Abstract
Already in the early 1960s, researchers noted the potential of mitochondria to take up large amounts of Ca2+. However, the physiological role and the molecular identity of the mitochondrial Ca2+ uptake mechanisms remained elusive for a long time. The identification of the individual components of the mitochondrial calcium uniporter complex (MCUC) in the inner mitochondrial membrane in 2011 started a new era of research on mitochondrial Ca2+ uptake. Today, many studies investigate mitochondrial Ca2+ uptake with a strong focus on function, regulation, and localization of the MCUC. However, on its way into mitochondria Ca2+ has to pass two membranes, and the first barrier before even reaching the MCUC is the outer mitochondrial membrane (OMM). The common opinion is that the OMM is freely permeable to Ca2+. This idea is supported by the presence of a high density of voltage-dependent anion channels (VDACs) in the OMM, forming large Ca2+ permeable pores. However, several reports challenge this idea and describe VDAC as a regulated Ca2+ channel. In line with this idea is the notion that its Ca2+ selectivity depends on the open state of the channel, and its gating behavior can be modified by interaction with partner proteins, metabolites, or small synthetic molecules. Furthermore, mitochondrial Ca2+ uptake is controlled by the localization of VDAC through scaffolding proteins, which anchor VDAC to ER/SR calcium release channels. This review will discuss the possibility that VDAC serves as a physiological regulator of mitochondrial Ca2+ uptake in the OMM.
Keywords: mitochondrial calcium uptake, VDAC, MCU
1. Introduction
Mitochondria are known as the cell’s powerhouses as they produce ATP by oxidative phosphorylation. However, already in the 1950s and 1960s, researchers noted the potential of mitochondria to sequester vast amounts of Ca2+ [1,2], while the role of this mitochondrial Ca2+ uptake and the involved molecular players remained elusive. Many proteins including RyR, LetM1, or UCP2/3 had been suggested to mediate mitochondrial Ca2+ uptake, before the molecular identity of the MCU complex (MCUC) in the inner mitochondrial membrane was identified as the main route of Ca2+ uptake in the early 2010s. Subsequently, research on mitochondrial Ca2+ uptake experienced a revival when molecular structures and knock-out models of the individual MCUC components became available. However, on its way into mitochondria Ca2+ has to pass two membranes, and the first barrier before Ca2+ even reaches the MCUC is the outer mitochondrial membrane (OMM), a fact that is still often neglected in current literature, where the OMM is described as freely permeable to ions. Indeed, the term mitochondrial Ca2+ uniport is often used synonymously for mitochondrial Ca2+ uptake, and some textbook representations do not even show the OMM but depict the MCUC such that it is directly facing the cytosol.
The OMM is widely occupied by large pore-forming structures, the voltage-dependent anion channels (VDACs), which mediate the flux of ions and metabolites over the OMM. While bacteria express only one form of VDAC, higher eukaryotes express multiple isoforms. In yeast, two isoforms are described, while plants and animals express three isoforms (VDAC1-3).
In a ground-setting publication in 2001, Shoshan-Barmatz and colleagues provided several lines of evidence that the voltage-dependent anion channel (VDAC) is the main carrier for Ca2+ over the OMM [3]: when inserted into planar lipid bilayers, VDAC1 was permeable to Ca2+, and VDAC1 reconstituted into liposomes mediated Ca2+ efflux from these vesicles. These in situ results are supported by the observations that VDAC1 overexpression enhanced Ca2+ uptake into mitochondria [4], and knock-down of VDAC1 reduced mitochondrial Ca2+ uptake [5] in vitro in cultured cells. Already in these early publications, it was noted that the Ca2+ flux through VDAC could be blocked by ruthenium red (RuR) and La3+ and that VDAC possesses a Ca2+ binding site, raising the intriguing hypothesis that VDAC is not freely permeable to Ca2+ but might present a regulated barrier for Ca2+ flux over the OMM.
However, considering that VDAC forms a pore large enough to allow the passage of metabolites such as ATP, two questions arise: how can such a big pore be regulated at the molecular level and is the regulation of mitochondrial Ca2+ uptake at the outer mitochondrial membrane physiologically relevant?
Several recent reports have argued in favor of a regulation of mitochondrial Ca2+ uptake at the OMM: (i) the tight connection between VDAC1 and the IP3 receptor (IP3R) was reported to be a key regulatory mechanism to either promote enhanced cellular metabolism or to induce apoptosis by modulating mitochondrial Ca2+ signaling [5,6], (ii) regulation of VDAC-mediated Ca2+ uptake through channel monoubiquitinylation [7] was demonstrated to be critically involved in the pathophysiology of Parkinson’s disease and to mediate programmed cell death after DNA damage [8], and iii) our lab has recently demonstrated that the small compound efsevin modulates VDAC2 and, thereby, amplifies mitochondrial Ca2+ uptake approx. 3-fold in cardiomyocytes [9,10]. This amplification induces a protective effect against cardiac arrhythmia, both in vitro and in vivo.
In this review, we will first discuss possible regulatory mechanisms of mitochondrial Ca2+ uptake through VDAC and then review their putative roles in cell physiology and pathophysiology.
2. Mechanisms to Control the Ca2+ Conductance of VDAC
2.1. Structure and Electrophysiological Properties of VDACs
The common perception that VDAC creates pores in the OMM that are freely permeable to ions is closely related to its structure. VDACs are 30–35 kD transmembrane proteins consisting of approximately 280–300 amino acids, depending on isoform and species. They form barrel-like pores consisting of 19 β-sheets aligned in an antiparallel orientation. On the N-terminal end, an α-helix lines the channel pore inside the barrel [11,12]. The odd-numbered 19 β-sheet geometry is unique for pores within the OMM and was postulated to possess superior properties regarding voltage sensitivity, mitochondrial targeting, and lipid-modulated stability over barrels with a higher or lower number of sheets [13]. The pore has a diameter of approx. 18–20Å [14]. Nevertheless, despite this large diameter, the channel can undergo conformational changes of a still unknown structural nature, which induce a significant change in the channel’s conductance and ion selectivity. When purified VDAC is inserted into artificial lipid bilayers the channel is in a high-conductance state at neutral potential that is classically also referred to as the open state. Upon gradual polarization of the membrane, the channel starts gating at around ±20–30mV between this high-conductance state and several gated states, which are also referred to as closed states (Figure 1 and Figure 2). In these states, the conductance of the channel is reduced to approximately 50% [15,16,17,18]. By using CaCl2 as the charge carrier, the channel was shown to be permeable for Ca2+. Interestingly, the ion selectivity of the channel changes upon gating from a higher anion selectivity in the high-conductance state to a lower selectivity and, thus, higher proportional conductance for Ca2+ in the gated states [3,19,20,21]. Though this observation is consistent, the degree of ion selectivity varies between distinct reports and experimental conditions depending on, e.g., the concentration of CaCl2 used or the composition of the bilayer making an estimation of VDACs ion selectivity in the native environment in the cell difficult. Nevertheless, the relative permeability of cations over anions in VDAC1 was identified experimentally and by molecular simulations to be in the range of 0.05 to 0.4 for the high-conductance state, while the relative permeability for cations over anions was almost 1 for the gated states [3,19,20,21] for VDAC1 and VDAC2 [10]. Ambiguity exists concerning the electrophysiological properties of VDAC3. While initially reported to form channels with low conductance and weak voltage-dependent gating [22], this isoform was later reported to have similar electrophysiological properties as VDAC1 and VDAC2 [23]. However, to our knowledge, no reports are available for the ion selectivity and Ca2+ conductance of VDAC3.
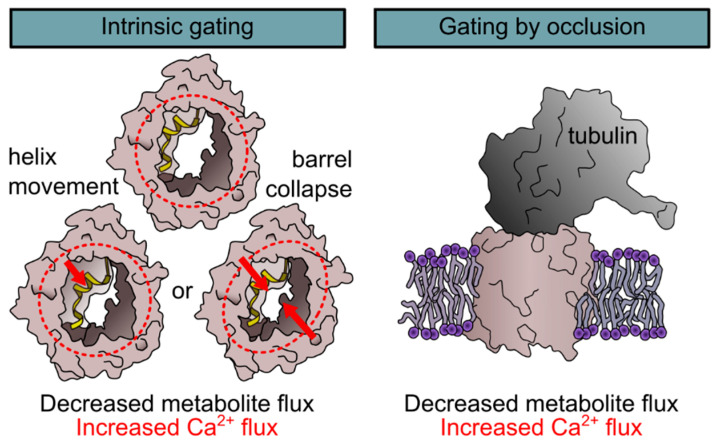
Figure 1.
Mechanisms of voltage-dependent anion channels (VDAC) gating. Two mechanisms for the gating of VDACs were described, namely gating by an intrinsic conformational change (left) and gating by occlusion (right). The first is experimentally induced by voltage but is modulated by extrinsic parameters such as protein interactions, the lipidic environment or posttranslational modifications. Intrinsic gating results in a conformational change of a yet unknown nature. Several models ranging from a movement of the N-terminal α-helix to a collapse of the barrel were suggested. The second mechanism that was observed for VDAC gating is gating by occlusion by, e.g., free tubulin through a molecular plug model. Both mechanisms were shown to block metabolite flux and to induce a lower anion selectivity and, thus, an increased Ca2+ flux.
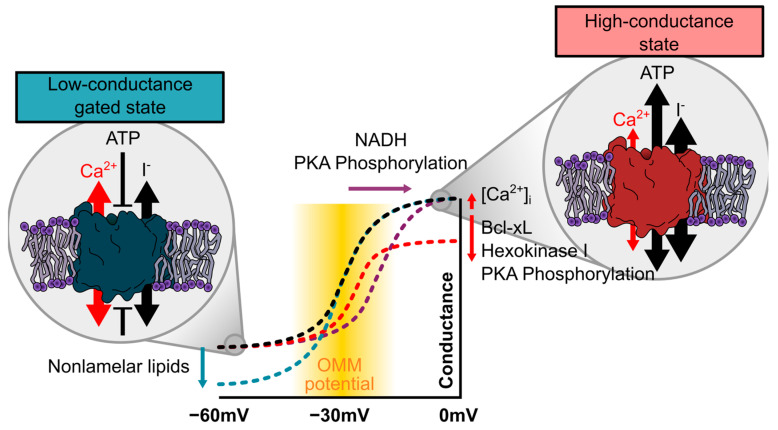
Figure 2.
Regulation of voltage-dependent gating of VDAC. While VDAC almost exclusively resides in its anion-selective and metabolite-permeable high-conductance state at neutral potentials, it starts gating within the range of −20 to −30 mV (black line), which is well within the predicted range of an OMM potential (yellow zone). Note that the conductance–voltage relation of VDAC is symmetrical, and only the negative side is depicted. At potentials below −40 mV, it almost exclusively resides in the low-conductance state that is impermeable for ATP but shows a higher permeability of Ca2+. Several factors were reported to modulate this voltage–conductance relationship. The phosphorylation status or binding of protein partners such as Bcl-xL or hexokinase were suggested to facilitate channel gating and, thus, induce both a right-shift of the voltage dependence and a reduction in the maximum conductance at potentials close to neutral (red line), while Ca2+ is suggested to facilitate channel opening and, thus, has an opposite effect. Nonlamellar lipids were demonstrated to promote channel closure at very negative potentials (blue).
