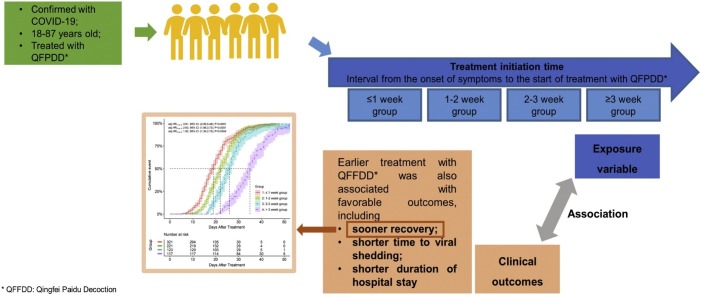
Graphical abstract
Abbreviations: QFPDD, Qingfei Paidu Decoction; COVID-19, coronavirus disease 2019; SARS-COV-2, severe acute respiratory syndrome-coronavirus 2; RT-PCR, reverse transcription-polymerase-chain-reaction; WISP, work information system platform; HR, hazard ratio; CI, confidence interval; No., number of patients; COPD, chronic obstructive pulmonary disease; WBC, white blood cell; RBC, red blood cell; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; SpO2, blood oxygen saturation; PO2, partial pressure of oxygen; Ref., reference; Adj., adjusted; Unadj., unadjusted; CT, computed tomography; IFN, interferon
Keywords: COVID-19, Qingfei Paidu decoction, Early treatment
Abstract
The coronavirus disease 2019 (COVID-19) epidemic has been almost controlled in China under a series of policies, including “early diagnosis and early treatment”. This study aimed to explore the association between early treatment with Qingfei Paidu decoction (QFPDD) and favorable clinical outcomes. In this retrospective multicenter study, we included 782 patients (males, 56 %; median age 46) with confirmed COVID-19 from 54 hospitals in nine provinces of China, who were divided into four groups according to the treatment initiation time from the first date of onset of symptoms to the date of starting treatment with QFPDD. The primary outcome was time to recovery; days of viral shedding, duration of hospital stay, and course of the disease were also analyzed. Compared with treatment initiated after 3 weeks, early treatment with QFPDD after less than 1 week, 1-2 weeks, or 2-3 weeks had a higher likelihood of recovery, with adjusted hazard ratio (HR) (95 % confidence interval [CI]) of 3.81 (2.65–5.48), 2.63 (1.86-3.73), and 1.92 (1.34-2.75), respectively. The median course of the disease decreased from 34 days to 24 days, 21 days, and 18 days when treatment was administered early by a week (P < 0.0001). Treatment within a week was related to a decrease by 1-4 days in the median duration of hospital stay compared with late treatment (P<0.0001). In conclusion, early treatment with QFPDD may serve as an effective strategy in controlling the epidemic, as early treatment with QFPDD was associated with favorable outcomes, including faster recovery, shorter time to viral shedding, and a shorter duration of hospital stay. However, further multicenter, prospective studies with a larger sample size should be conducted to confirm the benefits of early treatment with QFPDD.
1. Introduction
The outbreak of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has severely impacted public health and has become a global pandemic [1,2]. More than 8.7 million confirmed cases of COVID-19 have been reported in over 200 countries, areas, and territories, with over 461 thousand deaths due to COVID-19 as of June 21, 2020 [3].
Initially, many studies have reported the clinical characteristics of patients with COVID-19 [[4], [5], [6], [7]]. Some studies have explored risk factors for the illness that may influence the prognosis [[8], [9], [10]], all of which helped toward a better understanding of the physiology and pathology of COVID-19 for the further development of potential effective vaccines and drugs. Clinical trials on potential drugs are still ongoing, and some preliminary results have been reported [[11], [12], [13], [14], [15], [16]]. Many vaccine candidates were in phase II and one in phase III of clinical trials [17]. However, no effective medication is currently confirmed, and the vaccine is still months to years away from being used in clinical practice. Current treatments are supportive of relieving the severity of symptoms and avoiding critical illness. In China, traditional Chinese medicine has been included as an option for the treatment of COVID-19, and Qingfei Paidu decoction (QFPDD) is recommended for use in patients in the seventh edition of the COVID-19 guideline [18].
To date, few studies have focused on efficient policies for COVID-19 management. As the crisis has progressed, there has been an enormous burden on medical sources and health systems due to increasing demands. Hence, efficient policies for the management of COVID-19 are of great importance in coping with the challenges posed to people’s health and medical systems.
Different policies have been implemented. “Self-isolation” and “self-care” were recommended to keep people with minor ailments at home [19]. Policies, including “early diagnosis and early treatment”, have been implemented in China as preventive and therapeutic strategies, under which the COVID-19 epidemic has been almost controlled [18]. The benefits of early treatment, if proven, could help reduce hospital stay, lower intensive care use, and relieve the burden on the public health system, especially for those living in source-limited areas [20]. However, very few studies have provided data on the correlation between early treatment and clinical outcomes. This study aimed to examine whether early treatment with QFPDD results in favorable clinical outcomes.
2. Methods
2.1. Participants
A retrospective follow-up study design was adopted. Patients aged 18-87 years with confirmed COVID-19 from 54 hospitals in nine provinces of China (Anhui, Fujian, Guangxi, Hebei, Heilongjiang, Shaanxi, Sichuan, Shanxi, and Chongqing) were enrolled from January 21, 2020 to March 10, 2020. The final follow-up was conducted on March 17, 2020. Patients were eligible for inclusion if they self-reported signs and symptoms and were diagnosed with confirmed SARS-CoV-2 by the nasopharyngeal reverse transcription-polymerase chain reaction (RT-PCR) test [18].
This study was supported by the National Administration of Traditional Chinese Medicine, Administration of Traditional Chinese Medicine of the nine provinces, and the institutional board of the 54 participating hospitals. The study was approved by the ethics committee of the Chinese Clinical Trial Registry (ChiECRCT20200123). Due to the urgency of COVID-19 treatment, the requirement for written informed consent from the study participants was replaced by verbal consent.
