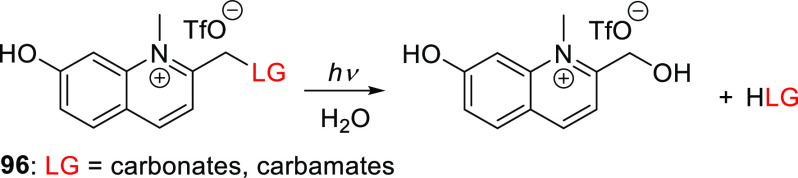
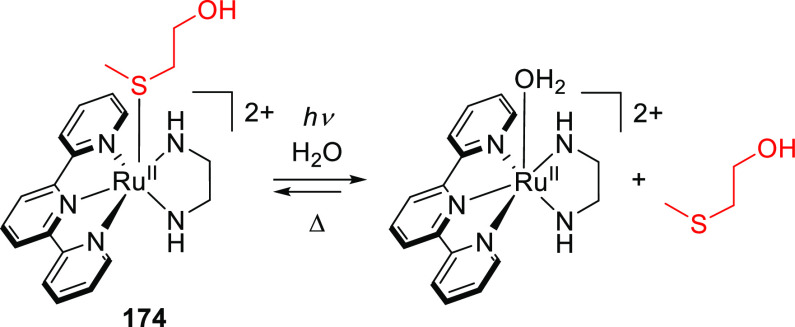
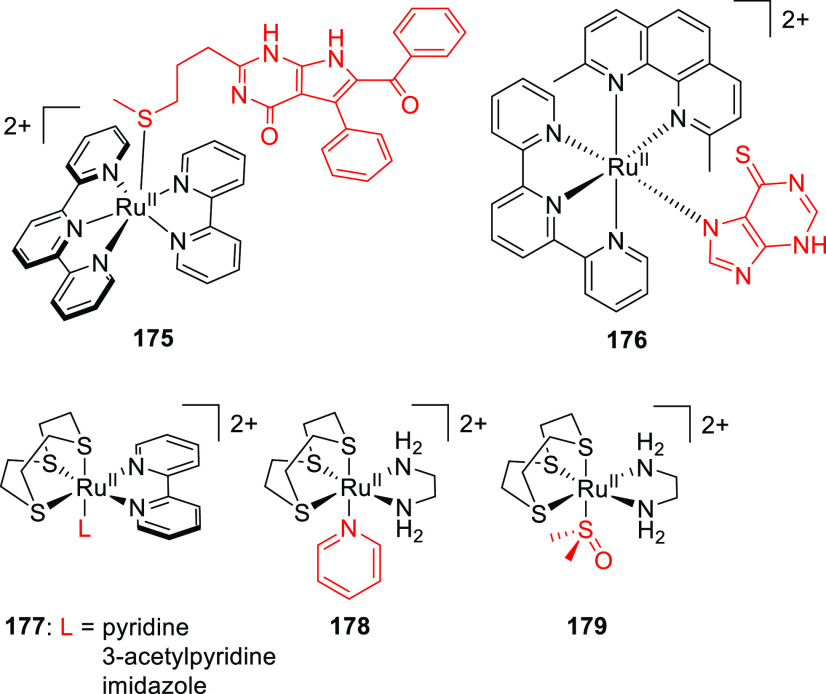
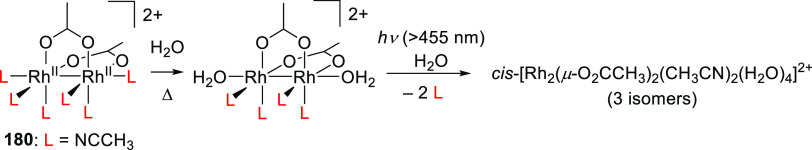
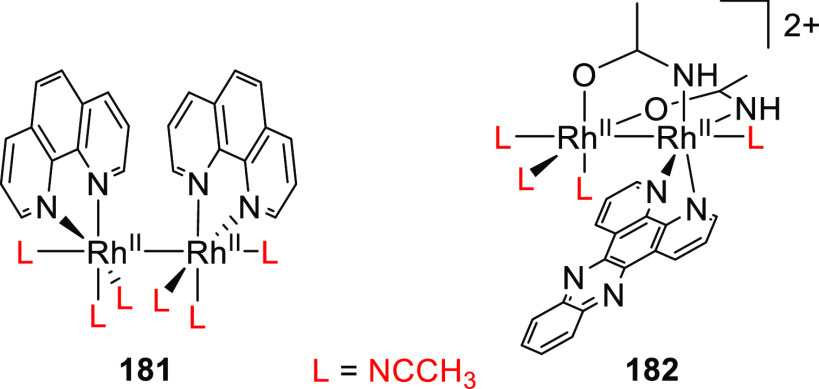
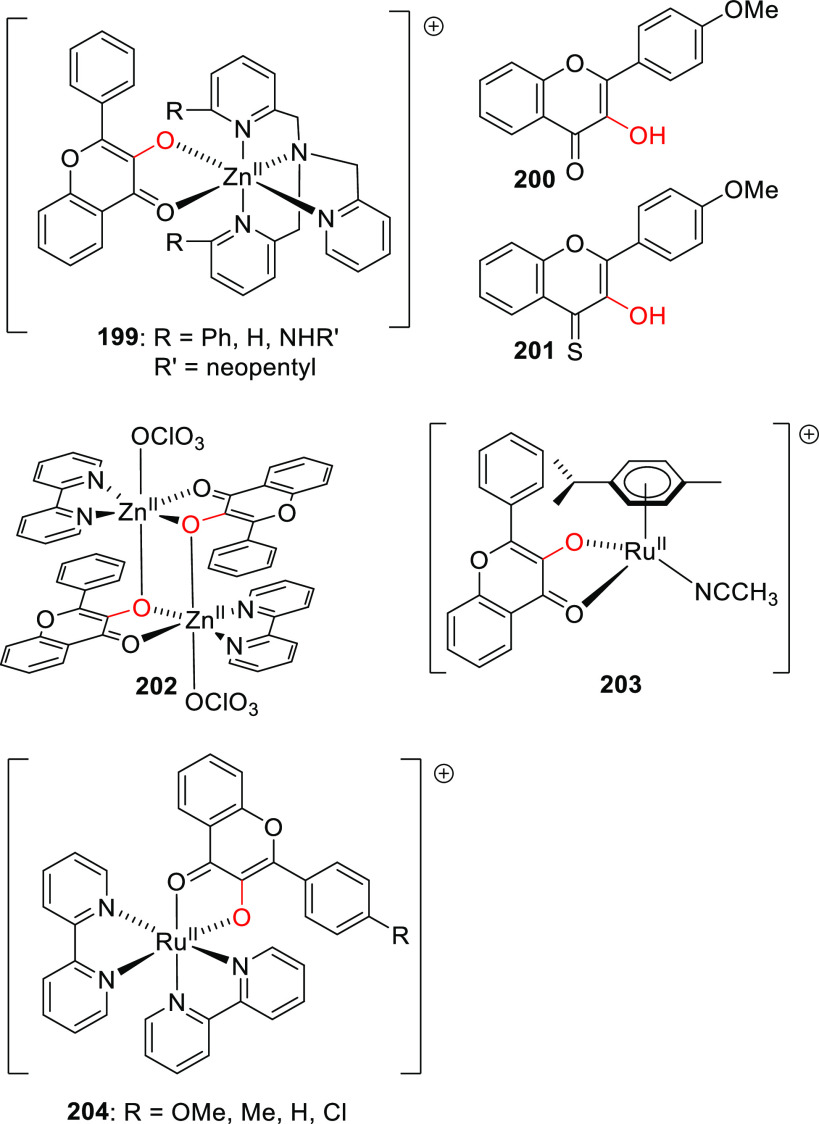
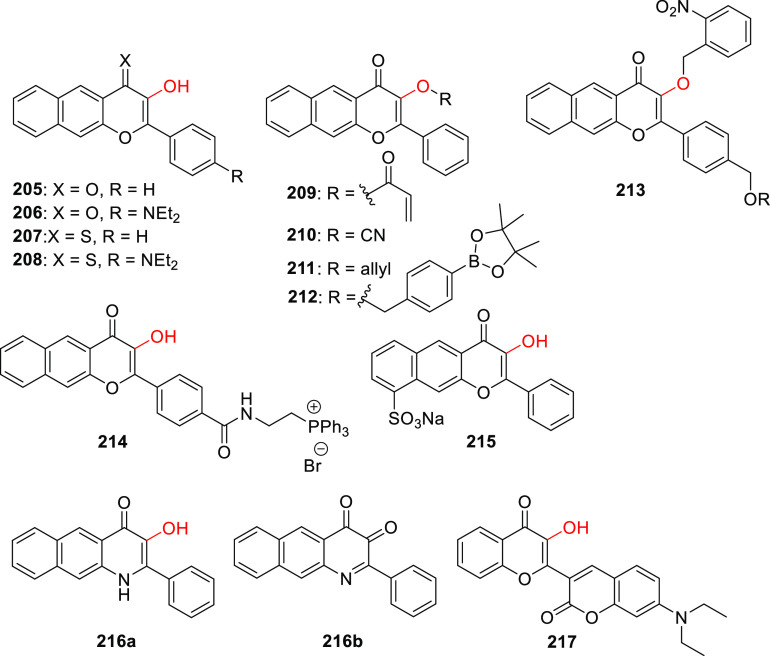
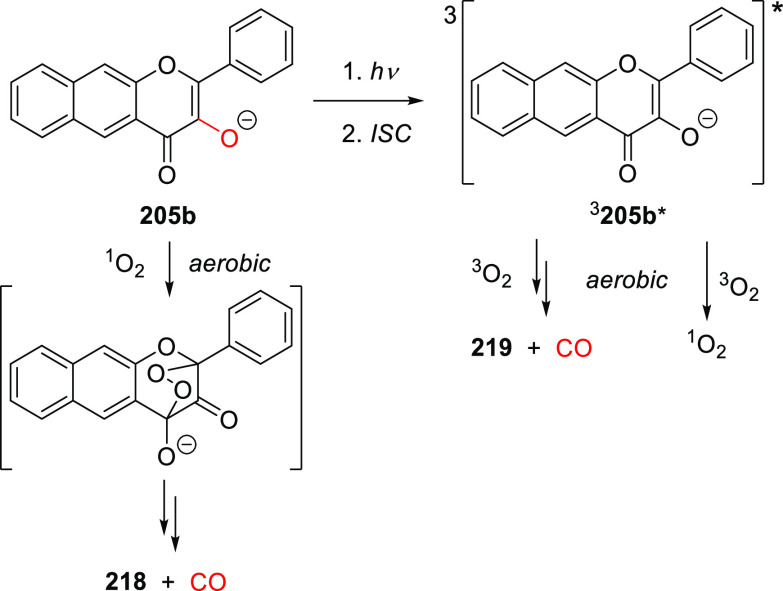
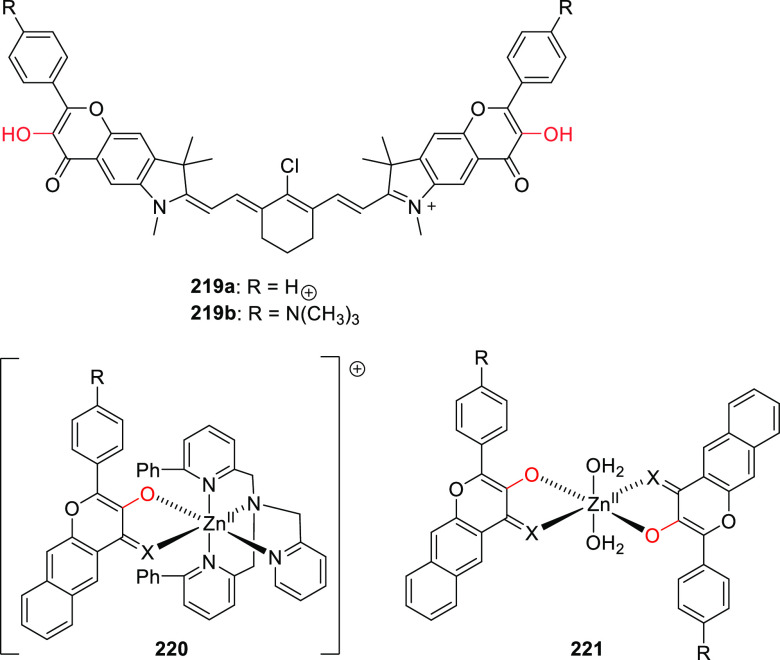
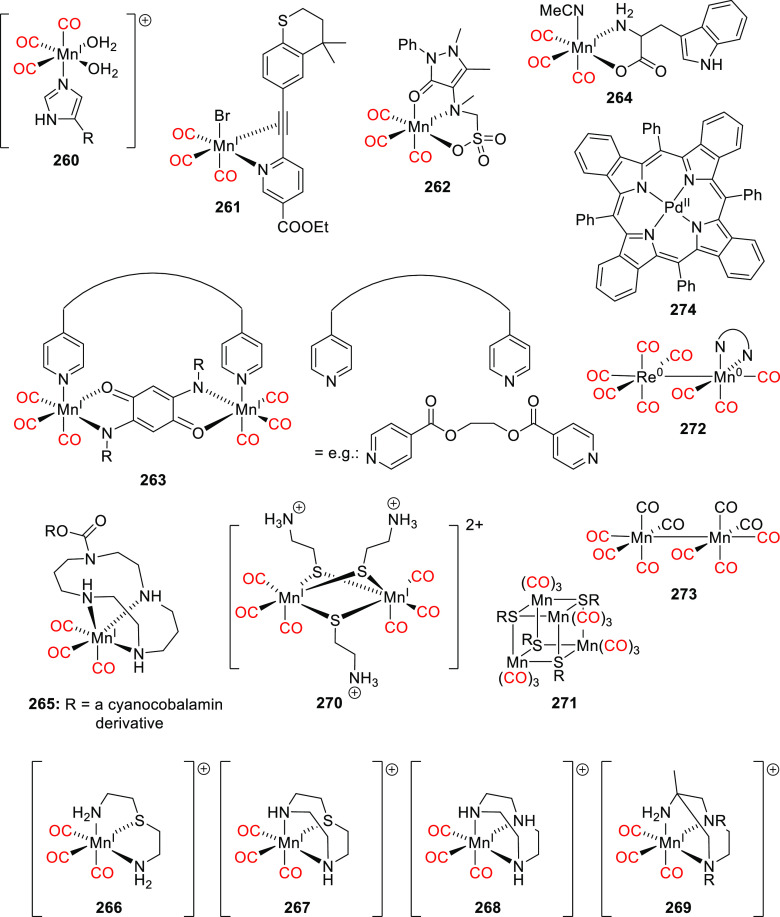
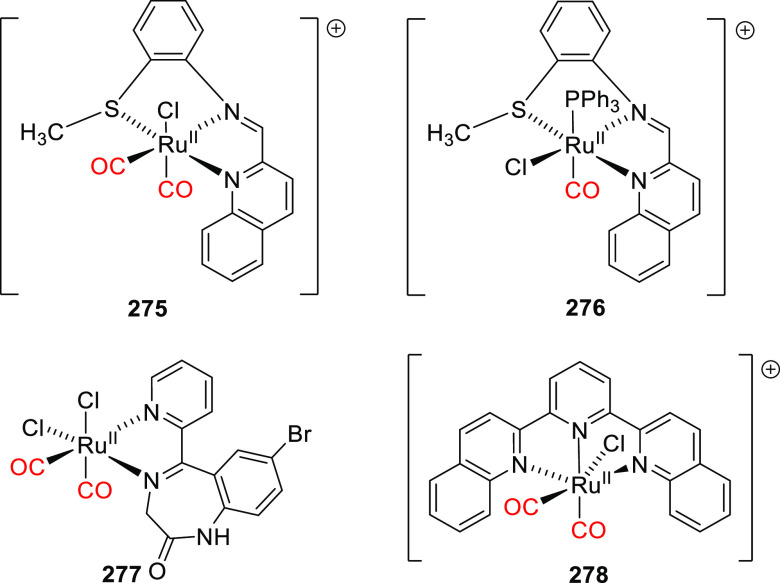
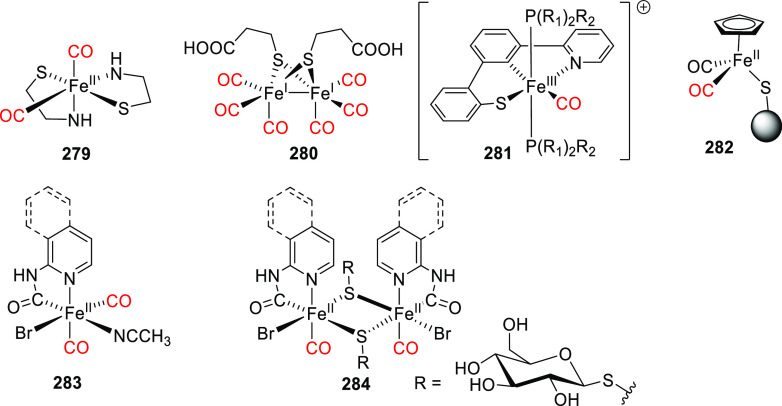
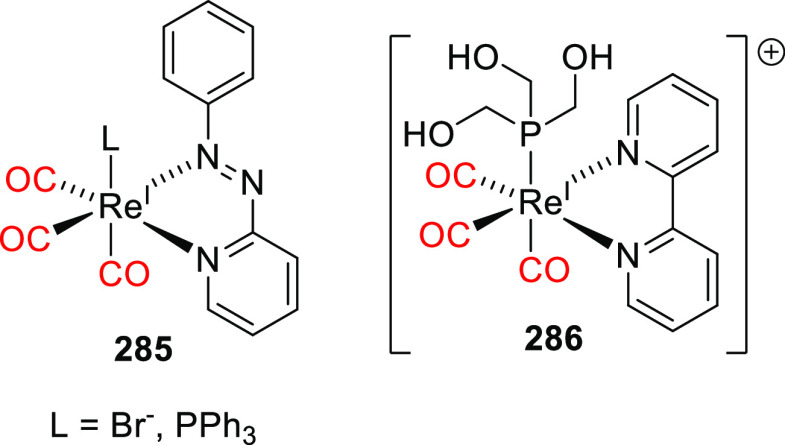
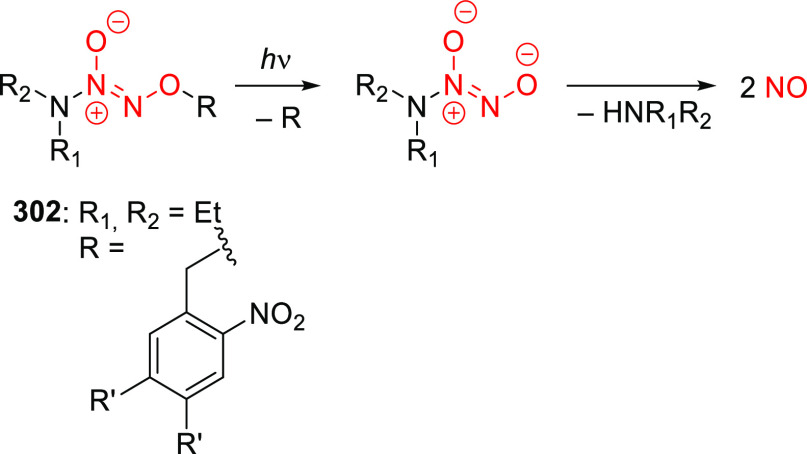
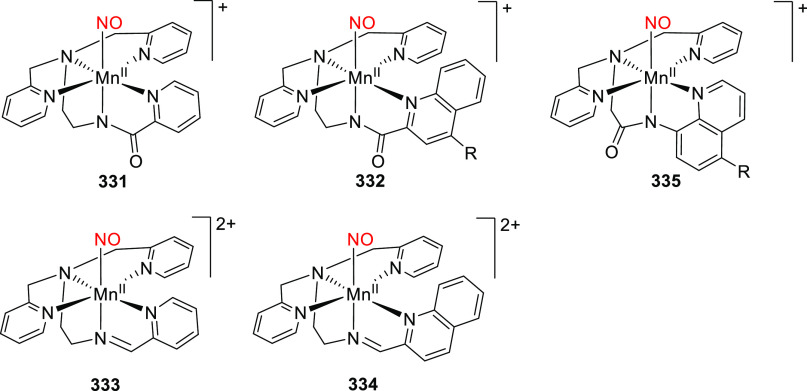
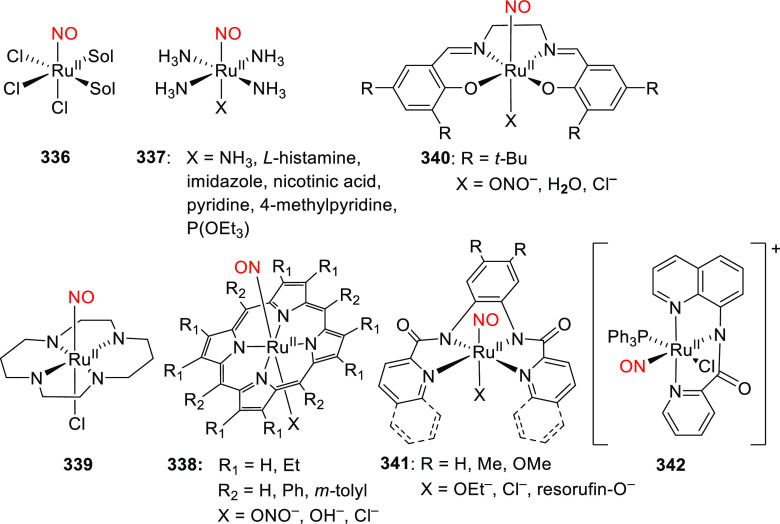
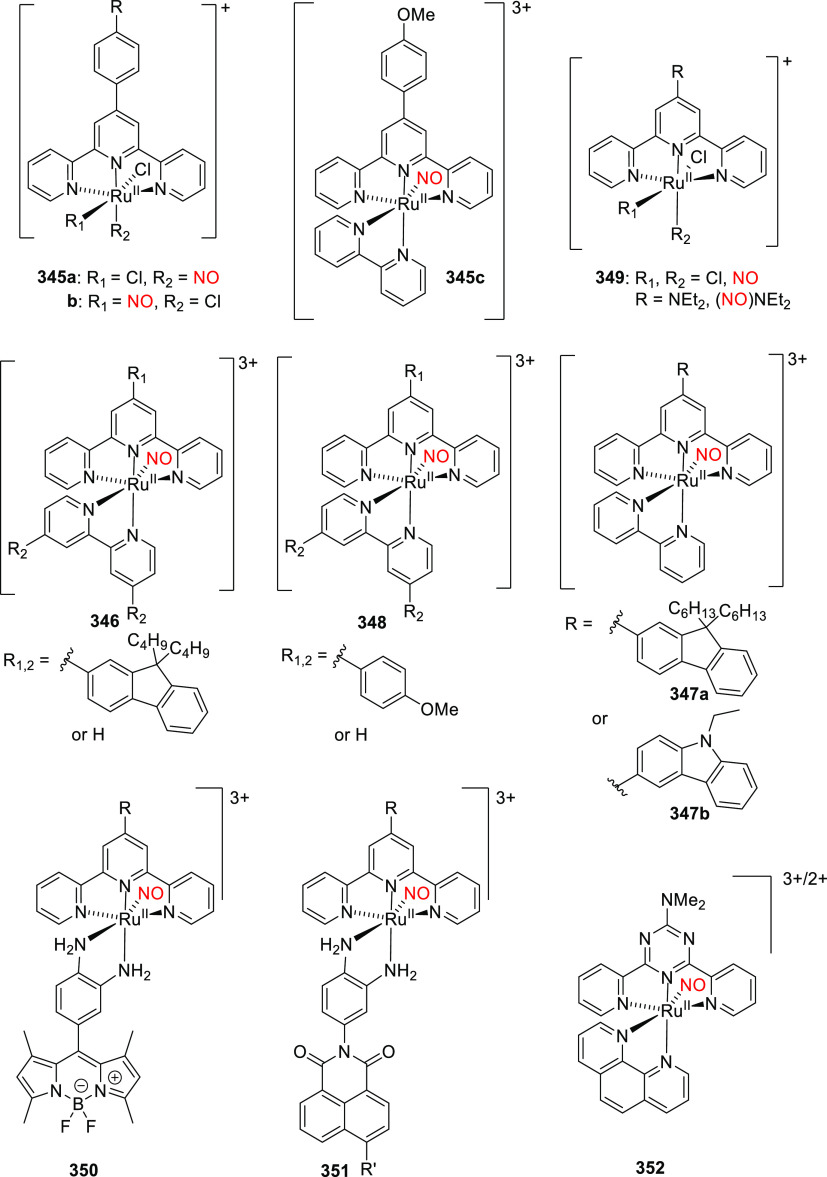
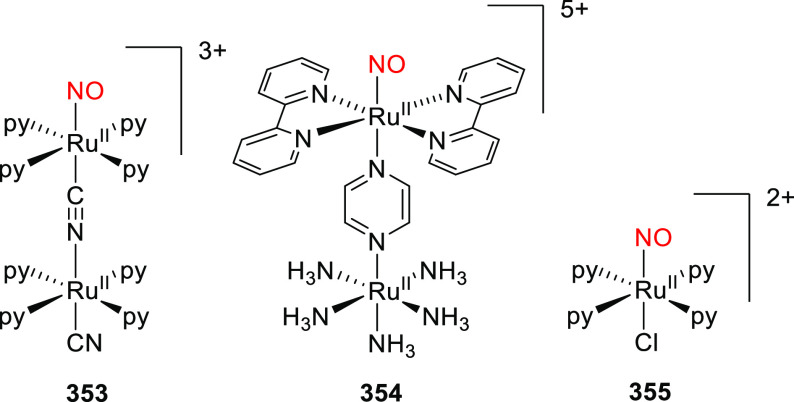
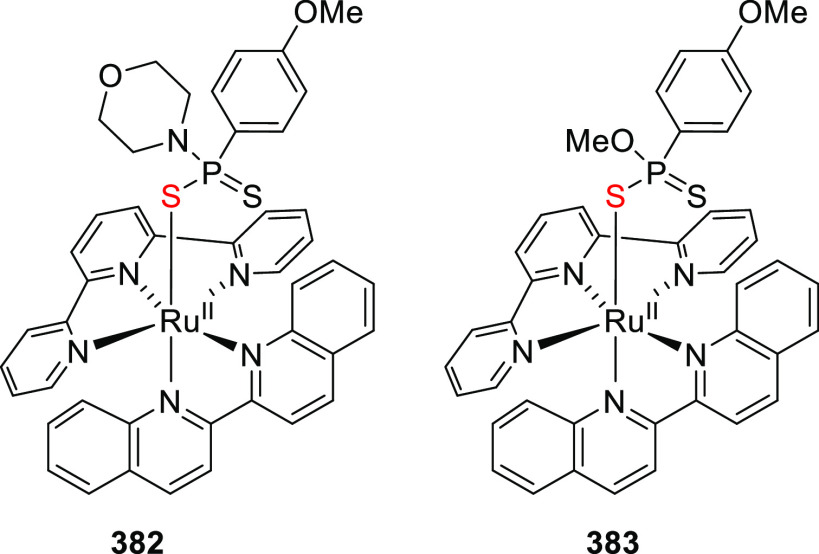
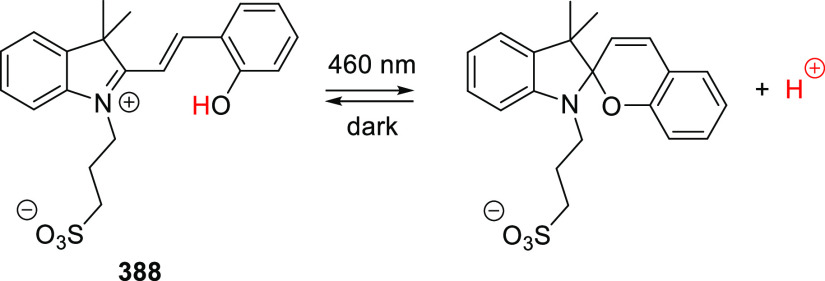
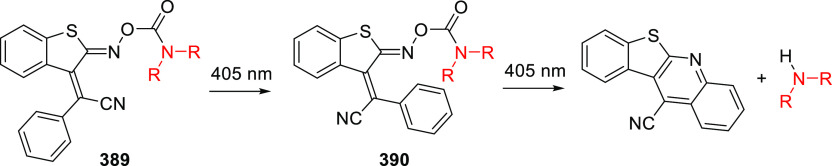
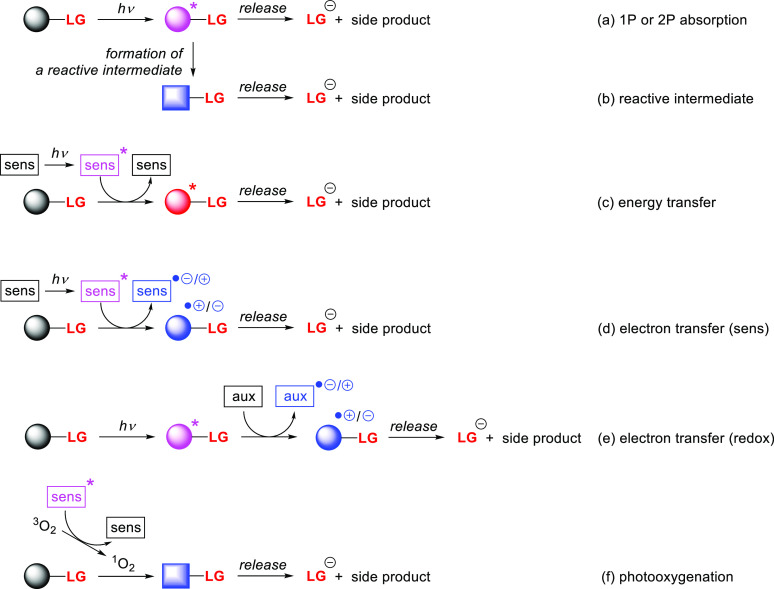
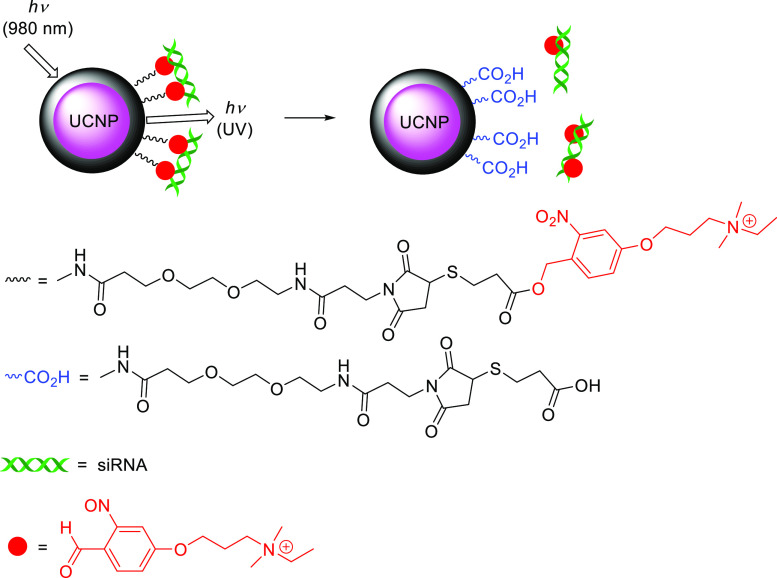
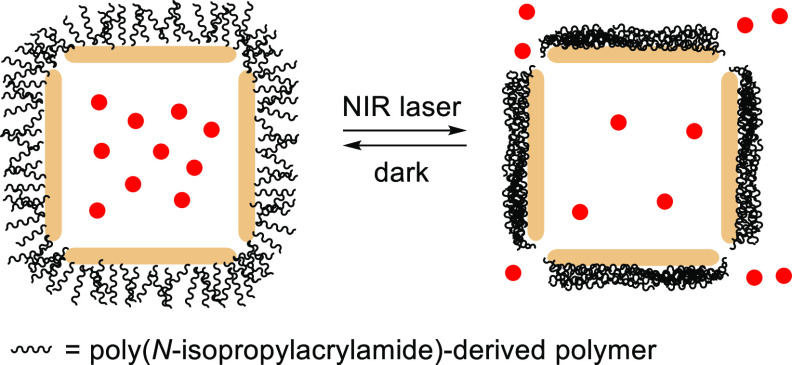
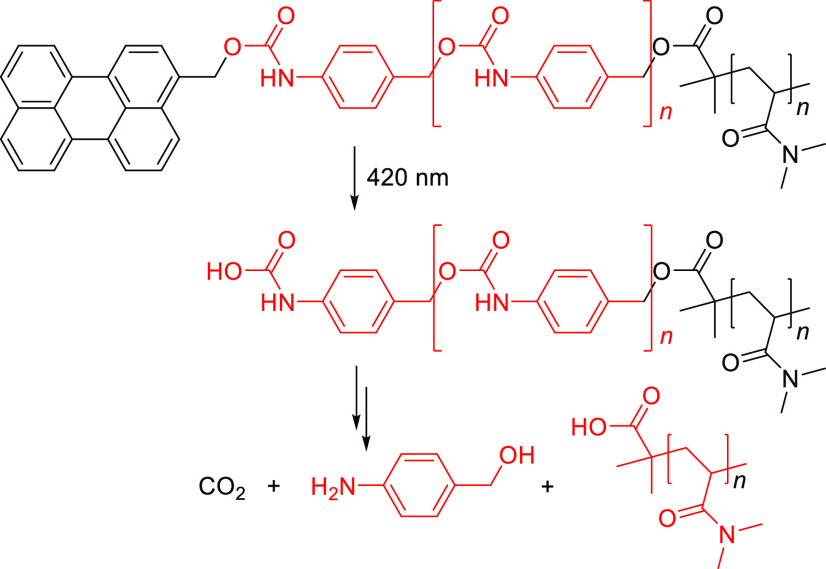
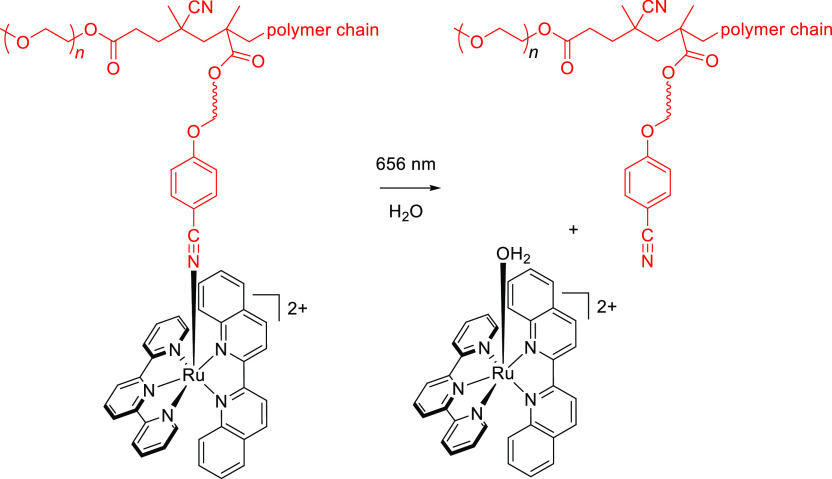
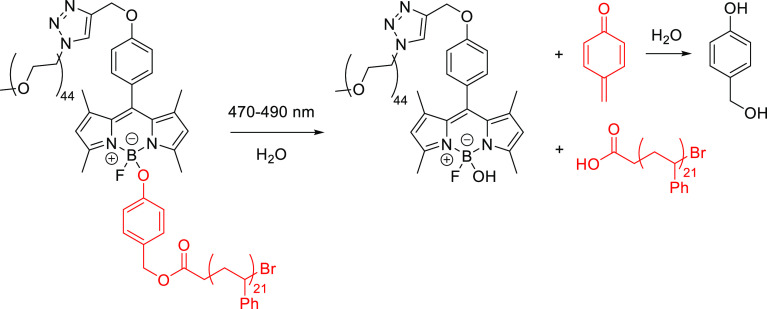
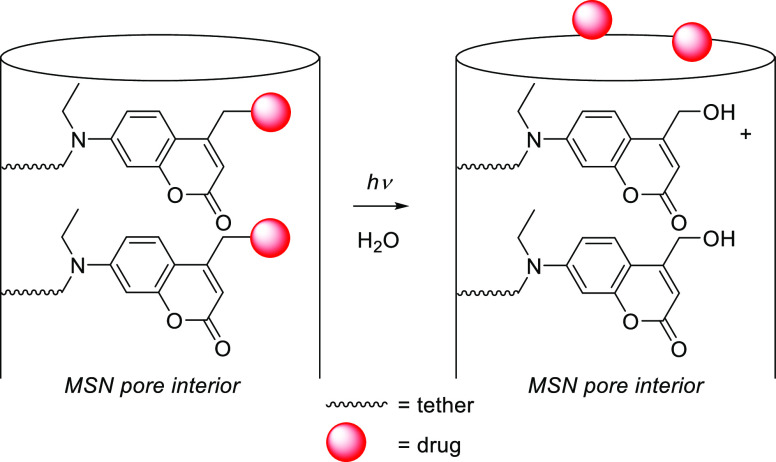
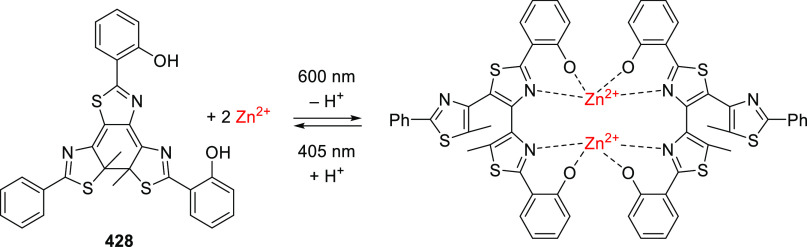
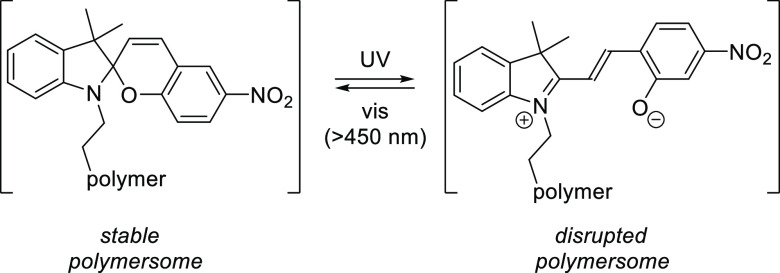
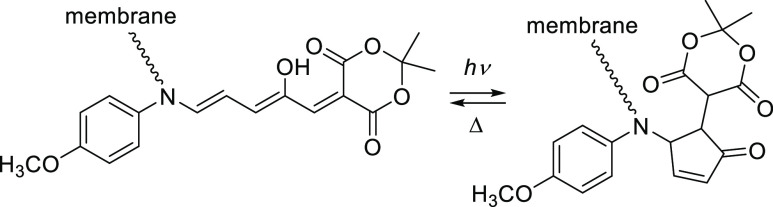
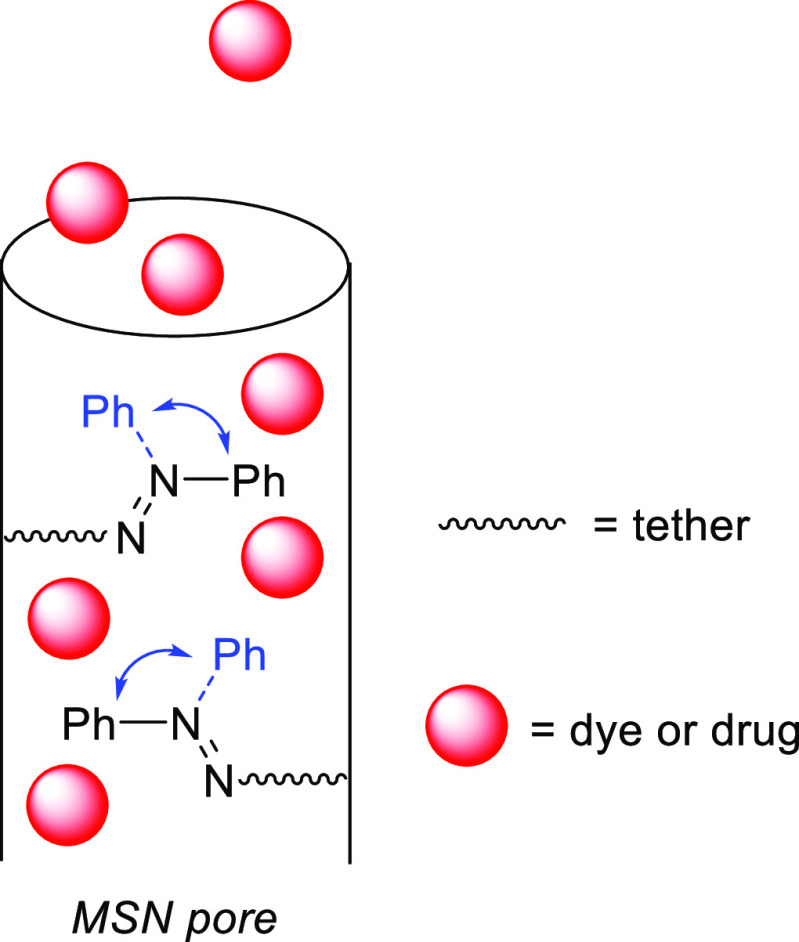
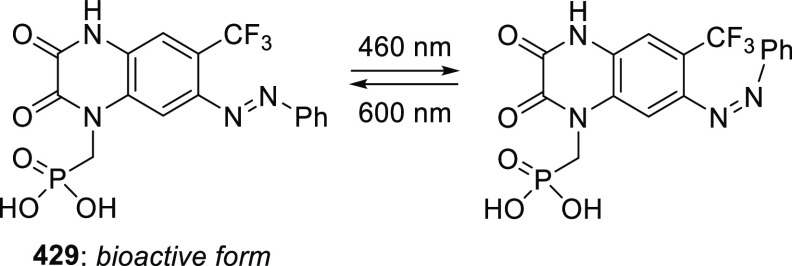
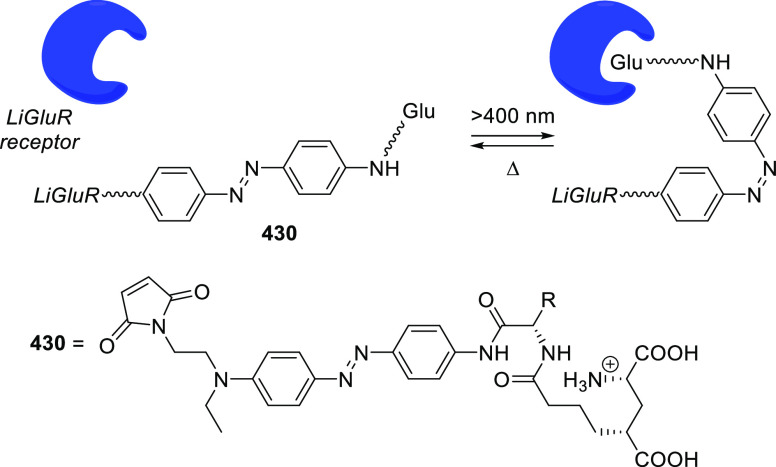
Abstract

Photoactivatable (alternatively, photoremovable, photoreleasable, or photocleavable) protecting groups (PPGs), also known as caged or photocaged compounds, are used to enable non-invasive spatiotemporal photochemical control over the release of species of interest. Recent years have seen the development of PPGs activatable by biologically and chemically benign visible and near-infrared (NIR) light. These long-wavelength-absorbing moieties expand the applicability of this powerful method and its accessibility to non-specialist users. This review comprehensively covers organic and transition metal-containing photoactivatable compounds (complexes) that absorb in the visible- and NIR-range to release various leaving groups and gasotransmitters (carbon monoxide, nitric oxide, and hydrogen sulfide). The text also covers visible- and NIR-light-induced photosensitized release using molecular sensitizers, quantum dots, and upconversion and second-harmonic nanoparticles, as well as release via photodynamic (photooxygenation by singlet oxygen) and photothermal effects. Release from photoactivatable polymers, micelles, vesicles, and photoswitches, along with the related emerging field of photopharmacology, is discussed at the end of the review.
1. Introduction
Photoactivatable (alternatively, photoremovable, photoreleasable, or photocleavable) protecting groups (PPGs) or caged compounds are used to achieve non-invasive spatiotemporal control over the release of molecules of interest including biologically active compounds, synthetic precursors, fluorescent probes, initiators of polymerization reactions, fragrances, and gasotransmitters. As such, they constitute one of the most important current applications of photochemistry in diverse research areas. The first PPGs were reported in the early works of Barltrop,1 Barton,2,3 Woodward,4 and Sheehan,5 and their first biological applications were presented by Engels and Schlaeger6 and Kaplan7 and co-workers. Since then, tens of photoactivatable molecules and systems have been developed. Several reviews and perspectives covering the applications of organic8−55 and (transition) metal-containing56−76 PPGs have been published in the past two decades. Special attention has been paid to compounds that release gasotransmitters such as nitric oxide (NO; photoactivatable NO-releasing moieties or photoNORMs), carbon monoxide (photoactivatable CO-releasing moieties or photoCORMs), and hydrogen sulfide (photoactivatable H2S-releasing molecules).77−114
Key criteria for the design and use of PPGs, as discussed at length in previous works,10,115−118 are often specific to individual applications. In general, however, a PPG (a) must exhibit sufficient absorption of the irradiated light, which must either not be absorbed by other molecules or not trigger unwanted photochemical transformations in the system of interest, (b) should release protected species within a time-frame compatible with the application, (c) must be soluble and stable in the targeted medium/environment (an aqueous solution in typical biological/medical applications), (d) should not produce reactive or toxic side-products upon irradiation, and (e) should be detectable in the medium, for example, by light emission. The overall efficiency of species release is evaluated using the quantity Φrε(λirr), sometimes called the uncaging cross section, which takes units of M–1 cm–1, where Φr is the reaction quantum yield and ε is the decadic molar absorption coefficient.10
Short-wavelength UV photons have sufficient energy to induce bond cleavage, isomerization, or rearrangement reactions in many organic and inorganic molecules. For example, the energy of a photon with a wavelength of λ ≈ 300 nm (NAhν = 95.6 kcal mol–1) is sufficient to induce homolytic cleavage of most single bonds in organic molecules. Most PPGs absorb light in the 300–400 nm region.10 However, excitation in the UV region presents several challenges, especially in biological settings; high-energy UV light has very limited tissue penetration due to high optical scattering and strong absorbance by endogenous chromophores (e.g., hemoglobin or melanin),119−121 can lead to sample overheating, and can cause phototoxic or photoallergic reactions resulting from its interactions with endogenous molecules such as DNA, RNA, and lipids.122−124 Visible and especially NIR light can penetrate deeper into tissues119,120,125−128 and is considerably less harmful to biological matter, opening the door to new applications in areas such as drug delivery.20,103,129,130 Encouragingly, some photoresponsive approaches are already used routinely in clinical applications.131−135 In addition, visible/NIR light sources, both coherent and non-coherent, are often cheaper, more common, and more accessible to non-specialist end-users than UV-light sources.
The desire to exploit these advantages has motivated several recent efforts to develop PPGs activated by visible/NIR light. Until recently, only a few PPGs activated directly by light of wavelengths above ∼600 nm were known, and the design of PPGs that undergo efficient photorelease upon irradiation at wavelengths above 500 nm was considered challenging.10,11 According to the gap law,136 nonradiative transition rate constants increase approximately exponentially as the associated energy gap contracts, which is one reason why π-extended organic PPGs absorbing visible or NIR light generally undergo inefficient photoreactions. However, while the quantum yields for release from such PPGs can be very small, their chromophores can have very large molar absorption coefficients, making their Φrε(λirr) values large enough for practical use.11 Alternatively, PPG activation by one (1P)-photon direct excitation using short-wavelength radiation can be replaced by alternative methods using substantially less energetic photons such as two (2P)-photon excitation or sensitization via photoinduced energy- or electron-transfer.
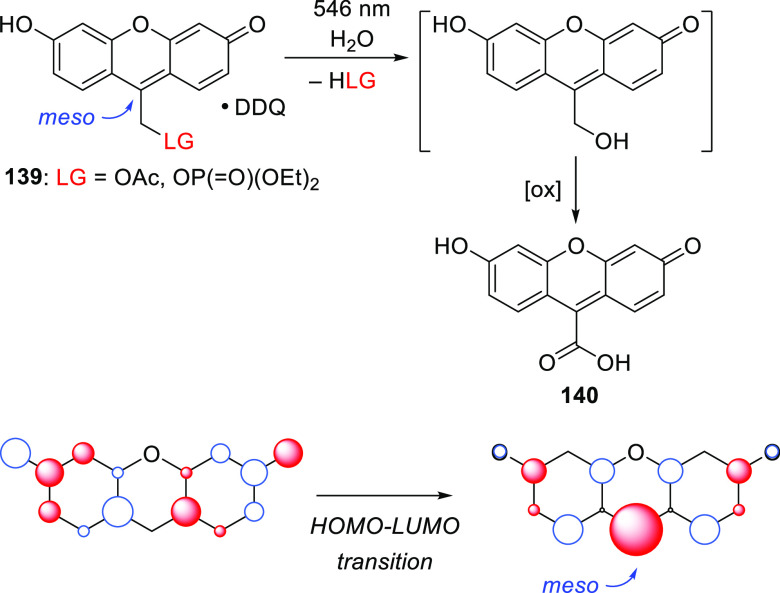
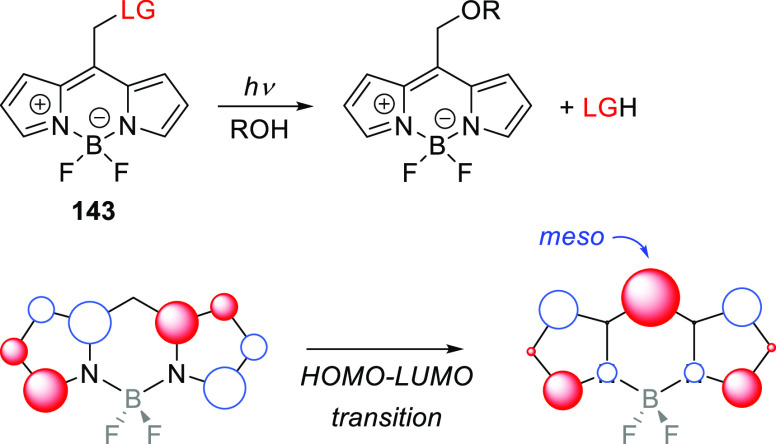
The applications of PPGs are not restricted to the release of a single species of interest. Careful selection of complementary photoactivatable moieties that undergo specific phototransformations can enable wavelength-selective release, which is often called chromatic orthogonality. Photochemical reactions are also in principle orthogonal to reagent- or thermally-initiated chemical processes. A unique and elegant approach exploiting this orthogonality was introduced by Bochet and co-workers,137,138 but the general concept remains somewhat underexplored. Multiple chromatically orthogonal systems including (among others) a monochromophoric system,139 a single multichromophoric entity,138 and mixtures of independent photoactivatable compounds140−144 have been reported. The latter approach is uniquely well-placed to benefit from the expansion of the photoexcitation window resulting from the development of visible- and NIR-light excitable PPGs. We discuss several orthogonal systems here, and further examples can be found in recent reviews.10,16,145−147
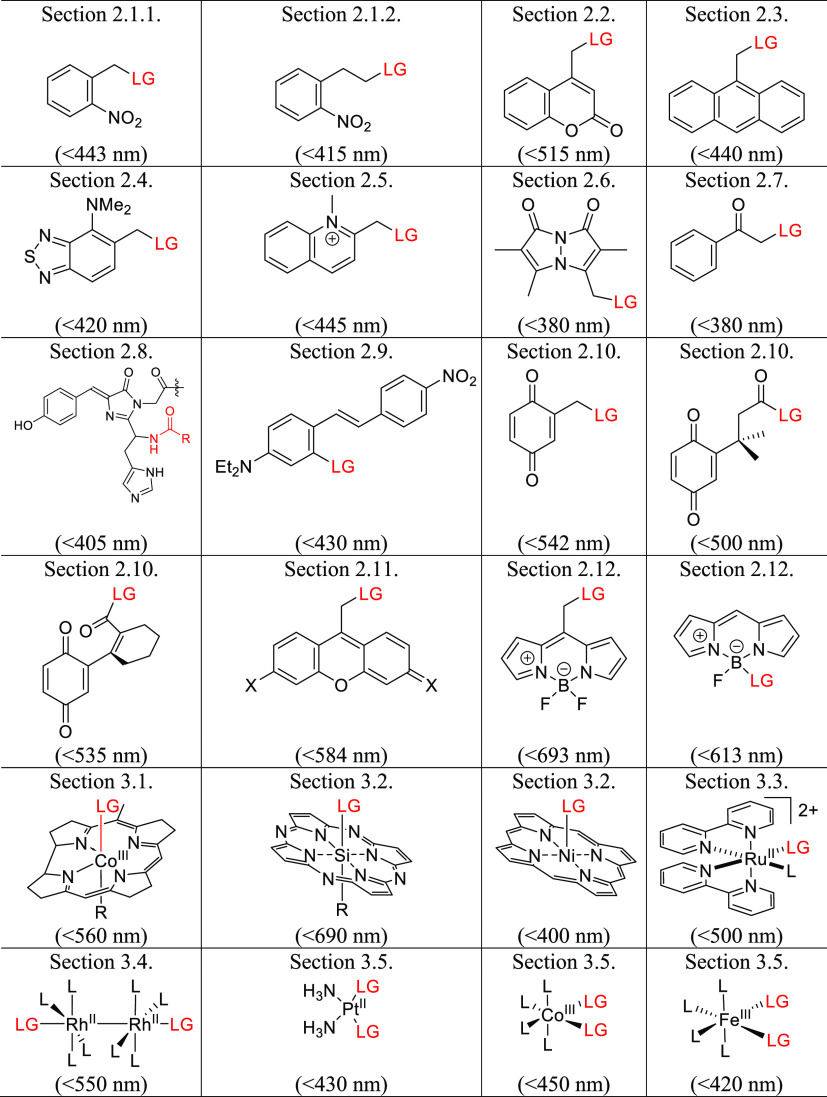
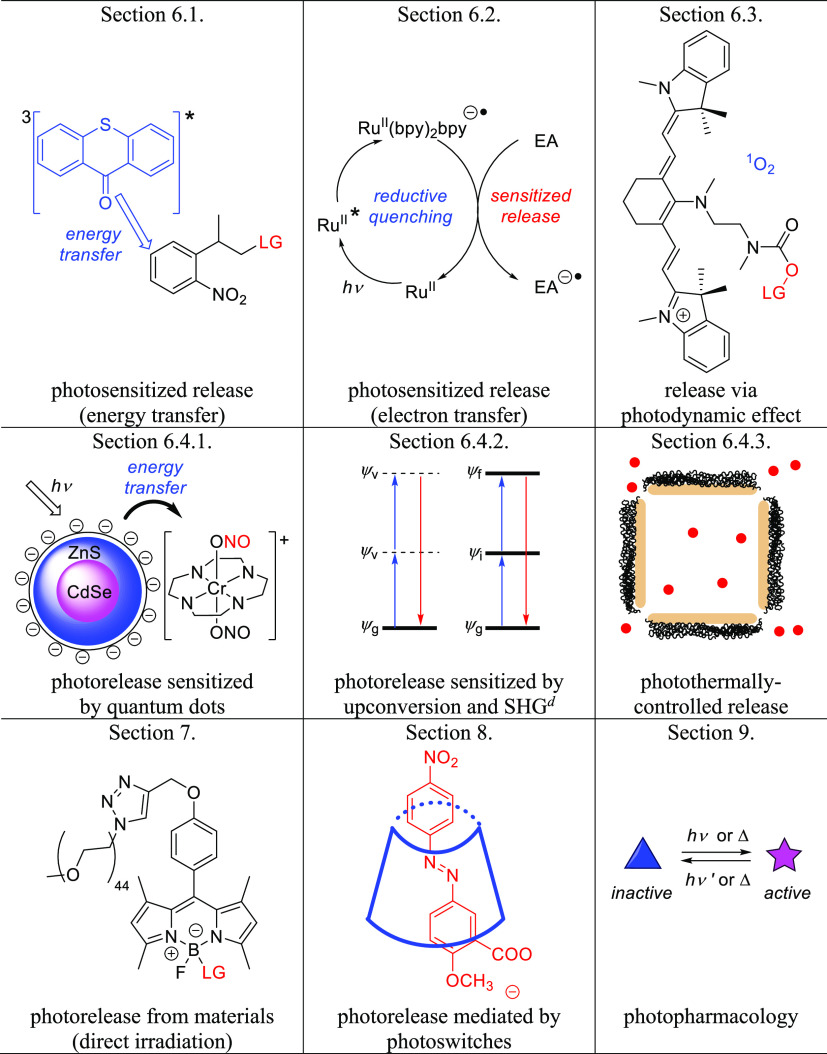
This review follows up on an earlier article that provided a comprehensive overview of the photochemistry and applications of PPGs known and used before 2013.10 We present a comprehensive list of PPGs absorbing in the visible and near-infrared (NIR) range including organic (section 2) and (transition) metal-containing molecular PPGs (section 3) that absorb photons directly (via 1P and (in several examples) 2P30,31,148 excitation) to release various leaving groups (LG) (Table 1), organic and metal-containing photoCORMs, photoNORMs, and photoactivatable H2S-releasing molecules (section 4, Table 2), and photoacids and photobases (section 5). These sections are followed by an overview of PPGs that use indirect methods of photoactivation, including photosensitization by molecular photosensitizers, quantum dots, upconversion, and second-harmonic nanoparticles, as well as photorelease by the photodynamic effect and photothermally-controlled release (section 6). The final sections discuss the chemistry of photoactivatable polymers, micelles, vesicles (section 7), and photoswitches (section 8), concluding with a brief discussion of the new concept of photopharmacology (section 9) (Table 3).
Table 1. Organic and Metal-Containing PPGs Covered in This Reviewab.
Values in parentheses indicate the longest wavelength that can be used for PPG activation.
Leaving groups (LG) are depicted in red.
Table 2. Organic and Transition Metal-Containing CO-, NO-, and H2S-Releasing Molecules Covered in This Reviewab.
Values in parentheses indicate the longest wavelength that can be used for PPG activation.
Leaving groups/moieties are depicted in red.
Table 3. Other Photoactivatable Systems Covered in This Review.
2. Photorelease from Organic Photoactivatable Compounds
2.1. Nitroaryl Groups
The nitroaryl motif has proven to be a fertile scaffold for the development of photoremovable protecting groups (PPGs), leading to the emergence of several structural families, including the o-nitrobenzyl, o-nitro-2-phenethyl, and o-nitroanilide groups.10 This section focuses on efforts to bathochromically shift the absorption spectra of o-nitrobenzyl and o-nitro-2-phenethyl PPGs toward the visible part of the spectrum. The absorption spectra of some representative nitroaryl PPGs are shown in Figure 1. A comprehensive review of UV-excitable nitroaryl derivatives covering their development and photochemical properties has been published.10
Figure 1.
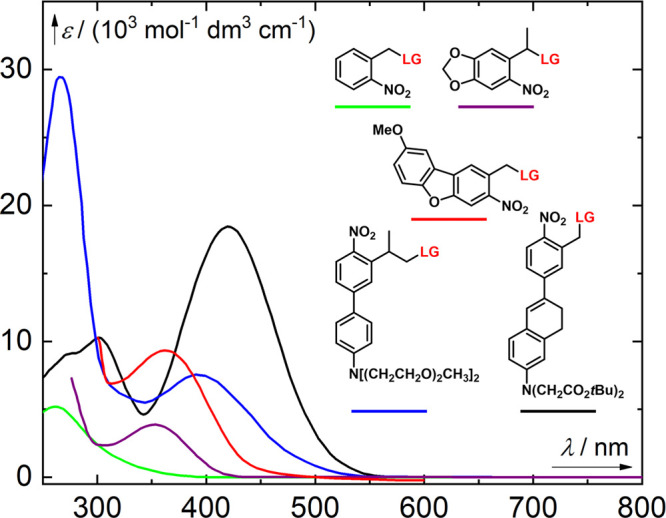
Absorption spectra of selected nitroaryl PPGs. Green line, a 2-nitrobenzyl derivative (LG = thymidine);149 purple line, an α-methyl-(6-nitropiperonyloxymethyl) derivative (LG = thymidine);150 red line, a nitrodibenzofuran derivative (LG = Fmoc-Cys-OH);151 blue line, an o-nitro-2-phenethyl derivative (LG = Boc-glutamate);152 black line, a π-extended 2-nitrobenzyl derivative (LG = GABA).153
2.1.1. The o-Nitrobenzyl Group
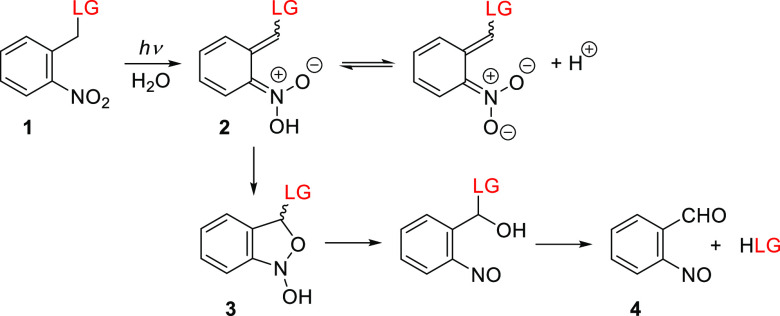
o-Nitrobenzyl derivatives (oNB) make up a family of general-purpose PPGs that have been developed since the 1960s4,154 and are still widely used.10 Their photorelease mechanism has been studied extensively.155−160 Briefly, the excitation of the ground state of an oNB derivative 1 (Figure 1) is followed by intramolecular hydrogen abstraction by the nitro group to form an aci-nitro intermediate (2, Scheme 1; LG = leaving group). The decay rate constant of the aci-nitro intermediate (∼102–104 s–1) depends on the substitution of the oNB group, the solvent, and the pH. An irreversible cyclization of the aci-nitro intermediate leads to a 1,3-dihydrobenz[c]isoxazol-1-ol (3). Subsequent ring-opening gives a hemiacetal intermediate that hydrolyzes to release the leaving group (LG) and form an o-nitrosobenzaldehyde byproduct (4). The photorelease of many functional groups including carboxylic acids,4 phosphates,161 thiols,162 alcohols,163 and amines164 has been demonstrated, although the latter two moieties are typically attached as carbonic acid derivatives.
Scheme 1. Proposed Photoreaction Mechanism of o-Nitrobenzyl PPGs159.
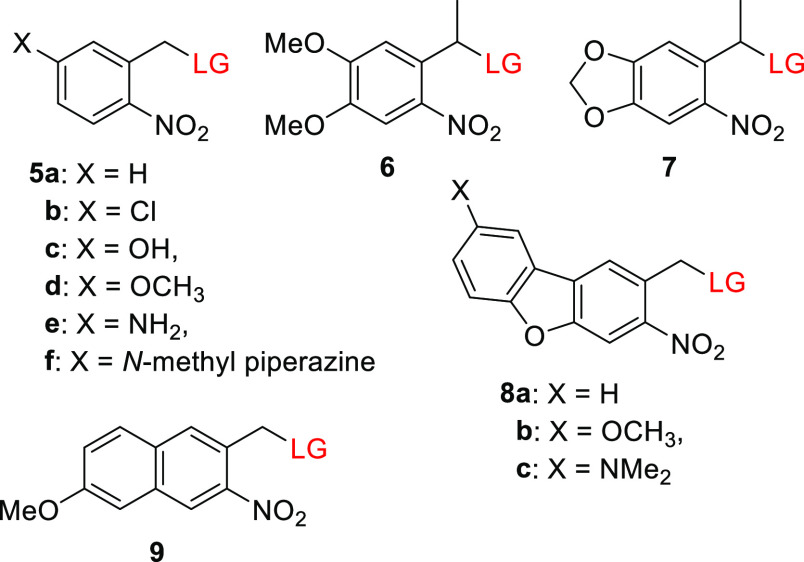
Efforts to bathochromically shift the absorption maxima of the parental oNB 5a (λmaxabs ≈ 260 nm) have generally met with limited success because of an inverse correlation between the bathochromic shifts of absorption bands and photochemical parameters such as the release quantum yield (Φr) and rate constant.137,163,165−167 For example, Jullien and co-workers examined a series of p-substituted nitrobenzyl derivatives 5b–5f and found that bathochromic shifts of their absorption maxima were associated with a decrease in Φr (Table 4).163 This loss of efficiency could be counteracted to some extent by substitution at the benzyl position,4,163,166−168 leading to the development of the red-shifted α-methyl-6-nitroveratryl (6)4 and α-methyl-(6-nitropiperonyloxymethyl) (7) PPGs (Figure 1).169 However, due to the reduction in quantum efficiency, the uncaging cross section (Φrε(λirr)) of the latter group tends to be comparable to that of the parent oNB 5a.4,170,171 Nitrodibenzofuran 8a (NDBF; Figure 1), introduced by Ellis-Davies and co-workers, is an exceptional red-shifted oNB derivative that releases LGs efficiently.172 The photolysis of ether,172 thioether,151 and phosphoester173,174 LGs caged with this group reportedly proceeded with Φr values of 0.5–0.7, although lower quantum yields were obtained in some cases (0.04–0.2).175−177 The tail absorption of 8a in the visible range (398–440 nm) was sufficient to promote the photoreaction.175,178 Introducing electron-donating groups (EDG) at the 7-position of NDBF (8b and 8c) led to a bathochromic shift in λmax but also reduced its photouncaging quantum efficiency (Table 4).151,174 The low quantum yield of 8c was attributed to a charge-transfer transition following photoexcitation that competes with LG release.174,179 Ball and co-workers recently reported that derivatives of 8a and 8c undergo efficient photocleavage of C(sp2)–N bonds.180 To explain this, a mechanism was proposed involving hydrogen-atom abstraction followed by selective nucleophilic attack of a solvent molecule on the resulting extended conjugated system. The absorption maximum of oNB-type PPGs can also be bathochromically shifted by extending the aromatic core,181−183 as in the 7-methoxynaphthalene derivative 9.183
Table 4. oNB Derivatives.
| PPG | λmaxabs (nm) | εmax (M–1 cm–1) | leaving groupsa | Φr (λirr/nm) | solventb | ref |
|---|---|---|---|---|---|---|
| 5a | 262 | 5.2 × 103 | thymidine (as carbonic acid) | 0.033 (365) | CH3OH/H2O, 1:1 | (167−170) |
| pivalic acid | 0.13 (254) | CH3CN | (149) | |||
| 5b | 272 | 6.0 × 103 | 4-nitrophenol | 0.1 (325) | CH3CN | (163) |
| 5c | 310 | 9.0 × 103 | 4-nitrophenol | not reported | CH3CN | (163) |
| 5d | 310 | 8.0 × 103 | 4-nitrophenol | 0.007 (325) | CH3CN | (163) |
| 5e | 367 | 1.6 × 104 | 4-nitrophenol | <0.001 (325) | CH3CN | (163) |
| 5f | 394 | 1.6 × 104 | 4-nitrophenol | <0.001 (325) | CH3CN | (163) |
| 6 | 352 | 4.0 × 103 | l-threo-β-benzyloxyaspartate | 0.005 (355) | PBS buffer, pH 7.4 | (184) |
| 7 | 351 | 3.5 × 103 (ε365) | thymidine (as carbonic acid) | 0.0075 (365) | CH3OH/H2O, 1:1 | (150) |
| 8a | 325 | 18.4 × 103 | EGTA (Ca2+), IP3 | 0.5–0.7 (350–400) | HEPES buffer, pH 7.2 | (172,173) |
| 8b | 362 | 9.3 × 104 | Fmoc-cysteine–OH | 0.51 (350) | phosphate buffer, pH 7.4 | (151) |
| 8c | 424 | 1.6 × 104 | nucleobases | 0.5–11 × 10–3 (420) | DMSO | (174) |
| 9 | 339 | 1.1 × 104 (ε350) | hippuric acid | 0.031 (420) | ethanol | (183) |
Only selected LGs are shown.
PBS = phosphate buffer saline. HEPES = 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; DMSO = dimethyl sulfoxide; EGTA = ethylene glycol tetraacetic acid; IP3 = inositol triphosphate.
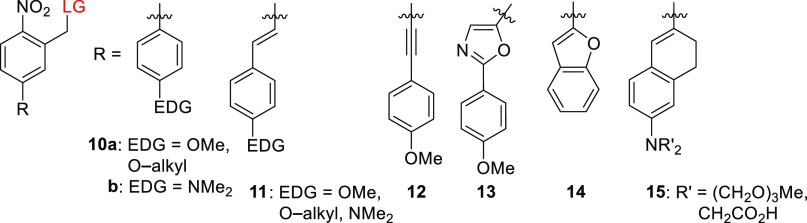
Jullien and co-workers also found that a bathochromic shift in λmaxabs relative to the parent PPG 5a could be achieved by substitution to form a π-extended donor–acceptor system containing an electron-donating group (EDG) such as a methoxy group (10–13, Table 5).163 These chromophores had λmax values of 336–371 nm but were photolyzed inefficiently to release a carboxylic acid (Φr = 0.001), in keeping with the previously mentioned inverse correlation between shifts in λmaxabs and Φr.163 Derivatives of biphenyl 10a exhibited a bathochromic shift in λmax of ∼70 nm relative to 5a,163,185,186 and an additional ∼60 nm shift was achieved by using a dialkylamine EDG (10b; Figure 1).187,188 The release of a carboxylic acid187 and the fragmentation of the selective Ca2+-chelator ethylene glycol tetraacetic acid27 (EGTA) with subsequent Ca2+ release were achieved at λirr = 400–405 nm using PPGs of this type.188 Stilbene-type derivatives 11, which bear various alkoxy EDGs, had λmaxabs values of 369–376 nm but released carboxylic acids with low quantum yields when irradiated above 400 nm.163,189,190 Relatively similar quantum yields were reported for release from a derivative of 11 bearing the dimethylamino group as an EDG (Φr = 0.8–2 × 10–4).191 It was proposed that a photoinduced reversible E–Z isomerization192−194 competes with photorelease in this case.190 Accordingly, rigid derivatives 14 and 15 (Figure 1) were photolyzed more efficiently than 11 to liberate carboxylic acid LGs or to cleave an ether bond (causing EGTA bifurcation leading to Ca2+ release).153,188,195,196 The π-extended 1,2-dihydronaphthalene 15, which has a dialkylamino EDG, is the chromophore with the longest absorption wavelength in this series.153 Visible-light uncaging from simple oNB derivatives has also been achieved through conjugation with silicon quantum dots197 or upconverting nanoparticles198−203 (see also sections 6.4.1 and 6.4.2). It should be noted that many oNB derivatives with absorption maxima in the near UV-region have proven very useful in diverse applications16,20,23,25,204−208 including in vivo experiments.209−215 Several genetically encoded amino acids caged by oNB derivatives have also been reported.216−218
Table 5. oNB Derivatives with Extended π-Systems.
| PPG | λmaxabs (nm) | εmax (M–1 cm–1) | leaving groupsa | Φr (λirr/nm) | solventb | ref |
|---|---|---|---|---|---|---|
| 10a | 335–342 | 7.3–14.0 × 103 | 4-nitrophenol, chlorambucil, celecoxib | 0.005–0.013 (325 or 355) | CH3CN or CH3CN/Tris pH 9.0, 1:1 or CH3CN/phosphate buffer pH 7.2, 1:1 | (163, 185, 186) |
| 10b | 403 | 8.8 × 103 | EGTA (Ca2+) | 0.05 (400) | C6D6 | (188) |
| 11 | 369–376 | 1.9–2.5 × 104 | coumarin, chlorambucil | 3.2–15.4 × 10–4 (325 or 400) | CH3CN or CH3CN/Tris pH 9.0, 1:1 | (163, 189) |
| 12 | 348 | 1.9 × 104 | 4-nitrophenol, coumarin | 0.001–0.005 (325) | CH3CN/Tris pH 9.0, 1:1 | (163) |
| 13 | 371 | 1.9 × 104 | coumarin | 0.001 (325) | CH3CN/Tris pH 9.0, 1:1 | (163) |
| 14 | 362–364 | 1.2–1.8 × 104 | benzoic acid, EGTA (Ca2+) | 0.09–0.3 (360) | CH3CN or DMSO | (188, 195) |
| 15 | 420–443 | 1.8–2.9 × 104 | Boc-glutamate | 0.01 (355) | CH3OH | (153) |
Only selected LGs are shown.
Tris = tris(hydroxymethyl)aminomethane; DMSO = dimethyl sulfoxide; EGTA = ethylene glycol tetraacetic acid.
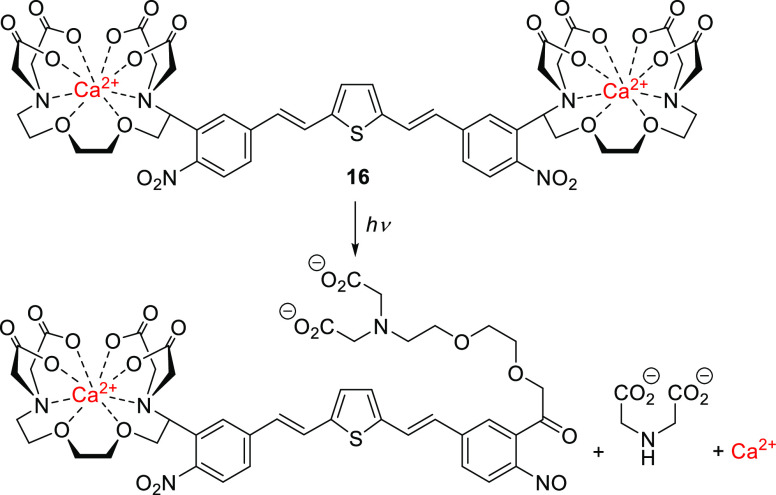
An outstanding 1-photon (1P)-absorbing oNB derivative is compound 16, a dinitro-derivative of bisstyrylthiophene (BIST) coupled to two units of EGTA, which was recently reported by Ellis-Davies and co-workers and used for visible-light-induced (λirr = 473 nm) calcium uncaging (Scheme 2).219 UV-excitable oNB derivatives are the PPGs most commonly used for photoscission of C–O or C–N bonds leading to the bifurcation of a chelator and the release of metal cations.27,213,220,221 The π-extended electron-poor compound 16 exhibited strong absorption maxima in the blue light region (λmaxabs = 440 nm, ε440 = 6.6 × 104 M–1 cm–1) and a large two-photon (2P) absorption cross section (δunc of >250 GM) in the 720–830 nm range.219 This compound is a strong Ca2+ chelator, but upon 1P (λirr = 473 nm, Φr = 0.23) or 2P excitation (λirr = 720 or 810 nm), its Ca2+ affinity falls markedly, leading to the release of free Ca2+. A BIST scaffold masked with PEG dendrons was also used to cage γ-butyric acid (GABA), although this species was found to be resilient to 1P photolysis (λirr = 470 nm) and released GABA only upon 2P excitation.222 Similar effects on uncaging have been reported previously.174
Scheme 2. Photouncaging of Ca2+ with Visible Light219.
Simple oNB derivatives tend to have rather low 2P-uncaging cross sections (δunc), ranging from 0.01 to 0.035 GM.163,165,223 Nevertheless, they have been used successfully in some biological applications.224−226 NDBF derivative 8a is an exception, with a reported δunc of 0.6 GM (at 720 nm).172 The 2P-uncaging cross sections of derivatives of 6 were improved by incorporating the chromophore into dyads (δunc = 0.1–1.0 GM).227,228 Jullien and co-workers observed that the δunc of derivatives 10–13 remained low for 2P uncaging of carboxylic acids (δunc = 0.02–0.05 GM, λirr = 730–800 nm).163 The same authors reported that substitution at the benzyl position has similar effects on both δunc and Φr.163 It was therefore suggested that the same excited state is involved in both 1P and 2P photolysis. Stilbene derivative 11 (OEt = EDG) exhibited 2P absorption of 20 GM and δunc = 0.014 GM for the release of chlorambucil,189 whereas rigid stilbene derivatives of 15 and the biphenyl 10 were reported to be photolyzed more efficiently, with δunc = 5–21 and 7.8 GM at 740 and 800 nm, respectively.
2.1.2. The o-Nitro-2-phenethyl Group
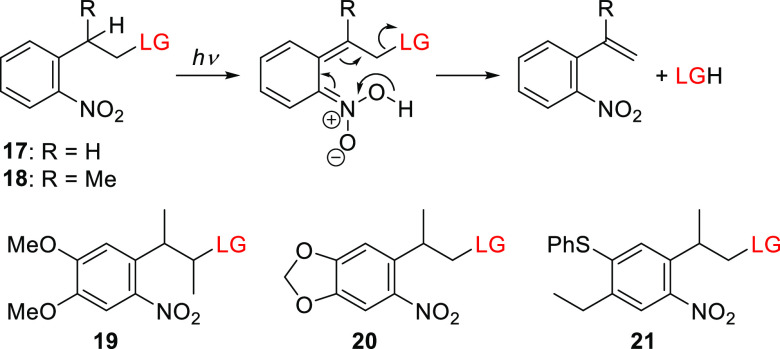
The 1-(2-nitrophenyl)ethyloxycarbonyl (NPEOC) group17017 and its α-methyl analog170,22918 (NPPOC; the “OC” stands for the −OC(=O) group, which is typically a part of the LG) constitute a separate class of nitroaryl PPGs. Despite its close structural similarity to oNB (5a), the proposed photoreaction mechanism of o-nitro-2-phenethyl derivatives is markedly different, involving a photoinduced elimination step (Scheme 3)170 reminiscent of that reported for (2-hydroxyethyl)benzophenone-type PPGs.118,230−235 The quantum yields obtained for o-nitro-2-phenethyl derivatives exceed those for their oNB analogs150,170 (for example, Φr = 0.35 and 0.033 for 5′-O-nucleoside carbonate photorelease from 18 and 5a, respectively), leading to their use in automated light-mediated oligonucleotide synthesis (DNA-chips),236,237 the preparation of peptide238−240 and RNA241,242 microarrays, the synthesis of aptamers243 and carbohydrates,244 and gene assembly.245
Scheme 3. Photorelease from o-Nitro-2-phenethyl PPGs170.
The parent compounds 17 and 18 were further modified to enhance their absorption at longer wavelengths, as exemplified by the 3-(4,5-dimethoxy-2-nitrophenyl)-2-butyl group (DMNPB, 19)246−248 and the analogous 2-(3,4-methylenedioxy-6-nitrophenyl)-propoxycarbonyl group (MNPPOC, 20).150 Both these groups have a λmaxabs at 350 nm but lack the associated decrease in Φr observed for oNB derivatives (Table 6). Bowman and co-workers showed that the tail absorption of 20 above 400 nm enables its use in visible-light photobase generation (see also section 5); the photorelease of tetramethylguanidine (TMG) at λirr = 405 and 455 nm proceeded with uncaging cross sections (Φrε(λirr)) of 38.5 and 4.6 M–1 cm–1, facilitating visible-light-mediated control over a thiol-Michael addition polymerization process.249 The thiophenyl-2-(2-nitrophenyl)propoxycarbonyl derivative 21 was shown to have spectroscopic properties comparable to those of 20 (Table 6).250,251 Additionally, Steiner and co-workers used intra- and intermolecular energy transfer from a triplet sensitizer (section 6.1) to initiate the release of LGs from NPPOC derivative 18 at λirr ≥ 400 nm.171,252,253
Table 6. Spectroscopic and Photochemical Properties of o-Nitro-2-phenethyl Derivatives.
| PPG | λmaxabs (nm) | εmax (M–1 cm–1) | leaving groupsa | Φr (λirr/nm) | solvent | ref |
|---|---|---|---|---|---|---|
| 17 | ∼260 | 0.29 × 103 (ε365) | thymidine (as carbonic acid) | 0.042 (365) | CH3OH/H2O, 1:1 | (170) |
| 18 | ∼260 | 0.26 × 103 (ε365) | thymidine (as carbonic acid) | 0.35 (365) | CH3OH/H2O, 1:1 | (170) |
| 19 | 350 | 3.5 × 103 | GABA | 0.26 (364) | phosphate buffer, pH 7.2 | (246) |
| 20 | 353 | 3.4 × 103 (ε365) | thymidine (as carbonic acid) | 0.035–0.037 (365) | CH3OH/H2O, 1:1 | (150) |
| 21 | ∼350 | 1.5 × 103 (ε365) | DNA phosphoramidites | 0.68 (365) | CH3OH | (250) |
| 22 | 317 | 9.9 × 103 | glutamate | 0.09 (364) | phosphate buffer, pH 7.4 | (254, 255) |
| 23 | 296–302 | 6.3–7.1 × 103 | glutamate | n.d. | phosphate buffer, pH 7.4 | (254, 255) |
| 24 | 397 | 7.5 × 103 | GABA | 0.15 (405) | phosphate buffer, pH 7.4 | (152) |
| 25 | 415 | 6.4 × 104 | glutamate | 0.25 (354) | phosphate buffer, pH 7.4 | (267) |
Only selected LGs are shown. GABA = γ-aminobutyric acid.
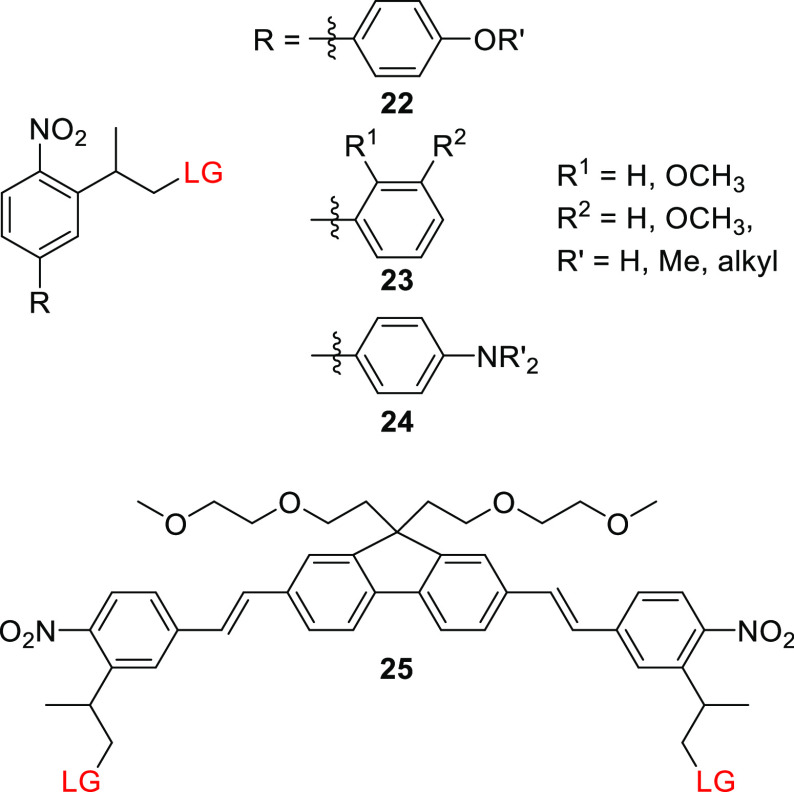
o-Nitro-2-phenethyl derivatives such as 17 and 18 typically have higher 2P δunc values than simple oNB derivatives such as 5 and 6 (δunc = 0.1–0.9233,246 vs 0.01–0.35163,165,223 GM, respectively).246 NPPOC biphenyl systems 22 (Figure 2) have been studied to determine whether extending the π-system of o-nitro-2-phenethyl moieties could improve their 2P-absorption sensitivity. Goeldner and co-workers showed that p-methoxynitrobiphenyl platform 22 exhibits a ∼60 nm bathochromic shift in λmaxabs relative to 18 while retaining a comparable 1P-photorelease quantum yield for glutamate (Table 6).254 This stands in contrast to the previously mentioned inverse correlation between bathochromic shifts of λmax and Φr in oNB derivatives (see section 2.1.1). The 2P-uncaging cross sections of glutamate from 22 were 3.2 and 0.45 GM at 740 and 800 nm, respectively,254,255 both of which are significantly higher than the corresponding values for 19 (δunc = 0.17 GM, 720 nm).246 Moving the methoxy EDG to the ortho or meta positions (23) did not affect 1P photorelease yield but reduced the 2P uncaging cross section (δunc = 2.2 and 1.8 GM, respectively, 740 nm).255 The introduction of a hydroxyl EDG was detrimental to the photouncaging of glutamate (reducing its chemical yield to <10%), presumably because it opened up photochemical pathways that compete with photorelease.254 The impact of varying the p-alkoxy substituent of 22 on the photorelease of various LGs at λirr = 300–365 nm was investigated, but no appreciable effects on photoreaction properties were observed.185,255−260 Specht, Goeldner, and co-workers further showed that dialkylamino substituents (24) caused an additional ∼90 nm bathochromic shift with no significant detrimental effects on the quantum yield of 1P GABA photorelease (Table 6) and also substantially increased the 2P-uncaging cross section, giving δunc values of up to 11 GM at 800 nm.152 The photorelease of carboxylates,152,255,261 amines260,262−264 (connected as carbamates), alcohols,265 and phosphates266 from various dialkylamino derivatives of 24 proceeded with Φr = 0.09–0.28 at λirr = 390–520 nm and with δunc values of up to 20.5 GM at 800 nm. To improve the water-solubility of these rather hydrophobic PPGs and enable their conjugation to (intra)cellular targeting groups, hydrophilic functional groups were attached to the amino152,262,263,266 or alkoxy256,260 moieties of 22 and 24.185,258
Figure 2.
o-Nitro-2-phenethyl derivatives (LG = alkoxide, carboxylate, carbonate, carbamates, or phosphate).
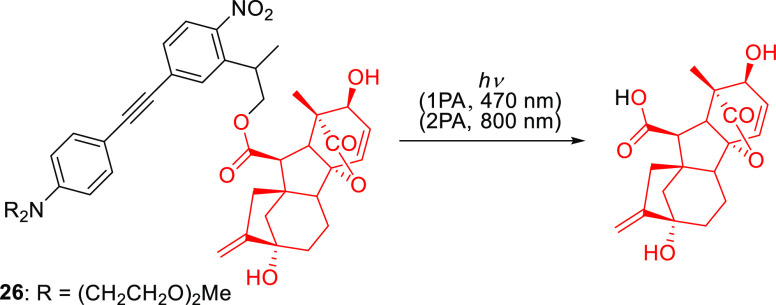
The extension of the π-system of NPPOC with styrene and phenylacetylene substituents was also explored.254,257,268 For example, Wombacher and co-workers synthesized 26 to cage the plant hormone gibberellic acid (GA3) via an ester linkage (Scheme 4).268 This conjugate had a λmaxabs of 400 nm and released GA3 upon 1P (λirr = 470 nm) or 2P (λirr = 800 nm) excitation in cultured COS-7 cells, enabling light-mediated control over a chemically-induced dimerization system based on the gibberellin perception mechanism.269,270 Symmetric biphenyl-substituted NPPOC structures such as 25 (Figure 2) exhibited significantly improved 1P- and 2P-absorption photorelease efficiencies (Φr = 0.25–0.30, δunc = 0.9–5.0 GM (at 840 nm),267 but their size and poor solubility make them more suitable for applications where they are incorporated into larger structures.271
Scheme 4. Photouncaging of Plant Hormone GA3 from π-Extended NPPOC Derivative268.
2.2. The (Coumarin-4-yl)methyl Group
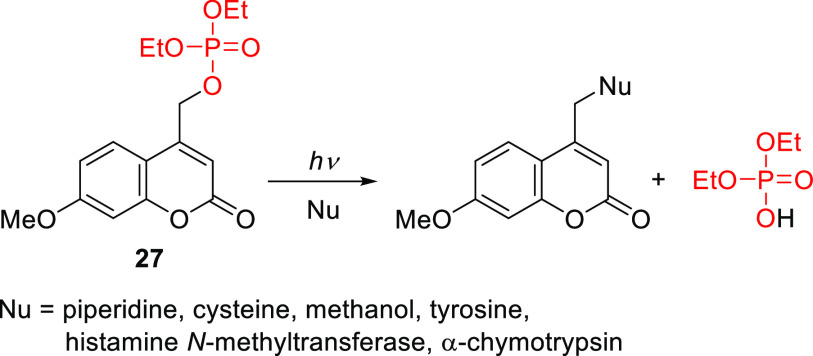
Coumarin (2H-chromen-2-on) is a secondary metabolite found in many plants that was first isolated from the Tonka bean, known in French as coumarou, in 1820.272−274 The development of coumarins as a new class of photoremovable protecting groups began with the discovery of Givens and Matuszewski that the (coumarin-4-yl)methyl group exhibits photoreactivity, enabling the release of phosphate esters (Scheme 5).275
Scheme 5. Release of Phosphate from 7-Methoxycoumarin 27(275).
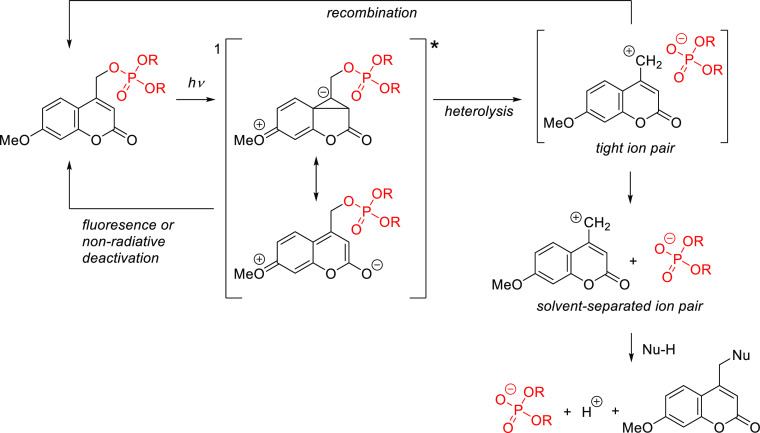
The mechanism of the photorelease from (coumarin-4-yl)methyl derivatives has been extensively studied276−278 and reviewed,10,279 and it is summarized in Scheme 6.276 Briefly, a heterolytic C–X bond cleavage takes place from the lowest 1π,π* singlet excited state, which competes with unproductive radiationless decay and fluorescence emission. A tight ion pair (TIP) was proposed to be the key intermediate in this process; the (coumarin-4-yl)methyl cation in this pair could react directly with adventitious nucleophiles or solvents to form a new stable (coumarin-4-yl)methyl product. Recombination of the TIP to regenerate the ground-state caged derivative would be an unproductive competing radiationless pathway in this mechanism. It should however be noted that ultrafast time-resolved visible-pump-infrared-probe spectroscopy experiments yielded no evidence of TIP formation during the photorelease of a (coumarin-4-yl)methyl azide.280 There are also evidences suggesting that some coumarin derivatives exhibit triplet-state reactivity.165,281−284
Scheme 6. Photocleavage Mechanism of (Coumarin-4-yl)methyl-Caged Phosphates276.
In general, coumarin-based PPGs offer several advantages: (1) high molar absorption coefficients at wavelengths above 350 nm, (2) high photorelease efficiencies, (3) acceptable stabilities in the dark, (4) fast photolysis kinetics, and (5) practically useful 2-photon excitation cross sections. Furthermore, their spectroscopic, photochemical, and other relevant properties (e.g., solubility and conjugation) can easily be tuned by varying the substituents on the coumarin ring. Given the high diversity of known coumarins, their synthesis is outside the scope of this review; interested readers are directed to reference works for extensive surveys.10,285 Similarly, comprehensive reviews of the biological and other applications of (coumarin-4-yl)methyl PPGs can be found elsewhere.16,19,21,22,26,50,285−289 The following section focuses on the evolution of coumarinyl PPGs that are excitable by light in the visible region of the spectrum. The absorption spectra of representative (coumarin-4-yl)methyl PPGs discussed in this section are shown in Figure 3.
Figure 3.

Absorption spectra of selected (coumarin-4-yl)methyl PPGs. Black line, a (coumarin-4-yl)methyl derivative (LG = cAMP);290 red line, a [7-(diethylamino)coumarin-4-yl]methyl derivative (LG = benzoate);290 magenta line, a thionated [7-(diethylamino)coumarin-4-yl]methyl derivative (LG = benzoate);291 orange line, a 3-[3-(methylamino)-3-oxoprop-1-en-1-yl] derivative (LG = glutamate);292 green line, a 7-styryl derivative (LG = 4-methoxybenzylcarbonate);293 cyan line, a bis-julolidine derivative (LG = 4-methoxybenzoate);294 blue line, a benzothiazolium derivative (LG = 3,5-dimethylbenzoate).295
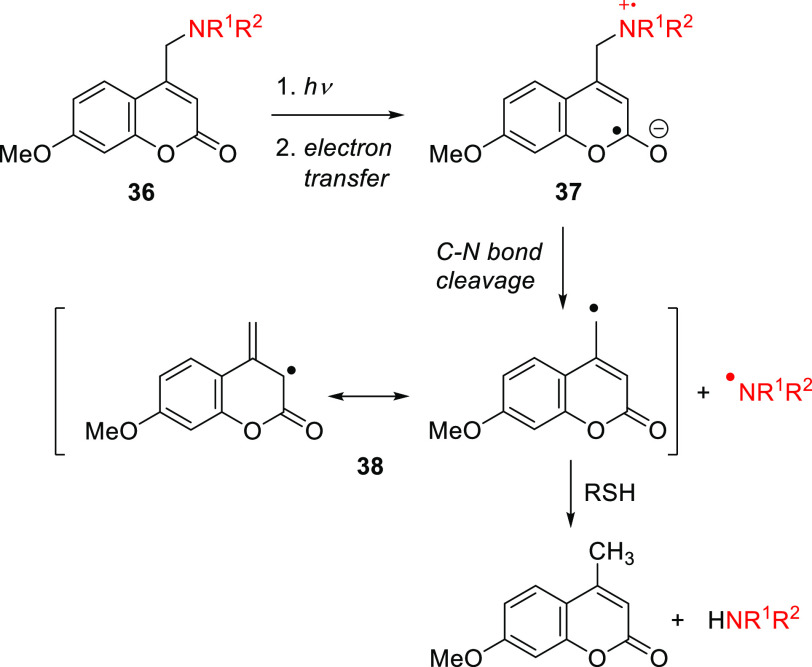
The parent (coumarin-4-yl)methyl 28a has an absorption maximum at 310 nm (Table 7; Figure 3) and was shown to photorelease cyclic adenosine monophosphate (cAMP) with Φr = 0.085.290 Introducing EDGs at the C7-position led to an increased intramolecular charge-transfer (ICT) character and a greater transition dipole moment, resulting in more intense and red-shifted absorption.278,290,296−302 The weakly electron-donating 7-methyl substituent (28b) caused a ∼7 nm bathochromic shift in λmaxabs,303,304 while derivatives with stronger EDGs such as hydroxy ((7-hydroxycoumarin-4-yl)methyl, 29a) and methoxy ((7-methoxycoumarin-4-yl)methyl, 29b) exhibited more pronounced effects (Table 7). The(7-carboxymethoxycoumarin-4-yl)methyl derivative 29c was designed to provide improved water solubility,184,301,305−307 while esters 29d ((7-acetoxycoumarin-4-yl)methyl) and 29e ((7-propionyloxycoumarin-4-yl)methyl) were introduced to improve membrane permeability.308−311 After penetration into live cells by diffusion, the esters of 29d and 29e are hydrolyzed by endogenous esterases to form the more polar phenolic derivative 29a, which has negligible membrane permeability and thus accumulates inside cells.309,310 A genetically encodable lysine caged by 29a was developed to control protein functions in cell cultures and in vivo.312−316 The photoexcitation of 29a–e and their derivatives is usually restricted to the 300–350 nm wavelength range. Photouncaging of phosphates, sulfonates, and quaternary amines from 29a–e and their derivatives typically occurs with Φr values of 0.05–0.39,276,278,281,290,301,309,317,318 whereas poorer leaving groups, such as carboxylic,184,276,278,319,320 carbonic,319,321−323 and carbamic305,313,319,324−326 acids are liberated less efficiently (Φr = 0.004–0.03). The photorelease efficiencies of amino acids connected to 29a and 29b through different linkers declined in the following order: anhydride > ester > carbamate > carbonate.319 The carbonic or carbamic acids initially liberated by photorelease from these linkers are unstable and undergo decarboxylation to give the corresponding free alcohol or amine, respectively. These decarboxylation reactions usually have quite low rates, with k-CO2 on the order of 10–3 s–1, and they are subject to both acid and base catalysis.327−330 A single example of a C–N bond cleavage from 29b was reported.331 This reaction proceeded efficiently only in the presence of an excess of a hydrogen-atom donor such as n-decanethiol or 1,4-cyclohexadiene. A radical mechanism was proposed (Scheme 7), involving electron transfer between the amine and coumarinylmethyl moieties in 36 to form the intramolecular radical ion pair 37. The subsequent cleavage of the C–N bond generates an aminyl radical and a resonance-stabilized coumarinylmethyl radical 38, both of which can be trapped by hydrogen-atom donors.
Table 7. Coumarin PPGs Substituted at the 7-Positiona.
| PPG | λmaxabs (nm) | εmax (M–1 cm–1) | solventb | ref |
|---|---|---|---|---|
| 28a | 310 | 5.1 × 103 | CH3OH/HEPES buffer pH 7.2, 1:1 | (290) |
| 28b | 317 | 3.92 × 103 | ethanol | (303, 304) |
| 29a–e | 314–328 | 1.0–1.6 × 104 | CH3OH/HEPES buffer pH 7.2, 1:1 or MOPS buffer, pH 7.2 | (165, 290, 301, 308) |
| 30a | 348 | 1.4 × 104 | PBS buffer, pH 7.4 | (324) |
| 30b | 378–398 | 1.5–1.8 × 104 | CH3OH/HEPES buffer pH 7.2, 1:1 | (290, 332, 333) |
| 30c | 387–406 | 1.5–2.1 × 104 | CH3CN/HEPES buffer pH 7.2, 1:20 or HEPES buffer pH 7.2 or CH3OH/HEPES buffer pH 7.2, 1:4 | (301, 334) |
| 31 | 399–403 | 1.8–4.4 × 104 | CH3OH/H2O, 9:1 or CH3OH/HEPES buffer pH 7.2, 4:1 | (294, 335, 336) |
| 32 | 371 | 1.6 × 104 | CH3CN/PBS buffer pH 7.4, 7:3 | (337) |
| 33 | 450 | not reported | CH3CN | (338) |
| 34a | 323 | 4.1 × 104 | CH3OH/HEPES buffer pH 7.2, 4:1 | (339) |
| 34b | 325–340 | 3.9-4.1 × 104 | CH3OH/HEPES buffer pH 7.2, 4:1 | (339) |
| 35a | 347–354 | 3.5–5.8 × 104 | CH3OH/HEPES buffer pH 7.2, 4:1 or CH3CN/H2O, 9:1 | (293, 339) |
| 35b | 366 | 2.8 × 104 | CH3CN/H2O, 9:1 | (293) |
| 35c | 407 | 2.9 × 104 | CH3CN/H2O, 9:1 | (293) |
LG = alkoxides, carboxylates, carbonates, carbamates, phosphates, thiols, sulfonates, azide, halides.
HEPES = 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; MOPS = 3-(N-morpholino)propanesulfonic acid; PBS = phosphate buffer saline.
Scheme 7. Photouncaging of Amines via Direct C–N Bond Cleavage331.
The introduction of a 7-NH2 substituent ((7-aminocoumarin-4-yl)methyl, 30a)324 caused a ∼40–45 nm bathochromic shift of λmaxabs (Figure 3), and the liberation of carboxylic acids and amines from the corresponding esters and carbamates of 30a proceeded with Φr values of 0.003–0.6 (λirr = 350 or 419 nm).308,324,339,340 Alkylation of the 7-amino moiety, which increases its electron-donating ability, resulted in a more red-shifted and intense absorption band in [7-(dimethylamino)coumarin-4-yl]methyl derivative 30b(290,334,341) and [7-(diethylamino)coumarin-4-yl]methyl analog 30c(301,334) (Table 7).278,290,301 The photorelease quantum yields for 30b and 30c exceeded those for all other compounds in this series. This was attributed to greater stabilization of the (coumarin-4-yl)methyl carbocation by the electron-donating dialkylamino substituents, leading to more efficient LG liberation from the TIP intermediate.276,278,290 For example, the Φr values for cAMP release from 30b and 30c were 0.28 and 0.21, respectively, around twice that for 29b (Φr = 0.13).278,290 The release of carboxylic acids from 30b and 30c occurred with Φr values of 0.003–0.12,291,321,332,333 whereas amines (as carbamic acids),278,342−346 alcohols (as carbonic acids),293,344,347 and thiols (as thiocarbonic acids)348−350 were liberated with Φr = 0.01–0.09. The direct release of phenols occurred with Φr = 0.02–0.26, but competing recombination of the primary products proceeded with similar or even higher efficiency with these LGs.345,351−353 The favorable spectroscopic and photochemical properties of 30c, such as its absorption above 400 nm,332,333 have made it one of the most popular PPGs. For more examples of its applications, the reader is referred to several review articles.10,16,19,21,22,26,285
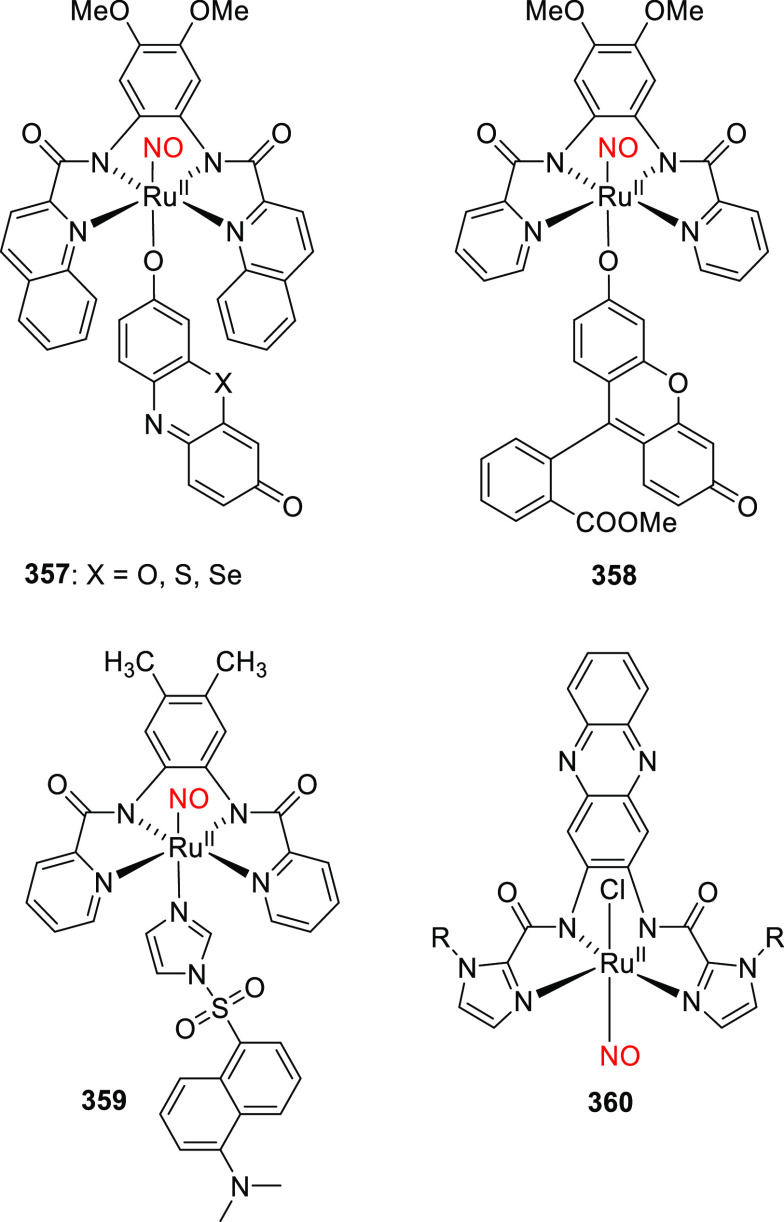
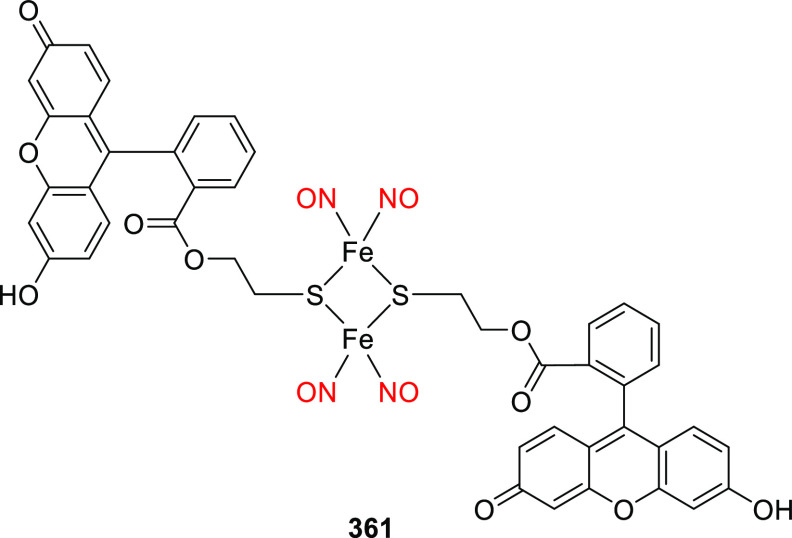
Derivative 30d was shown to have similar spectroscopic and photochemical properties to 30c (Table 7)354 while providing an additional derivatization point for further modulation of its properties and functions.354−360 The alkyl substituents of the (7-dialkylaminocoumaryl)methyl group can easily be replaced with other functional moieties without significantly affecting the molecule’s photophysical and photochemical properties,361 allowing other properties to be tuned to expand the PPG’s utility. For example, long alkyl chains have been appended to the 7-amino group to increase hydrophobicity,362−366 and highly polar or charged moieties such as bis(carboxymethyl),283,306,367−375 bis((dimethylamino)ethyl)carboxamide,376 and bis(ethylsulfonate)377,378 groups have been used to increase water solubility and control cellular permeability. Other functionalities have been appended to the 7-amino group to enable conjugation to (sub)cellular targeting motifs,377,379−382 binding to surfaces and nanoparticles,383−388 or incorporation into polymer backbones.389,390 Analyte-dependent photoactivatable derivatives have also been reported.383,391,392
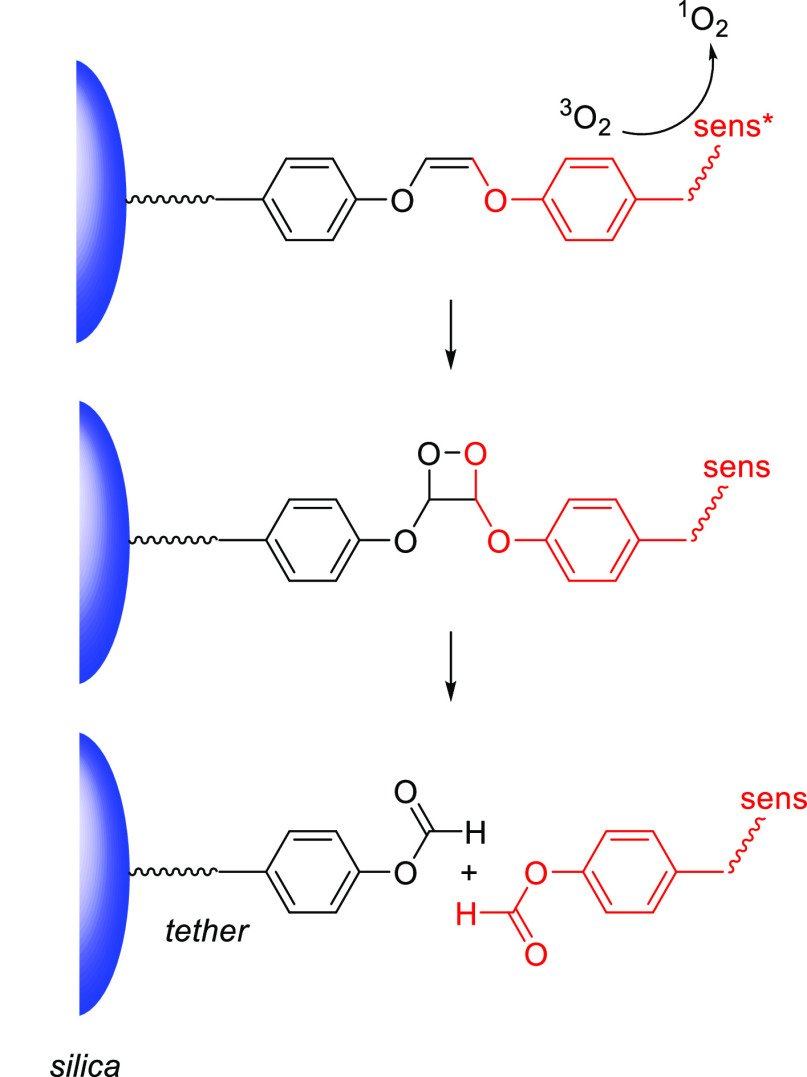
Derivatives bearing a conformationally locked electron-donating julolidine motif393,394 exhibited a 10–15 nm bathochromic shift of λmaxabs relative to their corresponding open-chain analogs (Table 7) and were photolyzed with higher quantum yields.294,335−337 For example, the liberation of benzoic acid derivatives from coumarin 31 was 5–7-times more efficient than from 30c under the same conditions (λirr = 405 nm).337 The 7-azetidinyl and 7-aziridinyl substitutions significantly increased fluorescence quantum yields in coumarin fluorophores, which was related to a decrease in the population of twisted intramolecular charge transfer (TICT) states395 upon excitation.313,396 Rivera-Fuentes and co-workers synthesized 7-azetidinyl coumarin 32, which released carboxylic acids with Φr = 1.4–1.6 × 10–2 upon irradiation at 405 nm.337 The authors suggested that this increase in photouncaging efficiency is not due to the substituent’s effect on the population of TICT states (as was suggested for the fluorescence enhancement313,396) but rather to suppression of an unproductive H-bond-induced non-radiative decay397−399 (HBIND) channel.337 Photouncaging (λirr = 405 nm) of a fluorescein derivative from 32 in live cells was demonstrated.337 Singh and co-workers synthesized the squaric acid–coumarin conjugate 33 (LG = the anticancer drug chlorambucil, Table 7). An organic nanoparticle formulation of this compound exhibited a hypsochromically shifted and broadened absorption spectrum (λmax ≈ 410 nm) relative to that of the free molecular species.338 Photoexcitation of 33–nanoparticle conjugates (λirr = 410 nm) led to the simultaneous release of chlorambucil (Φr = 0.083) and generation of singlet oxygen (ΦΔ = 0.51) from the excited squaraine moiety.400−402 This simultaneous release of a strong oxidant and an anticancer drug had synergistic effects on cell viability in cultured HeLa cells.338
Gonçalves and co-workers expanded the coumarin π-system by substituting the 7-position with phenyl (34a) or p-methoxyphenyl (34b) groups, resulting in bathochromic shifts in the absorption of 19 and 31 nm, respectively, relative to the parent coumarin 28a (Table 7). However, detectable carboxylic acid release from these derivatives occurred only upon irradiation below 350 nm.339 The introduction of a 7-styryl group293,339 in 35a caused a more significant bathochromic shift of λmaxabs that was further enhanced by substituting the para-position with EDGs (35b and 35c, Table 7; Figure 3).293 The liberation of alcohols (caged through a carbonate linker) from 35c proceeded with Φr = 8.3 × 10–4 (λirr = 420 nm), which is ∼50-times lower than the corresponding value for coumarin 30c (Φr = 4.5 × 10–2). Nevertheless, the uncaging cross section of 35c upon irradiation at 430 nm was around 4-times that of 30c (Φrε430 = 8.28 and 2.29 M–1 cm–1 for 35c and 30c, respectively).293 Because of its extended D−π–A backbone,26735c exhibited a much stronger 2P absorption than 30c (309 vs 2.3 GM at 800 nm) and a ∼2-fold higher 2P uncaging cross section (δunc = 0.26 vs 0.12 GM at 800 nm).293
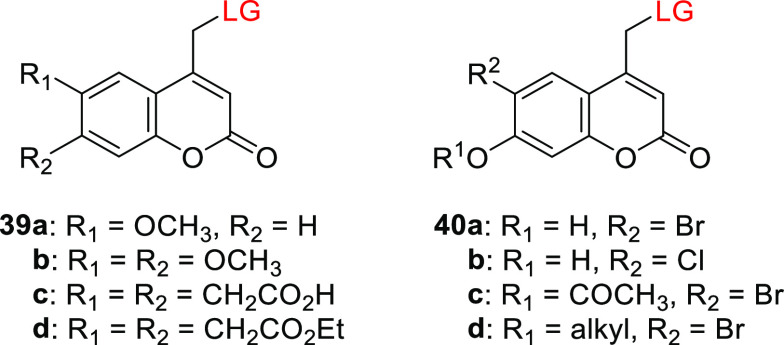
Coumarin derivatives bearing EDGs at the 6-position exhibited greater bathochromic shifts in absorption than their 7-EDG counterparts,278,290,405,406 but usually also exhibited less efficient photorelease (Table 8).278,290 For example, the (6-methoxycoumarin-4-yl)methyl compound 39a had a 20 nm bathochromic shift of λmaxabs relative to its 7-methoxy analog 29b and was photolyzed to release cAMP as an LG ∼4-times less efficiently.278,290 The spectroscopic and photochemical properties of the 6,7-dialkoxy derivatives 39b–d resembled those of their 6- or 7-monosubstituted analogs. Sulfonates and phosphates such as cAMP and cGMP were released from 39b–d with Φr = 0.08–0.14,278,290,301,407−410 while poorer LGs such as carboxylic and carbamic acids were released with Φr = 0.6–2.0 × 10–2.305,321 The uncaging of cysteine residues protected with 39b in proteins was used to study their folding kinetics on a sub-microsecond time-scale.411,412 The (6,7-dicarboxymethoxycoumarin-4-yl)methyl derivative 39c was designed to provide increased water solubility,301,305,410 and the diethyl ester 39d was synthesized to improve membrane permeability.413
Table 8. Coumarin PPGs Substituted at the 6-Position.
| PPG | λmaxabs (nm) | εmax (M–1 cm–1) | leaving groupsa | Φr (λirr/nm) | solventb | ref |
|---|---|---|---|---|---|---|
| 39a | 337–346 | 4.2–4.5 × 103 | cAMP | 0.02-0.055 (333) | CH3OH/HEPES buffer pH 7.2, 1:1 | (278, 290) |
| 39b | 341–349 | 1.1–1.2 × 104 | cAMP | 0.04 (333) | CH3OH/HEPES buffer pH 7.2, 1:1 | (278, 290) |
| 39c | 346–347 | 1.1–1.2 × 104 | cAMP | 0.08–0.10 (333) | CH3CN/HEPES buffer pH 7.2, 1:20 or HEPES buffer pH 7.2 | (301) |
| 40a | 370–375 | 1.5–1.7 × 104 | acetic acid | 0.37 (365) | MOPS buffer, pH | (165) |
| cAMP | 0.1 (350) | 7.2 | (403) | |||
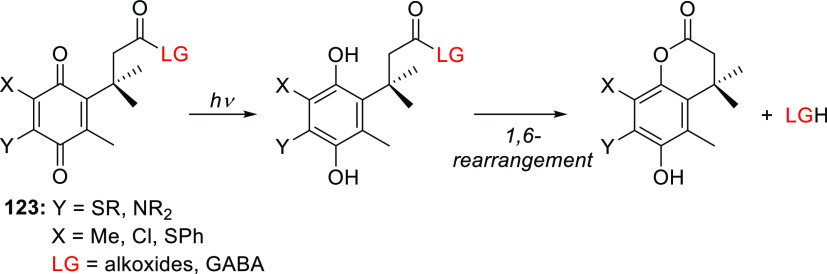
| 40b | 370 | 1.6 × 104 | acetic acid | 0.01 (365) | MOPS buffer, pH 7.2 | (165) |
| 40c | 320 | 0.6 × 104 | cAMP | 0.074 (350) | MOPS buffer, pH 7.2 | (403) |
| 40d | 329–330 | 0.5–1.0 × 104 | 2′-deoxycytidines | 0.24–0.30 (350) | MOPS buffer, pH 7.2 | (404) |
Only selected LGs are shown.
HEPES = 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; MOPS = 3-(N-morpholino)propanesulfonic acid; cAMP = cyclic adenosine monophosphate.
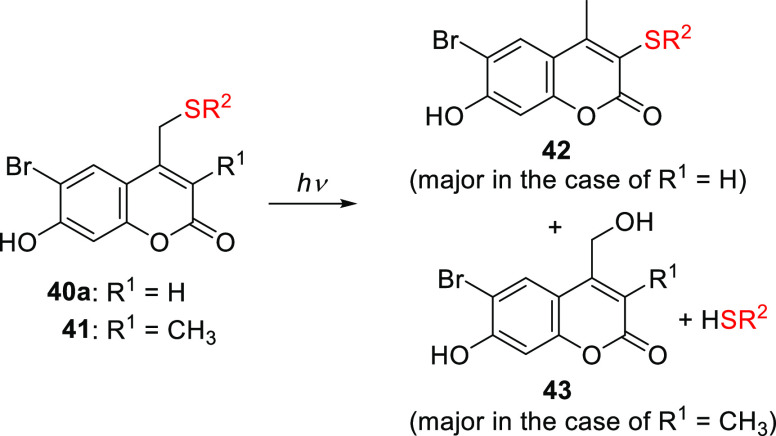
The effect of electron-withdrawing groups (EWGs) at the 6-position has mainly been explored in combination with an EDG at the 7-position. Introducing an EWG in the 6-position had only a minor effect on the absorption spectrum relative to the parent PPG (causing bathochromic shifts of ∼5–15 nm) and often led to reduced photouncaging quantum yields,165,285,303 presumably due to interference with through-bond electron transfer to the C2 carbonyl in the excited state.285 Two exceptions to these effects were observed for the (6-bromo-7-hydroxycoumarin-4-yl)methyl compound 40a by Tsien and co-workers.165 First, the 6-bromo substituent increased the acidity of the 7-OH group relative to 29a (pKa = 6.2 vs 7.9), causing 40a to be predominantly anionic at physiological pH. Consequently, 40a has an absorption maximum at 375 nm, compared to 330 nm for its protonated form and 325 nm for 29a, and is more water-soluble.165,414 These effects were also observed for the 6-chloro derivative 14b.165,415 Second, acetate was liberated from 40a ∼1.5-times more efficiently than from 29a (Φr = 0.037 vs 0.025).165 It was suggested that the heavy bromo substituent of 40a promotes ISC to the triplet excited state and that this effect outweighs its interference with through-bond electron transfer to the C2 carbonyl, resulting in increased quantum efficiency.165,285 The introduction of an electron-withdrawing chlorine atom at the 6-position led to a lower photorelease quantum efficiency in 40b, but the heavy atom effect of two additional bromo substituents at the 3- and 8-positions increased efficiency in the case of 40a (Φr = 0.065), suggesting that the triplet excited state is productive in these derivatives.165 Phosphates (e.g., cAMP, cGMP, deoxycytidines; Φr = 0.09–0.1),403,404 carboxylic acids (Φr = 0.02–0.13),165,416,417 amines (as carbamic acids; Φr = 0.04–0.16),418,419 alcohols (as carbonic acids; Φr = 0.01–0.4),321,342,420,421 diols (Φr = 0.004–0.06),422,423 and alkoxyamines424 have all been successfully released from 40a. The 2P uncaging cross section of 40a at 740 nm ranged from 0.35 to 2.0 GM depending on the caged substrate.165,404,417,419,423 Despite several reports of successful liberation of thiols from 40a-thioethers,425−428 photoisomerization of the by-product 42 occurred with higher efficiency (Scheme 8). Blocking the 3-position with a methyl group as in 41 prevented the formation of 42, facilitating the clean formation of 43 and the liberation of free thiols, albeit with lower quantum efficiencies than were achieved with 40a (Φr = 0.01 and 0.04, respectively).429,430
Scheme 8. Photochemistry of Thioethers 40a and 41(429,430).
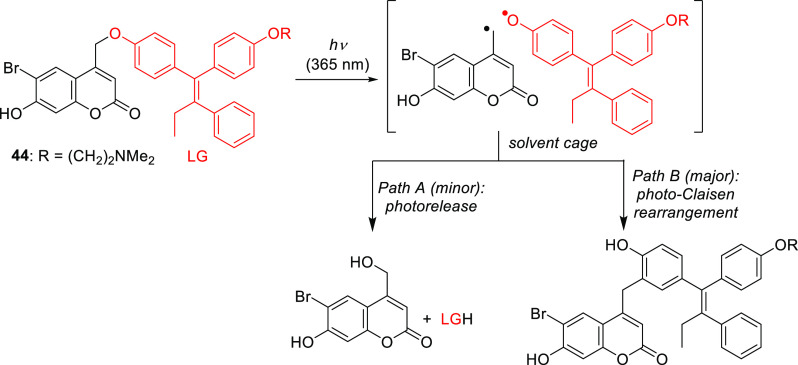
Similarly, phenols could be liberated directly from 40a, but competing recombination of the primary products was observed.345,352,431 For example, a photo-Claisen rearrangement was found to proceed ∼2.5-times more efficiently than LG photorelease from 44 (Scheme 9).345
Scheme 9. Photochemistry of 44(345).
The favorable spectroscopic and photochemical properties of 40a were found to be useful not only for the photorelease of bioactive small molecules421,427,432−438 but also in the development of photoresponsive polymers,426,439−446 dendrimers,447 and supramolecular materials.448−451 A genetically encodable lysine caged by 40a was also reported.325 Compound 40c (LG = acetate) was introduced as a more cell-permeable version of 40a, which can be trapped inside cells after hydrolysis of the ester bond.403 The 6-bromo-7-alkoxy derivatives of 40d had spectroscopic properties comparable to those of the protonated form of 40a, and were shown to release various LGs with Φr = 0.01–0.3 at λirr = 350 nm.342,347,404,421,452,453
Singh and co-workers developed coumarin 45 as a photoresponsive, dual-channel sensor for hypoxia and nitric oxide (NO; see also section 4.2) with λmaxabs = 410 nm and very weak fluorescence (ΦF = 0.01; Scheme 10).454 Reduction of the 6-NO2 group to an NH2 group (46) led to a hypsochromic-shift in the absorption maximum (λmax = 387 nm) and intense fluorescence emission centered at 535 nm (ΦF = 0.55). Further reaction of the diamino moiety in 46 with NO455−457 provided triazole 47 with λmaxabs = 355 nm and fluorescence emission at 500 nm. The liberation of chlorambucil from 47 took place with Φr = 0.04 (λirr ≥410 nm) and a chemical yield of 90%. Hypoxia-dependent detection of NO based on changes in fluorescence and subsequent light-mediated release of chlorambucil was demonstrated in cultured HeLa cells.454
Scheme 10. Photochemistry of the Hypoxia and NO Dual-Channel Sensor 45(454).