The transition between high-conductance and gated states induces drastic changes in the permeability and selectivity. Although, lipid bilayer experiments are performed in an artificial and rather unphysiological system and care must be taken when transferring them directly to the in vivo situation, it is conceivable that gating of the channel presents a mechanism to regulate not only ATP, but also Ca2+ flux over the OMM. However, gating of the channel was experimentally induced by voltage, and although several mechanisms to create and regulate a membrane potential over the OMM were postulated [24,25,26], it remains controversial whether this is a physiologically relevant trigger of gating. Several other factors including Ca2+ [27], metabolites [28], the lipidic environment [29,30,31,32], temperature [33], small molecules [10,34,35], interacting proteins [6,36,37], and biochemical modifications of the channel itself [38,39] were reported to either modify voltage-induced gating or to induce channel closure on their own. Among those is also the intriguing hypothesis of an entirely different mode of channel gating, namely a closure by occlusion of the large VDAC pore by a “molecular plug” mechanism (Figure 1). In the following, we will discuss the different modes of gating in respect to the Ca2+ conductance of the channel.
2.1.1. Regulation of Ca2+ Conductance by Gating
Evidence for the ability of the channel to undergo intrinsic conformational changes for gating was provided by lipid bilayer experiments on purified channels [15,16]. VDAC heterologously expressed in E. coli and, subsequently, purified and refolded from inclusion bodies forms functional channels in lipid bilayers, which are completely devoid of any regulatory partners that might be associated with the channel in its native environment [10,11]. These channels are able to reversibly gate between the high-conductance and gated states indicating that gating is an intrinsic property of the channel protein. Various models were proposed to explain the molecular movements underlying gating of VDACs (Figure 1). Many of these models involve participation of the N-terminal α-helix and include mechanisms with relatively small movements of the helix inside the barrel [17,40] and models in which the helix moves outside of the barrel and induces its collapse leading to channel closure [20,41]. However, while deletion of the helix abrogated voltage-gating of the channel, cross-linking of the helix against the channel wall did not [42]. This favors a model in which the helix stabilizes the barrel, while gating is induced by a yet uncharacterized deformation of the barrel.
As described earlier, gating of the channel was demonstrated to directly affect its Ca2+ conductance, hence gating would be an intriguing possibility to regulate VDAC Ca2+ flow. However, the existence of voltage-dependent gating in the native environment remains controversial. Several extrinsic factors were described to influence and modulate voltage-gating of the channel and are discussed later.
2.1.2. Regulation of Ca2+ Conductance by Occlusion
In addition to the intrinsic gating of the channel, another intriguing hypothesis that was more recently developed is a regulation of the channel by occlusion. Given the large pore size of the channel, which is wide enough for the passage of metabolites, it appears conceivable that metabolites or domains of interacting proteins enter the channel to serve as “channel plugs”. Among those suggested to serve as molecular plugs is NADH: while it was known for long that NADH closes VDAC [28,43], it was only recently found in a combination of high-resolution NMR with molecular dynamics simulations that NADH reduces conduction sterically by binding into the open pore at the hinge of the pore lining helix without a structural change of the channel [44]. Other partners suggested to mediate channel occlusion are free tubulin, which was demonstrated to interact with VDAC though its C-terminus [45,46,47] (Figure 1), and α-synuclein, which blocks the channel by occlusion but also translocates through the channel [48]. Although to our knowledge, no reports have selectively evaluated the influence of channel occlusion on Ca2+ specifically, the occluded state was also demonstrated to make the channel more cation selective [49], thus suggesting a higher Ca2+ permeability.
Interestingly, a recent study using NADH measurements combined with mathematical modeling has proposed that in cardiac cells 98% of all VDACs reside in a state that is impermeable for ATP, but it remains speculative whether this is the gated or occluded state [50]. It raises, however, the interesting possibility that 98% of VDACs are in a Ca2+ conducting state.
2.2. Modification of the Gating Profile of VDAC
In the following, we will summarize factors that were discussed as possible physiological triggers that control gating or occlusion of the channel.
2.2.1. An OMM Potential as a Trigger for VDAC Gating
The most straight-forward idea when bearing the lipid bilayer experiments in mind is a regulation of the channel by voltage. Since VDAC resides in its high-conductance state at 0 mV and only starts gating at potentials above 20 to 30 or below −20 to −30 mV, a physiologically relevant voltage-dependent gating of the apo-form of the channel would depend on a membrane potential across the OMM. Although the generation of a membrane potential has often been considered impossible due to the high abundance and high conductance of VDACs in the OMM, a series of modeling studies by Victor and Sergy Lemeshko provides reasonable evidence for a membrane potential over the OMM. This potential was suggested to be created by a spatial separation of VDAC from the electron transport chain and to be regulated through the local concentration of ADP within the contact sites and the Gibbs free energy of the hexokinase reaction bound to VDAC [51]. Most strikingly, it was calculated to be well within the range of VDAC gating [24,52,53]. Experimental studies using pH indicators selectively targeted into the intermembrane space (IMS) have found a pH in the IMS, which is approximately 0.5 to 0.7 lower than in the cytosol, suggesting an even more negative potential of approx. −40 mV and acidification of the IMS as a modulator of the membrane potential [26].
Another hypothesis suggests that at points where the IMM is in close contact to the OMM [54], possibly at sites of VDAC and MCUC interaction [55], the strong negative potential of the IMM induces a local OMM potential by capacitative coupling between the two membranes, which allows voltage-gating of VDAC [25]. This is particularly interesting, since these contact sites are believed to be the sites of mitochondrial Ca2+ uptake (see Section 2.4) and closure of the channel by a local negative potential could induce a higher Ca2+ conductance specifically in these areas.
Taken together, such a potential difference in the OMM could very likely be at least one factor contributing to the regulation of the channel. It is feasible that voltage induces gating of the channel, while interaction partners and channel modifications shift the channels open probability towards a preferentially high-conductance or gated state (Figure 2).
2.2.2. Effects of Ca2+ on VDAC Gating
Besides conducting Ca2+, several lines of evidence suggest that the channel is also regulated by Ca2+ and that Ca2+ can regulate its Ca2+ conductance. In their 2001 report, Shoshan-Barmatz and colleagues were among the first to suggest that VDAC1 has a Ca2+ binding site. This was based on several lines of evidence: (i) a Ca2+ induced shift in electrophoretic mobility of VDAC1, (ii) a blockade of the channel by La3+, which could be reversed by the addition of EGTA, and (iii) an inhibition of the channel by ruthenium derivatives such as RuR, Ru360 [3], or AzRu [56] that was reversed by the addition of Ca2+. More recently, a combination of NMR and single-molecule force spectroscopy revealed that VDAC barrels are highly flexible, but a significant reduction in conformational variability is induced when Ca2+ is bound to the channel [57]. In a very detailed work, the lab of György Hajnóczky showed that the channel resides in a non-conducting state, with openings to the low-conductance states in the absence of Ca2+, but starts gating and mainly resides in the high-conductance state as the concentration of Ca2+ increases [27], indicating a regulation of the channel through Ca2+. Indeed, the relationship between the external Ca2+ concentration and the uptake rate of Ca2+ into VDAC liposomes follows a non-linear relation, indicating the presence of a Ca2+-regulated Ca2+ flux through VDAC [27].
In an effort to localize the Ca2+ binding site, it was observed that the addition of an excess amount of Ca2+ prevented the blocking effect of RuR, which was reinstalled after the addition of EGTA. This suggests that ruthenium derivatives compete with Ca2+ for the same binding site [56]. Mutation of a glutamate residue at position 73 (E73) eliminated the blocking effect of RuR, establishing this residue as a primary candidate for the Ca2+-mediated control. However, E73 is located on the outside of the barrel facing the lipidic environment [11,12], and although molecular simulations predict a thinning of the nearby membrane due to the charged nature of E73 [58], it remains controversial whether Ca2+ can enter the lipidic environment of the membrane. Alternatively, the mere presence of E73 could stabilize the channel in a conformation that is required for Ca2+-mediated channel regulation.
Nevertheless, a regulation of the channels Ca2+ transport activity by E73 was recently confirmed in physiological experiments, where Ca2+ transfer from lysosomes into mitochondria after lysosomal Ca2+ release through TRPML1 was shown to depend on E73 [59]. In agreement with this, Ca2+ transfer from the SR into mitochondria in cardiomyocytes is also regulated through E73 [60]. These findings again highlight the presence of a Ca2+ regulated uptake of Ca2+ through VDAC.
2.2.3. Modulation of VDAC Gating by Posttranslational Modification
A common way to regulate ion channels are posttranslational modifications. Phosphorylation of all three VDAC isoforms was repeatedly reported, and several studies showed that PKA phosphorylation results in altered gating properties of VDAC. Interestingly, PKA phosphorylation affected channel gating asymmetrically with no influence at positive potentials but a reduction in both single channel conductance and open probability at negative potentials [61,62]. This is in line with the idea that, if a potential is established at all across the outer membrane, this would be a negative potential. Similar results were obtained for phosphorylation of VDAC by c-Jun N-terminal Kinase-3 (JNK3), which also led to a lower single-channel conductance and a reduction in open probability [63]. Although the authors of these studies only delineate a role of VDAC phosphorylation for the regulation of apoptosis, the modulatory effect of VDAC on apoptosis was critically linked to its Ca2+ conductance [5]. In later studies, both PKA-phosphorylation mediated by the 18 kDa translocator protein TSPO [64] and phosphorylation by PKCε reduced mitochondrial Ca2+ accumulation [65].
These experiments are difficult to interpret. Bilayer experiments indicate that phosphorylation of VDAC-induced voltage-dependent closure of the channel and in vitro phosphorylation of VDAC by either glycogen synthase kinase-3β (GSK3β) or cAMP-dependent protein kinase A (PKA) increased tubulin binding and, thereby, channel occlusion [66]. Both the voltage-closed state and the tubulin-bound occluded state were reported to induce Ca2+ flux, and the bilayer experiments are, thus, not in line with the physiological observations of a limited Ca2+ uptake after PKA phosphorylation [65]. This might indicate that results from lipid bilayers are too simplistic, and an additional degree of regulation exists in vivo.
A critical link between posttranslational modifications and Ca2+ conductance in vivo was established in a recent study where PINK1-dependent monoubiquitinylation of VDAC1 was directly shown to limit mitochondrial Ca2+ uptake. Abolishing VDAC1 monoubiquitinylation by introducing a K247R mutation in VDAC1 induced a Parkinson disease phenotype in fruitflies was associated with excessive apoptosis and could be relieved by MCU knock-out [7]. Although mechanistical data on how monoubiquitinylation decreases Ca2+ flux are missing in this report, these data directly indicate that reducing mitochondrial Ca2+ uptake through VDAC1 monoubiquitinylation is a critical mechanism to suppress apoptosis.
