Abstract
Objective
To perform a systematic review and meta-analysis to summarize and quantitatively evaluate the electroencephalogram (EEG) findings in patients with coronavirus disease 2019 (COVID-19).
Methods
The MEDLINE, CENTRAL, and ClinicalTrials.Gov databases were comprehensively assessed and searched for observational studies with EEG findings in patients with COVID-19. Pooled proportions of EEG findings with 95% confidence intervals (CIs) were assessed using a random effects model. The quality of assessment for each study, heterogeneity between the studies, and publication bias were also evaluated.
Results
In total, 12 studies with 308 patients were included in the meta-analysis. Abnormal background activity and generalized slowing in the pooled proportions were common findings among the patients with COVID-19 (96.1% [95% CI: 89.4–99.9]; I2 = 60%; p < 0.01 and 92.3% [95% CI: 81.2–99.3]; I2 = 74%; p < 0.01, respectively). The proportion of patients with epileptiform discharges (EDs) was 20.3% ([95% CI: 9.85–32.9]; I2 = 78%; p < 0.01). The proportion of EDs varied between patients with a history of epilepsy or seizures (59.5% [95% CI: 33.9–83.2]; I2 = 0%; p = 0.49) and patients without them (22.4% [95% CI: 10.4–36.4]; I2 = 46%; p = 0.07). The findings of seizures and status epilepticus on EEG were observed in 2.05% ([95% CI: 0.02–6.04]; I2 = 39%; p = 0.08) and 0.80% ([95% CI: 0.00.-3.69]; I2 = 28%; p = 0.17) of the patients, respectively.
Conclusion
The proportion of abnormal background activity in patients with COVID-19 was high (96.1%). Epileptiform discharges were present in 20.3% of the cases and the proportion varied between people who had a history of epilepsy/seizure and those who did not. However, the proportion of seizures and status epilepticus on EEG was low (2.05% and 0. 80%, respectively).
Keywords: COVID-19, EEG, Epilepsy, Seizure, Status epilepticus, Neurology
1. Introduction
Coronavirus disease 2019 (COVID-19) occurred in December 2019 in Wuhan, China and is currently one of the most important infectious diseases in the world. The common symptoms of patients with COVID-19 include fever, cough, and difficulty in breathing [1]. Some patients have neurological symptoms such as headache, dizziness, altered mental status, or seizures [2]. It has also been reported that COVID-19 causes serious neurological complications such as stroke, encephalitis, meningitis, encephalopathy, or status epilepticus [3], [4], [5].
Electroencephalography (EEG) is one of the simplest and most widely used neurological diagnostic tests. Moreover, EEG is an important test that can change clinical decisions. It is useful to evaluate whether patients have neurological complications. In particular, EEG can be used to assess encephalopathy, epileptogenicity, and any focal abnormalities in patients with COVID-19.
Some studies successfully described EEG findings in patients with COVID-19 [6], [7], [8]. However, the number of patients in previous studies has been limited, and the proportions of abnormal findings in EEG vary across studies. In addition, a comprehensive and quantitative analysis of each finding in these past studies has not been conducted. Therefore, the aim of our systematic review and meta-analysis was to evaluate the proportion of EEG findings in patients with COVID-19, systematically and quantitatively.
2. Methods
2.1. Retrieval of studies
We conducted a systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [9]. This review protocol has not been previously registered. MEDLINE (accessed from PubMed) and CENTRAL (accessed from the Cochrane Library) were searched systematically up to September 19, 2020. In the PubMed and Cochrane libraries, the following key words (in the title/abstract) were searched: (“COVID 19″ OR ”SARS CoV 2″) AND (“epilepsy” OR “seizure” OR “status epilepticus” OR “electroencephalogram” OR “EEG” OR “encephalopathy” OR “encephalitis”). We also used ClinicalTtrials.gov to search for unpublished, ongoing, terminated, or completed studies to avoid publication bias. We screened the reference lists of all relevant articles for additional data.
2.2. Eligibility and risk-of-bias assessment
Studies were included based on the following criteria: (1) reports of COVID-19 patients who underwent EEG; (2) original reports such as case series with more than 3 cases, case–control studies, or cohort studies; (3) studies with descriptions of EEG findings such as background activity, rhythmic/periodic discharges, epileptiform discharges, focal findings, seizures, or status epilepticus. To accurately evaluate the proportion and to avoid selection bias of the abnormal findings, we excluded case reports with 3 cases or less. We also excluded not yet recruiting, recruiting, or withdrawn studies in Clinical.Trials.gov. Studies written in languages other than English were excluded. We used the Joanna Briggs Institute checklist to assess the quality of the included case series and case–control studies [10], [11].
2.3. Data extraction and outcome measures
Two reviewers (TK and PG) independently screened the titles and abstracts, and evaluated the full texts of the selected articles. They included those that met the inclusion criteria and discussed any discrepancies with a 3rd reviewer (NK). The following variables were extracted: author, publication year, study design, age and sex of the subjects, country in which the study was conducted, diagnostic methods used, neurological comorbidity in the subjects, time from symptom onset until EEG, clinical setting, presentation/indication for EEG, need for intubation, need for sedation and antiepileptic/seizure drug, occurrence of cardiac arrest, brain imaging investigations, if any, type and findings of EEG, and clinical outcomes.
The outcome measures were the proportions of abnormal background activity, generalized slowing, discontinuous/burst attenuation or suppression/suppression, generalized periodic discharges (GPDs), lateralized periodic discharges (LPDs), generalized rhythmic delta activity (GRDA), lateralized rhythmic delta activity (LRDA), epileptiform discharges (EDs), focal slowing, seizures, and status epilepticus. We referred to the American Clinical Neurophysiology Society’s (ACNS) Standardized Critical Care EEG Terminology: 2012 version for the EEG terminology [12]. According to the ACNS’s EEG terminology, we defined any bilateral and bisynchronous pattern as generalized, sharp waves and spikes as epileptiform discharges, frontal intermittent rhythmic delta activity as GRDA, and triphasic waves such as GPDs [12]. We also defined background/diffuse slow as generalized slowing, seizure and status epilepticus as epileptiform discharges. We defined status epilepticus as one type of seizure, because based on the International League Against Epilepsy's definition of status epilepticus, status epilepticus is a prolonged seizure [13]. For findings that were not described, we used the following two approaches: (1) In studies with individual EEG reports, we included undescribed findings as negative findings in the meta-analysis; (2) in studies without individual EEG data, undescribed findings were not included in the meta-analysis as unavailable data. We excluded unclassifiable data in our meta-analysis (ex. severe diffuse slowing/discontinuous/electrocerebral inactivity).