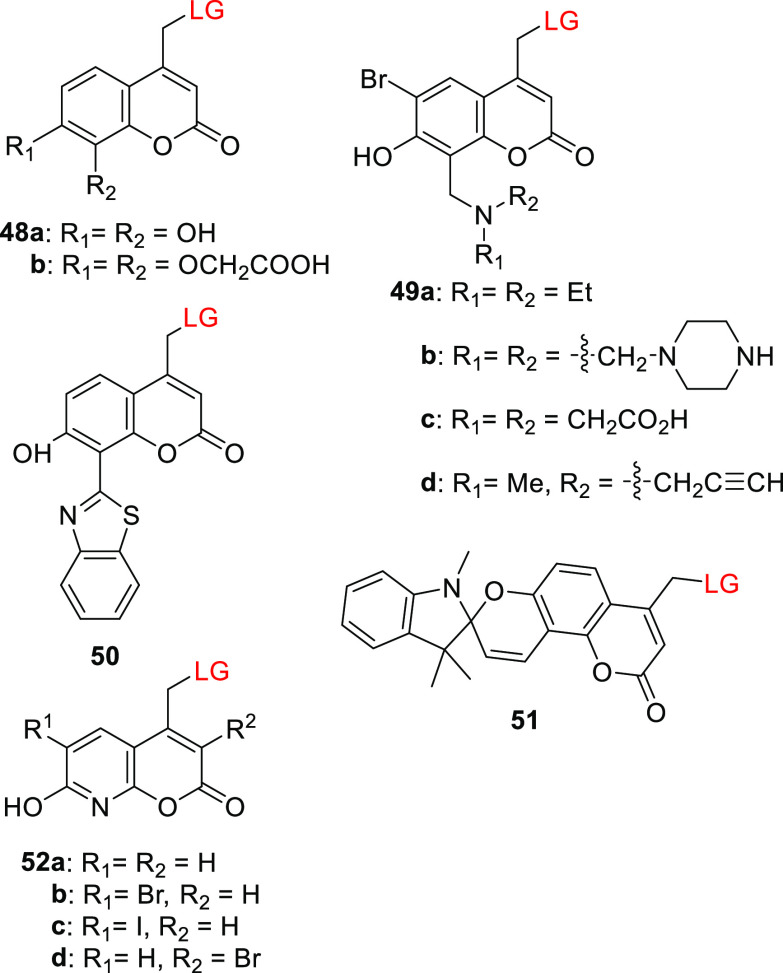
EWGs or EDGs at the 8-position do not significantly affect the absorption spectra of coumarins;406,458 thus, substitution at this position was used to tune the non-photochemical properties of coumarin-based PPGs. For example, the 7,8-dihydroxy derivative 48a and the bis(carboxymethoxy)-substituted coumarin 48b exhibited similar spectroscopic properties (Table 9) to their analogs 29a and 29c (Table 7).367,459 The catechol motif in 48a enabled its attachment to TiO2 nanoparticles; photorelease of chlorambucil (Cbl) from 48a (LG = Cbl) bound to such nanoparticles (λirr >410 nm) was accompanied by 1O2 generation by excited TiO2 (ΦΔ = 0.29).459 The bis(carboxymethoxy) moiety of 48b conferred increased water solubility (up to 2.7 mM in acetonitrile/HEPES buffer 5:95, pH 7.2).367 The dialkylaminomethyl C8 substituents of 49a–d (Table 9) significantly reduced the acidity of the 7-OH group in 49c (pKa = 4.9)460 and 49d (pKa = 3.8)461 relative to the parent 40a (pKa = 6.2), presumably because the aminomethyl group forms an intramolecular hydrogen bond with the phenolic hydroxyl group,462 leading to greater photouncaging efficiency at the lower end of the physiological pH range. The liberation of carboxylic acids, diols, amines (as carbamic acids), and phenols (as carbonic acids) from 49a–d occurred with quantum efficiencies similar to or slightly exceeding that for 40a upon both 1P (Φr = 0.06–0.014, λirr = 360 nm) and 2P excitation (δunc = 0.5–1.4 GM, 755 nm).418,460,461,463 The 8-bis(carboxymethyl)aminomethyl moiety of 49c increased water solubility418,460 (to >2 mM in acetonitrile/HEPES buffer 5:95, pH 7.2), and the appended alkyne of 49d enabled further conjugation of the PPG via copper-mediated click chemistry.461,463 Singh and co-workers developed the π-extended coumarin derivatives 50 and 51 (Table 9),464,465 which exhibited broad-range absorption extending to 400 or 550 nm, respectively. The 2-(2′-hydroxyphenyl)benzothiazole466 (HBT) moiety in 50 facilitated pH-dependent excited-state intramolecular proton transfer467 (ESIPT); at pH < 7.4, the 7-OH group enabled an ESIPT process resulting in emission at 528 nm, but at higher pH values, the hydroxy group was ionized and ESIPT was prevented, resulting in blue-shifted emission with λmaxem = 480 nm.464 The acidochromic spiropyran moiety468 in 51 allows this PPG to undergo a reversible pH-dependent transformation between two species with distinguishable absorption spectra (Scheme 11; see also section 8).465 The closed form of the spiropyran (51SP) has a Cspiro–O bond and has an absorption spectrum typical of 7-OR coumarin derivatives (λmax ≈ 325 nm). Under acidic conditions (pH < 5.4), the Cspiro–O bond was cleaved to form the zwitterionic merocyanine isomer (51MC), which has a more intense and red-shifted absorption spectrum extending up to 550 nm. The 51MC form can thus be selectively photolyzed at λirr >410 nm. Chlorambucil liberation was observed upon irradiation of 50 and 51 (LG = Cbl) at 365 and 410 nm, respectively.464,465
Table 9. Coumarin PPGs Substituted at the 8-Position.
| PPG | λmaxabs (nm) | εmax (M–1 cm–1) | leaving groupsa | Φr (λirr/nm) | solventb | ref |
|---|---|---|---|---|---|---|
| 48a | ∼325 | not reported | chlorambucil | 0.034 (410) | ethanol | (459) |
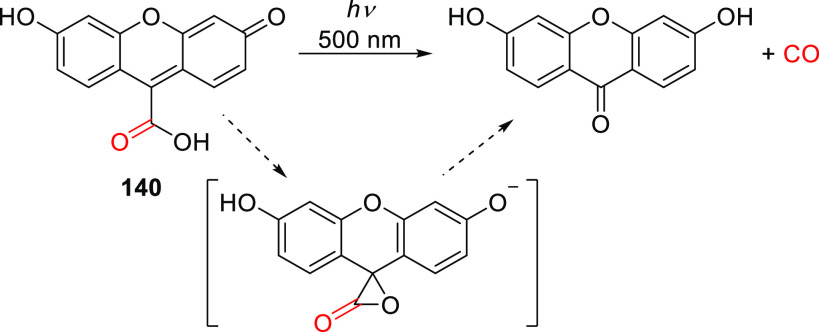
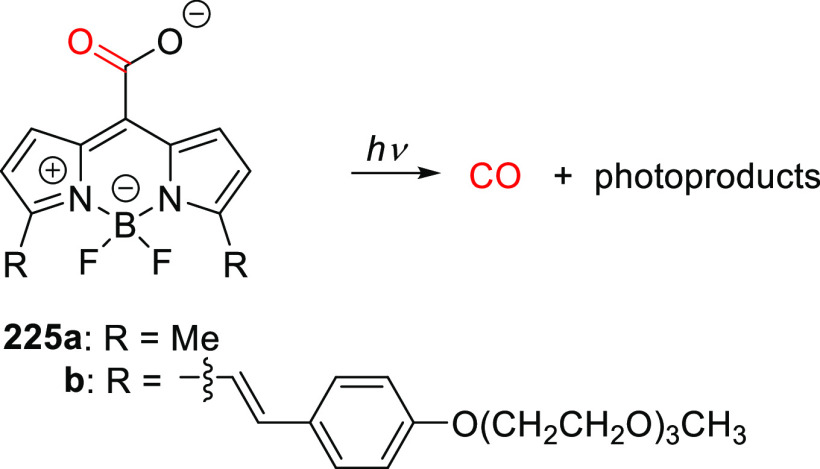
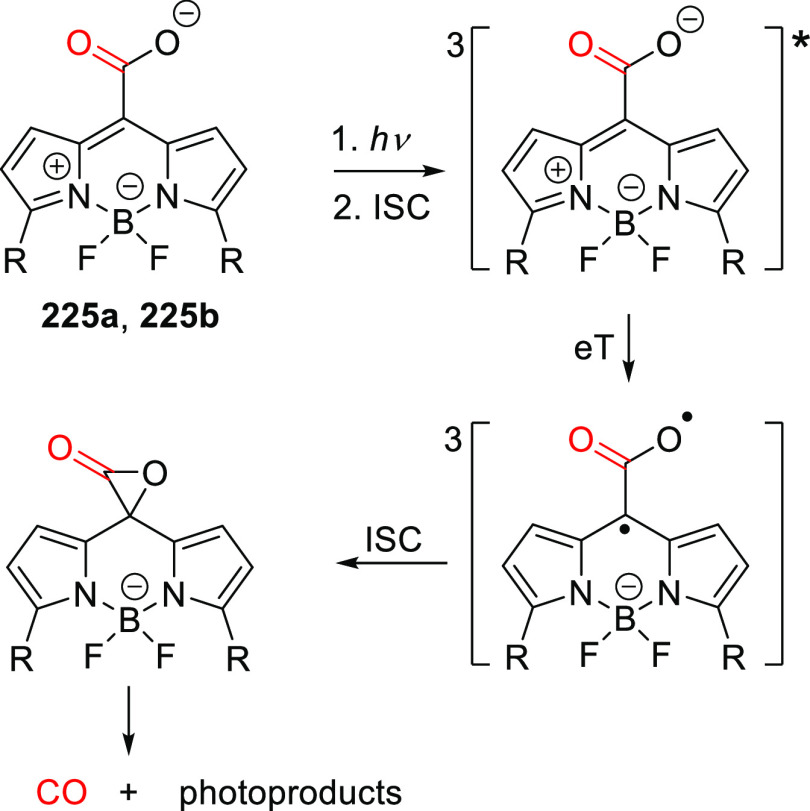
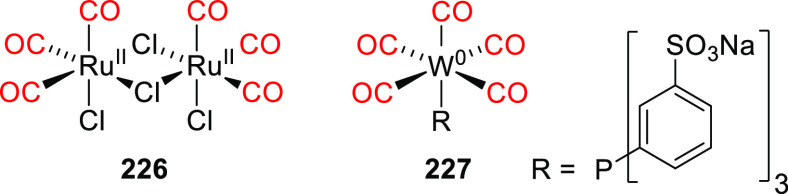
| 48b | 324 | 1.1 × 104 | Fmoc-cysteine | 0.06 (350) | CH3CN/HEPES buffer pH 7.2, 1:20 | (367) |
| 49a–c | 371–376 | 1.2–1.8 × 104 | benzoic acid, dopamine and octopamine (as carbamic acids), capsaicin (as a carbonic acid), benzaldehyde (as a diol) | 0.06–0.16 (360 or 365) | CH3CN/PBS buffer pH 7.2, 1:20 or CH3CN/HEPES buffer pH 7.2, 1:20 | (418, 460) |
| 49d | 359 | 0.9 × 104 | arachidonic acid, paclitaxels | 0.06–0.14 | MOPS buffer, pH 7.2 | (461) |
| 50 | 330 | not reported | chlorambucil | 0.006 (365) | ethanol | (464) |
| 51 | ∼330 | not reported | chlorambucil | (410) | CH3CN/H2O, 7:3 | (465) |
| 52a | 356 | 2.1 × 104 | acetic acid | 0.026 (350) | MOPS buffer, pH 7.2 | (416) |
| 52b | 362 | 2.3 × 104 | acetic acid | 0.059 (350) | MOPS buffer, pH 7.2 | (282, 416, 469) |
| 0.11 (365) | PBS buffer pH 7.4 | (131) | ||||
| 52c | 365 | 2.3 × 104 | acetic acid | 0.52 (365) | MOPS buffer, pH 7.2 | (282) |
| 52d | 378 | 2.7 × 104 | acetic acid, glutamate (as ester or as carbamic acid) | 0.17–0.43 | PBS buffer pH 7.4 | (282, 469) |
Only selected LGs are shown.
HEPES = 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; MOPS = 3-(N-morpholino)propanesulfonic acid; PBS = phosphate buffer saline.
Scheme 11. Photochemistry of Spiropyran-Coumarin 25(465).
Tamamura and co-workers developed 8-azacoumarin derivatives 52a–c, whose absorption maxima are bathochromically shifted by ∼30 nm relative to 29a (Table 9). These compounds have rather acidic phenolic OH groups (with pKas of 4.22–5.67) and high water solubility (5–10 mM in PBS buffer).282,416,469 The observed trend in the efficiency of acetic acid photorelease from 52a–c (c > b > a) was attributed to the heavy atom effect of the 6-substituents on the ISC rate.282,416,469 The bromine atom at the 3-position of 52d induced an additional bathochromic shift, approximately doubled the photouncaging efficiency, and increased the pKa of the phenolic OH group to 5.1.282,416
Extending the π-system at the 3-position is a well-establishedand useful way to bathochromically shift the absorption and emission maxima of coumarin fluorophores.474−479 Jullien and co-workers synthesized 3-cyano coumarins 53a and 53b, which exhibited bathochromic shifts in λmaxabs of 37 and 58 nm, respectively, relative to the parent coumarins 29b and 30c (Table 10).291 The photorelease of benzoic acid from 53b was ∼5-times less efficient than from 30c. A 3-iodo derivative of 30c had a similar λmax to 53b (441 nm) but released pyridine derivatives more efficiently than 30c (Φrε405 = 202.0 vs 0.3 M–1 cm–1, respectively), presumably due to less efficient PeT from the pyridine to the coumarin.480 Ellis-Davies and co-workers introduced the water-soluble 3-[3-(methylamino)-3-oxoprop-1-en-1-yl] coumarin derivative 54a (DEAC450; Figure 3), which strongly absorbs blue light.292 The release of carboxylic acids (e.g., glutamate, GABA), cyclic adenosine monophosphate (cAMP), and cyclic guanosine monophosphate (cGMP) from 54a proceeded quantitatively and efficiently upon either 1P (λirr = 473 nm, Φr = 0.18–0.78) or 2P (δunc = 0.5 GM, 900 nm) excitation, and a solvent-captured species was identified as the sole photoproduct (Scheme 12).140,292,481,482
Table 10. Coumarin PPGs Substituted at the 3-Position.
| PPG | λmaxabs (nm) | εmax (M–1 cm–1) | leaving groupsa | Φr (λirr/nm) | solventb | ref |
|---|---|---|---|---|---|---|
| 53a | 360 | 2.5 × 104 | benzoic acid | not reported | CH3CN/Tris buffer (1:1), pH 7.2 | (291) |
| 53b | 443 | 2.6 × 104 | benzoic acid | 0.04 | CH3CN/Tris buffer (1:1), pH 7.2 | (291) |
| 54a | 450 | 4.3 × 104 | glutamate | 0.39 (473) | phosphate buffer, pH 7.4 | (292) |
| 55a–f | 457–472 | 3.3–4.7 × 104 | Fmoc-Gly-OH | 0.09-0.45 (455) | DMSO | (470) |
| 55g–i | 482–538 | 3.0–4.0 × 104 | 3,5-dimethyl benzoic acid | 0.001–0.01 (544) | H2O | (295) |
| 55j | 470 | 3.5 × 104 | Boc-Phe-OH | 0.0044 (463) | CH3CN/HEPES buffer (2:1), pH 7.0 | (471) |
| 56a | 345 | 2.3 × 104 | benzoic acid | 0.09 (360) | DMSO | (472) |
| 56b | 369 | 1.7 × 104 | benzoic acid | 0.03 (360) | DMSO | (472) |
| 57a | 407 | not reported | glutamate | 0.05 (410) | HEPES buffer, pH 7.4 | (140) |
| 57b | 407 | 2.4 × 104 | benzoic acid | 0.16 (400) | DMSO | (473) |
| 58a–e | 430–456 | 3.0–4.4 × 104 | 4-methoxy benzoic acid | 0.04–0.45 (430–456) | CH3OH/H2O (9:1) | (294) |
| 59 | 467 | 3.5 × 104 | 4-methoxy benzoic acid | 0.41 (467) | CH3OH/H2O (9:1) | (294) |
Only selected LGs are shown.
Tris = tris(hydroxymethyl)aminomethane; DMSO = dimethyl sulfoxide; HEPES = 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid.
Scheme 12. Photochemistry of DEAC450 PPG 54a(292).
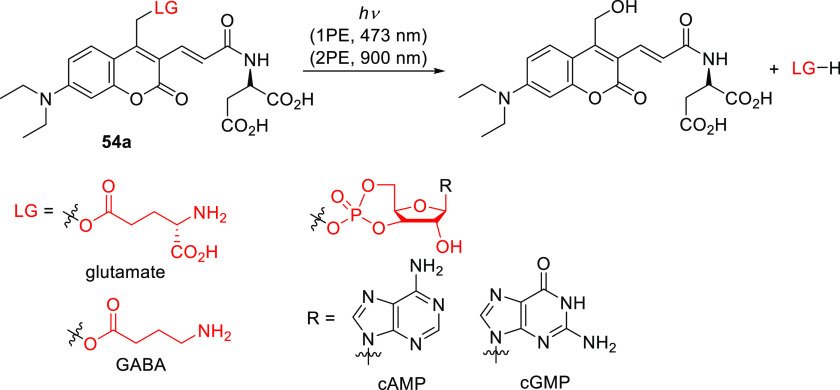
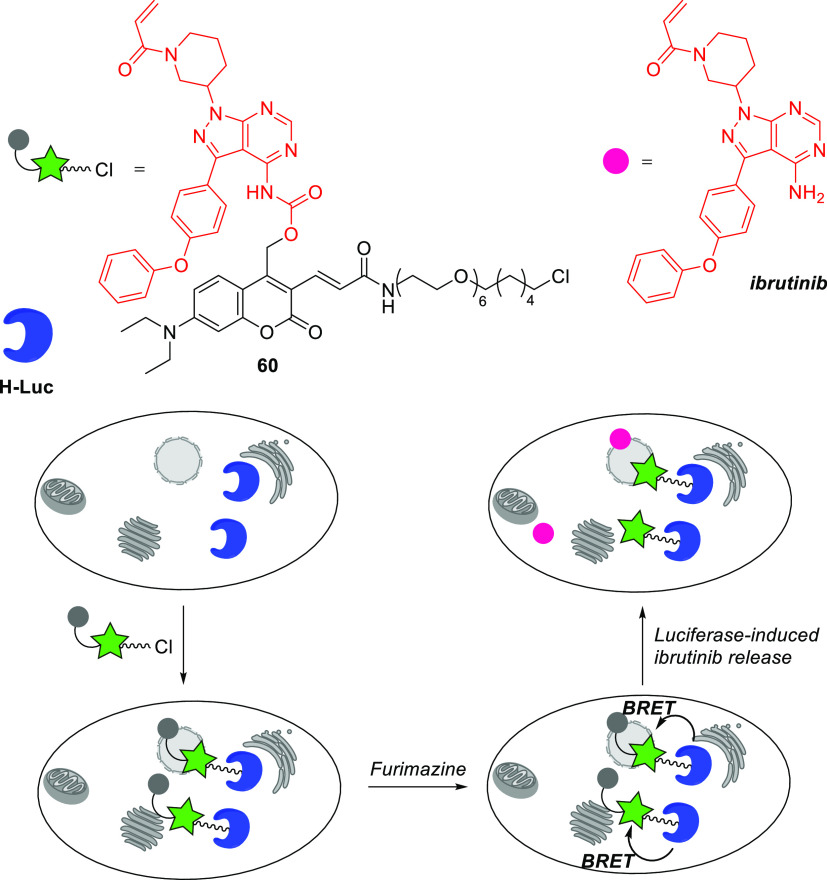
Coumarin 54a absorbs weakly in the UV region, especially in the range of 340–360 nm,140,292 which enabled its selective and orthogonal 1P (473 and. 355 nm) and 2P (900 and 720 nm) excitation in the presence of shorter-wavelength activatable PPGs such as 4-carboxymethoxy-7-nitroindolinyl (CDNI) or dicarboxylate 2-(p-phenyl-o-nitrophenyl)propyl (dcPNPP).140,481,483 Derivatives in which the 3-acrylamide moiety is conjugated with O-(aminoethyl)-2-azidoethyl-pentaethylene glycol (54b) or a PEG dendron (54c) were developed to increase the water solubility of caged GABA481,483,484 and reduce antagonism towards GABA-A receptors.481,485 Winssinger and co-workers reported that bioluminescence resonance energy transfer (BRET) from the Nanoluc-Halotag486 fusion protein (H-Luc) to coumarin 60 was sufficient to induce uncaging of the kinase inhibitor ibrutinib (Scheme 13).487 Accordingly, treatment with 60 caused furimazine-dependent covalent inhibition of the ErbB2 protein kinase in NanoLuc-HaloTag-expressing SKBR3 cells.487
Scheme 13. BRET-Induced Photouncaging of Ibrutinib in Live Cells487.
Blanchard-Desce, Kele, and co-workers independently developed a series of 3-π-extended 7-(diethylamino)coumarinylmethyl derivatives bearing electron-withdrawing end-groups (55a–i, Table 10).295,470 Coumarins 55a–f had absorption maxima at 457–472 nm, and the charged pyridinium and benzothiazolium derivatives 55g–i exhibited even more pronounced bathochromic shifts in λmaxabs.295,470 The 66 nm difference in λmax between 55c and 55i (472 and 538 nm, respectively; Figure 3) can be attributed to the effect of the N-alkyl moiety on the benzothiazolyl end-group. The release of carboxylic acids from 55a–c and 55e–f proceeded with Φr = 0.09–0.45, but 55d was photolyzed with Φr < 0.001. The authors explained this discrepancy by noting that the benzothiadiazolyl end-group of 55d is the strongest EWG in this series and suggesting that its strong electron-withdrawing effect gives rise to a strongly polarized excited state with pronounced photoinduced ICT that hinders photorelease.470,488 Accordingly, carboxylic acid liberation was less efficient from 55g–i, which have strong cationic EWGs.295 The 2PE cross section for these compounds was larger in the 700–750 nm region (δ = 175–1304 GM) than in the 940–970 nm region (δ = 59–371 GM).295,470 Coumarin 55f had the highest photoreaction efficiency for both 1PE (Φr = 0.45) and 2PE (δunc = 442 and 64 GM, at 730 and 940 nm, respectively) in this series.470 Visible-light activated liberation of carboxylic acid LGs from coumarin 55j was demonstrated to occur only after bioorthogonal transformation of the modulating tetrazine moiety.471
The introduction of 3-phenyl groups bearing either a strong EWG (56a,b and 57a) or a strong EDG (57b) in the para position to create D−π–A or D−π–D systems, respectively, resulted in a ∼25–30 nm bathochromic shift of λmaxabs relative to the parent coumarin (Table 10).140,472,473 Photolysis of 56a,b and 57a,b led to quantitative carboxylic acid release; D−π–D derivative 57b had the highest quantum yield in this series.140,472,473 The 2PE uncaging cross section was determined to be 3.4 GM (710 nm) for 56a and 2.1 GM (740 nm) for 56b;472 that for 57b was estimated to be 16 GM (680 nm).473 Aldehyde 61 was the major photoproduct (70%) formed upon irradiation of 57b in anhydrous DMSO; the expected alcohol 62 was obtained only in the presence of water.473 A proposed mechanism is shown in Scheme 14.
Scheme 14. Proposed Mechanism for the Uncaging Reaction of 57b(473).
Zhu and co-workers observed similar photochemical behavior in the D−π–A and D−π–D systems 58a–e and 59, which were formed by extending the π-systems of 3-styryl coumarins at the 3-position (Table 10).294 Coumarin 58a exhibited a 48 nm bathochromic shift in λmaxabs relative to 30c, and it was photolyzed to release p-methoxybenzoic acid with a similar quantum yield (Φr = 0.05 and 0.04, respectively) upon irradiation at the corresponding absorption maxima.294 The introduction of either an EDG or an EWG at the 3-styryl para position (58b–e) caused a further bathochromic shift. The uncaging quantum yields for D−π–D derivatives 58c–e were 5–10-times higher than that for D−π–A derivative 58b (Φr = 0.19–0.45 vs 0.04, respectively).294 The ∼40 nm difference between the absorption maxima of 58d and 58b is probably related to the presence of the π-bridge. Bis(julolidine) derivative 59, which bears the strongest electron donors in this series,393 also had the most bathochromically shifted λmax (Figure 3) and was photolyzed with the highest efficiency (Φrε467 = 14.3 M–1 cm–1).294 The 2PE uncaging cross sections determined for D−π–D systems 58c–e and 59 were ∼5–10-times larger than that for D−π–A system 58b (δunc = 17.7–39.6 vs 3.2 GM at 730 nm, respectively).
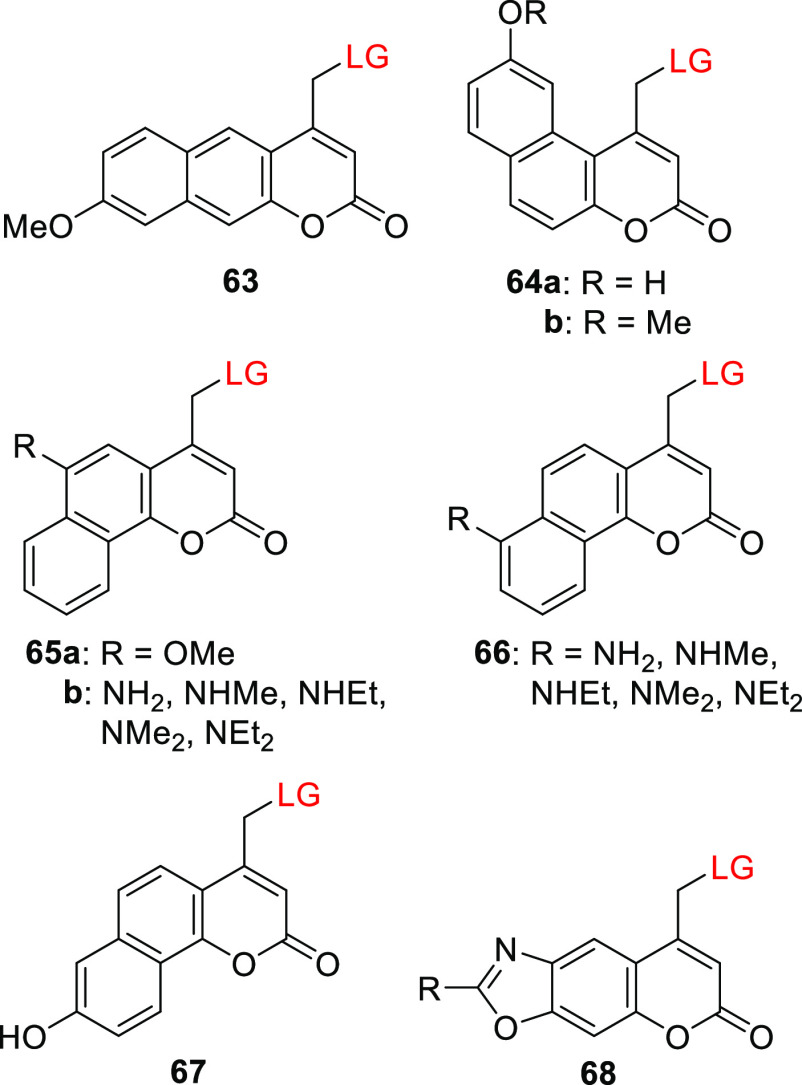
Benzocoumarin derivatives typically have similar or slightly hypsochromically shifted absorption maxima relative to 4-methylcoumarin406,489,490 (λmaxabs = 274–321 nm vs 310 nm), but their spectroscopic properties can be modified by introducing EDGs or EWGs to modulate their ICT states.490 Gonçalves, Costa, and co-workers reported on several benzocoumarin PPGs (63–68); their structures and photophysical and photochemical properties relating to the photorelease of various carboxylic acids are shown in Table 11.320,491−500
Table 11. Spectroscopic and Photochemical Properties of Benzocoumarin-Derived PPGs.
| PPG | λmaxabs (nm) | εmax (M–1 cm–1) | leaving groupsa | Φr (λirr/nm) | solventb | ref |
|---|---|---|---|---|---|---|
| 63 | 345 | 0.8 × 104 | GABA–OH | 0.7 × 10–5 (350) | ethanol | (320) |
| 64a | 360 | 1 × 104 | Phe-OH | ethanol | (491−493) | |
| 64b | 344–348 | 0.6–1.8 × 104 | various amino acids | 0.1–6.2 × 10–5 (350) | ethanol or CH3OH/HEPES pH 7.2, 4:1 | (320, 491−493) |
| 65a | 371−376 | 4.1 × 103 | GABA, various amino acids, 5-aminolevulinic acid | 2–24 × 10–5 (350) | CH3OH/HEPES pH 7.2, 4:1 | (494, 496, 497) |
| 0.4–16 × 10–5 (419) | (494, 496, 497) | |||||
| 65b | 377–418 | 0.7–7.7 × 103 | butyric acid, 5-aminolevulinic acid | 0.8–25.0 × 10–5 (350) | CH3OH/HEPES pH 7.2, 4:1 | (495, 497, 498) |
| 0.5–31.0 × 10–5 (419) | (495, 497, 498) | |||||
| 66 | 377–398 | 0.7–7.7 × 103 | butyric acid | 0.7–13.0 × 10–5 (350) | CH3OH/HEPES pH 7.2, 4:1 | (497, 498) |
| 0.6–7.0 × 10–5 (419) | (497, 498) | |||||
| 67 | 362 | 1.1 × 104 | glutamate | 0.006 (355) | CHCl3 | (499) |
| 68 | 339–361 | 0.3–1.1 × 104 | butyric acid | 8.6–12.0 × 10–5 (350) | CH3OH/HEPES pH 7.2, 4:1 | (497, 500) |
| 0.04–1.0 × 10–5 (419) | (497, 500) |
Only selected LGs are shown.
GABA–OH = 4-(benzyloxycarbonylamino)butanoic acid; Phe-OH = N-(carbobenzyloxy)-l-phenylalanine; HEPES = 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid.
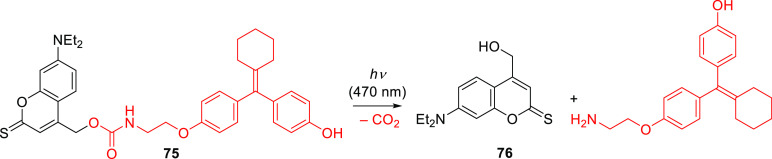
The absorption maxima of coumarins can be significantly red-shifted by increasing the electron-withdrawing capacity at the 2-position.501−504 For example, the singlet excited states of thiocarbonyls are lower in energy than those of their carbonyl analogs, so their light absorption is bathochromically shifted.505,506 This effect was also observed for coumarins.507−510 Costa, Jullien, and co-workers studied thionated coumarin 69a, which had a 73 nm bathochromic shift of λmaxabs relative to its carbonyl analog, yet it released carboxylic acids with a low quantum efficiency (Table 12).291,304,511 The absorption maximum of 7-NEt2 thiocoumarin 69b was bathochromically shifted by 87 nm relative to its carbonyl analog (Figure 3), and it photoreleased benzoic acid with Φr = 0.18, which was 2 orders of magnitude higher than the corresponding value for the carbonyl analog when both were irradiated at their absorption maxima.291 The liberation of benzoic acid from 69b was induced by irradiating Er3+- or Tm3+-based upconverting nanoparticles (see also section 6.4.2) at 974 nm.512 However, the release quantum yield of 69b was found to be concentration-dependent, decreasing ∼30-fold when its concentration in the irradiated solution was lowered from 25 to 4 μM.291 The photouncaging of a carbamate-linked cyclofen analog431,513 from 75 was demonstrated (Scheme 15).514 The solvent-captured derivative 76 was identified as the sole photoproduct of this reaction, and the cyclofen derivative was liberated in high chemical yield (90%, Φr = 5 × 10–3) at λirr = 470 nm. The chromatically orthogonal photoactivation of 75 and 13-cis-retinoic acid (using blue-cyan- and UV-light sources, respectively) was used to control the development of live zebrafish embryos.514 Additionally, the photouncaging of a Cas9 activator, trimethoprim, from 69b was used to control the activity of a CRISPR-Cas9 system in cell cultures.515
Table 12. Spectroscopic and Photochemical Properties of Thionated Coumarin PPGs.
| PPG | λmaxabs (nm) | εmax (M–1 cm–1) | leaving groupsa | Φr (λirr/nm) | solventb | ref |
|---|---|---|---|---|---|---|
| 69a | 395–398 | 1.4–1.7 × 104 | benzoic acid, Z-Phe-OH | 2–7 × 10–5 (365, 419) | ethanol or CH3CN/Tris buffer pH 7.5, 1:1 | (291, 304, 511) |
| 69b | 472 | 3.1 × 104 | benzoic acid | 0.12 (365), 0.18 (487) | CH3CN/Tris buffer pH 7.5, 1:1 | (291) |
| 70a–c | 400–431 | 0.7–2.7 × 104 | butyric acid, various amino acids | 1.0–13.0 × 10–5 (419) | CH3OH/HEPES pH 7.2, 4:1 | (335, 516, 517) |
| 71 | 427 | 1.8 × 104 | benzoic acid | CH3CN/Tris buffer pH 7.5, 1:1 | (291) | |
| 72 | 490 | 3.0 × 104 | 4-methoxybenzoic acid | 0.4 (490) | CH3OH/H2O 9:1 | (294) |
| 73 | 515 | 2.5 × 104 | 4-methoxybenzoic acid | 0.7 (515) | CH3OH/H2O 9:1 | (294) |
| 74 | 479 | 1.0 × 104 | acetic acid | 0.071 (475) | H2O | (284) |
Only selected LGs are shown.
Tris = tris(hydroxymethyl)aminomethane; HEPES = 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; Z-Phe-OH = N-(carbobenzyloxy)-l-phenylalanine.
Scheme 15. Photochemistry of Thiocoumarin 75(514).
Gonçalves and co-workers studied several thionated benzo[f]coumarins (70a–c) that exhibited bathochromic shifts of ∼65 nm relative to their carbonyl analogs and released carboxylic acids, with 3–20-times higher quantum yields upon irradiation at 419 nm (Table 12).335,516,517 The thiocarbonyl motif proved to be compatible with other structural modifications that caused further bathochromic shifts in absorption. For example, the 3-CN derivative 71 had a λmaxabs of 427 nm, although it was thermally unstable in a Tris buffer/acetonitrile solution (pH = 7.5).291 Conversely, coumarin derivatives 72 and 73 bearing electron-rich p-aminostyryl moieties in the 3-position had significantly bathochromically-shifted absorption spectra (λmax = 490 and 515 nm, respectively) but were thermally stable.294 Irradiation of 72 and 73 at their λmaxabs induced photorelease of p-methoxybenzoic acid with Φr = 0.4 and 0.7, respectively. The cyclized derivative 78, rather than the expected 4-hydroxymethyl-coumarin, was identified as the main photoproduct of photolysis of 77. The mechanism proposed to explain this observation is shown in Scheme 16. Similar photoproducts are formed from 72 and 73; in all three cases, the loss of π-conjugation at the 3-position in the photoproducts causes a hypsochromic shift of λmax (to 470 nm).294
Scheme 16. Proposed Photolysis Mechanism for 3-Styryl-Conjugated Thiocoumarins294.
Howorka and co-workers reported that the absorption maximum of thiocoumarin 74, which has an electron-rich p-diethylaminostyryl moiety at the 7-position, is at ∼480 nm (Table 12).284 Irradiation of 74 in DMSO or water liberated acetic acid with Φr = 0.024 and 0.071, respectively. On the basis of transient-absorption spectroscopy and steady-state kinetic studies in the presence and absence of oxygen, the authors proposed that the photorelease of the LG in DMSO proceeds through the triplet excited state, while a charge-separated state is more populated in water.284
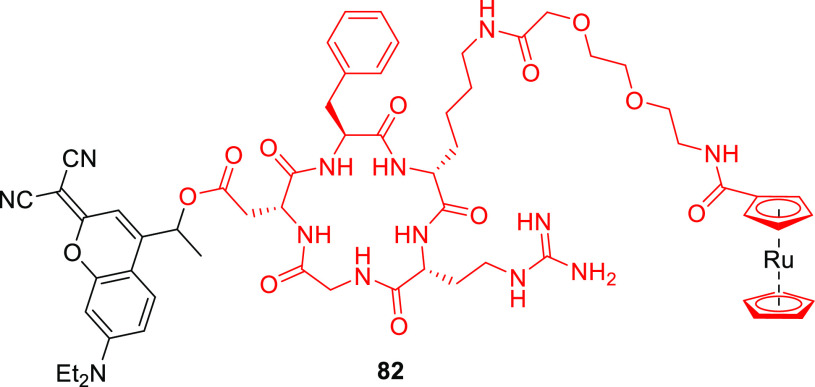
Imino and hydroxyimino derivatives 79a and 79b (Figure 4) exhibited only minimal shifts in λmaxabs relative to the carbonyl analog 29b.291 Compound 79a was thermally unstable in a tris buffer/acetonitrile solution (pH 7.5), while 79b was photochemically inactive; it did not release acetic acid as a LG upon irradiation at 365 nm.291 Conversely, 7-(N,N-diethylamino)-dicyano derivative 80a (λmax = 487 nm, ε487 = 3.3 × 104 M–1 cm–1)291 released carboxylic acids in high chemical yield (>90%) upon irradiation at 487 or 505 nm with Φr = 0.3–1.5 × 10–3.291,518,519 However, the liberation of an amine (as a carbamic acid) from 80a proceeded ∼3-times less efficiently than that of a comparable carboxylic acid.519 Marchán and co-workers reported that the release quantum yields of carboxylic acids and amines from dicyano derivative 80b (R = Me) were up to 2.5-fold higher than those for release from 80a.518,519 Additionally, 80b was more stable than its thiocarbonyl analog in the presence of acids and bases commonly used in Fmoc/t-Bu solid-phase peptide synthesis. It was therefore used to cage a cyclic RGD peptide-drug conjugate (82, Figure 5).518 Photouncaging of the peptide-drug conjugate from 82 (λirr = 505 nm) proceeded with Φr = 7.2 × 10–3.
Figure 4.
Structures of coumarin PPGs substituted at the 2-position. LG = alkoxide, carboxylate, or amine (as a carbamate).
Figure 5.
Structure of the 80b-caged c(RGDfK)-ruthenocene conjugate 82.
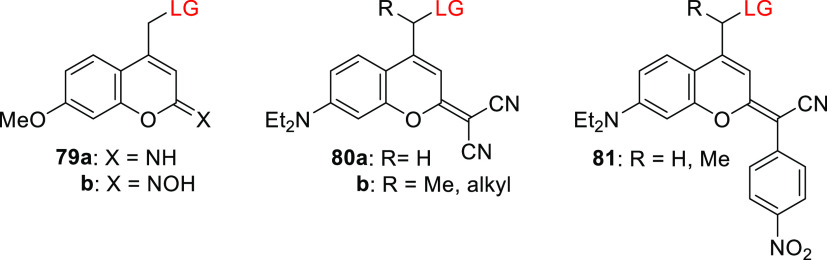
An analog of 80b (R = alkyl) was used to prepare caged morpholino oligonucleotides212,520−523 (cMOs) capable of perturbing targeted RNAs in vivo.356 Although the caged cMOs were successfully uncaged in live zebrafish embryos (λirr = 470 nm), their thermal stability in vivo was significantly lower than that of their carbonyl analogs.356 Increasing the system’s electron-withdrawing capacity at the 2-position by replacing one cyano group with a p-nitrophenyl moiety (81) caused an additional ∼15 nm bathochromic shift of the absorption maximum (λmaxabs ≈ 502 nm) but also significantly reduced the photouncaging quantum yield (Φr = 0.5–2.3 × 10–6).519
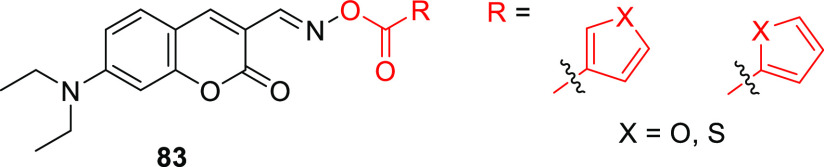
A different way of utilizing the coumarin scaffold for photorelease was demonstrated by introducing a photoreactive oxime ester524 in the 3-position (83, Figure 6).525 The excitation of oxime esters typically results in homolytic scission of the N–O bond and the formation of a caged radical pair.526−528 Photoexcitation of 83 (λmaxabs = 436 nm, ε436 = 3.9–4.2 × 104 M–1 cm–1) at 450 nm led to the formation of heterocyclic radicals, and the system was used as a photoinitiator for radical polymerization of acrylate monomers.525
Figure 6.
Structure of coumarin-oxime-ester PPG 83.
2.3. Arylmethyl and Arylcarbonylmethyl Groups
Polyaromatic cores provide convenient platforms for developing π-extended arylmethyl and arylcarbonylmethyl PPGs. For example, the (anthracen-9-yl)methyl group was introduced as a polyaromatic benzyl-type PPG for carboxylates, alcohols, and hydroxylamines with a bathochromically shifted absorption band (λmaxabs ≈ 385 nm)529 relative to those of benzyl1 (λmax ≈ 254 nm) and 2-naphthylmethyl317 (λmaxabs ≈ 280 nm) chromophores.323,529−531 Lam and co-workers prepared an (anthracen-9-yl)methyl that absorbs above 400 nm by extending its π-conjugation through the 10-position (84a–f, Figure 7).532
Figure 7.
π-Extended (anthracen-9-yl)methyl PPGs.
The absorption spectra of compounds 84b–d (λmaxabs ≈ 405 nm) are bathochromically shifted relative to that of 84a (λmax = 376 nm). Although these compounds have different substituents at the para position of the phenyl moiety, their spectra are similar. This was attributed to steric hindrance, which may cause the phenyl ring to be oriented orthogonally to the anthracenyl core.533 The presence of an acetylene bridge in 84e and 84f eliminates this steric hindrance;534 accordingly, the absorption maxima of these compounds are bathochromically shifted by ∼30–40 nm (λmaxabs = 425–440 nm). The photorelease of the diphenylphosphinothioester LG from 84b–f in a THF/water mixture (3:1, λirr >420 nm) was demonstrated, and 84f showed the highest release efficiency within this series. The photoinduced heterolytic cleavage of the (anthracen-9-yl)methyl–phosphorus bond in 84f at λirr = 366 and 416 nm occurred with Φr = 0.08 and 0.025, respectively; these values are comparable to those for previously reported 4,5-dimethoxy-2-nitrobenzyl535 (DMNB) and (anthracen-9-yl)methyl536 caged phosphines (λirr = 360–400 nm). A phototriggered (λirr > 420 nm) traceless Staudinger ligation537 of caged oligopeptides (85) with azide-containing amino acids was shown to form the expected oligopeptides in chemical yields of 31–43% (Scheme 17).
Scheme 17. Visible-Light Triggered Traceless Staudinger Ligation532.
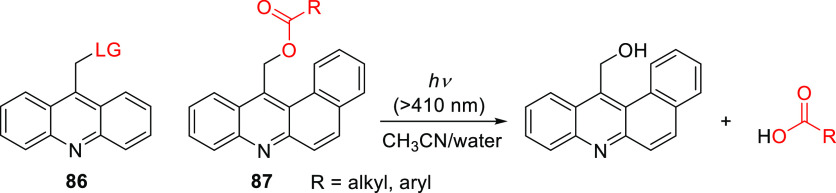
The (acridin-9-yl)methyl group (86) was introduced by Zhang and co-workers as a UV-activatable (λmaxabs ≈ 355 nm, ε360 ≈ 1 × 104 M–1 cm–1) PPG for alcohols538 and was later used with carboxylic acids.335,517,539,540 Its tail absorption in the visible range enabled photolysis at λirr = 419 nm, albeit with low quantum efficiency (Φr = 0.5–1.6 × 10–4).335,540 The photoreaction was proposed to proceed through an ion-pair intermediate.539 Singh and co-workers introduced the π-extended [benzo(a)acridin-12-yl]methyl derivative 87, which exhibits a bathochromic shift of ∼20 nm (λmax ≈ 374 nm, εmax ≈ 5 × 104 M–1 cm–1) and extended absorption up to ∼425 nm (Scheme 18).541 Photorelease (λirr ≥ 410 nm) of carboxylic acids from 87 proceeded in excellent chemical yields (80–92%) and with significantly higher quantum efficiencies (Φr = 0.08–0.13) than for 86; solvent-captured (benzo(a)acridin-12-yl)methylalcohol was identified as the sole side-photoproduct (Scheme 18).541 A (benzo(a)acridin-12-yl)methyl-caged chlorambucil derivative was also shown to accumulate in the nuclei of cultured HeLa cells, presumably due to the acridine scaffold’s capacity to intercalate with DNA,542−544 and exhibited light-dependent cytotoxicity.541
Scheme 18. (Acridin-9-yl)methyl PPGs 86 and 87(541).
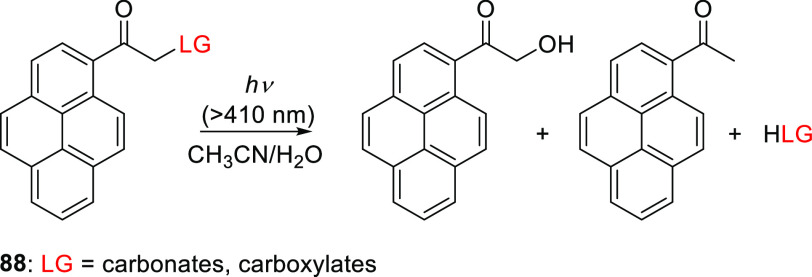
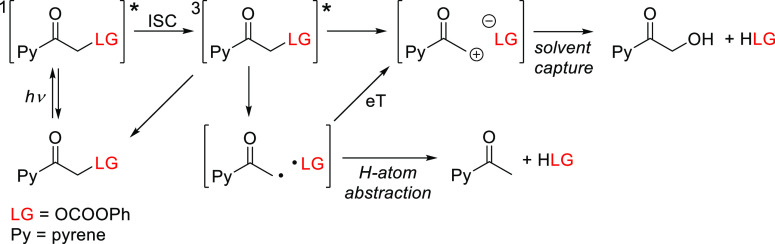
Singh and co-workers also introduced 1-(hydroxyacetyl)pyrene54588 as a variant of the well-established (pyren-1-yl)methyl PPG (Scheme 19).322,546−549 In contrast to (pyren-1-yl)methyl, which absorbs only in the UV region (with a solvent-dependent λmaxabs = 320–340 nm), the addition of a hydroxyacetyl group in 88 caused a bathochromic shift of λmax (∼355 nm), resulting in sufficient absorption above 400 nm (ε410 = 2.7–3.9 × 103 M–1 cm–1) to enable visible light-induced photolysis.545 The photorelease (λirr ≥ 410 nm) of carboxylic acids545,550 and alcohols551 (as carbonates) in a 1:1 acetonitrile/H2O solution proceeded with near-quantitative chemical yields (>94%) and high quantum efficiencies (Φr = 0.30–0.41 and 0.17–0.20, respectively; Scheme 19). These results can be compared to those achieved with a (pyren-1-yl)methyl group, which released carboxylic acids and alcohols with (solvent dependent) Φr = 0.0029–0.139 upon excitation at 350 nm.322,546−548 The inherent fluorescence of 1-acetylpyrene552 (λmaxabs = 439 nm, ΦF = 0.02) enabled imaging of 88 in fixed cells.551 The efficiency of photorelease from 88 depended strongly on the water content of the reaction mixtures and decreased in the presence of a triplet quencher (potassium sorbate). Three photoproducts (the leaving group, 1-hydroxyacetylpyrene, and acetylpyrene) were formed upon irradiation; Scheme 20 shows a mechanism explaining these results.545,551
Scheme 19. Photochemistry of 1-(Hydroxyacetyl)pyrene PPG551.
Scheme 20. Photorelease from 1-Acetylpyrene PPG (88)551.
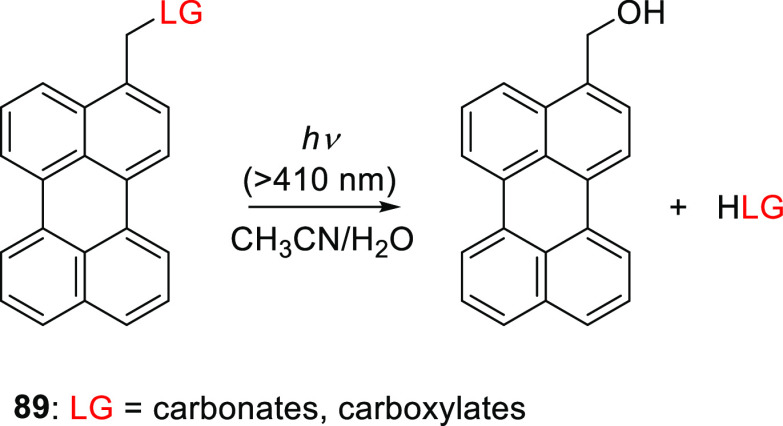
Another arylmethyl-type PPG studied by Singh and co-workers is the (perylen-3-yl)methyl group (89), which absorbs in the visible range (λmaxabs = 438 nm, εmax = 2.4–3.5 × 104) and displays characteristic fluorescence (λmax = 445 nm, ΦF = 0.9; Scheme 21).553 Carboxylic acids and alcohols (attached as carbonates) were successfully photoreleased from 89 (λirr ≥ 410 nm) in an acetonitrile/H2O (3:1) solution with high chemical yields (>89%) and moderate quantum efficiencies (Φr = 7.7–9.3 × 10–2).
Scheme 21. Photochemistry of the (Perylen-3-yl)methyl PPG553.
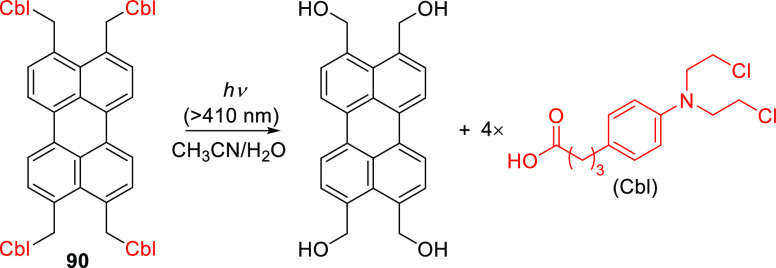
Similar to other polyaromatic arylmethyl-type PPGs,529 the photoreaction mechanism of 89 was proposed to proceed through the singlet excited state, followed by heterolysis of the C–O bond and solvent capture to afford the photoproducts.553 Heterolysis of the C–O bond was previously calculated to be energetically preferable to homolysis, especially when the carbocation is stabilized,554−556 although homolysis dominates in simple benzyl derivatives.557 Zhao and co-workers further demonstrated that carboxylic acid leaving groups (e.g., chlorambucil) can be released from perylene 90 (Scheme 22), although quantitative chemical yields were not reported.558
Scheme 22. Photorelease of Chlorambucil from a Single (Perylen-3,4,9,10-yl)tetramethyl PPG (90)558.
The inherent hydrophobicity of polyaromatic PPGs limits their applicability in aqueous media. However, their incorporation into larger molecular structures has been demonstrated. For example, 89 was used to prepare photodegradable hydrogels559,560 and polymer nanoparticles.561−563 Additionally, Singh and co-workers used a reprecipitation technique564 to formulate (perylen-3-yl)methyl-caged chloambucil565 and pesticide 2,4-D566 (91 and 92, Figure 8) as globular organic nanoparticles with an average particle size of 25–30 nm, broad absorption spectra extending into the visible range (350–550 nm), and fluorescence emission at 625 nm. These nanoparticles were photolyzed (λirr ≥ 410 nm) to release the parent compound (perylen-3-yl)methanol and the corresponding leaving group. In the absence of light, 2–5% of the starting material hydrolyzed upon incubation in water or 10% fetal bovine serum (FBS) at 35–37 °C over 2–7 days. Photorelease (λirr ≥410 nm) from nanoparticles of 91 and 92 was also demonstrated in cultured HeLa cells565 and plants566 (Cicer arietinum), respectively, and light-dependent biological effects of the corresponding bioactive leaving groups were observed. Leaving group release could be monitored in real time because it caused the fluorescence emission band to shift from 625 nm (nanoparticle) to 450 nm ((perylen-3-yl)methanol). Similarly, the antimicrobial compound salicylic acid was caged with 1-(hydroxyacetyl)pyrene via an ester linkage (93, Figure 8), and the resulting conjugate was formulated into light-responsive (λirr ≥ 410 nm) organic nanoparticles whose photoactivation was demonstrated.567
Figure 8.
Structures of (perylen-3-yl)methyl and 1-(hydroxyacetyl)pyrene derivatives.
2.4. The (Benzothiadiazol-6/7-yl)methyl Group
VanVeller and co-workers studied compounds 94 and 95 as benzyl-type PPGs with a π-expanded heteroaromatic benzothiadiazole core (Figure 9).568 Their photoreactivity was explained based on the redistribution of electron density from the EDGs in the o- and m-positions upon excitation.554,555,557 Although the meta effect was established for methoxy-substituted benzyl derivatives over 50 years ago,556 the corresponding NR2 analogs were only studied recently569−576 and achieved high photoreaction quantum yields (up to 0.45570). Derivatives 94 and 95 had broad absorption bands extending up to 500 nm with maxima at 420 nm. Acetate was photoreleased from 94a and 95 with comparable quantum yields (Φr = 0.067 and 0.061, respectively; λirr = 455 nm), and ethanol was liberated from 95 with Φr = 0.04.568 The presence of a bromo substituent in the 7-position (94b) roughly halved the quantum yield,568 presumably because of competing photochemical processes such as C–Br scission.577
Figure 9.
(Benzothiadiazol-6/7-yl)methyl PPGs.
2.5. The (N-Methyl-7-hydroxyquinolinium-2-yl)methyl Group
The photochemistry of the (7-hydroxyquinoline-2-yl)methyl group was initially explored and exploited by Dore and co-workers, who identified the (8-bromo-7-hydroxyquinoline-2-yl)methyl (BHQ)578 and (8-cyano-7-hydroxyquinoline-2-yl)methyl (CyHQ)579 groups (among others) as efficient PPGs for carboxylates, phosphates, diols, phenols, and amines.578,580−584 Derivatives of the (7-hydroxyquinoline-2-yl)methyl group can also be removed by 2P excitation, which reportedly proceeds with δunc values of up to 2.6 GM (740 nm) for carboxylate LGs.579,585 The photochemical properties of (7-hydroxyquinoline-2-yl)methyl could be adjusted by varying the substituents on the quinoline core, although the λmaxabs remained in the range of 320–385 nm.579,585,586 Singh and co-workers showed that attaching an (8-alkoxyquinoline-2-yl)methyl derivative to carbon dots enabled their photolysis with λirr ≥ 410 nm to release H2S (see also section 4.3).587 Additionally, Narumi and co-workers588,589 extended the absorption wavelengths of quinoline-derived PPGs above 400 nm by N-alkylation of the quinoline nitrogen, a modification known to shift the absorption spectrum bathochromically.590,591
The N-methyl-7-hydroxyquinolinium group 96 (N-Me-7HQm, λmaxabs = 418 nm) photoreleased acetic acid as an LG upon irradiation with blue light (λirr = 458 nm, Φr = 0.045), with concomitant formation of (N-methyl-7-hydroxyquinolinium-2-yl)methanol as the sole additional photoproduct (Scheme 23). It is not yet known whether the mechanism of this photoreaction is similar to that of other (7-hydroxyquinoline-2-yl)methyl derivatives, in which heterolysis of the C–O bond proceeds from the triplet-excited state to generate an ion pair that subsequently collapses into the free leaving group and a solvent-captured side product.578,582,592,593 Methylation of the 7-hydroxy group caused a 60 nm hypsochromic shift of λmax but terminated the photoreaction. 7-NMe2 derivatives exhibited a λmaxabs at ∼445 nm and were photolyzed with lower quantum yields (Φr = 1.2–2.8 × 10–3) than the 7-hydroxy derivative, presumably due to the contribution of the NMe2 group in the twisted intramolecular charge-transfer (TICT) excited state.395,594 Increasing the system’s electron density by introducing an ethyl group in the 4-position more than doubled the quantum yield. As a salt, 96 was highly soluble in water (up to 20 mM, depending on the leaving group) and was successfully used for the photorelease of several amino acids and neurotransmitters (caged via their amino groups as carbamates), achieving Φr values of 0.025–0.068 and Φrε(λirr) values at 458 nm in the range of 96–272 M–1 cm–1.588,589
Scheme 23. Photochemistry of N-Methyl-7-hydroxyquinolinium PPG588.
2.6. The Bimane Group
Singh and co-workers repurposed the fluorescent molecule bimane, often used in biochemistry for fluorescence labeling of proteins,595−597 as a photoremovable protecting group by introducing carboxylate leaving groups on the 3-methyl or 3,5-dimethyl substituents (97 and 98, respectively, Figure 10).598 These compounds had a λmaxabs at ∼380 nm with tail absorption above 400 nm and a fluorescence λmax between 460 and 480 nm (ΦF = 0.6–0.8). These values are similar to those for unsubstituted bimane.
Figure 10.
Bimane PPGs.
Photorelease (λirr ≥ 410 nm) of various aliphatic and aromatic carboxylic acids from bimane 97 proceeded with good chemical (75–85%) and moderate quantum yields (Φr = 0.06–0.07)598 that increased with the solvent’s water content.598 The authors proposed that the photoreaction proceeds through the singlet excited state and involves heterolytic C–O bond cleavage to generate an ion-pair that then undergoes solvent-mediated separation, leading to solvent capture of the bimane methyl cation (Scheme 24). In the case of 98, the captured product underwent a second photoreaction to release another equivalent of the LG with a quantum efficiency similar to that for the first LG liberation (Φr = 0.04).598
Scheme 24. Proposed Mechanism of Photorelease from Bimane-Derived PPGs598.
The photorelease of thiols from bimane 97 upon irradiation at 420 nm proceeded with a high chemical yield (70%) and Φr = 0.02.599 Because of the high nucleophilicity of thiols, the presence of strong electrophiles was required to prevent recombination of the resulting ion pair; in their absence, no photoproducts were detected. Similar behavior was observed for the release of thiols from 3-(hydroxymethyl)-2-naphthol derivatives.600 Photouncaging (λirr = 420 nm) of thiols from the corresponding tetra-bimane-protected dendrimeric monomer 99 in the presence of a dendritic monomer functionalized with strong electrophiles led to the formation of a photoinduced crosslinked polymeric network (Scheme 25) capable of entrapping live cells while preserving their viability.599
Scheme 25. Polymer Cross-Linking via a Light-Induced Thiol-Electrophile Reaction599.
2.7. Arylcarbonylmethyl Groups
Aromatic ketones are synthetically accessible, thermally stable, and photochemically reactive moieties that have been used extensively as PPGs.10 Both their lowest energy transition (n,π*) and the higher energy π,π* absorption bands are typically in the UV range. The liberation of leaving groups from arylcarbonylmethyl groups (Figure 11) can proceed via different reaction mechanisms depending on the substitution of the arene; for details, see an earlier review.10 Simple phenacyl PPGs (100) liberate LGs via H atom abstraction601−603 or electron transfer (see section 6.2), o-methylacetophenones (101) react through an intramolecular H-transfer process,235,604−610p-hydroxyphenacyl moieties (102) release LGs via a photo-Favorskii rearrangement,611−613 and benzoin derivatives (103) release LGs via photocyclization to form 2-phenylbenzofuran as a side-product.275,614−616 Here, we discuss only arylcarbonylmethyl PPGs that absorb above 400 nm.
Figure 11.
Arylcarbonylmethyl PPGs.
A phenacyl-based polyaromatic scaffold containing tetraphenylethylene617 was used by Singh and co-workers to create a PPG with an aggregation-induced emission chromophore618,619 by installing a pendant acetyl group on each aryl ring (104).620 Sequential photorelease (λirr ≥ 410 nm) of four carboxylic acid moieties (chlorambucil, Cbl) from an organic nanoparticle formulation of 104 was demonstrated (Scheme 26).620 The chemical yield and quantum efficiency of release depended strongly on the water fraction of the solution (fw): there was no appreciable release below fw = 85% but photorelease proceeded in chemical yields of up to 96% for fw = 99% (Φr = 0.52). It was suggested that the photoreaction requires a restriction of intramolecular rotation (RIR)621 that is imposed in the aggregated state. Singlet oxygen generation by both 104 as a nanoparticle and the photoproduct 105 (ΦΔ = 0.31 and 0.27, respectively) provided a complementary cell-killing mechanism whose effects were additive with those of the photoreleased drug in HeLa cells.620
Scheme 26. Photorelease of Chlorambucil from a Phenacyl-type Tetraphenylethylene PPG (104) via a Photoreaction That Occurs Only in an Aggregated State620.
The p-hydroxyphenacyl (pHP) group (102) is an established612 UV-excitable (λmaxabs ∼ 275 nm), arylcarbonylmethyl-type622 PPG with several favorable properties including high rate constants (107–108 s–1), quantum yields (0.2–1.0), and chemical yields (typically 60–90%) for LG release, and the formation of a major biocompatible photoproduct (p-hydroxyphenylacetic acid) with a hypsochromically-shifted absorption spectrum. It has therefore found many applications in chemistry and biology.22,622 The mechanism of the photoreaction has been studied extensively and previously reviewed.22,622 Briefly, photoexcitation and subsequent ISC are thought to generate an intermediate622−628 reminiscent of a Favorskii rearrangement624−626 cyclopropanone intermediate (106) that either hydrolyzes to form p-hydroxyphenylacetic acid or undergoes decarbonylation to form p-hydroxybenzylalcohol (Scheme 27).629,630
Scheme 27. Photochemistry of 102 (pHP) as a PPG613.
A bathochromic shift in the absorption spectrum of pHP was induced by extending its π-system at the 3-position (107 and 108, Scheme 12; λmaxabs ≈ 380 nm in polar protic solvents). The nitrogen-containing substituents in 107 and 108 are positioned in a way that was expected to facilitate an excited-state intramolecular proton transfer (ESIPT) that would assist in the deprotonation of the p-hydroxyl group. Specifically, it was proposed that upon excitation to the singlet excited state, an ESIPT would occur,631 followed by a transition to a triplet excited state, allowing the reaction to proceed as shown in Scheme 27.631−633 The hydrophobicity of the tetraphenylethylene617 moiety in 108 enabled the formation of organic nanoparticles633 using a reprecipitation technique.564 Visible light-mediated photorelease (λirr ≥ 410 nm) of a carboxylic acid631,633 (chlorambucil) and a hydroxylamine632 (an NO-donating NONOate;634 see section 4.2) from 107 and 108 proceeded in high chemical yields, with the corresponding p-hydroxyphenylacetic acid derivative being the sole additional photoproduct631,633 (Scheme 28). Uncaging was accompanied by a 70–100 nm blue-shift of the fluorescence emission spectrum, enabling real-time quantitative monitoring of the reaction’s progress in live cultured cells.631−633
Scheme 28. Photouncaging of Chlorambucil and DEA NONOate from 107(631,632,635).
A two-step activation system was developed by capping the p-hydroxyl group in 110 with a 4-benzylboronic acid pinacol ester.636 Phenylboronic acid and its esters react with hydrogen peroxide (H2O2) to form the corresponding phenol,637−639 which in turn can lead to the release of a leaving group from the p-benzyl position via 1,6-elimination.640−642 The photorelease of chlorambucil from 110 thus occurred only in the presence of H2O2 (Scheme 29).636
Scheme 29. Two-Step Sequential Uncaging of Chlorambucil (Cbl) from 110(636).
Goeldner and co-workers introduced donor–acceptor biphenyl pHP derivatives 109a and 109b (λmaxabs = 313 and 369 nm, respectively; Scheme 27).255 The photorelease of glutamate was more efficient from 109a than from 109b (Φr = 0.21 and 0.015, respectively). The 2P-uncaging cross section of glutamate liberation from 109a was 0.21 GM at 740 nm,255 which is comparable to the values previously observed for 2P release of diethyl phosphate and ATP from the parent pHP (0.5–1.1 GM at 550 nm).643
2.8. The 4-(p-Hydroxybenzylidene)-5-imidazolinone Group
Campbell and co-workers developed a photocleavable variant (PhoCl) of the photoconvertible fluorescent protein mMaple644 that exploits the phototransformation of the protein’s chromophore, a 4-(p-hydroxybenzylidene)-5-imidazolinone group formed autocatalytically from the tripeptide serine-tyrosine-glycine in the presence of oxygen.645 This moiety is non-fluorescent in its neutral form (111; Scheme 30). Upon excitation with UV or violet (∼400 nm) light, it is deprotonated to form an excited intermediate, 112*,646,647 which undergoes irreversible β-elimination to liberate a carboxamide-containing peptide.648 The photocleavage of PhoCl has been used in several applications, for example, to control protein function645,649−652 or modulate the mechanical properties of hydrogels.653
Scheme 30. Photocleavage of a Peptide in PhoCl648.
2.9. The Stilbene Group
Singh and co-workers harnessed the photocyclization of stilbenes to induce elimination of alcohol and carboxylic acid leaving groups from the 2-position of E-4-(N,N-dimethylamino)-4′-nitrostilbene654 (DANS, 113, Scheme 31). The light-mediated E–Z isomerization of stilbenes followed by a photochemically allowed conrotatory 6π-electrocyclization to form an E-dihydrophenanthrene is a well-studied process.655−657 This intermediate tends to spontaneously undergo a subsequent oxidative dehydrogenation to yield a phenanthrene derivative.656−658 However, in the absence of an oxidant, the dihydrophenanthrene intermediate may reversibly open to give the corresponding Z-stilbene; alternatively, if suitable substituents are present, hydrogen-shift processes may occur.659,660 However, the presence of substituents such as methoxy661−663 or halide663,664 groups in the 2-position resulted in thermal non-oxidative elimination to form a phenanthrene derivative 114 (Scheme 31).
Scheme 31. Photorelease from E-4-(N,N-Diethylamino)-4′-nitrostilbene (DANS) PPG654.
The spectroscopic properties of 113 were solvent-dependent: on going from non-polar to polar solvents, λabs shifted from 410 to 430 nm, λmaxem shifted from 502 to 720 nm, and there was a marked decrease in the fluorescence quantum yield (from 0.55 to <0.002), presumably because of the formation of a TICT state.665 The molar absorption coefficient remained relatively constant (εmax = 2.5–2.8 × 104 M–1 cm–1). Photorelease of alcohols and carboxylic acids from 113 was demonstrated in hexane, acetonitrile, and water. In acetonitrile, the Φr was in the range of 0.10–0.14 for all tested leaving groups, and the chemical yields of photorelease were between 88 and 93%. On the basis of previous studies,661,666−668 a photoreaction mechanism was proposed (Scheme 32) in which photoexcitation of 113 leads to a singlet excited state, allowing the E-stilbene to isomerize into its Z-isomer. The subsequent photoexcitation of the Z-isomer to its singlet state leads to a conrotatory 6π-electrocyclization to form E-dihydrophenanthrene. Orbital symmetry and energy considerations669 suggest that the relative configuration of the dihydrophenanthrene product is E. Spontaneous re-aromatization of the E-dihydrophenanthrene by elimination then yields phenanthrene 114 and releases the leaving group. The fluorescence of the phenanthrene photoproduct (λem = 450 nm) was monitored to follow the reaction in real time. The photorelease of chlorambucil from 113 (λirr ≥ 410 nm), giving rise to light-dependent cytotoxicity, was observed in MCF-7 breast cancer cell lines.654
Scheme 32. Proposed Release Mechanism from Stilbene Derivatives 113(654).
2.10. Quinones
The photoreduction of quinones has been studied extensively.670−675 The reaction can proceed intermolecularly in the presence of a hydrogen donor676−681 (e.g. an alcohol or amine) or intramolecularly via H atom transfer682−689 from a favorably positioned C–H bond on a side-chain. Examples of hydrogen abstraction from the C–H bonds of amino690−693 and sulfido694,695 substituents are also known.
Iwamura and co-workers were the first to exploit the solvent-assisted intermolecular photoreduction of quinones to release leaving groups.317 The (anthraquinon-2-yl)methyl derivative 115 (λmaxabs = 325 nm), originally developed as a protecting group for carboxylic acids that could be removed with reducing agents,696 efficiently released cyclic adenosine monophosphate (cAMP, Φr = 0.2) upon irradiation at 350 nm (Scheme 33). This photoreaction was proposed to involve two photochemical steps.697−699 The first is quinone photoreduction via H atom transfer from the solvent in the triplet excited state to form a ketyl radical that gives rise to the final 1,4-dihydroxyanthraquinone derivative. The second is the photorelease of the leaving group from the resulting dihydroxyanthraquinone via a mechanism similar to that of photorelease from o-hydroxybenzyl PPGs.570,700−702 Photochemical liberation (λirr = 350 nm) of alcohols,322,697 carboxylates,698,699 and ketones/aldehydes703 from 115 (and derivatives thereof) proceeded with Φr = 0.03–0.12. However, the solvent-dependence of the intermolecular photoreduction of 115 may limit its usefulness.322,697,698
Scheme 33. Photorelease of cAMP from an (Anthraquinon-2-yl)methyl PPG317.
The presence of electron donors on the quinone core can give rise to broad charge-transfer absorption bands in the visible range.704 Although 1,4-naphthoquinone and 1,4-anthraquinone appear to have more extended π-systems than 1,4-benzoquinone, a hypsochromic shift in λmaxabs of ∼50 nm was observed for each fused benzene ring.705 The photochemistry of naphthoquinone and anthraquinone derivatives resembles that of benzoquinones bearing aryl substituents. However, the presence of electron donors and acceptors on the distal rings causes substantial absorption spectrum shifts.706 For example, the absorption of 1,4-benzoquinone (λmax = 281 nm) was significantly extended into the visible range (400–600 nm) upon introducing electron-donating substituents.707−710 The magnitude of this shift correlated qualitatively with the magnitude of the HOMO and LUMO coefficients in the substituted quinone.711
Chen and Steinmetz used the photocyclization of 2-dialkylamino-1,4-benzoquinones690,691,712,713 into benzoxazoline photoproducts to drive 1,4-elimination of carboxylates and phenolates bound through the 5-methylene group (116, Scheme 34).714,715
Scheme 34. (1,4-Benzoquinon-5-yl)methyl Derivatives 116 as PPGs715.
2-Pyrrolidino-1,4-benzoquinone 116a exhibited a strong absorption band in the UV region and a weaker charge-transfer absorption band extending into the visible range (450–650 nm, ε = 1.9–2.6 × 103 M–1 cm–1),697,714−716 which is typical for amino-substituted quinones.717,718 Carboxylates were released from 116a (λirr = 458 or 532 nm) in chemical yields of 50–60% at full conversion. An additional photoproduct resulting from cycloaddition of an o-quinone methide photoproduct with 116a was formed with a chemical yield of 36%. The photorelease yield increased to 89–92% upon irradiation in the presence of 3-(dimethylamino)cyclohexen-1-one (0.1 M) as an o-quinone methide trapping agent.715 The quantum yield for the disappearance of 116a was similar to that for carboxylate formation and was sensitive to solvent polarity (dropping from 0.10 in dichloromethane to ∼0.005 in water) but not to the presence of oxygen. Kalow and co-workers improved the quantum efficiency of release in water by changing the substituents around the side-chain γ-hydrogen:697,716 replacing the pyrrolidine in 116a with an isoindoline (116c) or tetrahydroisoquinoline (116d), in which the C–H bond is weaker, led to an 8- to 10-fold increase in Φr in water (5.8 × 10–3 vs 4.7 × 10–2 and 5.7 × 10–2, respectively). The release efficiency correlated strongly with the leaving group pKa,714,715 suggesting that the thermal 1,4-elimination step is rate determining. Rate constants for the release of phenols in this step were in the range of k = 5.1–20.1 × 10–4 s–1,715 in keeping with previous measurements.719,720 The proposed photoreaction mechanism is shown in Scheme 35.
Scheme 35. Proposed Release Mechanism from a 2-Pyrrolidino-1,4-Benzoquinone PPG715.
The sensitivity of the reaction’s quantum yields to solvent polarity suggested the involvement of an intramolecular charge-transfer (ICT) excited state679,721,722 (117) formed prior to excited-state hydrogen transfer from the pyrrolidino group to benzoquinone (118). The initial ICT excited state undergoes rapid back electron transfer (eT) to regenerate the ground state 116a, competing with the hydrogen-transfer step. A possible immediate precursor to benzoxazoline 121 in the photocyclization is the ground-state zwitterionic species 119.684,685,723 In keeping with this hypothesis, photolysis of α-keto amides bearing leaving groups at the α-position yielded intermediates analogous to 119 that could cyclize with concomitant expulsion of a phenolate or carboxylate leaving group.724 Alternatively, leaving groups can be liberated from 121 via deprotonation and subsequent 1,4-elimination.725 Evidence for this pathway was obtained by isolating intermediate 121 (with LG = OPh) and showing that it could undergo elimination under sufficiently basic conditions.715 Additionally, nanosecond laser flash photolysis experiments provided no evidence supporting direct elimination of carboxylates from 119.715 Chromatically orthogonal photorelease from 116d (λirr = 626 nm) and a 2-nitrobenzyl derivative (λirr = 365 nm) has been demonstrated.716
Almutairi and co-workers overcame the inefficiency of photorelease from 116a in water by formulating 116a-caged drug systems (with drugs including paclitaxel, dexamethasone, and chlorambucil) by applying a microemulsion probe-sonication method to a mixture of poloxamer 407 (1% w/v) in water to form monodisperse water-dispersible nanoparticles with hydrophobic interiors. The particles had diameters of 108 ± 20 to 305 ± 101 nm, and their caged drug content ranged from 69 to 94 mol %.726 Photolysis of these nanoparticles (λirr > 590 nm) using various mouse tissue filters released the caged drugs, and the progress of the photorelease process was monitored by co-loading fluorescent dyes (DiD and IR780) into the nanoparticles.726
Dougherty and co-workers723,727 studied analogs of 123, an o-hydroxydihydrocinnamic acid derivative with a conformationally restrictive “trimethyl lock” that enables thermal uncaging and photoreduction to release alcohols and amines via a 1,6-rearrangement mechanism728−730 (Scheme 36). The UV-light-induced release of 2-nitrobenzyl-protected phenols to activate the trimethyl lock mechanism has been demonstrated previously.223,731−733
Scheme 36. Photochemistry of 1,4-Benzoquinone Trimethyl Lock PPGs723.
The broad visible absorption bands of 124, 125 (400–600 nm), and 126 (350–500 nm; Figure 12) are indicative of transitions to charge-transfer states.707 The absorption maxima of 124 and 125 are bathochromically shifted relative to that of 116,715,716 which was attributed to the steric effect of the trimethyl lock moiety. Photolysis of 126 (λirr = 455 nm), 124 (455 nm), and 125 (565 nm) in polar protic solvents liberated caged alcohols in quantitative chemical yield.723 Additionally, the orthogonal release of two different alcohols from 126 (R = Me) and 124a was demonstrated (λirr = 455 and 565 nm, respectively),727 and a mechanism for their photoreaction was proposed on the basis of studies on 126(723) (Scheme 37).
Figure 12.
Structures of 2-amino- and 2-sulfido-substituted 1,4-benzoquinone trimethyl lock PPGs.
Scheme 37. Proposed Uncaging Mechanism of Photoinduced Quinone Trimethyl Lock PPGs723.
Photochemical transformations are shown for R1 = R2 = H or Ph.
Zwitterion 127 was proposed to be the penultimate intermediate in this photoreaction; it can undergo nucleophilic capture at the carbocationic center by the solvent or an intramolecular nucleophile to give 128 or 129, respectively. Either of these species can then undergo trimethyl lock-facilitated ring closure to provide the final products in high chemical yield (>95%).723 The singlet excited state, 1126*, has a charge-transfer character. In contrast to the photoreactions of typical benzoquinones, which undergo ISC to their triplet excited state with a near-unity quantum yield,7341126* predominantly undergoes nonemissive return to the ground state (with >90% efficiency). The triplet excited state 3126* is formed with low efficiency (ΦISC = 1–5%) and was suggested to have a charge-transfer character based on a spin density calculation (DFT M06/6-311++G**),723 in contrast to the n,π* triplet-excited state of simple benzoquinones.735 It is still unclear whether the nonproductive triplet decay of 3126* to 127 occurs directly or via 3127*. Formation of 127 can thus occur through both the singlet and triplet manifolds. For simple S-alkyl derivatives, product formation via the singlet pathway dominated, but the triplet pathway contributed more substantially in the case of S-benzyl analogs.723 Although the triplet excited state is formed with low efficiency, it forms the product more efficiently than the singlet excited state.
The fluorophore 4-methylumbelliferone and the neurotransmitter GABA were both successfully photoreleased from derivatives of 126 under physiological conditions.723 Additionally, Forsythe and co-workers736 showed that S-substituted analogs of 126 can be formed in water under slightly basic conditions via a thio-bromo coupling.737,738 Dendrimeric monomers with bromo-substituted 1,4-benzoquinone trimethyl lock moieties (132) were reported to react with dendrimeric monomers bearing sulfhydryl end groups (133) to form a polymeric network with sulfido-substituted 1,4-benzoquinone trimethyl lock PPGs incorporated into its backbone (Scheme 38).736 Encapsulation of cells (mouse fibroblast L929, human foreskin fibroblast, and human mesenchymal stem cells) in this polymeric network and their subsequent release by photodegradation of the polymer (λirr > 420 nm) were demonstrated, with the cells retaining high viability throughout the process.736
Scheme 38. Photocleavable Polymer Based on a Sulfido-Group-Substituted 1,4-Benzoquinone Trimethyl Lock PPG736.
The 2-hydroxycinnamyl moiety (134, Scheme 39) was first utilized as a PPG by Porter and co-workers to achieve photochemical activation of thrombin.739,740 This system undergoes an initial photoinduced isomerization followed by cyclization to form a coumarin derivative, causing the release of caged substrates such as alcohols.741−750 The cyclization rates of 2-hydroxycinnamyl esters or amides approached those of the trimethyl lock system (k = 0.03–50 × 105 s–1).723,728,729,751−754 The absorption maxima of 134 could be further bathochromically shifted (up to λmaxabs = 394 nm) by introducing electron-donating substituents on the phenyl ring743,755,756 or extending the system’s π-conjugation.750,757 A small set of 2-hydroxycinnamic derivatives was synthesized to study and improve the 2P-absorption-mediated release process.755,756,758
Scheme 39. Photochemistry of o-Hydroxycinnamic Derivatives740.
Dougherty and co-workers incorporated conformationally locked Z-cinnamyl esters (with a cis-alkenyl lock759) into amino-substituted 1,4-benzoquinones 135 and 136, and 1,4-naphthoquinone 137 (Figure 13), enabling visible-light mediated quinone photoreduction to serve as an intramolecular cyclization initiator and leaving-group release trigger (Scheme 40).760
Figure 13.
Structures of amino-substituted 1,4-benzo- and naphthoquinone cis-alkenyl lock PPGs.
Scheme 40. Photochemistry of 1,4-Benzoquinone cis-Alkenyl Lock PPG 18(760).
Amino-substituted quinones 135–137 have broad charge-transfer absorption bands in the range of 495–535 nm (εmax = 2.9–3.6 × 103 M–1 cm–1). Upon irradiation at 565 nm, methanol or ethanol was liberated with Φr = 0.03–0.04 in quantitative chemical yield.760 Solvent effects on the photoreduction715 and cyclization759 processes were observed as expected; in polar protic solvents, photoreduction was less efficient and cyclization was more efficient, whereas the opposite was true in non-polar aprotic solvents.760
A different mechanism involving photoenolization609,761 followed by heterolytic elimination230,231,233,762 was demonstrated by Kamdzhilov and Wirz232 for substituted 5-(ethylene-2-yl)-1,4-naphthoquinone 138 (Scheme 41), which absorbs below 405 nm. This compound released HBr and diethyl phosphate upon irradiation at 365 nm (Φr = 0.35 and 0.70, respectively) in a neutral aqueous solution, but a poorer leaving group (acetic acid) was released less efficiently (Φr < 0.01). Unfortunately, the sensitivity of this photoreaction to acids and the instability of naphthoquinones toward bases limit the use of this photochemical protecting group.
Scheme 41. Photochemistry of 5-(Ethylene-2-yl)-1,4-naphthoquinone PPG232.
2.11. Xanthene and Pyronin Groups
Wirz, Klán, and co-workers showed that acetate and diethyl phosphate can be released from a 1:1 complex of 139 with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) upon irradiation at >500 nm with Φr = 0.3–2.4 × 10–2 (Scheme 42).763 This relatively low photorelease quantum yield was compensated for by a large molar absorption coefficient at the irradiation wavelength (ε ≈ 4 × 104 M–1 cm–1), resulting in an uncaging cross section Φrε(λirr) of ∼100 M–1 cm–1.763 Efforts to synthesize 139 by alternative methods or to separate this DDQ complex were unsuccessful.11,763 It was assumed that the primary photoproduct in aqueous solutions, a meso-methylhydroxy derivative formed from the corresponding cationic intermediate, is further oxidized by DDQ to give 6-hydroxy-3-oxo-3H-xanthene-9-carboxylic acid (140) as the major photoproduct. HMO calculations of the xanthene frontier molecular orbitals revealed that the HOMO and LUMO are disjoint at the meso-position, resulting in an increase in its negative charge upon HOMO–LUMO excitation. This behavior was linked to specific photoreactivity such as the extrusion of leaving groups (Scheme 42)11 (see also section 2.12).
Scheme 42. Photochemistry of Xanthene PPG 139(763) and Schematic Representation of the HOMO and LUMO of Its π-System11.
A different approach involved placing a 1,3-dithian-2-yl substituent at the meso-position of pyronin 141 (Scheme 43).764 The resulting compound exhibited strong absorption (λmaxabs = 584 nm) and emission (λmax = 607 nm) bands in aqueous solution, and irradiation in its major absorption band (Φr = 3 × 10–4) gave a stable photoproduct that absorbed at 382 nm and emitted at 448 nm. It was proposed that direct thiolate release could explain the observed meso C–C bond cleavage in the pyronin chromophore.764 Recently, Klán, Roithová, and co-workers subsequently showed that transformation of 141 is a multi-photon multi-step process. Many intermediates in this process were identified and their interrelationships were clarified using steady-state and time-resolved optical spectroscopy, mass spectrometry, and NMR spectroscopy.765Scheme 43 presents a simplified mechanism for this complex reaction, which involves at least three light-initiated steps. A different mechanism involving photooxidative cleavage by singlet oxygen was observed for the photolysis of exo-alkylidene xanthenes.766
Scheme 43. Photochemistry of Pyronin 141(764,765).
Jo and co-workers recently demonstrated the release of 10-N-carbamoyl substituents from a 3,7-bis(dimethylamino)-10H-phenothiazine chromophore upon excitation with red light in aqueous solution (142, Scheme 44).767 The side product of this transformation is the fluorescent indicator methylene blue. Because 3,7-bis(dimethylamino)-10H-phenothiazine is a UV-light absorbing chromophore,768 its excitation at 660 nm was unexpected. Although no mechanistic details were provided in the report, the authors suggested that the reaction starts with the heterolytic cleavage of the N–C(O) bond upon irradiation.
Scheme 44. Photorelease from 10H-Phenothiazine Derivatives767.
2.12. BODIPY Groups
4,4-Difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) and its derivatives are highly fluorescent dyes that are widely used in chemistry769−773 and biology.774−778 Their favorable spectroscopic properties are mainly due to their relatively compact, rigid, and planar structures, which make the potential energy surfaces of their S0 and S1 states very similar. Consequently, narrow Gaussian-shaped absorption and emission bands are typically observed for their lowest energy transition.779,780 These compounds have high ΦHF values (typically >0.5) because of inefficient nonradiative decay and ISC.781,782 Simple BODIPY chromophores typically have two narrow absorption bands in the visible range (λmax ≈ 490–540 nm, ε ≈ 3–9 × 104 M–1 cm–1): an intense 0–0 band due to the S0–S1 transition, and a shoulder attributed to the 0–1 vibrational transition.783−787 The BODIPY scaffold tends to resist photobleaching781,788,789 and degradation by acids/bases,790,791 but it is highly amenable to synthetic transformations that modulate its spectral properties.779,780,792,793 This section focuses on the different strategies reported to date utilizing the BODIPY chromophore as a photoreleasing system (Figure 14).
Figure 14.
Structures of BODIPY PPGs with LGs at different positions.
The groups of Winter795 and Weinstain794 independently studied the release of leaving groups from meso-methyl BODIPY derivatives upon photoexcitation with green light (a general structure 143 is shown in Scheme 45). The basis for this photoreactivity can be traced to the properties of the chromophore’s meso position. Heterolytic photodissociation to give a meso-methyl carbocation is favored when electronic excitation to the lowest singlet-excited state involves a substantial transfer of electron density to the sp2 carbon atom of a chromophore bearing a LG in the α-position such that the antibonding σ* orbital is mixed with the chromophore’s LUMO.11 In such cases, the promotion of an electron from the HOMO to the LUMO weakens the C–LG bond, facilitating heterolytic dissociation of the leaving group. This mechanism is responsible for, among other things, Zimmerman’s meta-effect in the photosolvolysis of methoxybenzyl acetates556 and the photochemical reactivity of coumarinyl-4-methyl PPGs (section 2.2).22,278 HMO calculations suggested that BODIPY also reacts in this way because its HOMO and LUMO are disjoint at the meso position (Scheme 45), and the negative charge at this position increases upon excitation,11,780 as also predicted for xanthene chromophores (Scheme 42). meso-Methyl carbocations were reported to have a low-lying excited state with a vertical S0–S1 energy gap of 8–13 kcal mol–1 (TD-DFT), suggesting a near-degenerate diradical configuration.795 Such low-energy diradicals can have a close-lying conical intersection between the closed-shell singlet and singlet diradical forms.796 The photoheterolysis of C–LG bonds to generate ion pairs was shown to be favored when the ion pair has access to a nearby productive conical intersection that provides an efficient channel for the excited state of the precursor to decay to the ground-state ion pair.796,797
Scheme 45. Photorelease of LG from meso-Methyl BODIPY Derivatives,794 and Schematic Representation of the HOMO and LUMO of the BODIPY π-System (BF2 Is Omitted)11.
The synthesis of meso-methyl functionalized BODIPYs generally follows known routes,481,779,780,792,793 and the attachment of a leaving group is achieved by one of (a) incorporation during BODIPY core synthesis,795,798−801 (b) displacement of a formerly installed good leaving group,295,798,801−803 (c) utilizing a meso-methylhydroxy derivative as a nucleophile to attack a pre-activated form of the leaving group,795,804−808 or (d) modifying a previously installed carbonic acid derivative794,805,809−811 (Scheme 46). The latter process was shown to compete with an undesired direct attack at the meso-methyl position.809
Scheme 46. Synthetic Pathways to meso-Methyl BODIPY PPGs.
meso-Methyl BODIPY PPGs such as 144, 145, and 146 (Figure 15) typically have absorption bands with λmaxabs at 490–545 nm (Figure 16), although a family of π-extended meso-methyl BODIPYs with λmax at 586–693 nm was also reported (147; Figure 16).807,812 The absorption spectra of representative meso-methyl BODIPY PPGs discussed in this section are shown in Figure 16.
Figure 15.
Structures of meso-methyl BODIPY PPGs.
Figure 16.

Absorption spectra of selected meso-methyl BODIPY PPGs. Black line, 1,3,5,7-tetramethyl derivative (LG = acetate);795 blue line, a 2,6-diethyl-1,3,5,7-tetramethyl derivative (LG = (4-nitrophenyl)carbamate);794 red line, a 2,6-diiodo-B-dimethyl derivative (LG = acetate);798 green line, a bis(p-methoxystyryl) derivative (LG = benzoate);807 cyan line, a bis(p-dimethylaminostyryl) derivative (LG = benzoate).807
The photorelease of diverse functional groups, including primary and secondary amines794,805,809,810,812 (as carbamates), carboxylic acids,295,794,795,798,802,804,806−808 alcohols794,798,805 (as carbonic acid esters794 or ethers798,801,805), halogens,798 hydroxylamines,803 thiocarbamic acid,799 thioacetic acid,798 and thiols800 (Figure 15) from BODIPY-based PPGs, has been reported. The liberation efficiency of LGs correlated well with the pKa of their conjugate acids,794,798,809 and the photoreaction quantum efficiency of unsubstituted BODIPYs (other than 1,3,5,7-tetramethyl derivatives) was moderate to low. The photoreaction quantum yields determined in aerated protic solvents were around half those measured in degassed solutions.798 The typically small values of Φr for these systems (Φraer = 1–800 × 10–4) are compensated by high molar absorption coefficients (typically, εmax = 3–7 × 104 M–1 cm–1), giving rise to uncaging cross sections, Φrε(λirr), of 1–100 M–1 cm–1, that are similar in magnitude to those of traditional UV-absorbing PPGs.10 The major side-photoproducts were identified as solvent-captured meso-methyl BODIPY derivatives (Scheme 45).719,794,795,798,803,808 These compounds (e.g., meso-methyl ethers formed when using methanol as the solvent) absorb at very similar wavelengths to the original PPG and may thus act as internal optical filters, but they are eventually degraded upon extensive irradiation.798
Klán, Weinstain, Winter, and co-workers showed that in addition to the nature of the LG, the efficiency of photorelease from BODIPY chromophores depended on their substitution: halogen substituents at the 2,6-positions increased both the reaction efficiency and ΦISC,795,798 with a substituent heavy-atom effect that decreased in the order I > Br > Cl > H.813,814 However, electronegative halogen substituents increased the electrophilicity of the meso-methyl position, making it prone to nucleophilic attacks that caused LG liberation even in the dark.809 Stronger EWGs such as aldehydes or sulfonates in the 2,6-positions substantially raised the barrier to C–O bond heterolysis on the triplet surface of 144 and thus impeded photorelease.811 For example, the calculated C–O bond (heterolytic) dissociation energies for derivatives of 144 bearing 2,6-disulfonate, -dihydrogen, and -diethyl substituents were 18.9, 15.7, and 13.5 kcal mol–1, respectively, with photouncaging quantum yields of 0, 0.6 × 10–4 and 3.9 × 10–4, respectively.811 The absence of the 1,7-methyl groups reduced Φr by a factor of ∼1.5, probably due to a reduced electron density in the BODIPY core and thus a reduced capacity to stabilize the putative cationic diradical intermediate.795,798 In addition, dialkylborano analogs had significantly higher Φr values than their BF2 counterparts (up to 30-fold).798,801,807,812 These substituent effects were additive: the Φr of 149 (Figure 16) was 100-times that of 148 (Figure 17).798
Figure 17.
Structures and quantum efficiencies of BODIPY PPGs 148 and 149, the latter of which combines the 2,6-diiodo and BMe2 modifications.
Winter and co-workers recently studied conformationally-restrained π-extended boron-methylated meso-methyl BODIPYs that absorb light at around 700 nm to liberate acetic acid with Φr = 0.018–0.037. These efficiencies are ∼50-times those reported for the comparable non-restrained 147 derivatives.815 It was suggested that conformational restriction inhibited competing excited state decay pathways such as internal conversion, leading to higher photorelease quantum yields. The Jabłoński diagram describing the photochemistry of 150 shown in Figure 18 indicates that photorelease proceeds from both the singlet and triplet excited states.798 The increase in quantum efficiency correlated with enhanced ISC,795,798 which can reduce competition from radiative (ΦF) and nonradiative (ΦNR) processes.798 It should be noted that the reaction efficiencies are relatively low for mediocre LGs such as carboxylates, presumably because of ion-pair recombination and nonradiative decay from the triplet excited state, which do not occur during photolysis of good LGs such as Cl–.798
Figure 18.
Jabłoński diagram of the photochemistry of 150.798
Photorelease from meso-methyl BODIPYs has been used in various biological and synthetic applications. For example, the uncaging of signaling molecules including the gasotransmitter H2S (see also section 4.3),799 histamine, and the neurotransmitter dopamine,794,811,812 has been demonstrated in cellular environments. Sortino and co-workers reported that light-dependent NO-induced vasodilatation of rat aorta can be achieved by uncaging the NO donor (see also section 4.2) N-nitroso-N-phenylhydroxylamine.803 Weinstain and co-workers introduced a protecting-group-free, late-stage functionalization of meso-methyl BODIPYs that enabled their targeting to specific cellular organelles805 and the development of water-soluble derivatives.811 Other notable applications include the selective photorelease of the protonophore 2,4-dinitrophenol in mitochondria and the protein synthesis inhibitor puromycin in the endoplasmic reticulum,805 as well as the light-dependent delivery of cytotoxic molecules including chlorambucil and a cathepsin B inhibitor (CA-074816) to cells.804,806 In both of the latter cases, the observed cytotoxicity was partly due to photosensitized 1O2 generation by the BODIPY PPG.804,806 Sebastián and co-workers used a meso-methyl BODIPY-caged diethylamine as an organic light-responsive nucleophilic cyanoacrylate initiator capable of fast on-demand photocuring of commercial formulations of various cyanoacrylates including biologically-relevant long alkyl chain monomers.810 Klán and co-workers reported that controlled photorelease of alkynoic acids was followed by efficient decarboxylation, giving terminal alkynes that could subsequently undergo CuI-catalyzed azide/alkyne cycloaddition reactions.802 Similarly, Truong and co-workers developed a phototriggered thiol-propiolate addition that is initiated by uncaging methyl-3-mercaptopropionate.719 The utility of this photochemical ligation strategy was demonstrated by fabricating hydrogels with specific architectures, photo-immobilization of biomacromolecules, and live-cell encapsulation within a hydrogel scaffold.800
The BODIPY boron center has also been identified as a photoreactive center. Urano, Nagano, and co-workers showed that the B–O bond in 4-aryloxy BODIPYs can be photolyzed (λirr = 475–490 nm) to release phenols (Scheme 47).817,818 The absorption/emission wavelengths (λabs = 495–525; λem = 506–541 nm) and molar absorption coefficients (εmax = 6.2–9.8 × 104 M–1 cm–1) of derivatives 151 were only slightly affected by modulation of the HOMO/LUMO energy gap. However, their fluorescence quantum yields, ΦF, decreased dramatically upon raising the HOMO energy of the aryl group or reducing the LUMO energy of the BODIPY core.817,818 This effect on ΦF was attributed to electron transfer (eT) from the adjacent aryl group to the BODIPY core.819 The release quantum efficiencies were generally inversely correlated with ΦF, suggesting that the photoreaction proceeds via a competing PeT process in which B–O bond solvolysis is preceded by the formation of a charge-separated intermediate with a cationic aryl group radical and an anionic radical BODIPY core. Derivatives with a high calculated PeT driving force820 had low uncaging efficiencies (Φr = 0.3–2 × 10–3). The same was true when the calculated PeT driving force was low (Φr = 0.7–2.6 × 10–3), presumably because fast reverse-electron transfer from a charge-separated intermediate821 competes with uncaging. Derivatives with moderate predicted PeT driving forces thus had the highest uncaging efficiencies (Φr = 1.4–5.4 × 10–3).817,818 Photouncaging of phenols from π-extended BODIPY analogs at λirr = ∼620 nm was demonstrated, albeit with very low quantum efficiencies (Φr = 1.5 × 10–7–7.4 × 10–5).818 This capability was used to achieve intracellular photorelease of the transient receptor potential cation channel V1 (TRPV1) agonist capsaicin in cultured HEK293 cells transiently transfected with TRPV1, leading to the induction of light- and ligand-dependent Ca2+ uptake.817
Scheme 47. Photorelease of Phenols by B–O Photolysis818.
The range of functionalities releasable via B–O bond photocleavage was expanded818,822−824 by introducing a benzyloxycarbonyl linker that undergoes a spontaneous 1,6-elimination to release a leaving group from its benzyl position after the phenol moiety is liberated (Scheme 48).825 For example, a carboxylic acid derivative of the fluoroquinolone antibiotic levofloxacin was directly photoreleased (λirr = 470 nm) from ester 152 in 31% chemical yield.822 As a result, this ester exhibited light-dependent bactericidal effects in E. coli and the Gram-positive S. aureus. The terminal primary amine of biogenic histamine was similarly caged through a carbamate bond (153a and 153b), and its subsequent photorelease (λirr = ∼480 nm) was achieved in 40% chemical yield with Φr = 3–3.9 × 10–4.818 Photoexcitation (λirr = 488 nm using an argon laser) of the cell-impermeable 153b in HeLa cells induced light- and H1-receptor-dependent Ca2+ oscillations. The photolysis of carbamothioate 154 (λirr = 470 nm) also resulted in the release of a free amine;823 the carbamothioic acid subsequently underwent thermal B–O bond cleavage (k = 0.02 min–1, pH 7.4) to release carbonyl sulfide (COS) and a free amine.823,826 COS is hydrolyzed into CO2 and H2S (spontaneously or under carbonic anhydrase catalysis827), making it a useful H2S generator for research with potential therapeutic applications (see also section 4.3).799,828−830
Scheme 48. Expansion of Chemical Functionalities Amenable to Uncaging from BODIPYs via B–O Photolysis Using a Self-Immolative Linker818,822−824.
Smith, Winter, and co-workers developed 155 as a photoswitchable probe831 for single-molecule localization spectroscopy,832 demonstrating that the B–C bond is also amenable to photocleavage. Photoexcitation of 155 (λirr = 488 or 532 nm) caused the cleavage of its two B–alkyl groups and their subsequent replacement by solvent adducts (156), which was accompanied by a ∼6-fold increase in ΦF (from 0.15 to 0.96; Scheme 49). Conjugation of 155 with paclitaxel via its styryl group enabled super-resolution imaging of microtubules in live HeLa cells with an average full-width-at-half maximum (FWHM) diameter of 94 ± 10 nm.831 The photoactivation of 155 required only a single laser wavelength and relatively low laser power.833−835
Scheme 49. Photoswitchable BODIPY Probe Based on B–C Bond Photocleavage831.
A different approach to using BODIPY scaffolds for photorelease is to introduce photoreactive functional groups in the 2,6-positions (157).836,837 This approach is exemplified by the photorelease of amines from sulfonamide groups in the BODIPY 2,6-positions (λirr = 470 ± 20 nm, chemical yields = 45–50%, Φr = 0.022–0.11).836 These photoreactions were insensitive to the presence of oxygen, required a carboxylic functional group in the α-position of the amide chain, and exhibited pH-dependent rates, proceeding less efficiently under acidic conditions. These observations are consistent with the known photochemistry of arylsulfonamides,838 which have been studied extensively in the context of their use as potential PPGs839−842 and due to their prevalence in pharmaceuticals.843−845 The proposed photoreaction mechanism involves a sequence of electron-transfer, decarboxylation, hydrogen-transfer, and fragmentation steps that are shown in Scheme 50.
Scheme 50. Proposed Mechanism for Photolysis of 157(836).
The key steps in this reaction are electron transfer from the donor (carboxylate) to the acceptor (sulfonamide)839,840,846 and the subsequent formation of a charge-separated state.845,846 In the case of 157, the ability of carboxylates to transfer a single electron to the excited BODIPY is yet to be established, but a similar process was proposed for the closely related compounds 225a,b (section 4.1.1).847 The formation of a charge-separated state was supported by the need for the carboxylate and sulfonamide groups to be in close proximity.836,848 Decarboxylation occurs from this state842,849 and is followed by a radical reaction that can proceed through competing pathways, resulting in either the cleavage of the S–N bond with the formation of a reduced BODIPY derivative (pathway a) or deboronation (pathway b).850 In both cases, back electron transfer terminates the reaction. The formation of equal amounts of amine and aldehyde in the pathway suggests the formation of an imine intermediate that is subsequently hydrolyzed.836 The observed chemical yields of pathways a and b were consistent with previous observations of competition between different reaction pathways in arylsulfonamides.849,850 When the carboxylate group is not ionized, the yields of the amine are reduced; this was attributed to a need for H atom transfer847,851 and makes other reaction pathways more competitive.850 Photolytic cleavage of GABA from 158 (λirr = 488 nm; Scheme 51) enabled the controlled induction of light-triggered membrane-potential responses in patch-clamped mouse basolateral amygdala brain slices.836
Scheme 51. Release of GABA upon Photolysis of 158(836).
Zhang and co-workers introduced a PPG based on a BODIPY derivative bearing photoreactive oxime esters524 at the 2-position (Scheme 52).837 Compounds 159a and 159b have strong absorption bands centered at 507 and 516 nm (εmax = 5.2–5.7 × 104 M–1 cm–1) with low ΦF values (0.09–0.12). This was attributed to the proximity of the 3n,π* state to the lowest 1π,π* excited state, suggesting that ISC competes with fluorescence. The quantum yields of photorelease for carboxylic acid LGs at λirr = 503 nm were comparable to those for other BODIPY PPGs (Φr = 5.2–7.2 × 10–4). The proposed photoreaction mechanism for this process is shown in Scheme 53. The excitation of oxime esters was shown to result in homolytic scission of the N–O bond and formation of a caged radical-pair.526−528 This process is considered to occur mainly from the triplet-excited state,852−856 although dissociation proceeded efficiently from the singlet-excited state in some oxime esters.854 The caged radical pair can undergo recombination or in-cage reactions,854 but escape of the carboxyl radical from the cage and subsequent hydrogen atom abstraction from a polar protic solvent creates a carboxylic acid leaving group.412,528,837,856 The corresponding iminyl radical can undergo several transformations796,857,858 or fast back-electron transfer to form an iminyl cation that is subsequently converted into a nitrile by deprotonation.859 In the case of 159, back electron transfer was found to be favorable; the 2-cyano BODIPY photoproduct was obtained in 60% chemical yield.837 The histone deacetylase inhibitor valproic acid (VPA) was caged as a BODIPY oxime ester in this way; in HeLa cells, this compound caused light- and dose-dependent toxicity with an IC50 100-times lower than that of free VPA.837
Scheme 52. Photorelease of Carboxylic Acids from BODIPY Oxime Esters837.
Scheme 53. Proposed Mechanism for Photolysis of 159(837).
3. Photorelease from Coordination Compounds
Coordination compounds, which consist of a central (usually metal) atom or ion surrounded by ligands, have rich and varied photochemistry.860,861 They often have unique ground- and excited-state properties that can be tuned by varying the central atom or the coordinating ligands to allow light-triggered release of chemically or biologically active species. Many organometallic complexes also exhibit photonuclease, photo-crosslinking, or photocytotoxic activities, accompanied by diverse types of photofragmentation reactions or photodynamic effects.862−869 This section surveys coordination compounds that have been used as photoreleasable systems activated by visible/NIR light in the past decade. However, comprehensive coverage of all their applications would be beyond the scope of this review. A more extensive discussion of these applications can be found in several review articles and perspectives that have been published in recent years.8,40,58,65,66,68,69,76,98,107,113,869−876 Representative spectra of selected PPGs discussed in this section are shown in Figure 19.
Figure 19.
Representative spectra of transition metal-containing PPGs: black line, a vitamin B12 derivative877 (LG = alkyl; section 3.1); red line, a phthalocyanine derivative (LG = aryloxy; section 3.2);878 green line, a ruthenium(II) bipyridyl derivative (L = tyramine; section 3.3);879 blue line, a dirhodium(II,II) derivative (L = acetonitrile; section 3.4; the ε values are 50-fold smaller than shown);880 and magenta line, a cobalt(III) derivative (section 3.5).881
Transition-metal complexes are usually colored compounds and are therefore readily excited using visible light. The accessibility of multiple excited states with different spin multiplicities882 and competing photophysical and chemical processes can result in complex and sometimes unpredictable photochemistry. Many different primary photophysical processes, including metal-to-ligand charge transfer (MLCT), ligand-to-metal charge transfer (LMCT), and ligand-to-ligand charge transfer (LLCT), may precede the release of species.883,884 However, ligand exchange is usually the key process in ligand (species) liberation.
3.1. Photochemistry of Vitamin B12 Derivatives
Vitamin B12 is a water-soluble metal complex bearing a cobalt ion in the center of a conjugated corrin ring; its photophysical and photochemical properties are relatively well understood.60−62,64,67,885,886 The corrin CoIII complex absorbs light below 580 nm,877 and its derivatives such as 160 (Scheme 54, Figure 19; LG = alkyl, CN, OH, oradenosyl) can undergo homolysis887 of the Co–C bond (BDE = 30–44 kcal mol–1)886 in the singlet excited state888 to give a charge-transfer CoIII intermediate within tens of picoseconds.877,889−896 The intermediate then dissociates into a close radical pair of LG• and CoII side-product radicals that either recombine within nanoseconds or escape the solvent cage (Scheme 54).61,67,885,886,892,897−900 The solvent’s properties affect the efficiency of recombination.901 Depending on the ligands, the relaxed singlet excited states have been characterized as either MLCT or LMCT states using DFT and TD-DFT methods.62,886,902−912 In addition, magnetic field effects on the photolysis of 5′-deoxyadenosylcobalamin have been reported.913,914 The rate of radical pair recombination was found to be sensitive to external magnetic fields on the order of tens to hundreds of mT in viscous solutions. Although the involvement of a triplet state in the dissociation of ligands from cobalamin complexes has not been precluded by calculations,886,909 the formation of a triplet radical pair seems inconsistent with the observed magnetic field effects.895,913 The photobiological role of vitamin B12 in the photoreception of photosynthetic and non-photosynthetic bacteria was studied by Kutta, Jones, and co-workers.915 In contrast to the mechanism described above, the photochemistry of the coenzyme B12-dependent photoreceptor protein, a bacterial transcriptional regulator that controls carotenoid biosynthesis, does not proceed via radical pair intermediates but through Co–C bond heterolysis.
Scheme 54. Vitamin B12 PPGs877.
It was demonstrated that visible-light-induced hydrogel formation can be facilitated using alkyl-cobalamin-based photoinitiators whose photochemistry induces radical photopolymerization.916 The production of free radicals from thiolato-Cob(III)alamins was also supported by electron paramagnetic resonance.917 Irradiation of alkylcobalamins using >500 nm light was shown to form carbon-centered radicals that cause DNA damage via strand scission of polynucleotides.918 Additionally, in the presence of reductants such as TiO2 or Zn/NH4Cl, cobalamin derivatives undergo photochemical reduction to strongly nucleophilic CoI complexes that can react with electrophiles via an SN2 mechanism.919
The quantum yields of LG release from cobalamin derivatives are often irradiation-wavelength dependent.892,894,920 For example, the Φr for •CH3 liberation from methylcobalamin in aerated aqueous solutions varies from 0.35 at λirr = 490 nm to 0.24 at Φr = 550 nm.921,922 Similarly, 5′-deoxyadenosylcobalamin undergoes Co–C bond cleavage with Φr = 0.20 under anaerobic conditions,923 although a near-unity quantum yield for bond homolysis in aqueous solution has also been reported for this compound.924 The CoII side-product can be trapped by oxygen.923,925,926 The photolysis mechanism and release quantum yields depend not only on the type of LG but also on experimental parameters including the solvent, the pH, the presence of specific enzymes,888,894,901,927−929 and the nature of the lower axial base.895,923 Cobalamin release mechanisms are discussed in more detail in a recent review by Jones.61
Photoactivatable vitamin B12 systems have been used for visible-light-initiated drug release. The absorption limit of the corrin ring (>580 nm) can be extended by appending a sensitizer absorbing in the NIR region, by exploiting 2P excitation, or via upconversion (see section 6.4.2).885,930 Building on the earlier studies on the photochemical decomposition of adenosylcobalamin and other vitamin B12 analogs discussed above, Lawrence and co-workers showed that the photochemical cleavage of the Co–C bond in cobalamins can be used in the design of caged compounds.885 Cobalamin 160, which bears a rhodamine fluorophore as an LG connected through an alkyl linker, was shown to undergo selective photochemical homolysis upon irradiation at 560 nm in high chemical yield (97%), even when mixed with two different caged compounds absorbing only UV light.931 The fluorescence of the appended rhodamine in 160 is quenched by the cobalamin, allowing its release to be monitored by observing its fluorescence under a confocal microscope in microwells and living cells.
The portfolio of leaving groups used with these PPGs was subsequently extended beyond fluorophore indicators by caging biologically active species including the anti-inflammatory agents methotrexate (161a), colchicine (161b), and dexamethasone (161c) with the cobalamin lipid conjugate 161 (Figure 20).932 These caged derivatives were loaded onto human erythrocytes and the agents (which were connected via an auxiliary linker that was subsequently removed by esterase hydrolysis) were released in quantitative yield upon irradiation at 525 nm. To shift the absorption into the phototherapeutic window, the C18 derivatives of pentamethine cyanine (Cy5; λirr = 646 nm), AlexaFluor700 (λirr = 700 nm), heptamethine cyanine (Cy7; λirr = 747 nm), and DyLight 800 (λirr = 784 nm) were used as sensitizers. Irradiation at the dyes’ maxima led to drug release and the induction of the expected biological responses.932 A similar strategy was used to release cAMP from conjugate 162a to control the activity of a cAMP-dependent protein kinase and to release the anticancer agent doxorubicin from 162b (Figure 21).930 In these studies, several commercially available sensitizers including 5-carboxytetramethylrhodamine, SulfoCy5, Atto725, DyLight800, Alexa700, and BODIPY650 were used to facilitate excitation of cobalamin conjugates with visible-to-NIR light.
Figure 20.
Cobalamin PPGs designed to release methotrexate (161a), colchicine (161b), and dexamethasone (161c).932
Figure 21.
Cobalamin PPGs designed to release cyclic adenosine monophosphate (162a) and doxorubicin (162b).930
A photorelease strategy for liberating membrane-permeable bioagents such as colchicine, paclitaxel, and methotrexate from cobalamin–bioagent conjugates confined within lipid-enclosed compartments in the interior of erythrocytes was reported by Lawrence and co-workers.933 Upon photolysis of the conjugates by visible-to-NIR light, enabled by a Cy5 sensitizer attached via a dimethylbenzimidazole ligand, the drugs were liberated inside red blood cells. Janovjak and co-workers recently used the 5′-deoxyadenosylcobalamin binding domains of bacterial CarH transcription factors to induce growth factor receptor 1 dissociation.934 Several other relevant biological applications of cobalamin photochemistry have also been reported.935−937
3.2. Photochemistry of Phthalocyanine and Porphyrin Derivatives
Si-phthalocyanine macrocycles (Figure 22) are photostable, hydrophobic, and non-toxic.938−940 Their usefulness in aqueous media is limited by their low aqueous solubility, although this can be overcome through structural modification.941−943 Their absorption spectra feature an intense Q-band at approximately 670 nm and a Soret band in the region of 300–400 nm,939,944 and the quantum yield of ISC is reduced by dye aggregation.944 Interestingly, however, the efficiency of singlet oxygen production by the triplet state is very similar to that for the singlet states.945 These complexes can thus serve as efficient oxygen photosensitizers in photodynamic therapy (see also section 6.3),938,940,946−948 although photobleaching by self-sensitized photooxidation can limit their usefulness.945 Interestingly, the properties of the axial ligands (Figure 22, R and R′) and the pH of the solution were found to profoundly affect their photophysics.939
Figure 22.
Si-phthalocyanine macrocycles as PPGs.
Axial alkyl ligands in various Si-porphyrin derivatives (Figure 22) undergo homolytic cleavage upon irradiation with visible light;949−951 the dissociation energy of the axial Si–C bond is relatively low950 (around 40 kcal mol–1).952 Accordingly, Ziady, Burda, and co-workers found that an axial alkyl tether used to link Si-phthalocyanines to Au nanoparticles (163) underwent efficient photochemical homolytic cleavage upon irradiation with 660 nm light (Scheme 55).953 This Au-drug delivery system is initially PDT-inactive because the excited state of the Si-phthalocyanine is quenched by the Au nanoparticle (see also section 6.4). Upon irradiation, the chromophore is liberated and undergoes ligand exchange950 with water to give a PDT-active species that produces singlet oxygen with a quantum yield of 49%. The homolytic photocleavage of axial alkyl groups was also investigated in methanol,954 and a mechanism was proposed involving the initial formation of a radical centered on the Si atom of the Si-phthalocyanine and an alkyl radical that subsequently abstracts hydrogen from methanol. Several Si-phthalocyanine derivatives bearing amino acrylate axial linkers cleavable by singlet oxygen produced by in situ phthalocyanine sensitization have been reported.955−957
Scheme 55. Si-Phthalocyanine Attached to Au Nanoparticle as a PPG953.
The dissociation energy of axial Si–O bonds in Si-phthalocyanines is much higher (≥80 kcal mol–1) than that of comparable Si–C bonds.952 Irradiation of Si-phthalocyanines bearing both axial alkyl and alkylsiloxy ligands (Figure 22, R = alkyl, R′ = alkylsiloxy groups) leads to the exclusive homolytic liberation of the alkyl group.952
The photochemistry of a series of Si-octaphenoxyphthalocyanines bearing aryloxy, siloxy, aminoalkoxy, carboxyl, and sulfonyloxy groups as axial ligands was studied by Nyokong and co-workers.945 Axial ligand exchange to give the corresponding hydroxy derivatives in DMSO solutions was suggested to proceed via intermolecular electron transfer between the phthalocyanine π,π* excited state and an electron acceptor. Schnermann and co-workers showed that the Si–OAr bond in Si-phthalocyanines with axial aryloxy ligands can be cleaved in aqueous solutions using NIR light.878Scheme 56 shows the synthesized aryloxy derivatives 164 (Figure 19), which liberated substituted coumarin and stilbene moieties in degassed (hypoxic) aqueous solutions upon irradiation at 690 nm. Complex 164a released the fluorescent reporter 4-methylumbelliferone, and the photorelease of combretastatin-A4 and its E-isomer from 164b and 164c, respectively, was used to study the effects of tubulin polymerization inhibition under low-O2 conditions typical of tumor microenvironments. Under normoxic (normal oxygen concentration) conditions, complexes 164 exhibited reactive oxygen species-mediated phototoxicity. Both spectroscopic and computational studies provided evidence of photoinduced electron transfer to the Si-phthalocyanine triplet to form a radical anion intermediate that undergoes ligand exchange with water958 (Scheme 57). DFT calculations indicated that the attack of water as a nucleophile on the radical-anion center is more feasible than an attack on the neutral complex.958
Scheme 56. Si-Phthalocyanine PPGs to Release Aryloxy Groups878.
Scheme 57. Ligand Exchange in a Si-Phthalocyanine PPG958.
A different way of exploiting photoinduced ligand release from Si phthalocyanines is embodied by the phthalocyanine derivative IR700 (165, Scheme 58), which releases a ligand upon irradiation at 676.5 nm in the presence of l-ascorbate as an electron donor.959,960 It was proposed that this reaction changes the dye’s hydrophilicity and propensity to aggregate in aqueous solutions, which contributes significantly to the induction of cell death.
Scheme 58. Ligand Exchange in the Phthalocyanine IR700959.
Herges and co-workers designed NiII-porphyrin systems 166, which undergo photochemical axial coordination/de-coordination (Scheme 59)961,962 in a manner that enables controlled coordination-induced spin-state switching.963 Photochemical isomerization of the E-azopyridine ligand to the Z form weakens its binding due to steric clashing between the substituents at the 2-positions of the pyridyl rings, resulting in ligand release. When the axial ligand is bound, the Ni complex is pentacoordinate, high spin, and paramagnetic; dissociation of the axial ligand causes switching to the diamagnetic tetracoordinate low spin state. Similarly, the spin state of FeIII porphyrins bridged with 1,2,3-triazole ligands can be changed by adding phenylazopyridine as a photodissociable ligand,964 or the pyridine-bearing dithienylethene (DTE) photoswitch can be used to induce metal–ligand interaction between two ZnII-porphyrin moieties connected through a diethyne linker.965
Scheme 59. NiII–Porphyrin PPGs.
3.3. Photochemistry of Ruthenium(II) Polypyridyl Complexes
The photochemical activity of metal polypyridyl complexes has been known for decades, and the archetypical chromophore of this type, the [Ru(bpy)3]2+ cation (bpy = 2,2′-bipyridyl; Figure 23), has received considerable attention because of its unique optical and physicochemical properties.56,70,860,966,967 It absorbs in the visible region (λmaxabs ≈ 450 nm) and the metal-to-ligand charge-transfer (MLCT) d → π* transition populates the singlet state, formally represented as a RuIII–bpy– state, which is converted into the triplet 3MLCT state in about 10 fs968 with an ISC quantum yield of almost unity.966 The long-lived triplet state 3MLCT can be deactivated by radiative, non-radiative, and electron transfer pathways or be thermally activated to give a low-lying triplet ligand-field state (3LF) with an Ru–ligand antibonding character that can lead to ligand release.70,72,969−971 A relationship between the π-accepting ability of the ligands and the photosubstitution efficiency has been demonstrated.972−975 In the triplet state, this complex is an efficient oxygen sensitizer.976 The photochemical applications of [Ru(bpy)3]2+ range from solar energy conversion, photocatalysis, and sensing to photochemotherapy and bioimaging.57,70,71,966
Figure 23.
RuII polypyridyl complexes.
The photoreactions of analogous [Ru(bpy)2L1L2]2+ complexes (where L1 and L2 may belong to the single or separate ligands; see Figure 23) have attracted great interest in the context of photocaging and are discussed at length in recent reviews and perspectives.70,72,977−979 The major advantage of these and many other transition metal-containing photoactivatable systems is that they can release neutral bioactive small molecules (ligands) including nitriles, amines, and aromatic heterocycles.980,981 The absorption spectra of [Ru(bpy)2L1L2]2+ feature strong bands in the visible region (λmaxabs ≈ 420 nm), but larger conjugated terpyridine or bisquinoline ligands shift the absorption maximum up to as much as 600 nm, with tail absorption extending into the phototherapeutic window in the NIR and IR regions.982 In an early study, Pinnick and Durham found that the quantum yields of photosubstitution (ligand exchange) in [Ru(bpy)2L1L2]2+ derivatives correlated with the energy of the lowest energy charge-transfer transition.983 It has been suggested that the direct population of the reactive 3LF state from 1MLCT along with the population of the emissive 3MLCT state are the first photophysical events to occur in these complexes.969,977,984 However, Dunbar and Turro showed that the population of 3MLCT competes with ligand liberation on time scales of fs to ps.985,986 In their work, irradiation of [Ru(bpy)2(CH3CN)2]2+ in water resulted in stepwise CH3CN release to give [Ru(bpy)2(CH3CN)(H2O)]2+ and [Ru(bpy)2(H2O)2]2+ as the first and second intermediates, with the former complex being detected after only 77 ps.
Etchenique and co-workers created a [Ru(bpy)2L1L2]2+ PPG by coordinating two K+ channel-blocking 4-aminopyridine (4AP) ligands to obtain complex 167 (Scheme 60). These ligands were released sequentially upon irradiation at 480987 or 800 nm (2P absorption).988 A similar strategy was used to cage nicotine,989 γ-aminobutyric acid (GABA, for which the release quantum yield was 0.036990) and other amines.879,991−993 When one of the monodentate ligands is triphenylphosphine, which is a weaker σ-donor than an amine but a stronger π-acceptor (168, Scheme 60, Figure 19), the RuII center becomes electronically depleted, resulting in more efficient GABA liberation (Φr > 0.21).990 A kinetic flash photolysis study showed that [Ru(bpy)2(PMe3)(glutamine)] photoreleases glutamine within 50 ns.994 The analogous [Ru(bpy)(dcbpy)py2]2+ and [Ru(dcbpy)2 py2]2+ complexes (bpy = 2,2′-bipyridine, dcbpy = 4,4′-dicarboxy-2,2′-bipyridine, and py = pyridine) released their pyridine ligands upon irradiation at 450 nm at physiological pH.995 Similarly, [Ru(ane)(chel)(py)]2+ (ane = 1,4,7-trithiacyclononane, chel = chelating diimine) photoreleased pyridine at 470 nm.996 In another application, a weakly fluorescent rhodamine-substituted RuII complex was shown to photorelease a rhodamine dye, increasing its fluorescence intensity almost six-fold.997
Scheme 60. [Ru(bpy)2L1L2]2+ Complexes 167(988) (4AP = 4-Aminopyridine) and 168(990) as PPGs.
Sterically bulky ligands were introduced to distort the pseudo-octahedral geometry of the RuII complexes, which reduces the energy of the locally-excited 3LE state, and increases the efficiency of ligand exchange.70 For example, 2,2′-biquinoline (biq) is photoreleased from [Ru(biq)(phen)2]2+ (phen = 1,10-phenathroline), whereas [Ru(phen)3]2+ is photochemically inactive.998 This phenomenon was demonstrated by Turro and co-workers in a series of Ru complexes bearing tridentate ligands, such as [Ru(tpy)(Me2 bpy)(py)]2+ (Me2 bpy = 6,6′-dimethyl-2,2′-bipyridine, tpy = terpyridine, py = pyridine).999 As shown in Scheme 61, this complex (169) undergoes ligand exchange with a quantum yield of 0.16 upon irradiation at 500 nm. This value is approximately 3 orders of magnitude higher than that for [Ru(tpy)(bpy)(py)]2+, which features the sterically undemanding bpy ligand rather than the sterically bulky Me2 bpy. The 3LE state was found to form within 3–7 ps, and it can be deactivated by ligand dissociation or non-radiative decay. Building on preceding theoretical studies,1000,1001 Alary and co-workers performed DFT calculations indicating that these results can be attributed to the formation of a quasi-degenerate triplet metal-centered state and triplet excited-state potential energy surfaces with differing topologies.1002,1003 An analogous photoactivatable ruthenium complex [Ru(tpy)(bpy)(L)]2+, where L is a rigidin derivative caged through its thioether group, released the caged ligand upon irradiation at 530 nm.1004 The rigidins are cytotoxic marine alkaloids known to kill cancer cells. A series of related Ru terpyridine complexes bearing acetylacetonate-based ligands (Scheme 61, 170, X = H or halogen) was synthesized to bathochromically shift the absorption of these systems (λmaxabs < 517 nm).1005 These complexes exhibited quantum yields of ligand release five- to seven-times higher than that of [Ru(tpy)(bpy)(CH3CN)]2+.
Scheme 61. RuII Complexes 169(999) and 170(1005) as PPGs.
Another class of RuII complexes features tetradentate ligands such as tris(2-pyridylmethyl)amine 171 (Figure 24).72,977,1006−1008 These stable complexes can cage a wide range of different ligands L, including the cathepsin K inhibitor Cbz-Leu-NHCH2CN and nicotinamide, which are released upon irradiation at >400 nm. The selective release quantum yields of cis-nitrile ligands (∼0.01) were higher than those for cis-heterocyclic ligands in water, which was attributed to aromatic heterocycles being stronger σ-donors than nitriles.977 Rigid complexes 172 and 173 bearing tetradentate piperidine ligands (Figure 24) also underwent photochemical ligand exchange with quantum yields of 0.001–0.03.1009 DFT studies on the two isomers of the tris(2-quinolinylmethyl)amine (TQA) complexes [Ru(TQA)(MeCN)2]2+172970 and 173(1009) showed that orbital mixing is crucial for effective ligand photodissociation.
Figure 24.
RuII complexes with the tetradentatetris(2-pyridylmethyl)amine ligand and its analogs as PPGs.
Bonnet and co-workers demonstrated the photorelease of 2-(methylthio)ethanol from RuII complexes such as 174 (Scheme 62).1010 This ligand was released in water upon irradiation at 465 nm with a quantum yield of 0.13. An analogous complex bearing 6,6′-dichloro-2,2′-bipyridine as a ligand was used to control light-responsive supramolecular interactions.1011 The photorelease of a microtubule-targeted rigidin analog from [Ru(tpy)(bpy)L]2+ derivative 175 (Figure 25) in hypoxic cancer cells is another notable practical application of RuII-based PPGs.1004 A series of RuII polypyridyl complexes bearing 6-mercaptopurine as a photocleavable ligand was prepared by Renfrew and co-workers.1012 The highest release quantum yield (0.6) in this series was achieved with complex 176 (Scheme 62), which liberates 6-mercaptopurine upon irradiation at 465 nm in acetonitrile. The 1,4,7-trithiacyclononane RuII complexes 177–179 (Figure 25), bearing photocleavable pyridine, DMSO, 3-acetylpyridine, and imidazole ligands, were designed and studied by Alessio, Sadler, and co-workers.1013−1015 These complexes release their ligands upon irradiation with blue light (400–490 nm).
Scheme 62. RuII Photoreleasable Complex 174(1010).
Figure 25.
RuII complexes as PPGs.
Many other applications of RuII complexes as photoactivatable groups have been reported. The liberation of neurotransmitters to enable control over receptor activity in neuronal cells was mentioned above.56,879 These complexes have also been used for controlled release of small molecule drugs and enzyme inhibitors. Enzymes successfully targeted in this way include proteases,1016 cathepsin B (inhibited with a novel dipeptidyl nitrile1017),980,1018 nicotinamide phosphoribosyl transferase,1019 cytochromes P450,1020 and CYP17A1.1021 Drugs successfully photoreleased from RuII complexes include the anticancer agent CHS-828,1022 the imidazole-based cytotoxic drug econazole,1023 the anti-tuberculosis drug isoniazid,1024 and 5-cyanouracil.1025,1026 Additionally, a photoactivatable histidine building block for Fmoc/t-Bu solid-phase peptide synthesis based on a RuII complex with an imidazole ligand was used to prepare caged histidine peptides.1027 A library of tetra- and pentadentate ligands was attached to a polystyrene resin to prepare the corresponding photolabile RuII complexes for a solid-phase synthesis application.1028 [Ru(bpy)2(4AMP)2] (4AMP = 4-(aminomethyl)pyridine) was incorporated into polyurea organo- and hydrogels and used as a photoremovable moiety to induce de-gelation upon 1P or 2P excitation.1029 Similarly, supramolecular crosslinked gels with a photosensitive ruthenium bipyridine complex functioning as a crosslinker and poly(4-vinylpyridine) as a macromolecular ligand were developed by Teasdale and Monkowius.1030 Photolysis of these organogels with visible (>395 nm) and NIR light (1028 nm; a multiphoton process) resulted in the liberation of the pyridine moieties and degelation.
