Abstract
Despite the efforts to develop new treatments against Ebola virus (EBOV) there is currently no antiviral drug licensed to treat patients with Ebola virus disease (EVD). Therefore, there is still an urgent need to find new drugs to fight against EBOV. In order to do this, a virtual screening was done on the druggable interaction between the EBOV glycoprotein (GP) and the host receptor NPC1 with a subsequent selection of compounds for further validation. This screening led to the identification of new small organic molecules with potent inhibitory action against EBOV infection using lentiviral EBOV-GP-pseudotype viruses. Moreover, some of these compounds have shown their ability to interfere with the intracellular cholesterol transport receptor NPC1 using an ELISA-based assay. These preliminary results pave the way to hit to lead optimization programs that lead to successful candidates.
Keywords: Ebola virus, NPC1, Antiviral, African swine fever virus, Virtual screening
Graphical abstract
Infectious diseases represent one of the major concerns for human and veterinary public health and, as a consequence, for the global economy. Due to climate and environmental changes and travel and trade globalization, infectious agents spread more rapidly and widely than in the last century. This is shown by the number of emerging and reemerging viral infections appeared since 2000, such as the 2009 pandemic H1N1 influenza virus, the widespread epidemic of Ebola virus (EBOV) from West Africa that generated cases also in Europe and America and the unprecedented pandemic caused by a novel coronavirus (SARS-CoV-2/COVID-19) in December 2019. Moreover, additional emerging viruses have been identified for the first time in Spain, most of them causing potentially lethal infections in humans, such as Crimean-Congo hemorrhagic fever, Dengue or West Nile viruses. Due to these sporadic outbreaks, these emerging viruses require a continuous surveillance activity that needs a significant effort of the National Health Systems and of the International Authorities (Wolfe et al., 2007; Zhou et al., 2020).
In December 2013, an outbreak caused by EBOV began in West Africa. The epidemic lasted until 2016 with 28,616 cases and 11,301 deaths (WHO Ebola Response Team et al., 2016). This was the largest and most complex outbreak so far, being declared by the WHO as a public health emergency of international concern. There were more cases and deaths in this outbreak than in all the others combined. In addition, it also spread between countries, starting in Guinea then moving across land borders to Sierra Leone and Liberia. For the first time, EBOV infected individuals appeared and were subsequently treated in the US and some European countries. Spain was the first country to ever have a case of EBOV transmission out of Africa (Lópaz et al., 2015). The 2014 outbreak highlighted the scarcity of antivirals and vaccines against this highly pathogenic virus which precludes not only medical care but also epidemic control. In 2016, the largest epidemic of Ebola virus disease finally ended. However, the risk of re-emergences was made evident by the 2018–2020 outbreak in the Democratic Republic of the Congo (DRC) and the current situation in this country (Rohan and McKay, 2020). Currently, despite the 280.000 persons have been vaccinated (Saphire, 2020), the epidemics continue and it seems necessary to concentrate research efforts in antivirals. Consequently, the WHO is trying to accelerate trials with experimental drugs in the expectation of reducing the number of deaths and protecting those who are in contact with the patients. Actually, the first clinical trials with the nucleoside analogue remdesivir (GS-5734), the single monoclonal antibody MAb 114 or combinations of monoclonal antibodies such as ZMapp (the control group) or the triple monoclonal antibody REGN-EB3 have been performed (Malvy et al., 2019; Mulangu et al., 2019).