2.4. Statistical analysis
In this systematic review and meta-analysis, we used a single-arm analysis. For categorical variables, the percentage, mean, and standard deviation were calculated. We used random-effects models with the DerSimonian-Laird estimator to consider the variance between and among the studies. We calculated the pooled proportions using the variance-stabilized Freeman–Tukey double arcsine transformation because some proportions from the original studies were extremely high or low. Confidence intervals (CIs) for individual studies were computed using the Wilson score CI method, adjusting for continuity. The I 2 statistic and Cochran Q tests were used to indicate heterogeneity between the studies. For the I 2 statistic, 25% to 50%, 50% to 75%, and ≥75% were considered as low, moderate, and high heterogeneity, respectively. For the Cochran Q test, P < 0.10 was considered as severe heterogeneity [14], [15]. Publication bias was assessed using a funnel plot and Egger’s test, which is a quantitative analysis of asymmetry in the funnel plot. For Egger’s test, P < 0.10 was considered significant publication bias [15], [16]. We did not assess publication bias for the outcomes reported in less than 10 studies. We conducted statistical analyses using the R software version 3.6.2 (R Development Core Team 2019), with packages meta version 4.15-0 and metaphor version 2.4-0.
3. Results
A total of 363 studies were retrieved (349 papers from MEDLINE, 3 papers from CENTRAL, and 11 studies from Clinical.Trials.gov) up to September 19, 2020. After removing duplicates and screening the titles and abstracts, 228 studies were identified. The full-text screening of these studies led to the exclusion of 216 studies that did not meet the inclusion criteria. A total of 12 studies with 308 patients fulfilled the eligibility criteria for inclusion in the meta-analysis [6], [7], [8], [17], [18], [19], [20], [21], [22], [23], [24], [25]. We referred to a case report [26] of patients who were included in a case series [19] to obtain more detailed EEG findings. Fig. 1 outlines the selection process. Table 1 summarizes the findings of the included studies. More details of the included studies are shown in Supplementary Table 1. The mean score for the quality of 10 case series and 2 case–control studies was 8.7 out of 10. (see Table 1 and Supplementary Table 2).
Fig. 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram of this research.
Table 1.
Summary of findings.
| First author and year [Reference] |
Study design | No | Age (SD or range) | Male (%) | Country | Quality score (out of 10) | Neurological Comorbidity | Presentation/EEG indication | Setting | Sedation | Cardiac arrest | Brain Image | EEG type | EEG findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Petrescu 2020 [17] | CS | 36 | 63.9 (12.1) | 80.5 | France | 9 | 10/36: Cognitive impairment, 2/36: Stroke, 2/36: SDH, 1/36: Brain tumor, 1/36: Epilepsy, 1/36: Hydrocephalus, 1/36: Anti-MAG Neuropathy |
36/36: ARDS, 6/18: Absence of awakening after stopping sedation, 6/18: AMS, 2/18: Suspicion of epileptic seizures |
18/36: ICU, 18/36: MU |
5/36 | N/A | 11/36: MRI (4: Atrophy, 3: Stroke, 2: SDH, 1: Gliosis, 1: Leptomeningeal enhancement), 15/36: CT (8: Atrophy, 5: Normal, 1: SDH, 1: Stroke, 1: Meningioma, 1: Focal resection) |
36/36: Routine EEG |
32/36: ABA, 27/36: Generalized slowing, 4/36: Discontinuous background, 6/36: GPDs, 0/36: LPDs, 6/36: GRDA, 0/36: LRDA, 0/36: Focal slowing, 0/36 EDs, N/A: EDs in PWE, N/A: EDs in PwoES, 0/36: Seizure, 0/36: SE |
| Ayub 2020 [18] | CS | 37 | 66.0 (59.5–76.3) | 73 | USA | 9 | 8/37: Past history of CNS pathology, 1/37: Past seizure history |
11/37: Possible seizure, 24/37: AMS, 2/37: Cardiac arrest |
N/A | 27/37 | 2/37 | 9/35: Neuroimaging acutely abnormal |
23/37: cEEG, 14/37: Routine EEGs |
N/A: Overall ABA, 34/37: Absent PDR, 31/37: Generalized slowing, 5/37: Burst suppression, 12/37: GPDs, 0/37: LPDs, 5/37: GRDA, 1/37: LRDA, 3/37: Focal slowing, 14/37 EDs, N/A: EDs in PWE, N/A: EDs in PwoES, 1/37: Seizure, 1/37: SE (NCSE) |
| Galanopoulou 2020 [6] | CC | 28 | 63.2 (11.9) | 63.6 | USA | 9 | 4/22: Epilepsy, 7/22: Other neurological disorders |
20/22: AMS, 14/22: Suspicion of clinical seizure-like events, 1/22: Confusion, 2/22: Gaze deviation |
ICU | 14/22 | N/A | 3/13: Neuroimaging: new findings (subcortical and mild periventricular white matter signal hyperintensity, SAH, SDH) |
20/22: 8ch‐EEG, 4/22: routine, 7/22: cEEG |
22/22: ABA, 22/22: Generalized slowing, 1/22: Discontinuous or burst suppression, 1/22 GPDs, N/A: LPDs, 3/22: GRDA, 1/22: LRDA, 5/22: Focal slowing, 9/22 EDs, 2/4: EDs in PWES, 7/18: EDs in PwoES, 0/22: Seizure, 0/22: SE |
| Pasini 2020 [7] | CS | 15 | 64.6 (47–79) | 40 | Italy | 8 | 2/15: Cognitive decline, 1/15: Previous limbic encephalitis, 1/15: Frontal metastasis |
15/15: AMS (11 confusion, 4 impairment of consciousness), 1/15: Aphasia |
N/A | N/A | 2/15 | 0/8 CT: Abnormality, 2/6: MRI: hyperintensity in white matter |
N/A | 11/15: ABA, 9/15: Generalized slowing, 2/15: Discontinued activity or background suppression, 10/15: No reactivity to external stimuli, N/A: GPDs, N/A: LPDs, 1/15: GRDA, 0/15: LRDA, 3/15: Focal slowing (frontal or central), 1/15 EDs, N/A: EDs in PWE, N/A: EDs in PwoES, 1/15: Seizure, 1/15: SE |
| Pastor 2020 [8] | CC | 20 | 63.9 (12.1) | 85 | Spain | 9 | 8/20: Stroke | 18/20: AMS (8 Stuporous, 10 Confused) |
Non-critical care unit | N/A | 0/20 | N/A | N/A | 20/20: ABA, 20/20: Generalized slowing (5/20: Excess of slow posterior activity, 5/20: Predominant theta activity in more than 50% of recording, 10/20 Predominant delta activity in more than 50% of recording), N/A: DC/BAS/S, 0/20: GPDs, 1/20: LPDs, GRDA: 0/20, LRDA: 0/20, 0/20: Focal slowing, 3/20 EDs, N/A: EDs in PWE, N/A: EDs in PwoES, 0/20: Seizure, 0/20: SE |