2.2.4. Modulation of VDAC Gating through the Lipidic Environment
Finally, the lipidic environment was shown to influence ion channels properties, and corresponding observations were also made for VDAC. When inserted into lipid bilayers, the addition of nonlamellar lipids such as phosphatidylethanolamine (PE) or cardiolipin induced asymmetry in voltage gating, evident by increased channel closure at negative potentials [29]. A similar effect was recently also reported for the neuroprotective cholesterol-like synthetic compound olesoxime, which also induced channel gating at more negative potentials [67]. This effect might again be relevant when VDAC is exposed to a negative potential in the OMM to facilitate or prevent gating of the channel. Additionally, the lipidic environment might also modulate the channels’ interaction with its molecular plugs. The neuroprotective cholesterol-like lipid olesoxime prevented the interaction of VDAC with α-synuclein and, thereby, affected not only VDAC voltage gating but also binding of α-synuclein to the channel [67]. As a third way to regulate VDAC through the lipidic environment, molecular dynamics simulations suggested that interactions of lipids with acidic residues of VDAC might modulate the anion selectivity of the channel [30].
Although lipid bilayer experiments and molecular dynamics simulations might not directly recapitulate channel function in vivo due to the simplicity of the system and the lack of additional regulatory factors present in its native environment, a regulation of the channel by membrane lipids might fine-tune channel activity in response to cellular stimuli also there. Indeed, a change in the membrane composition was reported, for example for the induction of apoptosis [68].
2.3. Regulation of VDAC Ca2+ Flux by Protein Partners
Many of the aforementioned mechanisms might be regulated by partner proteins. VDAC was shown to interact with many cellular proteins ranging from small signaling molecules to large enzymes and other ion channels, and these interactions are often associated with Ca2+ homeostasis or regulated by Ca2+.
In particular, members of the Bcl-2 family were described to influence mitochondrial Ca2+ uptake by binding to VDAC, presumably at a structure formed by the N-terminal helix and the loop connecting beta sheets 14 and 15 [36,69]. Bcl-xL was demonstrated to selectively interact with VDAC1 and VDAC3 but not VDAC2. Huang et al. report enhanced mitochondrial Ca2+ uptake, when Bcl-xL is associated with the channel [36] in permeabilized cells. Similarly, also the Bcl-2 member Mcl-1 interacts with VDAC1 and VDAC3 but only weakly with VDAC2 to promote the uptake of Ca2+ into mitochondria [70]. However, other reports in intact cells found that Bcl-xL limits Ca2+ uptake into mitochondria [71,72], differences that were explained by experimental disparities between whole cell experiments in which mitochondrial Ca2+ uptake is triggered by local IP3Rs in Ca2+ microdomains within contact sites of mitochondria and the SR and permeabilized cells in which mitochondrial Ca2+ uptake is triggered by a global rise in Ca2+ (see also Section 2.4). Interestingly, in lipid bilayers Bcl-xL induced a reduction in channel conductance, while preserving voltage gating [73], indicating that the Bxl-xL induced channel closure is distinct from voltage-gated closure but might also affect its Ca2+ conductance.
Furthermore, several enzymes have been found to interact with VDAC to regulate its Ca2+ flux. Among those are reports about GAPDH, which was recently shown to interact with VDAC1 to enhance the uptake of Ca2+ into VDAC1-containing protoliposomes [37] and the 18 kDa translocator protein TSPO, also referred to as the peripheral benzothiazepine receptor (PBR), a mitochondrial protein that forms a complex with VDAC, which, when inserted into lipid bilayers, was sensitive to its ligand hemin [74]. The addition of hemin closed VDAC and by this limited Ca2+ uptake into mitochondria. Although these interactions do not go into mechanistical detail, in particular, the question of why closure of the channel would decrease Ca2+ conductance, which is opposing to other results, they highlight the importance of direct or indirect protein–protein interactions for the regulation of VDAC.
Apart from interactions that were shown to directly alter VDAC electrophysiology or mitochondrial Ca2+ fluxes VDAC was repeatedly shown to interact with Ca2+ handling proteins, such as the SR Ca2+/K+ channel mitsugumin-23 [75], the L-type Ca2+ channel [76], the RyR [77], MCU [55], and the IP3R in a complex that is highly regulated through different proteins such as DJ-1 [78], grp75, or transglutaminase 2 [79]. Although these interactions were not directly shown to modulate VDAC, they still highlight its important role in Ca2+ handling.
Taken together, several proteins modulate VDAC directly or through higher protein complexes. However, only a small number of these reports specifically investigated Ca2+ conductance. Thus, ambiguity still exists about the Ca2+ conductivity of the gated and occluded states in vivo. Several reports indicate reduced Ca2+ uptake upon VDAC closure [74], while other reports do not observe changes on Ca2+ flux despite closure of the channel [80]. This might in part be explained by distinct modes of VDAC closure, whereof some affect Ca2+ conductivity and some do not, but it needs further research to selectively discriminate between them.
2.4. Regulation by Subcellular Localization in Ca2+ Microdomains
Finally, another powerful regulatory mechanism for cellular Ca2+ fluxes and Ca2+ signaling pathways is subcellular localization. In line with the idea that VDAC regulates the Ca2+ flux over the OMM, a distinct subcellular localization within sites of Ca2+ mobilization was also observed for VDAC.
Most eminently, mitochondria intimately and dynamically interact with the internal Ca2+ stores of the ER/SR in regions where the two organelles are densely tethered. Those sites of ER/SR-mitochondrial coupling, called mitochondrial-associated membranes (MAMs), are a key player in Ca2+ shuttling. MAMs cover approximately 5–20 percent of the mitochondrial surface in mammalian cells [81]. Interestingly, VDAC is not homogeneously distributed across the OMM and certain VDAC isoforms occur more frequently in MAMs and in close vicinity to ER/SR Ca2+ release sites, where local cytosolic Ca2+ can reach particularly high levels [54,77,82,83,84]. The IP3R, the Ca2+ release channel in the ER membrane of non-excitable cells is tethered to VDAC1 but not VDAC2 or VDAC3 [5,85], through the anchoring protein grp75 [85,86]. More recent studies have shown that in cardiomyocytes VDAC2 is analogously coupled to RyR2 [10,77]. For the subsequent transport of Ca2+ into mitochondria, the SR-OMM contact points align with contact points of OMM and IMM [54], and VDAC1 physically and functionally interacts with the MCUC [55].
These couplons of Ca2+ release (IP3R/RyR2), Ca2+ shuttling (VDACs), and Ca2+ uptake sites (MCUC) provide an efficient route for direct Ca2+ shuttling from high Ca2+ microdomains of the SR into mitochondria. They were shown to be dynamic, as they can change in response to cytosolic or extracellular triggers to regulate mitochondrial Ca2+ uptake [8,86] and are intensively regulated at different levels under physiological and pathophysiological conditions [8,78,87] (see Section 3 and Section 4).
However, intracellular Ca2+ transfer between organelles regulated through VDAC localization is not an exclusive mechanism limited to the contact sites with the ER/SR. The lysosomal Ca2+ release channel TRPML1 was shown to selectively interact with VDAC1 but not VDAC2 or VDAC3 to mediate Ca2+ transfer from lysosomes to mitochondria. Interestingly, this VDAC1-mediated lysosome–mitochondria Ca2+ transfer was shown to be regulated by Ca2+ through residue E73 [59].
It is tempting to speculate that the subcellular localization not only positions VDAC in an ideal environment for Ca2+ transfer from the ER into mitochondria but also enables a higher degree of regulation. As such, it is feasible that the high Ca2+ concentration within this microdomain, local changes in OMM membrane potential, or a different set of partner proteins in this micro-environment allow for distinct regulation of channels inside the MAM compared to channels outside of the MAM (Figure 3).
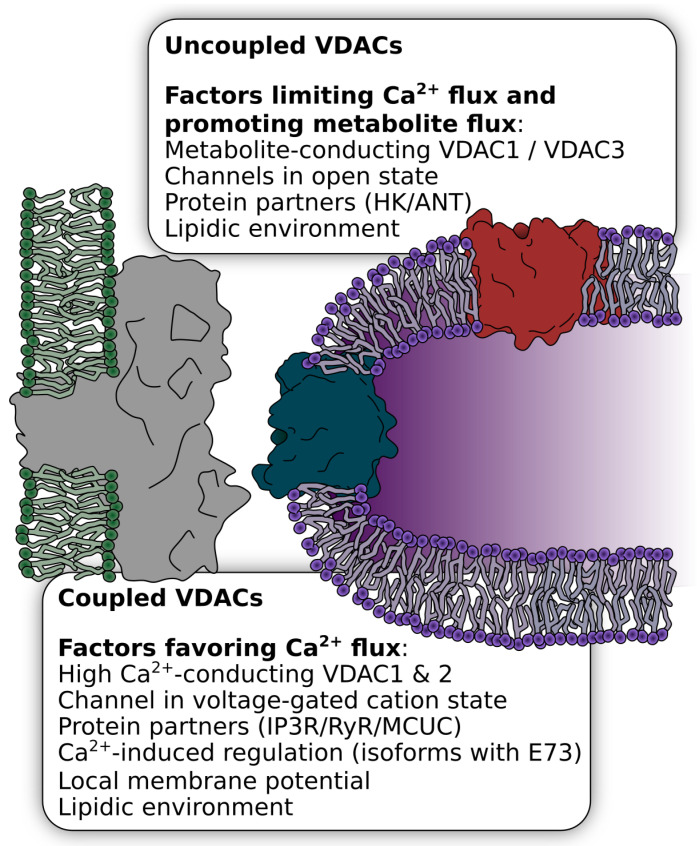
Figure 3.
Regulation of VDAC by subcellular localization. A fraction of VDAC channels was shown to be present in couplons with Ca2+ release channels of the ER/SR, in particular VDAC1 with the IP3R or VDAC2 with the RyR. These couplons represent hotspots of Ca2+-mediated regulation of mitochondrial activity. In addition to the strategic positioning of VDAC in close vicinity to the mouth of the release channels, other factors might regulate VDAC within and outside the couplons for the specialized tasks within these environments. These include isoform selectivity, protein partners, the lipidic environment, and the voltage-gated state of the channel. Depending on the VDAC localization, these factors might determine whether VDAC serves as a Ca2+ or metabolite channel.