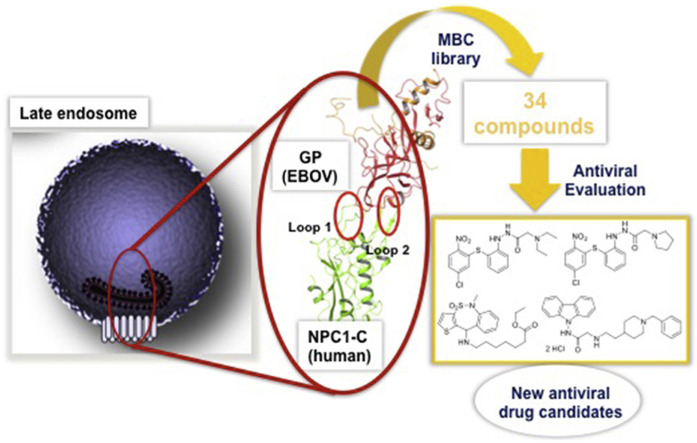
The objective of the present work is to identify new therapeutics to fight against highly pathogenic viruses with limited or non-available vaccines or treatment options and with the potential to produce emergences of catastrophic consequences such as those caused by EBOV. With the aim of developing anti-EBOV drugs, a host target-based approach was followed. Our search is focused on blocking the specific EBOV pathway for human infection. We have directed our efforts to the inhibition of the protein-protein interaction between the envelope glycoprotein of Ebola virus (EBOV-GP) and domain C of the late endosomes protein Niemann-Pick 1 (NPC1) as a drug target. Blocking this protein-protein interaction, the delivery of genetic viral material inside the host cells will be blocked at the stage of endosomal exit and the subsequent infection stopped. With this therapeutic action, the endosome containing EBOV would follow the degradative pathway and cleared.
EBOV binds a number of attachment factors at the plasma membrane mediating its entry by macropinocytosis. Once the virus is engulfed in endosomes, cysteine proteases, cathepsins B and L, mediate the priming of the EBOV-GP. The primed glycoprotein (GPcl) can then bind to the NPC1 that is an indispensable host receptor required for viral fusion/entry (Carette et al., 2011; Côté et al., 2011). EBOV-GPcl binding to NPC1 is the key in the lock that mediates fusion with the endosomal membrane and allows the viral nucleic acid to be delivered in the cytoplasm (Miller et al., 2012). Of note, this sequence of events is similar in a number of highly pathogenic emerging viruses affecting humans and animals (Del Campo et al., 2012; Cuesta-Geijo et al., 2016).
To identify the desired protein-protein interaction inhibitors as new antiviral agents, a virtual screening was performed initially. We used our in-house chemical library (MBC library) (Sebastian-Pérez et al., 2017) and the crystal structure of the complex EBOV-GPcl/NPC1 domain C (Wang et al., 2016) focusing the screening in the interface between both proteins. As filter, the interaction with the key residues Phe503 and Phe504 of NPC1 located in the protruding loop 2, which play a major role by contributing to the majority of the tight hydrophobic interactions with the GPcl head cavity was used (Wang et al., 2016). The druggability of this interaction was previously validated and reported with different antiviral compounds directed to inhibit the EBOV-GPcl/NPC1 binding, such as adamantane dipeptide piperazine 3.47, (Côté et al., 2011) triazole thioether MBX2270 and aminoacetamide sulfonamide MBX2254 (Basu et al., 2015). The study here presented has allowed the selection and identification of novel candidates to be evaluated as anti-EBOV agents.
From this initial virtual screening, 34 compounds were selected to be evaluated as antivirals based on best docking scores and chemical diversity (Table S1). All selected compounds were located between the interface of the GPcl/NPC1 domain C complex, The compounds with best scoring are located between loop 1 and loop 2 of NPC1 domain C showing interactions with key residues involved in the interaction such as Phe503, and Phe504 (Fig. S1). Noteworthy, compounds MBX2254 y MBX2270, (Basu et al., 2015) previously reported as inhibitors of the target interaction were used as reference controls being near the top of the ranking. Compounds 1–34 were tested in a first step using a lentiviral EBOV-GP-pseudotyped infection assay (Table S2) using as references of the assay (Basu et al., 2015) MBX2254 (IC50 = 2.5 μM) and MBX2270 (IC50 = 14.2 μM). This system of virus-like-particles expressing on their surface the EBOV-GP allowed us to explore the effects showed by the tested molecules in the viral entry under BSL-2 facilities. Initially, all the compounds were tested at 10 μM and those that inhibited the infection by more than 60% at this concentration were further analyzed for potency, selectivity and cytotoxicity in HeLa cells. Seven of the 34 tested compounds showed viral inhibition to merit further studies (Table 1 ). These seven compounds belong to three diverse chemical families, with no structural similarities to previously described anti-EBOV drugs: benzothiazepine (9, 12, 19 and 30), carbazole (2) and sulfide (14 and 26) derivatives.