| Louis 2020 [19] | CS | 22 | 66.5 (11.2) | 63.6 | USA | 9 | 2/22: Epilepsy, 1/22: Stroke, 1/22: Headache, TBI |
17/22: AMS, 5/22: seizure-like event |
N/A | 14/22 | N/A | N/A | 19/22: cEEG, 3/22: Routine EEG |
22/22: ABA, 22/22: Generalized slowing, 0/22: DC/BAS/S, 11/22: No PDR, 7/22: GPDs, N/A: LPDs, 11/22: GRDA, N/A: LRDA, 5/22 EDs, 2/2: EDs in PWES, 3/20: EDs in PwoES, 2/22: Seizure, 1/22: SE [26] |
| Canham 2020 [20] | CS | 10 | 55.9 (20.4) | 80 | UK | 8 | 1/10: Epilepsy, 1/10: Stroke |
9/9: AMS, 2/9: Delirium, 6/10 Seizure (4: GTCS, 1: Focal Motor, 2: Tonic) |
ICU | 1/10 | 0/10 | 10/10: CT (2 venography, 4: small vessel disease, 2: volume loss, 2: SAH), 3/10: MRI (1: small infarct in the left precentral gyrus, 1: frontal and medial temporal atrophy, 1: Normal) |
10/10: Routine EEG (a minimum of 9 electrodes) |
10/10: ABA, 10/10: Generalized slowing, 0/10: DC/BAS/S, 2/10: GPDs (Triphasic waves), 0/10: LPDs, 1/10: GRDA, 0/10: LRDA, 0/10: Focal slowing, 2/10 EDs, 0/1: EDs in PWES, 2/9: EDs in PwoES, 0/10: Seizure, 0/10: SE |
| Pellinen 2020 [21] | CS | 111 | 64 (17) | 71.2 | USA | 9 | 23/111: Remote ischemic stroke, 13/111: Epilepsy, 19/111: Other brain disorders |
42/111: Definite/suspected seizure, 78/111: Coma |
85/111: ICU | 67/111 | 11/111 | 71/90: Abnormal, 31/90: Acute focal cortical, 23/90: Acute diffuse, 10/90: Chronic focal cortical, 36/90: Chronic subcortical, 15/90: Chronic diffuse |
Majority: cEEG, Some cases: Reduced 8-bioplar channel montage |
106/111: ABA, N/A: Generalized slowing (17/111: Mild diffuse slowing, 60/111: Moderate diffuse slowing, 29/111: Severe diffuse slowing/discontinuous/ electrocerebral inactivity), N/A: DC/BAS/S, 11/111: GPDs, 3/111: LPDs, 4/111: GRDA, 7/111: LRDA, 27/111: Focal slowing, 35/111 EDs, 8/13: EDs in PWES, 20/85: EDs in PwoES, 8/111: Seizure, 2/111: SE |
| Cecchetti 2020 [22] | CS | 18 | 66.8 (10.7) | 61.1 | Italy | 9 | 1/18: Glioblastoma | 5/18: AMS, 5/18: Seizure/spasm, 3/18: Delirium, 5/18: Coma | N/A | N/A | N/A | 1/18: PRES, 1/18: Remote IPH, 1/18: Glioblastoma, 1/18: Brain metastasis, 1/18: Traumatic SDH, 1/18: Remote hemispheric stroke, 1/18: Anterior pontine demyelinating les. | N/A | 16/18: ABA, 16/18: Generalized slowing (5/18: Normal/mild EEG alteration, 9/18: Moderate EEG alteration, 4/18: Severe EEG alteration), N/A: DC/BAS/S, N/A: GPDs, N/A: LPDs, N/A: GRDA, N/A: LRDA, 7/18: Focal slowing, 2/18: EDs, N/A: EDs in PWES, 2/18: EDs in PwoES, 0/18 Seizure, 0/18: SE |
| Pilato 2020 [23] | CS | 8 | 63 (47–87) | 62.5 | USA | 8 | 5/8: Epilepsy, 2/8: Developmental delay, 2/8: Dementia, 1/8: TBI, 1/8: Anoxic brain injury |
6/8: AMS, 3/8: Seizure or seizure-like episode, 2/8: Weakness | N/A | 1/8 | 2/8 | 5/8: MRI (3: Atrophy, 3: Ventriculomegaly, 2: Stroke) |
3/8: Routine EEG, 5/8: cEEG |
8/8: ABA, 8/8: Generalized slowing, 1/8: Discontinuous background, 3/8: GPDs, 1/8: LPDs, 0/8: GRDA, 0/8: LRDA, 1/8: Focal slowing (Right posterior quadrant), 5/8 EDs, 3/5: EDs in PWES, 2/3: EDs in PwoES, 2/8: Seizure, 2/8: SE (NCSE) |
| Chen 2020 [24] | CS | 5 | 45.0 (9.8) | 40 | USA | 9 | 0/5 | 5/5: AMS (3/5: suspected encephalopathy), 3/5: Seizure-like episode | ICU | 5/5 | 0/5 | 1/1: CT (small subacute-chronic strokes) | 3/5: Rapid-EEG recording (10-electrode), 2/5: cEEG |
5/5: ABA, 5/5: Generalized slowing, 0/5: Discontinuous/Suppression, 2/5: GPDs, 0/5: LPDs, 5/5: GRDA, 0/5: LRDA, 0/5: Focal slowing, 2/5: EDs, N/A: EDs in PWES, 2/2: EDs in PwoES, 2/5: Seizure, 2/5: SE |
| Delorme 2020 [25] | CS | 4 | 66.8 (5.1) | 50 | France | 8 | 1/4: Epilepsy | 3/4: AMS (Agitation), 2/4: Frontal lobe syndrome, 1/4: Generalized convulsive status epilepticus, 1/4: Anxiety and Depressed mood, 1/4: Cerebellar syndrome | N/A | N/A | N/A | 4/4: MRI (1: Unremarkable, 1: Non-specific white matter hyperintensities, 1: Right mesial sclerosis (already known), 1: Right T2 orbitofrontal hyperintensity), 4/4: PET (4: hypometabolism within bilateral prefrontal or orbitofrontal cortex, 4: hypermetabolism within the cerebellar vermis) | N/A | 1/4: ABA, 1/4: Generalized slowing, 0/4: Discontinuous/Suppression, 1/4: GPDs, 1/4: LPDs, 0/4: GRDA, 0/4: LRDA, 0/4: Focal slowing, 0/4: EDs, N/A: EDs in PWES, N/A: EDs in PwoES, 0/4: Seizure, 0/4: SE |
CS: Case series. CC: Case–control study. No: Number of patients. SD: Standard deviation. EEG: Electroencephalogram. CNS: Central nervous system. ARDS: Acute respiratory distress syndrome. RT-PCR: Reverse transcription polymerase chain reaction. RNA: Ribonucleic acid. N/A: Not available. MU: Medical unit. ICU: Intensive care unit. LTAC: Long-term acute care hospital. AMS: Altered mental status. SAH: Subarachnoid hemorrhage. SDH: Subdural hematoma. MAG: Myelin-associated glycoprotein. TBI: Trauma brain injury. GTCS: Generalized tonic-clonic seizure. cEEG: Continuous electroencephalogram. routine EEG: Routine electroencephalogram. CT: Computed tomography. MRI: Magnetic resonance imaging. PRES: Posterior reversible encephalopathy syndrome. IPH: Intraparenchymal hemorrhage. ABA: Abnormal background activity. DC/BAS/S: Discontinuous/Burst-attenuation or suppression/Suppression. GPD: Generalized periodic discharges. LPD: Lateralized periodic discharge. PDR: Posterior dominant rhythm. GRDA: Generalized rhythmic delta activity. LRDA: Lateralized rhythmic delta activity. EDs: Epileptiform discharges. PWES: Patients with prior epilepsy or seizure history. PwoES: Patients without prior epilepsy or seizure history. SE: Status epilepticus. NCSE: Nonconvulsive status epilepticus.
