La Mancha suspended face to face consultations at the health centers and local offices. Patients' with mild symptoms were attended mainly by phone while patients with severe symptoms were treated in person at health centers or their homes. Many of these patients were only prescribed symptomatic treatment, and those who evolved to severe forms of the disease were referred to the hospital. Patients’ fear to go to health centers caused many patients to remain at home until the disease evolved into a critical situation. Many hospitals collapsed and had to restructure their Medical Services and Intensive Care Units (ICU) to facilitate the large volume of patients.
The Basic Zone of Health of Yepes belongs to the Area of Health of Toledo and provides health coverage to a population of approximately 10.000 inhabitants, and offers assistance to two nursing homes. The eighty-four patients living in these nursing homes are the patient population of focus for this study. These patients had a mean age of 85, and in most cases additional comorbidities which would predict poor COVID-19 disease outcomes.
Given the limited scientific knowledge existing in March 2020 about SARS-CoV-2 and effective COVID-19 treatments or protocols, we developed our own clinical guidelines for the management of patients with COVID-19. These guidelines are based on our clinical experience in the management of severe respiratory infections, especially in patients with underlying high risks pathologies such as chronic obstructive pulmonary disease (COPD), asthma, and bronchiolitis.
Antihistamines are old drugs commonly used in Primary Care to treat all kind of allergies. Second generation antihistamines such as cetirizine, loratadine, ebastine, bilastine, levocetirizine, fexofenadine, desloratadine, mizolastine and rupatadine, have a good safety profile without adverse effects when updosed up to four times the standard dose [2]. The use of antihistamines is widespread in our area due to the high prevalence of winter and spring allergies, and for the prevention of catarrhal relapses in children and adults at risk. We included antihistamines for the treatment of all patients after observing that when added to the initial treatment, our patients had a notable improvement in 24–48 h.
Azithromycin and quinolones are also part of the therapeutic arsenal of Primary Care. Azithromycin is an antibiotic widely used for the treatment of respiratory infections due to its safety profile, comfortable dosage, good tolerance and a low incidence of treatment-related adverse events. Furthermore, dosage adjustment is not necessary for elderly patients with mild to moderate renal impairment, and it has demonstrated effectiveness in the treatment of patients with community-acquired pneumonia [3].
2. Materials and methods
This is a retrospective observational study of a case series of 84 elderly patients diagnosed with COVID-19, living in two nursing homes in a rural area of Toledo. The study was classified by the AEMPS (Spanish Agency of Medicines and Medical Devices) and approved by the Clinical Research Ethics Committee of the IIS-FJD (Madrid). The study period runs from the beginning of March to the end of June 2020.
3. Objective
Evaluate the effectiveness of early COVID-19 treatment using a combination of antihistamines and azithromycin in elderly patients, to control the disease at Primary Health Care level. Fatality, hospital, and ICU admission rates from this case series are compared with Spanish official metrics for this patient population.
3.1. Study endpoints
The primary endpoint is the total fatality rate from COVID-19. Secondary endpoints are rates of hospital admission and ICU admission.
3.2. Complementary diagnostic tests
Until mid-April PCR or serology diagnostics were not available in health centers. Therefore, most patients who became symptomatic since the beginning of March were diagnosed based on symptoms. When available, PCR confirmation was performed for new cases that emerged (after April 28th).
Our health center does not have the facilities to perform chest X-rays. Due to the frailty of the elderly population coupled with the collapse of the health system, our patients were treated directly at the nursing homes without the possibility of complementary diagnostic tests. A rapid test serological study was carried out in mid April 2020 to all the surviving residents included in this study. The rapid tests used were 2019-nCoV IgG/IgM Detection Kit (Colloidal Gold-Based) Vazyme Biotech (nursing home A) and BIOZEK COVID-19 IGG/IGM rapid test (nursing home B). The results obtained indicate that all patients in this study had SARS-CoV-2 antibodies.
3.3. Inclusion criteria
Patients were included in this study if they:
-
-
Were residents in assisted living facilities A or B.
-
-
Exhibited two or more of the following symptoms: fever, cough, chest discomfort, dyspnea, anosmia/dysgeusia, or general malaise with polymyalgia, or tested positive for COVID-19 via PCR or serology testing.
3.4. Clinical management of COVID-19 used in the nursing homes of Yepes
-
1.
Early start of treatment, regardless of the severity of patient symptoms.
-
2.Patients with mild or recent-onset symptoms (cough, fever, general malaise, anosmia, polymyalgia):
-
-Antihistamines every 12 h: dexchlorpheniramine 2 mg, cetirizine 10 mg or loratadine 10 mg.
-
-Azithromycin 500 mg orally every 24 h for 3 days if there is rapid improvement, and for 6 days if the duration of symptoms is prolonged.