2.2. Treatment
All patients were treated with QFPDD. QFPDD is a Chinese formula comprising 21 herbs: má huáng (Herba Ephedrae) 9 g, zhì gān căo (Radix et Rhizoma Glycyrrhizae Praeparata cum Melle) 6 g, xìng rén (Semen Armeniacae Amarum) 9 g, shí gāo (Gypsum Fibrosum) 15-30 g (fried first), guì zhī (Ramulus Cinnamomi) 9 g, zé xiè (Rhizoma Alismatis) 9 g, zhū líng (Polyporus) 9 g, bái zhú (Rhizoma Atractylodis Macrocephalae) 9 g, fú líng (Poria) 15 g, chái hú (Radix Bupleuri) 16 g, huáng qín (Radix Scutellariae) 6 g, jiāng bàn xià (Rhizoma Pinelliae Praeparatum) 9 g, shēng jiāng (Rhizoma Zingiberis Recens) 9 g, zĭ wăn (Radix et Rhizoma Asteris) 9 g, kuăn dōng huā (Flos Farfarae) 9 g, shè gān (Rhizoma Belamcandae) 9 g, xì xīn (Radix et Rhizoma Asari) 6 g, shān yào (Rhizoma Dioscoreae) 12 g, zhĭ shí (Fructus Aurantii Immaturus) 6 g, chén pí (Pericarpium Citri Reticulatae) 6 g, and huò xiāng (Herba Agastachis) 9 g. The quality of the herbs conformed to the criteria set by the 2015 Chinese Pharmacopoeia. Hazardous substances, including pesticide residues, heavy metals, and microbial contamination, were detected in all herbs to ensure safety, and the results met the criteria in China. QFPDD was prepared by a pharmacist according to the standardized procedure in each hospital. The dose and mode of administration were based on the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia [18].
Additionally, antiviral drugs, antibiotics, corticosteroids, α-IFN inhalation, and symptomatic treatments were used based on the patients’ needs. The dose and mode of administration were based on the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia [18]. Supplemental oxygen was given to those with oxygen saturation levels dropping below 93 % or in patients who felt obvious chest tightness.
2.3. Laboratory procedures
Laboratory blood examinations included hematologic indices and infection-related indices. Chest imaging results were separately reviewed by two radiologists to assess image progression or absorption. Two evaluators independently assessed the chest computed tomography (CT) features of the patients without access to clinical or laboratory findings. Disagreements, if any, were resolved by discussion and consensus. Physicians determined the timings of all the above examinations based on the patients’ condition during hospitalization.
2.4. Definitions
Recovery was defined as: (1) body temperature returned to normal for more than 3 days; (2) respiratory symptoms clearly improved; (3) pulmonary imaging showed obvious absorption of inflammation; and (4) nucleic acid tests showed negative results twice consecutively with an interval of at least 24 h [18]. The primary outcome was time to recovery, which was defined as the interval from the first date of onset of symptoms to the date of recovery.
The secondary outcomes were days of viral shedding, course of the disease, and duration of hospital stay. Days of viral shedding was defined as the interval from the first positive RT-PCR test to the date of the second consecutive negative test. The course of the disease was calculated from the day of onset of symptoms to the day of discharge. The duration of hospital stay was calculated from the day of admission to the day of discharge.
The exposure variable was defined as the treatment initiation time, which was the interval from the onset of symptoms to the start of the treatment with QFPDD. Accordingly, patients were divided into four groups: the ≤1 week group (≤7 days), 1-2 week group (>7 days and ≤14 days), 2-3 week group (>14 days and ≤21 days), and >3 week group (>21 days).
The clinical classification of patients included mild, moderate, severe, and critical cases [18]. Mild cases were defined as those with mild clinical symptoms without any signs of pneumonia on imaging. Moderate cases were defined as those showing fever and respiratory symptoms with radiological findings of pneumonia. Severe cases were defined as those meeting any of the following criteria: (1) respiratory distress (≥30 breaths/min), (2) oxygen saturation ≤93 % at rest, and (3) arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≤300 mmHg (l mmHg = 0.133 kPa). Cases that met any of the following criteria were defined as critical cases: (1) respiratory failure and requiring mechanical ventilation, (2) shock, and (3) other organ failure requiring ICU care. In our study, mild and moderate cases were combined into a non-severe group, and severe and critical cases were combined into a severe group. Comorbidities included previously diagnosed diseases such as diabetes, hypertension, coronary heart disease, and cerebrovascular disease.
2.5. Data collection
A work information system platform (WISP) was developed for data collection and management. Demographic and clinical characteristics and treatment information were collected through WISP; the laboratory and imaging tests were scanned or photographed and uploaded to WISP. Demographic and clinical characteristics and treatment information were extracted from WISP using a standardized data collection form. Laboratory data were input by researchers in duplicate, and imaging data were reviewed by radiologists independently. If core data were missing, we contacted coordinators on the site who would contact the attending physicians.
2.6. Statistical analysis
Continuous and categorical variables are presented as medians (interquartile ranges) and percentages (%), respectively. The univariate analysis for demographic and clinical characteristics at baseline was conducted using the Chi-square test or Fisher’s exact method for categorical data; continuous variables were compared using ANOVA if data were normally distributed and by the Kruskal-Wallis test otherwise. Survival curves were generated by the Kaplan-Meier method. Univariate and multivariable Cox proportional hazard ratio (HR) models were used to estimate unadjusted and adjusted HRs and 95 % confidence intervals (CIs) for the association between treatment initiation time and clinical outcomes. As recovery was a beneficial event, an HR value >1 would increase the likelihood of an event, whereas an HR value <1 meant the factor would decrease the likelihood of an event. Proportional assumptions for the Cox proportional hazard model were examined using scaled Schoenfeld residuals. Similar analyses were then conducted in subgroups of patients with different classifications.
Multiple linear regression models were used to model the relationships between the continuous outcomes with logarithm transformation and treatment initiation time, adjusted by propensity score. A repeated measures ANOVA was used to estimate the trend and possible difference in body temperature among the four groups. For the categorical data, a multinomial logistic regression model was used.
To adjust for the potential bias inherent in the retrospective studies, such as unbalanced baseline clinical characteristics, propensity scores were derived from multivariable logistic regression that included covariates, including age, sex, clinical classification, history of visiting Wuhan in the past 14 days, days from onset of symptoms to hospital admission, fever and cough on admission, comorbidities, antiviral use, expectorant use, and CT imaging outcomes, which were selected by the stepwise selection method (P = 0.05). The propensity score was then included in the regression models as a continuous variable, along with the treatment groups and other significant covariates, but not selected in the stepwise selection method at baseline in the univariate analysis, to adjust the heterogeneity at the baseline among the four groups with different treatment initiation times.
All tests were two-sided, and a P-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using R software, version 4.0.0 (R Foundation for Statistical Computing).