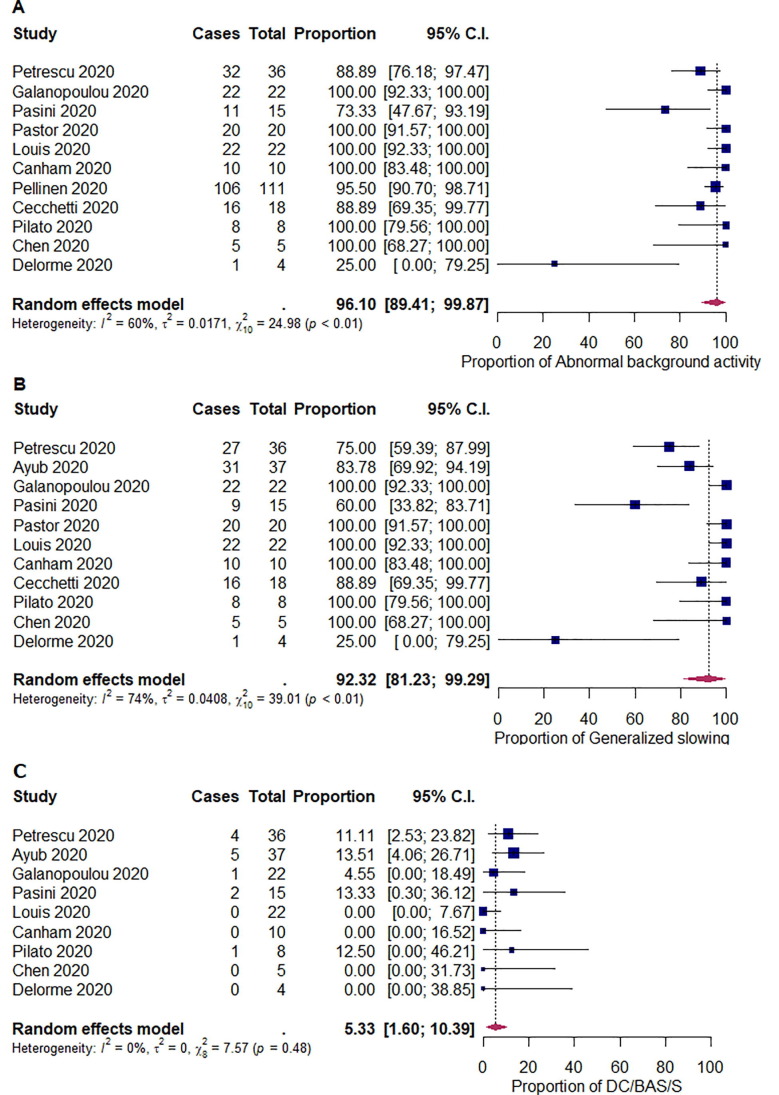
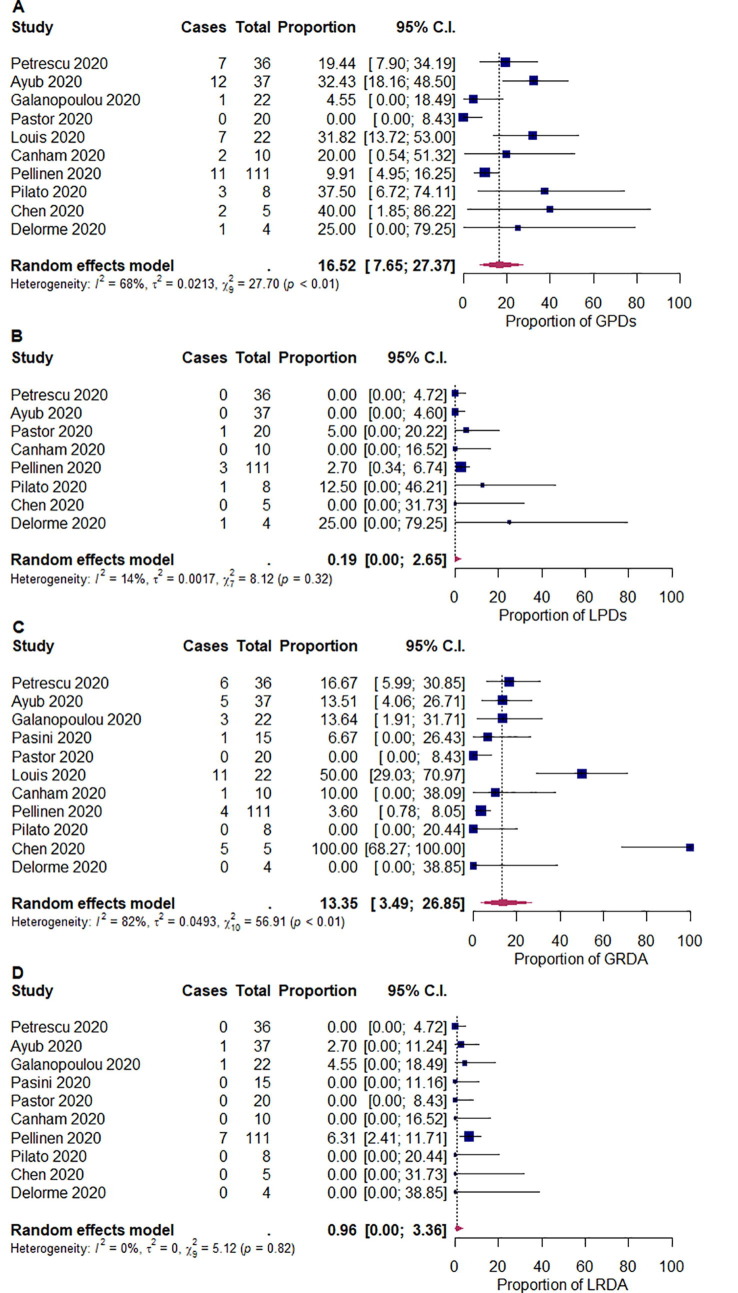
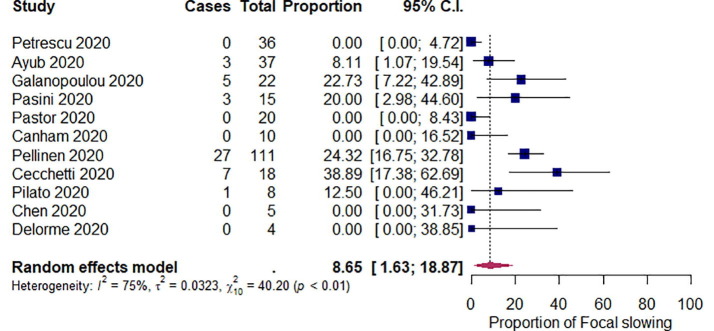
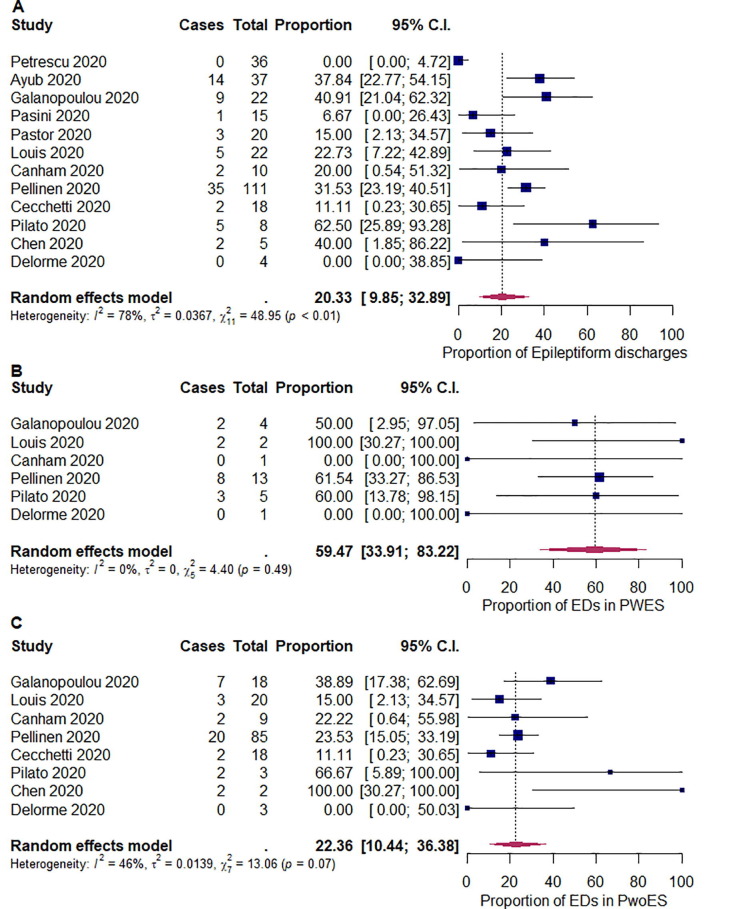
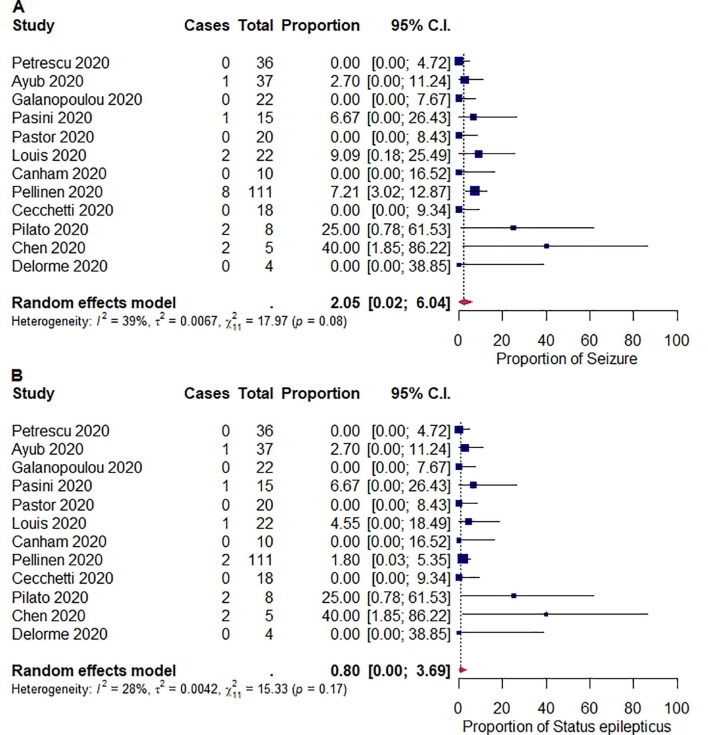
We summarized the outcomes of our meta-analysis in Table 2 . The pooled proportions of abnormal background activity, generalized slowing, and discontinuous/burst attenuation or suppression/suppression were 96.1% ([95% CI: 89.4–99.9]; I 2 = 60%; p < 0.01), 92.3% ([95% CI: 81.2–99.3]; I 2 = 74%; p < 0.01), and 5.33% ([95% CI: 1.60–10.4]; I 2 = 0%; p = 0.48), respectively (Fig. 2 ). The pooled proportions of GPDs, LPDs, GRDA, and LRDA were 16.5% ([95% CI: 7.65–27.4]; I 2 = 68%; p < 0.01), 0.19% ([95% CI: 0.00–2.65]; I 2 = 14%; p = 0.32), 13.4% ([95% CI: 3.49–26.9]; I 2 = 82%; p < 0.01), and 0.96% ([95% CI: 0.00–3.36]; I 2 = 0%; p < 0.82), respectively (Fig. 3 ). The pooled proportion of focal slowing was 8.65% ([95% CI: 1.63–18.9]; I 2 = 75%; p < 0.01) (Fig. 4 ). The pooled proportion of EDs was 20.3% ([95% CI: 9.85–32.9]; I 2 = 78%; p < 0.01) (Fig. 5 A). The proportion of EDs varied between patients with a history of epilepsy or seizure (59.5% [95% CI: 33.9–83.2]; I 2 = 0%; p = 0.49) and those without them (22.4% [95% CI: 10.4–36.4]; I 2 = 46%; p = 0.07) (Fig. 5B and C). The pooled proportion of seizures and status epilepticus was 2.05% ([95% CI: 0.02–6.04]; I 2 = 39%; p = 0.08) and 0.80% ([95% CI: 0.00–3.69]; I 2 = 28%; p = 0.17) of the patients, respectively (Fig. 6 ).