Photoinduced ligand dissociation from ruthenium complexes can also be accompanied by singlet oxygen production.70 Turro and co-workers showed that the triplet excited state of the [Ru(bpy)(dppn)(CH3CN)2]2+ (dppn = benzo[i]dipyridophenazine) complex efficiently sensitizes oxygen to give 1O2 in aqueous solution (ΦΔ = 0.72) and also releases acetonitrile in a less efficient competing process (Φr <0.01).1031 Similar dual reactivity was demonstrated for [Ru(tpy)(Me2 dppn)(py)]2+ (dppn = dimethylbenzo-[i]dipyridophenazine)1032 and [Ru(pydppn)(biq)(py)]2+ (pydppn = (pyrid-2-yl)benzo[i]dipyridophenazine)1033 complexes. Additionally, a structurally distinct nitrosyl phthalocyanine ruthenium complex was shown to produce singlet oxygen and release nitric oxide (see section 4.2).1034
RhIII complexes with polypyridyl and phenanthrene quinone diamine ligands are also photoactive and have been used to achieve photoinduced DNA cleavage. However, ligand exchange reactions are not the primary processes responsible for their photochemical activity.113
3.4. Photochemistry of Dirhodium(II,II) Complexes
Dirhodium (RhII–RhII) complexes have also attracted attention as photoactivatable species,71,113 although there have been few studies on this aspect of their behavior. The complex 180, reported by Turro and co-workers, has an absorption maximum at 525 nm (εmax = 218 M–1 cm–1; Scheme 63; Figure 19) in acetonitrile, which was attributed to a Rh2(π*) → Rh2(σ*) transition on the basis of TD-DFT calculations.880 This compound selectively exchanges its axial CH3CN ligands with H2O in aqueous solutions in the dark. Upon irradiation of the product with visible light, two equatorial CH3CN ligands dissociate and are replaced with water to give three different isomers of cis-[Rh2(μ-O2CCH3)2(CH3CN)2(H2O)4]2+, causing a slight bathochromic shift of the absorption maximum. The liberation quantum yields depended on the irradiation wavelength: Φ355 nm = 0.37 and Φ509 nm = 0.09. Irradiation of 180 in water in the presence of 2,2′-bipyridine or 9-ethylguanine led to the coordination of these ligands to the dirhodium core. Similar results were obtained with cis-[Rh2(HN(O)CCH3)2(CH3CN)6]2+, which releases two molecules of acetonitrile upon irradiation at >495 nm to form bis-aqua products,1035 and with [Rh2(O2CCH3)2(CH3CN)6]2+, which releases its axial CH3CN ligands upon irradiation at >455 nm.1036
Scheme 63. Dirhodium (RhII–RhII) Complex as a PPG880.
The 1,10-phenanthroline complex 181 was shown by Turro and Dunbar to release two equatorial CH3CN ligands in water upon irradiation with visible light (λirr >590 nm), whereas mononuclear radical RhII fragments were formed upon homolytic photocleavage of the metal–metal bond (Figure 26).1037 Remarkably, the release quantum yield measured upon irradiation at 550 nm exceeded unity (Φr = 1.38), suggesting that a dark release follows the initial photoreaction. Another photoactivatable dirhodium complex, 182, bearing a benzo[i]dipyridoquinoxaline ligand (Figure 26) was designed to serve as a DNA-intercalating singlet oxygen generator (ΦΔ = 0.22 at 477 nm) thanks to its low-lying dppn-centered 3π,π* state.1038 Upon irradiation in water, acetonitrile is released from this compound and replaced by H2O as a ligand (Φr = 0.0033 at 450 nm).
Figure 26.
3.5. Photochemistry of Pt-, Co-, and Fe-Containing Organometallic Complexes
Usually unreactive PtIV prodrugs are important anticancer compounds1039 that are designed to be converted into toxic PtII species in vivo by reducing agents such as ascorbic acid.1040 Well-known PtII drugs such as cisplatin and carboplatin have very narrow therapeutic indexes, so there is great interest in their controlled photochemical production in target tissues. Several visible-light absorbing photoactivatable PtIV complexes with the general structure trans,trans,trans-[Pt(N3)2(OH)2(N1)(N2)] (183, N1, N2 = pyridine or amines; Figure 27) were studied by Sadler and co-workers and shown to be cytotoxic to cancer cells upon irradiation with blue light.1041−1044 Compounds 183 do not liberate pyridine or amines upon excitation but do exhibit Pt–N3 bond elongation, eventually leading to the release of azidyl radicals.1041 This concept was also used in the design of a photoactivatable dopamine-conjugated PtIV anticancer complex that was incorporated into borate hydrogels,1045 as well as PtIV triazolato azido complexes that photorelease PtIV and PtII 5′-guanosine monophosphate species.1046 Additionally, the oxaliplatin-based photocaged PtIV prodrug coumaplatin (184), was shown to release an axial ligand upon irradiation at 450 nm, forming a cationic PtIV intermediate that oxidizes water and generates oxygen under biological conditions.1047
Figure 27.
PtIV complexes as PPGs.
Chakravarty and co-workers showed that curcumin (185, Figure 28), a compound with significant antioxidant, anti-inflammatory, antiseptic, and anticancer activities,59 can form photoactivatable PtII complexes.1048−1050 For example, [Pt(NH3)2(cur)](NO3) (186, cur = curcumin) exhibits a strong absorption band with a λmaxabs of ∼430 nm and releases two anticancer agents, curcumin and a cisplatin analog (which crosslinks DNA), upon irradiation with visible light.1048 The analogous [Pt(en)(cur)](NO3) and [Pt(dach)(cur)](NO3) (187, en = ethylenediamine, dach = 1R,2R-(−)-1,2-diaminocyclohexane) complexes also liberated curcumin under similar conditions.1049 The use of a photosensitizer as a ligand (see also section 6) can lead to dual photochemotherapeutic effects. This was demonstrated using [Pt(L)(R-BODIPY)]Cl complexes, where R-BODIPY is a distyryl-BODIPY derivative (sensitizer) and L are different terpyridine ligands. Irradiation of these species with red light (600–720 nm) caused both singlet oxygen production and the release of photoactive BODIPY ligands, resulting in appreciable photocytotoxicity.1051 Similarly, platinum(II) ferrocenylterpyridine (Fc-tpy) complexes [Pt(Fc-tpy)(L)]Cl (L = a biotin-containing ligand) released their biotinylated ligands upon irradiation with red light (647 nm) because of the photosensitizing behavior of the Fc-tpy ligand.1052 Finally, the very interesting heptamethine cyanine-based PtII complex 188 (Figure 28) was reported to undergo Pt–O bond scission and to generate singlet oxygen upon irradiation with near-IR light.1053
Figure 28.
Photoactivatable PtII complexes.
Unlike RuII and PtIV complexes, CoIII complexes (see also section 3.1) usually have very weak absorption bands in the visible region.875 Therefore, strongly absorbing ligands that can photoreduce the CoIII ion to induce ligand release have been developed. The first reported complex of this type was the RuII–CoIII heterodinuclear species 189 (Figure 29), which has an absorption maximum close to 400 nm.1054 Upon irradiation with visible light, the RuII moiety probably transfers an electron to the CoIII complex to produce a CoII species with concomitant release of the ethylenediamine ligands. Renfrew and co-workers reported the release of curcumin from CoIII curcumin complexes such as 190 (Figure 29, Figure 19).881 This complex absorbs at λmaxabs = 451 nm, and the authors hypothesized that irradiation at 520 nm causes electron transfer from curcumin to the cobalt ion. The photodegradation quantum yield for this compound was found to be 0.01. A similar strategy was demonstrated using ternary CoIII complexes of mitocurcumin (a water-soluble curcumin derivative) bearing a tetradentate phenolate-based ligand.1055 Mitocurcumin was released upon irradiation with visible light and was shown to act as a phototoxin that generated reactive oxygen species in cells.
Figure 29.
RuII–CoIII, CoIII, and FeIII complexes.
The analogous charge-neutral FeIII complex 191 (Figure 29) and two other high-spin iron complexes reportedly released curcumin upon irradiation with visible light, and thus exhibited cytotoxicity in multiple cell lines.1056 In addition, FeIII–polysaccharide hydrogels were found to be visible-light (405 nm) responsive because of the photoreduction of the FeIII ions to FeII, which rendered the Fe complexes incapable of functioning as cross-linkers for the polymer.1057
4. Photorelease of Gasotransmitters
Gasotransmitters are small gaseous endogenously-produced signaling molecules that are involved in the control of a vast array of physiological processes in the cardiovascular, nervous, gastrointestinal, excretory, and immune systems as well as cellular functions including apoptosis, proliferation, inflammation, metabolism, oxygen sensing, and gene transcription.1058 The most important gasotransmitters identified to date are nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S). These small molecules are freely permeable through membranes and are perceived without cognate receptors.1059 Their molecular targets can be divided into two groups. The first are metal-containing prosthetic groups of proteins that form coordination complexes with CO and NO; examples include heme-imidazoles (as in hemoglobin and cytochrome c oxidase), thiolated hemes (as in cytochromes P450), and non-heme iron complexes (as in prolyl hydroxylase and superoxide dismutase). The second group consists of organic thiols, which can be nitrosylated by NO and sulfhydrated by H2S.1058 Gasotransmitter perception can induce diverse macroscopic biological responses, many of which are therapeutically relevant such as vasodilatation,1060,1061 protection of tissues against hypoxia,1062 anti-inflammatory processes,1063 wound healing,1064,1065 platelet aggregation inhibition,1066 postsynaptic plasticity augmentation, and hormone secretion.1067 The simplest method of administering gasotransmitters is by direct inhalation of small quantities of the gaseous species. While this approach induces therapeutic effects in some contexts,1068,1069 its usefulness is limited by narrow pharmaceutical windows and it requires precise control of the gasotransmitter’s concentration, which is very difficult to achieve.1070 Consequently, there is considerable interest in the development of gasotransmitter-releasing molecules.80,82,94
Most known gasotransmitter-releasing molecules are based on metal complexes that release a weakly bound gasotransmitter ligand via simple hydrolytic ligand exchange upon dissolution in aqueous media. Such complexes are prone to rapid initial releases of the bound gasotransmitter prior to administration to the target organism but do not allow for precise control over the release. Enzymatically triggered reactions that offer more controlled release profiles have also been demonstrated.1071 An alternative activation strategy that enables precise spatial and temporal control over gasotransmitter release is to use photochemically activatable gasotransmitter-releasing molecules such as photoactivatable CO-releasing moieties (photoCORMs) or photoactivatable nitric oxide-releasing moieties (photoNORMs).77−114 In therapeutic applications, organic (transition-metal-free) photoCORMs can have important advantages over metal carbonyl complexes such as more favorable biodistribution and lower toxicity.80−82 Because of their distinct physicochemical properties (which typically include small size or neutral charge), the photorelease of gasotransmitters requires unique and highly specific strategies that may differ appreciably from those used for photorelease of larger ligands. Therefore, we present this research area in its own section. Research on photochemically activatable gasotransmitter-releasing molecules has advanced rapidly in the past decade, and many reviews are available.49,80−102,1072−1074 This section focuses solely on visible-light-absorbing photorelease systems. Figure 30 shows the absorption spectra of selected transition-metal-free molecules that release gasotransmitters upon excitation with visible or NIR light, and Figure 31 shows the absorption spectra of some transition metal complexes with such activity.
Figure 30.
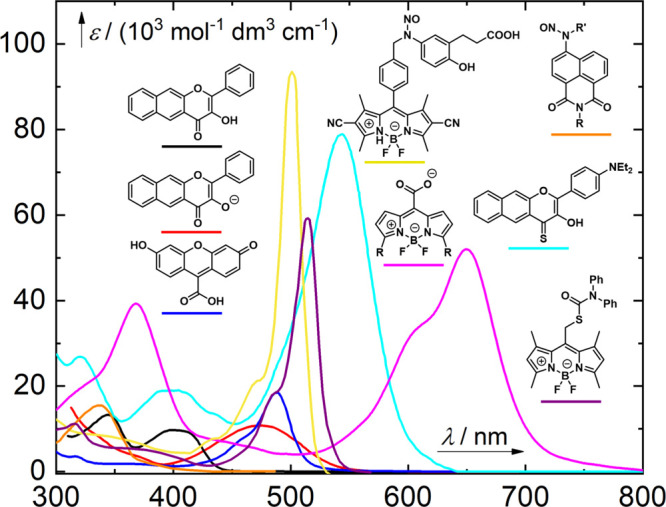
Representative absorption spectra of transition-metal-free molecules that photorelease CO, NO, and H2S. Black,1075 red,1075 cyan1076 lines, flavonol-based photoCORMs (section 4.1.1); blue line, a xanthene-based photoCORM (section 4.1.1);763 magenta line, a BODIPY-based photoCORM (R = a styryl group; section 4.1.1);847 yellow line, a BODIPY-based photoNORM (section 4.2.1);1077 orange line, a naphthalimide-based photoNORM (section 4.2.1);1078 violet line, a BODIPY-based H2S releasing molecule (section 4.3).799
Figure 31.

Representative spectra of transition-metal complexes photoreleasing CO, NO, and H2S. Red,1079 blue,1079 green,1080 cyan1080 lines, MnII tricarbonyl photoCORMs (section 4.1.2); magenta1081 and orange1082 lines, MnII photoNORMs (section 4.2.2); black line, a RuII-based H2S releasing complex (section 4.3).1083
4.1. Release of Carbon Monoxide
4.1.1. Transition-Metal-Free PhotoCORMs
The lowest excited state of simple ketones and aldehydes corresponds to the excitation of an electron from the n lone pair to the π* molecular orbital.136 n,π*-Transitions are generally weak and often hidden by the red tail of a stronger π,π*-absorption. Homolytic cleavage of the α-bond in ketones (α-cleavage; Norrish type I reaction) often results in decarbonylation, that is, carbon monoxide (CO) release. Therefore, carbonyl compounds have been widely used as photoCORMs.80,82
The release of CO from prototypical aliphatic acyclic and especially small cyclic ketones by radical decarbonylation occurs only at the edge of the vacuum UV range (e.g., λirr = 193 nm for 3-cyclopentenone).1084 However, an extension of the ketone π-system results in bathochromic shifts of their absorption maxima.136 1,2-Dicarbonyl compounds (which typically absorb above 300 nm) can also be photolyzed to produce CO.1085 Irradiation of bicyclo[2.2.2]octane-2,3-dione 192a in toluene at the edge of the visible region (395 ± 25 nm) resulted in the formation of aromatic side-products 193a–f and the release of two equivalents of CO (Φr = 0.02, εΦCO = 6 at 395 nm) (Scheme 64).1085 1,2-Diketone 192a was used as an additive in poly(ε-caprolactone) electrospun scaffolds designed for vascular tissue engineering1086 and was shown to release CO upon irradiation at λirr = 470 nm in this environment.1086,1087 To increase the hydrophilicity of the bicyclo[2.2.2]octane-2,3-dione scaffold, a derivative substituted with oligo-(ethylene)glycol side chains (192b) was prepared.1087 However, 192b did not release CO when dissolved in a water/DMSO (99:1) mixture due to hydration of the carbonyl groups. This was circumvented by encapsulating 192b in micelles of Pluronic F127, a biocompatible block copolymer of poly(ethylene oxide) and polypropylene oxide. The encapsulated compound efficiently released CO upon irradiation at 470 nm, and the system was successfully used in vitro.1087
Scheme 64. 1,2-Diketones as PhotoCORMs1085−1087.
Liao and co-workers recently overcame the hydration-induced deactivation of photoactivity in bicyclo[2.2.2]octane-2,3-dione by preparing derivative 192c, which carries t-butyl substituents (Scheme 64) that sterically hinder hydrate formation.1088 This compound was incorporated into poly(butyl cyanoacrylate) nanoparticles and used as a tissue adhesive with possible applications in CO delivery to the brain. Additionally, Raymo and co-workers have designed an autocatalytic reaction based on the photoinduced decarbonylation (λirr = 420 nm) of 192a and 192d, which is sensitized by its own photoproducts, the anthracene derivatives 193. The quantum yields of decarbonylation for 192a and 192d were 0.20 and 0.50, respectively.1089
The group of Yamada substituted the bicyclo[2.2.2]octane-2,3-dione scaffold with BODIPY antennas to obtain 192e and 192f (Figure 32).1090 These compounds have a major absorption maximum in the green-to-yellow region (λmaxabs = 534 nm for 192e and λmax = 605 nm for 192f) and release CO upon irradiation at 450 nm via initial photoinduced electron transfer from the BODIPY moiety to the 1,2-diketone functionality. Moreover, 192e releases 2 equiv of ethylene via a thermal process that occurs upon heating to 220 °C. The release of CO and ethylene are thus orthogonal and can be performed sequentially. Unfortunately, 192e and 192f are insoluble in polar media, which limits their biological applications. Diketones 194a and 194b (Figure 32) decarbonylate upon irradiation at λirr = 395 ± 25 nm, but their aromatic photoproducts (hexacene and heptacene, respectively) do not accumulate in the reaction system due to their fast oxidation and dimerization.1085,1091,1092
Figure 32.
1,2-Dicarbonyl PhotoCORMs.
2-Ketocarboxylic acids can also be used as photoCORMs. Visible light irradiation (>390 nm) of the tetra-(2-N-methylpyridyl)porphyrin-FeIII complex with 2-ketocarboxylic acid 195 led to photoinduced electron transfer from the carboxylate anion to the central metal ion, yielding an FeII complex and a carboxyl radical (Scheme 65)1093 that underwent simultaneous decarboxylation and decarbonylation. The released CO was then efficiently trapped by the FeII-porphyrin complex in the solvent cage.
Scheme 65. Photolysis of 2-Ketocarboxylic Acids (R = iPr, Ph)1093.
Flavonol or 3-hydroxyflavone (3-hydroxy-2-phenylchromen-4-one; 196, Scheme 66) belongs to the family of flavonoids, well-known natural antioxidants1094,1095 that have been recognized as CO-releasing molecules. Unsubstituted flavonols absorb only in the UV region and, thanks to their biological relevance, the photodecomposition mechanism responsible for the resulting CO release has been studied since the 1960s. The photosensitized oxygenation of 196 by singlet oxygen generated photochemically in situ was reported in the seminal work of Matsuura and co-workers,1096 who showed that it results in the formation of CO together with o-benzoyl salicylic acid 197 as a side-photoproduct. The reaction was suggested to proceed via an endoperoxide intermediate. In the absence of oxygen, 3-hydroxyflavone rearranges into the 3-arylphthalide derivative 198 with concomitant CO liberation. The 3-hydroxy group was found to be essential for this reaction because the analogous 3-methoxyflavone derivative is photostable. These mechanistic pathways were later studied in detail.1097−1100 Kubinyi and co-workers introduced the push-pull substituted 4′-diethylamino-3-hydroxyflavone and its MgII complex.1101 However, despite the ESIPT character of this compound and its absorption in the visible part of the spectrum, only UV-light-initiated CO release was studied. The photochemistry of flavonol-based CORMs was recently reviewed.92
Scheme 66. Photodecarbonylation of 3-Hydroxyflavone1096.
Flavonols are excellent ligands for d-block elements; complexation of metal cations with flavonolate anions causes bathochromic shifts of their lowest energy absorption bands into the visible part of the spectrum and also increases their molar absorption coefficients in some cases.1099 Because of their biological relevance in plant metabolism and occurrence in soil,1102 the photochemistry of these metal complexes has been studied in detail. The photoreactivity of PbII and AlIII flavonolato complexes is suppressed, whereas ZnII complexes exhibit similar reactivity to free 3-hydroxyflavone.1099 The flavonolate complex [(6-R2TPA)Zn(3-Hfl)]ClO4 (TPA = tris(2-pyridylmethyl)amine) 199 (Figure 33),1103 ZnII complexes bearing tetradentate tripodal nitrogen donor ligands and flavonol derivatives 200 or 201,1104 and the bipyridine-ligated ZnII complex 202 with a bridging flavonolate ligand1105 all released CO upon irradiation at λirr > 400 nm. In addition, the RuII cymene complex 203 released CO upon irradiation at either 300 or 419 nm.1106 Additionally, Farmer and co-workers synthesized and characterized a series of RuII bipyridine-substituted flavonolato complexes 204,1107,1108 and studied the mechanism of their photooxygenation by 1O2 at low temperatures. Their results suggest that this process occurs via 1,2- or 1,3-addition to the flavonol core.
Figure 33.
Metal flavonolato complexes and their ligands.
The π-extended 3-hydroxyflavone 205 (Figure 34) and its derivatives absorb in the visible region.1076,1109 Displaying photochemistry similar to that of 196, Berreau and co-workers have demonstrated that 1 equiv of CO is photoreleased from 205. The reported quantum yield of CO release for 205 (Φr = 0.007, λirr = 419 nm) in DMSO/aqueous buffer (1:1, v/v, pH = 7.4) increased by a factor of 2 upon complexation with ZnII but was reduced by an order of magnitude upon binding to bovine serum albumin.1110 The 4′-diethylamino-substituted 206 exhibits bathochromically shifted absorption (λmaxabs = 442 nm) but with an unchanged photochemical efficiency of CO release.1076 The structure of 205 was further modified to obtain 4-flavonothione analog 207 and the 4′-diethylamino derivative 208, which have bathochromically shifted absorption bands (Figure 30).1076 Compound 207 also had a higher quantum yield of CO release than 205 (Φr = 0.4 at 419 nm). Because a free 3-hydroxy group was found to be essential for the photoreactivity of 205, its substitution with a protecting group sensitive to an external trigger allowed Berreau and co-workers to construct a series of RS– co-triggered “AND logic gates” that release CO only in the simultaneous presence of oxygen, light, and a thiol. An acryloyl-protected derivative 209(1111,1112) was activated by thiols including cysteine, while the cyanate-substituted compound 210 proved suitable for intracellular H2S sensing.1111 When combined with PdCl2, the allyl-protected flavonol derivative 211 was shown to act as an OFF-ON fluorescent CO sensor that replenishes the CO consumed during detection.1113 A similar approach was used by Tang and co-workers, who designed the hydrogen peroxide-sensitive compound 212.1114 Oxidation of this compound’s pinacol boronate ester by hydrogen peroxide liberates free 205, which can subsequently photorelease CO. The 2-nitrobenzyl-protected flavonol 213 was used by Hu and co-workers to prepare CO-releasing micelles that undergo a tandem photochemical reaction in which 2-nitrobenzyl deprotection is followed by CO release, accompanied by a dual fluorescence response.1115,1116 Flavonol derivatives substituted with polar groups such as triphenylphosphonium (214) were found to target mitochondria and affect cellular bioenergetics.1117 However, the sulfonated analog 215 did not penetrate through the cell membrane and thus enabled extracellular CO release.1118 Both derivatives had photochemical properties similar to 205, allowing Berreau and co-workers to compare the effects of extracellular (215), cytosolic (205), and mitochondrial-localized (214) photoinduced CO release.1117,1118 The 3-hydroxybenzo[g]quinolone derivative 216a releases one equivalent of CO upon illumination at 465 nm under physiological conditions.1119216b, an oxidized form of 216a, is photochemically stable and can act as a prodrug that is activated by thiol-mediated reduction in vivo.1119 The group of Feng recently introduced a coumarin-flavone hybrid 217 that combines the excellent absorption properties of the 7-(diethylamino)coumarinyl moiety with the CO-releasing ability of 3-hydroxyflavone.1120 Upon irradiation at 460 nm, the excited coumarinyl moiety transfers energy to the fluorescent flavone CO-releasing group. Following CO release, the molecule is transformed into a coumarinyl-substituted salicylic acid derivative with fluorescence similar to that of free 7-diethylaminocoumarin.
Figure 34.
π-Extended flavonol derivatives.
The mechanism of the aerobic photodecarbonylation of 205 was the subject of several investigations.1109,1110 Like its parent flavonol 196,1110,1121,1122 the visible-light absorbing 205 exists in both acid (205a) and base (205b) forms (Schemes 67 and 68; Figure 30).1123 Klán and co-workers showed that the singlet excited state of 205a undergoes rapid excited-state intramolecular proton transfer (ESIPT) in methanol to give 1205z* (z = zwitterion), which intersystem crosses to the triplet 3205z*. The triplet then reacts with ground-state oxygen, possibly via an endoperoxide intermediate, to release CO (Φr = 0.031; Scheme 67).1075 The conjugate base 205b releases CO via an oxygenation reaction with singlet oxygen formed by the sensitizing action of the triplet 3205b* (ΦΔ = 0.07; Φr = 0.018), and partially via oxidation with 3O2 (Φr = 0.003; Scheme 68). There are thus three major orthogonal pathways of CO release. In addition, both forms undergo a very inefficient photorearrangement to release CO in the absence of oxygen. An isotopic labeling study with 18O2 revealed that the photoproduct 218 exclusively incorporates 18O atoms.1076
Scheme 67. Photochemistry of Conjugate Acid 205a(1075).
Scheme 68. Photochemistry of the Conjugate Base 205b(1075).
Štacko, Klán, and co-workers developed a new class of transition-metal-free photoCORMs by fusing two CO-releasing flavonol moieties with a heptamethine cyanine chromophore (219a,b, Figure 35).1124 The resulting hybrids released CO in high chemical yields of ∼130% (in principle, 2 equiv of CO can be liberated) upon activation with NIR light of up to 820 nm, with excellent uncaging cross sections (Φrε(λ793 nm) = 75 for 219b). The biocompatibility and applicability of these systems in vitro and in vivo were also demonstrated.
Figure 35.
Cyanine-flavonol hybrid 219(1124) and ZnII complexes 220 and 221.
Complexation of 205, 207, and 208 with [ZnII(Ph2TPA)]2+ (TPA = tris(2-pyridylmethyl)amine) in 220 (Figure 35) bathochromically shifts their absorption bands by 60–80 nm (e. g., λmaxabs = 600 nm for 220, X = S, R = NEt2) and increases the CO release quantum yield to almost unity (Φr = 0.95 for both 220, X = S, R = H, and 220, X = S, R = NEt2).1125,1126 One equivalent of CO is always released upon irradiation, and the complexes are active in both the solution and solid phases. In the absence of the TPA ligand, bis-flavonolato-ZnII complexes 221 are formed. These compounds have even more bathochromically shifted absorption spectra (by ∼10 nm) and can release, in principle, 2 equiv of CO originating from the two flavonolato ligands upon irradiation at >545 nm.1125
Cyclopropenones are strained systems that liberate CO upon irradiation by UV light.1127 Most 2,3-alkylcyclopropenones absorb only in the deep UV-region, but their absorption band can be bathochromically shifted by substitution, for example, 2,3-bis(4-methoxyphenyl)-cyclopropenone 222 has a λmaxabs of 340 nm (Figure 36).1127 2,3-Bis-naphthyl-cyclopropenone derivatives 223 have an absorption tail in the range of 400–440 nm, but 1P absorption leading to decarbonylation occurs only under illumination with UV light (λirr = 350–380 nm).1128 However, they also efficiently decarbonylate upon non-resonant two-photon absorption at 800 nm. Unfortunately, their strong π-stacking makes them poorly soluble in polar protic solvents, which often limits their usefulness as photoCORMs.
Figure 36.
Cyclopropenone photoCORMs.
The doubly 9-anthryl substituted cyclopropenone 224 absorbs in the visible region (λmaxabs = 465 nm), although excitation at these wavelengths does not induce decarbonylation.1129,1130 The lowest absorption band of this compound corresponds to an intramolecular excimer of the 9-anthryl substituents and does not weaken the C–C bonds in the cyclopropenone moiety. Derivative 224 thus releases CO only upon excitation with shorter wavelength light (λirr = 366 nm) due to a very fast adiabatic reaction from an upper excited state that is largely localized in the cyclopropenone chromophore.1131
Klán and co-workers discovered that xanthene-based carboxylic acid 140, isolated as a product from the photoreaction of 139 (Scheme 42, section 2.11), can release carbon monoxide via the triplet-excited state with Φr = 6.8 × 10–4 in aqueous solutions of pH 7.4 upon irradiation at 500 nm (Scheme 69).763 A 6-fold increase in the quantum yield was obtained at pH 5.7; under these conditions, 140 and its conjugate base exhibit equal absorbance at the irradiation wavelength (Figure 30). The photoreaction cross section Φrε(λirr) for 140 was on the order of 10 M–1 cm–1 at λirr ∼500 nm and pH 7.0 (Table 13). Irradiation of 140 at 503 nm in the presence of non-complexed methemoglobin (MetHb, FeII) in aqueous solution led to the formation of carbonylhemoglobin (COHb). Studies on isotopically labeled 140 (C18O2H) and DFT calculations suggested that an α-lactone intermediate is formed upon irradiation (via a mechanism analogous to that shown for BODIPY-based photoCORMs, vide infra), which subsequently thermally decarbonylates to release CO847,1132,1133 and form 3,6-dihydroxy-9H-xanthen-9-one as a photoproduct.763
Scheme 69. Photochemistry of photoCORM 140(763).
Table 13. Photophysical Properties of Some Organic PhotoCORMs.
| CORM | λmaxabs (nm) | εmax (M–1 cm–1) | nCO | Φr (λirr/nm) | solvent | ref |
|---|---|---|---|---|---|---|
| 140 | 488 | 18.6 × 103 | (1)a | 6.8 × 10–4 (500) | PBS | (763) |
| 191a | 461 | 0.3 × 103 | (2)a | 0.02 (395) | toluene | (1085) |
| 191e | 534 | 53.8 × 103 | (2)a | n.d. (450) | DCM | (1090) |
| 191f | 605 | 135 × 103 | (2)a | n.d. (450) | DCM | (1090) |
| 205 | 409 | 16.2 × 103 | 0.96 | 0.0073 (419) | CH3CN | (1075, 1076, 1109) |
| 208 | 543 | 79.4 × 103 | 1.00 | n.d. | CH3CN | (1076) |
| 224 | 465 (inactive) | 17.8 × 103 | ||||
| 374 (active) | 8.9 × 103 | (1)a | 0.14 (366) | cyclohexane | (1131) | |
| 225a | 502 | 49.0 × 103 | 0.87 | 2.7 × 10–4 (500) | PBS | (847) |
| 225b | 652 | 52.5 × 103 | 0.91 | 1.2 × 10–5 (365) | PBS | (847) |
Theoretical yield of CO equivalents. PBS = phosphate-buffered saline; DCM = dichloromethane.
Klán and co-workers also introduced two organic photoCORMs10,80 based on the BODIPY chromophore, 225a and 225b(847) (Scheme 70). The release of CO from 225a was achieved in 45% chemical yield with Φr = 1.1 × 10–4 in an aerated PBS solution (pH = 7.4) to give 2-methylpyrrole and 2H-pyrrole-4-carbaldehyde as the major additional photoproducts.847 These compounds are typical products of photochemical degradation of BODIPYs.1134 The release of CO from the π-extended BODIPY 225b(847) (λirr = 652 and 732 nm; Figure 30) occurred with a lower quantum efficiency (Φr = 1.4 × 10–5), presumably due to an enhancement of radiationless decay related to the presence of the two flexible styryl groups.1135,1136 Nevertheless, the release was efficient enough for use in vivo: white light-induced photoactivation of 225b in mice noticeably increased CO levels in the blood and some tissues.847 The involvement of a triplet excited state was established by transient spectroscopy, oxygen quenching experiments, and experiments using CsCl as a heavy-atom-effect mediator.1137 The benzyl ester derivative of 225a was photostable, and the photolysis of 225a at pH 2.5 proceeded with a ∼4-fold lower quantum efficiency than at pH = 7.0. This was in agreement with the calculated ΔGeT, which predicted a more efficient intramolecular electron transfer from the carboxylate to the triplet-excited BODIPY core than for the protonated form.847 The proposed mechanism of the photoreaction is shown in Scheme 71. Upon excitation, a strongly fluorescent singlet excited state of 225 undergoes relatively inefficient ISC to the triplet state followed by an exergonic electron transfer (eT) from the carboxylate to the BODIPY core to form an oxyallyl-type triplet diradical.1138 The diradical then intersystem crosses to an open-shell singlet state, followed by the formation of α-lactone on the singlet ground-state potential energy surface.847 Finally, thermal fragmentation of the lactone releases CO.1132,1133
Scheme 70. Photorelease of CO from BODIPY PhotoCORMs847.
Scheme 71. Proposed Mechanism of CO Photorelease from BODIPY PhotoCORMs 225a,b847.
4.1.2. Release of Carbon Monoxide from Transition-Metal-Containing PhotoCORMs
Most of the known photochemically activatable CO-releasing molecules (photoCORMs) are based on metal carbonyl complexes that undergo photoinduced cleavage of the carbonyl moiety followed by the addition of a solvent molecule to the vacant position in the metal’s coordination sphere.83 The mechanisms of photochemical CO release from the coordination sphere of a transition metal have been studied in detail.1139−1141 The carbonyl–metal bond is relatively strong (∼20–40 kcal mol–1)1142 because of its π-backbonding character. Delocalization of the LUMO on the carbonyl moiety is a general requirement for the photochemical liberation of CO.1140 The photocleavage is a reversible process; the recombination of the liberated CO molecule with the vacancy on the metal’s coordination sphere regenerates the photoCORM and thus reduces the quantum yield of CO release. This can be avoided by using ancillary ligands that shift electron density away from the metal center and reduce the amount of metal–CO backbonding.1139 It has been shown that not all carbonyl ligands are cleavable from complexes containing multiple carbonyl moieties and not all CO molecules are released in the primary photochemical step. CO can also be liberated by subsequent solvolysis or oxidative steps.82,1140,1143
The first report on a photoCORM was published by Motterlini and co-workers, who described the photodissociation of Fe(CO)5 and complex 226 (Figure 37).1144 The term photoCORM was introduced by Rimmer and co-workers in reference to W0 complex 227 (Figure 37), which releases one equivalent of CO upon irradiation.1143 The first transition-metal-containing photoCORMs required excitation at wavelengths in the range of 310–360 nm,1139,1145 but strategies for bathochromically shifting their spectra were introduced by Mascharak and co-workers.1146 The use of nitrogen-based ligands with extended π-conjugation can lower the energy of the LUMO, while strongly donating ancillary σ-and π-donors raise the HOMO energy. The combination of such ligands with highly thermostable carbonyl complexes of electron-rich d6 metal ions such as MnI, ReI, FeII, or RuII gives rise to bathochromically shifted MLCT absorption bands.
Figure 37.
Structures of some UV-absorbing photoCORMs.
Many visible-light-absorbing photoCORMs are based on MnI complexes. For example, MnI complexes with bidentate heteroaryl-imine ligands (Table 14) exhibit absorption maxima in the range of 390–700 nm. The absence of the σ-donating ligand Br–(as in 228b, 229b, and 231b) caused a hypsochromic shift of 60–110 nm (Figure 31) and reduced the CO release efficiency by a factor of ∼1.1–3.5 relative to the reference analogs (228a, 229a, and 231a).1079,1146,1147 Extending the conjugation of the aromatic ligand, for example, by replacing pyridine with quinoline (as in complexes 229a, 229b, and 234), bathochromically shifted the MLCT band absorption maximum by ∼35–45 nm and also increased the quantum yield of CO release. Complexes 232, 233, and 234 (Table 14) containing α-diimine ligands were designed to be more soluble in water.1148 Irradiation of 233 released 3 equiv of CO, but the quantum yield of this process declined by factors of ∼2 and 3 in dichloromethane and aqueous solutions, respectively. The CO release efficiency of these complexes increased in the order 232 < 233 < 234, paralleling the increase in the electron-donating abilities of their ligands. Studies on analogous MnI and ReI complexes containing 4-aminophenyl instead of adamantyl ligands revealed that only manganese complex 235 photoreleased CO upon visible light irradiation.1149 Zobi and co-workers studied a series of MnI-tricarbonyl complexes bearing azobipyridine ligands (236a–e, Table 14).1150 CO was liberated upon their illumination with red light (≥625 nm), and DFT calculations indicated that electron-withdrawing substituents lowered the LUMO energy more than that of the HOMO, resulting in a bathochromic shift of the MLCT band maximum. Complex 236e, which bore the strongest electron-withdrawing groups (CF3 and Cl) was even activatable at the tail of the absorption range (810 nm). In the dark, complexes 236a and 236b were stable but the electron-poor complexes 236c–e slowly released CO. A series of 8 benzimidazole-based photoCORMs 230 was recently studied by Schatzschneider.1151 These compounds rapidly released CO upon illumination, and their photochemistry was sensitive to their substitution. The 4-NO2 substituted derivative 230 exhibited the most bathochromically shifted absorption but released CO with the lowest observed chemical yield because of a competing photodecomposition process.
Table 14. MnI-Based photoCORMs Containing Bidentate Heteroaryl-Imine Ligandsa.
| CORM | λmaxabs (nm) | εmax (M–1 cm–1) | nCO | Φr (λirr/nm) | solvent | ref |
|---|---|---|---|---|---|---|
| 228a | 500 | 2.5 × 103 | <3 | 0.34 (509) | THF | (1079) |
| 228b | 390 | 3.6 × 103 | <3 | 0.12 (509) | CH3CN | (1079) |
| 229a | 535 | 2.2 × 103 | <3 | 0.37 (509) | THF | (1079) |
| 229b | 435 | 3.7 × 103 | <3 | 0.34 (509) | CH3CN | (1079) |
| 230 | 386–495 | 1.3–2.2 × 103 | 0.8–1.8 | n.d. (412 or 468) | DMSO | (1151) |
| 231a | 586 | 3.9 × 103 | n.d. | 0.48 (550) | CH3CN, DCM | (1146, 1147) |
| 231b | 520 | 4.1 × 103 | n.d. | ∼0.33b (420) | DCM | (1146, 1147) |
| 232 | 445 | 1.8 × 103 | n.d. | ∼0.18b(≥450)e | DCM | (1148) |
| 233 | 455 | 2.1 × 103 | 3 | 0.35 (≥450)e | DCM | (1148) |
| ∼0.16b | PBS | |||||
| ∼0.10b | H2O | |||||
| 234 | 490 | 1.9 × 103 | n.d. | ∼0.78b(≥450)e | DCM | (1148) |
| 235 | 437 | n.d. | 3 | n.d. (525 or 468) | DMSO | (1149) |
| 236a | 625 | 4.35 × 103 | n.d. | n.d. | DCM | (1150) |
| (τ1/2 = 3.52 h)c | (H2O)d | |||||
| 236b | 630 | 4.43 × 103 | n.d. | n.d. | DCM | (1150) |
| (τ1/2 = 3.60 h)c | (H2O)d | |||||
| 236c | 661 | 3.46 × 103 | n.d. | n.d. | DCM | (1150) |
| (τ1/2 = 1.21 h)c | (H2O)d | |||||
| 236d | 678 | 3.76 × 103 | n.d. | n.d. | DCM | (1150) |
| (τ1/2 = 0.48 h)c | (H2O)d | |||||
| 236e | 693 | 4.85 × 103 | n.d. | n.d. | DCM | (1150) |
| (τ1/2 = 0.41 h)c | (H2O)d |
n.d.: not determined, PBS = phosphate-buffered saline, THF = tetrahydrofuran, DMSO = dimethyl sulfoxide, DCM = dichloromethane.
Values estimated from the relative apparent CO release rates (kCO).
Relative half-lives in the series 236a–e; samples were irradiated at λmaxabs.
Aqueous solutions were used in the Mb assay1152 to determine CO release.
Broadband visible light with a cut-off filter was used.
α,α′-Diimines and related ligands can also be used to tune the properties of MnI-based photoCORMs. For example, the MnIfac-complex 237 (Table 15, L = Br−, Ar = 2,6-iPr2Ph) releases CO upon irradiation with green light.1153 Its photoactivity can be attributed to an MLCT transition from the MnI–CO π and Br-centered orbitals to the π* orbitals of the diamine ligand, which weakens Mn–CO π-backbonding and thus facilitates CO release. The substitution of the bromide ligand with a neutral molecule (237, L = CO, THF, CH3CN, tBuCN) afforded complexes absorbing at 420 nm. A tetracarbonyl complex (237, L = CO) was reactive in the dark and rapidly released CO upon dissolution in acetonitrile or THF. UV-photolysis of 237 (L = Br−) in THF released one equivalent of CO along with the Br– ligand isomerization, and the resulting dicarbonyl complex coordinated CO to form meridional isomer of 237. The complexation of highly conjugated ligands derived from α,α′-diimines with the [Mn(CO)3Br] moiety, as in 238a (Figure 31) and 238b (Table 15), led to exceptionally efficient CO release.1080,1154 Complex 238b reportedly released CO in the IR region upon irradiation above 780 nm, where the compound does not absorb noticeably.1154 The authors attributed this to a weak but not completely forbidden optical population of the lowest triplet excited state of the complex. Similar S0–T1 absorption was later observed for carbazole derivatives.1155 The complex 238b exhibited strong solvatochromism, which was rationalized by suggesting that its lowest-lying singlet excited state has a charge-transfer character. Complex 239 (Table 15), bearing an iminoketone ligand, has a significantly bathochromically-shifted absorption maximum at 630 nm (Figure 31) and retains CO releasing ability.1080 A series of thiourea- and thiazolyl-benzotriazolyl-carbothioamide-based MnI photoCORMs 240a, 240b, 241, and 242 (Table 15) were also reported to release 1-2 equiv of CO.1156,1157
Table 15. MnI-Based PhotoCORMs Containing α,α′-Diimino, Iminoketone, or Carbothioamide Ligandsa.
| CORM | λmaxabs (nm) | εmax (M–1 cm–1) | nCO | Φr (λirr/nm) | solvent | ref |
|---|---|---|---|---|---|---|
| 237 (L = Br–) | 582 | n.d. | <1 | n.d. (560) | THF | (1153) |
| 238a | 570 | 4.6 × 103 | 3 | 0.70 (545) | CH3CN | (1080) |
| 238b | 513 (H2O) | 2.1 × 103 | 1 | 0.54 (545) | H2O | (1154) |
| 568 (MeOH) | 0.30 (623) | |||||
| 0.38 (623, deg.)c | ||||||
| 239 | 630 | 3.7 × 103 | n.d. | ∼0.46b (>520) | CH3CN | (1080) |
| 240a | 398 | n.d. | 2 | n.d. (468) | DMSO | (1156) |
| 240b | 407 | n.d. | 2 | n.d. (468) | DMSO | (1156) |
| 241 | 387 | n.d. | 1 | n.d. (468) | DMSO | (1156) |
| 242 | 437 | 6.1 × 103 | 1.5 | n.d. (468 or 525) | DMSO | (1157) |
THF = tetrahydrofuran, DMSO = dimethyl sulfoxide.
Values estimated from relative apparent CO release rates (kCO).
deg.: degassed.
A MnI tricarbonyl structural motif was used to develop several bipyridine-based visible-light absorbing photoCORMs. Polypyridyl-containing metallodendrimers 243 (Table 16, n = 4, 8; R = 1,4-diaminobutane-poly(propyleneimine); DAB-PPI) released ∼65% of their total content of CO ligands upon irradiation with 410 nm light.1158 Complex 244 (Table 16) releases CO upon irradiation with blue light, while photoCORMs combining 244 and lanthanide ion-doped upconversion nanoparticles (see section 6.4.2) based on YbIII- and TmIII-doped GdIII salts coated with a polymer matrix consisting of phospholipid-functionalized poly(ethylene glycol) released CO upon irradiation at 980 nm.1159 Blakemore, Elles, and co-workers recently studied 4,4′-disubstituted 2,2′-bipyridyl complexes 245 (R1 = R2 = NO2, CF3, H, tBu; Table 16) and the influence of the ligand’s electronic properties on the CO release rate,1160 showing that irradiation into the MLCT band caused rapid CO release (τCO = 0.46–0.68 ps) followed by solvent coordination (τsolv = 18–39 ps). A recent mechanistic study by Pordel and White examined a series of tricarbonylmanganese(I) complexes with 4,4′-substituted 2,2′-bipyridine ligands (bpy′) fac-[Mn(bpy′)(CO)3L; L = Br– or py] 245 (Table 16).1161 In accordance with the findings of Mascharak and co-workers,86 substituting the electron-donating Br– ligand with a π-accepting pyridine stabilized the MnI-based HOMO, causing a hypsochromic shift of the absorption maxima (by 100–150 nm), and reduced the quantum yield of CO release. The regioselectivity of CO release could also be tuned: the CO ligand cis to L was liberated first when L = Br–, but the trans-CO was liberated first when L = py. Increasing the π-acidity of the bipyridine ligands also increased the efficiency of the CO release, although this effect was comparatively weak. The absorption spectra and energies of the MLCT states of 50 different fac-[M(CO)3]+ complexes (M = MnI, ReI) evaluated as potential photoCORMs were recently analyzed and mathematically correlated by the group of Zobi.1162
Table 16. MnI-Based PhotoCORMs with Heteroaryl Bidentate Ligands and Their Analogsa.
| CORM | λmaxabs (nm) | εmax (M–1 cm–1) | nCO | Φr (λirr/nm) | solvent | ref |
|---|---|---|---|---|---|---|
| 243, n = 4 | 410 | 10.3 × 103 | 7.56 | 2.66 × 10–3 (410) | DMSO:H2O | (1158) |
| n = 8 | 420 | 18.8 × 103 | 15.24 | 2.71 × 10–3 (410) | 1:9, v/v | |
| 244 | 400 | 5.4 × 103 | 1.85 | 0.26 (470) | DCM | (1159) |
| 245 | ||||||
| L = Br–, R1 = R2 = CO2Me | 460 | 3.2 × 103 | ∼3 | 0.32 (405) | CH3CN | (1161) |
| L = Br–, R1 = R2 = H | 416 | 2.9 × 103 | ∼3 | 0.22 (405) | CH3CN | |
| L = Br–, R1 = R2 = Me | 411 | 2.9 × 103 | ∼3 | 0.20 (405) | CH3CN | |
| L = py, R1 = R2 = CO2Me | 420 | 4.0 × 103 | ∼3 | 0.19 (405) | CH3CN | |
| L = py, R1 = R2 = H | 383 | 3.4 × 103 | ∼3 | 0.17 (405) | CH3CN | |
| L = py, R1 = R2 = Me | 378 | 3.4 × 103 | ∼3 | 0.15 (405) | CH3CN | |
| L = Br–, R1 = R2 = tBu | 412 | 2.4 × 103 | n.d. | n.d. (415) | CH3CN | (1160) |
| L = Br–, R1 = R2 = H | 415 | 2.3 × 103 | n.d. | n.d. (415) | CH3CN | |
| L = Br–, R1 = R2 = CF3 | 457 | 1.5 × 103 | n.d. | n.d. (415) | CH3CN | |
| L = Br–, R1 = R2 = NO2 | 510 | 0.2 × 103 | n.d. | n.d. (415) | CH3CN | |
| 246 | 410 | n.d. | 3 | n.d. (460) | DMSO | (1164) |
| 247 | 385 | n.d. | 2.84 | n.d. (456) | PBS, pH = 7 | (1165) |
| 248a, R = OH | 422 | n.d. | n.d. | (kCO = 0.07 min–1)b (410) | CH3CN | (1166) |
| R = O– | 490 | n.d. | (kCO = 0.81 min–1)b (410) | CH3CN | ||
| 248b, L = Br– | 428 | 35.3 × 103 | 3 | 0.19 (451)c | ethanol:PBS | (1167) |
| L = CF3SO3– | 428 | 28.6 × 103 | 3 | 0.04 (451)e | 2:1, v/v | |
| 0.22 (451)c | ||||||
| 0.06 (451)e | ||||||
| 249a | 396 | n.d. | ∼2.5 | (kCO = 16 × 10–4s–1)d(468) | DMSO | (1168) |
| 249b | 401 | n.d. | ∼2.5 | (kCO = 37 × 10–4s–1)d (468) | DMSO | (1168) |
| 250 | 450 | 3.2 × 103 | n.d. | n.d. (>440) | DCM | (1169) |
| 251 | 465 | 3.2 × 103 | 2.85 | n.d. (470) | DMSO | (1170) |
| 252 | 440–640 | 0.15–3.0 × 103 | n.d. | n.d. (400–700) | CH3CN | (1171) |
| 253 | 393 | n.d. | 0.09f | n.d. (480) | CH3OH | (1172) |
| 254a | 375 | 1.5 × 103 | 3 | 0.18 (405) | PBS | (1173) |
| 0.31 (470) | ||||||
| 254b | 379 | 1.4 × 103 | 3 | 0.17 (405) | PBS | (1173) |
| 0.43 (470) | ||||||
| 255 | 340 | 3.6 × 103 | 2.5 | n.d. (400) | DMSO | (1174) |
| 256a | 370 | 5 × 103 | 3 | 0.35 (380) | CH3CN | (1175) |
| 256b | 350 | 6.2 × 103 | 3 | 0.39 (>410) | CH3CN | (1176) |
DMSO = dimethyl sulfoxide, DCM = dichloromethane, PBS = phosphate buffer saline.
Relative rate of the CO release of 248a.
Quantum yield for the release of the first equivalent of CO.
Quantum yield for the release of the second equivalent of CO.
Relative rates of CO release from 249a and 249b.
Measured by irradiation of solid crystalline phase. dend. = 1,4-diaminobutane dendrimer. 247: the sphere represents the membrane-puncturing needle domain of bacteriophage T4.
Zobi and co-workers also developed hybrid systems referred to as quantum-CORMs that combine MnI-tricarbonyl complexes 245 (Table 16) with bipyridyl ligands containing anchoring groups (R1 = H, COOH; R2 = COOH, NH2, (4-carboxyphenyl)ethynyl, and (4-aminophenyl)ethynyl) that were used to bind the Mn complexes to the surfaces of CdSe/ZnS core/shell semiconductor quantum dots (see also section 6.4.1).1163 These quantum dots have a band-gap wavelength of 504 nm and bright emission at 512 nm. Upon irradiation at 510 nm, they sensitize the release of CO from the photoCORM, increasing its efficiency 2- to 6-fold compared to non-sensitized excitation.
Furukawa and co-workers developed light-responsive metal-organic frameworks as controllable CO-releasing cell-culture substrates.1164 These materials combine a MnI tricarbonyl bipyridyl complex 246 (Table 16) with a highly robust ZrIV-based MOF. The group of Ueno developed a construct containing an artificial protein needle by conjugating the membrane puncturing needle domain of bacteriophage T4 to the MnI carbonyl photoCORM complex 247 (Table 16) via a maleimide thiol linkage.1165 This system was used as an in vivo magnetic-resonance-imaging contrast reagent. Allosteric regulation of CO release was demonstrated in complex 248a (Table 16),1166 in which the phenolic substituent of the terpyridyl ligand responds to fluoride ions by undergoing deprotonation, leading to allosteric activation of CO release; the deprotonated complex released CO approximately 1 order of magnitude more efficiently than its neutral form. Ford and co-workers synthesized another terpyridine-based manganese tricarbonyl complex 248b (Table 16), which can release CO both by 1-photon excitation in the visible region and also by 2-photon excitation at 750 and 800 nm because the terpyridine ligand acts as an efficient 2-photon antenna.1167
Potentially bioactive MnI tricarbonyl complexes with 2-(2′-pyridyl)benzimidazole ligands bearing morpholino (249a) or phthalimido (249b, Table 16) substituents were studied spectroscopically and computationally by Mansour and Ragab.1168
Another MnI tricarbonyl photoCORM, 250 (Table 16), contains a benzothiazole ligand that functions as a turn-on fluorescent signal.1169 Upon photoexcitation, this complex releases both CO and the 2-(2-pyridyl)-benzothiazole ligand, whose fluorescence was successfully used to monitor the CO-induced death of human breast cancer cells treated with 250. The similar MnI complex 251 (Table 16), which has a ligand derived from the anti-anxiety drug bromazepam, was reported by Mansour.1170
A series of four photoCORMs 252 (Table 16) bearing 2-(benzo[d]thiazol-2-yl)phenol ligands, was developed by Roy and co-workers,1171 and tricarbonyl MnI complexes with 3-(2-pyridyl)pyrazole ligands 253 (Table 16) were shown to release CO independently of the choice of R substituents.1172
MnI tricarbonyl photoCORMs 254a and 254b (Table 16), containing substituted bispyrazolylmethane ligands, were prepared by the group of Westerhausen.1173 These complexes are initially neutral but their ligands have terminal acetyl groups that are hydrolyzed into carboxylates upon cellular uptake. As a result, the photoCORMs become anionic and are trapped inside the cells. Fairlamb, Lynam, and co-workers synthesized a series of biotin-conjugated MnI-based photoCORMs 255 (R = biotin; Table 16) that release CO upon irradiation at 400 nm and bind efficiently to avidin.1174
Fluorescent dansylimidazole-substituted complex 256a (Table 16) released CO upon irradiation with visible light.1175 Its analog 256b exhibited strong green luminescence that could be visualized in vitro.1176 This class of luminescent MnI-based photoCORMs was extended by preparing dansylimidazole complexes with diazabutadiene ligands bearing sterically similar adamantyl (lipophilic) or 1,3,5-triazaadamantyl (hydrophilic) substituents.1177 Changing the ligand’s lipophilicity altered the localization of the photoCORMs in cellular organelles.
Tridentate heteroaryl ligands form stable complexes with MnI and were used for the successful design of photoCORMs. Very recently, the group of Schiller introduced a novel class of 2-photon absorbing naphthalimide-containing photoCORMs 257 (Figure 38).1178 These compounds release CO by both 1- (405 nm) and 2-photon (800 nm) excitation. CO liberation is accompanied by the release of the naphthalimide ligands, which are fluorescent in solution, in non-woven fabrics, and in HeLa cells. Similar naphthalimide-substituted photoCORMs 257 (X = NR) were used to synthesize green light-responsive (550 nm) CO-releasing polymeric materials by ring-opening metathesis polymerization.1179
Figure 38.
Naphthalimide substituted photoCORM.
Schiller and co-workers developed the dabsyl-substituted MnI tricarbonyl complex 258 (Figure 39), which releases CO upon irradiation at 405 nm.1180 The complex was loaded onto paper strips to form a material whose light-triggered CO release could be monitored with the naked eye by observing the change in its color. An analogous approach was used in the design of the colorimetric and fluorometric dual response photoCORM 259 (Figure 39), which is based on the nitrobenzoxadiazole fluorophore and releases CO upon irradiation at 490 nm.1181
Figure 39.
Dabsyl- and nitrobenzoxadiazole-substituted photoCORMs.
MnI-based photoCORMs with other structures have also been reported (Table 17). For example, Ueno and co-workers developed a series of engineered protein crystals using the photoCORM Mn(CO)5Br.1182 Polyhedral crystals containing histidine as a ligand were used to immobilize the MnI carbonyl complex 260 (Table 17). Two proteins were prepared, a wild-type (WT) with 3 histidine units and mutants with hexahistidine tags containing 3 or 6 equiv of the photoCORM. The CORM loading and the corresponding quantum yields of CO release correlated with the number of histidine residues in the protein.
Table 17. Other Mn-Based PhotoCORMsa.
| CORM | λmaxabs (nm) | εmax (M–1 cm–1) | nCO | Φr (λirr/nm) | solvent | ref |
|---|---|---|---|---|---|---|
| 260 | n.d. | n.d. | 1.9b | 0.013b (456) | PBS | (1182) |
| 2.9c | 0.047c (456) | |||||
| 261 | 435 | n.d. | ∼1 | n.d. (468) | DMSO | (1183) |
| 262 | 395 | n.d. | ∼0.5 | n.d. (410) | DMSO | (1183) |
| 263 | 465–486 | n.d. | 1.7–1.9 | n.d. (520–560) | DCM | (1184) |
| 264 | 360 | 1.4 × 103 | 2 | n.d. (400) | CH3CN | (1185) |
| 1.4 | n.d. (465) | |||||
| 265 | 388 | n.d. | 3 | n.d. (470) | H2O | (1188) |
| 266 | 354 | n.d. | 2.86 (405) | 0.1 (365) | PBS | (1189) |
| 267 | 350 | n.d. | 2.61 (405) | 0.06 (365) | PBS | (1189) |
| 268 | 344 | 1.1 × 103 | 3 | 0.054 (385) | H2O | (1190) |
| 0.030 (410) | ||||||
| 269 | H2O | (1190) | ||||
| R = H | 349 | 1.3 × 103 | 3 | 0.058 (385) | ||
| 0.044 (410) | ||||||
| R = CH3 | 354 | 1.4 × 103 | 3 | 0.081 (385) | ||
| 0.033 (410) | ||||||
| 270 | 384 | n.d. | 6 | 0.11 (365) | PBS | (1191) |
| 0.06 (470) | ||||||
| 271 | (1193) | |||||
| R = nPr | 385 | 1.8 × 103 | n.d. | n.d. (405) | CHCl3 | |
| R = nBu | n.d. | n.d. | n.d. | n.d. (405) | CHCl3 | |
| 272 | (1194) | |||||
| N∩N = phe | 550 | 5.8 × 103 | 2 | 0.41 (659) | CH3CN | |
| N∩N = bpy | 550 | 4.9 × 103 | n.d. | 0.39 (659) | CH3CN | |
| N∩N = biq | 719 | 1.3 × 103 | n.d. | 0.24 (659) | CH3CN | |
| N∩N = phen–CHO | 652 | 7.6 × 103 | n.d. | 0.02 (659) | CH3CN | |
| 273 | 340 | n.d. | 5.3 | 0.24 (405)d | DMA | (1195) |
| 0.006 (635)e |
PBS = phosphate buffer saline, DMSO = dimethyl sulfoxide, DCM = dichloromethane, Phe = 1,10-phenanthroline, Bpy = 2,2′-bipyridyl, Biq = 2,2′-biquinoline, Phen-CHO = phenanthrolinecarboxaldehyde, DMA = dimethylacetamide.
Wild-type protein (3 histidine units).
Mutant protein with hexahistidine tag.
Direct irradiation.
Sensitized release.
MnI tricarbonyl complexes with tazarotene (261) and metamizole (262) as bidentate and tridentate ligands, respectively, were developed by Mansour and Shebab (Table 17).1183 Both ligands dissociate from the metal center upon the CO photorelease. In addition, Manimaran and co-workers developed a series of 10 MnI-based aminoquinonato-bridged dinuclear complexes 263 (Table 17) that release CO upon irradiation with green light.1184 The mechanism of CO release from the MnI tryptophanate complex 264 (Table 17)1185 was studied by time-resolved ultrafast infrared spectroscopy (TRIR) and TD-DFT,1186 which revealed that excitation leads to an LMCT from the indole moiety of the tryptophan ligand to the metal d-orbitals. The loss of CO then occurs within 3 ps, resulting in the formation of the triplet state of the dicarbonyl product 3[MnI(tryp)(CO)2(MeCN)], which is solvated within 20 ps. The mechanism of CO release from 264 was further studied by laser-interfaced mass spectrometry (LIMS) across a wide wavelength range (λirr = 234–580 nm).1187
Zobi and co-workers studied a fac-MnI tricarbonyl complex with a tetraazacyclotetradecane ligand attached to the 5′–OH ribose group of vitamin B12 (265, Table 17).1188 Vitamin B12 acts as a biocompatible water-soluble scaffold that allows the photoCORM to be actively transported into cells. Because of its remote attachment, it does not affect the photochemistry of the MnI complex. The compound was successfully delivered into fibroblasts, where the photoinduced release of CO protected them against death under conditions of hypoxia and metabolic depletion.
The group of Westerhausen developed a series of water-soluble manganese tricarbonyl complexes 266 and 267 (Table 17) based on tridentate aminoalkylsulfide ligands.1189 Accumulation of these complexes inside cells was observed by FT-IR imaging. Peralta and co-workers recently synthesized three analogous water-soluble complexes 268 and 269 (R = H, CH3) that sequentially release three equivalents of CO upon irradiation with blue light.1190 The thiolato-bridged MnI-dimer 270 (Table 17) prepared from cysteamine by Westerhausen and co-workers was shown to photochemically release all 6 of its bound CO ligands.1191
Schiller and co-workers demonstrated the photochemically controlled release of CO from non-woven polylactide fibers containing the Mn0 decacarbonyl complex (Mn2(CO)10, CORM-1).1192 They later constructed a device for remote-controlled delivery of CO using tetranuclear MnI-based complexes 271 (Table 17, R = nPr, nBu) embedded in non-woven polylactide or polymethacrylate fabrics1193 that were shown to be nontoxic towards 3T3 mouse fibroblast cells.
A new strategy for triggering the photochemical release of caged CO using long-wavelength and NIR light was described by Ford and co-workers using dinuclear Rh0–Mn0 carbonyl complexes 272 (Table 17).1194 These complexes have strong metal–metal-bond-to-ligand charge-transfer (MMLCT) absorption bands from ∼550 to ∼720 nm. Their photoexcitation leads to homolytic cleavage of the Re–Mn bond, yielding mononuclear metal radicals that tend to recombine in the absence of trapping agents but react with dioxygen to form active species that efficiently release CO via secondary thermal or photochemical processes in aerated solutions. Schiller and co-workers combined the classical UV-absorbing photoCORM Mn2(CO)10273 (Table 17) with the PdII tetraphenyltetrabenzoporphyrin complex 274, which is a triplet photosensitizer (see also section 6.1) excitable by red or NIR light.1195 The triplet-excited photosensitizer transfers its energy to the photoCORM, which then liberates CO. The authors combined these components in the solid state to prepare a CO-releasing material supported on electrospun non-woven fabrics.
RuII complexes have d6 configurations similar to those of MnI complexes and have also been successfully used as visible-light activatable photoCORMs. RuII analogs of 229a (Table 14), 275, and 276 (Figure 40) are carbonyl complexes where the 2-quinoline-N-(2′-methylthiophenyl)-methylenimine (qmtpm) moiety acts as a tridentate ligand due to the high affinity of RuII for sulfur. Complex 275 has a MLCT band at λmaxabs = 405 nm.1196 The replacement of a strongly π-accepting CO ligand with PPh3 resulted in a bathochromic shift of the MLCT band to 465 nm. Complex 275 releases CO only in acetonitrile upon UV-light (310 nm) irradiation, whereas phosphine-substituted complex 276 is active in the visible region and releases CO under visible light (≥440 nm). Mansour studied the RuII dicarbonyl complex 277 (Figure 40), which incorporates a ligand derived from the anti-anxiety drug bromazepam and liberated 2 equiv of CO upon excitation with 470 nm light.1170 The complexation of this photoCORM to bromozepam increased its antibacterial toxicity relative to the non-complexed drug. Complex 278, which has a bisquinoline ligand, sequentially releases two equivalents of CO upon irradiation at 350 or 420 nm, with the first equivalent being liberated more efficiently.1197 Finally, Oyama and co-workers recently introduced a series of RuII dicarbonyl photoCORM complexes with asymmetric bipyridine ligands.1198
Figure 40.
RuII-based photoCORMs.
Some FeII carbonyl complexes were reported as visible-light excitable photoCORMs. For example, irradiation of complex 279 (Figure 41) at 470 nm resulted in decarbonylation, which was monitored using a myoglobin assay and ion channels sensitive to CO.1199,1200 The diiron hexacarbonyl complex 280 (Figure 41)1201 is a water-soluble analog of an iron–iron hydrogenase model complex1202 and can liberate 6 equiv of CO upon irradiation at 390 nm. It is not clear whether all 6 CO ligands are liberated from the excited state. Nakajima and co-workers reported a series of N,C,S-pincer iron(III) carbonyl complexes 281 (Figure 41) with two phosphorous ligands (281a: R1 = Me, R2 = Ph; 281b: R1 = R2 = Me; 281c: R1 = R2 = OEt) in the trans-positions.1203 A detailed study of their wavelength dependence showed that all of these complexes released CO upon irradiation at <500 nm and that complex 281c was photoactive even at 800 nm. The release quantum yields were in the range of 0.03–0.01 for all complexes. A core-shell-based material that releases CO via an upconversion process (see section 6.4.2) was developed by Liu and co-workers.1204 The core, in this case, consists of upconverting nanoparticles of β-NaYF4:YbIII/ErIII, while the shell consists of [FeII(η5-Cp)(CO)2] complexes with the structure 282 (Figure 41) that are anchored to the surfaces of the nanoparticles via thiol groups. Irradiation of this material with a 980 nm laser led to the sensitization and subsequent decarbonylation of the CORM shell. Wright and co-workers introduced 2-aminopyridine and 1-aminoisoquinoline-based iron(II) complexes 283 bearing two CO molecules1205 that can be substituted by thioglucose to obtain the ferracyclic dimeric complexes 284, which exhibit enhanced water solubility and Φr values of 0.9–1.7 × 10–4.
Figure 41.
Iron-based photoCORMs.
ReI complexes 285 (L = Br–, PPh3, Figure 42), which are analogous to MnI-based complexes 231 (Table 14), were found to release CO only under UV illumination.1146 This was rationalized by TD-DFT calculations indicating that ISC to the triplet state promoted by strong spin-orbit coupling competed with CO release. Conversely, the water-soluble ReI-based photoCORM 286 (Figure 42) released 1 equiv of CO upon irradiation at 405 nm with Φr = 0.111206 (Φr (365 nm) = 0.024).1207 This complex and its photoproduct are both fluorescent, with distinguishable maxima at 515 (ΦF (365 nm) = 0.08) and 585 nm, respectively, enabling qualitative monitoring of CO release in cells using confocal fluorescence microscopy.
Figure 42.
ReI-based photoCORMs.
4.2. Release of Nitric Oxide
Nitric oxide (NO) is a gasotransmitter with many important biological roles1208 in processes including vasodilatation,1061,1209−1212 platelet aggregation inhibition,1066 wound healing,1065 postsynaptic plasticity augmentation, and hormone secretion.1067 The development of NO-releasing molecules (NORMs) is therefore of interest for both therapeutic applications1213 and mechanistic chemico-biological studies. Advances in the photochemical release of NO from photoNORMs have been reviewed previously.103−109
4.2.1. Transition-Metal-Free PhotoNORMs
There are three types of transition-metal-free photoactivatable nitric oxide (NO) donors (often termed photoactivatable nitric-oxide-releasing moieties, or photoNORMs): (i) N-nitroso amines, (ii) diazeniumdiolates (NONOates, R2N–(NO–)–N=O) and related structures, and (iii) o-substituted nitroarenes and their derivatives. NO release from these species can be induced by direct excitation or energy/electron transfer from an excited sensitizer.
N-Nitroso amines (Scheme 72) are popular NO donors because they are easily prepared by nitrosylation of amines.1214 The N–NO bond is typically very weak (∼39 kcal mol–1); its energy is comparable to that of a photon with a wavelength of ∼730 nm.103 However, the N–NO group exhibits very limited absorption in the visible/NIR part of the spectrum and is therefore always combined with a suitable sensitizer. Upon NO photorelease, N-nitroso amines form aminyl radicals that may abstract hydrogen atoms from other molecules or undergo reduction or oxidation.1215,1216 They can also transnitrosate with nucleophiles such as thiols.1217
Scheme 72. Photoreactions of N-Nitroso Amines1214,1215,1217.
The thermally stable and non-cytotoxic aza-BODIPY-based N-nitrosamine 287 (Figure 43) was shown to release NO upon irradiation at λirr = 700 nm in vitro and in vivo.1218 This molecule also acts as an excellent photoacoustic sensor, allowing the local, irradiation-dependent release of NO to be monitored in vivo by photoacoustic tomography. The BODIPY scaffold was also used to prepare 288,1077 which releases NO upon irradiation at wavelengths in the range λirr = 470–500 nm with a Φr of 0.0019 at 488 nm (Figure 30).
Figure 43.
Structures of BODIPY N-nitroso amine-based photoNORMs.
Like 288, the rosamine photoNORM 289 releases NO upon irradiation at λirr = 530–590 nm (Scheme 73).1219 The suggested mechanism involves photoinduced electron transfer from the N-nitrosoaminophenol group to the excited rosamine moiety to form unstable phenoxyl radical 290, which decomposes to release NO and form the stable quinoneimine 291.
Scheme 73. Mechanism of Photochemical Release of NO from 289(1219).
This approach was further developed by the synthesis of photoNORMs 292 and 293 (Figure 44), and it was found that the distance between the NO-releasing N-nitrosoaminophenol group and the rosamine sensitizer profoundly affects the efficiency of NO release.1220 Compound 293 exhibited the most efficient NO release (Φr = 1.01 × 10–3); the other derivatives were strongly fluorescent. This proximity effect was attributed to π–π stacking and the formation of conformational isomers that enable efficient electron transfer. Conjugation of d-galactose with the phenolic hydroxy group completely blocked NO release, which was restored by selective enzymatic hydrolysis with β-galactosidase, enabling NO release using two independent triggers that can be activated simultaneously. The formation of phenoxyl radical 290 (Scheme 73) was shown to be the key step in NO release. The release of NO from compound 293 upon irradiation at 530–590 nm was tested in HET293T cells and used to control the response of rat aortas to NO in an ex vivo system.1221 Compounds 288, 289, and 293 were subsequently evaluated as potential NO donors for treatment of erectile dysfunction.105
Figure 44.
Structures of rosamine-based photoNORMs.
Yang and co-workers recently developed a visible-light absorbing photoNORM based on N-nitroso rhodamine derivative 294 (Figure 45).1222 Upon irradiation with λirr = 532 nm, the weakly fluorescent molecule 294 was converted into NO and a fluorescent rhodamine-based photoproduct (ΦF = 0.43 at 560 nm in phosphate buffer, pH = 7.4), which was used to monitor the localization, flux, and dose of the released NO. NO release was found to occur within 7 ps after excitation. A sulfonate group was attached to the N-nitrosamino group in photoNORM 295 to increase its water solubility,1223 while the morpholine-substituted derivative 296 was designed to target the lysosomes and release NO in living cells and zebrafish.1224 Interestingly, a chromenylium analog of 296, 297, released NO in 91% yield only upon excitation with 365 nm light despite absorbing in the visible region (λmaxabs = 537 nm).1225 Additionally, the dihedral angle between the nitroso moiety and the rhodamine core was found to influence the efficiency of NO photorelease. The nitrosamine group is almost orthogonal to the plane of the chromophore in 294–296, whereas in the ring-restricted compound 298, the nitrosamine moiety is locked in a coplanar geometry.1216 The direct conjugation of the chromophore and nitrosamine systems enables more efficient photoinduced intramolecular charge transfer, leading to NO release at λirr = 532 nm, ∼20-times more efficiently than from 294. A doubly N-nitrosylated analog of 294, 299, released NO only upon UV light irradiation (λirr = 365 nm; Figure 45).1226 Unlike 294, which exists as an equilibrating mixture of a fluorescent visible-light-absorbing open form and a non-fluorescent UV-light-absorbing lactone form, photoNORM 299 exists exclusively as a lactone. This molecule was used to study changes in mitochondrial dynamics following NO release induced by irradiation at 375 nm.1227
Figure 45.
N-Nitroso amine-based photoNORMs.
The naphthalimide derivative 300 is another notable N-nitrosoamino photoNORM (Figures 46 and 30).1078 It releases NO only upon irradiation with UV light (λirr = 365 nm) or 2P excitation at λirr = 740 nm. Its coumarinyl-substituted analog 301 also releases NO upon UV irradiation or 2P excitation at λirr = 800 nm, with a chemical yield of 79%.1228
Figure 46.
Napththalimide-based photoNORMs.
Diazeniumdiolates (NONOates) release two equivalents of NO via thermal processes and have been investigated by Keefer and co-workers as potential liver-selective NO donating prodrugs.1229 NONOates release NO in neutral aqueous solutions at a rate that depends on their structure. Because of their simple preparation1230 and generation of predictable amounts of NO, they have attracted considerable attention as NORMs.1231,1232 NO release from NONOates is insensitive to biological factors, so they do not induce resistance.1233 The first attempt to control NO release from NONOates with light was reported by Tsien and co-workers, who used the o-nitrobenzyl protecting group (section 2.1.1) as a PPG in 302 (Scheme 74).1234 This group is activated only by UV-irradiation (λirr = 365 nm); upon deprotection of the NO group, it thermally releases two equivalents of NO and a secondary amine. Another approach was used by Sortino and co-workers, who used the thermal NO releaser cupferron (which exists in two resonance forms: phenyldiazenium diolate 303 and N-nitroso-N-phenylhydroxylaminate 304)634 in the BODIPY-based (see section 2.11) photoNORM 305 (Figure 47).803 Its irradiation at 530–550 nm resulted in the heterolytic cleavage of the BODIPY protecting group, unmasking cupferron, which thermally releases 1 equiv of NO (Φr = 0.008 ± 0.001 at 532 nm; Φrε(λirr) = 550 M–1 cm–1). Iodo analog 306 was introduced as a photosensitizer for photodynamic therapy that simultaneously photoreleases NO (Figure 47).798 The iodine atoms enhance the quantum yield of ISC, which increases singlet oxygen production in aerated solutions because of the sensitizing effect of the triplet-excited BODIPY group.
Scheme 74. Photochemical Deprotection of NONOates1234.
Figure 47.
Structures of cupferron and cupferron-based photoNORMs.
o-Substituted nitroarenes have also been identified as potential photoNORMs. The o-trifluoromethyl nitrobenzene derivatives 307 (R = H, Scheme 75) isomerize upon irradiation with UV light (λmaxabs ≈ 300 nm) to give arylnitrite derivatives 308,1235 which thermally release NO to form phenoxy radicals 309. These radicals subsequently abstract hydrogen atoms to give phenol derivatives 310. The substituent in the ortho-position plays a key role in this process because it forces the nitro group to adopt a twisted geometry. Introducing an amino substituent para to the nitro group yields a chromophore with push-pull character, bathochromically shifting the absorption bands by ∼80 nm (307, R = NHR′, R′ = alkyl, acyl).1235
Scheme 75. Photochemical Release of NO from o-Substituted Nitroarenes1235.
Hexadecylamine-substituted 4-nitro-3-(trifluoromethyl)aniline 311 (Figure 48) was used by Jose and co-workers to prepare nanoscale lipid vesicles for photoinduced NO delivery (λirr = 410 nm).1236 However, even push-pull-substituted nitroarenes are poor chromophores (εmax ≈ 1000 M–1 cm–1) and must be sensitized to enable visible-light activation. Sortino and co-workers introduced the anthracene-based photoNORM 312, which releases NO upon irradiation with 420 nm light.1237312 was used as a photoNORM in the construction of a fluorescein-labeled β-cyclodextrin-based supramolecular nanoassembly with a red-emitting singlet oxygen photosensitizer (zinc phthalocyanine).1238 This system exhibits “five-in-one” photochemical features: visible-light or 2P (740 nm) excitation, facile visualization due to distinct fluorescence, production of cytotoxic singlet oxygen, and NO release. The coumarin-based compound 313 was incorporated into photo-antimicrobial polymeric films to release NO upon irradiation at λirr > 400 nm.1239o-Trifluoromethyl-substituted nitroarenes were similarly used to prepare diketopyrrolopyrrole-based nanoplatforms for pH-responsive photodynamic/photothermal synergistic cancer therapy.1240
Figure 48.
Structures of o-trifluoromethyl substituted nitroarene-based photoNORMs.
Miyata and co-workers developed a series of 2,6-dimethylnitrobenzene-based photoNORMs with properties similar to their trifluoromethyl analogs.110,1241 However, π-extension of the aromatic system did not lead to visible-light activated NO release. The most active derivative 314 (Table 18) generated 0.55 equiv of NO upon irradiation with UV light. The attachment of fluorescein as a sensitizer generated the visible-light-absorbing molecule 315, which releases NO only upon UV-irradiation or 2P excitation (σTPA = 0.12 GM).1242,1243 The conjugated analog 316 releases NO both by 1P and 2P excitation with a 2P absorption cross section 8-times higher than that of 315 (σTPA = 0.98 GM).1244 A similar approach is embodied in rhodamine-based derivatives 317, which liberate NO upon irradiation with yellow light,1245 and in compound 318, which incorporates 6-bromo-7-hydroxycoumarin as a sensitizer.1246
Table 18. 2,6-Dimethylnitrobenzene-Based PhotoNORMs.
| NORM | λmaxabs (nm) | εmax (M–1 cm–1) | λirr (nm) | Φr (λirr/nm) | solvent | ref |
|---|---|---|---|---|---|---|
| 314 | 335 | n.d. | UVa | n.d. | H2O:DMSO | (110, 1241) |
| 3:1 (v/v) | ||||||
| 315 | 452 | n.d. | 330–380 | n.d. | H2O:DMSO | (1242) |
| 450–480 | 3:1 (v/v) | |||||
| 720–735c | ||||||
| 316 | 450 | n.d. | 450–480 | n.d. | H2O:DMSO | (1244) |
| 720c | 3:1 (v/v) | |||||
| 317, X = pyrrolidine | 563 | 26 300 | 530–590 | 0.0023 (550) | phosphate bufferb | (1245) |
| 317, X = morpholine | 553 | 15 500 | 530–590 | n.d. | phosphate bufferb | (1245) |
| 318 | 360 | 11 200 | 400–430 | 0.053 (358) | H2O:DMSO | (1246) |
| 1:1 (v/v) |
UV irradiation with a Pyrex filter.
Sodium phosphate buffer, c = 100 mM, 1% DMSO (v/v).
2-photon excitation.
Sortino and co-workers recently synthesized two hybrid fluorescent photoNORMs 319a and 320 (Figure 49) that combine an N-nitrosamine moiety with an o-trifluoromethyl nitroarene and a BODIPY or rhodamine sensitizer.1247 Both compounds released NO upon irradiation with green light (λirr = 510 nm for 319a and 532 nm for 320), but release was more efficient from the BODIPY-substituted derivative 319a (Φr = 0.031) than from the rhodamine-substituted compound 320 (Φr = 0.001). The reaction was suggested to be initiated by electron transfer from the N–NO group to the excited dye moiety. While these compounds should release 2 equiv of NO (one from the N-nitrosamine and one from the aryl nitro group), only the cleavage of the N-nitrosamine N–NO bond was observed. The iodo analog of 319a, 319b, releases NO upon irradiation with 532 nm light, which is accompanied by singlet oxygen production.1248
Figure 49.
Hybrid photoNORMs combining N-nitrosamines and o-trifluoromethyl nitroarenes.
4.2.2. Transition-Metal-Containing PhotoNORMs
The most accessible sources of NO are nitrosyl and nitrito transition metal complexes with weakly bound NO ligands that can be released by external triggers. The oldest and best known NO donors are pentacyanonitrosylmetallates such as disodium pentacyanonitrosylferrate (sodium nitroprusside), which has been known since the mid-19th century.1249−1251 The M–NO bond is readily cleaved by excitation into the MLCT (dM → π*NO) band of an organometallic complex. These bands exist in the visible region (above 400 nm); excitation weakens π-back-bonding to the NO ligand and facilitates electron transfer from the metal center to NO+, which is then liberated as a neutral NO molecule.1252 Transition metal complexes releasing NO have been reviewed by Ford77−79,98,107 Mascharak,63 and Liu1253 and their co-workers.
The nitrito complex trans-[CrIII(cyclam)(ONO)2]+321 (Scheme 76) was shown to release one equivalent of NO upon irradiation at 436 nm with Φr = 0.0092 in a degassed aqueous solution.1254 Upon irradiation in aerated aqueous solutions, the complex releases NO with Φr = 0.25. This dichotomy was attributed to oxidation of the CrIV complex formed upon NO release by O2 to give CrV species 322. The presence of glutathione increased the quantum yield of NO release to ΦNO = 0.25 by reducing an intermediate CrIV complex to a CrIII–OH complex.1255,1256 Complex 321 was combined with anthracene or pyrene “antenna” ligands (323a and 323b, Figure 50) that harvest light and act as fluorescent reporters.1255,1256
Scheme 76. CrIII-Nitrito Complexes and Their Photochemistry1254.
Figure 50.
CrIII-Nitrito complexes substituted with antenna ligands.
Ford and co-workers recently developed a CrIII nitrito complex 324 (Figure 51), which photoreleased NO upon irradiation at 451 nm1257 and also upon irradiation at 800 nm when loaded onto polymer disks containing Nd-sensitized upconverting nanoparticles (see also section 6.4.2).
Figure 51.
CrIII nitrito complex 324.
FeIII and FeII nitrosyl complexes have long been known as photoNORMs. The most established FeII nitrosyl complex, sodium nitroprusside 325 (Scheme 77), was shown to photochemically release both CN– and NO upon irradiation with 314–456 nm light.1250 When irradiated in aqueous solution at >480 nm, NO was the sole photoproduct together with the oxidized FeIII aqua complex as a side-product. NO release from nitrosyl complexes of electron-rich d-elements generally proceeds via electron transfer from the metal center to the NO+ ligand and subsequent release of the NO radical.
Scheme 77. Photochemistry of Sodium Nitroprusside1250.
Roussin’s black salt 326 (Figure 52) and Roussin’s red salt 327 were also found to release NO (5.9 equiv for 326 and 4 equiv for 327) upon photolysis at wavelengths of 313–546 nm.1141 The NO release quantum yield of 326 was Φr = 0.007, while that of 327 was one order of magnitude higher. Like CrIII complexes 321, the NO release efficiency was increased in the presence of oxygen. Encapsulation of 326 in NIR-absorbing nanocarriers resulted in efficient NO photorelease upon 980 nm excitation.1258,1259 Derivatives of 327 and 328 were used as photoNORMs to efficiently release NO in aerated solutions.1260−1263 Protoporphyrin IX was used to sensitize NO release from compound 328 (R = CH2CH2OH) at 436 and 546 nm with quantum yields of Φr = 5.2 × 10–4 and 2.5 × 10–4, respectively.1262 Ford and co-workers showed that 328 (R = CH2CH2OH) could also be activated by attaching either sensitizing fluorescein derivatives absorbing at λirr = 400 (1PE) and 800 nm (2PE)1260 or a benzothiazolyl-substituted fluorenyl two-photon antenna with a large 2P absorption cross section (σTPA = 246 GM).1264 Patra, Mascharak, and co-workers prepared FeIII complex 329 (Figure 52), which incorporates pentadentate carboxamide-containing ligands.63,1265,1266 This complex releases NO upon irradiation with visible light at 500 nm with Φr = 0.19. Lee, Chiang, Tsai, and co-workers recently introduced novel FeII-based photoCORMs with pendant thiols or thioethers (330, R = H, CH3).1267 The S-methylated complex releases NO upon irradiation with visible light (λirr > 400 nm), but the free thiol in 330 (R = H) interacts with the departing NO, generating HNO as the main photoproduct.
Figure 52.
PhotoNORMs based on iron–sulfur clusters.
Complex 331, a MnII analog of complex 329 (Table 19; Figure 31), irreversibly released NO upon visible-light activation1081,1268 and was used to construct NO-releasing polyurethane-coated sol-gel hybrid materials.1269 Replacing one pyridyl ligand of 331 with a quinoline unit yielded complex 332, in which the absorption band is bathochromically shifted but photoactivity upon irradiation is retained at up to 810 nm.1081 Additionally, the absorption maxima of the related complexes 333 and 334 (Figure 31), which contain imine nitrogens trans to the NO ligand, are bathochromically shifted by ∼100 nm relative to their carboxamide counterparts 331 and 332.1082
Table 19. Manganese(II) Multidentate Complexes Activatable in the Visible and NIR Region.
| NORM | λmaxabs (nm) | εmax (M–1 cm–1) | λirr (nm) | Φr (λirr/nm) | solvent | ref |
|---|---|---|---|---|---|---|
| 331 | 635 | 220 | 500, 550 | 0.326 (500) | CH3CN | (1081, 1268) |
| 0.309 (550) | CH3CN | |||||
| 0.400 (500) | H2O | |||||
| 0.385 (550) | H2O | |||||
| 332 | 650 | 450 | 500, 550 | 0.623 (500) | CH3CN | (1081) |
| 0.579 (550) | CH3CN | |||||
| 670 | 0.742 (500) | H2O | ||||
| 0.694 (550) | H2O | |||||
| 333 | 720 | 750 | 500, 550 | 0.41 (500) | CH3CN | (1082) |
| 0.58 (550) | ||||||
| 334 | 785 | 1200 | 500, 550 | 0.39 (500) | CH3CN | (1082) |
| 0.43 (550) | ||||||
| 335 | 460, 530, 650 | MESa | (1270) | |||
| R = OCH3 | 457 | 4740 | 0.58 (460) | |||
| 0.47 (530) | ||||||
| 0.49 (650) | ||||||
| R = H | 461 | 3120 | 0.61 (460) | |||
| 0.51 (530) | ||||||
| 0.47 (650) | ||||||
| R = Cl | 475 | 6940 | 0.66 (460) | |||
| 0.66 (530) | ||||||
| 0.73 (650) | ||||||
| R = NO2 | 523 | 13.6 × 103 | 0.61 (460) | |||
| 0.63 (530) | ||||||
| 0.78 (650) |
MES = a 2-(N-morpholino)ethanesulfonic acid-based buffer.
Hitomi and co-workers studied the effects of varying the electronic properties of the ligands and the irradiation wavelength on the NO photorelease quantum yields of substituted complexes 335.1270 Electron-neutral and electron-donating groups gave the highest NO liberation efficiency at 460 nm, whereas electron-withdrawing groups provided the most efficient release at 650 nm.
Thanks to their robustness, thermal stability, and photoreactivity, RuII nitrosyl complexes have become established as useful photoNORMs.113,1271−1286 The applications of these complexes are quite broad and beyond the scope of this review. Several representative complexes of this type, namely the nitrosyl-substituted RuII trichloride complex 336,1277trans-tetraamine RuII nitrosyl complex 337 substituted with N- or P-based ligands,1276 and theporphyrin-based RuII nitrosyl complexes 338,1278 are shown in Figure 53.
Figure 53.
Structures of some RuII-based photoNORMs, Sol = DMSO, CH3CN.
Cyclam RuII complex 339, prepared by Tfouni and co-workers, releases NO only upon irradiation with near-UV light (Φr = 0.14 at 355 nm).1287 Salen complexes 340 bearing π-extended ligands were extensively studied as potential photoNORMs because of their visible-light absorption.1275,1283,1288 Upon irradiation at λirr = 546 nm, 340 (in which X = Cl–) released NO more efficiently (Φr = 0.07) than complexes with other X ligands (ONO– or H2O).1275,1283
Mascharak and co-workers developed a series of RuII complexes 341 bearing tetradentate ligands.1272,1289,1290 A systematic study of complexes in this series with π-extended ligands revealed factors important for release in the visible region.1272 For example, 341 (R = OMe, X = Cl–) releases NO with Φr = 0.01 at 500 nm (λmaxabs = 420 nm), while its π-extended quinoline analogue (341, R = OMe, X = Cl–, quinoline ligand) is photolyzed more efficiently (Φr = 0.025 at 500 nm, λmax = 490 nm).1289 RuII nitrosyl complex 342 containing a tridentate N-(pyridin-2-ylmethylene)quinolin-8-amine ligand released NO upon irradiation with both 365 nm UV light and visible light with Φr = 0.004 (at 365 nm) in acetonitrile.1291 Similarly, RuII complexes 343 and 344 (Figure 54) released NO upon irradiation at 355 nm in water (Φr = 0.12 and 0.20, respectively) and at 410 nm in acetonitrile (Φr = 0.05 and 0.17, respectively).1292
Figure 54.
Structures of RuII NO complexes with pentadentate ligands.
Malfant and co-workers investigated the mechanism of NO photorelease in RuII nitrosyl complexes with terpyridyl ligands bearing substituents having different electron-donating abilities (345a–345c; Table 20),1293,1294 showing that low-lying electronic transitions that drive NO release exhibit strong charge-transfer interactions with the nitrosyl moiety. Upon excitation, the nitrosyl MLCT state is reduced to form a free NO radical and an oxidized RuIII metal complex. Additionally, the 9-dibutyl-9H-fluoren-2-yl substituted RuII terpyridine complexes 346 (Table 20) released NO upon irradiation with blue light.1295 The fluorenyl substituents of these complexes make them excellent chromophores, with a 2-photon absorption cross section of σTPA = (156 ± 23) GM. Substitution of the 9-dihexyl-9H-fluoren-2-yl groups in 347a with N-ethylcarbazol-3-yl ligands (as in 347b) strengthened the bathochromic shift of the charge-transfer transitions toward the electron-withdrawing Ru-NO fragment, resulting in excellent 2-photon absorption (σTPA = (159 ± 22) GM) but reducing the rate of NO release.1296 A similar group of complexes 348 (Table 20) bearing zero, one, two, or three 4′-(4-methoxyphenyl) electron-donating substituents was also investigated.1297 The degree of intramolecular charge-transfer toward the strongly electron-withdrawing nitrosyl ligand increased with the number of methoxyphenyl substituents. However, irradiation of these complexes in the charge-transfer absorption band revealed only minor differences in the quantum yield of NO release, indicating that the CT band is not the sole determinant of NO release efficiency and that other factors must be involved. Malfant and co-workers further extended the study of terpyridine RuII complexes by examining derivatives 349 (Table 20),1298 which released NO upon green-light irradiation. Similar complexes were used by Liu and co-workers to create NO-releasing RuII nitrosyl-containing nanoplatforms bearing BODIPY (350)1299 or naphthalimide (351) ligands.1300 Maji and co-workers recently synthesized two nitrosyl complexes 352, which can be classified as {RuNO}6 and {RuNO}7 complexes using Enemark-Feltham notation.1301 Irradiation with visible light caused NO release from both complexes, but the {RuNO}7 complex (3522+) was more active. This was attributed to the more efficient formation of the MLCT state in the {RuNO}7 complex, which contains a RuII–NO• fragment, than in the {RuNO}6 complex containing a RuII–NO+ fragment.
Table 20. Structures and Properties of RuII PhotoNORMs with Terpyridine Ligandsa.
| NORM | λmaxabs (nm) | εmax (M–1 cm–1) | λirr (nm) | Φr (λirr/nm) | solvent | ref |
|---|---|---|---|---|---|---|
| 345a – trans | 365 | CH3CN | (1294) | |||
| R = NO2 | 357 | 9900 | 0.05 | |||
| R = H | 350 | 18 000 | 0.12 | |||
| R = Br | 354 | 22 900 | 0.11 | |||
| R = OCH3 | 387 | 18 500 | 0.07 | |||
| 345b – cis | 365 | CH3CN | (1294) | |||
| R = NO2 | 352 | 6700 | 0.24 | |||
| R = H | 330 | 17 400 | 0.39 | |||
| R = Br | 340 | 23 600 | 0.32 | |||
| R = OCH3 | 366 | 15 600 | 0.28 | |||
| 345c | 420 | 12.4 × 103 | 365, 436 | 0.08 (365) | CH3CN | (1293) |
| 0.03 (436) | ||||||
| 346 | 400, 405 | CH3CN | (1295) | |||
| R1 = Fl, R2 = H | 455 | 16 700 | 0.06 (400) | |||
| R1 = H, R2 = Fl | 362 | 39 400 | 0.033 (400) | |||
| 347a | 453 | 16 700 | 405, 436 | 0.06 (405) | CH3CN | (1296) |
| 0.03 (436) | ||||||
| 347b | 517 | 14 600 | 436 | 0.01 | CH3CN | (1296) |
| 348 | 365, 436 | CH3CN | (1297) | |||
| R1 = H, R2 = H | 352 | n.d. | 0.086 (365) | |||
| R1 = Ar, R2 = H | 425 | n.d. | 0.011 (436) | |||
| R1 = H, R2 = Ar | 360 | 33 000 | 0.024 (365) | |||
| R1 = Ar, R2 = Ar | 365 | 39 000 | 0.002 (436) | |||
| 421 | n.d.b | |||||
| 349 – trans | 365, 546 | CH3CN | (1298) | |||
| R = NEt2 | 550 | 20 200 | 0.09 (365) | |||
| 0.01 (546) | ||||||
| R = (NO)NEt2 | 497 | 3200 | 0.13 (365) | |||
| 349 – cis | 365, 546 | CH3CN | (1298) | |||
| R = NEt2 | 516 | 17 200 | 0.12 (365) | |||
| 0.045 (546) | ||||||
| 350 | 548 | n.d. | >400, 470, 530, 672 | 0.034 (470) | H2O | (1299) |
| 0.083 (530) | ||||||
| 0.017 (627) | ||||||
| 351 | 519 | n.d. | 808 | 0.017 | salinec | (1300) |
| 352 | –d | n.d. | CH3CN | (1301) | ||
| {RuNO}6 | 298 | 34 500 | (74 min)e | |||
| {RuNO}7 | 479 | 15 400 | (17 min)e |
Fl = 9,9′-dibutyl-9H-fluoren-2-yl, Ar = 4′-(4-methoxyphenyl).
A shoulder in the absorption spectrum.
NaCl aqueous solution (c = 150 mM).
Unspecified wavelength, Xe light source.
Half-life of the NO release for 352.
Slep and co-workers studied complexes 353–355 (Figure 55), which release NO upon visible-light irradiation (λirr = 455 nm).1302 Their quantum yields of NO release span 3 orders of magnitude, ranging from Φr = 0.06 × 10–3 for 353, to Φr = 1.63 × 10–3 for 355, and Φr = 0.04 for 354. DFT analysis revealed that the presence of a second RuII center increases the molar absorption coefficient but does not necessarily influence the electronic distribution of the excited state responsible for NO release. Nikolaou and co-workers developed a ruthenium-based trinuclear complex [Ru3O(CH3COO)6(4-pic)2(NO)]PF6 ((4-pic) = 4-methylpyridine) that releases NO upon irradiation at λirr = 532 nm.1303
Figure 55.
RuII-based dinuclear photoNORMs.
Cho and co-workers recently synthesized a CoIII-nitrosyl complex 356 (Figure 56) that efficiently released NO upon white-light irradiation (λirr = 385–740 nm; Φr = 0.78)1304 and was used in a real-time simulation of cell signaling to study extracellular signal-regulated kinases.
Figure 56.

CoIII-nitrosyl photoNORM.
4.2.3. Sensitized Release of NO from Metal Nitrosyl Complexes
Transition metal nitrosyl complexes are often efficient photoNORMs that can be activated by visible light. However, their absorption bands have often lower molar absorption coefficients than common organic dyes (ε ≈ 103 M–1 cm–1)1082 and absorption maxima in the 400–500 nm region, which is unsuitable for deep tissue irradiation. Unfortunately, modifications that bathochromically shift their absorption into the 600–800 nm region often render these complexes unstable to hydrolysis (i.e., NO and ligand solvolysis).98 Many alternative strategies have therefore been developed to shift their absorption maxima into the red and infrared regions while maintaining good dark stability and enhancing their molar absorption coefficients. These include (i) conjugation of photoNORMs with antenna moieties,1081,1255,1261,1270,1272,1305,1306 (ii) multiphoton excitation of photoNORMs,78,79,1243,1260,1305 (iii) the use of semiconductor quantum dots (see also section 6.4.1),79,1305,1307 and (iv) combining photoNORMs with upconverting nanoparticles (see also section 6.4.2).1258,1259,1305
Chromium(III) nitrito complexes 323a and 323b (Figure 50) that release NO upon intramolecular sensitization by pyrene or anthracene antennae are representative implementations of the first strategy.1255 These complexes typically become fluorescent after releasing NO, enabling the reaction to be monitored. Roussin’s red salt derivatives 328 (R = CH2CH2OH, Figure 52) bearing protoporphyrin IX as a sensitizer are another notable implementation.1262 RuII complexes 357 (Figure 57), which have tetradentate quinoline-based ligands containing various antennae (X = O, resorufin; X = S, thionol; X = Se, selenophore) were developed by Mascharak and co-workers.1289,1308,1309 The attachment of the dye antenna introduces an absorption band (ε ≈ 28 000 M–1 cm–1) in the visible region (500–550 nm), and sensitized NO release occurs with Φr = 0.1–0.2. The derivative 357 (X = Se) was shown to be photoactive even at λirr = 600 nm with Φr = 0.04.1309 Fluorescein- and dansyl-substituted analogs 358(1310) and 359(1311) also efficiently released NO (358: Φr = 0.306 ± 0.01 at 500 nm; 359: Φr = 0.08 at 400 nm) upon irradiation with visible light. Complex 358 has an internal fluorescence turn-on indicator of NO release because release is accompanied by the photochemical formation of a highly emissive fluorescein methyl ester, while complex 359 acts as a fluorescence turn-off indicator of NO release because it is converted into a non-emissive paramagnetic RuIII-dansyl aqua complex upon irradiation.1310,1311 Schiller and co-workers reported a combined spectroscopic-theoretical investigation of RuII complex 360,1312 which has a tetradentate ligand and releases NO upon irradiation at 475 nm.
Figure 57.
RuII photoNORMs with antennae.
Multiphoton excitation is another approach for NIR activation of NO release.1258,1260,1263,1264 The fluorescein-conjugated iron–sulfur cluster 361 (Figure 58) releases 4 equiv of NO upon both 2P- (λirr = 800 nm) and 1P- (λirr = 436 nm; ΦNO = 0.014, calculated per NO molecule) excitation.1313 Similar complexes were used to deliver NO to cells and tissues.1258,1263
Figure 58.
PhotoNORM based on an iron–sulfur cluster sensitized with fluorescein.
Semiconductor quantum dots (see also section 6.4.1) and related nanoparticles can also be used to induce NO release upon irradiation with red or NIR light.1258,1259,1314−1319 This approach is exemplified by the photosensitized release of NO from CrIII nitrito complex 321 (Scheme 76) with CdSe(ZnS) core/shell quantum dots upon irradiation with 450 nm light.1307,1320 Tan and co-workers used a similar approach to design MnII-doped ZnS quantum dots that were encapsulated in the polysaccharide chitosan and conjugated to Roussin’s black salt 326 (Figure 52).1321,1322 NIR excitation (λirr = 1160 nm) of this system caused 2P-induced photoluminescence at λmaxem = 589 nm. The emitted photons were then absorbed by 326, inducing NO release.
A fourth way of inducing NO release with long-wavelength light is to use upconverting nanoparticles (see also section 6.4.2)1258,1323−1325 that are excited via sequential absorption of 2 or more NIR photons and then emit upconverted blue-shifted light that is absorbed by attached photoNORMs. Nanoparticles of this type have been used in combination with Roussin’s black salt 326(1258,1259) and chromium(III) nitrito complex 321.1326
4.2.4. NO-Photoreleasing Materials
NO-releasing materials (see also section 6.4) have attracted considerable research interest because of their potential to offer lower toxicity and better solubility and photoreactivity than molecular photoNORMs. This field has recently been thoroughly reviewed,98,104,106,107,803 and a comprehensive discussion would be beyond the scope of this review. In general, NO-releasing materials contain NO donors attached to an inert carrier, which increases water solubility and may influence many (bio)physical properties including in vivo stability, biodistribution, and pharmacokinetics. NO donors are often coupled with a visible-light absorbing sensitizer or a NIR-absorbing upconverting species to improve photorelease.104 The carriers are often biocompatible polymers,1257 and the NO donors may either be present in a mixture or covalently attached.1327 Polymeric gels are another common tool for delivering NO into organisms.1081,1288,1309,1328−1330 For example, a MnII nitrosyl complex-based sol-gel was demonstrated to release NO upon irradiation at 780 nm.1081 Additionally, self-assembled NO-releasing amphiphiles based on N-nitrosamine moieties have been used to achieve NO delivery with polymersomes.1331 Another common strategy is to combine polymers with NIR-active nanoparticles1322,1332 and upconverting nanoparticles.1258,1323,1325,1333 Finally, Furukawa and co-workers introduced an alternative approach for NO release using a zeolitic imidazole framework with nitroimidazole ligands.1334
4.3. Release of Hydrogen Sulfide and Sulfur-Based Small Molecules
Hydrogen sulfide is a gasotransmitter produced endogenously from cysteine and homocysteine.1335 It acts as a signaling agent involved in antioxidative, antiinflammatory, vasorelaxant, and cytoprotective processes,1336−1338 and there have been several efforts to develop methods for its controlled release. Various H2S-liberating systems activatable by pH, the presence of thiols, redox processes, and light, have been designed.826,828,1339,1340 A complementary approach for H2S release is to exploit photothermal effect using NIR light.1341,1342 Developments in this field have been summarized in several reviews.110−112
The first H2S-releasing systems activatable by UV light were reported only recently.1343 The o-nitrobenzyl caged geminal dithiol 362 (Figure 59) was prepared by the TiCl4-catalyzed condensation of the corresponding thiol with acetone. Upon irradiation of this compound at 365 nm in the presence of water, the free gem-dithiol is released and then hydrolyzed to liberate H2S.1344 The ketoprofenate-based donor 363 also releases H2S with simultaneous decarboxylation upon irradiation at 300–350 nm,1345 while its xanthone analog 364 liberates H2S under UVA irradiation (325–385 nm).1346 Other H2S-releasing systems are based on the Norrish type II hydrogen abstraction-induced photoproduction (λirr = 365 nm) of thiobenzaldehydes1347 from compounds such as 365. The thiobenzaldehydes formed in this way release H2S in the presence of amines (Scheme 78).1348 Another successful system was prepared by encapsulating the hydrosulfide-containing leuco-form of malachite green 366 into vesicles that released H2S upon irradiation with UV light (Φr = 0.01 at 365 nm; Φr = 0.22 at 254 nm).1349 Interesting results were also achieved with the meta-effect-based H2S photodonor 367, which bears water-solubilizing substituents (Φr = 0.14 at 365 nm).573 Compound 368 is an analog of 362 that also releases H2S upon irradiation with UV light. It was also used in combination with upconverting nanoparticles based on LiYF4:Yb/Tm coated with polyethylene glycol-octadecylamine, which convert NIR excitation (λirr = 980 nm) into UV photoemission (λem = 365 nm) to trigger H2S liberation.1350 Finally, compounds 369 release biologically active persulfides upon irradiation at 365 nm with Φr = 0.07 for 369 (R = H) and Φr = 0.36 for 369 (R = CH3).1351
Figure 59.
H2S-releasing systems.
Scheme 78. UV-Absorbing H2S Photodonors.
Chakrapani and co-workers were the first to develop an H2S photodonor activatable by direct excitation with visible light.823 Their BODIPY-based molecule 370 undergoes photoinduced B–O bond cleavage (section 2.12) to release a thiocarbamate-substituted phenolate upon irradiation with 470 nm light. Subsequent thermal self-immolation of this phenolate (kimmol = 0.02 min–1) then liberates carbonyl sulfide (COS), which is transformed to H2S (khydrol = 1.82 s–1) in the presence of carbonic anhydrase, an omnipresent enzyme that catalyzes the hydration of carbon dioxide and the dehydration of bicarbonate.1352 The H2S yield was 30–40% and its formation was tracked in vitro by monitoring the fluorescence enhancement due to the highly emissive photoproduct.
Štacko, Klán, and co-workers developed H2S-releasing molecules 371–373 (Figure 60; 371: Figure 30) based on a BODIPY PPG (section 2.12).799 Upon photochemical excitation of the BODIPY core, the thiocarbamate leaving group installed at its meso-methylene position dissociates, leading to the release of COS, which is then converted into H2S using carbonic anhydrase. Unlike 371, the polyethylene glycol-substituted analog 372 is water-soluble and efficiently releases COS together with Ph2NH (λmaxabs = 513 nm in degassed aq. PBS, Φr = 15.1 × 10–2 at 365 nm; yield ≈ 86%). The π-extended derivative 373 has a bathochromically shifted absorption band and photoreleases H2S upon irradiation at 700 nm (λmax = 688 nm in degassed aq. PBS, Φr = 9.7 × 10–2 at 365 nm, yield 69%). Oxygen quenches the productive triplet state, but H2S release can proceed through both the singlet and triplet states.798 The thiocarbamates are synthesized by the reaction of thiols with a suitable carbamoyl donor (4-nitrophenyl carbamate823 or carbamoyl chloride,799Figure 60). The strategy of thiocarbamate caging and COS release was originally conceived by Pluth and co-workers and implemented in the form of compound 374, which bears an o-nitrobenzyl PPG (section 2.1.1) and absorbs below 400 nm.1353
Figure 60.
H2S photoreleasing molecules.
Singh and co-workers developed tetraphenylethylene-conjugated p-hydroxyphenacyl H2S donors 375 (Scheme 79), which aggregate in aqueous media to form visible-light activatable (λirr >410 nm) nanoparticles that exhibit both aggregation-induced emission (AIE) and excited-state intramolecular proton transfer (ESIPT).1354 These nanoparticles material offer efficient H2S release (Φr = 0.18) that can be monitored in real time due to a fluorescence color change (λem = 549 nm for the starting material and 486 nm for the photoproduct).
Scheme 79. Tetraphenylethylene-Conjugated p-Hydroxyphenacyl-Based H2S Donor.
Singh and co-workers also reported H2S photorelease from the benzo[d]thiazol-2-yl-substituted p-hydroxyphenacyl compound 376 (Figure 61) upon irradiation with visible light (λirr > 410 nm).1355 The closely related derivative 377 liberated hydrogen persulfide (H2S2) under similar conditions,1356 while sulfide dimers analogous to 376 photoreleased H2S when formulated as organic nanoparticles.633
Figure 61.
p-Hydroxyphenacyl-based H2S photodonors.
Another H2S releasing system developed by Singh and co-workers is the visible light-responsive (λirr > 410 nm) nanocarrier system 378, which is based on a quinoline derivative (Figure 62) attached to a fluorescent carbon dot (see also section 6.4).587 The system fluoresces in the visible region (λem = 425 nm, ΦF = 0.078) and releases H2S with a quantum yield of Φr = 0.09.
Figure 62.
Fluorescent carbon dot-based H2S photodonor (CD = carbon dot).
The group of You used a hybrid approach to develop Pluronic F-127-based vesicles containing a photosensitizer that generates singlet oxygen upon irradiation with visible light.1357 Two such photosensitizers were tested, as shown in Scheme 80: PtII octaethylporphine 379 (Φr = 0.30) and [IrIII bis(2-(3-methoxyphenyl)pyridinate)(1,10-phenanthroline)]PF6380 (ΦΔ = 0.41). The singlet oxygen generated by these complexes upon irradiation (λirr = 500–550 nm for 379 and 380–500 nm for 380) reacts with 1,3-diphenylisobenzothiophene 381 to form an endoperoxide, which then undergoes thermal decomposition to release H2S (Φr = ∼2× 10–3).
Scheme 80. Sensitizer-Based System for Photorelease of H2S by Visible Light.
Visible light-induced H2S release can also be achieved using organometallic complexes such as 382 and 383 (Figures 63 and 31), as demonstrated by Wilson and co-workers. These RuII terpyridyl complexes have low energy metal-to-ligand charge transfer (MLCT) absorption bands in the red region (λmaxabs = 581 nm for 382 and 570 nm for 383).1083 Efficient ISC from 1MLCT* leads to a dissociative triplet ligand-field excited state (3LF) that liberates the monodentate ligand phosphinodithioate with near-quantitative quantum yields (Φr = 0.85 for 382, Φr = 1.02 for 383 at 626 nm). The released phosphinodithioate acts as a thermal H2S donor, undergoing hydrolytic decomposition to give two equivalents of H2S.1358,1359 Complexes 382 and 383 were used successfully in living cells both to protect H9c2 cardiomyoblasts and in an in vitro model of ischemia-reperfusion injury.
Figure 63.
RuII-based H2S donors.
Carbon disulfide is another small gaseous molecule that was recently identified as an important bioregulatory and therapeutic agent1360 and has thus become an interesting target for uncaging and triggered delivery. Ford and co-workers developed a photocatalytic method for CS2 production from potassium 1,1-dithiooxalate 384 (Figure 64) by oxidative cleavage photosensitized by CdSe quantum dots (see also section 6.4.1).1361 This system releases CS2 upon irradiation between 365 and 530 nm with Φr = 0.029–0.045. The mechanism of CS2 release involves photoinduced two-electron oxidation of 384 to give CS2 and CO2.
Figure 64.

Carbon disulfide donor.
5. Photoacid and Photobase Generators
Photopolymerization processes use light to initiate polymerization, usually via radical reactions. Alternatively, polymerization may be triggered by an acid or a base formed by irradiation of a photoacid or photobase generator. This field has been covered by several recent reviews,51,1362 so we discuss only a few particularly notable visible-light absorbing generators. Thiophene-containing oxime sulfonates 385 release sulfonic acids upon irradiation at 365–475 nm (Figure 65).1363 The first step in the release mechanism was proposed to be the liberation of the corresponding sulfonyl radical via homolytic cleavage of the N–O bond. Also notable are the BODIPY-based donor–acceptor triarylsulfonium salt-based photoacid generator systems 386 and 387, which are photoactivated by green and red LED light, respectively, and were used to trigger cationic polymerization (Figure 65).1364
Figure 65.
Photoacids 385–387.
Visible-light initiated polymerization in the presence of merocyanine-based photoacid 388 was demonstrated by Boyer and co-workers (Scheme 81).1365 The proton dissociation was reversible, enabling temporal control of the process.
Scheme 81. Photoconversion of Merocyanine-Based Photoacid 388(1365).
Scheme 82 shows a rare example of a visible-light absorbing photobase generator. In the first case, benzothiophene imino derivative 389 releases an amine base in a two-stage photoprocess.1366 The oxamic acid ester 390 then undergoes homolytic N–O bond cleavage, followed by decarboxylation and radical addition into the adjacent aryl ring. In another example, tetramethyl guanidine (a basic polymerization initiator) was liberated from a coumarinyl-4-methyl PPG (see also section 2.2) upon irradiation at 400–500 nm.346
Scheme 82. Visible-Light Absorbing Photobase Generator1366.
6. Photosensitized Release: From Small Molecules to Nanoparticles and Nanomaterials
Photochemically induced uncaging using visible/NIR light can be achieved by various approaches. Direct release following one-photon (1P) absorption is the most desirable but is also rather challenging to achieve. The low energy of red and near-infrared photons is usually insufficient to initiate chemical processes; thus, a major goal when developing photoactivatable moieties is to identify feasible photochemical transformations. Many additional criteria may also need to be addressed; in particular, useful compounds must have suitable photochemical (good quantum yields and release rate constants, non-absorbing side-products), chemical (non-reactive side-products), and biological (non-toxicity of all species in the photoreaction pathway, and potentially water solubility) properties.10 The release of a leaving group, usually an anion or neutral species, can generally proceed directly from an excited state of different multiplicity (Scheme 83a) or a reactive ground-state intermediate formed from the excited chromophore (Scheme 83b). Near-infrared absorption is often related to molecular overtone and combination vibrations that are forbidden by the quantum-physical selection rules, so the corresponding molar absorptivities are usually small.1367 Only dyes with extensive conjugated systems such as cyanines or squaraines1368 exhibit intense electronic transitions in the NIR region. A potentially expensive solution is to induce absorption of two (2P) or more photons (multi-photon absorption) by a single molecule using a high-power femtosecond laser, which enables access to excited states with energies equal to the sum of the absorbed photon energies.1369,1370 This method can thus be used to excite chromophores absorbing in the UV region with NIR or visible light, as discussed extensively in our previous review.10
Scheme 83. Direct Photorelease versus Photosensitized Release.
Another strategy for activating release with visible/NIR-light is to use two separate molecular components or bi-/multi-chromophoric systems, with one being a light-harvesting molecular or nanoscale sensitizer (see also section 6.1) that can transfer energy to10,1371−1373 (Scheme 83c) or exchange an electron with10,1374,1375 (Scheme 83d; the excited sensitizer is either an electron donor or acceptor) a separate molecule or complex bearing the leaving group. The excited chromophore can also be the photoremovable moiety, as in the case of Schemes 83a and 83b; in such cases, electron transfer to or from an auxiliary ground-state electron acceptor or donor, respectively, is responsible for leaving group release and the advantage of the auxiliary light-harvesting system is lost (Scheme 83e).
Scheme 83f depicts an alternative strategy that relies on a photosensitizer acting via the photodynamic effect: it generates singlet oxygen or another reactive oxygen species (ROS) upon irradiation,1376−1379 which then reacts with an oxidizable moiety bearing the leaving group.
6.1. Molecular Photosensitizers: Energy Transfer
Photoinduced energy transfer is a practical way to generate (usually) a triplet excited state, particularly when the desired state is not accessible by direct excitation or the molecule does not absorb sufficiently at the desired wavelength.136,1371 An efficient triplet–triplet energy transfer should be exergonic to avoid reverse transfer, and the sensitizer should have a high molar absorption coefficient, undergo efficient ISC, and have a sufficiently long triplet lifetime. Intramolecular energy transfer via either through-space or through-bond mechanisms might be preferred because it avoids the bimolecular entropic restrictions associated with diffusion and its efficiency can be finely tuned by adjusting the inter-chromophore distance.10,1372,1373,1380,1381 Intramolecular energy transfer necessarily involves the use of an “equimolar” quantity of the sensitizer.
Several bichromophoric photoremovable protecting groups containing various UV-absorbing light-harvesting chromophores have been designed and studied by Corrie and co-workers over the past decade.842,1382−1385 Benzophenone, which has substantially higher molar absorption coefficients above 300 nm than the photoactivatable nitroindoline group, was found to act as a triplet sensitizer to promote the nitroindoline moiety in compound 391 into its triplet state and trigger the subsequent release of a carboxylic acid (Scheme 84).1385
Scheme 84. Energy-Transfer-Mediated Release Involving the Nitroindoline Group1385.
Steiner and co-workers used 9H-thioxanthen-9-one, which absorbs at slightly above 400 nm, to improve the light sensitivity of the weakly absorbing o-nitro-2-phenethyl PPG (see also section 2.1.2).171,252,253,1386 For example, compounds 392 consisting of two chromophores connected via flexible tethers of different lengths were tested in the photolithographic synthesis of high-density DNA chips (Scheme 85).253 It was found that in addition to triplet–triplet energy transfer, the singlet excited state of the sensitizer was important, especially in systems with short tethers. Similarly, the photocleavage of the 2-(2-nitrophenyl)propyl group was sensitized intramolecularly by 9H-thioxanthen-9-one in the triplet excited state to release a fluorescent rhodamine dye.1387
Scheme 85. Energy-Transfer-Mediated Release Involving the o-Nitrobenzyl Group253.
The triplet excited state of 9H-thioxanthen-9-one was also shown to sensitize a linked benzothiophene-2-carboxanilide ring system (393, Scheme 86) via electrocyclic ring closure of the anilide moiety to liberate leaving groups including halides, thiolates, carboxylates, and phosphates (Φr = 0.14–0.41 at 395 nm).1388
Scheme 86. Energy-Transfer-Mediated Release Involving the Benzothiophene-2-carboxanilide Group1388.
Wang and co-workers recently demonstrated that intermolecular triplet–triplet energy transfer between a PtII tetraphenyltetrabenzoporphyrin sensitizer excited at 625 nm and a photoactivatable meso-methyl-substituted BODIPY derivative (394) (see also section 2.12) leads to the release of a carboxylate moiety (Scheme 87).808,1389 The use of photosensitizers with a higher T1 energy and a lower S1 energy than that of the photocleavable group was recommended to enable exergonic energy transfer from a sensitizer excited at longer wavelengths.
Scheme 87. Energy-Transfer-Mediated Release Involving the BODIPY PPG808.
6.2. Molecular Sensitizers and Photocatalysts: Electron Transfer
The liberation of a leaving group can also be facilitated by (inter-/intramolecular) photoinduced electron transfer (PET), where the excited species is either the sensitizer or the substrate itself (Scheme 83d,e).10,1374 For uncaging purposes, the sensitizer should have a high molar absorption coefficient and satisfy the other criteria mentioned in the previous section. If both reactants are neutral prior to the reaction, the resulting radical ion pair will undergo chemical transformations that eventually lead to leaving group release or recombination to restore the starting material. The Gibbs free energy of PET can be calculated from the corresponding redox potentials of both reactants and the excitation energy of the excited molecule.10,136,1390−1392
UV-light-initiated PET-assisted uncaging was reviewed several years ago.10,1374 Hamada’s pioneering photofragmentation of tosylamides in the presence of a reducing agent to give amines,840 and especially the work of Falvey and co-workers on photosensitized uncaging of phenacyl esters,1393,1394 picolinium esters,1393,1395−1397 or 9-phenyl-9-tritylone1398 were key studies in this area.
Falvey and co-workers also demonstrated that the sensitized release of carboxylic acids from phenacyl esters using a visible-light-absorbing electron donor (anthracen-2-amine; λirr > 400 nm) proceeds in near-quantitative chemical yield (Scheme 88).1399 The sacrificial sensitizer can be regenerated in the presence of ascorbic acid by donating a hydrogen atom (or electron) to the aryloxy radical. Their experiments indicated that the phenacyl moiety interacts with the singlet excited state of the sensitizer.1393 More recently, Speckmeier and Zeitler reported the catalytic deprotection of analogous phenacyl anddesyl (395) protecting groups using substoichiometric quantities of [Ru(bpy)3](PF6)2 (1 mol %) as a photocatalyst excited at 455 nm and ascorbic acid (Asc–H) as a sacrificial electron donor (Scheme 89).1400
Scheme 88. Sensitized Release of Carboxylic Acids from Phenacyl Esters1399.
Scheme 89. Photocatalyzed Release of Carboxylic Acids1400.
A similar strategy was used by Falvey’s group to release carboxylic acids, amino acids, and phosphates from N-alkylpicolinium, which has a favorable reduction potential of Ered = −1.1 V. Scheme 90 shows a bimolecular photodeprotection of a carboxylic acid using BODIPY and coumarin derivatives as photosensitizers absorbing at λmaxabs ≈ 500 and 467 nm, respectively.1401 The PET-induced uncaging of carboxylic acids from an N-alkylpicolinium derivative by visible light was also demonstrated in the presence of substoichiometric amounts of tris(bipyridyl)ruthenium(II) (λmax ≈ 450 nm) acting as both a sensitizer and a mediator of electron transfer between a good donor and the protecting group.1402 Ascorbic acid, N,N-dimethylaniline, or 1,4-diazabicyclo[2.2.2]octane served as sacrificial electron donors in this case. Fluorescence quenching and transient spectroscopy experiments showed that the reaction rate constants were near the diffusion limit. Analogous visible-light promoted reactions were performed with ketocoumarin derivatives (λmaxabs ≈ 450 nm) as sensitizers/mediators,1403N-methylpyridinium iodide esters that undergo charge-transfer excitation,1404 and an anthraquinone-based chromophore covalently attached to an N-alkylpicolinium ester.1405 Similarly, Boncella and co-workers used tris(bipyridyl)ruthenium(II) to mediate PET to N-methylpicolinium carbamates to release amines in very high chemical yields,1406 Cui reported the release of N-alkyl substituted 4-picolinium ions conjugated with self-assembled monolayers via an ester group using [Ru(bpy)3]2+ as a photocatalyst under irradiation at 452 nm,1407 and Anderson, Flamigni, and co-workers showed that electron-accepting N-methylpyridinium, phenacyl, or p-nitrobenzoate moieties can be activated via intramolecular PET via two-photon absorption if covalently attached to electron-donating fluorene derivatives (λabs < 450 nm).1408
Scheme 90. Photosensitized Release from N-Alkylpicolinium Ions1401.
A photoactivatable system based on a 9-phenyltritylone protecting group that releases alcohols upon irradiation at 447 nm in the presence of fac-(tris(2,2′-phenylpyridine))iridium(III) (fac-Ir(ppy)3) or tris(bipyridine)ruthenium(II) chloride ([Ru(bpy)3]2+) as photosensitizers and triethylamine as a sacrificial electron donor was reported by Falvey and co-workers.1409 The authors proposed photodeprotection mechanisms involving both oxidative and reductive quenching scenarios (Scheme 91) corresponding to the general mechanism shown in Scheme 83d. In another case, these authors demonstrated the efficient release of calcium ions (Ca2+) from an EDTA complex facilitated by photolysis of riboflavin photocatalysts at λirr > 440 nm.1410
Scheme 91. Photocatalyzed Release of Alcohols1409.
An interesting visible-light uncaging reaction using photocatalytic deboronative hydroxylation was recently reported by Chen and co-workers (one example is presented in Scheme 92.1411 Phenol, alcohol, and amine derivatives were liberated from the corresponding boronates in high chemical yields in bacteria and mammalian cells by reaction with transient hydrogen peroxides generated in the presence of molecular oxygen using fluorescein or rhodamine derivatives as photocatalysts and ascorbate as a reductant. In a different approach, Winssinger and co-workers used an azide-reduction-triggered immolative linker1412 to release rhodamine using a [Ru(bpy)2 phen]2+ conjugate as a photocatalyst in the presence of ascorbate.1413
Scheme 92. Photocatalytic Deboronative Hydroxylation1411.
6.3. Release via the Photodynamic Effect
A different way to release molecules of interest is to exploit the photodynamic effect, in which an excited photosensitizer and ground-state (triplet) oxygen (3O2) react to produce reactive oxygen species (ROS) or radicals that then react with molecules in the vicinity. The most common ROS is singlet oxygen (1O2) produced from 3O2 by the triplet–triplet annihilation mechanism using triplet-excited organic dyes such as porphyrins, phthalocyanines, cyanines, pyropheophorbide, rhodamine, methylene blue, or eosin, which typically absorb in the red or NIR regions.136 The use of this phenomenon to induce cell death in medical applications is known as photodynamic therapy. Diverse chemical functionalities and entities can be cleaved by reaction with singlet oxygen (Type II photooxygenation136) including olefins, vinyl ethers, vinyl disulfides, thioketals, and lipids.1414,1415 Such singlet oxygen-sensitive groups can be inserted into tethers connecting drug molecules to structures such as membranes, nanomaterials, surfaces, or supramolecular carriers. The drug can then be liberated in the presence of a sensitizer, oxygen, and light.1414−1417 The triplet-excited photosensitizer may also participate in electron exchange, that is, in Type I photooxygenation, as discussed in section 6.2.
In an early work, Anderson and Thompson demonstrated that singlet oxygen oxidation of liposome membranes by irradiating a membrane-incorporated sensitizing zinc phthalocyanine at 640 nm resulted in the release of encapsulated glucose.1418 The destruction of the membranes was attributed to a [2+2] cycloaddition reaction between 1O2 and membrane alkenyl groups to form dioxetanes that subsequently decompose into two aldehydes.1419 This mechanism was demonstrated to be responsible for the release of chlorin, which was used as a sensitizer (sens) that was covalently attached to silica via a tether containing a 1,2-diphenoxyethene unit (Scheme 93).1417 Many other studies have exploited the reaction between 1O2 and ethene derivatives such as vinyl ethers, bis(alkylthio)alkenes, or aminoacrylates for substrate liberation.955,956,1420−1437 The uncaging photooxidation of lipids or liposomes1438 in the presence of organic sensitizers may also proceed via mechanisms involving singlet oxygen.1439−1446
Scheme 93. Cleavage via the Photodynamic Effect (sens = Chlorin)1417.
The second example of uncaging via the photodynamic effect is the release of siRNA bearing a 9-anthracenyl group and a photosensitizer (pyropheophorbide or eosin Y derivatives) attached to the 3′-terminus of the lagging strand (Scheme 94).1447 Upon irradiation at 650 nm, singlet oxygen is formed by sensitization and attacks the 9-anthracene moiety to form an endoperoxide intermediate, which is then detached as anthracene-9,10-dione to liberate the siRNA strand. Similarly, methylene blue and alkoxyanthracene were used as a photosensitizer and a cleavable group, respectively, to disrupt micelles loaded with a chemotherapeutic agent.1448 Other singlet-oxygen sensitive groups including thioketals,1449−1462 thioethers,1463 imidazoles,1464 indolizines,1465 and hydrazones,1466 as well as selenium-1467−1477 and tellurium-containing1478−1480 moieties have also been used for uncaging. A qualitatively different photosensitizer, TiO2 nanotube-doped PbS quantum dots combined with S-nitrosocysteine, was found to generate singlet oxygen upon irradiation at <600 nm, leading to the release of nitric oxide (Scheme 94).1481
Scheme 94. Photodynamic Cleavage of a 9-Anthracenyl Group from siRNA1447.
An interesting approach to fluorophore photoactivation was recently reported by Wensel, Xiao, and co-workers,1482 who replaced the carbonyl groups in common fluorophores with thiocarbonyl groups. This significantly reduced their fluorescence because of a photoinduced electron transfer-quenching mechanism. Upon irradiation, these compounds generate singlet oxygen, with which they then react to form their oxo derivatives, thereby restoring their original strong fluorescence (only one example is shown in Scheme 95).
Scheme 95. Fluorescence Switch via Self-Oxygenation1482.
Two additional very interesting uncaging strategies involving photosensitized singlet-oxygen-mediated self-destruction of the photosensitizer leading to the release of a desired species are the liberation of carbon monoxide from flavonols (see section 4.1.1) and the use of cyanine dyes to release various leaving groups upon irradiation with red and NIR light (see below).