3. Results
3.1. Demographic and clinical characteristics
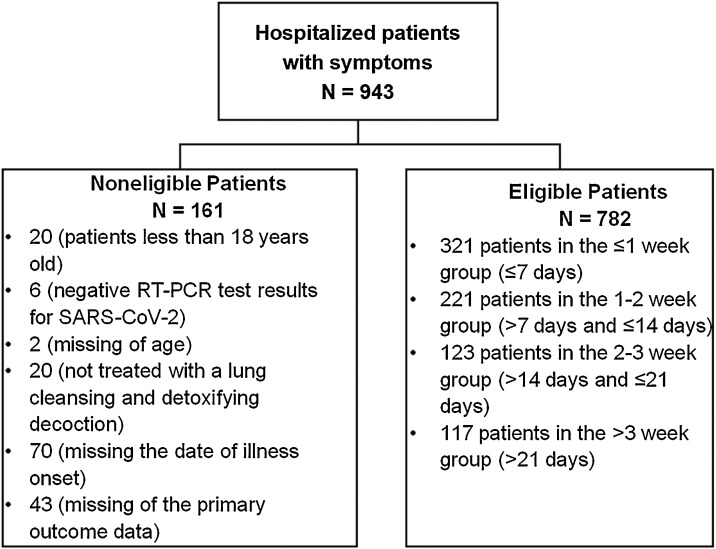
A total of 943 patients were recruited, and 782 patients (male, 52 %; median age, 46 years) from 54 hospitals in nine provinces were included in our analysis till the cut-off date of March 17, 2020. Patients were divided into ≤1 week (321 patients, 41 %), 1-2 week (221 patients, 28 %), 2-3 week (123 patients, 16 %), and >3 week (117 patients, 15 %) groups (Fig. 1 ).
Fig. 1.
Patient inclusion/exclusion criteria.
The patients included were predominantly non-severe (91 %); 34 % of the patients reported that they visited Wuhan within the past 14 days, and 66 % had contacted patients with confirmed COVID-19. The most common coexisting diseases included hypertension (15 %), diabetes (7%), and coronary heart disease (3%). The most common signs and symptoms on admission were cough (52 %), followed by fever (49 %), fatigue (31 %), and dry cough (29 %). The median body temperature was 37.7 °C. A total of 641 (90 %) patients had abnormal findings with unilateral or bilateral multiple mottling and ground-glass opacity of the lung. All 782 patients were treated with QFPDD (100 %); over 90 % of the patients received antiviral therapy, including arbidol, lopinavir/ritonavir, ribavirin, ganciclovir, and others. Antibiotics (53 %), α-IFN inhalation (43 %), corticosteroids (15 %), antipyretic drugs (10 %), and expectorants (4%) were also used. At baseline, there were significant differences among the four groups based on the clinical classification (P = 0.0016); history of visiting Wuhan (P = 0.0002); signs and symptoms on admission, including fever (P = 0.0022), sore throat (P = 0.0357), cough (P = 0.0008), shortness of breath (P < 0.0001), and fatigue (P = 0.0423); median body temperature of patients on admission (P = 0.0088) and during hospitalization (P = 0.0486); and the imaging results of CT (P = 0.0088), and use of expectorant (P = 0.0005) (Table 1 ). Significant differences were observed among the four groups in hematologic test results, including lymphocyte count (P = 0.0007), RBC count (P = 0.0012), hemoglobin (P < 0.0001), and platelet count (P< 0.0001), as well as in infection-related indices, including C-reactive protein (P = 0.0018), procalcitonin (P = 0.0017), and erythrocyte sedimentation rate (P = 0.0136) (Table 2 ).
Table 1.
Demographic and clinical characteristics of patients infected with coronavirus disease 2019 at baseline, China.
| Study population | No. (%) | Time from onset of symptoms to treatment with QFPDD# |
P* | |||
|---|---|---|---|---|---|---|
| ≤1 week | 1−2 weeks | 2−3 weeks | >3 weeks | |||
| No. of patients | 782 | 321(41 %) | 221(28 %) | 123(16 %) | 117(15 %) | – |
| Age, median, years | 46.0(23.0) | 44.0(24.0) | 47.0(22.0) | 45.0(24.0) | 46.0(18.0) | 0.5429 |
| Sex | 0.4246 | |||||
| Male | 405(52 %) | 156(49 %) | 120(54 %) | 69(56 %) | 60(51 %) | |
| Female | 377(48 %) | 165(51 %) | 101(46 %) | 54(44 %) | 57(49 %) | |
| Clinical classification | 0.0016 | |||||
| Nonseverea | 710(91 %) | 301(94 %) | 205(93 %) | 107(87 %) | 97(83 %) | |
| Severeb | 72(9%) | 20(6%) | 16(7%) | 16(13 %) | 20(17 %) | |
| Exposure to source of transmission within the past 14 days | ||||||
| Recently visited Wuhan, China | 0.0002 | |||||
| No | 518(66 %) | 231(72 %) | 154(70 %) | 71(58 %) | 62(53 %) | |
| Yes | 263(34 %) | 90(28 %) | 66(30 %) | 52(42 %) | 55(47 %) | |
| Had contact with the confirmed COVID-19 patients | 0.5594 | |||||
| No | 266(34 %) | 104(32 %) | 74(34 %) | 42(36 %) | 46(40 %) | |
| Yes | 508(66 %) | 216(68 %) | 146(66 %) | 76(64 %) | 70(60 %) | |
| Comorbidities | ||||||
| Hypertension | 120(15 %) | 54(17 %) | 39(18 %) | 12(10 %) | 15(13 %) | 0.1738 |
| Diabetes | 51(7%) | 22(7%) | 17(8%) | 4(3%) | 8(7%) | 0.4350 |
| Coronary heart disease | 24(3%) | 7(2%) | 10(5%) | 3(2%) | 4(3%) | 0.4508 |
| Cerebrovascular disease | 7(1%) | 4(1%) | 1(0%) | 2(2%) | 0 | 0.4349 |
| Liver disease | 8(1%) | 3(1%) | 1(0%) | 2(2%) | 2(2%) | 0.6322 |
| COPD | 14(2%) | 4(1%) | 4(2%) | 5(4%) | 1(1%) | 0.1916 |
| Signs and symptoms on a dmission |
||||||
| Fever | 383(49 %) | 132(41 %) | 117(53 %) | 72(59 %) | 62(53 %) | 0.0022 |
| Chills | 39(5%) | 15(5%) | 10(5%) | 10(8%) | 4(3%) | 0.3424 |
| Nasal congestion | 37(5%) | 13(4%) | 12(5%) | 6(5%) | 6(5%) | 0.8926 |
| Shed tears | 27(3%) | 8(2%) | 6(3%) | 7(6%) | 6(5%) | 0.2526 |
| Sore throat | 86(11 %) | 23(7%) | 31(14 %) | 15(12 %) | 17(15 %) | 0.0357 |
| Cough | 409(52 %) | 140(44 %) | 130(59 %) | 69(56 %) | 70(60 %) | 0.0008 |
| Dry cough | 228(29 %) | 88(27 %) | 67(30 %) | 37(30 %) | 36(31 %) | 0.8461 |
| Shortness of breath | 102(13 %) | 23(7%) | 26(12 %) | 21(17 %) | 32(27 %) | <0.0001 |
| Apocleisis | 98(13 %) | 39(12 %) | 31(14 %) | 12(10 %) | 16(14 %) | 0.6828 |
| Diarrhea | 34(4%) | 13(4%) | 8(4%) | 7(6%) | 6(5%) | 0.7879 |
| Fatigue | 245(31 %) | 83(26 %) | 74(33 %) | 46(37 %) | 42(36 %) | 0.0423 |