Table 2.
Results of Meta-analysis.
| Heterogenicity |
Egger's test | ||||||
|---|---|---|---|---|---|---|---|
| Outcomes | n | N | Proportion (%) | 95% CI | I2 (%) | p | p |
| Abnormal background activity | 11 | 271 | 96.1 | 89.4–99.9 | 60 | < 0.01 | 0.18 |
| Generalized slowing | 11 | 197 | 92.3 | 81.2–99.3 | 74 | < 0.01 | 0.48 |
| Discontinuous/Burst-attenuation or suppression/Suppression | 9 | 159 | 5.33 | 1.60–10.4 | 0 | 0.48 | - |
| GPDs | 10 | 275 | 16.5 | 7.65–27.4 | 68 | < 0.01 | 0.21 |
| LPDs | 8 | 231 | 0.19 | 0.00–2.65 | 14 | 0.32 | - |
| GRDA | 11 | 275 | 13.4 | 3.49–26.9 | 82 | < 0.01 | 0.21 |
| LRDA | 10 | 268 | 0.96 | 0.00–3.36 | 0 | 0.82 | 0.34 |
| Focal slowing | 11 | 286 | 8.65 | 1.63–18.9 | 75 | <0.01 | 0.55 |
| EDs | 12 | 308 | 20.3 | 9.85–32.9 | 78 | <0.01 | 0.83 |
| EDs in patients with prior history of epilepsy or seizure | 6 | 26 | 59.5 | 33.9–83.2 | 0 | 0.49 | - |
| EDs in patients without prior history of epilepsy or seizure | 8 | 155 | 22.4 | 10.4–36.4 | 46 | 0.07 | - |
| Seizure | 12 | 308 | 2.05 | 0.02–6.04 | 39 | 0.08 | 0.18 |
| Status epilepticus | 12 | 308 | 0.80 | 0.00–3.69 | 28 | 0.17 | 0.03 |
n: Number of studies. N: Number of patients. CI: Confidence interval. GPDs: Generalized periodic discharges. LPDs: Lateralized periodic discharges. GRDA: Generalized rhythmic delta activity. LRDA: Lateralized rhythmic delta activity. EDs: Epileptiform discharges.