Cyanine dyes are invaluable fluorophores in chemistry and biology.1483 They feature odd-numbered methine bridges connecting two nitrogen-containing heterocycles,1484 which are responsible for their unique photophysical properties.1485 In particular, heptamethine dyes with seven-carbon bridges are widely used as fluorescent tags in biological studies1486−1488 and as markers in medical diagnostic tests.1489,1490 Heptamethine cyanines (Cy7) have narrow absorption bands with high molar absorption coefficients (εmax = 0.5–2.5 × 105 M–1 cm–1) in the red to near-infrared (NIR) parts of the spectrum (650–800 nm). The peak absorption of heptamethine cyanines lies within the “first optical window” of mammalian tissue,125,1486 where light attenuation due to absorption and scattering is minimal, making them particularly suitable for in vivo applications. This section focuses on the use of cyanine chromophores, particularly Cy7, as photoreleasing systems. Several aspects of this topic have been addressed by various recent review articles and perspectives.20,21,50,878,890,1491−1494
The photodegradation of heptamethine and other cyanines is a known phenomenon1495 and has been shown to proceed via photooxidative cleavage of one or more heptamethine C=C bonds to form the corresponding carbonyl photoproducts.1496−1506 The most common mechanism of Cy7 photooxygenation involves photosensitization of ambient (ground-state) molecular oxygen by the triplet excited state of Cy7 (3396*) to form singlet oxygen (1O2), followed by a [2+2] cycloaddition to form dioxetanes 397 that undergo thermal decomposition to form the carbonyl photoproducts (Scheme 96). Supportive evidence for this pathway include the findings that (1) triplet–triplet annihilation is exergonic1507,1508 even though the quantum yields of 1O2 production (ΦΔ) tend to be low (∼0.01–0.001);1509,1510 (2) the extent of photooxygenation depends on the oxygen concentration;1501 (3) the reaction is suppressed in the presence of 1O2 quenchers1499,1501 or traps;1497,1504,1511 (4) reaction rates are higher in deuterated solvents, which extend the lifetime of 1O2;1504 and (5) dioxetane intermediates can be detected by mass spectrometry.1499,1512,1513 The generated singlet oxygen attacks the C2=C1′ or C2′=C3′ bonds to form the corresponding dioxetanes, which undergo thermal decomposition to give two carbonyl compounds.1499,1512−1514 For example, the paired carbonyl products 398 + 399 and 400 + 401 were formed in a ∼4:1 concentration ratio during the photooxidative cleavage of 396, accounting for 70% of the photodegradation chemical yield (Scheme 96).1513 Computational analysis (B3LYP/cc-pVTZ) of 396 suggested that the observed regioselectivity is determined by the energies of the dioxetane intermediates; it was found that only dioxetanes at the C2/C1′ and C2′/C3′ positions, which give rise to carbonyl compounds 398–401, are formed exergonically (ΔG = −2.8 and −0.7 kcal mol–1, relative to 396),1513 in agreement with the experimental findings.1494,1499,1512,1513 Cyanine photobleaching may also involve other pathways,1501 such as photoinduced electron transfer from the Cy7 triplet state to oxygen to form O2–, which may subsequently generate hydroxyl radicals or other reactive oxygen species (ROS), together with a potentially reactive cyanine-radical cation.1497,1498,1505,1515 The photodegradation of cyanines has mainly been studied to identify factors affecting their stability1495,1502,1505,1516−1520 to improve their performance as fluorescent imaging agents,1495,1521 although uses of their photodegradation, for example in sensing,1522,1523 have also been reported.
Scheme 96. Proposed Mechanism of Cy7 Photodegradation1513.
Schnermann and co-workers pioneered the repurposing of heptamethine cyanines as photocages by developing two Cy7 scaffolds (402 and 403, Figure 66) that harness the regioselectivity of the photooxidative degradation process to drive either C–N bond cleavage1491,1512,1524,1525 (402) or a β-elimination reaction1514 (403), both ultimately leading to leaving group release. Both scaffolds contain a cyclohexenyl moiety attached at the C3′/C5′ positions of the heptamethine bridge, which was originally used to increase the rigidity (and hence the fluorescence quantum efficiency) of Cy7.1526,1527
Figure 66.
General structures of Cy7 photocages undergoing photooxidative-cleavage and C4′–N bond hydrolysis (402) or β-elimination (403).
Cy7 derivatives 402 were synthesized from the corresponding cyanine dye featuring a chlorocyclohexenyl group.1491,1512,1524,1525 The chlorine atom of this group is conveniently displaced via an SNR1 reaction under mild conditions,1528 in this case using ethylenediamine as the nucleophile. The leaving groups, such as chloroformate or p-nitrophenylcarbonate, were introduced in the last step. Photoexcitation (λirr = 690 nm) of 404 (λmaxabs = 676 nm, ε = 5.15 × 104 M–1 cm–1) resulted in the formation of products 405 and 406 in a ∼4:1 ratio (Scheme 97), as also observed for Cy7 396. The Cy7 derivative 404 is less prone to the hydrolytic release of the tertiary amine than the photooxidative cleavage products 405 and 406, which was attributed to its more extensive π-conjugation, which reduces the electrophilicity of the key C4′–N bond (i.e., weakens its iminium character). Electrophilic reactivity at the C4′ position of heptamethine cyanines has previously been documented.1529−1531 The inertness of the resulting aldehydes to both light and reactive oxygen species further supports the assumption that hydrolysis is the main pathway of amine release.1512 The direct hydrolytic release of an aniline derivative (7-aminocoumarin) bound to the C4′ position was inefficient (with a chemical yield of <8%) despite rapid photooxidation of the corresponding heptamethine cyanine.1514 It was therefore proposed that hydrolysis requires the prior protonation of the amine, and that the difference in the efficiency of hydrolysis between the tertiary amine and the aniline is due to their different basicities.1514 Hydrolysis of the C4′–N bond is followed by intramolecular cyclization of the ethylenediamine linker,1532,1533 resulting in the release of an alcohol as a leaving group.1512,1524,1525,1534 Although the cyclization step is pH-dependent1533 (proceeding more slowly at low pH), leaving group release efficiency was only reduced by a factor of 1.5 upon lowering the pH from 7.4 to 5.0.1525 The overall chemical yield of uncaging (66–70%) correlated with the quantities of 405 and 406 formed during the reaction, suggesting that the light-independent steps are efficient.1512 The kinetics of leaving group release appear to depend mainly on the rate of C4′–N bond hydrolysis.1512
Scheme 97. Mechanism of Uncaging from Cy7 Photocages 404(1512).
The effects of structural variation of 404 on its spectroscopic and photochemical properties were also explored (Figure 67).1524 Replacing the butyl sulfonic acid substituents on the indolenine nitrogens with n-propyl (407) or n-butyl pentanoates (408) did not affect the compound’s spectroscopic and photochemical properties but significantly improved cellular penetration.1491,1524 Replacing the N,N′-dimethylethylenediamine linker with N,N′-diethylethylenediamine (409) caused a 40 nm bathochromic shift of the absorption band, reduced the background (dark) hydrolysis rate (krel = 0.73), and increased the photooxidation rate (krel = 2.8) under the experimental conditions, although overall uncaging efficiency was reduced (krel = 0.81).1524 Efforts to introduce more sterically demanding amines were hampered by the substantially lower reactivity of such amines in the chlorine substitution reaction.1524 The lower background hydrolysis rate of 409 was attributed to increased steric hindrance either around the amine-heptamethine bond or the carbamate group.1532 The introduction of sulfonates on the indolenine rings (410), which is often done to prevent aggregation,1535 reduced photooxidation efficiency (krel = 0.43) and increased the rate of background hydrolysis (krel = 1.3) but also improved the kinetics of release (krel = 4.2).1524 An alkyne group allowing the photocage to be connected to targeting molecules using click chemistry was introduced by using a branched carbamate linker (411).1525 Replacing one (412a) or both (412b) flanking heterocycles with benzothiazole rings significantly improved oxidation efficiencies (krel = 3.7 and 6.7, respectively), in accordance with earlier studies on Cy7 fluorophores.1504 However, this also dramatically increased the background hydrolysis rate (krel = 4.7 and 8.5, respectively).1524 Replacing the central cyclohexenyl ring with a cyclopentenyl moiety (413) also increased the photooxidation rate (krel = 3.5) but significantly reduced the uncaging rate (krel = 0.013) and the overall uncaging chemical yield (<20%). The reason for the decreased uncaging efficiency was determined to be inefficient hydrolysis of the carbonyl intermediates.1524 On the other hand, replacing the sulfonated indolenine rings with sulfonated benzindolenines and installing an alkoxy substituent on the cyclohexyl group (414a and 414b) yielded PPGs with properties comparable to those of the original 404 but with a significantly red-shifted absorption spectrum (λmaxabs = 690 and 732 nm, respectively).1524 The individual contributions of each of these modifications have not yet been determined. In principle, the flexibility of the synthesis1536−1538 and post-synthetic functionalization1494,1531,1539,1540,1510 of cyanines enables useful structural modifications of the bridge or terminal heterocycles, providing considerable scope for modulation of their spectroscopic and photoreaction properties.
Figure 67.
Structures of Cy7 photocage derivatives.
An analogous photooxidative cleavage mechanism was used by Schnermann and co-workers in the case of 403 (Figure 66) to drive the release of a leaving group through a β-elimination reaction.1514 Several examples utilizing similar photooxygenation/β-elimination sequences to drive leaving group release have been reported.187,1541−1545 Cy 7 photocages similar to 402 have mainly been used to release of phenols, while β-elimination of a carbamic acid functionality in 403 followed by spontaneous decarboxylation1546 was used to release a free amine. Photoexcitation (λirr = 780 nm) of 415 (λmaxabs = 781 nm, εmax = 3 × 105 M–1 cm–1, LG = coumarin 151) proceeded rapidly in PBS buffer to form two carbonyl intermediates, 416 and 417 (Scheme 98).1514 Only 417 underwent efficient β-elimination, however. The formation ratio of 416 and 417 (∼4:1; 70% chemical yield) explains the relatively low overall uncaging yield of this process (∼14%).1514
Scheme 98. Mechanism of Uncaging from Cy7 PPGs via a Photooxidative Cleavage/β-Elimination Sequence1514.
The photooxidative cleavage/β-elimination sequence was also applied to merocyanines such as 418 and 419 (λmax = 664 and 713 nm, respectively).1514 It was previously shown that oxidative cleavage takes place preferentially in the position adjacent to the more electron-rich heterocycle in unsymmetrical merocyanines.1505,1511 Accordingly, irradiation of 418 and 419 (λirr ≈ 660 and 690 nm, respectively, in PBS buffer, pH 7.4) resulted in the release of coumarin 151 with chemical yields of 33% and 22%, respectively1514 (Scheme 99). The difference in yield was attributed to differences in the extent of dye aggregation in solution.1514 For both compounds, additional release (4–5%) was observed after irradiation was stopped, suggesting that β-elimination is the rate-limiting step.1514
Scheme 99. Photochemistry of Merocyanine Photocages 418 and 419 Involving a Photooxidative Cleavage/β-Elimination Sequence1514.
The estrogen receptor antagonist/agonist 4-hydroxycyclofen was caged with a Cy7 derivative (420) and its light-induced release was used to regulate gene expression in cell cultures (λirr = 690 nm; Scheme 100).1491,1512 The reactive oxygen species generated during the photodecomposition process and the potentially reactive carbonyl photooxidation products were both well-tolerated in the studied cell cultures. Compound 420 also enabled light-mediated regulation of gene expression under similar irradiation conditions (λirr = 690 nm) in a CreER/LoxP system in transgenic mouse embryonic fibroblasts (MEFs).1512 Exchanging the butyl sulfonic acids on the indolenine nitrogens with n-butyl pentanoates (421) significantly improved cellular penetration and increased spatial control over photoactivation by causing intracellular entrapment of the photocage.1491 A prolonged irradiation time was required in experiments using this compound,1491,1512 which was a limitation in applications requiring efficient substrate release. Fluorescence imaging of 420 in MEF and HeLa cells revealed that its intracellular distribution displayed a distinct punctate pattern and that it co-localized with LysoTracker staining,1512 whereas 421 co-localized with MitoTracker.1491 This difference in subcellular localization was attributed to the different charges of the two compounds. The cellular uptake mechanism was not determined, but other non-sulfonated heptamethine cyanines were shown to be captured by cells via endosomal uptake.1547−1549
Scheme 100. Photouncaging of Cyclofen Derivatives from Cyanine Photocages1512.
Cy7 PPGs have also been used for antibody-targeted drug-release.1534,1550,1551 The two strategies discussed above were used to non-specifically conjugate an NHS ester, the caged combretastatin A41525 (CA4, a microtubule polymerization inhibitor) derivative 422, and the caged duocarmycin1524,1552 (a DNA alkylating agent) derivative 423 to panitumumab (Pan), a clinically used anti-human epidermal growth factor receptor (EGFR) monoclonal antibody (Figure 68). The latter conjugate was injected in vivo into mice bearing MDA-MB-468 EGFR+ tumor xenografts, and the tumor area was irradiated at 690 nm 4 days after its administration. This single-dose treatment was sufficient to significantly reduce tumor size and improve overall survival compared to control groups.1524 A combination therapy using 423 and Pan-IR700947 (a near-IR photodynamic therapy agent) exhibited greater treatment efficacy than either therapeutic agent alone.1552 The location of this antibody-drug conjugate was verified prior to its photoactivation by exploiting the fluorescence of the heptamethine cyanine photocage.1524,1525,1552
Figure 68.
Structures of CA4 and duocarmycin caged with Pan-targeted heptamethine cyanine photocages.
6.4. Photosensitization by Nanoparticles and Nanomaterials
Nanotechnology has found a remarkable array of applications in science and technology including biotechnology and biomedicine.1553 Nanoparticles (NPs) and nanocarriers are frequently used for diagnostics, biosensing, photodynamic therapy, photothermal therapy, and targeted and controlled drug delivery/release. Developments in this field have been reviewed extensively in the past decade.8,14,15,114,129,130,1415,1438,1554−1585 Various materials can be used in the design of nanocarriers including metal NPs, semiconductor NPs, nanocarbons, virus- and bacteriophage-based NPs, microcapsules, and hydrogel-based systems.1554,1556,1586 NPs can be both carriers (transporters) or active participants in drug (species) delivery. Photoactivatable NP systems may feature direct covalent bonds to drug molecules, or drugs may be encapsulated via non-covalent interactions. These systems must be stable in the relevant environment until an external trigger is applied to induce release. Light-activated release mechanisms include photochemical bond cleavage, photoreduction, photooxidation, photochemically-induced hydrophobicity switching, photo-cross-linking, photoisomerization, and photothermal processes.1554 The following paragraphs briefly review the fundamental principles of species release from NPs upon irradiation with visible/NIR light and present some notable examples of related NP systems. This review is not fully comprehensive because many more specific reviews already exist, as noted above.
6.4.1. Photosensitization by Quantum Dots
Quantum dots (QDs), nanoscale semiconductor particles with interesting optical and electronic properties, typically consist of binary compounds such as PbS, PbSe, or CdS,1587 although carbon1588 and silicon quantum dots1589 have also been used for uncaging purposes. They usually exhibit broad absorption spectra and narrow emission peaks and have large two-photon absorption cross sections. Their irradiation generally causes electron excitation from the valence band to the conduction band, and the resulting electron and hole can interact to produce an exciton. Their photophysics can be controlled using appropriate ligands.1590,1591 Photoinduced energy transfer (Förster resonance energy transfer, FRET) or electron exchange between an excited QD and a ligand that undergoes subsequent chemical change is another way of using QDs for photosensitization.1590,1592 QDs can interact with both electron acceptors and donors upon excitation. It should be noted that QDs are considerably larger than molecular species.
The QDs can serve as antennas that sensitize the photoreaction. Ford and co-workers reported that NO (section 4.2) is generated from electrostatic assemblies of water-soluble CdSe/ZnS and CdSeS/ZnS QDs loaded with negatively charged dihydrolipoic acid surface ligands and the cationic complex trans-CrIII(cyclam)(ONO)2+ (cyclam = 1,4,8,11-tetraazacyclotetradecane) upon irradiation with visible light (Scheme 101).1593
Scheme 101. QD-Mediated Photorelease1593.
Carbon quantum dots (CQDs) have high photostability and large two-photon cross sections.1588 CQDs covalently linked to a nitroaniline derivative as a NO photodonor were shown to release NO (section 4.2) upon one-photon (<450 nm) or two-photon (800 nm) absorption via anenergy-transfer mechanism.1594 Another example of drug delivery from a CQD using a quinoline-based phototrigger was reported by Ghosh, Singh, and co-workers.1595
Photoinduced release via electron transfer from QDs to a ligand requires rather small nanoparticles to enable close contact between the two species.1592 Bao, Zhu, and co-workers prepared water-soluble nanocrystalline CdSe/ZnS particles functionalized with an N-alkyl-4-picolinium ester linked to the anticancer drug 5-fluorouracil and l-cysteine (Scheme 102).1596 Upon irradiation at λirr > 400 nm, 5-fluorouracil was liberated via electron transfer from the QD to the picolinium moiety, with l-cysteine acting as an electron donor.
Scheme 102. QD-Mediated Photorelease1592.
Water-soluble CdTe QDs capped with mercaptopropionic acid and a ruthenium nitrosyl complex cis-[RuII(NO)(4AP)(bpy)2]3+ (bpy = 2,2′-bipyridine, and 4AP = 4-aminopyridine) were shown by Ford, da Silva, and co-workers to release NO (section 4.2) upon irradiation at 530 nm via a charge-transfer mechanism.1597 Visible light excitation of CdSe QDs was also demonstrated to trigger the release of coumarin from cinnamate surface ligands.1598 In this system, electron transfer from the excited nanocrystal to the surface-bound cinnamate triggers E–Z isomerization and subsequent lactonization. o-Nitrobenzyl (oNB) groups can also be liberated from CdTe/CdS core/shell QDs under UV illumination to control QD emission.1599 The possibility of exciton energy transfer was ruled out in this case, and because there was no overlap between QD emission and oNB absorption, it was suggested that an electron or hole transfer from the QD to the oNB occurred. Other examples of UV- or near-visible light-activated release via oNB group cleavage have also been reported.197,1600,1601
6.4.2. Photorelease Mediated by Upconversion and Second-Harmonic Nanoparticles
Several photophysical phenomena combine the energies of two or more photons to produce that of one higher-energy photon. Photon upconversion in organic molecules converts two or more low-energy photons into one higher-energy photon via two fundamental mechanisms: two (multi)-photon absorption (Figure 69b) or sensitized triplet–triplet annihilation (TTA).136,1602 The former mechanism leads to an excited state of higher energy (which would also be accessible by one-photon absorption; see Figure 69a) via a virtual state, whereas the latter intermolecular process typically involves two molecules in their triplet states that interact to leave one molecule in the ground state with the second molecule being excited to a higher electronic state. Some nonlinear crystal materials and non-centrosymmetric compounds and structures can exhibit second-harmonic generation (SHG), in which two photons with the same frequency interact with matter and coalesce to a virtual state (Figure 69c).1603 The resulting second-harmonic photon is generated practically instantaneously (within a few fs), so the signal is coherent (frequency doubling). Another interesting phenomenon is observed in so-called upconversion nanoparticles (UCNPs), in which two or more sequentially absorbed photons are converted into one emitted photon with higher energy via real metastable excited states (Figure 69d).1324,1604−1609 UCNPs typically absorb in the IR region and emit in the visible or ultraviolet regions. Most UCNPs consist of rare-earth-based lanthanide- or actinide-doped transition metals. The theoretical quantum yield of upconversion cannot exceed 0.5 because at least two photons are required to produce one upconverted photon.
Figure 69.
(a) Single-photon absorption; (b) two-photon absorption; (c) second-harmonic generation; (d) upconversion. ψg = ground state; ψf = final excited state; ψv = virtual state; ψi = intermediate state.
Because of their unique optical and chemical properties, UCNPs can be used for drug release/delivery.1610−1620 They are convenient and biologically favored “UV-vis lightbulbs”49,1621 because of their ability to convert NIR light into UV and visible photons. Many conventional UV-absorbing photoactivatable groups that undergo photocleavage or photoswitching processes can thus be activated through the tissue-transparent window. Because of the many recent excellent reviews cited above, we discuss only a few illustrative examples.
An application using o-nitrobenzyl derivatives was presented by Liu, Xing, and co-workers (Scheme 103).202 Monodispersed core-shell UCNPs consisting of NaYF4 nanocrystals doped with Yb3+ and Tm3+ were functionalized with cationic photoreleasable linkers via covalent bonding, enabling the adsorption of anionic siRNA molecules via electrostatic interactions. Upon NIR light irradiation (980 nm), the photolabile linker was cleaved by upconverted UV light, initiating the intracellular release of the siRNA.
Scheme 103. Release of siRNA from UCNPs202.
The literature provides many examples of applications in which different types of UCNPs serve as mediators in species release. Most works of this type published in recent years have used o-nitrobenzyl derivatives as photocleavable moieties.203,1622−1641 However, other systems have also been studied, including coumarin-4-ylmethyl,365 pyrenemethyl,1642 or o-hydroxycinnamic1643 PPGs as well as photoactivatable ruthenium,1333,1644 platinum,1645,1646 and manganese1159 complexes, and Roussin’s black salt.1647 Photochromic moieties have also been used for species liberation involving UCNPs, for example, by incorporating azobenzene1648−1650 or spiropyran/merocyanine1651 photoswitches.
Second-harmonic emission has recently been used for uncaging. Bismuth ferrite harmonic nanoparticles (HNPs) were used to release l-tryptophan linked to a coumarin-4-ylmethyl photoactivatable group via a carbamate functionality (Scheme 104).387 Light (790 nm) from a femtosecond pulsed laser was converted into emission at 395 nm, which was responsible for the excitation of the PPG.
Scheme 104. Release of l-Tryptophan from UCNPs387.
6.4.3. Photothermally Controlled Release
Visible or NIR irradiation of some nanoparticles consisting of noble metals (gold or silver), carbon (graphene derivatives or carbon nanotubes), metallic composites (CuS, MoS2), or polymers (polyanilines and liposomes) can result in the production of thermal energy (heat), which is dissipated into the surroundings of the nanostructure. This process is referred to as the photothermal effect,1652,1653 and it can be regarded as a distinct type of photosensitization. Photothermal effects have been used to achieve spatially and temporally controlled release of species such as drugs and metal ions.1653−1656
Upon excitation, noble metal nanoparticles (particularly those made of gold, AuNPs) exhibit localized surface plasmon resonance, that is, resonant oscillations of the conduction electrons, which are transformed into phonons, followed by rapid relaxation and heating.1654 The wavelengths of the absorption maxima of AuNPs are related to the size of the particles: 10–40 nm AuNPs absorb in the green region, while larger particles have bathochromically shifted maxima. AuNPs have been used to uncage, among other things, (a) drugs embedded in a polymeric matrix surrounding AuNPs, (b) drugs embedded in liposomes together with AuNPs, and (c) drugs covalently or non-covalently attached to AuNPs via a tether.1654 In all cases, photothermal heating disrupts the interactions confining the drug, leading to its release.
Scheme 105 shows a photothermally releasable system based on Au nanocages covered with poly(N-isopropylacrylamide) chains that undergo conformational changes when heated.1657 Upon irradiation with a NIR laser, the light is absorbed by the AuNPs and is converted into heat via the photothermal effect. When the polymer chains collapse, a pre-loaded drug such as doxorubicin is released through the resulting pores. The polymer chains return to their original conformation in the dark and the pores close. Many other photothermal release systems using AuNPs have been reported.1658−1672 Releasable systems consisting of cobalt nanowire-based particles,1673 NaYbF4:Er3+ UCNP nanocomposites,1674 carbon nanotube thermosensitive hydrogel,1675 biochar,1676 and photothermal heating of water droplets confined in polymeric particles1677 have also been reported.
Scheme 105. Au Nanocage Opening via the Photothermal Effect1657.
7. Photoactivatable Polymers, Micelles, and Vesicles
Many photoactivatable polymeric materials, micelles, and vesicles have been developed for drug/species delivery in recent decades.109,1573,1678−1689 Photocleavable polymer nanostructures are particularly interesting platforms for targeted drug delivery.14 Several release mechanisms are available: these systems can serve as photoresponsive/degradable nanocarriers for drug delivery, polymeric films can facilitate photochemical species detachment or patterning,1683 and hydrogels can alter the properties of biomaterials and affect the microenvironment.1575,1690
Photocleavage of covalent bonds in UV-absorbing chromophores such as o-nitrobenzyl or coumarin-4-ylmethyl groups10,1683 connected to polymers and vesicles is an appealing strategy because the photochemistry and applications of these chromophores are well known.1691 Their activation with red or NIR light is usually enabled by two-photon absorption or the use of upconverting nanoparticles (section 6.4.2). For example, two-photon or blue-light activation was used to remove o-nitrobenzyl-derived PPGs to release payloads from micelles1692−1694 or polymers.271,1695−1697 Similarly, coumarin-4-ylmethyl groups have been used to release drugs from micelles,445 polymers,1696,1698 hyaluronic acid nanogels,1699 nanoparticles,383,415 and nanocomposites.364 Applications of photodegradable micelles consisting of amphiphiles containing a diazonaphthoquinone group have also been reported.1700−1705
One-photon visible-light photoactivation (at 420 nm) of cascade depolymerization of self-immolative polymersomes with photoremovable perylen-3-yl protecting groups553 was shown to release encapsulated bioactive agents (section 2.3) via photosolvolysis (Scheme 106).563
Scheme 106. Photochemical Depolymerization of Self-Immolative Polymersomes563.
Wu and co-workers designed red-light-responsive RuII-containing block copolymers for anticancer phototherapy. These copolymers can be assembled into micelles, vesicles, or large compound micelles depending on their molecular weights (Scheme 107).1483 Upon excitation at 656 nm, they release the 1O2 generating anticancer agent [Ru(tpy)(biq)(H2O)]2+ via ligand exchange (see section 3). Similar strategies were used with block copolymers bearing [Ru(Biq)2(Hob)2](PF6)2 (Biq = 2,2′-biquinoline, Hob = 4-((6-hydroxyhexyl)oxy)benzonitrile)1706 or surface-grafted ruthenium complexes to release cytotoxic molecules into cancer cells from mesoporous silica nanoparticles.1274
Scheme 107. Release from RuII-Containing Block Copolymers1483.
The amino-1,4-benzoquinone (424; see also section 2.10) photoactivatable moiety was used to prepare a nanoparticle-bound photocage–drug conjugate. Upon irradiation with red light, the nanoparticles dissolved in aqueous media, releasing the drug (Scheme 108; drug = paclitaxel, dexamethasone, or chlorambucil).726 It was also shown that an encapsulated cyanine NIR-fluorescent dye such as DiD or IR780 could facilitate the location of the nanoparticles and monitoring of the photorelease process.
Scheme 108. Amino-1,4-benzoquinone Derivatives as PPGs for Drug Uncaging from Nanoparticles726.
Photorelease from light-responsive polymeric micelles made from an amphiphilic block polymer incorporating a BODIPY derivative (see section 2.12) is shown in Scheme 109.824 Upon irradiation, the micellar assembly of this polymer is disrupted due to the release of phenolate from the polymers to release a payload (Nile red). Another example of photoactivatable drug delivery is the photochemical release of dexamethasone from subcutaneously implanted polymeric particles, in which a π-extended o-nitrobenzyl derivative (section 2.1.1) absorbing below 500 nm serves as the photocleavable moiety.261
Scheme 109. Release from BODIPY-Containing Amphiphilic Block Polymer824.
Mesoporous silica nanoparticles (MSNs) are important drug delivery nanocarriers with high surface areas and large pore volumes for drug loading, and they are readily functionalized with light-responsive groups or photoswitches.1707−1710 Most known systems of this type rely on doped upconversion nanoparticles that convert NIR radiation into UV/vis radiation (section 6.4.2) or Au-based, CuS, or graphene oxide nanoparticles that absorb and convert NIR light into thermal energy via the photothermal effect (see section 6.4.3).
For example, 1P (420 nm) or 2P (800 nm) irradiation was used to release the anticancer drug chlorambucil, which was connected to a 7-amino-coumarin derivative and grafted onto the surface of aminopropyl-functionalized MSNs (Scheme 110).384 The drug is liberated by a photosolvolysis reaction. Multi-photon-absorption (808 nm) leading to dissociation of o-nitrobenzyl-containing poly(ethylene glycol) on the surface of gold nanostars coated with a mesoporous silica shell was also shown to release doxorubicin.1711
Scheme 110. Release from Aminopropyl-Functionalized MSNs384.
8. Release Mediated by Photoswitching
Host–guest interactions are affected by several factors, such as the nature of the host and guest molecules and the properties of the solvent.1712 The host entity can consist of a single molecule, usually a photoswitchable (photochromic) system whose photoreaction leads to the release of a guest molecule due to a change in binding affinity. The two isomers of a photoswitchable molecule have distinct chemical, physical and optical properties, which can be used to tune the properties of the host material. The most commonly used photoswitches1713 for this purpose are azobenzene,1714 spiropyran, and diarylethene derivatives (whose photoswitching involves ring-opening/closing) (Scheme 111), but stilbene and fumaric acid derivatives (which undergo light-induced E–Z isomerization) or anthracene and coumarin chromophores (which undergo reversible photodimerization) have also been used.1712 An alternative approach uses multi-component supramolecular cages or capsules that incorporate a photoactivatable moiety to control guest release. To achieve visible or NIR light absorption, UV-absorbing chromophores can be modified by π-extension to bathochromically shift their absorption bands. Alternatively, they can be excited via a 2-photon absorption, upconversion emission, or sensitization.1411,1715 Research in this area has been reviewed on several occasions in the past decade,1712,1714−1722 so here we present only some particularly notable systems bearing chromophores absorbing over 400 nm.
Scheme 111. Examples of Photochromic Systems.
Since Ueno and co-workers showed in 1978 that azobenzene-capped β-cyclodextrin can regulate the binding of various substrates (including toluene, cyclohexanol, and geraniol) upon irradiation at wavelengths of 320–390 nm,1723 the azobenzene unit has become one of the most widely used photoswitches. Azobenzene-bridged cryptand 425 is a typical example; its irradiation with visible light selectively triggers Z → E isomerization, while E → Z isomerization is triggered by UV light, enabling control over its binding affinity towards the guest 2,7-diazapyrenium ion (Scheme 112).1724
Scheme 112. Azobenzene Isomerization to Control Binding Affinity of a Guest1724.
Tian and co-workers observed room-temperature phosphorescence emission as a result of photochemically controlled complexation of 2-hydroxy-5-((4-nitrophenyl)-diazenyl)benzoate (426) in β-cyclodextrin (Scheme 113), displacing the fluorescent heavy-atom containing α-bromonaphthalene (427). The fluorescence of the bromonaphthalene was suppressed when complexed with the cyclodextrin but not when it was displaced by the E-isomer of the azadiene.1725 Similarly, Wang and Wu constructed supramolecular valves from tetra-o-methoxy-substituted azobenzene and β-cyclodextrin to control the release of doxorubicin from nanopores of mesoporous silica nanoparticles (see section 7) using red light.1726
Scheme 113. Photochemically Controlled Complexation in β-Cyclodextrin1725.
An application of a self-assembled coordination cage consisting of two square-planar-coordinated PdII ions and four photochromic dithienylethene-containing ligands was reported by Clever and co-workers.1727 The photorelease and encapsulation of the guest, [B12F12]2–, was accomplished using UV and white light, respectively. Photoactivatable metal-containing complexes40,870 can also serve as excellent platforms for metal ion photorelease. Metal ions can induce profound biological responses, so it is desirable to control their concentrations with a high spatiotemporal resolution using photoactivatable systems. To this end, Yu and collaborators introduced the terthiazole-based molecular switch 428, which enables photoswitchable release and uptake of Zn2+ ions based on a 6π-electrocyclization/cycloreversion reaction of the chromophore and excited-state intramolecular proton transfer (ESIPT; Scheme 114).1728 Additionally, visible-light triggered switching of the G-quadruplex ligand binding mode and G-tetrad structure formation using a pyridinium-substituted dithienylethene has been demonstrated under physiologically relevant conditions.1729
Scheme 114. Terthiazole-Based Molecular Photoswitch1728.
Another notable photoswitchable system for controlled metal ion release is a bisstyrylthiophene derivative that incorporates both a π-extended photoactivatable nitrobenzyl group and a conjugated Ca2+ chelator (see also section 2.1).219 This species has a large two-photon cross section (350 GM) at 775 nm. Ca2+ photorelease (/uptake) has also been accomplished using a photoswitchable diarylethene-containing chelator,1730 visible-light irradiation of flavin photosensitizers in the presence of Ca2+-EDTA,1410 and two-photon excitation of a 5-bromo-2-nitrobenzyl-substituted ethylene glycol tetraacetic acid chelator.188 The last example discussed here is a system that releases metal ions such as Ca2+1731 or Zn2+ from polymersomes, that is, bilayer vesicles that self-assemble from amphiphilic diblock copolymers.1732 A photoresponsive polymersome system containing an ethyne-bridged bis[(porphinato)zinc] fluorophore as a hydrophilic membrane solute and dextran in the aqueous core undergoes deformation upon irradiation at 488 nm, liberating the metal ion.1732
The incorporation of photoswitchable moieties into the backbones of polymer nanoparticles, micelles, polymersomes, vesicles, microgels, liposomes, mesoporous silica nanoparticles, and so on offers another way of controlling the photochemical delivery/release of cargoes encapsulated within the assembled nanocarriers.130,1581,1707−1709,1714,1715,1733−1738 As in the supramolecular systems discussed above, photoisomerization (e.g., azobenzene) and 6π-electrocyclization of triene systems (e.g., spiropyrans and diarylethenes; Scheme 111) are commonly used mechanisms for species release from nanocarriers. This approach was exemplified by photochromic polymersomes composed of self-assembled poly(ethylene oxide) diblock copolymers containing a spiropyran-based monomer, which exhibited reversible bilayer permeability upon photoisomerization of the hydrophobic spiropyran to give the zwitterionic merocyanine upon irradiation at >450 nm; this process could be reversed by irradiation at <420 nm (Scheme 115).1739 The authors assumed that the structure of the polymersomes is determined by multiple cooperative noncovalent interactions including hydrophobic, hydrogen bonding, π–π stacking, and electrostatic interactions, and that the isomerization changes the pattern of these interactions. This system was successfully used to release a nuclei-staining dye, 4′,6-diamidino-2-phenylindole, in living HeLa cells.
Scheme 115. Photoswitching of a Spiropyran/Merocyanine Pair in Polymersome1739.
Similarly, photoinduced isomerization of two different donor–acceptor Stenhouse adducts upon irradiation with visible light (Scheme 116) was used to switch the permeability of polymersome by inducing the isomerization of a nonpolar triene-enol into a polar cyclopentenone within amphiphilic block copolymers containing poly(pentafluorophenyl methacrylate).1740 The hydrophilic anticancer drug 2′-deoxy-5-fluorouridine and the DNA-intercalating dye 4′,6-diamidino-2-phenylindole were used as payloads in this system. Other photoswitchable systems have also been used for controlled delivery upon irradiation with visible or NIR light including a spiropyran–merocyanine-containing polymer,1741 a nanocarrier-based on hollow mesoporous silica (HMS) nanoparticles,1742 polymer nanoparticles incorporating a donor–acceptor Stenhouse adduct,1743 sulfonatocalix[4]arene with bound flavylium ions,1744 azobenzene-containing micelles,1745 β-cyclodextrin-grafted hyperbranched conjugated polymers,1746 and catanionic vesicles.1747 Additionally, the azobenzene chromophore has been used for the photochemical control of drug release from supramolecular1748 and poly(ethylene glycol)1749 hydrogels.
Scheme 116. Switching of a Stenhouse Adduct within Amphiphilic Block Copolymers1740.
Photoisomerization-induced release of luminescent dyes and anticancer drugs from functionalized azobenzene molecules attached to the interiors of MSN pores upon irradiation at 413 nm was reported by Tamanoi, Zink, and co-workers (Figure 70).1750 Another photoactivatable system was reported by Kneževič and co-workers,1751 who entrapped the model dye sulforhodamine 101 inside the mesopores of mercaptopropyl-functionalized MSNs in the presence of a Ru(bpy)2(PPh3) moiety coordinated to mercaptopropyl functional group. The dye was liberated upon irradiation at 455 nm via a ligand exchange reaction.
Figure 70.
Azobenzene-controlled release from the pores of mesoporous silica nanoparticles.1750
9. Photoactivation and Photodeactivation of Drugs: Photopharmacology
Most of the photochemical release systems discussed in this review rely on irreversible release (uncaging) from auxiliary photochemically active/reactive chromophores that undergo various photochemical side-reactions, leading to their destruction and thus potentially to the formation of unwanted materials (Scheme 117a). These processes must be carefully considered during the design and development of new photoactivatable systems, especially in terms of their compatibility with biological systems where relevant. Despite this limitation, irreversible uncaging remains the dominant approach to photorelease in both fundamental and practical research. However, as discussed in the preceding sections, reversible release systems using (photoswitchable) photochromic moieties also exist and can be incorporated into more complex systems, where their reversible (photo)reactions such as E–Z-photoisomerization can induce changes in the binding affinity of a releasable guest upon irradiation (Scheme 117b, section 7). Active species can also be incorporated into a tether whose conformation/length changes upon irradiation to bring an attached active molecule into close proximity with a site of interest (Scheme 117c).1752Scheme 117d shows a different approach involving a so-called photochromic ligand that can exist in two or more different isomeric forms that are interconvertible upon irradiation, of which only one is active in a specific application. The isomerization, in this case, may also be triggered by heat (Δ) in one direction, and may thus proceed spontaneously under certain conditions. The term “photopharmacology” is used in reference to bioactive molecular systems that undergo reversible photochemical transformations that alter their pharmacokinetic or pharmacodynamic properties. This is a relatively new concept but one that has attracted considerable attention and has therefore been reviewed several times in recent years.145,874,1752−1764 Consequently, we present only a few representative examples here.
Scheme 117. Irreversible versus Reversible Photoactivation.
Azobenzene and diarylethene photoswitches and their analogs (section 8) are the most frequently used photochromic systems in photopharmacology.1752,1753,1758,1763,1765 Most of them are activatable by UV light in one direction and by visible light in the reverse process. Substituents attached to a photochromic group can bathochromically shift its absorption maxima such that both forward and backward photoactivation occurs in the visible part of the spectrum.
Both E and Z isomers of azobenzenes can be pharmacologically active. Trauner and co-workers introduced azobenzene derivative 429, which is a freely diffusible photoswitchable antagonist of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptor (Schemes 117 and 118).1766 This compound is active in its stable E-form but considerably less active as the Z-isomer. Because of a significant bathochromic shift of its absorption maxima, the two isomers can be interconverted by irradiation at 460 and 600 nm, respectively.
Scheme 118. Photoswitchable Quinoxaline-2,3-dione Antagonist.
Another azobenzene-based protein ligand, 430, was developed by Yuste, Gorostiza, and co-workers to activate the light-gated glutamate receptor LiGluR in living cells (Scheme 119; Glu = glutamate).1767 The E-form of the tether is inactive, but E–Z isomerization induced by one- (>400 nm) or two-photon irradiation brings the glutamate residue at the end of the ligand chain into the vicinity of the receptor’s glutamate-binding site. In the dark, the receptor is inactivated by the rapid and spontaneous reverse isomerization of the ligand.
Scheme 119. Azobenzene-Based Protein Switch.
Szymanski, Feringa, and co-workers developed switchable antibacterial agents1768 based on the azobenzene chromophore whose activity is controlled by visible-light irradiation.1769 The Z-azobenzene derivative 431, formed upon photoisomerization of the corresponding E-isomer at 652 nm, exhibited at least an 8-fold increase in activity (Scheme 120).
Scheme 120. Photoactivation of Antibacterial Azobenzene Derivative.
A different photoswitch based on a photochromic dithienylethene (432) was studied by König and co-workers who showed that the open isomer of this compound activated the dopamine D2S receptor considerably more efficiently than the closed isomer (Scheme 121).1770 Notably, the photophysical properties of these dithienylethene dopamine ligands exhibited high fatigue resistance.
Scheme 121. Photochromic Dithienylethene Derivative.
Bifunctional molecules targeting proteins for ubiquitylation by an E3 ligase complex and subsequent degradation by the proteasome (PROTACs; proteolysis targeting chimeras) are powerful tools for regulating the levels of certain cellular proteins.1771 Photoswitchable PROTACs, or PHOTACs (photochemically targeting chimeras), that enable optical (photopharmacological) control of protein levels using azobenzene-containing photoswitches, were recently developed by Pagano and Trauner.1772 Conceptually similar photoswitchable azobenzene-proteolysis targeting chimeras (Azo-PROTACs) were introduced by You and Jiang.1773
Acknowledgments
This work was supported by the Czech Science Foundation (GA18-12477S, P.K.). We thank the CETOCOEN EXCELLENCE Teaming 2 project (supported by the Czech Ministry of Education, Youth and Sports: CZ.02.1.01/0.0/0.0/17_043/0009632 and EU H2020:857560) and the RECETOX research infrastructure (LM2018121) (P.K.). R.W. acknowledges support from the European Research Council (GAtransport) and the Binational Science Foundation (2016060). T.S. was supported by the Czech Science Foundation (GJ19-20467Y) as well as by MEYS CR (LTAIN19166). D.K. was supported in part by the Planning and Budgeting Committee (PBC) of the Israeli Council for Higher Education. The authors express their thanks to Daniel Falvey (University of Maryland), David Lawrence (University of North Carolina at Chapel Hill), Martin J. Schnermann (National Cancer Institute), Arthur Winter (Iowa State University), and Jakob Wirz (University of Basel) who read parts of the text and provided valued suggestions.
Glossary
Abbreviations
- λmaxabs
absorption maximum
- λmaxem
emission maximum
- λirr
irradiation wavelength
- Φr
reaction quantum yield
- ΦΔ
quantum yield of singlet oxygen production
- Φrε(λirr)
uncaging cross section
- σTPA
2-photon absorption cross section
- δunc
2-photon uncaging cross section
- 1P
one-photon
- 1PE
1-photon excitation
- 2P
two-photon
- 2PE
2-photon excitation
- 4AMP
4-(aminomethyl)pyridine)
- 4AP
4-aminopyridine
- 4-pic
4-methylpyridine
- AIE
aggregation-induced emission
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ATP
adenosine triphosphate
- AuNP
Au nanoparticle
- BDE
bond dissociation energy
- BHQ
(8-bromo-7-hydroxyquinoline-2-yl)methyl
- BIST
bisstyrylthiophene
- biq
2,2′-biquinoline
- BODIPY
4,4-difluoro-4-bora-3a,4a-diaza-s-indacene
- bpy
2,2′-bipyridyl
- BRET
bioluminescence resonance energy transfer
- cAMP
cyclic adenosine monophosphate
- Cbl
chlorambucil
- CD
carbon dot
- CDNI
4-carboxymethoxy-7-nitroindolinyl
- cGMP
cyclic guanosine monophosphate
- cMO
caged morpholino oligonucleotide
- COHb
carbonylhemoglobin
- COS
carbonyl sulfide
- CQD
carbon quantum dot
- CRISPR
clustered regularly interspaced short palindromic repeats
- CT
charge transfer
- cur
curcumin
- Cy5
pentamethine cyanine
- Cy7
heptamethine cyanine
- cyclam
1,4,8,11-tetraazacyclotetradecane
- CyHQ
(8-cyano-7-hydroxyquinoline-2-yl)methyl
- DANS
E-4-(N,N-dimethylamino)-4′-nitrostilbene
- dach
1R,2R-(−)-1,2-diaminocyclohexane
- DDQ
2,3-dichloro-5,6-dicyano-1,4-benzoquinone
- DEA
diethylamine
- DEAC
7-diethylaminocoumarin
- dend.
1,4-diaminobutane dendrimer
- DFT
density functional theory
- DMA
dimethylacetamide
- DMNB
4,5-dimethoxy-2-nitrobenzyl
- DMNPB
3-(4,5-dimethoxy-2-nitrophenyl)-2-butyl
- DMSO
dimethyl sulfoxide
- DNA
deoxyribonucleic acid
- dppn
benzo[i]dipyridophenazine
- DTE
dithienylethene
- EDG
electron-donating group
- EDTA
ethylenediaminetetraacetic acid
- EGFR
epidermal growth factor receptor
- EGTA
ethylene glycol tetraacetic acid
- en
ethylenediamine
- ESIPT
excited-state intramolecular proton transfer
- Et
ethyl
- EWG
electron-withdrawing group
- FBS
fetal bovine serum
- Fl
9,9′-dibutyl-9H-fluoren-2-yl
- FRET
Förster resonance energy transfer
- FT-IR
Fourier transform infrared
- FWHM
full-width-at-half maximum
- GA3
gibberellin A3
- GABA
γ-butyric acid
- glu
glutamate
- GM
Goeppert-Mayer
- HBIND
H-bond-induced non-radiative decay
- HBT
2-(2′-hydroxyphenyl)benzothiazole
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HMO
Hückel molecular orbital
- HMS
hollow mesoporous silica
- HNP
harmonic nanoparticle
- Hob
4-((6-hydroxyhexyl)oxy)benzonitrile)
- HOMO
highest occupied molecular orbital
- hydrol
hydrolysis
- ICT
intramolecular charge transfer
- immol
self-immolation
- IP3
inositol triphosphate
- IR
infrared
- ISC
intersystem crossing
- L
ligand
- LE
locally excited
- LED
light-emitting diode
- LF
ligand field
- LG
leaving group
- LLCT
ligand-to-ligand charge transfer
- LMCT
ligand-to-metal charge transfer
- LUMO
lowest unoccupied molecular orbital
- Me
methyl
- Me2bpy
6,6′-dimethyl-2,2′-bipyridine
- MetHb
methemoglobin
- MES
2-(N-morpholino)ethanesulfonic acid–based buffer
- MLCT
metal-to-ligand charge transfer
- MMLCT
metal–metal-bond-to-ligand charge-transfer
- MNPPOC
2-(3,4-methylenedioxy-6-nitrophenyl)-propoxycarbonyl
- MOF
metal–organic framework
- MOPS
3-(N-morpholino)propanesulfonic acid
- MSN
mesoporous silica nanoparticle
- NDBF
nitrodibenzofuran
- NHS
N-hydroxysuccinimide
- NIR
near-infrared
- NO
nitric oxide
- NONOate
diazeniumdiolate
- NP
nanoparticle
- NPEOC
1-(2-nitrophenyl)ethyloxycarbonyl
- NPPOC
1-(2-nitrophenyl)propyloxycarbonyl
- oNB
o-nitrobenzyl
- PBS
phosphate buffer saline
- PDT
photodynamic therapy
- PEG
polyethylene glycol
- PET
photoinduced electron transfer
- Ph
phenyl
- phen
1,10-phenanthroline
- phen-CHO
phenanthrolinecarboxaldehyde
- photoCORM
photoactivatable CO-releasing moiety
- photoNORM
photoactivatable NO-releasing moiety
- pHP
p-hydroxyphenacyl
- PPG
photoprotecting group
- py
pyridine
- Py
pyrene
- pydppn
(pyrid-2-yl)benzo[i]dipyridophenazine
- QD
quantum dot
- qmtpm
2-quinoline-N-(2′-methylthiophenyl)-methylenimine
- RNA
ribonucleic acid
- sens
sensitizer
- SHG
second-harmonic generation
- sol
solvent
- TD-DFT
time-dependent density functional theory
- TICT
twisted intramolecular charge transfer
- TIP
tight ion-pair
- TMG
tetramethylguanidine
- TPA
two photon absorption
- TPA
tris(2-pyridylmethyl)amine
- TPE
tetraphenylethylene
- tpy
terpyridine
- TQA
tris(2-quinolinylmethyl)amine
- TRIR
time-resolved ultrafast infrared spectroscopy
- Tris
tris(hydroxymethyl)aminomethane
- TRPV1
transient receptor potential cation channel V1
- TTA
triplet–triplet annihilation
- UCNP
upconversion nanoparticle
- UV
ultraviolet
- VPA
valproic acid
- WT
wild type
- z
zwitterion
Biographies
Roy Weinstain received his B.Sc. degree in chemistry and biology in 2005 from Tel Aviv University, Israel. He obtained his Ph.D. from the same institution in 2010 for the development of self-immolative molecular systems under the supervision of Prof. Doron Shabat. He then joined Prof. Roger Y. Tsien’s group at the University of California San Diego as a post-doctoral fellow, working on the synthesis and application of fluorescent probes to study dynamic processes in vivo. Since 2014, he is a Senior Lecturer in the School of Plant Science and Food Security at Tel Aviv University, Israel. His research focuses on the development and implementation of chemical-biology methods to study the functions and regulation mechanisms of plant signaling molecules.
Tomáš Slanina received his M.S. degree from Masaryk University, Brno, Czech Republic in 2012. He received his Ph.D. in organic chemistry in 2015 in a joint programme between Masaryk University and the University of Regensburg, Germany, under the supervision of Prof. Petr Klán and Prof. Burkhard König. He later worked as a postdoctoral researcher in Prof. Alexander Heckel’s group at Goethe University, Frankfurt am Main, Germany, and in the research group of Prof. Henrik Ottosson at Uppsala University, Sweden. He is currently a leader of the junior research group or redox photochemistry at the Institute of Organic Chemistry and Biochemistry in Prague, Czech Republic. His research interests include organic chemistry, photochemistry, physical organic chemistry, electrochemistry, time-resolved and steady-state spectroscopy, investigation of reaction mechanisms, and chemical biology.
Dnyaneshwar Kand received his B.Sc. and M.Sc. degrees in chemistry from Pune University, India. He then joined the Indian Institute of Science, Education and Research (IISER), Pune, India with Dr. Pinaki Talukdar, receiving his Ph.D. degree in organic chemistry in 2015 for the development of colorimetric and fluorescent selective thiols sensors. In 2015, he joined the group of Dr. Roy Weinstain at Tel Aviv University, Israel, as a post-doctoral fellow (and a PBC fellow), where he worked on the development of meso-methyl BODIPY photocages. When not doing chemistry, he enjoys playing cricket.
Petr Klán received an M.Sc. degree in organic chemistry from Masaryk University, Brno, Czech Republic in 1986. After working in the industry for five years, he stayed at Michigan State University to pursue a Ph.D. in chemistry under the tutelage of Prof. Peter J. Wagner. After receiving his Ph.D. in chemistry in 1998, he joined the faculty at Masaryk University where he is now a full professor. His current research focuses on photochemistry, mechanisms of organic reactions, kinetic flash photolysis, spectroscopy, photoremovable protecting groups, and environmental photochemistry. He co-authored the book “Photochemistry of Organic Compounds” (Wiley, 2009) with Prof. Jakob Wirz.
The authors declare no competing financial interest.
References
- Barltrop J. A.; Schofield P. Photosensitive Protecting Groups. Tetrahedron Lett. 1962, 16, 697–699. 10.1016/S0040-4039(00)70935-X. [DOI] [Google Scholar]
- Barton D. H. R.; Chow Y. L.; Cox A.; Kirby G. W. Photosensitive Protection of Functional Groups. Tetrahedron Lett. 1962, 23, 1055–1057. 10.1016/S0040-4039(00)70957-9. [DOI] [Google Scholar]
- Barton D. H. R.; Chow Y. L.; Cox A.; Kirby G. W. Photochemical Transformations. 19. Some Photosensitive Protecting Groups. J. Chem. Soc. 1965, 3571–3578. 10.1039/jr9650003571. [DOI] [Google Scholar]
- Patchornik A.; Amit B.; Woodward R. B. Photosensitive Protecting Groups. J. Am. Chem. Soc. 1970, 92, 6333–6335. 10.1021/ja00724a041. [DOI] [Google Scholar]
- Sheehan J. C.; Wilson R. M. Photolysis of Desyl Compounds. A New Photolytic Cyclization. J. Am. Chem. Soc. 1964, 86, 5277–5281. 10.1021/ja01077a046. [DOI] [Google Scholar]
- Engels J.; Schlaeger E. J. Synthesis, Structure, and Reactivity of Adenosine Cyclic 3′,5′-Phosphate-Benzyltriesters. J. Med. Chem. 1977, 20, 907–911. 10.1021/jm00217a008. [DOI] [PubMed] [Google Scholar]
- Kaplan J. H.; Forbush B. III; Hoffman J. F. Rapid Photolytic Release of Adenosine 5′-Triphosphate From a Protected Analog: Utilization by the Sodium/Potassium Pump of Human Red Blood Cell Ghosts. Biochemistry 1978, 17, 1929–1935. 10.1021/bi00603a020. [DOI] [PubMed] [Google Scholar]
- Yang Y. M.; Mu J.; Xing B. G. Photoactivated Drug Delivery and Bioimaging. Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol. 2017, 9, e1408 10.1002/wnan.1408. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Ye H.; Chen Y. B.; Zhu R. Y.; Yin L. C. Photoresponsive Drug/Gene Delivery Systems. Biomacromolecules 2018, 19, 1840–1857. 10.1021/acs.biomac.8b00422. [DOI] [PubMed] [Google Scholar]
- Klán P.; Šolomek T.; Bochet C. G.; Blanc A.; Givens R.; Rubina M.; Popik V.; Kostikov A.; Wirz J. Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2013, 113, 119–191. 10.1021/cr300177k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šolomek T.; Wirz J.; Klán P. Searching for Improved Photoreleasing Abilities of Organic Molecules. Acc. Chem. Res. 2015, 48, 3064–3072. 10.1021/acs.accounts.5b00400. [DOI] [PubMed] [Google Scholar]
- Bort G.; Gallavardin T.; Ogden D.; Dalko P. I. From One-Photon to Two-Photon Probes: “Caged” Compounds, Actuators, and Photoswitches. Angew. Chem., Int. Ed. 2013, 52, 4526–4537. 10.1002/anie.201204203. [DOI] [PubMed] [Google Scholar]
- Aubert S.; Bezagu M.; Spivey A. C.; Arseniyadis S. Spatial and Temporal Control of Chemical Processes. Nat. Rev. Chem. 2019, 3, 706–722. 10.1038/s41570-019-0139-6. [DOI] [Google Scholar]
- Beauté L.; McClenaghan N.; Lecommandoux S. Photo-Triggered Polymer Nanomedicines: From Molecular Mechanisms to Therapeutic Applications. Adv. Drug Delivery Rev. 2019, 138, 148–166. 10.1016/j.addr.2018.12.010. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Kohane D. S. External Triggering and Triggered Targeting Strategies for Drug Delivery. Nat. Rev. Mater. 2017, 2, 17020. 10.1038/natrevmats.2017.20. [DOI] [Google Scholar]
- Hansen M. J.; Velema W. A.; Lerch M. M.; Szymanski W.; Feringa B. L. Wavelength-Selective Cleavage of Photoprotecting Groups: Strategies and Applications in Dynamic Systems. Chem. Soc. Rev. 2015, 44, 3358–3377. 10.1039/C5CS00118H. [DOI] [PubMed] [Google Scholar]
- So W. H.; Wong C. T. T.; Xia J. Peptide Photocaging: A Brief Account of the Chemistry and Biological Applications. Chin. Chem. Lett. 2018, 29, 1058–1062. 10.1016/j.cclet.2018.05.015. [DOI] [Google Scholar]
- Spicer C. D.; Pashuck E. T.; Stevens M. M. Achieving Controlled Biomolecule–Biomaterial Conjugation. Chem. Rev. 2018, 118, 7702–7743. 10.1021/acs.chemrev.8b00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieke C.; Rohrbach F.; Gottschalk A.; Mayer G.; Heckel A. Light-Controlled Tools. Angew. Chem., Int. Ed. 2012, 51, 8446–8476. 10.1002/anie.201202134. [DOI] [PubMed] [Google Scholar]
- Silva J. M.; Silva E.; Reis R. L. Light-Triggered Release of Photocaged Therapeutics - Where Are We Now?. J. Controlled Release 2019, 298, 154–176. 10.1016/j.jconrel.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Ankenbruck N.; Courtney T.; Naro Y.; Deiters A. Optochemical Control of Biological Processes in Cells and Animals. Angew. Chem., Int. Ed. 2018, 57, 2768–2798. 10.1002/anie.201700171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givens R. S.; Rubina M.; Wirz J. Applications of p-Hydroxyphenacyl (pHP) and Coumarin-4-ylmethyl Photoremovable Protecting Groups. Photochem. Photobiol. Sci. 2012, 11, 472–488. 10.1039/c2pp05399c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H.; Sterner E. S.; Coughlin E. B.; Theato P. o-Nitrobenzyl Alcohol Derivatives: Opportunities in Polymer and Materials Science. Macromolecules 2012, 45, 1723–1736. 10.1021/ma201924h. [DOI] [Google Scholar]
- Ellis-Davies G. C. R. Caged Compounds: Photorelease Technology for Control of Cellular Chemistry and Physiology. Nat. Methods 2007, 4, 619–628. 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Q.; Xing B. Photoactive Molecules for Applications in Molecular Imaging and Cell Biology. Chem. Soc. Rev. 2010, 39, 2835–2846. 10.1039/b915574k. [DOI] [PubMed] [Google Scholar]
- Bardhan A.; Deiters A. Development of Photolabile Protecting Groups and their Application to the Optochemical Control of Cell Signaling. Curr. Opin. Struct. Biol. 2019, 57, 164–175. 10.1016/j.sbi.2019.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis-Davies G. C. R. Neurobiology with Caged Calcium. Chem. Rev. 2008, 108, 1603–1613. 10.1021/cr078210i. [DOI] [PubMed] [Google Scholar]
- Choi S. K.Photocleavable Linkers: Design and Applications in Nanotechnology. Photonanotechnology for Therapeutics and Imaging; Choi S. k., Ed.; Elsevier, 2020. [Google Scholar]
- Amatrudo J. M.; Olson J. P.; Agarwal H. K.; Ellis-Davies G. C. R. Caged Compounds for Multichromic Optical Interrogation of Neural Systems. Eur. J. Neurosci. 2015, 41, 5–16. 10.1111/ejn.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakkampudi S.; Abe M.. Caged Compounds for Two-Photon Uncaging. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier, 2018. [Google Scholar]
- Piant S.; Bolze F.; Specht A. Two-Photon Uncaging, from Neuroscience to Materials. Opt. Mater. Express 2016, 6, 1679–1691. 10.1364/OME.6.001679. [DOI] [Google Scholar]
- Sankaranarayanan J.; Muthukrishnan S.; Gudmundsdottir A. D. Photoremovable Protecting Groups Based on Photoenolization. Adv. Phys. Org. Chem. 2009, 43, 39–77. 10.1016/S0065-3160(08)00002-6. [DOI] [Google Scholar]
- Bochet C. G.; Blanc A.. Photolabile Protecting Groups in Organic Synthesis. Handbook of Synthetic Photochemistry, Ch. 13; Albini A., Fagnoni M., Eds.; Wiley: Weinheim, 2010. [Google Scholar]
- Kramer R. H.; Chambers J. J.; Trauner D. Photochemical Tools for Remote Control of Ion Channels in Excitable Cells. Nat. Chem. Biol. 2005, 1, 360–365. 10.1038/nchembio750. [DOI] [PubMed] [Google Scholar]
- Xiangming M.; Xiaoyun C.; Yao F.; Qingxiang G. Photolysis of Caged Compounds and Its Applications to Chemical Biology. Progr. Chem. 2008, 20, 2034–2044. [Google Scholar]
- Sjulson L.; Miesenböck G. Photocontrol of Neural Activity: Biophysical Mechanisms and Performance. Chem. Rev. 2008, 108, 1588–1602. 10.1021/cr078221b. [DOI] [PubMed] [Google Scholar]
- Lee H. M.; Larson D. R.; Lawrence D. S. Illuminating the Chemistry of Life: Design, Synthesis, and Applications of “Caged” and Related Photoresponsive Compounds. ACS Chem. Biol. 2009, 4, 409–427. 10.1021/cb900036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H.; Li J.; Wu D.; Qiu Z.; Zhang Y. Chemistry and Biological Applications of Photo-Labile Organic Molecules. Chem. Soc. Rev. 2010, 39, 464–473. 10.1039/B901255A. [DOI] [PubMed] [Google Scholar]
- Specht A.; Bolze F.; Omran Z.; Nicoud J.-F.; Goeldner M. Photochemical Tools to Study Dynamic Biological Processes. HFSP J. 2009, 3, 255–264. 10.2976/1.3132954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesienski K. L.; Franz K. J. Keys for Unlocking Photolabile Metal-Containing Cages. Angew. Chem., Int. Ed. 2011, 50, 814–824. 10.1002/anie.201002542. [DOI] [PubMed] [Google Scholar]
- Lovell J. F.; Liu T. W. B.; Chen J.; Zheng G. Activatable Photosensitizers for Imaging and Therapy. Chem. Rev. 2010, 110, 2839–2857. 10.1021/cr900236h. [DOI] [PubMed] [Google Scholar]
- Herrmann A. Controlled Release of Volatiles Under Mild Reaction Conditions: From Nature to Everyday Products. Angew. Chem., Int. Ed. 2007, 46, 5836–5863. 10.1002/anie.200700264. [DOI] [PubMed] [Google Scholar]
- Herrmann A. Using Photolabile Protecting Groups for the Controlled Release of Bioactive Volatiles. Photochem. Photobiol. Sci. 2012, 11, 446–459. 10.1039/C1PP05231D. [DOI] [PubMed] [Google Scholar]
- Suyama K.; Shirai M. Photobase Generators: Recent Progress and Application Trend in Polymer Systems. Prog. Polym. Sci. 2009, 34, 194–209. 10.1016/j.progpolymsci.2008.08.005. [DOI] [Google Scholar]
- Puliti D.; Warther D.; Orange C.; Specht A.; Goeldner M. Small Photoactivatable Molecules for Controlled Fluorescence Activation in Living Cells. Bioorg. Med. Chem. 2011, 19, 1023–1029. 10.1016/j.bmc.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Li W.-H.; Zheng G. Photoactivatable Fluorophores and Techniques for Biological Imaging Applications. Photochem. Photobiol. Sci. 2012, 11, 460–471. 10.1039/c2pp05342j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaminato T. Single-Molecule Fluorescence Photoswitching: Design and Synthesis of Photoswitchable Fluorescent Molecules. J. Photochem. Photobiol., C 2011, 12, 177–208. 10.1016/j.jphotochemrev.2011.08.006. [DOI] [Google Scholar]
- A Special Issue of Photochem. Photobiol. Sci. on Photoremovable Protecting Groups: Developments and Applications; Wirz J., Ed.; Royal Society of Chemistry: Cambridge, 2012; Vol. 11, pp 433–600. [DOI] [PubMed] [Google Scholar]
- Alabugin A. Near-IR Photochemistry for Biology: Exploiting the Optical Window of Tissue. Photochem. Photobiol. 2019, 95, 722–732. 10.1111/php.13068. [DOI] [PubMed] [Google Scholar]
- Olejniczak J.; Carling C. J.; Almutairi A. Photocontrolled Release Using One-Photon Absorption of Visible or NIR Light. J. Controlled Release 2015, 219, 18–30. 10.1016/j.jconrel.2015.09.030. [DOI] [PubMed] [Google Scholar]
- Martin C. J.; Rapenne G.; Nakashima T.; Kawai T. Recent Progress in Development of Photoacid Generators. J. Photochem. Photobiol., C 2018, 34, 41–51. 10.1016/j.jphotochemrev.2018.01.003. [DOI] [Google Scholar]
- Deiters A. Principles and Applications of the Photochemical Control of Cellular Processes. ChemBioChem 2010, 11, 47–53. 10.1002/cbic.200900529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis-Davies G. C. R. Useful Caged Compounds for Cell Physiology. Acc. Chem. Res. 2020, 53, 1593–1604. 10.1021/acs.accounts.0c00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.; Rizzo R.; Surman F.; Zenobi-Wong M. Guiding Lights: Tissue Bioprinting Using Photoactivated Materials. Chem. Rev. 2020, 120, 10950–11027. 10.1021/acs.chemrev.0c00077. [DOI] [PubMed] [Google Scholar]
- Dcona M. M.; Mitra K.; Hartman M. C. T. Photocontrolled Activation of Small Molecule Cancer Therapeutics. RSC Med. Chem. 2020, 11, 982–1002. 10.1039/D0MD00107D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayat L.; Filevich O.; Baraldo L. M.; Etchenique R. Ruthenium Polypyridyl Phototriggers: From Beginnings to Perspectives. Philos. Trans. R. Soc., A 2013, 371, 20120330. 10.1098/rsta.2012.0330. [DOI] [PubMed] [Google Scholar]
- Mari C.; Pierroz V.; Ferrari S.; Gasser G. Combination of Ru(II) Complexes and Light: New Frontiers in Cancer Therapy. Chem. Sci. 2015, 6, 2660–2686. 10.1039/C4SC03759F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. H.; Wang X. Y.; Jin S. X.; Muhammad N.; Guo Z. J. Stimuli-Responsive Therapeutic Metallodrugs. Chem. Rev. 2019, 119, 1138–1192. 10.1021/acs.chemrev.8b00209. [DOI] [PubMed] [Google Scholar]
- Banerjee S.; Chakravarty A. R. Metal Complexes of Curcumin for Cellular Imaging, Targeting, and Photoinduced Anticancer Activity. Acc. Chem. Res. 2015, 48, 2075–2083. 10.1021/acs.accounts.5b00127. [DOI] [PubMed] [Google Scholar]
- Chemaly S. M. New Light on Vitamin B-12: The Adenosylcobalamin-Dependent Photoreceptor Protein CarH. S. Afr. J. Sci. 2016, 112, 39–47. 10.17159/sajs.2016/20160106. [DOI] [Google Scholar]
- Jones A. R. The Photochemistry and Photobiology of Vitamin B-12. Photochem. Photobiol. Sci. 2017, 16, 820–834. 10.1039/C7PP00054E. [DOI] [PubMed] [Google Scholar]
- Kumar M.; Kozlowski P. M. Electronic and Structural Properties of Cobalamin: Ramifications for B-12-Dependent Processes. Coord. Chem. Rev. 2017, 333, 71–81. 10.1016/j.ccr.2016.11.010. [DOI] [Google Scholar]
- Heilman B.; Mascharak P. K. Light-Triggered Nitric Oxide Delivery to Malignant Sites and Infection. Philos. Trans. R. Soc., A 2013, 371, 20120368. 10.1098/rsta.2012.0368. [DOI] [PubMed] [Google Scholar]
- Padmanabhan S.; Jost M.; Drennan C. L.; Elias-Arnanz M.. A New Facet of Vitamin B-12: Gene Regulation by Cobalamin-Based Photoreceptors. Annu. Rev. Biochem.; Kornberg R. D., Ed.; Annual Reviews: Palo Alto, 2017; Vol. 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfrew A. K. Transition Metal Complexes With Bioactive Ligands: Mechanisms for Selective Ligand Release and Applications for Drug Delivery. Metallomics 2014, 6, 1324–1335. 10.1039/C4MT00069B. [DOI] [PubMed] [Google Scholar]
- Smith N. A.; Sadler P. J. Photoactivatable Metal Complexes: From Theory to Applications in Biotechnology and Medicine. Philos. Trans. R. Soc., A 2013, 371, 20120519. 10.1098/rsta.2012.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rury A. S.; Wiley T. E.; Sension R. J. Energy Cascades, Excited State Dynamics, and Photochemistry in Cob(III)alamins and Ferric Porphyrins. Acc. Chem. Res. 2015, 48, 860–867. 10.1021/ar5004016. [DOI] [PubMed] [Google Scholar]
- Crespy D.; Landfester K.; Schubert U. S.; Schiller A. Potential Photoactivated Metallopharmaceuticals: From Active Molecules to Supported Drugs. Chem. Commun. 2010, 46, 6651–6662. 10.1039/c0cc01887b. [DOI] [PubMed] [Google Scholar]
- Szacilowski K.; Macyk W.; Drzewiecka-Matuszek A.; Brindell M.; Stochel G. Bioinorganic Photochemistry: Frontiers and Mechanisms. Chem. Rev. 2005, 105, 2647–2694. 10.1021/cr030707e. [DOI] [PubMed] [Google Scholar]
- Knoll J. D.; Albani B. A.; Turro C. New Ru(II) Complexes for Dual Photoreactivity: Ligand Exchange and O-1(2) Generation. Acc. Chem. Res. 2015, 48, 2280–2287. 10.1021/acs.accounts.5b00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll J. D.; Turro C. Control and Utilization of Ruthenium and Rhodium Metal Complex Excited States for Photoactivated Cancer Therapy. Coord. Chem. Rev. 2015, 282, 110–126. 10.1016/j.ccr.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A.; Turro C.; Kodanko J. J. Ru(II) Polypyridyl Polypyridyl Complexes Derived from Tetradentate Ancillary Ligands for Effective Photocaging. Acc. Chem. Res. 2018, 51, 1415–1421. 10.1021/acs.accounts.8b00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynton F. E.; Bright S. A.; Blasco S.; Williams D. C.; Kelly J. M.; Gunnlaugsson T. The Development of Ruthenium(ii) Polypyridyl Complexes and Conjugates for in Vitro Cellular and in Vivo Applications. Chem. Soc. Rev. 2017, 46, 7706–7756. 10.1039/C7CS00680B. [DOI] [PubMed] [Google Scholar]
- Knör G. The Concept of Photochemical Enzyme Models – State of the Art. Coord. Chem. Rev. 2016, 325, 102–115. 10.1016/j.ccr.2016.06.006. [DOI] [Google Scholar]
- Priestman M. A.; Lawrence D. S. Light-Mediated Remote Control of Signaling Pathways. Biochim. Biophys. Acta, Proteins Proteomics 2010, 1804, 547–558. 10.1016/j.bbapap.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet S. Shifting the Light Activation of Metallodrugs to the Red and Near-Infrared Region in Anticancer Phototherapy. Comments Inorg. Chem. 2015, 35, 179–213. 10.1080/02603594.2014.979286. [DOI] [Google Scholar]
- Ford P. C.; Bourassa J.; Miranda K.; Lee B.; Lorkovic I.; Boggs S.; Kudo S.; Laverman L. Photochemistry of Metal Nitrosyl Complexes. Delivery of Nitric Oxide to Biological Targets. Coord. Chem. Rev. 1998, 171, 185–202. 10.1016/S0010-8545(98)90031-5. [DOI] [Google Scholar]
- Ford P. C. Polychromophoric Metal Complexes for Generating the Bioregulatory Agent Nitric Oxide by Single- and Two-Photon Excitation. Acc. Chem. Res. 2008, 41, 190–200. 10.1021/ar700128y. [DOI] [PubMed] [Google Scholar]
- Ostrowski A. D.; Ford P. C. Metal Complexes as Photochemical Nitric Oxide Precursors: Potential Applications in the Treatment of Tumors. Dalton Trans. 2009, 10660–10669. 10.1039/b912898k. [DOI] [PubMed] [Google Scholar]
- Slanina T.; Sebej P. Visible-Light-Activated photoCORMs: Rational Design of CO-Releasing Organic Molecules Absorbing in the Tissue-Transparent Window. Photochem. Photobiol. Sci. 2018, 17, 692–710. 10.1039/C8PP00096D. [DOI] [PubMed] [Google Scholar]
- Garcia-Gallego S.; Bernardes G. J. L. Carbon-Monoxide-Releasing Molecules for the Delivery of Therapeutic CO In Vivo. Angew. Chem., Int. Ed. 2014, 53, 9712–9721. 10.1002/anie.201311225. [DOI] [PubMed] [Google Scholar]
- Wright M. A.; Wright J. A. PhotoCORMs: CO Release Moves into the Visible. Dalton Trans. 2016, 45, 6801–6811. 10.1039/C5DT04849D. [DOI] [PubMed] [Google Scholar]
- Marhenke J.; Trevino K.; Works C. The Chemistry, Biology and Design of Photochemical CO Releasing Molecules and the Efforts to Detect CO for Biological Applications. Coord. Chem. Rev. 2016, 306, 533–543. 10.1016/j.ccr.2015.02.017. [DOI] [Google Scholar]
- Kautz A. C.; Kunz P. C.; Janiak C. CO-Releasing Molecule (CORM) Conjugate Systems. Dalton Trans. 2016, 45, 18045–18063. 10.1039/C6DT03515A. [DOI] [PubMed] [Google Scholar]
- Schatzschneider U. Novel Lead Structures and Activation Mechanisms for CO-Releasing Molecules (CORMs). Br. J. Pharmacol. 2015, 172, 1638–1650. 10.1111/bph.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales M. A.; Mascharak P. K. Photoactive Metal Carbonyl Complexes as Potential Agents for Targeted CO Delivery. J. Inorg. Biochem. 2014, 133, 127–135. 10.1016/j.jinorgbio.2013.10.015. [DOI] [PubMed] [Google Scholar]
- Steiger C.; Hermann C.; Meinel L. Localized Delivery of Carbon Monoxide. Eur. J. Pharm. Biopharm. 2017, 118, 3–12. 10.1016/j.ejpb.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Ismailova A.; Kuter D.; Bohle D. S.; Butler I. S. An Overview of the Potential Therapeutic Applications of CO-Releasing Molecules. Bioinorg. Chem. Appl. 2018, 2018, 8547364. 10.1155/2018/8547364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faizan M.; Muhammad N.; Niazi K. U. K.; Hu Y.; Wang Y.; Wu Y.; Sun H.; Liu R.; Dong W.; Zhang W.; Gao Z. CO-Releasing Materials: An Emphasis on Therapeutic Implications, as Release and Subsequent Cytotoxicity Are the Part of Therapy. Materials 2019, 12, 1643. 10.3390/ma12101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.; de Caestecker M.; Otterbein L. E.; Wang B. Carbon Monoxide: An Emerging Therapy for Acute Kidney Injury. Med. Res. Rev. 2020, 40, 1–31. 10.1002/med.21650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto M. N.; Mascharak P. K. Light-Assisted and Remote Delivery of Carbon Monoxide to Malignant Cells and Tissues: Photochemotherapy in the Spotlight. J. Photochem. Photobiol., C 2020, 42, 100341. 10.1016/j.jphotochemrev.2020.100341. [DOI] [Google Scholar]
- Soboleva T.; Berreau L. M. 3-Hydroxyflavones and 3-Hydroxy-4-oxoquinolines as Carbon Monoxide-Releasing Molecules. Molecules 2019, 24, 1252. 10.3390/molecules24071252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmer R. D.; Pierri A. E.; Ford P. C. Photochemically Activated Carbon Monoxide Release for Biological Targets. Toward Developing Air-Stable PhotoCORMs Labilized by Visible Light. Coord. Chem. Rev. 2012, 256, 1509–1519. 10.1016/j.ccr.2011.12.009. [DOI] [Google Scholar]
- Schatzschneider U. PhotoCORMs: Light-Triggered Release of Carbon Monoxide From the Coordination Sphere of Transition Metal Complexes for Biological Applications. Inorg. Chim. Acta 2011, 374, 19–23. 10.1016/j.ica.2011.02.068. [DOI] [Google Scholar]
- Kottelat E.; Zobi F. Visible Light-Activated PhotoCORMs. Inorganics 2017, 5, 24. 10.3390/inorganics5020024. [DOI] [Google Scholar]
- Ling K.; Men F.; Wang W. C.; Zhou Y. Q.; Zhang H. W.; Ye D. W. Carbon Monoxide and Its Controlled Release: Therapeutic Application, Detection, and Development of Carbon Monoxide Releasing Molecules (CORMs). J. Med. Chem. 2018, 61, 2611–2635. 10.1021/acs.jmedchem.6b01153. [DOI] [PubMed] [Google Scholar]
- Adach W.; Olas B. Carbon Monoxide and Its Donors – Their Implications for Medicine. Future Med. Chem. 2019, 11, 61–73. 10.4155/fmc-2018-0215. [DOI] [PubMed] [Google Scholar]
- Ford P. C. Metal Complex Strategies for Photo-Uncaging the Small Molecule Bioregulators Nitric Oxide and Carbon Monoxide. Coord. Chem. Rev. 2018, 376, 548–564. 10.1016/j.ccr.2018.07.018. [DOI] [Google Scholar]
- Dichiarante V.; Bergamaschi G.. Photochemistry of Transition Metal Complexes (2017–2018). Photochemistry; The Royal Society of Chemistry, 2020; Vol. 47. [Google Scholar]
- Nguyen D.; Boyer C. Macromolecular and Inorganic Nanomaterials Scaffolds for Carbon Monoxide Delivery: Recent Developments and Future Trends. ACS Biomater. Sci. Eng. 2015, 1, 895–913. 10.1021/acsbiomaterials.5b00230. [DOI] [PubMed] [Google Scholar]
- Lee L. C.-C.; Leung K.-K.; Lo K. K.-W. Recent Development of Luminescent Rhenium(I) Tricarbonyl Polypyridine Complexes as Cellular Imaging Reagents, Anticancer Drugs, and Antibacterial Agents. Dalton Trans. 2017, 46, 16357–16380. 10.1039/C7DT03465B. [DOI] [PubMed] [Google Scholar]
- Soboleva T.; Berreau L. M. Tracking CO Release in Cells via the Luminescence of Donor Molecules and/or their By-Products. Isr. J. Chem. 2019, 59, 339–350. 10.1002/ijch.201800172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobev A. Y.; Moskalensky A. E. Long-Wavelength Photoremovable Protecting Groups: On the Way to in Vivo Application. Comput. Struct. Biotechnol. J. 2020, 18, 27–34. 10.1016/j.csbj.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong F.; Tang Y.; Wang T.; Feng T.; Song J.; Li P.; Huang W. Nitric Oxide-Releasing Polymeric Materials for Antimicrobial Applications: A Review. Antioxidants 2019, 8, 556. 10.3390/antiox8110556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta Y.; Kataoka T.; Mori T.; Kimura K. Review of a Potential Novel Approach for Erectile Dysfunction: Light-Controllable Nitric Oxide Donors and Nanoformulations. Sex. Med. Rev. 2020, 8, 297–302. 10.1016/j.sxmr.2019.05.006. [DOI] [PubMed] [Google Scholar]
- Midgley A. C.; Wei Y.; Li Z.; Kong D.; Zhao Q. Nitric-Oxide-Releasing Biomaterial Regulation of the Stem Cell Microenvironment in Regenerative Medicine. Adv. Mater. 2020, 32, 1805818. 10.1002/adma.201805818. [DOI] [PubMed] [Google Scholar]
- Pierri A. E.; Muizzi D. A.; Ostrowski A. D.; Ford P. C.. Photo-Controlled Release of NO and CO with Inorganic and Organometallic Complexes. Luminescent and Photoactive Transition Metal Complexes as Biomolecular Probes and Cellular Reagents; Lo K. K. W., Ed.; Springer-Verlag Berlin: Berlin, 2015; Vol. 165. [Google Scholar]
- Mir J. M.; Malik B. A.; Maurya R. C. Nitric Oxide-Releasing Molecules at the Interface of Inorganic Chemistry and Biology: a Concise Overview. Rev. Inorg. Chem. 2019, 39, 91–112. 10.1515/revic-2018-0017. [DOI] [Google Scholar]
- Xiao P.; Zhang J.; Zhao J.; Stenzel M. H. Light-Induced Release of Molecules From Polymers. Prog. Polym. Sci. 2017, 74, 1–33. 10.1016/j.progpolymsci.2017.06.002. [DOI] [Google Scholar]
- Nakagawa H. Photo-Controlled Release of Small Signaling Molecules to Induce Biological Responses. Chem. Rec. 2018, 18, 1708–1716. 10.1002/tcr.201800035. [DOI] [PubMed] [Google Scholar]
- Powell C. R.; Dillon K. M.; Matson J. B. A Review of Hydrogen Sulfide (H2S) Donors: Chemistry and Potential Therapeutic Applications. Biochem. Pharmacol. 2018, 149, 110–123. 10.1016/j.bcp.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.; Ji X.; Ji K.; Wang B. Hydrogen Sulfide Prodrugs—A Review. Acta Pharm. Sin. B 2015, 5, 367–377. 10.1016/j.apsb.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzschneider U. Photoactivated Biological Activity of Transition-Metal Complexes. Eur. J. Inorg. Chem. 2010, 2010, 1451–1467. 10.1002/ejic.201000003. [DOI] [Google Scholar]
- Katz J. S.; Burdick J. A. Light-Responsive Biomaterials: Development and Applications. Macromol. Biosci. 2010, 10, 339–348. 10.1002/mabi.200900297. [DOI] [PubMed] [Google Scholar]
- Givens R. S.; Conrad P. G. I.; Yousef A. L.; Lee J.-I.. Photoremovable Protecting Groups; In CRC Handbook of Organic Photochemistry and Photobiology, 2nd ed.; CRC Press: Boca Raton, 2004. [Google Scholar]
- Goeldner M.; Givens R. S.. Dynamic Studies in Biology; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Mayer G.; Heckel A. Biologically Active Molecules with a “Light Switch. Angew. Chem., Int. Ed. 2006, 45, 4900–4921. 10.1002/anie.200600387. [DOI] [PubMed] [Google Scholar]
- Pelliccioli A. P.; Wirz J. Photoremovable Protecting Groups: Reaction Mechanisms and Applications. Photochem. Photobiol. Sci. 2002, 1, 441–458. 10.1039/b200777k. [DOI] [PubMed] [Google Scholar]
- Cheong W. F.; Prahl S. A.; Welch A. J. A Review of the Optical Properties of Biological Tissues. IEEE J. Quantum Electron. 1990, 26, 2166–2185. 10.1109/3.64354. [DOI] [Google Scholar]
- Tuchin V. V. Light Scattering Study of Tissues. Phys.-Usp. 1997, 40, 495–515. 10.1070/PU1997v040n05ABEH000236. [DOI] [Google Scholar]
- Tuchin V. V.Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnostics; SPIE Press: Bellingham, 2015. [Google Scholar]
- Mang R.; Stege H.; Krutmann J.. Mechanisms of Phototoxic and Photoallergic Reactions; In Contact Dermatitis; Frosch P. J., Menné T., Lepoittevin J. P., Eds.; Springer: Berlin, Heidelberg, 2006. [Google Scholar]
- Kim K.; Park H.; Lim K.-M. Phototoxicity: Its Mechanism and Animal Alternative Test Methods. Toxicol. Res. 2015, 31, 97–104. 10.5487/TR.2015.31.2.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman R. D. Ultraviolet Phototoxicity to the Retina. Eye Contact Lens 2011, 37, 196–205. 10.1097/ICL.0b013e31821e45a9. [DOI] [PubMed] [Google Scholar]
- Lim Y. T.; Kim S.; Nakayama A.; Stott N. E.; Bawendi M. G.; Frangioni J. V. Selection of Quantum Dot Wavelengths for Biomedical Assays and Imaging. Mol. Imaging 2003, 2, 50–64. 10.1162/153535003765276282. [DOI] [PubMed] [Google Scholar]
- Weissleder R. A Clearer Vision for in Vivo Imaging. Nat. Biotechnol. 2001, 19, 316–317. 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- Juzenas P.; Juzeniene A.; Kaalhus O.; Iani V.; Moan J. Noninvasive Fluorescence Excitation Spectroscopy during Application of 5-Aminolevulinic Acid in Vivo. Photochem. Photobiol. Sci. 2002, 1, 745–748. 10.1039/b203459j. [DOI] [PubMed] [Google Scholar]
- Bashkatov A. N.; Genina E. A.; Kochubey V. I.; Tuchin V. V. Optical Properties of Human Skin, Subcutaneous and Mucous Tissues in the Wavelength Range from 400 to 2000nm. J. Phys. D: Appl. Phys. 2005, 38, 2543–2555. 10.1088/0022-3727/38/15/004. [DOI] [Google Scholar]
- Ruskowitz E. R.; DeForest C. A. Photoresponsive Biomaterials for Targeted Drug Delivery and 4D Cell Culture. Nat. Rev. Mater. 2018, 3, 17087. 10.1038/natrevmats.2017.87. [DOI] [Google Scholar]
- Rwei A. Y.; Wang W.; Kohane D. S. Photoresponsive Nanoparticles for Drug Delivery. Nano Today 2015, 10, 451–467. 10.1016/j.nantod.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Straten D.; Mashayekhi V.; de Bruijn H. S.; Oliveira S.; Robinson D. J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. 10.3390/cancers9020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos A. F.; de Almeida D. R. Q.; Terra L. F.; Baptista M. S.; Labriola L. Photodynamic Therapy in Cancer Treatment - An Update Review. J. Cancer Metastasis Treat. 2019, 5, 25. 10.20517/2394-4722.2018.83. [DOI] [Google Scholar]
- Baskaran R.; Lee J.; Yang S.-G. Clinical Development of Photodynamic Agents and Therapeutic Applications. Biomater. Res. 2018, 22, 25. 10.1186/s40824-018-0140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursoy H.; Ozcakir-Tomruk C.; Tanalp J.; Yılmaz S. Photodynamic Therapy in Dentistry: A Literature Review. Clin. Oral Investig. 2013, 17, 1113–1125. 10.1007/s00784-012-0845-7. [DOI] [PubMed] [Google Scholar]
- Maisch T.; Szeimies R.-M.; Jori G.; Abels C. Antibacterial Photodynamic Therapy in Dermatology. Photochem. Photobiol. Sci. 2004, 3, 907–917. 10.1039/b407622b. [DOI] [PubMed] [Google Scholar]
- Klán P.; Wirz J.. Photochemistry of Organic Compounds: From Concepts to Practice, 1st ed.; John Wiley & Sons Ltd.: Chichester, 2009. [Google Scholar]
- Bochet C. G. Wavelength-Selective Cleavage of Photolabile Protecting Groups. Tetrahedron Lett. 2000, 41, 6341–6346. 10.1016/S0040-4039(00)01050-9. [DOI] [Google Scholar]
- Bochet C. G. Orthogonal Photolysis of Protecting Groups. Angew. Chem., Int. Ed. 2001, 40, 2071–2073. . [DOI] [PubMed] [Google Scholar]
- Kammari L.; Šolomek T.; Ngoy B. P.; Heger D.; Klán P. Orthogonal Photocleavage of a Monochromophoric Linker. J. Am. Chem. Soc. 2010, 132, 11431–11433. 10.1021/ja1047736. [DOI] [PubMed] [Google Scholar]
- Olson J. P.; Banghart M. R.; Sabatini B. L.; Ellis-Davies G. C. R. Spectral Evolution of a Photochemical Protecting Group for Orthogonal Two-Color Uncaging with Visible Light. J. Am. Chem. Soc. 2013, 135, 15948–15954. 10.1021/ja408225k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton-Humphreys M. N.; Taylor R. D. T.; McDougall C.; Hart M. L.; Brown C. T. A.; Emptage N. J.; Conway S. J. Wavelength-Orthogonal Photolysis of Neurotransmitters in Vitro. Chem. Commun. 2012, 48, 657–659. 10.1039/C1CC15135E. [DOI] [PubMed] [Google Scholar]
- Rodrigues-Correia A.; Weyel X. M. M.; Heckel A. Four Levels of Wavelength-Selective Uncaging for Oligonucleotides. Org. Lett. 2013, 15, 5500–5503. 10.1021/ol402657j. [DOI] [PubMed] [Google Scholar]
- Menge C.; Heckel A. Coumarin-Caged dG for Improved Wavelength-Selective Uncaging of DNA. Org. Lett. 2011, 13, 4620–4623. 10.1021/ol201842x. [DOI] [PubMed] [Google Scholar]
- Morihiro K.; Kodama T.; Mori S.; Tsunoda S.; Obika S. Wavelength-Selective Light-Triggered Strand Exchange Reaction. Org. Biomol. Chem. 2016, 14, 1555–1558. 10.1039/C5OB02369F. [DOI] [PubMed] [Google Scholar]
- Hoorens M. W. H.; Szymanski W. Reversible, Spatial and Temporal Control over Protein Activity Using Light. Trends Biochem. Sci. 2018, 43, 567–575. 10.1016/j.tibs.2018.05.004. [DOI] [PubMed] [Google Scholar]
- Li J.; Kong H.; Zhu C.; Zhang Y. Photo-Controllable Bioorthogonal Chemistry for Spatiotemporal Control of Bio-Targets in Living Systems. Chem. Sci. 2020, 11, 3390–3396. 10.1039/C9SC06540G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protti S.; Ravelli D.; Fagnoni M. Wavelength Dependence and Wavelength Selectivity in Photochemical Reactions. Photochem. Photobiol. Sci. 2019, 18, 2094–2101. 10.1039/C8PP00512E. [DOI] [PubMed] [Google Scholar]
- Warther D.; Gug S.; Specht A.; Bolze F.; Nicoud J. F.; Mourot A.; Goeldner M. Two-Photon Uncaging: New Prospects in Neuroscience and Cellular Biology. Bioorg. Med. Chem. 2010, 18, 7753–7758. 10.1016/j.bmc.2010.04.084. [DOI] [PubMed] [Google Scholar]
- Milburn T.; Matsubara N.; Billington A. P.; Udgaonkar J. B.; Walker J. W.; Carpenter B. K.; Webb W. W.; Marque J.; Denk W. Synthesis, Photochemistry, and Biological Activity of a Caged Photolabile Acetylcholine Receptor Ligand. Biochemistry 1989, 28, 49–55. 10.1021/bi00427a008. [DOI] [PubMed] [Google Scholar]
- Berroy P.; Viriot M. L.; Carré M. C. Photolabile Group for 5′-OH Protection of Nucleosides: Synthesis and Photodeprotection Rate. Sens. Actuators, B 2001, 74, 186–189. 10.1016/S0925-4005(00)00731-0. [DOI] [Google Scholar]
- Bader T. K.; Xu F.; Hodny M. H.; Blank D. A.; Distefano M. D. Methoxy-Substituted Nitrodibenzofuran-Based Protecting Group with an Improved Two-Photon Action Cross-Section for Thiol Protection in Solid Phase Peptide Synthesis. J. Org. Chem. 2020, 85, 1614–1625. 10.1021/acs.joc.9b02751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato L.; Mourot A.; Davenport C. M.; Herbivo C.; Warther D.; Léonard J.; Bolze F.; Nicoud J.-F.; Kramer R. H.; Goeldner M.; Specht A. Water-Soluble, Donor-Acceptor Biphenyl Derivatives in the 2-(o-Nitrophenyl)propyl Series: Highly Efficient Two-Photon Uncaging of the Neurotransmitter γ-Aminobutyric Acid at λ = 800 nm. Angew. Chem., Int. Ed. 2012, 51, 1840–1843. 10.1002/anie.201106559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boinapally S.; Huang B.; Abe M.; Katan C.; Noguchi J.; Watanabe S.; Kasai H.; Xue B.; Kobayashi T. Caged Glutamates with π-Extended 1,2-Dihydronaphthalene Chromophore: Design, Synthesis, Two-Photon Absorption Property, and Photochemical Reactivity. J. Org. Chem. 2014, 79, 7822–7830. 10.1021/jo501425p. [DOI] [PubMed] [Google Scholar]
- Barltrop J.; Plant P.; Schofield P. Photosensitive Protective Groups. Chem. Commun. 1966, 822–823. 10.1039/c19660000822. [DOI] [Google Scholar]
- Corrie J. E. T.; Barth A.; Munasinghe V. R. N.; Trentham D. R.; Hutter M. C. Photolytic Cleavage of 1-(2-Nitrophenyl)ethyl Ethers Involves Two Parallel Pathways and Product Release Is Rate-Limited by Decomposition of a Common Hemiacetal Intermediate. J. Am. Chem. Soc. 2003, 125, 8546–8554. 10.1021/ja034354c. [DOI] [PubMed] [Google Scholar]
- Dunkin I. R.; Gebicki J.; Kiszka M.; Sanín-Leira D. Phototautomerism of o-Nitrobenzyl Compounds: o-Quinonoid Aci-Nitro Species Studied by Matrix Isolation and DFT Calculations. J. Chem. Soc. Perk. T. 2001, 2, 1414–1425. 10.1039/b009630j. [DOI] [Google Scholar]
- Dunkin I. R.; Gebicki J.; Kiszka M.; Sanín-Leira D. The Matrix-Isolation IR Spectrum of the o-Quinonoid Intermediate in the Photolysis of 2-Nitrobenzyl Methyl Ether. Spectrochim. Acta, Part A 1997, 53, 2553–2557. 10.1016/S1386-1425(97)00186-8. [DOI] [Google Scholar]
- Il’ichev Y. V.; Schwörer M. A.; Wirz J. Photochemical Reaction Mechanisms of 2-Nitrobenzyl Compounds: Methyl Ethers and Caged ATP. J. Am. Chem. Soc. 2004, 126, 4581–4595. 10.1021/ja039071z. [DOI] [PubMed] [Google Scholar]
- Il’ichev Y. V.; Wirz J. Rearrangements of 2-Nitrobenzyl Compounds. 1. Potential Energy Surface of 2-Nitrotoluene and Its Isomers Explored with ab Initio and Density Functional Theory Methods. J. Phys. Chem. A 2000, 104, 7856–7870. 10.1021/jp000261v. [DOI] [Google Scholar]
- Walker J. W.; Reid G. P.; McCray J. A.; Trentham D. R. Photolabile 1-(2-Nitrophenyl)ethyl Phosphate Esters of Adenine Nucleotide Analogs. Synthesis and Mechanism of Photolysis. J. Am. Chem. Soc. 1988, 110, 7170–7177. 10.1021/ja00229a036. [DOI] [Google Scholar]
- Engels J.; Schlaeger E. J. Synthesis, Structure, and Reactivity of Adenosine Cyclic 3′,5′-Phosphate Benzyl Triesters. J. Med. Chem. 1977, 20, 907–911. 10.1021/jm00217a008. [DOI] [PubMed] [Google Scholar]
- Bayley H.; Chang C.-Y.; Todd Miller W.; Niblack B.; Pan P.. Caged Peptides and Proteins by Targeted Chemical Modification. Methods Enzymol.; Academic Press, 1998; Vol. 291. [DOI] [PubMed] [Google Scholar]
- Aujard I.; Benbrahim C.; Gouget M.; Ruel O.; Baudin J.-B.; Neveu P.; Jullien L. o-Nitrobenzyl Photolabile Protecting Groups with Red-Shifted Absorption: Syntheses and Uncaging Cross-Sections for One- and Two-Photon Excitation. Chem. - Eur. J. 2006, 12, 6865–6879. 10.1002/chem.200501393. [DOI] [PubMed] [Google Scholar]
- Kaplan J. H.; Ellis-Davies G. C. Photolabile Chelators for the Rapid Photorelease of Divalent Cations. Proc. Natl. Acad. Sci. U. S. A. 1988, 85, 6571–6575. 10.1073/pnas.85.17.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta T.; Wang S. S.; Dantzker J. L.; Dore T. M.; Bybee W. J.; Callaway E. M.; Denk W.; Tsien R. Y. Brominated 7-Hydroxycoumarin-4-ylmethyls: Photolabile Protecting Groups with Biologically Useful Cross-Sections for Two Photon Photolysis. Proc. Natl. Acad. Sci. U. S. A. 1999, 96, 1193–1200. 10.1073/pnas.96.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmanis E.; Gooden R.; Wilkins C. W. Jr.; Schonhorn H. A Study of the Photochemical Response of o-Nitrobenzyl Cholate Derivatives in P(MMA-MAA) Matrices. J. Polym. Sci., Polym. Chem. Ed. 1983, 21, 1075–1083. 10.1002/pol.1983.170210415. [DOI] [Google Scholar]
- Reichmanis E.; Smith B. C.; Gooden R. o-Nitrobenzyl Photochemistry: Solution vs. Solid-State Behavior. J. Polym. Sci., Polym. Chem. Ed. 1985, 23, 1–8. 10.1002/pol.1985.170230101. [DOI] [Google Scholar]
- Adams S. R.; Kao J. P. Y.; Grynkiewicz G.; Minta A.; Tsien R. Y. Biologically Useful Chelators That Release Ca2+ Upon Illumination. J. Am. Chem. Soc. 1988, 110, 3212–3220. 10.1021/ja00218a034. [DOI] [Google Scholar]
- Pease A. C.; Solas D.; Sullivan E. J.; Cronin M. T.; Holmes C. P.; Fodor S. P. Light-Generated Oligonucleotide Arrays for Rapid DNA Sequence Analysis. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 5022–5026. 10.1073/pnas.91.11.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A.; Stengele K.-P.; Giegrich H.; Cornwell P.; Isham K. R.; Sachleben R. A.; Pfleiderer W.; Foote R. S. Photolabile Protecting Groups for Nucleosides: Synthesis and Photodeprotection Rates. Tetrahedron 1997, 53, 4247–4264. 10.1016/S0040-4020(97)00154-3. [DOI] [Google Scholar]
- Wöll D.; Walbert S.; Stengele K.-P.; Albert T. J.; Richmond T.; Norton J.; Singer M.; Green R. D.; Pfleiderer W.; Steiner U. E. Triplet-Sensitized Photodeprotection of Oligonucleotides in Solution and on Microarray Chips. Helv. Chim. Acta 2004, 87, 28–45. 10.1002/hlca.200490015. [DOI] [Google Scholar]
- Momotake A.; Lindegger N.; Niggli E.; Barsotti R. J.; Ellis-Davies G. C. R. The Nitrodibenzofuran Chromophore: A New Caging Group for Ultra-Efficient Photolysis in Living Cells. Nat. Methods 2006, 3, 35–40. 10.1038/nmeth821. [DOI] [PubMed] [Google Scholar]
- Kantevari S.; Buskila Y.; Ellis-Davies G. C. R. Synthesis and Characterization of Cell-Permeant 6-Nitrodibenzofuranyl-Caged IP3. Photochem. Photobiol. Sci. 2012, 11, 508–513. 10.1039/C1PP05155E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker Y.; Unger E.; Fichte M. A. H.; Gacek D. A.; Dreuw A.; Wachtveitl J.; Walla P. J.; Heckel A. A Red-Shifted Two-Photon-Only Caging Group for Three-Dimensional Photorelease. Chem. Sci. 2018, 9, 2797–2802. 10.1039/C7SC05182D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer F.; Joshi K. B.; Fichte M. A. H.; Mack T.; Wachtveitl J.; Heckel A. Wavelength-Selective Uncaging of dA and dC Residues. Org. Lett. 2011, 13, 1450–1453. 10.1021/ol200141v. [DOI] [PubMed] [Google Scholar]
- Kennedy D. P.; Brown D. C.; Burdette S. C. Probing Nitrobenzhydrol Uncaging Mechanisms Using FerriCast. Org. Lett. 2010, 12, 4486–4489. 10.1021/ol101726a. [DOI] [PubMed] [Google Scholar]
- Mahmoodi M. M.; Abate-Pella D.; Pundsack T. J.; Palsuledesai C. C.; Goff P. C.; Blank D. A.; Distefano M. D. Nitrodibenzofuran: A One- and Two-Photon Sensitive Protecting Group That Is Superior to Brominated Hydroxycoumarin for Thiol Caging in Peptides. J. Am. Chem. Soc. 2016, 138, 5848–5859. 10.1021/jacs.5b11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sõrmus T.; Lavogina D.; Enkvist E.; Uri A.; Viht K. Efficient Photocaging of a Tight-Binding Bisubstrate Inhibitor of cAMP-Dependent Protein Kinase. Chem. Commun. 2019, 55, 11147–11150. 10.1039/C9CC04978A. [DOI] [PubMed] [Google Scholar]
- Riguet E.; Bochet C. G. New Safety-Catch Photolabile Protecting Group. Org. Lett. 2007, 9, 5453–5456. 10.1021/ol702327c. [DOI] [PubMed] [Google Scholar]
- Mangubat-Medina A. E.; Trial H. O.; Vargas R. D.; Setegne M. T.; Bader T.; Distefano M. D.; Ball Z. T. Red-Shifted Backbone N–H Photocaging Agents. Org. Biomol. Chem. 2020, 18, 5110–5114. 10.1039/D0OB00923G. [DOI] [PubMed] [Google Scholar]
- Bühler S.; Lagoja I.; Giegrich H.; Stengele K.-P.; Pfleiderer W. New Types of Very Efficient Photolabile Protecting Groups Based upon the [2-(2-Nitrophenyl)propoxy]carbonyl (NPPOC) Moiety. Helv. Chim. Acta 2004, 87, 620–659. 10.1002/hlca.200490060. [DOI] [Google Scholar]
- Singh A. K.; Khade P. K. Synthesis and Photochemical Properties of Nitro-Naphthyl Chromophore and the Corresponding Immunoglobulin Bioconjugate. Bioconjugate Chem. 2002, 13, 1286–1291. 10.1021/bc020021d. [DOI] [PubMed] [Google Scholar]
- Singh A. K.; Khade P. K. 7-Methoxy-3-nitro-2-naphthalenemethanol—A New Phototrigger for Caging Applications. Tetrahedron Lett. 2011, 52, 4899–4902. 10.1016/j.tetlet.2011.07.043. [DOI] [Google Scholar]
- Takaoka K.; Tatsu Y.; Yumoto N.; Nakajima T.; Shimamoto K. Synthesis and Photoreactivity of Caged Blockers for Glutamate Transporters. Bioorg. Med. Chem. Lett. 2003, 13, 965–970. 10.1016/S0960-894X(02)01042-9. [DOI] [PubMed] [Google Scholar]
- Anstaett P.; Pierroz V.; Ferrari S.; Gasser G. Two-Photon Uncageable Enzyme Inhibitors Bearing Targeting Vectors. Photochem. Photobiol. Sci. 2015, 14, 1821–1825. 10.1039/C5PP00245A. [DOI] [PubMed] [Google Scholar]
- Liu W.; Liang L.; Lo P. K.; Gou X. J.; Sun X. H. A Double Branched Photosensitive Prodrug: Synthesis and Characterization of Light Triggered Drug Release. Tetrahedron Lett. 2016, 57, 959–963. 10.1016/j.tetlet.2016.01.064. [DOI] [Google Scholar]
- Abou Nakad E.; Bolze F.; Specht A. o-Nitrobenzyl Photoremovable Groups with Fluorescence Uncaging Reporting Properties. Org. Biomol. Chem. 2018, 16, 6115–6122. 10.1039/C8OB01330F. [DOI] [PubMed] [Google Scholar]
- Jakkampudi S.; Abe M.; Komori N.; Takagi R.; Furukawa K.; Katan C.; Sawada W.; Takahashi N.; Kasai H. Design and Synthesis of a 4-Nitrobromobenzene Derivative Bearing an Ethylene Glycol Tetraacetic Acid Unit for a New Generation of Caged Calcium Compounds with Two-Photon Absorption Properties in the Near-IR Region and Their Application in Vivo. ACS Omega 2016, 1, 193–201. 10.1021/acsomega.6b00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao C.; Jin M.; Li B.; Xu Y.; Jin J.; Zhu L. Long Conjugated 2-Nitrobenzyl Derivative Caged Anticancer Prodrugs with Visible Light Regulated Release: Preparation and Functionalizations. Org. Biomol. Chem. 2012, 10, 5238–5244. 10.1039/c2ob25701g. [DOI] [PubMed] [Google Scholar]
- Jin M.; Xu H.; Hong H.; Bao C.; Pu H.; Wan D.; Zhu L. Micropatterning of Polymethacrylates by Single- or Two-Photon Irradiation Using π-Conjugated o-Nitrobenzyl Ester Phototrigger as Side Chains. J. Appl. Polym. Sci. 2013, 130, 4099–4106. 10.1002/app.39683. [DOI] [Google Scholar]
- Thapaliya E. R.; Mazza M. M. A.; Cusido J.; Baker J. D.; Raymo F. M. A Synthetic Strategy for the Structural Modification of Photoactivatable BODIPY-Oxazine Dyads. ChemPhotoChem. 2020, 4, 332–337. 10.1002/cptc.201900276. [DOI] [Google Scholar]
- Dyck R. H.; McClure D. S. Ultraviolet Spectra of Stilbene, p-Monohalogen Stilbenes, and Azobenzene and the trans to cis Photoisomerization Process. J. Chem. Phys. 1962, 36, 2326–2345. 10.1063/1.1732885. [DOI] [Google Scholar]
- Malkin S.; Fischer E. Temperature Dependence of Photoisomerization. Part II. Quantum Yields of cis ⇆ trans Isomerization in Azo-Compounds. J. Phys. Chem. 1962, 66, 2482–2486. 10.1021/j100818a038. [DOI] [Google Scholar]
- Waldeck D. H. Photoisomerization Dynamics of Stilbenes. Chem. Rev. 1991, 91, 415–436. 10.1021/cr00003a007. [DOI] [Google Scholar]
- Komori N.; Jakkampudi S.; Motoishi R.; Abe M.; Kamada K.; Furukawa K.; Katan C.; Sawada W.; Takahashi N.; Kasai H.; Xue B.; Kobayashi T. Design and Synthesis of a New Chromophore, 2-(4-Nitrophenyl)benzofuran, for Two-Photon Uncaging Using Near-IR Light. Chem. Commun. 2016, 52, 331–334. 10.1039/C5CC07664A. [DOI] [PubMed] [Google Scholar]
- Maurits E.; van de Graaff M. J.; Maiorana S.; Wander D. P. A.; Dekker P. M.; van der Zanden S. Y.; Florea B. I.; Neefjes J. J. C.; Overkleeft H. S.; van Kasteren S. I. Immunoproteasome Inhibitor–Doxorubicin Conjugates Target Multiple Myeloma Cells and Release Doxorubicin upon Low-Dose Photon Irradiation. J. Am. Chem. Soc. 2020, 142, 7250–7253. 10.1021/jacs.9b11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A.; Jana A.; Karthik S.; Bera M.; Zhao Y.; Singh N. D. P. Photoresponsive Real Time Monitoring Silicon Quantum Dots for Regulated Delivery of Anticancer Drugs. J. Mater. Chem. B 2016, 4, 521–528. 10.1039/C5TB02045J. [DOI] [PubMed] [Google Scholar]
- Wang W.; Liu Q.; Zhan C.; Barhoumi A.; Yang T.; Wylie R. G.; Armstrong P. A.; Kohane D. S. Efficient Triplet–Triplet Annihilation-Based Upconversion for Nanoparticle Phototargeting. Nano Lett. 2015, 15, 6332–6338. 10.1021/acs.nanolett.5b01325. [DOI] [PubMed] [Google Scholar]
- Yan B.; Boyer J.-C.; Branda N. R.; Zhao Y. Near-Infrared Light-Triggered Dissociation of Block Copolymer Micelles Using Upconverting Nanoparticles. J. Am. Chem. Soc. 2011, 133, 19714–19717. 10.1021/ja209793b. [DOI] [PubMed] [Google Scholar]
- Yan B.; Boyer J.-C.; Habault D.; Branda N. R.; Zhao Y. Near Infrared Light Triggered Release of Biomacromolecules from Hydrogels Loaded with Upconversion Nanoparticles. J. Am. Chem. Soc. 2012, 134, 16558–16561. 10.1021/ja308876j. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Lu G.; Yu Y.; Zhang H.; Gao J.; Sun Z.; Lu Y.; Zou H. NIR-Responsive Copolymer Upconversion Nanocomposites for Triggered Drug Release in Vitro and in Vivo. ACS Applied Bio Materials 2019, 2, 495–503. 10.1021/acsabm.8b00681. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Liu F.; Liu X.; Xing B. NIR Light Controlled Photorelease of SiRNA and Its Targeted Intracellular Delivery Based on Upconversion Nanoparticles. Nanoscale 2013, 5, 231–238. 10.1039/C2NR32835F. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Velmurugan B.; Liu X.; Xing B. NIR Photoresponsive Crosslinked Upconverting Nanocarriers Toward Selective Intracellular Drug Release. Small 2013, 9, 2937–2944. 10.1002/smll.201201765. [DOI] [PubMed] [Google Scholar]