2.4.1. Regulation of the OMM Ca2+ Flux by Different VDAC Isoforms
An alternative but also associated form of regulation might be achieved by isoform specificity. While the three VDAC isoforms are certainly able to fulfill to some degree redundant and compensatory functions between each other, they also have different channel properties, different expression levels, and specific protein interaction partners, indicating that the isoforms serve different physiological tasks [88]. Furthermore, the same VDAC isoform might perform distinct roles in different tissues or cell types depending on its microenvironment. The specific knock-out of individual VDAC isoforms provided first insights into their function: VDAC1 KO mice are viable and studies with VDAC1-/- mice confirmed that it is mainly involved in metabolite exchange and pro-apoptotic processes [89], whereof the latter is believed to be mediated by mitochondrial Ca2+ uptake. Indeed, in VDAC1-/- HeLa cells, the transfer of local, low-amplitude Ca2+ signals was selectively abolished. However, mitochondrial Ca2+ uptake after maximum agonist response was still measurable, indicating that other VDAC isoforms, presumably outside of the IP3R couplons, are able to take up Ca2+ from high-amplitude signals [5].
In contrast to VDAC1, a global VDAC2 KO, was demonstrated to be lethal at around E10.5 to E11.5 of embryonic development. Furthermore, a heart-specific VDAC2 knockout showed postnatal onset of progressive fibrosis and cardiomyopathy, resulting in early mortality indicating a, presumably cardiac, role of VDAC2, which cannot be fulfilled by the two other isoforms [90]. One intriguing possibility is that the other VDAC isoforms cannot form the specific interaction of VDAC2 with RyR2 in the highly specialized heart muscle cells [77]. This would result in decreased mitochondrial Ca2+ uptake via RyR2 and VDAC2 and associated downstream effects such as, for example, cytosolic Ca2+ overload, a lack of mitochondrial energy–demand matching, or an impairment in mitochondrial function.
It is, thus, conceivable that the IP3R-VDAC1 coupling is required for the control of apoptosis through local low-amplitude Ca2+ signals. In cardiomyocytes, however, where local RyR-mediated Ca2+ signals of the SR are of a higher magnitude and more frequent, the presence of VDAC1 within these couplons would induce immediate apoptosis due its interaction with pro-apoptotic protein partners and/or other regulatory mechanisms that are specific for VDAC1. Therefore, in the heart, VDAC2 mediates a more effective flux of Ca2+ into mitochondria in the MAM, where it shapes cytosolic Ca2+ signals and rapidly accommodates energy–demand matching, while VDAC1 is located outside of the couplons and mediates metabolite flux. Indeed, VDAC2 was shown to have a different distribution of charges within the pore, which mediates a lower anion selectivity [91]. Enhanced apoptosis that might be induced by this process could be suppressed by the anti-apoptotic inhibition of BAK activation by VDAC2 [92]. The existence of IP3R-VDAC1 couplons in cardiomyocytes is under debate but would not contradict this picture.
In contrast to VDAC1 and VDAC2, the role of VDAC3 in Ca2+ signaling is questionable. Indeed, VDAC3 lacks residue E73 that was reported to be important for Ca2+-dependent regulation of the channel and is conserved in both VDAC1 and VDAC2.
2.4.2. Regulation of OMM Ca2+ Flux by VDAC Expression Levels
A very direct way to regulate ion channel activity is the variation of the mere number of channels by adapting expression levels. Indeed, several reports have identified a variation in expression levels as a way to regulate VDAC activity. A first hint that expression levels of VDAC1 can control life and death of the cell was that downregulation of VDAC1 inhibited cell growth and overexpression lead to cell death [93]. This process seems to be physiologically relevant, since in podocytes, induction of apoptosis was shown to cause an increased expression of both VDAC1 and the IP3R, presumably to enhance mitochondrial Ca2+ uptake. Likewise, in diabetes, coronary endothelia cells display higher rates of apoptosis due to mitochondrial Ca2+ overload, which was linked to enhanced VDAC1 protein levels [94]. Vice-versa, a selective downregulation of VDAC1 but not VDAC2 was suggested to serve as a cardioprotective pathway during ischemia reperfusion [95].
Several pathways have been described to control VDAC expression. VDAC1 downregulation during ischemia reperfusion was shown to depend on liproxstatin-1 [95], and the transcription factors GATA1 and MYBL2 were reported to bind to and activate the VDAC2 promoter, to suppresses autophagy [96]. Another expression regulator could be Ca2+ itself. Interestingly, in a recent report, elevation of intracellular Ca2+ levels by Ca2+ mobilizing agents induced overexpression of VDAC1, while chelation of intracellular Ca2+ reduced VDAC1 expression [97].
3. Physiological Relevance of a Ca2+ Flux Regulation at the OMM
Mitochondria play a pivotal role in sensing, regulating, and decoding cellular Ca2+ signals. Mitochondrial Ca2+ can induce apoptotic signals and decode a “survival or death decision”, increase mitochondrial metabolism to adjust to a higher energy demand, or modulate cytosolic Ca2+ signals. While the main Ca2+ uptake route is supposed to be via MCUC in the IMM, a substantial part of the decoding could also be controlled by the permeability of the outer membrane for Ca2+ via VDACs and is regulated at different levels like isoform specificity, localization, and regulation of the individual channels.
One hypothesis of how VDAC mediates distinct Ca2+-induced signals is maybe best explained by taking highly specialized cells such as cardiomyocytes as an example. Cardiomyocytes are characterized by precisely regulated Ca2+ oscillations and critically depend on constant energy supply. Here, VDAC2 interacts with RyR2 in subsarcolemmal regions and at SR–mitochondria contact sites (see Section 2.4) and transfers beat-to-beat Ca2+ signals into mitochondria to respond to an increased workload with increased ATP synthesis. While VDAC2 is “occupied” with Ca2+ shuttling, VDAC1 could fulfill the function of a metabolite transporter across the outer membrane to ensure the supply of metabolites to the cytosol.
Although this is still only a hypothetical model, several experimental observations are in agreement with this split of specialized tasks between the isoforms and between coupled and uncoupled VDACs, respectively: VDAC2 was suggested to be less anion selective and, thus, more permeable to Ca2+. Furthermore, it was proposed that the close proximity of the IMM and OMM in the contact sites favors voltage-induced VDAC closure, which additionally favors Ca2+ flux, whereas VDACs that are distributed outside of these contact sites might be in an open state [25], thus favoring the flux of metabolites (Figure 3). A specific interaction of VDAC1 with ANT [98] and hexokinase (HK) might additionally favor the high-conductance state of VDAC1 and, thus, provide an effective metabolite exchange across the OMM. In this scenario, the lack of coupling of the pro-apoptotic VDAC1 with the high-efficiency Ca2+ release sites around RyR clusters and the anti-apoptotic features of VDAC2 prevent apoptosis induction. However, in addition to these highly efficient RyR2-VDAC2 clusters, the canonical VDAC1-IP3R clusters were also observed in cardiomyocytes [87], which might be located in areas distinct from the RyR2-VDAC2 clusters away from the SR Ca2+ release sites within the Z-bands and be important for mediating apoptosis.
Indeed, the VDAC1-mediated mitochondrial Ca2+ flux at IP3R clusters was shown to regulate apoptosis: here, VDAC1 significantly contributes to the determination of cell fate towards survival or death [99,100,101]. Mitochondrial Ca2+ signals can either boost mitochondrial respiration or induce apoptosis. Therefore, they need to be meticulously regulated, and it is assumed that numerous factors, in particular the interaction of VDAC1 with the IP3R, HK, and Bcl-2 family members, regulate this process. Apoptosis induction was shown to increase expression and interaction of VDAC1 with the IP3R, presumably to enhance mitochondrial Ca2+ uptake [5,94]. However, blocking Ca2+ release via IP3R or mitochondrial Ca2+ uptake leads to necrosis of cancer cells but not normal cells [102]. These results highlight once more the importance of Ca2+ signal fine tuning within nanodomains to control cellular metabolism and apoptosis but also show that more work is necessary to elucidate the exact mechanisms of VDAC-controlled mitochondrial Ca2+ uptake.
4. Regulation of VDAC-Mediated Ca2+ Flux under Pathophysiological Conditions
Considering the prominent role of VDAC-mediated Ca2+ signaling, it is only consistent that altered Ca2+ handling via VDAC is also associated with pathophysiology of many diseases, including cancer, cardiovascular diseases, and neurodegenerative diseases.
Cancer cell growth relies on adaptions in cell metabolism and bioenergetics, and VDACs significantly contribute to these alterations by regulating metabolite and ion flux into and out of mitochondria [103,104,105]. A central role in oncogenic behavior was ascribed to mitochondrial Ca2+ remodeling [106], and specifically, the VDAC1-mediated association between the ER and mitochondria was suggested to be essential for cancer cell viability. Indeed, both blocking of the IP3R Ca2+ release and blocking of mitochondrial Ca2+ uptake, respectively, lead to necrosis of cancer cells but not normal cells [102]. Strikingly, downregulation of VDAC1 via RNA interference was shown to limit cancer cell growth in vitro and tumor development in vivo without otherwise affecting the mice [107,108]. Several mechanisms were suggested to regulate VDACs Ca2+ conductance in cancer: hexokinase specifically interacts with VDAC1 in cancer cells to promote metabolism and to reduce apoptosis [6]. In non-small cell lung cancer cells Mcl-1, an anti-apoptotic member of the Bcl-2 family, interacts with VDAC to enhance mitochondrial Ca2+ uptake and ROS production resulting in an increased cancer cell migration [70].
Abnormal mitochondrial Ca2+ handling, resulting in energy failure, is a key factor of heart failure. Several reports describe the involvement of VDAC in this process. In ventricular cardiomyocytes isolated from failing rat hearts, the expression of the Bcl-2 family member BNIP3 was found to be significantly increased. An interaction of BNIP3 with VDAC and a consecutive shift of Ca2+ from the SR into mitochondria results in decreased SR Ca2+ content and mitochondrial damage [109]. Likewise, during remodeling after myocardial infarction, reactive aldehydes such as 4-hydroxynonenal are produced by mitochondria and by a yet not understood mechanism bind to VDAC1 and MCU to promote formation of ER–mitochondria contact sites and ultimately mitochondrial Ca2+ accumulation [87]. Another study showed that in diabetes, heart failure develops due to coronary microvascular rarefaction induced by apoptosis in coronary endothelia through increased VDAC1 expression [94]. Strikingly, overexpression of hexokinase 2 reduced mitochondrial overload and apoptosis [110]. Similarly, excessive VDAC-mediated Ca2+ transfer was also suggested in ischemia reperfusion (I/R), and a block of mitochondrial Ca2+ uptake was shown to be beneficial for the survival of cardiac tissue [111,112]. The close proximity between VDAC1 and SR Ca2+ handling proteins has been shown to be significant during I/R. Resveratrol, a polyphenolic compound in red wine, prevents VDAC1 upregulation in I/R, leading to a reduced infarct size in vivo [113]. Liproxstatin-1 was shown to be protective against I/R injury by decreasing VDAC1 expression and, thus, reducing low-amplitude apoptotic Ca2+ shuttling by the IP3R-Grp75-VDAC1 complex [95]. In adult mouse cardiomyocytes, the inhibition of GSK3β, which promotes an increased Ca2+ shuttling between IP3R and VDAC1, limits the Ca2+ transfer from SR/ER to mitochondria and prevents mitochondrial Ca2+ overload [114].