Fig. 2.
Random-effects meta-analysis of the pooled proportions of (A) abnormal background activity, (B) generalized slowing, and (C) discontinuous/burst-attenuation or suppression/suppression (DC/BAS/S). 95% C.I. = 95% confidence intervals.
Fig. 3.
Random-effects meta-analysis of the pooled proportions of (A) generalized periodic discharges (GPDs), (B) lateralized periodic discharges (LPDs), (C) generalized rhythmic delta activity (GRDA), and (D) lateralized rhythmic delta activity (LRDA). 95% C.I. = 95% confidence intervals.
Fig. 4.
Random-effects meta-analysis of the pooled proportion of focal slowing. 95% C.I. = 95% confidence intervals.
Fig. 5.
Random-effects meta-analysis of the pooled proportions of (A) epileptiform discharges (EDs), (B) EDs in patients with a history of epilepsy or seizure (PWES), and (C) EDs in patients without a history of epilepsy or seizure (PwoES). 95% C.I. = 95% confidence intervals.
Fig. 6.
Random-effects meta-analysis of the pooled proportions of (A) electrographic seizure and (B) electrographic status epilepticus. 95% C.I. = 95% confidence intervals.
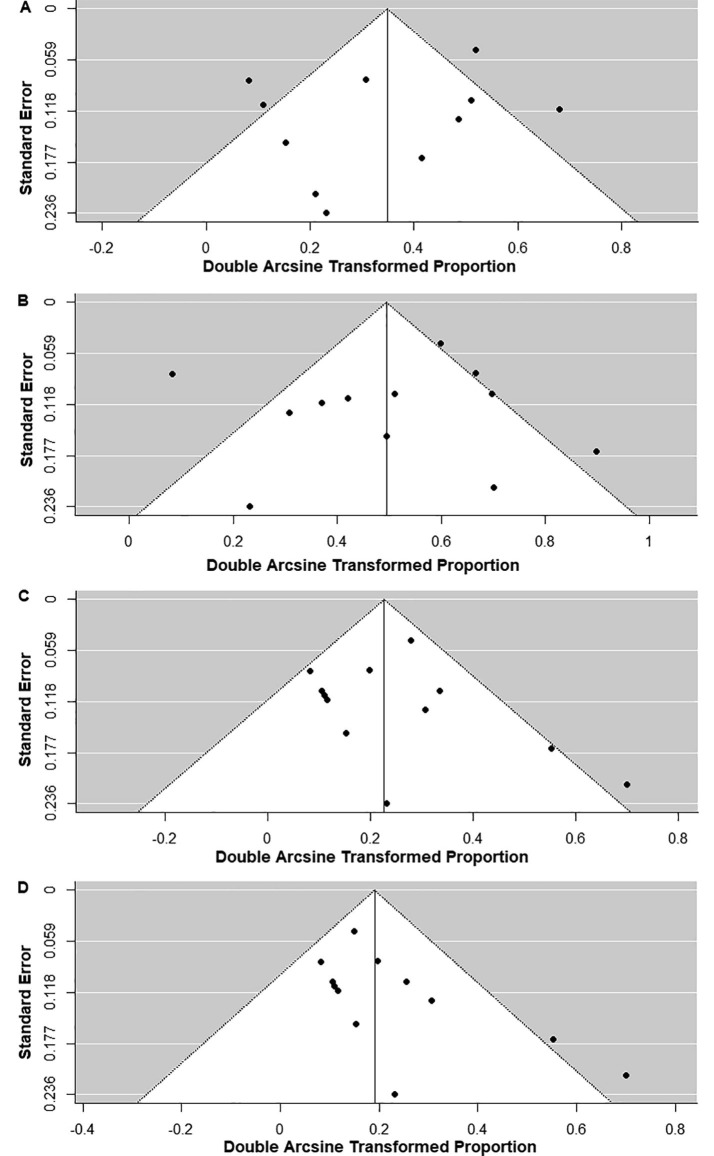
We looked for publication bias for abnormal background activity, generalized slowing, GPDs, GRDA, LRDA, focal slowing, EDs, seizures, and status epilepticus using a funnel plot and Egger’s test (Fig. 7, Fig. 8 and Table 2). There was a significant publication bias for status epilepticus (P = 0.03); however, there was no significant publication bias for abnormal background activity (P = 0.18), generalized slowing (P = 0.48), GPDs (P = 0.21), GRDA (P = 0.21), LRDA (P = 0.34), focal slowing (P = 0.55), EDs (P = 0.83), and seizures (P = 0.18) (Table 2).
Fig. 7.
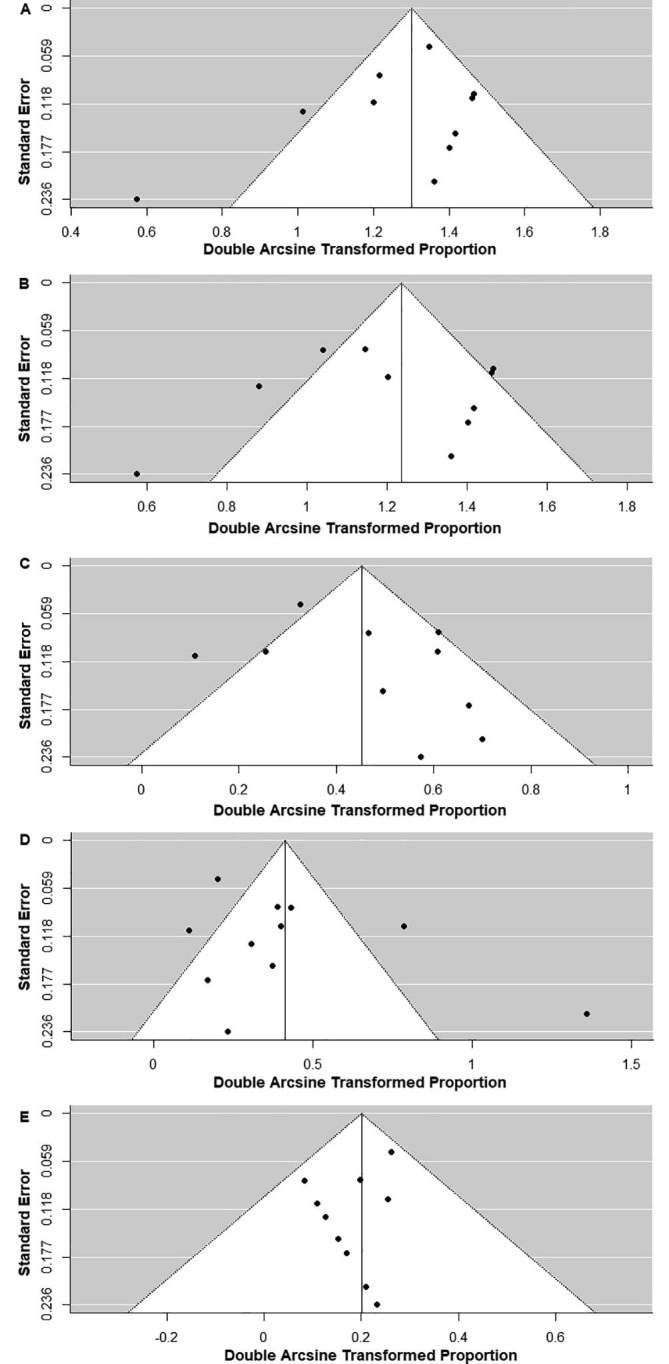
Funnel plots of the meta-analysis of (A) abnormal background activity, (B) generalized slowing, (C) generalized periodic discharges, (D) generalized rhythmic delta activity, and (E) lateralized rhythmic delta activity.