| Vital signs at admission | ||||||
| Respiratory rate, median, rpm | 20.0(1.0) | 20.0(1.0) | 20.0(1.0) | 20.0(1.0) | 20.0(1.5) | 0.6215 |
| Temperature at admission | ||||||
| median, °C | 37.7(1.3) | 37.5(1.2) | 37.8(1.2) | 37.8(1.4) | 37.8(1.5) | 0.0088 |
| <37.3 °C | 212(32 %) | 94(35 %) | 60(31 %) | 32(29 %) | 26(27 %) | 0.1258 |
| 37.3−38.0 °C | 261(39 %) | 111(41 %) | 76(39 %) | 36(32 %) | 38(40 %) | |
| 38.1−39.0 °C | 179(27 %) | 57(21 %) | 54(28 %) | 42(38 %) | 26(27 %) | |
| >39.0 °C | 21(3%) | 9(3%) | 5(3%) | 2(2%) | 5(5%) | |
| Highest temperature during hospitalization | ||||||
| median, °C | 37.4(1.1) | 37.3(1.1) | 37.5(1.2) | 37.5(0.8) | 37.4(0.9) | 0.0486 |
| <37.3 °C | 319(42 %) | 155(50 %) | 84(40 %) | 32(27 %) | 48(41 %) | |
| 37.3−38.0 °C | 296(39 %) | 102(33 %) | 81(38 %) | 58(49 %) | 55(48 %) | |
| 38.1−39.0 °C | 118(16 %) | 46(15 %) | 38(18 %) | 24(20 %) | 10(9%) | |
| >39.0 °C | 25(3%) | 8(3%) | 9(4%) | 5(4%) | 3(3%) | |
| CT imaging | ||||||
| Normalc | 68(9%) | 37(12 %) | 22(10 %) | 4(3%) | 5(4%) | 0.0088 |
| Abnormald | 641(90 %) | 248(87 %) | 184(89 %) | 106(96 %) | 103(95 %) | |
| Medication | ||||||
| QFPDD | 782(100 %) | 321(100 %) | 221(100 %) | 123(100 %) | 117(100 %) | 1.0000 |
| Anti-viruse | 712(91 %) | 284(88 %) | 202(91 %) | 115(94 %) | 111(95 %) | 0.1301 |
| Antibiotics | 411(53 %) | 161(50 %) | 126(57 %) | 62(50 %) | 62(53 %) | 0.4335 |
| α-IFN inhalation | 339(43 %) | 153(48 %) | 93(42 %) | 52(42 %) | 41(35 %) | 0.1154 |
| Corticosteroid | 118(15 %) | 51(16 %) | 37(17 %) | 11(9%) | 19(16 %) | 0.2234 |
| Antipyretic drugs | 81(10 %) | 33(10 %) | 18(8%) | 17(14 %) | 13(11 %) | 0.4189 |
| Expectorant | 32(4%) | 7(2%) | 10(5%) | 13(11 %) | 2(2%) | 0.0005 |
Abbreviations: QFPDD, Qingfei Paidu decoction; No., number of patients; COVID-19, coronavirus disease 2019; COPD, chronic obstructive pulmonary disease; CT, computed tomography; IFN, interferon. Data are expressed as n (%). Totals do not add up to 100 % because of rounding or missing data.
P-values were calculated using the Chi-square test, Fisher’s exact method, or Kruskal-Wallis test. Bold indicates statistically significant <0.05.
Patients were divided into four groups: the ≤1 week group (≤7 days), 1−2 week group (>7 days and ≤14 days), 2−3 week group (>14 days and ≤21 days), and >3 week group (>21 days).
Including mild and moderate cases.
Including severe and critical cases.
Without unilateral or bilateral abnormal lung lesions.
Unilateral or bilateral multiple mottling and ground-glass opacity of the lung.
Including arbidol, lopinavir/ritonavir, ribavirin, ganciclovir, etc.
Table 2.
Baseline laboratory indices of patients infected with coronavirus disease 2019, China.
| Tests in the study population | Total (n = 782) |
Time from onset of symptoms to treatment with QFPDD# |
P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤1 week (n = 321) |
1−2 weeks (n = 221) |
2−3 weeks (n = 123) |
>3 weeks (n = 117) |
||||||||
| No. | Value, median | No. | Value, median | No. | Value, median | No. | Value, median | No. | Value, median | ||
| Hematologic | |||||||||||
| Neutrophils, ×109/L | 708 | 3.4(2.4) | 308 | 3.4(2.4) | 203 | 3.2(2.3) | 108 | 3.2(2.7) | 109 | 3.6(2.1) | 0.3459 |
| Lymphocyte, ×109/L | 712 | 1.3(0.8) | 309 | 1.4(0.8) | 206 | 1.3(0.8) | 108 | 1.2(0.7) | 109 | 1.5(0.8) | 0.0007 |
| RBC count, ×1012/L | 675 | 4.5(0.8) | 278 | 4.61(0.8) | 201 | 4.5(0.7) | 106 | 4.5(0.7) | 108 | 4.3(0.8) | 0.0012 |
| Hemoglobin, g/L | 672 | 136.0(24.0) | 276 | 140.0(25.0) | 201 | 136.0(25.0) | 106 | 132.0(19.0) | 107 | 129.0(26.0) | <0.0001 |
| WBC count, ×109/L | 710 | 5.4(2.8) | 308 | 5.4(3.0) | 205 | 5.2(2.6) | 108 | 5.1(2.7) | 109 | 5.8(2.4) | 0.0530 |
| Platelet count, ×109/L | 672 | 197.5(94.0) | 279 | 190.0(84.0) | 198 | 195.0(96.0) | 105 | 202.0(108.0) | 108 | 233.0(74.5) | <0.0001 |
| Infection-related indices | |||||||||||
| CRP, mg/L | 534 | 10.0(15.2) | 237 | 5.1(10.0) | 148 | 10.0(26.4) | 86 | 10.0(23.3) | 80 | 10.0(13.6) | 0.0018 |
| Procalcitonin, ng/mL | 445 | 0.1(0.1) | 198 | 0.1(0.1) | 133 | 0.1(0.1) | 66 | 0.1(0.1) | 57 | 0.1(0) | 0.0017 |
| ESR, mm/hr | 299 | 27.0(34.0) | 104 | 20.5(24.5) | 87 | 26.0(37.0) | 58 | 30.5(39.0) | 59 | 34.0(43.0) | 0.0136 |
| Arterial blood gas analysis | |||||||||||
| SpO2, % | 266 | 97.0(3.4) | 85 | 97.0(3.0) | 75 | 97.0(4.0) | 47 | 97.3(4.0) | 64 | 97.1(4.0) | 0.4312 |
| PO2, mmHg | 213 | 87.0(28.5) | 74 | 87.0(24.9) | 54 | 86.5(28.0) | 38 | 85.8(29.0) | 51 | 91.8(42.3) | 0.2552 |
Abbreviations: QFPDD, Qingfei Paidu decoction; No., number of patients tested; WBC, white blood cell; RBC, red blood cell; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; SpO2, blood oxygen saturation; PO2, partial pressure of oxygen. The P-values of the comparison groups were calculated using the Kruskal-Wallis method. Bold indicates statistically significant <0.05.