-
-If pain or fever, acetaminophen 650 mg/6–8 h.
-
-Nasal washing and gargling with sodium bicarbonate water (half a glass of warm water with half a teaspoon of sodium bicarbonate).
-
-
-
3.If symptoms of severity (dyspnea, breathing difficulty, mild or moderate chest pain, with SpO2 >80%, heart rate <100 beats per minute at any time of the process):
-
-Antihistamines + Azithromycin (see mild treatment management)
-
-Levofloxacin 500 mg/12 h, up to 14 days of antibiotic treatment from diagnosis.
-
-Mepifilin solution, 50 mg/8 h as a bronchodilator, until subjective improvement. Patients with previous lung disease (asthma or COPD) used their usual bronchodilators.
-
-If the patient experienced increased breathing difficulty, prednisone 1 mg/kg/day divided into two doses until clinical improvement, and then it was slowly tapered down.
-
-
-
4.Prophylactic treatment for close contacts, including all asymptomatic residents:
-
-Antihistamines at the same dose as symptomatic patients.
-
-
A daily telephone assessment was performed until recovery. If recurrence of symptoms, azithromycin 500 mg/24h was prescribed for 3 more days.
All patients were trained in techniques of respiratory physiotherapy (mainly deep inspiration and expiration).
Caregivers were given home isolation instructions which included the use of facemask and were prescribed antihistamines at the same dose as patients.
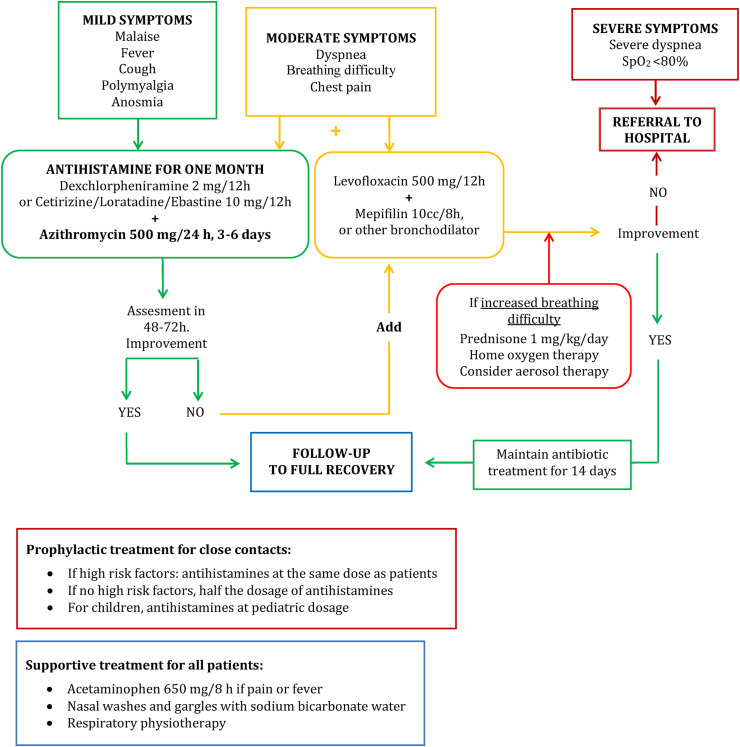
Fig. 1 represents the flow chart of the complete treatment protocol we used in Primary Care. This treatment protocol was used to treat patients residing in both nursing homes as well as the general community.
Fig. 1.
Clinical guidelines for the management of patients with COVID-19 at primary health care level.
3.5. Study population
The study population includes 84 elderly residents and 64 employees located in two separate nursing homes. Nursing home A had 64 elderly residents and 44 employees. Nursing home B had 20 elderly residents and 14 employees. We were not overseeing the health of patients in nursing home B until April 16th.
At the beginning of the pandemic and before residents were treated with antihistamine alone or with azithromycin, three elderly patients in each of the nursing homes died. In nursing home A, one of the three patients died of possible coronavirus pneumonia. In nursing home B, two women died in the hospital with a confirmed diagnosis by PCR and another one at the nursing home, diagnosed by serology rapid test.
After these first cases, 25 patients became symptomatic, were isolated in individual rooms and started treatment with antihistamines and azithromycin, while the remaining 59 asymptomatic nursing home residents were treated prophylactically only with antihistamines. Antihistamines (dexchlorpheniramine) were also prescribed prophylactically to caregivers. All residents stopped using common spaces such as dining rooms and patios, and occupational therapies were discontinued.
4. Results
During the first month and a half of the pandemic, we attended 84 elderly patients in two separate nursing homes (55 females and 29 males).