Fig. 8.
Funnel plots of the meta-analysis of (A) focal slowing, (B) epileptiform discharges, (C) electrographic seizure, and (D) electrographic status epilepticus.
4. Discussion
In this analysis, using the methodology of systematic review and meta-analysis, we have shown the comprehensive and quantitative analysis of the proportion of EEG findings in patients with COVID-19 who underwent EEG tests.
We found that the proportion of abnormal background activity was exceedingly high at 96.1%. This result suggests that patients infected with COVID-19 who required EEG may likely have encephalopathy. In fact, the most common indication for EEG was altered mental status (210/307; 68.4%). Symptoms such as fever, hypoxia, and sedation may cause encephalopathy. Furthermore, encephalopathy associated with COVID-19 might be caused by any of the three pathways as follows: (1) indirect immune-mediated responses such as a cytokine storm, (2) direct central nervous system invasion by the virus, and (3) damage to the neural cells through angiotensin-converting enzyme 2 (ACE2) receptors [27]. In addition, we found discontinuous/burst suppression/background suppression and GPDs in 5.33% and 16.5% of the patients, respectively. In general, GPDs are seen in only 1% of routine EEGs; however, these are seen in up to 20% of cases with severe post-anoxic encephalopathy [28]. Furthermore, many patients in the intensive care unit were included in our study. Hence, patients with COVID-19 who required EEG may likely have severe COVID-19 infection and/or severe encephalopathy. Alternatively, EEGs might have been selectively administered to more critically ill patients due to infection prevention to medical providers and medical resource limitations during the COVID-19 pandemic [29].
Our results showed that focal EEG abnormalities were not specific. Although focal slowing was seen in 8.65% of the cases, most of the included studies (9/12; 75.0%) did not describe the detailed location of the focal abnormalities. Some of them described that focal sharp/slow waves were seen in the frontal, central, and the right posterior quadrant; however, these were small in number and were inconsistent [7], [17], [23]. In addition, LPD and LRDA were infrequent. Complications of COVID-19, such as stroke, vasculitis, or encephalitis, might cause focal abnormalities [3]; however, focal findings on the EEG may also be affected by the patient’s preexisting chronic neurological diseases or past medical history of brain disease.
We also found that EDs were present in 20.3% of patients. The causes of the ED findings in patients with COVID-19 may include acute symptomatic seizure, de novo epileptogenicity, or preexisting epilepsy with or without exacerbation. In fact, the proportion of EDs varied between patients with a history of epilepsy or seizures (59.5%) and those without them (22.4%). One study showed that a history of epilepsy has an increased risk of EDs [21]; however, other included studies were not properly designed to compare epileptiform discharge rates between patients with epilepsy and those without epilepsy. In addition, there might be an EEG ordering bias in which patients with epilepsy are given priority to take EEG in order to avoid unnecessary exposure of health-care workers to COVID-19. Therefore, our study cannot conclude that epileptiform discharges are more common in patients with epilepsy. Furthermore, it is not known whether epileptiform discharges in patients with epilepsy are increased by the COVID-19 infection. However, infection is widely known as a common precipitating factor for seizures in patients with epilepsy [30]. A well-designed study to determine whether COVID-19 infection increases epileptiform discharges is needed.
The other major finding of our study was that the proportion of seizures and status epilepticus on EEG was low at 2.05% and 0.80%, respectively. The proportions of clinical seizures and status epilepticus in patients with COVID-19 have been reported to be 0.08–0.5% and 0.03%, respectively [2], [31]. On the contrary, suspicious seizure episodes were a common indication for EEG in the studies included in this meta-analysis. Although our study included a relatively large number of patients with clinical seizures or status epilepticus episodes, seizures and status epilepticus on EEG were rare. Hence, patients with COVID-19 having both clinical and electrographic seizures/status epilepticus might be rare. Although not to be taken lightly, the data showing low rates of seizures and status epilepticus on EEG might be helpful evidence for clinicians, especially in situations with limited medical resources, limiting the indication of EEG tests.
Two systematic review studies with similar clinical questions have been previously reported [32], [33]. Our meta-analysis provided additional value to those studies. Our findings were consistent with those of previous studies showing that abnormal background activity was the most common finding, and seizure/status epilepticus was infrequent.
However, this study has several limitations. First, we included only studies written in English. Moreover, all included studies were reported from either the USA or Europe. Hence, there might be a selection bias, and the results cannot be generalized to other regions. Second, we did not include preprint articles in this meta-analysis. Although we searched for unpublished studies using ClinicalTrials.gov, there might have been unpublished studies in MEDLINE or CENTRAL that could have been included. Third, we considered undescribed findings as negative findings in studies with individual EEG reports. Some infrequent or less critical findings such as focal slowing might have been overlooked or unreported. Hence, some findings might have been underestimated. Fourth, the patient characteristics and clinical settings, such as montage, timing, interpretation, and duration of EEG, COVID-19 severity, and treatment of COVID-19 patients varied across each study. In fact, there was heterogenicity in the ranges of abnormal background activity, generalized slowing, GPDs, GRDA, focal slowing, and EDs, EDs in patients without a history of epilepsy or seizure, seizure, which might support the possibility of these limitations. Finally, there was a significant publication bias for status epilepticus. This might be because small case series are likely to report status epilepticus.
5. Conclusion
In conclusion, our findings suggest that the proportion of abnormal background activity in patients with COVID-19 was high (96.1%). Epileptiform discharges were present in 20.3% of the cases and the proportion varied between people who had a history of epilepsy or seizures and those who did not. However, the proportion of seizures and status epilepticus on EEG was low (2.05% and 0.80%, respectively).
Declarations
Conflicts of interest/Competing interests: The authors declare that they have no conflicts of interest.
Ethics approval: Waived because of the nature of the article: A systematic review and meta-analysis.
Consent to participate: Waived because of the nature of the article: A systematic review and meta-analysis.
Consent for publication: All authors approve publication of the article. Consent from patients was waived because this article does not include personal details which allow patient identification.
Availability of data and material: Data will be made available on reasonable request.