- Ballister E. R.; Aonbangkhen C.; Mayo A. M.; Lampson M. A.; Chenoweth D. M. Localized Light-Induced Protein Dimerization in Living Cells Using a Photocaged Dimerizer. Nat. Commun. 2014, 5, 5475. 10.1038/ncomms6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler M.; Lo Giudice C.; Vogl P.; Ebner A.; Hinterdorfer P.; Gruber H. J.; Alsteens D. Control of Ligand-Binding Specificity Using Photocleavable Linkers in AFM Force Spectroscopy. Nano Lett. 2020, 20, 4038–4042. 10.1021/acs.nanolett.0c01426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C.; Schimelman J.; Wang P.; Miller K. L.; Ma X.; You S.; Guan J.; Sun B.; Zhu W.; Chen S. Photopolymerizable Biomaterials and Light-Based 3D Printing Strategies for Biomedical Applications. Chem. Rev. 2020, 120, 10695–10743. 10.1021/acs.chemrev.9b00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentzen N. B.; Mogaki R.; Otake S.; Okuro K.; Aida T. Intracellular Photoactivation of Caspase-3 by Molecular Glues for Spatiotemporal Apoptosis Induction. J. Am. Chem. Soc. 2020, 142, 8080–8084. 10.1021/jacs.0c01823. [DOI] [PubMed] [Google Scholar]
- Jedlitzke B.; Yilmaz Z.; Dörner W.; Mootz H. D. Photobodies: Light-Activatable Single-Domain Antibody Fragments. Angew. Chem., Int. Ed. 2020, 59, 1506–1510. 10.1002/anie.201912286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler S.; Schayek H.; Rajendar K.; Tal I.; Shani E.; Meroz Y.; Dobrovetsky R.; Weinstain R. Characterizing Gibberellin Flow in Planta Using Photocaged Gibberellins. Chem. Sci. 2019, 10, 1500–1505. 10.1039/C8SC04528C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raccuglia D.; Mueller U. Focal Uncaging of GABA Reveals a Temporally Defined Role for Gabaergic Inhibition During Appetitive Associative Olfactory Conditioning in Honeybees. Learn. Mem. 2013, 20, 410–416. 10.1101/lm.030205.112. [DOI] [PubMed] [Google Scholar]
- Shao Q.; Jiang T.; Ren G.; Cheng Z.; Xing B. Photoactivable Bioluminescent Probes for Imaging Luciferase Activity. Chem. Commun. 2009, 4028–4030. 10.1039/b908346d. [DOI] [PubMed] [Google Scholar]
- Shestopalov I. A.; Sinha S.; Chen J. K. Light-Controlled Gene Silencing in Zebrafish Embryos. Nat. Chem. Biol. 2007, 3, 650–651. 10.1038/nchembio.2007.30. [DOI] [PubMed] [Google Scholar]
- Warmutha R.; Grell E.; Lehn J.-M.; Bats J. W.; Quinkert G. Photo-Cleavable Cryptands: Synthesis and Structure. Helv. Chim. Acta 1991, 74, 671–681. 10.1002/hlca.19910740402. [DOI] [Google Scholar]
- Kolarski D.; Sugiyama A.; Breton G.; Rakers C.; Ono D.; Schulte A.; Tama F.; Itami K.; Szymanski W.; Hirota T.; Feringa B. L. Controlling the Circadian Clock with High Temporal Resolution through Photodosing. J. Am. Chem. Soc. 2019, 141, 15784–15791. 10.1021/jacs.9b05445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W.; Brown W.; Bardhan A.; Delaney M.; Ilk A. S.; Rauen R. R.; Kahn S. I.; Tsang M.; Deiters A. Spatiotemporal Control of CRISPR/Cas9 Function in Cells and Zebrafish using Light-Activated Guide RNA. Angew. Chem., Int. Ed. 2020, 59, 8998–9003. 10.1002/anie.201914575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A. S.; Deiters A. Optical Control of Protein Function through Unnatural Amino Acid Mutagenesis and Other Optogenetic Approaches. ACS Chem. Biol. 2014, 9, 1398–1407. 10.1021/cb500176x. [DOI] [PubMed] [Google Scholar]
- Courtney T.; Deiters A. Recent Advances in the Optical Control of Protein Function through Genetic Code Expansion. Curr. Opin. Chem. Biol. 2018, 46, 99–107. 10.1016/j.cbpa.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nödling A. R.; Spear L. A.; Williams T. L.; Luk L. Y. P.; Tsai Y.-H. Using Genetically Incorporated Unnatural Amino Acids to Control Protein Functions in Mammalian Cells. Essays Biochem. 2019, 63, 237–266. 10.1042/EBC20180042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal H. K.; Janicek R.; Chi S.-H.; Perry J. W.; Niggli E.; Ellis-Davies G. C. R. Calcium Uncaging with Visible Light. J. Am. Chem. Soc. 2016, 138, 3687–3693. 10.1021/jacs.5b11606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basa P. N.; Antala S.; Dempski R. E.; Burdette S. C. A Zinc(II) Photocage Based on a Decarboxylation Metal Ion Release Mechanism for Investigating Homeostasis and Biological Signaling. Angew. Chem., Int. Ed. 2015, 54, 13027–13031. 10.1002/anie.201505778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesienski K. L.; Haas K. L.; Dickens M. G.; Tesema Y. T.; Franz K. J. A Photolabile Ligand for Light-Activated Release of Caged Copper. J. Am. Chem. Soc. 2008, 130, 12246–12247. 10.1021/ja8047442. [DOI] [PubMed] [Google Scholar]
- Richers M. T.; Passlick S.; Agarwal H.; Ellis-Davies G. C. R. Dendrimer Conjugation Enables Multiphoton Chemical Neurophysiology Studies with an Extended π-Electron Caging Chromophore. Angew. Chem., Int. Ed. 2019, 58, 12086–12090. 10.1002/anie.201906067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigenaga A.; Yamamoto J.; Sumikawa Y.; Furuta T.; Otaka A. Development and Photo-Responsive Peptide Bond Cleavage Reaction of Two-Photon Near-Infrared Excitation-Responsive Peptide. Tetrahedron Lett. 2010, 51, 2868–2871. 10.1016/j.tetlet.2010.03.079. [DOI] [Google Scholar]
- Kantevari S.; Hoang C. J.; Ogrodnik J.; Egger M.; Niggli E.; Ellis-Davies G. C. R. Synthesis and Two-photon Photolysis of 6-(ortho-Nitroveratryl)-Caged IP3 in Living Cells. ChemBioChem 2006, 7, 174–180. 10.1002/cbic.200500345. [DOI] [PubMed] [Google Scholar]
- Neveu P.; Aujard I.; Benbrahim C.; Le Saux T.; Allemand J.-F.; Vriz S.; Bensimon D.; Jullien L. A Caged Retinoic Acid for One- and Two-Photon Excitation in Zebrafish Embryos. Angew. Chem., Int. Ed. 2008, 47, 3744–3746. 10.1002/anie.200800037. [DOI] [PubMed] [Google Scholar]
- Shi D. D.; Trigo F. F.; Semmelhack M. F.; Wang S. S. H. Synthesis and Biological Evaluation of Bis-CNB-GABA, a Photoactivatable Neurotransmitter with Low Receptor Interference and Chemical Two-Photon Uncaging Properties. J. Am. Chem. Soc. 2014, 136, 1976–1981. 10.1021/ja411082f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueto Díaz E. J.; Picard S.; Chevasson V.; Daniel J.; Hugues V.; Mongin O.; Genin E.; Blanchard-Desce M. Cooperative Dyads for Two-Photon Uncaging. Org. Lett. 2015, 17, 102–105. 10.1021/ol5033046. [DOI] [PubMed] [Google Scholar]
- Cueto Diaz E.; Picard S.; Klausen M.; Hugues V.; Pagano P.; Genin E.; Blanchard-Desce M. Cooperative Veratryle and Nitroindoline Cages for Two-Photon Uncaging in the NIR. Chem. - Eur. J. 2016, 22, 10848–10859. 10.1002/chem.201601109. [DOI] [PubMed] [Google Scholar]
- Giegrich H.; Eisele-Bühler S.; Hermann C.; Kvasyuk E.; Charubala R.; Pfleiderer W. New Photolabile Protecting Groups in Nucleoside and Nucleotide Chemistry—Synthesis, Cleavage Mechanisms and Applications. Nucleosides Nucleotides 1998, 17, 1987–1996. 10.1080/07328319808004738. [DOI] [Google Scholar]
- Tseng S.-S.; Ullman E. F. Elimination Reactions Induced by Photoenolization of o-Alkylbenzophenones. J. Am. Chem. Soc. 1976, 98, 541–544. 10.1021/ja00418a037. [DOI] [Google Scholar]
- Atemnkeng W. N.; Louisiana L. D.; Yong P. K.; Vottero B.; Banerjee A. 1-[2-(2-Hydroxyalkyl)phenyl]ethanone: A New Photoremovable Protecting Group for Carboxylic Acids. Org. Lett. 2003, 5, 4469–4471. 10.1021/ol035782q. [DOI] [PubMed] [Google Scholar]
- Kamdzhilov Y.; Wirz J. Synthesis and Reaction Mechanism of a Photoremovable Protecting Group Based on 1,4-Naphthoquinone. Photochem. Photobiol. Sci. 2007, 6, 865–872. 10.1039/b706318k. [DOI] [PubMed] [Google Scholar]
- Pirrung M. C.; Dore T. M.; Zhu Y.; Rana V. S. Sensitized Two-Photon Photochemical Deprotection. Chem. Commun. 2010, 46, 5313–5315. 10.1039/c0cc00782j. [DOI] [PubMed] [Google Scholar]
- Sobczak M.; Wagner P. J. Light-Induced Decarboxylation of (o-Acylphenyl)acetic Acids. Org. Lett. 2002, 4, 379–382. 10.1021/ol0170758. [DOI] [PubMed] [Google Scholar]
- Klán P.; Pelliccioli A. P.; Pospíšil T.; Wirz J. 2, 5-Dimethylphenacyl Esters: A Photoremovable Protecting Group for Phosphates and Sulfonic Acids. Photochem. Photobiol. Sci. 2002, 1, 920–923. 10.1039/B208171G. [DOI] [PubMed] [Google Scholar]
- Beier M.; Hoheisel J. D. Production by Quantitative Photolithographic Synthesis of Individually Quality Checked DNA Microarrays. Nucleic Acids Res. 2000, 28, e11 10.1093/nar/28.4.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrung M. C.; Wang L.; Montague-Smith M. P. 3’-Nitrophenylpropyloxycarbonyl (NPPOC) Protecting Groups for High-Fidelity Automated 5’ → 3’ Photochemical DNA Synthesis. Org. Lett. 2001, 3, 1105–1108. 10.1021/ol0069150. [DOI] [PubMed] [Google Scholar]
- Forsström B.; Axnäs B. B.; Stengele K.-P.; Bühler J.; Albert T. J.; Richmond T. A.; Hu F. J.; Nilsson P.; Hudson E. P.; Rockberg J.; Uhlen M. Proteome-Wide Epitope Mapping of Antibodies Using Ultra-dense Peptide Arrays. Mol. Cell. Proteomics 2014, 13, 1585–1597. 10.1074/mcp.M113.033308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen L. B.; Buus S.; Schafer-Nielsen C. Identification and Mapping of Linear Antibody Epitopes in Human Serum Albumin Using High-Density Peptide Arrays. PLoS One 2013, 8, e68902 10.1371/journal.pone.0068902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan K. R.; DeLisi C.; Laursen R. A. Synthesis of Photolabile 2-(2-Nitrophenyl)propyloxycarbonyl Protected Amino Acids. Tetrahedron Lett. 2003, 44, 8585–8588. 10.1016/j.tetlet.2003.09.155. [DOI] [Google Scholar]
- Lackey J. G.; Mitra D.; Somoza M. M.; Cerrina F.; Damha M. J. Acetal Levulinyl Ester (ALE) Groups for 2′-Hydroxyl Protection of Ribonucleosides in the Synthesis of Oligoribonucleotides on Glass and Microarrays. J. Am. Chem. Soc. 2009, 131, 8496–8502. 10.1021/ja9002074. [DOI] [PubMed] [Google Scholar]
- Wu C.-H.; Holden M. T.; Smith L. M. Enzymatic Fabrication of High-Density RNA Arrays. Angew. Chem., Int. Ed. 2014, 53, 13514–13517. 10.1002/anie.201408747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen-Van Hal N. L. W.; van der Putte P.; Hellmuth K.; Matysiak S.; Kretschy N.; Somoza M. M. Optimized Light-Directed Synthesis of Aptamer Microarrays. Anal. Chem. 2013, 85, 5950–5957. 10.1021/ac400746j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H.; Maisonneuve S.; Xie J. Synthesis, Glycosylation and Photolysis of Photolabile 2-(2-Nitrophenyl)propyloxycarbonyl (NPPOC) Protected Glycopyranosides. Org. Biomol. Chem. 2009, 7, 3847–3854. 10.1039/b908404e. [DOI] [PubMed] [Google Scholar]
- Wu C.-H.; Lockett M. R.; Smith L. M. RNA-Mediated Gene Assembly from DNA Arrays. Angew. Chem., Int. Ed. 2012, 51, 4628–4632. 10.1002/anie.201109058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht A.; Thomann J.-S.; Alarcon K.; Wittayanan W.; Ogden D.; Furuta T.; Kurakawa Y.; Goeldner M. New Photoremovable Protecting Groups for Carboxylic Acids with High Photolytic Efficiencies at Near-UV Irradiation. Application to the Photocontrolled Release of L-Glutamate. ChemBioChem 2006, 7, 1690–1695. 10.1002/cbic.200600111. [DOI] [PubMed] [Google Scholar]
- Salierno M. J.; García A. J.; del Campo A. Photo-Activatable Surfaces for Cell Migration Assays. Adv. Funct. Mater. 2013, 23, 5974–5980. 10.1002/adfm.201300902. [DOI] [Google Scholar]
- Lunzer M.; Shi L.; Andriotis O. G.; Gruber P.; Markovic M.; Thurner P. J.; Ossipov D.; Liska R.; Ovsianikov A. A Modular Approach to Sensitized Two-Photon Patterning of Photodegradable Hydrogels. Angew. Chem., Int. Ed. 2018, 57, 15122–15127. 10.1002/anie.201808908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Xi W.; Gao G.; Wang X.; Stansbury J. W.; Bowman C. N. o-Nitrobenzyl-Based Photobase Generators: Efficient Photoinitiators for Visible-Light Induced Thiol-Michael Addition Photopolymerization. ACS Macro Lett. 2018, 7, 852–857. 10.1021/acsmacrolett.8b00435. [DOI] [PubMed] [Google Scholar]
- Kretschy N.; Holik A.-K.; Somoza V.; Stengele K.-P.; Somoza M. M. Next-Generation o-Nitrobenzyl Photolabile Groups for Light-Directed Chemistry and Microarray Synthesis. Angew. Chem., Int. Ed. 2015, 54, 8555–8559. 10.1002/anie.201502125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack M.; Hölz K.; Holik A.-K.; Kretschy N.; Somoza V.; Stengele K.-P.; Somoza M. M. Express Photolithographic DNA Microarray Synthesis with Optimized Chemistry and High-Efficiency Photolabile Groups. J. Nanobiotechnol. 2016, 14, 14. 10.1186/s12951-016-0166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöll D.; Smirnova J.; Galetskaya M.; Prykota T.; Bühler J.; Stengele K.-P.; Pfleiderer W.; Steiner U. E. Intramolecular Sensitization of Photocleavage of the Photolabile 2-(2-Nitrophenyl)propoxycarbonyl (NPPOC) Protecting Group: Photoproducts and Photokinetics of the Release of Nucleosides. Chem. - Eur. J. 2008, 14, 6490–6497. 10.1002/chem.200800613. [DOI] [PubMed] [Google Scholar]
- Wöll D.; Smirnova J.; Pfleiderer W.; Steiner U. E. Highly Efficient Photolabile Protecting Groups with Intramolecular Energy Transfer. Angew. Chem., Int. Ed. 2006, 45, 2975–2978. 10.1002/anie.200504091. [DOI] [PubMed] [Google Scholar]
- Gug S.; Charon S.; Specht A.; Alarcon K.; Ogden D.; Zietz B.; Léonard J.; Haacke S.; Bolze F.; Nicoud J.-F.; Goeldner M. Photolabile Glutamate Protecting Group with High One- and Two-Photon Uncaging Efficiencies. ChemBioChem 2008, 9, 1303–1307. 10.1002/cbic.200700651. [DOI] [PubMed] [Google Scholar]
- Specht A.; Bolze F.; Donato L.; Herbivo C.; Charon S.; Warther D.; Gug S.; Nicoud J.-F.; Goeldner M. The Donor–Acceptor Biphenyl Platform: A Versatile Chromophore for the Engineering of Highly Efficient Two-Photon Sensitive Photoremovable Protecting Groups. Photochem. Photobiol. Sci. 2012, 11, 578–586. 10.1039/c2pp05360h. [DOI] [PubMed] [Google Scholar]
- Warther D.; Bolze F.; Léonard J.; Gug S.; Specht A.; Puliti D.; Sun X.-H.; Kessler P.; Lutz Y.; Vonesch J.-L.; Winsor B.; Nicoud J.-F.; Goeldner M. Live-Cell One- and Two-Photon Uncaging of a Far-Red Emitting Acridinone Fluorophore. J. Am. Chem. Soc. 2010, 132, 2585–2590. 10.1021/ja9074562. [DOI] [PubMed] [Google Scholar]
- Schelkle K. M.; Becht S.; Faraji S.; Petzoldt M.; Müllen K.; Buckup T.; Dreuw A.; Motzkus M.; Hamburger M. Emission Turn-On and Solubility Turn-Off in Conjugated Polymers: One- and Two-Photon-Induced Removal of Fluorescence-Quenching Solubilizing Groups. Macromol. Rapid Commun. 2015, 36, 31–37. 10.1002/marc.201400562. [DOI] [PubMed] [Google Scholar]
- Leonidova A.; Anstaett P.; Pierroz V.; Mari C.; Spingler B.; Ferrari S.; Gasser G. Induction of Cytotoxicity through Photorelease of Aminoferrocene. Inorg. Chem. 2015, 54, 9740–9748. 10.1021/acs.inorgchem.5b01332. [DOI] [PubMed] [Google Scholar]
- Farrukh A.; Paez J. I.; del Campo A. 4D Biomaterials for Light-Guided Angiogenesis. Adv. Funct. Mater. 2019, 29, 1807734. 10.1002/adfm.201807734. [DOI] [Google Scholar]
- García-Fernández L.; Herbivo C.; Arranz V. S. M.; Warther D.; Donato L.; Specht A.; del Campo A. Dual Photosensitive Polymers with Wavelength-Selective Photoresponse. Adv. Mater. 2014, 26, 5012–5017. 10.1002/adma.201401290. [DOI] [PubMed] [Google Scholar]
- Carling C.-J.; Viger M. L.; Nguyen Huu V. A.; Garcia A. V.; Almutairi A. In Vivo Visible Light-Triggered Drug Release From an Implanted Depot. Chem. Sci. 2015, 6, 335–341. 10.1039/C4SC02651A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrukh A.; Fan W.; Zhao S.; Salierno M.; Paez J. I.; del Campo A. Photoactivatable Adhesive Ligands for Light-Guided Neuronal Growth. ChemBioChem 2018, 19, 1271–1279. 10.1002/cbic.201800118. [DOI] [PubMed] [Google Scholar]
- Goegan B.; Terzi F.; Bolze F.; Cambridge S.; Specht A. Synthesis and Characterization of Photoactivatable Doxycycline Analogues Bearing Two-Photon-Sensitive Photoremovable Groups Suitable for Light-Induced Gene Expression. ChemBioChem 2018, 19, 1341–1348. 10.1002/cbic.201700628. [DOI] [PubMed] [Google Scholar]
- Farrukh A.; Zhao S.; Paez J. I.; Kavyanifar A.; Salierno M.; Cavalié A.; del Campo A. In Situ, Light-Guided Axon Growth on Biomaterials via Photoactivatable Laminin Peptidomimetic IK(HANBP)VAV. ACS Appl. Mater. Interfaces 2018, 10, 41129–41137. 10.1021/acsami.8b15517. [DOI] [PubMed] [Google Scholar]
- Fichte M. A. H.; Weyel X. M. M.; Junek S.; Schäfer F.; Herbivo C.; Goeldner M.; Specht A.; Wachtveitl J.; Heckel A. Three-Dimensional Control of DNA Hybridization by Orthogonal Two-Color Two-Photon Uncaging. Angew. Chem., Int. Ed. 2016, 55, 8948–8952. 10.1002/anie.201603281. [DOI] [PubMed] [Google Scholar]
- Herbivo C.; Omran Z.; Revol J.; Javot H.; Specht A. Synthesis and Characterization of Cell-Permeable Caged Phosphates that Can Be Photolyzed by Visible Light or 800 nm Two-Photon Photolysis. ChemBioChem 2013, 14, 2277–2283. 10.1002/cbic.201300425. [DOI] [PubMed] [Google Scholar]
- Gug S.; Bolze F.; Specht A.; Bourgogne C.; Goeldner M.; Nicoud J.-F. Molecular Engineering of Photoremovable Protecting Groups for Two-Photon Uncaging. Angew. Chem., Int. Ed. 2008, 47, 9525–9529. 10.1002/anie.200803964. [DOI] [PubMed] [Google Scholar]
- Schelkle K. M.; Griesbaum T.; Ollech D.; Becht S.; Buckup T.; Hamburger M.; Wombacher R. Light-Induced Protein Dimerization by One- and Two-Photon Activation of Gibberellic Acid Derivatives in Living Cells. Angew. Chem., Int. Ed. 2015, 54, 2825–2829. 10.1002/anie.201409196. [DOI] [PubMed] [Google Scholar]
- Lin Y.-C.; Nihongaki Y.; Liu T.-Y.; Razavi S.; Sato M.; Inoue T. Rapidly Reversible Manipulation of Molecular Activity with Dual Chemical Dimerizers. Angew. Chem., Int. Ed. 2013, 52, 6450–6454. 10.1002/anie.201301219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T.; DeRose R.; Suarez A.; Ueno T.; Chen M.; Sun T.-p.; Wolfgang M. J.; Mukherjee C.; Meyers D. J.; Inoue T. Rapid and Orthogonal Logic Gating with a Gibberellin-Induced Dimerization System. Nat. Chem. Biol. 2012, 8, 465–470. 10.1038/nchembio.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olejniczak J.; Sankaranarayanan J.; Viger M. L.; Almutairi A. Highest Efficiency Two-Photon Degradable Copolymer for Remote Controlled Release. ACS Macro Lett. 2013, 2, 683–687. 10.1021/mz400256x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guibourt N. J.-B. G.Histoire Abrégée Des Drogues Simples; Colas L., Ed.; Méquignon-Marvis: Paris, 1820. [Google Scholar]
- Guibort N.; Jean B.; Planchon G.. Histoire Naturelle des Drogues Simples ou Cours D’Histoire Naturelle; L’éCole de Pharmacie de Paris, 1869. [Google Scholar]
- Harborne J. B. The Natural Coumarins: Occurrence, Chemistry and Biochemistry. Plant, Cell Environ. 1982, 5, 435–436. 10.1111/1365-3040.ep11611630. [DOI] [Google Scholar]
- Givens R. S.; Matuszewski B. Photochemistry of Phosphate Esters: An Efficient Method for the Generation of Electrophiles. J. Am. Chem. Soc. 1984, 106, 6860–6861. 10.1021/ja00334a075. [DOI] [Google Scholar]
- Schade B.; Hagen V.; Schmidt R.; Herbrich R.; Krause E.; Eckardt T.; Bendig J. Deactivation Behavior and Excited-State Properties of (Coumarin-4-yl)methyl Derivatives. 1. Photocleavage of (7-Methoxycoumarin-4-yl)methyl-Caged Acids with Fluorescence Enhancement. J. Org. Chem. 1999, 64, 9109–9117. 10.1021/jo9910233. [DOI] [PubMed] [Google Scholar]
- Schmidt R.; Geissler D.; Hagen V.; Bendig J. Kinetics Study of the Photocleavage of (Coumarin-4-yl)methyl Esters. J. Phys. Chem. A 2005, 109, 5000–5004. 10.1021/jp050581k. [DOI] [PubMed] [Google Scholar]
- Schmidt R.; Geissler D.; Hagen V.; Bendig J. Mechanism of Photocleavage of (Coumarin-4-yl)methyl Esters. J. Phys. Chem. A 2007, 111, 5768–5774. 10.1021/jp071521c. [DOI] [PubMed] [Google Scholar]
- Givens R.; Kotala M. B.; Lee J.-I.. Mechanistic Overview of Phototriggers and Cage Release. Dynamic Studies in Biology; Goeldner M., Givens R., Eds., 2005. [Google Scholar]
- van Wilderen L. J. G. W.; Neumann C.; Rodrigues-Correia A.; Kern-Michler D.; Mielke N.; Reinfelds M.; Heckel A.; Bredenbeck J. Picosecond Activation of the DEACM Photocage Unravelled by Vis-Pump-IR-Probe Spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 6487–6496. 10.1039/C6CP07022A. [DOI] [PubMed] [Google Scholar]
- Sarker A. M.; Kaneko Y.; Neckers D. C. Photochemistry and Photophysics of Novel Photoinitiators: N,N,N-Tributyl-N-(4-methylene-7-methoxycoumarin) Ammonium Borates. J. Photochem. Photobiol., A 1998, 117, 67–74. 10.1016/S1010-6030(98)00315-3. [DOI] [Google Scholar]
- Takano H.; Narumi T.; Nomura W.; Furuta T.; Tamamura H. Utilization of the Heavy Atom Effect for the Development of a Photosensitive 8-Azacoumarin-Type Photolabile Protecting Group. Org. Lett. 2015, 17, 5372–5375. 10.1021/acs.orglett.5b02720. [DOI] [PubMed] [Google Scholar]
- Senda N.; Momotake A.; Nishimura Y.; Arai T. Synthesis and Photochemical Properties of a New Water-Soluble Coumarin, Designed as a Chromophore for Highly Water-Soluble and Photolabile Protecting Group. Bull. Chem. Soc. Jpn. 2006, 79, 1753–1757. 10.1246/bcsj.79.1753. [DOI] [Google Scholar]
- Offenbartl-Stiegert D.; Clarke T. M.; Bronstein H.; Nguyen H. P.; Howorka S. Solvent-Dependent Photophysics of a Red-Shifted, Biocompatible Coumarin Photocage. Org. Biomol. Chem. 2019, 17, 6178–6183. 10.1039/C9OB00632J. [DOI] [PubMed] [Google Scholar]
- Corrie J. E. T.; Furuta T.; Givens R.; Yousef A. L.; Goeldner M.. Photoremovable Protecting Groups Used for the Caging of Biomolecules. Dynamic Studies in Biology; Goeldner M., Givens R., Eds., 2005. [Google Scholar]
- Dore M. T.Multiphoton Phototriggers for Exploring Cell Physiology; In Dynamic Studies in Biology; Goeldner M., Givens R., Eds., 2005. [Google Scholar]
- Hagen V.; Benndorf K.; Kaupp U. B.; Pavlos M. C.; Xu H.; Toscano P. J.; Hess G. P.; Gillespie C. D.; Kim G.; Kandler K.. Control of Cellular Activity. Dynamic Studies in Biology; Goeldner M., Givens R., Eds., 2005. [Google Scholar]
- Loudwig S.; Bayley H.; Peng L.; Goeldner M.; Condeelis S. J.; Lawrence D. S.. Photoregulation of Proteins. Dynamic Studies in Biology; Goeldner M., Givens R., Eds., 2005. [Google Scholar]
- Tatsu Y.; Shigeri Y.; Yumoto N.. Caged Compounds and Solid-Phase Synthesis. Dynamic Studies in Biology; Goeldner M., Givens R., Eds., 2005. [Google Scholar]
- Eckardt T.; Hagen V.; Schade B.; Schmidt R.; Schweitzer C.; Bendig J. Deactivation Behavior and Excited-State Properties of (Coumarin-4-yl)methyl Derivatives. 2. Photocleavage of Selected (Coumarin-4-yl)methyl-Caged Adenosine Cyclic 3′,5′-Monophosphates with Fluorescence Enhancement. J. Org. Chem. 2002, 67, 703–710. 10.1021/jo010692p. [DOI] [PubMed] [Google Scholar]
- Fournier L.; Aujard I.; Le Saux T.; Maurin S.; Beaupierre S.; Baudin J.-B.; Jullien L. Coumarinylmethyl Caging Groups with Redshifted Absorption. Chem. - Eur. J. 2013, 19, 17494–17507. 10.1002/chem.201302630. [DOI] [PubMed] [Google Scholar]
- Olson J. P.; Kwon H.-B.; Takasaki K. T.; Chiu C. Q.; Higley M. J.; Sabatini B. L.; Ellis-Davies G. C. R. Optically Selective Two-Photon Uncaging of Glutamate at 900 nm. J. Am. Chem. Soc. 2013, 135, 5954–5957. 10.1021/ja4019379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao C.; Fan G.; Lin Q.; Li B.; Cheng S.; Huang Q.; Zhu L. Styryl Conjugated Coumarin Caged Alcohol: Efficient Photorelease by Either One-Photon Long Wavelength or Two-Photon NIR Excitation. Org. Lett. 2012, 14, 572–575. 10.1021/ol203188h. [DOI] [PubMed] [Google Scholar]
- Lin Q.; Yang L.; Wang Z.; Hua Y.; Zhang D.; Bao B.; Bao C.; Gong X.; Zhu L. Coumarin Photocaging Groups Modified with an Electron-Rich Styryl Moiety at the 3-Position: Long-Wavelength Excitation, Rapid Photolysis, and Photobleaching. Angew. Chem., Int. Ed. 2018, 57, 3722–3726. 10.1002/anie.201800713. [DOI] [PubMed] [Google Scholar]
- Bojtár M.; Kormos A.; Kis-Petik K.; Kellermayer M.; Kele P. Green-Light Activatable, Water-Soluble Red-Shifted Coumarin Photocages. Org. Lett. 2019, 21, 9410–9414. 10.1021/acs.orglett.9b03624. [DOI] [PubMed] [Google Scholar]
- Ito K.; Maruyama J. Studies on Stable Diazoalkanes as Potential Fluorogenic Reagents. I. 7-Substituted 4-Diazomethylcoumarins. Chem. Pharm. Bull. 1983, 31, 3014–3023. 10.1248/cpb.31.3014. [DOI] [Google Scholar]
- Lopez Arbeloa T.; Lopez Arbeloa F.; Tapia M. J.; Lopez Arbeloa I. Hydrogen-Bonding Effect on the Photophysical Properties of 7-Aminocoumarin Derivatives. J. Phys. Chem. 1993, 97, 4704–4707. 10.1021/j100120a024. [DOI] [Google Scholar]
- Rechthaler K.; Köhler G. Excited State Properties and Deactivation Pathways of 7-Aminocoumarins. Chem. Phys. 1994, 189, 99–116. 10.1016/0301-0104(94)80010-3. [DOI] [Google Scholar]
- Fabian W. M. F.; Niederreiter K. S.; Uray G.; Stadlbauer W. Substituent Effects on Absorption and Fluorescence Spectra of Carbostyrils. J. Mol. Struct. 1999, 477, 209–220. 10.1016/S0022-2860(98)00616-4. [DOI] [Google Scholar]
- Seixas de Melo J. S.; Becker R. S.; Macanita A. L. Photophysical Behavior of Coumarins as a Function of Substitution and Solvent: Experimental Evidence for the Existence of a Lowest Lying 1π,π* State. J. Phys. Chem. 1994, 98, 6054–6058. 10.1021/j100075a002. [DOI] [Google Scholar]
- Hagen V.; Bendig J.; Frings S.; Eckardt T.; Helm S.; Reuter D.; Kaupp U. B. Highly Efficient and Ultrafast Phototriggers for cAMP and cGMP by Using Long-Wavelength UV/Vis-Activation. Angew. Chem., Int. Ed. 2001, 40, 1045–1048. . [DOI] [PubMed] [Google Scholar]
- Donovalová J.; Cigán M.; Stankovičová H.; Gašpar J.; Danko M.; Gáplovský A.; Hrdlovič P. Spectral Properties of Substituted Coumarins in Solution and Polymer Matrices. Molecules 2012, 17, 3259–3276. 10.3390/molecules17033259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca A. S.; Goncalves M. S.; Costa S. P. Light-Induced Cleavage of Model Phenylalanine Conjugates Based on Coumarins and Quinolones. Amino Acids 2010, 39, 699–712. 10.1007/s00726-010-0492-8. [DOI] [PubMed] [Google Scholar]
- Fonseca A. S. C.; Soares A. M. S.; Gonçalves M. S. T.; Costa S. P. G. Thionated Coumarins and Quinolones in the Light Triggered Release of a Model Amino Acid: Synthesis and Photolysis Studies. Tetrahedron 2012, 68, 7892–7900. 10.1016/j.tet.2012.07.021. [DOI] [Google Scholar]
- Takaoka K.; Tatsu Y.; Yumoto N.; Nakajima T.; Shimamoto K. Synthesis of Carbamate-Type Caged Derivatives of a Novel Glutamate Transporter Blocker. Bioorg. Med. Chem. 2004, 12, 3687–3694. 10.1016/j.bmc.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Velema W. A.; van der Berg J. P.; Szymanski W.; Driessen A. J. M.; Feringa B. L. Orthogonal Control of Antibacterial Activity with Light. ACS Chem. Biol. 2014, 9, 1969–1974. 10.1021/cb500313f. [DOI] [PubMed] [Google Scholar]
- Velema W. A.; van der Berg J. P.; Szymanski W.; Driessen A. J. M.; Feringa B. L. Bacterial Patterning Controlled by Light Exposure. Org. Biomol. Chem. 2015, 13, 1639–1642. 10.1039/C4OB02483D. [DOI] [PubMed] [Google Scholar]
- Atta S.; Jana A.; Ananthakirshnan R.; Narayana Dhuleep P. S. Fluorescent Caged Compounds of 2,4-Dichlorophenoxyacetic Acid (2,4-D): Photorelease Technology for Controlled Release of 2,4-D. J. Agric. Food Chem. 2010, 58, 11844–11851. 10.1021/jf1027763. [DOI] [PubMed] [Google Scholar]
- Furuta T.; Iwamura M.. New Caged Groups: 7-Substituted Coumarinylmethyl Phosphate Esters. Methods Enzymol.; Academic Press, 1998; Vol. 291. [DOI] [PubMed] [Google Scholar]
- Furuta T.; Momotake A.; Sugimoto M.; Hatayama M.; Torigai H.; Iwamura M. Acyloxycoumarinylmethyl-Caged cAMP, the Photolabile and Membrane-Permeable Derivative of cAMP That Effectively Stimulates Pigment-Dispersion Response of Melanophores. Biochem. Biophys. Res. Commun. 1996, 228, 193–198. 10.1006/bbrc.1996.1638. [DOI] [PubMed] [Google Scholar]
- Yu P.-L.; Zhang Z.-H.; Hao B.-X.; Zhao Y.-J.; Zhang L.-H.; Lee H.-C.; Zhang L.; Yue J. A Novel Fluorescent Cell Membrane-permeable Caged Cyclic ADP-ribose Analogue. J. Biol. Chem. 2012, 287, 24774–24783. 10.1074/jbc.M111.329854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J.; Kong M.; Liu L.; Samanta S.; Van Houten B.; Deiters A. Optical Control of DNA Helicase Function through Genetic Code Expansion. ChemBioChem 2017, 18, 466–469. 10.1002/cbic.201600624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Hemphill J.; Samanta S.; Tsang M.; Deiters A. Genetic Code Expansion in Zebrafish Embryos and Its Application to Optical Control of Cell Signaling. J. Am. Chem. Soc. 2017, 139, 9100–9103. 10.1021/jacs.7b02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W.; Hankinson C. P.; Deiters A. Optical Control of Cellular ATP Levels with a Photocaged Adenylate Kinase. ChemBioChem 2020, 21, 1832–1836. 10.1002/cbic.201900757. [DOI] [PubMed] [Google Scholar]
- Brown W.; Liu J.; Tsang M.; Deiters A. Cell-Lineage Tracing in Zebrafish Embryos with an Expanded Genetic Code. ChemBioChem 2018, 19, 1244–1249. 10.1002/cbic.201800040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney T. M.; Deiters A. Controlling Phosphate Removal with Light: The Development of Optochemical Tools to Probe Protein Phosphatase Function. SLAS Discovery 2020, 25, 957–960. 10.1177/2472555220918519. [DOI] [PubMed] [Google Scholar]
- Furuta T.; Torigai H.; Sugimoto M.; Iwamura M. Photochemical Properties of New Photolabile cAMP Derivatives in a Physiological Saline Solution. J. Org. Chem. 1995, 60, 3953–3956. 10.1021/jo00118a008. [DOI] [Google Scholar]
- Hagen V.; Bendig J.; Frings S.; Wiesner B.; Schade B.; Helm S.; Lorenz D.; Benjamin Kaupp U. Synthesis, Photochemistry and Application of (7-Methoxycoumarin-4-yl)methyl-Caged 8-Bromoadenosine Cyclic 3′,5′-Monophosphate and 8-Bromoguanosine Cyclic 3′,5′-Monophosphate Photolyzed in the Nanosecond Time Region. J. Photochem. Photobiol., B 1999, 53, 91–102. 10.1016/S1011-1344(99)00131-1. [DOI] [PubMed] [Google Scholar]
- Fonseca A. S. C.; Gonçalves M. S. T.; Costa S. P. G. Photocleavage Studies of Fluorescent Amino Acid Conjugates Bearing Different Types of Linkages. Tetrahedron 2007, 63, 1353–1359. 10.1016/j.tet.2006.11.082. [DOI] [Google Scholar]
- Fernandes M. J. G.; Gonçalves M. S. T.; Costa S. P. G. Comparative Study of Polyaromatic and Polyheteroaromatic Fluorescent Photocleavable Protecting Groups. Tetrahedron 2008, 64, 3032–3038. 10.1016/j.tet.2008.01.032. [DOI] [Google Scholar]
- Suzuki A. Z.; Watanabe T.; Kawamoto M.; Nishiyama K.; Yamashita H.; Ishii M.; Iwamura M.; Furuta T. Coumarin-4-ylmethoxycarbonyls as Phototriggers for Alcohols and Phenols. Org. Lett. 2003, 5, 4867–4870. 10.1021/ol0359362. [DOI] [PubMed] [Google Scholar]
- Furuta T.; Hirayama Y.; Iwamura M. Anthraquinon-2-ylmethoxycarbonyl (Aqmoc): A New Photochemically Removable Protecting Group for Alcohols. Org. Lett. 2001, 3, 1809–1812. 10.1021/ol015787s. [DOI] [PubMed] [Google Scholar]
- Atta S.; Ikbal M.; Boda N.; Gauri S. S.; Singh N. D. P. Photoremovable Protecting Groups as Controlled-Release Device for Sex Pheromone. Photochem. Photobiol. Sci. 2013, 12, 393–403. 10.1039/C2PP25118C. [DOI] [PubMed] [Google Scholar]
- Cürten B.; Kullmann P. H. M.; Bier M. E.; Kandler K.; Schmidt B. F. Synthesis, Photophysical, Photochemical and Biological Properties of Caged GABA, 4-[[(2H-1-Benzopyran-2-one-7-amino-4-methoxy) carbonyl] amino] Butanoic Acid. Photochem. Photobiol. 2005, 81, 641–648. 10.1562/2004-07-08-RA-226.1. [DOI] [PubMed] [Google Scholar]
- Luo J.; Uprety R.; Naro Y.; Chou C.; Nguyen D. P.; Chin J. W.; Deiters A. Genetically Encoded Optochemical Probes for Simultaneous Fluorescence Reporting and Light Activation of Protein Function with Two-Photon Excitation. J. Am. Chem. Soc. 2014, 136, 15551–15558. 10.1021/ja5055862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardado-Alvarez T. M.; Sudha Devi L.; Russell M. M.; Schwartz B. J.; Zink J. I. Activation of Snap-Top Capped Mesoporous Silica Nanocontainers Using Two Near-Infrared Photons. J. Am. Chem. Soc. 2013, 135, 14000–14003. 10.1021/ja407331n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocker Y.; Davison B. L.; Deits T. L. Decarboxylation of Monosubstituted Derivatives of Carbonic Acid. Comparative Studies of Water- and Acid-Catalyzed Decarboxylation of Sodium Alkyl Carbonates in Water and Water-d2. J. Am. Chem. Soc. 1978, 100, 3564–3567. 10.1021/ja00479a045. [DOI] [Google Scholar]
- Rossi F. M.; Margulis M.; Tang C.-M.; Kao J. P. Y. N-Nmoc-L-Glutamate, a New Caged Glutamate with High Chemical Stability and Low Pre-photolysis Activity. J. Biol. Chem. 1997, 272, 32933–32939. 10.1074/jbc.272.52.32933. [DOI] [PubMed] [Google Scholar]
- Papageorgiou G.; Barth A.; Corrie J. E. T. Flash Photolytic Release of Alcohols From Photolabile Carbamates or Carbonates Is Rate-Limited by Decarboxylation of the Photoproduct. Photochem. Photobiol. Sci. 2005, 4, 216–220. 10.1039/b417153e. [DOI] [PubMed] [Google Scholar]
- Johnson S. L.; Morrison D. L. Kinetics and Mechanism of Decarboxylation of N-Arylcarbamates. Evidence for Kinetically Important Zwitterionic Carbamic Acid Species of Short Lifetime. J. Am. Chem. Soc. 1972, 94, 1323–1334. 10.1021/ja00759a045. [DOI] [PubMed] [Google Scholar]
- Schoenleber R. O.; Giese B. Photochemical Release of Amines by C, N-Bond Cleavage. Synlett 2003, 2003, 0501–0504. 10.1055/s-2003-37507. [DOI] [Google Scholar]
- Shembekar V. R.; Chen Y.; Carpenter B. K.; Hess G. P. A Protecting Group for Carboxylic Acids That Can Be Photolyzed by Visible Light. Biochemistry 2005, 44, 7107–7114. 10.1021/bi047665o. [DOI] [PubMed] [Google Scholar]
- Shembekar V. R.; Chen Y.; Carpenter B. K.; Hess G. P. Coumarin-Caged Glycine that Can Be Photolyzed within 3 μs by Visible Light. Biochemistry 2007, 46, 5479–5484. 10.1021/bi700280e. [DOI] [PubMed] [Google Scholar]
- Hagen V.; Frings S.; Wiesner B.; Helm S.; Kaupp U. B.; Bendig J. [7-(Dialkylamino)coumarin-4-yl]methyl-Caged Compounds as Ultrafast and Effective Long-Wavelength Phototriggers of 8Bromo-Substituted Cyclic Nucleotides. ChemBioChem 2003, 4, 434–442. 10.1002/cbic.200300561. [DOI] [PubMed] [Google Scholar]
- Piloto A. M.; Costa S. P. G.; Gonçalves M. S. T. Wavelength-Selective Cleavage of o-Nitrobenzyl and Polyheteroaromatic Benzyl Protecting Groups. Tetrahedron 2014, 70, 650–657. 10.1016/j.tet.2013.11.100. [DOI] [Google Scholar]
- Piloto A. M.; Hungerford G.; Costa S. P. G.; Gonçalves M. S. T. Photoinduced Release of Neurotransmitter Amino Acids from Coumarin-Fused Julolidine Ester Cages. Eur. J. Org. Chem. 2013, 2013, 7715–7723. 10.1002/ejoc.201300730. [DOI] [Google Scholar]
- Bassolino G.; Nançoz C.; Thiel Z.; Bois E.; Vauthey E.; Rivera-Fuentes P. Photolabile Coumarins with Improved Efficiency Through Azetidinyl Substitution. Chem. Sci. 2018, 9, 387–391. 10.1039/C7SC03627B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A.; Venkatesh Y.; Das J.; Behara K. K.; Mandal S.; Maiti T. K.; Singh N. D. P. Squaric Acid-Coumarin-Chlorambucil: Photoresponsive Single-Component Fluorescent Organic Nanoconjugates for Self-Monitored Therapeutics. ACS Appl. Nano Mater. 2018, 1, 6312–6319. 10.1021/acsanm.8b01533. [DOI] [Google Scholar]
- Conceição R.; Hungerford G.; Costa S. P. G.; Gonçalves M. S. T. Photolytic Release of Bioactive Carboxylic Acids From Fused Pyran Conjugates. Dyes Pigm. 2018, 148, 368–379. 10.1016/j.dyepig.2017.09.023. [DOI] [Google Scholar]
- Atta S.; Ikbal M.; Kumar A.; Pradeep Singh N. D. Application of Photoremovable Protecting Group for Controlled Release of Plant Growth Regulators by Sunlight. J. Photochem. Photobiol., B 2012, 111, 39–49. 10.1016/j.jphotobiol.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Geißler D.; Kresse W.; Wiesner B.; Bendig J.; Kettenmann H.; Hagen V. DMACM-Caged Adenosine Nucleotides: Ultrafast Phototriggers for ATP, ADP, and AMP Activated by Long-Wavelength Irradiation. ChemBioChem 2003, 4, 162–170. 10.1002/cbic.200390027. [DOI] [PubMed] [Google Scholar]
- Bourbon P.; Peng Q.; Ferraudi G.; Stauffacher C.; Wiest O.; Helquist P. Development of Carbamate-Tethered Coumarins as Phototriggers for Caged Nicotinamide. Bioorg. Med. Chem. Lett. 2013, 23, 6321–6324. 10.1016/j.bmcl.2013.09.067. [DOI] [PubMed] [Google Scholar]
- Herzig L. M.; Elamri I.; Schwalbe H.; Wachtveitl J. Light-Induced Antibiotic Release From a Coumarin-Caged Compound on the Ultrafast Timescale. Phys. Chem. Chem. Phys. 2017, 19, 14835–14844. 10.1039/C7CP02030A. [DOI] [PubMed] [Google Scholar]
- Nguyen H. P.; Stewart S.; Kukwikila M. N.; Jones S. F.; Offenbartl-Stiegert D.; Mao S.; Balasubramanian S.; Beck S.; Howorka S. A Photo-responsive Small-Molecule Approach for the Opto-epigenetic Modulation of DNA Methylation. Angew. Chem., Int. Ed. 2019, 58, 6620–6624. 10.1002/anie.201901139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. T.; Roberts E. W.; Tang S.; Mukherjee J.; Cannon J.; Nip A. J.; Corbin K.; Krummel M. F.; Choi S. K. Control of an Unusual Photo-Claisen Rearrangement in Coumarin Caged Tamoxifen through an Extended Spacer. ACS Chem. Biol. 2017, 12, 1001–1010. 10.1021/acschembio.6b00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Xi W.; Wang C.; Podgórski M.; Bowman C. N. Visible-Light-Initiated Thiol-Michael Addition Polymerizations with Coumarin-Based Photobase Generators: Another Photoclick Reaction Strategy. ACS Macro Lett. 2016, 5, 229–233. 10.1021/acsmacrolett.5b00923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbon P.; Peng Q.; Ferraudi G.; Stauffacher C.; Wiest O.; Helquist P. Synthesis and Photochemical Behavior of Coumarin-Caged Cholesterol. Bioorg. Med. Chem. Lett. 2013, 23, 2162–2165. 10.1016/j.bmcl.2013.01.095. [DOI] [PubMed] [Google Scholar]
- Franceschini C.; Scrimin P.; Prins L. J. Light-Triggered Thiol-Exchange on Gold Nanoparticles at Low Micromolar Concentrations in Water. Langmuir 2014, 30, 13831–13836. 10.1021/la5034965. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Liu T.; Lin Q.; Bao C.; Zhu L. Photoreleasable Thiol Chemistry for Facile and Efficient Bioconjugation. Chem. Commun. 2014, 50, 1256–1258. 10.1039/C3CC48263D. [DOI] [PubMed] [Google Scholar]
- San Miguel V.; Bochet C. G.; del Campo A. Wavelength-Selective Caged Surfaces: How Many Functional Levels Are Possible?. J. Am. Chem. Soc. 2011, 133, 5380–5388. 10.1021/ja110572j. [DOI] [PubMed] [Google Scholar]
- Lin W.; Long L.; Tan W.; Chen B.; Yuan L. Coumarin-Caged Rosamine Probes Based on a Unique Intramolecular Carbon–Carbon Spirocyclization. Chem. - Eur. J. 2010, 16, 3914–3917. 10.1002/chem.201000015. [DOI] [PubMed] [Google Scholar]
- Tang S.; Cannon J.; Yang K.; Krummel M. F.; Baker J. R.; Choi S. K. Spacer-Mediated Control of Coumarin Uncaging for Photocaged Thymidine. J. Org. Chem. 2020, 85, 2945–2955. 10.1021/acs.joc.9b02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaal J.; Kotzur N.; Dekowski B.; Quilitz J.; Klimakow M.; Wessig P.; Hagen V. A Novel Photorearrangement of (Coumarin-4-yl)methylphenyl Ethers. J. Photochem. Photobiol., A 2009, 208, 171–179. 10.1016/j.jphotochem.2009.09.012. [DOI] [Google Scholar]
- Weis S.; Shafiq Z.; Gropeanu R. A.; del Campo A. Ethyl Substituted Coumarin-4-yl Derivatives as Photoremovable Protecting Groups for Amino Acids with Improved Stability for SPSS. J. Photochem. Photobiol., A 2012, 241, 52–57. 10.1016/j.jphotochem.2012.05.014. [DOI] [Google Scholar]
- Fan L.; Lewis R. W.; Hess G. P.; Ganem B. A New Synthesis of Caged GABA Compounds for Studying GABAA Receptors. Bioorg. Med. Chem. Lett. 2009, 19, 3932–3933. 10.1016/j.bmcl.2009.03.065. [DOI] [PubMed] [Google Scholar]
- Yamazoe S.; Liu Q.; McQuade L. E.; Deiters A.; Chen J. K. Sequential Gene Silencing Using Wavelength-Selective Caged Morpholino Oligonucleotides. Angew. Chem., Int. Ed. 2014, 53, 10114–10118. 10.1002/anie.201405355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfried P.; Eiden L.; Grebenovsky N.; Mayer G.; Heckel A. Photo-Tethers for the (Multi-)Cyclic, Conformational Caging of Long Oligonucleotides. Angew. Chem., Int. Ed. 2017, 56, 359–363. 10.1002/anie.201610025. [DOI] [PubMed] [Google Scholar]
- Seyfried P.; Heinz M.; Pintér G.; Klötzner D.-P.; Becker Y.; Bolte M.; Jonker H. R. A.; Stelzl L. S.; Hummer G.; Schwalbe H.; Heckel A. Optimal Destabilization of DNA Double Strands by Single-Nucleobase Caging. Chem. - Eur. J. 2018, 24, 17568–17576. 10.1002/chem.201804040. [DOI] [PubMed] [Google Scholar]
- Kahlstatt J.; Reiß P.; Halbritter T.; Essen L. O.; Koert U.; Heckel A. A Light-Triggered Transmembrane Porin. Chem. Commun. 2018, 54, 9623–9626. 10.1039/C8CC05221B. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Jin S.; Piao X.; Devaraj N. K. Multiplexed Photoactivation of mRNA with Single-Cell Resolution. ACS Chem. Biol. 2020, 15, 1773–1779. 10.1021/acschembio.0c00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q.; Bao C.; Fan G.; Cheng S.; Liu H.; Liu Z.; Zhu L. 7-Amino Coumarin Based Fluorescent Phototriggers Coupled with Nano/Bio-Conjugated Bonds: Synthesis, Labeling and Photorelease. J. Mater. Chem. 2012, 22, 6680–6688. 10.1039/c2jm30357d. [DOI] [Google Scholar]
- Dong J.; Xun Z.; Zeng Y.; Yu T.; Han Y.; Chen J.; Li Y.-Y.; Yang G.; Li Y. A Versatile and Robust Vesicle Based on a Photocleavable Surfactant for Two-Photon-Tuned Release. Chem. - Eur. J. 2013, 19, 7931–7936. 10.1002/chem.201300526. [DOI] [PubMed] [Google Scholar]
- Ji W.; Li N.; Chen D.; Jiao Y.; Xu Q.; Lu J. A Hollow Porous Magnetic Nanocarrier for Efficient Near-Infrared Light- and pH-Controlled Drug Release. RSC Adv. 2014, 4, 51055–51061. 10.1039/C4RA07573K. [DOI] [Google Scholar]
- Ji W.; Li N.; Chen D.; Qi X.; Sha W.; Jiao Y.; Xu Q.; Lu J. Coumarin-Containing Photo-Responsive Nanocomposites for NIR Light-Triggered Controlled Drug Release via a Two-Photon Process. J. Mater. Chem. B 2013, 1, 5942–5949. 10.1039/c3tb21206h. [DOI] [PubMed] [Google Scholar]
- Zhao L.; Peng J.; Huang Q.; Li C.; Chen M.; Sun Y.; Lin Q.; Zhu L.; Li F. Near-Infrared Photoregulated Drug Release in Living Tumor Tissue via Yolk-Shell Upconversion Nanocages. Adv. Funct. Mater. 2014, 24, 363–371. 10.1002/adfm.201302133. [DOI] [Google Scholar]
- Ming Z.; Ruan X.; Bao C.; Lin Q.; Yang Y.; Zhu L. Micropatterned Protein for Cell Adhesion through Phototriggered Charge Change in a Polyvinylpyrrolidone Hydrogel. Adv. Funct. Mater. 2017, 27, 1606258. 10.1002/adfm.201606258. [DOI] [Google Scholar]
- Kotzur N.; Briand B.; Beyermann M.; Hagen V. Wavelength-Selective Photoactivatable Protecting Groups for Thiols. J. Am. Chem. Soc. 2009, 131, 16927–16931. 10.1021/ja907287n. [DOI] [PubMed] [Google Scholar]
- Hagen V.; Dekowski B.; Kotzur N.; Lechler R.; Wiesner B.; Briand B.; Beyermann M. {7-[Bis(carboxymethyl)amino]coumarin-4-yl}methoxycarbonyl Derivatives for Photorelease of Carboxylic Acids, Alcohols/Phenols, Thioalcohols/Thiophenols, and Amines. Chem. - Eur. J. 2008, 14, 1621–1627. 10.1002/chem.200701142. [DOI] [PubMed] [Google Scholar]
- Hagen V.; Dekowski B.; Nache V.; Schmidt R.; Geißler D.; Lorenz D.; Eichhorst J.; Keller S.; Kaneko H.; Benndorf K.; Wiesner B. Coumarinylmethyl Esters for Ultrafast Release of High Concentrations of Cyclic Nucleotides upon One- and Two-Photon Photolysis. Angew. Chem., Int. Ed. 2005, 44, 7887–7891. 10.1002/anie.200502411. [DOI] [PubMed] [Google Scholar]
- Senda N.; Momotake A.; Arai T. Synthesis and Photocleavage of 7-[{Bis(carboxymethyl)amino}coumarin-4-yl]methyl-Caged Neurotransmitters. Bull. Chem. Soc. Jpn. 2007, 80, 2384–2388. 10.1246/bcsj.80.2384. [DOI] [Google Scholar]
- Gilbert D.; Funk K.; Dekowski B.; Lechler R.; Keller S.; Möhrlen F.; Frings S.; Hagen V. Caged Capsaicins: New Tools for the Examination of TRPV1 Channels in Somatosensory Neurons. ChemBioChem 2007, 8, 89–97. 10.1002/cbic.200600437. [DOI] [PubMed] [Google Scholar]
- Taniguchi A.; Skwarczynski M.; Sohma Y.; Okada T.; Ikeda K.; Prakash H.; Mukai H.; Hayashi Y.; Kimura T.; Hirota S.; Matsuzaki K.; Kiso Y. Controlled Production of Amyloid β Peptide from a Photo-Triggered, Water-Soluble Precursor “Click Peptide. ChemBioChem 2008, 9, 3055–3065. 10.1002/cbic.200800503. [DOI] [PubMed] [Google Scholar]
- Priestman M. A.; Sun L.; Lawrence D. S. Dual Wavelength Photoactivation of cAMP- and cGMP-Dependent Protein Kinase Signaling Pathways. ACS Chem. Biol. 2011, 6, 377–384. 10.1021/cb100398e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyret A.; Ibarboure E.; Tron A.; Beauté L.; Rust R.; Sandre O.; McClenaghan N. D.; Lecommandoux S. Polymersome Popping by Light-Induced Osmotic Shock under Temporal, Spatial, and Spectral Control. Angew. Chem., Int. Ed. 2017, 56, 1566–1570. 10.1002/anie.201609231. [DOI] [PubMed] [Google Scholar]
- Banala S.; Arvin M. C.; Bannon N. M.; Jin X.-T.; Macklin J. J.; Wang Y.; Peng C.; Zhao G.; Marshall J. J.; Gee K. R.; Wokosin D. L.; Kim V. J.; McIntosh J. M.; Contractor A.; Lester H. A.; Kozorovitskiy Y.; Drenan R. M.; Lavis L. D. Photoactivatable Drugs for Nicotinic Optopharmacology. Nat. Methods 2018, 15, 347–350. 10.1038/nmeth.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi M.; Skwarczynski M.; Prakash H.; Hirota S.; Kimura T.; Hayashi Y.; Kiso Y. Development of Novel Water-Soluble Photocleavable Protective Group and Its Application for Design of Photoresponsive Paclitaxel Prodrugs. Bioorg. Med. Chem. 2008, 16, 5389–5397. 10.1016/j.bmc.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Nadler A.; Yushchenko D. A.; Müller R.; Stein F.; Feng S.; Mulle C.; Carta M.; Schultz C. Exclusive Photorelease of Signalling Lipids at the Plasma Membrane. Nat. Commun. 2015, 6, 10056. 10.1038/ncomms10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmacher M.; Grasskamp A. T.; Barahtjan P.; Wagner N.; Lombardot B.; Schuhmacher J. S.; Sala P.; Lohmann A.; Henry I.; Shevchenko A.; Coskun Ü.; Walter A. M.; Nadler A. Live-Cell Lipid Biochemistry Reveals a Role of Diacylglycerol Side-Chain Composition for Cellular Lipid Dynamics and Protein Affinities. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 7729. 10.1073/pnas.1912684117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S.; Harayama T.; Chang D.; Hannich J. T.; Winssinger N.; Riezman H. Lysosome-Targeted Photoactivation Reveals Local Sphingosine Metabolism Signatures. Chem. Sci. 2019, 10, 2253–2258. 10.1039/C8SC03614D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner N.; Stephan M.; Höglinger D.; Nadler A. A Click Cage: Organelle-Specific Uncaging of Lipid Messengers. Angew. Chem., Int. Ed. 2018, 57, 13339–13343. 10.1002/anie.201807497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S.; Harayama T.; Montessuit S.; David F. P. A.; Winssinger N.; Martinou J.-C.; Riezman H. Mitochondria-Specific Photoactivation to Monitor Local Sphingosine Metabolism and Function. eLife 2018, 7, e34555 10.7554/eLife.34555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarode B. R.; Kover K.; Friedman S. H. Visible-Light-Activated High-Density Materials for Controlled in Vivo Insulin Release. Mol. Pharmaceutics 2019, 16, 4677–4687. 10.1021/acs.molpharmaceut.9b00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q.; Bao C.; Yang Y.; Liang Q.; Zhang D.; Cheng S.; Zhu L. Highly Discriminating Photorelease of Anticancer Drugs Based on Hypoxia Activatable Phototrigger Conjugated Chitosan Nanoparticles. Adv. Mater. 2013, 25, 1981–1986. 10.1002/adma.201204455. [DOI] [PubMed] [Google Scholar]
- Lin Q.; Huang Q.; Li C.; Bao C.; Liu Z.; Li F.; Zhu L. Anticancer Drug Release from a Mesoporous Silica Based Nanophotocage Regulated by Either a One- or Two-Photon Process. J. Am. Chem. Soc. 2010, 132, 10645–10647. 10.1021/ja103415t. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Lin Q.; Sun Y.; Liu T.; Bao C.; Li F.; Zhu L. Spatiotemporally Controllable and Cytocompatible Approach Builds 3D Cell Culture Matrix by Photo-Uncaged-Thiol Michael Addition Reaction. Adv. Mater. 2014, 26, 3912–3917. 10.1002/adma.201306061. [DOI] [PubMed] [Google Scholar]
- Pahattuge T. N.; Jackson J. M.; Digamber R.; Wijerathne H.; Brown V.; Witek M. A.; Perera C.; Givens R. S.; Peterson B. R.; Soper S. A. Visible Photorelease of Liquid Biopsy Markers Following Microfluidic Affinity-Enrichment. Chem. Commun. 2020, 56, 4098. 10.1039/C9CC09598E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier J.; Gaulier G.; De Matos R.; Ortiz D.; Menin L.; Campargue G.; Mas C.; Constant S.; Le Dantec R.; Mugnier Y.; Bonacina L.; Gerber-Lemaire S. Two-Photon-Triggered Photorelease of Caged Compounds from Multifunctional Harmonic Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 27443–27452. 10.1021/acsami.9b07954. [DOI] [PubMed] [Google Scholar]
- Vuilleumier J.; Gaulier G.; De Matos R.; Mugnier Y.; Campargue G.; Wolf J.-P.; Bonacina L.; Gerber-Lemaire S. Photocontrolled Release of the Anticancer Drug Chlorambucil with Caged Harmonic Nanoparticles. Helv. Chim. Acta 2020, 103, e1900251 10.1002/hlca.201900251. [DOI] [Google Scholar]
- Gangopadhyay M.; Singh T.; Behara K. K.; Karwa S.; Ghosh S. K.; Singh N. D. P. Coumarin-Containing-Star-Shaped 4-Arm-Polyethylene Glycol: Targeted Fluorescent Organic Nanoparticles for Dual Treatment of Photodynamic Therapy and Chemotherapy. Photochem. Photobiol. Sci. 2015, 14, 1329–1336. 10.1039/C5PP00057B. [DOI] [PubMed] [Google Scholar]
- Huang Q.; Bao C.; Ji W.; Wang Q.; Zhu L. Photocleavable Coumarin Crosslinkers Based Polystyrene Microgels: Phototriggered Swelling and Release. J. Mater. Chem. 2012, 22, 18275–18282. 10.1039/c2jm33789d. [DOI] [Google Scholar]
- Lin Q.; Bao C.; Cheng S.; Yang Y.; Ji W.; Zhu L. Target-Activated Coumarin Phototriggers Specifically Switch on Fluorescence and Photocleavage upon Bonding to Thiol-Bearing Protein. J. Am. Chem. Soc. 2012, 134, 5052–5055. 10.1021/ja300475k. [DOI] [PubMed] [Google Scholar]
- Lin Q.; Du Z.; Yang Y.; Fang Q.; Bao C.; Yang Y.; Zhu L. Intracellular Thiols and Photo-Illumination Sequentially Activate Doubly Locked Molecular Probes for Long-Term Cell Highlighting and Tracking with Precise Spatial Accuracy. Chem. - Eur. J. 2014, 20, 16314–16319. 10.1002/chem.201403905. [DOI] [PubMed] [Google Scholar]
- Coe B. J.; Foxon S. P.; Harper E. C.; Harris J. A.; Helliwell M.; Raftery J.; Asselberghs I.; Clays K.; Franz E.; Brunschwig B. S.; Fitch A. G. The Syntheses, Structures and Nonlinear Optical and Related Properties of Salts with Julolidinyl Electron Donor Groups. Dyes Pigm. 2009, 82, 171–186. 10.1016/j.dyepig.2008.12.010. [DOI] [Google Scholar]
- Reynolds G. A.; Drexhage K. H. New Coumarin Dyes with Rigidized Structure for Flashlamp-Pumped Dye Lasers. Opt. Commun. 1975, 13, 222–225. 10.1016/0030-4018(75)90085-1. [DOI] [Google Scholar]
- Grabowski Z. R.; Rotkiewicz K.; Rettig W. Structural Changes Accompanying Intramolecular Electron Transfer: Focus on Twisted Intramolecular Charge-Transfer States and Structures. Chem. Rev. 2003, 103, 3899–4032. 10.1021/cr940745l. [DOI] [PubMed] [Google Scholar]
- Grimm J. B.; English B. P.; Chen J.; Slaughter J. P.; Zhang Z.; Revyakin A.; Patel R.; Macklin J. J.; Normanno D.; Singer R. H.; Lionnet T.; Lavis L. D. A General Method to Improve Fluorophores for Live-Cell and Single-Molecule Microscopy. Nat. Methods 2015, 12, 244–250. 10.1038/nmeth.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereka B.; Vauthey E. Direct Local Solvent Probing by Transient Infrared Spectroscopy Reveals the Mechanism of Hydrogen-Bond Induced Nonradiative Deactivation. Chem. Sci. 2017, 8, 5057–5066. 10.1039/C7SC00437K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fita P.; Fedoseeva M.; Vauthey E. Ultrafast Excited-State Dynamics of Eosin B: A Potential Probe of the Hydrogen-Bonding Properties of the Environment. J. Phys. Chem. A 2011, 115, 2465–2470. 10.1021/jp110849x. [DOI] [PubMed] [Google Scholar]
- Richert S.; Mosquera Vazquez S.; Grzybowski M.; Gryko D. T.; Kyrychenko A.; Vauthey E. Excited-State Dynamics of an Environment-Sensitive Push–Pull Diketopyrrolopyrrole: Major Differences between the Bulk Solution Phase and the Dodecane/Water Interface. J. Phys. Chem. B 2014, 118, 9952–9963. 10.1021/jp506062j. [DOI] [PubMed] [Google Scholar]
- Ramaiah D.; Joy A.; Chandrasekhar N.; Eldho N. V.; Das S.; George M. V. Halogenated Squaraine Dyes as Potential Photochemotherapeutic Agents. Synthesis and Study of Photophysical Properties and Quantum Efficiencies of Singlet Oxygen Generation. Photochem. Photobiol. 1997, 65, 783–790. 10.1111/j.1751-1097.1997.tb01925.x. [DOI] [Google Scholar]
- Salice P.; Arnbjerg J.; Pedersen B. W.; Toftegaard R.; Beverina L.; Pagani G. A.; Ogilby P. R. Photophysics of Squaraine Dyes: Role of Charge-Transfer in Singlet Oxygen Production and Removal. J. Phys. Chem. A 2010, 114, 2518–2525. 10.1021/jp911180n. [DOI] [PubMed] [Google Scholar]
- Avirah R. R.; Jayaram D. T.; Adarsh N.; Ramaiah D. Squaraine Dyes in PDT: From Basic Design to in Vivo Demonstration. Org. Biomol. Chem. 2012, 10, 911–920. 10.1039/C1OB06588B. [DOI] [PubMed] [Google Scholar]
- Furuta T.; Takeuchi H.; Isozaki M.; Takahashi Y.; Kanehara M.; Sugimoto M.; Watanabe T.; Noguchi K.; Dore T. M.; Kurahashi T.; Iwamura M.; Tsien R. Y. Bhc-cNMPs as either Water-Soluble or Membrane-Permeant Photoreleasable Cyclic Nucleotides for both One- and Two-Photon Excitation. ChemBioChem 2004, 5, 1119–1128. 10.1002/cbic.200300814. [DOI] [PubMed] [Google Scholar]
- Furuta T.; Watanabe T.; Tanabe S.; Sakyo J.; Matsuba C. Phototriggers for Nucleobases with Improved Photochemical Properties. Org. Lett. 2007, 9, 4717–4720. 10.1021/ol702106t. [DOI] [PubMed] [Google Scholar]
- Loarueng C.; Boekfa B.; Jarussophon S.; Pongwan P.; Kaewchangwat N.; Suttisintong K.; Jarussophon N. Theoretical and Experimental Investigation of NMR, IR and UV-Visible Spectra of Hydroxyl-Substituted-4-Chloromethylcoumarin Derivatives. ARKIVOC 2020, 6, 1–12. 10.24820/ark.5550190.p010.982. [DOI] [Google Scholar]
- Jivaramonaikul W.; Rashatasakhon P.; Wanichwecharungruang S. UVA Absorption and Photostability of Coumarins. Photochem. Photobiol. Sci. 2010, 9, 1120–1125. 10.1039/c0pp00057d. [DOI] [PubMed] [Google Scholar]
- Bradley J.; Reuter D.; Frings S. Facilitation of Calmodulin-Mediated Odor Adaptation by cAMP-Gated Channel Subunits. Science 2001, 294, 2176–2178. 10.1126/science.1063415. [DOI] [PubMed] [Google Scholar]
- Matsumoto M.; Solzin J.; Helbig A.; Hagen V.; Ueno S.-i.; Kawase O.; Maruyama Y.; Ogiso M.; Godde M.; Minakata H.; Kaupp U. B.; Hoshi M.; Weyand I. A Sperm-Activating Peptide Controls a cGMP-Signaling Pathway in Starfish Sperm. Dev. Biol. 2003, 260, 314–324. 10.1016/S0012-1606(03)00236-7. [DOI] [PubMed] [Google Scholar]
- Geißler D.; Antonenko Y. N.; Schmidt R.; Keller S.; Krylova O. O.; Wiesner B.; Bendig J.; Pohl P.; Hagen V. (Coumarin-4-yl)methyl Esters as Highly Efficient, Ultrafast Phototriggers for Protons and Their Application to Acidifying Membrane Surfaces. Angew. Chem., Int. Ed. 2005, 44, 1195–1198. 10.1002/anie.200461567. [DOI] [PubMed] [Google Scholar]
- Hagen V.; Frings S.; Bendig J.; Lorenz D.; Wiesner B.; Kaupp U. B. Fluorescence Spectroscopic Quantification of the Release of Cyclic Nucleotides from Photocleavable [Bis(carboxymethoxy)coumarin-4-yl]methyl Esters inside Cells. Angew. Chem., Int. Ed. 2002, 41, 3625–3628. . [DOI] [PubMed] [Google Scholar]
- Chen H.-L.; Hsu J. C.-C.; Viet M. H.; Li M. S.; Hu C.-K.; Liu C.-H.; Luh F. Y.; Chen S. S.-W.; Chang E. S.-H.; Wang A. H.-J.; Hsu M.-F.; Fann W.; Chen R. P.-Y. Studying Submicrosecond Protein Folding Kinetics Using a Photolabile Caging Strategy and Time-Resolved Photoacoustic Calorimetry. Proteins: Struct., Funct., Genet. 2010, 78, 2973–2983. 10.1002/prot.22823. [DOI] [PubMed] [Google Scholar]
- Chen E. H. L.; Lu T. T. Y.; Hsu J. C. C.; Tseng Y. J.; Lim T. S.; Chen R. P. Y. Directly Monitor Protein Rearrangement on a Nanosecond-To-Millisecond Time-Scale. Sci. Rep. 2017, 7, 8691. 10.1038/s41598-017-08385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp U. B.; Solzin J.; Hildebrand E.; Brown J. E.; Helbig A.; Hagen V.; Beyermann M.; Pampaloni F.; Weyand I. The Signal Flow and Motor Response Controling Chemotaxis of Sea Urchin Sperm. Nat. Cell Biol. 2003, 5, 109–117. 10.1038/ncb915. [DOI] [PubMed] [Google Scholar]
- Bourbon P.; Peng Q.; Ferraudi G.; Stauffacher C.; Wiest O.; Helquist P. Synthesis, Photophysical, Photochemical, and Computational Studies of Coumarin-Labeled Nicotinamide Derivatives. J. Org. Chem. 2012, 77, 2756–2762. 10.1021/jo2025527. [DOI] [PubMed] [Google Scholar]
- Shibu E. S.; Ono K.; Sugino S.; Nishioka A.; Yasuda A.; Shigeri Y.; Wakida S.-i.; Sawada M.; Biju V. Photouncaging Nanoparticles for MRI and Fluorescence Imaging in Vitro and in Vivo. ACS Nano 2013, 7, 9851–9859. 10.1021/nn4043699. [DOI] [PubMed] [Google Scholar]
- Narumi T.; Takano H.; Ohashi N.; Suzuki A.; Furuta T.; Tamamura H. Isostere-Based Design of 8-Azacoumarin-Type Photolabile Protecting Groups: A Hydrophilicity-Increasing Strategy for Coumarin-4-ylmethyls. Org. Lett. 2014, 16, 1184–1187. 10.1021/ol5000583. [DOI] [PubMed] [Google Scholar]
- Fedoryak O. D.; Dore T. M. Brominated Hydroxyquinoline as a Photolabile Protecting Group with Sensitivity to Multiphoton Excitation. Org. Lett. 2002, 4, 3419–3422. 10.1021/ol026524g. [DOI] [PubMed] [Google Scholar]
- Schaal J.; Dekowski B.; Wiesner B.; Eichhorst J.; Marter K.; Vargas C.; Keller S.; Eremina N.; Barth A.; Baumann A.; Eisenhardt D.; Hagen V. Coumarin-Based Octopamine Phototriggers and their Effects on an Insect Octopamine Receptor. ChemBioChem 2012, 13, 1458–1464. 10.1002/cbic.201200110. [DOI] [PubMed] [Google Scholar]
- Goard M.; Aakalu G.; Fedoryak O. D.; Quinonez C.; St. Julien J.; Poteet S. J.; Schuman E. M.; Dore T. M. Light-Mediated Inhibition of Protein Synthesis. Chem. Biol. 2005, 12, 685–693. 10.1016/j.chembiol.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Robu V. G.; Pfeiffer E. S.; Robia S. L.; Balijepalli R. C.; Pi Y.; Kamp T. J.; Walker J. W. Localization of Functional Endothelin Receptor Signaling Complexes in Cardiac Transverse Tubules. J. Biol. Chem. 2003, 278, 48154–48161. 10.1074/jbc.M304396200. [DOI] [PubMed] [Google Scholar]
- Nomura W.; Narumi T.; Ohashi N.; Serizawa Y.; Lewin N. E.; Blumberg P. M.; Furuta T.; Tamamura H. Synthetic Caged DAG-lactones for Photochemically Controlled Activation of Protein Kinase C. ChemBioChem 2011, 12, 535–539. 10.1002/cbic.201000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W.; Lawrence D. S. A Strategy for the Construction of Caged Diols Using a Photolabile Protecting Group. J. Org. Chem. 2002, 67, 2723–2726. 10.1021/jo0163851. [DOI] [PubMed] [Google Scholar]
- Lu M.; Fedoryak O. D.; Moister B. R.; Dore T. M. Bhc-diol as a Photolabile Protecting Group for Aldehydes and Ketones. Org. Lett. 2003, 5, 2119–2122. 10.1021/ol034536b. [DOI] [PubMed] [Google Scholar]
- Baron M.; Morris J. C.; Telitel S.; Clément J.-L.; Lalevée J.; Morlet-Savary F.; Spangenberg A.; Malval J.-P.; Soppera O.; Gigmes D.; Guillaneuf Y. Light-Sensitive Alkoxyamines as Versatile Spatially- and Temporally- Controlled Precursors of Alkyl Radicals and Nitroxides. J. Am. Chem. Soc. 2018, 140, 3339–3344. 10.1021/jacs.7b12807. [DOI] [PubMed] [Google Scholar]
- Wosnick J. H.; Shoichet M. S. Three-dimensional Chemical Patterning of Transparent Hydrogels. Chem. Mater. 2008, 20, 55–60. 10.1021/cm071158m. [DOI] [Google Scholar]
- Wylie R. G.; Ahsan S.; Aizawa Y.; Maxwell K. L.; Morshead C. M.; Shoichet M. S. Spatially Controlled Simultaneous Patterning of Multiple Growth Factors in Three-Dimensional Hydrogels. Nat. Mater. 2011, 10, 799–806. 10.1038/nmat3101. [DOI] [PubMed] [Google Scholar]
- Abate-Pella D.; Zeliadt N. A.; Ochocki J. D.; Warmka J. K.; Dore T. M.; Blank D. A.; Wattenberg E. V.; Distefano M. D. Photochemical Modulation of Ras-Mediated Signal Transduction Using Caged Farnesyltransferase Inhibitors: Activation by One- and Two-Photon Excitation. ChemBioChem 2012, 13, 1009–1016. 10.1002/cbic.201200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam R. Y.; Fisher S. A.; Baker A. E. G.; Shoichet M. S. Transparent Porous Polysaccharide Cryogels Provide Biochemically Defined, Biomimetic Matrices for Tunable 3D Cell Culture. Chem. Mater. 2016, 28, 3762–3770. 10.1021/acs.chemmater.6b00627. [DOI] [Google Scholar]
- Fisher S. A.; Tam R. Y.; Fokina A.; Mahmoodi M. M.; Distefano M. D.; Shoichet M. S. Photo-Immobilized EGF Chemical Gradients Differentially Impact Breast Cancer Cell Invasion and Drug Response in Defined 3D Hydrogels. Biomaterials 2018, 178, 751–766. 10.1016/j.biomaterials.2018.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoodi M. M.; Fisher S. A.; Tam R. Y.; Goff P. C.; Anderson R. B.; Wissinger J. E.; Blank D. A.; Shoichet M. S.; Distefano M. D. 6-Bromo-7-hydroxy-3-methylcoumarin (mBhc) Is an Efficient Multi-Photon Labile Protecting Group for Thiol Caging and Three-Dimensional Chemical Patterning. Org. Biomol. Chem. 2016, 14, 8289–8300. 10.1039/C6OB01045H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha D. K.; Neveu P.; Gagey N.; Aujard I.; Benbrahim-Bouzidi C.; Le Saux T.; Rampon C.; Gauron C.; Goetz B.; Dubruille S.; Baaden M.; Volovitch M.; Bensimon D.; Vriz S.; Jullien L. Photocontrol of Protein Activity in Cultured Cells and Zebrafish with One- and Two-Photon Illumination. ChemBioChem 2010, 11, 653–663. 10.1002/cbic.201000008. [DOI] [PubMed] [Google Scholar]
- Kim Y. A.; Ramirez D. M. C.; Costain W. J.; Johnston L. J.; Bittman R. A New Tool to Assess Ceramide Bioactivity: 6-Bromo-7-hydroxycoumarinyl-Caged Ceramide. Chem. Commun. 2011, 47, 9236–9238. 10.1039/c1cc12345a. [DOI] [PubMed] [Google Scholar]
- Mizukami S.; Hosoda M.; Satake T.; Okada S.; Hori Y.; Furuta T.; Kikuchi K. Photocontrolled Compound Release System Using Caged Antimicrobial Peptide. J. Am. Chem. Soc. 2010, 132, 9524–9525. 10.1021/ja102167m. [DOI] [PubMed] [Google Scholar]
- Kawakami T.; Cheng H.; Hashiro S.; Nomura Y.; Tsukiji S.; Furuta T.; Nagamune T. A Caged Phosphopeptide-Based Approach for Photochemical Activation of Kinases in Living Cells. ChemBioChem 2008, 9, 1583–1586. 10.1002/cbic.200800116. [DOI] [PubMed] [Google Scholar]
- Perdicakis B.; Montgomery H. J.; Abbott G. L.; Fishlock D.; Lajoie G. A.; Guillemette J. G.; Jervis E. Photocontrol of Nitric Oxide Production in Cell Culture Using a Caged Isoform Selective Inhibitor. Bioorg. Med. Chem. 2005, 13, 47–57. 10.1016/j.bmc.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Ando H.; Furuta T.; Tsien R. Y.; Okamoto H. Photo-Mediated Gene Activation Using Caged RNA/DNA in Zebrafish Embryos. Nat. Genet. 2001, 28, 317–325. 10.1038/ng583. [DOI] [PubMed] [Google Scholar]
- Rudd A. K.; Devaraj N. K. Traceless Synthesis of Ceramides in Living Cells Reveals Saturation-Dependent Apoptotic Effects. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, 7485. 10.1073/pnas.1804266115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery H. J.; Perdicakis B.; Fishlock D.; Lajoie G. A.; Jervis E.; Guy Guillemette J. Photo-Control of Nitric Oxide Synthase Activity Using a Caged Isoform Specific Inhibitor. Bioorg. Med. Chem. 2002, 10, 1919–1927. 10.1016/S0968-0896(02)00050-0. [DOI] [PubMed] [Google Scholar]
- Feeney M. J.; Hu X.; Srinivasan R.; Van N.; Hunter M.; Georgakoudi I.; Thomas S. W. UV and NIR-Responsive Layer-by-Layer Films Containing 6-Bromo-7-hydroxycoumarin Photolabile Groups. Langmuir 2017, 33, 10877–10885. 10.1021/acs.langmuir.7b01469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gracia Lux C.; Lux J.; Collet G.; He S.; Chan M.; Olejniczak J.; Foucault-Collet A.; Almutairi A. Short Soluble Coumarin Crosslinkers for Light-Controlled Release of Cells and Proteins from Hydrogels. Biomacromolecules 2015, 16, 3286–3296. 10.1021/acs.biomac.5b00950. [DOI] [PubMed] [Google Scholar]
- Owen S. C.; Fisher S. A.; Tam R. Y.; Nimmo C. M.; Shoichet M. S. Hyaluronic Acid Click Hydrogels Emulate the Extracellular Matrix. Langmuir 2013, 29, 7393–7400. 10.1021/la305000w. [DOI] [PubMed] [Google Scholar]
- Fomina N.; McFearin C. L.; Sermsakdi M.; Morachis J. M.; Almutairi A. Low Power, Biologically Benign NIR Light Triggers Polymer Disassembly. Macromolecules 2011, 44, 8590–8597. 10.1021/ma201850q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman N.; Purpura K. A.; Wylie R. G.; Zandstra P. W.; Shoichet M. S. The Use of Vascular Endothelial Growth Factor Functionalized Agarose to Guide Pluripotent Stem Cell Aggregates Toward Blood Progenitor Cells. Biomaterials 2010, 31, 8262–8270. 10.1016/j.biomaterials.2010.07.040. [DOI] [PubMed] [Google Scholar]
- Wylie R. G.; Shoichet M. S. Two-Photon Micropatterning of Amines within an Agarose Hydrogel. J. Mater. Chem. 2008, 18, 2716–2721. 10.1039/b718431j. [DOI] [Google Scholar]
- Kumar S.; Allard J.-F.; Morris D.; Dory Y. L.; Lepage M.; Zhao Y. Near-Infrared Light Sensitive Polypeptide Block Copolymer Micelles for Drug Delivery. J. Mater. Chem. 2012, 22, 7252–7257. 10.1039/c2jm16380b. [DOI] [Google Scholar]
- Zhu C.; Bettinger C. J. Light-Induced Remodeling of Physically Crosslinked Hydrogels Using Near-IR Wavelengths. J. Mater. Chem. B 2014, 2, 1613–1618. 10.1039/C3TB21689F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomina N.; McFearin C. L.; Almutairi A. Increasing Materials’ Response to Two-Photon Nir Light via Self-Immolative Dendritic Scaffolds. Chem. Commun. 2012, 48, 9138–9140. 10.1039/c2cc00072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M.; Tanida T.; Yoshii T.; Hamachi I. Rational Molecular Design of Stimulus-Responsive Supramolecular Hydrogels Based on Dipeptides. Adv. Mater. 2011, 23, 2819–2822. 10.1002/adma.201004658. [DOI] [PubMed] [Google Scholar]
- Subramaniam R.; Xiao Y.; Li Y.; Qian S. Y.; Sun W.; Mallik S. Light-Mediated and H-Bond Facilitated Liposomal Release: The Role of Lipid Head Groups in Release Efficiency. Tetrahedron Lett. 2010, 51, 529–532. 10.1016/j.tetlet.2009.11.084. [DOI] [Google Scholar]
- Carter Ramirez D. M.; Pitre S. P.; Kim Y. A.; Bittman R.; Johnston L. J. Photouncaging of Ceramides Promotes Reorganization of Liquid-Ordered Domains in Supported Lipid Bilayers. Langmuir 2013, 29, 3380–3387. 10.1021/la3039158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii T.; Ikeda M.; Hamachi I. Two-Photon-Responsive Supramolecular Hydrogel for Controlling Materials Motion in Micrometer Space. Angew. Chem., Int. Ed. 2014, 53, 7264–7267. 10.1002/anie.201404158. [DOI] [PubMed] [Google Scholar]
- Furuta T.; Manabe K.; Teraoka A.; Murakoshi K.; Ohtsubo A.; Suzuki A. Design, Synthesis, and Photochemistry of Modular Caging Groups for Photoreleasable Nucleotides. Org. Lett. 2012, 14, 6182–6185. 10.1021/ol3029093. [DOI] [PubMed] [Google Scholar]
- Katayama K.; Tsukiji S.; Furuta T.; Nagamune T. A Bromocoumarin-Based Linker for Synthesis of Photocleavable Peptidoconjugates with High Photosensitivity. Chem. Commun. 2008, 5399–5401. 10.1039/b812058g. [DOI] [PubMed] [Google Scholar]
- Biswas S.; Rajesh Y.; Barman S.; Bera M.; Paul A.; Mandal M.; Pradeep Singh N. D. A Dual-Analyte Probe: Hypoxia Activated Nitric Oxide Detection with Phototriggered Drug Release Ability. Chem. Commun. 2018, 54, 7940–7943. 10.1039/C8CC01854E. [DOI] [PubMed] [Google Scholar]
- Tarpey M. M.; Wink D. A.; Grisham M. B. Methods for Detection of Reactive Metabolites of Oxygen and Nitrogen: In Vitro and in Vivo Considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R431–R444. 10.1152/ajpregu.00361.2003. [DOI] [PubMed] [Google Scholar]
- Zhegalova N. G.; Gonzales G.; Berezin M. Y. Synthesis of Nitric Oxide Probes with Fluorescence Lifetime Sensitivity. Org. Biomol. Chem. 2013, 11, 8228–8234. 10.1039/c3ob41498a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H.; Hirotani M.; Nakatsubo N.; Kikuchi K.; Urano Y.; Higuchi T.; Hirata Y.; Nagano T. Bioimaging of Nitric Oxide with Fluorescent Indicators Based on the Rhodamine Chromophore. Anal. Chem. 2001, 73, 1967–1973. 10.1021/ac001136i. [DOI] [PubMed] [Google Scholar]
- Christie R. M.; Lui C.-H. Studies of Fluorescent Dyes: Part 1. An Investigation of the Electronic Spectral Properties of Substituted Coumarins. Dyes Pigm. 1999, 42, 85–93. 10.1016/S0143-7208(99)00012-1. [DOI] [Google Scholar]
- Gangopadhyay M.; Mukhopadhyay S. K.; Karthik S.; Barman S.; Pradeep Singh N. D. Targeted Photoresponsive TiO2–Coumarin Nanoconjugate for Efficient Combination Therapy in MDA-MB-231 Breast Cancer Cells: Synergic Effect of Photodynamic Therapy (PDT) and Anticancer Drug Chlorambucil. MedChemComm 2015, 6, 769–777. 10.1039/C4MD00481G. [DOI] [Google Scholar]
- Hagen V.; Kilic F.; Schaal J.; Dekowski B.; Schmidt R.; Kotzur N. [8-[Bis(carboxymethyl)aminomethyl]-6-bromo-7-hydroxycoumarin-4-yl]methyl Moieties as Photoremovable Protecting Groups for Compounds with COOH, NH2, OH, and C=O Functions. J. Org. Chem. 2010, 75, 2790–2797. 10.1021/jo100368w. [DOI] [PubMed] [Google Scholar]
- Suzuki A. Z.; Sekine R.; Takeda S.; Aikawa R.; Shiraishi Y.; Hamaguchi T.; Okuno H.; Tamamura H.; Furuta T. A Clickable Caging Group as a New Platform for Modular Caged Compounds with Improved Photochemical Properties. Chem. Commun. 2019, 55, 451–454. 10.1039/C8CC07981A. [DOI] [PubMed] [Google Scholar]
- Adamczyk M.; Cornwell M.; Huff J.; Rege S.; Rao T. V. S. Novel 7-Hydroxycoumarin Based Fluorescent Labels. Bioorg. Med. Chem. Lett. 1997, 7, 1985–1988. 10.1016/S0960-894X(97)00345-4. [DOI] [Google Scholar]
- Suzuki A. Z.; Shiraishi Y.; Aoki H.; Sasaki H.; Watahiki R.; Furuta T. Design, Synthesis, and Photochemical Properties of Clickable Caged Compounds. J. Visualized Exp. 2019, e60021 10.3791/60021. [DOI] [PubMed] [Google Scholar]
- Barman S.; Mukhopadhyay S. K.; Gangopadhyay M.; Biswas S.; Dey S.; Singh N. D. P. Coumarin–Benzothiazole–Chlorambucil (Cou–Benz–Cbl) Conjugate: An ESIPT Based pH Sensitive Photoresponsive Drug Delivery System. J. Mater. Chem. B 2015, 3, 3490–3497. 10.1039/C4TB02081B. [DOI] [PubMed] [Google Scholar]
- Barman S.; Das J.; Biswas S.; Maiti T. K.; Pradeep Singh N. D. A Spiropyran–Coumarin Platform: An Environment Sensitive Photoresponsive Drug Delivery System for Efficient Cancer Therapy. J. Mater. Chem. B 2017, 5, 3940–3944. 10.1039/C7TB00379J. [DOI] [PubMed] [Google Scholar]
- Ikegami M.; Arai T. Photoinduced Intramolecular Hydrogen Atom Transfer in 2-(2-Hydroxyphenyl)benzoxazole and 2-(2-Hydroxyphenyl)benzothiazole Studied by Laser Flash Photolysis. J. Chem. Soc. Perk. T. 2002, 2, 1296–1301. 10.1039/b202559k. [DOI] [Google Scholar]
- Zhao J.; Ji S.; Chen Y.; Guo H.; Yang P. Excited State Intramolecular Proton Transfer (ESIPT): From Principal Photophysics to the Development of New Chromophores and Applications in Fluorescent Molecular Probes and Luminescent Materials. Phys. Chem. Chem. Phys. 2012, 14, 8803–8817. 10.1039/C2CP23144A. [DOI] [PubMed] [Google Scholar]
- Wojtyk J. T. C.; Wasey A.; Xiao N.-N.; Kazmaier P. M.; Hoz S.; Yu C.; Lemieux R. P.; Buncel E. Elucidating the Mechanisms of Acidochromic Spiropyran-Merocyanine Interconversion. J. Phys. Chem. A 2007, 111, 2511–2516. 10.1021/jp068575r. [DOI] [PubMed] [Google Scholar]
- Takano H.; Narumi T.; Ohashi N.; Suzuki A.; Furuta T.; Nomura W.; Tamamura H. Development of the 8-Aza-3-bromo-7-hydroxycoumarin-4-ylmethyl Group as a New Entry of Photolabile Protecting Groups. Tetrahedron 2014, 70, 4400–4404. 10.1016/j.tet.2014.04.063. [DOI] [Google Scholar]
- Klausen M.; Dubois V.; Clermont G.; Tonnelé C.; Castet F.; Blanchard-Desce M. Dual-Wavelength Efficient Two-Photon Photorelease of Glycine by π-Extended Dipolar Coumarins. Chem. Sci. 2019, 10, 4209–4219. 10.1039/C9SC00148D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojtár M.; Németh K.; Domahidy F.; Knorr G.; Verkman A.; Kállay M.; Kele P. Conditionally Activatable Visible-Light Photocages. J. Am. Chem. Soc. 2020, 142, 15164–15171. 10.1021/jacs.0c07508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitose Y.; Abe M.; Furukawa K.; Katan C. Design, Synthesis, and Reaction of π-Extended Coumarin-based New Caged Compounds with Two-photon Absorption Character in the Near-IR Region. Chem. Lett. 2016, 45, 1186–1188. 10.1246/cl.160586. [DOI] [Google Scholar]
- Chitose Y.; Abe M.; Furukawa K.; Lin J.-Y.; Lin T.-C.; Katan C. Design and Synthesis of a Caged Carboxylic Acid with a Donor-π–Donor Coumarin Structure: One-photon and Two-photon Uncaging Reactions Using Visible and Near-Infrared Lights. Org. Lett. 2017, 19, 2622–2625. 10.1021/acs.orglett.7b00957. [DOI] [PubMed] [Google Scholar]
- Balaiah V.; Seshadri T. R.; Venkateswarlu V. Visible Fluorescence and Chemical Constitution of Compounds of the Benzopyrone Group. Proc. - Indian Acad. Sci., Sect. A 1942, 16, 68. 10.1007/BF03177739. [DOI] [Google Scholar]
- Drexhage K. H.Structure and Properties of Laser Dyes; In Dye Lasers; Schäfer F. P., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 1990. [Google Scholar]
- Kuznetsova N. y. A.; Kaliya O. L. The Photochemistry of Coumarins. Russ. Chem. Rev. 1992, 61, 683–696. 10.1070/RC1992v061n07ABEH000992. [DOI] [Google Scholar]
- Griffiths J.; Millar V.; Bahra G. S. The Influence of Chain Length and Electron Acceptor Residues in 3-Substituted 7-N,N-Diethylaminocoumarin Dyes. Dyes Pigm. 1995, 28, 327–339. 10.1016/0143-7208(95)00024-A. [DOI] [Google Scholar]
- Schiedel M.-S.; Briehn C. A.; Bäuerle P. Single-Compound Libraries of Organic Materials: Parallel Synthesis and Screening of Fluorescent Dyes. Angew. Chem., Int. Ed. 2001, 40, 4677–4680. . [DOI] [PubMed] [Google Scholar]
- Schill H.; Nizamov S.; Bottanelli F.; Bierwagen J.; Belov V. N.; Hell S. W. 4-Trifluoromethyl-Substituted Coumarins with Large Stokes Shifts: Synthesis, Bioconjugates, and Their Use in Super-Resolution Fluorescence Microscopy. Chem. - Eur. J. 2013, 19, 16556–16565. 10.1002/chem.201302037. [DOI] [PubMed] [Google Scholar]
- Tang X.-J.; Wu Y.; Zhao R.; Kou X.; Dong Z.; Zhou W.; Zhang Z.; Tan W.; Fang X. Photorelease of Pyridines Using a Metal-Free Photoremovable Protecting Group. Angew. Chem., Int. Ed. 2020, 59, 18386. 10.1002/anie.202005310. [DOI] [PubMed] [Google Scholar]
- Amatrudo J. M.; Olson J. P.; Lur G.; Chiu C. Q.; Higley M. J.; Ellis-Davies G. C. R. Wavelength-Selective One- and Two-Photon Uncaging of GABA. ACS Chem. Neurosci. 2014, 5, 64–70. 10.1021/cn400185r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal H. K.; Zhai S.; Surmeier D. J.; Ellis-Davies G. C. R. Intracellular Uncaging of cGMP with Blue Light. ACS Chem. Neurosci. 2017, 8, 2139–2144. 10.1021/acschemneuro.7b00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passlick S.; Kramer P. F.; Richers M. T.; Williams J. T.; Ellis-Davies G. C. R. Two-Color, One-Photon Uncaging of Glutamate and GABA. PLoS One 2017, 12, e0187732 10.1371/journal.pone.0187732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richers M. T.; Amatrudo J. M.; Olson J. P.; Ellis-Davies G. C. R. Cloaked Caged Compounds: Chemical Probes for Two-Photon Optoneurobiology. Angew. Chem., Int. Ed. 2017, 56, 193–197. 10.1002/anie.201609269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M.; Hayama T.; Kasai H.; Ellis-Davies G. C. R. Two-Photon Uncaging of γ-Aminobutyric Acid in Intact Brain Tissue. Nat. Chem. Biol. 2010, 6, 255–257. 10.1038/nchembio.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiblot J.; Yu Q.; Sabbadini M. D. B.; Reymond L.; Xue L.; Schena A.; Sallin O.; Hill N.; Griss R.; Johnsson K. Luciferases with Tunable Emission Wavelengths. Angew. Chem., Int. Ed. 2017, 56, 14556–14560. 10.1002/anie.201708277. [DOI] [PubMed] [Google Scholar]
- Chang D.; Lindberg E.; Feng S.; Angerani S.; Riezman H.; Winssinger N. Luciferase-Induced Photouncaging: Bioluminolysis. Angew. Chem., Int. Ed. 2019, 58, 16033–16037. 10.1002/anie.201907734. [DOI] [PubMed] [Google Scholar]
- Belfield K. D.; Morales A. R.; Kang B.-S.; Hales J. M.; Hagan D. J.; Van Stryland E. W.; Chapela V. M.; Percino J. Synthesis, Characterization, and Optical Properties of New Two-Photon-Absorbing Fluorene Derivatives. Chem. Mater. 2004, 16, 4634–4641. 10.1021/cm049872g. [DOI] [Google Scholar]
- Preat J.; Jacquemin D.; Perpète E. A. Theoretical Investigations of the UV Spectra of Coumarin Derivatives. Chem. Phys. Lett. 2005, 415, 20–24. 10.1016/j.cplett.2005.08.135. [DOI] [Google Scholar]
- Tasior M.; Kim D.; Singha S.; Krzeszewski M.; Ahn K. H.; Gryko D. T. π-Expanded Coumarins: Synthesis, Optical Properties and Applications. J. Mater. Chem. C 2015, 3, 1421–1446. 10.1039/C4TC02665A. [DOI] [Google Scholar]
- Piloto A. M.; Rovira D.; Costa S. P. G.; Gonçalves M. S. T. Oxobenzo[f]benzopyrans as New Fluorescent Photolabile Protecting Groups for the Carboxylic Function. Tetrahedron 2006, 62, 11955–11962. 10.1016/j.tet.2006.09.085. [DOI] [Google Scholar]
- Fernandes M. J. G.; Costa S. P. G.; Gonçalves M. S. T. Phototriggering of Neuroactive Amino Acids From 5,6-Benzocoumarinyl Conjugates. Tetrahedron 2011, 67, 2422–2426. 10.1016/j.tet.2011.01.090. [DOI] [Google Scholar]
- Fernandes M. J. G.; Gonçalves M. S. T.; Costa S. P. G. Neurotransmitter Amino Acid—Oxobenzo[f]benzopyran Conjugates: Synthesis and Photorelease Studies. Tetrahedron 2008, 64, 11175–11179. 10.1016/j.tet.2008.09.050. [DOI] [Google Scholar]
- Soares A. M. S.; Costa S. P. G.; Gonçalves M. S. T. 2-Oxo-2H-Benzo[h]benzopyran as a New Light Sensitive Protecting Group for Neurotransmitter Amino Acids. Amino Acids 2010, 39, 121–133. 10.1007/s00726-009-0383-z. [DOI] [PubMed] [Google Scholar]
- Soares A. M. S.; Hungerford G.; Costa S. P. G.; Gonçalves M. S. T. Photoactivation of Butyric Acid from 6-Aminobenzocoumarin Cages. Eur. J. Org. Chem. 2015, 2015, 5979–5986. 10.1002/ejoc.201500396. [DOI] [Google Scholar]
- Piloto A. M.; Soares A. M. S.; Hungerford G.; Costa S. P. G.; Gonçalves M. S. T. Long-Wavelength Photolysis of Amino Acid 6-(Methoxy-2-oxo-2H-naphtho[1,2-b]pyran-4-yl)methyl Esters. Eur. J. Org. Chem. 2011, 2011, 5447–5451. 10.1002/ejoc.201100391. [DOI] [Google Scholar]
- Soares A. M. S.; Hungerford G.; Gonçalves M. S. T.; Costa S. P. G. Light Triggering of 5-Aminolevulinic Acid From Fused Coumarin Ester Cages. New J. Chem. 2017, 41, 2997–3005. 10.1039/C6NJ03787A. [DOI] [Google Scholar]
- Soares A. M. S.; Hungerford G.; Costa S. P. G.; Gonçalves M. S. T. Aminobenzocoumarinylmethyl Esters as Photoactive Precursors for the Release of Butyric Acid. New J. Chem. 2015, 39, 7227–7233. 10.1039/C5NJ00699F. [DOI] [Google Scholar]
- Sakamoto Y.; Boinapally S.; Katan C.; Abe M. Synthesis and Photochemical Reactivity of Caged Glutamates with a π-Extended Coumarin Chromophore as a Photolabile Protecting Group. Tetrahedron Lett. 2013, 54, 7171–7174. 10.1016/j.tetlet.2013.10.107. [DOI] [Google Scholar]
- Soares A. M. S.; Hungerford G.; Costa S. P. G.; Gonçalves M. S. T. Photoactivatable Prodrugs of Butyric Acid Based on New Coumarin Fused Oxazole Heterocycles. Dyes Pigm. 2017, 137, 91–100. 10.1016/j.dyepig.2016.10.001. [DOI] [Google Scholar]
- Yu J.; Shirota Y. A New Class of High-Performance Red-Fluorescent Dyes for Organic Electroluminescent Devices, [7-Diethylamino-3-(2-thienyl)chromen-2-ylidene]-2,2-dicyanovinylamine and {10-(2-Thienyl)-2,3,6,7-tetrahydro-1H,5H-chromeno[8,7,6-ij]quinolizin-11-ylidene}-2,2-dicyanovinylamine. Chem. Lett. 2002, 31, 984–985. 10.1246/cl.2002.984. [DOI] [Google Scholar]
- Tkach I. I.; Reznichenko A. V.; Luk’yanets E. A. Reaction of 4-Diethylaminosalicylaldehyde with Malononitrile. Chem. Heterocycl. Compd. 1992, 28, 872–880. 10.1007/BF00531317. [DOI] [Google Scholar]
- Gandioso A.; Bresolí-Obach R.; Nin-Hill A.; Bosch M.; Palau M.; Galindo A.; Contreras S.; Rovira A.; Rovira C.; Nonell S.; Marchán V. Redesigning the Coumarin Scaffold into Small Bright Fluorophores with Far-Red to Near-Infrared Emission and Large Stokes Shifts Useful for Cell Imaging. J. Org. Chem. 2018, 83, 1185–1195. 10.1021/acs.joc.7b02660. [DOI] [PubMed] [Google Scholar]
- Kirpichenok M. A.; Gorozhankin S. K.; Grandberg I. I. Reactions of 7-Aminocoumarins Leading to Alkylidenebenzopyrans. Chem. Heterocycl. Compd. 1988, 24, 611–616. 10.1007/BF00475593. [DOI] [Google Scholar]
- Maciejewski A.; Steer R. P. The Photophysics, Physical Photochemistry, and Related Spectroscopy of Thiocarbonyls. Chem. Rev. 1993, 93, 67–98. 10.1021/cr00017a005. [DOI] [Google Scholar]
- Steer R. P.; Ramamurthy V. Photophysics and Intramolecular Photochemistry of Thiones in Solution. Acc. Chem. Res. 1988, 21, 380–386. 10.1021/ar00154a005. [DOI] [Google Scholar]
- Becker R. S.; Chakravorti S.; Gartner C. A.; de Graca Miguel M. Photosensitizers: Comprehensive Photophysics/Photochemistry and Theory of Coumarins, Chromones, Their Homologues and Thione Analogues. J. Chem. Soc., Faraday Trans. 1993, 89, 1007–1019. 10.1039/ft9938901007. [DOI] [Google Scholar]
- Bhattacharyya K.; Das P. K.; Ramamurthy V.; Rao V. P. Triplet-State Photophysics and Transient Photochemistry of Cyclic Enethiones. A Laser Flash Photolysis Study. J. Chem. Soc., Faraday Trans. 2 1986, 82, 135–147. 10.1039/f29868200135. [DOI] [Google Scholar]
- Burdzinski G.; Buntinx G.; Poizat O.; Lapouge C. Time-Resolved Resonance Raman Investigation and Ab Initio Calculations of the T1-State Structure of Thiocoumarin. J. Mol. Struct. 2005, 735-736, 115–122. 10.1016/j.molstruc.2004.10.079. [DOI] [Google Scholar]
- Devanathan S.; Ramamurthy V. Photochemistry of α,β-Unsaturated Thiones: Cycloaddition of Thiocoumarin to Electron-Rich and Electron-Deficient Olefins From T1. J. Org. Chem. 1988, 53, 741–744. 10.1021/jo00239a007. [DOI] [Google Scholar]
- Fonseca A. S. C.; Gonçalves M. S. T.; Costa S. P. G. Phenacyl Ester Derivatives Bearing Heterocycles as Models for Photocleavable Linkers: Synthesis and Photolysis Studies. Tetrahedron 2012, 68, 8024–8032. 10.1016/j.tet.2012.06.100. [DOI] [Google Scholar]
- Chen Z.; Sun W.; Butt H.-J.; Wu S. Upconverting-Nanoparticle-Assisted Photochemistry Induced by Low-Intensity Near-Infrared Light: How Low Can We Go?. Chem. - Eur. J. 2015, 21, 9165–9170. 10.1002/chem.201500108. [DOI] [PubMed] [Google Scholar]
- Sinha D. K.; Neveu P.; Gagey N.; Aujard I.; Le Saux T.; Rampon C.; Gauron C.; Kawakami K.; Leucht C.; Bally-Cuif L.; Volovitch M.; Bensimon D.; Jullien L.; Vriz S. Photoactivation of the CreERT2 Recombinase for Conditional Site-Specific Recombination with High Spatiotemporal Resolution. Zebrafish 2010, 7, 199–204. 10.1089/zeb.2009.0632. [DOI] [PubMed] [Google Scholar]
- Fournier L.; Gauron C.; Xu L.; Aujard I.; Le Saux T.; Gagey-Eilstein N.; Maurin S.; Dubruille S.; Baudin J.-B.; Bensimon D.; Volovitch M.; Vriz S.; Jullien L. A Blue-Absorbing Photolabile Protecting Group for in Vivo Chromatically Orthogonal Photoactivation. ACS Chem. Biol. 2013, 8, 1528–1536. 10.1021/cb400178m. [DOI] [PubMed] [Google Scholar]
- Manna D.; Maji B.; Gangopadhyay S. A.; Cox K. J.; Zhou Q.; Law B. K.; Mazitschek R.; Choudhary A. A Singular System with Precise Dosing and Spatiotemporal Control of CRISPR-Cas9. Angew. Chem., Int. Ed. 2019, 58, 6285–6289. 10.1002/anie.201900788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piloto A. M.; Soares A. M. S.; Costa S. P. G.; Gonçalves M. S. T. Photorelease of Amino Acids From Novel Thioxobenzo[f]benzopyran Ester Conjugates. Amino Acids 2012, 42, 2275–2282. 10.1007/s00726-011-0969-0. [DOI] [PubMed] [Google Scholar]
- Piloto A. M.; Hungerford G.; Sutter J. U.; Soares A. M. S.; Costa S. P. G.; Gonçalves M. S. T. Photoactivable Heterocyclic Cages in a Comparative Release Study of Butyric Acid as a Model Drug. J. Photochem. Photobiol., A 2015, 299, 44–53. 10.1016/j.jphotochem.2014.10.016. [DOI] [Google Scholar]
- Gandioso A.; Cano M.; Massaguer A.; Marchán V. A Green Light-Triggerable RGD Peptide for Photocontrolled Targeted Drug Delivery: Synthesis and Photolysis Studies. J. Org. Chem. 2016, 81, 11556–11564. 10.1021/acs.joc.6b02415. [DOI] [PubMed] [Google Scholar]
- Gandioso A.; Contreras S.; Melnyk I.; Oliva J.; Nonell S.; Velasco D.; García-Amorós J.; Marchán V. Development of Green/Red-Absorbing Chromophores Based on a Coumarin Scaffold That Are Useful as Caging Groups. J. Org. Chem. 2017, 82, 5398–5408. 10.1021/acs.joc.7b00788. [DOI] [PubMed] [Google Scholar]
- Deiters A.; Garner R. A.; Lusic H.; Govan J. M.; Dush M.; Nascone-Yoder N. M.; Yoder J. A. Photocaged Morpholino Oligomers for the Light-Regulation of Gene Function in Zebrafish and Xenopus Embryos. J. Am. Chem. Soc. 2010, 132, 15644–15650. 10.1021/ja1053863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang X.; Shestopalov I. A.; Sinha S.; Zheng G.; Pitt C. L. W.; Li W.-H.; Olson A. J.; Chen J. K. Versatile Synthesis and Rational Design of Caged Morpholinos. J. Am. Chem. Soc. 2009, 131, 13255–13269. 10.1021/ja809933h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallafuss A.; Gibson D.; Morcos P.; Li Y.; Seredick S.; Eisen J.; Washbourne P. Turning Gene Function ON and OFF Using Sense and Antisense Photo-Morpholinos in Zebrafish. Development 2012, 139, 1691–1699. 10.1242/dev.072702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazoe S.; Shestopalov I. A.; Provost E.; Leach S. D.; Chen J. K. Cyclic Caged Morpholinos: Conformationally Gated Probes of Embryonic Gene Function. Angew. Chem., Int. Ed. 2012, 51, 6908–6911. 10.1002/anie.201201690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H.; Tokumaru K. Photolysis of Aromatic Oxime Esters. Finding of Aromatic Substitution by Diphenylmethyleneimino Radicals. Bull. Chem. Soc. Jpn. 1975, 48, 2393–2394. 10.1246/bcsj.48.2393. [DOI] [Google Scholar]
- Qiu W.; Li M.; Yang Y.; Li Z.; Dietliker K. Cleavable Coumarin-Based Oxime Esters with Terminal Heterocyclic Moieties: Photobleachable Initiators for Deep Photocuring Under Visible LED Light Irradiation. Polym. Chem. 2020, 11, 1356–1363. 10.1039/C9PY01690B. [DOI] [Google Scholar]
- Hwu J. R.; Tsay S.-C.; Hong S. C.; Leu Y.-J.; Liu C.-F.; Chou S.-S. P. Oxime Esters of Anthraquinone as Photo-Induced DNA-Cleaving Agents for Single- and Double-Strand Scissions. Tetrahedron Lett. 2003, 44, 2957–2960. 10.1016/S0040-4039(03)00375-7. [DOI] [Google Scholar]
- Hwu J. R.; Yang J.-R.; Tsay S.-C.; Hsu M.-H.; Chen Y.-C.; Chou S.-S. P. Photo-Induced DNA Cleavage by (Heterocyclo)carbonyl Oxime Esters of Anthraquinone. Tetrahedron Lett. 2008, 49, 3312–3315. 10.1016/j.tetlet.2008.03.056. [DOI] [Google Scholar]
- Fast D. E.; Lauer A.; Menzel J. P.; Kelterer A.-M.; Gescheidt G.; Barner-Kowollik C. Wavelength-Dependent Photochemistry of Oxime Ester Photoinitiators. Macromolecules 2017, 50, 1815–1823. 10.1021/acs.macromol.7b00089. [DOI] [Google Scholar]
- Singh A. K.; Khade P. K. Anthracene-9-methanol—A Novel Fluorescent Phototrigger for Biomolecular Caging. Tetrahedron Lett. 2005, 46, 5563–5566. 10.1016/j.tetlet.2005.06.026. [DOI] [Google Scholar]
- Boháčová S.; Ludvíková L.; Poštová Slavĕtínská L.; Vaníková Z.; Klán P.; Hocek M. Protected 5-(Hydroxymethyl)uracil Nucleotides Bearing Visible-Light Photocleavable Groups as Building Blocks for Polymerase Synthesis of Photocaged DNA. Org. Biomol. Chem. 2018, 16, 1527–1535. 10.1039/C8OB00160J. [DOI] [PubMed] [Google Scholar]
- Vittorino E.; Cicciarella E.; Sortino S. A “Dual-Function” Photocage Releasing Nitric Oxide and an Anthrylmethyl Cation with a Single Wavelength Light. Chem. - Eur. J. 2009, 15, 6802–6806. 10.1002/chem.200901037. [DOI] [PubMed] [Google Scholar]
- Hu P.; Berning K.; Lam Y.-W.; Ng I. H.-M.; Yeung C.-C.; Lam M. H.-W. Development of a Visible Light Triggerable Traceless Staudinger Ligation Reagent. J. Org. Chem. 2018, 83, 12998–13010. 10.1021/acs.joc.8b01370. [DOI] [PubMed] [Google Scholar]
- Nikitin K.; Müller-Bunz H.; Ortin Y.; Muldoon J.; McGlinchey M. J. Restricted Rotation in 9-Phenyl-anthracenes: A Prediction Fulfilled. Org. Lett. 2011, 13, 256–259. 10.1021/ol102665y. [DOI] [PubMed] [Google Scholar]
- Nazir R.; Thorsted B.; Balčiunas E.; Mazur L.; Deperasińska I.; Samoć M.; Brewer J.; Farsari M.; Gryko D. T. π-Expanded 1,3-Diketones – Synthesis, Optical Properties and Application in Two-Photon Polymerization. J. Mater. Chem. C 2016, 4, 167–177. 10.1039/C5TC03334A. [DOI] [Google Scholar]
- Shah L.; Laughlin S. T.; Carrico I. S. Light-Activated Staudinger–Bertozzi Ligation within Living Animals. J. Am. Chem. Soc. 2016, 138, 5186–5189. 10.1021/jacs.5b13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P.; Feng T.; Yeung C.-C.; Koo C.-K.; Lau K.-C.; Lam M. H. W. A Photo-Triggered Traceless Staudinger–Bertozzi Ligation Reaction. Chem. - Eur. J. 2016, 22, 11537–11542. 10.1002/chem.201601807. [DOI] [PubMed] [Google Scholar]
- Saxon E.; Armstrong J. I.; Bertozzi C. R. A “Traceless” Staudinger Ligation for the Chemoselective Synthesis of Amide Bonds. Org. Lett. 2000, 2, 2141–2143. 10.1021/ol006054v. [DOI] [PubMed] [Google Scholar]
- Zhuang H.-B.; Tang W.-J.; Yu J.-Y.; Song Q.-H. Acridin-9-ylmethoxycarbonyl (Amoc): A New Photochemically Removable Protecting Group for Alcohols. Chin. J. Chem. 2006, 24, 1465–1468. 10.1002/cjoc.200690276. [DOI] [Google Scholar]
- Jana A.; Saha B.; Karthik S.; Barman S.; Ikbal M.; Ghosh S. K.; Pradeep Singh N. D. Fluorescent Photoremovable Precursor (Acridin-9-ylmethyl)ester: Synthesis, Photophysical, Photochemical and Biological Applications. Photochem. Photobiol. Sci. 2013, 12, 1041–1052. 10.1039/c3pp25362g. [DOI] [PubMed] [Google Scholar]
- Piloto A. M.; Hungerford G.; Costa S. P. G.; Gonçalves M. S. T. Acridinyl Methyl Esters as Photoactive Precursors in the Release of Neurotransmitteramino Acids. Photochem. Photobiol. Sci. 2013, 12, 339–347. 10.1039/C2PP25261A. [DOI] [PubMed] [Google Scholar]
- Ikbal M.; Saha B.; Barman S.; Atta S.; Banerjee D. R.; Ghosh S. K.; Singh N. D. P. Benzo[a]Acridinylmethyl Esters as pH Sensitive Fluorescent Photoactive Precursors: Synthesis, Photophysical, Photochemical and Biological Applications. Org. Biomol. Chem. 2014, 12, 3459–3469. 10.1039/c3ob42600a. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J.; Darzynkiewicz Z. Interactions of Acridine Orange with Double Stranded Nucleic Acids. Spectral and Affinity Studies. J. Biomol. Struct. Dyn. 1987, 5, 127–143. 10.1080/07391102.1987.10506381. [DOI] [PubMed] [Google Scholar]
- Sayed M.; Krishnamurthy B.; Pal H. Unraveling Multiple Binding Modes of Acridine Orange to DNA Using a Multispectroscopic Approach. Phys. Chem. Chem. Phys. 2016, 18, 24642–24653. 10.1039/C6CP03716J. [DOI] [PubMed] [Google Scholar]
- von Tscharner V.; Schwarz G. Complex Formation of Acridine Orange with Single-Stranded Polyriboadenylic Acid and 5′-AMP: Cooperative Binding and Intercalation Between Bases. Biophys. Struct. Mech. 1979, 5, 75–90. 10.1007/BF00535774. [DOI] [PubMed] [Google Scholar]
- Jana A.; Atta S.; Sarkar S. K.; Singh N. D. P. 1-Acetylpyrene with Dual Functions as an Environment-Sensitive Fluorophore and Fluorescent Photoremovable Protecting Group. Tetrahedron 2010, 66, 9798–9807. 10.1016/j.tet.2010.10.090. [DOI] [Google Scholar]
- Furuta T.; Torigai H.; Osawa T.; Iwamura M. New Photochemically Labile Protecting Group for Phosphates. Chem. Lett. 1993, 22, 1179–1182. 10.1246/cl.1993.1179. [DOI] [Google Scholar]
- Fernandes M. J. G.; Gonçalves M. S. T.; Costa S. P. G. Photorelease of Amino Acid Neurotransmitters From Pyrenylmethyl Ester Conjugates. Tetrahedron 2007, 63, 10133–10139. 10.1016/j.tet.2007.07.107. [DOI] [Google Scholar]
- Iwamura M.; Hodota C.; Ishibashi M. 1-(α-Diazobenzyl)pyrene: A Reagent for Photolabile and Fluorescent Protection of Carboxyl Groups of Amino Acids and Peptides. Synlett 1991, 1991, 35–36. 10.1055/s-1991-20619. [DOI] [Google Scholar]
- Okada S.; Yamashita S.; Furuta T.; Iwamura M. (1-Pyrenyl)methyl Carbamates for Fluorescent “Caged” Amino Acids and Peptides. Photochem. Photobiol. 1995, 61, 431–434. 10.1111/j.1751-1097.1995.tb02340.x. [DOI] [Google Scholar]
- Pukenas L.; Prompinit P.; Nishitha B.; Tate D. J.; Singh N. D. P.; Wälti C.; Evans S. D.; Bushby R. J. Soft Ultraviolet (UV) Photopatterning and Metallization of Self-Assembled Monolayers (SAMs) Formed from the Lipoic Acid Ester of α-Hydroxy-1-acetylpyrene: The Generality of Acid-Catalyzed Removal of Thiol-on-Gold SAMs using Soft UV Light. ACS Appl. Mater. Interfaces 2017, 9, 18388–18397. 10.1021/acsami.7b04708. [DOI] [PubMed] [Google Scholar]
- Jana A.; Saha B.; Ikbal M.; Ghosh S. K.; Singh N. D. P. 1-(Hydroxyacetyl)pyrene a New Fluorescent Phototrigger for Cell Imaging and Caging of Alcohols, Phenol and Adenosine. Photochem. Photobiol. Sci. 2012, 11, 1558–1566. 10.1039/c2pp25091h. [DOI] [PubMed] [Google Scholar]
- Armbruster C.; Knapp M.; Rechthaler K.; Schamschule R.; Parusel A. B. J.; Köhler G.; Wehrmann W. Fluorescence Properties of 1-Heptanoylpyrene: A Probe for Hydrogen Bonding in Microaggregates and Biological Membranes. J. Photochem. Photobiol., A 1999, 125, 29–38. 10.1016/S1010-6030(99)00099-4. [DOI] [Google Scholar]
- Jana A.; Ikbal M.; Singh N. D. P. Perylen-3-ylmethyl: Fluorescent Photoremovable Protecting Group (FPRPG) for Carboxylic Acids and Alcohols. Tetrahedron 2012, 68, 1128–1136. 10.1016/j.tet.2011.11.074. [DOI] [Google Scholar]
- Zimmerman H. E. The Meta Effect in Organic Photochemistry: Mechanistic and Exploratory Organic Photochemistry. J. Am. Chem. Soc. 1995, 117, 8988–8991. 10.1021/ja00140a014. [DOI] [Google Scholar]
- Zimmerman H. E. Meta-Ortho Effect in Organic Photochemistry: Mechanistic and Exploratory Organic Photochemistry. J. Phys. Chem. A 1998, 102, 5616–5621. 10.1021/jp9803182. [DOI] [Google Scholar]
- Zimmerman H. E.; Sandel V. R. Mechanistic Organic Photochemistry. II. Solvolytic Photochemical Reactions. J. Am. Chem. Soc. 1963, 85, 915–922. 10.1021/ja00890a019. [DOI] [Google Scholar]
- Pincock J. A. Photochemistry of Arylmethyl Esters in Nucleophilic Solvents: Radical Pair and Ion Pair Intermediates. Acc. Chem. Res. 1997, 30, 43–49. 10.1021/ar960177r. [DOI] [Google Scholar]
- Jana A.; Nguyen K. T.; Li X.; Zhu P.; Tan N. S.; Ågren H.; Zhao Y. Perylene-Derived Single-Component Organic Nanoparticles with Tunable Emission: Efficient Anticancer Drug Carriers with Real-Time Monitoring of Drug Release. ACS Nano 2014, 8, 5939–5952. 10.1021/nn501073x. [DOI] [PubMed] [Google Scholar]
- Truong V. X.; Li F.; Forsythe J. S. Photolabile Hydrogels Responsive to Broad Spectrum Visible Light for Selective Cell Release. ACS Appl. Mater. Interfaces 2017, 9, 32441–32445. 10.1021/acsami.7b11517. [DOI] [PubMed] [Google Scholar]
- Truong V. X. Break Up to Make Up: Utilization of Photocleavable Groups in Biolabeling of Hydrogel Scaffolds. ChemPhotoChem. 2020, 4, 564–570. 10.1002/cptc.202000072. [DOI] [Google Scholar]
- Dong J.; Zhang R.; Wu H.; Zhan X.; Yang H.; Zhu S.; Wang G. Polymer Nanoparticles for Controlled Release Stimulated by Visible Light and pH. Macromol. Rapid Commun. 2014, 35, 1255–1259. 10.1002/marc.201400078. [DOI] [PubMed] [Google Scholar]
- Wang G.; Dong J.; Yuan T.; Zhang J.; Wang L.; Wang H. Visible Light and pH Responsive Polymer-Coated Mesoporous Silica Nanohybrids for Controlled Release. Macromol. Biosci. 2016, 16, 990–994. 10.1002/mabi.201600008. [DOI] [PubMed] [Google Scholar]
- Liu G.; Wang X.; Hu J.; Zhang G.; Liu S. Self-Immolative Polymersomes for High-Efficiency Triggered Release and Programmed Enzymatic Reactions. J. Am. Chem. Soc. 2014, 136, 7492–7497. 10.1021/ja5030832. [DOI] [PubMed] [Google Scholar]
- Kasai H.; Oikawa H.; Okada S.; Nakanishi H. Crystal Growth of Perylene Microcrystals in the Reprecipitation Method. Bull. Chem. Soc. Jpn. 1998, 71, 2597–2601. 10.1246/bcsj.71.2597. [DOI] [Google Scholar]
- Jana A.; Devi K. S. P.; Maiti T. K.; Singh N. D. P. Perylene-3-ylmethanol: Fluorescent Organic Nanoparticles as a Single-Component Photoresponsive Nanocarrier with Real-Time Monitoring of Anticancer Drug Release. J. Am. Chem. Soc. 2012, 134, 7656–7659. 10.1021/ja302482k. [DOI] [PubMed] [Google Scholar]
- Atta S.; Bera M.; Chattopadhyay T.; Paul A.; Ikbal M.; Maiti M. K.; Singh N. D. P. Nano-Pesticide Formulation Based on Fluorescent Organic Photoresponsive Nanoparticles: For Controlled Release of 2,4-D and Real Time Monitoring of Morphological Changes Induced by 2,4-D in Plant Systems. RSC Adv. 2015, 5, 86990–86996. 10.1039/C5RA17121K. [DOI] [Google Scholar]
- Barman S.; Mukhopadhyay S. K.; Behara K. K.; Dey S.; Singh N. D. P. 1-Acetylpyrene–Salicylic Acid: Photoresponsive Fluorescent Organic Nanoparticles for the Regulated Release of a Natural Antimicrobial Compound, Salicylic Acid. ACS Appl. Mater. Interfaces 2014, 6, 7045–7054. 10.1021/am500965n. [DOI] [PubMed] [Google Scholar]
- Norris S.; Warner C. C.; Thooft A. M.; Demirci S. K.; Lampkin B. J.; Miner K.; Ellern A.; VanVeller B. Blue-Light Photocleavable Protecting Groups Based on Benzothiadiazole Scaffolds. Org. Lett. 2020, 22, 270–273. 10.1021/acs.orglett.9b04268. [DOI] [PubMed] [Google Scholar]
- Perrotta R. R.; Winter A. H.; Falvey D. E. Photochemical Heterolysis of 3,5-Bis(dimethylamino)benzyl Alcohols and Esters: Generation of a Benzyl Cation with a Low-Energy Triplet State. Org. Lett. 2011, 13, 212–215. 10.1021/ol102606m. [DOI] [PubMed] [Google Scholar]
- Škalamera Đ.; Blažek Bregović V.; Antol I.; Bohne C.; Basarić N. Hydroxymethylaniline Photocages for Carboxylic Acids and Alcohols. J. Org. Chem. 2017, 82, 12554–12568. 10.1021/acs.joc.7b02314. [DOI] [PubMed] [Google Scholar]
- Winter A. H.; Falvey D. E.; Cramer C. J.; Gherman B. F. Benzylic Cations with Triplet Ground States: Computational Studies of Aryl Carbenium Ions, Silylenium Ions, Nitrenium Ions, and Oxenium Ions Substituted with Meta π Donors. J. Am. Chem. Soc. 2007, 129, 10113–10119. 10.1021/ja070143m. [DOI] [PubMed] [Google Scholar]
- Reinfelds M.; von Cosel J.; Falahati K.; Hamerla C.; Slanina T.; Burghardt I.; Heckel A. A New Photocage Derived from Fluorene. Chem. - Eur. J. 2018, 24, 13026–13035. 10.1002/chem.201802390. [DOI] [PubMed] [Google Scholar]
- Bera M.; Maji S.; Paul A.; Ray S.; Maiti T. K.; Singh N. D. P. A Water Soluble Light Activated Hydrogen Sulfide Donor Induced by an Excited State meta Effect. Org. Biomol. Chem. 2019, 17, 9059–9064. 10.1039/C9OB01502G. [DOI] [PubMed] [Google Scholar]
- Wang P.; Lu W.; Devalankar D.; Ding Z. Photochemical Formation and Cleavage of C–N Bond. Org. Lett. 2015, 17, 170–172. 10.1021/ol503473c. [DOI] [PubMed] [Google Scholar]
- Wang P.; Lu W.; Devalankar D. A.; Ding Z. Structurally Simple Benzyl-Type Photolabile Protecting Groups for Direct Release of Alcohols and Carboxylic Acids. Org. Lett. 2015, 17, 2114–2117. 10.1021/acs.orglett.5b00699. [DOI] [PubMed] [Google Scholar]
- Wang P.; Devalankar D. A.; Lu W. Photochemical Cleavage of Benzylic C–N Bond To Release Amines. J. Org. Chem. 2016, 81, 6195–6200. 10.1021/acs.joc.6b00508. [DOI] [PubMed] [Google Scholar]
- Freccero M.; Fagnoni M.; Albini A. Homolytic vs Heterolytic Paths in the Photochemistry of Haloanilines. J. Am. Chem. Soc. 2003, 125, 13182–13190. 10.1021/ja036000r. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Pavlos C. M.; Toscano J. P.; Dore T. M. 8-Bromo-7-hydroxyquinoline as a Photoremovable Protecting Group for Physiological Use: Mechanism and Scope. J. Am. Chem. Soc. 2006, 128, 4267–4276. 10.1021/ja0555320. [DOI] [PubMed] [Google Scholar]
- Davis M. J.; Kragor C. H.; Reddie K. G.; Wilson H. C.; Zhu Y.; Dore T. M. Substituent Effects on the Sensitivity of a Quinoline Photoremovable Protecting Group to One- and Two-Photon Excitation. J. Org. Chem. 2009, 74, 1721–1729. 10.1021/jo802658a. [DOI] [PubMed] [Google Scholar]
- Asad N.; Deodato D.; Lan X.; Widegren M. B.; Phillips D. L.; Du L.; Dore T. M. Photochemical Activation of Tertiary Amines for Applications in Studying Cell Physiology. J. Am. Chem. Soc. 2017, 139, 12591–12600. 10.1021/jacs.7b06363. [DOI] [PubMed] [Google Scholar]
- Deodato D.; Asad N.; Dore T. M. Photorearrangement of Quinoline-Protected Dialkylanilines and the Photorelease of Aniline-Containing Biological Effectors. J. Org. Chem. 2019, 84, 7342–7353. 10.1021/acs.joc.9b01031. [DOI] [PubMed] [Google Scholar]
- Huang J.; Muliawan A. P.; Ma J.; Li M. D.; Chiu H. K.; Lan X.; Deodato D.; Phillips D. L.; Dore T. M. A Spectroscopic Study of the Excited State Proton Transfer Processes of (8-Bromo-7-hydroxyquinolin-2-yl)methyl-Protected Phenol in Aqueous Solutions. Photochem. Photobiol. Sci. 2017, 16, 575–584. 10.1039/C6PP00377J. [DOI] [PubMed] [Google Scholar]
- Rea A. C.; Vandenberg L. N.; Ball R. E.; Snouffer A. A.; Hudson A. G.; Zhu Y.; McLain D. E.; Johnston L. L.; Lauderdale J. D.; Levin M.; Dore T. M. Light-Activated Serotonin for Exploring Its Action in Biological Systems. Chem. Biol. 2013, 20, 1536–1546. 10.1016/j.chembiol.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asad N.; McLain D. E.; Condon A. F.; Gore S.; Hampton S. E.; Vijay S.; Williams J. T.; Dore T. M. Photoactivatable Dopamine and Sulpiride to Explore the Function of Dopaminergic Neurons and Circuits. ACS Chem. Neurosci. 2020, 11, 939–951. 10.1021/acschemneuro.9b00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig A.-L. K.; Deodato D.; Asad N.; Herbivo C.; Dore T. M. Two-Photon Excitable Photoremovable Protecting Groups Based on the Quinoline Scaffold for Use in Biology. J. Org. Chem. 2020, 85, 726–744. 10.1021/acs.joc.9b02780. [DOI] [PubMed] [Google Scholar]
- Li Y.-M.; Shi J.; Cai R.; Chen X.-Y.; Guo Q.-X.; Liu L. Development of New Quinoline-Based Photo-Labile Groups for Photo-Regulation of Bioactive Molecules. Tetrahedron Lett. 2010, 51, 1609–1612. 10.1016/j.tetlet.2010.01.071. [DOI] [Google Scholar]
- Bera M.; Maji S.; Paul A.; Sahoo B. K.; Maiti T. K.; Singh N. D. P. Quinoline H2S Donor Decorated Fluorescent Carbon Dots: Visible Light Responsive H2S Nanocarriers. J. Mater. Chem. B 2020, 8, 1026–1032. 10.1039/C9TB02157D. [DOI] [PubMed] [Google Scholar]