The mean age of the 64 patients in nursing home A was 85 years, range (52–97), with a median of 88 years. After the first three deaths, five patients exhibited two or more COVID-19 symptoms, and they were treated with dexchlorpheniramine and azithromycin. Two of these patients also received levofloxacin due to high risk for underlying pathologies. These suspected patients were isolated in single rooms when possible, and subsequently in double rooms. During the first weeks of the pandemic it was not possible to carry out diagnostic confirmation using molecular or rapid tests, due to lack of supply, so the remaining 59 patients, all of them asymptomatic, were treated as close contacts and only prescribed dexchlorphemiramine. All residents were isolated for more than 14 days.
When available, rapid tests were performed on all residents and confirmed as positive cases by rapid test or PCR. PCR was also carried out on the emerging suspected cases.
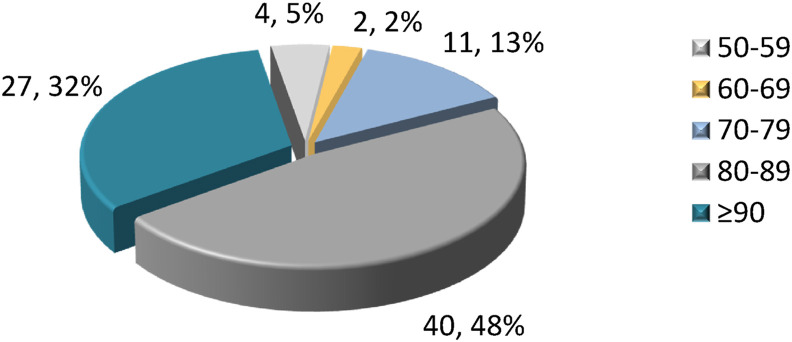
Fig. 2. Pie chart of the pooled study population ages. Patients over the age of 80 represent 80% of residents.
Fig. 2.
Number and percentage of nursing home patients by age group (both nursing homes together).
Table 1 shows the pooled clinical characteristics and administered COVID-19 treatments of the elderly patients included in this study.
Table 1.
Clinical characteristics of the 84 residents treated.
| Nº patients and percentage (%) |
Nº patients with COVID-19 symptoms and percentage (%) |
||
|---|---|---|---|
| (n = 84) | yes (n = 25) | No (n = 59) | |
| Age (mean and SD) | 85 (9.5) | 86.1 (7.1) | 84.1 (10.7) |
| Female sex | 55 (65.5) | 17 (68) | 38 (64.4) |
| Male sex | 29 (34.5) | 8 (32) | 21 (35.6) |
| Number of chronic diseases | |||
| • Hypertension | 51 (60.7) | 15 (60) | 36 (61) |
| • Diabetes | 20 (23.8) | 5 (20) | 15 (25.4) |
| • COPD/Asthma | 11 (13.1) | 6 (24) | 5 (8.5) |
| • Dementia | 8 (9.5) | 4 (16) | 4 (6.8) |
| • Cardiovascular disease | 64 (76.2) | 17 (68) | 47 (79.7) |
| Drugs | |||
| Polypharmacy (>5) | 55 (65.5) | 19 (76) | 36 (61) |
| Chronic consumed drugs | |||
| • ACE inhibitors | 16 (19) | 5 (20) | 11 (18.6) |
| • ARB | 27 (32.1) | 3 (12) | 24 (40.7) |
| • Antihypertensive drugs | 18 (21.4) | 13 (52) | 5 (8.5) |
| • Statins | 28 (33.3) | 10 (40) | 18 (30.5) |
| • Oral anticoagulants | 11 (13.1) | 6 (24) | 5 (8.5) |
| • Antiplatelet agents | 41 (48.8) | 16 (64) | 25 (42.4) |
| • NSAIDs | 4 (4.8) | 1 (4) | 3 (5.1) |
| • Hypoglicemiants | 15 (17.9) | 5 (20) | 10 (16.9 |
| • Neuroleptics | 35 (41.7) | 8 (32) | 27 (45.8) |
| • Benzodiazepines | 31 (36.9) | 11 (44) | 20 (33.9) |
| • AChEI/memantine | 9 (10.7) | 5 (20) | 4 (6.8) |
| • Inhalation drugs | 12 (14.3) | 6 (24) | 6 (10.2) |
| • Corticosteroids | 1 (1.2) | 1 (4) | 0 (0) |
| • Proton pump inhibitors | 48 (57.1) | 12 (48) | 36 (61) |
| • Antihistamines H1* | 8 (9.5) | 7 (28) | 1 (1.7) |
| • Antihistamine H2 (famotidine) | 1 (1.2) | 1 (4) | 0 (0) |
| Hospitalization | 0 | ||
| Diagnostic tests | ** | ||
| Treatment | |||
| • Oxygen therapy*** | 3 (3.6) | ||
| • Acetaminophen/metamizole | 19 (22.6) | ||
| • Azithromycin | 25 (29.8) | ||
| • Levofloxacin | 7 (8.3) | ||
| • Deflazacort/prednisone p.o. | 3 (3.6) | ||
| • H1 antihistamines | 84 (100) | 25 (100) | 59 (100) |
| Dexchlorpheniramine | 77 (91.7) | 18 (72) | 59 (100) |
| Cetirizine | 5 (6.0) | 5 (20) | 0 (0) |
| Loratadine | 2 (2.4) | 2 (8) | 0 (0) |
COPD: Chronic Obstructive Pulmonary Disease. ACE: Angiotensin converting enzyme. ARB: Angiotensin receptor blocker. NSAIDs: Non-steroidal anti-inflammatory drugs. AChEI: Acetylcholinesterase inhibitors. PO: oral administration. *Cetirizine (5 patients), loratadine (2) and dexchlorpheniramine (1) ** No X ray, no laboratory analysis available. *** Low flow oxygen 3 to 5 lpm.