Patients were divided into four groups: the ≤1 week group (≤7 days), 1−2 week group (>7 days and ≤14 days), 2−3 week group (>14 days and ≤21 days), and >3 week group (>21 days).
3.2. Primary outcome
The univariate Cox HR models showed that groups with different treatment initiation times with QFPDD were statistically associated with recovery (P < 0.0001). Compared with treatment initiated after 3 weeks, the patients who received treatment within a week after the onset of symptoms showed more likelihood of recovery (unadjusted HR 4.56, 95 % CI: 3.61-5.76). Similar higher odds were also found for those in the groups treated at 1-2 weeks (unadjusted HR 3.36, 95 % CI: 2.62-4.30) and 2-3 weeks (unadjusted HR 2.24, 95 % CI: 1.71-2.93) (Table 3 ). This negative association between treatment initiation time and recovery was also observed in the subgroup of non-severe patients (≤1 week vs. > 3 weeks: unadjusted HR 4.34, 95 % CI: 3.40-5.55; 1-2 weeks vs. > 3 weeks: unadjusted HR 3.16, 95 % CI: 2.44-4.08; 2-3 weeks vs. > 3 weeks: unadjusted HR 2.26, 95 % CI: 1.69-3.01). In the subgroup of severe patients, there were significant differences between the ≤1 week group and the >3 week group (unadjusted HR 5.59, 95 % CI: 2.02-15.48) and between the 1-2 week group and the >3 week group (unadjusted HR 5.80, 95 % CI: 1.83-18.45); however, the difference between the 2-3 week group and the >3 week group (unadjusted HR 2.39, 95 % CI: 0.95-5.98) was not significant (Sup Table 1).
Table 3.
Cox proportional hazards models for the primary outcome of recovery in patients infected with coronavirus disease 2019, China.
| Time from onset of symptoms to treatment with QFPDD# | Univariate |
Multivariable |
||||
|---|---|---|---|---|---|---|
| Unadj. HR (95 % CI) | Pa | Adj. HR (95 % CI) | Pb | Adj. HR (95 % CI) | Pc | |
| ≤1 week | 4.56(3.61,5.76) | <0.0001 | 3.16(2.27,4.41) | <0.0001 | 3.81(2.65,5.48) | <0.0001 |
| 1−2 weeks | 3.36(2.62,4.30) | <0.0001 | 2.75(2.00,3.79) | <0.0001 | 2.63(1.86,3.73) | <0.0001 |
| 2−3 weeks | 2.24(1.71,2.93) | <0.0001 | 2.08(1.50,2.89) | <0.0001 | 1.92(1.34,2.75) | 0.0004 |
| >3 weeks | ref. = 1 | ref. = 1 | ref. = 1 | |||
Abbreviations: QFPDD, Qingfei Paidu decoction; ref., reference; Unadj., unadjusted; Adj., adjusted; CI, confidence interval; HR, hazard ratio. HRs and their 95 % CIs were calculated using the Cox proportional risk model. The proportional hazards assumption was not violated (P > 0.05). Bold indicates statistically significant <0.05.
Patients were divided into four groups: the ≤1 week group (≤7 days), 1−2 week group (>7 days and ≤14 days), 2−3 week group (>14 days and ≤21 days), and >3 week group (>21 days).
Unadjusted result.
Adjusted by covariates at baseline, including age, sex, clinical classification, history of visiting Wuhan in the past 14 days, days from the onset of symptoms to hospital admission, fever and cough on admission, any comorbidity, antiviral, expectorant, and computed tomography imaging, which were selected by the stepwise selection method (P = 0.05).
Adjusted by propensity score and other covariates. Propensity scores were derived from multivariable logistic regression that included covariates selected by the stepwise selection method at baseline. The covariates that were significant in univariate analysis but not selected in the stepwise method were included in the model as other covariates.
In multivariable Cox HR models adjusted for age, sex, clinical classification, history of visiting Wuhan within the past 14 days, days from the onset of symptoms to hospital admission, whether fever or cough were present on admission, any comorbidities, treatment with antiviral therapy and expectorant therapy, and imaging results of CT selected by the stepwise selection method, treatment initiation time still showed a significant association with recovery. Compared with the >3 week group, the ≤1 week group, 1-2 week group, and the 2-3 week group showed two to three times more likelihood of recovery (adjusted HR 3.16, 95 % CI: 2.27-4.41; adjusted HR 2.75, 95 % CI: 2.00-3.79; adjusted HR 2.08, 95 % CI: 1.50-2.89, respectively) (Table 3).