Table 1.
Biological activity and chemical structure of active compounds from the first selection in EBOV-GP-pseudotype virus (pEBOV) and ASFV.
| Comp. | Chemical structure | IC50a (pEBOV) | CC50b (HeLa) | SIc (pEBOV) (CC50/IC50) | IC50a (ASFV) | CC50b (Vero) | SIc (ASFV) (CC50/IC50) |
|---|---|---|---|---|---|---|---|
| 2 | 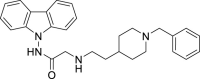 |
0.37 μM | 7 μM | 19 | 2.54 | 16.56 | 6.5 |
| 9 | 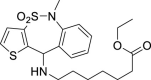 |
0.37 μM | 96 μM | 258 | inactive | >100 | – |
| 12 | 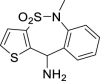 |
64.9%@10 μM | – | – | inactive | >100 | – |
| 14 | 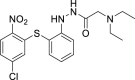 |
4.69 μM | 30 μM | 11 | 35.17 | >100 | >2.8 |
| 19 | 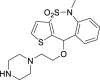 |
70.9%@10 μM | – | – | inactive | >100 | – |
| 26 | 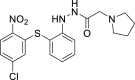 |
2.04 μM | 60 μM | 26 | 15.75 | >100 | >6.34 |
| 30 | 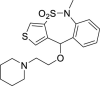 |
61.2%@10 μM | – | – | inactive | >100 | – |
IC50: 50% inhibitory concentration.
CC50: 50% cytotoxic concentration.
SI: Selectivity Index.
The best compounds in terms of percentage of inhibition were selected to calculate the IC50 (50% inhibitory concentration) and CC50 (50% cytotoxic concentration). Benzothiazepine 9 and carbazole 2 showed an IC50 of 0.37 μM, while sulfides 14 and 26, 4.69 μM and 2.04 μM respectively. With regard to cytotoxicity in HeLa cells, although the four selected compound showed cytotoxic concentrations under their respective inhibitory concentrations (Table 1) is remarkable the high selectivity index (CC50/CI50) of 9.
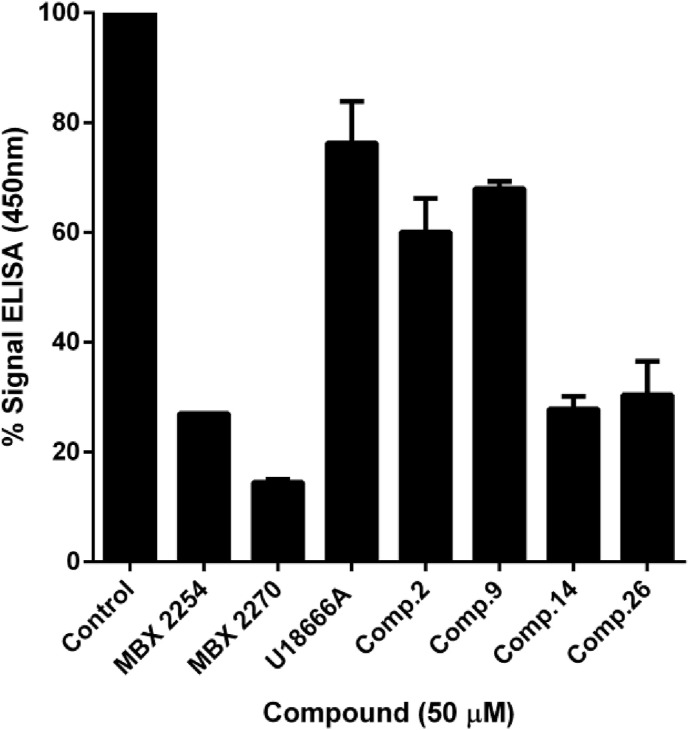
In order to confirm the mechanism of action of these novel classes of antiviral compounds, we investigated the effect of the four hits in the binding of EBOV-GPcl to NPC1 domain C using an enzyme-linked immunosorbent assay (ELISA)-based assay and compounds MBX2254 and MBX2270, with a reported inhibition of this interaction, as positive controls (Basu et al., 2015), while the cholesterol synthesis inhibitor U18666A was used as negative control (Côté et al., 2011). As the interaction between EBOV-GP and NPC1 requires the removal of a part of the EBOV-GP (mucin and glycan cap domains), we use thermolysin to mimic this process. Lentiviral EBOV-GP-particles previously cleaved by thermolysin, were then captured on an ELISA plate using the anti-EBOV GP monoclonal antibody KZ52 (Lee et al., 2008). Then, a purified human NPC1-domain C with a Flag tag was added, and the binding to GPcl detected by anti-Flag antibody conjugated to horseradish peroxidase (HRP) using a colorimetric substrate (TMB) and quantified by absorbance at 450 nm.