The mean age of the surviving 20 patients in nursing home B was 85 years, range (69–96), with a median of 85 years. All of these patients were symptomatic and were treated early with antihistamines (dexchlorpheniramine, loratadine or cetirizine) and azithromycin. Five of these residents were also treated with levofloxacin due to mild or moderate symptoms and high-risk pathologies. Two residents with moderate symptoms also required low-concentration oxygen therapy and oral corticosteroids. None of the patients treated progressed to severe disease, required hospital referral, or had adverse effects related to treatment. All residents recovered in a few days after beginning treatment, and were confirmed later as COVID-19 cases by seroconversion by rapid test when available.
Among the 44 caregivers in nursing home A, 18 had positive serology, and 12 of them had mild symptoms.
Of the 14 caregivers in nursing home B - all under 50 years of age - 7 presented positive serology by rapid test: 4 were asymptomatic and 3 were mildly symptomatic. The protocol of treatment and home isolation was applied to all, with recovery in a few days and without known sequelae.
All of our patients evolved satisfactorily and were recovered at the beginning of June. No adverse effects were recorded in any patient and no one required hospital admission. At the end of June, 100% of the residents and almost half of the workers had positive serology for COVID-19, most of them with past infection.
Table 2. Comparison of the data from a published study that summarized the characteristics and endpoints of patients from six nursing homes in Albacete, Spain with the corresponding pooled metrics from our sample population. After starting our treatment protocol we observed a mortality rate of 0%. The comparator retirement homes had a mortality rate of 28%.
Table 2.
Comparison between the epidemiology of COVID -19 in the two nursing homes of Yepes and the pooled data from 6 nursing homes in Albacete (Mas Romero et al). [8] Data from Albacete are from March 6th to April 5th, while Yepes’ are from March 1st to June 30th, 2020.
| Pooled data - Albacete | Nursing homes - Yepes | |
|---|---|---|
| Number of residents | 1084 | 90 |
| Date of first case | March 07, 2020 | March 18, 2020 |
| PCR positive | 134 | 27 |
| Rapid serological test (RST) positive | – | 81 |
| Total confirmed cases by PRC/RST | 134 | 84 |
| Typical symptoms + contact | 364 | 84 |
| Mortality from March 01, 2020 until June 30, 2020: nº (%) | 303 (28.0%) | 6*(6.67%) |
* All of them died before the establishment of the treatment with antihistamines + azithromycin. Four covid confirmed deaths, one in nursing home A and three in nursing home B (one palliative patient and two in the hospital). Two residents in nursing home A died with no covid-19 symptoms and no test performed.
5. Discussion
The situation we lived in our country in the period from March to May 2020 was catastrophic for our health system, with thousands of daily infections and patients worsening within a few hours, saturating the emergency services and requiring hospital admission. Due to the lack of beds, it was not possible to admit all the patients who required admission. Family doctors had to use their expertise in other respiratory diseases to try and save as many patients as possible. This work reflects one of these experiences.
According to data from the National Epidemiology Centre, the case fatality rate among identified confirmed cases in Spain was 11.2% [4], due in large part to the collapse of the healthcare system. Castilla-La Mancha was one of the hardest hit in the first wave of the pandemic, registering 10.67% of the deaths nationwide, and being the third Autonomous Community in the number of deaths, only behind the Communities of Madrid and Catalonia [4]. Of the total COVID-19 mortality, 36.5% were in the population aged over 70 [5]. In our region, the population aged 65–74 registered an excess of mortality of 124%, and for over 74 years old, it was 98% [6]. Deaths in nursing homes would be equivalent to 69.1% of all deaths in our country reported by the Ministry of Health [7]. Data available from six nursing homes in Albacete (Castilla-La Mancha) suggest that between March 6th and June 5th, 28% of the residents died [8]. Asturias, another Spanish region, reported a fatality rate from COVID-19 of 33.38% in its nursing homes [9].