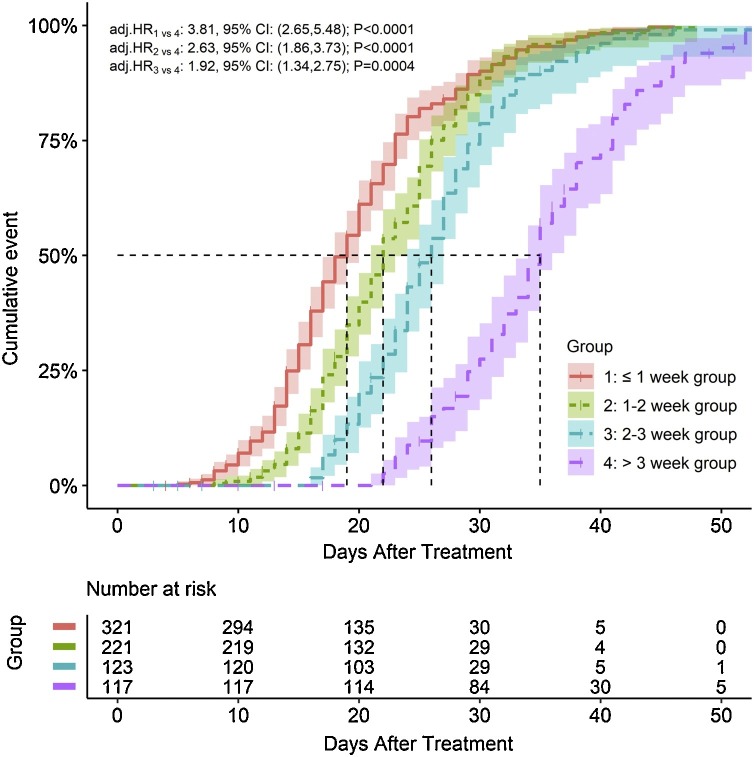
Furthermore, after adjusting for significant baseline variables and propensity score, treatment initiation time also showed a statistically significant association with a clinically effective outcome, suggesting that a longer time from the onset of symptoms to the start of treatment with QFPDD was negatively associated with the time to recovery (P < 0.0001) (Table 3). The Kaplan-Meier plot showed that the median time to recovery of the ≤1 week, 1-2 week, 2-3 week, and >3 week groups were 19 days (95 % CI: 17-20), 22 days (95 % CI: 21-23), 26 days (95 % CI: 24-27), and 35 days (95 % CI: 33-36), respectively (Fig. 2 ). Compared with the patients in the >3 week group, the patients in the ≤1 week, 1-2 week, and 2-3 week groups demonstrated approximately two to three times higher odds of recovery as adjusted by propensity score and other covariates (adjusted HR 3.81, 95 % CI: 2.65-5.48; adjusted HR 2.63, 95 % CI: 1.86-3.73; adjusted HR 1.92, 95 % CI: 1.34-2.75) (Fig. 2). This negative impact of treatment initiation time on recovery was consistent in the subgroup of non-severe patients (≤1 week vs. > 3 weeks: adjusted HR 3.75, 95 % CI: 2.56-5.49; 1-2 weeks vs. > 3 weeks: adjusted HR 2.47, 95 % CI: 1.72-3.56; 2-3 weeks vs. > 3 weeks: adjusted HR 1.80, 95 % CI: 1.23-2.64). However, there was no significant association in the subgroup of severe patients (P > 0.05) (Sup Table 1).
Fig. 2.
Kaplan-Meier plot for the primary outcome of time to recovery in patients infected with coronavirus disease 2019 by time from the onset of symptoms to treatment groups with 95 % CIs. Abbreviations: COVID-19, coronavirus disease 2019; Adj., adjusted; CI, confidence interval; HR, hazard ratio. The primary outcome was time to events, which was defined as the days from the onset of COVID-19 disease symptoms to clinical effectiveness for the treatment. If no event had occurred at the time of the last record, the patient’s survival time was censored at that time. Covariates were selected by the stepwise selection method (P = 0.05) and then were used to estimate propensity scores in multivariable logistic regression. HR and its 95 % CI were calculated using the Cox proportional risk model adjusted by propensity score and other significant covariates in the univariate analysis.
3.3. Secondary outcomes
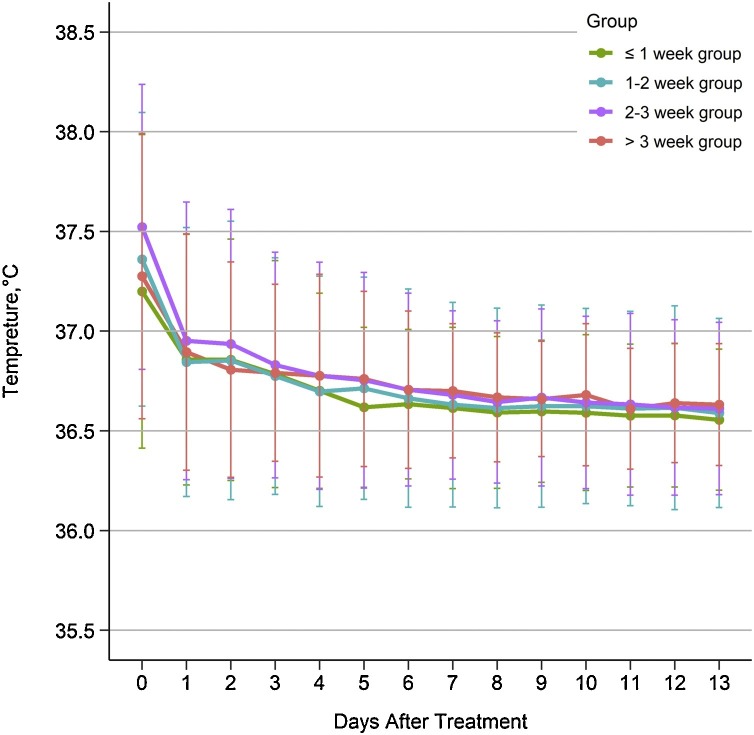
The multivariate linear regression models showed that there was a significant difference among the four groups in days of viral shedding (P = 0.0137), duration of hospital stay (P < 0.0001), and course of the disease (P<0.0001), along with early treatment with QFPDD after the onset of symptoms. There was no significant difference in the CT imaging results among the four groups (P = 0.3065) (Table 4 ). Approximately half of the patients (49 %) had a fever on admission; the results demonstrated that the body temperature of the patients in the four groups significantly decreased after treatment (P < 0.0001), showing a trend of better remission of fever in the early treatment group, with no significant difference among the four groups (P > 0.05) (Fig. 3 ).
Table 4.
Multivariate analysis for the secondary outcomes in patients infected with coronavirus disease 2019, China.
| Outcomes | All patients | Time from onset of symptoms to treatment with QFPDD# |
P value | |||
|---|---|---|---|---|---|---|
| No. (%) (n = 782) |
≤1 week (n = 321) |
1−2 week (n = 221) |
2−3 week (n = 123) |
>3 week (n = 117) |
||
| Days of viral sheddinga | ||||||
| median, days | 13.0(9.0) | 12.0(7.0) | 12.0(7.0) | 13.0(8.0) | 17.0(10.0) | 0.0137 |
| Duration of hospital staya | ||||||
| median, days | 15.0(9.0) | 14.0(8.5) | 15.0(9.0) | 15.0(9.0) | 18.0(12.0) | <0.0001 |
| Course of diseasea | ||||||
| median, days | 22.0(12.0) | 18.0(8.0) | 21.0(10.0) | 24.0(9.0) | 34.0(12.0) | <0.0001 |
| CT imagingb | ||||||
| Foci absorption, or no abnormal lesions | 457(77 %) | 176(75 %) | 126(74 %) | 78(80 %) | 77(83 %) | 0.3065 |
| No significant change | 103(17 %) | 37(16 %) | 34(20 %) | 16(16 %) | 16(17 %) | |
| Progress | 37(6%) | 22(9%) | 11(6%) | 4(4%) | 0 | |
Abbreviations: QFPDD, Qingfei Paidu decoction; CT, computed tomography; No., number of patients. Totals do not add up to 100 % because of rounding or missing data. Bold indicates statistically significant <0.05.