As it can be observed, benzothiazepine 9 is the less potent by interfering the EBOV-GPcl/NPC1 interaction. Sulfides 14 and 26 clearly inhibited this interaction, while carbazole 2 does it to a lesser extent (Fig. 1 ).
Fig. 1.
Inhibitory effect of compounds on EBOV-GPcl/NPC1-domain C interaction. The effect of selected compounds on this interaction was quantified by an ELISA method. ELISA plate previously coated with monoclonal antibody KZ52 was incubated with EBOV-GPcl particles. Unbound viral particles were washed off, and purified NPC1-domain C Flag protein was added in the absence (control) or in presence of 50 μM of each compound. After that, samples were incubated with an Anti-Flag-Peroxidase monoclonal antibody and absorbance was measured at 450 nm after addition of TMB substrate. The values of percentage of binding presented on the graph correspond to the mean of 3 independent experiments with error bars corresponding to the standard errors of the mean with respect to the control (no compound added). Analyses were performed using the GraphPad Prism v6.0 software.
Based on the antiviral effect found in EBOV-GP-pseudotyped virus, we decided to test the seven most active compounds (Table 1) in another highly pathogenic virus, described to require also NPC1 integrity and function for a successful infection, such as African swine fever virus (ASFV) (Cuesta-Geijo et al., 2016), which has a great sanitary and socioeconomic impact. Compounds were first evaluated for cell viability and cytotoxicity to select non-cytotoxic working concentrations. Then, compounds were added to Vero cell monolayers before infection with BPP30GFP, a recombinant ASFV expressing the GFP gene fused to the promoter of the early viral p30 protein at a moi of 1 pfu/cell. Viral infectivity was analyzed by flow cytometry. Results depicted in Table 1, showed that carbazole 2 and sulfides 14 and 26, also presented antiviral activity in this virus at micromolar range (non-cytotoxic). By contrast, members of the benzothiazepine family showed no activity at all.