The mortality and fatality rates in other nursing homes were higher than our patient population (Table 2). We had 4 confirmed COVID-19 deaths in our patient population before starting treatment with antihistamines and azithromycin. The expected mortality in our population with 84 patients with positive diagnosis confirmed by serology or PCR, and whose mean age was of 85 years, should have been about 25 deaths (considering a fatality rate of 30%). It is worth mentioning that in the most serious cases, we successfully treated patients by adding levofloxacin. For this patient population, no symptoms improvement was observed after 48–72 h when treated with cefixime or ciprofloxacin.
Although this work focuses solely on the results obtained in the nursing home population, we applied the algorithm in Fig. 1 to all patients in our health area. Between May and August 2020 there were no new cases, no new deaths attributable to COVID-19, nor excess of mortality in our population.
We searched the literature for publications that could give support to our results. Numerous studies describe a possible antiviral activity of azithromycin against viruses as diverse as influenza viruses (Orthomyxoviridae), rhinovirus (Picornaviridae) [10], respiratory syncytial virus (Paramyxoviridae) and zika (Flaviviridae) [11]. Tran et al.[12] demonstrated the possible mechanism of antiviral action of azithromycin that blocks internalization into human lung epithelial cells during the early phase of infection of influenza virus A(H1N1)/pdm09 in vitro. They subsequently showed in mice that a single treatment with azithromycin via the intranasal route suppressed the virus load in the lungs, thereby preventing hypothermia during A(H1N1)pdm09 virus infection.
Azithromycin appears to act as a lysosomotropic agent, affecting lysosomal traffic and pH [13,14]. A similar mechanism has been proposed for the now controversial hydroxychloroquine [15], which alkalinizes phagolysosomes by inhibiting viral segmentation mediated by pH-dependent proteases, interfering with the fusion process and viral replication. Perhaps it is needed to correctly determine the minimum not toxic effective dose. Possible effects of azithromycin would include interference with ACE2 receptor and decrease virus binding [16], increase of the intra-vesicular pH of endosomes and lysosomes up to around neutrality, thereby inhibiting the lysosomal enzyme activities responsible in virus entry and replication cycle [17]. In RSV infected mice, prophylactic administration of azithromycin produced a reduction in weight loss, inflammation of the airways, cytokine levels and mortality [18]. Different publications collect evidence of the anti-inflammatory properties of azithromycin in the airway, decreasing the levels of cytokines - closely related to lung damage [19] - and various interleukins. Murphy et al. showed that azithromycin alters the macrophage phenotype, reducing the production of proinflammatory cytokines IL-12 and IL-6 and increasing that of IL-10, which has an anti-inflammatory effect [20]. Furthermore, it would provoke a global amplification of the host antiviral responses mediated by interferon [14,16], and it seems to modify the airway microbiome [21]. In vitro inhibition of SARS-CoV-2 replication by azithromycin has recently been demonstrated [22]. Although our experience and research are about azithromycin, other macrolides may have similar properties [[23], [24], [25]]. In patients with cystic fibrosis, many of whom have chronic treatment with azithromycin and other antibiotics, low morbidity and mortality are being registered, in contrast to what would be expected as a high risk pathology [26]. The Patient Registry of the European Cystic Fibrosis Society has so far collected data from 180 documented cases from 20 countries, of which 70 were hospitalized, 12 in the ICU and have only recorded 5 deaths [27].
Regarding antihistamines, in recent years molecules with antihistamine activity have been identified as having powerful antiviral properties, inhibiting the entry of certain viruses into the target cell, such as the Ebola virus (filovirus) [28], or the hepatitis C virus (flavivirus) [[29], [30], [31]], or by other mechanisms [32]. Several H1 receptor antagonists have demonstrated inhibitory properties on the production and expression of interleukins, chemokines, and other cytokines [33]. Specifically, cetirizine decreases interleukin production [34,35].
According to the mechanisms of action described, these drugs would act synergistically in the early stages of the disease, which is why we consider it essential to start the treatment as soon as possible. Once the virus has colonized the respiratory system, the effectiveness is probably more limited, and hence the failure of these treatments in more advanced stages of the disease, when hospital admission is necessary. In our experience, early double antibiotics were effective to control the process in cases with moderate symptoms.
Although this study is descriptive, our results bear some similarities with other studies [26,36,37]. Mast cells seem to play an important role in the inflammatory responses by releasing granules of histamine, in addition to synthesizing and secrete inflammatory lipid mediators and pro-inflammatory cytokines TNF-α and IL-6 [38]. The use of antihistamines may help to minimize the histamine-mediated cytokine storm [39].