Patients were divided into four groups: the ≤1 week group (≤7 days), 1−2 week group (>7 days and ≤14 days), 2−3 week group (>14 days and ≤21 days), and >3 week group (>21 days).
Outcomes were used by logarithm transformation and P-values were calculated using a multiple linear regression model. Covariates were selected by the stepwise selection method (P = 0.05) and were then used to estimate propensity scores in multivariable logistic regression. P-values were adjusted by propensity score and other significant covariates at baseline in the univariate analysis.
P-values were calculated using a multinomial logistic regression model adjusted by propensity score and other covariate variables.
Fig. 3.
Daily temperature (℃) variations of patients infected with coronavirus disease 2019 by time from the onset of symptoms to treatment during a 14-day hospitalization period. Data are expressed as the Mean ± SD. Abbreviation: SD, standard deviation.
Favorable secondary outcomes were shown in the case of early treatment with QFPDD in the subgroup of non-severe patients in days of viral shedding (P = 0.0279), duration of hospital stay (P < 0.0001), and course of the disease (P<0.0001); however, the differences were not significant in the subgroup of severe patients (P = 0.0750; P = 0.2145; P = 0.4562, respectively) (Sup Table 2).
As of March 17, 2020, 715 patients (91 %) had recovered, 37 patients (5%) continued treatment in a stable condition, 28 patients (4%) deteriorated and were transferred to the ICU or superior hospitals to receive invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO), and 2 patients died. The median age for the 28 deteriorated patients was 55 years; 12 (43 %) were males, and 20 (71 %) were severe cases on admission. The coexisting diseases were hypertension, diarrhea, coronary heart disease, and chronic obstructive pulmonary disease. The allocation of deteriorated patients was 10 (10/321, 3%) in the ≤1 week group, 9 (9/221, 4%) in the 1-2 week group, 5 (5/123, 4%) in the 2-3 week group, and 4 (4/117, 3%) in the >3 week group. Two female patients died; the first patient was 72 years old with a severe case on admission combined with diarrhea, deteriorated 2 days after admission, and was transferred to the ICU. The patient died of respiratory failure and multiple organ failure 6 days later. The second patient’s condition—who was 75 years old with a severe case on admission, combined with diarrhea and coronary heart disease—deteriorated 13 days after admission, and died of respiratory failure and multiple organ failure 2 days after transfer to the ICU. Two deaths in the study were not treatment-related adverse events judged by clinical experts.
4. Discussion
We analyzed data from 782 patients with COVID-19 from nine provinces in China to examine the association between various treatment timings with QFPDD and clinical outcomes. We found that early treatment with QFPDD is linked to favorable clinical outcomes of quicker recovery, a shorter period of viral shedding, a shorter course of the disease, and a shorter duration of hospital stay. Meanwhile, the mortality in our study was 0.3 % (2/754), lower than the global mortality of 5.3 % (461,715/8,708,008)3, indicating that the combination treatment with QFPDD against COVID-19 in China served an advantage.
The present study showed that compared with treatment initiated 3 weeks after the onset of symptoms, treatment initiated within 2-3 weeks, 1-2 weeks, or less than 1 week had two to three times more likelihood of quicker recovery; the median days decreased from 35 days to 26 days, 22 days, and 19 days, respectively. The early treatment group (within 7 days) had a lower proportion of patients with severe COVID-19 than the other three treatment groups. These findings are similar to those of the previous studies; one showed that treatment with lopinavir/ritonavir within 12 days from the onset of symptoms could promote the recovery of patients with COVID-19 [12]. One study showed that treatment with remdesivir within 10 days from the onset of symptoms could promote clinical improvement [11]; another revealed that treatment initiated during the first 10 days had higher odds of recovery than treatment initiated 10 days after the onset of symptoms [14]. The present study also explored the relationship between treatment initiation time and recovery in the severe and non-severe subgroups of patients. Similar results of early treatment with QFPDD having favorable outcomes were found in patients with non-severe COVID-19, suggesting that for patients with mild and moderate COVID-19, early treatment with QFPDD was related to a shorter time to recovery.
Early treatment with QFPDD was also found to be associated with fewer days of viral shedding, a shorter course of the disease, and a shorter duration of hospital stay. Compared with patients in whom the treatment was initiated after 3 weeks, the median days of viral shedding decreased by approximately 5 days in patients in whom the treatment was initiated within 3 weeks. A similar trend of a decrease in the days of viral shedding from 12 days to 7 days after early triple treatment was found by Hung et al. in a subgroup analysis of an open-label, randomized, phase II trial [13]. Early treatment was related to a shortened course of the disease by 10 days or more. Compared with late treatment, treatment within a week after the onset of symptoms was related to a decrease of 1-4 days in the duration of hospital stay, consistent with a previous study that reported a decrease by 5 days [13]. The correlation between early treatment and fewer days of viral shedding, a shorter course of the disease, and a shorter duration of hospital stay were also found in patients with non-severe COVID-19. The differences were not significant in patients with severe COVID-19, possibly due to the small sample size of severe patients in our study. Symptoms of fever and CT images of the patients improved significantly at the end of the treatment and showed a trend of better outcomes in relation to early treatment.
As included in the core outcome set of COVID-19, the time to recovery, duration of hospital stay, and days of viral shedding were considered as significant clinical outcomes for patients [21]. The improvement in these outcomes may indicate better effects of treatment and prognosis of patients with COVID-19. A shorter duration of the disease and hospital stay could result in a decreased demand for hospital beds and relieve the burden on health systems.
Our study has some notable strengths. First, the current study is a large sample multicenter study and is one of the few studies to address the relationship between treatment initiation time and clinical outcomes. Second, we applied the propensity score method to balance the patients’ baseline demographic and clinical characteristics to reduce the bias caused by these potential confounding factors. Third, we revealed that early treatment with QFPDD resulted in favorable clinical outcomes.