- Narumi T.; Miyata K.; Nii A.; Sato K.; Mase N.; Furuta T. 7-Hydroxy-N-Methylquinolinium Chromophore: A Photolabile Protecting Group for Blue-Light Uncaging. Org. Lett. 2018, 20, 4178–4182. 10.1021/acs.orglett.8b01505. [DOI] [PubMed] [Google Scholar]
- Narumi T. Creation and Application of Quinolinium-Type Caged Neurotransmitters Capable of Uncaging with Visible Light. Yakugaku Zasshi 2019, 139, 263–271. 10.1248/yakushi.18-00174-1. [DOI] [PubMed] [Google Scholar]
- Barni E.; Savarino P. Quaternary Salts From 2-(Methylpyridyl or Quinolyl)benzimidazoles and Related Polymethine Dyes. J. Heterocycl. Chem. 1979, 16, 1583–1587. 10.1002/jhet.5570160814. [DOI] [Google Scholar]
- Sutherland D.; Compton C. The Absorption Spectra of some Substituted Quinolines and their Methiodides. J. Org. Chem. 1952, 17, 1257–1261. 10.1021/jo50009a011. [DOI] [Google Scholar]
- Ma J.; Mewes J.-M.; Harris K. T.; Dore T. M.; Phillips D. L.; Dreuw A. Unravelling the Early Photochemical Behavior of (8-Substituted-7-hydroxyquinolinyl)methyl Acetates Through Electronic Structure Theory and Ultrafast Transient Absorption Spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 1089–1096. 10.1039/C6CP05499D. [DOI] [PubMed] [Google Scholar]
- Ma J.; Rea A. C.; An H.; Ma C.; Guan X.; Li M.-D.; Su T.; Yeung C. S.; Harris K. T.; Zhu Y.; Nganga J. L.; Fedoryak O. D.; Dore T. M.; Phillips D. L. Unraveling the Mechanism of the Photodeprotection Reaction of 8-Bromo- and 8-Chloro-7-hydroxyquinoline Caged Acetates. Chem. - Eur. J. 2012, 18, 6854–6865. 10.1002/chem.201200366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler G.; Rechthaler K.; Rotkiewicz K.; Rettig W. Formation and Stabilization of Twisted Intramolecular Charge Transfer States in Binary Mixed Solvents. Chem. Phys. 1996, 207, 85–101. 10.1016/0301-0104(96)00054-7. [DOI] [Google Scholar]
- Kosower N. S.; Kosower E. M.; Newton G. L.; Ranney H. M. Bimane Fluorescent Labels: Labeling of Normal Human Red Cells Under Physiological Conditions. Proc. Natl. Acad. Sci. U. S. A. 1979, 76, 3382–3386. 10.1073/pnas.76.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavis L. D.; Raines R. T. Bright Ideas for Chemical Biology. ACS Chem. Biol. 2008, 3, 142–155. 10.1021/cb700248m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraska J. W.; Puljung M. C.; Zagotta W. N. Short-Distance Probes for Protein Backbone Structure Based on Energy Transfer Between Bimane and Transition Metal Ions. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 16227–16232. 10.1073/pnas.0905207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A.; Venkatesh Y.; Behara K. K.; Singh N. D. Bimane: A Visible Light Induced Fluorescent Photoremovable Protecting Group for the Single and Dual Release of Carboxylic and Amino Acids. Org. Lett. 2017, 19, 1598–1601. 10.1021/acs.orglett.7b00416. [DOI] [PubMed] [Google Scholar]
- Truong V. X.; Li F.; Forsythe J. S. Visible Light Activation of Nucleophilic Thiol-X Addition via Thioether Bimane Photocleavage for Polymer Cross-Linking. Biomacromolecules 2018, 19, 4277–4285. 10.1021/acs.biomac.8b01153. [DOI] [PubMed] [Google Scholar]
- Arumugam S.; Guo J.; Mbua N. E.; Friscourt F.; Lin N.; Nekongo E.; Boons G.-J.; Popik V. V. Selective and Reversible Photochemical Derivatization of Cysteine Residues in Peptides and Proteins. Chem. Sci. 2014, 5, 1591–1598. 10.1039/C3SC51691A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A.; Falvey D. E. Direct Photolysis of Phenacyl Protecting Groups Studied by Laser Flash Photolysis: An Excited State Hydrogen Atom Abstraction Pathway Leads to Formation of Carboxylic Acids and Acetophenone. J. Am. Chem. Soc. 1998, 120, 2965–2966. 10.1021/ja971431t. [DOI] [Google Scholar]
- Literák J.; Dostálová A.; Klán P. Chain Mechanism in the Photocleavage of Phenacyl and Pyridacyl Esters in the Presence of Hydrogen Donors. J. Org. Chem. 2006, 71, 713–723. 10.1021/jo0521551. [DOI] [PubMed] [Google Scholar]
- Sheehan J. C.; Umezawa K. Phenacyl Photosensitive Blocking Groups. J. Org. Chem. 1973, 38, 3771–3774. 10.1021/jo00961a027. [DOI] [Google Scholar]
- Bergmark W. R. Photolysis of α-Chloro-o-methylacetophenones. J. Chem. Soc., Chem. Commun. 1978, 61–62. 10.1039/C39780000061. [DOI] [Google Scholar]
- Haag R.; Wirz J.; Wagner P. J. The Photoenolization of 2-Methylacetophenone and Related Compounds. Helv. Chim. Acta 1977, 60, 2595–2607. 10.1002/hlca.19770600813. [DOI] [Google Scholar]
- Kammari L.; Plíštil L.; Wirz J.; Klán P. 2,5-Dimethylphenacyl Carbamate: A Photoremovable Protecting Group for Amines and Amino Acids. Photochem. Photobiol. Sci. 2007, 6, 50–56. 10.1039/B612233G. [DOI] [PubMed] [Google Scholar]
- Klán P.; Zabadal M.; Heger D. 2, 5-Dimethylphenacyl as a New Photoreleasable Protecting Group for Carboxylic Acids. Org. Lett. 2000, 2, 1569–1571. 10.1021/ol005789x. [DOI] [PubMed] [Google Scholar]
- Literák J.; Wirz J.; Klán P. 2,5-Dimethylphenacyl Carbonates: A Photoremovable Protecting Group for Alcohols and Phenols. Photochem. Photobiol. Sci. 2005, 4, 43–46. 10.1039/B408851D. [DOI] [PubMed] [Google Scholar]
- Zabadal M.; Pelliccioli A. P.; Klán P.; Wirz J. 2,5-Dimethylphenacyl Esters: A Photoremovable Protecting Group for Carboxylic Acids. J. Phys. Chem. A 2001, 105, 10329–10333. 10.1021/jp010220e. [DOI] [Google Scholar]
- Ruzicka R.; Zabadal M.; Klán P. Photolysis of Phenacyl Esters in a Two-Phase System. Synth. Commun. 2002, 32, 2581–2590. 10.1081/SCC-120005943. [DOI] [Google Scholar]
- Anderson J. C.; Reese C. B. A Photo-Induced Rearrangement Involving Aryl Participation. Tetrahedron Lett. 1962, 3, 1–4. 10.1016/S0040-4039(00)62031-2. [DOI] [Google Scholar]
- Givens R. S.; Park C.-H. p-Hydroxyphenacyl ATP1: A New Phototrigger. Tetrahedron Lett. 1996, 37, 6259–6262. 10.1016/0040-4039(96)01390-1. [DOI] [Google Scholar]
- Park C.-H.; Givens R. S. New Photoactivated Protecting Groups. 6. p-Hydroxyphenacyl: A Phototrigger for Chemical and Biochemical Probes. J. Am. Chem. Soc. 1997, 119, 2453–2463. 10.1021/ja9635589. [DOI] [Google Scholar]
- Corrie J. E. T.; Trentham D. R. Synthetic, Mechanistic and Photochemical Studies of Phosphate Esters of Substituted Benzoins. J. Chem. Soc., Perkin Trans. 1 1992, 1, 2409–2417. 10.1039/p19920002409. [DOI] [Google Scholar]
- Sheehan J. C.; Wilson R. M.; Oxford A. W. Photolysis of Methoxy-Substituted Benzoin Esters. Photosensitive Protecting Group for Carboxylic Acids. J. Am. Chem. Soc. 1971, 93, 7222–7228. 10.1021/ja00755a017. [DOI] [Google Scholar]
- Shi Y.; Corrie J. E.; Wan P. Mechanism of 3’,5’-Dimethoxybenzoin Ester Photochemistry: Heterolytic Cleavage Intramolecularly Assisted by the Dimethoxybenzene Ring Is the Primary Photochemical Step. J. Org. Chem. 1997, 62, 8278–8279. 10.1021/jo971121t. [DOI] [PubMed] [Google Scholar]
- Dong Y.; Lam J. W. Y.; Qin A.; Liu J.; Li Z.; Tang B. Z.; Sun J.; Kwok H. S. Aggregation-Induced Emissions of Tetraphenylethene Derivatives and Their Utilities as Chemical Vapor Sensors and in Organic Light-Emitting Diodes. Appl. Phys. Lett. 2007, 91, 011111. 10.1063/1.2753723. [DOI] [Google Scholar]
- Hong Y.; Lam J. W. Y.; Tang B. Z. Aggregation-Induced Emission. Chem. Soc. Rev. 2011, 40, 5361–5388. 10.1039/c1cs15113d. [DOI] [PubMed] [Google Scholar]
- Luo J.; Xie Z.; Lam J. W. Y.; Cheng L.; Chen H.; Qiu C.; Kwok H. S.; Zhan X.; Liu Y.; Zhu D.; Tang B. Z. Aggregation-Induced Emission of 1-Methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. 10.1039/b105159h. [DOI] [PubMed] [Google Scholar]
- Parthiban C.; M P.; Vinod Kumar Reddy L.; Sen D.; Singh N. D. P. Single-Component Fluorescent Organic Nanoparticles with Four-Armed Phototriggers for Chemo-Photodynamic Therapy and Cellular Imaging. ACS Appl. Nano Mater. 2019, 2, 3728–3734. 10.1021/acsanm.9b00630. [DOI] [Google Scholar]
- Hong Y.; Lam J. W. Y.; Tang B. Z. Aggregation-Induced Emission: Phenomenon, Mechanism and Applications. Chem. Commun. 2009, 4332–4353. 10.1039/b904665h. [DOI] [PubMed] [Google Scholar]
- Kammath V. B.; Šolomek T.; Ngoy B. P.; Heger D.; Klán P.; Rubina M.; Givens R. S. A Photo-Favorskii Ring Contraction Reaction: The Effect of Ring Size. J. Org. Chem. 2013, 78, 1718–1729. 10.1021/jo300850a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favorskii A. Favorskii Rearrangement. Russ. J. Gen. Chem. 1894, 26, 590. [Google Scholar]
- Aston J. G.; Newkirk J. D. α-Halo Ketones. IV. The Isomeric α-Chloroketones Derived from 3-Heptanone. J. Am. Chem. Soc. 1951, 73, 3900–3902. 10.1021/ja01152a102. [DOI] [Google Scholar]
- Bordwell F. G.; Scamehorn R. G.; Springer W. R. Favorskii Rearrangements. III. Evidence for an Ionization-π-Participation Mechanism. J. Am. Chem. Soc. 1969, 91, 2087–2093. 10.1021/ja01036a037. [DOI] [Google Scholar]
- Burr J. G.; Dewar M. J. S. The Mechanism of the Favorski Reaction. J. Chem. Soc. 1954, 1201–1203. 10.1039/jr9540001201. [DOI] [Google Scholar]
- Loftfield R. B. On the Mechanism of the Faworskii Rearrangement of α-Halo Ketones. J. Am. Chem. Soc. 1950, 72, 632–633. 10.1021/ja01157a515. [DOI] [Google Scholar]
- Loftfield R. B. The Alkaline Rearrangement of α-Haloketones. II. The Mechanism of the Faworskii Reaction. J. Am. Chem. Soc. 1951, 73, 4707–4714. 10.1021/ja01154a066. [DOI] [Google Scholar]
- Chiang Y.; Kresge A. J.; Zhu Y. Flash Photolytic Generation and Study of p-Quinone Methide in Aqueous Solution. An Estimate of Rate and Equilibrium Constants for Heterolysis of the Carbon-Bromine Bond in p-Hydroxybenzyl Bromide. J. Am. Chem. Soc. 2002, 124, 6349–6356. 10.1021/ja020020w. [DOI] [PubMed] [Google Scholar]
- Givens R. S.; Heger D.; Hellrung B.; Kamdzhilov Y.; Mac M.; Conrad P. G.; Cope E.; Lee J. I.; Mata-Segreda J. F.; Schowen R. L.; Wirz J. The Photo-Favorskii Reaction of p-Hydroxyphenacyl Compounds Is Initiated by Water-Assisted, Adiabatic Extrusion of a Triplet Biradical. J. Am. Chem. Soc. 2008, 130, 3307–3309. 10.1021/ja7109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman S.; Mukhopadhyay S. K.; Biswas S.; Nandi S.; Gangopadhyay M.; Dey S.; Anoop A.; Pradeep Singh N. D. A p-Hydroxyphenacyl–Benzothiazole–Chlorambucil Conjugate as a Real-Time-Monitoring Drug-Delivery System Assisted by Excited-State Intramolecular Proton Transfer. Angew. Chem., Int. Ed. 2016, 55, 4194–4198. 10.1002/anie.201508901. [DOI] [PubMed] [Google Scholar]
- Luo X.; Wu J.; Lv T.; Lai Y.; Zhang H.; Lu J.-J.; Zhang Y.; Huang Z. Synthesis and Evaluation of Novel O2-Derived Diazeniumdiolates as Photochemical and Real-Time Monitoring Nitric Oxide Delivery Agents. Org. Chem. Front. 2017, 4, 2445–2449. 10.1039/C7QO00695K. [DOI] [Google Scholar]
- Parthiban C.; M P.; L V. K. R.; Sen D.; S M. S.; Singh N. D. P. Visible-Light -Triggered Fluorescent Organic Nanoparticles for Chemo-Photodynamic Therapy with Real-Time Cellular Imaging. ACS Appl. Nano Mater. 2018, 1, 6281–6288. 10.1021/acsanm.8b01495. [DOI] [Google Scholar]
- Hrabie J. A.; Keefer L. K. Chemistry of the Nitric Oxide-Releasing Diazeniumdiolate (“Nitrosohydroxylamine”) Functional Group and Its Oxygen-Substituted Derivatives. Chem. Rev. 2002, 102, 1135–1154. 10.1021/cr000028t. [DOI] [PubMed] [Google Scholar]
- Korman A.; Sun H.; Hua B.; Yang H.; Capilato J. N.; Paul R.; Panja S.; Ha T.; Greenberg M. M.; Woodson S. A. Light-Controlled Twister Ribozyme with Single-Molecule Detection Resolves RNA Function in Time and Space. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 12080–12086. 10.1073/pnas.2003425117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S.; Das J.; Barman S.; Rao Pinninti B.; Maiti T. K.; Singh N. D. P. Environment Activatable Nanoprodrug: Two-Step Surveillance in the Anticancer Drug Release. ACS Appl. Mater. Interfaces 2017, 9, 28180–28184. 10.1021/acsami.7b05132. [DOI] [PubMed] [Google Scholar]
- Chang M. C. Y.; Pralle A.; Isacoff E. Y.; Chang C. J. A Selective, Cell-Permeable Optical Probe for Hydrogen Peroxide in Living Cells. J. Am. Chem. Soc. 2004, 126, 15392–15393. 10.1021/ja0441716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuivila H. G.; Armour A. G. Electrophilic Displacement Reactions. IX. Effects of Substituents on Rates of Reactions Between Hydrogen Peroxide and Benzeneboronic Acid. J. Am. Chem. Soc. 1957, 79, 5659–5662. 10.1021/ja01578a020. [DOI] [Google Scholar]
- Kuivila H. G.; Wiles R. A. Electrophilic Displacement Reactions. VII. Catalysis by Chelating Agents in the Reaction Between Hydrogen Peroxide and Benzeneboronic Acid. J. Am. Chem. Soc. 1955, 77, 4830–4834. 10.1021/ja01623a043. [DOI] [Google Scholar]
- Noh J.; Kwon B.; Han E.; Park M.; Yang W.; Cho W.; Yoo W.; Khang G.; Lee D. Amplification of Oxidative Stress by a Dual Stimuli-Responsive Hybrid Drug Enhances Cancer Cell Death. Nat. Commun. 2015, 6, 6907. 10.1038/ncomms7907. [DOI] [PubMed] [Google Scholar]
- Ye M.; Han Y.; Tang J.; Piao Y.; Liu X.; Zhou Z.; Gao J.; Rao J.; Shen Y. A Tumor-Specific Cascade Amplification Drug Release Nanoparticle for Overcoming Multidrug Resistance in Cancers. Adv. Mater. 2017, 29, 1702342. 10.1002/adma.201702342. [DOI] [PubMed] [Google Scholar]
- Major Jourden J. L.; Cohen S. M. Hydrogen Peroxide Activated Matrix Metalloproteinase Inhibitors: A Prodrug Approach. Angew. Chem., Int. Ed. 2010, 49, 6795–6797. 10.1002/anie.201003819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk A. L.; Givens R. S.; Elles C. G. Two-Photon Activation of p-Hydroxyphenacyl Phototriggers: Toward Spatially Controlled Release of Diethyl Phosphate and ATP. J. Phys. Chem. B 2016, 120, 3178–3186. 10.1021/acs.jpcb.5b12150. [DOI] [PubMed] [Google Scholar]
- McEvoy A. L.; Hoi H.; Bates M.; Platonova E.; Cranfill P. J.; Baird M. A.; Davidson M. W.; Ewers H.; Liphardt J.; Campbell R. E. mMaple: A Photoconvertible Fluorescent Protein for Use in Multiple Imaging Modalities. PLoS One 2012, 7, e51314 10.1371/journal.pone.0051314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.; Lohman A. W.; Zhuravlova Y.; Lu X.; Wiens M. D.; Hoi H.; Yaganoglu S.; Mohr M. A.; Kitova E. N.; Klassen J. S.; Pantazis P.; Thompson R. J.; Campbell R. E. Optogenetic Control with a Photocleavable Protein, PhoCl. Nat. Methods 2017, 14, 391–394. 10.1038/nmeth.4222. [DOI] [PubMed] [Google Scholar]
- Chattoraj M.; King B. A.; Bublitz G. U.; Boxer S. G. Ultrafast Excited State Dynamics in Green Fluorescent Protein: Multiple States and Proton Transfer. Proc. Natl. Acad. Sci. U. S. A. 1996, 93, 8362. 10.1073/pnas.93.16.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAnaney T. B.; Shi X.; Abbyad P.; Jung H.; Remington S. J.; Boxer S. G. Green Fluorescent Protein Variants as Ratiometric Dual Emission pH Sensors. 3. Temperature Dependence of Proton Transfer. Biochemistry 2005, 44, 8701–8711. 10.1021/bi050132a. [DOI] [PubMed] [Google Scholar]
- Mizuno H.; Mal T. K.; Tong K. I.; Ando R.; Furuta T.; Ikura M.; Miyawaki A. Photo-Induced Peptide Cleavage in the Green-to-Red Conversion of a Fluorescent Protein. Mol. Cell 2003, 12, 1051–1058. 10.1016/S1097-2765(03)00393-9. [DOI] [PubMed] [Google Scholar]
- Endo M.; Iwawaki T.; Yoshimura H.; Ozawa T. Photocleavable Cadherin Inhibits Cell-to-Cell Mechanotransduction by Light. ACS Chem. Biol. 2019, 14, 2206–2214. 10.1021/acschembio.9b00460. [DOI] [PubMed] [Google Scholar]
- Meador K.; Wysoczynski C. L.; Norris A. J.; Aoto J.; Bruchas M. R.; Tucker C. L. Achieving Tight Control of a Photoactivatable Cre Recombinase Gene Switch: New Design Strategies and Functional Characterization in Mammalian Cells and Rodent. Nucleic Acids Res. 2019, 47, e97 10.1093/nar/gkz585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollech D.; Pflästerer T.; Shellard A.; Zambarda C.; Spatz J. P.; Marcq P.; Mayor R.; Wombacher R.; Cavalcanti-Adam E. A. An Optochemical Tool for Light-Induced Dissociation of Adherens Junctions to Control Mechanical Coupling Between Cells. Nat. Commun. 2020, 11, 472. 10.1038/s41467-020-14390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadish J. A.; Strange A. C.; DeForest C. A. Genetically Encoded Photocleavable Linkers for Patterned Protein Release from Biomaterials. J. Am. Chem. Soc. 2019, 141, 15619–15625. 10.1021/jacs.9b07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang D.; Wu X.; Cao W.; Xue B.; Qin M.; Cao Y.; Wang W. Hydrogels With Tunable Mechanical Properties Based on Photocleavable Proteins. Front. Chem. 2020, 8, 7. 10.3389/fchem.2020.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A.; Biswas A.; Sinha S.; Shah S. S.; Bera M.; Mandal M.; Singh N. D. P. Push–Pull Stilbene: Visible Light Activated Photoremovable Protecting Group for Alcohols and Carboxylic Acids with Fluorescence Reporting Employed for Drug Delivery. Org. Lett. 2019, 21, 2968–2972. 10.1021/acs.orglett.9b00124. [DOI] [PubMed] [Google Scholar]
- Lewis G. N.; Magel T. T.; Lipkin D. The Absorption and Re-emission of Light by cis- and trans-Stilbenes and the Efficiency of their Photochemical Isomerization. J. Am. Chem. Soc. 1940, 62, 2973–2980. 10.1021/ja01868a024. [DOI] [Google Scholar]
- Moore W. M.; Morgan D. D.; Stermitz F. R. The Photochemical Conversion of Stilbene to Phenanthrene. The Nature of the Intermediate. J. Am. Chem. Soc. 1963, 85, 829–830. 10.1021/ja00889a050. [DOI] [Google Scholar]
- Muszkat K. A.; Fischer E. Structure, Spectra, Photochemistry, and Thermal Reactions of the 4a,4b-Dihydrophenanthrenes. J. Chem. Soc. B 1967, 662–678. 10.1039/j29670000662. [DOI] [Google Scholar]
- Mallory F. B.; Gordon J. T.; Wood C. S. Photochemistry of Stilbenes. II. Substituent Effects on the Rates of Phenanthrene Formation. J. Am. Chem. Soc. 1963, 85, 828–829. 10.1021/ja00889a049. [DOI] [Google Scholar]
- Lapouyade R.; Koussini R.; Rayez J.-C. Photocyclisation of 1,1-Diarylethylenes; The Novel Formation of a Five-Membered Ring. J. Chem. Soc., Chem. Commun. 1975, 676–677. 10.1039/c39750000676. [DOI] [Google Scholar]
- Sargent M. V.; Timmons C. J. Studies in Photochemistry. Part I. The Stilbenes. J. Chem. Soc. 1964, 5544–5552. 10.1039/jr9640005544. [DOI] [Google Scholar]
- Giles R. G. F.; Sargent M. V. Photochemical-Synthesis of Phenanthrenes from 2-Methoxystilbenes. J. Chem. Soc., Perkin Trans. 1 1974, 1, 2447–2450. 10.1039/p19740002447. [DOI] [Google Scholar]
- Wood C. S.; Mallory F. B. Photochemistry of Stilbenes. IV. The Preparation of Substituted Phenanthrenes. J. Org. Chem. 1964, 29, 3373–3377. 10.1021/jo01034a059. [DOI] [Google Scholar]
- Cava M. P.; Mitchell M. J.; Havlicek S. C.; Lindert A.; Spangler R. J. Photochemical Routes to Aporphines. New Syntheses of Nuciferine and Glaucine. J. Org. Chem. 1970, 35, 175–179. 10.1021/jo00826a038. [DOI] [PubMed] [Google Scholar]
- Cava M. P.; Stern P.; Wakisaka K. An Improved Photochemical Aporphine Synthesis: New Syntheses of Dicentrine and Cassameridine. Tetrahedron 1973, 29, 2245–2249. 10.1016/S0040-4020(01)93344-7. [DOI] [Google Scholar]
- Lin C.-K.; Wang Y.-F.; Cheng Y.-C.; Yang J.-S. Multisite Constrained Model of trans-4-(N, N-Dimethylamino)-4′-nitrostilbene for Structural Elucidation of Radiative and Nonradiative Excited States. J. Phys. Chem. A 2013, 117, 3158–3164. 10.1021/jp310770s. [DOI] [PubMed] [Google Scholar]
- Galvan-Gonzalez A.; Belfield K. D.; Stegeman G. I.; Canva M.; Marder S. R.; Staub K.; Levina G.; Twieg R. J. Photodegradation of Selected π-Conjugated Electro-Optic Chromophores. J. Appl. Phys. 2003, 94, 756–763. 10.1063/1.1578703. [DOI] [Google Scholar]
- Mallory F. B.; Rudolph M. J.; Oh S. M. Photochemistry of Stilbenes. 8. Eliminative Photocyclization of Ortho-Methoxystilbenes. J. Org. Chem. 1989, 54, 4619–4626. 10.1021/jo00280a032. [DOI] [Google Scholar]
- Cuppen T. J. H. M.; Laarhoven W. H. Photodehydrocyclizations of Stilbene-Like Compounds. VI. Chemical Evidence of an Excited State Mechanism. J. Am. Chem. Soc. 1972, 94, 5914–5915. 10.1021/ja00771a074. [DOI] [Google Scholar]
- Mallory F. B.; Wood C. S.; Gordon J. T.; Lindquist L. C.; Savitz M. L. Photochemistry of Stilbenes I. J. Am. Chem. Soc. 1962, 84, 4361–4362. 10.1021/ja00881a044. [DOI] [Google Scholar]
- Baxter I.; Phillips W. R. Reactions Between 2,5-Di-t-butyl-1,4-benzoquinone and Certain Primary Aliphatic Amines. J. Chem. Soc., Perkin Trans. 1 1973, 1, 268–272. 10.1039/p19730000268. [DOI] [Google Scholar]
- Bruce J. M. Light-Induced Reactions of Quinones. Q. Rev., Chem. Soc. 1967, 21, 405–428. 10.1039/qr9672100405. [DOI] [Google Scholar]
- El’tsov A. V.; Studzinskii O. P.; Grebenkina V. M. Photoinitiation of the Reactions of Quinones. Russ. Chem. Rev. 1977, 46, 93–114. 10.1070/RC1977v046n02ABEH002115. [DOI] [Google Scholar]
- Görner H. Photoprocesses of p-Benzoquinones in Aqueous Solution. J. Phys. Chem. A 2003, 107, 11587–11595. 10.1021/jp030789a. [DOI] [Google Scholar]
- Görner H.; von Sonntag C. Photoprocesses of Chloro-Substituted p-Benzoquinones. J. Phys. Chem. A 2008, 112, 10257–10263. 10.1021/jp805046p. [DOI] [PubMed] [Google Scholar]
- Ando Y.; Suzuki K. Photoredox Reactions of Quinones. Chem. - Eur. J. 2018, 24, 15955–15964. 10.1002/chem.201801064. [DOI] [PubMed] [Google Scholar]
- Amada I.; Yamaji M.; Tsunoda S. i.; Shizuka H. Laser Photolysis Studies of Electron Transfer Between Triplet Naphthoquinones and Amines. J. Photochem. Photobiol., A 1996, 95, 27–32. 10.1016/1010-6030(95)04235-0. [DOI] [Google Scholar]
- Görner H. Photoreduction of 9,10-Anthraquinone Derivatives: Transient Spectroscopy and Effects of Alcohols and Amines on Reactivity in Solution. Photochem. Photobiol. 2003, 77, 171–179. 10.1562/0031-8655(2003)0770171POADTS2.0.CO2. [DOI] [PubMed] [Google Scholar]
- Görner H. Photoreduction of p-Benzoquinones: Effects of Alcohols and Amines on the Intermediates and Reactivities in Solution. Photochem. Photobiol. 2003, 78, 440–448. . [DOI] [PubMed] [Google Scholar]
- Jones G.; Mouli N.; Haney W. A.; Bergmark W. R. Photoreduction of Chloranil by Benzhydrol and Related Compounds. Hydrogen Atom Abstraction vs Sequential Electron-Proton Transfer via Quinone Triplet Radical Ion-Pairs. J. Am. Chem. Soc. 1997, 119, 8788–8794. 10.1021/ja970271i. [DOI] [Google Scholar]
- Mac M.; Wirz J. Salt Effects on the Reactions of Radical Ion Pairs Formed by Electron Transfer Quenching of Triplet 2-Methyl-1,4-naphthoquinone by Amines. Optical Flash Photolysis and Step-Scan FTIR Investigations. Photochem. Photobiol. Sci. 2002, 1, 24–29. 10.1039/b106798b. [DOI] [PubMed] [Google Scholar]
- Wakisaka A.; Ebbesen T. W.; Sakuragi H.; Tokumaru K. Effect of Water Concentration on Photoreduction of Anthraquinone-2-sulfonate by 2-Propanol in Aqueous Acetonitrile Solution. J. Phys. Chem. 1987, 91, 6547–6551. 10.1021/j100310a025. [DOI] [Google Scholar]
- Bruce J. M.; Chaudhry A.-U.-H.; Dawes K. Light-Induced and Related Reactions of Quinones. Part X. Further Studies with Hydroxymethyl-, Vinyl-, and (2-Ethoxycarbonylethyl)-1,4-benzoquinones. J. Chem. Soc., Perkin Trans. 1 1974, 1, 288–294. 10.1039/p19740000288. [DOI] [Google Scholar]
- Farid S. Photolysis of t-Butyl-p-quinones: Competing 1,4- and 1,5-Dipolar Cycloadditions of the Photoproduct to Nitriles and Ketones. J. Chem. Soc. D 1970, 303–304. 10.1039/c29700000303. [DOI] [Google Scholar]
- Görner H. Photoreactions of 2-Methyl-5-isopropyl-1,4-benzoquinone. J. Photochem. Photobiol., A 2004, 165, 215–222. 10.1016/j.jphotochem.2004.03.020. [DOI] [Google Scholar]
- Görner H. Photoreactions of 2,5-Dibromo-3-methyl-6-isopropyl-1,4-benzoquinone. J. Photochem. Photobiol., A 2005, 175, 138–145. 10.1016/j.jphotochem.2005.03.028. [DOI] [Google Scholar]
- Orlando C. M.; Mark H.; Bose A. K.; Manhas M. S. Photoreactions: Rearrangement of Thymoquinone. Chem. Commun. 1966, 714–715. 10.1039/c19660000714. [DOI] [Google Scholar]
- Orlando C. M.; Mark H.; Bose A. K.; Manhas M. S. Photoreactions. IV. Photolysis of tert-Butyl-Substituted p-Benzoquinones. J. Am. Chem. Soc. 1967, 89, 6527–6532. 10.1021/ja01001a027. [DOI] [Google Scholar]
- Orlando C. M.; Mark H.; Bose A. K.; Manhas M. S. Photoreactions. V. Mechanism of the Photorearrangement of Alkyl-p-Benzoquinones. J. Org. Chem. 1968, 33, 2512–2516. 10.1021/jo01270a076. [DOI] [Google Scholar]
- Stidham M. A.; Siedow J. N. Photochemical Reactions of Dibromothymoquinone: Structure and Inhibitory Properties of the Photoproduct. Photochem. Photobiol. 1983, 38, 537–539. 10.1111/j.1751-1097.1983.tb03379.x. [DOI] [Google Scholar]
- Cameron D. W.; Giles R. G. F. A Photochemical Rearrangement Involving Aminated Quinones. Chem. Commun. 1965, 573–574. 10.1039/c19650000573. [DOI] [Google Scholar]
- Cameron D. W.; Giles R. G. F. Photochemical Formation of Benzoxazoline Derivatives from Aminated Quinones. J. Chem. Soc. C 1968, 1461–1464. 10.1039/j39680001461. [DOI] [Google Scholar]
- Giles R. G. F. The Photochemistry of an Aminated 1,4-Benzoquinone. Tetrahedron Lett. 1972, 13, 2253–2254. 10.1016/S0040-4039(01)84819-X. [DOI] [Google Scholar]
- Maruyama K.; Kozuka T.; Otsuki T. The Intramolecular Hydrogen Abstraction Reaction in the Photolysis of Aminated 1,4-Naphthoquinones. Bull. Chem. Soc. Jpn. 1977, 50, 2170–2173. 10.1246/bcsj.50.2170. [DOI] [Google Scholar]
- Iwamoto H. Photoinduced Intramolecular Cyclization Reaction of 3-Substituted 2-Alkenoyl-1,4-benzoquinones. Bull. Chem. Soc. Jpn. 1989, 62, 3479–3487. 10.1246/bcsj.62.3479. [DOI] [Google Scholar]
- Iwamoto H.; Takuwa A. Photoinduced Intramolecular Cyclization Reaction of 2-(2-Alkenoyl)-3-isopropylthio-1,4-benzoquinones to Heterocyclic Compounds. Bull. Chem. Soc. Jpn. 1991, 64, 724–726. 10.1246/bcsj.64.724. [DOI] [Google Scholar]
- Kemp D. S.; Reczek J. New Protective Groups for Peptide Synthesis--III the Maq Ester Group Mild Reductive Cleavage of 2-Acyloxymethyleneanthraquinones. Tetrahedron Lett. 1977, 18, 1031–1034. 10.1016/S0040-4039(01)92820-5. [DOI] [Google Scholar]
- Guo Y.; Song Q.; Wang J.; Ma J.; Zhang X.; Phillips D. L. Unraveling the Photodeprotection Mechanism of Anthraquinon-2-ylmethoxycarbonyl-Caged Alcohols Using Time-Resolved Spectroscopy. J. Org. Chem. 2018, 83, 13454–13462. 10.1021/acs.joc.8b02252. [DOI] [PubMed] [Google Scholar]
- Guo Y.; Song Q.; Xu T.; Ma J.; Phillips D. L. The Solvent Effect on the Photodeprotection of Anthraquinone Protected Carboxylic Acid Unravelled by Time-Resolved Spectroscopic Studies. Phys. Chem. Chem. Phys. 2019, 21, 14598–14604. 10.1039/C9CP01227C. [DOI] [PubMed] [Google Scholar]
- Ren M.-G.; Bi N.-M.; Mao M.; Song Q.-H. 2-(1′-Hydroxyethyl)-anthraquinone as a Photolabile Protecting Group for Carboxylic Acids. J. Photochem. Photobiol., A 2009, 204, 13–18. 10.1016/j.jphotochem.2009.02.006. [DOI] [Google Scholar]
- Arumugam S.; Popik V. V. Photochemical Generation and the Reactivity of o-Naphthoquinone Methides in Aqueous Solutions. J. Am. Chem. Soc. 2009, 131, 11892–11899. 10.1021/ja9031924. [DOI] [PubMed] [Google Scholar]
- Arumugam S.; Popik V. V. Dual Reactivity of Hydroxy- and Methoxy- Substituted o-Quinone Methides in Aqueous Solutions: Hydration versus Tautomerization. J. Org. Chem. 2010, 75, 7338–7346. 10.1021/jo101613t. [DOI] [PubMed] [Google Scholar]
- Kulikov A.; Arumugam S.; Popik V. V. Photolabile Protection of Alcohols, Phenols, and Carboxylic Acids with 3-Hydroxy-2-naphthalenemethanol. J. Org. Chem. 2008, 73, 7611–7615. 10.1021/jo801302m. [DOI] [PubMed] [Google Scholar]
- Yu J.-y.; Tang W.-J.; Wang H.-B.; Song Q.-H. Anthraquinon-2-ylethyl-1′,2′-diol (Aqe-Diol) as a New Photolabile Protecting Group for Aldehydes and Ketones. J. Photochem. Photobiol., A 2007, 185, 101–105. 10.1016/j.jphotochem.2006.05.010. [DOI] [Google Scholar]
- Perpète E. A.; Lambert C.; Wathelet V.; Preat J.; Jacquemin D. Ab Initio Studies of the λmax of Naphthoquinones Dyes. Spectrochim. Acta, Part A 2007, 68, 1326–1333. 10.1016/j.saa.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Russkikh V. V. Synthesis and Efficiency of Photolysis of 2-Dialkylamino-1,4-benzoquinones and 2-Dialkylamino-1,4-anthraquinones. Zhurnal Organicheskoi Khimii 1995, 31, 380–384. [Google Scholar]
- Takagi K.; Kawabe M.; Matsuoka M.; Kitao T. Syntheses of Deep Coloured Aminonaphthoquinonoid Dyes. Reaction of Dichloronaphthazarins with 2-Aminobenzenethiol and Related Compounds. Dyes Pigm. 1985, 6, 177–188. 10.1016/0143-7208(85)80016-4. [DOI] [Google Scholar]
- Braude E. A. Studies in Light Absorption. Part I. p-Benzoquinones. J. Chem. Soc. 1945, 490–497. 10.1039/jr9450000490. [DOI] [Google Scholar]
- Eugster C. H.; Bosshard P. Abnormale Diels-Alder-Reaktionen zwischen Acylchinonen und Furanen. Helv. Chim. Acta 1963, 46, 815–851. 10.1002/hlca.19630460315. [DOI] [Google Scholar]
- Hammond P. R. Substituent Effects on the Acceptor Properties of 1,4-Benzoquinone. J. Chem. Soc. 1964, 471–479. 10.1039/jr9640000471. [DOI] [Google Scholar]
- Ukai S.; Hirose K. Reaction of Phenol Derivatives with Sulfoxides. II. A New Method of Synthesis of Monothio Derivatives of p-Benzoquinone. Chem. Pharm. Bull. 1968, 16, 195–201. 10.1248/cpb.16.195. [DOI] [Google Scholar]
- Fabian J.; Hartmann H.. Quinoid Dyes. Light Absorption of Organic Colorants: Theoretical Treatment and Empirical Rules; Fabian J., Hartmann H., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 1980. [Google Scholar]
- Falci K. J.; Franck R. W.; Smith G. P. Approaches to Mitomycins - Photochemistry of Aminoquinones. J. Org. Chem. 1977, 42, 3317–3319. 10.1021/jo00440a033. [DOI] [PubMed] [Google Scholar]
- Giles R. G. F.; Mitchell P. R.; Roos G. H. P.; Baxter I. Photochemical Reaction of 2-Acetyl-3-Alkylamino-1,4-Benzoquinones - Formation of Benzoxazoles. J. Chem. Soc., Perkin Trans. 1 1973, 1, 493–496. 10.1039/p19730000493. [DOI] [Google Scholar]
- Chen Y. G.; Steinmetz M. G. Photochemical Cyclization with Release of Carboxylic Acids and Phenol from Pyrrolidino-Substituted 1,4-Benzoquinones Using Visible Light. Org. Lett. 2005, 7, 3729–3732. 10.1021/ol051362k. [DOI] [PubMed] [Google Scholar]
- Chen Y. G.; Steinmetz M. G. Photoactivation of Amino-Substituted 1,4-Benzoquinones for Release of Carboxylate and Phenolate Leaving Groups Using Visible Light. J. Org. Chem. 2006, 71, 6053–6060. 10.1021/jo060790g. [DOI] [PubMed] [Google Scholar]
- Wang X. D.; Kalow J. A. Rapid Aqueous Photouncaging by Red Light. Org. Lett. 2018, 20, 1716–1719. 10.1021/acs.orglett.8b00100. [DOI] [PubMed] [Google Scholar]
- Ramachandran M. S.; Singh U. C.; Subbaratnam N. R.; Kelkar V. K. Spectral Properties of Some Amino Substituted p-Benzoquinones. Proc. Indian Acad. Sci., Chem. Sci. 1979, 88, 155–162. [Google Scholar]
- Wallenfels K.; Draber W. Der Einfluss der Substituenten auf Elektronen- und Schwingungsspektren von Aminochinonen. Tetrahedron 1964, 20, 1889–1912. 10.1016/S0040-4020(01)98457-1. [DOI] [Google Scholar]
- Alouane A.; Labruère R.; Le Saux T.; Aujard I.; Dubruille S.; Schmidt F.; Jullien L. Light Activation for the Versatile and Accurate Kinetic Analysis of Disassembly of Self-Immolative Spacers. Chem. - Eur. J. 2013, 19, 11717–11724. 10.1002/chem.201301298. [DOI] [PubMed] [Google Scholar]
- Labruère R.; Alouane A.; Le Saux T.; Aujard I.; Pelupessy P.; Gautier A.; Dubruille S.; Schmidt F.; Jullien L. Self-Immolative” Spacer for Uncaging with Fluorescence Reporting. Angew. Chem., Int. Ed. 2012, 51, 9344–9347. 10.1002/anie.201204032. [DOI] [PubMed] [Google Scholar]
- Schaefer C. G.; Peters K. S. Picosecond Dynamics of the Photoreduction of Benzophenone by Triethylamine. J. Am. Chem. Soc. 1980, 102, 7566–7567. 10.1021/ja00545a030. [DOI] [Google Scholar]
- Bruce J. M.The Chemistry of the Quinonoid Compounds; Wiley and Sons: New York, 1974. [Google Scholar]
- Regan C. J.; Walton D. P.; Shafaat O. S.; Dougherty D. A. Mechanistic Studies of the Photoinduced Quinone Trimethyl Lock Decaging Process. J. Am. Chem. Soc. 2017, 139, 4729–4736. 10.1021/jacs.6b12007. [DOI] [PubMed] [Google Scholar]
- Ma C.; Kwok W. M.; Chan W. S.; Du Y.; Kan J. T. W.; Toy P. H.; Phillips D. L. Ultrafast Time-Resolved Transient Absorption and Resonance Raman Spectroscopy Study of the Photodeprotection and Rearrangement Reactions of p-Hydroxyphenacyl Caged Phosphates. J. Am. Chem. Soc. 2006, 128, 2558–2570. 10.1021/ja0532032. [DOI] [PubMed] [Google Scholar]
- Toteva M. M.; Richard J. P. The Generation and Reactions of Quinone Methides. Adv. Phys. Org. Chem. 2011, 45, 39–91. 10.1016/B978-0-12-386047-7.00002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carling C.-J.; Olejniczak J.; Foucault-Collet A.; Collet G.; Viger M. L.; Nguyen Huu V. A.; Duggan B. M.; Almutairi A. Efficient Red Light Photo-Uncaging of Active Molecules in Water Upon Assembly Into Nanoparticles. Chem. Sci. 2016, 7, 2392–2398. 10.1039/C5SC03717D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton D. P.; Dougherty D. A. A General Strategy for Visible-Light Decaging Based on the Quinone Trimethyl Lock. J. Am. Chem. Soc. 2017, 139, 4655–4658. 10.1021/jacs.7b01548. [DOI] [PubMed] [Google Scholar]
- Milstien S.; Cohen L. A. Rate Acceleration by Stereopopulation Control: Models for Enzyme Action. Proc. Natl. Acad. Sci. U. S. A. 1970, 67, 1143–1147. 10.1073/pnas.67.3.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstien S.; Cohen L. A. Stereopopulation Control. I. Rate Enhancement in the Lactonizations of o-Hydroxyhydrocinnamic Acids. J. Am. Chem. Soc. 1972, 94, 9158–9165. 10.1021/ja00781a029. [DOI] [PubMed] [Google Scholar]
- Okoh O. A.; Klahn P. Trimethyl Lock: A Multifunctional Molecular Tool for Drug Delivery, Cellular Imaging, and Stimuli-Responsive Materials. ChemBioChem 2018, 19, 1668–1694. 10.1002/cbic.201800269. [DOI] [PubMed] [Google Scholar]
- Huvelle S.; Alouane A.; Le Saux T.; Jullien L.; Schmidt F. Syntheses and Kinetic Studies of Cyclisation-Based Self-Immolative Spacers. Org. Biomol. Chem. 2017, 15, 3435–3443. 10.1039/C7OB00121E. [DOI] [PubMed] [Google Scholar]
- Shigenaga A.; Morishita K.; Yamaguchi K.; Ding H.; Ebisuno K.; Sato K.; Yamamoto J.; Akaji K.; Otaka A. Development of UV-Responsive Catch-and-Release System of a Cysteine Protease Model Peptide. Tetrahedron 2011, 67, 8879–8886. 10.1016/j.tet.2011.09.062. [DOI] [Google Scholar]
- Shigenaga A.; Tsuji D.; Nishioka N.; Tsuda S.; Itoh K.; Otaka A. Synthesis of a Stimulus-Responsive Processing Device and Its Application to a Nucleocytoplasmic Shuttle Peptide. ChemBioChem 2007, 8, 1929–1931. 10.1002/cbic.200700442. [DOI] [PubMed] [Google Scholar]
- Barbafina A.; Latterini L.; Carlotti B.; Elisei F. Characterization of Excited States of Quinones and Identification of Their Deactivation Pathways. J. Phys. Chem. A 2010, 114, 5980–5984. 10.1021/jp911734x. [DOI] [PubMed] [Google Scholar]
- Kemp D. R.; Porter G. Photochemistry of Methylated p-Benzoquinones. Proc. R. Soc. London, A 1971, 326, 117–130. 10.1098/rspa.1971.0195. [DOI] [Google Scholar]
- Truong V. X.; Li F.; Ercole F.; Forsythe J. S. Visible-Light-Mediated Cleavage of Polymer Chains Under Physiological Conditions via Quinone Photoreduction and Trimethyl Lock. Chem. Commun. 2017, 53, 12076–12079. 10.1039/C7CC07257K. [DOI] [PubMed] [Google Scholar]
- Rosen B. M.; Lligadas G.; Hahn C.; Percec V. Synthesis of Dendrimers Through Divergent Iterative Thio-Bromo “Click” Chemistry. J. Polym. Sci., Part A: Polym. Chem. 2009, 47, 3931–3939. 10.1002/pola.23519. [DOI] [Google Scholar]
- Rosen B. M.; Lligadas G.; Hahn C.; Percec V. Synthesis of Dendritic Macromolecules Through Divergent Iterative Thio-Bromo “Click” Chemistry and SET-LRP. J. Polym. Sci., Part A: Polym. Chem. 2009, 47, 3940–3948. 10.1002/pola.23518. [DOI] [Google Scholar]
- Turner A. D.; Pizzo S. V.; Rozakis G.; Porter N. A. Photoreactivation of Irreversibly Inhibited Serine Proteinases. J. Am. Chem. Soc. 1988, 110, 244–250. 10.1021/ja00209a040. [DOI] [Google Scholar]
- Turner A. D.; Pizzo S. V.; Rozakis G. W.; Porter N. A. Photochemical Activation of Acylated α-Thrombin. J. Am. Chem. Soc. 1987, 109, 1274–1275. 10.1021/ja00238a062. [DOI] [Google Scholar]
- Shiono H.; Nohta H.; Utsuyama C.; Hiramatsu M. New Method for Adding Reagents: An Application of Caged Molecules to Analytical Chemistry. Anal. Chim. Acta 2000, 405, 17–21. 10.1016/S0003-2670(99)00746-1. [DOI] [Google Scholar]
- Norris J. L.; Hangauer M. J.; Porter N. A.; Caprioli R. M. Nonacid Cleavable Detergents Applied to MALDI Mass Spectrometry Profiling of Whole Cells. J. Mass Spectrom. 2005, 40, 1319–1326. 10.1002/jms.914. [DOI] [PubMed] [Google Scholar]
- Duan X.-Y.; Zhai B.-C.; Song Q.-H. Water-Soluble o-Hydroxycinnamate as an Efficient Photoremovable Protecting Group of Alcohols with Fluorescence Reporting. Photochem. Photobiol. Sci. 2012, 11, 593–598. 10.1039/c2pp05309h. [DOI] [PubMed] [Google Scholar]
- Thuring J. W.; Li H.; Porter N. A. Comparative Study of the Active Site Caging of Serine Proteases: Thrombin and Factor Xa. Biochemistry 2002, 41, 2002–2013. 10.1021/bi0106078. [DOI] [PubMed] [Google Scholar]
- Porter N. A.; Bruhnke J. D. Acyl Thrombin Photochemistry: Kinetics for Deacylation of Enzyme Cinnamate Geometric Isomers. J. Am. Chem. Soc. 1989, 111, 7616–7618. 10.1021/ja00201a054. [DOI] [Google Scholar]
- Porter N. A.; Bruhnke J. D. Photocoagulation of Human Plasma: Acyl Serine Proteinase Photochemistry. Photochem. Photobiol. 1990, 51, 37–43. 10.1111/j.1751-1097.1990.tb01681.x. [DOI] [PubMed] [Google Scholar]
- Porter N. A.; Bush K. A.; Kinter K. S. Photo-Reversible Binding of Thrombin to Avidin by Means of a Photolabile Inhibitor. J. Photochem. Photobiol., B 1997, 38, 61–69. 10.1016/S1011-1344(96)07427-1. [DOI] [PubMed] [Google Scholar]
- Stoddard B. L.; Bruhnke J.; Porter N.; Ringe D.; Petsko G. A. Structure and Activity of Two Photoreversible Cinnamates Bound to Chymotrypsin. Biochemistry 1990, 29, 4871–4879. 10.1021/bi00472a017. [DOI] [PubMed] [Google Scholar]
- Schelkle K. M.; Bender M.; Jeltsch K.; Buckup T.; Müllen K.; Hamburger M.; Bunz U. H. F. Light-Induced Solubility Modulation of Polyfluorene To Enhance the Performance of OLEDs. Angew. Chem., Int. Ed. 2015, 54, 14545–14548. 10.1002/anie.201505141. [DOI] [PubMed] [Google Scholar]
- Venkatesh Y.; Srivastava H. K.; Bhattacharya S.; Mehra M.; Datta P. K.; Bandyopadhyay S.; Singh N. D. P. One- and Two-Photon Uncaging: Carbazole Fused o-Hydroxycinnamate Platform for Dual Release of Alcohols (Same or Different) with Real-Time Monitoring. Org. Lett. 2018, 20, 2241–2244. 10.1021/acs.orglett.8b00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett E. R.; Lippold B. C.; Mielck J. B. Kinetics and Mechanisms of Lactonization of Coumarinic Acids and Hydrolysis of Coumarins I. J. Pharm. Sci. 1971, 60, 396–405. 10.1002/jps.2600600312. [DOI] [PubMed] [Google Scholar]
- Hershfield R.; Schmir G. L. Lactonization of Ring-Substituted Coumarinic Acids. Structural Effects on the Partitioning of Tetrahedral Intermediates in Esterification. J. Am. Chem. Soc. 1973, 95, 7359–7369. 10.1021/ja00803a025. [DOI] [PubMed] [Google Scholar]
- Hershfield R.; Schmir G. L. Lactonization of Coumarinic Acids. Kinetic Evidence for Three Species of the Tetrahedral Intermediate. J. Am. Chem. Soc. 1973, 95, 8032–8040. 10.1021/ja00805a014. [DOI] [PubMed] [Google Scholar]
- Lippold B. C.; Garrett E. R. Kinetics and Mechanisms of Lactonization of Coumarinic Acids and Hydrolysis of Coumarins II. J. Pharm. Sci. 1971, 60, 1019–1027. 10.1002/jps.2600600704. [DOI] [PubMed] [Google Scholar]
- Gagey N.; Emond M.; Neveu P.; Benbrahim C.; Goetz B.; Aujard I.; Baudin J.-B.; Jullien L. Alcohol Uncaging with Fluorescence Reporting: Evaluation of o-Acetoxyphenyl Methyloxazolone Precursors. Org. Lett. 2008, 10, 2341–2344. 10.1021/ol800284g. [DOI] [PubMed] [Google Scholar]
- Gagey N.; Neveu P.; Benbrahim C.; Goetz B.; Aujard I.; Baudin J.-B.; Jullien L. Two-Photon Uncaging with Fluorescence Reporting: Evaluation of the o-Hydroxycinnamic Platform. J. Am. Chem. Soc. 2007, 129, 9986–9998. 10.1021/ja0722022. [DOI] [PubMed] [Google Scholar]
- Paul A.; Mengji R.; Chandy O. A.; Nandi S.; Bera M.; Jana A.; Anoop A.; Singh N. D. P. ESIPT-Induced Fluorescent o-Hydroxycinnamate: A Self-Monitoring Phototrigger for Prompt Image-Guided Uncaging of Alcohols. Org. Biomol. Chem. 2017, 15, 8544–8552. 10.1039/C7OB02280H. [DOI] [PubMed] [Google Scholar]
- Gagey N.; Neveu P.; Jullien L. Two-Photon Uncaging with the Efficient 3,5-Dibromo-2,4-dihydroxycinnamic Caging Group. Angew. Chem., Int. Ed. 2007, 46, 2467–2469. 10.1002/anie.200604598. [DOI] [PubMed] [Google Scholar]
- Tremblay M. S.; Sames D. A New Fluorogenic Transformation: Development of an Optical Probe for Coenzyme Q. Org. Lett. 2005, 7, 2417–2420. 10.1021/ol0507569. [DOI] [PubMed] [Google Scholar]
- Walton D. P.; Dougherty D. A. A General Strategy for Visible-Light Decaging Based on the Quinone cis-Alkenyl Lock. Chem. Commun. 2019, 55, 4965–4968. 10.1039/C9CC01073D. [DOI] [PubMed] [Google Scholar]
- Pelliccioli A. P.; Klán P.; Zabadal M.; Wirz J. Photorelease of HCl from o-Methylphenacyl Chloride Proceeds through the Z-Xylylenol. J. Am. Chem. Soc. 2001, 123, 7931–7932. 10.1021/ja016088d. [DOI] [PubMed] [Google Scholar]
- Chiang Y.; Kresge A. J.; Hellrung B.; Schünemann P.; Wirz J. Flash Photolysis of 5-Methyl-1,4-naphthoquinone in Aqueous Solution: Kinetics and Mechanism of Photoenolization and of Enol Trapping. Helv. Chim. Acta 1997, 80, 1106–1121. 10.1002/hlca.19970800408. [DOI] [Google Scholar]
- Antony L. A. P.; Slanina T.; Šebej P.; Šolomek T.; Klán P. Fluorescein Analogue Xanthene-9-Carboxylic Acid: A Transition-Metal-Free CO Releasing Molecule Activated by Green Light. Org. Lett. 2013, 15, 4552–4555. 10.1021/ol4021089. [DOI] [PubMed] [Google Scholar]
- Štacko P.; Šebej P.; Veetil A. T.; Klán P. Carbon–Carbon Bond Cleavage in Fluorescent Pyronin Analogues Induced by Yellow Light. Org. Lett. 2012, 14, 4918–4921. 10.1021/ol302244f. [DOI] [PubMed] [Google Scholar]
- Martínek M.; Váňa J.; Šebej P.; Navrátil R.; Slanina T.; Ludvíková L.; Roithová J.; Klán P. Photochemistry of a 9-Dithianyl-Pyronin Derivative: A Cornucopia of Reaction Intermediates Lead to Common Photoproducts. ChemPlusChem 2020, 85, 2230–2242. 10.1002/cplu.202000370. [DOI] [PubMed] [Google Scholar]
- Katori A.; Kuramochi K.; Tsubaki K. Oxidative Cleavage of exo-Alkylidene Xanthenes. Tetrahedron 2016, 72, 2997–3002. 10.1016/j.tet.2016.04.019. [DOI] [Google Scholar]
- Dao H. M.; Whang C.-H.; Shankar V. K.; Wang Y.-H.; Khan I. A.; Walker L. A.; Husain I.; Khan S. I.; Murthy S. N.; Jo S. Methylene Blue as a Far-Red Light-Mediated Photocleavable Multifunctional Ligand. Chem. Commun. 2020, 56, 1673–1676. 10.1039/C9CC08916K. [DOI] [PubMed] [Google Scholar]
- Sun J.; Feng F. An S-Alkyl Thiocarbamate-Based Biosensor for Highly Sensitive and Selective Detection of Hypochlorous Acid. Analyst 2018, 143, 4251–4255. 10.1039/C8AN01027G. [DOI] [PubMed] [Google Scholar]
- Benstead M.; Mehl G. H.; Boyle R. W. 4,4′-Difluoro-4-bora-3a,4a-diaza-s-indacenes (BODIPYs) as Components of Novel Light Active Materials. Tetrahedron 2011, 67, 3573–3601. 10.1016/j.tet.2011.03.028. [DOI] [Google Scholar]
- Bessette A.; Hanan G. S. Design, Synthesis and Photophysical Studies of Dipyrromethene-Based Materials: Insights Into Their Applications in Organic Photovoltaic Devices. Chem. Soc. Rev. 2014, 43, 3342–3405. 10.1039/C3CS60411J. [DOI] [PubMed] [Google Scholar]
- El-Khouly M. E.; Fukuzumi S.; D’Souza F. Photosynthetic Antenna–Reaction Center Mimicry by Using Boron Dipyrromethene Sensitizers. ChemPhysChem 2014, 15, 30–47. 10.1002/cphc.201300715. [DOI] [PubMed] [Google Scholar]
- Klfout H.; Stewart A.; Elkhalifa M.; He H. BODIPYs for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 39873–39889. 10.1021/acsami.7b07688. [DOI] [PubMed] [Google Scholar]
- Li W.; Xie Z.; Jing X. BODIPY Photocatalyzed Oxidation of Thioanisole Under Visible Light. Catal. Commun. 2011, 16, 94–97. 10.1016/j.catcom.2011.09.007. [DOI] [Google Scholar]
- Boens N.; Leen V.; Dehaen W. Fluorescent Indicators Based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. 10.1039/C1CS15132K. [DOI] [PubMed] [Google Scholar]
- Kamkaew A.; Lim S. H.; Lee H. B.; Kiew L. V.; Chung L. Y.; Burgess K. BODIPY Dyes in Photodynamic Therapy. Chem. Soc. Rev. 2013, 42, 77–88. 10.1039/C2CS35216H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowada T.; Maeda H.; Kikuchi K. BODIPY-Based Probes for the Fluorescence Imaging of Biomolecules in Living Cells. Chem. Soc. Rev. 2015, 44, 4953–4972. 10.1039/C5CS00030K. [DOI] [PubMed] [Google Scholar]
- Bertrand B.; Passador K.; Goze C.; Denat F.; Bodio E.; Salmain M. Metal-Based BODIPY Derivatives as Multimodal Tools for Life Sciences. Coord. Chem. Rev. 2018, 358, 108–124. 10.1016/j.ccr.2017.12.007. [DOI] [Google Scholar]
- Ni Y.; Wu J. Far-Red and Near Infrared BODIPY Dyes: Synthesis and Applications for Fluorescent pH Probes and Bio-Imaging. Org. Biomol. Chem. 2014, 12, 3774–3791. 10.1039/c3ob42554a. [DOI] [PubMed] [Google Scholar]
- Ulrich G.; Ziessel R.; Harriman A. The Chemistry of Fluorescent Bodipy Dyes: Versatility Unsurpassed. Angew. Chem., Int. Ed. 2008, 47, 1184–1201. 10.1002/anie.200702070. [DOI] [PubMed] [Google Scholar]
- Lu H.; Mack J.; Yang Y.; Shen Z. Structural Modification Strategies for the Rational Design of Red/Nir Region BODIPYs. Chem. Soc. Rev. 2014, 43, 4778–4823. 10.1039/C4CS00030G. [DOI] [PubMed] [Google Scholar]
- Hinkeldey B.; Schmitt A.; Jung G. Comparative Photostability Studies of BODIPY and Fluorescein Dyes by Using Fluorescence Correlation Spectroscopy. ChemPhysChem 2008, 9, 2019–2027. 10.1002/cphc.200800299. [DOI] [PubMed] [Google Scholar]
- Singh-Rachford T. N.; Haefele A.; Ziessel R.; Castellano F. N. Boron Dipyrromethene Chromophores: Next Generation Triplet Acceptors/Annihilators for Low Power Upconversion Schemes. J. Am. Chem. Soc. 2008, 130, 16164–16165. 10.1021/ja807056a. [DOI] [PubMed] [Google Scholar]
- Kollmannsberger M.; Rurack K.; Resch-Genger U.; Daub J. Ultrafast Charge Transfer in Amino-Substituted Boron Dipyrromethene Dyes and Its Inhibition by Cation Complexation: A New Design Concept for Highly Sensitive Fluorescent Probes. J. Phys. Chem. A 1998, 102, 10211–10220. 10.1021/jp982701c. [DOI] [Google Scholar]
- Qin W.; Baruah M.; Stefan A.; Van der Auweraer M.; Boens N. Photophysical Properties of BODIPY-Derived Hydroxyaryl Fluorescent pH Probes in Solution. ChemPhysChem 2005, 6, 2343–2351. 10.1002/cphc.200500341. [DOI] [PubMed] [Google Scholar]
- Röhr H.; Trieflinger C.; Rurack K.; Daub J. Proton- and Redox-Controlled Switching of Photo- and Electrochemiluminescence in Thiophenyl-Substituted Boron–Dipyrromethene Dyes. Chem. - Eur. J. 2006, 12, 689–700. 10.1002/chem.200500729. [DOI] [PubMed] [Google Scholar]
- Sazanovich I. V.; Kirmaier C.; Hindin E.; Yu L.; Bocian D. F.; Lindsey J. S.; Holten D. Structural Control of the Excited-State Dynamics of Bis(dipyrrinato)zinc Complexes: Self-Assembling Chromophores for Light-Harvesting Architectures. J. Am. Chem. Soc. 2004, 126, 2664–2665. 10.1021/ja038763k. [DOI] [PubMed] [Google Scholar]
- Kee H. L.; Kirmaier C.; Yu L.; Thamyongkit P.; Youngblood W. J.; Calder M. E.; Ramos L.; Noll B. C.; Bocian D. F.; Scheidt W. R.; Birge R. R.; Lindsey J. S.; Holten D. Structural Control of the Photodynamics of Boron-Dipyrrin Complexes. J. Phys. Chem. B 2005, 109, 20433–20443. 10.1021/jp0525078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wael E. V.; Pardoen J. A.; van Koeveringe J. A.; Lugtenburg J. Pyrromethene-BF2 Complexes (4,4′-Difluoro-4-bora-3a,4a-diaza-s-indacenes). Synthesis and Luminescence Properties. Recl. Trav. Chim. Pays-Bas 1977, 96, 306–309. 10.1002/recl.19770961205. [DOI] [Google Scholar]
- Yogo T.; Urano Y.; Ishitsuka Y.; Maniwa F.; Nagano T. Highly Efficient and Photostable Photosensitizer Based on BODIPY Chromophore. J. Am. Chem. Soc. 2005, 127, 12162–12163. 10.1021/ja0528533. [DOI] [PubMed] [Google Scholar]
- Rumyantsev E. V.; Alyoshin S. N.; Marfin Y. S. Kinetic Study of BODIPY Resistance to Acids and Alkalis: Stability Ranges in Aqueous and Non-Aqueous Solutions. Inorg. Chim. Acta 2013, 408, 181–185. 10.1016/j.ica.2013.08.015. [DOI] [Google Scholar]
- Yang L.; Simionescu R.; Lough A.; Yan H. Some Observations Relating to the Stability of the BODIPY Fluorophore Under Acidic and Basic Conditions. Dyes Pigm. 2011, 91, 264–267. 10.1016/j.dyepig.2011.03.027. [DOI] [Google Scholar]
- Boens N.; Verbelen B.; Ortiz M. J.; Jiao L.; Dehaen W. Synthesis of BODIPY Dyes Through Postfunctionalization of the Boron Dipyrromethene Core. Coord. Chem. Rev. 2019, 399, 213024. 10.1016/j.ccr.2019.213024. [DOI] [Google Scholar]
- Loudet A.; Burgess K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. 10.1021/cr078381n. [DOI] [PubMed] [Google Scholar]
- Rubinstein N.; Liu P.; Miller E. W.; Weinstain R. meso-Methylhydroxy BODIPY: a scaffold for photo-labile protecting groups. Chem. Commun. 2015, 51, 6369–6372. 10.1039/C5CC00550G. [DOI] [PubMed] [Google Scholar]
- Goswami P. P.; Syed A.; Beck C. L.; Albright T. R.; Mahoney K. M.; Unash R.; Smith E. A.; Winter A. H. BODIPY-Derived Photoremovable Protecting Groups Unmasked with Green Light. J. Am. Chem. Soc. 2015, 137, 3783–3786. 10.1021/jacs.5b01297. [DOI] [PubMed] [Google Scholar]
- Albright T. R.; Winter A. H. A Fine Line Separates Carbocations from Diradical Ions in Donor-Unconjugated Cations. J. Am. Chem. Soc. 2015, 137, 3402–3410. 10.1021/jacs.5b00707. [DOI] [PubMed] [Google Scholar]
- Buck A. T.; Beck C. L.; Winter A. H. Inverted Substrate Preferences for Photochemical Heterolysis Arise from Conical Intersection Control. J. Am. Chem. Soc. 2014, 136, 8933–8940. 10.1021/ja501777r. [DOI] [PubMed] [Google Scholar]
- Slanina T.; Shrestha P.; Palao E.; Kand D.; Peterson J. A.; Dutton A. S.; Rubinstein N.; Weinstain R.; Winter A. H.; Klán P. In Search of the Perfect Photocage: Structure–Reactivity Relationships in meso-Methyl BODIPY Photoremovable Protecting Groups. J. Am. Chem. Soc. 2017, 139, 15168–15175. 10.1021/jacs.7b08532. [DOI] [PubMed] [Google Scholar]
- Štacko P.; Muchovaá L.; Vítek L.; Klán P. Visible to NIR Light Photoactivation of Hydrogen Sulfide for Biological Targeting. Org. Lett. 2018, 20, 4907–4911. 10.1021/acs.orglett.8b02043. [DOI] [PubMed] [Google Scholar]
- Li M.; Dove A. P.; Truong V. X. Additive-Free Green Light-Induced Ligation Using BODIPY Triggers. Angew. Chem., Int. Ed. 2020, 59, 2284–2288. 10.1002/anie.201912555. [DOI] [PubMed] [Google Scholar]
- Peterson J. A.; Fischer L. J.; Gehrmann E. J.; Shrestha P.; Yuan D.; Wijesooriya C.; Smith E. A.; Winter A. H. Direct Photorelease of Alcohols from Boron-Alkylated BODIPY Photocages. J. Org. Chem. 2020, 85, 5712–5717. 10.1021/acs.joc.0c00044. [DOI] [PubMed] [Google Scholar]
- Vohradská N.; Sánchez-Carnerero E. M.; Pastierik T.; Mazal C.; Klán P. Controlled Photorelease of Alkynoic Acids and Their Decarboxylative Deprotection for Copper-Catalyzed Azide/Alkyne Cycloaddition. Chem. Commun. 2018, 54, 5558–5561. 10.1039/C8CC03341B. [DOI] [PubMed] [Google Scholar]
- Blangetti M.; Fraix A.; Lazzarato L.; Marini E.; Rolando B.; Sodano F.; Fruttero R.; Gasco A.; Sortino S. A Nonmetal-Containing Nitric Oxide Donor Activated with Single-Photon Green Light. Chem. - Eur. J. 2017, 23, 9026–9029. 10.1002/chem.201701889. [DOI] [PubMed] [Google Scholar]
- Liu M.; Meng J.; Bao W.; Liu S.; Wei W.; Ma G.; Tian Z. Single-Chromophore-Based Therapeutic Agent Enables Green-Light-Triggered Chemotherapy and Simultaneous Photodynamic Therapy to Cancer Cells. ACS Applied Bio Materials 2019, 2, 3068–3076. 10.1021/acsabm.9b00356. [DOI] [PubMed] [Google Scholar]
- Kand D.; Pizarro L.; Angel I.; Avni A.; Friedmann-Morvinski D.; Weinstain R. Organelle-Targeted BODIPY Photocages: Visible-Light-Mediated Subcellular Photorelease. Angew. Chem., Int. Ed. 2019, 58, 4659–4663. 10.1002/anie.201900850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toupin N. P.; Arora K.; Shrestha P.; Peterson J. A.; Fischer L. J.; Rajagurubandara E.; Podgorski I.; Winter A. H.; Kodanko J. J. BODIPY-Caged Photoactivated Inhibitors of Cathepsin B Flip the Light Switch on Cancer Cell Apoptosis. ACS Chem. Biol. 2019, 14, 2833–2840. 10.1021/acschembio.9b00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. A.; Wijesooriya C.; Gehrmann E. J.; Mahoney K. M.; Goswami P. P.; Albright T. R.; Syed A.; Dutton A. S.; Smith E. A.; Winter A. H. Family of BODIPY Photocages Cleaved by Single Photons of Visible/Near-Infrared Light. J. Am. Chem. Soc. 2018, 140, 7343–7346. 10.1021/jacs.8b04040. [DOI] [PubMed] [Google Scholar]
- Lv W.; Li Y.; Li F.; Lan X.; Zhang Y.; Du L.; Zhao Q.; Phillips D. L.; Wang W. Upconversion-like Photolysis of BODIPY-Based Prodrugs via a One-Photon Process. J. Am. Chem. Soc. 2019, 141, 17482–17486. 10.1021/jacs.9b09034. [DOI] [PubMed] [Google Scholar]
- Sitkowska K.; Feringa B. L.; Szymański W. Green-Light-Sensitive BODIPY Photoprotecting Groups for Amines. J. Org. Chem. 2018, 83, 1819–1827. 10.1021/acs.joc.7b02729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggi E.; Aguilera J.; Sáez R.; Pujol F.; Marquet J.; Hernando J.; Sebastián R. M. Wavelength-Tunable Light-Induced Polymerization of Cyanoacrylates Using Photogenerated Amines. Macromolecules 2019, 52, 2329–2339. 10.1021/acs.macromol.8b02318. [DOI] [Google Scholar]
- Kand D.; Liu P.; Navarro M. X.; Fischer L. J.; Rousso-Noori L.; Friedmann-Morvinski D.; Winter A. H.; Miller E. W.; Weinstain R. Water-Soluble BODIPY Photocages with Tunable Cellular Localization. J. Am. Chem. Soc. 2020, 142, 4970–4974. 10.1021/jacs.9b13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitkowska K.; Hoes M. F.; Lerch M.; Lameijer L.; van der Meer P.; Szymanski W.; Feringa B. L. Red – Light – Sensitive BODIPY Photoprotecting Groups for Amines and Their Biological Application in Controlling Heart Rhythm. Chem. Commun. 2020, 56, 5480. 10.1039/D0CC02178D. [DOI] [PubMed] [Google Scholar]
- Al Anshori J.; Slanina T.; Palao E.; Klán P. The Internal Heavy-Atom Effect on 3-Phenylselanyl and 3-Phenyltellanyl BODIPY Derivatives Studied by Transient Absorption Spectroscopy. Photochem. Photobiol. Sci. 2016, 15, 250–259. 10.1039/C5PP00366K. [DOI] [PubMed] [Google Scholar]
- Ludvíková L.; Friš P.; Heger D.; Šebej P.; Wirz J.; Klán P. Photochemistry of Rose Bengal in Water and Acetonitrile: A Comprehensive Kinetic Analysis. Phys. Chem. Chem. Phys. 2016, 18, 16266–16273. 10.1039/C6CP01710J. [DOI] [PubMed] [Google Scholar]
- Shrestha P.; Dissanayake K. C.; Gehrmann E. J.; Wijesooriya C. S.; Mukhopadhyay A.; Smith E. A.; Winter A. H. Efficient Far-Red/Near-IR Absorbing BODIPY Photocages by Blocking Unproductive Conical Intersections. J. Am. Chem. Soc. 2020, 142, 15505–15512. 10.1021/jacs.0c07139. [DOI] [PubMed] [Google Scholar]
- Murata M.; Miyashita S.; Yokoo C.; Tamai M.; Hanada K.; Hatayama K.; Towatari T.; Nikawa T.; Katunuma N. Novel Epoxysuccinyl Peptides Selective Inhibitors of Cathepsin B, in Vitro. FEBS Lett. 1991, 280, 307–310. 10.1016/0014-5793(91)80318-W. [DOI] [PubMed] [Google Scholar]
- Kawatani M.; Kamiya M.; Takahashi H.; Urano Y. Factors Affecting the Uncaging Efficiency of 500 nm Light-Activatable BODIPY Caging Group. Bioorg. Med. Chem. Lett. 2018, 28, 1–5. 10.1016/j.bmcl.2017.11.030. [DOI] [PubMed] [Google Scholar]
- Umeda N.; Takahashi H.; Kamiya M.; Ueno T.; Komatsu T.; Terai T.; Hanaoka K.; Nagano T.; Urano Y. Boron Dipyrromethene As a Fluorescent Caging Group for Single-Photon Uncaging with Long-Wavelength Visible Light. ACS Chem. Biol. 2014, 9, 2242–2246. 10.1021/cb500525p. [DOI] [PubMed] [Google Scholar]
- Sunahara H.; Urano Y.; Kojima H.; Nagano T. Design and Synthesis of a Library of BODIPY-Based Environmental Polarity Sensors Utilizing Photoinduced Electron-Transfer-Controlled Fluorescence ON/OFF Switching. J. Am. Chem. Soc. 2007, 129, 5597–5604. 10.1021/ja068551y. [DOI] [PubMed] [Google Scholar]
- Rehm D.; Weller A. Kinetics of Fluorescence Quenching by Electron and H-Atom Transfer. Isr. J. Chem. 1970, 8, 259–271. 10.1002/ijch.197000029. [DOI] [Google Scholar]
- Miura T.; Urano Y.; Tanaka K.; Nagano T.; Ohkubo K.; Fukuzumi S. Rational Design Principle for Modulating Fluorescence Properties of Fluorescein-Based Probes by Photoinduced Electron Transfer. J. Am. Chem. Soc. 2003, 125, 8666–8671. 10.1021/ja035282s. [DOI] [PubMed] [Google Scholar]
- Kumari P.; Kulkarni A.; Sharma A. K.; Chakrapani H. Visible-Light Controlled Release of a Fluoroquinolone Antibiotic for Antimicrobial Photopharmacology. ACS Omega 2018, 3, 2155–2160. 10.1021/acsomega.7b01906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A. K.; Nair M.; Chauhan P.; Gupta K.; Saini D. K.; Chakrapani H. Visible-Light-Triggered Uncaging of Carbonyl Sulfide for Hydrogen Sulfide (H2S) Release. Org. Lett. 2017, 19, 4822–4825. 10.1021/acs.orglett.7b02259. [DOI] [PubMed] [Google Scholar]
- Patil N. G.; Basutkar N. B.; Ambade A. V. Visible Light-Triggered Disruption of Micelles of an Amphiphilic Block Copolymer With BODIPY at the Junction. Chem. Commun. 2015, 51, 17708–17711. 10.1039/C5CC06820G. [DOI] [PubMed] [Google Scholar]
- Dyer R. G.; Turnbull K. D. Hydrolytic Stabilization of Protected p-Hydroxybenzyl Halides Designed as Latent Quinone Methide Precursors. J. Org. Chem. 1999, 64, 7988–7995. 10.1021/jo991085t. [DOI] [Google Scholar]
- Chauhan P.; Bora P.; Ravikumar G.; Jos S.; Chakrapani H. Esterase Activated Carbonyl Sulfide/Hydrogen Sulfide (H2S) Donors. Org. Lett. 2017, 19, 62–65. 10.1021/acs.orglett.6b03336. [DOI] [PubMed] [Google Scholar]
- Schenk S.; Kesselmeier J.; Anders E. How Does the Exchange of One Oxygen Atom with Sulfur Affect the Catalytic Cycle of Carbonic Anhydrase?. Chem. - Eur. J. 2004, 10, 3091–3105. 10.1002/chem.200305754. [DOI] [PubMed] [Google Scholar]
- Steiger A. K.; Pardue S.; Kevil C. G.; Pluth M. D. Self-Immolative Thiocarbamates Provide Access to Triggered H2S Donors and Analyte Replacement Fluorescent Probes. J. Am. Chem. Soc. 2016, 138, 7256–7259. 10.1021/jacs.6b03780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell C. R.; Foster J. C.; Okyere B.; Theus M. H.; Matson J. B. Therapeutic Delivery of H2S via COS: Small Molecule and Polymeric Donors with Benign Byproducts. J. Am. Chem. Soc. 2016, 138, 13477–13480. 10.1021/jacs.6b07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson K. R. The Therapeutic Potential of Hydrogen Sulfide: Separating Hype From Hope. Am. J. Physiol. Reg. I. 2011, 301, R297–R312. 10.1152/ajpregu.00045.2011. [DOI] [PubMed] [Google Scholar]
- Wijesooriya C. S.; Peterson J. A.; Shrestha P.; Gehrmann E. J.; Winter A. H.; Smith E. A. A Photoactivatable BODIPY Probe for Localization-Based Super-Resolution Cellular Imaging. Angew. Chem., Int. Ed. 2018, 57, 12685–12689. 10.1002/anie.201805827. [DOI] [PubMed] [Google Scholar]
- Li H.; Vaughan J. C. Switchable Fluorophores for Single-Molecule Localization Microscopy. Chem. Rev. 2018, 118, 9412–9454. 10.1021/acs.chemrev.7b00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey G. T.; Vaughan J. C.; Chen K. H.; Bates M.; Zhuang X. Evaluation of Fluorophores for Optimal Performance in Localization-Based Super-Resolution Imaging. Nat. Methods 2011, 8, 1027–1036. 10.1038/nmeth.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Linde S.; Löschberger A.; Klein T.; Heidbreder M.; Wolter S.; Heilemann M.; Sauer M. Direct Stochastic Optical Reconstruction Microscopy with Standard Fluorescent Probes. Nat. Protoc. 2011, 6, 991–1009. 10.1038/nprot.2011.336. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Raymo F. M. Live-Cell Imaging at the Nanoscale with Bioconjugatable and Photoactivatable Fluorophores. Bioconjugate Chem. 2020, 31, 1052–1062. 10.1021/acs.bioconjchem.0c00073. [DOI] [PubMed] [Google Scholar]
- Takeda A.; Komatsu T.; Nomura H.; Naka M.; Matsuki N.; Ikegaya Y.; Terai T.; Ueno T.; Hanaoka K.; Nagano T.; Urano Y. Unexpected Photo-instability of 2,6-Sulfonamide-Substituted BODIPYs and Its Application to Caged GABA. ChemBioChem 2016, 17, 1233–1240. 10.1002/cbic.201600097. [DOI] [PubMed] [Google Scholar]
- Sambath K.; Zhao T.; Wan Z.; Zhang Y. Photo-Uncaging of BODIPY Oxime Ester for Histone Deacetylases Induced Apoptosis in Tumor Cells. Chem. Commun. 2019, 55, 14162–14165. 10.1039/C9CC07199G. [DOI] [PubMed] [Google Scholar]
- Umezawa B.; Hoshino O.; Sawaki S. Organic Photochemistry. I. 3, 4-Dihydroisoquinolines from Tetrahydroisoquinoline N-Tosylates. Chem. Pharm. Bull. 1969, 17, 1115–1119. 10.1248/cpb.17.1115. [DOI] [Google Scholar]
- Hamada T.; Nishida A.; Matsumoto Y.; Yonemitsu O. Photohydrolysis of Sulfonamides via Donor-Acceptor Ion Pairs with Electron-Donating Aromatics and Its Application to the Selective Detosylation of Lysine Peptides. J. Am. Chem. Soc. 1980, 102, 3978–3980. 10.1021/ja00531a064. [DOI] [Google Scholar]
- Hamada T.; Nishida A.; Yonemitsu O. Selective Removal of Electron-Accepting p-Toluene- and Naphthalenesulfonyl Protecting Groups for Amino Function via Photoinduced Donor Acceptor Ion Pairs With Electron-Donating Aromatics. J. Am. Chem. Soc. 1986, 108, 140–145. 10.1021/ja00261a023. [DOI] [Google Scholar]
- Hamada T.; Nishida A.; Yonemitsu O. A New Amino Protecting Group Readily Removable with Near Ultraviolet Light as an Application of Electron-Transfer Photochemistry. Tetrahedron Lett. 1989, 30, 4241–4244. 10.1016/S0040-4039(01)80700-0. [DOI] [Google Scholar]
- Papageorgiou G.; Corrie J. E. T. Synthetic and Photochemical Studies of N-Arenesulfonyl Amino Acids. Tetrahedron 1999, 55, 237–254. 10.1016/S0040-4020(98)01029-1. [DOI] [Google Scholar]
- Boreen A. L.; Arnold W. A.; McNeill K. Photochemical Fate of Sulfa Drugs in the Aquatic Environment: Sulfa Drugs Containing Five-Membered Heterocyclic Groups. Environ. Sci. Technol. 2004, 38, 3933–3940. 10.1021/es0353053. [DOI] [PubMed] [Google Scholar]
- Boreen A. L.; Arnold W. A.; McNeill K. Triplet-Sensitized Photodegradation of Sulfa Drugs Containing Six-Membered Heterocyclic Groups: Identification of an SO2 Extrusion Photoproduct. Environ. Sci. Technol. 2005, 39, 3630–3638. 10.1021/es048331p. [DOI] [PubMed] [Google Scholar]
- Weiss B.; Dürr H.; Haas H. J. Photochemistry of Sulfonamides and Sulfonylureas: A Contribution to the Problem of Light-Induced Dermatoses. Angew. Chem., Int. Ed. Engl. 1980, 19, 648–650. 10.1002/anie.198006481. [DOI] [PubMed] [Google Scholar]
- Jones G.; Lu L. N.; Fu H.; Farahat C. W.; Oh C.; Greenfield S. R.; Gosztola D. J.; Wasielewski M. R. Intramolecular Electron Transfer across Amino Acid Spacers in the Picosecond Time Regime. Charge-Transfer Interaction through Peptide Bonds. J. Phys. Chem. B 1999, 103, 572–581. 10.1021/jp9832802. [DOI] [Google Scholar]
- Palao E.; Slanina T.; Muchová L.; Šolomek T.; Vítek L.; Klán P. Transition-Metal-Free CO-Releasing BODIPY Derivatives Activatable by Visible to NIR Light as Promising Bioactive Molecules. J. Am. Chem. Soc. 2016, 138, 126–133. 10.1021/jacs.5b10800. [DOI] [PubMed] [Google Scholar]
- Fukuzumi S. New Perspective of Electron Transfer Chemistry. Org. Biomol. Chem. 2003, 1, 609–620. 10.1039/b300053b. [DOI] [PubMed] [Google Scholar]
- Corrie J. E. T.; Papageorgiou G. Synthesis and Evaluation of Photolabile Sulfonamides as Potential Reagents for Rapid Photorelease of Neuroactive Amines. J. Chem. Soc., Perkin Trans. 1 1996, 1, 1583–1592. 10.1039/p19960001583. [DOI] [Google Scholar]
- Hill R. R.; Jeffs G. E.; Roberts D. R.; Wood S. A. Photodegradation of Aryl Sulfonamides: N-Tosylglycine. Chem. Commun. 1999, 1735–1736. 10.1039/a905358a. [DOI] [Google Scholar]
- Griesbeck A. G. Photochemical Transformations of Proteinogenic and Non-Proteinogenic Amino Acids. Chimia 1998, 52, 272–283. [Google Scholar]
- Bucher G.; Scaiano J. C.; Sinta R.; Barclay G.; Cameron J. Laser Flash Photolysis of Carbamates Derived from 9-Fluorenone Oxime. J. Am. Chem. Soc. 1995, 117, 3848–3855. 10.1021/ja00118a021. [DOI] [Google Scholar]
- Hwang H.; Jang D.-J.; Chae K. H. Photolysis Reaction Mechanism of Dibenzophenoneoxime Hexamethylenediurethane, a New Type of Photobase Generator. J. Photochem. Photobiol., A 1999, 126, 37–42. 10.1016/S1010-6030(99)00129-X. [DOI] [Google Scholar]
- Lalevée J.; Allonas X.; Fouassier J. P.; Tachi H.; Izumitani A.; Shirai M.; Tsunooka M. Investigation of the Photochemical Properties of an Important Class of Photobase Generators: The O-Acyloximes. J. Photochem. Photobiol., A 2002, 151, 27–37. 10.1016/S1010-6030(02)00174-0. [DOI] [Google Scholar]
- McCarroll A. J.; Walton J. C. Exploitation of Aldoxime Esters as Radical Precursors in Preparative and EPR Spectroscopic Roles. J. Chem. Soc. Perk. T. 2000, 2, 2399–2409. 10.1039/b007212p. [DOI] [Google Scholar]
- Chowdhury N.; Dutta S.; Dasgupta S.; Singh N. D. P.; Baidya M.; Ghosh S. K. Synthesis, Photophysical, Photochemical, Dna Cleavage/Binding and Cytotoxic Properties of Pyrene Oxime Ester Conjugates. Photochem. Photobiol. Sci. 2012, 11, 1239–1250. 10.1039/c2pp25033k. [DOI] [PubMed] [Google Scholar]
- Jiang H.; An X.; Tong K.; Zheng T.; Zhang Y.; Yu S. Visible-Light-Promoted Iminyl-Radical Formation from Acyl Oximes: A Unified Approach to Pyridines, Quinolines, and Phenanthridines. Angew. Chem., Int. Ed. 2015, 54, 4055–4059. 10.1002/anie.201411342. [DOI] [PubMed] [Google Scholar]
- An X.-D.; Yu S. Visible-Light-Promoted and One-Pot Synthesis of Phenanthridines and Quinolines from Aldehydes and O-Acyl Hydroxylamine. Org. Lett. 2015, 17, 2692–2695. 10.1021/acs.orglett.5b01096. [DOI] [PubMed] [Google Scholar]
- Matsubara R.; Idros U. M.; Yabuta T.; Ma H.; Hayashi M.; Eda K. Photoinduced Nitrile Formation from O-(Arylcarbonyl) oxime: Usage as a Photoremovable Protecting Group. ChemPhotoChem. 2018, 2, 1012–1016. 10.1002/cptc.201800167. [DOI] [Google Scholar]
- Photochemistry and Photophysics of Coordination Compounds I; Balzani V., Campagna S., Eds.; Springer-Verlag: Berlin, Heidelberg, 2007. [Google Scholar]
- Photochemistry and Photophysics of Coordination Compounds II; Balzani V., Campagna S., Eds.; Springer-Verlag: Berlin, Heidelberg, 2007. [Google Scholar]
- Martins P.; Marques M.; Coito L.; Pombeiro A. J. L.; Baptista P. V.; Fernandes A. R. Organometallic Compounds in Cancer Therapy: Past Lessons and Future Directions. Anti-Cancer Agents Med. Chem. 2014, 14, 1199–1212. 10.2174/1871520614666140829124925. [DOI] [PubMed] [Google Scholar]
- Simpson P. V.; Desai N. M.; Casari I.; Massi M.; Falasca M. Metal-Based Antitumor Compounds: Beyond Cisplatin. Future Med. Chem. 2019, 11, 119–135. 10.4155/fmc-2018-0248. [DOI] [PubMed] [Google Scholar]
- Ndagi U.; Mhlongo N.; Soliman M. E. Metal Complexes in Cancer Therapy - an Update From Drug Design Perspective. Drug Des., Dev. Ther. 2017, 11, 599–616. 10.2147/DDDT.S119488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maity B.; Chakravarty A. R. Photocytotoxic Organometallic Compounds. Indian J. Chem. A 2012, 51, 69–82. [Google Scholar]
- Lo K. K. W. Luminescent Rhenium(I) and Iridium(III) Polypyridine Complexes as Biological Probes, Imaging Reagents, and Photocytotoxic Agents. Acc. Chem. Res. 2015, 48, 2985–2995. 10.1021/acs.accounts.5b00211. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski S.; Knap B.; Przystupski D.; Saczko J.; Kedzierska E.; Knap-Czop K.; Kotlinska J.; Michel O.; Kotowski K.; Kulbacka J. Photodynamic Therapy - Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. 10.1016/j.biopha.2018.07.049. [DOI] [PubMed] [Google Scholar]
- Yip A. M. H.; Lo K. K. W. Luminescent Rhenium(I), Ruthenium(II), and Iridium(III) Polypyridine Complexes Containing a Poly(Ethylene Glycol) Pendant or Bioorthogonal Reaction Group as Biological Probes and Photocytotoxic Agents. Coord. Chem. Rev. 2018, 361, 138–163. 10.1016/j.ccr.2018.01.021. [DOI] [Google Scholar]
- Imberti C.; Zhang P.; Huang H.; Sadler P. J. New Designs for Phototherapeutic Transition Metal Complexes. Angew. Chem., Int. Ed. 2020, 59, 61–73. 10.1002/anie.201905171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas K. L.; Franz K. J. Application of Metal Coordination Chemistry To Explore and Manipulate Cell Biology. Chem. Rev. 2009, 109, 4921–4960. 10.1021/cr900134a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelosevic A.; Pages B. J.; Spare L. K.; Deo K. M.; Ang D. L.; Aldrich-Wright J. R. Exposing “Bright” Metals: Promising Advances in Photoactivated Anticancer Transition Metal Complexes. Curr. Med. Chem. 2018, 25, 478–492. 10.2174/0929867324666170530085123. [DOI] [PubMed] [Google Scholar]
- Bonnet S. Why Develop Photoactivated Chemotherapy?. Dalton Trans. 2018, 47, 10330–10343. 10.1039/C8DT01585F. [DOI] [PubMed] [Google Scholar]
- Jia P. P.; Ouyang R. Z.; Cao P. H.; Tong X.; Zhou X.; Lei T.; Zhao Y. F.; Guo N.; Chang H. Z.; Miao Y. Q.; Zhou S. Review: Recent Advances and Future Development of Metal Complexes as Anticancer Agents. J. Coord. Chem. 2017, 70, 2175–2201. 10.1080/00958972.2017.1349313. [DOI] [Google Scholar]
- Reessing F.; Szymanski W. Beyond Photodynamic Therapy: Light-Activated Cancer Chemotherapy. Curr. Med. Chem. 2018, 24, 4905–4950. 10.2174/0929867323666160906103223. [DOI] [PubMed] [Google Scholar]
- Renfrew A. K.; O’Neill E. S.; Hambley T. W.; New E. J. Harnessing the Properties of Cobalt Coordination Complexes for Biological Application. Coord. Chem. Rev. 2018, 375, 221–233. 10.1016/j.ccr.2017.11.027. [DOI] [Google Scholar]
- Ruggiero E.; Alonso-De Castro S.; Habtemariam A.; Salassa L.. The Photochemistry of Transition Metal Complexes and Its Application in Biology and Medicine. Luminescent and Photoactive Transition Metal Complexes as Biomolecular Probes and Cellular Reagents; Lo K. K. W., Ed., 2015; Vol. 165. [Google Scholar]
- Miller N. A.; Wiley T. E.; Spears K. G.; Ruetz M.; Kieninger C.; Krautler B.; Sension R. J. Toward the Design of Photoresponsive Conditional Antivitamins B-12: A Transient Absorption Study of an Arylcobalamin and an Alkynylcobalamin. J. Am. Chem. Soc. 2016, 138, 14250–14256. 10.1021/jacs.6b05299. [DOI] [PubMed] [Google Scholar]
- Anderson E. D.; Gorka A. P.; Schnermann M. J. Near-Infrared Uncaging or Photosensitizing Dictated by Oxygen Tension. Nat. Commun. 2016, 7, 13378. 10.1038/ncomms13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayat L.; Salierno M.; Etchenique R. Ruthenium(II) Bipyridyl Complexes as Photolabile Caging Groups for Amines. Inorg. Chem. 2006, 45, 1728–1731. 10.1021/ic0512983. [DOI] [PubMed] [Google Scholar]
- Lutterman D. A.; Fu P. K. L.; Turro C. cis-[Rh2(μ-O2CCH3)2(CH3CN)6]2+ as a Photoactivated Cisplatin Analog. J. Am. Chem. Soc. 2006, 128, 738–739. 10.1021/ja057620q. [DOI] [PubMed] [Google Scholar]
- Renfrew A. K.; Bryce N. S.; Hambley T. Cobalt(III) Chaperone Complexes of Curcumin: Photoreduction, Cellular Accumulation and Light-Selective Toxicity towards Tumour Cells. Chem. - Eur. J. 2015, 21, 15224–15234. 10.1002/chem.201502702. [DOI] [PubMed] [Google Scholar]
- Hennig H. Homogeneous Photo Catalysis by Transition Metal Complexes. Coord. Chem. Rev. 1999, 182, 101–123. 10.1016/S0010-8545(98)00201-X. [DOI] [Google Scholar]
- Vlcek A. The Life and Times of Excited States of Organometallic and Coordination Compounds. Coord. Chem. Rev. 2000, 200, 933–977. 10.1016/S0010-8545(00)00308-8. [DOI] [Google Scholar]
- Vogler A.; Kunkely H. Charge Transfer Excitation of Organometallic Compounds - Spectroscopy and Photochemistry. Coord. Chem. Rev. 2004, 248, 273–278. 10.1016/j.ccr.2004.01.005. [DOI] [Google Scholar]
- Shell T. A.; Lawrence D. S. Vitamin B-12: A Tunable, Long Wavelength, Light-Responsive Platform for Launching Therapeutic Agents. Acc. Chem. Res. 2015, 48, 2866–2874. 10.1021/acs.accounts.5b00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski P. M.; Garabato B. D.; Lodowski P.; Jaworska M. Photolytic Properties of Cobalamins: A Theoretical Perspective. Dalton Trans. 2016, 45, 4457–4470. 10.1039/C5DT04286K. [DOI] [PubMed] [Google Scholar]
- Barker H. A.; Weissbach H.; Smyth R. D. A Coenzyme Containing Pseudovitamin B12. Proc. Natl. Acad. Sci. U. S. A. 1958, 44, 1093–1097. 10.1073/pnas.44.11.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. A.; Stickrath A. B.; Carroll E. C.; Sension R. J. Influence of Environment on the Electronic Structure of Cob(III)alamins: Time-Resolved Absorption Studies of the S1 State Spectrum and Dynamics. J. Am. Chem. Soc. 2007, 129, 7578–7585. 10.1021/ja066197y. [DOI] [PubMed] [Google Scholar]
- Subramanian G.; Zhang X. Y.; Kodis G.; Kong Q. Y.; Liu C. M.; Chizmeshya A.; Weierstall U.; Spence J. Direct Structural and Chemical Characterization of the Photolytic Intermediates of Methylcobalamin Using Time-Resolved X-ray Absorption Spectroscopy. J. Phys. Chem. Lett. 2018, 9, 1542–1546. 10.1021/acs.jpclett.8b00083. [DOI] [PubMed] [Google Scholar]
- Miller N. A.; Deb A.; Alonso-Mori R.; Garabato B. D.; Glownia J. M.; Kiefer L. M.; Koralek J.; Sikorski M.; Spears K. G.; Wiley T. E.; Zhu D. L.; Kozlowski P. M.; Kubarych K. J.; Penner-Hahn J. E.; Sension R. J. Polarized XANES Monitors Femtosecond Structural Evolution of Photoexcited Vitamin B-12. J. Am. Chem. Soc. 2017, 139, 1894–1899. 10.1021/jacs.6b11295. [DOI] [PubMed] [Google Scholar]
- Miller N. A.; Deb A.; Alonso-Mori R.; Glownia J. M.; Kiefer L. M.; Konar A.; Michocki L. B.; Sikorski M.; Sofferman D. L.; Song S.; Toda M. J.; Wiley T. E.; Zhu D. L.; Kozlowski P. M.; Kubarych K. J.; Penner-Hahn J. E.; Sension R. J. Ultrafast X-ray Absorption Near Edge Structure Reveals Ballistic Excited State Structural Dynamics. J. Phys. Chem. A 2018, 122, 4963–4971. 10.1021/acs.jpca.8b04223. [DOI] [PubMed] [Google Scholar]
- Shiang J. J.; Walker L. A.; Anderson N. A.; Cole A. G.; Sension R. J. Time-Resolved Spectroscopic Studies of B-12 Coenzymes: The Photolysis of Methylcobalamin Is Wavelength Dependent. J. Phys. Chem. B 1999, 103, 10532–10539. 10.1021/jp992358r. [DOI] [Google Scholar]
- Shiang J. J.; Cole A. G.; Sension R. J.; Hang K.; Weng Y. X.; Trommel J. S.; Marzilli L. G.; Lian T. Q. Ultrafast Excited-State Dynamics in Vitamin B-12 and Related Cob(III)alamins. J. Am. Chem. Soc. 2006, 128, 801–808. 10.1021/ja054374+. [DOI] [PubMed] [Google Scholar]
- Sension R. J.; Harris D. A.; Stickrath A.; Cole A. G.; Fox C. C.; Marsh E. N. G. Time-Resolved Measurements of the Photolysis and Recombination of Adenosylcobalamin Bound to Glutamate Mutase. J. Phys. Chem. B 2005, 109, 18146–18152. 10.1021/jp052492d. [DOI] [PubMed] [Google Scholar]
- Peng J. A.; Tang K. C.; McLoughlin K.; Yang Y.; Forgach D.; Sension R. J. Ultrafast Excited-State Dynamics and Photolysis in Base-Off B-12 Coenzymes and Analogues: Absence of the trans-Nitrogenous Ligand Opens a Channel for Rapid Nonradiative Decay. J. Phys. Chem. B 2010, 114, 12398–12405. 10.1021/jp104641u. [DOI] [PubMed] [Google Scholar]
- Wiley T. E.; Miller N. A.; Miller W. R.; Sofferman D. L.; Lodowski P.; Toda M. J.; Jaworska M.; Kozlowski P. M.; Sension R. J. Off to the Races: Comparison of Excited State Dynamics in Vitamin B12 Derivatives Hydroxocobalamin and Aquocobalamin. J. Phys. Chem. A 2018, 122, 6693–6703. 10.1021/acs.jpca.8b06103. [DOI] [PubMed] [Google Scholar]
- Bussandri A. P.; Kiarie C. W.; Van Willigen H. Photoinduced Bond Homolysis of B12 Coenzymes. An FT-EPR Study. Res. Chem. Intermed. 2002, 28, 697–710. 10.1163/15685670260469366. [DOI] [Google Scholar]
- Schwartz P. A.; Frey P. A. 5’-Peroxyadenosine and 5’-Peroxyadenosylcobalamin as Intermediates in the Aerobic Photolysis of Adenosylcobalamin. Biochemistry 2007, 46, 7284–7292. 10.1021/bi700077v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruppa A. I.; Taraban M. B.; Leshina T. V.; Natarajan E.; Grissom C. B. CIDNP in the Photolysis of Coenzyme B-12 Model Compounds Suggesting That C-CO Bond Homolysis Occurs From the Singlet State. Inorg. Chem. 1997, 36, 758–759. 10.1021/ic960562c. [DOI] [Google Scholar]
- Padmakumar R.; Banerjee R. Evidence That Cobalt-Carbon Bond Homolysis Is Coupled to Hydrogen Atom Abstraction From Substrate in Methylmalonyl-CoA Mutase. Biochemistry 1997, 36, 3713–3718. 10.1021/bi962503g. [DOI] [PubMed] [Google Scholar]
- Yoder L. M.; Cole A. G.; Walker L. A.; Sension R. J. Time-Resolved Spectroscopic Studies of B-12 Coenzymes: Influence of Solvent on the Photolysis of Adenosylcobalamin. J. Phys. Chem. B 2001, 105, 12180–12188. 10.1021/jp012157z. [DOI] [Google Scholar]
- Lodowski P.; Ciura K.; Toda M. J.; Jaworska M.; Kozlowski P. M. Photodissociation of Ethylphenylcobalamin Antivitamin B-12. Phys. Chem. Chem. Phys. 2017, 19, 30310–30315. 10.1039/C7CP06589B. [DOI] [PubMed] [Google Scholar]
- Lodowski P.; Jaworska M.; Andruniow T.; Garabato B. D.; Kozlowski P. M. Mechanism of Co-C Bond Photolysis in the Base-On Form of Methylcobalamin. J. Phys. Chem. A 2014, 118, 11718–11734. 10.1021/jp508513p. [DOI] [PubMed] [Google Scholar]
- Lodowski P.; Jaworska M.; Andruniow T.; Kumar M.; Kozlowski P. M. Photodissociation of Co-C Bond in Methyl- and Ethylcobalamin: An Insight from TD-DFT Calculations. J. Phys. Chem. B 2009, 113, 6898–6909. 10.1021/jp810223h. [DOI] [PubMed] [Google Scholar]
- Lodowski P.; Jaworska M.; Garabato B. D.; Kozowski P. M. Mechanism of Co-C Bond Photolysis in Methylcobalamin: Influence of Axial Base. J. Phys. Chem. A 2015, 119, 3913–3928. 10.1021/jp5120674. [DOI] [PubMed] [Google Scholar]
- Lodowski P.; Toda M. J.; Ciura K.; Jaworska M.; Kozlowski P. M. Photolytic Properties of Antivitamins B-12. Inorg. Chem. 2018, 57, 7838–7850. 10.1021/acs.inorgchem.8b00956. [DOI] [PubMed] [Google Scholar]
- Andruniow T.; Lodowski P.; Garabato B. D.; Jaworska M.; Kozlowski P. M. The Role of Spin-Orbit Coupling in the Photolysis of Methylcobalamin. J. Chem. Phys. 2016, 144, 124305. 10.1063/1.4943184. [DOI] [PubMed] [Google Scholar]
- Garabato B. D.; Lodowski P.; Jaworska M.; Kozlowski P. M. Mechanism of Co-C Photodissociation in Adenosylcobalamin. Phys. Chem. Chem. Phys. 2016, 18, 19070–19082. 10.1039/C6CP02136K. [DOI] [PubMed] [Google Scholar]
- Jaworska M.; Lodowski P.; Andruniow T.; Pawel M. Photolysis of Methylcobalamin: Identification of the Relevant Excited States Involved in CO-C Bond Scission. J. Phys. Chem. B 2007, 111, 2419–2422. 10.1021/jp0685840. [DOI] [PubMed] [Google Scholar]
- Kozlowski P. M.; Kumar M.; Piecuch P.; Li W.; Bauman N. P.; Hansen J. A.; Lodowski P.; Jaworska M. The Cobalt–Methyl Bond Dissociation in Methylcobalamin: New Benchmark Analysis Based on Density Functional Theory and Completely Renormalized Coupled-Cluster Calculations. J. Chem. Theory Comput. 2012, 8, 1870–1894. 10.1021/ct300170y. [DOI] [PubMed] [Google Scholar]
- Mamun A. A.; Toda M. J.; Kozlowski P. M. Can Photolysis of the Co-C Bond in Coenzyme B12-Dependent Enzymes Be Used to Mimic the Native Reaction?. J. Photochem. Photobiol., B 2019, 191, 175–184. 10.1016/j.jphotobiol.2018.12.018. [DOI] [PubMed] [Google Scholar]
- Mamun A. A.; Toda M. J.; Lodowski P.; Jaworska M.; Kozlowski P. M. Mechanism of Light Induced Radical Pair Formation in Coenzyme B12-Dependent Ethanolamine Ammonia-Lyase. ACS Catal. 2018, 8, 7164–7178. 10.1021/acscatal.8b00120. [DOI] [Google Scholar]
- Jones A. R.; Woodward J. R.; Scrutton N. S. Continuous Wave Photolysis Magnetic Field Effect Investigations with Free and Protein-Bound Alkylcobalamins. J. Am. Chem. Soc. 2009, 131, 17246–17253. 10.1021/ja9059238. [DOI] [PubMed] [Google Scholar]
- Jones A. R.; Hay S.; Woodward J. R.; Scrutton N. S. Magnetic Field Effect Studies Indicate Reduced Geminate Recombination of the Radical Pair in Substrate-Bound Adenosylcobalamin-Dependent Ethanolamine Ammonia Lyase. J. Am. Chem. Soc. 2007, 129, 15718–15727. 10.1021/ja077124x. [DOI] [PubMed] [Google Scholar]
- Kutta R. J.; Hardman S. J. O.; Johannissen L. O.; Bellina B.; Messiha H. L.; Ortiz-Guerrero J. M.; Elias-Arnanz M.; Padmanabhan S.; Barran P.; Scrutton N. S.; Jones A. R. The Photochemical Mechanism of a B-12-Dependent Photoreceptor Protein. Nat. Commun. 2015, 6, 7907. 10.1038/ncomms8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers Z. L.; Hughes R. M.; Doherty L. M.; Shell J. R.; Molesky B. P.; Brugh A. M.; Forbes M. D. E.; Moran A. M.; Lawrence D. S. B-12-Mediated, Long Wavelength Photopolymerization of Hydrogels. J. Am. Chem. Soc. 2015, 137, 3372–3378. 10.1021/jacs.5b00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers Z. L.; Shell T. A.; Brugh A. M.; Nowotarski H. L.; Forbes M. D. E.; Lawrence D. S. Fluorophore Assisted Photolysis of Thiolato-Cob(III)alamins. Inorg. Chem. 2016, 55, 1962–1969. 10.1021/acs.inorgchem.5b02036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue A. C.; Moreau W. M.; Shell T. A. Visible Light-Induced Radical Mediated DNA Damage. Photochem. Photobiol. 2018, 94, 545–551. 10.1111/php.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedyk M.; Turkowska J.; Lepak S.; Marculewicz M.; Proinsias K. O.; Gryko D. Photoinduced Vitamin B-12-Catalysis for Deprotection of (Allyloxy)arenes. Org. Lett. 2017, 19, 2670–2673. 10.1021/acs.orglett.7b01012. [DOI] [PubMed] [Google Scholar]
- Walker L. A.; Jarrett J. T.; Anderson N. A.; Pullen S. H.; Matthews R. G.; Sension R. J. Time-Resolved Spectroscopic Studies of B-12 Coenzymes, the Identification of a Metastable Cob(III)Alamin Photoproduct in the Photolysis of Methylcobalamin. J. Am. Chem. Soc. 1998, 120, 3597–3603. 10.1021/ja974024q. [DOI] [Google Scholar]
- Pratt J. M. Chemistry of Vitamin B12. 2. Photochemical Reactions. J. Chem. Soc. 1964, 5154–5160. 10.1039/jr9640005154. [DOI] [Google Scholar]
- Pratt J. M.; Whitear B. R. D. Photolysis of Methylcobalamin. J. Chem. Soc. A 1971, 252–255. 10.1039/j19710000252. [DOI] [Google Scholar]
- Chen E.; Chance M. R. Continuous-Wave Quantum Yields of Various Cobalamins Are Influenced by Competition Between Geminate Recombination and Cage Escape. Biochemistry 1993, 32, 1480–1487. 10.1021/bi00057a011. [DOI] [PubMed] [Google Scholar]
- Walker L. A.; Shiang J. J.; Anderson N. A.; Pullen S. H.; Sension R. J. Time-Resolved Spectroscopic Studies of B12 Coenzymes: The Photolysis and Geminate Recombination of Adenosylcobalamin. J. Am. Chem. Soc. 1998, 120, 7286–7292. 10.1021/ja981029u. [DOI] [Google Scholar]
- Endicott J. F.; Ferraudi G. J. Flash Photolytic Investigation of Low-Energy Homolytic Processes in Methylcobalamin. J. Am. Chem. Soc. 1977, 99, 243–245. 10.1021/ja00443a043. [DOI] [PubMed] [Google Scholar]
- Shell T. A.; Lawrence D. S. A New Trick (Hydroxyl Radical Generation) for an Old Vitamin (B-12). J. Am. Chem. Soc. 2011, 133, 2148–2150. 10.1021/ja111585c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sension R. J.; Cole A. G.; Harris A. D.; Fox C. C.; Woodbury N. W.; Lin S.; Marsh E. N. G. Photolysis and Recombination of Adenosylcobalamin Bound to Glutamate Mutase. J. Am. Chem. Soc. 2004, 126, 1598–1599. 10.1021/ja0396910. [DOI] [PubMed] [Google Scholar]
- Vaid F. H. M.; Zahid S.; Faiyaz A.; Qadeer K.; Gul W.; Anwar Z.; Ahmad I. Photolysis of Methylcobalamin in Aqueous Solution: A Kinetic Study. J. Photochem. Photobiol., A 2018, 362, 40–48. 10.1016/j.jphotochem.2018.05.011. [DOI] [Google Scholar]
- Ahmad I.; Qadeer K.; Hafeez A.; Zahid S.; Sheraz M. A.; Khattak S. U. R. Effect of Ascorbic Acid on the Photolysis of Cyanocobalamin and Aquocobalamin/Hydroxocobalamin in Aqueous Solution: A Kinetic Study. J. Photochem. Photobiol., A 2017, 332, 92–100. 10.1016/j.jphotochem.2016.08.004. [DOI] [Google Scholar]
- Shell T. A.; Shell J. R.; Rodgers Z. L.; Lawrence D. S. Tunable Visible and Near-IR Photoactivation of Light-Responsive Compounds by Using Fluorophores as Light-Capturing Antennas. Angew. Chem., Int. Ed. 2014, 53, 875–878. 10.1002/anie.201308816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestman M. A.; Shell T. A.; Sun L.; Lee H. M.; Lawrence D. S. Merging of Confocal and Caging Technologies: Selective Three-Color Communication with Profluorescent Reporters. Angew. Chem., Int. Ed. 2012, 51, 7684–7687. 10.1002/anie.201202820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. J.; Oien N. P.; Hughes R. M.; Marvin C. M.; Rodgers Z. L.; Lee J.; Lawrence D. S. Cell-Mediated Assembly of Phototherapeutics. Angew. Chem., Int. Ed. 2014, 53, 10945–10948. 10.1002/anie.201406216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. M.; Marvin C. M.; Rodgers Z. L.; Ding S.; Oien N. P.; Smith W. J.; Lawrence D. S. Phototriggered Secretion of Membrane Compartmentalized Bioactive Agents. Angew. Chem., Int. Ed. 2016, 55, 16080–16083. 10.1002/anie.201609731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainrath S.; Stadler M.; Reichhart E.; Distel M.; Janovjak H. Green-Light-Induced Inactivation of Receptor Signaling Using Cobalamin-Binding Domains. Angew. Chem., Int. Ed. 2017, 56, 4608–4611. 10.1002/anie.201611998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelle C.; Ochoa-Fernandez R.; Engesser R.; Schneider N.; Beyer H. M.; Jones A. R.; Timmer J.; Zurbriggen M. D.; Weber W. A Green-Light-Responsive System for the Control of Transgene Expression in Mammalian and Plant Cells. ACS Synth. Biol. 2018, 7, 1349–1358. 10.1021/acssynbio.7b00450. [DOI] [PubMed] [Google Scholar]
- Jost M.; Fernandez-Zapata J.; Polanco M. C.; Ortiz-Guerrero J. M.; Chen P. Y. T.; Kang G.; Padmanabhan S.; Elias-Arnanz M.; Drennan C. L. Structural Basis for Gene Regulation by a B-12-Dependent Photoreceptor. Nature 2015, 526, 536–U167. 10.1038/nature14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost M.; Simpson J. H.; Drennan C. L. The Transcription Factor CarH Safeguards Use of Adenosylcobalamin as a Light Sensor by Altering the Photolysis Products. Biochemistry 2015, 54, 3231–3234. 10.1021/acs.biochem.5b00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claessens C. G.; Hahn U.; Torres T. Phthalocyanines: From Outstanding Electronic Properties to Emerging Applications. Chem. Rec. 2008, 8, 75–97. 10.1002/tcr.20139. [DOI] [PubMed] [Google Scholar]
- Doane T. L.; Chuang C. H.; Chomas A.; Burda C. Photophysics of Silicon Phthalocyanines in Aqueous Media. ChemPhysChem 2013, 14, 321–330. 10.1002/cphc.201200962. [DOI] [PubMed] [Google Scholar]
- Moreira L. M.; Vieira dos Santos F.; Lyon J. P.; Maftoum-Costa M.; Pacheco-Soares C.; Soares da Silva N. Photodynamic Therapy: Porphyrins and Phthalocyanines as Photosensitizers. Aust. J. Chem. 2008, 61, 741–754. 10.1071/CH08145. [DOI] [Google Scholar]
- Nyokong T. Effects of Substituents on the Photochemical and Photophysical Properties of Main Group Metal Phthalocyanines. Coord. Chem. Rev. 2007, 251, 1707–1722. 10.1016/j.ccr.2006.11.011. [DOI] [Google Scholar]
- Wang K. K.-H.; Wilson J. D.; Kenney M. E.; Mitra S.; Foster T. H. Irradiation-Induced Enhancement of Pc 4 Fluorescence and Changes in Light Scattering are Potential Dosimeters for Pc 4-PDT. Photochem. Photobiol. 2007, 83, 1056–1062. 10.1111/j.1751-1097.2007.00128.x. [DOI] [PubMed] [Google Scholar]
- Li Z. Y.; Lieberman M. Axial Reactivity of Soluble Silicon(IV) Phthalocyanines. Inorg. Chem. 2001, 40, 932–939. 10.1021/ic000968w. [DOI] [Google Scholar]
- Fujitsuka M.; Ito O.; Konami H. Photoexcited State Properties of Silicon Phthalocyanine Monomer, Dimer, and Trimer. Bull. Chem. Soc. Jpn. 2001, 74, 1823–1829. 10.1246/bcsj.74.1823. [DOI] [Google Scholar]
- Maree M. D.; Kuznetsova N.; Nyokong T. Silicon Octaphenoxyphthalocyanines: Photostability and Singlet Oxygen Quantum Yields. J. Photochem. Photobiol., A 2001, 140, 117–125. 10.1016/S1010-6030(01)00409-9. [DOI] [Google Scholar]
- Allen C. M.; Sharman W. M.; van Lier J. E. Current Status of Phthalocyanines in the Photodynamic Therapy of Cancer. J. Porphyrins Phthalocyanines 2001, 5, 161–169. 10.1002/jpp.324. [DOI] [Google Scholar]
- Mitsunaga M.; Ogawa M.; Kosaka N.; Rosenblum L. T.; Choyke P. L.; Kobayashi H. Cancer Cell–Selective in Vivo Near Infrared Photoimmunotherapy Targeting Specific Membrane Molecules. Nat. Med. 2011, 17, 1685. 10.1038/nm.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Zheng B.-D.; Peng X.-H.; Li S.-Z.; Ying J.-W.; Zhao Y.; Huang J.-D.; Yoon J. Phthalocyanines as Medicinal Photosensitizers: Developments in the Last Five Years. Coord. Chem. Rev. 2019, 379, 147–160. 10.1016/j.ccr.2017.08.003. [DOI] [Google Scholar]
- Oleinick N. L.; Antunez A. R.; Clay M. E.; Rihter B. D.; Kenney M. E. New Phthalocyanine Photosensitizers for Photodynamic Therapy. Photochem. Photobiol. 1993, 57, 242–247. 10.1111/j.1751-1097.1993.tb02282.x. [DOI] [PubMed] [Google Scholar]
- Maiti B.; Manna A. K.; McCleese C.; Doane T. L.; Chakrapani S.; Burda C.; Dunietz B. D. Photoinduced Homolytic Bond Cleavage of the Central Si-C Bond in Porphyrin Macrocycles Is a Charge Polarization Driven Process. J. Phys. Chem. A 2016, 120, 7634–7640. 10.1021/acs.jpca.6b05610. [DOI] [PubMed] [Google Scholar]
- Zheng J. Y.; Konishi K.; Aida T. A Photoresponsive Silicon Radical Within a Porphyrin (-Cloud: Photolysis of Organo- and Nitroxysilicon Porphyrins With Visible Light. J. Am. Chem. Soc. 1998, 120, 9838–9843. 10.1021/ja980273i. [DOI] [Google Scholar]
- Li J.; Yang Y.; Zhang P.; Sounik J. R.; Kenney M. E. Synthesis, Properties and Drug Potential of the Photosensitive Alkyl- and Alkylsiloxy-Ligated Silicon Phthalocyanine Pc 227. Photochem. Photobiol. Sci. 2014, 13, 1690–1698. 10.1039/C4PP00321G. [DOI] [PubMed] [Google Scholar]
- Cheng Y.; Doane T. L.; Chuang C.-H.; Ziady A.; Burda C. Near Infrared Light-Triggered Drug Generation and Release from Gold Nanoparticle Carriers for Photodynamic Therapy. Small 2014, 10, 1799–1804. 10.1002/smll.201303329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane T.; Cheng Y.; Sodhi N.; Burda C. NIR Photocleavage of the Si-C Bond in Axial Si-Phthalocyanines. J. Phys. Chem. A 2014, 118, 10587–10595. 10.1021/jp505656e. [DOI] [PubMed] [Google Scholar]
- Rajaputra P.; Bio M.; Nkepang G.; Thapa P.; Woo S.; You Y. Anticancer Drug Released From Near IR-Activated Prodrug Overcomes Spatiotemporal Limits of Singlet Oxygen. Bioorg. Med. Chem. 2016, 24, 1540–1549. 10.1016/j.bmc.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bio M.; Rajaputra P.; Nkepang G.; You Y. J. Far-Red Light Activatable, Multifunctional Prodrug for Fluorescence Optical Imaging and Combinational Treatment. J. Med. Chem. 2014, 57, 3401–3409. 10.1021/jm5000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa P.; Li M.; Bio M.; Rajaputra P.; Nkepang G.; Sun Y.; Woo S.; You Y. Far-Red Light-Activatable Prodrug of Paclitaxel for the Combined Effects of Photodynamic Therapy and Site-Specific Paclitaxel Chemotherapy. J. Med. Chem. 2016, 59, 3204–3214. 10.1021/acs.jmedchem.5b01971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E. D.; Sova S.; Ivanic J.; Kelly L.; Schnermann M. J. Defining the Conditional Basis of Silicon Phthalocyanine Near-IR Ligand Exchange. Phys. Chem. Chem. Phys. 2018, 20, 19030–19036. 10.1039/C8CP03842B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K.; Ando K.; Okuyama S.; Moriguchi S.; Ogura T.; Totoki S.; Hanaoka H.; Nagaya T.; Kokawa R.; Takakura H.; Nishimura M.; Hasegawa Y.; Choyke P. L.; Ogawa M.; Kobayashi H. Photoinduced Ligand Release from a Silicon Phthalocyanine Dye Conjugated with Monoclonal Antibodies: A Mechanism of Cancer Cell Cytotoxicity after Near-Infrared Photoimmunotherapy. ACS Cent. Sci. 2018, 4, 1559–1569. 10.1021/acscentsci.8b00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H.; Choyke P. L. Near-Infrared Photoimmunotherapy of Cancer. Acc. Chem. Res. 2019, 52, 2332–2339. 10.1021/acs.accounts.9b00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies S.; Sell H.; Bornholdt C.; Schutt C.; Kohler F.; Tuczek F.; Herges R. Light-Driven Coordination-Induced Spin-State Switching: Rational Design of Photodissociable Ligands. Chem. - Eur. J. 2012, 18, 16358–16368. 10.1002/chem.201201698. [DOI] [PubMed] [Google Scholar]
- Thies S.; Sell H.; Schütt C.; Bornholdt C.; Näther C.; Tuczek F.; Herges R. Light-Induced Spin Change by Photodissociable External Ligands: A New Principle for Magnetic Switching of Molecules. J. Am. Chem. Soc. 2011, 133, 16243–16250. 10.1021/ja206812f. [DOI] [PubMed] [Google Scholar]
- Thies S.; Bornholdt C.; Köhler F.; Sönnichsen F. D.; Näther C.; Tuczek F.; Herges R. Coordination-Induced Spin Crossover (CISCO) through Axial Bonding of Substituted Pyridines to Nickel–Porphyrins: σ-Donor versus π-Acceptor Effects. Chem. - Eur. J. 2010, 16, 10074–10083. 10.1002/chem.201000603. [DOI] [PubMed] [Google Scholar]
- Peters M. K.; Hamer S.; Jakel T.; Rohricht F.; Sonnichsen F. D.; von Essen C.; Lahtinen M.; Naether C.; Rissanen K.; Herges R. Spin Switching with Triazolate-Strapped Ferrous Porphyrins. Inorg. Chem. 2019, 58, 5265–5272. 10.1021/acs.inorgchem.9b00349. [DOI] [PubMed] [Google Scholar]
- Nilsson J. R.; O’Sullivan M. C.; Li S.; Anderson H. L.; Andreasson J. A Photoswitchable Supramolecular Complex With Release-And-Report Capabilities. Chem. Commun. 2015, 51, 847–850. 10.1039/C4CC08513B. [DOI] [PubMed] [Google Scholar]
- Juris A.; Balzani V.; Barigelletti F.; Campagna S.; Belser P.; von Zelewsky A. Ru(II) Polypyridine Complexes: Photophysics, Photochemistry, Eletrochemistry, and Chemiluminescence. Coord. Chem. Rev. 1988, 84, 85–277. 10.1016/0010-8545(88)80032-8. [DOI] [Google Scholar]
- Campagna S.; Puntoriero F.; Nastasi F.; Bergamini G.; Balzani V.. Photochemistry and Photophysics of Coordination Compounds: Ruthenium. Photochemistry and Photophysics of Coordination Compounds I; Balzani V., Campagna S., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2007. [Google Scholar]
- Cannizzo A.; van Mourik F.; Gawelda W.; Zgrablic G.; Bressler C.; Chergui M. Broadband Femtosecond Fluorescence Spectroscopy of [Ru(bpy)3]2+. Angew. Chem., Int. Ed. 2006, 45, 3174–3176. 10.1002/anie.200600125. [DOI] [PubMed] [Google Scholar]
- Durham B.; Caspar J. V.; Nagle J. K.; Meyer T. J. Photochemistry of tris(2,2′-Bipyridine)ruthenium2+ ion. J. Am. Chem. Soc. 1982, 104, 4803–4810. 10.1021/ja00382a012. [DOI] [Google Scholar]
- Tu Y. J.; Mazumder S.; Endicott J. F.; Turro C.; Kodanko J. J.; Schlegel H. B. Selective Photodissociation of Acetonitrile Ligands in Ruthenium Polypyridyl Complexes Studied by Density Functional Theory. Inorg. Chem. 2015, 54, 8003–8011. 10.1021/acs.inorgchem.5b01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmel A. C.; Collin J. P.; Sauvage J. P. Efficient and Selective Photochemical Labilization of a Given Bidentate Ligand in Mixed Ruthenium(II) Complexes of the Ru(phen)2L2+ and Ru(bipy)2L2+ family (L = sterically hindering chelate). Eur. J. Inorg. Chem. 1999, 1999, 383–386. . [DOI] [Google Scholar]
- Wacholtz W. F.; Auerbach R. A.; Schmehl R. H. Independent Control of Charge-Transfer and Metal-Centered Excited States in Mixed-Ligand Polypyridine Ruthenium(II) Complexes via Specific Ligand Design. Inorg. Chem. 1986, 25, 227–234. 10.1021/ic00222a027. [DOI] [Google Scholar]
- Rillema D. P.; Blanton C. B.; Shaver R. J.; Jackman D. C.; Boldaji M.; Bundy S.; Worl L. A.; Meyer T. J. MLCT-dd Energy Gap in Pyridyl-Pyrimidine and Bis(Pyridine) Complexes of Ruthenium(II). Inorg. Chem. 1992, 31, 1600–1606. 10.1021/ic00035a016. [DOI] [Google Scholar]
- Allen G. H.; White R. P.; Rillema D. P.; Meyer T. J. Synthetic Control of Excited-State Properties. Tris-Chelate Complexes Containing the Ligands 2,2′-Bipyrazine, 2,2′-Bipyridine, and 2,2′-Bipyrimidine. J. Am. Chem. Soc. 1984, 106, 2613–2620. 10.1021/ja00321a020. [DOI] [Google Scholar]
- Cruz A. J.; Kirgan R.; Siam K.; Heiland P.; Rillema D. P. Photochemical and Photophysical Properties of Ruthenium(II) bis-Bipyridine bis-Nitrile Complexes: Photolability. Inorg. Chim. Acta 2010, 363, 2496–2505. 10.1016/j.ica.2010.04.014. [DOI] [Google Scholar]
- Sun Y.; Joyce L. E.; Dickson N. M.; Turro C. Efficient DNA Photocleavage by [Ru(bpy)2(dppn)]2+ with Visible Light. Chem. Commun. 2010, 46, 2426–2428. 10.1039/b925574e. [DOI] [PubMed] [Google Scholar]
- Li A.; Turro C.; Kodanko J. J. Ru(II) Polypyridyl Complexes as Photocages for Bioactive Compounds Containing Nitriles and Aromatic Heterocycles. Chem. Commun. 2018, 54, 1280–1290. 10.1039/C7CC09000E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. K.; Schmehl R. H.; Turro C. An Overview of Photosubstitution Reactions of RU(II) Imine Complexes and Their Application in Photobiology and Photodynamic Therapy. Inorg. Chim. Acta 2017, 454, 7–20. 10.1016/j.ica.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soupart A.; Alary F.; Heully J.-L.; Elliott P. I. P.; Dixon I. M. Recent Progress in Ligand Photorelease Reaction Mechanisms: Theoretical Insights Focusing on RuII3MC States. Coord. Chem. Rev. 2020, 408, 213184. 10.1016/j.ccr.2020.213184. [DOI] [Google Scholar]
- Huisman M.; White J. K.; Lewalski V. G.; Podgorski I.; Turro C.; Kodanko J. J. Caging the Uncageable: Using Metal Complex Release for Photochemical Control Over Irreversible Inhibition. Chem. Commun. 2016, 52, 12590–12593. 10.1039/C6CC07083C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming F. F.; Yao L. H.; Ravikumar P. C.; Funk L.; Shook B. C. Nitrile-Containing Pharmaceuticals: Efficacious Roles of the Nitrile Pharmacophore. J. Med. Chem. 2010, 53, 7902–7917. 10.1021/jm100762r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W.; Thiramanas R.; Slep L. D.; Zeng X. L.; Mailander V.; Wu S. Photoactivation of Anticancer Ru Complexes in Deep Tissue: How Deep Can We Go?. Chem. - Eur. J. 2017, 23, 10832–10837. 10.1002/chem.201701224. [DOI] [PubMed] [Google Scholar]
- Pinnick D. V.; Durham B. Photosubstitution Reactions of Ru(bpy)2XYn+ Complexes. Inorg. Chem. 1984, 23, 1440–1445. 10.1021/ic00178a028. [DOI] [Google Scholar]
- Wacholtz W. M.; Auerbach R. A.; Schmehl R. H.; Ollino M.; Cherry W. R. Correlation of Ligand Field Excited-State Energies With Ligand Field Strength in (Polypyridine)Ruthenium(II) Complexes. Inorg. Chem. 1985, 24, 1758–1760. 10.1021/ic00206a009. [DOI] [Google Scholar]
- Liu Y.; Turner D. B.; Singh T. N.; Angeles-Boza A. M.; Chouai A.; Dunbar K. R.; Turro C. Ultrafast Ligand Exchange: Detection of a Pentacoordinate Ru(II) Intermediate and Product Formation. J. Am. Chem. Soc. 2009, 131, 26–27. 10.1021/ja806860w. [DOI] [PubMed] [Google Scholar]
- Sun Q.; Mosquera-Vazquez S.; Suffren Y.; Hankache J.; Amstutz N.; Lawson Daku L. M.; Vauthey E.; Hauser A. On the Role of Ligand-Field States for the Photophysical Properties of Ruthenium(II) Polypyridyl Complexes. Coord. Chem. Rev. 2015, 282-283, 87–99. 10.1016/j.ccr.2014.07.004. [DOI] [Google Scholar]
- Zayat L.; Calero C.; Albores P.; Baraldo L.; Etchenique R. A New Strategy for Neurochemical Photodelivery: Metal-Ligand Heterolytic Cleavage. J. Am. Chem. Soc. 2003, 125, 882–883. 10.1021/ja0278943. [DOI] [PubMed] [Google Scholar]
- Nikolenko V.; Yuste R.; Zayat L.; Baraldo L. M.; Etchenique R. Two-Photon Uncaging of Neurochemicals Using Inorganic Metal Complexes. Chem. Commun. 2005, 1752–1754. 10.1039/b418572b. [DOI] [PubMed] [Google Scholar]
- Filevich O.; Salierno M.; Etchenique R. A Caged Nicotine With Nanosecond Range Kinetics and Visible Light Sensitivity. J. Inorg. Biochem. 2010, 104, 1248–1251. 10.1016/j.jinorgbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Zayat L.; Noval M. G.; Campi J.; Calero C. I.; Calvo D. J.; Etchenique R. A New Inorganic Photolabile Protecting Group for Highly Efficient Visible Light GABA Uncaging. ChemBioChem 2007, 8, 2035–2038. 10.1002/cbic.200700354. [DOI] [PubMed] [Google Scholar]
- Ruiu T.; Garino C.; Salassa L.; Pizarro A. M.; Nervi C.; Gobetto R.; Sadler P. J. Spectroscopic and Computational Study of Ligand Photodissociation from [Ru(dipyrido[3,2-a:2′,3′-c]phenazine)(4-aminopyridine)4]2+. Eur. J. Inorg. Chem. 2010, 2010, 1186–1195. 10.1002/ejic.200900990. [DOI] [Google Scholar]
- Salierno M.; Fameli C.; Etchenique R. Caged Amino Acids for Visible-Light Photodelivery. Eur. J. Inorg. Chem. 2008, 2008, 1125–1128. 10.1002/ejic.200700963. [DOI] [Google Scholar]
- Rial Verde E. M.; Zayat L.; Etchenique R.; Yuste R. Photorelease of GABA with Visible Light Using an Inorganic Caging Group. Front. Neural Circuits 2008, 2, 2. 10.3389/neuro.04.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salierno M.; Marceca E.; Peterka D. S.; Yuste R.; Etchenique R. A Fast Ruthenium Polypyridine Cage Complex Photoreleases Glutamate With Visible or IR Light in One and Two Photon Regimes. J. Inorg. Biochem. 2010, 104, 418–422. 10.1016/j.jinorgbio.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraballo R. M.; Rosi P.; Hodak J. H.; Baraldo L. M. Photosubstitution of Monodentate Ligands from Ru-II-Dicarboxybipyridine Complexes. Eur. J. Inorg. Chem. 2017, 2017, 3612–3621. 10.1002/ejic.201700439. [DOI] [Google Scholar]
- Battistin F.; Balducci G.; Wei J. H.; Renfrew A. K.; Alessio E. Photolabile Ru Model Complexes with Chelating Diimine Ligands for Light-Triggered Drug Release. Eur. J. Inorg. Chem. 2018, 2018, 1469–1480. 10.1002/ejic.201701392. [DOI] [Google Scholar]
- del Marmol J.; Filevich O.; Etchenique R. A Ruthenium-Rhodamine Complex as an Activatable Fluorescent Probe. Anal. Chem. 2010, 82, 6259–6264. 10.1021/ac1012128. [DOI] [PubMed] [Google Scholar]