All these data indicate that mitochondrial dysfunction through Ca2+ overload is a key player in heart failure and I/R. However, contrarily to these results, other studies propose heart failure to be associated with a reduced ER–mitochondria coupling: in diabetes type 2, where a reduced Ca2+ shuttling in MAM regions was repeatedly demonstrated for many tissues [115,116,117], a reduction in IP3R-VDAC1 interaction and, thus, a decrease in IP3 stimulated Ca2+ transfer to mitochondria was observed for cardiomyocytes. The impaired SR–mitochondrial Ca2+ coupling resulted in an impairment of mitochondrial energy supply, reduced cell contraction, and thus, cardiomyopathy [118].
These data are in line with the idea that an enhanced mitochondrial Ca2+ uptake can be protective in pressure overload induced heart failure: TSPO closely interacts with VDAC1, acts as a negative regulator, and decreases mitochondrial Ca2+ uptake by inhibiting VDAC1 expression and mediating PKA phosphorylation [74,119]. Mice that underwent transverse aortic banding surgery showed significantly higher expression levels of TSPO, and cardiac TSPO-ko substantially limited the progression of pressure-induced HF and maintained cardiac function in vivo [120]. Taken together, myocardial remodeling during heart failure was repeatedly shown to affect SR–mitochondria coupling and in particular VDAC-mediated Ca2+ transfer; however, further research is needed to clarify opposing results of a diminished or increased ER/SR–mitochondria coupling.
Considering the important role of VDAC in physiological and especially pathophysiological modulation of mitochondrial Ca2+ uptake in the heart, it is, however, tempting to investigate the potential of drugs that interfere with this process. Indeed, our lab has recently described a very prominent role of the small molecular VDAC2 modulator efsevin. In lipid bilayer experiments, efsevin induced gating of VDAC2 and promoted the closed state of the channel [10]. In cardiomyocytes, this specifically increased Ca2+ transfer from the SR into mitochondria and, thereby, spatially and temporally restricted diastolic Ca2+ sparks and prevented the formation of arrhythmogenic Ca2+ waves [9]. Most importantly, efsevin significantly reduced ventricular tachycardia in mice.
Additionally, for neurodegenerative diseases disruption of functional ER–mitochondrial Ca2+ homeostasis was shown to be associated with diseases such as Parkinson’s disease (PD), Alzheimer’s disease (AD), and amyotrophic lateral sclerosis (ALS) [121]. One protein that has been associated with the early-onset of PD is DJ-1, which was recently shown to interact with the IP3R-Grp75-VDAC1 complex [78,122]. DJ-1 reduction functionally correlates with decreased mitochondrial Ca2+ uptake and cells expressing PD-associated DJ-1 mutants showed impaired mitochondrial function [78,123]. A recent study has further demonstrated that functional ER–mitochondria interactions are of great importance for axon regeneration after injury. The results suggest that increased Grp75 expression enhances ER–mitochondria tethering, which leads to an increase in mitochondrial Ca2+ uptake that seems to be beneficial for axon regeneration [124].
5. Conclusions
Taken together, the compiled data indicate that VDAC-mediated mitochondrial Ca2+ uptake, in particular, though its close interaction with the ER/SR calcium release sites, is an important modulator of physiology and pathophysiology of the cell. However, discrepancies exist between results obtained from lipid bilayer experiments and experiments performed in cells, in particular concerning the flux of Ca2+ upon channel closure, indicating that in the native environment other or additional levels of VDAC regulation exist and lipid bilayer experiments should not be overinterpreted. VDAC-mediated mitochondrial Ca2+ uptake represents a critical component to decipher cellular Ca2+ signals and to mediate either enhanced energy production or induction of apoptosis but also to fine-tune intracellular Ca2+ signals. Additionally, at least in cardiomyocytes, it further shapes cytosolic Ca2+ signals and acts as a buffer to blunt cytosolic Ca2+ spikes. VDAC-mediated Ca2+ uptake is regulated at different levels, ranging from expression to protein partners as well as intrinsic properties of VDAC, which can adapt multiple states of conductance. However, the specific molecular mechanisms leading to these different conductance states have not been fully resolved and care should be taken when using the term “channel closure”, since voltage-induced closure, as well as occlusion, prevents the flux of metabolites but increases Ca2+ flux. Future studies are needed to resolve open questions such as the role of voltage gating in vivo the relevance of VDAC gating cellular physiology and the selective contribution of the regulation of mitochondrial Ca2+ uptake at the OMM and IMM, respectively.
Acknowledgments
The authors thank Annette Nicke and Simon Huber for critical comments on the manuscripts.
Author Contributions
P.S. and J.S. performed literature review and wrote the manuscript. T.G. and J.S. provided resources and funding. All authors revised and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was in part funded by the German Research Foundation (DFG), grant number SCHR 1471/1-1 to J.S.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Slater E.C., Cleland K.W. The calcium content of isolated heart-muscle sarcosomes. Biochem. J. 1953;54:xxii. [PubMed] [Google Scholar]
- 2.DeLuca H.F., Engstrom G.W. Calcium uptake by rat kidney mitochondria. Proc. Natl. Acad. Sci. USA. 1961;47:1744–1750. doi: 10.1073/pnas.47.11.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gincel D., Zaid H., Shoshan-Barmatz V. Calcium binding and translocation by the voltage-dependent anion channel: A possible regulatory mechanism in mitochondrial function. Biochem. J. 2001;358:147–155. doi: 10.1042/bj3580147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rapizzi E., Pinton P., Szabadkai G., Wieckowski M.R., Vandecasteele G., Baird G., Tuft R.A., Fogarty K.E., Rizzuto R. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J. Cell Biol. 2002;159:613–624. doi: 10.1083/jcb.200205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Stefani D., Bononi A., Romagnoli A., Messina A., De Pinto V., Pinton P., Rizzuto R. VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria. Cell Death Differ. 2012;19:267–273. doi: 10.1038/cdd.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azoulay-Zohar H., Israelson A., Abu-Hamad S., Shoshan-Barmatz V. In self-defence: Hexokinase promotes voltage-dependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. Biochem. J. 2004;377:347–355. doi: 10.1042/bj20031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ham S.J., Lee D., Yoo H., Jun K., Shin H., Chung J. Decision between mitophagy and apoptosis by Parkin via VDAC1 ubiquitination. Proc. Natl. Acad. Sci. USA. 2020;117:4281–4291. doi: 10.1073/pnas.1909814117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng P., Chen Q., Tian X., Qian N., Chai P., Liu B., Hu J., Blackstone C., Zhu D., Teng J., et al. DNA damage triggers tubular endoplasmic reticulum extension to promote apoptosis by facilitating ER-mitochondria signaling. Cell Res. 2018;28:833–854. doi: 10.1038/s41422-018-0065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schweitzer M.K., Wilting F., Sedej S., Dreizehnter L., Dupper N.J., Tian Q., Moretti A., My I., Kwon O., Priori S.G., et al. Suppression of Arrhythmia by Enhancing Mitochondrial Ca2+ Uptake in Catecholaminergic Ventricular Tachycardia Models. JACC Basic Transl. Sci. 2017;2:737–746. doi: 10.1016/j.jacbts.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilting F., Kopp R., Gurnev P.A., Schedel A., Dupper N.J., Kwon O., Nicke A., Gudermann T., Schredelseker J. The antiarrhythmic compound efsevin directly modulates voltage-dependent anion channel 2 by binding to its inner wall and enhancing mitochondrial Ca2+ uptake. Br. J. Pharmacol. 2020;177:2947–2958. doi: 10.1111/bph.15022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ujwal R., Cascio D., Colletier J.-P., Faham S., Zhang J., Toro L., Ping P., Abramson J. The crystal structure of mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into metabolite gating. Proc. Natl. Acad. Sci. USA. 2008;105:17742–17747. doi: 10.1073/pnas.0809634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schredelseker J., Paz A., López C.J., Altenbach C., Leung C.S., Drexler M.K., Chen J.-N., Hubbell W.L., Abramson J. High-Resolution Structure and Double Electron-Electron Resonance of the Zebrafish Voltage Dependent Anion Channel 2 Reveal an Oligomeric Population. J. Biol. Chem. 2014;289:12566–12577. doi: 10.1074/jbc.M113.497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastava S.R., Mahalakshmi R. Evolutionary selection of a 19-stranded mitochondrial β-barrel scaffold bears structural and functional significance. J. Biol. Chem. 2020;295:14653–14665. doi: 10.1074/jbc.RA120.014366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choudhary O.P., Paz A., Adelman J., Colletier J.-P., Abramson J., Grabe M. Characterizing ATP Permeation through the Voltage-Dependent Anion Channel VDAC. Biophys. J. 2014;106:147a–148a. doi: 10.1016/j.bpj.2013.11.851. [DOI] [Google Scholar]
- 15.Colombini M. Voltage gating in the mitochondrial channel, VDAC. J. Membr. Biol. 1989;111:103–111. doi: 10.1007/BF01871775. [DOI] [PubMed] [Google Scholar]
- 16.Menzel V.A., Cassará M.C., Benz R., De Pinto V., Messina A., Cunsolo V., Saletti R., Hinsch K., Hinsch E. Molecular and functional characterization of VDAC2 purified from mammal spermatozoa. Biosci. Rep. 2009;29:351–362. doi: 10.1042/BSR20080123. [DOI] [PubMed] [Google Scholar]