Authors’ contributions: All authors contributed to study conception and design. Takafumi Kubota and Prasannakumar Gajera obtained and analyzed the data. All authors discussed any discrepancies found and results obtained. Takafumi Kubota drafted the manuscript. Takafumi Kubota and Prasannakumar Gajera made the tables and figures. Naoto Kuroda and Prasannakumar Gajera critically revised the work. All authors read and approved the final version of the manuscript.
Acknowledgments
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.yebeh.2020.107682.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Guan W., Ni Z., Hu Y.H.Y.Y.H., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varatharaj A., Thomas N., Ellul M.A., Davies N.W.S., Pollak T.A., Tenorio E.L., et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020 doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed M.U., Hanif M., Ali M.J., Haider M.A., Kherani D., Memon G.M., et al. Neurological manifestations of COVID-19 (SARS-CoV-2): a review. Front Neurol. 2020;11:518. doi: 10.3389/fneur.2020.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., et al. Neurological associations of COVID-19. Lancet Neurol. 2020;4422:2–3. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galanopoulou A.S., Ferastraoaru V., Correa D.J., Cherian K., Duberstein S., Gursky J., et al. EEG findings in acutely ill patients investigated for SARS-CoV-2/COVID-19: A small case series preliminary report. Epilepsia Open. 2020;5:314–324. doi: 10.1002/epi4.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasini E., Bisulli F., Volpi L., Minardi I., Tappatà M., Muccioli L., et al. EEG findings in COVID-19 related encephalopathy. Clin Neurophysiol. 2020;131:2265–2267. doi: 10.1016/j.clinph.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pastor J., Vega-Zelaya L., Martín Abad E. Specific EEG encephalopathy pattern in SARS-CoV-2 patients. J Clin Med. 2020;9 doi: 10.3390/jcm9051545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. vol. 62. 2009. https://doi.org/10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed]
- 10.The Joanna Briggs Institute Checklist for case control studies. Joanna Briggs Inst Crit Apprais Tools. 2016:1–6. [Google Scholar]
- 11.Joanna Briggs Institute Checklist for case series. Joanna Briggs Inst Crit Apprais Tools Use JBI Syst Rev. 2017:1–6. doi: 10.17221/96/2009-CJGPB. [DOI] [Google Scholar]
- 12.Hirsch L.J., Laroche S.M., Gaspard N., Gerard E., Svoronos A., Herman S.T., et al. American clinical neurophysiology society’s standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- 13.Trinka E., Cock H., Hesdorffer D., Rossetti A.O., Scheffer I.E., Shinnar S., et al. A definition and classification of status epilepticus - Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56:1515–1523. doi: 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 14.Lau J., Ioannidis J.P.A., Schmid C.H. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 15.Haidich A. Meta-analysis in medical research. Hippokratia. 2010;14:29–37. [PMC free article] [PubMed] [Google Scholar]
- 16.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrescu A.-M., Taussig D., Bouilleret V. Electroencephalogram (EEG) in COVID-19: a systematic retrospective study. Neurophysiol Clin. 2020;50:155–165. doi: 10.1016/j.neucli.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayub N., Cohen J., Jing J., Jain A., Tesh R., Mukerji S.S., et al. Clinical electroencephalography findings and considerations in hospitalized patients with coronavirus SARS-CoV-2. MedRxiv 2020. 2020 doi: 10.1101/2020.07.13.20152207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louis S., Dhawan A., Newey C., Nair D., Jehi L., Hantus S., Punia V. Continuous electroencephalography characteristics and acute symptomatic seizures in COVID-19 patients. Clin Neurophysiol. 2020;131:2651–2656. doi: 10.1016/j.clinph.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canham L.J.W., Staniaszek L.E., Mortimer A.M., Nouri L.F., Kane N.M. Electroencephalographic (EEG) features of encephalopathy in the setting of Covid-19: a case series. Clin Neurophysiol Pract. 2020;5 doi: 10.1016/j.cnp.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pellinen J, Carroll E, Friedman D, Boffa M, Dugan P, Friedman DE, et al. Continuous EEG findings in patients with COVID‐19 infection admitted to a New York academic hospital system. Epilepsia 2020:epi.16667. https://doi.org/10.1111/epi.16667. [DOI] [PubMed]
- 22.Cecchetti G., Vabanesi M., Chieffo R., Fanelli G., Minicucci F., Agosta F., et al. Cerebral involvement in COVID-19 is associated with metabolic and coagulation derangements: an EEG study. J Neurol. 2020:1–5. doi: 10.1007/s00415-020-09958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pilato MS, Urban A, Alkawadri R, Barot NV, Castellano JF, Rajasekaran V, et al. EEG findings in coronavirus disease. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc 2020. https://doi.org/10.1097/WNP.0000000000000752. [DOI] [PubMed]
- 24.Chen W., Toprani S., Werbaneth K., Falco-Walter J. Status epilepticus and other EEG findings in patients with COVID-19: a case series. Seizure. 2020;81:198–200. doi: 10.1016/j.seizure.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delorme C., Paccoud O., Kas A., Hesters A., Bombois S., Shambrook P., et al. Covid-19-related encephalopathy: a case series with brain FDG-PET/CT findings. Eur J Neurol. 2020:1–7. doi: 10.1111/ene.14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hepburn M., Mullaguri N., George P., Hantus S., Punia V., Bhimraj A., et al. Acute symptomatic seizures in critically ill patients with COVID-19: is there an association? Neurocrit Care. 2020 doi: 10.1007/s12028-020-01006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammadi S., Moosaie F., Aarabi M.H. Understanding the immunologic characteristics of neurologic manifestations of SARS-CoV-2 and potential immunological mechanisms. Mol Neurobiol. 2020;57 doi: 10.1007/s12035-020-02094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Putten M.J.A.M., Hofmeijer J. Generalized periodic discharges: pathophysiology and clinical considerations. Epilepsy Behav. 2015;49:228–233. doi: 10.1016/j.yebeh.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Assenza G., Lanzone J., Ricci L., Boscarino M., Tombini M., Galimberti C.A., et al. Electroencephalography at the time of Covid-19 pandemic in Italy. Neurol Sci. 2020;41:1999–2004. doi: 10.1007/s10072-020-04546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frucht M.M., Quigg M., Schwaner C., Fountain N.B. Distribution of seizure precipitants among epilepsy syndromes. Epilepsia. 2008;41:1534–1539. doi: 10.1111/j.1528-1167.2000.01534.x. [DOI] [PubMed] [Google Scholar]
- 31.Emami A., Fadakar N., Akbari A., Lotfi M., Farazdaghi M., Javanmardi F., et al. Seizure in patients with COVID-19. Neurol Sci. 2020;41:3057–3061. doi: 10.1007/s10072-020-04731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberto K.T., Espiritu A.I., Laurence M., Fernandez L., Gutierrez C. Electroencephalographic findings in COVID-19 patients: a systematic review. Seizure Eur J Epilepsy. 2020;82:17–22. doi: 10.1016/j.seizure.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antony A.R., Haneef Z. Systematic review of EEG findings in 617 patients diagnosed with COVID-19. Seizure. 2020 doi: 10.1016/j.seizure.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.