- Wachter E.; Heidary D. K.; Howerton B. S.; Parkin S.; Glazer E. C. Light-Activated Ruthenium Complexes Photobind DNA and Are Cytotoxic in the Photodynamic Therapy Window. Chem. Commun. 2012, 48, 9649–9651. 10.1039/c2cc33359g. [DOI] [PubMed] [Google Scholar]
- Knoll J. D.; Albani B. A.; Durr C. B.; Turro C. Unusually Efficient Pyridine Photodissociation from Ru(II) Complexes with Sterically Bulky Bidentate Ancillary Ligands. J. Phys. Chem. A 2014, 118, 10603–10610. 10.1021/jp5057732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salassa L.; Garino C.; Salassa G.; Gobetto R.; Nervi C. Mechanism of Ligand Photodissociation in Photoactivable Ru(bpy)2L22+ Complexes: A Density Functional Theory Study. J. Am. Chem. Soc. 2008, 130, 9590–9597. 10.1021/ja8025906. [DOI] [PubMed] [Google Scholar]
- Salassa L.; Garino C.; Salassa G.; Nervi C.; Gobetto R.; Lamberti C.; Gianolio D.; Bizzarri R.; Sadler P. J. Ligand-Selective Photodissociation from Ru(bpy)(4AP)42+: A Spectroscopic and Computational Study. Inorg. Chem. 2009, 48, 1469–1481. 10.1021/ic8015436. [DOI] [PubMed] [Google Scholar]
- Gottle A. J.; Alary F.; Boggio-Pasqua M.; Dixon I. M.; Heully J. L.; Bahreman A.; Askes S. H. C.; Bonnet S. Pivotal Role of a Pentacoordinate (MC)-M-3 State on the Photocleavage Efficiency of a Thioether Ligand in Ruthenium(II) Complexes: A Theoretical Mechanistic Study. Inorg. Chem. 2016, 55, 4448–4456. 10.1021/acs.inorgchem.6b00268. [DOI] [PubMed] [Google Scholar]
- Loftus L. M.; Al-Afyouni K. F.; Rohrabaugh T. N.; Gallucci J. C.; Moore C. E.; Rack J. J.; Turro C. Unexpected Role of Ru(II) Orbital and Spin Contribution on Photoinduced Ligand Exchange: New Mechanism To Access the Photodynamic Therapy Window. J. Phys. Chem. C 2019, 123, 10291–10299. 10.1021/acs.jpcc.9b01576. [DOI] [Google Scholar]
- van Rixel V. H. S.; Ramu V.; Auyeung A. B.; Beztsinna N.; Leger D. Y.; Lameijer L. N.; Hilt S. T.; Le Dévédec S. E.; Yildiz T.; Betancourt T.; Gildner M. B.; Hudnall T. W.; Sol V.; Liagre B.; Kornienko A.; Bonnet S. Photo-Uncaging of a Microtubule-Targeted Rigidin Analogue in Hypoxic Cancer Cells and in a Xenograft Mouse Model. J. Am. Chem. Soc. 2019, 141, 18444–18454. 10.1021/jacs.9b07225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus L. M.; Al-Afyouni K. F.; Turro C. New RuII Scaffold for Photoinduced Ligand Release with Red Light in the Photodynamic Therapy (PDT) Window. Chem. - Eur. J. 2018, 24, 11550–11553. 10.1002/chem.201802405. [DOI] [PubMed] [Google Scholar]
- Li A.; White J. K.; Arora K.; Herroon M. K.; Martin P. D.; Schlegel H. B.; Podgorski I.; Turro C.; Kodanko J. J. Selective Release of Aromatic Heterocycles from Ruthenium Tris(2-pyridylmethyl)amine with Visible Light. Inorg. Chem. 2016, 55, 10–12. 10.1021/acs.inorgchem.5b02600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R.; Knoll J. D.; Martin P. D.; Podgorski I.; Turro C.; Kodanko J. J. Ruthenium Tris(2-pyridylmethyl)amine as an Effective Photocaging Group for Nitriles. Inorg. Chem. 2014, 53, 3272–3274. 10.1021/ic500299s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora K.; White J. K.; Sharma R.; Mazumder S.; Martin P. D.; Schlegel H. B.; Turro C.; Kodanko J. J. Effects of Methyl Substitution in Ruthenium Tris(2-pyridylmethyl)amine Photocaging Groups for Nitriles. Inorg. Chem. 2016, 55, 6968–6979. 10.1021/acs.inorgchem.6b00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus L. M.; Li A.; Fillman K. L.; Martin P. D.; Kodanko J. J.; Turro C. Unusual Role of Excited State Mixing in the Enhancement of Photoinduced Ligand Exchange in Ru(II) Complexes. J. Am. Chem. Soc. 2017, 139, 18295–18306. 10.1021/jacs.7b09937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahreman A.; Limburg B.; Siegler M. A.; Bouwman E.; Bonnet S. Spontaneous Formation in the Dark, and Visible Light-Induced Cleavage, of a Ru-S Bond in Water: A Thermodynamic and Kinetic Study. Inorg. Chem. 2013, 52, 9456–9469. 10.1021/ic401105v. [DOI] [PubMed] [Google Scholar]
- Bahreman A.; Limburg B.; Siegler M. A.; Koning R.; Koster A. J.; Bonnet S. Ruthenium Polypyridyl Complexes Hopping at Anionic Lipid Bilayers through a Supramolecular Bond Sensitive to Visible Light. Chem. - Eur. J. 2012, 18, 10271–10280. 10.1002/chem.201200624. [DOI] [PubMed] [Google Scholar]
- Chan H.; Ghrayche J. B.; Wei J. H.; Renfrew A. K. Photolabile Ruthenium(II)-Purine Complexes: Phototoxicity, DNA Binding, and Light-Triggered Drug Release. Eur. J. Inorg. Chem. 2017, 2017, 1679–1686. 10.1002/ejic.201601137. [DOI] [Google Scholar]
- Ragazzon G.; Bratsos I.; Alessio E.; Salassa L.; Habtemariam A.; McQuitty R. J.; Clarkson G. J.; Sadler P. J. Design of Photoactivatable Metallodrugs: Selective and Rapid Light-Induced Ligand Dissociation From Half-Sandwich [Ru([9]aneS3)(N–N′)(py)]2+ Complexes. Inorg. Chim. Acta 2012, 393, 230–238. 10.1016/j.ica.2012.06.031. [DOI] [Google Scholar]
- Finazzi I.; Bratsos I.; Gianferrara T.; Bergamo A.; Demitri N.; Balducci G.; Alessio E. Photolabile Ru-II Half-Sandwich Complexes Suitable for Developing “Caged” Compounds: Chemical Investigation and Unexpected Dinuclear Species with Bridging Diamine Ligands. Eur. J. Inorg. Chem. 2013, 2013, 4743–4753. 10.1002/ejic.201300792. [DOI] [Google Scholar]
- Bratsos I.; Alessio E. The Pivotal Role of Ru-DMSO Compounds in the Discovery of Well-Behaved Precursors. Eur. J. Inorg. Chem. 2018, 2018, 2996–3013. 10.1002/ejic.201800469. [DOI] [Google Scholar]
- Respondek T.; Garner R. N.; Herroon M. K.; Podgorski I.; Turro C.; Kodanko J. J. Light Activation of a Cysteine Protease Inhibitor: Caging of a Peptidomimetic Nitrile with RuII(bpy)2. J. Am. Chem. Soc. 2011, 133, 17164–17167. 10.1021/ja208084s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho S. D.; Sharma R.; White J. K.; Aggarwal N.; Chalasani A.; Sameni M.; Moin K.; Vieira P. C.; Turro C.; Kodanko J. J.; Sloane B. F. Imaging Sites of Inhibition of Proteolysis in Pathomimetic Human Breast Cancer Cultures by Light-Activated Ruthenium Compound. PLoS One 2015, 10, e0142527. 10.1371/journal.pone.0142527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herroon M. K.; Sharma R.; Rajagurubandara E.; Turro C.; Kodanko J. J.; Podgorski I. Photoactivated Inhibition of Cathepsin K in a 3D Tumor Model. Biol. Chem. 2016, 397, 571–582. 10.1515/hsz-2015-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lameijer L. N.; Ernst D.; Hopkins S. L.; Meijer M. S.; Askes S. H. C.; Le Devedec S. E.; Bonnet S. A Red-Light-Activated Ruthenium-Caged NAMPT Inhibitor Remains Phototoxic in Hypoxic Cancer Cells. Angew. Chem., Int. Ed. 2017, 56, 11549–11553. 10.1002/anie.201703890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora A.; Denning C. A.; Heidary D. K.; Wachter E.; Nease L. A.; Ruiz J.; Glazer E. C. Ruthenium-Containing p450 Inhibitors for Dual Enzyme Inhibition and DNA Damage. Dalton Trans. 2017, 46, 2165–2173. 10.1039/C6DT04405K. [DOI] [PubMed] [Google Scholar]
- Li A.; Yadav R.; White J. K.; Herroon M. K.; Callahan B. P.; Podgorski I.; Turro C.; Scott E. E.; Kodanko J. J. Illuminating Cytochrome P450 Binding: Ru(II)-Caged Inhibitors of CYP17A1. Chem. Commun. 2017, 53, 3673–3676. 10.1039/C7CC01459G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J. H.; Renfrew A. K. Photolabile Ruthenium Complexes to Cage and Release a Highly Cytotoxic Anticancer Agent. J. Inorg. Biochem. 2018, 179, 146–153. 10.1016/j.jinorgbio.2017.11.018. [DOI] [PubMed] [Google Scholar]
- Karaoun N.; Renfrew A. K. A Luminescent Ruthenium(II) Complex for Light-Triggered Drug Release and Live Cell Imaging. Chem. Commun. 2015, 51, 14038–14041. 10.1039/C5CC05172J. [DOI] [PubMed] [Google Scholar]
- Smith N. A.; Zhang P. Y.; Greenough S. E.; Horbury M. D.; Clarkson G. J.; McFeely D.; Habtemariam A.; Salassa L.; Stavros V. G.; Dowson C. G.; Sadler P. J. Combatting AMR: Photoactivatable Ruthenium(II)-Isoniazid Complex Exhibits Rapid Selective Antimycobacterial Activity. Chem. Sci. 2017, 8, 395–404. 10.1039/C6SC03028A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner R. N.; Gallucci J. C.; Dunbar K. R.; Turro C. Ru(bpy)2(5-cyanouracil)22+ as a Potential Light-Activated Dual-Action Therapeutic Agent. Inorg. Chem. 2011, 50, 9213–9215. 10.1021/ic201615u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgambellone M. A.; David A.; Garner R. N.; Dunbar K. R.; Turro C. Cellular Toxicity Induced by the Photorelease of a Caged Bioactive Molecule: Design of a Potential Dual-Action Ru(II) Complex. J. Am. Chem. Soc. 2013, 135, 11274–11282. 10.1021/ja4045604. [DOI] [PubMed] [Google Scholar]
- Mosquera J.; Sanchez M. I.; Mascarenas J. L.; Vazquez M. E. Synthetic Peptides Caged on Histidine Residues With a Bisbipyridyl Ruthenium(II) Complex That Can Be Photolyzed by Visible Light. Chem. Commun. 2015, 51, 5501–5504. 10.1039/C4CC08049A. [DOI] [PubMed] [Google Scholar]
- Sharma R.; Knoll J. D.; Ancona N.; Martin P. D.; Turro C.; Kodanko J. J. Solid-Phase Synthesis as a Platform for the Discovery of New Ruthenium Complexes for Efficient Release of Photocaged Ligands with Visible Light. Inorg. Chem. 2015, 54, 1901–1911. 10.1021/ic502791y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis S.; Iturmendi A.; Gorsche C.; Orthofer M.; Lunzer M.; Baudis S.; Ovsianikov A.; Liska R.; Monkowius U.; Teasdale I. Metallo-Supramolecular Gels that are Photocleavable with Visible and Near-Infrared Irradiation. Angew. Chem., Int. Ed. 2017, 56, 15857–15860. 10.1002/anie.201707321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale I.; Theis S.; Iturmendi A.; Strobel M.; Hild S.; Jacak J.; Mayrhofer P.; Monkowius U. Dynamic Supramolecular Ruthenium-Based Gels Responsive to Visible/NIR Light and Heat. Chem. - Eur. J. 2019, 25, 9851–9855. 10.1002/chem.201902088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albani B. A.; Peña B.; Leed N. A.; de Paula N. A. B. G.; Pavani C.; Baptista M. S.; Dunbar K. R.; Turro C. Marked Improvement in Photoinduced Cell Death by a New Tris-heteroleptic Complex with Dual Action: Singlet Oxygen Sensitization and Ligand Dissociation. J. Am. Chem. Soc. 2014, 136, 17095–17101. 10.1021/ja508272h. [DOI] [PubMed] [Google Scholar]
- Knoll J. D.; Albani B. A.; Turro C. Excited State Investigation of a New Ru(II) Complex for Dual Reactivity With Low Energy Light. Chem. Commun. 2015, 51, 8777–8780. 10.1039/C5CC01865J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus L. M.; White J. K.; Albani B. A.; Kohler L.; Kodanko J. J.; Thummel R. P.; Dunbar K. R.; Turro C. New Ru-II Complex for Dual Activity: Photoinduced Ligand Release and O-1(2) Production. Chem. - Eur. J. 2016, 22, 3704–3708. 10.1002/chem.201504800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro Z. A.; de Moraes J. C. B.; Rodrigues F. P.; de Lima R. G.; Curti C.; da Rocha Z. N.; Paulo M.; Bendhack L. M.; Tedesco A. C.; Formiga A. L. B.; da Silva R. S. Photocytotoxic Activity of a Nitrosyl Phthalocyanine Ruthenium Complex - A System Capable of Producing Nitric Oxide and Singlet Oxygen. J. Inorg. Biochem. 2011, 105, 1035–1043. 10.1016/j.jinorgbio.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Burya S. J.; Palmer A. M.; Gallucci J. C.; Turro C. Photoinduced Ligand Exchange and Covalent DNA Binding by Two New Dirhodium Bis-Amidato Complexes. Inorg. Chem. 2012, 51, 11882–11890. 10.1021/ic3017886. [DOI] [PubMed] [Google Scholar]
- Chifotides H. T.; Lutterman D. A.; Dunbar K. R.; Turro C. Insight into the Photoinduced Ligand Exchange Reaction Pathway of cis- Rh2((-O2CCH3)2(CH3CN)62+ with a DNA Model Chelate. Inorg. Chem. 2011, 50, 12099–12107. 10.1021/ic201645b. [DOI] [PubMed] [Google Scholar]
- Li Z. Y.; Burya S. J.; Turro C.; Dunbar K. R. Photochemistry and DNA Photocleavage by a new Unsupported Dirhodium(II, II) Complex. Philos. Trans. R. Soc., A 2013, 371, 20120128. 10.1098/rsta.2012.0128. [DOI] [PubMed] [Google Scholar]
- Akhimie R. N.; White J. K.; Turro C. Dual Photoreactivity of a New Rh2(II, II) Complex for Biological Applications. Inorg. Chim. Acta 2017, 454, 149–154. 10.1016/j.ica.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarski P. J.; Mackay F. S.; Sadler P. J. Photoactivatable Platinum Complexes. Anti-Cancer Agents Med. Chem. 2007, 7, 75–93. 10.2174/187152007779314053. [DOI] [PubMed] [Google Scholar]
- Wilson J. J.; Lippard S. J. Synthetic Methods for the Preparation of Platinum Anticancer Complexes. Chem. Rev. 2014, 114, 4470–4495. 10.1021/cr4004314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer N. J.; Woods J. A.; Salassa L.; Zhao Y.; Robinson K. S.; Clarkson G.; Mackay F. S.; Sadler P. J. A Potent Trans-Diimine Platinum Anticancer Complex Photoactivated by Visible Light. Angew. Chem., Int. Ed. 2010, 49, 8905–8908. 10.1002/anie.201003399. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Woods J. A.; Farrer N. J.; Robinson K. S.; Pracharova J.; Kasparkova J.; Novakova O.; Li H. L.; Salassa L.; Pizarro A. M.; Clarkson G. J.; Song L. J.; Brabec V.; Sadler P. J. Diazido Mixed-Amine Platinum(IV) Anticancer Complexes Activatable by Visible-Light Form Novel DNA Adducts. Chem. - Eur. J. 2013, 19, 9578–9591. 10.1002/chem.201300374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Farrer N. J.; Li H. L.; Butler J. S.; McQuitty R. J.; Habtemariam A.; Wang F. Y.; Sadler P. J. De Novo Generation of Singlet Oxygen and Ammine Ligands by Photoactivation of a Platinum Anticancer Complex. Angew. Chem., Int. Ed. 2013, 52, 13633–13637. 10.1002/anie.201307505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J. S.; Woods J. A.; Farrer N. J.; Newton M. E.; Sadler P. J. Tryptophan Switch for a Photoactivated Platinum Anticancer Complex. J. Am. Chem. Soc. 2012, 134, 16508–16511. 10.1021/ja3074159. [DOI] [PubMed] [Google Scholar]
- Venkatesh V.; Mishra N. K.; Romero-Canelón I.; Vernooij R. R.; Shi H.; Coverdale J. P. C.; Habtemariam A.; Verma S.; Sadler P. J. Supramolecular Photoactivatable Anticancer Hydrogels. J. Am. Chem. Soc. 2017, 139, 5656–5659. 10.1021/jacs.7b00186. [DOI] [PubMed] [Google Scholar]
- Yao K.; Bertran A.; Howarth A.; Goicoechea J. M.; Hare S. M.; Rees N. H.; Foroozandeh M.; Bowen A. M.; Farrer N. J. A Visible-Light Photoactivatable di-Nuclear PtIV Triazolato Azido Complex. Chem. Commun. 2019, 55, 11287–11290. 10.1039/C9CC05310G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z.; Wang N.; Liu Y.; Xu Z.; Wang Z.; Lau T.-C.; Zhu G. A Photocaged, Water-Oxidizing, and Nucleolus-Targeted Pt(IV) Complex with a Distinct Anticancer Mechanism. J. Am. Chem. Soc. 2020, 142, 7803–7812. 10.1021/jacs.0c00221. [DOI] [PubMed] [Google Scholar]
- Mitra K.; Gautam S.; Kondaiah P.; Chakravarty A. R. The cis-Diammineplatinum(II) Complex of Curcumin: A Dual Action DNA Crosslinking and Photochemotherapeutic Agent. Angew. Chem., Int. Ed. 2015, 54, 13989–13993. 10.1002/anie.201507281. [DOI] [PubMed] [Google Scholar]
- Mitra K.; Gautam S.; Kondaiah P.; Chakravarty A. R. Platinum(II) Complexes of Curcumin Showing Photocytotoxicity in Visible Light. Eur. J. Inorg. Chem. 2017, 2017, 1753–1763. 10.1002/ejic.201601078. [DOI] [Google Scholar]
- Bhattacharyya A.; Dixit A.; Mitra K.; Banerjee S.; Karande A. A.; Chakravarty A. R. BODIPY Appended Copper(II) Complexes of Curcumin Showing Mitochondria Targeted Remarkable Photocytotoxicity in Visible Light. MedChemComm 2015, 6, 846–851. 10.1039/C4MD00425F. [DOI] [Google Scholar]
- Ramu V.; Gautam S.; Garai A.; Kondaiah P.; Chakravarty A. R. Glucose-Appended Platinum(II)-BODIPY Conjugates for Targeted Photodynamic Therapy in Red Light. Inorg. Chem. 2018, 57, 1717–1726. 10.1021/acs.inorgchem.7b02249. [DOI] [PubMed] [Google Scholar]
- Mitra K.; Shettar A.; Kondaiah P.; Chakravarty A. R. Biotinylated Platinum(II) Ferrocenylterpyridine Complexes for Targeted Photoinduced Cytotoxicity. Inorg. Chem. 2016, 55, 5612–5622. 10.1021/acs.inorgchem.6b00680. [DOI] [PubMed] [Google Scholar]
- Mitra K.; Lyons C. E.; Hartman M. C. T. A Platinum(II) Complex of Heptamethine Cyanine for Photoenhanced Cytotoxicity and Cellular Imaging in Near-IR Light. Angew. Chem., Int. Ed. 2018, 57, 10263–10267. 10.1002/anie.201806911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward A. M.; Moore E. G.; Hartshorn R. M. Photoinduced Ligand Release in a Ruthenium(II)-Cobalt(III) Heterodinuclear Systembasic Understanding of the Lanthanide Related Upconversion Emissions. Chem. Commun. 2011, 47, 7692–7694. 10.1039/c1cc12729b. [DOI] [PubMed] [Google Scholar]
- Garai A.; Pant I.; Banerjee S.; Banik B.; Kondaiah P.; Chakravarty A. R. Photorelease and Cellular Delivery of Mitocurcumin from Its Cytotoxic Cobalt(III) Complex in Visible Light. Inorg. Chem. 2016, 55, 6027–6035. 10.1021/acs.inorgchem.6b00554. [DOI] [PubMed] [Google Scholar]
- Jana A.; Verma B. K.; Garai A.; Kondaiah P.; Chakravarty A. R. Mitochondria Localizing High-Spin Iron Complexes of Curcumin for Photo-Induced Drug Release. Inorg. Chim. Acta 2018, 483, 571–578. 10.1016/j.ica.2018.09.008. [DOI] [Google Scholar]
- Giammanco G. E.; Sosnofsky C. T.; Ostrowski A. D. Light-Responsive Iron(III)-Polysaccharide Coordination Hydrogels for Controlled Delivery. ACS Appl. Mater. Interfaces 2015, 7, 3068–3076. 10.1021/am506772x. [DOI] [PubMed] [Google Scholar]
- Donald J. A.Chapter 103 - Gasotransmitter Family. Handbook of Hormones; Takei Y., Ando H., Tsutsui K., Eds.; Academic Press: San Diego, 2016. [Google Scholar]
- Wareham L. K.; Southam H. M.; Poole R. K. Do Nitric Oxide, Carbon Monoxide and Hydrogen Sulfide Really Qualify as ‘Gasotransmitters’ in Bacteria?. Biochem. Soc. Trans. 2018, 46, 1107–1118. 10.1042/BST20170311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorup C.; Jones C. L.; Gross S. S.; Moore L. C.; Goligorsky M. S. Carbon Monoxide Induces Vasodilation and Nitric Oxide Release but Suppresses Endothelial NOS. Am. J. Physiol.-Renal 1999, 277, F882–F889. 10.1152/ajprenal.1999.277.6.F882. [DOI] [PubMed] [Google Scholar]
- Romao C. C.; Blattler W. A.; Seixas J. D.; Bernardes G. J. L. Developing Drug Molecules for Therapy With Carbon Monoxide. Chem. Soc. Rev. 2012, 41, 3571–3583. 10.1039/c2cs15317c. [DOI] [PubMed] [Google Scholar]
- Queiroga C. S. F.; Tomasi S.; Widerøe M.; Alves P. M.; Vercelli A.; Vieira H. L. A. Preconditioning Triggered by Carbon Monoxide (CO) Provides Neuronal Protection Following Perinatal Hypoxia-Ischemia. PLoS One 2012, 7, e42632 10.1371/journal.pone.0042632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterbein L. E.; Bach F. H.; Alam J.; Soares M.; Tao Lu H.; Wysk M.; Davis R. J.; Flavell R. A.; Choi A. M. K. Carbon Monoxide has Anti-Inflammatory Effects Involving the Mitogen-Activated Protein Kinase Pathway. Nat. Med. 2000, 6, 422–428. 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- Brandi C.; Grimaldi L.; Nisi G.; Brafa A.; Campa A.; Calabrò M.; Campana M.; D’Aniello C. The Role of Carbon Dioxide Therapy in the Treatment of Chronic Wounds. In Vivo 2010, 24, 223–226. [PubMed] [Google Scholar]
- Schäffer M. R.; Tantry U.; Gross S. S.; Wasserkrug H. L.; Barbul A. Nitric Oxide Regulates Wound Healing. J. Surg. Res. 1996, 63, 237–240. 10.1006/jsre.1996.0254. [DOI] [PubMed] [Google Scholar]
- Radomski M. W.; Palmer R. M. J.; Moncada S. The Role of Nitric Oxide and cGMP in Platelet Adhesion to Vascular Endothelium. Biochem. Biophys. Res. Commun. 1987, 148, 1482–1489. 10.1016/S0006-291X(87)80299-1. [DOI] [PubMed] [Google Scholar]
- Garthwaite J.; Boulton C. L. Nitric Oxide Signaling in the Central Nervous System. Annu. Rev. Physiol. 1995, 57, 683–706. 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- Gullotta F.; Masi A. d.; Ascenzi P. Carbon Monoxide: An Unusual Drug. IUBMB Life 2012, 64, 378–386. 10.1002/iub.1015. [DOI] [PubMed] [Google Scholar]
- Bloch K. D.; Ichinose F.; Roberts J. D. Jr.; Zapol W. M. Inhaled NO as a Therapeutic Agent. Cardiovasc. Res. 2007, 75, 339–348. 10.1016/j.cardiores.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motterlini R.; Otterbein L. E. The Therapeutic Potential of Carbon Monoxide. Nat. Rev. Drug Discovery 2010, 9, 728–U724. 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- Heinemann S. H.; Hoshi T.; Westerhausen M.; Schiller A. Carbon Monoxide - Physiology, Detection and Controlled Release. Chem. Commun. 2014, 50, 3644–3660. 10.1039/C3CC49196J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.-X.; Ke B.-W.; Lu W.; Wang B.-H. CO as a Therapeutic Agent: Discovery and Delivery Forms. Chin. J. Nat. Medicines 2020, 18, 284–295. 10.1016/S1875-5364(20)30036-4. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Yu W.; Cao J.; Gao H. Harnessing Carbon Monoxide-Releasing Platforms for Cancer Therapy. Biomaterials 2020, 255, 120193. 10.1016/j.biomaterials.2020.120193. [DOI] [PubMed] [Google Scholar]
- Stucki D.; Stahl W. Carbon Monoxide – Beyond Toxicity?. Toxicol. Lett. 2020, 333, 251–260. 10.1016/j.toxlet.2020.08.010. [DOI] [PubMed] [Google Scholar]
- Russo M.; Štacko P.; Nachtigallová D.; Klán P. Mechanisms of Orthogonal Photodecarbonylation Reactions of 3-Hydroxyflavone-Based Acid–Base Forms. J. Org. Chem. 2020, 85, 3527–3537. 10.1021/acs.joc.9b03248. [DOI] [PubMed] [Google Scholar]
- Anderson S. N.; Richards J. M.; Esquer H. J.; Benninghoff A. D.; Arif A. M.; Berreau L. M. A Structurally-Tunable 3-Hydroxyflavone Motif for Visible Light-Induced Carbon Monoxide-Releasing Molecules (CORMs). ChemistryOpen 2015, 4, 590–594. 10.1002/open.201500167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieda N.; Hotta Y.; Miyata N.; Kimura K.; Nakagawa H. Photomanipulation of Vasodilation with a Blue-Light-Controllable Nitric Oxide Releaser. J. Am. Chem. Soc. 2014, 136, 7085–7091. 10.1021/ja5020053. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Wu J.; Shang Z.; Wang C.; Cheng J.; Qian X.; Xiao Y.; Xu Z.; Yang Y. Photocalibrated NO Release from N-Nitrosated Napthalimides upon One-Photon or Two-Photon Irradiation. Anal. Chem. 2016, 88, 7274–7280. 10.1021/acs.analchem.6b01603. [DOI] [PubMed] [Google Scholar]
- Gonzalez M. A.; Carrington S. J.; Fry N. L.; Martinez J. L.; Mascharak P. K. Syntheses, Structures, and Properties of New Manganese Carbonyls as Photoactive CO-Releasing Molecules: Design Strategies that Lead to CO Photolability in the Visible Region. Inorg. Chem. 2012, 51, 11930–11940. 10.1021/ic3018216. [DOI] [PubMed] [Google Scholar]
- Carrington S. J.; Chakraborty I.; Mascharak P. K. Exceptionally Rapid CO Release from a Manganese(I) Tricarbonyl Complex Derived from Bis(4-chloro-phenylimino)acenaphthene upon Exposure to Visible Light. Dalton Trans. 2015, 44, 13828–13834. 10.1039/C5DT01007A. [DOI] [PubMed] [Google Scholar]
- Eroy-Reveles A. A.; Leung Y.; Beavers C. M.; Olmstead M. M.; Mascharak P. K. Near-Infrared Light Activated Release of Nitric Oxide from Designed Photoactive Manganese Nitrosyls: Strategy, Design, and Potential as NO Donors. J. Am. Chem. Soc. 2008, 130, 4447–4458. 10.1021/ja710265j. [DOI] [PubMed] [Google Scholar]
- Hoffman-Luca C. G.; Eroy-Reveles A. A.; Alvarenga J.; Mascharak P. K. Syntheses, Structures, and Photochemistry of Manganese Nitrosyls Derived from Designed Schiff Base Ligands: Potential NO Donors That Can Be Activated by Near-Infrared Light. Inorg. Chem. 2009, 48, 9104–9111. 10.1021/ic900604j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods J. J.; Cao J.; Lippert A. R.; Wilson J. J. Characterization and Biological Activity of a Hydrogen Sulfide-Releasing Red Light-Activated Ruthenium(II) Complex. J. Am. Chem. Soc. 2018, 140, 12383–12387. 10.1021/jacs.8b08695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez R.; Kable S. H.; Loison J. C.; Simpson C. J. S. M.; Adam W.; Houston P. L. Photodissociation Dynamics of 3-Cyclopentenone: Using the Impact Parameter Distribution as a Criterion for Concertedness. J. Phys. Chem. 1992, 96, 4188–4195. 10.1021/j100190a018. [DOI] [Google Scholar]
- Mondal R.; Okhrimenko A. N.; Shah B. K.; Neckers D. C. Photodecarbonylation of α-Diketones: A Mechanistic Study of Reactions Leading to Acenes. J. Phys. Chem. B 2008, 112, 11–15. 10.1021/jp076738l. [DOI] [PubMed] [Google Scholar]
- Michael E.; Abeyrathna N.; Patel A. V.; Liao Y.; Bashur C. A. Incorporation of Photo-Carbon Monoxide Releasing Materials into Electrospun Scaffolds for Vascular Tissue Engineering. Biomed. Mater. 2016, 11, 025009. 10.1088/1748-6041/11/2/025009. [DOI] [PubMed] [Google Scholar]
- Peng P.; Wang C.; Shi Z.; Johns V. K.; Ma L.; Oyer J.; Copik A.; Igarashi R.; Liao Y. Visible-Light Activatable Organic CO-Releasing Molecules (PhotoCORMs) That Simultaneously Generate Fluorophores. Org. Biomol. Chem. 2013, 11, 6671–6674. 10.1039/c3ob41385c. [DOI] [PubMed] [Google Scholar]
- Elgattar A.; Washington K. S.; Talebzadeh S.; Alwagdani A.; Khalil T.; Alghazwat O.; Alshammri S.; Pal H.; Bashur C.; Liao Y. Poly(butyl Cyanoacrylate) Nanoparticle Containing an Organic PhotoCORM. Photochem. Photobiol. Sci. 2019, 18, 2666–2672. 10.1039/C9PP00287A. [DOI] [PubMed] [Google Scholar]
- Thapaliya E. R.; Swaminathan S.; Captain B.; Raymo F. M. Autocatalytic Fluorescence Photoactivation. J. Am. Chem. Soc. 2014, 136, 13798–13804. 10.1021/ja5068383. [DOI] [PubMed] [Google Scholar]
- Aotake T.; Suzuki M.; Tahara K.; Kuzuhara D.; Aratani N.; Tamai N.; Yamada H. An Optically and Thermally Switchable Electronic Structure Based on an Anthracene–BODIPY Conjugate. Chem. - Eur. J. 2015, 21, 4966–4974. 10.1002/chem.201406384. [DOI] [PubMed] [Google Scholar]
- Mondal R.; Shah B. K.; Neckers D. C. Photogeneration of Heptacene in a Polymer Matrix. J. Am. Chem. Soc. 2006, 128, 9612–9613. 10.1021/ja063823i. [DOI] [PubMed] [Google Scholar]
- Mondal R.; Adhikari R. M.; Shah B. K.; Neckers D. C. Revisiting the Stability of Hexacenes. Org. Lett. 2007, 9, 2505–2508. 10.1021/ol0709376. [DOI] [PubMed] [Google Scholar]
- Gilbert B. C.; Hodges G. R.; Smith J. R. L.; MacFaul P.; Taylor P. Photodecarboxylation of Substituted Alkylcarboxylic Acids Brought about by Visible Light and Iron(III) tetra(2-N-Methylpyridyl)porphyrin in Aqueous Solution. J. Chem. Soc., Perkin Trans. 2 1996, 2, 519–524. 10.1039/p29960000519. [DOI] [Google Scholar]
- Griffiths H. R.; Gao D.; Pararasa C. Redox Regulation in Metabolic Programming and Inflammation. Redox Biol. 2017, 12, 50–57. 10.1016/j.redox.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R. Redox outside the Box: Linking Extracellular Redox Remodeling with Intracellular Redox Metabolism. J. Biol. Chem. 2012, 287, 4397–4402. 10.1074/jbc.R111.287995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura T.; Matsushima H.; Sakamoto H. Photosensitized Oxygenation of 3-Hydroxyflavones. Possible Model for Biological Oxygenation. J. Am. Chem. Soc. 1967, 89, 6370–6371. 10.1021/ja01000a078. [DOI] [PubMed] [Google Scholar]
- Studer S. L.; Brewer W. E.; Martinez M. L.; Chou P. T. Time-Resolved Study of the Photooxygenation of 3-Hydroxyflavone. J. Am. Chem. Soc. 1989, 111, 7643–7644. 10.1021/ja00201a071. [DOI] [Google Scholar]
- Tournaire C.; Croux S.; Maurette M.-T.; Beck I.; Hocquaux M.; Braun A. M.; Oliveros E. Antioxidant Activity of Flavonoids: Efficiency of Singlet Oxygen (1Δg) Quenching. J. Photochem. Photobiol., B 1993, 19, 205–215. 10.1016/1011-1344(93)87086-3. [DOI] [PubMed] [Google Scholar]
- Protti S.; Mezzetti A.; Lapouge C.; Cornard J.-P. Photochemistry of Metal Complexes of 3-Hydroxyflavone: Towards a Better Understanding of the Influence of Solar Light on the Metal–Soil Organic Matter Interactions. Photochem. Photobiol. Sci. 2008, 7, 109–119. 10.1039/B709682H. [DOI] [PubMed] [Google Scholar]
- Matsuura T.; Takemoto T.; Nakashima R. Photoinduced Reactions-LXXI: Photorearrangement of 3-Hydroxyflavones to 3-Aryl-3-Hydroxy-1,2-Indandiones. Tetrahedron 1973, 29, 3337–3340. 10.1016/S0040-4020(01)93485-4. [DOI] [Google Scholar]
- Szakács Z.; Bojtár M.; Drahos L.; Hessz D.; Kállay M.; Vidóczy T.; Bitter I.; Kubinyi M. The Kinetics and Mechanism of Photooxygenation of 4′-Diethylamino-3-hydroxyflavone. Photochem. Photobiol. Sci. 2016, 15, 219–227. 10.1039/C5PP00358J. [DOI] [PubMed] [Google Scholar]
- Mierziak J.; Kostyn K.; Kulma A. Flavonoids as Important Molecules of Plant Interactions with the Environment. Molecules 2014, 19, 16240–16265. 10.3390/molecules191016240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. N.; Noble M.; Grubel K.; Marshall B.; Arif A. M.; Berreau L. M. Influence of Supporting Ligand Microenvironment on the Aqueous Stability and Visible Light-Induced CO-Release Reactivity of Zinc Flavonolato Species. J. Coord. Chem. 2014, 67, 4061–4075. 10.1080/00958972.2014.977272. [DOI] [Google Scholar]
- Su Y.; Yang W.; Yang X.; Zhang R.; Zhao J. Visible Light-Induced CO-Release Reactivity of a Series of ZnII–Flavonolate Complexes. Aust. J. Chem. 2018, 71, 549–558. 10.1071/CH18192. [DOI] [Google Scholar]
- Sorenson S.; Popova M.; Arif A. M.; Berreau L. M. A Bipyridine-Ligated Zinc(II) Complex with Bridging Flavonolate Ligation: Synthesis, Characterization, and Visible-Light-Induced CO Release Reactivity. Acta Crystallogr., Sect. C: Struct. Chem. 2017, 73, 703–709. 10.1107/S2053229617011366. [DOI] [PubMed] [Google Scholar]
- Saraf S. L.; Fish T. J.; Benninghoff A. D.; Buelt A. A.; Smith R. C.; Berreau L. M. Photochemical Reactivity of RuII(η6-p-cymene) Flavonolato Compounds. Organometallics 2014, 33, 6341–6351. 10.1021/om5006337. [DOI] [Google Scholar]
- Han X.; Klausmeyer K. K.; Farmer P. J. Characterization of the Initial Intermediate Formed during Photoinduced Oxygenation of the Ruthenium(II) Bis(bipyridyl)flavonolate Complex. Inorg. Chem. 2016, 55, 7320–7322. 10.1021/acs.inorgchem.6b00852. [DOI] [PubMed] [Google Scholar]
- Han X.; Kumar M. R.; Hoogerbrugge A.; Klausmeyer K. K.; Ghimire M. M.; Harris L. M.; Omary M. A.; Farmer P. J. Mechanistic Investigations of Photoinduced Oxygenation of Ru(II) Bis-bipyridyl Flavonolate Complexes. Inorg. Chem. 2018, 57, 2416–2424. 10.1021/acs.inorgchem.7b01384. [DOI] [PubMed] [Google Scholar]
- Grubel K.; Laughlin B. J.; Maltais T. R.; Smith R. C.; Arif A. M.; Berreau L. M. Photochemically-Induced Dioxygenase-Type CO-Release Reactivity of Group 12 Metal Flavonolate Complexes. Chem. Commun. 2011, 47, 10431–10433. 10.1039/c1cc13961d. [DOI] [PubMed] [Google Scholar]
- Popova M.; Soboleva T.; Arif A. M.; Berreau L. M. Properties of a Flavonol-Based PhotoCORM in Aqueous Buffered Solutions: Influence of Metal Ions, Surfactants and Proteins on Visible Light-Induced CO Release. RSC Adv. 2017, 7, 21997–22007. 10.1039/C7RA02653F. [DOI] [Google Scholar]
- Soboleva T.; Benninghoff A. D.; Berreau L. M. An H2S-Sensing/CO-Releasing Flavonol That Operates via Logic Gates. ChemPlusChem 2017, 82, 1408–1412. 10.1002/cplu.201700524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboleva T.; Esquer H. J.; Benninghoff A. D.; Berreau L. M. Sense and Release: A Thiol-Responsive Flavonol-Based Photonically Driven Carbon Monoxide-Releasing Molecule That Operates via a Multiple-Input AND Logic Gate. J. Am. Chem. Soc. 2017, 139, 9435–9438. 10.1021/jacs.7b04077. [DOI] [PubMed] [Google Scholar]
- Popova M.; Soboleva T.; Benninghoff A. D.; Berreau L. M. CO Sense and Release Flavonols: Progress toward the Development of an Analyte Replacement PhotoCORM for Use in Living Cells. ACS Omega 2020, 5, 10021–10033. 10.1021/acsomega.0c00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Shu Y.; Liang M.; Xie X.; Jiao X.; Wang X.; Tang B. A Two-Photon H2O2-Activated CO Photoreleaser. Angew. Chem., Int. Ed. 2018, 57, 12415–12519. 10.1002/anie.201805806. [DOI] [PubMed] [Google Scholar]
- Cheng J.; Zheng B.; Cheng S.; Zhang G.; Hu J. Metal-Free Carbon Monoxide-Releasing Micelles Undergo Tandem Photochemical Reactions for Cutaneous Wound Healing. Chem. Sci. 2020, 11, 4499–4507. 10.1039/D0SC00135J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.; Cheng J.; Huang X.; Zhang G.; Ding S.; Hu J.; Qiao R. Photo-Degradable Micelles Capable of Releasing of Carbon Monoxide under Visible Light Irradiation. Macromol. Rapid Commun. 2020, 41, 2000323. 10.1002/marc.202000323. [DOI] [PubMed] [Google Scholar]
- Soboleva T.; Esquer H. J.; Anderson S. N.; Berreau L. M.; Benninghoff A. D. Mitochondrial-Localized Versus Cytosolic Intracellular CO-Releasing Organic PhotoCORMs: Evaluation of CO Effects Using Bioenergetics. ACS Chem. Biol. 2018, 13, 2220–2228. 10.1021/acschembio.8b00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboleva T.; Simons C. R.; Arcidiacono A.; Benninghoff A. D.; Berreau L. M. Extracellular vs Intracellular Delivery of CO: Does It Matter for a Stable, Diffusible Gasotransmitter?. J. Med. Chem. 2019, 62, 9990–9995. 10.1021/acs.jmedchem.9b01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova M.; Soboleva T.; Ayad S.; Benninghoff A. D.; Berreau L. M. Visible-Light-Activated Quinolone Carbon-Monoxide-Releasing Molecule: Prodrug and Albumin-Assisted Delivery Enables Anticancer and Potent Anti-Inflammatory Effects. J. Am. Chem. Soc. 2018, 140, 9721–9729. 10.1021/jacs.8b06011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W.; Feng S.; Feng G. CO Release with Ratiometric Fluorescence Changes: a Promising Visible-Light-Triggered Metal-Free CO-Releasing Molecule. Chem. Commun. 2019, 55, 8987–8990. 10.1039/C9CC04026A. [DOI] [PubMed] [Google Scholar]
- Schwartz B. J.; Peteanu L. A.; Harris C. B. Direct Observation of Fast Proton Transfer: Femtosecond Photophysics of 3-Hydroxyflavone. J. Phys. Chem. 1992, 96, 3591–3598. 10.1021/j100188a009. [DOI] [Google Scholar]
- Dick B.; Ernsting N. P. Excited-State Intramolecular Proton Transfer in 3-Hydroxylflavone Isolated in Solid Argon: Fluoroescence and Fluorescence-Excitation Spectra and Tautomer Fluorescence Rise Time. J. Phys. Chem. 1987, 91, 4261–4265. 10.1021/j100300a012. [DOI] [Google Scholar]
- Dávila Y. A.; Sancho M. I.; Almandoz M. C.; Blanco S. E. Solvent Effects on the Dissociation Constants of Hydroxyflavones in Organic–Water Mixtures. Determination of the Thermodynamic pKa Values by UV–Visible Spectroscopy and DFT Calculations. J. Chem. Eng. Data 2013, 58, 1706–1716. 10.1021/je400153r. [DOI] [Google Scholar]
- Štacková L.; Russo M.; Muchová L.; Orel V.; Vítek L.; Štacko P.; Klán P. Cyanine-Flavonol Hybrid for Near-Infrared Light-Activated Delivery of Carbon Monoxide. Chem. - Eur. J. 2020, 26, 13184–13190. 10.1002/chem.202003272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. N.; Larson M. T.; Berreau L. M. Solution or Solid – It Doesn’t Matter: Visible Light-Induced CO Release Reactivity of Zinc Flavonolato Complexes. Dalton Trans. 2016, 45, 14570–14580. 10.1039/C6DT01709F. [DOI] [PubMed] [Google Scholar]
- An S.-Y.; Su Y.-Y.; Qi X.; Zhang R.-L.; Ma Y.-L.; Zhao J.-S. Photoinduced Reactivity and Cytotoxicity of a Series of Zinc(II)–Flavonolate Derivative Complexes. Transition Met. Chem. 2020, 45, 253–266. 10.1007/s11243-020-00377-w. [DOI] [Google Scholar]
- Poloukhtine A.; Popik V. V. Highly Efficient Photochemical Generation of a Triple Bond: Synthesis, Properties, and Photodecarbonylation of Cyclopropenones. J. Org. Chem. 2003, 68, 7833–7840. 10.1021/jo034869m. [DOI] [PubMed] [Google Scholar]
- Urdabayev N. K.; Poloukhtine A.; Popik V. V. Two-Photon Induced Photodecarbonylation Reaction of Cyclopropenones. Chem. Commun. 2006, 454–456. 10.1039/B513248G. [DOI] [PubMed] [Google Scholar]
- Wadsworth D. H.; Donatelli B. A. Preparation of Diarylacetylenes via Cyclopropenones. Synthesis 1981, 1981, 285–286. 10.1055/s-1981-29417. [DOI] [Google Scholar]
- Becker H. D.; Andersson K. On the Relationship between Molecular Geometry and Photochemical Properties of 1,2-Substituted 1,2-Di-9-anthrylethylenes. J. Org. Chem. 1987, 52, 5205–5213. 10.1021/jo00232a027. [DOI] [Google Scholar]
- Kuzmanich G.; Gard M. N.; Garcia-Garibay M. A. Photonic Amplification by a Singlet-State Quantum Chain Reaction in the Photodecarbonylation of Crystalline Diarylcyclopropenones. J. Am. Chem. Soc. 2009, 131, 11606–11614. 10.1021/ja9043449. [DOI] [PubMed] [Google Scholar]
- L’Abbé G. Heterocyclic Analogues of Methylenecyclopropanes. Angew. Chem., Int. Ed. Engl. 1980, 19, 276–289. 10.1002/anie.198002761. [DOI] [Google Scholar]
- Showalter B. M.; Toscano J. P. Time-Resolved IR Studies of α-Lactones. J. Phys. Org. Chem. 2004, 17, 743–748. 10.1002/poc.789. [DOI] [Google Scholar]
- Mula S.; Ray A. K.; Banerjee M.; Chaudhuri T.; Dasgupta K.; Chattopadhyay S. Design and Development of a New Pyrromethene Dye with Improved Photostability and Lasing Efficiency: Theoretical Rationalization of Photophysical and Photochemical Properties. J. Org. Chem. 2008, 73, 2146–2154. 10.1021/jo702346s. [DOI] [PubMed] [Google Scholar]
- Costela A.; García-Moreno I.; Pintado-Sierra M.; Amat-Guerri F.; Liras M.; Sastre R.; Arbeloa F. L.; Prieto J. B.; Arbeloa I. L. New Laser Dye Based on the 3-Styryl Analog of the BODIPY Dye PM567. J. Photochem. Photobiol., A 2008, 198, 192–199. 10.1016/j.jphotochem.2008.03.010. [DOI] [Google Scholar]
- Ortiz M. J.; Garcia-Moreno I.; Agarrabeitia A. R.; Duran-Sampedro G.; Costela A.; Sastre R.; López Arbeloa F.; Bañuelos Prieto J.; López Arbeloa I. Red-Edge-Wavelength Finely-Tunable Laser Action from New BODIPY Dyes. Phys. Chem. Chem. Phys. 2010, 12, 7804–7811. 10.1039/b925561c. [DOI] [PubMed] [Google Scholar]
- Saito F.; Tobita S.; Shizuka H. Photoionization of Aniline in Aqueous Solution and its Photolysis in Cyclohexane. J. Chem. Soc., Faraday Trans. 1996, 92, 4177–4185. 10.1039/ft9969204177. [DOI] [Google Scholar]
- Šolomek T.; Heger D.; Ngoy B. P.; Givens R. S.; Klán P. The Pivotal Role of Oxyallyl Diradicals in Photo-Favorskii Rearrangements: Transient Spectroscopic and Computational Studies. J. Am. Chem. Soc. 2013, 135, 15209–15215. 10.1021/ja407588p. [DOI] [PubMed] [Google Scholar]
- Chakraborty I.; Carrington S. J.; Mascharak P. K. Design Strategies To Improve the Sensitivity of Photoactive Metal Carbonyl Complexes (photoCORMs) to Visible Light and Their Potential as CO-Donors to Biological Targets. Acc. Chem. Res. 2014, 47, 2603–2611. 10.1021/ar500172f. [DOI] [PubMed] [Google Scholar]
- Rudolf P.; Kanal F.; Knorr J.; Nagel C.; Niesel J.; Brixner T.; Schatzschneider U.; Nuernberger P. Ultrafast Photochemistry of a Manganese-Tricarbonyl CO-Releasing Molecule (CORM) in Aqueous Solution. J. Phys. Chem. Lett. 2013, 4, 596–602. 10.1021/jz302061q. [DOI] [PubMed] [Google Scholar]
- Garino C.; Salassa L. The Photochemistry of Transition Metal Complexes Using Density Functional Theory. Philos. Trans. R. Soc., A 2013, 371, 20120134. 10.1098/rsta.2012.0134. [DOI] [PubMed] [Google Scholar]
- Sunderlin L. S.; Wang D.; Squires R. R. Bond Strengths in First-Row-Metal Carbonyl Anions. J. Am. Chem. Soc. 1993, 115, 12060–12070. 10.1021/ja00078a051. [DOI] [Google Scholar]
- Rimmer R. D.; Richter H.; Ford P. C. A Photochemical Precursor for Carbon Monoxide Release in Aerated Aqueous Media. Inorg. Chem. 2010, 49, 1180–1185. 10.1021/ic902147n. [DOI] [PubMed] [Google Scholar]
- Motterlini R.; Clark J. E.; Foresti R.; Sarathchandra P.; Mann B. E.; Green C. J. Carbon Monoxide-Releasing Molecules. Circ. Res. 2002, 90, e17-e24 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- Zhang W.-Q.; Atkin A. J.; Fairlamb I. J. S.; Whitwood A. C.; Lynam J. M. Synthesis and Reactivity of Molybdenum Complexes Containing Functionalized Alkynyl Ligands: A Photochemically Activated CO-Releasing Molecule (PhotoCO-RM). Organometallics 2011, 30, 4643–4654. 10.1021/om200495h. [DOI] [Google Scholar]
- Chakraborty I.; Carrington S. J.; Mascharak P. K. Photodelivery of CO by Designed PhotoCORMs: Correlation between Absorption in the Visible Region and Metal-CO Bond Labilization in Carbonyl Complexes. ChemMedChem 2014, 9, 1266–1274. 10.1002/cmdc.201402007. [DOI] [PubMed] [Google Scholar]
- Carrington S. J.; Chakraborty I.; Mascharak P. K. Rapid CO Release from a Mn(I) Carbonyl Complex Derived from Azopyridine upon Exposure to Visible Light and Its Phototoxicity Toward Malignant Cells. Chem. Commun. 2013, 49, 11254–11256. 10.1039/c3cc46558f. [DOI] [PubMed] [Google Scholar]
- Jimenez J.; Chakraborty I.; Carrington S. J.; Mascharak P. K. Light-Triggered CO Delivery by a Water-Soluble and Biocompatible Manganese PhotoCORM. Dalton Trans. 2016, 45, 13204–13213. 10.1039/C6DT01358A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A. M. Green-Light-Induced PhotoCORM: Lysozyme Binding Affinity towards MnI and ReI Carbonyl Complexes and Biological Activity Evaluation. Eur. J. Inorg. Chem. 2018, 2018, 4805–4811. 10.1002/ejic.201801055. [DOI] [Google Scholar]
- Kottelat E.; Ruggi A.; Zobi F. Red-Light Activated PhotoCORMs of Mn(I) Species Bearing Electron Deficient 2,2′-Azopyridines. Dalton Trans. 2016, 45, 6920–6927. 10.1039/C6DT00858E. [DOI] [PubMed] [Google Scholar]
- Mansour A. M.; Steiger C.; Nagel C.; Schatzschneider U. Wavelength-Dependent Control of the CO Release Kinetics of Manganese(I) Tricarbonyl PhotoCORMs with Benzimidazole Coligands. Eur. J. Inorg. Chem. 2019, 2019, 4572–4581. 10.1002/ejic.201900894. [DOI] [Google Scholar]
- Atkin A. J.; Lynam J. M.; Moulton B. E.; Sawle P.; Motterlini R.; Boyle N. M.; Pryce M. T.; Fairlamb I. J. S. Modification of the Deoxy-Myoglobin/Carbonmonoxy-Myoglobin UV-vis Assay for Reliable Determination of CO-Release Rates from Organometallic Carbonyl Complexes. Dalton Trans. 2011, 40, 5755–5761. 10.1039/c0dt01809k. [DOI] [PubMed] [Google Scholar]
- Yempally V.; Kyran S. J.; Raju R. K.; Fan W. Y.; Brothers E. N.; Darensbourg D. J.; Bengali A. A. Thermal and Photochemical Reactivity of Manganese Tricarbonyl and Tetracarbonyl Complexes with a Bulky Diazabutadiene Ligand. Inorg. Chem. 2014, 53, 4081–4088. 10.1021/ic500025k. [DOI] [PubMed] [Google Scholar]
- Kianfar E.; Apaydin D. H.; Knor G. Spin-Forbidden Excitation: A New Approach for Triggering Photopharmacological Processes with Low-Intensity NIR Light. ChemPhotoChem. 2017, 1, 378–382. 10.1002/cptc.201700086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J.; Chen R.; Tang X.; Tao Y.; Xu S.; Jin L.; Chen C.; Zhou X.; Zheng C.; Huang W. Direct Population of Triplet Excited States through Singlet–Triplet Transition for Visible-Light Excitable Organic Afterglow. Chem. Sci. 2019, 10, 5031–5038. 10.1039/C8SC05198D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A. M.; Friedrich A. Blue-Light Induced CO Releasing Properties of Thiourea Based Manganese(I) Carbonyl Complexes. Polyhedron 2017, 131, 13–21. 10.1016/j.poly.2017.04.021. [DOI] [Google Scholar]
- Mansour A. M.; Shehab O. R. Reactivity of Visible-Light Induced CO Releasing Thiourea-Based Mn(I) Tricarbonyl Bromide (CORM-NS1) towards Lysozyme. Inorg. Chim. Acta 2018, 480, 159–165. 10.1016/j.ica.2018.05.009. [DOI] [Google Scholar]
- Govender P.; Pai S.; Schatzschneider U.; Smith G. S. Next Generation PhotoCORMs: Polynuclear Tricarbonylmanganese(I)-Functionalized Polypyridyl Metallodendrimers. Inorg. Chem. 2013, 52, 5470–5478. 10.1021/ic400377k. [DOI] [PubMed] [Google Scholar]
- Pierri A. E.; Huang P. J.; Garcia J. V.; Stanfill J. G.; Chui M.; Wu G.; Zheng N.; Ford P. C. A photoCORM Nanocarrier for CO Release Using NIR Light. Chem. Commun. 2015, 51, 2072–2075. 10.1039/C4CC06766E. [DOI] [PubMed] [Google Scholar]
- Henke W. C.; Otolski C. J.; Moore W. N. G.; Elles C. G.; Blakemore J. D. Ultrafast Spectroscopy of [Mn(CO)3] Complexes: Tuning the Kinetics of Light-Driven CO Release and Solvent Binding. Inorg. Chem. 2020, 59, 2178–2187. 10.1021/acs.inorgchem.9b02758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pordel S.; White J. K. Impact of Mn(I) PhotoCORM Ligand Set on Photochemical Intermediate Formation During Visible Light-Activated CO Release. Inorg. Chim. Acta 2020, 500, 119206. 10.1016/j.ica.2019.119206. [DOI] [Google Scholar]
- Kottelat E.; Lucarini F.; Crochet A.; Ruggi A.; Zobi F. Correlation of MLCTs of Group 7 fac-[M(CO)3]+ Complexes (M = Mn, Re) with Bipyridine, Pyridinylpyrazine, Azopyridine, and Pyridin-2-ylmethanimine Type Ligands for Rational photoCORM Design. Eur. J. Inorg. Chem. 2019, 2019, 3758–3768. 10.1002/ejic.201900568. [DOI] [Google Scholar]
- Ruggi A.; Zobi F. Quantum-CORMs: Quantum Dot Sensitized CO Releasing Molecules. Dalton Trans. 2015, 44, 10928–10931. 10.1039/C5DT01681A. [DOI] [PubMed] [Google Scholar]
- Diring S.; Carné-Sánchez A.; Zhang J.; Ikemura S.; Kim C.; Inaba H.; Kitagawa S.; Furukawa S. Light Responsive Metal–Organic Frameworks as Controllable CO-Releasing Cell Culture Substrates. Chem. Sci. 2017, 8, 2381–2386. 10.1039/C6SC04824B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo F.; Sho T.; Maity B.; Fujita K.; Tachibana Y.; Akashi S.; Mano M.; Hishikawa Y.; Matsuo M.; Ueno T. Photoinduced in Vivo Magnetic Resonance Imaging (MRI) with Rapid CO Release from an MnCO-Protein Needle Composite. Chem. - Eur. J. 2018, 24, 11578–11583. 10.1002/chem.201802445. [DOI] [PubMed] [Google Scholar]
- Sakla R.; Singh A.; Kaushik R.; Kumar P.; Jose D. A. Allosteric Regulation in Carbon Monoxide (CO) Release: Anion Responsive CO-Releasing Molecule (CORM) Derived from (Terpyridine)phenol Manganese Tricarbonyl Complex with Colorimetric and Fluorescence Monitoring. Inorg. Chem. 2019, 58, 10761–10768. 10.1021/acs.inorgchem.9b00984. [DOI] [PubMed] [Google Scholar]
- Jiang Q.; Xia Y.; Barrett J.; Mikhailovsky A.; Wu G.; Wang D.; Shi P.; Ford P. C. Near-Infrared and Visible Photoactivation to Uncage Carbon Monoxide from an Aqueous-Soluble PhotoCORM. Inorg. Chem. 2019, 58, 11066–11075. 10.1021/acs.inorgchem.9b01581. [DOI] [PubMed] [Google Scholar]
- Mansour A. M.; Ragab M. S. Spectroscopic and DFT Studies of Photoactivatable Mn(I) Tricarbonyl Complexes. Appl. Organomet. Chem. 2019, 33, e4944 10.1002/aoc.4944. [DOI] [Google Scholar]
- Carrington S. J.; Chakraborty I.; Bernard J. M. L.; Mascharak P. K. Synthesis and Characterization of a “Turn-On” photoCORM for Trackable CO Delivery to Biological Targets. ACS Med. Chem. Lett. 2014, 5, 1324–1328. 10.1021/ml500399r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A. M. Rapid Green and Blue Light-Induced CO Release from Bromazepam Mn(I) and Ru(II) Carbonyls: Synthesis, Density Functional Theory and Biological Activity Evaluation. Appl. Organomet. Chem. 2017, 31, e3564 10.1002/aoc.3564. [DOI] [Google Scholar]
- Musib D.; Raza M. K.; Martina K.; Roy M. Mn(I)-Based PhotoCORMs for Trackable, Visible Light-Induced CO Release and Photocytotoxicity to Cancer Cells. Polyhedron 2019, 172, 125–131. 10.1016/j.poly.2019.04.008. [DOI] [Google Scholar]
- Mede R.; Gläser S.; Suchland B.; Schowtka B.; Mandel M.; Görls H.; Krieck S.; Schiller A.; Westerhausen M. Manganese(I)-Based CORMs with 5-Substituted 3-(2-Pyridyl)Pyrazole Ligands. Inorganics 2017, 5, 8. 10.3390/inorganics5010008. [DOI] [Google Scholar]
- Mede R.; Hoffmann P.; Neumann C.; Gorls H.; Schmitt M.; Popp J.; Neugebauer U.; Westerhausen M. Acetoxymethyl Concept for Intracellular Administration of Carbon Monoxide with Mn(CO)3-Based PhotoCORMs. Chem. - Eur. J. 2018, 24, 3321–3329. 10.1002/chem.201705686. [DOI] [PubMed] [Google Scholar]
- Ward J. S.; De Palo A.; Aucott B. J.; Moir J. W. B.; Lynam J. M.; Fairlamb I. J. S. A Biotin-Conjugated Photo-Activated CO-Releasing Molecule (BiotinCORM): Efficient CO-Release from an Avidin–BiotinCORM Protein Adduct. Dalton Trans. 2019, 48, 16233–16241. 10.1039/C9DT03429C. [DOI] [PubMed] [Google Scholar]
- Jimenez J.; Chakraborty I.; Dominguez A.; Martinez-Gonzalez J.; Sameera W. M. C.; Mascharak P. K. A Luminescent Manganese PhotoCORM for CO Delivery to Cellular Targets under the Control of Visible Light. Inorg. Chem. 2018, 57, 1766–1773. 10.1021/acs.inorgchem.7b02480. [DOI] [PubMed] [Google Scholar]
- Jimenez J.; Pinto M. N.; Martinez-Gonzalez J.; Mascharak P. K. Photo-Induced Eradication of Human Colorectal Adenocarcinoma HT-29 Cells by Carbon Monoxide (CO) Delivery from a Mn-Based Green Luminescent PhotoCORM. Inorg. Chim. Acta 2019, 485, 112–117. 10.1016/j.ica.2018.09.088. [DOI] [Google Scholar]
- Pinto M. N.; Chakraborty I.; Jimenez J.; Murphy K.; Wenger J.; Mascharak P. K. Therapeutic Potential of Two Visible Light Responsive Luminescent photoCORMs: Enhanced Cellular Internalization Driven by Lipophilicity. Inorg. Chem. 2019, 58, 14522–14531. 10.1021/acs.inorgchem.9b02121. [DOI] [PubMed] [Google Scholar]
- Ramu V.; Upendar Reddy G.; Liu J.; Hoffmann P.; Sollapur R.; Wyrwa R.; Kupfer S.; Spielmann C.; Bonnet S.; Neugebauer U.; Schiller A. Two-Photon-Induced CO-Releasing Molecules as Molecular Logic Systems in Solution, Polymers, and Cells. Chem. - Eur. J. 2019, 25, 8453–8458. 10.1002/chem.201901396. [DOI] [PubMed] [Google Scholar]
- Gandra U. R.; Sinopoli A.; Moncho S.; NandaKumar M.; Ninković D. B.; Zarić S. D.; Sohail M.; Al-Meer S.; Brothers E. N.; Mazloum N. A.; Al-Hashimi M.; Bazzi H. S. Green Light-Responsive CO-Releasing Polymeric Materials Derived from Ring-Opening Metathesis Polymerization. ACS Appl. Mater. Interfaces 2019, 11, 34376–34384. 10.1021/acsami.9b12628. [DOI] [PubMed] [Google Scholar]
- Reddy G U.; Liu J.; Hoffmann P.; Steinmetzer J.; Görls H.; Kupfer S.; Askes S. H. C.; Neugebauer U.; Gräfe S.; Schiller A. Light-Responsive Paper Strips as CO-Releasing Material with a Colourimetric Response. Chem. Sci. 2017, 8, 6555–6560. 10.1039/C7SC01692A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Hoffmann P.; Steinmetzer J.; Askes S. H. C.; Kupfer S.; Görls H.; Gräfe S.; Neugebauer U.; Gandra U. R.; Schiller A. Visible Light-Activated Biocompatible Photo-CORM for CO-Release with Colorimetric and Fluorometric Dual Turn-On Response. Polyhedron 2019, 172, 175–181. 10.1016/j.poly.2019.04.031. [DOI] [Google Scholar]
- Tabe H.; Shimoi T.; Boudes M.; Abe S.; Coulibaly F.; Kitagawa S.; Mori H.; Ueno T. Photoactivatable CO Release from Engineered Protein Crystals to Modulate NF-κB Activation. Chem. Commun. 2016, 52, 4545–4548. 10.1039/C5CC10440H. [DOI] [PubMed] [Google Scholar]
- Mansour A. M.; Shehab O. R. Experimental and Quantum Chemical Calculations of Novel Photoactivatable Manganese(I) Tricarbonyl Complexes. J. Organomet. Chem. 2016, 822, 91–99. 10.1016/j.jorganchem.2016.08.018. [DOI] [Google Scholar]
- Divya D.; Nagarajaprakash R.; Vidhyapriya P.; Sakthivel N.; Manimaran B. Single-Pot Self-Assembly of Heteroleptic Mn(I)-Based Aminoquinonato-Bridged Ester/Amide-Functionalized Dinuclear Metallastirrups: Potential Anticancer and Visible-Light-Triggered CORMs. ACS Omega 2019, 4, 12790–12802. 10.1021/acsomega.9b01438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. S.; Lynam J. M.; Moir J.; Fairlamb I. J. S. Visible-Light-Induced CO Release from a Therapeutically Viable Tryptophan-Derived Manganese(I) Carbonyl (TryptoCORM) Exhibiting Potent Inhibition against E. coli. Chem. - Eur. J. 2014, 20, 15061–15068. 10.1002/chem.201403305. [DOI] [PubMed] [Google Scholar]
- Aucott B. J.; Eastwood J. B.; Anders Hammarback L.; Clark I. P.; Sazanovich I. V.; Towrie M.; Fairlamb I. J. S.; Lynam J. M. Insight into the Mechanism of CO-Release from Trypto-CORM Using Ultra-Fast Spectroscopy and Computational Chemistry. Dalton Trans. 2019, 48, 16426–16436. 10.1039/C9DT03343B. [DOI] [PubMed] [Google Scholar]
- Dessent C. E. H.; Cercola R.; Fischer K. C.; Sherman S. L.; Garand E.; Wong N. G. K.; Hammerback L. A.; Lynam J. M.; Fairlamb I. J. S. Direct Measurement of the Visible to UV Photodissociation Processes for the PhotoCORM TryptoCORM. Chem. - Eur. J. 2020, 26, 10297–10306. 10.1002/chem.202001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobi F.; Quaroni L.; Santoro G.; Zlateva T.; Blacque O.; Sarafimov B.; Schaub M. C.; Bogdanova A. Y. Live-Fibroblast IR Imaging of a Cytoprotective PhotoCORM Activated with Visible Light. J. Med. Chem. 2013, 56, 6719–6731. 10.1021/jm400527k. [DOI] [PubMed] [Google Scholar]
- Mede R.; Hoffmann P.; Klein M.; Görls H.; Schmitt M.; Neugebauer U.; Gessner G.; Heinemann S. H.; Popp J.; Westerhausen M. A Water-Soluble Mn(CO)3-Based and Non-Toxic PhotoCORM for Administration of Carbon Monoxide Inside of Cells. Z. Anorg. Allg. Chem. 2017, 643, 2057–2062. 10.1002/zaac.201700349. [DOI] [Google Scholar]
- Weiss V. C.; Amorim A. L.; Xavier F. R.; Bortoluzzi A. J.; Neves A.; Peralta R. A. Light Response of Three Water-Soluble MnI PhotoCORMs: Spectroscopic Features and CO Release Investigation. J. Braz. Chem. Soc. 2019, 30, 2649–2659. 10.21577/0103-5053.20190183. [DOI] [Google Scholar]
- Mede R.; Klein M.; Claus R. A.; Krieck S.; Quickert S.; Görls H.; Neugebauer U.; Schmitt M.; Gessner G.; Heinemann S. H.; Popp J.; Bauer M.; Westerhausen M. CORM-EDE1: A Highly Water-Soluble and Nontoxic Manganese-Based photoCORM with a Biogenic Ligand Sphere. Inorg. Chem. 2016, 55, 104–113. 10.1021/acs.inorgchem.5b01904. [DOI] [PubMed] [Google Scholar]
- Bohlender C.; Gläser S.; Klein M.; Weisser J.; Thein S.; Neugebauer U.; Popp J.; Wyrwa R.; Schiller A. Light-Triggered CO Release from Nanoporous Non-Wovens. J. Mater. Chem. B 2014, 2, 1454–1463. 10.1039/C3TB21649G. [DOI] [PubMed] [Google Scholar]
- Gläser S.; Mede R.; Görls H.; Seupel S.; Bohlender C.; Wyrwa R.; Schirmer S.; Dochow S.; Reddy G. U.; Popp J.; Westerhausen M.; Schiller A. Remote-Controlled Delivery of CO via Photoactive CO-Releasing Materials on a Fiber Optical Device. Dalton Trans. 2016, 45, 13222–13233. 10.1039/C6DT02011A. [DOI] [PubMed] [Google Scholar]
- Li Z.; Pierri A. E.; Huang P. J.; Wu G.; Iretskii A. V.; Ford P. C. Dinuclear PhotoCORMs: Dioxygen-Assisted Carbon Monoxide Uncaging from Long-Wavelength -Absorbing Metal-Metal-Bonded Carbonyl Complexes. Inorg. Chem. 2017, 56, 6094–6104. 10.1021/acs.inorgchem.6b03138. [DOI] [PubMed] [Google Scholar]
- Askes S. H. C.; Reddy G. U.; Wyrwa R.; Bonnet S.; Schiller A. Red Light-Triggered CO Release from Mn2(CO)10 Using Triplet Sensitization in Polymer Nonwoven Fabrics. J. Am. Chem. Soc. 2017, 139, 15292–15295. 10.1021/jacs.7b07427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M. A.; Carrington S. J.; Chakraborty I.; Olmstead M. M.; Mascharak P. K. Photoactivity of Mono- and Dicarbonyl Complexes of Ruthenium(II) Bearing an N,N,S-Donor Ligand: Role of Ancillary Ligands on the Capacity of CO Photorelease. Inorg. Chem. 2013, 52, 11320–11331. 10.1021/ic4016004. [DOI] [PubMed] [Google Scholar]
- Kubeil M.; Joshi T.; Wood B. R.; Stephan H. Synthesis, Structural Characterization and Photodecarbonylation Study of a Dicarbonyl Ruthenium(II)-Bisquinoline Complex. ChemistryOpen 2019, 8, 637–642. 10.1002/open.201900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akatsuka K.; Abe R.; Takase T.; Oyama D. Coordination Chemistry of Ru(II) Complexes of an Asymmetric Bipyridine Analogue: Synergistic Effects of Supporting Ligand and Coordination Geometry on Reactivities. Molecules 2020, 25, 27. 10.3390/molecules25010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takács J.; Soós E.; Nagy-Magos Z.; Markó L.; Gervasio G.; Hoffmann T. Synthesis and Molecular Structure of Carbonyl Derivatives of Iron(II) Thiolates Containing Nitrogen-Donor Ligands. Inorg. Chim. Acta 1989, 166, 39–46. 10.1016/S0020-1693(00)80784-7. [DOI] [Google Scholar]
- Kretschmer R.; Gessner G.; Görls H.; Heinemann S. H.; Westerhausen M. Dicarbonyl-Bis(cysteamine)iron(II): A Light Induced Carbon Monoxide Releasing Molecule Based on Iron (CORM-S1). J. Inorg. Biochem. 2011, 105, 6–9. 10.1016/j.jinorgbio.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Poh H. T.; Sim B. T.; Chwee T. S.; Leong W. K.; Fan W. Y. The Dithiolate-Bridged Diiron Hexacarbonyl Complex Na2[(μ-SCH2CH2COO)Fe(CO)3]2 as a Water-Soluble PhotoCORM. Organometallics 2014, 33, 959–963. 10.1021/om401013a. [DOI] [Google Scholar]
- Marhenke J.; Pierri A. E.; Lomotan M.; Damon P. L.; Ford P. C.; Works C. Flash and Continuous Photolysis Kinetic Studies of the Iron–Iron Hydrogenase Model (μ-pdt)[Fe(CO)3]2 in Different Solvents. Inorg. Chem. 2011, 50, 11850–11852. 10.1021/ic201523r. [DOI] [PubMed] [Google Scholar]
- Nakae T.; Hirotsu M.; Nakajima H. CO Release from N, C, S-Pincer Iron(III) Carbonyl Complexes Induced by Visible-to-NIR Light Irradiation: Mechanistic Insight into Effects of Axial Phosphorus Ligands. Inorg. Chem. 2018, 57, 8615–8626. 10.1021/acs.inorgchem.8b01407. [DOI] [PubMed] [Google Scholar]
- Kawatani M.; Kamiya M.; Takahashi H.; Urano Y. Factors Affecting the Ucaging Eficiency of 500nm Lght-Ativatable BODIPY Caging Group. Bioorg. Med. Chem. Lett. 2018, 28, 1–5. 10.1016/j.bmcl.2017.11.030. [DOI] [PubMed] [Google Scholar]
- Wright M. A.; Wooldridge T.; O’Connell M. A.; Wright J. A. Ferracyclic Carbonyl Complexes as Anti-inflammatory Agents. Chem. Commun. 2020, 56, 4300–4303. 10.1039/D0CC01449D. [DOI] [PubMed] [Google Scholar]
- Pierri A. E.; Pallaoro A.; Wu G.; Ford P. C. A Luminescent and Biocompatible PhotoCORM. J. Am. Chem. Soc. 2012, 134, 18197–18200. 10.1021/ja3084434. [DOI] [PubMed] [Google Scholar]
- Pierri A. E.; Pallaoro A.; Wu G.; Ford P. C. Correction to “A Luminescent and Biocompatible PhotoCORM. J. Am. Chem. Soc. 2018, 140, 525–525. 10.1021/jacs.7b11882. [DOI] [PubMed] [Google Scholar]
- Rosselli M.; Keller R.; Dubey R. Role of Nitric Oxide in the Biology, Physiology and Pathophysiology of Reproduction. Hum. Reprod. Update 1998, 4, 3–24. 10.1093/humupd/4.1.3. [DOI] [PubMed] [Google Scholar]
- Arnold W. P.; Mittal C. K.; Katsuki S.; Murad F. Nitric Oxide Activates Guanylate Cyclase and Increases Guanosine 3′:5′-Cyclic Monophosphate Levels in Various Tissue Preparations. Proc. Natl. Acad. Sci. U. S. A. 1977, 74, 3203–3207. 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F.; Zawadzki J. V. The Obligatory Role of Endothelial Cells in the Relaxation of Arterial Smooth Muscle by Acetylcholine. Nature 1980, 288, 373–376. 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J.; Buga G. M.; Wood K. S.; Byrns R. E.; Chaudhuri G. Endothelium-Derived Relaxing Factor Produced and Released from Artery and Vein Is Nitric Oxide. Proc. Natl. Acad. Sci. U. S. A. 1987, 84, 9265–9269. 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M. J.; Ferrige A. G.; Moncada S. Nitric Oxide Release Accounts for the Biological Activity of Endothelium-Derived Relaxing Factor. Nature 1987, 327, 524–526. 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Hermann M.; Flammer A.; Lüscher T. F. Nitric Oxide in Hypertension. J. Clin. Hypertens. 2006, 8, 17–29. 10.1111/j.1524-6175.2006.06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S.; Guo C.; Wang M.; Sun Z.; Li H.; Qian X.; Yang Y. Mild Dealkylative N-Nitrosation of N,N-Dialkylaniline Derivatives for Convenient Preparation of Photo-Triggered and Photo-Calibrated NO Donors. Org. Chem. Front. 2018, 5, 3206–3209. 10.1039/C8QO00818C. [DOI] [Google Scholar]
- Piech K.; Bally T.; Sikora A.; Marcinek A. Mechanistic Aspects of the Oxidative and Reductive Fragmentation of N-Nitrosoamines: A New Method for Generating Nitrenium Cations, Amide Anions, and Aminyl Radicals. J. Am. Chem. Soc. 2007, 129, 3211–3217. 10.1021/ja066855e. [DOI] [PubMed] [Google Scholar]
- He H.; He T.; Zhang Z.; Xu X.; Yang H.; Qian X.; Yang Y. Ring-Restricted N-Nitrosated Rhodamine as a Green-Light Triggered, Orange-Emission Calibrated and Fast-Releasing Nitric Oxide Donor. Chin. Chem. Lett. 2018, 29, 1497–1499. 10.1016/j.cclet.2018.08.019. [DOI] [Google Scholar]
- Yanagimoto T.; Toyota T.; Matsuki N.; Makino Y.; Uchiyama S.; Ohwada T. Transnitrosation of Thiols from Aliphatic N-Nitrosamines: S-Nitrosation and Indirect Generation of Nitric Oxide. J. Am. Chem. Soc. 2007, 129, 736–737. 10.1021/ja0658259. [DOI] [PubMed] [Google Scholar]
- Zhou E. Y.; Knox H. J.; Reinhardt C. J.; Partipilo G.; Nilges M. J.; Chan J. Near-Infrared Photoactivatable Nitric Oxide Donors with Integrated Photoacoustic Monitoring. J. Am. Chem. Soc. 2018, 140, 11686–11697. 10.1021/jacs.8b05514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno H.; Ieda N.; Hotta Y.; Kawaguchi M.; Kimura K.; Nakagawa H. A Yellowish-Green-Light-Controllable Nitric Oxide Donor Based on N-Nitrosoaminophenol Applicable for Photocontrolled Vasodilation. Org. Biomol. Chem. 2017, 15, 2791–2796. 10.1039/C7OB00245A. [DOI] [PubMed] [Google Scholar]
- Ieda N.; Oka Y.; Yoshihara T.; Tobita S.; Sasamori T.; Kawaguchi M.; Nakagawa H. Structure-Efficiency Relationship of Photoinduced Electron Transfer-Triggered Nitric Oxide Releasers. Sci. Rep. 2019, 9, 1430. 10.1038/s41598-018-38252-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieda N.; Hotta Y.; Kawaguchi M.; Kimura K.; Nakagawa H. In Cellullo and ex Vivo Availability of a Yellowish-Green-Light-Controllable NO Releaser. Chem. Pharm. Bull. 2019, 67, 576–579. 10.1248/cpb.c19-00112. [DOI] [PubMed] [Google Scholar]
- He H.; Liu Y.; Zhou Z.; Guo C.; Wang H.-Y.; Wang Z.; Wang X.; Zhang Z.; Wu F.-G.; Wang H.; Chen D.; Yang D.; Liang X.; Chen J.; Zhou S.; Liang X.; Qian X.; Yang Y. A Photo-Triggered and Photo-Calibrated Nitric Oxide Donor: Rational Design, Spectral Characterizations, and Biological Applications. Free Radical Biol. Med. 2018, 123, 1–7. 10.1016/j.freeradbiomed.2018.04.563. [DOI] [PubMed] [Google Scholar]
- He H.; Xia Y.; Qi Y.; Wang H.-Y.; Wang Z.; Bao J.; Zhang Z.; Wu F.-G.; Wang H.; Chen D.; Yang D.; Liang X.; Chen J.; Zhou S.; Liang X.; Qian X.; Yang Y. A Water-Soluble, Green-Light Triggered, and Photo-Calibrated Nitric Oxide Donor for Biological Applications. Bioconjugate Chem. 2018, 29, 1194–1198. 10.1021/acs.bioconjchem.7b00821. [DOI] [PubMed] [Google Scholar]
- Shen R.; Qian Y. A Turn-on and Lysosome-Targeted Fluorescent NO Releaser in Water Media and Its Application in Living Cells and Zebrafishes. Spectrochim. Acta, Part A 2020, 230, 118024. 10.1016/j.saa.2019.118024. [DOI] [PubMed] [Google Scholar]
- Shen R.; Qian Y. A Efficient Light-Controlled Nitric Oxide Releaser in Aqueous Solution and Its Red Fluorescence Imaging in Lysosome. Dyes Pigm. 2020, 176, 108247. 10.1016/j.dyepig.2020.108247. [DOI] [Google Scholar]
- Zhang S.; Wang Q.; Yang J.; Yang X.-F.; Li Z.; Li H. A Photocalibrated NO Donor Based on N-Nitrosorhodamine 6G upon UV Irradiation. Chin. Chem. Lett. 2019, 30, 454–456. 10.1016/j.cclet.2018.03.011. [DOI] [Google Scholar]
- He H.; Ye Z.; Xiao Y.; Yang W.; Qian X.; Yang Y. Super-Resolution Monitoring of Mitochondrial Dynamics upon Time-Gated Photo-Triggered Release of Nitric Oxide. Anal. Chem. 2018, 90, 2164–2169. 10.1021/acs.analchem.7b04510. [DOI] [PubMed] [Google Scholar]
- Xie X.; Fan J.; Liang M.; Li Y.; Jiao X.; Wang X.; Tang B. A Two-Photon Excitable and Ratiometric Fluorogenic Nitric Oxide Photoreleaser and Its Biological Applications. Chem. Commun. 2017, 53, 11941–11944. 10.1039/C7CC06820D. [DOI] [PubMed] [Google Scholar]
- Saavedra J. E.; Billiar T. R.; Williams D. L.; Kim Y.-M.; Watkins S. C.; Keefer L. K. Targeting Nitric Oxide (NO) Delivery in Vivo. Design of a Liver-Selective NO Donor Prodrug That Blocks Tumor Necrosis Factor-α-Induced Apoptosis and Toxicity in the Liver. J. Med. Chem. 1997, 40, 1947–1954. 10.1021/jm9701031. [DOI] [PubMed] [Google Scholar]
- Kauser N. I.; Weisel M.; Zhong Y.-L.; Lo M. M.-C.; Ali A. Calcium Dialkylamine Diazeniumdiolates: Synthesis, Stability, and Nitric Oxide Generation. J. Org. Chem. 2020, 85, 4807–4812. 10.1021/acs.joc.0c00020. [DOI] [PubMed] [Google Scholar]
- Miller M. R.; Megson I. L. Recent Developments in Nitric Oxide Donor Drugs. Br. J. Pharmacol. 2007, 151, 305–321. 10.1038/sj.bjp.0707224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefer L. K. Progress Toward Clinical Application of the Nitric Oxide–Releasing Diazeniumdiolates. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 585–607. 10.1146/annurev.pharmtox.43.100901.135831. [DOI] [PubMed] [Google Scholar]
- Brilli R. J.; Krafte-Jacobs B.; Smith D. J.; Roselle D.; Passerini D.; Vromen A.; Moore L.; Szabó C.; Salzman A. L. Intratracheal Instillation of a Novel NO/Nucleophile Adduct Selectively Reduces Pulmonary Hypertension. J. Appl. Physiol. 1997, 83, 1968–1975. 10.1152/jappl.1997.83.6.1968. [DOI] [PubMed] [Google Scholar]
- Makings L. R.; Tsien R. Y. Caged Nitric Oxide. Stable Organic Molecules from which Nitric Oxide can be Photoreleased. J. Biol. Chem. 1994, 269, 6282–6285. [PubMed] [Google Scholar]
- Caruso E. B.; Petralia S.; Conoci S.; Giuffrida S.; Sortino S. Photodelivery of Nitric Oxide from Water-Soluble Platinum Nanoparticles. J. Am. Chem. Soc. 2007, 129, 480–481. 10.1021/ja067568d. [DOI] [PubMed] [Google Scholar]
- Sharma N.; Dhyani A. K.; Marepally S.; Jose D. A. Nanoscale Lipid Vesicles Functionalized with a Nitro-Aniline Derivative for Photoinduced Nitric Oxide (NO) Delivery. Nanoscale Adv. 2020, 2, 463–469. 10.1039/C9NA00532C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittorino E.; Sciortino M. T.; Siracusano G.; Sortino S. Light-Activated Release of Nitric Oxide with Fluorescence Reporting in Living Cells. ChemMedChem 2011, 6, 1551–1554. 10.1002/cmdc.201100198. [DOI] [PubMed] [Google Scholar]
- Fraix A.; Kirejev V.; Malanga M.; Fenyvesi É.; Béni S.; Ericson M. B.; Sortino S. A Three-Color Fluorescent Supramolecular Nanoassembly of Phototherapeutics Activable by Two-Photon Excitation with Near-Infrared Light. Chem. - Eur. J. 2019, 25, 7091–7095. 10.1002/chem.201900917. [DOI] [PubMed] [Google Scholar]
- Marino N.; Perez-Lloret M.; Blanco A. R.; Venuta A.; Quaglia F.; Sortino S. Photo-Antimicrobial Polymeric Films Releasing Nitric Oxide with Fluorescence Reporting under Visible Light. J. Mater. Chem. B 2016, 4, 5138–5143. 10.1039/C6TB01388K. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Huang X.; Tang Y.; Zou J.; Wang P.; Zhang Y.; Si W.; Huang W.; Dong X. A Light-Induced Nitric Oxide Controllable Release Nano-Platform Based on Diketopyrrolopyrrole Derivatives for pH-Responsive Photodynamic/Photothermal Synergistic Cancer Therapy. Chem. Sci. 2018, 9, 8103–8109. 10.1039/C8SC03386B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T.; Nagae O.; Kato Y.; Nakagawa H.; Fukuhara K.; Miyata N. Photoinduced Nitric Oxide Release from Nitrobenzene Derivatives. J. Am. Chem. Soc. 2005, 127, 11720–11726. 10.1021/ja0512024. [DOI] [PubMed] [Google Scholar]
- Hishikawa K.; Nakagawa H.; Furuta T.; Fukuhara K.; Tsumoto H.; Suzuki T.; Miyata N. Photoinduced Nitric Oxide Release from a Hindered Nitrobenzene Derivative by Two-Photon Excitation. J. Am. Chem. Soc. 2009, 131, 7488–7489. 10.1021/ja8093668. [DOI] [PubMed] [Google Scholar]
- Nakagawa H.; Hishikawa K.; Eto K.; Ieda N.; Namikawa T.; Kamada K.; Suzuki T.; Miyata N.; Nabekura J.-i. Fine Spatiotemporal Control of Nitric Oxide Release by Infrared Pulse-Laser Irradiation of a Photolabile Donor. ACS Chem. Biol. 2013, 8, 2493–2500. 10.1021/cb400361m. [DOI] [PubMed] [Google Scholar]
- Ieda N.; Hishikawa K.; Eto K.; Kitamura K.; Kawaguchi M.; Suzuki T.; Fukuhara K.; Miyata N.; Furuta T.; Nabekura J.; Nakagawa H. A Double Bond-Conjugated Dimethylnitrobenzene-Type Photolabile Nitric Oxide Donor with Improved Two-Photon Cross Section. Bioorg. Med. Chem. Lett. 2015, 25, 3172–3175. 10.1016/j.bmcl.2015.05.095. [DOI] [PubMed] [Google Scholar]
- Kitamura K.; Kawaguchi M.; Ieda N.; Miyata N.; Nakagawa H. Visible Light-Controlled Nitric Oxide Release from Hindered Nitrobenzene Derivatives for Specific Modulation of Mitochondrial Dynamics. ACS Chem. Biol. 2016, 11, 1271–1278. 10.1021/acschembio.5b00962. [DOI] [PubMed] [Google Scholar]
- Kitamura K.; Ieda N.; Hishikawa K.; Suzuki T.; Miyata N.; Fukuhara K.; Nakagawa H. Visible Light-Induced Nitric Oxide Release from a Novel Nitrobenzene Derivative Cross-Conjugated with a Coumarin Fluorophore. Bioorg. Med. Chem. Lett. 2014, 24, 5660–5662. 10.1016/j.bmcl.2014.10.075. [DOI] [PubMed] [Google Scholar]
- Parisi C.; Failla M.; Fraix A.; Rolando B.; Gianquinto E.; Spyrakis F.; Gazzano E.; Riganti C.; Lazzarato L.; Fruttero R.; Gasco A.; Sortino S. Fluorescent Nitric Oxide Photodonors Based on BODIPY and Rhodamine Antennae. Chem. - Eur. J. 2019, 25, 11080–11084. 10.1002/chem.201902062. [DOI] [PubMed] [Google Scholar]
- Parisi C.; Failla M.; Fraix A.; Rescifina A.; Rolando B.; Lazzarato L.; Cardile V.; Graziano A. C. E.; Fruttero R.; Gasco A.; Sortino S. A Molecular Hybrid Producing Simultaneously Singlet Oxygen and Nitric Oxide by Single Photon Excitation with Green Light. Bioorg. Chem. 2019, 85, 18–22. 10.1016/j.bioorg.2018.12.027. [DOI] [PubMed] [Google Scholar]
- Butler A. R.; Glidewell C. Recent Chemical Studies of Sodium Nitroprusside Relevant to its Hypotensive Action. Chem. Soc. Rev. 1987, 16, 361–380. 10.1039/cs9871600361. [DOI] [Google Scholar]
- Shishido S. M.; de Oliveira M. G. Photosensitivity of Aqueous Sodium Nitroprusside Solutions: Nitric Oxide Release versus Cyanide Toxicity. Prog. React. Kinet. Mech. 2001, 26, 239–261. 10.3184/007967401103165271. [DOI] [Google Scholar]
- Feelisch M.; Noack E. Nitric Oxide (NO) Formation from Nitrovasodilators Occurs Independently of Hemoglobin or Non-Heme Iron. Eur. J. Pharmacol. 1987, 142, 465–469. 10.1016/0014-2999(87)90090-2. [DOI] [PubMed] [Google Scholar]
- Holloway L. R.; Li L.. The Preparation, Structural Characteristics, and Physical Chemical Properties of Metal-Nitrosyl Complexes. Nitrosyl Complexes in Inorganic Chemistry, Biochemistry and Medicine II; Mingos D. M. P., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2014. [Google Scholar]
- Xiang H.-J.; Guo M.; Liu J.-G. Transition-Metal Nitrosyls for Photocontrolled Nitric Oxide Delivery. Eur. J. Inorg. Chem. 2017, 2017, 1586–1595. 10.1002/ejic.201601135. [DOI] [Google Scholar]
- Ostrowski A. D.; Absalonson R. O.; Leo M. A. D.; Wu G.; Pavlovich J. G.; Adamson J.; Azhar B.; Iretskii A. V.; Megson I. L.; Ford P. C. Photochemistry of trans-Cr(cyclam)(ONO)2+, a Nitric Oxide Precursor. Inorg. Chem. 2011, 50, 4453–4462. 10.1021/ic200094x. [DOI] [PubMed] [Google Scholar]
- DeRosa F.; Bu X.; Ford P. C. Chromium(III) Complexes for Photochemical Nitric Oxide Generation from Coordinated Nitrite: Synthesis and Photochemistry of Macrocyclic Complexes with Pendant Chromophores, trans-[Cr(L)(ONO)2]BF4. Inorg. Chem. 2005, 44, 4157–4165. 10.1021/ic048311o. [DOI] [PubMed] [Google Scholar]
- Ostrowski A. D.; Lin B. F.; Tirrell M. V.; Ford P. C. Liposome Encapsulation of a Photochemical NO Precursor for Controlled Nitric Oxide Release and Simultaneous Fluorescence Imaging. Mol. Pharmaceutics 2012, 9, 2950–2955. 10.1021/mp300139y. [DOI] [PubMed] [Google Scholar]
- Huang P.-J.; Garcia J. V.; Fenwick A.; Wu G.; Ford P. C. Nitric Oxide Uncaging from a Hydrophobic Chromium(III) PhotoNORM: Visible and Near-Infrared Photochemistry in Biocompatible Polymer Disks. ACS Omega 2019, 4, 9181–9187. 10.1021/acsomega.9b00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks P. T.; Garcia J. V.; GonzalezIrias R.; Tillman J. T.; Niu M. T.; Mikhailovsky A. A.; Zhang J. P.; Zhang F.; Ford P. C. Nitric Oxide Releasing Materials Triggered by Near-Infrared Excitation Through Tissue Filters. J. Am. Chem. Soc. 2013, 135, 18145–18152. 10.1021/ja408516w. [DOI] [PubMed] [Google Scholar]
- San Miguel V.; Alvarez M.; Filevich O.; Etchenique R.; del Campo A. Multiphoton Reactive Surfaces Using Ruthenium(II) Photocleavable Cages. Langmuir 2012, 28, 1217–1221. 10.1021/la2033687. [DOI] [PubMed] [Google Scholar]
- Wecksler S. R.; Mikhailovsky A.; Korystov D.; Ford P. C. A Two-Photon Antenna for Photochemical Delivery of Nitric Oxide from a Water-Soluble, Dye-Derivatized Iron Nitrosyl Complex Using NIR Light. J. Am. Chem. Soc. 2006, 128, 3831–3837. 10.1021/ja057977u. [DOI] [PubMed] [Google Scholar]
- Conrado C. L.; Wecksler S.; Egler C.; Magde D.; Ford P. C. Synthesis and Photochemical Properties of a Novel Iron-Sulfur-Nitrosyl Cluster Derivatized with the Pendant Chromophore Protoporphyrin IX. Inorg. Chem. 2004, 43, 5543–5549. 10.1021/ic049459a. [DOI] [PubMed] [Google Scholar]
- Conrado C. L.; Bourassa J. L.; Egler C.; Wecksler S.; Ford P. C. Photochemical Investigation of Roussin’s Red Salt Esters: Fe2(μ-SR)2(NO)4. Inorg. Chem. 2003, 42, 2288–2293. 10.1021/ic020309e. [DOI] [PubMed] [Google Scholar]
- Zheng Q.; Bonoiu A.; Ohulchanskyy T. Y.; He G. S.; Prasad P. N. Water-Soluble Two-Photon Absorbing Nitrosyl Complex for Light-Activated Therapy through Nitric Oxide Release. Mol. Pharmaceutics 2008, 5, 389–398. 10.1021/mp700117s. [DOI] [PubMed] [Google Scholar]
- Wecksler S. R.; Mikhailovsky A.; Korystov D.; Buller F.; Kannan R.; Tan L.-S.; Ford P. C. Single- and Two-Photon Properties of a Dye-Derivatized Roussin’s Red Salt Ester (Fe2(μ-RS)2(NO)4) with a Large TPA Cross Section. Inorg. Chem. 2007, 46, 395–402. 10.1021/ic0607336. [DOI] [PubMed] [Google Scholar]
- Patra A. K.; Afshar R.; Olmstead M. M.; Mascharak P. K. The First Non-Heme Iron(III) Complex with a Ligated Carboxamido Group That Exhibits Photolability of a Bound NO Ligand. Angew. Chem., Int. Ed. 2002, 41, 2512–2515. . [DOI] [PubMed] [Google Scholar]
- Patra A. K.; Rowland J. M.; Marlin D. S.; Bill E.; Olmstead M. M.; Mascharak P. K. Iron Nitrosyls of a Pentadentate Ligand Containing a Single Carboxamide Group: Syntheses, Structures, Electronic Properties, and Photolability of NO. Inorg. Chem. 2003, 42, 6812–6823. 10.1021/ic0301627. [DOI] [PubMed] [Google Scholar]
- Chiang C.-K.; Chu K.-T.; Lin C.-C.; Xie S.-R.; Liu Y.-C.; Demeshko S.; Lee G.-H.; Meyer F.; Tsai M.-L.; Chiang M.-H.; Lee C.-M. Photoinduced NO and HNO Production from Mononuclear {FeNO}6 Complex Bearing a Pendant Thiol. J. Am. Chem. Soc. 2020, 142, 8649–8661. 10.1021/jacs.9b13837. [DOI] [PubMed] [Google Scholar]
- Ghosh K.; Eroy-Reveles A. A.; Avila B.; Holman T. R.; Olmstead M. M.; Mascharak P. K. Reactions of NO with Mn(II) and Mn(III) Centers Coordinated to Carboxamido Nitrogen: Synthesis of a Manganese Nitrosyl with Photolabile NO. Inorg. Chem. 2004, 43, 2988–2997. 10.1021/ic030331n. [DOI] [PubMed] [Google Scholar]
- Eroy-Reveles A. A.; Leung Y.; Mascharak P. K. Release of Nitric Oxide From a Sol-Gel Hybrid Material Containing a Photoactive Manganese Nitrosyl Upon Illumination With Visible Light. J. Am. Chem. Soc. 2006, 128, 7166–7167. 10.1021/ja061852n. [DOI] [PubMed] [Google Scholar]
- Hitomi Y.; Iwamoto Y.; Kodera M. Electronic Tuning of Nitric Oxide Release from Manganese Nitrosyl Complexes by Visible Light Irradiation: Enhancement of Nitric Oxide Release Efficiency by the Nitro-Substituted Quinoline Ligand. Dalton Trans. 2014, 43, 2161–2167. 10.1039/C3DT51719E. [DOI] [PubMed] [Google Scholar]
- Rose M. J.; Mascharak P. K. Photoactive Ruthenium Nitrosyls: Effects of Light and Potential Application as NO Donors. Coord. Chem. Rev. 2008, 252, 2093–2114. 10.1016/j.ccr.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry N. L.; Mascharak P. K. Photoactive Ruthenium Nitrosyls as NO Donors: How To Sensitize Them toward Visible Light. Acc. Chem. Res. 2011, 44, 289–298. 10.1021/ar100155t. [DOI] [PubMed] [Google Scholar]
- de Lima R. G.; Sauaia M. G.; Bonaventura D.; Tedesco A. C.; Bendhack L. M.; da Silva R. S. Influence of Ancillary Ligand L in the Nitric Oxide Photorelease by the [Ru(L)(tpy)NO]3+ Complex and its Vasodilator Activity Based on visible Light Irradiation. Inorg. Chim. Acta 2006, 359, 2543–2549. 10.1016/j.ica.2006.02.020. [DOI] [Google Scholar]
- Frasconi M.; Liu Z.; Lei J.; Wu Y.; Strekalova E.; Malin D.; Ambrogio M. W.; Chen X.; Botros Y. Y.; Cryns V. L.; Sauvage J.-P.; Stoddart J. F. Photoexpulsion of Surface-Grafted Ruthenium Complexes and Subsequent Release of Cytotoxic Cargos to Cancer Cells from Mesoporous Silica Nanoparticles. J. Am. Chem. Soc. 2013, 135, 11603–11613. 10.1021/ja405058y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordini J.; Hughes D. L.; Da Motta Neto J. D.; Jorge da Cunha C. Nitric Oxide Photorelease from Ruthenium Salen Complexes in Aqueous and Organic Solutions. Inorg. Chem. 2002, 41, 5410–5416. 10.1021/ic011273d. [DOI] [PubMed] [Google Scholar]
- Carlos R. M.; Ferro A. A.; Silva H. A. S.; Gomes M. G.; Borges S. S. S.; Ford P. C.; Tfouni E.; Franco D. W. Photochemical Reactions of trans-[Ru(NH3)4L(NO)]3+ Complexes. Inorg. Chim. Acta 2004, 357, 1381–1388. 10.1016/j.ica.2003.11.023. [DOI] [Google Scholar]
- Paula Q. A.; Batista A. A.; Castellano E. E.; Ellena J. On the Lability of Dimethylsulfoxide (DMSO) Coordinated to the {RuII–NO+} Species: X-Ray Structures of mer-[RuCl3(DMSO)2(NO)] and mer-[RuCl3(CD3CN)(DMSO)(NO)]. J. Inorg. Biochem. 2002, 90, 144–148. 10.1016/S0162-0134(02)00409-9. [DOI] [PubMed] [Google Scholar]
- Miranda K. M.; Bu X.; Lorković I.; Ford P. C. Synthesis and Structural Characterization of Several Ruthenium Porphyrin Nitrosyl Complexes. Inorg. Chem. 1997, 36, 4838–4848. 10.1021/ic970065b. [DOI] [PubMed] [Google Scholar]
- Ferreira K. Q.; Tfouni E. Chemical and Photochemical Properties of a Ruthenium Nitrosyl Complex with the N-Monosubstituted Cyclam 1-(3-Propylammonium)-1,4,8,11-Tetraazacyclotetradecane. J. Braz. Chem. Soc. 2010, 21, 1349–1358. 10.1590/S0103-50532010000700022. [DOI] [Google Scholar]
- Oliveira F. d. S.; Togniolo V.; Pupo T. T.; Tedesco A. C.; da Silva R. S. Nitrosyl Ruthenium Complex as Nitric Oxide Delivery Agent: Synthesis, Characterization and Photochemical Properties. Inorg. Chem. Commun. 2004, 7, 160–164. 10.1016/j.inoche.2003.10.025. [DOI] [Google Scholar]
- Sauaia M. G.; de Lima R. G.; Tedesco A. C.; da Silva R. S. Photoinduced NO Release by Visible Light Irradiation from Pyrazine-Bridged Nitrosyl Ruthenium Complexes. J. Am. Chem. Soc. 2003, 125, 14718–14719. 10.1021/ja0376801. [DOI] [PubMed] [Google Scholar]
- Marquele-Oliveira F.; de Almeida Santana D. C.; Taveira S. F.; Vermeulen D. M.; Moraes de Oliveira A. R.; da Silva R. S.; Lopez R. F. V. Development of Nitrosyl Ruthenium Complex-Loaded Lipid Carriers for Topical Administration: Improvement in Skin Stability and in Nitric Oxide Release by Visible Light Irradiation. J. Pharm. Biomed. Anal. 2010, 53, 843–851. 10.1016/j.jpba.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Works C. F.; Jocher C. J.; Bart G. D.; Bu X.; Ford P. C. Photochemical Nitric Oxide Precursors: Synthesis, Photochemistry, and Ligand Substitution Kinetics of Ruthenium Salen Nitrosyl and Ruthenium Salophen Nitrosyl Complexes. Inorg. Chem. 2002, 41, 3728–3739. 10.1021/ic020248k. [DOI] [PubMed] [Google Scholar]
- Tfouni E.; Krieger M.; McGarvey B. R.; Franco D. W. Structure, Chemical and Photochemical Reactivity and Biological Activity of Some Ruthenium Amine Nitrosyl Complexes. Coord. Chem. Rev. 2003, 236, 57–69. 10.1016/S0010-8545(02)00177-7. [DOI] [Google Scholar]
- Lorković I. M.; Miranda K. M.; Lee B.; Bernhard S.; Schoonover J. R.; Ford P. C. Flash Photolysis Studies of the Ruthenium(II) Porphyrins Ru(P)(NO)(ONO). Multiple Pathways Involving Reactions of Intermediates with Nitric Oxide. J. Am. Chem. Soc. 1998, 120, 11674–11683. 10.1021/ja981907o. [DOI] [Google Scholar]
- Vorobyev V.; Budkina D. S.; Tarnovsky A. N. Femtosecond Excited-State Dynamics and Nitric Oxide Photorelease in a Prototypical Ruthenium Nitrosyl Complex. J. Phys. Chem. Lett. 2020, 11, 4639–4643. 10.1021/acs.jpclett.0c01105. [DOI] [PubMed] [Google Scholar]
- Oliveira F. d. S.; Ferreira K. Q.; Bonaventura D.; Bendhack L. M.; Tedesco A. C.; Machado S. d. P.; Tfouni E.; Silva R. S. d. The Macrocyclic Effect and Vasodilation Response Based on the Photoinduced Nitric Oxide Release from trans-[RuCl(tetraazamacrocycle)NO]2+. J. Inorg. Biochem. 2007, 101, 313–320. 10.1016/j.jinorgbio.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Bordini J.; Ford P. C.; Tfouni E. Photochemical Release of Nitric Oxide from a Regenerable, Sol-Gel Encapsulated Ru–Salen–Nitrosyl Complex. Chem. Commun. 2005, 4169–4171. 10.1039/b507407j. [DOI] [PubMed] [Google Scholar]
- Fry N. L.; Heilman B. J.; Mascharak P. K. Dye-Tethered Ruthenium Nitrosyls Containing Planar Dicarboxamide Tetradentate N4 Ligands: Effects of In-Plane Ligand Twist on NO Photolability. Inorg. Chem. 2011, 50, 317–324. 10.1021/ic1019873. [DOI] [PubMed] [Google Scholar]
- Patra A. K.; Rose M. J.; Murphy K. A.; Olmstead M. M.; Mascharak P. K. Photolabile Ruthenium Nitrosyls with Planar Dicarboxamide Tetradentate N4 Ligands: Effects of In-Plane and Axial Ligand Strength on NO Release. Inorg. Chem. 2004, 43, 4487–4495. 10.1021/ic040030t. [DOI] [PubMed] [Google Scholar]
- Kumar R.; Kumar S.; Bala M.; Ratnam A.; Singh U. P.; Ghosh K. Unprecedented Oxidation of Aldimine to Carboxamido Function During Reactivity Studies on Ruthenium Complex with Acidified Nitrite Solution: Synthesis of Ruthenium Nitrosyl Complex Having {RuNO}6 Moiety and Photorelease of Coordinated NO. J. Organomet. Chem. 2018, 863, 77–83. 10.1016/j.jorganchem.2018.02.010. [DOI] [Google Scholar]
- Rose M. J.; Olmstead M. M.; Mascharak P. K. Photoactive Ruthenium Nitrosyls Derived from Quinoline- and Pyridine-Based Ligands: Accelerated Photorelease of NO Due to Quinoline Ligation. Polyhedron 2007, 26, 4713–4718. 10.1016/j.poly.2007.03.010. [DOI] [Google Scholar]
- Bukhanko V.; Lacroix P. G.; Sasaki I.; Tassé M.; Mallet-Ladeira S.; Voitenko Z.; Malfant I. Mechanism and Oxidation State Involved in the Nitric Oxide (NO) Photorelease in a Terpyridine-Bipyridine-Based Ruthenium Nitrosyl Complex. Inorg. Chim. Acta 2018, 482, 195–205. 10.1016/j.ica.2018.05.038. [DOI] [Google Scholar]
- Amabilino S.; Tasse M.; Lacroix P. G.; Mallet-Ladeira S.; Pimienta V.; Akl J.; Sasaki I.; Malfant I. Photorelease of Nitric Oxide (NO) on Ruthenium Nitrosyl Complexes with Phenyl Substituted Terpyridines. New J. Chem. 2017, 41, 7371–7383. 10.1039/C7NJ00866J. [DOI] [Google Scholar]
- Roose M.; Sasaki I.; Bukhanko V.; Mallet-Ladeira S.; Barba-Barba R. M.; Ramos-Ortiz G.; Enriquez-Cabrera A.; Farfán N.; Lacroix P. G.; Malfant I. Nitric Oxide Photo-Release from a Ruthenium Nitrosyl Complex with a 4,4′-Bisfluorenyl-2,2′-Bipyridine Ligand. Polyhedron 2018, 151, 100–111. 10.1016/j.poly.2018.05.028. [DOI] [Google Scholar]
- Enriquez-Cabrera A.; Lacroix P. G.; Sasaki I.; Mallet-Ladeira S.; Farfán N.; Barba-Barba R. M.; Ramos-Ortiz G.; Malfant I. Comparison of Carbazole and Fluorene Donating Effects on the Two-Photon Absorption and Nitric Oxide Photorelease Capabilities of a Ruthenium–Nitrosyl Complex. Eur. J. Inorg. Chem. 2018, 2018, 531–543. 10.1002/ejic.201700895. [DOI] [Google Scholar]
- Roose M.; Tassé M.; Lacroix P. G.; Malfant I. Nitric Oxide (NO) Photo-Release in a Series of Ruthenium–Nitrosyl Complexes: New Experimental Insights in the Search for a Comprehensive Mechanism. New J. Chem. 2019, 43, 755–767. 10.1039/C8NJ03907K. [DOI] [Google Scholar]
- Sasaki I.; Amabilino S.; Mallet-Ladeira S.; Tassé M.; Sournia-Saquet A.; Lacroix P. G.; Malfant I. Further Studies on the Photoreactivities of Ruthenium–Nitrosyl Complexes with Terpyridyl Ligands. New J. Chem. 2019, 43, 11241–11250. 10.1039/C9NJ02398D. [DOI] [Google Scholar]
- Xiang H.-J.; An L.; Tang W.-W.; Yang S.-P.; Liu J.-G. Photo-Controlled Targeted Intracellular Delivery of Both Nitric Oxide and Singlet Oxygen Using a Fluorescence-Trackable Ruthenium Nitrosyl Functional Nanoplatform. Chem. Commun. 2015, 51, 2555–2558. 10.1039/C4CC09869B. [DOI] [PubMed] [Google Scholar]
- Xiang H.-J.; Deng Q.; An L.; Guo M.; Yang S.-P.; Liu J.-G. Tumor Cell Specific and Lysosome-Targeted Delivery of Nitric Oxide for Enhanced Photodynamic Therapy Triggered by 808 nm Near-Infrared Light. Chem. Commun. 2016, 52, 148–151. 10.1039/C5CC07006F. [DOI] [PubMed] [Google Scholar]
- Giri B.; Kumbhakar S.; Kalai Selvan K.; Muley A.; Maji S. Formation, Reactivity, Photorelease, and Scavenging of NO in Ruthenium Nitrosyl Complexes. Inorg. Chim. Acta 2020, 502, 119360. 10.1016/j.ica.2019.119360. [DOI] [PubMed] [Google Scholar]
- De Candia A. G.; Marcolongo J. P.; Etchenique R.; Slep L. D. Widely Differing Photochemical Behavior in Related Octahedral (Ru-NO)6 Compounds: Intramolecular Redox Isomerism of the Excited State Controlling the Photodelivery of NO. Inorg. Chem. 2010, 49, 6925–6930. 10.1021/ic100491g. [DOI] [PubMed] [Google Scholar]
- Carneiro Z. A.; Biazzotto J. C.; Alexiou A. D. P.; Nikolaou S. Nitric Oxide Photorelease from a Trinuclear Ruthenium Nitrosyl Complex and its in Vitro Cytotoxicity Against Melanoma Cells. J. Inorg. Biochem. 2014, 134, 36–38. 10.1016/j.jinorgbio.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Shin S.; Choe J.; Park Y.; Jeong D.; Song H.; You Y.; Seo D.; Cho J. Artificial Control of Cell Signaling Using a Photocleavable Cobalt(III)–Nitrosyl Complex. Angew. Chem., Int. Ed. 2019, 58, 10126–10131. 10.1002/anie.201903106. [DOI] [PubMed] [Google Scholar]
- Ford P. C. Photochemical Delivery of Nitric Oxide. Nitric Oxide 2013, 34, 56–64. 10.1016/j.niox.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Fraix A.; Sortino S. Combination of PDT Photosensitizers with NO Photodononors. Photochem. Photobiol. Sci. 2018, 17, 1709–1727. 10.1039/C8PP00272J. [DOI] [PubMed] [Google Scholar]
- Neuman D.; Ostrowski A. D.; Absalonson R. O.; Strouse G. F.; Ford P. C. Photosensitized NO Release from Water-Soluble Nanoparticle Assemblies. J. Am. Chem. Soc. 2007, 129, 4146–4147. 10.1021/ja070490w. [DOI] [PubMed] [Google Scholar]
- Rose M. J.; Fry N. L.; Marlow R.; Hinck L.; Mascharak P. K. Sensitization of Ruthenium Nitrosyls to Visible Light via Direct Coordination of the Dye Resorufin: Trackable NO Donors for Light-Triggered NO Delivery to Cellular Targets. J. Am. Chem. Soc. 2008, 130, 8834–8846. 10.1021/ja801823f. [DOI] [PubMed] [Google Scholar]
- Rose M. J.; Mascharak P. K. Photosensitization of Ruthenium Nitrosyls to Red Light with an Isoelectronic Series of Heavy-Atom Chromophores: Experimental and Density Functional Theory Studies on the Effects of O-, S- and Se-Substituted Coordinated Dyes. Inorg. Chem. 2009, 48, 6904–6917. 10.1021/ic900899j. [DOI] [PubMed] [Google Scholar]
- Fry N. L.; Wei J.; Mascharak P. K. Triggered Dye Release via Photodissociation of Nitric Oxide from Designed Ruthenium Nitrosyls: Turn-ON Fluorescence Signaling of Nitric Oxide Delivery. Inorg. Chem. 2011, 50, 9045–9052. 10.1021/ic201242d. [DOI] [PubMed] [Google Scholar]
- Rose M. J.; Mascharak P. K. A Photosensitive {Ru–NO}6 Nitrosyl Bearing Dansyl Chromophore: Novel NO Donor with a Fluorometric On/Off Switch. Chem. Commun. 2008, 3933–3935. 10.1039/b805332d. [DOI] [PubMed] [Google Scholar]
- Becker T.; Kupfer S.; Wolfram M.; Görls H.; Schubert U. S.; Anslyn E. V.; Dietzek B.; Gräfe S.; Schiller A. Sensitization of NO-Releasing Ruthenium Complexes to Visible Light. Chem. - Eur. J. 2015, 21, 15554–15563. 10.1002/chem.201502091. [DOI] [PubMed] [Google Scholar]
- Wecksler S. R.; Hutchinson J.; Ford P. C. Toward Development of Water Soluble Dye Derivatized Nitrosyl Compounds for Photochemical Delivery of NO. Inorg. Chem. 2006, 45, 1192–1200. 10.1021/ic051723s. [DOI] [PubMed] [Google Scholar]
- Levy E. S.; Morales D. P.; Garcia J. V.; Reich N. O.; Ford P. C. Near-IR Mediated Intracellular Uncaging of NO from Cell Targeted Hollow Gold Nanoparticles. Chem. Commun. 2015, 51, 17692–17695. 10.1039/C5CC07989F. [DOI] [PubMed] [Google Scholar]
- Li Y.-H.; Guo M.; Shi S.-W.; Zhang Q.-L.; Yang S.-P.; Liu J.-G. A Ruthenium-Nitrosyl-Functionalized Nanoplatform for the Targeting of Liver Cancer Cells and NIR-Light-Controlled Delivery of Nitric Oxide Combined with Photothermal Therapy. J. Mater. Chem. B 2017, 5, 7831–7838. 10.1039/C7TB02059G. [DOI] [PubMed] [Google Scholar]
- Guo M.; Xiang H.-J.; Wang Y.; Zhang Q.-L.; An L.; Yang S.-P.; Ma Y.; Wang Y.; Liu J.-G. Ruthenium Nitrosyl Functionalized Graphene Quantum Dots as an Efficient Nanoplatform for NIR-Light-Controlled and Mitochondria-Targeted Delivery of Nitric Oxide Combined with Photothermal Therapy. Chem. Commun. 2017, 53, 3253–3256. 10.1039/C7CC00670E. [DOI] [PubMed] [Google Scholar]
- Yu Y.-T.; Shi S.-W.; Wang Y.; Zhang Q.-L.; Gao S.-H.; Yang S.-P.; Liu J.-G. A Ruthenium Nitrosyl-Functionalized Magnetic Nanoplatform with Near-Infrared Light-Controlled Nitric Oxide Delivery and Photothermal Effect for Enhanced Antitumor and Antibacterial Therapy. ACS Appl. Mater. Interfaces 2020, 12, 312–321. 10.1021/acsami.9b18865. [DOI] [PubMed] [Google Scholar]
- Deng Q.; Xiang H.-J.; Tang W.-W.; An L.; Yang S.-P.; Zhang Q.-L.; Liu J.-G. Ruthenium Nitrosyl Grafted Carbon Dots as a Fluorescence-Trackable Nanoplatform for Visible Light-Controlled Nitric Oxide Release and Targeted Intracellular Delivery. J. Inorg. Biochem. 2016, 165, 152–158. 10.1016/j.jinorgbio.2016.06.011. [DOI] [PubMed] [Google Scholar]
- Shi S.-W.; Li Y.-H.; Zhang Q.-L.; Yang S.-P.; Liu J.-G. Targeted and NIR Light-Controlled Delivery of Nitric Oxide Combined with a Platinum(IV) Prodrug for Enhanced Anticancer Therapy. J. Mater. Chem. B 2019, 7, 1867–1874. 10.1039/C8TB02743A. [DOI] [PubMed] [Google Scholar]
- Neuman D.; Ostrowski A. D.; Mikhailovsky A. A.; Absalonson R. O.; Strouse G. F.; Ford P. C. Quantum Dot Fluorescence Quenching Pathways with Cr(III) Complexes. Photosensitized NO Production from trans-Cr(cyclam)(ONO)2+. J. Am. Chem. Soc. 2008, 130, 168–175. 10.1021/ja074164s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L.; Wan A.; Zhu X.; Li H. Nitric Oxide Release Triggered by Two-Photon Excited Photoluminescence of Engineered Nanomaterials. Chem. Commun. 2014, 50, 5725–5728. 10.1039/c4cc01126k. [DOI] [PubMed] [Google Scholar]
- Tan L.; Wan A.; Zhu X.; Li H. Visible Light-Triggered Nitric Oxide Release from Near-Infrared Fluorescent Nanospheric Vehicles. Analyst 2014, 139, 3398–3406. 10.1039/c4an00275j. [DOI] [PubMed] [Google Scholar]
- Heilman B. J.; St. John J.; Oliver S. R. J.; Mascharak P. K. Light-Triggered Eradication of Acinetobacter baumannii by Means of NO Delivery from a Porous Material with an Entrapped Metal Nitrosyl. J. Am. Chem. Soc. 2012, 134, 11573–11582. 10.1021/ja3022736. [DOI] [PubMed] [Google Scholar]
- Chen G.; Qiu H.; Prasad P. N.; Chen X. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. 10.1021/cr400425h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Tu D.; Zhu H.; Chen X. Lanthanide-Doped Luminescent Nanoprobes: Controlled Synthesis, Optical Spectroscopy, and Bioapplications. Chem. Soc. Rev. 2013, 42, 6924–6958. 10.1039/c3cs60060b. [DOI] [PubMed] [Google Scholar]
- Mase J. D.; Razgoniaev A. O.; Tschirhart M. K.; Ostrowski A. D. Light-Controlled Release of Nitric Oxide from Solid Polymer Composite Materials using Visible and Near Infra-Red Light. Photochem. Photobiol. Sci. 2015, 14, 775–785. 10.1039/C4PP00441H. [DOI] [PubMed] [Google Scholar]
- Welbes L. L.; Borovik A. S. Confinement of Metal Complexes within Porous Hosts: Development of Functional Materials for Gas Binding and Catalysis. Acc. Chem. Res. 2005, 38, 765–774. 10.1021/ar0402513. [DOI] [PubMed] [Google Scholar]
- Halpenny G. M.; Olmstead M. M.; Mascharak P. K. Incorporation of a Designed Ruthenium Nitrosyl in PolyHEMA Hydrogel and Light-Activated Delivery of NO to Myoglobin. Inorg. Chem. 2007, 46, 6601–6606. 10.1021/ic700694b. [DOI] [PubMed] [Google Scholar]
- Robbins M. E.; Schoenfisch M. H. Surface-Localized Release of Nitric Oxide via Sol-Gel Chemistry. J. Am. Chem. Soc. 2003, 125, 6068–6069. 10.1021/ja034019o. [DOI] [PubMed] [Google Scholar]
- Heilman B. J.; Halpenny G. M.; Mascharak P. K. Synthesis, Characterization, and Light-Controlled Antibiotic Application of a Composite Material Derived from Polyurethane and Silica Xerogel with Embedded Photoactive Manganese Nitrosyl. J. Biomed. Mater. Res., Part B 2011, 99B, 328–337. 10.1002/jbm.b.31904. [DOI] [PubMed] [Google Scholar]
- Duan Y.; Wang Y.; Li X.; Zhang G.; Zhang G.; Hu J. Light-Triggered Nitric Oxide (NO) Release From Photoresponsive Polymersomes for Corneal Wound Healing. Chem. Sci. 2020, 11, 186–194. 10.1039/C9SC04039K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocito G.; Petralia S.; Malanga M.; Béni S.; Calabrese G.; Parenti R.; Conoci S.; Sortino S. Biofriendly Route to Near-Infrared-Active Gold Nanotriangles and Nanoflowers through Nitric Oxide Photorelease for Photothermal Applications. ACS Appl. Nano Mater. 2019, 2, 7916–7923. 10.1021/acsanm.9b01925. [DOI] [Google Scholar]
- Chen Z.; Thiramanas R.; Schwendy M.; Xie C.; Parekh S. H.; Mailänder V.; Wu S. Upconversion Nanocarriers Encapsulated with Photoactivatable Ru Complexes for Near-Infrared Light-Regulated Enzyme Activity. Small 2017, 13, 1700997. 10.1002/smll.201700997. [DOI] [PubMed] [Google Scholar]
- Diring S.; Wang D. O.; Kim C.; Kondo M.; Chen Y.; Kitagawa S.; Kamei K.-i.; Furukawa S. Localized Cell Stimulation by Nitric Oxide Using a Photoactive Porous Coordination Polymer Platform. Nat. Commun. 2013, 4, 2684. 10.1038/ncomms3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabil O.; Banerjee R. Enzymology of H2S Biogenesis, Decay and Signaling. Antioxid. Redox Signaling 2014, 20, 770–782. 10.1089/ars.2013.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L.; Wang R. Hydrogen Sulfide-Based Therapeutics: Exploiting a Unique but Ubiquitous Gasotransmitter. Nat. Rev. Drug Discovery 2015, 14, 329–345. 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- Lefer D. J. A New Gaseous Signaling Molecule Emerges: Cardioprotective Role of Hydrogen Sulfide. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 17907–17908. 10.1073/pnas.0709010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polhemus D. J.; Lefer D. J. Emergence of Hydrogen Sulfide as an Endogenous Gaseous Signaling Molecule in Cardiovascular Disease. Circ. Res. 2014, 114, 730–737. 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.; Li Z.; Organ C. L.; Park C.-M.; Yang C.-t.; Pacheco A.; Wang D.; Lefer D. J.; Xian M. pH-Controlled Hydrogen Sulfide Release for Myocardial Ischemia-Reperfusion Injury. J. Am. Chem. Soc. 2016, 138, 6336–6339. 10.1021/jacs.6b01373. [DOI] [PubMed] [Google Scholar]
- Benavides G. A.; Squadrito G. L.; Mills R. W.; Patel H. D.; Isbell T. S.; Patel R. P.; Darley-Usmar V. M.; Doeller J. E.; Kraus D. W. Hydrogen Sulfide Mediates the Vasoactivity of Garlic. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 17977–17982. 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Yao Y.; Shi H.; Lei Y.; Huang Y.; Wang K.; He X.; Liu J. A Near-Infrared Light-Responsive Nanocomposite for Photothermal Release of H2S and Suppression of Cell Viability. J. Mater. Chem. B 2019, 7, 5992–5997. 10.1039/C9TB01611B. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Li H.; He X.; Yang X.; Li L.; Liu S.; Zou Z.; Wang K.; Liu J. Near-Infrared Photothermal Release of Hydrogen Sulfide from Nanocomposite Hydrogels for Anti-Inflammation Applications. Chin. Chem. Lett. 2020, 31, 787–791. 10.1016/j.cclet.2019.05.025. [DOI] [Google Scholar]
- Levinn C. M.; Cerda M. M.; Pluth M. D. Development and Application of Carbonyl Sulfide-Based Donors for H2S Delivery. Acc. Chem. Res. 2019, 52, 2723–2731. 10.1021/acs.accounts.9b00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarie-Baez N. O.; Bagdon P. E.; Peng B.; Zhao Y.; Park C.-M.; Xian M. Light-Induced Hydrogen Sulfide Release from “Caged” gem-Dithiols. Org. Lett. 2013, 15, 2786–2789. 10.1021/ol401118k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima N.; Ieda N.; Sasakura K.; Nagano T.; Hanaoka K.; Suzuki T.; Miyata N.; Nakagawa H. Synthesis of a Photocontrollable Hydrogen Sulfide Donor Using Ketoprofenate Photocages. Chem. Commun. 2014, 50, 587–589. 10.1039/C3CC47421F. [DOI] [PubMed] [Google Scholar]
- Fukushima N.; Ieda N.; Kawaguchi M.; Sasakura K.; Nagano T.; Hanaoka K.; Miyata N.; Nakagawa H. Development of Photo-Controllable Hydrogen Sulfide Donor Applicable in Live Cells. Bioorg. Med. Chem. Lett. 2015, 25, 175–178. 10.1016/j.bmcl.2014.11.084. [DOI] [PubMed] [Google Scholar]
- Fox M. A.; Triebel C. A. A New Pathway for Cleavage of Some Phenacyl and Styryl Thioethers. J. Org. Chem. 1983, 48, 835–840. 10.1021/jo00154a017. [DOI] [Google Scholar]
- Xiao Z.; Bonnard T.; Shakouri-Motlagh A.; Wylie R. A. L.; Collins J.; White J.; Heath D. E.; Hagemeyer C. E.; Connal L. A. Triggered and Tunable Hydrogen Sulfide Release from Photogenerated Thiobenzaldehydes. Chem. - Eur. J. 2017, 23, 11294–11300. 10.1002/chem.201701206. [DOI] [PubMed] [Google Scholar]
- Tron A.; Peyret A.; Thevenot J.; Bofinger R.; Lecommandoux S.; McClenaghan N. D. A Prototype Reversible Polymersome-Stabilized H2S Photoejector Operating under Pseudophysiological Conditions. Org. Biomol. Chem. 2016, 14, 6394–6397. 10.1039/C6OB01155A. [DOI] [PubMed] [Google Scholar]
- Chen W.; Chen M.; Zang Q.; Wang L.; Tang F.; Han Y.; Yang C.; Deng L.; Liu Y.-N. NIR Light Controlled Release of Caged Hydrogen Sulfide Based on Upconversion Nanoparticles. Chem. Commun. 2015, 51, 9193–9196. 10.1039/C5CC02508G. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A.; Venkatesh Y.; Das J.; Gangopadhyay M.; Maiti T. K.; Singh N. D. P. One- and Two-Photon-Activated Cysteine Persulfide Donors for Biological Targeting. J. Org. Chem. 2019, 84, 11441–11449. 10.1021/acs.joc.9b01224. [DOI] [PubMed] [Google Scholar]
- Supuran C. T.; Scozzafava A. Carbonic Anhydrases as Targets for Medicinal Chemistry. Bioorg. Med. Chem. 2007, 15, 4336–4350. 10.1016/j.bmc.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Bolton S. G.; Pluth M. D. Light-Activated COS/H2S Donation from Photocaged Thiocarbamates. Org. Lett. 2017, 19, 2278–2281. 10.1021/acs.orglett.7b00808. [DOI] [PubMed] [Google Scholar]
- Parthiban C.; M P.; Reddy L. V. K.; Sen D.; Samuel S M.; Singh N. D. P. Tetraphenylethylene Conjugated p-Hydroxyphenacyl: Fluorescent Organic Nanoparticles for the Release of Hydrogen Sulfide under Visible Light with Real-Time Cellular Imaging. Org. Biomol. Chem. 2018, 16, 7903–7909. 10.1039/C8OB01629A. [DOI] [PubMed] [Google Scholar]
- Venkatesh Y.; Das J.; Chaudhuri A.; Karmakar A.; Maiti T. K.; Singh N. D. P. Light Triggered Uncaging of Hydrogen Sulfide (H2S) with Real-Time Monitoring. Chem. Commun. 2018, 54, 3106–3109. 10.1039/C8CC01172A. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A.; Venkatesh Y.; Jena B. C.; Behara K. K.; Mandal M.; Singh N. D. P. Real-Time Monitoring of a Photoactivated Hydrogen Persulfide Donor for Biological Entities. Org. Biomol. Chem. 2019, 17, 8800–8805. 10.1039/C9OB01982K. [DOI] [PubMed] [Google Scholar]
- Yi S. Y.; Moon Y. K.; Kim S.; Kim S.; Park G.; Kim J. J.; You Y. Visible Light-Driven Photogeneration of Hydrogen Sulfide. Chem. Commun. 2017, 53, 11830–11833. 10.1039/C7CC06990A. [DOI] [PubMed] [Google Scholar]
- Li L.; Whiteman M.; Guan Y. Y.; Neo K. L.; Cheng Y.; Lee S. W.; Zhao Y.; Baskar R.; Tan C.-H.; Moore P. K. Characterization of a Novel, Water-Soluble Hydrogen Sulfide-Releasing Molecule (GYY4137). Circulation 2008, 117, 2351–2360. 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- Alexander B. E.; Coles S. J.; Fox B. C.; Khan T. F.; Maliszewski J.; Perry A.; Pitak M. B.; Whiteman M.; Wood M. E. Investigating the Generation of Hydrogen Sulfide from the Phosphonamidodithioate Slow-Release Donor GYY4137. MedChemComm 2015, 6, 1649–1655. 10.1039/C5MD00170F. [DOI] [Google Scholar]
- DeMartino A. W.; Zigler D. F.; Fukuto J. M.; Ford P. C. Carbon Disulfide. Just Toxic or Also Bioregulatory and/or Therapeutic?. Chem. Soc. Rev. 2017, 46, 21–39. 10.1039/C6CS00585C. [DOI] [PubMed] [Google Scholar]
- Bernt C. M.; Burks P. T.; DeMartino A. W.; Pierri A. E.; Levy E. S.; Zigler D. F.; Ford P. C. Photocatalytic Carbon Disulfide Production via Charge Transfer Quenching of Quantum Dots. J. Am. Chem. Soc. 2014, 136, 2192–2195. 10.1021/ja4083599. [DOI] [PubMed] [Google Scholar]
- Zivic N.; Kuroishi P. K.; Dumur F.; Gigmes D.; Dove A. P.; Sardon H. Recent Advances and Challenges in the Design of Organic Photoacid and Photobase Generators for Polymerizations. Angew. Chem., Int. Ed. 2019, 58, 10410–10422. 10.1002/anie.201810118. [DOI] [PubMed] [Google Scholar]
- Sun X.; Jin M.; Wu X.; Pan H.; Wan D.; Pu H. Bis-Substituted Thiophene-Containing Oxime Sulfonates Photoacid Generators for Cationic Polymerization Under UV–Visible LED Irradiation. J. Polym. Sci., Part A: Polym. Chem. 2018, 56, 776–782. 10.1002/pola.28951. [DOI] [Google Scholar]
- Sambath K.; Wan Z.; Wang Q.; Chen H.; Zhang Y. BODIPY-Based Photoacid Generators for Light-Induced Cationic Polymerization. Org. Lett. 2020, 22, 1208–1212. 10.1021/acs.orglett.0c00118. [DOI] [PubMed] [Google Scholar]
- Fu C.; Xu J.; Boyer C. Photoacid-Mediated Ring Opening Polymerization Driven by Visible Light. Chem. Commun. 2016, 52, 7126–7129. 10.1039/C6CC03084J. [DOI] [PubMed] [Google Scholar]
- Hayes C. O.; Bell W. K.; Cassidy B. R.; Willson C. G. Synthesis and Characterization of a Two Stage, Nonlinear Photobase Generator. J. Org. Chem. 2015, 80, 7530–7535. 10.1021/acs.joc.5b01078. [DOI] [PubMed] [Google Scholar]
- Kaye W. Near-Infrared Spectroscopy: I. Spectral Identification and Analytical Applications. Spectrochim. Acta 1954, 6, 257. 10.1016/0371-1951(54)80011-7. [DOI] [Google Scholar]