- 17.Mertins B., Psakis G., Grosse W., Back K.C., Salisowski A., Reiss P., Koert U., Essen L.-O. Flexibility of the N-Terminal mVDAC1 Segment Controls the Channel’s Gating Behavior. PLoS ONE. 2012;7:e47938. doi: 10.1371/journal.pone.0047938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guardiani C., Magrì A., Karachitos A., Di Rosa M.C., Reina S., Bodrenko I., Messina A., Kmita H., Ceccarelli M., De Pinto V. yVDAC2, the second mitochondrial porin isoform of Saccharomyces cerevisiae. Biochim. Biophys. Acta Bioenerg. 2018;1859:270–279. doi: 10.1016/j.bbabio.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Tan W., Colombini M. VDAC closure increases calcium ion flux. Biochim. Biophys. Acta. 2007;1768:2510–2515. doi: 10.1016/j.bbamem.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zachariae U., Schneider R., Briones R., Gattin Z., Demers J.-P., Giller K., Maier E., Zweckstetter M., Griesinger C., Becker S., et al. β-Barrel Mobility Underlies Closure of the Voltage-Dependent Anion Channel. Structure. 2012;20:1540–1549. doi: 10.1016/j.str.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavlov E., Grigoriev S.M., Dejean L.M., Zweihorn C.L., Mannella C.A., Kinnally K.W. The mitochondrial channel VDAC has a cation-selective open state. Biochim. Biophys. Acta Bioenerg. 2005;1710:96–102. doi: 10.1016/j.bbabio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Checchetto V., Reina S., Magrì A., Szabo I., De Pinto V. Recombinant human voltage dependent anion selective channel isoform 3 (hVDAC3) forms pores with a very small conductance. Cell. Physiol. Biochem. 2014;34:842–853. doi: 10.1159/000363047. [DOI] [PubMed] [Google Scholar]
- 23.Queralt-Martín M., Bergdoll L., Teijido O., Munshi N., Jacobs D., Kuszak A.J., Protchenko O., Reina S., Magrì A., De Pinto V., et al. A lower affinity to cytosolic proteins reveals VDAC3 isoform-specific role in mitochondrial biology. J. Gen. Physiol. 2020;152 doi: 10.1085/jgp.201912501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemeshko V.V. Model of the Outer Membrane Potential Generation by the Inner Membrane of Mitochondria. Biophys. J. 2002;82:684–692. doi: 10.1016/S0006-3495(02)75431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams V., Bosch W., Schlegel J., Wallimann T., Brdiczka D. Further characterization of contact sites from mitochondria of different tissues: Topology of peripheral kinases. Biochim. Biophys. Acta Biomembr. 1989;981:213–225. doi: 10.1016/0005-2736(89)90031-X. [DOI] [PubMed] [Google Scholar]
- 26.Porcelli A.M., Ghelli A., Zanna C., Pinton P., Rizzuto R., Rugolo M. pH difference across the outer mitochondrial membrane measured with a green fluorescent protein mutant. Biochem. Biophys. Res. Commun. 2005;326:799–804. doi: 10.1016/j.bbrc.2004.11.105. [DOI] [PubMed] [Google Scholar]
- 27.Báthori G., Csordás G., Garcia-Perez C., Davies E., Hajnóczky G. Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC) J. Biol. Chem. 2006;281:17347–17358. doi: 10.1074/jbc.M600906200. [DOI] [PubMed] [Google Scholar]
- 28.Lee A.C., Xu X., Colombini M. The role of pyridine dinucleotides in regulating the permeability of the mitochondrial outer membrane. J. Biol. Chem. 1996;271:26724–26731. doi: 10.1074/jbc.271.43.26724. [DOI] [PubMed] [Google Scholar]
- 29.Rostovtseva T.K., Kazemi N., Weinrich M., Bezrukov S.M. Voltage gating of VDAC is regulated by nonlamellar lipids of mitochondrial membranes. J. Biol. Chem. 2006;281 doi: 10.1074/jbc.M602548200. [DOI] [PubMed] [Google Scholar]
- 30.Van Liefferinge F., Krammer E.-M., Sengupta D., Prévost M. Lipid composition and salt concentration as regulatory factors of the anion selectivity of VDAC studied by coarse-grained molecular dynamics simulations. Chem. Phys. Lipids. 2019;220:66–76. doi: 10.1016/j.chemphyslip.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Budelier M.M., Cheng W.W.L., Bergdoll L., Chen Z.-W., Janetka J.W., Abramson J., Krishnan K., Mydock-McGrane L., Covey D.F., Whitelegge J.P., et al. Photoaffinity labeling with cholesterol analogues precisely maps a cholesterol-binding site in voltage-dependent anion channel-1. J. Biol. Chem. 2017;292:9294–9304. doi: 10.1074/jbc.M116.773069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Briones R., Weichbrodt C., Paltrinieri L., Mey I., Villinger S., Giller K., Lange A., Zweckstetter M., Griesinger C., Becker S., et al. Voltage Dependence of Conformational Dynamics and Subconducting States of VDAC-1. Biophys. J. 2016;111:1223–1234. doi: 10.1016/j.bpj.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Queralt-Martín M., Hoogerheide D.P., Noskov S.Y., Berezhkovskii A.M., Rostovtseva T.K., Bezrukov S.M. VDAC gating thermodynamics, but not gating kinetics, are virtually temperature-independent. Biophys. J. 2020;119:2584–2592. doi: 10.1016/j.bpj.2020.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tewari D., Ahmed T., Chirasani V.R., Singh P.K., Maji S.K., Senapati S., Bera A.K. Modulation of the mitochondrial voltage dependent anion channel (VDAC) by curcumin. Biochim. Biophys. Acta. 2015;1848:151–158. doi: 10.1016/j.bbamem.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 35.Tewari D., Majumdar D., Vallabhaneni S., Bera A.K. Aspirin induces cell death by directly modulating mitochondrial voltage-dependent anion channel (VDAC) Sci. Rep. 2017;7:45184. doi: 10.1038/srep45184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang H., Hu X., Eno C.O., Zhao G., Li C., White C. An interaction between Bcl-xL and the voltage-dependent anion channel (VDAC) promotes mitochondrial Ca2+ uptake. J. Biol. Chem. 2013;288:19870–19881. doi: 10.1074/jbc.M112.448290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarze A., Deniaud A., Le Bras M., Maillier E., Molle D., Larochette N., Zamzami N., Jan G., Kroemer G., Brenner C. GAPDH, a novel regulator of the pro-apoptotic mitochondrial membrane permeabilization. Oncogene. 2007;26:2606–2620. doi: 10.1038/sj.onc.1210074. [DOI] [PubMed] [Google Scholar]
- 38.Okazaki M., Kurabayashi K., Asanuma M., Saito Y., Dodo K., Sodeoka M. VDAC3 gating is activated by suppression of disulfide-bond formation between the N-terminal region and the bottom of the pore. Biochim. Biophys. Acta Biomembr. 2015;1848:3188–3196. doi: 10.1016/j.bbamem.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Maurya S.R., Mahalakshmi R. N-helix and Cysteines Inter-regulate Human Mitochondrial VDAC-2 Function and Biochemistry. J. Biol. Chem. 2015;290:30240–30250. doi: 10.1074/jbc.M115.693978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shuvo S.R., Ferens F.G., Court D.A. The N-terminus of VDAC: Structure, mutational analysis, and a potential role in regulating barrel shape. Biochim. Biophys. Acta Biomembr. 2016;1858:1350–1361. doi: 10.1016/j.bbamem.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Choudhary O.P., Ujwal R., Kowallis W., Coalson R., Abramson J., Grabe M. The Electrostatics of VDAC: Implications for Selectivity and Gating. J. Mol. Biol. 2010;396:580–592. doi: 10.1016/j.jmb.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teijido O., Ujwal R., Hillerdal C.-O., Kullman L., Rostovtseva T.K., Abramson J. Affixing N-terminal α-helix to the wall of the voltage-dependent anion channel does not prevent its voltage gating. J. Biol. Chem. 2012;287:11437–11445. doi: 10.1074/jbc.M111.314229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zizi M., Forte M., Blachly-Dyson E., Colombini M. NADH regulates the gating of VDAC, the mitochondrial outer membrane channel. J. Biol. Chem. 1994;269:1614–1616. doi: 10.1016/S0021-9258(17)42070-9. [DOI] [PubMed] [Google Scholar]
- 44.Böhm R., Amodeo G.F., Murlidaran S., Chavali S., Wagner G., Winterhalter M., Brannigan G., Hiller S. The Structural Basis for Low Conductance in the Membrane Protein VDAC upon β-NADH Binding and Voltage Gating. Structure. 2020;28:206–214.e4. doi: 10.1016/j.str.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rostovtseva T.K., Gurnev P.A., Hoogerheide D.P., Rovini A., Sirajuddin M., Bezrukov S.M. Sequence diversity of tubulin isotypes in regulation of the mitochondrial voltage-dependent anion channel. J. Biol. Chem. 2018;293:10949–10962. doi: 10.1074/jbc.RA117.001569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puurand M., Tepp K., Timohhina N., Aid J., Shevchuk I., Chekulayev V., Kaambre T. Tubulin βII and βIII Isoforms as the Regulators of VDAC Channel Permeability in Health and Disease. Cells. 2019;8:239. doi: 10.3390/cells8030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rostovtseva T.K., Sheldon K.L., Hassanzadeh E., Monge C., Saks V., Bezrukov S.M., Sackett D.L. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc. Natl. Acad. Sci. USA. 2008;105:18746–18751. doi: 10.1073/pnas.0806303105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoogerheide D.P., Gurnev P.A., Rostovtseva T.K., Bezrukov S.M. Mechanism of α-synuclein translocation through a VDAC nanopore revealed by energy landscape modeling of escape time distributions. Nanoscale. 2017;9:183–192. doi: 10.1039/C6NR08145B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gurnev P.A., Rostovtseva T.K., Bezrukov S.M. Tubulin-blocked state of VDAC studied by polymer and ATP partitioning. FEBS Lett. 2011;585:2363–2366. doi: 10.1016/j.febslet.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simson P., Jepihhina N., Laasmaa M., Peterson P., Birkedal R., Vendelin M. Restricted ADP movement in cardiomyocytes: Cytosolic diffusion obstacles are complemented with a small number of open mitochondrial voltage-dependent anion channels. J. Mol. Cell. Cardiol. 2016;97:197–203. doi: 10.1016/j.yjmcc.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 51.Lemeshko V.V. VDAC electronics: 1. VDAC-hexo(gluco)kinase generator of the mitochondrial outer membrane potential. Biochim. Biophys. Acta Biomembr. 2014;1838:1362–1371. doi: 10.1016/j.bbamem.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Lemeshko V.V. VDAC electronics: 5. Mechanism and computational model of hexokinase-dependent generation of the outer membrane potential in brain mitochondria. Biochim. Biophys. Acta Biomembr. 2018;1860:2599–2607. doi: 10.1016/j.bbamem.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Lemeshko V.V. Electrical control of the cell energy metabolism at the level of mitochondrial outer membrane. Biochim. Biophys. Acta Biomembr. 2021;1863:183493. doi: 10.1016/j.bbamem.2020.183493. [DOI] [PubMed] [Google Scholar]