Interestingly, recent clinical studies have focused not only on the potential effect of H1 receptor antagonists but on the H2 receptor antagonist famotidine, which has shown improvement in disease progression when added to treatment. Two retrospective studies in hospitalized patients treated with famotidine found a lower risk of mortality, lower risk of combined outcome of mortality and intubation, and lower levels of serum markers for severe disease in hospitalized patients with COVID-19 [40,41]. A more recent cohort study used cetirizine and famotidine in hospitalized patients with severe to critical pulmonary symptoms. This study confirmed beneficial reductions in inpatient mortality and symptom progression, probably by minimizing the histamine-mediated cytokine storm [39]. As for non-hospitalized patients, another study showed improvements of disease symptoms after starting high dose oral famotidine [42]. Two phase III clinical trials have been initiated to investigate the clinical efficacy of famotidine on the recovery process of COVID-19 patients [43,44].
Given the low fatality rate observed in our patient population, this treatment protocol merits immediate consideration for the treatment of COVID-19 and future evaluation in randomized controlled clinical trials, taking into account the probably decisive role of antihistamines, which was the only treatment most of our patients received.
5.1. Limitations
This study has the limitations of all observational studies. Hypotheses inferred by observational studies must be subsequently confirmed by randomized controlled clinical trials that verify the causality of a certain effect. These types of studies are currently used on a large scale as a basis for pharmacoepidemiological research in Primary Care [45]. However, randomized, placebo-controlled clinical trials are the ‘gold standard’ for assessing the efficacy of repositioning drugs. Therefore, randomized controlled trials and observational approaches are complementary, and not interchangeable.
6. Conclusions
Early treatment of symptomatic COVID-19 patients with antihistamines and azithromycin, and administration of antihistamines in asymptomatic and high risk patients, close contacts and relatives, had excellent outcomes in our population reducing fatality rate, hospital admissions and ICU admissions in this elderly population, regardless of patient's age and risk factors.
This safe and inexpensive treatment protocol could have a crucial impact on morbidity and mortality rates of patients with COVID-19 and ease the burden of these patients on hospitals. Treatment should be started at the Primary Health Care level, as early as possible when the first symptoms appear. Antihistamines and azithromycin are drugs with extensive experience of use, good safety profile, good tolerance, low cost and wide availability, so this combined treatment regimen may respond to the global therapeutic needs for COVID-19 for all age groups. Clinical trials are necessary to determine its efficacy. As there are no commercial interests, they should be promoted by national health systems as a social responsibility.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
We thank Ma del Mar Cruz Acquaroni (MD) and Mercedes Mota Pérez (Pharmaceutical Inspector) for their support and suggestions on improving this manuscript.
Abbreviations
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IL
Interleukin
- DM
Diabetes mellitus
- HT
Hypertension
- PCR
Polymerase chain reaction
- ICU
Intensive Care Unit
- COPD
Chronic obstructive pulmonary disease
- HCV
Hepatitis C virus
- NEC
National Epidemiology Centre
- RSV
Respiratory syncytial virus
- SpO2
Oxygen saturation (SpO2) levels
- WHO
World Health Organization
Authors' contributions
JIMB devised the clinical guidance. JIMB and JAB carried out patients' diagnosis, prescription of treatments and the follow-up until their full recovery. JAB performed data extraction from the medical records. KVGH wrote the first draft, made the data analysis and graphs, reviewed the bibliography and wrote the argument. SH, KS, JAB and PFS reviewed and refined the final document. PFS was supported under Air Force Contract No. FA8702-15-D-0001. Any opinions, findings, conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the U.S. Air Force. All authors reviewed and approved this manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cataldi M., Maurer M., Taglialatela M., Church M.K. Cardiac safety of second-generation H1-antihistamines when updosed in chronic spontaneous urticaria. Clin. Exp. Allergy. 2019;49:1615–1623. doi: 10.1111/cea.13500. [DOI] [PubMed] [Google Scholar]
- 3.MINISTERIO DE SANIDAD POLÍTICA SOCIAL E IGUALDAD. Ficha Técnica de Zitromax. 2019. https://cima.aemps.es/cima/pdfs/es/ft/61272/61272_ft.pdf Available at: (Accessed: 15th November 2020)
- 4.Centro de Coordinación de Alertas y Emergencias Sanitarias (CCAES). Ministerio de Sanidad Actualización no 158. Enfermedad por el coronavirus (COVID-19). 08.07.2020. 2020. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_158_COVID-19.pdf Available at: (Accessed: 15th November 2020), 1-4.