The different antiviral profile of the three chemical classes could be explained on basis to the binding of thermolysin-cleaved EBOV-GP to NPC1-domain C determination using ELISA-based assay. Results pointed to the fact that carbazoles and sulfides potentially act through inhibition of NPC1-GP interaction, while benzothiazepines do not affect this interaction. The two groups of chemicals able to inhibit the infection of both viruses, carbazoles and sulfides, probably share a common mechanism of action relevant for both viruses. In contrast, benzothiazepines could act against EBOV through a novel specific mechanism that is currently under study. While further studies to assess experimentally the mechanism of action of these new compounds are in progress, a hit to lead optimization program is ongoing to show the therapeutic potential in in vivo EBOV infections and to evaluate their possible clinical value.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Funding from “la Caixa” Banking Foundation under the project code HR18-00469 is acknowledged. This research was partially supported through Instituto de Salud Carlos III (FIS PI 181007 and ISCIII-COV20/01007), CSIC (201980E024 and 202020E079), Spanish Ministry of Science and Innovation (RTI2018-097305-R-I00) and the European Commission Horizon 2020 Framework Programme (Project VIRUSCAN FETPROACT-2016 and VACDIVA-SFS-12-2019-1-862874).
Footnotes
Supplementary data to this article: Table S1 (top-ranked compounds selection), Table S2 (biological evaluation of compounds initially selected), Figure S1 (binding poses of top-ranked compounds), Figures S2 and S3 (dose-response curves for top compounds) and Material and Methods section.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.antiviral.2021.105011.
Appendix A. Supplementary data
The following is the supplementary data to this article:
Supplementary data to this article: Table S1 (top-ranked compounds selection), Table S2 (biological evaluation of compounds initially selected), Figure S1 (binding poses of top-ranked compounds), Figures S2 and S3 (dose-response curves for top compounds) and Material and Methods section.
References
- Basu A., Mills D.M., Mitchell D., Ndungo E., Williams J.D., Herbert A.S., Dye J.M., Moir D.T., Chandran K., Patterson J.L., Rong L., Bowlin T.L. Novel small molecule entry inhibitors of Ebola virus. J. Infect. Dis. 2015;212(Suppl. 2):S425–S434. doi: 10.1093/infdis/jiv223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carette J.E., Raaben M., Wong A.C., Herbert A.S., Obernosterer G., Mulherkar N., Kuehne A.I., Kranzusch P.J., Griffin A.M., Ruthel G., Dal Cin P., Dye J.M., Whelan S.P., Chandran K., Brummelkamp T.R. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 2011;477(7364):340–343. doi: 10.1038/nature10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté M., Misasi J., Ren T., Bruchez A., Lee K., Filone C.M., Hensley L., Li Q., Ory D., Chandran K., Cunningham J. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature. 2011;477(7364):344–348. doi: 10.1038/nature10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta-Geijo M.A., Chiappi M., Galindo I., Barrado-Gil L., Muñoz-Moreno R., Carrascosa J.L., Alonso C. Cholesterol flux is required for endosomal progression of african swine fever virions during the initial establishment of infection. J. Virol. 2016;90(3):1534–1543. doi: 10.1128/JVI.02694-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campo J.A., Rojas A., Romero-Gomez M. Entry of hepatitis C virus into the cell: a therapeutic target. World J. Gastroenterol. 2012;18(33):4481–4485. doi: 10.3748/wjg.v18.i33.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.E., Fusco M.L., Hessell A.J., Oswald W.B., Burton D.R., Saphire E.O. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature. 2008;454(7201):177–182. doi: 10.1038/nature07082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lópaz M.A., Amela C., Ordobas M., Domínguez-Berjón M.F., Álvarez C., Martínez M., Sierra M.J., Simon F., Jansá J.M., Plachouras D., Astray J. Working group of Ebola outbreak investigation team of Madrid. First secondary case of Ebola outside Africa: epidemiological characteristics and contact monitoring, Spain, September to November 2014. Euro Surveill. 2015;20(1):pii=2100. doi: 10.2807/1560-7917.es2015.20.1.21003. [DOI] [PubMed] [Google Scholar]
- Malvy D., McElroy A.K., de Clerck H., Gunther S., van Griensven J. Ebola virus disease. Lancet. 2019;393(10174):936–948. doi: 10.1016/S0140-6736(18)33132-5. [DOI] [PubMed] [Google Scholar]
- Miller E.H., Obernosterer G., Raaben M., Herbert A.S., Deffieu M.S., Krishnan A., Ndungo E., Sandesara R.G., Carette J.E., Kuehne A.I., Ruthel G., Pfeffer S.R., Dye J.M., Whelan S.P., Brummelkamp T.R., Chandran K. Ebola virus entry requires the host-programmed recognition of an intracellular receptor. EMBO J. 2012;31(8):1947–1960. doi: 10.1038/emboj.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulangu S., Dodd L.E., Davey R.T., Jr., Tshiani Mbaya O., Proschan M., Mukadi D., Lusakibanza Manzo M., Nzolo D., Tshomba Oloma A., Ibanda A., Ali R., Coulibaly S., Levine A.C., Grais R., Diaz J., Lane H.C., Muyembe-Tamfum J.J., Sivahera B., Camara M., Kojan R., Walker R., Dighero-Kemp B., Cao H., Mukumbayi P., Mbala-Kingebeni P., Ahuka S., Albert S., Bonnett T., Crozier I., Duvenhage M., Proffitt C., Teitelbaum M., Moench T., Aboulhab J., Barrett K., Cahill K., Cone K., Eckes R., Hensley L., Herpin B., Higgs E., Ledgerwood J., Pierson J., Smolskis M., Sow Y., Tierney J., Sivapalasingam S., Holman W., Gettinger N., Vallée D., Nordwall J. A randomized, controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan H., McKay G. The Ebola outbreak in the Democratic Republic of the Congo: why there is no 'silver bullet. Nat. Immunol. 2020;21(6):591–594. doi: 10.1038/s41590-020-0675-8. [DOI] [PubMed] [Google Scholar]
- Saphire E.O. A vaccine against Ebola virus. Cell. 2020;181(1):6. doi: 10.1016/j.cell.2020.03.011. [DOI] [PubMed] [Google Scholar]
- Sebastian-Pérez V., Roca C., Awale M., Reymond J.-L., Martínez A., Gil C., Campillo N.E. The Medicinal and Biological Chemistry (MBC) Library: an efficient source on new hits. J. Chem. Inf. Model. 2017;57(9):2143–2151. doi: 10.1021/acs.jcim.7b00401. [DOI] [PubMed] [Google Scholar]
- Wang H., Shi Y., Song J., Qi J., Lu G., Yan J., Gao G.F. Ebola viral glycoprotein bound to its endosomal receptor Niemann-Pick C1. Cell. 2016;164(1–2):258–268. doi: 10.1016/j.cell.2015.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Ebola Response Team, Agua-Agum J., Allegranzi B., Ariyarajah A., Aylward R., Blake I.M., Barboza P., Bausch D., Brennan R.J., Clement P., Coffey P., Cori A., Donnelly C.A., Dorigatti I., Drury P., Durski K., Dye C., Eckmanns T., Ferguson N.M., Fraser C., Garcia E., Garske T., Gasasira A., Gurry C., Hamblion E., Hinsley W., Holden R., Holmes D., Hugonnet S., Jaramillo Gutierrez G., Jombart T., Kelley E., Santhana R., Mahmoud N., Mills H.L., Mohamed Y., Musa E., Naidoo D., Nedjati-Gilani G., Newton E., Norton I., Nouvellet P., Perkins D., Perkins M., Riley S., Schumacher D., Shah A., Tang M., Varsaneux O., Van Kerkhove M.D. After Ebola in west Africa--Unpredictable risks, preventable epidemics. N. Engl. J. Med. 2016;375(6):587–596. doi: 10.1056/NEJMsr1513109. [DOI] [PubMed] [Google Scholar]
- Wolfe N.D., Dunavan C.P., Diamond J. Origins of major human infectious diseases. Nature. 2007;447(7142):279–283. doi: 10.1038/nature05775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.