However, there were several limitations to the present study. First, the data did not include patients who were unable to be hospitalized at the early stage of the outbreak or were not treated with QFPDD during hospitalization, thereby affecting the representativeness of the sample and statistical results. Second, the percentage of patients with severe COVID-19 in the present study was 9%, which is lower than the 19 % reported by the Chinese Center for Disease Control and Prevention in February 2020 [22]. The lower proportion of patients with severe COVID-19 may be explained by our study, as data from Wuhan city was not included, where there were more patients with severe COVID-19 than in other provinces. Additionally, because of the retrospective study design, not all laboratory tests, including lactate dehydrogenase and IL-6, were performed in all patients, and certain laboratory reports were not uploaded to the system and could not be obtained after contacting the coordinators. Moreover, deteriorated patients in our study were transferred to a superior hospital, and we could not obtain data on their outcomes at that time when physicians invested more energy on patient care and were unable to follow-up. Lastly, although we have tried to use certain statistical methods to control the influence of the confounding variables on the results, it cannot be eliminated; the evidence from this retrospective study is therefore at a lower level when compared with evidence from a well-conducted prospective and randomized controlled trial, as this design could only detect association between factors and outcomes, while the latter can confirm the association.
Early treatment with QFPDD is associated with favorable outcomes for patient recovery, viral shedding, hospital stay, and course of the disease. It demonstrated that early treatment with QFPDD could be an effective strategy for controlling the epidemic and can provide evidence that government and international organizations should adopt such COVID-19 policies. Further multicenter, prospective studies with larger samples should be conducted to confirm the benefits of early treatment with QFPDD.
Contributors
YW, YW, HZ, WW, GL, NS, BL, NL, YM, YG and HW participated the study design. JB, HC, LC, QF, TG, YH, GH, XH, YH, JH, QH, SH, LJ, JW, HJ, XL, CL, JL, ML, QL, XL, HL, JL, ZL, YM, YM, LM, HN, FS, SS, DW, JW, MW, XW, YW, YW, GW, WW, LW, YX, HX, HX, SX, RX, CY, KY, PY, SY, GZ, JZ, LZ, SZ, WZ, KZ, YZ, JZ and TZ were responsible for recruiting patients. WB, RC, YF, HG, RH, LJ, JW, YL, HL, LL, JL, SL, ZS, YT, LT, ZW, YX, CZ, YZ and XZ were responsible for inputting and checking data. HY, HG, YK, ST and YZ participated in data management and statistical analysis. YW, HZ, GL, NS, BL, NL, YM, YG and HW participated in drafting the manuscript. YW, YW, HZ and WW revised the final paper. All authors reviewed and approved the final version of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding
This study was supported by “National Science and Technology Major Project” (2018ZX10101001-005-003, 2018ZX10101001-005-004).
Disclaimer
The funder of the study had no role in the study design, data collection, statistical analysis, results interpretation or reports writing. The corresponding authors had full access to all the data and had final responsibility for the decision to submit for publication.
Provenance and peer review
Not commissioned; externally peer reviewed.
Data availability statement
All relevant data to the study are included in the article or uploaded as supplementary information. Data are available upon reasonable request.
Open access
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Transparency document
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
We acknowledged all participants’ works in the study. And particularly we thank Prof. Dongshan Zhu for language editing on the manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.phrs.2020.105290.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. 2020-03-11. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (’accessed’ May 18th 2020).
- 2.World Health Organization. Statement on the third meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of coronavirus disease (COVID-19). 2020-05-01. https://www.who.int/news-room/detail/01-05-2020-statement-on-the-third-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-coronavirus-disease-(covid-19) (’accessed’ May 18th 2020).
- 3.World Health Organization . 2020. Coronavirus Disease (COVID-19) Situation Report-153; pp. 06–21.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200621-covid-19-sitrep-153.pdf?sfvrsn=c896464d_2 (’Accessed’ June 21th 2020) [Google Scholar]
- 4.Escalera-Antezana J.P., Lizon-Ferrufino N.F., Maldonado-Alanoca A., Alarcón-De-la-Vega G., Alvarado-Arnez L.E., Balderrama-Saavedra M.A. Clinical features of the first cases and a cluster of Coronavirus Disease 2019 (COVID-19) in Bolivia imported from Italy and Spain. Travel Med. Infect. Di. 2020;35:101653. doi: 10.1016/j.tmaid.2020.101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lechien J.R., Chiesa Estomba C.M., Place S., Laethem Y.V., Cabaraux P., Mat Q. Clinical and epidemiological characteristics of 1420 European patients with mild‐to‐moderate coronavirus disease 2019. J. Intern. Med. 2020;288:335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao L., Jin H., Wang M., Hu Y., Chen S., H Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;16:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long L., Zeng X., Zhang X., Xiao W., Guo E., Zhan W. Short-term outcomes of coronavirus disease 2019 and risk factors for progression. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00990-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holman N., Knighton P., Kar P., O’Keefe J., Curley M., Weaver A. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020 doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of Lopinavir–Ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung I.F., Lung K.C., Tso E.Y., Liu R., Chung T.W., Chu M. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. LANCET. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of Covid-19 - preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 15.Wu R., Wang L., Kuo H.C.D., Shannar A., Peter R., Chou P.J. An update on current therapeutic drugs treating COVID-19. Curr. Pharmacol. Rep. 2020:1–15. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xin S., Cheng X., Zhu B., Liao X., Yang F., Song L. Clinical retrospective study on the efficacy of Qingfei Paidu decoction combined with Western medicine for COVID-19 treatment. Biomed. Pharmacother. 2020;129:110500. doi: 10.1016/j.biopha.2020.110500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin M.D., Shukla S., Chung Y.H., Beiss V., Chan S.K., Ortega-Rivera O.A. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020;15:646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 18.National health commission and national administration of traditional Chinese Medicine. Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7) Chin. Med. J. 2020;133:1087–1095. doi: 10.1097/CM9.0000000000000819.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goyal D.K., Mansab F., Iqbal A., Bhatti S. Early intervention likely improves mortality in COVID-19 infection. Clin. Med. 2020;20:248–250. doi: 10.7861/clinmed.2020-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipsitch M., Perlman S., Waldor M.K. Testing COVID-19 therapies to prevent progression of mild disease. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin X., Pang B., Zhang J., Liu Q., Yang Z., Feng J. Core outcome set for clinical trials on coronavirus disease 2019 (COS-COVID) Engineering. 2020 doi: 10.1016/j.eng.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) — China, 2020. China CDC Weekly. 2020;8:113–122. doi: 10.46234/ccdcw2020.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data to the study are included in the article or uploaded as supplementary information. Data are available upon reasonable request.