- 54.García-Pérez C., Schneider T.G., Hajnóczky G., Csordás G. Alignment of sarcoplasmic reticulum-mitochondrial junctions with mitochondrial contact points. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H1907-15. doi: 10.1152/ajpheart.00397.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liao Y., Hao Y., Chen H., He Q., Yuan Z., Cheng J. Mitochondrial calcium uniporter protein MCU is involved in oxidative stress-induced cell death. Protein Cell. 2015;6:434–442. doi: 10.1007/s13238-015-0144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Israelson A., Abu-Hamad S., Zaid H., Nahon E., Shoshan-Barmatz V. Localization of the voltage-dependent anion channel-1 Ca2+-binding sites. Cell Calcium. 2007;41:235–244. doi: 10.1016/j.ceca.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 57.Ge L., Villinger S., Mari S.A., Giller K., Griesinger C., Becker S., Müller D.J., Zweckstetter M. Molecular Plasticity of the Human Voltage-Dependent Anion Channel Embedded Into a Membrane. Structure. 2016;24:585–594. doi: 10.1016/j.str.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Villinger S., Briones R., Giller K., Zachariae U., Lange A., de Groot B.L., Griesinger C., Becker S., Zweckstetter M. Functional dynamics in the voltage-dependent anion channel. Proc. Natl. Acad. Sci. USA. 2010;107:22546–22551. doi: 10.1073/pnas.1012310108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng W., Wong Y.C., Krainc D. Mitochondria-lysosome contacts regulate mitochondrial Ca2+ dynamics via lysosomal TRPML1. Proc. Natl. Acad. Sci. USA. 2020;117:19266–19275. doi: 10.1073/pnas.2003236117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schredelseker J., Shimizu H., Huang J., Lin B., Chen J.-N. Regulation of Voltage-Dependent Anion Channel 2 at Glutamate 73 is Critical for its Role in Cardiac Calcium Handling. Biophys. J. 2012;102:312a. doi: 10.1016/j.bpj.2011.11.1720. [DOI] [Google Scholar]
- 61.Bera A.K., Ghosh S. Dual mode of gating of voltage-dependent anion channel as revealed by phosphorylation. J. Struct. Biol. 2001;135:67–72. doi: 10.1006/jsbi.2001.4399. [DOI] [PubMed] [Google Scholar]
- 62.Banerjee J., Ghosh S. Phosphorylation of rat brain mitochondrial voltage-dependent anion as a potential tool to control leakage of cytochrome c. J. Neurochem. 2006;98:670–676. doi: 10.1111/j.1471-4159.2006.03853.x. [DOI] [PubMed] [Google Scholar]
- 63.Gupta R., Ghosh S. Phosphorylation of purified mitochondrial Voltage-Dependent Anion Channel by c-Jun N-terminal Kinase-3 modifies channel voltage-dependence. Biochim. Open. 2017;4:78–87. doi: 10.1016/j.biopen.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gatliff J., East D.A., Singh A., Alvarez M.S., Frison M., Matic I., Ferraina C., Sampson N., Turkheimer F., Campanella M. A role for TSPO in mitochondrial Ca2+ homeostasis and redox stress signaling. Cell Death Dis. 2017;8:e2896. doi: 10.1038/cddis.2017.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baines C.P., Song C.-X., Zheng Y.-T., Wang G.-W., Zhang J., Wang O.-L., Guo Y., Bolli R., Cardwell E.M., Ping P. Protein kinase Cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ. Res. 2003;92:873–880. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sheldon K.L., Maldonado E.N., Lemasters J.J., Rostovtseva T.K., Bezrukov S.M. Phosphorylation of Voltage-Dependent Anion Channel by Serine/Threonine Kinases Governs Its Interaction with Tubulin. PLoS ONE. 2011;6:e25539. doi: 10.1371/journal.pone.0025539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rovini A., Gurnev P.A., Beilina A., Queralt-Martín M., Rosencrans W., Cookson M.R., Bezrukov S.M., Rostovtseva T.K. Molecular mechanism of olesoxime-mediated neuroprotection through targeting α-synuclein interaction with mitochondrial VDAC. Cell. Mol. Life Sci. 2020;77:3611–3626. doi: 10.1007/s00018-019-03386-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crimi M., Esposti M.D. Apoptosis-induced changes in mitochondrial lipids. Biochim. Biophys. Acta. 2011;1813:551–557. doi: 10.1016/j.bbamcr.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 69.Arbel N., Shoshan-Barmatz V. Voltage-dependent Anion Channel 1-based Peptides Interact with Bcl-2 to Prevent Antiapoptotic Activity. J. Biol. Chem. 2010;285:6053–6062. doi: 10.1074/jbc.M109.082990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang H., Shah K., Bradbury N.A., Li C., White C. Mcl-1 promotes lung cancer cell migration by directly interacting with VDAC to increase mitochondrial Ca2+ uptake and reactive oxygen species generation. Cell Death Dis. 2014;5:e1482. doi: 10.1038/cddis.2014.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monaco G., Decrock E., Arbel N., van Vliet A.R., La Rovere R.M., De Smedt H., Parys J.B., Agostinis P., Leybaert L., Shoshan-Barmatz V., et al. The BH4 domain of anti-apoptotic Bcl-XL, but not that of the related Bcl-2, limits the voltage-dependent anion channel 1 (VDAC1)-mediated transfer of pro-apoptotic Ca2+ signals to mitochondria. J. Biol. Chem. 2015;290:9150–9161. doi: 10.1074/jbc.M114.622514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tornero D., Posadas I., Ceña V. Bcl-xL Blocks a Mitochondrial Inner Membrane Channel and Prevents Ca2+ Overload-Mediated Cell Death. PLoS ONE. 2011;6:e20423. doi: 10.1371/journal.pone.0020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arbel N., Ben-Hail D., Shoshan-Barmatz V. Mediation of the antiapoptotic activity of Bcl-xL protein upon interaction with VDAC1 protein. J. Biol. Chem. 2012;287:23152–23161. doi: 10.1074/jbc.M112.345918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tamse C., Lu X., Mortel E., Cabrales E., Feng W., Schaefer S. The Peripheral Benzodiazepine Receptor Modulates Ca2+ Transport through the VDAC in Rat Heart Mitochondria. J. Clin. Basic Cardiol. 2008;11:24–29. [Google Scholar]
- 75.Hein M.Y., Hubner N.C., Poser I., Cox J., Nagaraj N., Toyoda Y., Gak I.A., Weisswange I., Mansfeld J., Buchholz F., et al. A Human Interactome in Three Quantitative Dimensions Organized by Stoichiometries and Abundances. Cell. 2015;163:712–723. doi: 10.1016/j.cell.2015.09.053. [DOI] [PubMed] [Google Scholar]
- 76.Viola H.M., Adams A.M., Davies S.M.K., Fletcher S., Filipovska A., Hool L.C. Impaired functional communication between the L-type calcium channel and mitochondria contributes to metabolic inhibition in the mdx heart. Proc. Natl. Acad. Sci. USA. 2014;111:E2905–E2914. doi: 10.1073/pnas.1402544111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Min C.K., Yeom D.R., Lee K.-E., Kwon H.-K., Kang M., Kim Y.-S., Park Z.Y., Jeon H., Kim D.H. Coupling of ryanodine receptor 2 and voltage-dependent anion channel 2 is essential for Ca2+ transfer from the sarcoplasmic reticulum to the mitochondria in the heart. Biochem. J. 2012;447:371–379. doi: 10.1042/BJ20120705. [DOI] [PubMed] [Google Scholar]
- 78.Liu Y., Ma X., Fujioka H., Liu J., Chen S., Zhu X. DJ-1 regulates the integrity and function of ER-mitochondria association through interaction with IP3R3-Grp75-VDAC1. Proc. Natl. Acad. Sci. USA. 2019;116:25322–25328. doi: 10.1073/pnas.1906565116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.D’Eletto M., Rossin F., Occhigrossi L., Farrace M.G., Faccenda D., Desai R., Marchi S., Refolo G., Falasca L., Antonioli M., et al. Transglutaminase Type 2 Regulates ER-Mitochondria Contact Sites by Interacting with GRP75. Cell Rep. 2018;25:3573–3581.e4. doi: 10.1016/j.celrep.2018.11.094. [DOI] [PubMed] [Google Scholar]
- 80.Israelson A., Arbel N., Da Cruz S., Ilieva H., Yamanaka K., Shoshan-Barmatz V., Cleveland D.W. Misfolded Mutant SOD1 Directly Inhibits VDAC1 Conductance in a Mouse Model of Inherited ALS. Neuron. 2010;67:575–587. doi: 10.1016/j.neuron.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rizzuto R., Pinton P., Carrington W., Fay F.S., Fogarty K.E., Lifshitz L.M., Tuft R.A., Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 82.Csordás G., Renken C., Várnai P., Walter L., Weaver D., Buttle K.F., Balla T., Mannella C.A., Hajnóczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 2006;174:915. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sharma V.K., Ramesh V., Franzini-Armstrong C., Sheu S.-S. Transport of Ca2+ from Sarcoplasmic Reticulum to Mitochondria in Rat Ventricular Myocytes. J. Bioenerg. Biomembr. 2000;32:97–104. doi: 10.1023/A:1005520714221. [DOI] [PubMed] [Google Scholar]
- 84.Brdiczka D.G., Zorov D.B. Mitochondrial contact sites: Their role in energy metabolism and apoptosis. Biochim. Biophys. Acta Mol. Basis Dis. 2006;1762:148–163. doi: 10.1016/j.bbadis.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 85.Szabadkai G., Bianchi K., Várnai P., De Stefani D., Wieckowski M.R., Cavagna D., Nagy A.I., Balla T., Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu H., Guan N., Ren Y.-L., Wei Q.-J., Tao Y.-H., Yang G.-S., Liu X.-Y., Bu D.-F., Zhang Y., Zhu S.-N. IP3R-Grp75-VDAC1-MCU calcium regulation axis antagonists protect podocytes from apoptosis and decrease proteinuria in an Adriamycin nephropathy rat model. BMC Nephrol. 2018;19:140. doi: 10.1186/s12882-018-0940-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Santin Y., Fazal L., Sainte-Marie Y., Sicard P., Maggiorani D., Tortosa F., Yücel Y.Y., Teyssedre L., Rouquette J., Marcellin M., et al. Mitochondrial 4-HNE derived from MAO-A promotes mitoCa2+ overload in chronic postischemic cardiac remodeling. Cell Death Differ. 2020;27:1907–1923. doi: 10.1038/s41418-019-0470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Caterino M., Ruoppolo M., Mandola A., Costanzo M., Orrù S., Imperlini E. Protein-protein interaction networks as a new perspective to evaluate distinct functional roles of voltage-dependent anion channel isoforms. Mol. Biosyst. 2017;13:2466–2476. doi: 10.1039/C7MB00434F. [DOI] [PubMed] [Google Scholar]
- 89.Anflous K., Armstrong D.D., Craigen W.J. Altered mitochondrial sensitivity for ADP and maintenance of creatine-stimulated respiration in oxidative striated muscles from VDAC1-deficient mice. J. Biol. Chem. 2001;276:1954–1960. doi: 10.1074/jbc.M006587200. [DOI] [PubMed] [Google Scholar]