- 5.Centro Nacional de Epidemiología Instituto de Salud Carlos III - Ministerio de Sanidad. Informe sobre la situación de COVID-19 en España. Informe COVID-19 no 32. 21 de mayo de 2020. 2020. https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/Informes COVID-19/Informe n_32_Situación_de_COVID-19_en_España_a_21_de_mayo_de_2020.pdf Available at: (Accessed: 15th November 2020), 1-15.
- 6.Centro Nacional de Epidemiología Vigilancia de los excesos de mortalidad por todas las causas: MoMo. Situación a 4 de junio de 2020. 2020. https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/MoMo/Documents/informesMoMo2020/MoMo_Situacion_a_4_de_junio_CNE.pdf Available at: (Accessed: 15th November 2020), 1-21.
- 7.Médicos sin Fronteras. MSF TOO LITTLE , TOO LATE : the unacceptable neglect of the elderly in care. 2020. https://msfcovid19.org/wp-content/uploads/2020/08/msf-report-too-little-too-late-elderly-and-covid-in-ltcf-english.pdf Available at: (Accessed: 15th November 2020)
- 8.Mas Romero M., et al. COVID-19 outbreak in long-term care facilities from Spain. Many lessons to learn. PloS One. 2020;15 doi: 10.1371/journal.pone.0241030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Observass Observatorio Asturiano de Servicios Sociales. Situación COVID-19 en Centros residenciales para personas mayores. 2020. https://www.socialasturias.es/v_portal/inc/clicklink.asp?t=3&cod=5440&c=2&s=354674608 Available at: (Accessed: 15th November 2020)
- 10.Menzel M., Akbarshahi H., Bjermer L., Uller L. Azithromycin induces anti-viral effects in cultured bronchial epithelial cells from COPD patients. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep28698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X., Xia S., Zou P., Lu L. Erythromycin estolate inhibits zika virus infection by blocking viral entry as a viral inactivator. Viruses. 2019;11 doi: 10.3390/v11111064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran D.H., et al. Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A(H1N1)pdm09 virus infection by interfering with virus internalization process. J. Antibiot. (Tokyo). 2019;72:759–768. doi: 10.1038/s41429-019-0204-x. [DOI] [PubMed] [Google Scholar]
- 13.Homolak J., Kodvanj I. Widely available lysosome targeting agents should be considered as potential therapy for COVID-19. Int. J. Antimicrob. Agents. 2020;56:106044. doi: 10.1016/j.ijantimicag.2020.106044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damle B., Vourvahis M., Wang E., Leaney J., Corrigan B. Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID-19. Clin. Pharmacol. Ther. 2020;108:1–11. doi: 10.1002/cpt.1857. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machiels J.D., et al. Reply to Gautret et al: hydroxychloroquine sulfate and azithromycin for COVID-19 : what is the evidence and what are the risks ? Int. J. Antimicrob. Agents. 2020;56:106056. doi: 10.1016/j.ijantimicag.2020.106056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mégarbane B., Scherrmann J.M. Hydroxychloroquine and azithromycin to treat patients with COVID-19: both friends and foes? J. Clin. Pharmacol. 2020;60:808–814. doi: 10.1002/jcph.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scherrmann J. Intracellular ABCB1 as a possible mechanism to explain the synergistic effect of hydroxychloroquine-azithromycin combination in COVID-19 therapy. AAPS J. 2020;22:86. doi: 10.1208/s12248-020-00465-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosquera R.A., et al. Role of prophylactic azithromycin to reduce airway inflammation and mortality in a RSV mouse infection model. Pediatr. Pulmonol. 2018;53:567–574. doi: 10.1002/ppul.23956. [DOI] [PubMed] [Google Scholar]
- 19.Zhou F., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy B.S., et al. Azithromycin alters macrophage phenotype. J. Antimicrob. Chemother. 2008;61:554–560. doi: 10.1093/jac/dkn007. [DOI] [PubMed] [Google Scholar]
- 21.Beigelman A., et al. Does azithromycin modify viral load during severe RSV bronchiolitis? J. Allergy Clin. Immunol. 2015;136:1129–1131. doi: 10.1016/j.jaci.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Touret F., et al. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci. Rep. 2020;10:1–8. doi: 10.1038/s41598-020-70143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaya M., et al. Clarithromycin inhibits type A seasonal influenza virus infection in human airway epithelial cells. J. Pharmacol. Exp. Therapeut. 2010;333:81–90. doi: 10.1124/jpet.109.162149. [DOI] [PubMed] [Google Scholar]
- 24.Min J.Y., Jang Y.J. Macrolide therapy in respiratory viral infections. Mediat. Inflamm. 2012 doi: 10.1155/2012/649570. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohe M., et al. Macrolide treatment for COVID-19: will this be the way forward? Biosci. Trends. 2020;14:159–160. doi: 10.5582/bst.2020.03058. [DOI] [PubMed] [Google Scholar]