- Pansare V. J.; Hejazi S.; Faenza W. J.; Prud’homme R. K. Review of Long-Wavelength Optical and NIR Imaging Materials: Contrast Agents, Fluorophores, and Multifunctional Nano Carriers. Chem. Mater. 2012, 24, 812–827. 10.1021/cm2028367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlicki M.; Collins H. A.; Denning R. G.; Anderson H. L. Two-Photon Absorption and the Design of Two-Photon Dyes. Angew. Chem., Int. Ed. 2009, 48, 3244–3266. 10.1002/anie.200805257. [DOI] [PubMed] [Google Scholar]
- Klausen M.; Dubois V.; Verlhac J.-B.; Blanchard-Desce M. Tandem Systems for Two-Photon Uncaging of Bioactive Molecules. ChemPlusChem 2019, 84, 589–598. 10.1002/cplu.201900139. [DOI] [PubMed] [Google Scholar]
- Speiser S. Photophysics and Mechanisms of Intramolecular Electronic Energy Transfer in Bichromophoric Molecular Systems: Solution and Supersonic Jet Studies. Chem. Rev. 1996, 96, 1953–1976. 10.1021/cr941193+. [DOI] [PubMed] [Google Scholar]
- Dietliker K.; Broillet S.; Hellrung B.; Rzadek P.; Rist G.; Wirz J.; Neshchadin D.; Gescheidt G. Photophysical Investigations on Photoinitiators with Covalently Linked Thioxanthone Sensitizer Moieties. Helv. Chim. Acta 2006, 89, 2211–2225. 10.1002/hlca.200690207. [DOI] [Google Scholar]
- Wagner P. J.; Klán P. Intramolecular Triplet Energy Transfer in Flexible Molecules: Electronic, Dynamic, and Structural Aspects. J. Am. Chem. Soc. 1999, 121, 9626–9635. 10.1021/ja990224l. [DOI] [Google Scholar]
- Falvey D. E.; Sundararajan C. Photoremovable Protecting Groups Based on Electron Transfer Chemistry. Photochem. Photobiol. Sci. 2004, 3, 831–838. 10.1039/b406866a. [DOI] [PubMed] [Google Scholar]
- Houmam A. Electron Transfer Initiated Reactions: Bond Formation and Bond Dissociation. Chem. Rev. 2008, 108, 2180–2237. 10.1021/cr068070x. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J.; Gomer C. J.; Henderson B. W.; Jori G.; Kessel D.; Korbelik M.; Moan J.; Peng Q. Photodynamic Therapy. JNCI-J. Natl. Cancer Inst. 1998, 90, 889–905. 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRosa M. C.; Crutchley R. J. Photosensitized Singlet Oxygen and Its Applications. Coord. Chem. Rev. 2002, 233, 351–371. 10.1016/S0010-8545(02)00034-6. [DOI] [Google Scholar]
- Detty M. R.; Gibson S. L.; Wagner S. J. Current Clinical and Preclinical Photosensitizers for Use in Photodynamic Therapy. J. Med. Chem. 2004, 47, 3897–3915. 10.1021/jm040074b. [DOI] [PubMed] [Google Scholar]
- Ormond A. B.; Freeman H. S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. 10.3390/ma6030817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klán P.; Wagner P. J. Intramolecular Triplet Energy Transfer in Bichromophores With Long Flexible Tethers. J. Am. Chem. Soc. 1998, 120, 2198–2199. 10.1021/ja974016+. [DOI] [Google Scholar]
- Vrbka L.; Klán P.; Kříž Z.; Koča J.; Wagner P. J. Computer Modeling and Simulations on Flexible Bifunctional Systems: Intramolecular Energy Transfer Implications. J. Phys. Chem. A 2003, 107, 3404–3413. 10.1021/jp026890h. [DOI] [Google Scholar]
- Papageorgiou G.; Corrie J. E. T. Optimised Synthesis and Photochemistry of Antenna-Sensitised 1-Acyl-7-Nitroindolines. Tetrahedron 2005, 61, 609–616. 10.1016/j.tet.2004.10.094. [DOI] [Google Scholar]
- Papageorgiou G.; Lukeman M.; Wan P.; Corrie J. E. T. An Antenna Triplet Sensitiser for 1-Acyl-7-Nitroindolines Improves the Efficiency of Carboxylic Acid Photorelease. Photochem. Photobiol. Sci. 2004, 3, 366–373. 10.1039/b316251f. [DOI] [PubMed] [Google Scholar]
- Papageorgiou G.; Ogden D.; Corrie J. E. T. An Antenna-Sensitized Nitroindoline Precursor to Enable Photorelease of l-Glutamate in High Concentrations. J. Org. Chem. 2004, 69, 7228–7233. 10.1021/jo049071x. [DOI] [PubMed] [Google Scholar]
- Papageorgiou G.; Ogden D.; Corrie J. E. T. An Antenna-Sensitised 1-Acyl-7-Nitroindoline That Has Good Solubility Properties in the Presence of Calcium Ions and Is Suitable for Use as a Caged L-Glutamate in Neuroscience. Photochem. Photobiol. Sci. 2008, 7, 423–432. 10.1039/b800683k. [DOI] [PubMed] [Google Scholar]
- Wöll D.; Laimgruber S.; Galetskaya M.; Smirnova J.; Pfleiderer W.; Heinz B.; Gilch P.; Steiner U. E. On the Mechanism of Intramolecular Sensitization of Photocleavage of the 2-(2-Nitrophenyl)propoxycarbonyl (NPPOC) Protecting Group. J. Am. Chem. Soc. 2007, 129, 12148–12158. 10.1021/ja072355p. [DOI] [PubMed] [Google Scholar]
- Röthlingshöfer M.; Gorska K.; Winssinger N. Nucleic Acid-Templated Energy Transfer Leading to a Photorelease Reaction and its Application to a System Displaying a Nonlinear Response. J. Am. Chem. Soc. 2011, 133, 18110–18113. 10.1021/ja2086504. [DOI] [PubMed] [Google Scholar]
- Ndzeidze G. N.; Li L.; Steinmetz M. G. Reversible Triplet Excitation Transfer in a Trimethylene-Linked Thioxanthone and Benzothiophene-2-Carboxanilide that Photochemically Expels Leaving Group Anions. J. Org. Chem. 2018, 83, 8995–9007. 10.1021/acs.joc.8b01173. [DOI] [PubMed] [Google Scholar]
- Lv W.; Wang W. One-Photon Upconversion-Like Photolysis: A New Strategy to Achieve Long-Wavelength Light-Excitable Photolysis. Synlett 2020, 31, 1129–1134. 10.1055/s-0040-1707100. [DOI] [Google Scholar]
- Rehm D.; Weller A. Kinetik und Mechanismus der Elektronübertragung bei der Fluoreszenzlöschung in Acetonitril. Berich. Bunsen. Gesell. 1969, 73, 834–839. [Google Scholar]
- Hoffmann N. Efficient Photochemical Electron Transfer Sensitization of Homogeneous Organic Reactions. J. Photochem. Photobiol., C 2008, 9, 43–60. 10.1016/j.jphotochemrev.2008.04.002. [DOI] [Google Scholar]
- Romero N. A.; Nicewicz D. A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- Banerjee A.; Falvey D. E. Protecting Groups That Can Be Removed through Photochemical Electron Transfer: Mechanistic and Product Studies on Photosensitized Release of Carboxylates from Phenacyl Esters. J. Org. Chem. 1997, 62, 6245–6251. 10.1021/jo970495j. [DOI] [Google Scholar]
- Lee K.; Falvey D. E. Photochemically Removable Protecting Groups Based on Covalently Linked Electron Donor-Acceptor Systems. J. Am. Chem. Soc. 2000, 122, 9361–9366. 10.1021/ja9939441. [DOI] [Google Scholar]
- Banerjee A.; Lee K.; Falvey D. E. Photoreleasable Protecting Groups Based on Electron Transfer Chemistry. Donor Sensitized Release of Phenacyl Groups From Alcohols, Phosphates and Diacids. Tetrahedron 1999, 55, 12699–12710. 10.1016/S0040-4020(99)00754-1. [DOI] [Google Scholar]
- Sundararajan C.; Falvey D. E. Photorelease of Carboxylic and Amino Acids From N-Methyl-4-Picolinium Esters by Mediated Electron Transfer. Photochem. Photobiol. Sci. 2006, 5, 116–121. 10.1039/B511269A. [DOI] [PubMed] [Google Scholar]
- Sundararajan C.; Falvey D. E. Photolytic Release of Carboxylic Acids Using Linked Donor-Acceptor Molecules: Direct versus Mediated Photoinduced Electron Transfer to N-Alkyl-4-picolinium Esters. Org. Lett. 2005, 7, 2631–2634. 10.1021/ol050744n. [DOI] [PubMed] [Google Scholar]
- Zeppuhar A. N.; Hill-Byrne K.; Falvey D. E. Mechanism of the Photorelease of Alcohols From the 9-Phenyl-9-Tritylone Protecting Group. Photochem. Photobiol. Sci. 2019, 18, 1990–1995. 10.1039/C9PP00183B. [DOI] [PubMed] [Google Scholar]
- Banerjee A.; Lee K.; Yu Q.; Fang A. G.; Falvey D. E. Protecting Group Release Through Photoinduced Electron Transfer: Wavelength Control Through Sensitized Irradiation. Tetrahedron Lett. 1998, 39, 4635–4638. 10.1016/S0040-4039(98)00857-0. [DOI] [Google Scholar]
- Speckmeier E.; Zeitler K. Desyl and Phenacyl as Versatile, Photocatalytically Cleavable Protecting Groups: A Classic Approach in a Different (Visible) Light. ACS Catal. 2017, 7, 6821–6826. 10.1021/acscatal.7b02117. [DOI] [Google Scholar]
- Sundararajan C.; Falvey D. E. Photorelease of Carboxylic Acids, Amino Acids, and Phosphates from N-Alkylpicolinium Esters Using Photosensitization by High Wavelength Laser Dyes. J. Am. Chem. Soc. 2005, 127, 8000–8001. 10.1021/ja050760f. [DOI] [PubMed] [Google Scholar]
- Borak J. B.; Falvey D. E. A New Photolabile Protecting Group for Release of Carboxylic Acids by Visible-Light-Induced Direct and Mediated Electron Transfer. J. Org. Chem. 2009, 74, 3894–3899. 10.1021/jo900182x. [DOI] [PubMed] [Google Scholar]
- Borak J. B.; Falvey D. E. Ketocoumarin Dyes as Electron Mediators for Visible Light Induced Carboxylate Photorelease. Photochem. Photobiol. Sci. 2010, 9, 854–860. 10.1039/c0pp00072h. [DOI] [PubMed] [Google Scholar]
- Kunsberg D. J.; Kipping A. H.; Falvey D. E. Visible Light Photorelease of Carboxylic Acids via Charge-Transfer Excitation of N-Methylpyridinium Iodide Esters. Org. Lett. 2015, 17, 3454–3457. 10.1021/acs.orglett.5b01490. [DOI] [PubMed] [Google Scholar]
- Thum M. D.; Falvey D. E. Photoreleasable Protecting Groups Triggered by Sequential Two-Photon Absorption of Visible Light: Release of Carboxylic Acids from a Linked Anthraquinone-N-Alkylpicolinium Ester Molecule. J. Phys. Chem. A 2018, 122, 3204–3210. 10.1021/acs.jpca.8b00657. [DOI] [PubMed] [Google Scholar]
- Edson J. B.; Spencer L. P.; Boncella J. M. Photorelease of Primary Aliphatic and Aromatic Amines by Visible-Light-Induced Electron Transfer. Org. Lett. 2011, 13, 6156–6159. 10.1021/ol202456d. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Kang X.; Yang X.; Yuan L.; Feng W.; Cui S. Surface Charge Inversion of Self-Assembled Monolayers by Visible Light Irradiation: Cargo Loading and Release by Photoreactions. Chem. Commun. 2013, 49, 3431–3433. 10.1039/c3cc39081k. [DOI] [PubMed] [Google Scholar]
- Ciuciu A. I.; Korzycka K. A.; Lewis W. J. M.; Bennett P. M.; Anderson H. L.; Flamigni L. Model Dyads for 2PA Uncaging of a Protecting Group via Photoinduced Electron Transfer. Phys. Chem. Chem. Phys. 2015, 17, 6554–6564. 10.1039/C4CP05812G. [DOI] [PubMed] [Google Scholar]
- Denning D. M.; Pedowitz N. J.; Thum M. D.; Falvey D. E. Uncaging Alcohols Using UV or Visible Light Photoinduced Electron Transfer to 9-Phenyl-9-tritylone Ethers. Org. Lett. 2015, 17, 5986–5989. 10.1021/acs.orglett.5b02924. [DOI] [PubMed] [Google Scholar]
- Heymann R. R.; Thum M. D.; Hardee A. L.; Falvey D. E. Visible Light Initiated Release of Calcium Ions Through Photochemical Electron Transfer Reactions. Photochem. Photobiol. Sci. 2017, 16, 1003–1008. 10.1039/C6PP00469E. [DOI] [PubMed] [Google Scholar]
- Wang H.; Li W.-G.; Zeng K.; Wu Y.-J.; Zhang Y.; Xu T.-L.; Chen Y. Photocatalysis Enables Visible-Light Uncaging of Bioactive Molecules in Live Cells. Angew. Chem., Int. Ed. 2019, 58, 561–565. 10.1002/anie.201811261. [DOI] [PubMed] [Google Scholar]
- Gorska K.; Manicardi A.; Barluenga S.; Winssinger N. DNA-Templated Release of Functional Molecules With an Azide-Reduction-Triggered Immolative Linker. Chem. Commun. 2011, 47, 4364–4366. 10.1039/c1cc10222b. [DOI] [PubMed] [Google Scholar]
- Rothlingshofer M.; Gorska K.; Winssinger N. Nucleic Acid Templated Uncaging of Fluorophores Using Ru-Catalyzed Photoreduction with Visible Light. Org. Lett. 2012, 14, 482–485. 10.1021/ol203029t. [DOI] [PubMed] [Google Scholar]
- Dariva C. G.; Coelho J. F. J.; Serra A. C. Near Infrared Light-Triggered Nanoparticles Using Singlet Oxygen Photocleavage for Drug Delivery Systems. J. Controlled Release 2019, 294, 337–354. 10.1016/j.jconrel.2018.12.042. [DOI] [PubMed] [Google Scholar]
- Shim G.; Ko S.; Kim D.; Le Q.-V.; Park G. T.; Lee J.; Kwon T.; Choi H.-G.; Kim Y. B.; Oh Y.-K. Light-Switchable Systems for Remotely Controlled Drug Delivery. J. Controlled Release 2017, 267, 67–79. 10.1016/j.jconrel.2017.09.009. [DOI] [PubMed] [Google Scholar]
- Zamadar M.; Ghosh G.; Mahendran A.; Minnis M.; Kruft B. I.; Ghogare A.; Aebisher D.; Greer A. Photosensitizer Drug Delivery via an Optical Fiber. J. Am. Chem. Soc. 2011, 133, 7882–7891. 10.1021/ja200840p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh G.; Belh S. J.; Chiemezie C.; Walalawela N.; Ghogare A. A.; Vignoni M.; Thomas A. H.; McFarland S. A.; Greer E. M.; Greer A. S, S-Chiral Linker Induced U Shape with a Syn-facial Sensitizer and Photocleavable Ethene Group. Photochem. Photobiol. 2019, 95, 293–305. 10.1111/php.13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson V. C.; Thompson D. H. Triggered Release of Hydrophilic Agents From Plasmologen Liposomes Using Visible Light or Acid. Biochim. Biophys. Acta, Biomembr. 1992, 1109, 33–42. 10.1016/0005-2736(92)90183-M. [DOI] [PubMed] [Google Scholar]
- Murthy R. S.; Bio M.; You Y. Low Energy Light-Triggered Oxidative Cleavage of Olefins. Tetrahedron Lett. 2009, 50, 1041–1044. 10.1016/j.tetlet.2008.12.069. [DOI] [Google Scholar]
- Baugh S. D. P.; Yang Z.; Leung D. K.; Wilson D. M.; Breslow R. Cyclodextrin Dimers as Cleavable Carriers of Photodynamic Sensitizers. J. Am. Chem. Soc. 2001, 123, 12488–12494. 10.1021/ja011709o. [DOI] [PubMed] [Google Scholar]
- Ruebner A.; Yang Z.; Leung D.; Breslow R. A Cyclodextrin Dimer With a Photocleavable Linker as a Possible Carrier for the Photosensitizer in Photodynamic Tumor Therapy. Proc. Natl. Acad. Sci. U. S. A. 1999, 96, 14692–14693. 10.1073/pnas.96.26.14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M. Y.; Dolphin D. Site-Specific Prodrug Release Using Visible Light. J. Am. Chem. Soc. 2008, 130, 4236–4237. 10.1021/ja800140g. [DOI] [PubMed] [Google Scholar]
- Liu J.; Yang G.; Zhu W.; Dong Z.; Yang Y.; Chao Y.; Liu Z. Light-Controlled Drug Release From Singlet-Oxygen Sensitive Nanoscale Coordination Polymers Enabling Cancer Combination Therapy. Biomaterials 2017, 146, 40–48. 10.1016/j.biomaterials.2017.09.007. [DOI] [PubMed] [Google Scholar]
- Wang H.; Han R.-l.; Yang L.-m.; Shi J.-h.; Liu Z.-j.; Hu Y.; Wang Y.; Liu S.-j.; Gan Y. Design and Synthesis of Core–Shell–Shell Upconversion Nanoparticles for NIR-Induced Drug Release, Photodynamic Therapy, and Cell Imaging. ACS Appl. Mater. Interfaces 2016, 8, 4416–4423. 10.1021/acsami.5b11197. [DOI] [PubMed] [Google Scholar]
- Chai S.; Guo Y.; Zhang Z.; Chai Z.; Ma Y.; Qi L. Cyclodextrin-Gated Mesoporous Silica Nanoparticles as Drug Carriers for Red Light-Induced Drug Release. Nanotechnology 2017, 28, 145101. 10.1088/1361-6528/aa5e74. [DOI] [PubMed] [Google Scholar]
- Yang G.; Sun X.; Liu J.; Feng L.; Liu Z. Light-Responsive, Singlet-Oxygen-Triggered On-Demand Drug Release from Photosensitizer-Doped Mesoporous Silica Nanorods for Cancer Combination Therapy. Adv. Funct. Mater. 2016, 26, 4722–4732. 10.1002/adfm.201600722. [DOI] [Google Scholar]
- Ghosh G.; Minnis M.; Ghogare A. A.; Abramova I.; Cengel K. A.; Busch T. M.; Greer A. Photoactive Fluoropolymer Surfaces That Release Sensitizer Drug Molecules. J. Phys. Chem. B 2015, 119, 4155–4164. 10.1021/acs.jpcb.5b00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Huang J.; Lyu Y.; Huang J.; Jiang Y.; Xie C.; Pu K. Photoactivatable Organic Semiconducting Pro-Nanoenzymes. J. Am. Chem. Soc. 2019, 141, 4073–4079. 10.1021/jacs.8b13507. [DOI] [PubMed] [Google Scholar]
- Bartusik D.; Aebisher D.; Ghosh G.; Minnis M.; Greer A. Fluorine End-Capped Optical Fibers for Photosensitizer Release and Singlet Oxygen Production. J. Org. Chem. 2012, 77, 4557–4565. 10.1021/jo3006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Park J.; Singha K.; Kim W. J. Mesoporous Silica Nanoparticle Facilitated Drug Release Through Cascade Photosensitizer Activation and Cleavage of Singlet Oxygen Sensitive Linker. Chem. Commun. 2013, 49, 1545–1547. 10.1039/c2cc38510d. [DOI] [PubMed] [Google Scholar]
- Dinache A.; Smarandache A.; Simon A.; Nastasa V.; Tozar T.; Pascu A.; Enescu M.; Khatyr A.; Sima F.; Pascu M. L.; Staicu A. Photosensitized Cleavage of Some Olefins as Potential Linkers to Be Used in Drug Delivery. Appl. Surf. Sci. 2017, 417, 136–142. 10.1016/j.apsusc.2017.03.177. [DOI] [Google Scholar]
- Saravanakumar G.; Lee J.; Kim J.; Kim W. J. Visible Light-Induced Singlet Oxygen-Mediated Intracellular Disassembly of Polymeric Micelles Co-loaded With a Photosensitizer and an Anticancer Drug for Enhanced Photodynamic Therapy. Chem. Commun. 2015, 51, 9995–9998. 10.1039/C5CC01937K. [DOI] [PubMed] [Google Scholar]
- Thapa P.; Li M.; Karki R.; Bio M.; Rajaputra P.; Nkepang G.; Woo S.; You Y. Folate-PEG Conjugates of a Far-Red Light-Activatable Paclitaxel Prodrug to Improve Selectivity toward Folate Receptor-Positive Cancer Cells. ACS Omega 2017, 2, 6349–6360. 10.1021/acsomega.7b01105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkepang G.; Bio M.; Rajaputra P.; Awuah S. G.; You Y. Folate Receptor-Mediated Enhanced and Specific Delivery of Far-Red Light-Activatable Prodrugs of Combretastatin A-4 to FR-Positive Tumor. Bioconjugate Chem. 2014, 25, 2175–2188. 10.1021/bc500376j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bio M.; Rajaputra P.; Nkepang G.; You Y. Far-Red Light Activatable, Multifunctional Prodrug for Fluorescence Optical Imaging and Combinational Treatment. J. Med. Chem. 2014, 57, 3401–3409. 10.1021/jm5000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bio M.; Nkepang G.; You Y. Click and Photo-Unclick Chemistry of Aminoacrylate for Visible Light-Triggered Drug Release. Chem. Commun. 2012, 48, 6517–6519. 10.1039/c2cc32373g. [DOI] [PubMed] [Google Scholar]
- Hossion A. M. L.; Bio M.; Nkepang G.; Awuah S. G.; You Y. Visible Light Controlled Release of Anticancer Drug through Double Activation of Prodrug. ACS Med. Chem. Lett. 2013, 4, 124–127. 10.1021/ml3003617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavlovich A.; Smith B.; Gupta K.; Blumenthal R.; Puri A. Light-Sensitive Lipid-Based Nanoparticles for Drug Delivery: Design Principles and Future Considerations for Biological Applications. Mol. Membr. Biol. 2010, 27, 364–381. 10.3109/09687688.2010.507788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitgupi U.; Shao S.; Carter K. A.; Huang W.-C.; Lovell J. F. Multicolor Liposome Mixtures for Selective and Selectable Cargo Release. Nano Lett. 2018, 18, 1331–1336. 10.1021/acs.nanolett.7b05025. [DOI] [PubMed] [Google Scholar]
- Carter K. A.; Shao S.; Hoopes M. I.; Luo D.; Ahsan B.; Grigoryants V. M.; Song W.; Huang H.; Zhang G.; Pandey R. K.; Geng J.; Pfeifer B. A.; Scholes C. P.; Ortega J.; Karttunen M.; Lovell J. F. Porphyrin–Phospholipid Liposomes Permeabilized by Near-Infrared Light. Nat. Commun. 2014, 5, 3546. 10.1038/ncomms4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda D.; Carter K.; Luo D.; Shao S.; Geng J.; Li C.; Chitgupi U.; Turowski S. G.; Li N.; Atilla-Gokcumen G. E.; Spernyak J. A.; Lovell J. F. Multifunctional Liposomes for Image-Guided Intratumoral Chemo-Phototherapy. Adv. Healthcare Mater. 2017, 6, 1700253. 10.1002/adhm.201700253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D.; Geng J.; Li N.; Carter K. A.; Shao S.; Atilla-Gokcumen G. E.; Lovell J. F. Vessel-Targeted Chemophototherapy with Cationic Porphyrin-Phospholipid Liposomes. Mol. Cancer Ther. 2017, 16, 2452–2461. 10.1158/1535-7163.MCT-17-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D.; Carter K. A.; Razi A.; Geng J.; Shao S.; Giraldo D.; Sunar U.; Ortega J.; Lovell J. F. Doxorubicin Encapsulated in Stealth Liposomes Conferred With Light-Triggered Drug Release. Biomaterials 2016, 75, 193–202. 10.1016/j.biomaterials.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter K. A.; Wang S.; Geng J.; Luo D.; Shao S.; Lovell J. F. Metal Chelation Modulates Phototherapeutic Properties of Mitoxantrone-Loaded Porphyrin–Phospholipid Liposomes. Mol. Pharmaceutics 2016, 13, 420–427. 10.1021/acs.molpharmaceut.5b00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng P.-C.; Hong R.-L.; Tsai Y.-J.; Li P.-T.; Tsai T.; Chen C.-T. Dual-Effect Liposomes Encapsulated With Doxorubicin and Chlorin e6 Augment the Therapeutic Effect of Tumor Treatment. Lasers Surg. Med. 2015, 47, 77–87. 10.1002/lsm.22312. [DOI] [PubMed] [Google Scholar]
- Luo D.; Li N.; Carter K. A.; Lin C.; Geng J.; Shao S.; Huang W.-C.; Qin Y.; Atilla-Gokcumen G. E.; Lovell J. F. Rapid Light-Triggered Drug Release in Liposomes Containing Small Amounts of Unsaturated and Porphyrin–Phospholipids. Small 2016, 12, 3039–3047. 10.1002/smll.201503966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A.; Mokhir A. RNA Interference Controlled by Light of Variable Wavelength. Angew. Chem., Int. Ed. 2014, 53, 12840–12843. 10.1002/anie.201405885. [DOI] [PubMed] [Google Scholar]
- Brega V.; Scaletti F.; Zhang X.; Wang L.-S.; Li P.; Xu Q.; Rotello V. M.; Thomas S. W. Polymer Amphiphiles for Photoregulated Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2019, 11, 2814–2820. 10.1021/acsami.8b18099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Y.; He S.; Li J.; Jiang Y.; Sun H.; Miao Y.; Pu K. A Photolabile Semiconducting Polymer Nanotransducer for Near-Infrared Regulation of CRISPR/Cas9 Gene Editing. Angew. Chem., Int. Ed. 2019, 58, 18197–18201. 10.1002/anie.201909264. [DOI] [PubMed] [Google Scholar]
- Xu X.; Zeng Z.; Huang Z.; Sun Y.; Huang Y.; Chen J.; Ye J.; Yang H.; Yang C.; Zhao C. Near-Infrared Light-Triggered Degradable Hyaluronic Acid Hydrogel for On-Demand Drug Release and Combined Chemo-Photodynamic Therapy. Carbohydr. Polym. 2020, 229, 115394. 10.1016/j.carbpol.2019.115394. [DOI] [PubMed] [Google Scholar]
- Wang C.; Huang B.; Yang G.; Ouyang Y.; Tian J.; Zhang W. NIR-Triggered Multifunctional and Degradable Nanoplatform Based on an ROS-Sensitive Block Copolymer for Imaging-Guided Chemo-Phototherapy. Biomacromolecules 2019, 20, 4218–4229. 10.1021/acs.biomac.9b01123. [DOI] [PubMed] [Google Scholar]
- Wilson D. S.; Dalmasso G.; Wang L.; Sitaraman S. V.; Merlin D.; Murthy N. Orally Delivered Thioketal Nanoparticles Loaded with TNF-α–siRNA Target Inflammation and Inhibit Gene Expression in the Intestines. Nat. Mater. 2010, 9, 923–928. 10.1038/nmat2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim M. S.; Xia Y. A Reactive Oxygen Species (ROS)-Responsive Polymer for Safe, Efficient, and Targeted Gene Delivery in Cancer Cells. Angew. Chem., Int. Ed. 2013, 52, 6926–6929. 10.1002/anie.201209633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.; Liu J.; Liu B. Conjugated-Polyelectrolyte-Based Polyprodrug: Targeted and Image-Guided Photodynamic and Chemotherapy with On-Demand Drug Release upon Irradiation with a Single Light Source. Angew. Chem., Int. Ed. 2014, 53, 7163–7168. 10.1002/anie.201402189. [DOI] [PubMed] [Google Scholar]
- Liu L.-H.; Qiu W.-X.; Li B.; Zhang C.; Sun L.-F.; Wan S.-S.; Rong L.; Zhang X.-Z. A Red Light Activatable Multifunctional Prodrug for Image-Guided Photodynamic Therapy and Cascaded Chemotherapy. Adv. Funct. Mater. 2016, 26, 6257–6269. 10.1002/adfm.201602541. [DOI] [Google Scholar]
- Pei Q.; Hu X.; Zheng X.; Liu S.; Li Y.; Jing X.; Xie Z. Light-Activatable Red Blood Cell Membrane-Camouflaged Dimeric Prodrug Nanoparticles for Synergistic Photodynamic/Chemotherapy. ACS Nano 2018, 12, 1630–1641. 10.1021/acsnano.7b08219. [DOI] [PubMed] [Google Scholar]
- Seah G. L.; Yu J. H.; Yang M. Y.; Kim W. J.; Kim J.-H.; Park K.; Cho J.-W.; Kim J. S.; Nam Y. S. Low-Power and Low-Drug-Dose Photodynamic Chemotherapy via the Breakdown of Tumor-Targeted Micelles by Reactive Oxygen Species. J. Controlled Release 2018, 286, 240–253. 10.1016/j.jconrel.2018.07.046. [DOI] [PubMed] [Google Scholar]
- Sun C.-Y.; Zhang B.-B.; Zhou J.-Y. Light-Activated Drug Release from a Hyaluronic Acid Targeted Nanoconjugate for Cancer Therapy. J. Mater. Chem. B 2019, 7, 4843–4853. 10.1039/C9TB01115C. [DOI] [PubMed] [Google Scholar]
- Yue C.; Zhang C.; Alfranca G.; Yang Y.; Jiang X.; Yang Y.; Pan F.; Fuente J. M. d. l.; Cui D. Near-Infrared Light Triggered ROS-activated Theranostic Platform based on Ce6-CPT-UCNPs for Simultaneous Fluorescence Imaging and Chemo-Photodynamic Combined Therapy. Theranostics 2016, 6, 456–469. 10.7150/thno.14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Meng G.; Zhang S.; Liu X. A Reactive 1O2 - Responsive Combined Treatment System of Photodynamic and Chemotherapy for Cancer. Sci. Rep. 2016, 6, 29911. 10.1038/srep29911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Wei K.; Zuo S.; Xu Y.; Zha Z.; Ke W.; Chen H.; Ge Z. Light-Triggered Clustered Vesicles with Self-Supplied Oxygen and Tissue Penetrability for Photodynamic Therapy against Hypoxic Tumor. Adv. Funct. Mater. 2017, 27, 1702108. 10.1002/adfm.201702108. [DOI] [Google Scholar]
- Phua S. Z. F.; Xue C.; Lim W. Q.; Yang G.; Chen H.; Zhang Y.; Wijaya C. F.; Luo Z.; Zhao Y. Light-Responsive Prodrug-Based Supramolecular Nanosystems for Site-Specific Combination Therapy of Cancer. Chem. Mater. 2019, 31, 3349–3358. 10.1021/acs.chemmater.9b00439. [DOI] [Google Scholar]
- Li Y.; Wang S.; Huang Y.; Chen Y.; Wu W.; Liu Y.; Zhang J.; Feng Y.; Jiang X.; Gou M. Light-Activated Drug Release From Prodrug Nanoassemblies by Structure Destruction. Chem. Commun. 2019, 55, 13128–13131. 10.1039/C9CC06673J. [DOI] [PubMed] [Google Scholar]
- Li X.; Gao M.; Xin K.; Zhang L.; Ding D.; Kong D.; Wang Z.; Shi Y.; Kiessling F.; Lammers T.; Cheng J.; Zhao Y. Singlet Oxygen-Responsive Micelles for Enhanced Photodynamic Therapy. J. Controlled Release 2017, 260, 12–21. 10.1016/j.jconrel.2017.05.025. [DOI] [PubMed] [Google Scholar]
- Watanabe K.; Terao N.; Kii I.; Nakagawa R.; Niwa T.; Hosoya T. Indolizines Enabling Rapid Uncaging of Alcohols and Carboxylic Acids by Red Light-Induced Photooxidation. Org. Lett. 2020, 22, 5434–5438. 10.1021/acs.orglett.0c01799. [DOI] [PubMed] [Google Scholar]
- Nunes E. D.; Villela A. D.; Basso L. A.; Teixeira E. H.; Andrade A. L.; Vasconcelos M. A.; do Nascimento Neto L. G.; Gondim A. C. S.; Diógenes I. C. N.; Romo A. I. B.; Nascimento O. R.; Zampieri D.; Paulo T. F.; de Carvalho I. M. M.; de França Lopes L. G.; Sousa E. H. S. Light-Induced Disruption of an Acyl Hydrazone Link as a Novel Strategy for Drug Release and Activation: Isoniazid as a Proof-of-Concept Case. Inorg. Chem. Front. 2020, 7, 859–870. 10.1039/C9QI01172B. [DOI] [Google Scholar]
- Xu H.; Cao W.; Zhang X. Selenium-Containing Polymers: Promising Biomaterials for Controlled Release and Enzyme Mimics. Acc. Chem. Res. 2013, 46, 1647–1658. 10.1021/ar4000339. [DOI] [PubMed] [Google Scholar]
- Cao W.; Li Y.; Yi Y.; Ji S.; Zeng L.; Sun Z.; Xu H. Coordination-Responsive Selenium-Containing Polymer Micelles for Controlled Drug Release. Chem. Sci. 2012, 3, 3403–3408. 10.1039/c2sc21315j. [DOI] [Google Scholar]
- Deepagan V. G.; Kwon S.; You D. G.; Nguyen V. Q.; Um W.; Ko H.; Lee H.; Jo D.-G.; Kang Y. M.; Park J. H. In situ Diselenide-Crosslinked Polymeric Micelles for ROS-Mediated Anticancer Drug Delivery. Biomaterials 2016, 103, 56–66. 10.1016/j.biomaterials.2016.06.044. [DOI] [PubMed] [Google Scholar]
- Han P.; Ma N.; Ren H.; Xu H.; Li Z.; Wang Z.; Zhang X. Oxidation-Responsive Micelles Based on a Selenium-Containing Polymeric Superamphiphile. Langmuir 2010, 26, 14414–14418. 10.1021/la102837a. [DOI] [PubMed] [Google Scholar]
- Ma N.; Li Y.; Ren H.; Xu H.; Li Z.; Zhang X. Selenium-Containing Block Copolymers and Their Oxidation-Responsive Aggregates. Polym. Chem. 2010, 1, 1609–1614. 10.1039/c0py00144a. [DOI] [Google Scholar]
- Zhou W.; Wang L.; Li F.; Zhang W.; Huang W.; Huo F.; Xu H. Selenium-Containing Polymer@Metal-Organic Frameworks Nanocomposites as an Efficient Multiresponsive Drug Delivery System. Adv. Funct. Mater. 2017, 27, 1605465. 10.1002/adfm.201605465. [DOI] [Google Scholar]
- Ren H.; Wu Y.; Ma N.; Xu H.; Zhang X. Side-Chain Selenium-Containing Amphiphilic Block Copolymers: Redox-Controlled Self-Assembly and Disassembly. Soft Matter 2012, 8, 1460–1466. 10.1039/C1SM06673K. [DOI] [Google Scholar]
- Zhai S.; Hu X.; Hu Y.; Wu B.; Xing D. Visible Light-Induced Crosslinking and Physiological Stabilization of Diselenide-Rich Nanoparticles for Redox-Responsive Drug Release and Combination Chemotherapy. Biomaterials 2017, 121, 41–54. 10.1016/j.biomaterials.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Han P.; Li S.; Cao W.; Li Y.; Sun Z.; Wang Z.; Xu H. Red Light Responsive Diselenide-Containing Block Copolymer Micelles. J. Mater. Chem. B 2013, 1, 740–743. 10.1039/C2TB00186A. [DOI] [PubMed] [Google Scholar]
- Ren H.; Wu Y.; Li Y.; Cao W.; Sun Z.; Xu H.; Zhang X. Visible-Light-Induced Disruption of Diselenide-Containing Layer-by-Layer Films: Toward Combination of Chemotherapy and Photodynamic Therapy. Small 2013, 9, 3981–3986. 10.1002/smll.201300628. [DOI] [PubMed] [Google Scholar]
- Sun C.; Ji S.; Li F.; Xu H. Diselenide-Containing Hyperbranched Polymer with Light-Induced Cytotoxicity. ACS Appl. Mater. Interfaces 2017, 9, 12924–12929. 10.1021/acsami.7b02367. [DOI] [PubMed] [Google Scholar]
- Fang R.; Xu H.; Cao W.; Yang L.; Zhang X. Reactive Oxygen Species (ROS)-Responsive Tellurium-Containing Hyperbranched Polymer. Polym. Chem. 2015, 6, 2817–2821. 10.1039/C5PY00050E. [DOI] [Google Scholar]
- Cao W.; Wang L.; Xu H. Selenium/Tellurium Containing Polymer Materials in Nanobiotechnology. Nano Today 2015, 10, 717–736. 10.1016/j.nantod.2015.11.004. [DOI] [Google Scholar]
- Li F.; Li T.; Cao W.; Wang L.; Xu H. Near-Infrared Light Stimuli-Responsive Synergistic Therapy Nanoplatforms Based on the Coordination of Tellurium-Containing Block Polymer and Cisplatin for Cancer Treatment. Biomaterials 2017, 133, 208–218. 10.1016/j.biomaterials.2017.04.032. [DOI] [PubMed] [Google Scholar]
- Ratanatawanate C.; Chyao A.; Balkus K. J. S-Nitrosocysteine-Decorated PbS QDs/TiO2 Nanotubes for Enhanced Production of Singlet Oxygen. J. Am. Chem. Soc. 2011, 133, 3492–3497. 10.1021/ja109328a. [DOI] [PubMed] [Google Scholar]
- Tang J.; Robichaux M. A.; Wu K.-L.; Pei J.; Nguyen N. T.; Zhou Y.; Wensel T. G.; Xiao H. Single-Atom Fluorescence Switch: A General Approach toward Visible-Light-Activated Dyes for Biological Imaging. J. Am. Chem. Soc. 2019, 141, 14699–14706. 10.1021/jacs.9b06237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W.; Parowatkin M.; Steffen W.; Butt H.-J.; Mailänder V.; Wu S. Ruthenium-Containing Block Copolymer Assemblies: Red-Light-Responsive Metallopolymers with Tunable Nanostructures for Enhanced Cellular Uptake and Anticancer Phototherapy. Adv. Healthcare Mater. 2016, 5, 467–473. 10.1002/adhm.201500827. [DOI] [PubMed] [Google Scholar]
- Kachkovskii A. D. The Nature of Electronic Transitions in Linear Conjugated Systems. Russ. Chem. Rev. 1997, 66, 647–664. 10.1070/RC1997v066n08ABEH000274. [DOI] [Google Scholar]
- Bricks J. L.; Kachkovskii A. D.; Slominskii Y. L.; Gerasov A. O.; Popov S. V. Molecular Design of Near Infrared Polymethine Dyes: A Review. Dyes Pigm. 2015, 121, 238–255. 10.1016/j.dyepig.2015.05.016. [DOI] [Google Scholar]
- Frangioni J. V. In Vivo Near-Infrared Fluorescence Imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Luo S.; Zhang E.; Su Y.; Cheng T.; Shi C. A Review of Nir Dyes in Cancer Targeting and Imaging. Biomaterials 2011, 32, 7127–7138. 10.1016/j.biomaterials.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Shi C.; Wu J. B.; Pan D. Review on Near-Infrared Heptamethine Cyanine Dyes as Theranostic Agents for Tumor Imaging, Targeting, and Photodynamic Therapy. J. Biomed. Opt. 2016, 21, 50901. 10.1117/1.JBO.21.5.050901. [DOI] [PubMed] [Google Scholar]
- Alander J. T.; Kaartinen I.; Laakso A.; Patila T.; Spillmann T.; Tuchin V. V.; Venermo M.; Valisuo P. A Review of Indocyanine Green Fluorescent Imaging in Surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. 10.1155/2012/940585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart M. B.; Huntington C. R.; Blair L. J.; Heniford B. T.; Augenstein V. A. Indocyanine Green: Historical Context, Current Applications, and Future Considerations. Surg. Innov. 2016, 23, 166–175. 10.1177/1553350615604053. [DOI] [PubMed] [Google Scholar]
- Gorka A. P.; Yamamoto T.; Zhu J.; Schnermann M. J. Cyanine Photocages Enable Spatial Control of Inducible Cre-Mediated Recombination. ChemBioChem 2018, 19, 1239–1243. 10.1002/cbic.201800061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q.; Tong R. Anticancer Nanoparticulate Polymer-Drug Conjugate. Bioeng. Transl. Med. 2016, 1, 277–296. 10.1002/btm2.10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalik L.; Chen J. K. Illuminating Developmental Biology Through Photochemistry. Nat. Chem. Biol. 2017, 13, 587–598. 10.1038/nchembio.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka A. P.; Nani R. R.; Schnermann M. J. Cyanine Polyene Reactivity: Scope and Biomedical Applications. Org. Biomol. Chem. 2015, 13, 7584–7598. 10.1039/C5OB00788G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q.; Juette M. F.; Jockusch S.; Wasserman M. R.; Zhou Z.; Altman R. B.; Blanchard S. C. Ultra-Stable Organic Fluorophores for Single-Molecule Research. Chem. Soc. Rev. 2014, 43, 1044–1056. 10.1039/C3CS60237K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers G. W.; Gross S.; Henrichs P. M. Direct and Sensitized Photooxidation of Cyanine Dyes. Photochem. Photobiol. 1976, 23, 37–43. 10.1111/j.1751-1097.1976.tb06768.x. [DOI] [PubMed] [Google Scholar]
- Chen P.; Li J.; Qian Z.; Zheng D.; Okasaki T.; Hayami M. Study on the Photooxidation of a Near-Infrared-Absorbing Benzothiazolone Cyanine Dye. Dyes Pigm. 1998, 37, 213–222. 10.1016/S0143-7208(97)00059-4. [DOI] [Google Scholar]
- Chen X.; Peng X.; Cui A.; Wang B.; Wang L.; Zhang R. Photostabilities of Novel Heptamethine 3H-Indolenine Cyanine Dyes with Different N-Substituents. J. Photochem. Photobiol., A 2006, 181, 79–85. 10.1016/j.jphotochem.2005.11.004. [DOI] [Google Scholar]
- Engel E.; Schraml R.; Maisch T.; Kobuch K.; Koenig B.; Szeimies R. M.; Hillenkamp J.; Baumler W.; Vasold R. Light-Induced Decomposition of Indocyanine Green. Invest. Ophthalmol. Visual Sci. 2008, 49, 1777–1783. 10.1167/iovs.07-0911. [DOI] [PubMed] [Google Scholar]
- Henary M.; Mojzych M.. Stability and Reactivity of Polymethine Dyes in Solution. Heterocyclic Polymethine Dyes: Synthesis, Properties and Applications; Strekowski L., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2008. [Google Scholar]
- Lepaja S.; Strub H.; Lougnot D. J. Photophysical Study of a Series of Cyanines. 3. The Direct Photo-Oxidation Reaction. Z. Naturforsch., A: Phys. Sci. 1983, 38, 56–60. 10.1515/zna-1983-0110. [DOI] [Google Scholar]
- Renikuntla B. R.; Rose H. C.; Eldo J.; Waggoner A. S.; Armitage B. A. Improved Photostability and Fluorescence Properties Through Polyfluorination of a Cyanine Dye. Org. Lett. 2004, 6, 909–912. 10.1021/ol036081w. [DOI] [PubMed] [Google Scholar]
- Samanta A.; Vendrell M.; Das R.; Chang Y.-T. Development of Photostable Near-Infrared Cyanine Dyes. Chem. Commun. 2010, 46, 7406–7408. 10.1039/c0cc02366c. [DOI] [PubMed] [Google Scholar]
- Toutchkine A.; Kraynov V.; Hahn K. Solvent-Sensitive Dyes to Report Protein Conformational Changes in Living Cells. J. Am. Chem. Soc. 2003, 125, 4132–4145. 10.1021/ja0290882. [DOI] [PubMed] [Google Scholar]
- Toutchkine A.; Nguyen D. V.; Hahn K. M. Merocyanine Dyes with Improved Photostability. Org. Lett. 2007, 9, 2775–2777. 10.1021/ol070780h. [DOI] [PubMed] [Google Scholar]
- Patel N. J.; Manivannan E.; Joshi P.; Ohulchanskyy T. J.; Nani R. R.; Schnermann M. J.; Pandey R. K. Impact of Substituents in Tumor Uptake and Fluorescence Imaging Ability of Near-Infrared Cyanine-like Dyes. Photochem. Photobiol. 2015, 91, 1219–1230. 10.1111/php.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouassier J. P.; Lougnot D. J.; Faure J. Transient Absorptions in a Polymethine Laser Dye. Chem. Phys. Lett. 1975, 35, 189–194. 10.1016/0009-2614(75)85311-5. [DOI] [Google Scholar]
- Kuzmin V. A.; Tatikolov A. S.; Borisevich Y. E. Charge-Transfer Complexing in Course of Triplet-State Quenching of Carbo-Cyanine Dyes by Nitroxyl Radical. Chem. Phys. Lett. 1978, 53, 52–55. 10.1016/0009-2614(78)80387-X. [DOI] [Google Scholar]
- Delaey E.; van Laar F.; De Vos D.; Kamuhabwa A.; Jacobs P.; de Witte P. A Comparative Study of the Photosensitizing Characteristics of Some Cyanine Dyes. J. Photochem. Photobiol., B 2000, 55, 27–36. 10.1016/S1011-1344(00)00021-X. [DOI] [PubMed] [Google Scholar]
- Redmond R. W.; Gamlin J. N. A Compilation of Singlet Oxygen Yields From Biologically Relevant Molecules. Photochem. Photobiol. 1999, 70, 391–475. . [DOI] [PubMed] [Google Scholar]
- Franck B.; Schneider U. Photooxidation Products of Merocyanine 540 Formed Under Preactivation Conditions for Tumor Therapy. Photochem. Photobiol. 1992, 56, 271–276. 10.1111/j.1751-1097.1992.tb02157.x. [DOI] [PubMed] [Google Scholar]
- Gorka A. P.; Nani R. R.; Zhu J.; Mackem S.; Schnermann M. J. A Near-IR Uncaging Strategy Based on Cyanine Photochemistry. J. Am. Chem. Soc. 2014, 136, 14153–14159. 10.1021/ja5065203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nani R. R.; Kelley J. A.; Ivanic J.; Schnermann M. J. Reactive Species Involved in the Regioselective Photooxidation of Heptamethine Cyanines. Chem. Sci. 2015, 6, 6556–6563. 10.1039/C5SC02396C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T.; Caldwell D. R.; Gandioso A.; Schnermann M. J. A Cyanine Photooxidation/β-Elimination Sequence Enables Near-Infrared Uncaging of Aryl Amine Payloads. Photochem. Photobiol. 2019, 95, 951–958. 10.1111/php.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.; Tian H.; Xiao H.; Shang X.; Gong X.; Yao S.; Chen K. Photodegradation of Cyanine and Merocyanine Dyes. Dyes Pigm. 2001, 49, 93–101. 10.1016/S0143-7208(01)00012-2. [DOI] [Google Scholar]
- Altman R. B.; Zheng Q.; Zhou Z.; Terry D. S.; Warren J. D.; Blanchard S. C. Enhanced Photostability of Cyanine Fluorophores Across the Visible Spectrum. Nat. Methods 2012, 9, 428–429. 10.1038/nmeth.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velde J. H. M.; Oelerich J.; Huang J.; Smit J. H.; Hiermaier M.; Ploetz E.; Herrmann A.; Roelfes G.; Cordes T. The Power of Two: Covalent Coupling of Photostabilizers for Fluorescence Applications. J. Phys. Chem. Lett. 2014, 5, 3792–3798. 10.1021/jz501874f. [DOI] [PubMed] [Google Scholar]
- van der Velde J. H. M.; Ploetz E.; Hiermaier M.; Oelerich J.; de Vries J. W.; Roelfes G.; Cordes T. Mechanism of Intramolecular Photostabilization in Self-Healing Cyanine Fluorophores. ChemPhysChem 2013, 14, 4084–4093. 10.1002/cphc.201300785. [DOI] [PubMed] [Google Scholar]
- Widengren J.; Chmyrov A.; Eggeling C.; Löfdahl P.-Å.; Seidel C. A. M. Strategies to Improve Photostabilities in Ultrasensitive Fluorescence Spectroscopy. J. Phys. Chem. A 2007, 111, 429–440. 10.1021/jp0646325. [DOI] [PubMed] [Google Scholar]
- Zheng Q.; Jockusch S.; Zhou Z.; Blanchard S. C. The Contribution of Reactive Oxygen Species to the Photobleaching of Organic Fluorophores. Photochem. Photobiol. 2014, 90, 448–454. 10.1111/php.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.; Zhu W. Stability Enhancement of Fluorophores for Lighting Up Practical Application in Bioimaging. Chem. Soc. Rev. 2015, 44, 4179–4184. 10.1039/C4CS00152D. [DOI] [PubMed] [Google Scholar]
- Oushiki D.; Kojima H.; Terai T.; Arita M.; Hanaoka K.; Urano Y.; Nagano T. Development and Application of a Near-Infrared Fluorescence Probe for Oxidative Stress Based on Differential Reactivity of Linked Cyanine Dyes. J. Am. Chem. Soc. 2010, 132, 2795–2801. 10.1021/ja910090v. [DOI] [PubMed] [Google Scholar]
- Sun M.; Yu H.; Zhu H.; Ma F.; Zhang S.; Huang D.; Wang S. Oxidative Cleavage-Based Near-Infrared Fluorescent Probe for Hypochlorous Acid Detection and Myeloperoxidase Activity Evaluation. Anal. Chem. 2014, 86, 671–677. 10.1021/ac403603r. [DOI] [PubMed] [Google Scholar]
- Nani R. R.; Gorka A. P.; Nagaya T.; Yamamoto T.; Ivanic J.; Kobayashi H.; Schnermann M. J. In Vivo Activation of Duocarmycin-Antibody Conjugates by Near-Infrared Light. ACS Cent. Sci. 2017, 3, 329–337. 10.1021/acscentsci.7b00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nani R. R.; Gorka A. P.; Nagaya T.; Kobayashi H.; Schnermann M. J. Near-IR Light-Mediated Cleavage of Antibody-Drug Conjugates Using Cyanine Photocages. Angew. Chem., Int. Ed. 2015, 54, 13635–13638. 10.1002/anie.201507391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds G. A.; Drexhage K. H. Stable Heptamethine Pyrylium Dyes That Absorb in the Infrared. J. Org. Chem. 1977, 42, 885–888. 10.1021/jo00425a027. [DOI] [Google Scholar]
- Slominskii Y. L.; Radchenko I.; Tolmachev A. Polymethine Dyes with Hydrocarbon Bridges. Effect of Substituents in the Chromophore on the Color of Tricarbocyanines. Zhurnal. Organicheskoi Khimii 1979, 10, 400–407. [Google Scholar]
- Strekowski L.; Lipowska M.; Patonay G. Substitution-Reactions of a Nucleofugal Group in Heptamethine Cyanine Dyes - Synthesis of an Isothiocyanato Derivative for Labeling of Proteins with a Near-Infrared Chromophore. J. Org. Chem. 1992, 57, 4578–4580. 10.1021/jo00043a009. [DOI] [Google Scholar]
- Lim S. Y.; Hong K. H.; Kim D. I.; Kwon H.; Kim H. J. Tunable Heptamethine-Azo Dye Conjugate as an Nir Fluorescent Probe for the Selective Detection of Mitochondrial Glutathione Over Cysteine and Homocysteine. J. Am. Chem. Soc. 2014, 136, 7018–7025. 10.1021/ja500962u. [DOI] [PubMed] [Google Scholar]
- Zaheer A.; Wheat T. E.; Frangioni J. V. IRDye78 Conjugates for Near-Infrared Fluorescence Imaging. Mol. Imaging 2002, 1, 354–364. 10.1162/153535002321093963. [DOI] [PubMed] [Google Scholar]
- Nani R. R.; Shaum J. B.; Gorka A. P.; Schnermann M. J. Electrophile-Integrating Smiles Rearrangement Provides Previously Inaccessible C4′-O-Alkyl Heptamethine Cyanine fluorophores. Org. Lett. 2015, 17, 302–305. 10.1021/ol503398f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari W. S.; Schwering J. E.; Lyle P. A.; Smith S. J.; Engelhardt E. L. Cyclization-Activated Prodrugs - Basic Carbamates of 4-Hydroxyanisole. J. Med. Chem. 1990, 33, 97–101. 10.1021/jm00163a016. [DOI] [PubMed] [Google Scholar]
- Dal Corso A.; Borlandelli V.; Corno C.; Perego P.; Belvisi L.; Pignataro L.; Gennari C. Fast Cyclization of a Proline-Derived Self-Immolative Spacer Improves the Efficacy of Carbamate Prodrugs. Angew. Chem., Int. Ed. 2020, 59, 4176–4181. 10.1002/anie.201916394. [DOI] [PubMed] [Google Scholar]
- Guo Z.; Ma Y.; Liu Y.; Yan C.; Shi P.; Tian H.; Zhu W.-H. Photocaged Prodrug Under NIR Light-Triggering with Dual-Channel Fluorescence: In Vivo Real-Time Tracking for Precise Drug Delivery. Sci. China: Chem. 2018, 61, 1293–1300. 10.1007/s11426-018-9240-6. [DOI] [Google Scholar]
- Mujumdar R. B.; Ernst L. A.; Mujumdar S. R.; Lewis C. J.; Waggoner A. S. Cyanine Dye Labeling Reagents - Sulfoindocyanine Succinimidyl Esters. Bioconjugate Chem. 1993, 4, 105–111. 10.1021/bc00020a001. [DOI] [PubMed] [Google Scholar]
- Levitz A.; Marmarchi F.; Henary M. Introduction of Various Substitutions to the Methine Bridge of Heptamethine Cyanine Dyes via Substituted Dianil Linkers. Photochem. Photobiol. Sci. 2018, 17, 1409–1416. 10.1039/C8PP00218E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojzych M.; Henary M.. Synthesis of Cyanine Dyes. Heterocyclic Polymethine Dyes: Synthesis, Properties and Applications; Strekowski L., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2008. [Google Scholar]
- Štacková L.; Štacko P.; Klán P. Approach to a Substituted Heptamethine Cyanine Chain by the Ring Opening of Zincke Salts. J. Am. Chem. Soc. 2019, 141, 7155–7162. 10.1021/jacs.9b02537. [DOI] [PubMed] [Google Scholar]
- Hilderbrand S. A.; Kelly K. A.; Weissleder R.; Tung C.-H. Monofunctional Near-Infrared Fluorochromes for Imaging Applications. Bioconjugate Chem. 2005, 16, 1275–1281. 10.1021/bc0501799. [DOI] [PubMed] [Google Scholar]
- Lee H.; Mason J. C.; Achilefu S. Heptamethine Cyanine Dyes with a Robust C-C Bond at the Central Position of the Chromophore. J. Org. Chem. 2006, 71, 7862–7865. 10.1021/jo061284u. [DOI] [PubMed] [Google Scholar]
- Mahajan S. S.; Deu E.; Lauterwasser E. M.; Leyva M. J.; Ellman J. A.; Bogyo M.; Renslo A. R. A Fragmenting Hybrid Approach for Targeted Delivery of Multiple Therapeutic Agents to the Malaria Parasite. ChemMedChem 2011, 6, 415–419. 10.1002/cmdc.201100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi D. V.; Schneider E. L.; Reid R.; Robinson L.; Ashley G. W. Predictable and Tunable Half-Life Extension of Therapeutic Agents by Controlled Chemical Release From Macromolecular Conjugates. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 6211–6216. 10.1073/pnas.1117147109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler B.; Morgan C. W.; Fontaine S. D.; Vander Wal M. N.; Chang C. J.; Wells J. A.; Renslo A. R. A Reactivity-Based Probe of the Intracellular Labile Ferrous Iron Pool. Nat. Chem. Biol. 2016, 12, 680. 10.1038/nchembio.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu J.; Xu M.; Parvez S.; Peterson R. T.; Franzini R. M. Bioorthogonal Removal of 3-Isocyanopropyl Groups Enables the Controlled Release of Fluorophores and Drugs in Vivo. J. Am. Chem. Soc. 2018, 140, 8410–8414. 10.1021/jacs.8b05093. [DOI] [PubMed] [Google Scholar]
- Luciano M. P.; Nourian S.; Gorka A. P.; Nani R. R.; Nagaya T.; Kobayashi H.; Schnermann M. J.. A Near-Infrared Light-Mediated Cleavable Linker Strategy Using the Heptamethine Cyanine Chromophore. Methods Enzymol.; Academic Press, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. L.; Morrison D. L. Kinetics and Mechanism of Decarboxylation of N-Arylcarbamates - Evidence for Kinetically Important Zwitterionic Carbamic Acid Species of Short Lifetime. J. Am. Chem. Soc. 1972, 94, 1323–1334. 10.1021/ja00759a045. [DOI] [PubMed] [Google Scholar]
- Conceição S. D.; Ferreira P. D.; Ferreira F. V. L. Photochemistry and Cytotoxicity Evaluation of Heptamethinecyanine Near Infrared (NIR) Dyes. Int. J. Mol. Sci. 2013, 14, 18557–18571. 10.3390/ijms140918557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M.-C.; Freire V.; Asumendi A.; Araiz J.; Herrera I.; Castiella G.; Corcóstegui I.; Corcóstegui G. Comparative Effects of Six Intraocular Vital Dyes on Retinal Pigment Epithelial Cells. Invest. Ophthalmol. Visual Sci. 2010, 51, 6018–6029. 10.1167/iovs.09-4916. [DOI] [PubMed] [Google Scholar]
- Yang X.; Shi C.; Tong R.; Qian W.; Zhau H. E.; Wang R.; Zhu G.; Cheng J.; Yang V. W.; Cheng T.; Henary M.; Strekowski L.; Chung L. W. K. Near IR Heptamethine Cyanine Dye–Mediated Cancer Imaging. Clin. Cancer Res. 2010, 16, 2833. 10.1158/1078-0432.CCR-10-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.; Goetsch L.; Dumontet C.; Corvaïa N. Strategies and Challenges for the Next Generation of Antibody–Drug Conjugates. Nat. Rev. Drug Discovery 2017, 16, 315. 10.1038/nrd.2016.268. [DOI] [PubMed] [Google Scholar]
- Birrer M. J.; Moore K. N.; Betella I.; Bates R. C. Antibody-Drug Conjugate-Based Therapeutics: State of the Science. J. Natl. Cancer Inst. 2019, 111, 538–549. 10.1093/jnci/djz035. [DOI] [PubMed] [Google Scholar]
- Nagaya T.; Gorka A. P.; Nani R. R.; Okuyama S.; Ogata F.; Maruoka Y.; Choyke P. L.; Schnermann M. J.; Kobayashi H. Molecularly Targeted Cancer Combination Therapy with Near-Infrared Photoimmunotherapy and Near-Infrared Photorelease with Duocarmycin-Antibody Conjugate. Mol. Cancer Ther. 2018, 17, 661–670. 10.1158/1535-7163.MCT-17-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G.; Wang Y.. Nanostructures and Nanomaterials, 2nd ed.; World Scientific Publishing Company, 2010. [Google Scholar]
- Doane T. L.; Burda C. The Unique Role of Nanoparticles in Nanomedicine: Imaging, Drug Delivery and Therapy. Chem. Soc. Rev. 2012, 41, 2885–2911. 10.1039/c2cs15260f. [DOI] [PubMed] [Google Scholar]
- Li Y.; Zhang Y.; Wang W. Phototriggered Targeting of Nanocarriers for Drug Delivery. Nano Res. 2018, 11, 5424–5438. 10.1007/s12274-018-2132-7. [DOI] [Google Scholar]
- Karimi M.; Zangabad P. S.; Baghaee-Ravari S.; Ghazadeh M.; Mirshekari H.; Hamblin M. R. Smart Nanostructures for Cargo Delivery: Uncaging and Activating by Light. J. Am. Chem. Soc. 2017, 139, 4584–4610. 10.1021/jacs.6b08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai W.-F.; Rogach A. L.; Wong W.-T. Molecular Design of Upconversion Nanoparticles for Gene Delivery. Chem. Sci. 2017, 8, 7339–7358. 10.1039/C7SC02956J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Q.; Lim Y.; Li D.; Pu K.; Liang L.; Duan H. Photoactive Nanocarriers for Controlled Delivery. Adv. Funct. Mater. 2020, 30, 1903896. 10.1002/adfm.201903896. [DOI] [Google Scholar]
- Alvarez-Lorenzo C.; Bromberg L.; Concheiro A. Light-Sensitive Intelligent Drug Delivery Systems. Photochem. Photobiol. 2009, 85, 848–860. 10.1111/j.1751-1097.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- Bansal A.; Zhang Y. Photocontrolled Nanoparticle Delivery Systems for Biomedical Applications. Acc. Chem. Res. 2014, 47, 3052–3060. 10.1021/ar500217w. [DOI] [PubMed] [Google Scholar]
- Hou Y.; Zhou Z.; Huang K.; Yang H.; Han G. Long Wavelength Light Activated Prodrug Conjugates for Biomedical Applications. ChemPhotoChem. 2018, 2, 1005–1011. 10.1002/cptc.201800147. [DOI] [Google Scholar]
- De Jong W. H.; Borm P. J. A. Drug Delivery and Nanoparticles: Applications and Hazards. Int. J. Nanomed. 2008, 3, 133–149. 10.2147/IJN.S596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W.; Zhao Y.; Wang Q.; Liu T.; Sun J.; Zhang R. Remote Light-Responsive Nanocarriers for Controlled Drug Delivery: Advances and Perspectives. Small 2019, 15, 1903060. 10.1002/smll.201903060. [DOI] [PubMed] [Google Scholar]
- Singh R.; Lillard J. W. Nanoparticle-Based Targeted Drug Delivery. Exp. Mol. Pathol. 2009, 86, 215–223. 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhi M.; Mottaghitalab F.; Saeb M. R.; Thomas S. Functionalized Theranostic Nanocarriers With Bio-Inspired Polydopamine for Tumor Imaging and Chemo-Photothermal Therapy. J. Controlled Release 2019, 309, 203–219. 10.1016/j.jconrel.2019.07.036. [DOI] [PubMed] [Google Scholar]
- He L.; Liu Y.; Lau J.; Fan W.; Li Q.; Zhang C.; Huang P.; Chen X. Recent Progress in Nanoscale Metal-Organic Frameworks for Drug Release and Cancer Therapy. Nanomedicine 2019, 14, 1343–1365. 10.2217/nnm-2018-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F.; Xiong W.; Sun S.; Zhang P.; Zhu Jun J. Recent Advances in Drug Release Monitoring. Nanophotonics 2019, 8, 391–413. 10.1515/nanoph-2018-0219. [DOI] [Google Scholar]
- Denkova A. G.; de Kruijff R. M.; Serra-Crespo P. Nanocarrier-Mediated Photochemotherapy and Photoradiotherapy. Adv. Healthcare Mater. 2018, 7, 1701211. 10.1002/adhm.201701211. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Shan Y.; Cong H.; Shen Y.; Yu B. Advanced Carbon-Based Nanoplatforms Combining Drug Delivery and Thermal Therapy for Cancer Treatment. Curr. Pharm. Des. 2019, 24, 4060–4076. 10.2174/1381612825666181120160959. [DOI] [PubMed] [Google Scholar]
- Sagar V.; Nair M. Near-Infrared Biophotonics-Based Nanodrug Release Systems and Their Potential Application for Neuro-Disorders. Expert Opin. Drug Delivery 2018, 15, 137–152. 10.1080/17425247.2017.1297794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaid G.; Broekgaarden M.; Bulin A.-L.; Huang H.-C.; Kuriakose J.; Liu J.; Hasan T. Photonanomedicine: A Convergence of Photodynamic Therapy and Nanotechnology. Nanoscale 2016, 8, 12471–12503. 10.1039/C5NR08691D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum A. P.; Kammeyer J. K.; Rush A. M.; Callmann C. E.; Hahn M. E.; Gianneschi N. C. Stimuli-Responsive Nanomaterials for Biomedical Applications. J. Am. Chem. Soc. 2015, 137, 2140–2154. 10.1021/ja510147n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahoui N.; Jiang B.; Taloub N.; Huang Y. D. Spatio-Temporal Control Strategy of Drug Delivery Systems Based Nano Structures. J. Controlled Release 2017, 255, 176–201. 10.1016/j.jconrel.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Zhan C.; Kohane D. S. Phototriggered Drug Delivery Using Inorganic Nanomaterials. Bioconjugate Chem. 2017, 28, 98–104. 10.1021/acs.bioconjchem.6b00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.; Scheiger J. M.; Levkin P. A. Design and Applications of Photoresponsive Hydrogels. Adv. Mater. 2019, 31, 1807333. 10.1002/adma.201807333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C. D.; Tong L.; Feng J.; Fu J. J. Recent Advances in Stimuli-Responsive Release Function Drug Delivery Systems for Tumor Treatment. Molecules 2016, 21, 1715. 10.3390/molecules21121715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A.; Hayat U.; Rasheed T.; Bilal M.; Iqbal H. M. N. Smart” Materials-Based Near-Infrared Light-Responsive Drug Delivery Systems for Cancer Treatment: A Review. J. Mater. Res. Technol. 2019, 8, 1497–1509. 10.1016/j.jmrt.2018.03.007. [DOI] [Google Scholar]
- Yu Z.; Chan W. K.; Tan T. T. Y. Neodymium-Sensitized Nanoconstructs for Near-Infrared Enabled Photomedicine. Small 2020, 16, 1905265. 10.1002/smll.201905265. [DOI] [PubMed] [Google Scholar]
- Mohamed M. S.; Veeranarayanan S.; Maekawa T.; Kumar D. S. External Stimulus Responsive Inorganic Nanomaterials for Cancer Theranostics. Adv. Drug Delivery Rev. 2019, 138, 18–40. 10.1016/j.addr.2018.10.007. [DOI] [PubMed] [Google Scholar]
- Guo M.; Song H.; Li K.; Ma M.; Liu Y.; Fu Q.; He Z. A New Approach to Developing Diagnostics and Therapeutics: Aggregation-Induced Emission-Based Fluorescence Turn-on. Med. Res. Rev. 2020, 40, 27–53. 10.1002/med.21595. [DOI] [PubMed] [Google Scholar]
- Swaminathan S.; Garcia-Amoros J.; Fraix A.; Kandoth N.; Sortino S.; Raymo F. M. Photoresponsive Polymer Nanocarriers With Multifunctional Cargo. Chem. Soc. Rev. 2014, 43, 4167–4178. 10.1039/C3CS60324E. [DOI] [PubMed] [Google Scholar]
- Tan P.; Jiang Y.; Liu X.-Q.; Sun L.-B. Making Porous Materials Respond to Visible Light. ACS Energy Lett. 2019, 4, 2656–2667. 10.1021/acsenergylett.9b01970. [DOI] [Google Scholar]
- Wells C. M.; Harris M.; Choi L.; Murali V. P.; Guerra F. D.; Jennings J. A. Stimuli-Responsive Drug Release from Smart Polymers. J. Funct. Biomater. 2019, 10, 34. 10.3390/jfb10030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehtani D.; Seth A.; Sharma P.; Maheshwari N.; Kapoor D.; Shrivastava S. K.; Tekade R. K.. Biomaterials for Sustained and Controlled Delivery of Small Drug Molecules. Biomaterials and Bionanotechnology; Tekade R. K., Ed.; Academic Press, 2019. [Google Scholar]
- Li J.; Pu K. Semiconducting Polymer Nanomaterials as Near-Infrared Photoactivatable Protherapeutics for Cancer. Acc. Chem. Res. 2020, 53, 752–762. 10.1021/acs.accounts.9b00569. [DOI] [PubMed] [Google Scholar]
- Panwar N.; Soehartono A. M.; Chan K. K.; Zeng S.; Xu G.; Qu J.; Coquet P.; Yong K.-T.; Chen X. Nanocarbons for Biology and Medicine: Sensing, Imaging, and Drug Delivery. Chem. Rev. 2019, 119, 9559–9656. 10.1021/acs.chemrev.9b00099. [DOI] [PubMed] [Google Scholar]
- Tartakovskii A.Quantum Dots: Optics, Electron Transport and Future Applications; Cambridge University Press: Cambridge, 2012. [Google Scholar]
- Wang Y.; Hu A. Carbon Quantum Dots: Synthesis, Properties and Applications. J. Mater. Chem. C 2014, 2, 6921–6939. 10.1039/C4TC00988F. [DOI] [Google Scholar]
- Chinnathambi S.; Chen S.; Ganesan S.; Hanagata N. Silicon Quantum Dots for Biological Applications. Adv. Healthcare Mater. 2014, 3, 10–29. 10.1002/adhm.201300157. [DOI] [PubMed] [Google Scholar]
- Sansalone L.; Tang S.; Zhang Y.; Thapaliya E. R.; Raymo F. M.; Garcia-Amorós J. Semiconductor Quantum Dots with Photoresponsive Ligands. Top. Curr. Chem. 2016, 374, 73. 10.1007/s41061-016-0073-8. [DOI] [PubMed] [Google Scholar]
- Michalet X.; Pinaud F. F.; Bentolila L. A.; Tsay J. M.; Doose S.; Li J. J.; Sundaresan G.; Wu A. M.; Gambhir S. S.; Weiss S. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks P. T.; Ford P. C. Quantum Dot Photosensitizers. Interactions With Transition Metal Centers. Dalton Trans. 2012, 41, 13030–13042. 10.1039/c2dt30465a. [DOI] [PubMed] [Google Scholar]
- Burks P. T.; Ostrowski A. D.; Mikhailovsky A. A.; Chan E. M.; Wagenknecht P. S.; Ford P. C. Quantum Dot Photoluminescence Quenching by Cr(III) Complexes. Photosensitized Reactions and Evidence for a FRET Mechanism. J. Am. Chem. Soc. 2012, 134, 13266–13275. 10.1021/ja300771w. [DOI] [PubMed] [Google Scholar]
- Fowley C.; McHale A. P.; McCaughan B.; Fraix A.; Sortino S.; Callan J. F. Carbon Quantum Dot–NO Photoreleaser Nanohybrids for Two-Photon Phototherapy of Hypoxic Tumorsphotochemistry of Metal Nitrosyl Complexes. Delivery of Nitric Oxide to Biological Targets. Chem. Commun. 2015, 51, 81–84. 10.1039/C4CC07827F. [DOI] [PubMed] [Google Scholar]
- Karthik S.; Saha B.; Ghosh S. K.; Pradeep Singh N. D. Photoresponsive Quinoline Tethered Fluorescent Carbon Dots for Regulated Anticancer Drug Delivery. Chem. Commun. 2013, 49, 10471–10473. 10.1039/c3cc46078a. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Lin Q.; Huang Q.; Liu H.; Bao C.; Zhang W.; Zhong X.; Zhu L. Semiconductor Quantum Dots Photosensitizing Release of Anticancer Drug. Chem. Commun. 2011, 47, 1482–1484. 10.1039/C0CC04676K. [DOI] [PubMed] [Google Scholar]
- Franco L. P.; Cicillini S. A.; Biazzotto J. C.; Schiavon M. A.; Mikhailovsky A.; Burks P.; Garcia J.; Ford P. C.; Silva R. S. d. Photoreactivity of a Quantum Dot–Ruthenium Nitrosyl Conjugate. J. Phys. Chem. A 2014, 118, 12184–12191. 10.1021/jp5111218. [DOI] [PubMed] [Google Scholar]
- Wijtmans M.; Rosenthal S. J.; Zwanenburg B.; Porter N. A. Visible Light Excitation of CdSe Nanocrystals Triggers the Release of Coumarin from Cinnamate Surface Ligands. J. Am. Chem. Soc. 2006, 128, 11720–11726. 10.1021/ja063562c. [DOI] [PubMed] [Google Scholar]
- Han G.; Mokari T.; Ajo-Franklin C.; Cohen B. E. Caged Quantum Dots. J. Am. Chem. Soc. 2008, 130, 15811–15813. 10.1021/ja804948s. [DOI] [PubMed] [Google Scholar]
- Impellizzeri S.; McCaughan B.; Callan J. F.; Raymo F. M. Photoinduced Enhancement in the Luminescence of Hydrophilic Quantum Dots Coated with Photocleavable Ligands. J. Am. Chem. Soc. 2012, 134, 2276–2283. 10.1021/ja209873g. [DOI] [PubMed] [Google Scholar]
- Miesch C.; Emrick T. Photo-Sensitive Ligands on Nanoparticles for Achieving Triggered Emulsion Inversion. J. Colloid Interface Sci. 2014, 425, 152–158. 10.1016/j.jcis.2014.03.036. [DOI] [PubMed] [Google Scholar]
- Ye C.; Zhou L.; Wang X.; Liang Z. Photon Upconversion: From Two-Photon Absorption (TPA) to Triplet–Triplet Annihilation (TTA). Phys. Chem. Chem. Phys. 2016, 18, 10818–10835. 10.1039/C5CP07296D. [DOI] [PubMed] [Google Scholar]
- Bonacina L. Nonlinear Nanomedecine: Harmonic Nanoparticles toward Targeted Diagnosis and Therapy. Mol. Pharmaceutics 2013, 10, 783–792. 10.1021/mp300523e. [DOI] [PubMed] [Google Scholar]
- Dong H.; Sun L.-D.; Yan C.-H. Basic Understanding of the Lanthanide Related Upconversion Emissions. Nanoscale 2013, 5, 5703–5714. 10.1039/c3nr34069d. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Liu Q.; Feng W.; Sun Y.; Li F. Upconversion Luminescent Materials: Advances and Applications. Chem. Rev. 2015, 115, 395–465. 10.1021/cr400478f. [DOI] [PubMed] [Google Scholar]
- Jafari M.; Rezvanpour A. Upconversion Nano-Particles From Synthesis to Cancer Treatment: A Review. Adv. Powder Technol. 2019, 30, 1731–1753. 10.1016/j.apt.2019.05.027. [DOI] [Google Scholar]
- Auzel F. Upconversion and Anti-Stokes Processes with f and d Ions in Solids. Chem. Rev. 2004, 104, 139–174. 10.1021/cr020357g. [DOI] [PubMed] [Google Scholar]
- Gulzar A.; Xu J.; Yang P.; He F.; Xu L. Upconversion Processes: Versatile Biological Applications and Biosafety. Nanoscale 2017, 9, 12248–12282. 10.1039/C7NR01836C. [DOI] [PubMed] [Google Scholar]
- Lingeshwar Reddy K.; Balaji R.; Kumar A.; Krishnan V. Lanthanide Doped Near Infrared Active Upconversion Nanophosphors: Fundamental Concepts, Synthesis Strategies, and Technological Applications. Small 2018, 14, 1801304. 10.1002/smll.201801304. [DOI] [PubMed] [Google Scholar]
- Lin M.; Gao Y.; Hornicek F.; Xu F.; Lu T. J.; Amiji M.; Duan Z. Near-Infrared Light Activated Delivery Platform for Cancer Therapy. Adv. Colloid Interface Sci. 2015, 226, 123–137. 10.1016/j.cis.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. J.; Chung M.; Shim M. S. Engineered Photo-Responsive Materials for Near-Infrared-Triggered Drug Delivery. J. Ind. Eng. Chem. 2015, 31, 15–25. 10.1016/j.jiec.2015.07.016. [DOI] [Google Scholar]
- Yang G. B.; Liu J. J.; Wu Y. F.; Feng L. Z.; Liu Z. Near-Infrared-Light Responsive Nanoscale Drug Delivery Systems for Cancer Treatment. Coord. Chem. Rev. 2016, 320, 100–117. 10.1016/j.ccr.2016.04.004. [DOI] [Google Scholar]
- Li K.; Hong E.; Wang B.; Wang Z.; Zhang L.; Hu R.; Wang B. Advances in the Application of Upconversion Nanoparticles for Detecting and Treating Cancers. Photodiagn. Photodyn. Ther. 2019, 25, 177–192. 10.1016/j.pdpdt.2018.12.007. [DOI] [PubMed] [Google Scholar]
- Bagheri A.; Arandiyan H.; Boyer C.; Lim M. Lanthanide-Doped Upconversion Nanoparticles: Emerging Intelligent Light-Activated Drug Delivery Systems. Adv. Sci. 2016, 3, 1500437. 10.1002/advs.201500437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Béjar M.; Francés-Soriano L.; Pérez-Prieto J. Upconversion Nanoparticles for Bioimaging and Regenerative Medicine. Front. Bioeng. Biotechnol. 2016, 4, 47. 10.3389/fbioe.2016.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.; Wei R.; Feng J.; Zhang H. Tailored Lanthanide-Doped Upconversion Nanoparticles and Their Promising Bioapplication Prospects. Coord. Chem. Rev. 2018, 364, 10–32. 10.1016/j.ccr.2018.03.007. [DOI] [Google Scholar]
- Fan W.; Bu W.; Shi J. On The Latest Three-Stage Development of Nanomedicines based on Upconversion Nanoparticles. Adv. Mater. 2016, 28, 3987–4011. 10.1002/adma.201505678. [DOI] [PubMed] [Google Scholar]
- Yang D.; Ma P. a.; Hou Z.; Cheng Z.; Li C.; Lin J. Current Advances in Lanthanide Ion (Ln3+)-Based Upconversion Nanomaterials for Drug Delivery. Chem. Soc. Rev. 2015, 44, 1416–1448. 10.1039/C4CS00155A. [DOI] [PubMed] [Google Scholar]
- Carling C.-J.; Nourmohammadian F.; Boyer J.-C.; Branda N. R. Remote-Control Photorelease of Caged Compounds Using Near-Infrared Light and Upconverting Nanoparticles. Angew. Chem., Int. Ed. 2010, 49, 3782–3785. 10.1002/anie.201000611. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Thang D. C.; Han Q.; Zhao X.; Xie X.; Wang Z.; Lin J.; Xing B. Near-Infrared Photocontrolled Therapeutic Release via Upconversion Nanocomposites. J. Controlled Release 2020, 324, 104–123. 10.1016/j.jconrel.2020.05.011. [DOI] [PubMed] [Google Scholar]
- Li H.; Wang X.; Huang D. X.; Chen G. Y. Recent Advances of Lanthanide-Doped Upconversion Nanoparticles for Biological Applications. Nanotechnology 2020, 31, 29. 10.1088/1361-6528/ab4f36. [DOI] [PubMed] [Google Scholar]
- Jayakumar M. K. G.; Idris N. M.; Zhang Y. Remote Activation of Biomolecules in Deep Tissues Using Near-Infrared-to-UV Upconversion Nanotransducers. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 8483–8488. 10.1073/pnas.1114551109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael Dcona M.; Yu Q.; Capobianco J. A.; Hartman M. C. T. Near Infrared Light Mediated Release of Doxorubicin Using Upconversion Nanoparticles. Chem. Commun. 2015, 51, 8477–8479. 10.1039/C5CC01795E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoryshin L. L.; Tavares A. J.; Petryayeva E.; Doughan S.; Krull U. J. Near-Infrared-Triggered Anticancer Drug Release from Upconverting Nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 13600–13606. 10.1021/am503039f. [DOI] [PubMed] [Google Scholar]
- Chen G.; Jaskula-Sztul R.; Esquibel C. R.; Lou I.; Zheng Q.; Dammalapati A.; Harrison A.; Eliceiri K. W.; Tang W.; Chen H.; Gong S. Neuroendocrine Tumor-Targeted Upconversion Nanoparticle-Based Micelles for Simultaneous NIR-Controlled Combination Chemotherapy and Photodynamic Therapy, and Fluorescence Imaging. Adv. Funct. Mater. 2017, 27, 1604671. 10.1002/adfm.201604671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Shao Q.; Deng R.; Wang C.; Teng X.; Cheng K.; Cheng Z.; Huang L.; Liu Z.; Liu X.; Xing B. In Vitro and In Vivo Uncaging and Bioluminescence Imaging by Using Photocaged Upconversion Nanoparticles. Angew. Chem., Int. Ed. 2012, 51, 3125–3129. 10.1002/anie.201107919. [DOI] [PubMed] [Google Scholar]
- Chien Y.-H.; Chou Y.-L.; Wang S.-W.; Hung S.-T.; Liau M.-C.; Chao Y.-J.; Su C.-H.; Yeh C.-S. Near-Infrared Light Photocontrolled Targeting, Bioimaging, and Chemotherapy with Caged Upconversion Nanoparticles in Vitro and in Vivo. ACS Nano 2013, 7, 8516–8528. 10.1021/nn402399m. [DOI] [PubMed] [Google Scholar]
- Li J.; Leung C. W. T.; Wong D. S. H.; Xu J.; Li R.; Zhao Y.; Yung C. Y. Y.; Zhao E.; Tang B. Z.; Bian L. Photocontrolled SiRNA Delivery and Biomarker-Triggered Luminogens of Aggregation-Induced Emission by Up-Conversion NaYF4:Yb3+Tm3+@SiO2 Nanoparticles for Inducing and Monitoring Stem-Cell Differentiation. ACS Appl. Mater. Interfaces 2019, 11, 22074–22084. 10.1021/acsami.7b00845. [DOI] [PubMed] [Google Scholar]
- Pan Y.; Yang J.; Luan X.; Liu X.; Li X.; Yang J.; Huang T.; Sun L.; Wang Y.; Lin Y.; Song Y. Near-Infrared Upconversion-Activated CRISPR-Cas9 System: A Remote-Controlled Gene Editing Platform. Sci. Adv. 2019, 5, eaav7199 10.1126/sciadv.aav7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H.; Zhang K.; Pan Q.; Lin S.; Wong D. S. H.; Li J.; Lee W. Y.-W.; Yang B.; Han F.; Li G.; Li B.; Bian L. Remote Control of Intracellular Calcium Using Upconversion Nanotransducers Regulates Stem Cell Differentiation In Vivo. Adv. Funct. Mater. 2018, 28, 1802642. 10.1002/adfm.201802642. [DOI] [Google Scholar]
- Xiang J.; Tong X.; Shi F.; Yan Q.; Yu B.; Zhao Y. Near-Infrared Light-Triggered Drug Release From UV-Responsive Diblock Copolymer-Coated Upconversion Nanoparticles With High Monodispersity. J. Mater. Chem. B 2018, 6, 3531–3540. 10.1039/C8TB00651B. [DOI] [PubMed] [Google Scholar]
- Li J.; Lee W. Y.-W.; Wu T.; Xu J.; Zhang K.; Hong Wong D. S.; Li R.; Li G.; Bian L. Near-Infrared Light-Triggered Release of Small Molecules for Controlled Differentiation and Long-Term Tracking of Stem Cells in Vivo Using Upconversion Nanoparticles. Biomaterials 2016, 110, 1–10. 10.1016/j.biomaterials.2016.09.011. [DOI] [PubMed] [Google Scholar]
- Wong P. T.; Chen D.; Tang S.; Yanik S.; Payne M.; Mukherjee J.; Coulter A.; Tang K.; Tao K.; Sun K.; Baker J. R. Jr.; Choi S. K. Modular Integration of Upconverting Nanocrystal–Dendrimer Composites for Folate Receptor-Specific NIR Imaging and Light-Triggered Drug Release. Small 2015, 11, 6078–6090. 10.1002/smll.201501575. [DOI] [PubMed] [Google Scholar]
- Liu G.; Liu N.; Zhou L.; Su Y.; Dong C.-M. NIR-Responsive Polypeptide Copolymer Upconversion Composite Nanoparticles for Triggered Drug Release and Enhanced Cytotoxicity. Polym. Chem. 2015, 6, 4030–4039. 10.1039/C5PY00479A. [DOI] [Google Scholar]
- Yuan Y.; Min Y.; Hu Q.; Xing B.; Liu B. NIR Photoregulated Chemo- and Photodynamic Cancer Therapy Based on Conjugated Polyelectrolyte–Drug Conjugate Encapsulated Upconversion Nanoparticles. Nanoscale 2014, 6, 11259–11272. 10.1039/C4NR03302G. [DOI] [PubMed] [Google Scholar]
- Liu G.; Zhou L.; Su Y.; Dong C.-M. An NIR-Responsive and Sugar-Targeted Polypeptide Composite Nanomedicine for Intracellular Cancer Therapy. Chem. Commun. 2014, 50, 12538–12541. 10.1039/C4CC05983B. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Ren K.; Zhang X.; Chao Z.; Yang Y.; Ye D.; Dai Z.; Liu Y.; Ju H. Photo-Tearable Tape Close-Wrapped Upconversion Nanocapsules for Near-Infrared Modulated Efficient Sirna Delivery and Therapy. Biomaterials 2018, 163, 55–66. 10.1016/j.biomaterials.2018.02.019. [DOI] [PubMed] [Google Scholar]
- Wong P. T.; Tang S.; Cannon J.; Chen D.; Sun R.; Lee J.; Phan J.; Tao K.; Sun K.; Chen B.; Baker J. R.; Choi S. K. Photocontrolled Release of Doxorubicin Conjugated through a Thioacetal Photocage in Folate-Targeted Nanodelivery Systems. Bioconjugate Chem. 2017, 28, 3016–3028. 10.1021/acs.bioconjchem.7b00614. [DOI] [PubMed] [Google Scholar]
- Zhao H.; Hu W.; Ma H.; Jiang R.; Tang Y.; Ji Y.; Lu X.; Hou B.; Deng W.; Huang W.; Fan Q. Photo-Induced Charge-Variable Conjugated Polyelectrolyte Brushes Encapsulating Upconversion Nanoparticles for Promoted siRNA Release and Collaborative Photodynamic Therapy under NIR Light Irradiation. Adv. Funct. Mater. 2017, 27, 1702592. 10.1002/adfm.201702592. [DOI] [Google Scholar]
- Liu J.; Bu W.; Pan L.; Shi J. NIR-Triggered Anticancer Drug Delivery by Upconverting Nanoparticles with Integrated Azobenzene-Modified Mesoporous Silica. Angew. Chem., Int. Ed. 2013, 52, 4375–4379. 10.1002/anie.201300183. [DOI] [PubMed] [Google Scholar]
- Jayakumar M. K. G.; Bansal A.; Huang K.; Yao R.; Li B. N.; Zhang Y. Near-Infrared-Light-Based Nano-Platform Boosts Endosomal Escape and Controls Gene Knockdown in Vivo. ACS Nano 2014, 8, 4848–4858. 10.1021/nn500777n. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Shi J.; Wang Y.; Gan Y.; Wan P. Facile Preparation of Pyrenemethyl Ester-Based Nanovalve on Mesoporous Silica Coated Upconversion Nanoparticle for NIR Light-Triggered Drug Release With Potential Monitoring Capability. Colloids Surf., A 2019, 568, 436–444. 10.1016/j.colsurfa.2019.02.027. [DOI] [Google Scholar]
- Li H.; Lei W.; Wu J.; Li S.; Zhou G.; Liu D.; Yang X.; Wang S.; Li Z.; Zhang J. An Upconverting Nanotheranostic Agent Activated by Hypoxia Combined With NIR Irradiation for Selective Hypoxia Imaging and Tumour Therapy. J. Mater. Chem. B 2018, 6, 2747–2757. 10.1039/C8TB00637G. [DOI] [PubMed] [Google Scholar]
- Ruggiero E.; Habtemariam A.; Yate L.; Mareque-Rivas J. C.; Salassa L. Near Infrared Photolysis of a Ru Polypyridyl Complex by Upconverting Nanoparticles. Chem. Commun. 2014, 50, 1715–1718. 10.1039/c3cc47601d. [DOI] [PubMed] [Google Scholar]
- Dai Y.; Bi H.; Deng X.; Li C.; He F.; Ma P. a.; Yang P.; Lin J. 808 nm Near-Infrared Light Controlled Dual-Drug Release and Cancer Therapy in Vivo by Upconversion Mesoporous Silica Nanostructures. J. Mater. Chem. B 2017, 5, 2086–2095. 10.1039/C7TB00224F. [DOI] [PubMed] [Google Scholar]
- Dai Y.; Xiao H.; Liu J.; Yuan Q.; Ma P. a.; Yang D.; Li C.; Cheng Z.; Hou Z.; Yang P.; Lin J. In Vivo Multimodality Imaging and Cancer Therapy by Near-Infrared Light-Triggered trans-Platinum Pro-Drug-Conjugated Upconverison Nanoparticles. J. Am. Chem. Soc. 2013, 135, 18920–18929. 10.1021/ja410028q. [DOI] [PubMed] [Google Scholar]
- Xu Z.; Wu Z.; Sun J.; Gui R. Glutathione Capped Mn2+-Doped ZnSe Quantum Dots-Photodonors Nanocomposites for Two-Photon Excited Fluorescence-Induced Nitric Oxide Release. Mater. Chem. Phys. 2015, 162, 286–290. 10.1016/j.matchemphys.2015.05.069. [DOI] [Google Scholar]
- Chen Y.; Hao Y.; Huang Y.; Wu W.; Liu X.; Li Y.; Gou M.; Qian Z. An Injectable, Near-Infrared Light-Responsive Click Cross-Linked Azobenzene Hydrogel for Breast Cancer Chemotherapy. J. Biomed. Nanotechnol. 2019, 15, 1923–1936. 10.1166/jbn.2019.2821. [DOI] [PubMed] [Google Scholar]
- Yao C.; Wang P.; Li X.; Hu X.; Hou J.; Wang L.; Zhang F. Near-Infrared-Triggered Azobenzene-Liposome/Upconversion Nanoparticle Hybrid Vesicles for Remotely Controlled Drug Delivery to Overcome Cancer Multidrug Resistance. Adv. Mater. 2016, 28, 9341–9348. 10.1002/adma.201503799. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Jayakumar M. K. G.; Zheng X.; Shikha S.; Zhang Y.; Bansal A.; Poon D. J. J.; Chu P. L.; Yeo E. L. L.; Chua M. L. K.; Chee S. K.; Zhang Y. Upconversion Superballs for Programmable Photoactivation of Therapeutics. Nat. Commun. 2019, 10, 4586. 10.1038/s41467-019-12506-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.; Gao Y.; Cao Z.; Wu B.; Wang L.; Wang H.; Dang Z.; Wang G. Nanocomposites of Spiropyran-Functionalized Polymers and Upconversion Nanoparticles for Controlled Release Stimulated by Near-Infrared Light and pH. Macromolecules 2016, 49, 7490–7496. 10.1021/acs.macromol.6b01760. [DOI] [Google Scholar]
- Yang Z. Z.; Sun Z. R.; Ren Y.; Chen X.; Zhang W.; Zhu X. H.; Mao Z. W.; Shen J. L.; Nie S. N. Advances in Nanomaterials for Use in Photothermal and Photodynamic Therapeutics. Mol. Med. Rep. 2019, 20, 5–15. 10.3892/mmr.2019.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. W.; Yao K.; Xu Z. K. Nanomaterials With a Photothermal Effect for Antibacterial Activities: An Overview. Nanoscale 2019, 11, 8680–8691. 10.1039/C9NR01833F. [DOI] [PubMed] [Google Scholar]
- Guerrero A. R.; Hassan N.; Escobar C. A.; Albericio F.; Kogan M. J.; Araya E. Gold Nanoparticles for Photothermally Controlled Drug Release. Nanomedicine 2014, 9, 2023–2039. 10.2217/nnm.14.126. [DOI] [PubMed] [Google Scholar]
- Liao H.; Nehl C. L.; Hafner J. H. Biomedical Applications of Plasmon Resonant Metal Nanoparticles. Nanomedicine 2006, 1, 201–208. 10.2217/17435889.1.2.201. [DOI] [PubMed] [Google Scholar]
- Cobley C. M.; Au L.; Chen J. Y.; Xia Y. N. Targeting Gold Nanocages to Cancer Cells for Photothermal Destruction and Drug Delivery. Expert Opin. Drug Delivery 2010, 7, 577–587. 10.1517/17425240903571614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavuz M. S.; Cheng Y.; Chen J.; Cobley C. M.; Zhang Q.; Rycenga M.; Xie J.; Kim C.; Song K. H.; Schwartz A. G.; Wang L. V.; Xia Y. Gold Nanocages Covered by Smart Polymers for Controlled Release With Near-Infrared Light. Nat. Mater. 2009, 8, 935–939. 10.1038/nmat2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borak J. B.; López-Sola S.; Falvey D. E. Photorelease of Carboxylic Acids Mediated by Visible-Light-Absorbing Gold-Nanoparticles. Org. Lett. 2008, 10, 457–460. 10.1021/ol702813w. [DOI] [PubMed] [Google Scholar]
- Bergamini L.; Voliani V.; Cappello V.; Nifosì R.; Corni S. Non-Linear Optical Response by Functionalized Gold Nanospheres: Identifying Design Principles to Maximize the Molecular Photorelease. Nanoscale 2015, 7, 13345–13357. 10.1039/C5NR03037D. [DOI] [PubMed] [Google Scholar]
- Song J.; Pu L.; Zhou J.; Duan B.; Duan H. Biodegradable Theranostic Plasmonic Vesicles of Amphiphilic Gold Nanorods. ACS Nano 2013, 7, 9947–9960. 10.1021/nn403846v. [DOI] [PubMed] [Google Scholar]
- Voliani V.; Ricci F.; Signore G.; Nifosì R.; Luin S.; Beltram F. Multiphoton Molecular Photorelease in Click-Chemistry-Functionalized Gold Nanoparticles. Small 2011, 7, 3271–3275. 10.1002/smll.201101753. [DOI] [PubMed] [Google Scholar]
- Agarwal A.; Mackey M. A.; El-Sayed M. A.; Bellamkonda R. V. Remote Triggered Release of Doxorubicin in Tumors by Synergistic Application of Thermosensitive Liposomes and Gold Nanorods. ACS Nano 2011, 5, 4919–4926. 10.1021/nn201010q. [DOI] [PubMed] [Google Scholar]
- Li M.; Yan H.; Teh C.; Korzh V.; Zhao Y. NIR-Triggered Drug Release From Switchable Rotaxane-Functionalized Silica-Covered Au Nanorods. Chem. Commun. 2014, 50, 9745–9748. 10.1039/C4CC02966F. [DOI] [PubMed] [Google Scholar]
- Luo G.; Chen W.; Jia H.; Sun Y.; Cheng H.; Zhuo R.; Zhang X. An Indicator-Guided Photo-Controlled Drug Delivery System Based on Mesoporous Silica/Gold Nanocomposites. Nano Res. 2015, 8, 1893–1905. 10.1007/s12274-014-0698-2. [DOI] [Google Scholar]
- Cai H.; Shen T.; Kirillov A. M.; Zhang Y.; Shan C.; Li X.; Liu W.; Tang Y. Self-Assembled Upconversion Nanoparticle Clusters for NIR-controlled Drug Release and Synergistic Therapy after Conjugation with Gold Nanoparticles. Inorg. Chem. 2017, 56, 5295–5304. 10.1021/acs.inorgchem.7b00380. [DOI] [PubMed] [Google Scholar]
- Zandberg W. F.; Bakhtiari A. B. S.; Erno Z.; Hsiao D.; Gates B. D.; Claydon T.; Branda N. R. Photothermal Release of Small Molecules From Gold Nanoparticles in Live Cells. Nanomedicine 2012, 8, 908–915. 10.1016/j.nano.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Basuki J. S.; Qie F.; Mulet X.; Suryadinata R.; Vashi A. V.; Peng Y. Y.; Li L.; Hao X.; Tan T.; Hughes T. C. Photo-Modulated Therapeutic Protein Release from a Hydrogel Depot Using Visible Light. Angew. Chem. 2017, 129, 986–991. 10.1002/ange.201610618. [DOI] [PubMed] [Google Scholar]
- You J.; Shao R.; Wei X.; Gupta S.; Li C. Near-Infrared Light Triggers Release of Paclitaxel from Biodegradable Microspheres: Photothermal Effect and Enhanced Antitumor Activity. Small 2010, 6, 1022–1031. 10.1002/smll.201000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju E.; Li Z.; Liu Z.; Ren J.; Qu X. Near-Infrared Light-Triggered Drug-Delivery Vehicle for Mitochondria-Targeted Chemo-Photothermal Therapy. ACS Appl. Mater. Interfaces 2014, 6, 4364–4370. 10.1021/am5000883. [DOI] [PubMed] [Google Scholar]
- Yang X.; Liu Z.; Li Z.; Pu F.; Ren J.; Qu X. Near-Infrared-Controlled, Targeted Hydrophobic Drug-Delivery System for Synergistic Cancer Therapy. Chem. - Eur. J. 2013, 19, 10388–10394. 10.1002/chem.201204624. [DOI] [PubMed] [Google Scholar]
- Han S.; Samanta A.; Xie X.; Huang L.; Peng J.; Park S. J.; Teh D. B. L.; Choi Y.; Chang Y.-T.; All A. H.; Yang Y.; Xing B.; Liu X. Gold and Hairpin DNA Functionalization of Upconversion Nanocrystals for Imaging and In Vivo Drug Delivery. Adv. Mater. 2017, 29, 1700244. 10.1002/adma.201700244. [DOI] [PubMed] [Google Scholar]
- Xiong H.; Li X.; Kang P.; Perish J.; Neuhaus F.; Ploski J. E.; Kroener S.; Ogunyankin M. O.; Shin J. E.; Zasadzinski J. A.; Wang H.; Slesinger P. A.; Zumbuehl A.; Qin Z. Near-Infrared Light Triggered-Release in Deep Brain Regions Using Ultra-photosensitive Nanovesicles. Angew. Chem., Int. Ed. 2020, 59, 8608–8615. 10.1002/anie.201915296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H.; Deng J.; Yang Y.; Li Y.; Shi J.; Zhao J.; Deng Y.; Chen X.; Yang W. Cobalt Nanowire-Based Multifunctional Platform for Targeted Chemo-Photothermal Synergistic Cancer Therapy. Colloids Surf., B 2019, 180, 401–410. 10.1016/j.colsurfb.2019.05.005. [DOI] [PubMed] [Google Scholar]
- Li N.; Wen X.; Liu J.; Wang B.; Zhan Q.; He S. Yb3+-Enhanced UCNP@SiO2 Nanocomposites for Consecutive Imaging, Photothermal-Controlled Drug Delivery and Cancer Therapy. Opt. Mater. Express 2016, 6, 1161–1171. 10.1364/OME.6.001161. [DOI] [Google Scholar]
- Zhou M.; Liu S.; Jiang Y.; Ma H.; Shi M.; Wang Q.; Zhong W.; Liao W.; Xing M. M. Q. Doxorubicin-Loaded Single Wall Nanotube Thermo-Sensitive Hydrogel for Gastric Cancer Chemo-Photothermal Therapy. Adv. Funct. Mater. 2015, 25, 4730–4739. 10.1002/adfm.201501434. [DOI] [Google Scholar]
- Liu B.; Chen C.; Wang R.; Dong S.; Li J.; Zhang G.; Cai D.; Zhai S.; Wu Z. Near-Infrared Light-Responsively Controlled-Release Herbicide Using Biochar as a Photothermal Agent. ACS Sustainable Chem. Eng. 2019, 7, 14924–14932. 10.1021/acssuschemeng.9b03123. [DOI] [Google Scholar]
- Viger M. L.; Sheng W.; Doré K.; Alhasan A. H.; Carling C.-J.; Lux J.; de Gracia Lux C.; Grossman M.; Malinow R.; Almutairi A. Near-Infrared-Induced Heating of Confined Water in Polymeric Particles for Efficient Payload Release. ACS Nano 2014, 8, 4815–4826. 10.1021/nn500702g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercole F.; Davis T. P.; Evans R. A. Photo-Responsive Systems and Biomaterials: Photochromic Polymers, Light-Triggered Self-Assembly, Surface Modification, Fluorescence Modulation and Beyond. Polym. Chem. 2010, 1, 37–54. 10.1039/B9PY00300B. [DOI] [Google Scholar]
- Gohy J.-F.; Zhao Y. Photo-Responsive Block Copolymer Micelles: Design and Behavior. Chem. Soc. Rev. 2013, 42, 7117–7129. 10.1039/c3cs35469e. [DOI] [PubMed] [Google Scholar]
- Grimm O.; Wendler F.; Schacher F. H. Micellization of Photo-Responsive Block Copolymers. Polymers 2017, 9, 396. 10.3390/polym9090396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lino M. M.; Ferreira L. Light-Triggerable Formulations for the Intracellular Controlled Release of Biomolecules. Drug Discovery Today 2018, 23, 1062–1070. 10.1016/j.drudis.2018.01.019. [DOI] [PubMed] [Google Scholar]
- Linsley C. S.; Wu B. M. Recent Advances in Light-Responsive On-Demand Drug- Delivery Systems. Ther. Delivery 2017, 8, 89–107. 10.4155/tde-2016-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X. L.; Zhou X. C.; Wu S. Red and Near-Infrared Light-Cleavable Polymers. Macromol. Rapid Commun. 2018, 39, 1800034. 10.1002/marc.201800034. [DOI] [PubMed] [Google Scholar]
- Fomina N.; Sankaranarayanan J.; Almutairi A. Photochemical Mechanisms of Light-Triggered Release From Nanocarriers. Adv. Drug Delivery Rev. 2012, 64, 1005–1020. 10.1016/j.addr.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q.; Han D.; Zhao Y. Main-Chain Photoresponsive Polymers With Controlled Location of Light-Cleavable Units: From Synthetic Strategies to Structural Engineering. Polym. Chem. 2013, 4, 5026–5037. 10.1039/c3py00804e. [DOI] [Google Scholar]
- Dai Y.; Chen X.; Zhang X. Recent Advances in Stimuli-Responsive Polymeric Micelles via Click Chemistry. Polym. Chem. 2019, 10, 34–44. 10.1039/C8PY01174E. [DOI] [Google Scholar]
- Liu G.; Liu W.; Dong C.-M. UV- and NIR-Responsive Polymeric Nanomedicines for On-Demand Drug Delivery. Polym. Chem. 2013, 4, 3431–3443. 10.1039/c3py21121e. [DOI] [Google Scholar]
- Tong R.; Kohane D. S. Shedding Light on Nanomedicine. WIREs Nanomed. Nanobi. 2012, 4, 638–662. 10.1002/wnan.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boase N. R. B. Shining a Light on Bioorthogonal Photochemistry for Polymer Science. Macromol. Rapid Commun. 2020, 41, 2000305. 10.1002/marc.202000305. [DOI] [PubMed] [Google Scholar]
- Griffin D. R.; Kasko A. M. Photoselective Delivery of Model Therapeutics from Hydrogels. ACS Macro Lett. 2012, 1, 1330–1334. 10.1021/mz300366s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhoumi A.; Liu Q.; Kohane D. S. Ultraviolet Light-Mediated Drug Delivery: Principles, Applications, and Challenges. J. Controlled Release 2015, 219, 31–42. 10.1016/j.jconrel.2015.07.018. [DOI] [PubMed] [Google Scholar]
- Zhao H.; Hou B.; Tang Y.; Hu W.; Yin C.; Ji Y.; Lu X.; Fan Q.; Huang W. o-Nitrobenzyl-Alt-(Phenylethynyl)Benzene Copolymer-Based Nanoaggregates With Highly Efficient Two-Photon-Triggered Degradable Properties via a FRET Process. Polym. Chem. 2016, 7, 3117–3125. 10.1039/C6PY00420B. [DOI] [Google Scholar]
- Peng K.; Tomatsu I.; van den Broek B.; Cui C.; Korobko A. V.; van Noort J.; Meijer A. H.; Spaink H. P.; Kros A. Dextran Based Photodegradable Hydrogels Formed via a Michael Addition. Soft Matter 2011, 7, 4881–4887. 10.1039/c1sm05291h. [DOI] [Google Scholar]
- Cao J.; Huang S.; Chen Y.; Li S.; Li X.; Deng D.; Qian Z.; Tang L.; Gu Y. Near-Infrared Light-Triggered Micelles for Fast Controlled Drug Release in Deep Tissue. Biomaterials 2013, 34, 6272–6283. 10.1016/j.biomaterials.2013.05.008. [DOI] [PubMed] [Google Scholar]
- de Gracia Lux C.; McFearin C. L.; Joshi-Barr S.; Sankaranarayanan J.; Fomina N.; Almutairi A. Single UV or Near IR Triggering Event Leads to Polymer Degradation into Small Molecules. ACS Macro Lett. 2012, 1, 922–926. 10.1021/mz3002403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.; Wu Y.; Du F.-S.; Li Z.-C. Modular Synthesis of Photodegradable Polymers With Different Sensitive Wavelengths As UV/NIR Responsive Nanocarriers. J. Polym. Sci., Part A: Polym. Chem. 2019, 57, 334–341. 10.1002/pola.29185. [DOI] [Google Scholar]
- Kehrloesser D.; Behrendt P. J.; Hampp N. Two-Photon Absorption Triggered Drug Delivery From a Polymer for Intraocular Lenses in Presence of an UV-Absorber. J. Photochem. Photobiol., A 2012, 248, 8–14. 10.1016/j.jphotochem.2012.08.012. [DOI] [Google Scholar]
- Babin J.; Pelletier M.; Lepage M.; Allard J.-F.; Morris D.; Zhao Y. A New Two-Photon-Sensitive Block Copolymer Nanocarrier. Angew. Chem., Int. Ed. 2009, 48, 3329–3332. 10.1002/anie.200900255. [DOI] [PubMed] [Google Scholar]
- Hang C.; Zou Y.; Zhong Y.; Zhong Z.; Meng F. NIR and UV-Responsive Degradable Hyaluronic Acid Nanogels for CD44-Targeted and Remotely Triggered Intracellular Doxorubicin Delivery. Colloids Surf., B 2017, 158, 547–555. 10.1016/j.colsurfb.2017.07.041. [DOI] [PubMed] [Google Scholar]
- Goodwin A. P.; Mynar J. L.; Ma Y.; Fleming G. R.; Fréchet J. M. J. Synthetic Micelle Sensitive to IR Light via a Two-Photon Process. J. Am. Chem. Soc. 2005, 127, 9952–9953. 10.1021/ja0523035. [DOI] [PubMed] [Google Scholar]
- Mynar J. L.; Goodwin A. P.; Cohen J. A.; Ma Y.; Fleming G. R.; Fréchet J. M. J. Two-Photon Degradable Supramolecular Assemblies of Linear-Dendritic Copolymers. Chem. Commun. 2007, 2081–2082. 10.1039/B701681F. [DOI] [PubMed] [Google Scholar]
- Liu G.-Y.; Chen C.-J.; Li D.-D.; Wang S.-S.; Ji J. Near-Infrared Light-Sensitive Micelles for Enhanced Intracellular Drug Delivery. J. Mater. Chem. 2012, 22, 16865–16871. 10.1039/c2jm00045h. [DOI] [Google Scholar]
- Yuan Y.; Wang Z.; Cai P.; Liu J.; Liao L.-D.; Hong M.; Chen X.; Thakor N.; Liu B. Conjugated Polymer and Drug Co-encapsulated Nanoparticles for Chemo- and Photo-Thermal Combination Therapy With Two-Photon Regulated Fast Drug Release. Nanoscale 2015, 7, 3067–3076. 10.1039/C4NR06420H. [DOI] [PubMed] [Google Scholar]
- Sun L.; Ma X.; Dong C.-M.; Zhu B.; Zhu X. NIR-Responsive and Lectin-Binding Doxorubicin-Loaded Nanomedicine from Janus-Type Dendritic PAMAM Amphiphiles. Biomacromolecules 2012, 13, 3581–3591. 10.1021/bm3010325. [DOI] [PubMed] [Google Scholar]
- Sun L.; Yang Y.; Dong C.-M.; Wei Y. Two-Photon-Sensitive and Sugar-Targeted Nanocarriers from Degradable and Dendritic Amphiphiles. Small 2011, 7, 401–406. 10.1002/smll.201001729. [DOI] [PubMed] [Google Scholar]
- Sun W.; Li S.; Häupler B.; Liu J.; Jin S.; Steffen W.; Schubert U. S.; Butt H.-J.; Liang X.-J.; Wu S. An Amphiphilic Ruthenium Polymetallodrug for Combined Photodynamic Therapy and Photochemotherapy In Vivo. Adv. Mater. 2017, 29, 1603702. 10.1002/adma.201603702. [DOI] [PubMed] [Google Scholar]
- Zhao T.; Chen L.; Li Q.; Li X. Near-Infrared Light Triggered Drug Release From Mesoporous Silica Nanoparticles. J. Mater. Chem. B 2018, 6, 7112–7121. 10.1039/C8TB01548A. [DOI] [PubMed] [Google Scholar]
- Chen F.; Hableel G.; Zhao E. R.; Jokerst J. V. Multifunctional Nanomedicine With Silica: Role of Silica in Nanoparticles for Theranostic, Imaging, and Drug Monitoring. J. Colloid Interface Sci. 2018, 521, 261–279. 10.1016/j.jcis.2018.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayir S.; Barras A.; Boukherroub R.; Szunerits S.; Raehm L.; Richeter S.; Durand J.-O. Mesoporous Silica Nanoparticles in Recent Photodynamic Therapy Applications. Photochem. Photobiol. Sci. 2018, 17, 1651–1674. 10.1039/C8PP00143J. [DOI] [PubMed] [Google Scholar]
- Croissant J. G.; Zink J. I.; Raehm L.; Durand J.-O. Two-Photon-Excited Silica and Organosilica Nanoparticles for Spatiotemporal Cancer Treatment. Adv. Healthcare Mater. 2018, 7, 1701248. 10.1002/adhm.201701248. [DOI] [PubMed] [Google Scholar]
- Hernández-Montoto A.; Gorbe M.; Llopis-Lorente A.; Terrés J. M.; Montes R.; Cao-Milán R.; Díaz de Greñu B.; Alfonso M.; Orzaez M.; Marcos M. D.; Martínez-Máñez R.; Sancenón F. A NIR Light-Triggered Drug Delivery System Using Core–Shell Gold Nanostars–Mesoporous Silica Nanoparticles Based on Multiphoton Absorption Photo-Dissociation of 2-Nitrobenzyl PEG. Chem. Commun. 2019, 55, 9039–9042. 10.1039/C9CC04260A. [DOI] [PubMed] [Google Scholar]
- Qu D. H.; Wang Q. C.; Zhang Q. W.; Ma X.; Tian H. Photoresponsive Host-Guest Functional Systems. Chem. Rev. 2015, 115, 7543–7588. 10.1021/cr5006342. [DOI] [PubMed] [Google Scholar]
- Bleger D.; Hecht S. Visible-Light-Activated Molecular Switches. Angew. Chem., Int. Ed. 2015, 54, 11338–11349. 10.1002/anie.201500628. [DOI] [PubMed] [Google Scholar]
- Wang D.; Zhao W.; Wei Q.; Zhao C.; Zheng Y. Photoswitchable Azobenzene/Cyclodextrin Host-Guest Complexes: From UV- to Visible/Near-IR-Light-Responsive Systems. ChemPhotoChem. 2018, 2, 403–415. 10.1002/cptc.201700233. [DOI] [Google Scholar]
- Yao X.; Li T.; Wang J.; Ma X.; Tian H. Recent Progress in Photoswitchable Supramolecular Self-Assembling Systems. Adv. Opt. Mater. 2016, 4, 1322–1349. 10.1002/adom.201600281. [DOI] [Google Scholar]
- Lee S.; Flood A. H. Photoresponsive Receptors for Binding and Releasing Anions. J. Phys. Org. Chem. 2013, 26, 79–86. 10.1002/poc.2973. [DOI] [Google Scholar]
- Kim T. Y.; Vasdev R. A. S.; Preston D.; Crowley J. D. Strategies for Reversible Guest Uptake and Release from Metallosupramolecular Architectures. Chem. - Eur. J. 2018, 24, 14878–14890. 10.1002/chem.201802081. [DOI] [PubMed] [Google Scholar]
- Diaz-Moscoso A.; Ballester P. Light-Responsive Molecular Containers. Chem. Commun. 2017, 53, 4635–4652. 10.1039/C7CC01568B. [DOI] [PubMed] [Google Scholar]
- Ramamurthy V. Photochemistry within a Water-Soluble Organic Capsule. Acc. Chem. Res. 2015, 48, 2904–2917. 10.1021/acs.accounts.5b00360. [DOI] [PubMed] [Google Scholar]
- Abendroth J. M.; Bushuyev O. S.; Weiss P. S.; Barrett C. J. Controlling Motion at the Nanoscale: Rise of the Molecular Machines. ACS Nano 2015, 9, 7746–7768. 10.1021/acsnano.5b03367. [DOI] [PubMed] [Google Scholar]
- Marturano V.; Cerruti P.; Giamberini M.; Tylkowski B.; Ambrogi V. Light-Responsive Polymer Micro- and Nano-Capsules. Polymers 2017, 9, 8. 10.3390/polym9010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncelsi G.; Ballester P. Photoswitchable Host-Guest Systems Incorporating Hemithioindigo and Spiropyran Units. ChemPhotoChem. 2019, 3, 304–317. 10.1002/cptc.201800249. [DOI] [Google Scholar]
- Ueno A.; Yoshimura H.; Saka R.; Osa T. Photocontrol of Binding Ability of Capped Cyclodextrin. J. Am. Chem. Soc. 1979, 101, 2779–2780. 10.1021/ja00504a070. [DOI] [Google Scholar]
- Liu M.; Yan X.; Hu M.; Chen X.; Zhang M.; Zheng B.; Hu X.; Shao S.; Huang F. Photoresponsive Host-Guest Systems Based on a New Azobenzene-Containing Crytpand. Org. Lett. 2010, 12, 2558–2561. 10.1021/ol100770j. [DOI] [PubMed] [Google Scholar]
- Ma X.; Cao J.; Wang Q.; Tian H. Photocontrolled Reversible Room Temperature Phosphorescence (RTP) Encoding β-Cyclodextrin Pseudorotaxane. Chem. Commun. 2011, 47, 3559–3561. 10.1039/c0cc05488g. [DOI] [PubMed] [Google Scholar]
- Wang D.; Wu S. Red-Light-Responsive Supramolecular Valves for Photocontrolled Drug Release from Mesoporous Nanoparticles. Langmuir 2016, 32, 632–636. 10.1021/acs.langmuir.5b04399. [DOI] [PubMed] [Google Scholar]
- Han M. X.; Michel R.; He B.; Chen Y. S.; Stalke D.; John M.; Clever G. H. Light-Triggered Guest Uptake and Release by a Photochromic Coordination Cage. Angew. Chem., Int. Ed. 2013, 52, 1319–1323. 10.1002/anie.201207373. [DOI] [PubMed] [Google Scholar]
- Guerin J.; Leaustic A.; Berthet J.; Metivier R.; Guillot R.; Delbaere S.; Nakatani K.; Yu P. Light-Controlled Release and Uptake of Zinc Ions in Solution by a Photochromic Terthiazole-Based Ligand. Chem. - Asian J. 2017, 12, 853–859. 10.1002/asia.201700028. [DOI] [PubMed] [Google Scholar]
- O’Hagan M. P.; Ramos-Soriano J.; Haldar S.; Sheikh S.; Morales J. C.; Mulholland A. J.; Galan M. C. Visible-Light Photoswitching of Ligand Binding Mode Suggests G-Quadruplex DNA as a Target for Photopharmacology. Chem. Commun. 2020, 56, 5186–5189. 10.1039/D0CC01581D. [DOI] [PubMed] [Google Scholar]
- Dozova N.; Pousse G.; Barnych B.; Mallet J.-M.; Cossy J.; Valeur B.; Plaza P. A Novel Diarylethene-Based Photoswitchable Chelator for Reversible Release and Capture of Ca2+ in Aqueous Media. J. Photochem. Photobiol., A 2018, 360, 181–187. 10.1016/j.jphotochem.2018.04.029. [DOI] [Google Scholar]
- Ma G. L.; Wen S. F.; He L.; Huang Y.; Wang Y. J.; Zhou Y. B. Optogenetic Toolkit for Precise Control of Calcium Signaling. Cell Calcium 2017, 64, 36–46. 10.1016/j.ceca.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griepenburg J. C.; Sood N.; Vargo K. B.; Williams D.; Rawson J.; Therien M. J.; Hammer D. A.; Dmochowski I. J. Caging Metal Ions with Visible Light-Responsive Nanopolymersomes. Langmuir 2015, 31, 799–807. 10.1021/la5036689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S.; Fong W.-K.; Graham B.; Boyd B. J. Photoswitchable Molecules in Long-Wavelength Light-Responsive Drug Delivery: From Molecular Design to Applications. Chem. Mater. 2018, 30, 2873–2887. 10.1021/acs.chemmater.8b00357. [DOI] [Google Scholar]
- Huang L.; Han G. Near Infrared Boron Dipyrromethene Nanoparticles for Optotheranostics. Small Methods 2018, 2, 1700370. 10.1002/smtd.201700370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basílio N.; García-Río L. Photoswitchable Vesicles. Curr. Opin. Colloid Interface Sci. 2017, 32, 29–38. 10.1016/j.cocis.2017.09.004. [DOI] [Google Scholar]
- Gautier A.; Gauron C.; Volovitch M.; Bensimon D.; Jullien L.; Vriz S. How to Control Proteins with Light in Living Systems. Nat. Chem. Biol. 2014, 10, 533–541. 10.1038/nchembio.1534. [DOI] [PubMed] [Google Scholar]
- Kataoka K.; Harada A.; Nagasaki Y. Block Copolymer Micelles for Drug Delivery: Design, Characterization and Biological Significance. Adv. Drug Delivery Rev. 2001, 47, 113–131. 10.1016/S0169-409X(00)00124-1. [DOI] [PubMed] [Google Scholar]
- Puri A. Phototriggerable Liposomes: Current Research and Future Perspectives. Pharmaceutics 2014, 6, 1–25. 10.3390/pharmaceutics6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Hu J.; Liu G.; Tian J.; Wang H.; Gong M.; Liu S. Reversibly Switching Bilayer Permeability and Release Modules of Photochromic Polymersomes Stabilized by Cooperative Noncovalent Interactions. J. Am. Chem. Soc. 2015, 137, 15262–15275. 10.1021/jacs.5b10127. [DOI] [PubMed] [Google Scholar]
- Rifaie-Graham O.; Ulrich S.; Galensowske N. F. B.; Balog S.; Chami M.; Rentsch D.; Hemmer J. R.; Read de Alaniz J.; Boesel L. F.; Bruns N. Wavelength-Selective Light-Responsive DASA-Functionalized Polymersome Nanoreactors. J. Am. Chem. Soc. 2018, 140, 8027–8036. 10.1021/jacs.8b04511. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Johns V. K.; Liao Y. Controlled Release of Fragrant Molecules with Visible Light. Chem. - Eur. J. 2014, 20, 14637–14640. 10.1002/chem.201404203. [DOI] [PubMed] [Google Scholar]
- Xing Q.; Li N.; Chen D.; Sha W.; Jiao Y.; Qi X.; Xu Q.; Lu J. Light-Responsive Amphiphilic Copolymer Coated Nanoparticles as Nanocarriers and Real-Time Monitors for Controlled Drug Release. J. Mater. Chem. B 2014, 2, 1182–1189. 10.1039/c3tb21269f. [DOI] [PubMed] [Google Scholar]
- Senthilkumar T.; Zhou L.; Gu Q.; Liu L.; Lv F.; Wang S. Conjugated Polymer Nanoparticles with Appended Photo-Responsive Units for Controlled Drug Delivery, Release, and Imaging. Angew. Chem., Int. Ed. 2018, 57, 13114–13119. 10.1002/anie.201807158. [DOI] [PubMed] [Google Scholar]
- Romero M. A.; Mateus P.; Matos B.; Acuña Á.; García-Río L.; Arteaga J. F.; Pischel U.; Basílio N. Binding of Flavylium Ions to Sulfonatocalix[4]arene and Implication in the Photorelease of Biologically Relevant Guests in Water. J. Org. Chem. 2019, 84, 10852–10859. 10.1021/acs.joc.9b01420. [DOI] [PubMed] [Google Scholar]
- Hu X.-Y.; Jia K.; Cao Y.; Li Y.; Qin S.; Zhou F.; Lin C.; Zhang D.; Wang L. Dual Photo- and pH-Responsive Supramolecular Nanocarriers Based on Water-Soluble Pillar[6]arene and Different Azobenzene Derivatives for Intracellular Anticancer Drug Delivery. Chem. - Eur. J. 2015, 21, 1208–1220. 10.1002/chem.201405095. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Shen L.; Guo D.; Yasen W.; Wu Y.; Su Y.; Chen D.; Qiu F.; Yan D.; Zhu X. A NIR-Triggered Gatekeeper of Supramolecular Conjugated Unimicelles With Two-Photon Absorption for Controlled Drug Release. Chem. Commun. 2019, 55, 6735–6738. 10.1039/C9CC02901J. [DOI] [PubMed] [Google Scholar]
- Liu Y.-C.; Le Ny A.-L. M.; Schmidt J.; Talmon Y.; Chmelka B. F.; Lee C. T. Photo-Assisted Gene Delivery Using Light-Responsive Catanionic Vesicles. Langmuir 2009, 25, 5713–5724. 10.1021/la803588d. [DOI] [PubMed] [Google Scholar]
- Pianowski Z. L.; Karcher J.; Schneider K. Photoresponsive Self-Healing Supramolecular Hydrogels for Light-Induced Release of Dna and Doxorubicin. Chem. Commun. 2016, 52, 3143–3146. 10.1039/C5CC09633B. [DOI] [PubMed] [Google Scholar]
- Nehls E. M.; Rosales A. M.; Anseth K. S. Enhanced User-Control of Small Molecule Drug Release From a Poly(Ethylene Glycol) Hydrogel via Azobenzene/Cyclodextrin Complex Tethers. J. Mater. Chem. B 2016, 4, 1035–1039. 10.1039/C5TB02004B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J.; Choi E.; Tamanoi F.; Zink J. I. Light-Activated Nanoimpeller-Controlled Drug Release in Cancer Cells. Small 2008, 4, 421–426. 10.1002/smll.200700903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knežević N. Ž.; Trewyn B. G.; Lin V. S. Y. Functionalized Mesoporous Silica Nanoparticle-Based Visible Light Responsive Controlled Release Delivery System. Chem. Commun. 2011, 47, 2817–2819. 10.1039/c0cc04424e. [DOI] [PubMed] [Google Scholar]
- Hull K.; Morstein J.; Trauner D. In Vivo Photopharmacology. Chem. Rev. 2018, 118, 10710–10747. 10.1021/acs.chemrev.8b00037. [DOI] [PubMed] [Google Scholar]
- Velema W. A.; Szymanski W.; Feringa B. L. Photopharmacology: Beyond Proof of Principle. J. Am. Chem. Soc. 2014, 136, 2178–2191. 10.1021/ja413063e. [DOI] [PubMed] [Google Scholar]
- Arrue L.; Ratjen L. Internal Targeting and External Control: Phototriggered Targeting in Nanomedicine. ChemMedChem 2017, 12, 1908–1916. 10.1002/cmdc.201700621. [DOI] [PubMed] [Google Scholar]
- Bregestovski P.; Maleeval G. Photopharmacology: Mini Review by Example of Potassium Channels Modulation by Light. Zh. Vyssh. Nerv. Deyat. 2017, 67, 41–52. [Google Scholar]
- Lubbe A. S.; Szymanski W.; Feringa B. L. Recent Developments in Reversible Photoregulation of Oligonucleotide Structure and Function. Chem. Soc. Rev. 2017, 46, 1052–1079. 10.1039/C6CS00461J. [DOI] [PubMed] [Google Scholar]
- Dong M. X.; Babalhavaeji A.; Samanta S.; Beharry A. A.; Woolley G. A. Red-Shifting Azobenzene Photoswitches for in Vivo Use. Acc. Chem. Res. 2015, 48, 2662–2670. 10.1021/acs.accounts.5b00270. [DOI] [PubMed] [Google Scholar]
- Broichhagen J.; Frank J. A.; Trauner D. A Roadmap to Success in Photopharmacology. Acc. Chem. Res. 2015, 48, 1947–1960. 10.1021/acs.accounts.5b00129. [DOI] [PubMed] [Google Scholar]
- Meisel P.; Jahrig D.; Jahrig K. What Is Photopharmacology, and What Can It Do?. Pharmazie 1980, 35, 377–388. [PubMed] [Google Scholar]
- Albert L.; Vazquez O. Photoswitchable Peptides for Spatiotemporal Control of Biological Functions. Chem. Commun. 2019, 55, 10192–10213. 10.1039/C9CC03346G. [DOI] [PubMed] [Google Scholar]
- Curcic S.; Tiapko O.; Groschner K. Photopharmacology and Opto-Chemogenetics of Trpc Channels-Some Therapeutic Visions. Pharmacol. Ther. 2019, 200, 13–26. 10.1016/j.pharmthera.2019.04.003. [DOI] [PubMed] [Google Scholar]
- Morstein J.; Trauner D. New Players in Phototherapy: Photopharmacology and Bio-Integrated Optoelectronics. Curr. Opin. Chem. Biol. 2019, 50, 145–151. 10.1016/j.cbpa.2019.03.013. [DOI] [PubMed] [Google Scholar]
- Szymański W.; Beierle J. M.; Kistemaker H. A. V.; Velema W. A.; Feringa B. L. Reversible Photocontrol of Biological Systems by the Incorporation of Molecular Photoswitches. Chem. Rev. 2013, 113, 6114–6178. 10.1021/cr300179f. [DOI] [PubMed] [Google Scholar]
- Fuchter M. J. On the Promise of Photopharmacology Using Photoswitches: A Medicinal Chemist’s Perspective. J. Med. Chem. 2020, 63, 11436–11447. 10.1021/acs.jmedchem.0c00629. [DOI] [PubMed] [Google Scholar]
- Mafy N. N.; Matsuo K.; Hiruma S.; Uehara R.; Tamaoki N. Photoswitchable CENP-E Inhibitor Enabling the Dynamic Control of Chromosome Movement and Mitotic Progression. J. Am. Chem. Soc. 2020, 142, 1763–1767. 10.1021/jacs.9b12782. [DOI] [PubMed] [Google Scholar]
- Barber D. M.; Liu S.-A.; Gottschling K.; Sumser M.; Hollmann M.; Trauner D. Optical Control of AMPA Receptors Using a Photoswitchable Quinoxaline-2,3-dione Antagonist. Chem. Sci. 2017, 8, 611–615. 10.1039/C6SC01621A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Serra M.; Gascón-Moya M.; Hirtz J. J.; Pittolo S.; Poskanzer K. E.; Ferrer È.; Alibés R.; Busqué F.; Yuste R.; Hernando J.; Gorostiza P. Two-Photon Neuronal and Astrocytic Stimulation with Azobenzene-Based Photoswitches. J. Am. Chem. Soc. 2014, 136, 8693–8701. 10.1021/ja5026326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velema W. A.; van der Berg J. P.; Hansen M. J.; Szymanski W.; Driessen A. J. M.; Feringa B. L. Optical Control of Antibacterial Activity. Nat. Chem. 2013, 5, 924–928. 10.1038/nchem.1750. [DOI] [PubMed] [Google Scholar]
- Wegener M.; Hansen M. J.; Driessen A. J. M.; Szymanski W.; Feringa B. L. Photocontrol of Antibacterial Activity: Shifting from UV to Red Light Activation. J. Am. Chem. Soc. 2017, 139, 17979–17986. 10.1021/jacs.7b09281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann D.; Studte C.; Männel B.; Hübner H.; Gmeiner P.; König B. Photochromic Dopamine Receptor Ligands Based on Dithienylethenes and Fulgides. Chem. - Eur. J. 2017, 23, 13423–13434. 10.1002/chem.201702147. [DOI] [PubMed] [Google Scholar]
- Skaar J. R.; Pagan J. K.; Pagano M. SCF Ubiquitin Ligase-Targeted Therapies. Nat. Rev. Drug Discovery 2014, 13, 889–903. 10.1038/nrd4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynders M.; Matsuura B. S.; Bérouti M.; Simoneschi D.; Marzio A.; Pagano M.; Trauner D. PHOTACs Enable Optical Control of Protein Degradation. Sci. Adv. 2020, 6, eaay5064 10.1126/sciadv.aay5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y.-H.; Lu M.-C.; Wang Y.; Shan W.-X.; Wang X.-Y.; You Q.-D.; Jiang Z.-Y. Azo-PROTAC: Novel Light-Controlled Small-Molecule Tool for Protein Knockdown. J. Med. Chem. 2020, 63, 4644–4654. 10.1021/acs.jmedchem.9b02058. [DOI] [PubMed] [Google Scholar]