- 90.Raghavan A., Sheiko T.V., Graham B.H., Craigen W.J. Voltage-dependant anion channels: Novel insights into isoform function through genetic models. Biochim. Biophys. Acta Biomembr. 2012;1818:1477–1485. doi: 10.1016/j.bbamem.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Amodeo G.F., Scorciapino M.A., Messina A., De Pinto V., Ceccarelli M. Charged Residues Distribution Modulates Selectivity of the Open State of Human Isoforms of the Voltage Dependent Anion-Selective Channel. PLoS ONE. 2014;9:e103879. doi: 10.1371/journal.pone.0103879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cheng E.H.Y., Sheiko T.V., Fisher J.K., Craigen W.J., Korsmeyer S.J. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 93.Abu-Hamad S., Sivan S., Shoshan-Barmatz V. The expression level of the voltage-dependent anion channel controls life and death of the cell. Proc. Natl. Acad. Sci. USA. 2006;103:5787–5792. doi: 10.1073/pnas.0600103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sasaki K., Donthamsetty R., Heldak M., Cho Y.-E., Scott B.T., Makino A. VDAC: Old protein with new roles in diabetes. Am. J. Physiol. Physiol. 2012;303:C1055–C1060. doi: 10.1152/ajpcell.00087.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Feng Y., Madungwe N.B., Imam Aliagan A.D., Tombo N., Bopassa J.-C. Liproxstatin-1 protects the mouse myocardium against ischemia/reperfusion injury by decreasing VDAC1 levels and restoring GPX4 levels. Biochem. Biophys. Res. Commun. 2019;520 doi: 10.1016/j.bbrc.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yuan J., Zhang Y., Sheng Y., Fu X., Cheng H., Zhou R. MYBL2 guides autophagy suppressor VDAC2 in the developing ovary to inhibit autophagy through a complex of VDAC2-BECN1-BCL2L1 in mammals. Autophagy. 2015;11:1081–1098. doi: 10.1080/15548627.2015.1040970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weisthal S., Keinan N., Ben-Hail D., Arif T., Shoshan-Barmatz V. Ca2+-mediated regulation of VDAC1 expression levels is associated with cell death induction. Biochim. Biophys. Acta Mol. Cell Res. 2014;1843:2270–2281. doi: 10.1016/j.bbamcr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 98.Allouche M., Pertuiset C., Robert J.L., Martel C., Veneziano R., Henry C., el dein O.S., Saint N., Brenner C., Chopineau J. ANT-VDAC1 interaction is direct and depends on ANT isoform conformation in vitro. Biochem. Biophys. Res. Commun. 2012;429:12–17. doi: 10.1016/j.bbrc.2012.10.108. [DOI] [PubMed] [Google Scholar]
- 99.Zaid H., Abu-Hamad S., Israelson A., Nathan I., Shoshan-Barmatz V. The voltage-dependent anion channel-1 modulates apoptotic cell death. Cell Death Differ. 2005;12:751–760. doi: 10.1038/sj.cdd.4401599. [DOI] [PubMed] [Google Scholar]
- 100.Bobba A., Amadoro G., La Piana G., Petragallo V.A., Calissano P., Atlante A. Glucose-6-phosphate tips the balance in modulating apoptosis in cerebellar granule cells. FEBS Lett. 2015;589:651–658. doi: 10.1016/j.febslet.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 101.Dubey A.K., Godbole A., Mathew M.K. Regulation of VDAC trafficking modulates cell death. Cell Death Discov. 2016;2:16085. doi: 10.1038/cddiscovery.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cárdenas C., Müller M., McNeal A., Lovy A., Jaňa F., Bustos G., Urra F., Smith N., Molgó J., Diehl J.A., et al. Selective Vulnerability of Cancer Cells by Inhibition of Ca2+ Transfer from Endoplasmic Reticulum to Mitochondria. Cell Rep. 2016;14:2313–2324. doi: 10.1016/j.celrep.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ge Y., Yan D., Deng H., Chen W., An G. Novel Molecular Regulators of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)-Induced Apoptosis in NSCLC Cells. Clin. Lab. 2015;61 doi: 10.7754/Clin.Lab.2015.150328. [DOI] [PubMed] [Google Scholar]
- 104.Simamura E., Shimada H., Ishigaki Y., Hatta T., Higashi N., Hirai K.-I. Bioreductive activation of quinone antitumor drugs by mitochondrial voltage-dependent anion channel 1. Anat. Sci. Int. 2008;83:261–266. doi: 10.1111/j.1447-073X.2008.00241.x. [DOI] [PubMed] [Google Scholar]
- 105.Grills C., Jithesh P.V., Blayney J., Zhang S.-D., Fennell D.A. Gene Expression Meta-Analysis Identifies VDAC1 as a Predictor of Poor Outcome in Early Stage Non-Small Cell Lung Cancer. PLoS ONE. 2011;6:e14635. doi: 10.1371/journal.pone.0014635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rimessi A., Patergnani S., Bonora M., Wieckowski M.R., Pinton P. Mitochondrial Ca2+ Remodeling is a Prime Factor in Oncogenic Behavior. Front. Oncol. 2015;5:143. doi: 10.3389/fonc.2015.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koren I., Raviv Z., Shoshan-Barmatz V. Downregulation of voltage-dependent anion channel-1 expression by RNA interference prevents cancer cell growth in vivo. Cancer Biol. Ther. 2010;9:1046–1052. doi: 10.4161/cbt.9.12.11879. [DOI] [PubMed] [Google Scholar]
- 108.Arif T., Krelin Y., Nakdimon I., Benharroch D., Paul A., Dadon-Klein D., Shoshan-Barmatz V. VDAC1 is a molecular target in glioblastoma, with its depletion leading to reprogrammed metabolism and reversed oncogenic properties. Neuro. Oncol. 2017;19:951–964. doi: 10.1093/neuonc/now297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chaanine A.H., Gordon R.E., Kohlbrenner E., Benard L., Jeong D., Hajjar R.J. Potential Role of BNIP3 in Cardiac Remodeling, Myocardial Stiffness, and Endoplasmic Reticulum. Circ. Hear. Fail. 2013;6:572–583. doi: 10.1161/CIRCHEARTFAILURE.112.000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pan M., Han Y., Basu A., Dai A., Si R., Willson C., Balistrieri A., Scott B.T., Makino A. Overexpression of hexokinase 2 reduces mitochondrial calcium overload in coronary endothelial cells of type 2 diabetic mice. Am. J. Physiol. Physiol. 2018;314:C732–C740. doi: 10.1152/ajpcell.00350.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miyamae M., Camacho S.A., Weiner M.W., Figueredo V.M. Attenuation of postischemic reperfusion injury is related to prevention of [Ca2+]m overload in rat hearts. Am. J. Physiol. 1996 doi: 10.1152/ajpheart.1996.271.5.H2145. [DOI] [PubMed] [Google Scholar]
- 112.de J García-Rivas G., Carvajal K., Correa F., Zazueta C. Ru360, a specific mitochondrial calcium uptake inhibitor, improves cardiac post-ischaemic functional recovery in rats in vivo. Br. J. Pharmacol. 2006;149:829–837. doi: 10.1038/sj.bjp.0706932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liao Z., Liu D., Tang L., Yin D., Yin S., Lai S., Yao J., He M. Long-term oral resveratrol intake provides nutritional preconditioning against myocardial ischemia/reperfusion injury: Involvement of VDAC1 downregulation. Mol. Nutr. Food Res. 2015;59:454–464. doi: 10.1002/mnfr.201400730. [DOI] [PubMed] [Google Scholar]
- 114.Gomez L., Thiebaut P.-A., Paillard M., Ducreux S., Abrial M., Crola Da Silva C., Durand A., Alam M.R., Van Coppenolle F., Sheu S.-S., et al. The SR/ER-mitochondria calcium crosstalk is regulated by GSK3β during reperfusion injury. Cell Death Differ. 2016;23:313–322. doi: 10.1038/cdd.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rieusset J., Fauconnier J., Paillard M., Belaidi E., Tubbs E., Chauvin M.-A., Durand A., Bravard A., Teixeira G., Bartosch B., et al. Disruption of calcium transfer from ER to mitochondria links alterations of mitochondria-associated ER membrane integrity to hepatic insulin resistance. Diabetologia. 2016;59:614–623. doi: 10.1007/s00125-015-3829-8. [DOI] [PubMed] [Google Scholar]
- 116.Thivolet C., Vial G., Cassel R., Rieusset J., Madec A.-M. Reduction of endoplasmic reticulum- mitochondria interactions in beta cells from patients with type 2 diabetes. PLoS ONE. 2017;12:e0182027. doi: 10.1371/journal.pone.0182027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tubbs E., Chanon S., Robert M., Bendridi N., Bidaux G., Chauvin M.-A., Ji-Cao J., Durand C., Gauvrit-Ramette D., Vidal H., et al. Disruption of Mitochondria-Associated Endoplasmic Reticulum Membrane (MAM) Integrity Contributes to Muscle Insulin Resistance in Mice and Humans. Diabetes. 2018;67:636–650. doi: 10.2337/db17-0316. [DOI] [PubMed] [Google Scholar]
- 118.Dia M., Gomez L., Thibault H., Tessier N., Leon C., Chouabe C., Ducreux S., Gallo-Bona N., Tubbs E., Bendridi N., et al. Reduced reticulum–mitochondria Ca2+ transfer is an early and reversible trigger of mitochondrial dysfunctions in diabetic cardiomyopathy. Basic Res. Cardiol. 2020;115:74. doi: 10.1007/s00395-020-00835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Joo H.K., Lee Y.R., Lim S.Y., Lee E.J., Choi S., Cho E.J., Park M.S., Ryoo S., Park J.B., Jeon B.H. Peripheral benzodiazepine receptor regulates vascular endothelial activations via suppression of the voltage-dependent anion channel-1. FEBS Lett. 2012;586:1349–1355. doi: 10.1016/j.febslet.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 120.Thai P.N., Daugherty D.J., Frederich B.J., Lu X., Deng W., Bers D.M., Dedkova E.N., Schaefer S. Cardiac-specific Conditional Knockout of the 18-kDa Mitochondrial Translocator Protein Protects from Pressure Overload Induced Heart Failure. Sci. Rep. 2018;8:16213. doi: 10.1038/s41598-018-34451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Paillusson S., Stoica R., Gomez-Suaga P., Lau D.H.W., Mueller S., Miller T., Miller C.C.J. There’s Something Wrong with my MAM; the ER–Mitochondria Axis and Neurodegenerative Diseases. Trends Neurosci. 2016;39:146–157. doi: 10.1016/j.tins.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Basso V., Marchesan E., Ziviani E. A trio has turned into a quartet: DJ-1 interacts with the IP3R-Grp75-VDAC complex to control ER-mitochondria interaction. Cell Calcium. 2020;87:102186. doi: 10.1016/j.ceca.2020.102186. [DOI] [PubMed] [Google Scholar]
- 123.Wang X., Petrie T.G., Liu Y., Liu J., Fujioka H., Zhu X. Parkinson’s disease-associated DJ-1 mutations impair mitochondrial dynamics and cause mitochondrial dysfunction. J. Neurochem. 2012;121:830–839. doi: 10.1111/j.1471-4159.2012.07734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee S., Wang W., Hwang J., Namgung U., Min K.-T. Increased ER–mitochondria tethering promotes axon regeneration. Proc. Natl. Acad. Sci. USA. 2019;116:16074–16079. doi: 10.1073/pnas.1818830116. [DOI] [PMC free article] [PubMed] [Google Scholar]