- 26.Mondejar-Lopez P., et al. Impact of SARS-CoV-2 infection in patients with cystic fibrosis in Spain: incidence and results of the national CF-COVID19-Spain survey. Respir. Med. 2020;170 doi: 10.1016/j.rmed.2020.106062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Cystic Fibrosis Society COVID-19 in people with CF in europe. Data up to 29 july 2020. 2020. 2020. https://www.ecfs.eu/covid-cf-project-europe Available at:
- 28.Cheng H., et al. Identification of a coumarin-based antihistamine-like small molecule as an anti-filoviral entry inhibitor. Antivir. Res. 2017;145:24–32. doi: 10.1016/j.antiviral.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Z., Charles M., R. Repurposing an old drug: a low-cost Allergy medication provides new Hope for hepatitis C patients. Hepatology. 2015;62:1911–1913. doi: 10.1002/hep.28028. [DOI] [PubMed] [Google Scholar]
- 30.He S., et al. Repurposing of the antihistamine chlorcyclizine and related compounds for treatment of hepatitis C virus infection. Sci. Transl. Med. 2015;7:1–22. doi: 10.1126/scitranslmed.3010286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mingorance L., et al. Selective inhibition of hepatitis c virus infection by hydroxyzine and benztropine. Antimicrob. Agents Chemother. 2014;58:3451–3460. doi: 10.1128/AAC.02619-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papi A., et al. Effect of desloratadine and loratadine on rhinovirus-induced intercellular adhesion molecule 1 upregulation and promoter activation in respiratory epithelial cells. J. Allergy Clin. Immunol. 2001;108:221–228. doi: 10.1067/mai.2001.116861. [DOI] [PubMed] [Google Scholar]
- 33.Aydin Suna, Aydin Suleyman. Could Antihistamines Help in the Treatment and Spread of COVID-19 Via Re-Modulating Cytokines and by Reducing Sneezing? Acta Scientific Nutritional Health. 2020;4.4:172–173. https://actascientific.com/ASNH/ASNH-04-0684.php [Google Scholar]
- 34.Ambrosch A., Borgmann S., Rihoux J.-P., König W. Effect of the H1 receptor antagonist cetirizine on the stimulated expression of adhesion molecules and the activation of NFκB in human endothelial cells. Int. Arch. Allergy Immunol. 2001;124:362–364. doi: 10.1159/000053758. [DOI] [Google Scholar]
- 35.Arnold R., Rihoux J.P., König W. Cetirizine counter-regulates interleukin-8 release from human epithelial cells (A549) Clin. Exp. Allergy. 1999;29:1681–1691. doi: 10.1046/j.1365-2222.1999.00630.x. [DOI] [PubMed] [Google Scholar]
- 36.Gautret P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56:1059. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Schwartz R.A., Suskind R.M. Azithromycin and COVID-19: prompt early use at first signs of this infection in adults and children, an approach worthy of consideration. Dermatol. Ther. 2020;33:2–4. doi: 10.1111/dth.13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang H.W., et al. A common signaling pathway leading to degranulation in mast cells and its regulation by CCR1-ligand. Allergy Eur. J. Allergy Clin. Immunol. 2020;75:1371–1381. doi: 10.1111/all.14186. [DOI] [PubMed] [Google Scholar]
- 39.Hogan R.B., et al. Dual-histamine receptor blockade with cetirizine - famotidine reduces pulmonary symptoms in COVID-19 patients. Pulm. Pharmacol. Therapeut. 2020;63 doi: 10.1016/j.pupt.2020.101942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mather J.F., Seip R.L., McKay R.G. Impact of famotidine use on clinical outcomes of hospitalized patients with COVID-19. Am. J. Gastroenterol. 2020;115:1617–1623. doi: 10.14309/ajg.0000000000000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freedberg D.E., et al. Famotidine use is Associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study. Gastroenterology. 2020;159:1129–1131. doi: 10.1053/j.gastro.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janowitz T., et al. Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: a case series. Gut. 2020;69:1592–1597. doi: 10.1136/gutjnl-2020-321852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samimagham H.R., et al. The Efficacy of Famotidine in improvement of outcomes in Hospitalized COVID-19 Patients: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21 doi: 10.1186/s13063-020-04773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Multi-site Adaptive trials for COVID-19. 2020. https://clinicaltrials.gov/ct2/show/NCT04370262 Available at: Accessed: 15th November 2020.
- 45.Maciá-Martínez M.-A., et al. Base de Datos para la Investigación Farmacoepidemiológica en Atención Primaria (BIFAP): a data resource for pharmacoepidemiology in Spain. Pharmacoepidemiol. Drug Saf. 2020;29:1236–1245. doi: 10.1002/pds.5006. [DOI] [PubMed] [Google Scholar]